Abstract
Nitric Oxide (NO) regulates important physiological functions. Garlic (Allium sativum) is an important food component consumed fresh and processed for thousands of years. It has high L-arginine, which contributes to the NO system in the body. Both garlic and NO impact important physiological processes. Here we produced brown garlic, with significantly higher nutritional and therapeutic value compared to fresh and black garlic. Lower exhaled NO was recorded in asthmatic mice fed with brown garlic but with higher blood SNOs and no change in eNOS and iNOS expression. Lung biopsy showed reduced eosinophil accumulation in asthmatic mice fed with brown garlic. Real-time PCR and Western blot analyses indicated high expression of antioxidant genes but reduced interleukin genes, IL-4, IL-5, IL-6, IL-13, IL1β, and TNF-α brown garlic-fed asthmatic mice as compared to that in fresh and black garlic-fed asthmatic mice. This study provides the first comprehensive and conclusive insight into the nutritional benefits of brown garlic and its therapeutic value for the treatment of asthma in animals.
Keywords: Brown garlic, Nitric oxide, Asthma, eNOS, iNOS
Highlights
-
•
A processing method was developed for the production of brown garlic at 65 °C for 95 h.
-
•
Brown garlic carries a significantly higher nutritional and therapeutic value than fresh and black garlic.
-
•
Brown garlic has high quantities of L-arginine, free amino acids, and modulates nitric oxide synthase (NOS)-like activity.
-
•
Brown garlic protects against asthma by modulation of NOS activity, and global S-Nitrosothiols.
-
•
Brown garlic activates antioxidant defense and expression of inflammation-related genes in the lungs.
1. Introduction
In animals, nitric oxide (NO) is produced by the nitric oxide synthase (NOS) via a two-step oxidation of L-Arginine, producing citrulline as a by-product. The reaction uses reduced NADPH as the electron donor, (6 R-)-5,6,7,8-tetrahydrobiopterin (BH4), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and calmodulin (CaM) as cofactors, and oxygen as co-substrate [1]. This indicates that the bioavailability of Arginine is an important requirement for NO production. The discovery that NO acts as a signaling molecule and is involved in vasodilation, allowed scientists to understand in part the process of blood vessel relaxation. On the other hand, black garlic (Allium sativum L., Alliaceae) is produced by slowly heating regular garlic for several weeks under high humidity and then drying it. This process results in a dark color and sticky texture, while also removing the sharp flavor of regular garlic and replacing it with a more subtle and mellow taste. Fermented or black garlic originated in Asia, although the exact location is not clearly known. In their research paper published in the Journal of Functional Foods, Kim, Kang [2] report that black garlic has higher levels of antioxidants than regular garlic due to the heating process involved in its production, which increases free-radical-fighting phenolic acids and flavonoids. It is pertinent to mention that the nutrient composition of black garlic is significantly higher than that of fresh garlic. Furthermore, the useful effects of garlic (whether used raw, aged, or powdered) have been attributed to the presence of bioactive metabolites including S-allyl-cysteine (SAC), E/Z-ajoene, diallyl thiosulfonate (allicin), S-allyl-cysteine sulfoxide (alliin), S-allylmercaptocysteine (SAMC), gallic acid, rutin, protocatechuic acid, diallyl trisulfide (DATS), quercetin, diallyl sulfide (DAS), and diallyl disulfide (DADS) among others. These water, oil, and alcohol soluble metabolites are responsible for antioxidant activity via activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway [3]. Black garlic can be produced under a variety of conditions involving variable heat, humidity, and time of treatment each resulting in unique shades of brown to black and nutrient composition. However, as a general rule, the time required decreases as the incubation temperature increases. Garlic has been an essential part of the human diet for centuries. Apart from its dietary use, garlic has been used in preventive and curative medicine for long [4]. The medicinal properties of Garlic are associated with its wide array of phenolic and other bioactive compounds [5] and sulfur compounds including hydrogen sulfide (H2S) [3].
Black garlic is the most sold garlic among processed garlic products. It is easy to eat and has improved functionality by reducing the pungent taste and smell of garlic. Also, black garlic can contribute to improving hyperlipidemia by scavenging cholesterol [6] and inhibiting the progression of atherosclerosis by reducing body weight and blood lipids [7,8]. Additionally, the strong antioxidant activity of black garlic has been proven both in vitro and in vivo [9]. However, due to the immature manufacturing process, there is a possibility that substances harmful to the human body such as benzopyrene, a carcinogen belonging to polyaromatic hydrocarbons may be generated at high temperatures.
In animals, three different isoforms of nitric oxide synthase (NOS) enzyme are responsible for NO production from L-arginine [10,11]. NOS enzymes have been reported from different bacterial genera including Staphylococcus, Bacillus, Streptomyces, and Deinococcus though structurally not very similar to the animal NOS vitro [12,13]. Though a canonical NOS enzyme has not bee identified in plants, sveral NOS-like enzymes in plants have been reported (reviewed by Hussain, Imran [14]). In plants, NO is produced through at least eight prominent enzymatic (oxidative and reductive) and non-enzymatic pathways and in different cellular compartments [14]. The nitrate reductase (NR)-dependent NO production from NO3− and NO2− appears to be a major reductive route in the thylakoids and peroxisomes [14]. Garlic is known as a significantly rich source of L-arginine [10,11], the main source of NO production in animals and an important NO source in plants [[15], [16], [17]] which is why garlic is known to increase NO production in animals [18]. Fermented or aged garlic contains NO2− produced via bioconversion by bacteria during the fermentation process which is why fermented garlic is known to improve vascular function by improving NO availability in the vasculature [19]. In rats, L-arginine augments the protective antioxidant effects of garlic during acetic acid-induced ulcerative colitis [20]. Garlic activates calcium-dependent NOS activity and NO production in a dose-dependent manner [21,22] providing a strong base for its therapeutic effects on cardiovascular problems.
In simple terms, asthma is a chronic respiratory disease that causes the air passages in the lungs to become inflamed and constricted, making it difficult to breathe. Common symptoms include coughing, shortness of breath, chest tightness, and wheezing. Asthma can be triggered by various factors such as allergens, irritants, respiratory infections, physical activity, and weather changes. Asthma may be a minor nuisance for some people. Yet, it can be a major life-threatening situation for others. While there is no permanent cure for asthma, it can be managed with medication and by avoiding the triggers. In animals, the pulmonary epithelium and the lower and upper airways are a rich source of NOS enzyme (eNOS, iNOS, and nNOS) and NO is usually present in the exhaled breath. Inflammation of the airways is usually associated with an increase in the expression of the inducible NOS (iNOS) subsequently increasing NO production which results in higher levels of NO in exhaled breath [23]. NO measurement in exhaled air is rapidly becoming a non-invasive test for diagnosing, treating, and monitoring asthma [24]. However, NO bioactivity in the lungs and its role in asthma is much more complex and depend on several factors such as the activity of NO-producing enzymes, the presence and activity of competitive enzymes that use the same substrate, for example, the arginase enzyme, the quantity of substrate available for NO production, the presence of various reactive oxygen species (ROS), NO detoxification processes and others [25].
As described above, arginine is the main substrate for NO production by the NOS enzymes. However, it is also a substrate for the Arginase enzyme. In the lungs, the expression of arginase is strongly induced by cytokines, especially the interleukin (IL)-4 and IL-13 produced in asthmatic airways [23]. Hence, arginine appears to be a limiting factor for the production of NOS-dependent NO production during asthma. In this study, we investigated brown garlic as a new, improved, and high-efficiency arginine-rich food source. We also investigated the therapeutic value of brown garlic for treating asthma in animals.
2. Materials and methods
2.1. Garlic cooking under pressure and preparation of garlic extracts
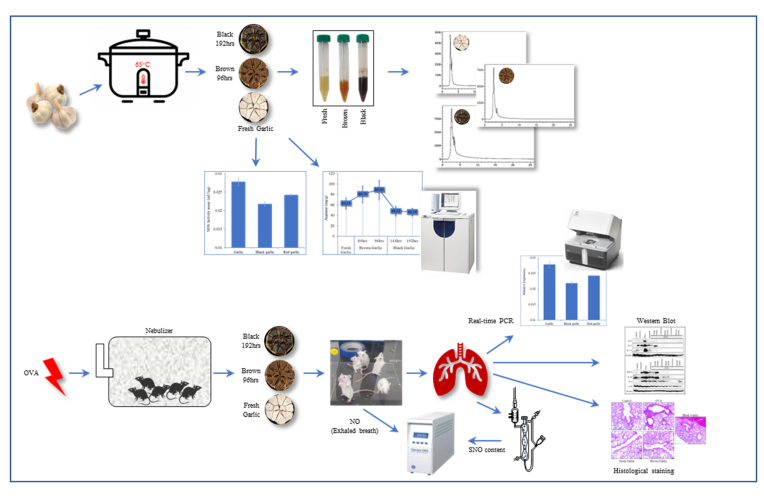
Whole garlic bulbs (Cultivar Uiseong) were purchased from the open market. Brown and black garlic bulbs were prepared by cooking under pressure in an electric pressure cooker (Cuchen CJS-FC0609K, Korea) at 65 °C. Garlic bulbs were cooked for 48 h s, 84 h s, 96 h s (producing brown garlic), 144 h s, and 192 h s (producing black garlic). Next, 30 g of garlic from each time point was crushed and squeezed thoroughly in 4 ml of distilled water in a muslin cloth and centrifuged at 10,000 rpm for 1 min. The supernatant was used for inductively coupled plasma (ICP) analysis, NOS-like activity assay, and mouse feeding. Fresh uncooked garlic bulbs were used as a comparative control during the experiments (Supplementary Fig. 1).
2.2. High-performance liquid chromatography (HPLC) analysis
HPLC analysis was performed using the Shimadzu Prominence HPLC system (Shimadzu, Kyoto, Japan) to detect benzopyrene in the cooked garlic. For this purpose, the uncooked and cooked garlic extracts were diluted 10 times and filter sterilized by passing through a 0.2 μm syringe filter Sterlitech, Auburn, WA, USA). Next, 10 μl of filtered samples and 100 ppm of benzopyrene (95 % HPLC grade, Sigma-Aldrich, USA) as a standard, were injected in the HPLC machine using a C18 column (4.6 × 250 mm, 5 μm). Mobile phase (DW: acetonitrile) was applied at a ratio of 2–8 with a flow rate of 1 ml/min. The temperature of the column was adjusted to 35 °C for a total of 40 min s running time. The detection wavelengths were set at 294 nm and 404 nm.
2.3. Determination of constituent and free amino acids
The quantification of constituent amino acids was carried out according to the method described by Ref. [26]. Briefly, 100 mg of uncooked and cooked finely ground garlic bulb samples were hydrolyzed under vacuum in 6N HCl at 110 °C followed by 80 °C for 24 h s. The dried-up remains were homogenized in 0.02N HCl and were passed through a 0.45-mm PTFE syringe filter (Sterlitech, Auburn, WA, USA). A total of 20 μl samples were injected and analyzed in High-speed amino acid analyzer L-8900 (Hitachi, Japan) fixed with a Hitachi custom ion exchange resin column (4.6 mm; ID’ 60 mm L) and column temperature of 50 °C. Buffers of pH range 1, 2, 3, and 4 were used as a mobile phase and detected at 570 and 440 nm. Free amino acids do not form peptides and exist as single molecules. Free amino acid contents were analyzed by adding 1 ml of each of the garlic extracts to 10 ml of 6N HCL in a test tube and filtered through nitrogen gas for 1 min. After that, the samples were hydrolyzed in a 100 °C dry oven for 24 h and cooled at room temperature in the dark. The samples were briefly centrifuged and 5 ml of the supernatant was transferred into 50 ml of glass volumetric flask and massed up with pure distilled water before filtering through Advantec filter paper (No. 2, Advantec, Japan). Next, the solution was filtered through a 0.2-μm syringe filter and 20 μl samples were injected and analyzed as described earlier.
2.4. Nitric oxide synthase-like activity assay
The calorimetric NOS activity assay kit (Cat. No. AB211083: Abcam, Cambridge, USA) was used for measuring the NOS-like activity according to the manufacturer's instructions. Based on this colorimetric assay, the NO produced by NOS participates in a series of reactions with Griess reagent and generates a colored product. Absorbance was determined at 540 nm in a microplate reader (Multiskan GO, Thermo Scientific,
Germany). Briefly, 100 mg of uncooked and cooked finely ground garlic bulb samples were homogenized in 200 μl of ice-cold NOS assay buffer and centrifuged at 10,000 rpm for 10min. Next, 50 μl (containing around 200–300 μg protein) supernatant was used for reaction with NOS cofactors, NOS substrate, nitrate reductase, and Griess reagents 1 and 2. Colorimetric detection was carried out by recording the absorbance at 540 nm in a microplate reader (Multiskan GO, Thermo Scientific, Germany).
2.5. Mice experiments
A brief flow chart of the mice experiments has been shown in Supplementary Fig. 1. A total of 25 male BALB/c mice (age, 5 weeks; weight, 18–20 g) were supplied by RaonBio, Inc., South Korea, and grown at 22 ± 2 °C and 50 ± 10 % humidity in a 12-h light/dark cycle. The Kyungpook National University Industry Foundation approved this study for animal experiments (approval no. 2018–0140). For research purposes, ovalbumin (OVA) is commonly used to induce asthma in mice. Several published studies report the use of OVA to study acute models of hypereosinophilic asthma [[27], [28], [29]]. The mice were divided into 5 groups: Control, OVA (Ovalbumin), OVA with fresh garlic, OVA with brown garlic, and OVA with black garlic. A total of 5 mice were used for each treatment. The mice were intraperitoneally injected with 50 μl of OVA (Sigma-Aldrich, USA) and 1 mg of aluminum hydroxide (Sigma-Aldrich, USA) in 200 μl of PBS on days 0 and 14. For the garlic treatments, mice were given 100 mg/kg body weight of uncooked, brown, and black garlic 2 h before OVA application. Subsequent OVA treatments were done in a nebulizer for 20 min on the 21st, 22nd, and 23rd day. The control groups were treated with sterile PBS during all experiment steps. Afterward, the mice were euthanized by cervical dislocation after 24 h s of the final OVA treatment.
2.6. Measurement of NO in exhaled breath of mice
After 24 h of the treatment, 5 mice from each treatment group were shifted to separate containers and left to acclimatize for 10min after which the containers were sealed airtight and the mice were allowed to breathe for 15min to collect NO gas inside the container. The collected NO gas from the containers was measured for 10 min continuously through the connected Sievers NO analyzer (NOA280i, Sievers, USA).
2.7. SNO measurements in mouse blood
Blood samples were drawn from the abdominal aorta of the mice after anesthetizing and before euthanizing and centrifuged at 750 g for 7 min. Next, 500 μl of the supernatant was transferred to the same volume of PBS in a fresh tube. These were then passed through Sephadex G25 (NAP-25, Amersham) columns via gravity flow to remove nitrate and small thiols. S-nitrosothiols (SNOs) were measured in the 100 μl filtered samples. Acidified KI buffer (5 ml of acetic acid, 50 mg of KI, 200 mM CuSO4) was used as a reducing agent in the purge vessel.
2.8. Lung histological staining
After the termination of the experiments, we performed lung biopsy of the mice to detect OVA-induced asthma and the effects of garlic supplementation in the diet. For this purpose, the staining was performed as described by Kim, Jang [30]. Briefly, the right lung lobes were fixed with 4 % formalin at 4 °C for 48 h, embedded in paraffin, and sliced into 4 μm thin sections. The sections were stained with hematoxylin and eosin (H&E) for 5 min, respectively, and observed using a Leica AF6000 CRTD microscope.
2.9. Real-time PCR
The lung tissues were homogenized in Trizol (BioScience Technology, Korea) for extraction of total RNA. Next, cDNA was synthesized using a PrimeScript™ 1st strand cDNA synthesis kit (Takara, Japan) with oligo-dT primers according to the manufacturer's instructions. Quantitative PCR was performed using the StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific, USA) with 10 μl Power SYBR®-Green PCR Master mix (Thermo Fisher Scientific, USA) following the manufacturer's instructions. Gene expression values were normalized with β-actin. The sequences of all the primers used are listed in Table 1.
Table 1.
List of primers used for real-time quantitative PCR.
| Gene | Primer Sequence (5′-3′) |
|---|---|
| IL-4 | F: 5′-CCACGGAT GCGACAAAAATC-3′ R: 5′-GACGTTTGGCACATCCAT CTC-3′ |
| IL-5 | F: 5′-GATGGACGCAGGAGGATCAC-3′ R: 5′-GTGTGGCATCCCTCAGCAA-3′ |
| IL-6 | F: 5′-GTTGTGCAATGGCAATTCTGA-3′ R: 5′-TTGGTAGCATCCATCATTTCTTTG-3′ |
| IL-13 | F: 5′-GGCCAGCCCACAGTTCTACA-3′ R: 5′-ACCACCAAGGCAAGCAAGAG-3′ |
| IL-1β | F: F: 5′-CCCCAGGGCATGTTAAGG A-3′ R: 5′-TGACCCTGAGCGACCTGTCT-3′ |
| TNF-α | F: 5′-AGGACCCAGTGTGGGAAGCT-3′ R: 5′-AAAGAGGCAACAAGGTAGAGA-3′ |
| CAT | F: 5′-CGA CCAGGGCATCAAAAACT-3′ R: 5′-ATTGGCGATGGCATTGAAA-3′ |
| SOD1 | F: 5′-GACTTGGGCAAAGGTGGAAA-3′ R: 5′-CAGGGAATGTTTACTGCGCAAT-3′ |
| SOD2 | F: 5′-TGCTCTTGATTGAACATTTTCGTTA-3′ R: 5′-GCCCCCCAAAACAGAGATG-3′ |
| GPX1 | F: 5′-AGAAAGCGATGCCACGTGAT-3′ R: 5′-GGAGATGTTGGGACTCAAACG-3′ |
| eNOS | F: 5′-CAACGCTACCACGAGGACATT-3′ R: 5′-CTCCTGCAAAGAAAAGCTCTGG-3′ |
| iNOS | F: 5′-CTTGGAGCGAGTTGTGGATTGTC-3′ R: 5′-TAGGTGAGGGCTTGGCTGAGTG-3′ |
| β-actin | F: 5′-GGCTCTTTTCCAGCCTTCCT-3′ R: 5′-GTCTTTACGGATGTCAACGTCACA-3′ |
2.10. Western blot
Lung tissues from all the mice groups were lysed in PRO-PREP™ for Cell/Tissue Protein Extraction Solution (iNtRron Biotechnology, Korea) according to the manufacturer's instructions. The extracted proteins were quantified using a Pierce™ BCA protein assay kit (Thermo Fisher Scientific, USA). A total of 20 μg proteins from each sample were separated in 10 % SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5 % skimmed milk in a 1 × TBST (Tris-buffered saline, 0.1 % Tween 20) containing 0.05 % Tween-20 at room temperature for 1hr and incubated with primary antibodies (Supplementary Table 1) at 4 °C overnight. Next, the blots were washed three times with 1x TBST buffer and incubated with anti-mouse HRP-conjugated secondary antibodies (goat anti-mouse IgG-HRP; Santa Cruz Biotechnology, USA) diluted at 1:5000 in 3 % skimmed milk in 1x TBST buffer at room temperature for 1 h. The membranes were washed three times with 1x TBST and the protein bands were detected with the SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Cat. no. 34580; Thermo Fisher Scientific, USA) and quantified in ImageQuant™ 500 (Amersham, USA). The protein expression level of β-actin was used as a control.
2.11. Statistical analysis
Data were collected from ≥3 independent experiments. Data were analyzed to determine the means and standard deviations in SPSS (Version 20; IBM Corp, USA). Data were analyzed using one-way ANOVA and Bonferroni's multiple comparison test at P = 0.05.
3. Results
3.1. Brown and black garlic production
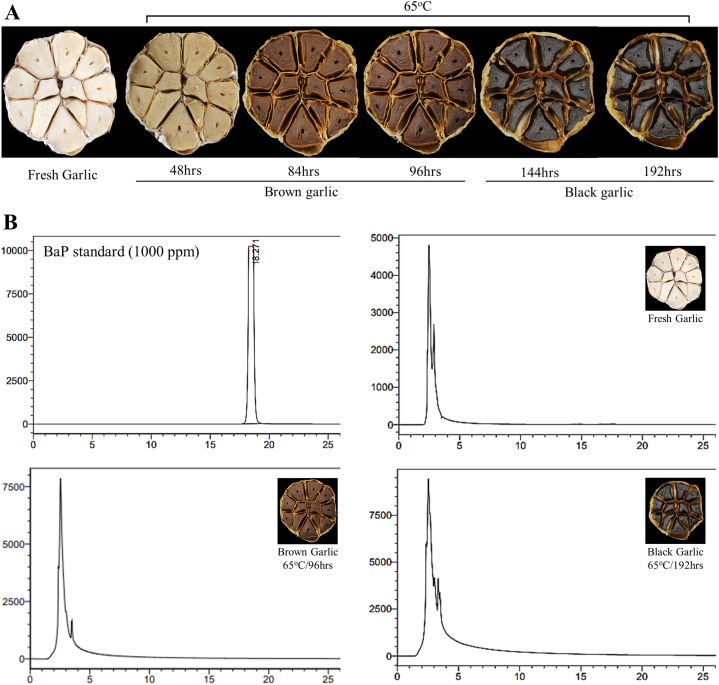
The cooking process involved heating whole garlic bulbs at 65 °C for 48 h s, 84 h s, 96 h s, 144 h s, and 192 h s in a pressure cooker. As expected, the fresh or uncooked garlic was off-white to beige or candlelit beige colored (Hex: f1ede1). However, the bulbs turned to an earthy cane (Hex: c4b08 b) color after cooking for 48 h s, and brown (Hex: 6a482c), and black bean (Hex: 4f4b48) color after being cooked for 84 to 96, and 144–192 h s, respectively (Fig. 1A). Benzo[a]pyrene (BaP) is the main polycyclic aromatic hydrocarbon found in cigarette smoke as well as smoked and grilled food products. Growing evidence shows the toxic effects of BaP in grilled food. Therefore, to determine the suitability of our cooking process and detect any BaP, we performed an HPLC analysis of the fresh and cooked garlic bulbs against a 1000 ppm BaP standard. For this purpose, we used fresh, and brown garlic bulbs cooked for 96 h s and black garlic bulbs cooked for 192 h s. We did not find BaP in any of the fresh or cooked garlic bulbs as opposed to a sharp peak observed for BaP standard after 18.2 min (Fig. 1B). These results indicate a significant utility and suitability of the cooking procedure adopted in this study.
Fig. 1.
Brown and black garlic cooking temperatures.
Garlic samples were heated at 65 °C for different time points to produce brown and black garlic (A). The garlic fresh, brown (65 °C/96 h s), and black (65 °C/192 h s) samples were subsequently analyzed to detect Benzo[a]pyrene (BaP) by HPLC analysis and compared to fresh garlic and 1000 ppm of BaP standard (B).
3.2. Amino acid profile of fresh, brown, and black garlic
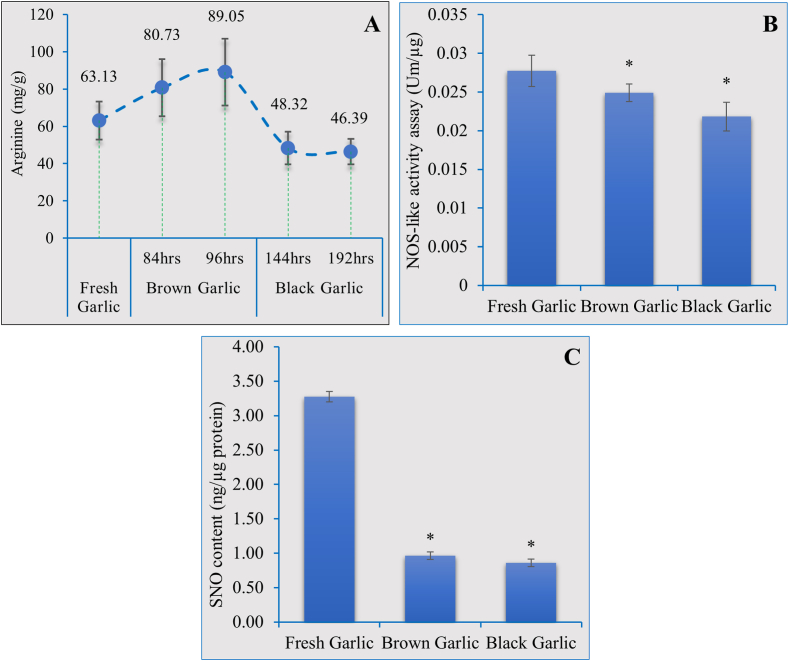
To determine the impact of the cooking process on the amino acid profile of garlic, the constituent (Table 2) and free amino acid (Table 3) content of fresh, brown, and black garlic bulbs was measured. Significant differences between the constituent and free amino acids of fresh, brown, and black garlic were recorded with respect to the different cooking times. To our interest, the arginine content of garlic increased significantly as the heat treatment under pressure proceeded, with an increase of up to 80.73 mg/g of brown garlic after 84 h s, ultimately reaching its peak value of 89 mg per gram of brown garlic after 96 h s (Table 2 and Fig. 2A) as compared to a maximum value of 48.3 mg/g of black garlic. Similarly, other amino acids such as glutamic acid, and aspartic acid were also found to be significantly higher in the brown garlic compared to the fresh and black garlic bulbs. Yet, the concentration of amino acids such as threonine, serine, alanine, and others decreased in both the brown and black garlic bulbs (Table 2). With the above results, we concluded that cooking garlic bulbs at 65 °C for 96 h s and 192 h s was the optimum condition for making brown and black garlic, respectively, and for attaining the maximum quantity of several essential amino acids. Further, we also measured the free amino acid content of the garlic bulbs cooked for 94 and 192 h (Table 3). Interestingly, we recorded the lowest concentration of free arginine (0.95 mg/g) in black garlic compared to 1.07 mg/g in brown garlic and 1.28 mg/g in fresh garlic bulbs with the highest quantity of total free amino acids recorded in the brown garlic followed by black and fresh garlic bulbs (Table 3).
Table 2.
Constituent amino acid profile of fresh, brown, and black garlic.
| Constituent amino acid quantity (mg/g) | Fresh Garlic | Brown Garlic 84 h s |
Brown Garlic 96 h s |
Black Garlic 144 h s |
Black Garlic 192 h s |
|---|---|---|---|---|---|
| Aspartic acid | 20.77 ± 0.63 a | 21.20 ± 3.42 a | 19.25 ± 7.78 a | 9.81 ± 2.33 b | 11.20 ± 2.86 b |
| Threonine | 4.41 ± 0.72 a | 3.36 ± 0.30 ab | 3.02 ± 1.06 bc | 1.91 ± 0.41 c | 2.02 ± 0.46 c |
| Serine | 4.53 ± 2.22 a | 3.99 ± 0.46 a | 3.31 ± 1.38 a | 2.39 ± 0.62 a | 2.54 ± 0.84 a |
| Glutamic acid | 30.48 ± 5.23 a | 38.02 ± 2.76 a | 34.31 ± 12.02 a | 24.91 ± 3.98 a | 25.97 ± 9.88 a |
| Glycine | 2.61 ± 1.69 a | 1.31 ± 0.16 a | 1.72 ± 0.40 a | 1.61 ± 0.87 a | 1.68 ± 0.79 a |
| Alanine | 8.05 ± 2.23 a | 3.50 ± 0.29 b | 4.63 ± 1.25 b | 3.30 ± 0.47 b | 3.52 ± 0.45 b |
| Cystine | 5.02 ± 6.61 a | 10.86 ± 0.55 a | 8.57 ± 6.13 a | 5.29 ± 3.69 a | 5.23 ± 3.92 a |
| Valine | 6.67 ± 1.83 a | 6.43 ± 0.22 ab | 5.55 ± 1.99 ab | 3.59 ± 0.96 b | 4.21 ± 0.86 ab |
| Methionine | 1.88 ± 0.67 a | 1.54 ± 0.18 ab | 1.63 ± 0.28 ab | 0.98 ± 0.31 b | 1.11 ± 0.35 ab |
| Isoleucine | 3.38 ± 1.17 a | 1.76 ± 0.25 b | 1.51 ± 0.23 b | 1.17 ± 0.29 b | 1.18 ± 0.29 b |
| Leucine | 6.72 ± 1.68 a | 2.82 ± 1.07 b | 2.09 ± 0.52 b | 1.58 ± 0.42 b | 1.62 ± 0.47 b |
| Tyrosine | 4.66 ± 2.20 a | 2.64 ± 0.51 a | 3.44 ± 0.68 a | 2.60 ± 0.91 a | 2.80 ± 0.76 a |
| Phenylalanine | 7.95 ± 0.73 a | 7.43 ± 0.86 a | 7.91 ± 1.11 a | 5.87 ± 0.99 a | 6.00 ± 1.04 a |
| Lysine | 9.77 ± 2.08 ab | 10.67 ± 1.39 ab | 11.17 ± 2.32 a | 7.12 ± 0.89 b | 6.98 ± 1.14 b |
| Ammonia | 8.17 ± 2.31 | 6.34 ± 0.09 | 7.16 ± 1.05 | 4.83 ± 0.58 | 5.30 ± 0.75 |
| Histidine | 4.24 ± 0.50 a | 2.67 ± 0.40 b | 2.87 ± 0.61 b | 1.54 ± 0.27 c | 1.49 ± 0.33 c |
| Arginine | 63.13 ± 10.20 bc | 80.73 ± 15.24 ab | 89.050 ± 17.92 a | 48.32 ± 8.73 c | 46.39 ± 6.83 c |
| Proline | 9.45 ± 2.91 a | 8.29 ± 0.93 a | 8.34 ± 3.72 a | 7.09 ± 1.10 a | 6.80 ± 1.09 a |
| Total amino acid | 197.08 ± 20.04 a | 207.23 ± 24.36 a | 205.04 ± 54.76 a | 129.11 ± 17.57 b | 130.76 ± 28.22 b |
All values represent means ± SD of three replications. The different letters indicate significant differences between the means according to Duncan's multiple range test (DMRT) at P = 0.05.
Table 3.
Free amino acid profile of fresh, brown, and black garlic.
| Free amino acids (mg/g) | Fresh Garlic | Brown Garlic (96 h s) | Black Garlic (192 h s) |
|---|---|---|---|
| Taurine | 0.01 | 0.13 | 0.11 |
| Aspartic acid | 0.32 | 1.54 | 0.40 |
| Glutamic acid | 0.06 | 0.30 | 0.18 |
| Alanine | 0.10 | 0.61 | 0.62 |
| Citrulline | 0.10 | 0.16 | 0.12 |
| Cystine | 0.03 | 0.65 | 0.26 |
| Leucine | 0.07 | 0.23 | 0.21 |
| Tyrosine | 0.38 | 0.62 | 0.44 |
| Ethanol amine | 0.01 | 0.05 | 0.06 |
| Serine | 0.33 | 0.49 | 0.29 |
| Lysine | 0.94 | 1.78 | 1.27 |
| Arginine | 1.28 | 1.07 | 0.95 |
| Hydroxy proline | 0.04 | 0.10 | 0.00 |
| Proline | 0.61 | 1.16 | 0.98 |
| Total free amino acids | 5.89 | 12.92 | 10.51 |
Fig. 2.
NOS-like activity and the accumulation of S-Nitrosothiols in garlic.
3.3. NOS-like activity assay and S-nitrosothiol accumulation
In plants, Nitric Oxide Synthase (NOS)-like activity has been reported where arginine is converted to NO and citrulline [31,32]. As we recorded a significantly higher quantity of constituent arginine and free citrulline in brown garlic bulbs as compared to the fresh and black garlic bulbs (Table 3), we performed NOS-like activity assay to determine whether the significantly higher arginine content of brown garlic contributes to NO production via the NOS-like activity. Results indicated that the NOS-like activity in brown garlic is significantly lower than in fresh garlic but higher than in the black garlic bulbs (Fig. 2B). NO exerts its effect via the canonical post-translational modification (PTM) of cysteine thiols called S-Nitrosation to form S-nitrosothiols (SNOs). We, therefore, measured the global SNO content in the garlic samples. Results showed significantly lower SNO contents in the brown and black garlic bulbs (Fig. 2C).
Amino acid analysis indicated the highest quantity of Arginine in the brown garlic samples which is the main substrate for nitric oxide synthesis via NOS-like activity (A). Subsequently, NOS activity was measured in the fresh and brown garlic samples (B). Furthermore, the contribution of high arginine and subsequence NOS-like activity to the global S-Nitrosothiol levels was also determined by SNO measurements through Siever's NO-analyzer (C). Each data point represents the mean ± SD of three independent replications. Asterisk (*) indicates significant differences between the means of the brown and garlic compared to fresh garlic samples at P = 0.05.
3.4. Impact of garlic consumption on exhaled NO and serum S-Nitrosothiols in mice
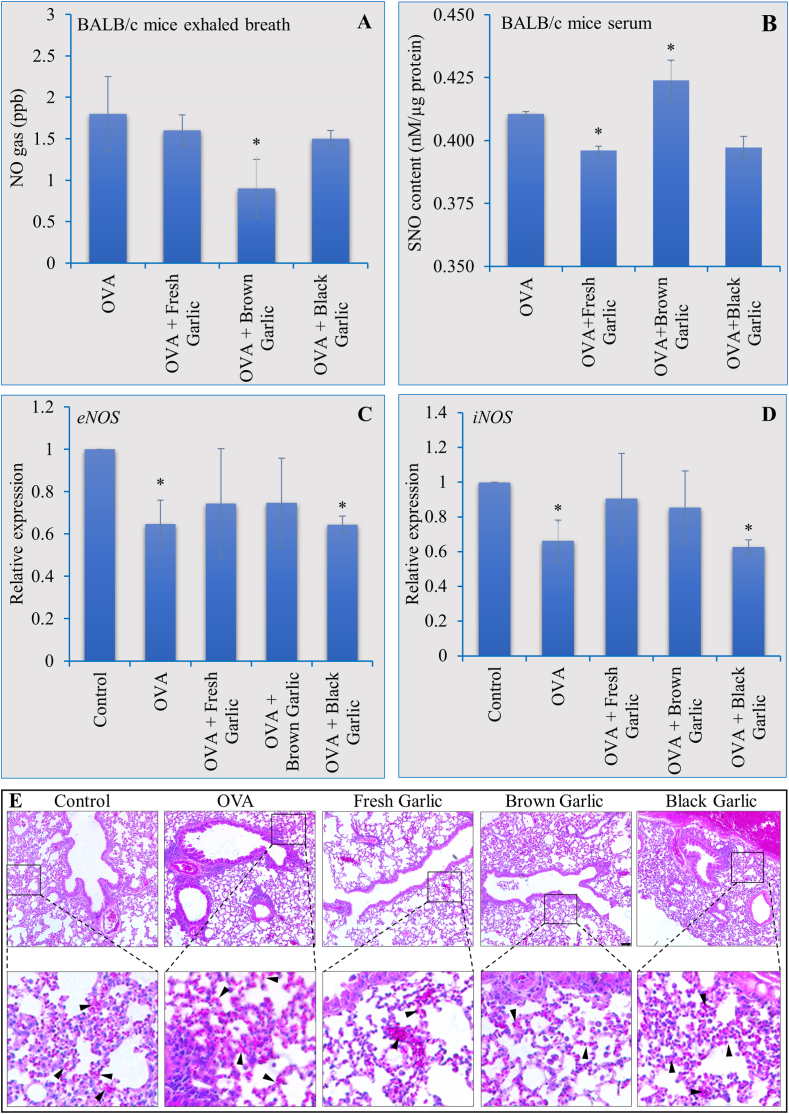
Garlic is a highly important component of daily food for humans. The higher arginine contents recorded in the brown and black garlic blubs tempted us to investigate whether its consumption would have an impact on NO production and subsequent S-Nitrosothiols (SNOs) in animal systems. Furthermore, as the role of NO in asthma is well-known (see section 1), we also wanted to know if the brown garlic-mediated increase in NO production regulates host responses during asthma. For this purpose, we used BALB/c mice as a model. Results showed a significantly lower concentration of NO gas in the exhaled breath when treated with OVA and brown garlic together (Fig. 3A). Fresh and black garlic application together with OVA also reduced the NO gas in exhaled breath as compared to the OVA treatment alone although the difference was not significant (Fig. 3A). Furthermore, SNO measurement of blood serum samples indicated significantly higher SNO levels in the serum of mice fed with brown garlic as compared to the serum of fresh and black garlic-fed mice (Fig. 3B). Next, we analyzed the expression patterns of the endothelial NOS (eNOS) and inducible NOS (iNOS) genes following the application of OVA and garlic. Interestingly, although the OVA application reduced the expression of both eNOS (Fig. 3C) and iNOS (Fig. 3D), none of the garlic samples had a statistically significant impact on the expression of eNOS or iNOS genes.
Fig. 3.
Impact of brown and black garlic on exhaled NO, SNOs, and NOS gene expression during asthma.
3.5. Impact of brown and black garlic on NO, global SNOs, and NOS gene expression during asthma
Lung biopsy and histological staining-based analysis of the mice's lung tissues indicated a significant increase in the accumulation of eosinophils (black arrows in Fig. 3E) in the airways of mice fed only with OVA as compared to mice fed with normal food only (Fig. 3E). Furthermore, significantly fewer eosinophils were observed in mice fed with either fresh or brown garlic combined with OVA (Fig. 3E). Interestingly, however, the lung tissues of mice fed with black garlic and OVA showed a significantly higher accumulation of eosinophils. These results indicate the mitigatory therapeutic potential of brown garlic during asthma.
NO gas in the exhaled breath of asthmatic mice fed with brown and black garlic was measured (A) along with the global SNO levels in the blood serum (B). Real-time PCR analysis was conducted to quantify changes in the expression of two nitric oxide synthase (NOS) isoforms eNSO and iNOS (C-D). Lung biopsy showed reduced accumulation of eosinophils (black arrowheads) after feeding with brown garlic compared to increased eosinophil accumulation with OVA only or combined with black garlic (E). Each data point represents the mean ± SD of three independent replications. Asterisk (*) indicates significant differences between the means of the brown and garlic compared to fresh garlic samples at P = 0.05.
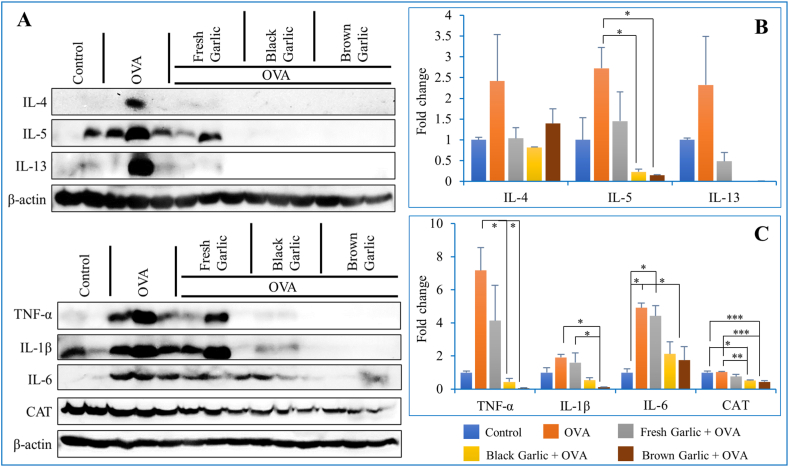
3.6. Brown garlic regulates the expression of inflammation-related genes during asthma
We also performed Western blot and quantitative real-time PCR analysis to measure the transcriptomic changes of genes regulating responses to inflammation in the lungs during asthma. Results of both experiments corroborated with each other as they indicated a significantly high increase in the expression of IL-4 after 4 days of OVA application only (Fig. 4A–B). On the other hand, IL-4 expression was lowest in the lungs of mice fed with black and brown garlic in addition to OVA, followed by those fed with fresh garlic along with OVA (Fig. 4A–B). Relatively same expression patterns were observed for the other interleukin gene IL-5, where the lowest expression was observed with brown garlic followed by black garlic as compared to fresh garlic and OVA only (Fig. 4B). Brown and black garlic combined with OVA resulted in the lowest expression of IL13 whereas OVA and Fresh garlic + OVA resulted in highest but statistically same expression (Fig. 4B). Similarly, the Brown garlic + OVA resulted in the lowest expression of TNF-α, IL-1β, IL-6 and CAT genes followed by Black garlic + OVA. On the other hand, Fresh garlic combined with OVA and OVA only resulted in statistically similar expression of TNF-α and IL-1β (Fig. 4C). In summary, the genes described above are used as markers of inflmation during asthma. Results confirmed that their expression was significantly increased in samples treated with OVA only, but decreased in treatment groups that were administered with brown (or black) garlic combined with OVA. The expression of the antioxidant gene catalase was similar in control conditions and OVA-treated groups, but was markedly reduced in groups treated with brown garlic. These results suggest that brown garlic plays a positive role in reducing inflammation and also has antioxidant pottential.
Fig. 4.
The impact of brown and black garlic on the expression of inflammation-related genes during asthma.
Following the treatment of asthmatic mice with brown and black garlic, Western blot was performed to measure the quantity of the translated proteins of these genes along with the antioxidant enzyme catalase (A). β-actin was used as a comparative control (consult Additional file for raw uncropped gel images). Furthermore, real-time PCR analysis was performed to determine the changes in the expression of inflammation-related genes IL-4, IL-5, IL-13 (B), TNF-α, IL-1β, and IL-6 (C). Each data point represents the mean ± SD of three independent replications. Asterisk (*) indicates significant differences between the means of the brown and garlic compared to fresh garlic samples at P = 0.05 (*), 0.01 (**), and 0.005 (***). Raw/un-cropped gel images can be found in the.
3.7. Brown garlic activates the antioxidant machinery during asthma
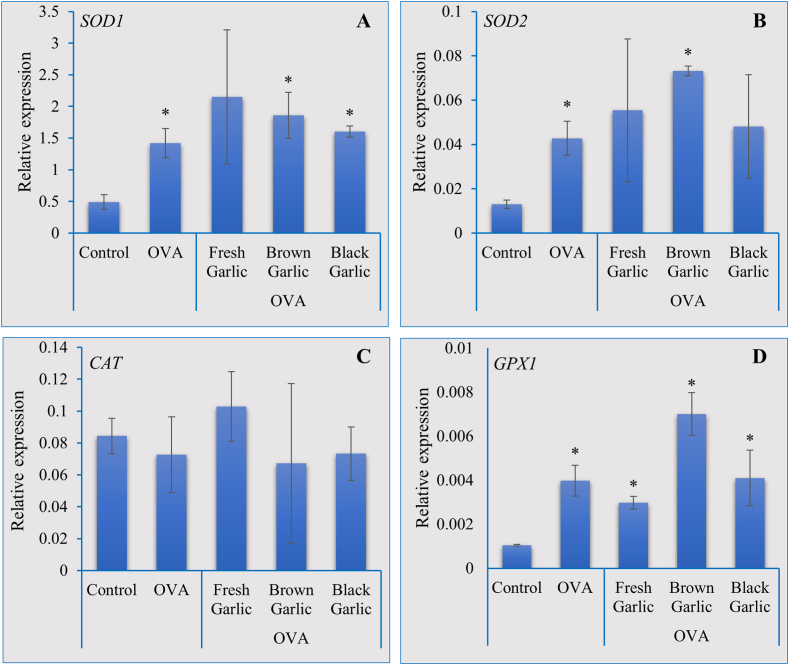
Inflammation and the activation of antioxidant responses are complex interconnected processes in animals. Inflammation is a natural and necessary response to injury or infection and may also lead to the generation of reactive oxygen species (ROS) and oxidative stress, which necessitates the activation of antioxidant defense via antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX). We, therefore, performed real-time PCR analysis to determine the impact of fresh, brown, and black garlic on the expression of these antioxidant genes in mice lungs during OVA-induced asthma. Results indicated a significant increase in the expression of all the antioxidant genes in the lungs of asthmatic mice fed with brown garlic followed by black and fresh garlic (Fig. 5A–D). Furthermore, feeding asthmatic mice with fresh and brown garlic resulted in a significantly higher accumulation of catalase proteins indicating an increase in the overall antioxidant activity at the cellular level (Fig. 4G).
Fig. 5.
Brown garlic activates the antioxidant machinery during asthma.
Real-time PCR analysis was conducted to determine changes in the expression of antioxidant genes SOD1 (A), SOD2 (B), CAT (C) and GPX1 (D) in asthmatic mice fed with brown and black garlic. Each data point represents the mean ± SD of three independent replications. Asterisk (*) indicates significant differences between the means of the brown and garlic compared to fresh garlic samples at P = 0.05.
4. Discussion
Several sources identify the surprising benefits of garlic. The University of Rochester Medical Center describes garlic as containing proteins, fats, carbohydrates, fiber, and essential minerals (https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=76&contentid=11215-4). It can be used to treat common colds and coughs, problems with blood pressure, cholesterol, heart health, immunity, and sexual health. Several food processing techniques are used to transform different food items into more valuable, nutrient-rich forms, though the type of food processing techniques varies according to objectives and the desired outcome. Trends in modern food processing include the reduction of fat content, maintaining the natural taste, increasing hygiene according to certain officially endorsed standards (e.g. HACCP), and making food items more efficient.
Processing regular or raw garlic on constant heat for several weeks to make black garlic is a ubiquitous method of garlic processing. However, a wide variety of proprietary methods have been reported for making black garlic. In this study, we made brown garlic and black garlic by heating fresh garlic at a fixed 65 °C but for different time points. We compared the nutritive value especially the arginine content of brown garlic with the fresh and black garlic bulbs and the potential of brown garlic to serve as a source of nitric oxide and a therapeutic for the treatment of asthma using the BALB/c mice model. As described earlier, different cooking methods affect the nutritional contents of garlic. Still, to our knowledge, there is no information on the relationship of the cooking process with changes in the L-arginine contents, especially for brown garlic.
Our results (and from the published literature) indicate several intermediate colors between fresh and fully cooked black garlic. Interestingly, brown garlic has been specially investigated by different researchers from South Korea [33,34] where it is customarily called “red garlic” although the color of the garlic is not red. Hence studies reporting research on red garlic from South Korea mean Brown garlic. During the cooking process, the intermediate color between the fresh and black garlic is brown (refer to 1st section of the results for exact color names and Hex values), here we call it brown garlic instead of red garlic. In the Korean Journal of Food Preservation published by the Korean Society of Food Preservation Kang, Kim [33] report the antioxidant and anti-inflammatory effects of red garlic and describe a significant increase in the Alliin and other phenolic compounds as compared to fresh garlic. They prepared the red garlic samples by using the patented method (10–1178,592, 2012.08.24) of Namhae Garlic Research Institute by first deep freezing the garlic samples at −80 °C for 24 h s and then slowly thawing to room temperature and aging at 1–60 °C for 80-58 h s. Similarly, there are two more patents (10-2011-0117,380, and 10-2016-0075,047) concerning the proprietorship of methods used for making red garlic. However, this is the first study proposing the use of brown garlic not only for culinary and dietary purposes but also as a medicine for highly important diseases such as Asthma.
Incomplete combustion of organic compounds in food products may sometimes lead to the production of harmful metabolites such as benzopyrene (BaP - a carcinogen), especially those cooked on live flame or grilled. BaP has been reported as a potential contaminant of tar, coal, tobacco smoke, and consumable food items such as grilled meats [35]. While analyzing 200 food items for BaP estimation, Kazerouni, Sinha [35] recorded up to 0.5 ng/g BaP in certain cereals. Furthermore, bread/cereal/grain, and grilled/barbecued meat, respectively, contributed 29 and 21 percent BaP to the mean daily intake of BaP by the human population under study. However, the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) reports 52–59 ng/cigarette of BaP in sidestream cigarette smoke [IARC-Monograph [36] mono100F-14.pdf (who.int)]. Although epidemiological data on BaP-induced cancers in humans are not available, it has been reported to cause cancers in several species of animals after being administered through different routes (oral, dermal, inhalation, intratracheal, intrabronchial, subcutaneous, intraperitoneal, and intravenous) in ng or μg quantities [37]. This is why we performed HPLC analyses to determine if there is any BaP produced in the garlic bulbs against a commercial BaP standard. We did not detect BaP in any of the garlic samples (fresh, brown and black). However, we did observe two sharp peaks in all the garlic samples. The two peaks were more concentrated in the brown and black garlic samples. One of these two peaks is more pronounced and taller than the other. Here we assume that these two peaks may represent allicin and alliin which are naturally occurring organic compounds found abundant in garlic and other plants of the genus Allium such as shallots and onions. Both organic compounds have been reported by several researchers in garlic via HPLC analysis with more or less the same retention times in their chromatograms (between 3 and 15min – depending upon the methodology and equipment) [[38], [39], [40], [41]].
As such, multiple routes for the production of NO in plants have been described including the NO synthase (NOS)-dependent NO production which utilizes Arginine as a substrate [15]. The brown garlic samples were found to have significantly higher arginine content, which is the basic source of nitric oxide in animals. The NOS enzyme produces NO by converting arginine to citrulline. Once produced NO exerts its function via S-nitrosylation to produce SNOs. By feeding fresh, brown, and black garlic samples to lab-grown mice, we observed significantly high SNOs in the blood serum of mice fed with brown garlic. However, we did not detect a major difference in the nitric oxide content of the exhaled breath in mice fed with different garlic samples. This indicates that the brown garlic-induced increase in NO production in mice contributes more to SNOs than being emitted in the exhaled breath. This was particularly expected due to the nature of the NO gas itself, as it is highly reactive and once generated it instantly reacts to form S-Nitrosothiols. Though fresh and black garlic reduced the quantity of NO in exhaled breath, the maximum significant reduction in breath-NO was caused by Brown garlic. As described above, the garlic-induced increase in NO contributes more to the global S-nitrosothiols in the blood serum rather than to the gas NO quantity in the exhaled breath for which the exact mechanism and biological significance still need to be investigated. However, at least this further strengthens our hypothesis that changes in the exhaled NO and blood SNO contents may be due to the high arginine content of brown garlic samples, rather than the related perturbations in the endogenous eNOS or iNOS expression for which we did not find any significant changes in the lungs of garlic-fed asthmatic mice. These findings are parallel to the results of Das, Hirani [42] from the Charing Cross and Westminster Medical School, London who reported that arginine is not responsible for the activation of the NOS enzymes by Garlic. In addition, besides the wide array of phenolic and other bioactive compounds, the medicinal properties of Garlic are associated with its wide array of sulfur compounds including hydrogen sulfide (H2S) [3]. The H2S and NO metabolic pathways are intertwined with each other which could be an important mechanism underlying the NO-related effects of garlic.
Eosinophils; the immune system defenders, are bone marrow-drawn white blood cells (WBC) found in various tissues. A higher number of eosinophils in the lungs generally indicates an inflammatory condition or response. While a small number of these are normally present in healthy lungs (less than 5 % of the total WBC), an increase in their number suggests an immune response or ongoing inflammation and underlying conditions such as asthma [43]. The lung biopsy samples of asthmatic mice fed with brown garlic showed a marked reduction in the number of eosinophils as compared to the black garlic. This indicates a significantly higher therapeutic value of brown garlic as compared to black garlic, especially for the treatment of asthma. These results were also supported by the expression of pro and anti-inflammatory interleukin cytokines, secreted by the white blood cells, that play an important role in the activation, differentiation, proliferation, migration, and adhesion of various immune cells [44]. In animals, the ILs perform both pro-inflammatory and anti-inflammatory functions. IL-4, IL-5, IL-6, IL-13, and IL1β are important genes regulating host responses during inflammation and allergic asthma. Particularly, asthma symptoms are often closely associated with the expression of IL-4, IL-5, IL-9, and IL13 in walls of the air passage and are standard asthma marker genes in the airways [45]. Furthermore, interferons (INF) are molecules produced by animal cells to resist virus infections, and tumors or cancers, for example, the tumor necrosis factor-α (TNF-α). The TNF- α is a pro-inflammatory cytokine implicated in the regulation of inflammation-related responses during asthma [46]. This study provides the first comprehensive and conclusive insight into the nutritional benefits of brown garlic and its therapeutic value for the treatment of asthma in animals.
Funding
This study was funded by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, by the Ministry of Education through the Grant Number RS-2023-00245922 to Byung-Wook Yun.
Ethics statement
This study involves the use of BALB/c laboratory-grown mice. Prior approval for the study was taken from the Kyungpook National University Industry Foundation for animal experiments (approval no. 2018–0140).
Data availability statement
All the relevant data is available within the manuscript. Any additional information will be provided by the corresponding author upon request.
CRediT authorship contribution statement
Geun-Mo Lee: Investigation, Data curation. Bong-Gyu Mun: Investigation, Data curation. Adil Hussain: Writing – review & editing, Writing – original draft, Resources, Formal analysis, Data curation. Eungyung Kim: Resources, Formal analysis, Data curation. Da-Sol Lee: Resources, Formal analysis, Data curation. Myoung Ok Kim: Writing – review & editing, Writing – original draft, Resources. Byung-Wook Yun: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Byung-Wook Yun reports financial support was provided by National Research Foundation of Korea. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36976.
Contributor Information
Geun-Mo Lee, Email: looxia@knu.ac.kr.
Bong-Gyu Mun, Email: munbg@chungbuk.ac.kr.
Adil Hussain, Email: adilhussain@awkum.edu.pk.
Eungyung Kim, Email: kime8@uthscsa.edu.
Da-Sol Lee, Email: giftanna@naver.com.
Myoung Ok Kim, Email: ok4325@knu.ac.kr.
Byung-Wook Yun, Email: bwyun@knu.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2011;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J.-S., Kang O.-J., Gweon O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct.Foods. 2013;5(1):80–86. doi: 10.1016/j.jff.2012.08.006. [DOI] [Google Scholar]
- 3.Pérez-Torres I., et al. Deodorized garlic decreases oxidative stress caused by lipopolysaccharide in rat heart through hydrogen sulfide: preliminary findings. Int. J. Mol. Sci. 2022;23(20) doi: 10.3390/ijms232012529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivlin R.S. Historical perspective on the use of garlic. J. Nutr. 2001;131(3):951S–954S. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 5.Lanzotti V. The analysis of onion and garlic. J. Chromatogr. A. 2006;1112(1):3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Seo Y.-J., et al. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. Preventive Nutrition and Food Science. 2009;14(1):1–7. [Google Scholar]
- 7.Kim I., et al. The beneficial effects of aged black garlic extract on obesity and hyperlipidemia in rats fed a high-fat diet. J. Med. Plants Res. 2011;5(14):3159–3168. [Google Scholar]
- 8.Dillon S.A., et al. Antioxidant properties of aged garlic extract: an in vitro study incorporating human low density lipoprotein. Life Sci. 2003;72(14):1583–1594. doi: 10.1016/s0024-3205(02)02475-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y.-M., et al. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr. Res. Prac. 2009;3(2):156–161. doi: 10.4162/nrp.2009.3.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judita L., et al. In: Herbs and Spices. Eva I., editor. IntechOpen; Rijeka: 2022. Garlic (<em>Allium sativum</em> L.): characterization of bioactive compounds and related health benefits. Ch. 6. [Google Scholar]
- 11.Shang A., et al. Bioactive compounds and biological functions of garlic (Allium sativum L.) Foods. 2019;8(7) doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buddha M.R., et al. Regioselective nitration of tryptophan by a complex between bacterial nitric-oxide synthase and tryptophanyl-tRNA synthetase. J. Biol. Chem. 2004;279(48):49567–49570. doi: 10.1074/jbc.C400418200. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z.Q., et al. Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase. J. Biol. Chem. 2007;282(4):2196–2202. doi: 10.1074/jbc.M608206200. [DOI] [PubMed] [Google Scholar]
- 14.Hussain A., et al. In: 2 - Nitric Oxide Synthase in the Plant Kingdom in Nitric Oxide in Plant Biology. Pratap Singh V., et al., editors. Academic Press; 2022. pp. 43–52. [Google Scholar]
- 15.Hussain A., et al. In: Nitric Oxide Synthase in the Plant Kingdom in Nitric Oxide in Plant Biology. Pratap Singh V., et al., editors. Academic Press; 2022. pp. 43–52. [Google Scholar]
- 16.Corpas F.J., et al. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta. 2006;224(2):246–254. doi: 10.1007/s00425-005-0205-9. [DOI] [PubMed] [Google Scholar]
- 17.Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993;329(27):2002–2012. doi: 10.1056/nejm199312303292706. [DOI] [PubMed] [Google Scholar]
- 18.Morihara N., et al. Aged garlic extract enhances production of nitric oxide. Life Sci. 2002;71(5):509–517. doi: 10.1016/s0024-3205(02)01706-x. [DOI] [PubMed] [Google Scholar]
- 19.Baik J.S., et al. Effects of fermented garlic extract containing nitric oxide metabolites on blood flow in healthy participants: a randomized controlled trial. Nutrients. 2022;14(24) doi: 10.3390/nu14245238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harisa G.E., et al. L-arginine augments the antioxidant effect of garlic against acetic acid-induced ulcerative colitis in rats. Pak. J. Pharm. Sci. 2009;22(4):373–380. [PubMed] [Google Scholar]
- 21.Das I., Khan N.S., Sooranna S.R. Potent activation of nitric oxide synthase by garlic: a basis for its therapeutic applications. Curr. Med. Res. Opin. 1995;13(5):257–263. doi: 10.1185/03007999509111550. [DOI] [PubMed] [Google Scholar]
- 22.Das I., Khan N.S., Sooranna S.R. Nitric oxide synthase activation is a unique mechanism of garlic action. Biochem. Soc. Trans. 1995;23(1) doi: 10.1042/bst023136s. [DOI] [PubMed] [Google Scholar]
- 23.Lewandowicz A.M., Pawliczak R. [Arginine metabolism in bronchial asthma] Postepy Hig. Med. Dosw. 2007;61:156–166. [PubMed] [Google Scholar]
- 24.Ashutosh K. Nitric oxide and asthma: a review. Curr. Opin. Pulm. Med. 2000;6(1):21–25. doi: 10.1097/00063198-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Antosova M., et al. Physiology of nitric oxide in the respiratory system. Physiol. Res. 2017;66(Suppl 2):S159–s172. doi: 10.33549/physiolres.933673. [DOI] [PubMed] [Google Scholar]
- 26.Shim Y.S., et al. Method validation of 16 types of structural amino acids using an automated amino acid analyzer. Food Sci. Biotechnol. 2013;22(6):1567–1571. doi: 10.1007/s10068-013-0252-0. [DOI] [Google Scholar]
- 27.Xu F., et al. Generation of IL10 and TGFB1 coexpressed mice displaying resistance to ovalbumin-induced asthma. Transgenic Res. 2016;25(6):829–837. doi: 10.1007/s11248-016-9972-2. [DOI] [PubMed] [Google Scholar]
- 28.Cai Z., et al. Albiflorin alleviates ovalbumin (OVA)-induced pulmonary inflammation in asthmatic mice. Am J Transl Res. 2019;11(12):7300–7309. [PMC free article] [PubMed] [Google Scholar]
- 29.Daubeuf F., Frossard N. Eosinophils and the ovalbumin mouse model of asthma. Methods Mol. Biol. 2014;1178:283–293. doi: 10.1007/978-1-4939-1016-8_24. [DOI] [PubMed] [Google Scholar]
- 30.Kim E., et al. Ginger-derived compounds exert in vivo and in vitro anti-asthmatic effects by inhibiting the T-helper 2 cell-mediated allergic response. Exp. Ther. Med. 2022;23(1):49. doi: 10.3892/etm.2021.10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., et al. Localization of NOS-like protein in guard cells of Vicia faba L. and its possible function. Chin. Sci. Bull. 2007;52(1):84–90. doi: 10.1007/s11434-007-0024-4. [DOI] [Google Scholar]
- 32.Astier J., Gross I., Durner J. Nitric oxide production in plants: an update. J. Exp. Bot. 2017;69(14):3401–3411. doi: 10.1093/jxb/erx420%. [DOI] [PubMed] [Google Scholar]
- 33.Kang M.J., Kim D.-G., Shin J.H. Antioxidant and anti-inflammatory effects of red garlic compositions. Korean Journal of Food Preservation. 2017;24(3):446–454. doi: 10.11002/KJFP.2017.24.3.446. [DOI] [Google Scholar]
- 34.Kang M.-J., et al. Physicochemical characteristics of red garlic during processing. Korean Journal of Food Preservation. 2011;18(6):898–906. doi: 10.11002/KJFP.2011.18.6.898. [DOI] [Google Scholar]
- 35.Kazerouni N., et al. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001;39(5):423–436. doi: 10.1016/S0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 36.IARC-Monograph . IARC Monogr Eval Carcinog Risks Hum. 2010. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures; pp. 1–853. [PMC free article] [PubMed] [Google Scholar]
- 37.IARC-Monograph-Vol-3 . IARC Monogr Eval Carcinog Risks Hum. 2010. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures; pp. 1–853. [PMC free article] [PubMed] [Google Scholar]
- 38.Mochizuki E., et al. Simultaneous determination of alliin and allicin in Allium plants and their products by liquid chromatography. J. AOAC Int. 2020;80(5):1052–1056. doi: 10.1093/jaoac/80.5.1052. [DOI] [Google Scholar]
- 39.Iberl B., et al. Quantitative determination of allicin and alliin from garlic by HPLC. Planta Med. 1990;56(3):320–326. doi: 10.1055/s-2006-960969. [DOI] [PubMed] [Google Scholar]
- 40.Rosen R.T., et al. Determination of allicin, S-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J. Nutr. 2001;131(3s) doi: 10.1093/jn/131.3.968S. 968s-71s. [DOI] [PubMed] [Google Scholar]
- 41.Rybak M.E., Calvey E.M., Harnly J.M. Quantitative determination of allicin in garlic: supercritical fluid extraction and standard addition of alliin. J. Agric. Food Chem. 2004;52(4):682–687. doi: 10.1021/jf034853x. [DOI] [PubMed] [Google Scholar]
- 42.Das I., Hirani J., Sooranna S. Arginine is not responsible for the activation of nitric oxide synthase by garlic. J. Ethnopharmacol. 1996;53(1):5–9. doi: 10.1016/0378-8741(96)01416-x. [DOI] [PubMed] [Google Scholar]
- 43.Carr T.F., Zeki A.A., Kraft M. Eosinophilic and noneosinophilic asthma. Am. J. Respir. Crit. Care Med. 2018;197(1):22–37. doi: 10.1164/rccm.201611-2232PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Justiz Vaillant A.A., Qurie A. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; Treasure Island (FL): 2023. Interleukin in StatPearls. [Google Scholar]
- 45.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 46.Babu K.S., Davies D.E., Holgate S.T. Role of tumor necrosis factor alpha in asthma. Immunol. Allergy Clin. 2004;24(4):583–597. doi: 10.1016/j.iac.2004.06.010. v-vi. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the relevant data is available within the manuscript. Any additional information will be provided by the corresponding author upon request.