Abstract
Cardiac glycogen-autophagy (‘glycophagy’) is disturbed in cardiometabolic pathologies. The physiological role of cardiac glycophagy is unclear. Exercise induces transient cardiac glycogen accumulation. Thus, this study experimentally examined glycophagy involvement during recovery from an exhaustive exercise protocol. Peak myocardial glycogen accumulation in mice was evident at 2 h post-exercise, preceded by transient activation of glycogen synthase. At 4 and 16 h post-exercise, glycogen degradation was associated with decreased STBD1 (glycophagy tagging protein) and increased GABARAPL1 (Atg8 protein), suggesting that glycophagy activity was increased. These findings provide the first evidence that glycophagy is involved in cardiac glycogen physiologic homeostasis post-exercise.
Keywords: Glycogen-autophagy, GABARAPL1, STBD1, GAA, Glycogen synthase, Glycogen phosphorylase
Highlights
-
•
A single event of exhaustive exercise in mice induces transient glycogen accumulation (‘supercompensation’) in the heart.
-
•
Increased myocardial glycogen content is linked with relieved inhibition of glycogen synthase early post-exercise.
-
•
Glycogen-autophagy (‘glycophagy’) is a glycogen degradation pathway via autophagosome capture and lysosome degradation.
-
•
Upregulation of glycophagy post-exercise is associated with glycogen degradation following glycogen supercompensation.
-
•
These findings suggest that glycophagy is important for glycogen homeostasis in the heart in response to metabolic stress.
1. Introduction
Cardiac glycogen accumulation is evident in both physiological and pathological settings (Chandramouli et al., 2015). Occurrence of cardiac failure in genetic disease states involving glycogen handling enzyme mutations indicate that glycogen plays a role in maintaining cardiac function (Chandramouli et al., 2015). As yet, an understanding of cardiac glycogen handling and regulation in physiological contexts is lacking. Cardiac glycogen content increases in response to exercise (Oliveira et al., 2019), in a manner similar to the well-established skeletal muscle concept of ‘glycogen supercompensation’. Exercise thus provides a relevant physiological setting of transient cardiac glycogen perturbation for experimentation.
The canonical pathway of glycogen handling involves synthesis via glycogen synthase and glycogen branching enzyme, and degradation via glycogen phosphorylase and glycogen debranching enzyme (Prats et al., 2018). Recently, a non-canonical pathway of glycogen degradation involving autophagy has been characterized, termed ‘glycophagy’ (Koutsifeli et al., 2022; Delbridge et al., 2017). Glycogen is encapsulated into autophagosomes by tagging and transfer involving partner proteins (starch binding domain 1 (STBD1) and the Atg8 homologue GABA type A receptor associated protein like 1 (GABARAPL1)), and then degraded in the lysosome by α-acid glucosidase (GAA) (Koutsifeli et al., 2022). We have previously demonstrated that glycophagy is responsive to insulin and glucose modulation in cardiomyocytes in vitro (Mellor et al., 2014) and is impaired in the diabetic heart in vivo (Mellor et al., 2024). In skeletal muscle, reduced GAA activity is linked with chronic exercise-induced increased glycogen content in human subjects and mice (Heden et al., 2022). Glycophagy involvement in the cardiac glycogen response to exercise has not been previously investigated.
Given that the importance of glycogen and glycophagy in the heart has been demonstrated in pathological settings (Mellor et al., 2024), and that glycophagy has been shown to have a physiological role in skeletal muscle (Heden et al., 2022), the experimental goal of this study was to investigate glycophagy involvement in exercise-induced cardiac glycogen perturbation.
2. Materials and methods
2.1. Animals
All animal experiments were conducted in the Vernon Jensen Unit at the University of Auckland and approved by the University of Auckland animal ethics committee. The care of animals was in accordance with the Good Practice Guide for the Use of Animals in Research, Testing and Teaching. Mice were maintained in a temperature-controlled environment and a 12:12 h light/dark cycle, with access to standard chow diet and water ad libitum. Male C57BL/6 mice aged 12 weeks were subjected to a 3-step treadmill ‘exhaustion’ protocol. From an initial treadmill speed of 18 cm/s, the speed was increased to 28 cm/s (over 10 min), to 42 cm/s (over 110 min) and then to 50 cm/s (over 30 min). Exhaustion status was identified when the latency of response to mild electrical stimulation (0.3 mA) delivered to mice located at the base of the treadmill exceeded 5.0 s. Following exhaustive exercise mice were euthanized by cervical dislocation at 0, 2, 4, and 16 h post-exercise (n = 8 mice/group), and heart tissues were collected (Fig. 1A). Tissues were snap-frozen in liquid N2 and ventricular samples were later homogenized in lysis buffer [100 mM Tris-HCl (pH 7.0), 5 mM EGTA, 5 mM EDTA, protease and phosphatase inhibitors (1x solution, 1 tablet/10 mL lysis buffer, #04693159001, #04906837001, Roche)] for molecular analysis.
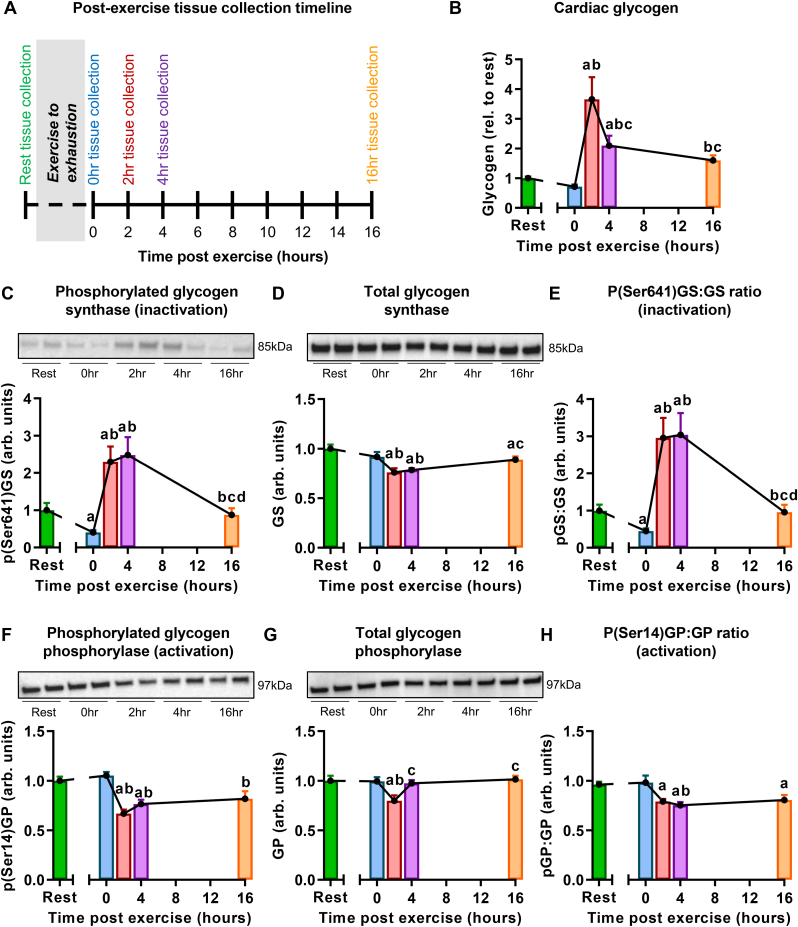
Fig. 1.
A single bout of exhaustive exercise induces transient cardiac glycogen accumulation. A. Schematic of the tissue collection timeline post-exercise. B. Cardiac glycogen normalized to protein and expressed relative to the rest group. n = 7–8 mice/group. C. Cardiac phosphorylated glycogen synthase (pGS, Ser641), normalized to coomassie stain intensity and expressed relative to the rest group. n = 7–8 mice/group. D. Cardiac total glycogen synthase (GS) protein content normalized to coomassie stain intensity and expressed relative to the rest group. n = 7–8 mice/group. E. Ratio of phosphorylated glycogen synthase to total glycogen synthase. n = 7–8 mice/group. F. Cardiac phosphorylated glycogen phosphorylase (pGP, Ser14) normalized to coomassie stain intensity and expressed relative to the rest group. n = 7–8 mice/group. G. Cardiac total glycogen phosphorylase (GP) normalized to coomassie stain intensity and expressed relative to the rest group. n = 7–8 mice/group. H. Ratio of phosphorylated glycogen phosphorylase to total glycogen phosphorylase. Data presented as mean ± SEM, ap<0.05 rel to rest, bp < 0.05 rel to 0hr, cp < 0.05 rel to 2hr and dp < 0.05 rel to 4hr.
2.2. Glycogen assay
Homogenized ventricular heart tissue was incubated in the absence or presence of amyloglucosidase. The homogenate was centrifuged at 16,000 g (2 min, 4 °C) and the supernatant was incubated with peroxidase-glucose oxidase and o-dianisdine dihydreochloride. Absorbance values, measured at 450 nm, were used to determine the amount of glucose via comparison with a standard curve. Glycogen content was determined by subtraction of glucose present in samples without amyloglucosidase incubation from those digested with amyloglucosidase.
2.3. Immunoblot
Equal amounts of protein for each sample were loaded into polyacrylamide gels. Protein was separated by gel electrophoresis and transferred onto a polyvinylidene difluoride membrane (PVDF). PVDF membranes were blocked and incubated overnight in the following primary antibodies: phosphorylated (Ser641) glycogen synthase (ab81230, Abcam), glycogen synthase (3893, Cell Signaling), phosphorylated (Ser14) glycogen phosphorylase (gift from Dr David Stapleton), glycogen phosphorylase (gift from Dr David Stapleton), STBD1 (gift from Dr David Stapleton), GAA (14367-1-AP, Protein Tech) and GABARAPL1 (26632, Cell Signaling). Membranes were incubated in anti-rabbit secondary antibody. Proteins were visualized using Amersham™ ECL Prime and imaged using a ChemiDocTM MP Imaging System. Protein expression of the proteins of interest were normalized to the total amount of protein in the sample as determined by Coomassie staining.
2.4. Statistical analysis
Statistical analyses were conducted using GraphPad Prism v8.2.1. One-way ANOVA was employed for analyzing the difference between groups, with Fisher's LSD post-hoc tests. All datasets were tested for normal distribution using Shapiro-Wilk tests, and for equal variances using Brown-Forsythe tests. If the assumptions for parametric testing were not met, data were log-transformed or non-parametric Kruskal-Wallis tests were used. Animals were euthanized at each timepoint to obtain tissues. Results are presented as mean ± standard error of the mean (SEM). Statistical significance was defined as p < 0.05.
3. Results and discussion
To establish the time-course of the cardiac glycogen response to exercise, glycogen content was measured at rest and 0, 2, 4 and 16 h after a single bout of exhaustive treadmill exercise (Fig. 1A). A trend for decreased cardiac glycogen content was evident immediately post-exercise (time 0 vs. rest, p = 0.11, Fig. 1B), followed by a dramatic 3.7-fold increase at 2 h post-exercise. Glycogen content remained significantly elevated at 4 h post-exercise, returning to near baseline by 16 h (Fig. 1B). The pattern of initial glycogen depletion, followed by glycogen accumulation and return to baseline is similar to that observed in skeletal muscle and described as ‘glycogen supercompensation’ (Jensen et al., 2021). Previous studies have reported that exercise-induced glycogen supercompensation is evident in the heart (Segel et al., 1975; Conlee et al., 1981; Segel and Mason, 1978), but the underlying mechanisms have not been fully elucidated.
To determine whether exercise-induced cardiac glycogen accumulation could be explained by changes in the canonical cytosolic glycogen handling pathway, phosphorylation status of glycogen synthase and phosphorylase was measured. Activation of glycogen synthase (decreased Ser641 phosphorylation) was evident immediately post-exercise (time 0, Fig. 1C–E), preceding the observed increase in glycogen at 2 h post-exercise. It could be speculated that activation of glycogen synthesis (via dephosphorylation of synthase Ser641) compensates for cardiac glycogen utilization during exercise, translating to delayed glycogen accumulation evident at the 2 h timepoint. At 2 and 4 h post-exercise, glycogen synthase was inactivated (hyperphosphorylated at Ser641 (Fig. 1C) with small but significant decreased glycogen synthase protein content (Fig. 1D)) and returned to rest levels by 16 h post-exercise (Fig. 1C–E). These findings are consistent with an early study reporting transient activation of glycogen synthase immediately post-exercise (Segel and Mason, 1978). Inactivation of glycogen phosphorylase was observed from 2 to 16 h post-exercise (reduced phosphorylation at Ser14, Fig. 1F–H). This result was unexpected given that glycogen degradation was evident during this time period (glycogen decreased by 56 ± 21% from peak at 2 h–16 h post-exercise). These data suggest that glycogen recovery towards basal levels is not driven by augmented glycogen phosphorylase phosphorylation and alternative routes of glycogen degradation were therefore investigated.
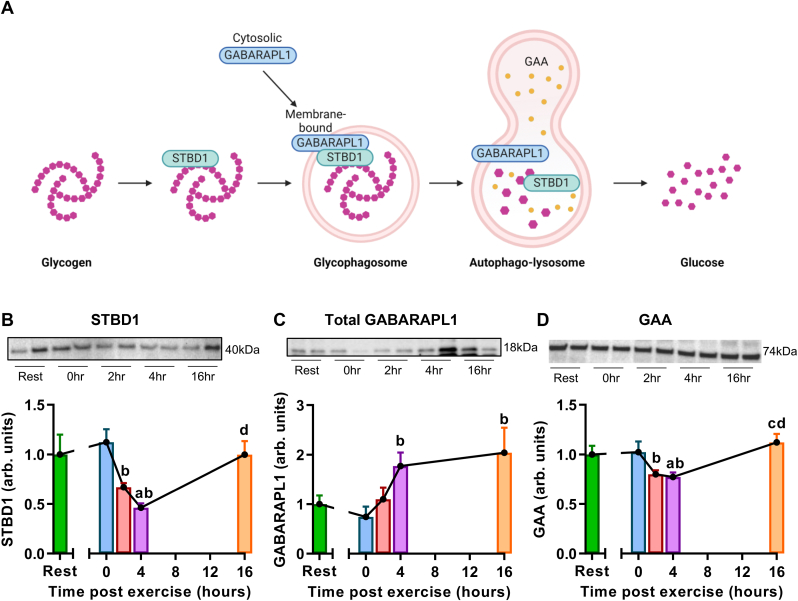
Recently, a non-canonical pathway of glycogen degradation involving autophagy has been characterized, termed ‘glycophagy’ (Koutsifeli et al., 2022). Glycogen is encapsulated into the autophagosome via starch binding domain 1 (STBD1) partnering with the Atg8 homologue GABARAPL1, and degraded in the lysosome via α-acid glucosidase (GAA) (schematic shown in Fig. 2A). We have previously visualized glycogen punctate inclusions in autophagosomes in diabetic and non-disease cardiac settings (Mellor et al., 2024). To determine whether glycophagy plays a role in the cardiac glycogen response to exercise, protein expression of glycophagy markers STBD1, GABARAPL1 and GAA was measured by immunoblot. STBD1 expression was unchanged immediately post-exercise and significantly decreased at 2 and 4 h post-exercise (33 ± 21% and 54 ± 22% decrease respectively, Fig. 2B). GABARAPL1 antibodies can be problematic due to high sequence similarity between proteins in the GABARAP subfamily of Atg8s (Le Grand et al., 2011). We confirmed the identity of the 18 kDa GABARAPL1-specific immunoblot band by inclusion of a global GABARAPL1 knockout mouse heart homogenate alongside the study samples (Supplementary Fig. S4). Total GABARAPL1 expression was increased at 4 and 16 h post-exercise relative to 0 h post-exercise (1.8- and 2-fold increase respectively, Fig. 2C). GAA expression was significantly decreased at 2 and 4 h post-exercise, returning to baseline levels at 16 h (Fig. 2D).
Fig. 2.
Glycophagy is involved in the cardiac glycogen response to exhaustive exercise. A. Schematic of the glycophagy pathway, created with Biorender. B. Cardiac starch binding domain protein 1 (STBD1) protein content, normalized to coomassie stain intensity and expressed relative to the rest group. n = 7–8 mice/group. C. Cardiac gamma-aminobutyric acid type A receptor-associated protein like 1 (GABARAPL1) protein content, normalized to coomassie stain intensity and expressed relative to the rest group. n = 7–8 mice/group. D. Cardiac acid alpha-glucosidase (GAA) protein content, normalized to coomassie stain intensity and expressed relative to the rest group. n = 8 mice/group. Data presented as mean ± SEM, ap<0.05 rel to rest, bp < 0.05 rel to 0hr, cp < 0.05 rel to 2hr and dp < 0.05 rel to 4hr.
In summary, during the glycogen degradation phase of the post-exercise period (i.e. 4-16 h) STBD1 and GAA were transiently decreased, and the Atg8 protein GABARAPL1 was increased. Atg8 proteins (such as GABARAPL1, light chain protin 3B (LC3B)) are considered to be at least partially recycled back into the cytosol after autophagosome-lysosome fusion, thus an increase in Atg8 protein content could be expected to be linked to upregulation of protein synthesis (Klionsky et al., 2021). In contrast, autophagy receptors (such as STBD1, p62) are generally considered to be degraded in the lysosome. Decreased autophagy receptor protein content may reflect either increased degradation rate (i.e. autophagy flux) or decreased protein synthesis. In the present study, the combination of decreased STBD1 with increased GABARAPL1 protein content would be consistent with increased flux through the glycophagy pathway (in a similar manner to studies reporting exercise-induced autophagy evidenced by decreased myocardial p62 with increased LC3B (Yuan et al., 2018)). Thus glycogen degradation observed during the later timepoints of the study (4 & 16 h post-exercise) may be explained by upregulation of glycophagy-mediated glycogenolysis. Interestingly, at the 16 h time point STBD1 and GAA expression have returned to basal levels, and GABARAPL1 expression remains elevated. This may suggest differences in the degradation rate of these three glycophagy proteins, or perhaps GABARAPL1 remains elevated to facilitate response to further stress. In addition to their well-documented role in autophagy processes, GABARAP family proteins also play a role in neuronal function, inflammatory response, tumor growth and metastasis (Chen et al., 2024). By extrapolation from non-cardiac studies, it is feasible that GABARAPL1 may also be involved in an exercise-induced cardiac inflammatory response in the present study, but this has not been directly investigated. Further investigation, including direct measurement of glycophagy flux in post-exercise hearts, is warranted.
Only one previous study has investigated the role of glycophagy in exercise. In skeletal muscle from chronically trained human subjects and mice, increased glycogen content was associated with lower GAA activity (Heden et al., 2022). These findings suggest that downregulation of glycophagy may be a long-term compensatory response to chronic exercise training to augment skeletal muscle glycogen content. In contrast, in the single exercise bout of the present study, transient glycophagy upregulation in cardiac muscle was observed. Understanding how adaptations relating to chronic exercise training affect the glycophagy response to a single exercise bout in both cardiac and skeletal muscle would be informative.
In the present study, male animals only were investigated. We have previously identified that fasting-induced glycogen accumulation and glycophagy regulation is accentuated in female hearts (Reichelt et al., 2013), and early studies documented that the GABARAPL1 gene contains an estrogen response element (Pellerin et al., 1993). Thus it is likely that female mice may exhibit a heightened cardiac glycophagy response to exercise, and this proposition requires investigation. Exercise-induced cardiac autophagy activation has been linked with cardioprotection from short-term cardiovascular stress (Yuan et al., 2018), but the involvement of glycophagy in cardioprotection is unknown. Given that glycophagy flux is impaired in the diabetic heart (Mellor et al., 2024), establishing whether exercise-related cardiac glycophagy induction is operational in a diabetic setting is an important next step. The potential for glycophagy to play a role in the cardiac benefit observed with exercise in diabetes is an exciting proposition.
In conclusion, this study demonstrates that a single event of exhaustive exercise induces transient glycogen accumulation (‘supercompensation’). Early in the post-exercise period, increased glycogen content is linked with relieved inhibition of glycogen synthase (decreased Ser641 phosphorylation). Following a peak in glycogen content at 2 h post-exercise, upregulation of glycophagy (and not glycogen phosphorylase) is associated with a period of glycogen degradation. These findings suggest that glycophagy is important for maintaining glycogen homeostasis in the heart, particularly in response to an acute metabolic stress. Myocardial glycophagy may represent a rapid and localized yield of glycolytic substrate following a high energy demand exercise setting.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Correspondence and requests for materials should be addressed to K.M.M (k.mellor@auckland.ac.nz).
Declaration of generative AI and AI assisted technologies in the writing process
The authors did not use generative AI or AI-assisted technologies in the development of this manuscript.
Sources of funding
This work was supported by funding from the New Zealand Marsden Fund (19-UOA-268), and the Health Research Council of New Zealand (19/190).
CRediT authorship contribution statement
Samuel L. James: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Visualization. Parisa Koutsifeli: Methodology, Validation. Randall F. D'Souza: Validation, Writing – review & editing, Supervision. Stewart WC. Masson: Investigation, Writing – review & editing. Jonathan ST. Woodhead: Investigation. Troy L. Merry: Conceptualization, Methodology, Validation, Resources, Supervision, Writing – review & editing. Lea MD. Delbridge: Conceptualization, Methodology, Validation, Resources, Supervision, Writing – review & editing. Kimberley M. Mellor: Methodology, Validation, Resources, Supervision, Writing – review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge David Stapleton for provision of glycogen phosphorylase and phosphorylated glycogen phosphorylase antibodies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crphys.2024.100131.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Chandramouli C., Varma U., Stevens E.M., Xiao R.-P., Stapleton D.I., Mellor K.M., Delbridge L.M.D. Myocardial glycogen dynamics: new perspectives on disease mechanisms. Clin. Exp. Pharmacol. Physiol. 2015;42:415–425. doi: 10.1111/1440-1681.12370. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhao H., Liu M., Chen L. A new perspective on the autophagic and non-autophagic functions of the GABARAP protein family: a potential therapeutic target for human diseases. Mol. Cell. Biochem. 2024;479:1415–1441. doi: 10.1007/s11010-023-04800-5. [DOI] [PubMed] [Google Scholar]
- Conlee R.K., Dalsky G.P., Robinson K.C. The influence of free fatty acids on glycogen recovery in rat heart after exercise. Eur. J. Appl. Physiol. 1981;47:377–383. doi: 10.1007/BF02332965. [DOI] [PubMed] [Google Scholar]
- Delbridge L.M.D., Mellor K.M., Taylor D.J., Gottlieb R.A. Myocardial stress and autophagy: mechanisms and potential therapies. Nat. Rev. Cardiol. 2017;14:412–425. doi: 10.1038/nrcardio.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heden T.D., Chow L.S., Hughey C.C., Mashek D.G. Regulation and role of glycophagy in skeletal muscle energy metabolism. Autophagy. 2022;18:1078–1089. doi: 10.1080/15548627.2021.1969633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Ørtenblad N., Stausholm M.-L.H., Skjaerbaek M.C., Larsen D.N., Hansen M., Holmberg H.-C., Plomgaard P., Nielsen J. Glycogen supercompensation is due to increased number, not size, of glycogen particles in human skeletal muscle. Exp. Physiol. 2021;106:1272–1284. doi: 10.1113/EP089317. [DOI] [PubMed] [Google Scholar]
- Klionsky D.J., Abdel-Aziz A.K., Abdelfatah S., Abdellatif M., Abdoli A., Abel S., Abeliovich H., Abildgaard M.H., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition) Autophagy. 2021;17:1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsifeli P., Varma U., Daniels L.J., Annandale M., Li X., Neale J.P.H., Hayes S., Weeks K.L., James S., Delbridge L.M.D., Mellor K.M. Glycogen-autophagy: molecular machinery and cellular mechanisms of glycophagy. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand J.N., Chakrama F.Z., Seguin-Py S., Fraichard A., Delage-Mourroux R., Jouvenot M., Risold P.-Y., Boyer-Guittaut M. GABARAPL1 antibodies: target one protein, get one free. Autophagy. 2011;7:1302–1307. doi: 10.4161/auto.7.11.16723. [DOI] [PubMed] [Google Scholar]
- Mellor K.M., Varma U., Stapleton D.I., Delbridge L.M.D. Cardiomyocyte glycophagy is regulated by insulin and exposure to high extracellular glucose. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H1240–H1245. doi: 10.1152/ajpheart.00059.2014. [DOI] [PubMed] [Google Scholar]
- Mellor K.M., Varma U., Koutsifeli P., Daniels L.J., Benson V.L., Annandale M., Li X., Nursalim Y., Janssens J.V., Weeks K.L., Powell K.L., O'Brien T.J., Katare R., Ritchie R.H., Bell J.R., Gottlieb R.A., Delbridge L.M.D. Myocardial glycophagy flux dysregulation and glycogen accumulation characterize diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 2024;189:83–89. doi: 10.1016/j.yjmcc.2024.02.009. [DOI] [PubMed] [Google Scholar]
- Oliveira L. da C., de Morais G.P., da Rocha A.L., Teixeira G.R., Pinto A.P., de Vicente L.G., Pauli J.R., de Moura L.P., Mekary R.A., Ropelle E.R., Cintra D.E., da Silva A.S.R. Excessive treadmill training enhances the insulin signaling pathway and glycogen deposition in mice hearts. J. Cell. Biochem. 2019;120:1304–1317. doi: 10.1002/jcb.27092. [DOI] [PubMed] [Google Scholar]
- Pellerin I., Vuillermoz C., Jouvenot M., Ordener C., Royez M., Adessi G.L. Identification and characterization of an early estrogen-regulated RNA in cultured Guinea-pig endometrial cells. Mol. Cell. Endocrinol. 1993;90:R17–R21. doi: 10.1016/0303-7207(93)90161-c. [DOI] [PubMed] [Google Scholar]
- Prats C., Graham T.E., Shearer J. The dynamic life of the glycogen granule. J. Biol. Chem. 2018;293:7089–7098. doi: 10.1074/jbc.R117.802843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt M.E., Mellor K.M., Curl C.L., Stapleton D., Delbridge L.M.D. Myocardial glycophagy - a specific glycogen handling response to metabolic stress is accentuated in the female heart. J. Mol. Cell. Cardiol. 2013;65:67–75. doi: 10.1016/j.yjmcc.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Segel L.D., Mason D.T. Effects of exercise and conditioning on rat heart glycogen and glycogen synthase. J. Appl. Physiol. 1978;44:183–189. doi: 10.1152/jappl.1978.44.2.183. [DOI] [PubMed] [Google Scholar]
- Segel L.D., Chung A., Mason D.T., Amsterdam E.A. Cardiac glycogen in long-evans rats: diurnal pattern and response to exercise. Am. J. Physiol. 1975;229:398–401. doi: 10.1152/ajplegacy.1975.229.2.398. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Pan S.-S., Shen Y.-J. Cardioprotection of exercise preconditioning involving heat shock protein 70 and concurrent autophagy: a potential chaperone-assisted selective macroautophagy effect. J. Physiol. Sci. JPS. 2018;68:55–67. doi: 10.1007/s12576-016-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Correspondence and requests for materials should be addressed to K.M.M (k.mellor@auckland.ac.nz).
Data will be made available on request.