Abstract
Background
Patients with alcohol use disorder (AUD) and high-risk opioid use are at risk of serious complications. The purpose of this study was to estimate the prevalence of and factors associated with high-risk opioid use in patients with an alcohol use problem from 2005 to 2018.
Methods
This repeated cross-sectional study analyzed data from first admissions for alcohol treatment (2005–2018) to the NYS Office of Addiction Services and Supports merged with Medicaid Claims Data. High-risk opioid use was defined as opioid dose ≥50 morphine mg equivalents (MME) per day; opioid prescriptions overlapping ≥7 days; opioids for chronic pain >90 days or opioids for acute pain >7 days.
Results
Patients receiving ≥50 MME increased from 690 to 3226 from 2005 to 2010; then decreased to 2330 in 2018. From 2005–2011, patients with opioid prescriptions overlapping ≥7 days increased from 226 to 1594 then decreased to 892 in 2018. From 2005–2010, opioid use >7 days for acute pain increased from 133 to 970 and plateaued after 2010. From 2005–2018, patients who received opioids >90 days for chronic pain trended from 186 to 1655. White patients, females, age 36–55, patients with chronic and acute pain diagnoses had the highest rates of high-risk use.
Conclusions
The prevalence of high-risk opioid use in patients with alcohol use problems increased from 2005 to 2011, and generally decreased after 2010. However, prevalence of opioids >90 days for chronic pain trended up from 2005 to 2018. High-risk opioid use among patients with AUD emphasizes the need to develop interventional strategies to improve patient care.
Keywords: Alcohol use disorder, Opioids, High-risk use, Pain
Highlights
-
•
Examined trends in high-risk opioid use in patients with alcohol use problems.
-
•
High-dose opioid usage peaked in 2010 during the study period (2005–2018).
-
•
The trend of opioid use >90 days for chronic pain increased from 2005 to 2018; opioid use >7 days for acute pain plateaued after 2010.
-
•
Most commonly used opioid was oxycodone for all predefined high-risk use indicators.
-
•
Patients with chronic and acute pain had the highest rates of high-risk opioid use.
1. Introduction
Although recently, concerns about opioid-related overdoses has focused on fentanyl, deaths from prescription opioids have remained high for more than 10 years. From 1999 to 2017, there was more than a 4-fold increase in overdose deaths, increasing from 1.2 to 5.2 per 100,000, and culminating in 17,029 U.S. deaths (Centers for Disease Control and Prevention, 2019). These deaths often arise in the context of high-risk opioid prescriptions. High-risk prescriptions have been defined as a daily dose of ≥50 morphine milligram equivalents (MME), and/or concurrent opioid and benzodiazepine use (McCormick et al., 2021). Similarly, having ≥2 of the following practices in each year was considered potentially problematic prescription behavior, including opioid dose ≥120 MME for ≥90 days, opioid prescriptions overlapped for ≥7 days, co-use of opioid and benzodiazepines, and opioid prescriptions from ≥3 prescribers among individuals with private insurance and Medicaid from 2005 to 2015 (Ali et al., 2019). Bohnert et al. (2016) reported that among VA patients receiving opioids for chronic pain from 2004 to 2009, about half of the overdose cases were prescribed more than 60 MME/day. Among deaths from opioid overdose, the average dose prescribed was 98 MME/day, while overdose survivors were prescribed a mean of 48 MME/day. Similarly, patients who received initial treatment with long-acting opioids, ≥50 MME per day, and >7-day supply had a higher probability of high-risk use in the six quarters following the first prescription (Zhang et al., 2018).
The potential for high risk prescribing to result in an adverse outcome is particularly elevated among heavy drinkers, those with alcohol problems and those with an alcohol use disorder (AUD). Patients with AUD are at significantly higher risk of having pain disorders than individuals without AUD and are more likely to use opioids for pain and require larger dose of opioid and extensive duration of therapy (Hung et al., 2021). Past alcohol dependence and higher level of alcohol consumption correlate with increased pain severity and development of chronic pain after severe injury (Castillo et al., 2006, Holmes et al., 2010), possibly because of neural adaptation resulting from chronic alcohol use that sensitizes the person to pain (Egli et al., 2012). Patients with pain conditions commonly turn to alcohol to cope with pain (Riley and King, 2009). Consequently, patients with an AUD may be more likely to use alcohol and opioid concurrently and excessively, with co-use leading to greater risk of overdose death than either alone (Witkiewitz and Vowles, 2018). Indeed, among patients receiving long-term opioid therapy for chronic non-cancer pain, patients with AUD experienced increased rates of opioid overdose, accident, and injury compared with patients without AUD (Landsman-Blumberg et al., 2017).
Previous studies have examined the prevalence of high-risk opioid use among the general population and opioid use for chronic and acute pain (McCormick et al., 2021, Mikosz et al., 2020, Zhang et al., 2018). However, there is a gap in research on high-risk opioid use among patients with AUD. Landsman-Blumberg et al. (2017) investigated adverse outcomes and healthcare costs associated with long-term opioid use for chronic pain in patients with and without AUD, but their study included only 750 patients with AUD (3.5 % of the sample). Our prior research used a similar dataset to assess the impact of New York State’s (NYS) Internet System for Tracking Over-Prescribing (I-STOP) on the prescription rates of opioids and benzodiazepines, along with their co-prescription trends (Jacobs et al., 2022). However, Jacobs et al. (2022) did not specifically address high-risk opioid prescribing practices. Our current research extends this work by examining the prevalence and trends of high-risk opioid prescribing for both (1) acute and (2) chronic pain in individuals with significant alcohol problems, assessing changes from 2005 to 2018, and identify patient factors associated with high-risk opioid use.
2. Methods
2.1. Data source
De-identified data extracted from the NYS Office of Addiction Services and Supports (OASAS) Client Data System (CDS) was merged with data extracted from the NYS Department of Health (DOH) Medicaid Data Warehouse (MDW) using a unique client identifier to anonymously track subjects (Lu et al., 2023). The CDS collects admission and discharge information for patients receiving treatment services for substance use disorder (SUD). A patient may have multiple admissions throughout the course of their treatment. For each admission, demographic characteristics such as age, sex, marital status, and living situation were collected. Client’s primary, secondary, and tertiary substance use (e.g., alcohol, opioids, benzodiazepines, marijuana, etc.) as well as age of first use are assessed at each admission. The MDW provides individual-level Medicaid enrollment, healthcare utilization, diagnoses, and outpatient prescription claim data. The SUNY at Buffalo institutional review board determined the study exempt.
2.2. Study population
Patients with a first admission to an OASAS treatment program from January 1, 2005 to December 31, 2018 were identified. We included patients presenting with primary alcohol use problems and no opioid use problems reported within the CDS and who were 18 years or older at the time of index OASAS admission. Most of our patients were assumed to have met criteria for AUD, as 79 % of them received an International Classification of Disease Clinical Modification (ICD-CM) 9 or 10 codes for AUD diagnosis in subsequent Medicaid records (Jacobs et al., 2022). Patients were included if they had at least 1 MDW prescription claim within the same year as their index OASAS admission and for each subsequent year. Patients with cancer and sickle cell disease were excluded from this study.
2.3. High-risk opioid use
High-risk opioid use was defined as one of the following: 1) opioid daily dose of ≥50 MME per day (Bohnert et al., 2016); 2) opioid prescriptions overlapping for ≥7 days (Zhang et al., 2018); 3) opioid duration of therapy for chronic pain > 90 days (Dowell et al., 2016, Raman et al., 2019); and 4) opioid duration of therapy for acute pain >7 days (Dowell et al., 2016, NYS DOH, 2016, The NYS Senate, 2018). Additionally, we identified the most common high-risk-used opioids in each year.
Among patients prescribed with opioids ≥50 MME, we categorized them into 50 to <90 MME, 90 to <120 MME, ≥120 MME. MME per day was calculated using the conversion factors from U.S. Department of Health & Human Services, (Centers for Medicare and Medicaid Services, 2020) (see Supplementary Material, Table 2). Opioid duration of therapy >90 days for chronic pain and >7 days for acute pain was defined as <7 days gap between each claim for a total duration >90 days and >7 days, respectively. Opioid prescriptions overlap was identified as patients filling an opioid when they still had ≥7-day supplies.
Table 2.
Rates of opioid use per day.
| High-risk use indicators (MME/day) | ≥50 MME | 50≤MME<90 | 90≤MME<120 | ≥120 MME | |
|---|---|---|---|---|---|
| Sex | Female | 2996 (2923,3070) | 1621 (1568, 1676) | 554 (523, 586) | 821 (783, 860) |
| Male | 2341 (2296, 2386) | 1179 (1147, 1211) | 419 (400, 439) | 743 (717, 769) | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Age | 18–35 years of age | 1974 (1925, 2025) | 1076 (1039, 1113) |
349 (328, 370) | 550 (524, 577) |
| 36–55 years of age | 3282 (3216, 3349) | 1635 (1588, 1682) |
604 (576, 633) | 1043 (1006, 1081) | |
| >55 years of age | 2075 (1964, 2190) | 1116 (1035, 1202) |
380 (333,430) | 579 (522, 641) | |
| P value | <0.001a | <0.001a | <0.001a | <0.001a | |
| Race | White NH | 3058 (2997, 3121) | 1620 (1576, 1667) | 575 (549, 603) | 863 (830, 897) |
| Black NH | 2309 (2238, 2379) | 1096 (1049, 1146) | 388 (360, 418) | 825 (784, 868) | |
| Other NH | 1705 (1575, 1844) | 895 (802, 997) | 292 (240, 352) | 518 (448, 596) | |
| Hispanic | 1970 (1895, 2046) | 1072 (1017, 1129) | 355 (324, 389) | 542 (504, 583) | |
| P value | <0.005 | <0.05b | <0.05b,c | <0.001c,d | |
| Pain diagnoses | Acute pain only | 1874 (1753, 2001) | 1361 (1259, 1470) | 246 (204, 294) | 267 (223, 317) |
| Chronic pain only | 4885 (4776, 4997) | 2279 (2205, 2356) | 923 (876, 972) | 1683 (1619, 1748) | |
| Acute pain and chronic pain | 7563 (7377, 7753) | 3961 (3827, 4099) | 1432 (1352, 1515) | 2170 (2071, 2273) | |
| No pain diagnosis | 540 (517, 564) | 327 (308, 345) | 80 (72, 90) | 134 (122, 146) | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
MME= morphine mg equivalents; NH=non-Hispanic.
Rates are expressed in number of patients per 100,000 people.
a P-value of 18–35 vs. >55 years of age >0.05.
b P-value of Black NH vs. Hispanic >0.05.
c P-value of other NH vs. Hispanic >0.05.
d P-value of White NH vs. Black NH >0.05.
We categorized high-risk opioid use population into 4 groups based on pain diagnoses as the following: 1) acute pain diagnoses only 2) chronic pain diagnoses only 3) both chronic and acute pain diagnoses 4) without pain diagnoses. Chronic pain was classified as 1) neuropathies and neuralgias 2) headaches or migraines 3) arthritis or joint pain 4) back or cervical pain 5) unclassified pain (Janakiram et al., 2019, Romanelli et al., 2017). Acute pain was classified as 1) abdominal pain 2) dental pain 3) rib fracture 4) musculoskeletal sprains and strains 5) kidney stone (Mikosz et al., 2020). Both chronic and acute pain diagnoses were identified in our claim database using the ICD 9 and 10 codes (see Supplementary Material Tables 3-6). The ICD 9 and 10 codes for pain were from previous literature, the AHFS ICD conversion tool, and physician team expert panel (AHFS Drug Information - Stat!Ref, 2023, Janakiram et al., 2019, Mikosz et al., 2020, Romanelli et al., 2017). NYS regulations require controlled substances prescriptions to be dispensed within 30 days from the date signed by the practitioner (NY Codes Rules and Regulations, 2013). Thus, we looked back 30 days from an opioid fill to identify an ICD code for acute and chronic pain documented closest to the fill date.
2.4. Covariates
Primary exposure is the receipt of an opioid prescription. Opioid medications that were identified were buprenorphine, codeine, dihydrocodeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, pentazocine, opium, oxymorphone, oxycodone, tapentadol, and tramadol. Buprenorphine and methadone are used for treatment for opioid use disorder (OUD) and chronic pain. However, these drugs carry black-box warnings from the FDA underscoring the risk of profound sedation, respiratory depression, and death when used with other CNS depressants, including alcohol and benzodiazepines (Mallinckrodt Pharmaceuticals, 2021). Buprenorphine and methadone misuse is observed in patients with or without prescriptions (Han et al., 2021, Lugoboni et al., 2019). Given the possibility of misuse, co-use of buprenorphine, methadone or alcohol, and NYS limits on early refill of control substances, we included methadone and buprenorphine in our high-risk use analysis. We evaluated prevalence of a concurrent of OUD and buprenorphine or methadone prescription in the given year. From 2005–2013, there were no buprenorphine or methadone prescriptions concurrent with an OUD. From 2014–2018, the percentage of buprenorphine or methadone prescriptions concurrent with an OUD diagnosis increase from 0.1 % to 2.9 % of all buprenorphine or methadone prescriptions. Methadone prescriptions are for pain because methadone prescribed for OUD is only available from an opioid treatment program; methadone prescription is not billed under Medicaid but is a bundled billing for all opioid treatment procedures. Thus, we did not separate those prescribed for OUD from those for chronic pain. Buprenorphine and methadone were assigned 0 in the daily MME dose conversion. As a result, medications prescribed for OUD were not included in our ≥50 MME high-risk use analysis.
Patient level factors include age, sex assigned at birth, ethnicity, pain diagnoses. Age was stratified into 18–35 years, 36–55 years, and >55 years groups. Race is categorized as White non-Hispanic (NH), Black NH, Other NH, or Hispanic.
2.5. Statistical analysis
We calculated the overall annual rates of high-risk opioid use, with the prevalence expressed as the number of patients with high-risk use per 100,000 population. Temporal prevalence trends of high-risk opioid use from 2005 to 2018 were estimated using the Kendall-tau test. Sampling was done annually using a repeated cross-sectional approach.
The high-risk use rates were stratified by pre-defined patient level factors. The significance of differences in rates of pre-defined high-risk opioid use based on patient level factors was examined using the chi-square test. This was evaluated in different age, sex, race, and pain diagnoses groups. All analyses were performed in Excel, Python, SPSS 28.0 (IBM Corp, 2021), and all hypothesis testing were two-sided with a significance set at α <0.05.
3. Results
3.1. Prevalence trends of high-risk opioid use
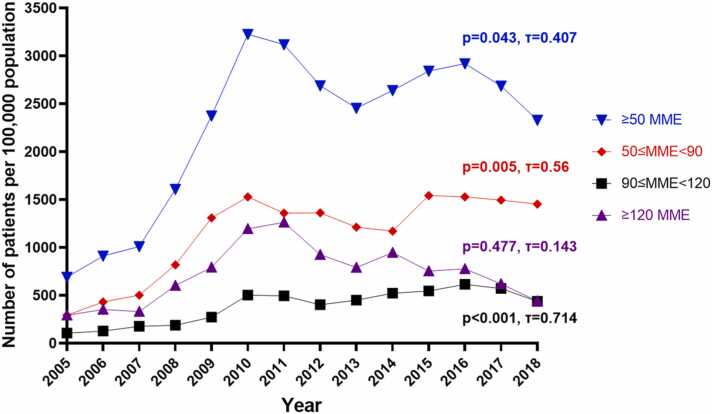
Patient baseline characteristics are presented in Table 1. Within the study period, 142,978 patients met the inclusion criteria. The prevalence trends of opioid use from 2005 to 2018 are presented in Fig. 1. The prevalence of patients who received ≥50 MME of opioids per day increased from 690 per 100,000 population in 2005 to a peak of 3226 in 2010 (p<0.01, τ=1). The prevalence declined in the following years to 2454 in 2013 (with a sub-peak of 2919 in 2016) then decreased to 2330 in 2018 (from 2011 to 2018: p=0.322, τ=-0.286).
Table 1.
Baseline Characteristics of Patients with alcohol use problems from 2005 to 2018.
| Year |
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total patient number | 7532 | 14,149 | 19,309 | 25,053 | 32,307 | 39,181 | 45,037 | 49,760 | 54,605 | 62,804 | 70,494 | 72,908 | 76,015 | 80,342 | |
| Sex | Female | 30 % | 30 % | 32 % | 33 % | 33 % | 33 % | 33 % | 33 % | 33 % | 33 % | 33 % | 32 % | 33 % | 33 % |
| Male | 70 % | 70 % | 68 % | 67 % | 67 % | 67 % | 67 % | 67 % | 67 % | 67 % | 67 % | 68 % | 67 % | 67 % | |
| Age, years | 18–35 | 44 % | 45 % | 45 % | 46 % | 47 % | 48 % | 47 % | 47 % | 47 % | 47 % | 47 % | 47 % | 47 % | 46 % |
| 36–55 | 48 % | 47 % | 47 % | 46 % | 45 % | 44 % | 44 % | 44 % | 44 % | 44 % | 43 % | 42 % | 43 % | 43 % | |
| >55 | 9 % | 8 % | 8 % | 9 % | 8 % | 8 % | 9 % | 9 % | 9 % | 9 % | 10 % | 10 % | 11 % | 11 % | |
| Race | White NH | 45 % | 45 % | 45 % | 45 % | 46 % | 46 % | 46 % | 46 % | 46 % | 47 % | 47 % | 47 % | 47 % | 47 % |
| Black NH | 30 % | 30 % | 30 % | 29 % | 29 % | 29 % | 29 % | 28 % | 28 % | 27 % | 26 % | 26 % | 27 % | 27 % | |
| Other NH | 5 % | 5 % | 5 % | 5 % | 5 % | 5 % | 5 % | 5 % | 6 % | 6 % | 6 % | 6 % | 6 % | 6 % | |
| Hispanic | 20 % | 20 % | 21 % | 21 % | 20 % | 20 % | 20 % | 20 % | 20 % | 20 % | 20 % | 20 % | 20 % | 20 % | |
| Pain diagnoses | Acute pain only | 7 % | 8 % | 8 % | 8 % | 8 % | 8 % | 8 % | 8 % | 7 % | 7 % | 7 % | 7 % | 7 % | 7 % |
| Chronic pain only | 15 % | 17 % | 19 % | 20 % | 21 % | 22 % | 23 % | 24 % | 25 % | 26 % | 27 % | 24 % | 25 % | 24 % | |
| Chronic and acute pain | 6 % | 9 % | 10 % | 11 % | 13 % | 14 % | 15 % | 15 % | 14 % | 14 % | 14 % | 12 % | 11 % | 10 % | |
| No pain diagnosis | 73 % | 65 % | 62 % | 60 % | 58 % | 56 % | 54 % | 53 % | 53 % | 53 % | 53 % | 58 % | 57 % | 59 % | |
NH=non-Hispanic.
Fig. 1.
Annual prevalence of patients receiving ≥50 MME of opioids from 2005 to 2018. MME=morphine mg equivalents.
Prescriptions between 50 and 90 MME per day increased from 292 to 1453 per 100,000 from 2005 to 2018, with subsequent sub-peaks in 2010 and 2015 at 1529 and 1542 (2005–2010: p<0.01, τ=1; 2011–2018: p=0.458, τ=0.241). Prescriptions for 90–120 MME per day increased significantly over the study period (p<0.001, τ=0.714), with increases from 106 to 437 from 2005 to 2018, and a peak in 2016 at 614. Finally, patients receiving ≥120 MME per day increased from 292 to 1263 from 2005 to 2011 (p=0.004, τ=0.905), then decreased in the following years to 441 in 2018 (p=0.024, τ=-0.714 from 2012 to 2018).
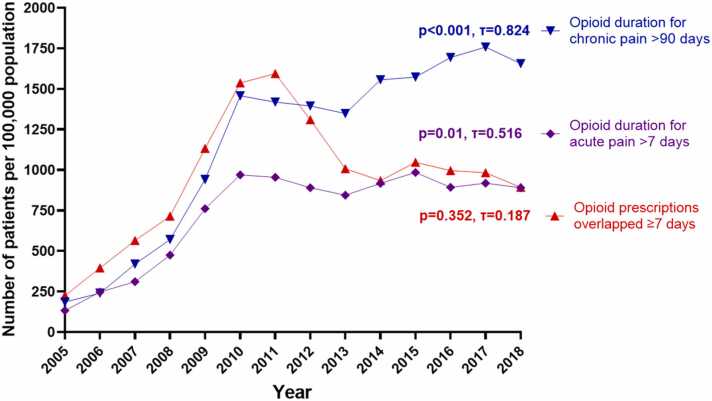
Fig. 2 presents the prevalence of opioid overlap for ≥7 days, >90 days for chronic pain, and >7 days for acute pain from 2005 to 2018. Overlapped prescriptions for ≥7 days increased from 226 to a peak of 1594 per 100,000 in 2011 (p<0.01, τ=1). This overlap then decreased to 892 in 2018 (from 2012 to 2018: p=0.051, τ=-0.619). From 2005–2010, opioid use >7 days for acute pain increased from 133 to 970 (p<0.01, τ=1), then plateaued after 2010 (from 2011 to 2018: p=0.805, τ=-0.071). Lastly, the trend of opioids use >90 days for chronic pain increased from 186 to 1457 from 2005 to 2010 (p<0.01, τ=1) and slightly decreased to 1348 in 2013. This frequency further increased to 1655 in the following years (from 2011 to 2018: p=0.026, τ=0.643). The coefficient showed significant increases in the prevalence trends across 2005–2018 (p<0.001, τ=0.824).
Fig. 2.
Annual prevalence of patients receiving high risk opioids.
Oxycodone was the most commonly identified opioid used in nearly every year across all predefined high-risk use indicators (Supplementary materials, Tables 7–13).
3.2. High-risk opioid use factors in patients with alcohol use problems
3.2.1. ≥50 MME of opioids per day
Rates of opioid use ≥50 MME among subgroups are presented in Table 2. We observed significantly higher opioid use in females than males in patients using ≥50 MME per day. Utilization was highest among patients aged 36–55. No significant differences were observed among patients aged 18–35 and >55 years. Highest rates occurred in White NH, followed by Black NH, Hispanics, and other NH. Patients with both chronic and acute pain diagnoses had the highest rates compared with patients with chronic pain only, or acute pain only, or without pain diagnosis (p<0.001 between each group). Rates of opioid use between 50 and 90 MME, 90 and 120 MME, and ≥120 MME showed a similar pattern of demographic differences.
3.2.2. Opioid prescriptions overlapped ≥7 days
Rates of opioids overlapped ≥7 days, opioid for acute pain >7 days and for chronic pain >90 days among subgroups are shown in Table 3. Significantly more females received overlapping opioid prescriptions than males. Rates varied significantly among age groups, with the highest rates observed in patients aged 36–55 years, followed by 18–35 years, and >55. Opioid prescription overlap was significantly higher among NH Whites compared to all other racial groups. Patients with both chronic and acute pain diagnoses had the highest rates of receiving overlapping opioids for ≥7 days, followed by patients with chronic pain only, acute pain only, and without pain diagnosis.
Table 3.
Rates of high-risk opioid use.
| High-risk use indicators | Opioid prescriptions overlapped ≥ 7 days | Opioid for acute pain >7 days | Opioid for chronic pain >90 days | |
|---|---|---|---|---|
| Sex | Female | 1316 (1268, 1365) | 1128 (1083, 1173) | 1745 (1690, 1802) |
| Male | 908 (880, 937) | 714 (690, 740) | 1257 (1224, 1291) | |
| P value | <0.001 | <0.001 | <0.001 | |
| Age | 18–35 years of age | 912 (878, 946) | 786 (755, 818) | 1015 (980, 1051) |
| 36–55 years of age | 1230 (1189, 1271) | 977 (941, 101) | 1880 (1830, 1931) | |
| >55 years of age | 814 (745, 887) | 579 (522, 641) | 1264 (1178, 1355) | |
| P value | <0.05 | <0.001 | <0.001 | |
| Race | White NH | 1418 (1376, 1461) | 1142 (1104, 1180) | 1775 (1728, 1823) |
| Black NH | 712 (673, 751) | 591 (556, 627) | 1131 (1083, 1181) | |
| Other NH | 661 (581, 749) | 507 (438, 584) | 893 (799, 994) | |
| Hispanic | 731 (686, 778) | 627 (585, 671) | 1129 (1073, 1188) | |
| P value | <0.001a,b,c | <0.05a,c | <0.001a | |
| Pain diagnoses | With acute pain | 532 (469, 601) | N/A | |
| With chronic pain | 2012 (1942, 2084) | |||
| With acute pain and chronic pain | 3142 (3023, 3265) | |||
| Without acute and chronic pain | 228 (212.7, 243.6) | |||
| P value | <0.001 | |||
NH=non-Hispanic.
Rates are expressed in number of patients per 100,000 people.
a P-value of Black NH vs. Hispanic >0.05.
b P-value of other NH vs. Hispanic >0.05.
c P-value of other NH vs. Black NH >0.05.
3.2.3. Opioids for acute pain >7 days
The frequency of using opioids for acute pain >7 days in females was significantly higher than in males. Consistent with other predefined high-risk indicators, patients aged 36–55 years had significantly highest rates of opioid use for acute pain >7 days, followed by 18–35 and >55 years. Significantly higher rates occurred among the White NH group and other racial groups. Hispanics had a higher rate than other NH. No significant differences were observed between Black NH vs. Hispanic and other NH groups.
3.2.4. Opioids for chronic pain >90 days
Females and patients 36–55 years of age had significantly higher rates as we observed in other high-risk indicators. Rates varied significantly among race groups, except for Black NH compared to Hispanic group. White NH group had the highest rates, followed by Black NH, Hispanic, and other NH.
4. Discussion
This study merged two NYS administrative databases to provide trends of high-risk use of prescription opioids among patients treated for alcohol use problems and characterize patient level factors associated with high-risk use. The prevalence of ≥50 MME increased from 2005 to 2010 and decreased in the following years to a trough in 2013. This further increased to another peak in 2016. Likewise, prevalence of opioid prescriptions with an overlap ≥7 days increased from 2005 to a peak in 2011 and decreased in the following years. The trend of opioid use for chronic pain >90 days increased significantly over the study period. The trend of opioid use for acute pain >7 days plateaued after 2010.
Our findings in decreasing trends in receiving ≥50 MME from 2010 to 2013 and overlapping prescriptions for ≥7 days align with our previous research, demonstrating a consistent decrease in opioid prescribing patterns and reaffirming the impact of the NYS I-STOP program (Jacobs et al., 2022). The introduction of an online Prescription Monitoring Program (PMP) in 2010 to prescribers within NYS (Community Health Care Association of NYS, 2020, NYS DOH, 2013) may have influenced this. Beginning in 2013, use of the PMP prior to prescribing a controlled became mandatory (NYS DOH, 2013). At the same time, pharmacies were also required to report controlled substance prescription data daily. Prior to mandated consultation of the PMP, there were 950,000 searches by 19,000 users for 202,714 patients from February 2010 to August 2013 (Community Health Care Association of NYS, 2020). After the implementation of mandated PMP consults, the rate rose to 47 searches per second. This suggests that the decrease in the annual prevalence of high-risk opioid use after 2010 was influenced by the awareness and then enforcement of this regulation. Guy et al. (2017) presented that the total number of opioid prescriptions and dispensing rate per 100 persons were the highest between 2010 and 2012 in the US, which was similar to the results observed in our study. The decline and plateau observed in yearly prevalence for all high-risk opioid use after 2016 may be resulted from the publication of 2016 CDC guideline for pain management and the courses on opioids required for licensure renewal. Changes in physician prescribing behavior associated with the CDC guideline are reported in literature. Following the 2016 CDC updates, reduction in opioid prescribing for chronic pain, increased in non-opioid analgesic alternatives, and tapering dosage of opioids were observed (Encinosa et al., 2022). Previous literature also highlights the benefits of policies on reducing rates of high-risk prescribing of initial opioid analgesics across multiple states, as well as reducing the rates of opioid misuse (Lee et al., 2021, Stein et al., 2022).
However, when examining patients grouped by MME levels, we observed mixed trends. Between 2010 and 2014, there was a slight decrease among the group receiving 50–90 MME, yet the overall trend still showed an increase. Additionally, for those patients receiving 90–120 MME, no clear decreasing trends were evident. Concurrently, the long-duration use of opioids for both chronic and acute pain increased from 2005 to 2010 and remained stable for the rest of the study period. This contrasts with our previous observations and suggests that despite the overall decrease in opioid prescribing due to the PMP system (Jacobs et al., 2022), it has not significantly reduced high-risk opioid use among this population, which remains a major concern. This may be partly due to the overlapping mechanisms between alcohol and opioid dependence, leading to the complex interaction observed in pain management and substance use behaviors. For instance, as revealed in a retrospective analysis of analgesic use among 4143 patients, frequent alcohol consumption has been associated with increased opioid use for postoperative pain control (Kao et al., 2017). Moreover, patients experiencing alcohol withdrawal syndrome were reported to have heightened pain sensitivity, which means people would perceive normal pain as more intense, necessitating higher doses of analgesics for pain relief (Jochum et al., 2010). Repeated exposure to alcohol also leads to tolerance of its analgesic effects through physiological mechanisms, and extended alcohol use could induce and exacerbate pain symptoms from other sources (Egli et al., 2012). Consequently, problem drinkers often report more severe pain symptoms than nondrinkers and are more likely to use alcohol to manage their pain, highlighting the intricate relationship between alcohol use, pain perception, and opioid consumption.
Our findings indicate the highest rates of high-risk opioid use among patients with both acute and chronic pain diagnoses, contrasting with the results from 4 states' Medicaid programs between 2017 and 2019, where high-risk opioid use was most common among patients with acute pain only (Heins et al., 2018). These varying results suggest that differences in time periods, the presence or absence of AUD comorbidity, and the scope of the sample may influence observational findings. Individuals with an AUD are at greater risk for accidents that can result in acute pain, even when sober (Hingson et al., 2009, Vinson et al., 2003). They are also at greater risk for chronic pain conditions (Boissoneault et al., 2019). As a consequence, pain management is an important issue for this population. Previous research highlighted the increased risks associated with high-dose opioid use. For example, the risk of opioid-related overdose death increased 11-fold when a patient used more than 100 MME of opioids daily (Gwira Baumblatt et al., 2014). The combination of alcohol and opioids significantly raises the likelihood of adverse events, overdoses, and fatalities (Gudin et al., 2013). This underscores the critical need for targeted interventions for individuals with AUD, who are at increased risk of severe or fatal outcomes.
High-risk opioid use among patients with AUD highlighted the necessity of education for patients, caregivers, and healthcare professionals. The NYS DOH website provides addiction and SUD educational resources and naloxone co-payment assistance program (NYS DOH, 2023). Online, multi-professional opioid prescriber training programs have been mandated for prescribers in NYS since 2016. The training was required to be completed by July 2017, with an ongoing mandate for retraining at 3-year intervals (Bednarczyk et al., 2021, The NYS Senate, 2016). Following this legislative update requiring training on opioid prescribing, the prevalence of high-risk use either decreased or plateaued. More recently, the Drug Enforcement Administration (DEA) has mandated training of the management of patients with opioid or other substance use disorders for all DEA-registered practitioners nationally (Drug Enforcement Administration, 2023). The new CDC guidelines provide guidance on opioid prescribing for patients with SUD (Dowell et al., 2022). Changes in opioid prescribing and prevalence of overdose following the guideline update warrant future research.
In this study, we found that among patients with alcohol use problems, females had a higher rate of high-risk opioid use compared to males. An analysis of a large commercial claims and encounters database revealed that females had significantly more opioid overlap incidents and numbers of different indicators of inappropriate use (Logan et al., 2013). Furthermore, an examination of the IQVIA Longitudinal Prescription Data also indicated that high-risk opioid use was more prevalent among female patients (McCormick et al., 2021). Previous research also suggests that women are at increased risk for a number of painful medical conditions and tend to experience more severe adverse effects from alcohol consumption, including a higher susceptibility to liver disease and greater cognitive and motor impairment (Zale et al., 2015). However, gender disparities in high-risk opioid use are not always consistent. Analyses of multi-state Medicaid databases showed that high-risk opioid use was more prevalent among males than females (Ali et al., 2019, Mack et al., 2015). The NYS opioid data for general population showed consistent higher percentage of patients prescribed ≥ 90 MME/day in male than female NYS DOH, 2018, NYS DOH, 2021.
Discrepancy in prevalence of high risk opioid use among different age groups is observed between our findings and previous literature. Patients aged 36–55 displayed the highest rates within our study group. This was different from the broader NYS population reported by NYS DOH, 2018, NYS DOH, 2021 and national trends reported by McCormick et al. (2021) where high-risk use was the most prevalent among those aged ≥55. These findings underscore the importance of careful interpretation of data due to differences in study designs and data sources that can yield contrasting results, particularly when examining high-risk opioid use among patients with or without alcohol use problems.
Racial differences in opioid prescriptions for pain are commonly reported in the literature (Joynt et al., 2013, Lee et al., 2019, Ly, 2019, Morden et al., 2021). Black and Hispanic patients are less likely to receive opioid prescriptions for pain management in outpatient and emergency department settings, pointing toward potential racial bias in prescribing practices (Joynt et al., 2013, Lee et al., 2019, Ly, 2019). Among short-term and long-term opioid recipients, Black patients received lower annual opioid doses than White patients (Morden et al., 2021). Consistent with previous reports on high-risk use among general population (Ali et al., 2019, Heins et al., 2018), in patients with alcohol use problems, our study found the greatest rates of all pre-defined high-risk use indicators among White NH.
This study has several limitations. First, our definitions of high-risk opioid use followed the 2016 CDC guidelines (e.g., ≥50 MME, >7 days for acute pain and >90 days for chronic pain), which may not reflect the 2022 updates that removed specific cutoffs for high MME and therapy duration (Dowell et al., 2016, Dowell et al., 2022). Despite this, our definitions are still supported by studies cited by the guideline. Second, until 2017, OASAS did not specifically indicate whether individuals with primary alcohol use problem met criteria for an AUD diagnosis. However, all of the clients reported that alcohol use was a problem and were admitted for treatment specifically for their drinking. In order to get further validity, we looked for AUD diagnoses in the Medicaid data and found corroboration in 79 % of the cases. For the other 21 %, they may not have experienced any further alcohol problems or none within the timeframe of the study. Future studies could improve validity by employing a matched control group with confirmed ICD codes for AUD. Additionally, researchers should consider including a broader Medicaid population to investigate patterns of high-risk opioid prescribing among patients who seek treatment for AUD versus those who do not. Third, using the merged database may introduce biases due to the possibilities of missing data. Limitations of the database were described in a previous study (Lu et al., 2023). This may be mitigated by integrating electronic health records. Fourth, the sample is comprised of NYS Medicaid patients who sought treatment for a primary alcohol use problem, which might not represent the entire population with AUD or patients who are not in Medicaid programs. The generalizability of the study results to other states or countries may be limited, but our study remains one of the only studies to evaluate high-risk use of opioids among patients with alcohol use problems. The patient level characteristics were self-reported, so misclassification is possible. However, we still detected statistically significant differences in high-risk opioids use in certain populations. Fifth, we did not analyze methadone or buprenorphine use for OUD separately, as their concomitant prescription rates remained low. Lastly, diagnoses for acute and chronic pain were captured via ICD codes, and the variable definitions used in practice guidelines and these codes may not comprehensively reflect all pain diagnoses. Despite these challenges, we detected significant differences in high-risk opioid use across different patient groups, highlighting the study's contribution to understanding opioid use in populations with alcohol use problems.
5. Conclusions
The temporal prevalence trend of high-risk opioid use in NYS Medicaid patients treated for alcohol use problems increased from 2005 to 2011. This increase was characterized by patient factors associated with high-risk opioid use. There were significant differences in rates of high-risk use based on certain patient characteristics including female sex, age 36–55, and combined acute and chronic pain diagnoses. While not a surprising finding, the rising use of high-risk opioids among patients with alcohol use problems and the continued high level emphasizes the need to raise awareness of concomitant use and develop intervention strategies to improve patient outcomes.
Funding
This work was supported in part by the National Institutes of Health NLM T15LM012495, NIAAA R21AA026954, R33AA0226954, NIDA K01DA056690, and NCATS UL1TR001412. This study was funded in part by the Department of Veterans Affairs. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
CRediT authorship contribution statement
Tzu-Yin Kuo: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Chi-Hua Lu: Writing – review & editing, Writing – original draft, Conceptualization. Zackary Falls: Writing – review & editing, Visualization, Software, Formal analysis, Data curation, Conceptualization. Gail Jette: Writing – review & editing, Data curation. Walter Gibson: Writing – review & editing, Data curation. Peter L. Elkin: Writing – review & editing, Funding acquisition. Kenneth E. Leonard: Writing – review & editing, Funding acquisition. Edward M. Bednarczyk: Writing – review & editing, Writing – original draft, Supervision. David M. Jacobs: Writing – review & editing, Supervision, Conceptualization.
Declaration of Competing Interest
None.
Acknowledgements
The authors acknowledge Barbara Roger, PharmD, MS and Steven Feuerstein, MS for assistance in data interpretation, Debanjan Paul, MS for data cleaning, and Maha Rauf and Ume Farwa Wasim for ICD code conversion.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dadr.2024.100278.
Appendix A. Supplementary material
Supplementary material.
References
- AHFS Drug Information - Stat!Ref, 2023. STAT!RefICD Conversion Tool. 〈https://online.statref.com/tools/icdconvert〉.
- Ali M.M., Tehrani A.B., Mutter R., Henke R.M., O'Brien M., Pines J.M., Mazer-Amirshahi M. Potentially problematic opioid prescriptions among individuals with private insurance and Medicaid. Psychiatr. Serv. 2019;70(8):681–688. doi: 10.1176/appi.ps.201800555. [DOI] [PubMed] [Google Scholar]
- Bednarczyk E.M., Blondell R.D., Wahler R.G., Fiebelkorn K.D., Waghmarae R., Lu C.H., Rogler B.A., Dunn T.E. A large-scale, online, multiprofessional opioid prescriber training program. J. Am. Coll. Clin. Pharm. 2021;5(2):123–131. doi: 10.1002/jac5.1546. [DOI] [Google Scholar]
- Bohnert A.S., Logan J.E., Ganoczy D., Dowell D. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med. Care. 2016;54(5):435–441. doi: 10.1097/mlr.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault J., Lewis B., Nixon S.J. Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol. 2019;75:47–54. doi: 10.1016/j.alcohol.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Castillo R.C., MacKenzie E.J., Wegener S.T., Bosse M.J. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124(3):321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2019. 2019 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report. 〈https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf〉.
- Centers for Medicare & Medicaid Services, 2020. Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors Table for Prescription Drug Coverage. Centers for Medicare & Medicaid Services. 〈https://www.hhs.gov/guidance/document/opioid-oral-morphine-milligram-equivalent-mme-conversion-factors-0〉.
- Community Health Care Association of NYS, 2020. The New York State Prescription Monitoring Program and Controlled Substance Management. 2020 Digital Health Workshop. 〈https://www.chcanys.org/sites/default/files/2020-11/DOH%20PMP.pdf〉.
- Dowell D., Haegerich T.M., Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D., Ragan K.R., Jones C.M., Baldwin G.T., Chou R. CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm. Rep. 2022;71(3):1–95. doi: 10.15585/mmwr.rr7103a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2023. Medication Assisted Treatment. 〈https://www.deadiversion.usdoj.gov/pubs/docs/MATE_training.html〉.
- Egli M., Koob G.F., Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci. Biobehav. Rev. 2012;36(10):2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinosa W., Bernard D., Selden T.M. Opioid and non-opioid analgesic prescribing before and after the CDC's 2016 opioid guideline. Int. J. Health Econ. Manag. 2022;22(1):1–52. doi: 10.1007/s10754-021-09307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudin J.A., Mogali S., Jones J.D., Comer S.D. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad. Med. 2013;125(4):115–130. doi: 10.3810/pgm.2013.07.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy G.P., Jr., Zhang K., Bohm M.K., Losby J., Lewis B., Young R., Murphy L.B., Dowell D. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb. Mortal. Wkly. Rep. 2017;66(26):697–704. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwira Baumblatt J.A., Wiedeman C., Dunn J.R., Schaffner W., Paulozzi L.J., Jones T.F. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern. Med. 2014;174(5):796–801. doi: 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- Han B., Jones C.M., Einstein E.B., Compton W.M. Trends in and characteristics of buprenorphine misuse among adults in the US. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.29409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins S.E., Sorbero M.J., Jones C.M., Dick A.W., Stein B.D. High-risk prescribing to medicaid enrollees receiving opioid analgesics: individual- and county-level factors. Subst. Use Misuse. 2018;53(10):1591–1601. doi: 10.1080/10826084.2017.1416407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R.W., Edwards E.M., Heeren T., Rosenbloom D. Age of drinking onset and injuries, motor vehicle crashes, and physical fights after drinking and when not drinking. Alcohol. Clin. Exp. Res. 2009;33(5):783–790. doi: 10.1111/j.1530-0277.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Williamson O., Hogg M., Arnold C., Prosser A., Clements J., Konstantatos A., O'Donnell M. Predictors of pain 12 months after serious injury. Pain Med. 2010;11(11):1599–1611. doi: 10.1111/j.1526-4637.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- Hung H.Y., Chien W.C., Chung C.H., Kao L.T., Chow L.H., Chen Y.H., Kotlinska J.H., Silberring J., Huang E.Y. Patients with alcohol use disorder increase pain and analgesics use: a nationwide population-based cohort study. Drug Alcohol Depend. 2021;229(Pt A)) doi: 10.1016/j.drugalcdep.2021.109102. [DOI] [PubMed] [Google Scholar]
- IBM Corp, 2021. IBM SPSS Statistics for Windows. Armonk, NY. In (Version 28.0).
- Jacobs D.M., Tober R., Yu C., Gibson W., Dunn T., Lu C.H., Bednzarczyk E., Jette G., Lape-Newman B., Falls Z., Elkin P.L., Leonard K.E. Trends in prescribing opioids, benzodiazepines, and both among adults with alcohol use disorder in New York state. J. Gen. Intern. Med. 2022 doi: 10.1007/s11606-022-07682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiram C., Fontelo P., Huser V., Chalmers N.I., Lopez Mitnik G., Brow A.R., Iafolla T.J., Dye B.A. Opioid prescriptions for acute and chronic pain management among medicaid beneficiaries. Am. J. Prev. Med. 2019;57(3):365–373. doi: 10.1016/j.amepre.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum T., Boettger M.K., Burkhardt C., Juckel G., Bär K.-J. Increased pain sensitivity in alcohol withdrawal syndrome. Eur. J. Pain. 2010;14(7):713–718. doi: 10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Joynt M., Train M.K., Robbins B.W., Halterman J.S., Caiola E., Fortuna R.J. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J. Gen. Intern. Med. 2013;28(12):1604–1610. doi: 10.1007/s11606-013-2516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S.-C., Tsai H.-I., Cheng C.-W., Lin T.-W., Chen C.-C., Lin C.-S. The association between frequent alcohol drinking and opioid consumption after abdominal surgery: a retrospective analysis. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0171275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman-Blumberg P.B., Katz N., Gajria K., Coutinho A.D., Yeung P.P., White R. Burden of alcohol abuse or dependence among long-term opioid users with chronic noncancer pain. J. Manag. Care Spec. Pharm. 2017;23(7):718–724. doi: 10.18553/jmcp.2017.23.7.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Le Saux M., Siegel R., Goyal M., Chen C., Ma Y., Meltzer A.C. Racial and ethnic disparities in the management of acute pain in US emergency departments: meta-analysis and systematic review. Am. J. Emerg. Med. 2019;37(9):1770–1777. doi: 10.1016/j.ajem.2019.06.014. [DOI] [PubMed] [Google Scholar]
- Lee B., Zhao W., Yang K.C., Ahn Y.Y., Perry B.L. Systematic evaluation of state policy interventions targeting the US opioid epidemic, 2007-2018. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.36687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Liu Y., Paulozzi L., Zhang K., Jones C. Opioid prescribing in emergency departments: the prevalence of potentially inappropriate prescribing and misuse. Med. Care. 2013;51(8):646–653. doi: 10.1097/MLR.0b013e318293c2c0. [DOI] [PubMed] [Google Scholar]
- Lu C.H., Jette G., Falls Z., Jacobs D.M., Gibson W., Bednarczyk E.M., Kuo T.Y., Lape-Newman B., Leonard K.E., Elkin P.L. A cohort of patients in New York state with an alcohol use disorder and subsequent treatment information - a merging of two administrative data sources. J. Biomed. Inf. 2023 doi: 10.1016/j.jbi.2023.104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugoboni F., Zamboni L., Cibin M., Tamburin S., Gruppo Inter S. d C.S. Intravenous misuse of methadone, buprenorphine and buprenorphine-naloxone in patients under opioid maintenance treatment: a cross-sectional multicentre study. Eur. Addict. Res. 2019;25(1):10–19. doi: 10.1159/000496112. [DOI] [PubMed] [Google Scholar]
- Ly D.P. Racial and ethnic disparities in the evaluation and management of pain in the outpatient setting, 2006-2015. Pain Med. 2019;20(2):223–232. doi: 10.1093/pm/pny074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack K.A., Zhang K., Paulozzi L., Jones C. Prescription practices involving opioid analgesics among Americans with Medicaid, 2010. J. Health Care Poor Underserved. 2015;26(1):182–198. doi: 10.1353/hpu.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinckrodt Pharmaceuticals, 2021. Methadose (Methadone Hydrochloride) [Package insert] Webster Groves, MO. 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/017116Orig1s043Lbl.pdf〉.
- McCormick C.D., Dadiomov D., Trotzky-Sirr R., Qato D.M. Prevalence and distribution of high-risk prescription opioid use in the United States, 2011-2016. Pharmacoepidemiol. Drug Saf. 2021;30(11):1532–1540. doi: 10.1002/pds.5349. [DOI] [PubMed] [Google Scholar]
- Mikosz C.A., Zhang K., Haegerich T., Xu L., Losby J.L., Greenspan A., Baldwin G., Dowell D. Indication-specific opioid prescribing for US patients with Medicaid or private insurance, 2017. JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden N.E., Chyn D., Wood A., Meara E. Racial inequality in prescription opioid receipt - role of individual health systems. N. Engl. J. Med. 2021;385(4):342–351. doi: 10.1056/NEJMsa2034159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NY Codes Rules and Regulations, 2013. VOLUME A-1a. Title, 10. SubChapter K - Controlled Substances. Part 80 Rules And Regulations On Controlled Substances. Prescribing and Dispensing Controlled Substances. Title: Section 80.73 - Pharmacists; dispensing schedule II substances and certain other controlled substances. 〈https://regs.health.ny.gov/content/section-8073-pharmacists-dispensing-schedule-ii-substances-and-certain-other-controlled〉.
- NYS DOH, 2013. I-STOP/PMP-Internet System for Tracking Over-Prescribing-Prescription Monitoring Program. 〈https://www.health.ny.gov/professionals/narcotic/prescription_monitoring/〉.
- NYS DOH, 2016. New Legislation Enacted to Limit Initial Opioid Prescribing to a Seven Day Supply for Acute Pain. New York State Medicaid Update - July 2016 Volume 32 - Number 7. 〈https://www.health.ny.gov/health_care/medicaid/program/update/2016/2016-07.htm#opioid〉.
- NYS DOH, 2018. NYS Opioid Annual Data Report. 〈https://www.health.ny.gov/statistics/opioid/data/pdf/nys_opioid_annual_report_2018.pdf〉.
- NYS DOH, 2021. NYS Opioid Annual Data Report. 〈https://www.health.ny.gov/statistics/opioid/data/pdf/nys_opioid_annual_report_2021.pdf〉.
- NYS DOH, 2023. Addressing the Opioid Epidemic in New York State. 〈https://www.health.ny.gov/community/opioid_epidemic/〉.
- Raman S.R., Bush C., Karmali R.N., Greenblatt L.H., Roberts A.W., Skinner A.C. Characteristics of new opioid use among medicare beneficiaries: identifying high-risk patterns. J. Manag. Care Spec. Pharm. 2019;25(9):966–972. doi: 10.18553/jmcp.2019.25.9.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J.L., 3rd, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. J. Pain. 2009;10(9):944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli R.J., Shah S.N., Ikeda L., Lynch B., Craig T.L., Cappelleri J.C., Jukes T., Ishisaka D. Patient characteristics and healthcare utilization of a chronic pain population within an integrated healthcare system. Am. J. Manag Care. 2017;23(2):e50–e56. 〈https://www.ncbi.nlm.nih.gov/pubmed/28245659〉 [PubMed] [Google Scholar]
- Stein B.D., Sheng F., Taylor E.A., Dick A.W., Sorbero M., Pacula R.L. The effect of state policies on rates of high-risk prescribing of an initial opioid analgesic. Drug Alcohol Depend. 2022;231 doi: 10.1016/j.drugalcdep.2021.109232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The NYS Senate, 2016. SECTION 3309-A Prescription Pain Medication Awareness Program. 〈https://www.nysenate.gov/legislation/laws/PBH/3309-A〉.
- The NYS Senate, 2018. New York State Public Health Law. Article 33. Controlled Substances. Title 4 Dispensing to Ultimate Users. Section 3331. 5. (b), (c). 〈https://www.nysenate.gov/legislation/laws/PBH/3331〉.
- Vinson D.C., Maclure M., Reidinger C., Smith G.S. A population-based case-crossover and case-control study of alcohol and the risk of injury. J. Stud. Alcohol. 2003;64(3):358–366. doi: 10.15288/jsa.2003.64.358. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K., Vowles K.E. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: a critical review. Alcohol. Clin. Exp. Res. 2018;42(3):478–488. doi: 10.1111/acer.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale E.L., Maisto S.A., Ditre J.W. Interrelations between pain and alcohol: an integrative review. Clin. Psychol. Rev. 2015;37:57–71. doi: 10.1016/j.cpr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Johnson P., Jeng P.J., Reid M.C., Witkin L.R., Schackman B.R., Ancker J.S., Bao Y. First opioid prescription and subsequent high-risk opioid use: a national study of privately insured and medicare advantage adults. J. Gen. Intern. Med. 2018;33(12):2156–2162. doi: 10.1007/s11606-018-4628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.