Summary
Obesity and type 2 diabetes mellitus (T2DM) present major global health challenges, with an increasing prevalence worldwide. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have emerged as a pivotal treatment option for both conditions, demonstrating efficacy in blood glucose management, weight reduction, cardiovascular disease prevention, and kidney health improvement. GLP-1, an incretin hormone, plays a crucial role in glucose metabolism and appetite regulation, influencing insulin secretion, insulin sensitivity, and gastric emptying. The therapeutic use of GLP-1RAs has evolved significantly, offering various formulations that provide different efficacy, routes of administration, and flexibility in dosing. These agents reduce HbA1c levels, facilitate weight loss, and exhibit cardiovascular protective effects, making them an integral component of T2DM and obesity management. This review will discuss the currently approved medication for T2DM and obesity, and will also highlight the advent of novel agents which are dual and triple hormonal agonists which represent the future direction of incretin-based therapy.
Funding
National Institutes of HealthNIDDKU24 DK132733 (FCS), UE5 DK137285 (FCS), and P30 DK040561 (FCS).
Keywords: Obesity, Type 2 diabetes, GLP-1RA, Incretin therapy, Pharmacotherapy for obesity, Weight management, GLP1/GIP dual agonists, GLP-1/Glucagon dual agonists, GLP1/GIP/Glucagon triple agonists, Metabolism, Energy expenditure, Insulin secretion
Introduction
Obesity and type 2 diabetes (T2DM) are two of the most common noncommunicable diseases (NCDs) in the world. It has been reported that the global age-standardized prevalence of diabetes worldwide is 6.1%, estimating 521 million individuals globally living with the disease.1 Additionally, obesity has emerged as a worldwide health concern, with multiple epidemiologic studies having identified pre-obesity and obesity as predisposing factors to several NCDs, T2DM, cardiovascular disease (CVD), and cancer. Obesity is also a contributor to 120 million disability-adjusted life-years (DALYs).2 It is estimated that 1 billion people worldwide are affected by the disease, with an alarming steep increase in the prevalence of obesity since the 1970s among all age groups.3
Glucagon-like peptide 1 Receptor Agonists (GLP-1RA) have emerged as valuable therapeutic agents for managing obesity and T2DM. Since its initial approval for treating T2DM in 2005, this category of drugs has undergone considerable developments. Initially requiring twice-daily dosing, they have evolved to once-weekly, and oral formulations now exist. GLP1-RAs have demonstrated benefits in managing blood sugar levels and weight, cardiovascular disease prevention, and kidney health.4
This review aims to explore the mechanisms of action of GLP1-RAs in the treatment of T2DM and obesity. It will cover GLP1-RAs currently in clinical use and those still under investigation, detailing their main clinical benefits, potential side effects, and evaluating their cost-effectiveness. Additionally, the review will explore prospective formulations and combinations within this class of drugs. Based on the data available, we also propose a potential treatment algorithm for effectively managing obesity and T2DM with these agents.
Search strategy.
We obtained citations for this publication through searches of PubMed up to April 2024, with no lower limit set for the date, using both MeSH and free-text terms to identify relevant articles. The terms “glucagon-like peptide-1 receptor agonist (GLP-1RA)”, “Gastric inhibitory polypeptide (GIP)”, and “Glucagon” (including “exenatide”, “lixisenatide”, “albiglutide”, “dulaglutide”, “liraglutide”, “semaglutide”, “Tirzepatide”, “survodutide”, “pemvidutide”, “retatrutide”, “orforglipron” and “MariTide”) were searched for. We reviewed guidelines for the management of type 2 diabetes and obesity management published by the American Diabetes Association, American Association of Clinical Endocrinologists, Obesity Canada, European Association for the Study of Diabetes, and European Association for the Study of Obesity. We also searched ClinicalTrials.gov, EudraCT, and conference abstracts for additional eligible studies and trial information. We also reviewed relevant references cited in retrieved articles and review articles. We restricted the search to human studies and used no language restrictions.
Mechanism of action of GLP1-RAs
GLP-1 is a peptide, an incretin hormone that is continuously secreted by the enteroendocrine L cells of the small intestine; the secretion is enhanced several times after the ingestion of meals. GLP-1 has also been identified in sweet- and umami-taste receptor cells in the oral cavity, suggesting that GLP-1 signaling might also be involved in taste perception.5
GLP-1 receptors (GLP-1Rs) are present in islet beta cells and the central nervous system (CNS). In the brain, GLP-1Rs are found in several hindbrain and forebrain sites, including areas that are implicated in the control of food intake and regulation of energy balance, such as the area postrema (AP), the nucleus tractus solitarius (NTS), hypothalamus, amygdala, and in the mesolimbic reward system. The canonical GLP-1R is also expressed in the blood vessels, Brunner’s glands, and the sinoatrial node.6
Mechanism in diabetes
GLP-1 directly acts on pancreatic β-cells to enhance insulin secretion. GLP-1 also decreases hepatic glucose production by inhibiting glucagon secretion from α cells, and increases insulin sensitivity in skeletal muscle through an indirect mechanism by increasing microvascular recruitment in skeletal muscle which may potentiate local insulin action.7 Several studies have also demonstrated the integral role that the glucose portal sensor plays in glucose homeostasis, and the association of GLP-1Rs in portal glucose sensing where GLP-1 is required for portal glucose to stimulate insulin response.8
Additionally, due to the transient -short term effects -effect of GLP-1RAs on slowing gastric emptying, there is a marked decrease in post-meal glycemic excursions with their use in T2DM. While additional research is necessary to explore the potential role of GLP-1Rs expressed outside of the beta cell, including within the enteric and peripheral nervous systems, in enhancing beta cell function through enteral glucose mediation, current evidence indicates that the central nervous system (CNS) GLP-1Rs are not essential for the physiological regulation of blood sugar levels by endogenous GLP-1 or the pharmacological control of glucose homeostasis dependent on GLP-1Rs.6
In individuals with T2DM, there is a decrease in the incretin effect which primarily attributed to the diminished effects of gastric inhibitory polypeptide (GIP).9 Current research indicates that although GLP-1 secretion is preserved in individuals with T2DM, there is an impairment of GLP-1 function. This includes reduced ability to secrete insulin, heightened insulin resistance, and elevated blood sugar levels, potentially resulting in diminished expression of GLP-1 receptors and developing GLP-1 resistance.10
Finally, the effect of GLP-1RAs on weight may contribute to an increase in insulin sensitivity and better control of blood glucose.
Mechanism in obesity
GLP-1 has a central anorexigenic effect. GLP-1 role in regulating feeding behavior and energy balance has been studied in rats. These studies demonstrate that administering the GLP-1 receptor antagonist exendin (9–39) directly into the brain increases food consumption in satiated rats. Conversely, reducing the expression of the proglucagon gene, responsible for encoding GLP-1, specifically in the nucleus tractus solitarius (NTS), results in heightened appetite and weight gain in rats.11 In humans, the administration of GLP-1RAs reduces appetite, decreases energy intake, and increases satiety. Functional magnetic resonance imaging (MRI) shows that GLP-1 R activation decreases anticipatory food reward (the anticipated pleasure of eating certain meals).12
GLP-1 RA used in diabetes
Commercially available GLP-1RA for management of T2DM
Short acting agents
Exenatide (twice daily)
Exenatide administered by twice-daily subcutaneous injection was the first GLP-1 RA in clinical use. It is a synthetic peptide-based form of the incretin mimetic exendin-4. At maximal doses, it reduced HbA1c by −0.78%.13 Once daily lixisenatide, structurally similar to exenatide and administered by subcutaneous injection, was later approved and found to reduce HbA1c by 0.8–0.9%.14 Exenatide twice daily is commercially available in Europe and the US, while lixisenatide is available only outside of the US. The GetGoal-X trial examined lixisenatide and twice-daily exenatide, finding lixisenatide non-inferior to exenatide in reducing HbA1c (−0.79% vs. −0.96%, respectively) (Table 1).15 Long acting agents typically have better glycemic efficacy (as noted below). It is important to note that the use of exenatide twice daily and lixisenatide should be avoided in patients with decreased kidney function (estimated glomerular filtration rate [eGFR], 30 mL/min/1.73 m2, as these agents are excreted by the kidneys, and exposure may be increased in patients with decreased function.26
Table 1.
RCTs of GLP1-RA use in T2DM.
| Trial | Number of patients | Duration | Inclusion criteria | Change in HbA1C (%) |
|---|---|---|---|---|
| GLP-1 RA | ||||
| AMIGO13 | Total = 336 Exenatide 5 μg twice daily = 110 Exenatide 10 μg twice daily = 113 Placebo = 113 |
30 weeks | 19–78 year- old adults treated with metformin monotherapy with HbA1C 7.1–11%%, BMI 27–45 kg/m2 | Exenatide 5 μg twice daily = −0.4 Exenatide 10 μg twice daily = −0.78 Placebo = +0.1 |
| LEAD-615 | Total = 464 Liraglutide 1.8 mg daily = 233 Exenatide 10 μg twice daily = 231 |
26 weeks | 18–80-year-old adults treated with metformin and/or a sulfonylurea with HbA1C 7.0–11.0%, BMI ≤ 45 kg/m2 | Liraglutide 1.8 mg daily = −1.12 Exenatide 10 μg twice daily = −0.79 |
| DURATION-116 | Total = 295 Exenatide 2 mg weekly = 148 Exenatide 10 μg twice daily = 147 |
30 weeks | Individuals ≥16 years-old treated with lifestyle modifications, metformin, sulfonylureas and/or thiazolidinediones with a HbA1C 7.1–11.0%%, BMI ≥ 25 kg/m2 | Exenatide 2 mg weekly = −1.9 Exenatide 10 μg twice daily = −1.5 |
| DURATION-517 | Total = 252 Exenatide 2 mg weekly = 129 exenatide 10 μg twice daily = 123 |
24 weeks | Adults ≥18 years-old treated with lifestyle modifications, metformin, a sulfonylurea and/or thiazolidinedione with a HbA1C 7.1–11.0%, BMI 25–45 kg/m2 | Exenatide 2 mg weekly = −0.9 exenatide 10 μg twice daily = −1.6 |
| GetGoal-X15 | Total = 634 Lixisenatide 20 μg daily = 318 exenatide 10 μg twice daily = 316 |
24 weeks | 21–84-year-old adults on 1.5 g/day of metformin with HbA1C 7–10% | Lixisenatide 20 μg daily = −0.96 exenatide 10 μg twice daily = −0.79 |
| Liraglutide vs. lixisenatide15 | Total = 404 Liraglutide 1.8 mg = 202 lixisenatide 20 μg daily = 202 |
26 weeks | Adults ≥18 years-old with HbA1C 7.5–10.5%, BMI ≥ 20 kg/m2 | Liraglutide 1.8 mg = −1.8 lixisenatide 20 μg daily = −1.2 |
| AWARD-118 | Total = 978 Dulaglutide 0.75 mg weekly = 280 Dulaglutide 1.5 mg weekly = 279 Exenatide 10 μg twice daily = 276 Placebo = 141 |
52 weeks | Adults ≥18 years-old on oral antihyperglycemic medications (metformin or pioglitazone) with HbA1C 7.0–11.0%, BMI 23–45 kg/m2 | Dulaglutide 0.75 mg weekly = −1.3 Dulaglutide 1.5 mg weekly = −1.51 Exenatide 10 μg twice daily = −0.99 Placebo = −0.46 |
| AWARD-615 | Total = 599 Dulaglutide 1.5 mg weekly = 299 Liraglutide 1.8 mg daily = 300 |
26 weeks | Adults ≥18 years-old on metformin (≥1.5 g/day) HbA1C 7.0–10.0%, BMI ≤ 45 kg/m2 | Dulaglutide 1.5 mg weekly = −1.42 Liraglutide 1.8 mg daily = −1.36 |
| SUSTAIN-319 | Total = 813 Semaglutide 1.0 mg weekly = 406 Exenatide 2 mg weekly = 407 |
56 weeks | Adults ≥18 years-old on metformin, a sulfonylurea and/or thiazolidinedione HbA1C 7.0–10.5%, BMI ≤ 45 kg/m2 | Semaglutide 1.0 mg weekly = −1.5 Exenatide 2 mg weekly = −0.9 |
| SUSTAIN-715 | Total = 1201 Semaglutide 0.5 mg weekly = 301 Semaglutide 1.0 mg weekly = 300 Dulaglutide 0.75 mg weekly = 299 Dulaglutide 1.0 mg weekly = 299 |
40 weeks | Adults ≥18 years-old on metformin witha HbA1C 7.0–10.5% | Semaglutide 0.5 mg weekly = −1.5 semaglutide 1.0 mg weekly = −1.8 Dulaglutide 0.75 mg weekly = −1.1 Dulaglutide 1.0 mg weekly = −1.4 |
| SUSTAIN-1020 | Total = 577 Semaglutide 1 mg weekly = 287 Liraglutide 1.2 mg daily = 282 |
30 weeks | Adults ≥18 years-old on metformin, sulfonylurea, and/or SGLT-2 inhibitor with a HbA1C 7.0–11.0% | Semaglutide 1 mg weekly = −1.7 Liraglutide 1.2 mg daily = −1.0 |
| PIONEER-415 | Total = 711 Oral semaglutide 14 mg daily = 285 Liraglutide 1.8 mg daily = 284 Placebo = 142 |
52 weeks | Adults ≥18 years-old on metformin with or without an SGLT-2 inhibitor with a HbA1C 7.0–9.5% |
Oral semaglutide 14 mg daily = −1.2 Liraglutide 1.8 mg daily = −1.1 Placebo = −0.2 |
| PIONEER-921 | Total = 243 Oral semaglutide 3 mg daily = 49 Oral semaglutide 7 mg daily = 49 Oral semaglutide 14 mg daily = 48 Liraglutide 0.9 mg daily = 48 Placebo = 49 |
52 weeks | Japanese adults ≥20 years-old on oral monotherapy with a HbA1C 6.5–9.5% |
Oral semaglutide 3 mg daily = −1.1 Oral semaglutide 7 mg daily = −1.5 Oral semaglutide 14 mg daily = −1.7 Liraglutide 0.9 mg daily = −1.4 Placebo = −0.1 |
| PIONEER-1021 | Total = 458 Oral semaglutide 3 mg daily = 131 Oral semaglutide 7 mg daily = 132 Oral semaglutide 14 mg daily = 130 Dulaglutide 0.75 mg weekly = 65 |
57 weeks | Japanese adults ≥20 years-old on oral monotherapy with a HbA1C 7.0–10.5% | Oral semaglutide 3 mg daily = −0.9 Oral semaglutide 7 mg daily = −1.4 Oral semaglutide 14 mg daily = −1.7 Dulaglutide 0.75 mg weekly = −1.4 |
| ACHIEVE22 | Total = 383 Orforglipron 3 mg daily = 51 Orforglipron 12 mg daily = 56 Orforglipron 24 mg daily = 47 Orforglipron 36 mg = 61 Orforglipron 45 mg daily = 63 Dulaglutide 1.5 mg weekly = 50 Placebo = 55 |
26 weeks | Adults ≥18 years-old treated with lifestyle modifications and/or metformin with a HbA1C 7.0–10.5%, BMI ≥ 23 kg/m2 | Orforglipron 3 mg daily = −1.2 Orforglipron 12 mg daily = −1.9 Orforglipron 24 mg daily = −1.8 Orforglipron 36 mg = −2.0 Orforglipron 45 mg daily = −2.1 Dulaglutide 1.5 mg weekly = −1.10 Placebo = −0.43 |
| Dual (GLP-1/GIP) agonist | ||||
| SURPASS-123 | Total = 478 Tirzepatide 5 mg weekly = 121 Tirzepatide 10 mg weekly = 121 Tirzepatide 15 mg weekly = 121 Placebo = 115 |
40 weeks | Adults ≥ 18 years-old treated with lifestyle modifications and/or metformin with a HbA1C 7.0–9.5%, BMI ≥ 23 kg/m2 |
Tirzepatide 5 mg weekly = −1.87 Tirzepatide 10 mg weekly = −1.89 Tirzepatide 15 mg weekly = −2.01 Placebo = +0.04 |
| CagriSema24 | Total = 92 Semaglutide/cagrilintide 2.4 mg weekly = 31 Semaglutide 2.4 mg weekly = 31 Cagrilintide 2.4 mg weekly = 30 |
32 weeks | Adults ≥18 years-old treated metformin with or without an SGLT-2 inhibitor with a HbA1C 7.5–10.0%, BMI ≥ 27 kg/m2 | Semaglutide/cagrilintide 2.4/2.4 mg weekly = −2.2 Semaglutide 2.4 mg weekly = −1.8 Cagrilintide 2.4 mg weekly = −0.9 |
| Triple (GLP-1/GIP/glucagon) Agonist | ||||
| TRIUMPH25 | Total = 281 Retatrutide 0.5 mg weekly = 47 Retatrutide 4 mg weekly (escalation from 2 mg) = 23 Retatrutide 4 mg weekly (no escalation) = 24 Retatrutide 8 mg weekly (slow escalation) = 26 Retatrutide 8 mg weekly (fast escalation) = 24 Retatrutide 12 mg weekly = 46 Dulaglutide 1.5 mg weekly = 46 Placebo = 45 |
24 weeks | 18–75-year-old adults treated with lifestyle modifications and/or metformin and/or a sulfonylurea with HbA1C 7.0–10.5%, BMI 25–50 kg/m2 | Retatrutide 0.5 mg weekly = −0.43 Retatrutide 4 mg weekly (escalation from 2 mg) = −1.39 Retatrutide 4 mg weekly (no escalation) = −1.30 Retatrutide 8 mg weekly (slow escalation) = −1.99 Retatrutide 8 mg weekly (fast escalation) = 1–0.88 Retatrutide 12 mg weekly = −2.02 Dulaglutide 1.5 mg weekly = −1.41 Placebo = −0.01 |
Long acting agents
Exenatide (once weekly)
Exanatide was later formulated in extended release, administered once weekly. In the DURATION-1 and -5 trials, exenatide once weekly was shown to reduce HbA1c more significantly than twice daily administration (−1.9% vs. −1.5%, and −1.6% vs. −0.9%. respectively) (Table 1).15
Liraglutide
Liraglutide administered once daily by subcutaneous injection, is an analog of human GLP-1, resistant to dipeptidyl peptidase-4 (DPP-4) inactivation. It was also found to be superior to exenatide twice daily in reducing HbA1c, −1.12% vs. −0.79%.15 Additionally, liraglutide decreased HbA1C more significantly than lixisenatide (−1.8% vs. −1.2%) (Table 1).27
Dulaglutide
Another analog of human of GLP-1, but in once weekly administration, was similarly found to be superior to exenatide twice daily in reducing HbA1C, −1.51% vs. −0.99%.18 In the AWARD-6 trial, dulaglutide was found non-inferior to liraglutide in reducing HbA1c, −1.42% vs. −1.36%, respectively (Table 1).15
Semaglutide
Semaglutide was the first GLP1-RA agonist available in subcutaneous injection and oral formulations. It is structurally similar to liraglutide but with modifications, making it even more resistant to degradation by DDP-4 and with a longer half-life. Semaglutide by subcutaneous injection was shown to be superior to exenatide once weekly, dulaglutide, and liraglutide in lowering HbA1c.19 In its daily oral formulation dose, semaglutide was non-inferior to liraglutide in decreasing HbA1c, in the PIONEER-4 trial.15 However, oral semaglutide more significantly reduced HbA1c than dulaglutide, −1.7% vs. 1.4% (Table 1).21
Comparative trials favor long acting agents (exenatide once weekly, liraglutide, dulaglutide, semaglutide) over short acting agents (exenatide twice daily and lixisenatide) in providing superior glycemic control. Among long acting agents, semaglutide by subcutaneous injection provides the greatest reduction in HbA1c. Liraglutide and dulaglutide seem to provide similar glycemic control, as do oral semaglutide and liraglutide. While oral semaglutide may be a preferred agent for patients adverse to injections, its strict administration requirements, that is 30 min before the first meal, beverage, or other medications, may limit its effective use.
Dual GLP-1/GIP RA
Tirzepatide
A novel, dual GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) RA, is a synthetic peptide analog of GIP with activity at both receptors. The activation of both receptors seems to lead to greater efficacy in glycemic control. Administered in a once-weekly subcutaneous injection, it was shown to significantly decrease HbA1c, up to 2.07% at a maximum dose of 15 mg weekly, compared to placebo (Table 1).23 Tirzepatide provides greater glycemic control compared to available single agonist agents.28
GLP-1 RA in development
Oral orfoglipron, a nonpeptide partial GLP-1 RA, enhances cyclic AMP signaling more than β-arrestin recruitment, indicating less receptor desensitization compared to full GLP-1 RAs.29 Can be administered without respect to food unlike, peptide based oral semaglutide. In a phase 2 study clinical trial evaluating its use in T2DM, orforglipron significantly decreased HbA1c to −2.1% compared to −1.1% for dulaglutide and −0.43% for placebo.22
Twice daily danuglipron, In a phase 2 study decreased HbA1c by −0.49% to −1.18% in a dose dependent manner, compared to −0.02% for placebo.30
Dual GLP-1 RA agonists
Semaglutide 2.4 mg, combined with cagrilintide (CagriSema), a long-acting amylin analogue, is being developed for treating T2DM. It is administered in a once-weekly subcutaneous injection. In a phase 2 clinical trial, CagriSema significantly reduced HbA1c by −2.2%, compared to −1.8% for semaglutide 2.4 mg alone (p = 0.075) (Table 1).24
Dual GLP-1 RA and glucagon agonists
Mazdutide is administered once weekly by subcutaneous injection. In a phase 2 clinical trial among Chinese patients, mazdutide reduced HbA1c by −1.41% to −1.67% in a dose dependent manner compared to +0.03% for placebo.31 Survotutide was studied in once and twice weekly administration by subcutaneous injection in a phase II trial, and was shown to significantly reduce HbA1c compared to placebo (Table 1).32
Triple GLP-1/GIP/glucagon RA
Retatrutide, a once weekly, triple GLP-1, GIP, and glucagon RA administered by subcutaneous injection, is under development for treating type 2 diabetes. In a phase 2 clinical trial, retatrutide reduced HbA1c to −2.02%, compared to −1.41% for dulaglutide 1.5 mg and −0.01% for placebo (Table 1).25
GLP-1 RA in combination with insulin
The use of GLP-1 RA with insulin, particularly basal insulin, has been shown to improve glycemic control and may be especially effective in treating advanced T2DM.15 For instance, in SUSTAIN-5 among patients on basal insulin, the addition of semaglutide 0.5 mg or 1 mg weekly lead to a −1.% and −1.8% reduction in HbA1c, respectively. Combination therapy may also decrease the risk of hypoglycemia and weight gain.15 Though the risk of hypoglycemia is higher than when treated with GLP-1 RA alone, it is lower than insulin-only treatment.15
GLP-1 RA in type 1 Diabetes (T1DM)
Currently, no GLP-1 RA has been approved for treating T1DM. Still, several studies support their use in this population, with positive effects on glycemic control and a reduction in bolus insulin doses and weight.33,34 In a meta-analysis by Wang and colleagues, a combination of GLP-1 RA and insulin therapy leads to a small but significantly more significant reduction in HbA1c than traditional treatment (−0.21 [−0.40 to −0.02]).34 While several studies included in their analysis did not find significant changes in HbA1c, they did find a reduction in insulin requirements.15,35,36 Furthermore, differences in hypoglycemic events were not substantial or reduced in combination treatment, suggesting that combination treatment does not increase the risk of hypoglycemia.34 More research is needed to evaluate the potential benefits and possible adverse effects of using GLP-1 RA in individuals with T1DM.
GLP-1 RA and obesity
Commercially available GLP-1 RA for management of obesity
Liraglutide
Liraglutide is approved for chronic weight management in adult and pediatric patients with obesity or overweight with at least one weight-related condition (Tables 2 and 3). Daily subcutaneous liraglutide 3.0 mg, alongside lifestyle therapy, reduces body weight by around 8% in adults with obesity, which can increase to 11.5–15.7% with added intensive behavior therapy, exercise, and caloric restriction (Table 2).40 Liraglutide counteracts the increased appetite after weight loss, and with exercise improves cognitive restraint and reduces sedentary time, helping to prevent weight regain.40,54 In adults with overweight or obesity at high cardiovascular disease risk (without diabetes), liraglutide 3.0 mg plus lifestyle intervention lowered visceral adipose tissue over 40 weeks compared to placebo (12.5% vs. 1.6%) (Table 2).41 In adolescents with obesity, liraglutide 3.0 mg reduced BMI by 4.3% over 56 weeks compared to 0.3% with placebo, with early treatment response predicting long-term effectiveness.39
Table 2.
RCTs of GLP1-RAs use in Obesity.
| Trial name or authors | Number of patients | Duration | Inclusion criteria | Change in weight (%) | Other information |
|---|---|---|---|---|---|
| GLP-1 RA | |||||
| SCALE Obesity and Prediabetesa,37 | Total = 3731 Liraglutide 3.0 mg = 2487 Placebo = 1244 |
56 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with at least one weight-related condition, without diabetes | Liraglutide 3.0 mg = −8.0% Placebo = −2.6% |
Bodyweight reduction threshold (≥5%; ≥10%): Liraglutide 3.0 mg = 63%; 33% Placebo = 27%; 10% |
| Wadden et al.a38 | Total = 150 IBT alone: 50 IBT + Liraglutide 3.0 mg: 50 Multicomponentb: 50 |
52 weeks | 21–70-year-old adults with at least one unsuccessful weight loss attempt, and a BMI between 30 and 55 kg/m2, without diabetes | At week 24: IBT alone: −5.4% IBT + Liraglutide 3.0 mg: −10.1% Multicomponentb: −12.2% At week 52: IBT alone: −6.1% IBT + Liraglutide 3.0 mg: −11.5% Multicomponentb: −11.8% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%): At week 24: IBT alone: 46%; 20%; 6% IBT + Liraglutide 3.0 mg: 78%; 46%; 20% Multicomponenta: 82%; 60%; 32% At week 52: IBT alone: 44%; 26%; 12% IBT + Liraglutide 3.0 mg: 70%; 46%; 28% Multicomponenta: 74%; 52%; 36% |
| SCALE Teensa39 | Total = 251 Liraglutide 3.0 mg = 125 Placebo = 126 |
82 weeks: 56 weeks (liraglutide or placebo) + 26 weeks (follow-up without treatment) |
12–18-year-old adolescents with at least one unsuccessful weight loss attempt, and a BMI in the 95th percentile or higher (according to sex and age-specific growth charts), including T2DM | At week 56: Liraglutide 3.0 mg = −3.2% Placebo = +2.2% At week 82: Liraglutide 3.0 mg = +1.5% Placebo = +4.6% |
BMI reduction threshold at week 56 (≥5%; ≥10%): Liraglutide 3.0 mg = 43%; 26% Placebo = 19%; 8% Changes in BMI at week 56 (%): Liraglutide 3.0 mg = −4.3% Placebo = +0.3% |
| SCALE IBTa40 | Total = 282 IBT + Liraglutide 3.0 mg = 142 IBT + Placebo = 140 |
56 weeks | ≥18-year-old adults with a BMI ≥ 30 kg/m2, without diabetes | IBT + Liraglutide 3.0 mg = −7.5% IBT + Placebo = −4.0% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%): IBT + Liraglutide 3.0 mg = 61%; 30%; 18% IBT + Placebo = 39%; 20%; 9% |
| Neeland et al.a41 | Total = 128 Liraglutide 3.0 mg = 73 Placebo = 55 |
40 weeks | ≥35-year-old adults with a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with metabolic syndrome, without diabetes | Liraglutide 3.0 mg = −6.6% Placebo = −1.2% |
Bodyweight reduction threshold (≥5%; ≥10%): Liraglutide 3.0 mg = 63%; 19% Placebo = 22%; 3% Visceral adipose tissue changes: Liraglutide 3.0 mg = −12.5% Placebo = −1.6% |
| Lundgren et al.a42 | Total = 195 Liraglutide 3.0 mg = 49 Exercise = 48 Liraglutide 3.0 mg + exercise = 49 Placebo = 49 |
60 weeks: 8 weeks of low-calorie diet + 52 weeks of treatment (randomization) |
18–65-year-old adults with a BMI between 32 and 43 kg/m2, without diabetes | From week −8 to 52: Liraglutide 3.0 mg = −13.4% Exercise = −10.9% Liraglutide 3.0 mg + exercise = −15.7% Placebo = −6.7% |
Bodyweight reduction threshold from week −8 to 52 (≥5%; ≥10%; ≥15%; ≥20%): Liraglutide 3.0 mg = 88%; 59%; 29%; 22% Exercise = 80%; 45%; 30%; 18% Liraglutide 3.0 mg + exercise = 87%; 69%; 49%; 33% Placebo = 70%; 28%; 10%; 2% |
| STEP 1a40 | Total = 1961 Semaglutide 2.4 mg = 1306 Placebo = 655 |
68 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with at least one weight-related condition, without diabetes | Semaglutide 2.4 mg = −14.9% Placebo = −2.4% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 2.4 mg = 86%; 69%; 50%; 32% Placebo = 31%; 12%; 5%; 2% |
| STEP 2a40 | Total = 1210 Semaglutide 1.0 mg = 403 Semaglutide 2.4 mg = 404 Placebo = 403 |
68 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 27 kg/m2, and T2DM | Semaglutide 1.0 mg = −6.9% Semaglutide 2.4 mg = −9.6% Placebo = −3.4% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 1.0 mg = 57%; 28%; 14%; 5% Semaglutide 2.4 mg = 69%; 45%; 26%; 13% Placebo = 28%; 8%; 3%; 1% |
| STEP 3a40 | Total = 611 IBT + Semaglutide 2.4 mg = 407 IBT + Placebo = 204 |
68 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with at least one weight-related condition, without diabetes | Semaglutide 2.4 mg = −16.0% Placebo = −5.7% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 2.4 mg = 86%; 75%; 56%; 36% Placebo = 47%; 27%; 13%; 4% |
| STEP 4a40 | Semaglutide 2.4 (week 0–20) = 803 Semaglutide 2.4 mg (week 20–68) = 535 Placebo (week 20–68) = 268 |
68 weeks: 20 weeks (semaglutide open-label) + 48 weeks (semaglutide or placebo) |
≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with at least one weight-related condition, without diabetes | From week 0–20 Semaglutide 2.4 = −10.6% From week 20–68: Semaglutide 2.4= −7.9% Placebo = +6.9% |
Bodyweight reduction threshold from week 0–68 (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 2.4 mg = 89%; 79%; 64%; 39% Placebo = 47%; 20%; 9%; 5% |
| STEP 5a40 | Total = 304 Semaglutide 2.4 mg = 152 Placebo = 152 |
104 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with at least one weight-related condition, without diabetes | Semaglutide 2.4 mg = −15.2% Placebo = −2.6% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 2.4 mg = 77%; 62%; 52%; 36% Placebo = 34%; 13%; 7%; 2% |
| STEP 5 (Control of eating)a43 | Total = 174 Semaglutide 2.4 mg = 88 Placebo = 86 |
104 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥27 kg/m2 with at least one weight-related condition, without diabetes | Semaglutide 2.4 mg = −14.8% Placebo = −2.4% |
Significant improvement of semaglutide compared to placebo: Craving control, craving for savory foods, difficulty in resisting cravings, and difficulty controlling eating at weeks 20, 52, and 104; Positive mood and craving for sweet foods at weeks 20 and 52; Hunger and feelings of fullness at week 20. |
| STEP 6a32 | Total = 401 Semaglutide 1.7 mg = 101 Semaglutide 2.4 mg = 199 Placebo = 101 |
68 weeks | East Asian (Japan and South Korea) ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 35 kg/m2 with at least one weight-related comorbidity or ≥27 kg/m2 with at least two weight-related comorbidities. At least one comorbidity had to be hypertension or dyslipidemia, or, in Japan only, T2DM | Semaglutide 1.7 mg = −9.6% Semaglutide 2.4 mg = −13.2% Placebo = −2.1% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 1.7 mg = 72%; 42%; 24%; 11% Semaglutide 2.4 mg = 83%; 61%; 41%; 20% Placebo = 21%; 5%; 3%; 2% |
| STEP 8a40 | Total = 338 Semaglutide 2.4 mg = 126 Liraglutide 3.0 mg = 127 Placebo = 85 |
68 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with at least one weight-related condition, without diabetes | Semaglutide 2.4 mg = −15.8% Liraglutide 3.0 mg = −6.4% Placebo = −1.9% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 2.4 mg = 87%; 71%; 55%; 38% Liraglutide 3.0 mg = 58%; 25%; 12%; 6% Placebo = 29%; 15%; 6%; 2% |
| STEP TEENSa40 | Total = 201 Semaglutide 2.4 mg = 134 Placebo = 67 |
68 weeks | 12–18-year-old adolescents with at least one unsuccessful weight loss attempt, a BMI in the 95th percentile or higher (according to sex and age-specific growth charts), or in the 85th percentile or higher with at least one weight-related condition, including T2DM | Semaglutide 2.4 mg = −14.7% Placebo = +2.7% |
BMI reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 2.4 mg = 76%; 63%; 57%; 40% Placebo = 23%; 10%; 5%; 3% Changes in BMI (%): Semaglutide 2.4 mg = −16.1% Placebo = +0.6% |
| OASIS 1a44 | Total = 667 Semaglutide 50 mg (oral) = 334 Placebo = 333 |
68 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with at least one weight-related condition, without diabetes | Semaglutide 50 mg = −15.1% Placebo = −2.4% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%): Semaglutide 50 mg = 85%; 69%; 54%; 34% Placebo = 26%; 12%; 6%; 3% |
| STEP-HFpEF45 | Total = 529 Semaglutide 2.4 mg = 263 Placebo = 266 |
52 weeks | ≥18-year-old adults with a left ventricular ejection fraction of at least 45%, a BMI ≥ 30 kg/m2, New York Heart Association functional class II, III, or IV, a Kansas City Cardiomyopathy Questionnaire clinical summary score of less than 90 points, and a 6-min walk distance of at least 100 m, without diabetes | Semaglutide 2.4 mg = −13.3% Placebo = −2.6% |
Bodyweight reduction threshold (≥10%; ≥15%; ≥20%): Semaglutide 2.4 mg = 66%; 44%; 23% Placebo = 9%; 2%; 0% |
| SELECT46 | Total = 17,604 Semaglutide 2.4 mg = 8803 Placebo = 8801 |
∼40 months | ≥45-year-old adults with a BMI ≥ 27 kg/m2 with established cardiovascular disease, without diabetes | Semaglutide 2.4 mg = −9.4% Placebo = −0.9% |
Primary cardiovascular end-point event: Semaglutide 2.4 mg = 6.5% Placebo = 8.0% |
| GZGIa29 | Total = 272 Orforglipron 12 mg = 50 Orforglipron 24 mg = 53 Orforglipron 36 mg = 58 Orforglipron 45 mg = 61 Placebo = 50 |
36 weeks | 18–75-year-old adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with at least one weight-related condition, without diabetes | At week 26 Orforglipron 12 mg = −8.6% Orforglipron 24 mg = −11.2% Orforglipron 36 mg = −12.3% Orforglipron 45 mg = −12.6% Placebo = −2.0% At week 36 Orforglipron 12 mg = −9.4% Orforglipron 24 mg = −12.5% Orforglipron 36 mg = −13.5% Orforglipron 45 mg = −14.7% Placebo = −2.3% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%): By week 26 Orforglipron 12 mg = 74%; 39%; 21% Orforglipron 24 mg = 89%; 57%; 26% Orforglipron 36 mg = 90%; 71%; 34% Orforglipron 45 mg = 87%; 70%; 34% Orforglipron Placebo = 23%; 2%; 0% By week 36 Orforglipron 12 mg = 72%; 46%; 22% Orforglipron 24 mg = 90%; 62%; 33% Orforglipron 36 mg = 92%; 75%; 43% Orforglipron 45 mg = 90%; 69%; 48% Placebo = 24%; 9%; 1% |
| Dual (GLP-1/GIP or GLP-1/glucagon) RA | |||||
| SURMOUNT-1a47 | Total = 2539 Tirzepatide 5 mg = 630 Tirzepatide 10 mg = 636 Tirzepatide 15 mg = 630 Placebo = 643 |
72 weeks | ≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with at least one weight-related condition, without diabetes | TZP 5 mg = −15.0% TZP 10 mg = −19.5% TZP 15 mg = −20.9% Placebo = −3.1% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%; ≥25%): TZP 5 mg = 85%; 68%; 48%; 30%; 15% TZP 10 mg = 89%; 78%; 66%; 50%; 32% TZP 15 mg = 91%; 83%; 70%; 56%; 36% Placebo = 34%; 19%; 9%; 3%; 1% |
| SURMOUNT-248 | Total = 938 Tirzepatide 10 mg = 312 Tirzepatide 15 mg = 311 Placebo = 315 |
72 weeks | ≥18-year-old adults with a BMI ≥ 27 kg/m2 with at least one weight-related condition, including diabetes | Tirzepatide 10 mg = −12.8% Tirzepatide 15 mg = −14.7% Placebo = −3.2% |
Bodyweight reduction threshold (≥5%; ≥10%; ≥15%; ≥20%; ≥25%): Tirzepatide 10 mg = 79%; 60%; 39%; 21%; 9% Tirzepatide 15 mg = 83%; 65%; 48%; 31%; 15% Placebo = 32%; 9%; 3%; 1%; 0% |
| SURMOUNT-3a40 | Total = 579 ILI + Tirzepatide MTD = 287 ILI + placebo = 292 |
84 weeks: 12 weeks of ILI + 72 weeks (Tirzepatide or placebo) |
≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with at least one weight-related condition, without diabetes | 72 weeks with Tirzepatide: Tirzepatide = −18.4% Placebo = +2.5% 84 weeks (12 weeks of lLI + 72 weeks of tirzepatide or placebo): Tirzepatide = −24.3% Placebo = −4.5% |
Bodyweight reduction threshold, from randomization to week 72 (≥5%; ≥10%; ≥15%; ≥20%; ≥25%): Tirzepatide = 87%; 77%; 65%; 45%; 29% Placebo = 16%; 9%; 4%; 2%; 1% |
| SURMOUNT-4a49 | Total = 670 Tirzepatide MTD = 335 Placebo = 335 |
88 weeks: 36 weeks of open-label Tirzepatide + 52 weeks (Tirzepatide or placebo) |
≥18-year-old adults with at least one unsuccessful weight loss attempt, a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 with at least one weight-related condition, without diabetes | At week 36 Tirzepatide = −20.9% From week 36–88 Tirzepatide = −5.5% Placebo = +14.0% |
Bodyweight reduction threshold, from week 0–88 (≥5%; ≥10%; ≥15%; ≥20%; ≥25%): Tirzepatide = 97%; 92%; 84%; 69%; 54% Placebo = 70%; 46%; 26%; 12%; 5% |
| le Roux et al.a50 | Total = 384 Survodutide 0.6 mg = 77 Survodutide 2.4 mg = 78 Survodutide 3.6 mg = 76 Survodutide 4.8 mg = 76 Placebo = 77 |
46 weeks | 18–75-year-old adults with at least one unsuccessful weight loss attempt, a BMI of 27 kg/m2 or greater, without diabetes | Survodutide 0.6 mg = −6.2 Survodutide 2.4 mg = −12.5 Survodutide 3.6 mg = −13.2% Survodutide 4.8 mg = −14.9% Placebo = −2.8% |
Bodyweight reduction threshold at week 46 (≥5%; ≥10%; ≥15%; ≥20%): Survodutide 0.6 mg = 60%; 34%; 12%; 0% Survodutide 2.4 mg = 81%; 65%; 38%; 21% Survodutide 3.6 mg = 82%; 65%; 46%; 30% Survodutide 4.8 mg = 83%; 69%; 55%; 33% Placebo = 26%; 11%; 5%; 0% |
| Ji et al.51 | Total = 248 Mazdutide 3.0 mg = 62 Mazdutide 4.5 mg = 63 Mazdutide 6.0 mg = 61 Placebo = 62 |
24 weeks | Chinese 18–75-year-old adults with overweight (BMI ≥ 24 kg/m2) with hyperphagia and/or at least one weight-related comorbidity or with obesity (BMI ≥ 28 kg/m2), without diabetes | Mazdutide 3.0 mg = −6.7% Mazdutide 4.5 mg = −10.4% Mazdutide 6.0 mg = −11.3% Placebo = −1.0% |
Bodyweight reduction threshold at week 24 (≥5%; ≥10%; ≥15%): Mazdutide 3.0 mg = 58%; 19%; 10% Mazdutide 4.5 mg = 82%; 49%; 16% Mazdutide 6.0 mg = 80%; 51%; 26% Placebo = 5%; 0%; 0% |
| Véniant et al.52 | Total = 26 MariTide 140 mg = 6 MariTide 280 mg = 6 MariTide 420 mg = 8 Placebo = 6 |
12 weeks (3 doses -monthly injection) | 18–65-year-old adults with a BMI between 30 and 40 kg/m2, without diabetes | MariTide 140 mg = −7.2% MariTide 280 mg = −9.9% MariTide 420 mg = −14.5% Placebo = −1.5% |
– |
| Triple (GLP-1/GIP/glucagon) RA | |||||
| Retatrutide Phase 2 Obesitya53 | Total = 338 Retatrutide 1 mg = 69 Retatrutide 4 mg (ID 2 mg) = 33 Retatrutide 4 mg (ID 4 mg) = 34 Retatrutide 8 mg (ID 2 mg) = 35 Retatrutide 8 mg (ID 4 mg) = 35 Retatrutide 12 mg (ID 2 mg) = 62 Placebo = 70 |
48 weeks | 18–75-year-old adults with at least one unsuccessful weight loss attempt, a BMI between 30 and 50 kg/m2 or ≥ 27 kg/m2 with at least one weight-related condition, without diabetes | At week 24: Retatrutide 1 mg = −7.2% Retatrutide 4 mg (ID 2 mg) = −11.8% Retatrutide 4 mg (ID 4 mg) = −13.9% Retatrutide 8 mg (ID 2 mg) = −16.7% Retatrutide 8 mg (ID 4 mg) = −17.9% Retatrutide 12 mg (ID 2 mg) = −17.5% Placebo = −1.6% At week 48: Retatrutide 1 mg = −8.7% Retatrutide 4 mg (ID 2 mg) = −16.3% Retatrutide 4 mg (ID 4 mg) = −17.8% Retatrutide 8 mg (ID 2 mg) = −21.7% Retatrutide 8 mg (ID 4 mg) = −23.9% Retatrutide 12 mg (ID 2 mg) = −24.2% Placebo = 2.1% |
Bodyweight reduction threshold at week 48 (≥5%; ≥10%; ≥15%; ≥20%; ≥25%; 30%): Retatrutide 1 mg = 64%; 27%; 16%; 6%; 6%; 1% Retatrutide 4 mg (ID 2 mg) = 87%; 73%; 55%; 31; 14%; 6% Retatrutide 4 mg (ID 4 mg) = 91%; 76%; 64%; 29%; 19%; 10% Retatrutide 8 mg (ID 2 mg) = 100%; 90%; 73%; 50%; 36%; 16% Retatrutide 8 mg (ID 4 mg) = 100%; 91%; 77%; 70%; 43%; 17% Retatrutide 12 mg (ID 2 mg) = 100%; 93%; 83%; 63%; 48%; 26% Placebo = 27%; 9%; 2%; 1%; 0%; 0% |
GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide; RA, receptor agonist; MTD, maximum tolerated dose (10-mg or 15-mg); ILI, intensive lifestyle intervention; IBT, intensive behavioral therapy; ID, initial dose.
Adjunct to lifestyle/behavioral intervention in all groups, with counseling, and diet and exercise recommendations.
Intensive behavioral therapy + Liraglutide 3.0 mg + 12 weeks of 1000–1200 kcal diet (from week 4 to week 16).
Table 3.
Currently commercially available GLP-1 receptor agonists for the treatment of obesity.
| Agent | Category | Type of use | Frequency of use | Indicationa | Commercial names | Doses (mg) |
|---|---|---|---|---|---|---|
| Liraglutide | Single GLP-1 RA | Injectable | Daily | Pediatric and adult | Saxendab, Victozac | 0.6, 1.2, 1.8, 2.4, 3.0 |
| Semaglutide | Single GLP-1 RA | Injectable | Weekly | Pediatric and adult | Wegovyb, Ozempicc | 0.25, 0.5, 1.0, 1.7, 2.4 |
| Tirzepatide | Dual GLP-1/GIP RA | Injectable | Weekly | Adult | Zepboundb, Mounjaroc | 2.5, 5.0, 7.5, 10.0, 12.5, 15.0 |
GLP-1, glucagon-like peptide-1; GIP, glucose-dependent insulinotropic polypeptide; RA, receptor agonist.
Approved by FDA.
FDA-approved for chronic weight management in patients with obesity or overweight with at least one weight-related condition.
FDA-approved for the treatment of type 2 diabetes and approved for chronic weight management in patients with obesity or overweight with at least one weight-related condition, mainly outside the USA.
Semaglutide
Subcutaneous semaglutide 2.4 mg taken once per week is approved for chronic weight management in adult and pediatric patients with obesity or preobesity with at least one weight-related condition (Table 3). Semaglutide 2.4 mg not only promotes weight loss but also improves eating control, reduces food cravings, and reduces the usual appetite increase after significant weight loss, supporting ongoing weight management.43
The Semaglutide Treatment Effect in People with obesity (STEP) Program trials, with semaglutide 2.4 mg and lifestyle intervention, showed weight reductions of 14.7–17.4% on average in persons with pre-obesity or obesity (without diabetes), with improvements in cardiometabolic risk factors and sustained effects for two years (Table 2).40 A lower bodyweight reduction of 9.6% was reported in persons with pre-obesity or obesity and T2DM.40 In adolescents with obesity, semaglutide 2.4 mg substantially reduced total body weight loss by 16.1% compared to 0.6% with placebo.40 In the East Asian population with preobesity or obesity, with or without T2DM, semaglutide 2.4 mg promoted a bodyweight reduction of 13.2%.40 In comparison with once-daily liraglutide 3.0 mg, once-weekly subcutaneous semaglutide 2.4 mg resulted in greater weight loss after 68 weeks (−15.8% vs. −6.4%).40 It is important to note that weight regain occurs after stopping semaglutide, even with lifestyle changes, highlighting the chronic nature of obesity treatment.40
Tirzepatide
Tirzepatide is approved for chronic weight management in adults with obesity or preobesity with at least one weight-related condition (Table 3). Its action on GIP and GLP-1 receptors enhances appetite control and metabolic function, suggesting higher efficacy compared to single GLP-1RAs.55
The SURMOUNT Program includes four global phase 3, randomized, placebo-controlled clinical trials with tirzepatide administered subcutaneously once weekly in conjunction with lifestyle intervention.47, 48, 49 There was a substantial degree of weight reduction with 10 mg and 15 mg doses of 18.4–20.9% in persons with obesity or preobesity (without diabetes), with improvements in cardiometabolic risk factors (Table 2).47, 48, 49 Lower bodyweight reductions of 12.8% and 14.7% with 10 mg and 15 mg doses of tirzepatide, respectively, were observed in persons with preobesity or obesity and T2DM.48
GLP-1 RA injectable agents in development for obesity treatment
Survodutide
Studied in a phase 2 randomized, double-blind trial in persons with pre-obesity and obesity (without diabetes) for 46 weeks.50 At its highest dose of 4.8 mg, study participants lost 14.9% of their weight vs. 2.8% in the placebo group (Table 2).50
Mazdutide
Promoted weight reduction in Chinese patients with preobesity or obesity (without diabetes) in a phase 2, randomized, double-blind, placebo-controlled trial.51 After 24 the placebo, and improved cardiometabolic risk factors (Table 2).51
Maridebart/cafraglutide (MariTide)
A monthly subcutaneous injection combining GIP receptor antagonism with GLP-1 RA, and seems to protect against diet-induced obesity as evidenced by models lacking GIP receptor activity.52 In a phase 1, randomized, double-blind placebo-controlled trial in individuals with obesity (without diabetes), this drug resulted in weight loss of 15% over 12 weeks at its highest dose of 420 mg (Table 2).52
Retatrutide
Enhances obesity treatment by regulating body fat mass, energy homeostasis, and energy intake.53 In a phase 2 randomized, double-blind trial, weekly injections of retatrutide (1 mg–12 mg) led to weight reductions of 7.2–17.5% at 24 weeks and 8.7–24.2% at 48 weeks in individuals with obesity or pre-obesity (without diabetes), compared to 1.6% and 2.1% with placebo, respectively, showing no plateau in this period (Table 2).53
GLP-1 RA oral agents in development for obesity treatment
Semaglutide
In a phase 3, randomized, double-blind trial, once-daily 50 mg of oral semaglutide an adjunct to diet and physical activity in adults with pre-obesity or obesity (without diabetes) for 68 weeks induced significant weight reduction compared to placebo (15.1% vs. 2.4%) (Table 2).44
Orforglipron
In a phase 2, randomized, double-blind trial, daily oral orforglipron (12, 24, 36, or 45 mg) for 36 weeks led to weight reductions of 9.4–14.7% in adults with pre-obesity or obesity (without diabetes), compared to 2.3% with placebo, and showed no signs of plateau (Table 2).29
Comparative analysis
Comparisons of multiple peptide GLP-1 RAs with respect to weight loss and adverse effects have found that these medications are more effective than placebo in reducing body weight (BW) (3.11 kg, CI: −3.64 to 2.57 kg).56 The largest statistically significant effects on weight reduction have been found for semaglutide, liraglutide and oral orforglipron.29,56 In the SUSTAIN trials, semaglutide use has been shown to lower HbA1c, reduce BW and lower blood pressure when compared to placebo.57 Out of the most effective peptide GLP-1 RAs for weight loss, exenatide IR and liraglutide had higher discontinuation rates than semaglutide due to adverse effects.56
Monotherapy with cagrilintide, an amylin receptor agonist produced a 10.6% weight loss at maximum titrated dose compared to placebo (2.8%) and liraglutide 3.0 mg (8.4%).58 When combined with semaglutide 2.4 mg (a.k.a. cagri-sema) the combination induced 43% more weight loss than semaglutide alone.59 In the SURPASS trials, tirzepatide decreased baseline HbA1c by ∼2.0% at doses of 5, 10, and 15 mg.23 The SURMOUNT-1 trial revealed a mean percent change in BW over placebo of 15%, 19.5% and 20.9% in those receiving tirzepatide at doses of 5, 10, and 15 mg, respectively.47
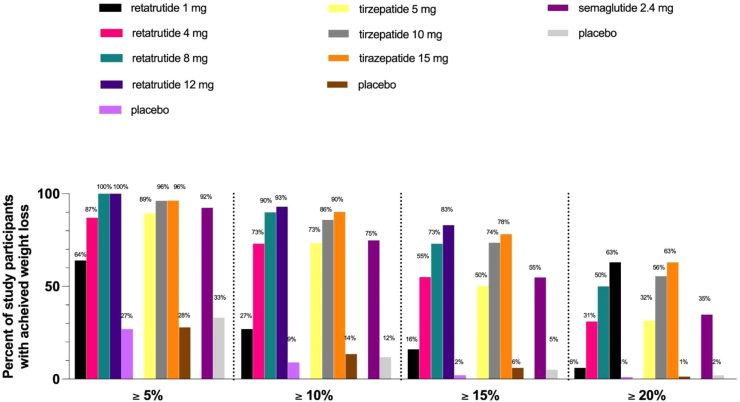
Retatrutide, the only triple GIP/GLP-1/glucagon RA under investigation for obesity and/or T2DM produced significant dose related improvements in glycemic control and BW when compared to placebo. When compared to dulaglutide 1.5 mg, the greatest reductions in HbA1c and BW were noted for the highest doses of retatrutide (8 mg and 12 mg).25 The average proportion of individuals experiencing gastrointestinal side effects in the retatrutide group was similar to that in those receiving dulaglutide25 though greater than placebo.53 Weight loss comparisons between semaglutide, tirzepatide, and retatrutide are shown in Fig. 1.
Fig. 1.
Weight loss with retatrutide1, tirazepatide2, and semaglutide3 compared to placebo.
Safety profile
Cardiorenal effects
Slight increases in heart rate have been reported with the GLP-1 RAs in certain populations,60 but the cardiac benefits of using these drugs have been widely investigated. In a meta-analysis of seven cardiovascular outcome trials (CVOTs), GLP-1RAs compared to placebo decreased major adverse cardiovascular events (MACEs) and all-cause mortality by 12%.61 The ongoing SURPASS trials evaluating tirzepatide also evidenced a trend towards cardiovascular safety with decreased incidence of MACEs (hazard ratio 0.80; 95% CI, 0.57–1.11).62 It is important to mention that the SURPASS-CVOT looking at this effect is still ongoing. Furthermore, it is noteworthy that in the SELECT trial, a 39.8-month follow-up trial in patients with cardiovascular disease and overweight or obesity, but not diabetes,46 semaglutide 2.4 mg was superior to placebo in reducing the incidence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (HR 0.80; 95% CI, 0.72–0.90, p < 0.001).63 Semaglutide also improved symptoms, physical limitations, exercise function, and weight loss in patients with obesity and heart failure with preserved ejection fraction.45 Additionally, Systolic blood pressure and lipid profiles show improvements with cagri-sema and retatrutide.25,59 A recent review aiming to evaluate the effects of GLP1-RAs on blood pressure found modest reductions in systolic blood pressure (i.e., semaglutide, liraglutide, dulaglutide, exenatide) and diastolic blood pressure (exenatide only), but suggested that the results were likely due to GLP1-RAs effects on weight reduction and glycemic control.64 The FLOW trial (NCT03819153) warranted early termination due to evidence that the use of weekly 1 mg semaglutide reduced the risk of kidney-disease progression as well as cardiovascular and kidney death by 24% compared with placebo.65 A meta-analysis of CVOTs also found a 17% reduction in a composite renal disease risk score, and post hoc analyses of SURPASS-4 revealed less GFR decline and reduced urine albumin to creatinine ratios in individuals with T2DM and high cardiovascular risk.66 Concerns for acute kidney injury, however, have been raised among those with higher body weight (>99 kg) and older age (45–84 yrs).67
Pancreatitis and pancreatic cancer
Amongst those with T2DM, findings from meta-analyses of the CVOTs suggest no increased risk of either disorder with use of individual GLP-1 RAs or the medications as a class.68 However, in a population using GLP-1 RAs for obesity management without history of diabetes, increased risk of pancreatitis associated with the GLP-1 RAs was found compared to bupropion-naltrexone (HR 9.1, 95%CI 1.25–66.0) suggesting the risk benefit ratio of these medications is influenced by underlying disease state.69 Studies evaluating the potential risk from dual and triple agonists are needed.
Thyroid disorders and thyroid cancer
The relationship between risk of thyroid cancer and use of GLP-1 RAs is not well understood and the mechanisms promoting potential unfavorable effects of the drug on the thyroid is not well defined. When specific GLP-1 RAs were evaluated compared to placebo and other antidiabetic drugs, liraglutide was found to increase risk of thyroid disorders by 37% and dulaglutide in similar comparisons increased risk by 96%.70 No effects on risk were noted for semaglutide, lixisenatide, exenatide, nor albiglutide in which relative risks were not significant.70 During its meeting in October 2023, The European Medical Association (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) concluded that the available evidence does not support a causal association between the GLP-1RA)–exenatide, liraglutide, dulaglutide, semaglutide, and lixisenatide–and cancer of the thyroid.71
Gastrointestinal effects
Gastrointestinal (GI) effects are the most reported by study participants and include nausea, vomiting, diarrhea, constipation, bowel obstruction, biliary disease and slowed gastric emptying. However, early exit from studies due to gastrointestinal disturbances may in part bias reported side effect findings at clinical trial termination. Non-use of validated assessment tools (e.g., stable 13C-labeled fatty acid breath test) and reliance on self-reported symptoms instead of validated GI disorder symptom indices are also limitations.72 Further, variations in baseline gastric emptying as a determinant of efficacy of GLP-1 RAs likely differentially impacts their effectiveness amongst those with obesity vs. those with diabetes in addition to affecting pre-procedural intragastric food retention post GLP-RA1 use.72 Although based primarily on anecdotal and case reports, concerns regarding risk of regurgitation and pulmonary aspiration due to delayed gastric emptying have resulted in guidance from the American Society of Anesthesiologists (ASA) Task Force on Preoperative Fasting for use of these medications prior to non-urgent or emergent procedures requiring general anesthesia.73 Fewer studies have evaluated the risk of biliary disease, possibly due to impaired gallbladder emptying,74 however, tirzepatide was shown to portend an increased risk (RR 1.97, 95% CI 1.14–3.42, I2 = 0.0%, p = 0.558).75
Regarding Metabolic Associated Steatotic Liver Disease (MASLD), both liraglutide and exenatide have shown significant reductions in liver fat as well as liver enzymes and indices of fibrosis, while semaglutide compared to placebo resulted in MASLD resolution in 59% and improvements in hepatic fibrosis, decreasing disease progression.76 Other recent analyses have revealed survodutide, tirzepatide, and retatrutide to each significantly reduce liver fat with no evidence of worsening in markers of liver fibrosis.77, 78, 79
Depression and suicidality
Most studies evaluating depression and GLP-1 RA use were conducted in those with T2DM and indicate no increased risk.80 Adverse drug reporting from FAERS indicates increased reporting of depression and suicidal ideation but not suicidal attempts/behavior with semaglutide and liraglutide. Due to confounding and the pharmacovigilance analytical approach used, causality is inconclusive.81 Most studies have evaluated monotherapeutic agonists. Further investigation of dual and triple agonists is warranted.
Cost effectiveness
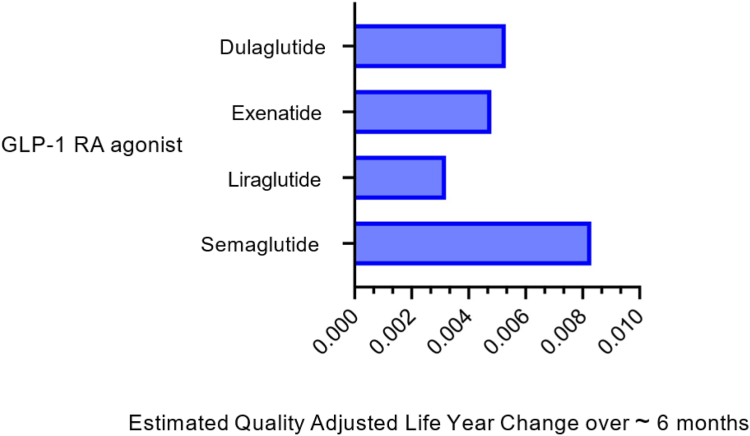
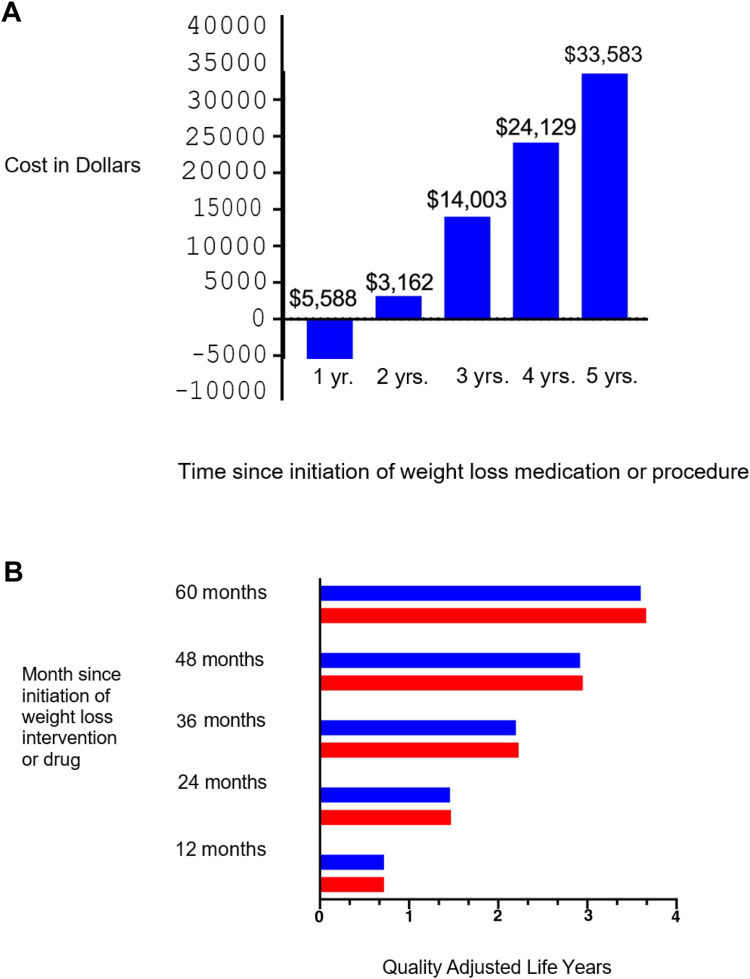
The novelty of the GLP-1 RAs and the dual and triple agonist anti-obesity medications and the demand for the substantial degree of weight loss they induce contribute to their high cost. In short term analyses (∼1/2 year) evaluating quality adjusted life years (QALYs) and incremental cost effectiveness ratios (ICERs), semaglutide was the most cost-effective compared to exenatide, dulaglutide, liraglutide or no treatment82 (Fig. 2). However, at the current annual price of ∼$13,618, semaglutide is less cost effective using these same outcome parameters when compared to endoscopic sleeve gastroplasty over 5 years83 (Fig. 3A and B).
Fig. 2.
Change in quality adjusted life years with use of GLP-1Ras compared to No treatment.
Fig. 3.
A: Cost savings with use of endoscopic sleeve gastroplasty vs. semaglutide over 5 years. B: Quality adjusted life years according to use of endoscopic sleeve gastroplasty vs. subcutaneous semaglutide 2.4 mg for weight loss at different time points.
Future agents
GLP-1/glucagon dual agonists
GLP-1/glucagon dual agonists promote glucose and body weight reduction via regulation of hunger/appetite and increasing energy expenditure. Glucagon agonism additionally acts on hepatocytes to stimulate fatty acid oxidation making GLP-1/glucagon dual agonists a treatment option for metabolic dysfunction-association steatohepatitis (MASH).84
Survodutide
Survodutide is a weekly subcutaneous GLP-1/glucagon dual agonist structurally modified from native glucagon currently in development for treatment of obesity, T2DM and MASH.84
A phase II trial enrolled participants with T2DM and BMI of 25–50 kg/m2 taking Metformin therapy and assigned them to receive survodutide, semaglutide or placebo. Survodutide was found to be equally effective at lowering HbA1c in comparison to semaglutide (Table 4).
Table 4.
Future agents in clinical trials.
| Trial name or authors | Number of patients | Enrollment criteria | Duration of trial | Results | Other information |
|---|---|---|---|---|---|
| Survodutide | |||||
| Blüher M, Rosenstock J et al.32,a | Total = 413 (411 treated) Survodutide DG1 = 50, DG2 = 50, DG3 = 52, DG4 = 50, DG5 = 51, DG6 = 50 Semaglutide = 50 Placebo = 59 |
|
16 weeks | A1c reduction DG1 = −0.91%. DG2 = −1.46%. DG3 = −1.71% DG4 = −1.56% DG5 = −1.63% DG6 = −1.86% Body weight reduction-up to −8.7% (DG6) |
AE 77.7% of survodutide treated participants (mainly gastrointestinal) |
| SYNCHRONIZE-1 (NCT06066515) | Estimated 600 |
|
76 weeks | PENDING |
RECRUITING 1:1:1 Survodutide 3.6 mg v 6.0 mg v. placebo Primary endpoint:
|
| SYNCHRONIZE-2 (NCT06066528) | Estimated 600 |
|
76 weeks | PENDING |
RECRUITING 1:1:1 Survodutide 3.6 mg v. 6.0 mg v. placebo Primary endpoint:
|
| Pemvidutide (ALT-801) | |||||
| MOMENTUM | Total = 391 Pemvidutide 1.2 mg = 40 Pemvidutide 1.8 mg = 40 Pemvidutide 2.4 mg = 41 Placebo = 39 |
|
48 weeks | Body weight reduction Pemvidutide 1.2 mg = −10.3% Pemvidutide 1.8 mg = −11.2% Pemvidutide 2.4 mg = −15.6% Placebo = −2.2% |
|
| IMPACT (NCT05989711) | Estimated 190 |
|
24 weeks |
RECRUITING 1:1:1 Pemvidutide 1.2 mg v. Pemvidutide 1.8 mg v. placebo Primary endpoint:
|
|
| Efinopegdutide | |||||
| Alba, M, Yee, J et al.85 | Total = 343 Efinopegdutide 5.0 mg = 43 Efinopegdutide 7.4 mg = 81 Efinopegdutide 10.0 mg = 72 Liraglutide 3.0 mg = 95 Placebo = 52 |
|
26 weeks | Body weight reduction Efinopegdutide 5.0 mg = −8.5% Efinopegdutide 7.4 mg = −9.8% Efinopegdutide 10.0 mg = −11.8% Liraglutide 3.0 mg = −7.0% Placebo = −1.8% |
The most common AE with treatment of efinopegdutide were gastrointestinal related (mainly nausea) |
| Di Prospero, N, Yee, J et al.86 | Total = 195 (144 completed) Efinopegdutide 5.0 mg = 33 Efinopegdutide 7.4 mg = 30 Efinopegdutide 10.0 mg = 32 Placebo = 47 |
|
12 weeks | Body weight reduction Efinopegdutide 5.0 mg = −5.3% Efinopegdutide 7.4 mg = −6.5% Efinopegdutide 10.0 mg = −7.9% |
The most commone AE with treatment of efinopegdutide were gastrointestinal related (mainly nausea) |
| AMG 133 (MariTide) | |||||
| NCT05669599 | Total = 592 |
|
52 weeks |
ACTIVE NOT RECRUITING Cohort A (without diagnosis of type 1 or T2DM): receive AMG 133 or placebo in 1 of 7 dose cohorts Cohort B (with diagnosis of T2DM): receive AMG 133 or placebo in 1 of 7 dose cohorts Primary endpoint
|
|
| Danuglipron | |||||
| Saxena AR, Frias JP et al.30 | Total = 411 (316 completed treatment) Danuglipron 2.5 mg BID = 68 Danuglipron 10 mg BID = 68 Danuglipron 40 mg BID = 71 Danuglipron 80 mg BID = 67 Danuglipron 120 mg BID = 71 Placebo = 66 |
|
16 weeks | HgB A1c reduction: −0.49% to −1.18% v. −0.02% for placebo Body weight reduction: 80 mg BID: mean −2.04 kg 120 mg BID: mean −4.17 kg Body weight not statistically significant in lower doses |
Safety
|
| NCT04707313 | Total = 630 Cohort 1 (1 week titration to target dose) Danuglipron 40 mg BID Danuglipron 80 mg BID Danuglipron 120 mg BID Danuglipron 160 mg BID Danuglipron 200 mg BID Placebo Cohort 2 (2 week titration to target dose) Danuglipron 120 mg BID Danuglipron 160 mg BID Danuglipron 200 mg BID Placebo Cohort 3 (4 week titration to target dose) Danuglipron 80 mg BID Danuglipron 140 mg BID Danuglipron 200 mg BID |
|
Cohorts 1&2: approximately 9 months Cohorts 3: Approximately 10 months |
Body weight reduction: −6.9% to −11.7% v. +1.4% for placebo (at 32 weeks) −4.8 to −9.4% v. + 0.17% (at 26 weeks)87 |
Most common AE were mild (up to 73% nausea, up to 47% vomiting, up to 25% diarrhea)87
|
| Efpeglenatide | |||||
| AMPLITUDE-M88 | Total = 406 Efpeglenatide 2 mg = 100 Efpeglenatide 4 mg = 101 Efpeglenatide 6 mg = 103 Placebo = 102 |
Inadequately controlled T2DM (A1c ≥ 7 and ≤10%) | 56 weeks | Baseline to week 30, A1c reduction 2 mg: −0.5% 4 mg: −0.8% 6 mg: −1.0% A1c reduction seen at week 30 was maintained at week 56 Body weight reduction, more significant in both 4 mg and 6 mg dose, −2.3 kg and −2.2 kg respectively |
GI events most commonly reported AE. Incidence increased with dose. |
AE, adverse events; T2DM, type 2 diabetes mellitus; NASH, non-alcoholic steatohepatitis; MRI-PDFF, magnetic resonance imaging-proton density fat fraction; HTN, hypertension; OSA, obstructive sleep apnea; CVD, cardiovascular disease; BID, twice daily; TEAE, treatment-emergent adverse eve.
DG (dose group) 1–4, up to 0.3, 0.9, 1.8 or 2.7 mg once weekly; DG 5 and 6, 1.2 or 1.8 mg twice weekly.
Another phase II study, randomized adults with BMI > 27 kg/m2 to receive survodutide (0.6, 2.4, 3.6 or 4.8 mg weekly) vs. placebo over the course of 46 weeks. At the completion of the trial period, body weight loss regardless of dose did not reach a plateau, suggesting more weight loss is possible with longer treatment duration.
SYNCHRONIZE is a global program comprised of phase III clinical trials studying the efficacy of survodutide.89 Similar to other programs studying anti-obesity medications, SYNCHRONIZE-1 and 2 will evaluate survodutide on patients with preobesity and obesity with and without T2DM. SYNCHRONIZE-CVOT is an event driven cardiovascular outcome trial enrolling patients with preobesity or obesity with cardiovascular disease, chronic kidney disease, or risk factors for cardiovascular disease.
Pemvidutide is a weekly subcutaneous GLP-1/glucagon receptor agonist in development for treatment of obesity and MASH.84 In mice models, pemvidutide lowered body weight and liver enzymes while promoting better blood glucose control, improved liver steatosis, inflammation and fibrosis superior to semaglutide.84
MOMENTUM, a phase II clinical trial that enrolled about 320 patients with preobesity and obesity, demonstrated clinically significant weight loss > 5% was observed in all participants receiving pemvidutide 1.2, 1.8 and 2.4 mg weekly. There was a significant reduction in cardiometabolic risk factors with pemvidutide.90 Adverse events were comparable to other GLPI- RA therapies.
GLP-1 RA/GIP receptor antagonist
MariTide is a monthly subcutaneous glucose-dependent insulinotropic polypeptide receptor (GIPR) antagonist conjugated to analog peptide of GLP-1.84 GIP receptor antagonism is thought to promote weight loss due to potential desensitization of GIP receptors by GIP agonsist exposure.84
A phase I trial assigned approximately 50 participants with a BMI between 30 and 40 kg/m2 without diabetes to single ascending dose (SAD), multiple ascending doses (MAD) or placebo.91 The MAD cohort randomized to 420 mg monthly lost 14.5% of their total body weight after receiving 3 doses of treatment and maintained 10% total weight loss approximately 2.5 months after last dose. The most frequent AE noted were gastrointestinal. No discontinuations were observed.
Oral GLP-1 RA
Orfoglipron and danuglipron are being studied for T2DM and obesity treatment. The phase IIb clinical trial studying danuglipron twice daily in participants with obesity had over 50% of patients discontinue treatment in comparison to about 40% with placebo.84 To increase patient adherence, danugliprion will be studied in a once daily formulation.
Future directions
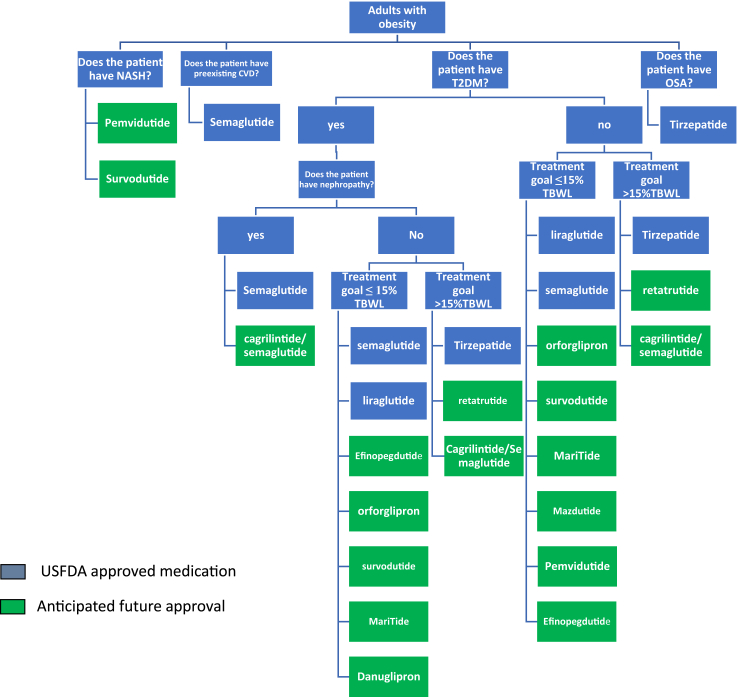
Considering the GLP-1 RAs currently available for treating T2DM and obesity, along with the numerous new GLP-1 RAs, dual, and triple agonists in development, we anticipate a shift in clinical practice towards more personalized and tailored use of these agents to meet individual patient needs. In Fig. 4, we present a proposed algorithm for the management of patients, which reflects this personalized approach.
Fig. 4.
Suggested treatment algorithm for the choice of GLP1-RA, dual, and triple agonists in the treatment of obesity.
Conclusion
The mechanism of action of GLP-1 receptor agonists (GLP-1RAs) offers a multifaceted approach to addressing both type 2 diabetes mellitus (T2DM) and obesity. By enhancing insulin secretion, improving insulin sensitivity, and decreasing hepatic glucose production, GLP-1RAs play a crucial role in regulating blood glucose levels. Additionally, their effects on appetite suppression and satiety contribute to weight loss and management. The wide array of available GLP-1RAs, from short-acting to long-acting formulations, provide clinicians with various options to tailor treatment to individual patient needs. Furthermore, the emergence of novel agents such as tirzepatide, dual and triple hormonal agonists, expands the therapeutic landscape for managing T2DM effectively. While the potential of GLP-1RAs in type 1 diabetes mellitus (T1DM) treatment shows promise, further research is warranted to elucidate their efficacy and safety profile in this population fully. Overall, GLP-1RAs represent a valuable class of medications with diverse applications in diabetes management, offering hope for improved outcomes and better quality of life for patients with diabetes and obesity.
GLP-1 receptor agonists (GLP-1 RAs) in managing obesity have demonstrated significant efficacy in promoting weight loss and improving cardiometabolic parameters. Liraglutide, semaglutide, tirzepatide, orforglipron, retatrutide, and other emerging agents have shown substantial reductions in body weight when used as monotherapy or combined with lifestyle interventions. These agents aid in weight reduction and exhibit beneficial effects on appetite control, glycemic control, and cardiovascular health.
Furthermore, dual and triple receptor agonists, such as tirzepatide and retatrutide, offer additional advantages by targeting multiple pathways involved in energy regulation, resulting in enhanced weight loss and improved metabolic outcomes compared to traditional GLP-1 RAs. Despite the promising efficacy of these agents, it is essential to consider their safety profiles, particularly regarding gastrointestinal adverse effects, which are common but generally transient and manageable. Additionally, concerns regarding the risk of pancreatitis, thyroid disorders, and depression with the use of GLP-1 RAs require careful monitoring and further investigation. While current evidence suggests no significant increase in these risks, ongoing research is needed to understand better the long-term safety and efficacy of these medications in diverse patient populations.
Overall, GLP-1 RAs represent a valuable therapeutic option for individuals with obesity, offering not only significant weight loss but also potential benefits for cardiometabolic health. Continued research and clinical monitoring will help optimize their use and maximize patient outcomes in managing obesity and related metabolic conditions.
Contributors
N.A served as the first author and her contributions included the initial literature search, crafting the outline of the review, as well as the writing and extensive editing of the manuscript. S. W worked on the writing of the comparative analysis section and its figures, in addition to her role in editing. V.J focused on future directions and worked on the literature search and writing and editing of that section. B.B and K.K worked on the role of GLP-1 RA in the treatment of diabetes and in the treatment of obesity with a literature search, writing the specific sections, and the development of tables that enhanced the presentation of our data. F.C.S was the senior author and assisted in the final editing of the manuscript.
Declaration of interests
F.C.S reports grants from the NIH NDDK, she also reports personal consulting fees from Eli Lilly, Novo Nordisk, Boehringer Ingelheim, Pfizer, Rhythm, Currax, and Gelesis. F.C.S also serves on the board of directors of The Obesity Society, she also serves as an obesity section member of the American Heart Association and the American Academy of Pediatrics. Finally, she heads the obesity section at the American Medical Woman’s Association. N.A. reports receiving personal consulting fees from Eli Lilly and Novo Nordisk. And serves on advisory board for Eli Lilly and receiving travel support from Novo Nordisk. K.K. reports research grants (HRSA T32HP32715 (July 2020–June 2022)). V.R.J reports consulting fees from Novo Nordisk and receiving honoraria for lectures given for Eli Lilly and educational institutes. None is related to the work presented herein. All other authors declare no conflict of interest.
References
- 1.Ong K.L., Stafford L.K., McLaughlin S.A., et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfaris N., Alqahtani A.M., Alamuddin N., Rigas G. Global impact of obesity. Gastroenterol Clin. 2023;52(2):277–293. doi: 10.1016/j.gtc.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 3.https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity
- 4.Sattar N., Lee M.M., Kristensen S.L., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 5.Egan J.M. Physiological integration of taste and metabolism. N Engl J Med. 2024;390(18):1699–1710. doi: 10.1056/NEJMra2304578. [DOI] [PubMed] [Google Scholar]
- 6.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabol. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Richards P., Parker H.E., Adriaenssens A.E., et al. Identification and characterization of GLP-1 receptor–expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malbert C.-H., Chauvin A., Horowitz M., Jones K.L. Glucose sensing mediated by portal glucagon-like peptide 1 receptor is markedly impaired in insulin-resistant obese animals. Diabetes. 2021;70(1):99–110. doi: 10.2337/db20-0361. [DOI] [PubMed] [Google Scholar]
- 9.Nauck M.A., Heimesaat M.M., Orskov C., Holst J.J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91(1):301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calanna S., Christensen M., Holst J.J., et al. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care. 2013;36(10):3346–3352. doi: 10.2337/dc13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrera J.G., Sandoval D.A., D’alessio D.A., Seeley R.J. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7(9):507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Bloemendaal L., Veltman D.J., ten Kulve J.S., et al. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metabol. 2015;17(9):878–886. doi: 10.1111/dom.12506. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo R.A., Ratner R.E., Han J., Kim D.D., Fineman M.S., Baron A.D. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 14.Ahren B., Leguizamo Dimas A., Miossec P., Saubadu S., Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M) Diabetes Care. 2013;36(9):2543–2550. doi: 10.2337/dc12-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauck M.A., Quast D.R., Wefers J., Meier J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46 doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker D.J., Buse J.B., Taylor K., et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 17.Blevins T., Pullman J., Malloy J., et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301–1310. doi: 10.1210/jc.2010-2081. [DOI] [PubMed] [Google Scholar]
- 18.Wysham C., Blevins T., Arakaki R., et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) Diabetes Care. 2014;37(8):2159–2167. doi: 10.2337/dc13-2760. [DOI] [PubMed] [Google Scholar]
- 19.Ahmann A.J., Capehorn M., Charpentier G., et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 20.Capehorn M.S., Catarig A.M., Furberg J.K., et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10) Diabetes Metab. 2020;46(2):100–109. doi: 10.1016/j.diabet.2019.101117. [DOI] [PubMed] [Google Scholar]
- 21.Yabe D., Nakamura J., Kaneto H., et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8(5):392–406. doi: 10.1016/S2213-8587(20)30074-7. [DOI] [PubMed] [Google Scholar]
- 22.Frias J.P., Hsia S., Eyde S., et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: a multicentre, randomised, dose-response, phase 2 study. Lancet. 2023;402(10400):472–483. doi: 10.1016/S0140-6736(23)01302-8. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstock J., Wysham C., Frias J.P., et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 24.Frias J.P., Deenadayalan S., Erichsen L., et al. Efficacy and safety of co-administered once-weekly cagrilintide 2.4 mg with once-weekly semaglutide 2.4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402(10403):720–730. doi: 10.1016/S0140-6736(23)01163-7. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstock J., Frias J., Jastreboff A.M., et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402(10401):529–544. doi: 10.1016/S0140-6736(23)01053-X. [DOI] [PubMed] [Google Scholar]
- 26.Filippatos T.D., Elisaf M.S. Effects of glucagon-like peptide-1 receptor agonists on renal function. World J Diabetes. 2013;4(5):190. doi: 10.4239/wjd.v4.i5.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauck M., Rizzo M., Johnson A., Bosch-Traberg H., Madsen J., Cariou B. Once-daily liraglutide versus lixisenatide as add-on to metformin in type 2 diabetes: a 26-week randomized controlled clinical trial. Diabetes Care. 2016;39(9):1501–1509. doi: 10.2337/dc15-2479. [DOI] [PubMed] [Google Scholar]
- 28.Frías J.P., Davies M.J., Rosenstock J., et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 29.Wharton S., Blevins T., Connery L., et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N Engl J Med. 2023;389(10):877–888. doi: 10.1056/NEJMoa2302392. [DOI] [PubMed] [Google Scholar]
- 30.Saxena A.R., Frias J.P., Brown L.S., et al. Efficacy and safety of oral small molecule glucagon-like peptide 1 receptor agonist danuglipron for glycemic control among patients with type 2 diabetes: a randomized clinical trial. JAMA Netw Open. 2023;6(5) doi: 10.1001/jamanetworkopen.2023.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B., Cheng Z., Chen J., et al. Efficacy and safety of mazdutide in Chinese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 2 trial. Diabetes Care. 2024;47(1):160–168. doi: 10.2337/dc23-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blüher M., Rosenstock J., Hoefler J., Manuel R., Hennige A.M. Dose–response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial. Diabetologia. 2024;67(3):470–482. doi: 10.1007/s00125-023-06053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyton J., Jeon M., Brooks A. Glucagon-like peptide 1 receptor agonists in type 1 diabetes mellitus. Am J Health Syst Pharm. 2019;76(21):1739–1748. doi: 10.1093/ajhp/zxz179. [DOI] [PubMed] [Google Scholar]
- 34.Wang W., Liu H., Xiao S., Liu S., Li X., Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2017;8(4):727–738. doi: 10.1007/s13300-017-0282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frandsen C.S., Dejgaard T.F., Holst J.J., Andersen H.U., Thorsteinsson B., Madsbad S. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care. 2015;38(12):2250–2257. doi: 10.2337/dc15-1037. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar G., Alattar M., Brown R.J., Quon M.J., Harlan D.M., Rother K.I. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care. 2014;37(3):666–670. doi: 10.2337/dc13-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau D.C., Krempf M., Astrup A., et al. Liraglutide 3.0 mg reduces body weight and improves cardiometabolic risk factors in adults with overweight/obesity: the SCALE obesity and prediabetes randomised trial. Can J Diabetes. 2015;39:S48–S49. [Google Scholar]
- 38.Wadden T.A., Walsh O.A., Berkowitz R.I., et al. Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity. 2019;27(1):75–86. doi: 10.1002/oby.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly A.S., Auerbach P., Barrientos-Perez M., et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117–2128. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 40.Blüher M., Aras M., Aronne L.J., et al. New insights into the treatment of obesity. Diabetes Obes Metabol. 2023;25(8):2058–2072. doi: 10.1111/dom.15077. [DOI] [PubMed] [Google Scholar]
- 41.Neeland I.J., Marso S.P., Ayers C.R., et al. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes Endocrinol. 2021;9(9):595–605. doi: 10.1016/S2213-8587(21)00179-0. [DOI] [PubMed] [Google Scholar]
- 42.Lundgren J.R., Janus C., Jensen S.B., et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18):1719–1730. doi: 10.1056/NEJMoa2028198. [DOI] [PubMed] [Google Scholar]
- 43.Wharton S., Batterham R.L., Bhatta M., et al. Two-year effect of semaglutide 2.4 mg on control of eating in adults with overweight/obesity: STEP 5. Obesity. 2023;31(3):703–715. doi: 10.1002/oby.23673. [DOI] [PubMed] [Google Scholar]
- 44.Knop F.K., Aroda V.R., do Vale R.D., et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402(10403):705–719. doi: 10.1016/S0140-6736(23)01185-6. [DOI] [PubMed] [Google Scholar]
- 45.Kosiborod M.N., Abildstrøm S.Z., Borlaug B.A., et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–1084. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- 46.Lincoff A.M., Brown-Frandsen K., Colhoun H.M., et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 47.Jastreboff A.M., Aronne L.J., Ahmad N.N., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 48.Garvey W.T., Frias J.P., Jastreboff A.M., et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402(10402):613–626. doi: 10.1016/S0140-6736(23)01200-X. [DOI] [PubMed] [Google Scholar]
- 49.Aronne L.J., Sattar N., Horn D.B., et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA. 2024;331(1):38–48. doi: 10.1001/jama.2023.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.le Roux C.W., Steen O., Lucas K.J., Startseva E., Unseld A., Hennige A.M. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: a randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 2024;12(3):162–173. doi: 10.1016/S2213-8587(23)00356-X. [DOI] [PubMed] [Google Scholar]
- 51.Ji L., Jiang H., Cheng Z., et al. A phase 2 randomised controlled trial of mazdutide in Chinese overweight adults or adults with obesity. Nat Commun. 2023;14(1):8289. doi: 10.1038/s41467-023-44067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Véniant M.M., Lu S.-C., Atangan L., et al. A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nat Metab. 2024;6:290–303. doi: 10.1038/s42255-023-00966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jastreboff A.M., Kaplan L.M., Frías J.P., et al. Triple–hormone-receptor agonist retatrutide for obesity—a phase 2 trial. N Engl J Med. 2023;389(6):514–526. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 54.Jensen S.B.K., Janus C., Lundgren J.R., et al. Exploratory analysis of eating-and physical activity-related outcomes from a randomized controlled trial for weight loss maintenance with exercise and liraglutide single or combination treatment. Nat Commun. 2022;13(1):4770. doi: 10.1038/s41467-022-32307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammoud R., Drucker D.J. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat Rev Endocrinol. 2023;19(4):201–216. doi: 10.1038/s41574-022-00783-3. [DOI] [PubMed] [Google Scholar]
- 56.Vosoughi K., Atieh J., Khanna L., et al. Association of glucagon-like peptide 1 analogs and agonists administered for obesity with weight loss and adverse events: a systematic review and network meta-analysis. EClinicalMedicine. 2021;42 doi: 10.1016/j.eclinm.2021.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown E., Heerspink H.J.L., Cuthbertson D.J., Wilding J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398(10296):262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 58.Lau D.C.W., Erichsen L., Francisco A.M., et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398(10317):2160–2172. doi: 10.1016/S0140-6736(21)01751-7. [DOI] [PubMed] [Google Scholar]
- 59.Enebo L.B., Berthelsen K.K., Kankam M., et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2.4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397(10286):1736–1748. doi: 10.1016/S0140-6736(21)00845-X. [DOI] [PubMed] [Google Scholar]
- 60.Jones K.L., Rigda R.S., Buttfield M.D.M., et al. Effects of lixisenatide on postprandial blood pressure, gastric emptying and glycaemia in healthy people and people with type 2 diabetes. Diabetes Obes Metab. 2019;21(5):1158–1167. doi: 10.1111/dom.13633. [DOI] [PubMed] [Google Scholar]
- 61.Kristensen S.L., Rørth R., Jhund P.S., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 62.Sattar N., McGuire D.K., Pavo I., et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28(3):591–598. doi: 10.1038/s41591-022-01707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryan D.H., Lingvay I., Colhoun H.M., et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–69. doi: 10.1016/j.ahj.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Rivera F.B., Lumbang G.N.O., Gaid D.R.M., et al. Glucagon-like peptide-1 receptor agonists modestly reduced blood pressure among patients with and without diabetes mellitus: a meta-analysis and meta-regression. Diabetes Obes Metab. 2024;26(6):2209–2228. doi: 10.1111/dom.15529. [DOI] [PubMed] [Google Scholar]
- 65.Gragnano F., De Sio V., Calabrò P. FLOW trial stopped early due to evidence of renal protection with semaglutide. Eur Heart J Cardiovasc Pharmacother. 2024;10(1):7–9. doi: 10.1093/ehjcvp/pvad080. [DOI] [PubMed] [Google Scholar]
- 66.Scheen A.J. Dual GIP/GLP-1 receptor agonists: new advances for treating type-2 diabetes. Ann Endocrinol. 2023;84(2):316–321. doi: 10.1016/j.ando.2022.12.423. [DOI] [PubMed] [Google Scholar]
- 67.Dong S., Sun C. Can glucagon-like peptide-1 receptor agonists cause acute kidney injury? An analytical study based on post-marketing approval pharmacovigilance data. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1032199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abd El Aziz M., Cahyadi O., Meier J.J., Schmidt W.E., Nauck M.A. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: a meta-analysis based on cardiovascular outcomes trials. Diabetes Obes Metab. 2020;22(4):699–704. doi: 10.1111/dom.13924. [DOI] [PubMed] [Google Scholar]
- 69.Sodhi M., Rezaeianzadeh R., Kezouh A., Etminan M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA. 2023;330(18):1795–1797. doi: 10.1001/jama.2023.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu W., Song R., Cheng R., et al. Use of GLP-1 receptor agonists and occurrence of thyroid disorders: a meta-analysis of randomized controlled trials. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.927859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.2023. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-23-26-october-2023
- 72.Jalleh R.J., Jones K.L., Nauck M., Horowitz M. Accurate measurements of gastric emptying and gastrointestinal symptoms in the evaluation of glucagon-like peptide-1 receptor agonists. Ann Intern Med. 2023;176(11):1542–1543. doi: 10.7326/M23-2019. [DOI] [PubMed] [Google Scholar]
- 73.Joshi G.P., Abdelmalak B.B., Weigel W.A., et al. 2023 American society of Anesthesiologists practice guidelines for preoperative fasting: carbohydrate-containing clear liquids with or without protein, chewing gum, and pediatric fasting duration-A modular update of the 2017 American society of Anesthesiologists practice guidelines for preoperative fasting. Anesthesiology. 2023;138(2):132–151. doi: 10.1097/ALN.0000000000004381. [DOI] [PubMed] [Google Scholar]
- 74.He L., Wang J., Ping F., et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2022;182(5):513–519. doi: 10.1001/jamainternmed.2022.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng Q., Xu J., Mu X., Shi Y., Fan H., Li S. Safety issues of tirzepatide (pancreatitis and gallbladder or biliary disease) in type 2 diabetes and obesity: a systematic review and meta-analysis. Front Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1214334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newsome P.N., Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79(6):1557–1565. doi: 10.1016/j.jhep.2023.07.033. [DOI] [PubMed] [Google Scholar]
- 77.Sanyal A.J., Bedossa P., Fraessdorf M., et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. 2024;391(4):311–319. doi: 10.1056/NEJMoa2401755. [DOI] [PubMed] [Google Scholar]
- 78.Sanyal A.J., Kaplan L.M., Frias J.P., et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat Med. 2024;30(7):2037–2048. doi: 10.1038/s41591-024-03018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loomba R., Hartman M.L., Lawitz E.J., et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391(4):299–310. doi: 10.1056/NEJMoa2401943. [DOI] [PubMed] [Google Scholar]
- 80.Cooper D.H., Ramachandra R., Ceban F., et al. Glucagon-like peptide 1 (GLP-1) receptor agonists as a protective factor for incident depression in patients with diabetes mellitus: a systematic review. J Psychiatr Res. 2023;164:80–89. doi: 10.1016/j.jpsychires.2023.05.041. [DOI] [PubMed] [Google Scholar]
- 81.McIntyre R.S., Mansur R.B., Rosenblat J.D., Kwan A.T.H. The association between glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: reports to the food and drug administration adverse event reporting system (FAERS) Expert Opin Drug Saf. 2024;23(1):47–55. doi: 10.1080/14740338.2023.2295397. [DOI] [PubMed] [Google Scholar]
- 82.Hu Y., Zheng S.L., Ye X.L., et al. Cost-effectiveness analysis of 4 GLP-1RAs in the treatment of obesity in a US setting. Ann Transl Med. 2022;10(3):152. doi: 10.21037/atm-22-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haseeb M., Chhatwal J., Xiao J., Jirapinyo P., Thompson C.C. Semaglutide vs endoscopic sleeve gastroplasty for weight loss. JAMA Netw Open. 2024;7(4) doi: 10.1001/jamanetworkopen.2024.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melson E., Ashraf U., Papamargaritis D., Davies M.J. What is the pipeline for future medications for obesity? Int J Obes. 2024 doi: 10.1038/s41366-024-01473-y. [DOI] [PubMed] [Google Scholar]
- 85.Alba M., Yee J., Frustaci M.E., Samtani M.N., Fleck P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose-ranging study. Clin Obes. 2021;11(2) doi: 10.1111/cob.12432. [DOI] [PubMed] [Google Scholar]
- 86.Di Prospero N.A., Yee J., Frustaci M.E., Samtani M.N., Alba M., Fleck P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with type 2 diabetes mellitus and obesity: a randomized dose-ranging study. Clin Obes. 2021;11(2) doi: 10.1111/cob.12433. [DOI] [PubMed] [Google Scholar]
- 87.Pfizer . 2023. Pfizer announces topline phase 2b results of oral GLP-1R agonist, danuglipron, in adults with obesity. [Google Scholar]
- 88.Frias J.P., Choi J., Rosenstock J., et al. Efficacy and safety of once-weekly efpeglenatide monotherapy versus placebo in type 2 diabetes: the AMPLITUDE-M randomized controlled trial. Diabetes Care. 2022;45(7):1592–1600. doi: 10.2337/dc21-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ingelheim B. 2023. Phase III studies to investigate survodutide for people living with obesity and overweight, with and without diabetes, Cardiovascular disease and chronic kidney disease. [Google Scholar]
- 90.Altimmune . 2023. Altimmune announces positive topline results from MOMENTUM 48-week phase 2 obesity trial of pemvidutide. [Google Scholar]
- 91.Klein S., Nestor J.J., Harris M.S., et al. 334-OR: pemvidutide (ALT-801), a balanced (1: 1) GLP-1/glucagon dual receptor agonist, induces rapid and marked weight loss without the need for dose titration in people with overweight/obesity. Diabetes. 2022;71(Supplement_1):334. [Google Scholar]