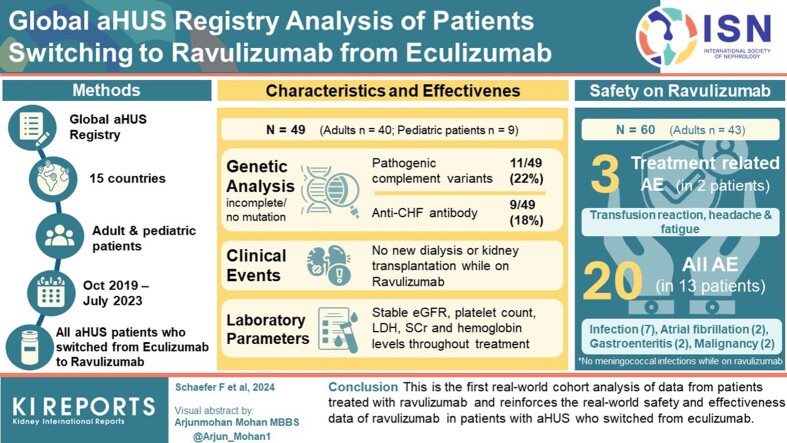
Abstract
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a progressive rare disease that, if untreated, can result in severe organ damage and death. Ravulizumab, a next-generation terminal complement inhibitor, provides immediate, complete, and sustained complement C5 inhibition. Real-world data in patients with aHUS who switched to ravulizumab from eculizumab are lacking.
Methods
The Global aHUS Registry is a multicenter study (NCT01522183) collecting data on adult or pediatric patients with an aHUS diagnosis, regardless of treatment. Patient characteristics, genetic data, hematological and renal parameters, clinical events (e.g., dialysis and kidney transplantation), and adverse events (AEs) were extracted from patients who switched to ravulizumab from eculizumab up to July 3, 2023.
Results
Overall, 60 patients switched to ravulizumab (adult: n = 43; pediatric: n = 17); 11 patients were excluded from effectiveness and genetic analyses (N = 49; adult: n = 40; pediatric: n = 9) because they received <3 months ravulizumab treatment and/or had >1 month between eculizumab discontinuation and ravulizumab initiation. Pathogenic complement variants were identified in 11 of 49 patients (22%); the most common was a complement factor H variant (n = 5/49 [10%]). During ravulizumab treatment, 20 AEs occurred in 13 patients, with no unexpected AEs and only 3 treatment-related AEs (infusion reaction, headaches, and fatigue). No meningococcal infections or deaths were reported. No new events of dialysis, kidney transplantation, or thrombotic microangiopathy were reported. Renal and hematological parameters remained stable after switching to ravulizumab.
Conclusion
This is the first real-world cohort analysis of data from patients treated with ravulizumab and reinforces the real-world safety and effectiveness data of ravulizumab in patients with aHUS who switched from eculizumab.
Keywords: atypical hemolytic uremic syndrome, eculizumab, real-world data, ravulizumab, outcomes, patient characteristics
Graphical abstract
Atypical hemolytic uremic syndrome (aHUS) is a progressive rare disease that, if untreated, can result in severe organ damage and death.1 aHUS is a form of thrombotic microangiopathy caused by complement dysregulation that leads to uncontrolled terminal complement activation, and it can manifest in the presence or absence of a trigger or associated condition.1, 2, 3 The annual incidence of aHUS, as estimated by a systematic review, ranges from 0.23 to 1.9 per million individuals overall, and between 0.26 and 0.75 per million individuals aged ≤ 20 years.4
Eculizumab, a humanized monoclonal antibody that blocks terminal complement activation by inhibiting cleavage of complement C5, was approved for the treatment of aHUS in 2011 and revolutionized the clinical management of aHUS.5, 6, 7 Ravulizumab, a next-generation terminal complement inhibitor,8 introduces 4 amino acid changes into the eculizumab frame at the complementary binding and neonatal Fc regions, resulting in efficient recycling and augmented endosomal dissociation of C5. This means that ravulizumab is associated with an extended duration of C5 inhibition while retaining the proven efficacy and safety of eculizumab.9 It is administered once every 4 to 8 weeks using individualized weight-based dosing, and is approved for the treatment of aHUS (among other indications) in the USA (2019), Europe (2020), Japan (2020), and other regions.10, 11, 12 The efficacy and safety of ravulizumab in patients with aHUS have been demonstrated in clinical trials, in which ravulizumab was shown to provide immediate, complete, and sustained complement C5 inhibition.13, 14, 15, 16
Cohort-level real-world evidence, such as claims studies and surveys, for ravulizumab in patients with aHUS has only recently emerged,17,18 yet the efficacy and safety of ravulizumab in a single large cohort has not been reported beyond clinical trials. In addition, clinical trials did not include adult patients with aHUS who switched to ravulizumab from eculizumab. To address this important data gap for ravulizumab, we assessed the real-world clinical characteristics and outcomes of patients with aHUS who switched to ravulizumab from eculizumab, by using data from the Global aHUS Registry.
Methods
Study Design
The Global aHUS Registry is a multicenter study (ClinicalTrials.gov: NCT01522183) sponsored by Alexion Pharmaceuticals, Inc. collecting both prospective and retrospective data on demographics, characteristics, natural history, and treatment outcomes in patients with aHUS.19 The registry was initiated in April 2012 and has collected data from patients in 23 countries (Australia, Austria, Belgium, Canada, Czechia, Denmark, Finland, France, Germany, Israel, Italy, Norway, Poland, Republic of Korea, Russia, Spain, Sweden, Switzerland, Taiwan, Turkey, UAE, UK, and USA). Patients who are ongoing in the registry are from 15 countries (Australia, Belgium, Canada, Denmark, France, Germany, Israel, Italy, Poland, Republic of Korea, Spain, Taiwan, Turkey, UK, and USA). The registry has the largest real-world cohort of patients with aHUS and is open to all patients, regardless of treatment status, providing reasonable representation of the global aHUS patient population. The safety analysis population for the current study included all patients with aHUS who switched to ravulizumab from eculizumab. The main analysis population included all patients with aHUS who switched to ravulizumab from eculizumab with at least 3 months of ravulizumab treatment (i.e., sufficient treatment duration to attain at least 1 maintenance dose) and less than 1 month between eculizumab discontinuation and ravulizumab initiation, with an initiation date on or after October 1, 2019. These criteria ensured inclusion of patients had a clinically meaningful ravulizumab treatment duration and exclusion of patients who discontinued eculizumab, relapsed, and then initiated treatment with ravulizumab. Age at ravulizumab initiation was used to determine the adult (≥18 years) or pediatric (<18 years) classification for each patient. Safety analyses were performed on all patients who switched to ravulizumab, regardless of treatment duration or time after switching from eculizumab. The data cut-off for this analysis was July 3, 2023.
Patient Characteristics
The patient characteristics extracted for analysis were as follows: sex, age at ravulizumab initiation, geographic location, extrarenal manifestations, family history of aHUS, aHUS triggers or associated conditions (occurring at any time during the analysis), time from aHUS onset to eculizumab initiation, ravulizumab and eculizumab treatment duration, time from eculizumab discontinuation to ravulizumab initiation, the number of patients who switched from ravulizumab back to eculizumab (and reasons for doing so), complement gene variants, and anti-complement factor H (CFH) antibody status. Information regarding vaccination status was not recorded.
Outcomes
Both effectiveness and safety outcomes were evaluated. Laboratory parameters included estimated glomerular filtration rate (eGFR; calculated using the Chronic Kidney Disease Epidemiology Collaboration method for adult patients [normal range for adults: >90 ml/min per 1.73 m2] and the Schwartz method for pediatric patients), platelet count (normal range: 150–450 × 106), lactate dehydrogenase level, creatinine, and hemoglobin. Assessed clinical events included kidney transplantation, dialysis, and thrombotic microangiopathy symptoms, measured before and after ravulizumab initiation. The safety analysis included AEs, meningococcal infections, and deaths.
Statistical Analysis
This was a descriptive study. Continuous data were summarized as median (range). Categorical data were summarized as number and percentage of patients. Laboratory parameters were presented as median (interquartile range).
Results
Demographic and Clinical Characteristics
Overall, data for 60 patients with aHUS who switched to ravulizumab from eculizumab (adult: n = 43, pediatric patients: n = 17) were available in the Global aHUS Registry database. All patients were included in the safety analysis set; following application of inclusion and exclusion criteria, 49 patients were included in the main analysis set (Table 1). At ravulizumab initiation, most patients in the main analysis set were adult (n = 40/49 [82%]) and female (n = 36/49 [73%]), with a median age of 35 years (range: 2–72 years; Table 2); 9 of 49 patients (18%) reported a family history of aHUS. The median (range) time on treatment was 66 (11–155) months for eculizumab and 23 (3–41) months for ravulizumab. The most common extrarenal manifestations at any time were gastrointestinal (n = 22/49 [45%]). Most patients (n = 43/49 [88%]) had no identified aHUS trigger or associated condition; the most common aHUS trigger or associated condition was “autoimmune disease” (n = 3/49 [6%]) (Supplementary Table S1).
Table 1.
Study analysis populations
| Study analysis populations and reasons for exclusion from the main analysis set | All patients (N = 60) | Adult patients (n = 43) | Pediatric patients (n = 17) |
|---|---|---|---|
| Safety analysis set, n (%) | 60 (100) | 43 (100) | 17 (100) |
| Main analysis set, n (%) | 49 (82) | 40 (93) | 9 (53) |
| Patients excluded from the main analysis set, n (%) | 11 (18) | 3 (7) | 8 (47) |
| Ravulizumab treatment duration of <3 mo | 6 (10) | 1 (2) | 5 (29) |
| Gap between eculizumab discontinuation and ravulizumab initiation > 1 mo | 5 (8) | 2 (5) | 3 (18) |
Table 2.
Demographic and clinical characteristics
| Characteristics | All patients (N = 49) | Adult patients (n = 40) | Pediatric patients (n = 9) |
|---|---|---|---|
| Sex, female, n (%) | 36 (73) | 29 (73) | 7 (78) |
| Age at ravulizumab initiation, years, median (range) | 35 (2–72) | 37 (19–72) | 8 (2–15) |
| Geographic location, n (%) | |||
| Germany | 26 (53) | 22 (55) | 4 (44) |
| USA | 7 (14) | 4 (10) | 3 (33) |
| Denmark | 6 (12) | 5 (13) | 1 (11) |
| UK | 5 (10) | 5 (13) | 0 |
| Spain | 3 (6) | 3 (8) | 0 |
| Israel | 2 (4) | 1 (3) | 1 (11) |
| Extrarenal manifestations at any time, n (%) | |||
| Gastrointestinal | 22 (45) | 19 (48) | 3 (33) |
| Central nervous system | 17 (35) | 14 (35) | 3 (33) |
| Cardiovascular | 15 (31) | 11 (28) | 4 (44) |
| Pulmonary | 6 (12) | 4 (10) | 2 (22) |
| Family history of aHUS, n (%) | |||
| Yes | 9 (18) | 6 (15) | 3 (33) |
| Unknown | 7 (14) | 7 (18) | 0 |
| Missing | 1 (2) | 1 (3) | 0 |
| Time from aHUS onset to eculizumab initiation, median (range), mo | 1 (0–304) | 2 (0–304) | <1 (<1–28) |
| Eculizumab treatment duration, median (range), mo | 66 (11–155) | 66 (11–155) | 62 (15–89) |
| Ravulizumab treatment duration, median (range), mo | 23 (3–41) | 23 (3–40) | 20 (7–41) |
aHUS, atypical hemolytic uremic syndrome.
Genetic Analyses
Overall, 11 of 49 patients (22%) had pathogenic complement variants, 9 of 49 patients (18%) were anti-CFH antibody tested and positive, and 18 of 49 patients (37%) had pathogenic complement variants or were anti-CFH antibody positive. In addition, 4 of 49 patients (8%) were tested for ≥5 variants with no pathogenic complement variant found, and 31 of 49 patients (63%) had incomplete data, were not tested, or had no identified pathogenic complement variant or anti-CFH antibodies (Table 3). The most common pathogenic variant was a CFH mutation (n = 5/49 [10%]) (Table 4).
Table 3.
Pathogenic variants in complement genes and anti-CFH antibody status
| Genotype and anti-CFH antibody status, n (%) | All patients (N = 49) | Adult patients (n = 40) | Pediatric patients (n = 9) |
|---|---|---|---|
| Any pathogenic variant found | 11 (22) | 9 (23) | 2 (22) |
| Tested for ≥5 variants with no pathogenic variant found | 4 (8) | 4 (10) | 0 |
| Anti-CFH antibody tested and positive | 9 (18) | 7 (18) | 2 (22) |
| Any pathogenic variant found or anti-CFH antibody positive | 18 (37) | 15 (38) | 3 (33) |
| Data incomplete/not tested/no pathogenic variant or anti-CFH antibody found | 31 (63) | 25 (63) | 6 (67) |
CFH, complement factor H.
Table 4.
Genotype status stratified by age
| Pathogenic variant, n (%) | All patients (N = 49) | Adult patients (n = 40) | Pediatric patients (n = 9) |
|---|---|---|---|
| Complement factor H variant | 5 (10) | 4 (10) | 1 (11) |
| Complement C3 variant | 3 (6) | 3 (8) | 0 |
| Complement factor I variant | 3 (6) | 2 (5) | 1 (11) |
| Complement CD46 (MCP) variant | 0 | 0 | 0 |
CD46, cluster of differentiation 46; MCP, membrane cofactor protein.
Clinical Events
Overall, 28 patients (57%) had a history of dialysis before or during eculizumab treatment; 24 were not receiving dialysis at the point of switching to ravulizumab, and the remaining 4 patients were on dialysis before eculizumab initiation for at least 1 year and had ongoing dialysis during treatment with eculizumab and ravulizumab (Table 5). No new events of dialysis were reported while receiving ravulizumab. In total, 15 patients received a kidney transplant before ravulizumab initiation, and there were no new events of kidney transplantation reported during ravulizumab treatment. One additional patient who switched to ravulizumab while receiving dialysis for the management of their extrarenal manifestation switched back to eculizumab (per local guidelines) to undergo a planned kidney transplantation, with the intention to reinitiate ravulizumab posttransplantation. There were no new events of thrombotic microangiopathy symptoms reported after ravulizumab initiation.
Table 5.
Clinical events
| Clinical event, n (%) | All patients (N = 49) | Adult patients (n = 40) | Pediatric patients (n = 9) |
|---|---|---|---|
| Kidney transplantation | |||
| Before or at ravulizumab initiation | 15 (31) | 15 (38) | 0 |
| After ravulizumab initiationa | 0 | 0 | 0 |
| Last follow-up | 0 | 0 | 0 |
| Dialysis | |||
| Before or at ravulizumab initiation | 28 (57) | 26 (65) | 2 (22) |
| Ongoing at ravulizumab initiation | 4 (8) | 4 (10) | 0 |
| After ravulizumab initiation | 0 | 0 | 0 |
| Ongoing at last follow-up | 4 (8) | 4 (10) | 0 |
One patient reverted to eculizumab (per local guidelines) for a planned kidney transplantation with the intention to return to ravulizumab post transplantation.
Changes in Laboratory Parameters Over Time
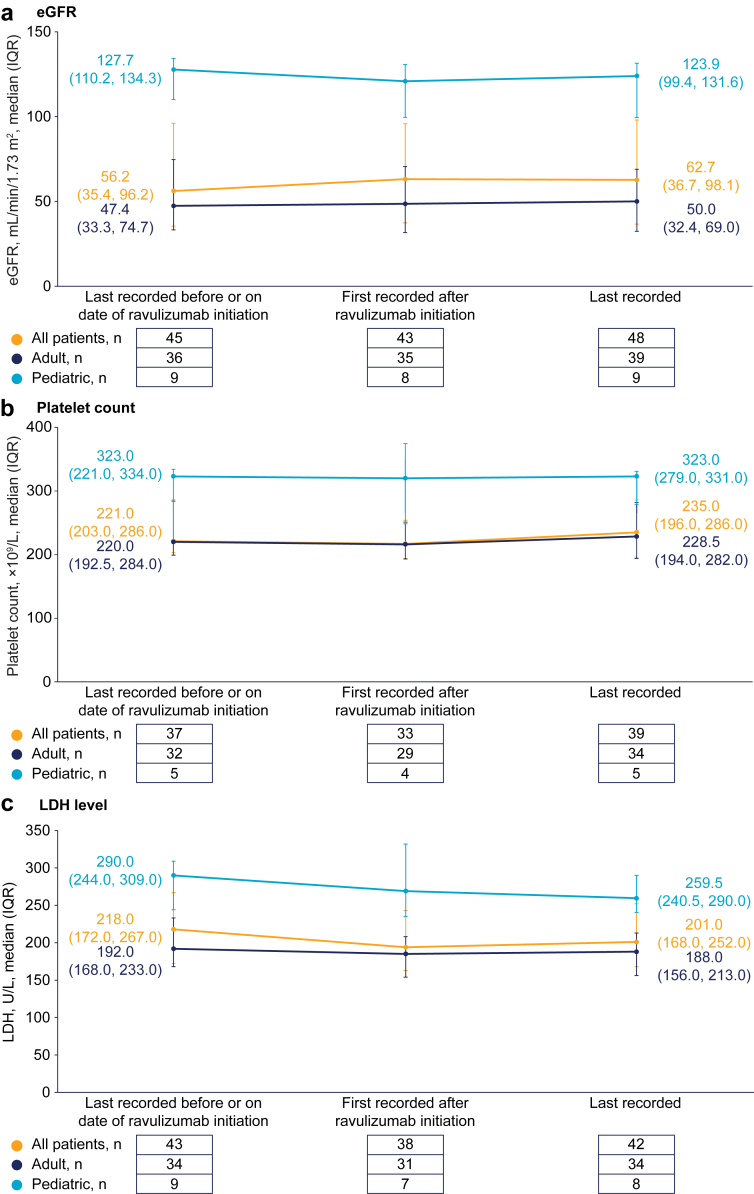
Estimated glomerular filtration rate, platelet count, and lactate dehydrogenase levels remained stable following the switch from eculizumab and during subsequent treatment with ravulizumab (Figure 1). Similarly, creatinine and hemoglobin levels remained stable after switching to ravulizumab from eculizumab (Supplementary Table S2).
Figure 1.
Laboratory parameters: change over time in (a) estimated glomerular filtration rate, (b) platelet count, and (c) LDH levels. eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDH, lactate dehydrogenase.
Safety
In the safety analysis population (N = 60), 20 AEs were reported in 13 patients (Table 6). Of those, 3 serious AEs were reported in 3 patients (1 event each of squamous cell carcinoma, atrial fibrillation, and SARS-CoV-2 infection). Ten of the AEs were reported in 8 patients who received a kidney transplant before ravulizumab initiation (these were infection [4 events], atrial fibrillation [2 events], gastroenteritis [2 events], malignancy [1 event], and seizures [1 event]). Overall, no unexpected AEs were reported. Three treatment-related AEs occurred in 2 patients during ravulizumab treatment. In 1 patient, an infusion reaction was assessed to be probably related to ravulizumab treatment by the reporting investigator; the event resolved within 24 hours and the patient continued ravulizumab treatment. Another patient experienced headaches and fatigue during ravulizumab treatment, which resolved after switching back to eculizumab. The most common AE was infection (7 events), including SARS-CoV2 infection (n = 4), influenza (n = 1), infection without focus (n = 1), and pulmonary nodular infiltration (n = 1); all infections were resolved except for 1 event of SARS-CoV2 infection, which was ongoing or persistent at data cut-off. After infection, the most common AEs were atrial fibrillation (2 events), gastroenteritis (2 events), and malignancy (2 events). Overall, 3 patients switched back to eculizumab; 1 due to AEs (headaches and fatigue, as described above) and 2 due to physician decision. No meningococcal infections or deaths were reported during ravulizumab treatment.
Table 6.
Adverse events reported on or after ravulizumab initiation
| Adverse event | All patients (N = 60), n (%) | Events, n |
|---|---|---|
| Infection | 7 (12) | 7 |
| Atrial fibrillation | 2 (3) | 2 |
| Malignancya | 2 (3) | 2 |
| Gastroenteritis | 1 (2) | 2 |
| Fatigue | 1 (2) | 1 |
| Fever | 1 (2) | 1 |
| Headache | 1 (2) | 1 |
| Infusion reaction | 1 (2) | 1 |
| Seizures | 1 (2) | 1 |
| Testicular torsion | 1 (2) | 1 |
| Transient cerebral ischemia | 1 (2) | 1 |
One event of squamous cell carcinoma and one event of malignant melanoma.
Discussion
This Global aHUS Registry study is the first cohort-level analysis to include safety and efficacy data from patients with aHUS treated with ravulizumab and reports the longest real-world treatment exposure and follow-up data to date. During ravulizumab treatment, no meningococcal infections or deaths were reported, only 3 AEs were assessed as related to ravulizumab treatment, and no unexpected AEs were reported, which confirmed the favorable safety profile of ravulizumab demonstrated in clinical trials.13, 14, 15, 16 Further, both kidney function (as measured by estimated glomerular filtration rate and creatinine levels) and hematological parameters remained stable after switching to ravulizumab from eculizumab. These results align with data from a real-world study of 6 pediatric patients with aHUS20 and a clinical trial of 10 pediatric patients with aHUS16 who maintained stable renal and hematological parameters after switching to ravulizumab from eculizumab.
Importantly, switching to ravulizumab from eculizumab did not necessitate any new events of dialysis or kidney transplantation. These results align with those from a retrospective analysis of claims data from patients with aHUS in the Clarivate Real World Database who switched to ravulizumab from eculizumab, in which the proportion of patients with claims for key procedures, such as dialysis and transplantation, remained low after treatment switch.18
An ongoing concern with complement C5 inhibitors is the risk of meningococcal infection.17,21 Previous studies have reported rates of meningococcal infection in patients with aHUS treated with eculizumab, including an aHUS Registry study that reported a rate of 0.17 cases per 100 patient years in adults, and a 10-year pharmacovigilance study that reported a rate of 0.29 cases per 100 patient years.21,22 Although no meningococcal infections were reported during treatment with ravulizumab in clinical trials of patients with aHUS,13, 14, 15 real-world data are lacking. Notably, in this study no meningococcal infections were reported during ravulizumab treatment. Furthermore, recent analyses of pharmacovigilance data for ravulizumab across approved indications demonstrated a real-world rate of 0.12 cases per 100 patient years, with no suggestion of any increased risk compared with eculizumab.23
Of note, collection of genetic data is not mandated by the Global aHUS Registry; thus, there was a high proportion of patients with incomplete data (63%), which prevented categorization. This may have contributed to the lower-than-expected proportion of patients with any pathogenic variant found.24,25 In addition, the proportions of patients who were anti-CFH antibody tested and positive was 18% and 22% in the overall, adult, and pediatric cohorts, respectively. These values may be higher than anticipated and we suspect that the heterogenous population and high proportion of patients with incomplete data may have contributed to this.25,26
Previous Global aHUS Registry studies have provided valuable insights into multiple aspects of the use of eculizumab in the management of patients with aHUS, and further registry studies of ravulizumab could yield similar insights. For example, outcomes for 188 kidney transplant recipients with aHUS treated with eculizumab enrolled in the Global aHUS Registry were improved in comparison with previous reports of patients with aHUS who were not treated with eculizumab.27 In contrast, published outcomes for ravulizumab are currently limited to case reports of 2 adult patients (both switched to ravulizumab from eculizumab) and 1 pediatric patient (complement C5 inhibitor-naive) which demonstrated that ravulizumab is also effective in kidney transplant recipients with aHUS.28, 29, 30 Our study included 15 patients (31%) who had kidney transplantation before ravulizumab initiation, indicating a substantial opportunity for further analysis in this subpopulation. The real-world findings from the Global aHUS Registry also suggest clinically meaningful improvements in fatigue and other patient-reported outcomes with eculizumab after enrollment into the registry.31 Notably, these data are lacking for patients who switched and those who are naive to ravulizumab treatment; therefore, comparable real-world data for these subpopulations would provide valuable insights into the overall effect of ravulizumab on patients’ quality of life. This may be particularly important in the switch population owing to the reduced dosing frequency associated with switching to ravulizumab from eculizumab, which may benefit patient quality of life.
Limitations of this study include those inherent to registry-derived data, such as missing data and variable lengths of follow-up. Further, the main analysis population did not include patients initiating complement C5 inhibition with ravulizumab only, owing to low numbers at the time of data collection; analysis of these patients is important to comprehensively determine the real-world effectiveness of ravulizumab.
In conclusion, this analysis from the Global aHUS Registry is the first cohort-level analysis of real-world data from adult and pediatric patients with aHUS who switched from eculizumab to ravulizumab, with the longest real-world treatment exposure and follow-up data to date. Overall, no unexpected AEs, meningococcal infections, or deaths were reported during treatment with ravulizumab. These data provide further evidence for the safety and effectiveness of ravulizumab treatment in patients with aHUS who switched from eculizumab and reinforce a positive risk-benefit profile of ravulizumab. Further studies in other aHUS subpopulations, including treatment-naive patients, would be beneficial to guide clinical practice.
Disclosure
FS received consultant and lecture fees from, and was a scientific advisory board member for, Alexion AstraZeneca Rare Disease; and is a member of the International Society of Nephrology. IA-D is an employee of Alexion, AstraZeneca Rare Disease and owns stock/options in Alexion, AstraZeneca Rare Disease. KA is an employee of Alexion, AstraZeneca Rare Disease and owns stock/options in Alexion, AstraZeneca Rare Disease. DC received consultant and lecture fees from, and was a scientific advisory board member for, Alexion AstraZeneca Rare Disease; and is a member of the International Society of Nephrology. LAG received consultant and lecture fees from, and was a scientific advisory board member for, Alexion AstraZeneca Rare Disease; and is a member of the International Society of Nephrology. GA received consultant and lecture fees from, and was a scientific advisory board member for, Alexion AstraZeneca Rare Disease.
Data from this study were published as an abstract (Schaefer F, Al-Dakkak I, Anokhina K, et al. Characteristics and outcomes of patients with atypical hemolytic uremic syndrome switching to ravulizumab from eculizumab: a global registry analysis. J Am Soc Nephrol 2023;34: abstract SA-PO922).
The Global aHUS Registry study is registered on ClinicalTrials.gov (ClinicalTrials.gov: NCT01522183).
Acknowledgments
The authors acknowledge the contributions to this study from the following investigators: Coralie Bingham (Royal Devon & Exeter NHS Foundation Trust, Exeter, UK), Catherine Broome (Georgetown University Hospital, Washington DC, USA), Virginia Cabello (Hospital Virgen del Rocio, Sevilla, Spain), Hans Dieperink (Odense University Hospital, Odense, Denmark), Anja Gäckler (Universitätsklinikum Essen, Essen, Germany), Daniel Gale (Royal Free Hospital School of Medicine, London, UK), Martina Guthoff (Universitätsklinikum Tübingen, Tübingen, Germany), Hermann Haller (Medizinische Hochschule Hannover, Hannover, Germany), Sally Jonhson (Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK), Martin Konrad (Universitätsklinikum Münster, Münster, Germany), Craig Langman (Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, USA), Elaine Majerus (Washington University School Of Medicine, St. Louis, USA), Steven Marks (Great Ormond Street Hospital, London, UK), Martin Nitschke (Universitätsklinikum Schleswig-Holstein, Lübeck, Germany), Ruth Schreiber (Soroka University Medical Center, Beersheba, Israel), Marie Ann Scully (University College London Hospitals NHS Foundation Trust, London, UK), Mohan Shenoy (Royal Manchester Children’s Hospital, Manchester), and Elke Wuehl (Universitätsklinikum Heidelberg, Heidelberg, Germany). Medical writing support was provided by Jess Healy, PhD, and Matthew Reynolds, BSc, of Oxford PharmaGenesis Ltd, Oxford, UK, and was funded by Alexion, AstraZeneca Rare Disease, Boston, MA, USA.
Data Availability Statement
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data deidentification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study Informed Consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at http://alexion.com/research-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
Author Contributions
All authors provided substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafted the work or revised it critically for important intellectual content; and provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Table S1. aHUS triggers or associated conditions at ravulizumab initiation.
Table S2. Creatinine and hemoglobin levels before and after ravulizumab initiation and at last follow-up.
STROBE checklist.
Supplementary Material
Table S1. aHUS triggers or associated conditions at ravulizumab initiation. Table S2. Creatinine and hemoglobin levels before and after ravulizumab initiation and at last follow-up. STROBE checklist.
References
- 1.Raina R., Krishnappa V., Blaha T., et al. Atypical hemolytic-uremic syndrome: an update on pathophysiology, diagnosis, and treatment. Ther Apher Dial. 2019;23:4–21. doi: 10.1111/1744-9987.12763. [DOI] [PubMed] [Google Scholar]
- 2.Goodship T.H., Cook H.T., Fakhouri F., et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Asif A., Nayer A., Haas C.S. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J Nephrol. 2017;30:347–362. doi: 10.1007/s40620-016-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan K., Desai K., Gullapalli L., Druyts E., Balijepalli C. Epidemiology of atypical hemolytic uremic syndrome: A systematic literature review. Clin Epidemiol. 2020;12:295–305. doi: 10.2147/CLEP.S245642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhouri F., Zuber J., Frémeaux-Bacchi V., Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390:681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 6.Menne J., Delmas Y., Fakhouri F., et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol. 2019;20:125. doi: 10.1186/s12882-019-1314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariceta G., Fakhouri F., Sartz L., et al. Eculizumab discontinuation in atypical haemolytic uraemic syndrome: TMA recurrence risk and renal outcomes. Clin Kidney J. 2021;14:2075–2084. doi: 10.1093/ckj/sfab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheridan D., Yu Z.-X., Zhang Y., et al. Design and preclinical characterization of ALXN1210: A novel anti-C5 antibody with extended duration of action. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Röth A., Rottinghaus S.T., Hill A., et al. Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies. Blood Adv. 2018;2:2176–2185. doi: 10.1182/bloodadvances.2018020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravulizumab prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761108s023lbl.pdf
- 11.Ultomiris. Japanese prescribing information. 2023. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/870056_6399427A1027_1_10
- 12.Ravulizumab summary of product characteristics. Ultomiris, INN-ravulizumab. 2023. https://www.ema.europa.eu/en/documents/product-information/ultomiris-epar-product-information_en.pdf
- 13.Ariceta G., Dixon B.P., Kim S.H., et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2021;100:225–237. doi: 10.1016/j.kint.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 14.Barbour T., Scully M., Ariceta G., et al. Long-term efficacy and safety of the long-acting complement C5 inhibitor ravulizumab for the treatment of atypical hemolytic uremic syndrome in adults. Kidney Int Rep. 2021;6:1603–1613. doi: 10.1016/j.ekir.2021.03.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rondeau E., Scully M., Ariceta G., et al. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020;97:1287–1296. doi: 10.1016/j.kint.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K., Adams B., Aris A.M., et al. The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab. Pediatr Nephrol. 2021;36:889–898. doi: 10.1007/s00467-020-04774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauch T.J., Chladek M.R., Cataland S., et al. Treatment preference and quality of life impact: ravulizumab vs eculizumab for atypical hemolytic uremic syndrome. J Comp Eff Res. 2023;12 doi: 10.57264/cer-2023-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Al-Dakkak I., Garlo K., Ong M.L., Tomazos I., Mahajerin A. Atypical hemolytic uremic syndrome treated with ravulizumab or eculizumab: a claims-based evaluation of health care resource utilization and clinical outcomes in the United States. Kidney Med. 2023;5 doi: 10.1016/j.xkme.2023.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Licht C., Ardissino G., Ariceta G., et al. The global aHUS registry: methodology and initial patient characteristics. BMC Nephrol. 2015;16:207. doi: 10.1186/s12882-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehren R., Habbig S. Real-world data of six patients with atypical hemolytic uremic syndrome switched to ravulizumab. Pediatr Nephrol. 2021;36:3281–3282. doi: 10.1007/s00467-021-05203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rondeau E., Cataland S.R., Al-Dakkak I., Miller B., Webb N.J.A., Landau D. Eculizumab safety: five-year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep. 2019;4:1568–1576. doi: 10.1016/j.ekir.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Socié G., Caby-Tosi M.-P., Marantz J.L., et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. 2019;185:297–310. doi: 10.1111/bjh.15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fam S., Werneburg B., Pandya S., et al. Clinical and real-world pharmacovigilance data of meningococcal infections in eculizumab- or ravulizumab-treated patients. J Neurol Sci. 2023;455 doi: 10.1016/j.jns.2023.121883. [DOI] [Google Scholar]
- 24.Delvaeye M., Noris M., De Vriese A., et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spasiano A., Palazzetti D., Dimartino L., et al. Underlying genetics of aHUS: which connection with outcome and treatment discontinuation? Int J Mol Sci. 2023;24 doi: 10.3390/ijms241914496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Ghiringhelli Borsa N., Shao D., et al. Factor H autoantibodies and complement-mediated diseases. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.607211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siedlecki A.M., Isbel N., Vande Walle J., James Eggleston J., Cohen D.J., Registry Global aHUS. Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. Kidney Int Rep. 2019;4:434–446. doi: 10.1016/j.ekir.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jehn U., Altuner U., Pavenstädt H., Reuter S. First report on successful conversion of long-term treatment of recurrent atypical hemolytic uremic syndrome with eculizumab to ravulizumab in a renal transplant patient. Transpl Int. 2022;35 doi: 10.3389/ti.2022.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sancho P.A., González O.D., Rodríguez A.C.A., et al. Ravulizumab “de novo” in pediatric patients with atypical hemolitic uremic syndrome: first worldwide cases. Nephrol Dial Transplant. 2023;5816:38. [Google Scholar]
- 30.Schmidt T., Gödel M., Mahmud M., et al. Ravulizumab in preemptive living donor kidney transplantation in hereditary atypical hemolytic uremic syndrome. Transplant Direct. 2022;8 doi: 10.1097/TXD.0000000000001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenbaum L.A., Licht C., Nikolaou V., et al. Functional assessment of fatigue and other patient-reported outcomes in patients enrolled in the global aHUS registry. Kidney Int Rep. 2020;5:1161–1171. doi: 10.1016/j.ekir.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. aHUS triggers or associated conditions at ravulizumab initiation. Table S2. Creatinine and hemoglobin levels before and after ravulizumab initiation and at last follow-up. STROBE checklist.
Data Availability Statement
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data deidentification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study Informed Consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at http://alexion.com/research-development. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).