Abstract
The use of solar energy for photocatalysis holds great potential for sustainable pollution reduction. Titanium dioxide (TiO2) is a benchmark material, effective under ultraviolet light but limited in visible light utilization, restricting its application in solar-driven photocatalysis. Previous studies have shown that semiconductor heterojunctions and nanostructuring can broaden the TiO2’s photocatalytic spectral range. Semiconductor heterojunctions are interfaces formed between two different semiconductor materials that can be engineered. Especially, type II heterojunctions facilitate charge separation, and they can be obtained by combining TiO2 with, for example, iron(III) oxide (Fe2O3). Nanostructuring in the form of 3D inverse opals (IOs) demonstrated increased TiO2 light absorption efficiency of the material, by tailoring light-matter interactions through their photonic crystal structure and specifically their photonic stopband, which can give rise to a slow photon effect. Such effect is hypothesized to enhance the generation of free charges. This work focuses on the above-described effects simultaneously, through the synthesis of TiO2–Fe2O3 IOs via multilayer atomic layer deposition (ALD) and the characterization of their photocatalytic activities. Our results reveal that the complete functionalization of TiO2 IOs with Fe2O3 increases the photocatalytic activity through the slow photon effect and semiconductor heterojunction formation. We systematically explore the influence of Fe2O3 thickness on photocatalytic performance, and a maximum photocatalytic rate constant of 1.38 ± 0.09 h–1 is observed for a 252 nm template TiO2–Fe2O3 bilayer IO consisting of 16 nm TiO2 and 2 nm Fe2O3. Further tailoring the performance by overcoating with additional TiO2 layers enhances photoinduced crystallization and tunes photocatalytic properties. These findings highlight the potential of TiO2–Fe2O3 IOs for efficient water pollutant removal and the importance of precise nanostructuring and heterojunction engineering in advancing photocatalytic technologies.
Keywords: atomic layer deposition, inverse opal, photocatalysis, photoinduced crystallization, semiconductor heterostructure, multilayer thin films
1. Introduction
Solar-driven photocatalysis has emerged as a promising self-sustainable technology for removing water pollutants by harnessing solar energy to decompose organic contaminants.1−3 Among various photocatalysts, titanium dioxide (TiO2) is a benchmark material based on its excellent chemical stability, biocompatibility, and photocatalytic activity under ultraviolet (UV) light irradiation.4−7 However, its wide band gap prevents the utilization of visible light, which constitutes the majority of the sunlight spectrum, and thereby limits its practical applications. In recent years, efforts have been made to improve the light harvesting of TiO2 by various strategies, such as doping with other elements, formation of semiconductor heterostructures, or nanostructuring of the material.6−8 The latter approach is based on increasing the surface area and light trapping in such structures.
Inverse opals (IOs) are an example of a nanostructured material characterized by a periodically ordered porous structure. They offer the possibility to tune light-matter interactions within the structure based on their photonic crystal (PhC) structure.9,10 PhCs feature so-called photonic stopbands (PSBs), i.e., spectral regions in which light of the respective wavelength cannot propagate through the structure and thus, the light is reflected by the PhC three-dimensional (3D) structure.9,11 The PSB position is determined by the composition and geometry of the PhC, namely the refractive indices of the utilized materials and the structural parameters, such as template size and spacing.9,12,13 Hence, modifications of the IO’s structural parameters enable the tuning of the PSB position and allow, for instance, to position it across the whole UV to infrared range. Note, the group velocity of photons inside a PhC is strongly reduced at the PSB edges due to the slow photon effect.14,15 This effect leads to an increment of the interaction probability of photons with the PhC material.
Consequently, the generation of free charge carriers by absorption of photons in a semiconductor photocatalyst nanostructured within an IO 3D structure can be enhanced when the material’s electronic band gap is aligned with the PhC’s PSB edge and, thus, potentially boost its’ photocatalytic performance. Another approach to further improve the activity of a photocatalyst is to facilitate charge separation. Combining different semiconductors results in heterojunctions at the interfaces, which tune the migration of free charge carriers through the structure.8,16 Specifically, type II heterojunctions of two semiconductors direct electrons (e–) and holes (h+) to the different materials, thereby, separating them and reducing their recombination. For example, iron(III) oxide (Fe2O3) as a visible light-active semiconductor photocatalyst can be combined with TiO2 to improve charge separation and to widen the light absorption range, potentially leading to a further increase in photocatalytic performance. Fe2O3 is abundant on Earth, cheap, and nontoxic, rendering it a promising candidate for photocatalytic applications.17,18 However, inherent limitations such as inefficient charge carrier generation or fast recombination of photogenerated charge carriers need to be overcome.19−21 Previous reports about TiO2–Fe2O3 heterostructure thin films and Fe2O3 coated TiO2 nanostructures prove the concept of enhancing the photocatalytic properties by adding Fe2O3 as visible light absorbing material to TiO2 due to semiconductor heterojunction formation.19,22−32
Moreover, Liu et al. and Pylarinou et al. reported further improvement of the photocatalytic activity of TiO2 IOs when decorating them with Fe2O3 nanoparticles or nanoclusters, respectively, based on the slow photon effect when the PSB edge is aligned with the Fe2O3 band gap.33,34 Liu et al. synthesized the nanoparticles at the TiO2 IOs by a hydrothermal method, while Pylarinou et al. utilized a chemisorption-calcination-cycle technique to deposit Fe2O3 nanoclusters.33,34 However, coating TiO2 IOs with Fe2O3 films to encapsulate the complete TiO2 film has not been reported yet. Such structure could affect the photocatalytic performance because only either Fe2O3 or TiO2 is in contact with the environment, thereby further increasing the importance of charge carrier separation. Since Fe2O3 often suffers from a short hole diffusion length of only a few nanometers and, thus, limited charge carrier separation, precise control over the film thickness is essential.6,18 Besides forming semiconductor heterojunctions to facilitate charge separation, the fabrication of Fe2O3 thin films by atomic layer deposition (ALD) allows for very defined thicknesses based on the self-limiting reactions during the ALD process.35 Hence, the Fe2O3 film thickness can be optimized for maximum photocatalytic performance.
Here, we report on the synthesis of TiO2–Fe2O3 multilayer inverse opals by ALD and assessment of their photocatalytic properties. We demonstrate that the complete functionalization of TiO2 IOs with Fe2O3 by ALD enhances their photocatalytic properties by concomitantly forming semiconductor heterojunctions (material combination) and activating the slow photon effect (nanostructuring into IOs). In addition, the influence of the Fe2O3 thickness on the photocatalytic performance of TiO2–Fe2O3 bilayer IOs is studied to further improve the efficient utilization of photogenerated charge carriers. Moreover, TiO2–Fe2O3 IOs are overcoated with another TiO2 thin film by ALD to investigate the effect on the photocatalytic performance. These TiO2–Fe2O3–TiO2 multilayer IOs exhibit reduced photocatalytic activities compared to the bilayer IOs due to nonoptimal heterojunction configuration leading to the charge carriers’ trapping. However, the TiO2–Fe2O3–TiO2 multilayer IOs provoke photoinduced crystallization of the amorphous TiO2 layers to anatase, which enhances their photocatalytic properties.
2. Experimental Section
2.1. Materials
Mucasol solution was purchased from Brand GmbH (Germany), and 5 w/v% aqueous polystyrene (PS) particles’ dispersions with particle sizes of 150 ± 3 nm and 252 ± 6 nm were acquired from microParticles GmbH (Germany). Ultrapure “Milli-Q” water (>16 MΩ cm, H2O) was utilized as oxidant precursor for the ALD cycles and to prepare aqueous dispersions for colloidal self-assembly performed on borosilicate glass cover slides from Paul Marienfeld GmbH (Germany). Methylene blue (C16H18ClN3S, MB, CAS 122965–43–9), and hydrogen peroxide (H2O2, CAS 7722–84–1) were supplied by Sigma-Aldrich (Germany), while titanium tetraisopropoxide (TTIP, CAS 546–68–9) and ferrocene (C10H10Fe, Cp2Fe, CAS 102–54–5) were purchased from Alfa Aesar (Germany). Nitrogen (6.0) was received from SOL (Germany), and oxygen (5.0) was supplied by Westfalen (Germany), respectively.
2.2. Fabrication of TiO2–Fe2O3 Inverse Opals
Preparation of TiO2–Fe2O3 IOs starts with the colloidal self-assembly of PS particles, followed by coating of the self-assembled direct opal structures with TiO2 by ALD, removal of the PS template, functionalization with Fe2O3 by ALD, and optionally depositing another TiO2 layer by ALD (Figure 1). The colloidal self-assembly process is performed by vertical convective self-assembly of PS particles on top of glass substrates that are immersed into PTFE beakers containing 25 mL of PS particle dispersion (0.75 mg/mL) and placed inside a humidity chamber (HCP108, Memmert) at 55 °C and 70% relative humidity for 90 h. Previously to immersion, the glass substrates were ultrasonically cleaned in 0.1 vol % aqueous mucasol solution for 1 h, brushed with mucasol solution, and rinsed with ultrapure H2O. The clean substrates were dried with a nitrogen stream and plasma treated using a RF plasma barrel etcher for 20 min (Polaron PT7160, VG Microtech). The resulting colloidal self-assembled PS template structures were coated with TiO2 by ALD in a custom-built reactor (Hamburg University of Technology, Integrated Materials Systems Group). The ALD process was operated in stop-flow mode at 95 °C and with 2 Nl/h nitrogen flow, starting after 3 h of prevacuum. TTIP as titanium precursor was heated to 85 °C, and H2O as oxygen precursor was kept at room temperature. During an ALD cycle, the precursors were pulsed, exposed, and purged for 1, 30, and 90 s (TTIP) and 0.2, 30, and 90 s (H2O), respectively, resulting in a growth per cycle (GPC) of 0.4 Å. TiO2 cycles were repeated until the desired coating thicknesses of 16 and 20 nm were obtained. After ALD coating, the PS templates were removed by burn-out in a muffle furnace in air, where samples were heated to 500 °C at a rate of 0.3 °C/min, kept at 500 °C for 30 min, and naturally cooled down to room temperature. The obtained TiO2 IOs were further functionalized with Fe2O3 in another custom-built ALD reactor (Universität Hamburg, CHyN). The Fe2O3 ALD process utilized Cp2Fe at 100 °C and O3 at room temperature (generated from O2 by an OzoneLab OL80W ozone generator; Ozone Services, Canada) as precursors and was operated in stop-flow mode at 200 °C. Pulse, exposure, and purge times were 2, 60, and 90 s for Cp2Fe and 0.08, 30, and 90 s for O3, respectively. The O3 half-cycle was twice repeated within one ALD cycle, and the GPC of Fe2O3 deposition was determined to be 0.16 Å. Fe2O3 coating thicknesses targeted 10 ALD pulses, 2 and 4 nm. To prepare TiO2–Fe2O3–TiO2 multilayer IOs, another TiO2 ALD process with the same parameters described above was applied. Here, TiO2 thicknesses of only 2 nm were deposited.
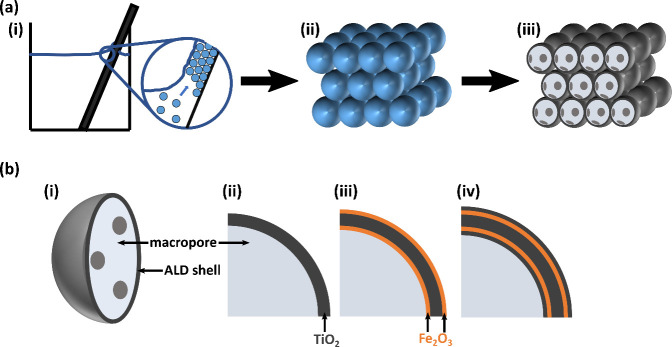
Figure 1.
Schematic drawing of the fabrication of TiO2–Fe2O3 IOs and their shell composition. (a) Different steps in the fabrication process show (i) self-assembly of PS spheres, (ii) an assembled PS sphere opal template, and (iii) a TiO2 inverse opal after ALD coating and burn-out of the polymer template. The latter scheme presents cuts through the front row of spheres to visualize the hollow inside and gaps connecting neighboring macropores. (b) The TiO2 IO structure presented in (i) and (ii) is further modified by ALD functionalization to produce (iii) TiO2–Fe2O3 bilayer IOs and (iv) TiO2–Fe2O3–TiO2 multilayer IOs.
2.3. Structural and Optical Characterization
Microstructural characterization was conducted with a Zeiss Supra 55 VP scanning electron microscope (SEM), both in top and cross-section view, obtained after sectioning the IOs’ substrates with a glass cutter. Energy-dispersive X-ray spectroscopy (EDX) measurements were acquired with an Oxford Instruments EDX SDD detector. Optical properties were analyzed with UV/vis spectroscopy in reflection mode utilizing a Flame Extended Range Spectrometer while irradiating the samples with a deuterium-halogen light source DH-2000 (OceanOptics, Germany). Reflection measurements were conducted at normal incidence for IOs filled with air and H2O. Their PSB positions were analyzed with OriginPro 2021 software by applying Gaussian fits to obtain the PSB central wavelength, while the PSB edges were determined as inflection points of the PSBs and obtained from reflection data smoothed using 200 data points. X-ray diffraction patterns were obtained with a Bruker D8 Discover diffractometer. Grazing incidence diffraction (GID) configuration was used with the X-ray source fixed at an angle of 0.5°, and the detector moved along the range from 10° to 60° with a step size of 0.01° and a step time of 5 s. Phase identification was performed using commercial software from Bruker (Diffrac.EVA 5.1) and the powder diffraction file database (PDF-2 Release 2020 RDB).
2.4. Photocatalytic Characterization
The photocatalytic performance of TiO2–Fe2O3 IOs was assessed by monitoring the photocatalytic degradation of methylene blue (MB) as a model pollutant of water. A sample was mounted in a custom-built photocatalysis cell consisting of polyether ether ketone (PEEK) and a soda-lime glass window. The cell was filled with 8 mL MB solution (2.5 mg/L), which included 200 mM H2O2 and was kept in darkness for 1 h to obtain the adsorption–desorption equilibrium of molecules at the sample surface. Afterward, the cell was illuminated with UV–visible light from a Euromex LE.5211 light source equipped with a Philips 64230 FO halogen bulb and the MB absorbance was measured every 5 min. This analysis was conducted by UV–vis spectroscopy after pipetting 1 mL of the MB solution into a cuvette and placing it in the UV–vis absorbance setup consisting of a halogen light source HL-2000 (OceanOptics, Germany), glass fibers, a cuvette holder, and a Flame Extended Range Spectrometer (OceanOptics, Germany). The analyzed volume was then transferred back into the photocatalysis cell. Irradiation of the photocatalysis cell was blocked during the absorbance measurements. For further studying the MB degradation pathway, 100 mM isopropyl alcohol (IPA) as a hole scavenger was added to 8 mL MB solution (2.5 mg/L). The further processing was the same as for the H2O2-containing solution. Based on Lambert–Beer’s law, the measured MB absorbance was converted to the concentration and the photocatalytic MB degradation was examined by assuming Langmuir–Hinshelwood kinetics:36,37
In this equation, c describes the concentration of the MB solution at the time t, c0 is the concentration at the measurement start (t = 0 h), and k denotes the apparent photocatalytic rate constant, which measures the photocatalytic activity of a sample. Unless otherwise stated, photocatalytic measurements were repeated three times for each sample to calculate the photocatalytic activity’s mean value and standard deviation. Note, the samples stayed in the same photocatalysis cell for the consecutive measurements to ensure the same positioning for all measurements.
A 400 nm long-pass filter and a 425 nm short-pass filter were installed between the light source and a sample, respectively, to assess the influence of the irradiation spectrum on the photocatalytic performance. For these measurements and the study with IPA containing solutions the samples and photocatalysis cells were new assembled. Their activity was normalized to the measured performance under the standard conditions (2.5 mg/L MB, 200 mM H2O2, full illumination spectrum) in this assembly.
3. Results and Discussion
3.1. Structural and Optical Characterization
The fabricated IOs show, in general, good structural integrity, as exemplarily depicted in Figure 2. SEM images of all samples are presented in Figure S1 in the Supporting Information. Top-view and cross-section SEM images reveal a 3D PhC structure with ordered domains and hollow shells, characteristic of the IOs (Figures 2a and 2b). However, vacancies and stacking faults are also visible. These are typical defects of IO structures originating from the self-assembly of the PS particle templates.38,39 EDX analysis of the TiO2–Fe2O3 IOs demonstrates coherent signals of iron and titanium throughout the structure (Figure 2c). Since the hereby practiced ALD processes cannot produce elemental iron and titanium, these signals originate from their oxides.35,40 For the TiO2–Fe2O3 bilayer IOs, bigger flakes and needles at the top surface of the PhC are observed (Figure 2d). EDX analysis indicates that they consist of iron oxide, probably arising from the Fe2O3 ALD process as detachments from the ALD reactor walls. The template with a smaller PS particle diameter of 150 nm presents a challenge for the ALD precursor penetration and homogeneous diffusion within the 3D structure. Hence, structural defects of the IO structure are observed at some spots (Figure S1).
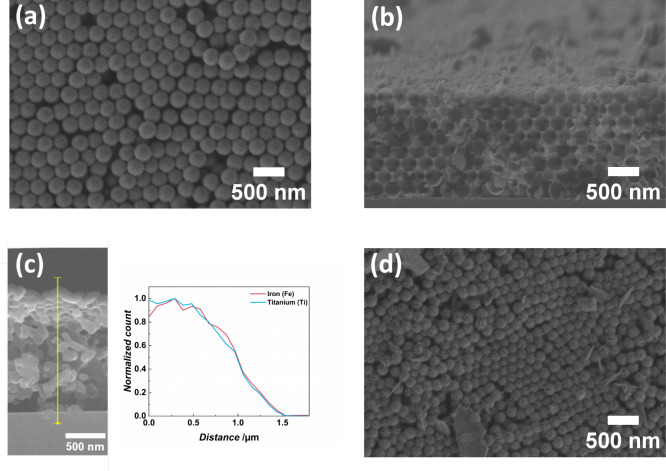
Figure 2.
Characterization of the structural integrity and composition by SEM and EDX. (a) and (b) demonstrate the typical IO structure for (a) 16 nm TiO2 IO and (b) 20 nm TiO2–2 nm Fe2O3, both fabricated with 252 nm PS template size. (c) an EDX scan along a 20 nm TiO2–2 nm Fe2O3 cross-section reveals a homogeneous distribution of iron and titanium. (d) Fe2O3-coated IOs present needle-like structures and larger particles at their top surface.
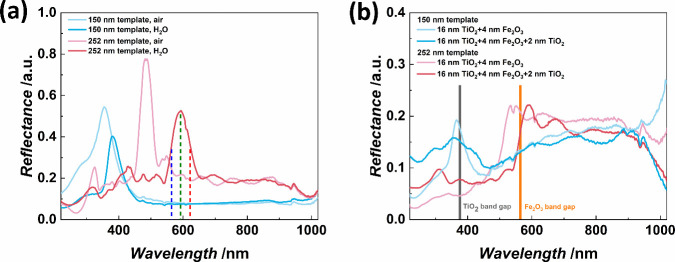
The fabricated IOs feature PSBs in the UV to the visible range of the electromagnetic spectrum corresponding to their structural characteristics, i.e., PS template particle size defining the template size, composition of the shell, and thicknesses of the ALD coated materials (shell thickness of the IO). Figure 3a displays the PSB positions of pure TiO2 IOs at normal incidence and their dependence on both the size of the PS spheres utilized as templates and the medium inside the pores. Increasing the PS particle diameter drives a redshift of the PSB position based on the increased spacing of the structure. The characterization of the optical properties not only in air, but also in an aqueous environment is crucial as the photocatalytic reactions will also take place in aqueous media and the PSB position needs to be tailored for this. Comparing the reflection spectra of TiO2 IOs in air to the TiO2 IOs filled with H2O reveals a PSB redshift as the refractive index of H2O is higher (1.33)41 than the refractive index of air (1.00).42,43 For the TiO2–Fe2O3 bilayer IOs, the layer composition determines their PSB position, as presented in Figure 3b. Nevertheless, only slight shifts of the PSB positions are observed for TiO2–Fe2O3–TiO2 multilayer IOs compared to TiO2–Fe2O3 bilayer IOs. The PSBs of all samples overlap with the respective semiconductor band gaps, i.e., TiO2 for 150 nm template size and Fe2O3 for 252 nm template size, as indicated in Figure 3b and Figure S2.
Figure 3.
Optical properties of the prepared IOs. (a) The template size of 16 nm TiO2 IOs determines the PSB position, which is characterized by the PSB central wavelength (green dashed line), the PSB blue edge (blue dashed line), and the PSB red edge (red dashed line) as exemplarily shown for one measurement. Infiltrating the IOs with H2O redshifts the PSB due to the higher refractive index of the pore-filling medium. (b) TiO2–Fe2O3 IOs and TiO2–Fe2O3–TiO2 multilayer IOs with 150 nm template size feature PSBs around the electronic band gap of TiO2. Templates of 252 nm lead to TiO2–Fe2O3 IOs and TiO2–Fe2O3–TiO2 multilayer IOs with PSBs overlapping with the Fe2O3 band gap. The measurements were conducted in aqueous environment.
3.2. Photocatalytic Performance
3.2.1. TiO2 and TiO2–Fe2O3 Inverse Opals
Pure 16 nm TiO2 IOs with 150 nm template size exhibit a higher photocatalytic activity of 0.98 ± 0.01 h–1 than their counterparts with 252 nm template size (0.86 ± 0.02 h–1) due to the expected activity enhancement by the slow photon effect (Figure 4a). The individual photocatalytic activities during three consecutive measurements are shown in Figure S3 and the MB concentration decline of the individual measurements is depicted in Figure S4. The PSB blue edge of the 150 nm template size TiO2 IO overlaps with the band edge of TiO2 at around 376 nm44 (as illustrated in Figure 3a) and thus, the slow photon effect results in an improved photocatalytic performance. Although larger template sizes should lead to facilitated mass transfer of dye molecules into and reaction products out of the structure, this effect is overweighed by the mismatch of the PSB position concerning the TiO2 band gap. Hence, as expected, the slow photon effect does not enhance the photocatalytic performance in 252 nm template TiO2 IOs.
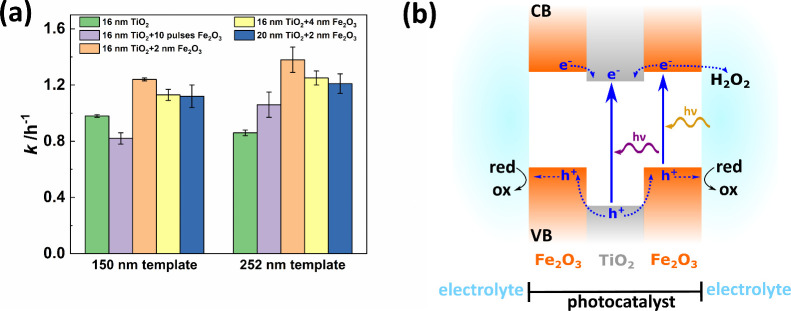
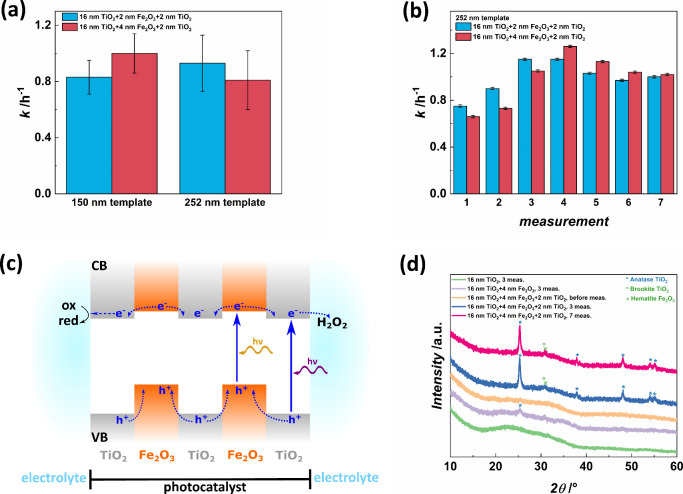
Figure 4.
(a) The photocatalytic activity of TiO2–Fe2O3 bilayer IOs depends on the TiO2 and Fe2O3 coating thicknesses and the template size due to the alignment of the PSB with the semiconductor band gap to utilize the slow photon effect for performance enhancement. Each sample was measured three times. (b) Schematic drawing of the band structure and charge carrier movement in TiO2–Fe2O3 bilayer IOs. Based on the type II heterojunction, photogenerated holes inside the valence band (VB) migrate toward the Fe2O3 layers and can induce an oxidation reaction at the catalyst’s surface. Electrons in the conduction band (CB) either get inside the TiO2 layer or are scavenged by H2O2, which is added to the reaction solution.
Additional layers of Fe2O3 significantly increase the photocatalytic activity of the IOs compared to the reference IO with only TiO2 (Figure 4a). This is associated with increased light absorption and facilitation of charge carrier separation. The location of the Fe2O3 band gap at ∼2.2 eV expands the absorption spectrum of the IOs to wavelengths smaller than ∼564 nm.20,21 Furthermore, coating Fe2O3 onto the TiO2 IOs results in the formation of heterojunctions at the materials interfaces, which should allow for efficient separation of the generated electron/hole pairs. The band alignment of Fe2O3 and TiO2 is ambiguous in literature since both type I and type II heterojunctions have been reported for such heterostructures.22,23,25,27−29,34,45 Accordingly, the heterojunction type depends on the fabrication method and further sample specifications, such as geometry. Based on the publications by Cao et al. and Yang et al. about ALD-based functionalization of TiO2 powders coated with Fe2O3 thin films and synthesis of TiO2–Fe2O3 thin film heterostructures, respectively, we assume that our samples feature type II heterojunctions (Figure 4b).22,31 Hence, photogenerated electrons move toward the conduction band (CB) of TiO2. At the same time, holes migrate to the valence band (VB) of Fe2O3 and can induce oxidation reactions in molecules adsorbed at the material surface. This charge carrier separation reduces the recombination of the free charge carriers and results in an improved photocatalytic performance. Free electrons can, in principle, also contribute to the degradation of organic pollutants by inducing reactions in adsorbed molecules. H2O2 was added to the reaction solution to aid the generation of radicals necessary for the photocatalytic decomposition. However, H2O2 also acts as an electron scavenger; thus, H2O2 molecules at the photocatalysts’ surface could trap free electrons.19 These electrons will then contribute to the photocatalytic degradation of the organic dye instead of moving toward the CB of the inner TiO2 layer. Although these two competing processes (electron migration to the TiO2 layer and electron trapping by H2O2 at the surface) cannot be clearly distinguished by the dye degradation measurements, both of them lead to a better separation of photogenerated charge carriers in the photocatalyst and hence, to an enhancement of the photocatalytic activity. Assessment of the dye degradation of the 252 nm template sample with 16 nm TiO2–2 nm Fe2O3 coating with a MB solution containing 100 mM IPA revealed a decrease of the activity to 26% compared to the H2O2-containing solution (Figure S5). IPA is used as hole scavenger and the decreasing activity upon its’ presence demonstrates that photogenerated holes are crucial for inducing the MB destruction in our samples.
A general increase of the photocatalytic activity for the 252 nm template compared to the smaller one is expected for TiO2–Fe2O3 bilayer IOs due to the slow photon effect, as in this case, the IO structural PSB was designed to match the band gap of the Fe2O3. The PSB edge of the larger template size samples overlaps with the band gap of Fe2O3, which allows for enhancement of the photocatalytic performance by the slow photon effect in Fe2O3 as observed for all TiO2–Fe2O3 bilayer IOs (Figure 4a).
Furthermore, the performance also depends on the Fe2O3 coating thickness. A rise of the Fe2O3 coating thickness from 10 ALD pulses to 2 nm improves the sample’ photocatalytic activity based on the material’s additional light absorption. An optimum activity of 1.38 ± 0.09 h–1 is demonstrated for TiO2–Fe2O3 bilayer IOs composed of 16 nm TiO2 and 2 nm Fe2O3 in comparison to 0.86 ± 0.02 h–1 of the single TiO2 IO. The photocatalytic performance is reduced when the illumination spectrum is limited to specific spectral regions (Figure S6). Specifically, the utilization of a 400 nm long-pass filter eliminates UV radiation. In this case, the photocatalytic performance of the 252 nm template sample consisting of 16 nm TiO2 and 4 nm Fe2O3 is reduced to 75% compared to the standard conditions. Further modification is observed when a 425 nm short-pass filter is applied. Here, the spectral range between 425 and 530 nm is suppressed and wavelengths higher than 530 nm are attenuated, while wavelengths shorter than 425 nm are transmitted without intensity alteration. With this short-pass filter, the samples’ activity decreases to 66%, demonstrating the importance of visible light radiation for inducing photocatalytic reactions by the presented heterostructure IOs. Please note that the sample was only tested once in each measurement configuration.
Nevertheless, a further increase to 4 nm Fe2O3 thickness reduces the photocatalytic activity. Although the thicker coating could absorb more light, it simultaneously reduces the gap size between neighboring shells, which might limit the diffusion of dye molecules and reaction products within the IO structure.46 Thus, the photocatalytic performance declines as measured for both template sizes. The higher diffusion path length for charge carriers within the 4 nm Fe2O3 coating could also result in higher charge carrier recombination rates, leading to decreasing activities with increasing thickness. Such performance decline with increasing Fe2O3 content was also observed by Pylarinou et al.34 for TiO2–Fe2O3 thin film heterostructure samples. Similar photocatalytic activities to the TiO2–Fe2O3 bilayer IOs of 16 nm TiO2 and 4 nm Fe2O3 are obtained for samples consisting of 20 nm TiO2 and 2 nm Fe2O3. ALD coating onto an assembled opal template structure presents a maximum coating thickness of ∼7.7% of the template sphere diameter because the tetrahedral gaps, i.e., the smallest interconnecting pores between neighboring macropores, close at this thickness.47 Hence, the template sizes used herein correspond to a theoretically estimated maximum coating of 11.6 and 19.4 nm for 150 and 252 nm templates, respectively. For the TiO2 deposition onto the opal templates, further material deposition can only occur by material transport through the octahedral gaps or at the outer surfaces of the IO, which are in contact with the environment. Since both template size IOs studied herein are coated with the same TiO2 thickness, the 150 nm template IO already reached the theoretical estimated maximum coating after the first ALD coating, while IOs consisting of 252 nm templates still have open tetrahedral gaps after the TiO2 deposition. During the Fe2O3 ALD process, the tetrahedral gaps of the 252 nm template size also get very small or even close.
Nevertheless, since the Fe2O3 coating is conducted by utilizing TiO2 IO structures as template instead of the PS opal, this template provides open channels between neighboring shells at the shell contact points.39 Thus, material diffusion through these contact points is still possible after the closure of the tetrahedral gaps by the Fe2O3 coating. Additional Fe2O3 coating may influence the charge carrier separation in the structure because the diffusion of molecules within the structure is reduced due to the tetrahedral gap closures. The slightly decreasing activities for the thicker coating, i.e., 20 nm TiO2 and 2 nm Fe2O3, support the assumption that diffusion limitation affects the photocatalytic properties because 2 nm Fe2O3 was the best-performing thickness for TiO2 IOs of 16 nm.
Functionalizing TiO2 IOs with Fe2O3 by ALD outperforms the photocatalytic performance of previously reported structures, namely TiO2 IOs modified with Fe2O3 by hydrothermal methods or chemisorption-calcination and Fe2O3-functionalized TiO2 nanostructures.22,23,26,28,33,34 This comparison considers samples of Fe2O3-coated TiO2 particles or inverse opals tested by photocatalytic dye degradation. Nevertheless, the exact value of the photocatalytic activity k depends strongly on the reaction conditions, such as illumination power, illumination spectrum, temperature, catalyst loading, type of dye, and additives in the reaction solution, which are summarized in Table S1. Hence, it is setup-specific and we, therefore, compare here the qualitative evolution of the photocatalytic activities upon Fe2O3 functionalization within publications on Fe2O3-modified TiO2 IOs. Our results of enhanced photocatalytic performances of TiO2 IOs upon functionalization with Fe2O3 agree with previous reports by Liu et al. and Pylarinou et al.33,34 In detail, Liu et al. observed an increase in the photocurrent density by up to 50% when TiO2 IOs were modified with Fe2O3 nanoparticles by the hydrothermal method. Similarly, Pylarinou et al. reported increased photocatalytic activities and photocurrent densities when they modified TiO2 IOs with FeOx nanoclusters by chemisorption-calcination cycles. Moreover, they showed that the enhancement depends on the utilized iron oxide content. They attributed the maximum improvement for low iron oxide contents to the efficient charge carrier separation in combination with the utilization of the slow photon effect. High iron oxide loadings resulted in a performance decline due to increased surface recombination of photogenerated charge carriers. However, the processes involved in the photocatalytic and photoelectrochemical reaction with the structures of the two aforementioned publications differ from those in this work. Since both references functionalized TiO2 IOs with iron oxide particles or clusters, TiO2 surfaces are still in contact with the reaction solution and charges accumulated at the TiO2 film can induce reactions in the aqueous surrounding.
In contrast, our TiO2–Fe2O3 bilayer IOs prepared by ALD consist of continuously capped TiO2 by the Fe2O3 layers. Thus, charge transfer from the photocatalyst structure toward the solution is only possible via the Fe2O3 surfaces. To the best of our knowledge, such configuration of Fe2O3-modified TiO2 IOs has yet to be reported. Significant enhancement of the photocatalytic performance of TiO2 powder coated with Fe2O3 by ALD was reported by Cao et al.22 Similar to our results, they observed an optimum coating thickness of ∼2.6 nm Fe2O3 for the photocatalytic degradation of methyl orange as an organic dye. The structures formed type II heterojunctions, effectively improving the separation of photogenerated charge carriers by reducing their recombination. Further, the IOs fabricated herein present nanostructured materials that could prevent potentially hazardous leaching of photocatalytic nanoparticles into the environment.48 The interconnected porous structure of IOs provides a stable framework and thus, can be considered as nanostructured solids with “bulk-like” properties regarding the high stability of the structure and adhesion to the substrate during operation.49
3.2.2. TiO2–Fe2O3–TiO2 Multilayer Inverse Opals
Depositing an additional TiO2 thin film onto the previously presented TiO2–Fe2O3 IOs leads to TiO2–Fe2O3–TiO2 multilayer IOs exhibiting unstable photocatalytic activities over consecutive measurements with a reduced average performance (Figure 5a). Specifically, the activities increase within the first four measurements, then slightly decrease for two measurements, and are stable for the following trial (Figure 5b). This behavior is observed for all studied TiO2–Fe2O3–TiO2 multilayer IOs independent of the Fe2O3 coating thickness and the template size. Note, the average performance is calculated from the first three measurements to compare the multilayer IOs to the TiO2–Fe2O3 bilayer IOs. The multilayer IOs with 150 nm template size feature average activities of 0.83 ± 0.12 h–1 and 1.00 ± 0.14 h–1 for samples composed of 16 nm TiO2–2 nm Fe2O3–2 nm TiO2 and 16 nm TiO2–4 nm Fe2O3–2 nm TiO2, respectively. In contrast, bilayer IOs of the same template size exhibit higher activities of 1.24 ± 0.01 h–1 (16 nm TiO2–2 nm Fe2O3) and 1.13 ± 0.04 h–1 (16 nm TiO2–4 nm Fe2O3). The MB concentration decrease within the seven photocatalysis measurements of TiO2–Fe2O3–TiO2 multilayer IOs is shown in Figure S8. The individual photocatalytic performances for the 150 nm template multilayer IOs are depicted in Figure S7. Template sizes of 252 nm also result in decreased activities of 0.93 ± 0.20 h–1 and 0.81 ± 0.21 h–1 for samples consisting of 16 nm TiO2–2 nm Fe2O3–2 nm TiO2 and 16 nm TiO2–4 nm Fe2O3–2 nm TiO2, respectively, compared to the bilayer samples of this template size which show photocatalytic activities of 1.38 ± 0.09 h–1 for 16 nm TiO2–2 nm Fe2O3 and 1.25 ± 0.05 h–1 for 16 nm TiO2–4 nm Fe2O3.
Figure 5.
(a) The mean photocatalytic activity of TiO2–Fe2O3–TiO2 multilayer IOs after three measurements depends on the composition and template size but shows a significant standard deviation. (b) The individual activities during seven consecutive measurements of 16 nm TiO2–2 nm Fe2O3–2 nm TiO2 and 16 nm TiO2–4 nm Fe2O3–2 nm TiO2 multilayer IOs for the 252 nm template increase during the first four measurements, slightly decrease in the following two measurements and are stable afterward. (c) The band structure of TiO2–Fe2O3–TiO2 multilayer IOs depicts trapping of photogenerated holes inside the Fe2O3 layers due to adding another TiO2 layer. Electrons in the CB move toward the TiO2 layers, and those located in the outer layers can induce reductive reactions in the surrounding electrolyte or get scavenged by H2O2 molecules. (d) XRD patterns show anatase TiO2 peaks for Fe2O3 functionalized TiO2 IOs after photocatalysis measurements. Multilayer structures exhibit significantly higher peak intensities, indicating that this composition provokes photoinduced crystallization of the TiO2 layers.
These results demonstrate that the photocatalytic properties of a multilayer arrangement of TiO2 and Fe2O3 not depend on the thicknesses of the individual layers but rather on the template size. The TiO2–Fe2O3–TiO2 multilayer IOs exhibit lower activities than those observed for TiO2–Fe2O3 bilayer IOs. The significant decrease in the average photocatalytic activity of multilayer IOs after three measurements compared to the TiO2–Fe2O3 bilayer IOs can be attributed to two effects. First, photo-Fenton reactions at the Fe2O3 surface can no longer contribute to MB degradation because TiO2 overcoats Fe2O3.1 Second, the nonoptimal heterojunction configuration in the multilayer IOs could lead to a performance decline. Although electrons migrate toward the TiO2 layers and the 2 nm outer TiO2 film, the holes could be trapped inside the Fe2O3 layers (see schematic drawing in Figure 5c). Organic dye degradation is often mainly driven by oxidative processes involving holes, but both charge carrier types can contribute to the decomposition. The H2O2 added to the dye solution herein also acts as an electron scavenger and, therefore, could use the electrons generated in the outer TiO2 layers to induce photocatalytic reactions. However, electrons that migrate from the Fe2O3 layers toward the inner TiO2 layer and the holes trapped in the Fe2O3 layers are not available for photocatalytic reactions. Correspondingly, the photocatalytic activity is strongly reduced compared to TiO2–Fe2O3 bilayer IOs. IPA was utilized as hole scavenger for the 16 nm TiO2–4 nm Fe2O3–2 nm TiO2 trilayer IO to elucidate the influence of holes on the photocatalytic performance (Figure S5). With 100 mM IPA, the activity decreases to 27%, revealing that holes that migrate from the outer TiO2 layer to the TiO2 surface significantly contribute to the dye degradation in the surrounding MB solution.
3.2.3. In Situ Photoinduced Crystallization of TiO2
The configuration of TiO2–Fe2O3–TiO2 multilayer IOs provokes photoinduced crystallization of TiO2 as characterized by XRD measurements (Figure 5d). The above-mentioned trapped holes contribute to the photoinduced crystallization of the inner TiO2 layer, which is enhanced by multilayer IOs (Figure 5d). While the TiO2 IO is still amorphous after the photocatalytic performance measurements, the TiO2–Fe2O3 bilayer IO with 4 nm Fe2O3 coating features a slight peak at 25° corresponding to the anatase main peak (PDF 00–064–0863). Utilizing the Scherrer equation with a shape factor of 0.9 gives an estimated average crystallite size of 9.2 nm.50 The TiO2–Fe2O3–TiO2 multilayer IO reveals intense peaks of the anatase phase and one peak, which indicates either brookite or hematite. The anatase (101) peak at ∼25° indicates crystallite sizes of 19.2 and 20.4 nm for the sample composed of 16 nm TiO2–4 nm Fe2O3–2 nm TiO2 after three and seven measurements, respectively. All peaks are present after three photocatalysis measurements and remain constant after seven measurements.
Moreover, the crystallite size is in the same range as the thickness of the inner TiO2 layer. Control experiments with Fe2O3 coated samples before the photocatalysis measurements (Figure 5d) and another control after 17 h in the reaction solution in darkness (Figure S9), i.e., the accumulated duration of 7 measurements, showed only shallow intense peaks in the XRD patterns corresponding to a crystallite size of 3.3 nm. As indicated by the crystallite sizes, crystallite growth occurs mainly within the first three measurements for which increasing activities are observed. Thus, we assume that Fe2O3 incorporation triggers the crystallization under illumination. The photoinduced crystallization is amplified for TiO2–Fe2O3–TiO2 multilayer IOs as the additional material interfaces and charge carrier trapping can facilitate the crystallization. It was previously reported that Fe ions inside the TiO2 lattice can create oxygen vacancies.51,52 These defects can serve as nucleation sites for the crystallization of the TiO2 film due to charge imbalances and structural distortion. In the TiO2–Fe2O3–TiO2 multilayer IOs, Fe ions present local defects in the amorphous TiO2 lattice at the interfaces of the TiO2 and Fe2O3 layers. In addition, the energy absorbed by the material during photoexcitation can also contribute to the crystallization process by providing the energy required for the crystallization.53 The photogenerated charge carriers can transfer energy to neighboring atoms, which can promote structural rearrangement such as crystallization.
TiO2 crystallization observed in this work is probably promoted by the band alignment of the individual materials. For TiO2–Fe2O3 bilayer IOs, the material interfaces, oxygen vacancies, and charge carriers already elicit crystallization of small parts of the TiO2 as indicated by the minor peak in the XRD pattern. Adding another TiO2 layer to the structure, i.e., the outer TiO2 layers in case of the TiO2–Fe2O3–TiO2 trilayer IOs, increases the number of material interfaces and confines photogenerated charge carriers to certain areas of the photocatalyst structure. Since holes migrate to the VB of Fe2O3 (Figure 5c), they are trapped inside the trilayer structure. Hence, these holes increase charge imbalances at the Fe2O3/TiO2 interfaces. In this way, they create additional nucleation sites for crystallization, and the required activation energy can be obtained from further photogenerated charge carriers in the material. Note, in contrast to the trilayer IOs, holes are not trapped in bilayer IO structures because they can move toward the Fe2O3 surface surrounded by the electrolyte and release their energy by inducing oxidation reactions in molecules adsorbed at the Fe2O3 surface. Both effects, the increased number of interfaces and the charge carrier trapping inside the multilayer structure are hypothesized to contribute to the strong photoinduced crystallization of the inner TiO2 layer in the TiO2–Fe2O3–TiO2 multilayer IOs. This photoinduced crystallization into the anatase phase improves the photocatalytic performance of the structures due to the higher inherent photocatalytic activity of anatase compared to amorphous TiO2.4 In addition, photoinduced crystallization could help to avoid shrinkage of porous structures and strong atom diffusion at interfaces, which are typical structural alterations induced by thermal crystallization.54 The increasing photocatalytic activities of the TiO2–Fe2O3–TiO2 multilayer IOs within the first four measurements correspond to the crystallization of the TiO2. The presence of oxygen vacancies, as introduced by the Fe2O3 layers, promotes charge carrier transport in TiO2 and improves the photocatalytic properties.51,52,55−57 Assuming that oxygen vacancies trigger the crystallization and are responsible for the high photocatalytic activity, a decline in their concentration would result in a reduced photocatalytic performance. Assuming that the oxygen vacancy content reaches a maximum during the crystallization process and decreases and vanishes in the final stage of the TiO2 crystallization, fits with the fact that the photocatalytic activity first increases and then slightly decreases until a stable performance is observed for the anatase structure. In situ XRD at a synchrotron during the photocatalysis characterization of the trilayer IOs could shed light on the details of the crystallization mechanism.
The emergence of photoinduced crystallization of TiO2 in TiO2–Fe2O3 multilayered structures was not previously reported. It could enable the fabrication of crystalline materials on templates unsuited for high-temperature treatments. This could, for example, be realized by incorporating ultrathin Fe2O3 layers into thicker TiO2 films to generate oxygen vacancies inside the complete TiO2 layer effectively. ALD is a commonly used technique to fabricate delta-doped structures based on self-limiting reactions. Moreover, ALD-based processing allows further combining Fe2O3-incorporated TiO2 with other semiconductor photocatalyst layers to separate photogenerated charge carriers as presented herein for the TiO2–Fe2O3 bilayer IOs. The observed photoinduced crystallization also emphasizes the influence of semiconductor heterojunctions on photocatalytic performance, structural stability, and possible tailoring. Hence, the formation of semiconductor heterostructures could further expand the application of photoinduced crystallization in various fields.58
4. Conclusion
Modifying TiO2 inverse opals with conformal Fe2O3 layers prepared by ALD significantly enhanced the photocatalytic properties due to additional visible light absorption and efficient separation of photogenerated charges with a photocatalytic degradation rate improvement of 27% compared to pure TiO2 IOs. Aligning the IOs’ PSB edge with the electronic band gap of Fe2O3 enabled further improvement of the photocatalytic performance by 60% due to the slow photon effect. Optimization of the Fe2O3 thickness resulted in a maximum activity of 1.38 ± 0.09 h–1 for TiO2–Fe2O3 bilayer IOs consisting of 16 nm TiO2 and 2 nm Fe2O3 coating. TiO2–Fe2O3–TiO2 multilayer IOs demonstrated reduced photocatalytic activities due to the nonoptimal band structure alignment of the individual layers. Nevertheless, the band structure provoked photoinduced crystallization of TiO2, resulting in an increase of the photocatalytic activity within the first four photocatalysis measurements due to anatase formation, which is known to enhance the performance compared to amorphous TiO2. In the future, in situ XRD at a synchrotron during the photocatalysis characterization could be conducted to elucidate the mechanism of photoinduced crystallization in detail. Moreover, fine-tuning the structural and optical properties of PhCs, e.g., by optimizing the IO thickness, in combination with precise adjustment of semiconductor heterostructures could further improve photocatalysts’ performance.
Acknowledgments
The authors gratefully acknowledge the financial support from the German Academic Exchange Service (DAAD) and the Coordination for the Improvement of Higher Education Personnel (CAPES foundation) in the framework of the German-Brazilian bilateral research projects “Advanced nanostructured materials for sustainable pollutant abatement and energy production” project ID 57598489 and “Development of catalytic materials systems for non-intermittent green hydrogen production” project ID 57680884. This work was also financially supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–Projektnummer 192346071–SFB 986 (projects C4 and C8). The authors further acknowledge the Electron Microscopy Unit (BeEM) of TUHH for providing access to the microscopy analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.4c10831.
SEM images of all samples, optical properties of the prepared IOs, individual photocatalytic activities of TiO2 IOs and TiO2–Fe2O3 bilayer IOs during three consecutive measurements, dye concentration decrease during three consecutive photocatalysis measurements for TiO2 IOs and TiO2–Fe2O3 bilayer IOs, photocatalytic activities over seven measurements of multilayer IOs prepared with 150 nm template size, dye concentration decrease during seven consecutive photocatalysis measurements for TiO2–Fe2O3–TiO2 multilayer IOs, and XRD pattern of a multilayer IO exposed to the reaction solution without illumination for 17 h (PDF)
Author Contributions
Carina Hedrich: methodology (equal); data curation; formal analysis and visualization (equal); validation; writing original draft (lead); writing-review and editing (lead). Nithin T. James: data curation (lead); formal analysis and visualization (equal); validation; writing-review and editing. Laura G. Maragno: data curation; formal analysis and visualization; validation; writing-review and editing. Valéria de Lima: formal analysis and visualization; validation; writing-review and editing. Sérgio Y. G. González: validation; project administration (equal); supervision; funding acquisition and resources; writing-review and editing. Robert H. Blick: supervision; funding acquisition and resources. Robert Zierold: conceptualization (equal); methodology (equal); validation; project administration; supervision (equal); funding acquisition and resources; writing-review and editing. Kaline P. Furlan: conceptualization (equal); methodology (lead); validation; project administration (equal); supervision (lead); funding acquisition and resources; writing-review and editing. All authors have approved the final version of the manuscript.
Author Contributions
∥ C.H. and N.T.J. contributed equally.
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Applied Materials & Interfacesspecial issue “Porous Semiconductor Science and Technology Conference - PSST 2024”.
Supplementary Material
References
- Jack R. S.; Ayoko G. A.; Adebajo M. O.; Frost R. L. A Review of Iron Species for Visible-Light Photocatalytic Water Purification. Environ. Sci. Pollut. Res. 2015, 22 (10), 7439–7449. 10.1007/s11356-015-4346-5. [DOI] [PubMed] [Google Scholar]
- Kumar N.; Kumbhat S.. Essentials in Nanoscience and Nanotechnology; John Wiley & Sons Inc.: Hoboken, NJ, 2016. [Google Scholar]
- Teoh W. Y.; Scott J. A.; Amal R. Progress in Heterogeneous Photocatalysis: From Classical Radical Chemistry to Engineering Nanomaterials and Solar Reactors. J. Phys. Chem. Lett. 2012, 3 (5), 629–639. 10.1021/jz3000646. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhou P.; Liu J.; Yu J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16 (38), 20382–20386. 10.1039/C4CP02201G. [DOI] [PubMed] [Google Scholar]
- Carp O.; Huisman C. L.; Reller A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32 (1–2), 33–177. 10.1016/j.progsolidstchem.2004.08.001. [DOI] [Google Scholar]
- Li J.; Wu N. Semiconductor-Based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: A Review. Catal. Sci. Technol. 2015, 5 (3), 1360–1384. 10.1039/C4CY00974F. [DOI] [Google Scholar]
- Zhang J.; Tian B.; Wang L.; Xing M.. Photocatalysis Fundamentals, Materials and Applications; Springer Open Ltd: Singapore, 2018. [Google Scholar]
- Li H.; Zhou Y.; Tu W.; Ye J.; Zou Z. State-of-the-Art Progress in Diverse Heterostructured Photocatalysts toward Promoting Photocatalytic Performance. Adv. Funct. Mater. 2015, 25 (7), 998–1013. 10.1002/adfm.201401636. [DOI] [Google Scholar]
- Aguirre C. I.; Reguera E.; Stein A. Tunable Colors in Opals and Inverse Opal Photonic Crystals. Adv. Funct. Mater. 2010, 20 (16), 2565–2578. 10.1002/adfm.201000143. [DOI] [Google Scholar]
- Galisteo-Lõpez J. F.; Ibisate M.; Sapienza R.; Froufe-Pérez L. S.; Blanco Ú.; Lõpez C. Self-Assembled Photonic Structures. Adv. Mater. 2011, 23 (1), 30–69. 10.1002/adma.201000356. [DOI] [PubMed] [Google Scholar]
- Joannopoulos J. D.; Villeneuve P. R.; Fan S. Photonic Crystals: Putting a New Twist on Light. Nature 1997, 386 (6621), 143–149. 10.1038/386143a0. [DOI] [Google Scholar]
- López C. Materials Science Aspects of Photonic Crystals. Adv. Mater. 2003, 15 (20), 1679–1704. 10.1002/adma.200300386. [DOI] [Google Scholar]
- Schroden R. C.; Al-Daous M.; Blanford C. F.; Stein A. Optical Properties of Inverse Opal Photonic Crystals. Chem. Mater. 2002, 14 (8), 3305–3315. 10.1021/cm020100z. [DOI] [Google Scholar]
- Chen J. I. L.; Von Freymann G.; Choi S. Y.; Kitaev V.; Ozin G. A. Amplified Photochemistry with Slow Photons. Adv. Mater. 2006, 18 (14), 1915–1919. 10.1002/adma.200600588. [DOI] [Google Scholar]
- Chen J. I. L.; Von Freymann G.; Choi S. Y.; Kitaev V.; Ozin G. A. Slow Photons in the Fast Lane in Chemistry. J. Mater. Chem. 2008, 18 (4), 369–373. 10.1039/B708474A. [DOI] [Google Scholar]
- Grushevskaya S.; Belyanskaya I.; Kozaderov O. Approaches for Modifying Oxide-Semiconductor Materials to Increase the Efficiency of Photocatalytic Water Splitting. Materials 2022, 15, 4915. 10.3390/ma15144915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P.; Sharma K.; Hasija V.; Sharma V.; Sharma S.; Raizada P.; Singh M.; Saini A.K.; Hosseini-Bandegharaei A.; Thakur V.K. Systematic Review on Applicability of Magnetic Iron-Oxides Integrated Photocatalysts for Degradation of Organic Pollutants in Water. Mater. Today 2019, 14, 100186. 10.1016/j.mtchem.2019.08.005. [DOI] [Google Scholar]
- Wheeler D. A.; Wang G.; Ling Y.; Li Y.; Zhang J. Z. Nanostructured Hematite: Synthesis, Characterization, Charge Carrier Dynamics, and Photoelectrochemical Properties. Energy Environ. Sci. 2012, 5, 6682–6702. 10.1039/c2ee00001f. [DOI] [Google Scholar]
- Akhavan O. Thickness Dependent Activity of Nanostructured TiO 2 /α- Fe 2 O 3 Photocatalyst Thin Films. Appl. Surf. Sci. 2010, 257 (5), 1724–1728. 10.1016/j.apsusc.2010.09.005. [DOI] [Google Scholar]
- Hardee K. I.; Bard A. J. Semiconductor Electrodes: X. Photoelectrochemical Behavior of Several Polycrystalline Metal Oxide Electrodes in Aqueous Solutions. J. Electrochem. Soc. 1977, 124 (124), 215–224. 10.1149/1.2133269. [DOI] [Google Scholar]
- Leland J. K.; Bard A. J. Photochemistry of Colloidal Semiconducting Iron Oxide Polymorphs. J. Phys. Chem. 1987, 91, 5076–5083. 10.1021/j100303a039. [DOI] [Google Scholar]
- Cao Y.-Q.; Zi T.-Q.; Zhao X.-R.; Liu C.; Ren Q.; Fang J.-B.; Li W.-M.; Li A.-D. Enhanced Visible Light Photocatalytic Activity of Fe2O3 Modified TiO2 Prepared by Atomic Layer Deposition. Sci. Rep. 2020, 10, 13437. 10.1038/s41598-020-70352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenczek-Zajac A.; Synowiec M.; Zakrzewska K.; Zazakowny K.; Kowalski K.; Dziedzic A.; Radecka M. Scavenger-Supported Photocatalytic Evidence of an Extended Type I Electronic Structure of the TiO2@Fe2O3Interface. ACS Appl. Mater. Interfaces 2022, 14 (33), 38255–38269. 10.1021/acsami.2c06404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves da Silva J.; Borges dos Santos G.; Stumpf Madeira V.; de Almeida Ramalho M. L.; Ouriques Brasilero I. L.; Marinho Cahino A. Use of Fe2O3-TiO2 in Solar Photo-Fenton Process for the Phenol Degradation. Engevista 2018, 757–771. [Google Scholar]
- Baldovi H. G. Optimization of α-Fe2O3 Nanopillars Diameters for Photoelectrochemical Enhancement of α-Fe2O3-TiO2 Heterojunction. Nanomaterials 2021, 11 (8), 2019. 10.3390/nano11082019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen A.; Ruoko T.-P.; Iivonen T.; Lahtonen K.; Ali-Löytty H.; Sarlin E.; Valden M.; Leskelä M.; Tkachenko N. Design Aspects of All Atomic Layer Deposited TiO 2 – Fe 2 O 3 Scaffold-Absorber Photoanodes for Water Splitting. Sustain. Energy Fuels 2018, 2 (9), 2124–2130. 10.1039/C8SE00252E. [DOI] [Google Scholar]
- Hitam C. N. C.; Jalil A. A. A Review on Exploration of Fe 2 O 3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. J. Environ. Manage. 2020, 258, 110050 10.1016/j.jenvman.2019.110050. [DOI] [PubMed] [Google Scholar]
- Peng L.; Xie T.; Lu Y.; Fan H.; Wang D. Synthesis, Photoelectric Properties and Photocatalytic Activity of the Fe2O3/TiO2 Heterogeneous Photocatalysts. Phys. Chem. Chem. Phys. 2010, 12 (28), 8033. 10.1039/c002460k. [DOI] [PubMed] [Google Scholar]
- dela Rosa F. M.; Popovic M.; Papac Zjacic J.; Radic G.; Kraljic Rokovic M.; Kovacic M.; Farre M. J.; Genorio B.; Lavrencic Stangar U.; Kusic H.; Loncaric Bozic A.; Petrovic M. Visible-Light Activation of Persulfate or H2O2 by Fe2O3/TiO2 Immobilized on Glass Support for Photocatalytic Removal of Amoxicillin: Mechanism, Transformation Products, and Toxicity Assessment. Nanomaterials 2022, 12, 4328. 10.3390/nano12234328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aritonang A. B.; Selpiana H.; Wibowo M. A.; Warsidah W.; Adhitiawarman A. PHOTOCATALYTIC DEGRADATION OF METHYLENE BLUE USING Fe2O3-TiO2/KAOLINITE UNDER VISIBLE LIGHT. J. Kim. Dan Pendidik. Kim. 2022, 7 (3), 277–286. 10.20961/jkpk.v7i3.66567. [DOI] [Google Scholar]
- Yang X.; Liu R.; Du C.; Dai P.; Zheng Z.; Wang D. Improving Hematite-Based Photoelectrochemical Water Splitting with Ultrathin TiO 2 by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2014, 6 (15), 12005–12011. 10.1021/am500948t. [DOI] [PubMed] [Google Scholar]
- Zandi O.; Klahr B. M.; Hamann T. W. Highly Photoactive Ti-Doped α-Fe2O3 Thin Film Electrodes: Resurrection of the Dead Layer. Energy Environ. Sci. 2013, 6 (2), 634–642. 10.1039/C2EE23620F. [DOI] [Google Scholar]
- Liu J.; Sun C.; Fu M.; Long J.; He D.; Wang Y. Enhanced Photochemical Catalysis of TiO2 Inverse Opals by Modification with ZnO or Fe2O3 Using ALD and the Hydrothermal Method. Mater. Res. Express 2018, 5, 025509 10.1088/2053-1591/aaabe9. [DOI] [Google Scholar]
- Pylarinou M.; Toumazatou A.; Sakellis E.; Xenogiannopoulou E.; Gardelis S.; Boukos N.; Dimoulas A.; Likodimos V. Visible Light Trapping against Charge Recombination in FeOx-TiO2 Photonic Crystal Photocatalysts. Materials 2021, 14, 7117. 10.3390/ma14237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson A. B. F.; Devries M. J.; Libera J. A.; Christensen S. T.; Hupp J. T.; Pellin M. J.; Elam J. W. Atomic Layer Deposition of Fe2O3 Using Ferrocene and Ozone. J. Phys. Chem. C 2011, 115 (10), 4333–4339. 10.1021/jp110203x. [DOI] [Google Scholar]
- Ollis D. F. Kinetic Disguises in Heterogeneous Photocatalysis. Top. Catal. 2005, 35 (3–4), 217–223. 10.1007/s11244-005-3827-z. [DOI] [Google Scholar]
- Gaya U. I.Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids; Springer: Berlin Heidelberg, 2014. [Google Scholar]
- Vogel N.; Retsch M.; Fustin C.-A.; Del Campo A.; Jonas U. Advances in Colloidal Assembly: The Design of Structure and Hierarchy in Two and Three Dimensions. Chem. Rev. 2015, 115 (13), 6265–6311. 10.1021/cr400081d. [DOI] [PubMed] [Google Scholar]
- Furlan K. P.; Larsson E.; Diaz A.; Holler M.; Krekeler T.; Ritter M.; Petrov A. Y.; Eich M.; Blick R.; Schneider G. A.; Greving I.; Zierold R.; Janßen R. Photonic Materials for High-Temperature Applications: Synthesis and Characterization by X-Ray Ptychographic Tomography. Appl. Mater. Today 2018, 13, 359–369. 10.1016/j.apmt.2018.10.002. [DOI] [Google Scholar]
- Rahtu A.; Ritala M. Reaction Mechanism Studies on Titanium Isopropoxide–Water Atomic Layer Deposition Process. Chem. Vap. Depos. 2002, 8 (1), 21.. [DOI] [Google Scholar]
- Daimon M.; Masumura A. Measurement of the Refractive Index of Distilled Water from the Near-Infrared Region to the Ultraviolet Region. Appl. Opt. 2007, 46 (18), 3811–3820. 10.1364/AO.46.003811. [DOI] [PubMed] [Google Scholar]
- Birch K. P.; Downs M. J. An Updated Edlén Equation for the Refractive Index of Air. Metrologia 1993, 30 (3), 155–162. 10.1088/0026-1394/30/3/004. [DOI] [Google Scholar]
- Lee J.; Bae K.; Kang G.; Choi M.; Baek S.; Yoo D. S.; Lee C. W.; Kim K. Graded-Lattice AAO Photonic Crystal Heterostructure for High Q Refractive Index Sensing. RSC Adv. 2015, 5 (88), 71770–71777. 10.1039/C5RA15890G. [DOI] [Google Scholar]
- Lim S. Y.; Hedrich C.; Jiang L.; Law C. S.; Chirumamilla M.; Abell A. D.; Blick R. H.; Zierold R.; Santos A. Harnessing Slow Light in Optoelectronically Engineered Nanoporous Photonic Crystals for Visible Light-Enhanced Photocatalysis. ACS Catal. 2021, 11 (21), 12947–12962. 10.1021/acscatal.1c03320. [DOI] [Google Scholar]
- Kment S.; Riboni F.; Pausova S.; Wang L.; Wang L.; Han H.; Hubicka Z.; Krysa J.; Schmuki P.; Zboril R. Photoanodes Based on TiO2 and Alpha-Fe2O3 for Solar Water Splitting - Superior Role of 1D Nanoarchitectures and of Combined Heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. 10.1039/C6CS00015K. [DOI] [PubMed] [Google Scholar]
- Hedrich C.; Burson A. R.; González-García S.; Vega V.; Prida V. M.; Santos A.; Blick R. H.; Zierold R. Enhancing the Photocatalytic Activity by Tailoring an Anodic Aluminum Oxide Photonic Crystal to the Semiconductor Catalyst: At the Example of Iron Oxide. Adv. Mater. Interfaces 2023, 10, 2300615. 10.1002/admi.202300615. [DOI] [Google Scholar]
- Míguez H.; Tétreault N.; Yang S. M.; Kitaev V.; Ozin G. A. A New Synthetic Approach to Silicon Colloidal Photonic Crystals with a Novel Topology and an Omni-Directional Photonic Bandgap: Micromolding in Inverse Silica Opal (MISO). Adv. Mater. 2003, 15 (7–8), 597–600. 10.1002/adma.200304043. [DOI] [Google Scholar]
- Eremin D. B.; Ananikov V. P. Understanding Active Species in Catalytic Transformations: From Molecular Catalysis to Nanoparticles, Leaching, “Cocktails” of Catalysts and Dynamic Systems. Coord. Chem. Rev. 2017, 346, 2–19. 10.1016/j.ccr.2016.12.021. [DOI] [Google Scholar]
- Namigata H.; Watanabe K.; Okubo S.; Hasegawa M.; Suga K.; Nagao D. Double-Inverse-Opal-Structured Particle Assembly as a Novel Immobilized Photocatalytic Material. Materials 2021, 14 (1), 28. 10.3390/ma14010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth U.; Gibson N. The Scherrer Equation versus the “Debye-Scherrer Equation.. Nat. Nanotechnol. 2011, 6 (9), 534–534. 10.1038/nnano.2011.145. [DOI] [PubMed] [Google Scholar]
- Nair R. V.; Gummaluri V. S.; Matham M. V.; C V. A Review on Optical Bandgap Engineering in TiO 2 Nanostructures via Doping and Intrinsic Vacancy Modulation towards Visible Light Applications. J. Phys. Appl. Phys. 2022, 55 (31), 313003. 10.1088/1361-6463/ac6135. [DOI] [Google Scholar]
- Pan X.; Yang M.-Q.; Fu X.; Zhang N.; Xu Y.-J. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale 2013, 5 (9), 3601. 10.1039/c3nr00476g. [DOI] [PubMed] [Google Scholar]
- Krylova G.; Na C. Photoinduced Crystallization and Activation of Amorphous Titanium Dioxide. J. Phys. Chem. C 2015, 119 (22), 12400–12407. 10.1021/acs.jpcc.5b02048. [DOI] [Google Scholar]
- Pasquarelli R. M.; Lee H. S.; Kubrin R.; Zierold R.; Petrov A. Y.; Nielsch K.; Schneider G. A.; Eich M.; Janssen R. Enhanced Structural and Phase Stability of Titania Inverse Opals. J. Eur. Ceram. Soc. 2015, 35 (11), 3103–3109. 10.1016/j.jeurceramsoc.2015.04.041. [DOI] [Google Scholar]
- Li J.; Su W.; Li J.; Wang L.; Ren J.; Zhang S.; Cheng P.; Hong H.; Wang D.; Zhou Y.; Mi W.; Du Y. Orientational Alignment of Oxygen Vacancies: Electric-Field-Inducing Conductive Channels in TiO 2 Film to Boost Photocatalytic Conversion of CO 2 into CO. Nano Lett. 2021, 21 (12), 5060–5067. 10.1021/acs.nanolett.1c00897. [DOI] [PubMed] [Google Scholar]
- Pham H. H.; Wang L.-W. Oxygen Vacancy and Hole Conduction in Amorphous TiO 2. Phys. Chem. Chem. Phys. 2015, 17 (1), 541–550. 10.1039/C4CP04209C. [DOI] [PubMed] [Google Scholar]
- Andronic L.; Enesca A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 565489 10.3389/fchem.2020.565489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretos I.; Jiménez R.; Ricote J.; Calzada M. L. Low-Temperature Crystallization of Solution-Derived Metal Oxide Thin Films Assisted by Chemical Processes. Chem. Soc. Rev. 2018, 47 (2), 291–308. 10.1039/C6CS00917D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.