Abstract
Glomerulonephritis (GN) is a progressive inflammation that may be caused by a variety of underlying disorders. It is the primary cause of chronic renal failure and end-stage renal disease, which require dialysis and transplantation worldwide. Immunosuppressive therapy has been used to treat GN clinically, but this treatment has had insufficient therapeutic effects. Here, we show that protein kinase CK2 is a key molecule in the progression of GN. cDNA microarray analysis identified CK2α, the catalytic subunit of CK2, as a GN-related, differentially expressed gene. Overexpression of CK2α was noted in the proliferative glomerular lesions in rat GN models and in renal biopsy specimens from lupus nephritis or IgA nephropathy patients. Administration of either antisense oligodeoxynucleotide against CK2α or low molecular weight CK2-specific inhibitors effectively prevented the progression of renal pathology in the rat GN models. The resolution of GN by CK2 inhibition may result from its suppression of extracellular signal-regulated kinase-mediated cell proliferation, and its suppression of inflammatory and fibrotic processes that are enhanced in GN. Our results show that CK2 plays a critical role in the progression of immunogenic renal injury, and therefore, CK2 is a potential target for GN therapy.
Keywords: antisense oligodeoxynucleotide, microarray, pharmacology
Glomerulonephritis (GN) is a disease characterized by renal inflammation, causing destruction of glomeruli and adjacent structures, as well as loss of renal function. It is associated with conditions such as hematuria and proteinuria. Current treatment is still limited to supportive therapy, with or without nonspecific immunosuppressive drugs (1–3). Early cellular proliferation followed by subsequent fibrosis is a prominent hallmark of proliferative GN, and it may ultimately lead to end-stage renal disease (4). The involvement of extracellular stimuli, such as growth factors, cytokines, activated complement, and immune complexes in the pathogenesis of experimental and human GN has been known for many years. However, only recently have the intracellular mediators that transduce signals from noxious extracellular stimuli to unfettered cellular proliferation and accompanying excess extracellular matrix deposition begun to be unraveled (5). Experiments with cultured glomerular cells and certain animal models of experimental GN implicate the activation of extracellular signal-regulated kinase (ERK), which results in glomerular cellular proliferation (6).
Protein kinase CK2 (formerly known as casein kinase II) is an extremely well conserved pleiotropic protein kinase with a growing list of >300 substrates, the majority of which are proteins implicated in signal transduction, gene expression, and transcription-related functions (7–11). Protein kinase CK2 is a ubiquitous heterotetrameric serine/threonine protein kinase made up of two α or α′ catalytic subunits and two β-regulatory subunits. CK2 is activated during cell division, cellular differentiation, and embryogenesis, and it plays an important role in transducing signals between extracellular growth factors and nuclear responses (7–11). Overexpression or inhibition of CK2 has been shown to affect proliferation; however, results varied greatly with cell type (12).
In the present study, we have undertaken a cDNA microarray strategy to isolate the GN-related gene, and these experiments identified CK2α, the catalytic subunit of CK2. Administration of either antisense oligodeoxynucleotide (AS-ODN) against CK2α, or low molecular weight CK2-specific inhibitors revealed that in vivo inhibition of CK2 ameliorates the renal dysfunction and histological progression. Our results show that CK2 plays a critical role in the progression of immunogenic renal injury.
Materials and Methods
Animals. Specific pathogen-free male Wistar–Kyoto rats weighing 300–350 g (Charles River Japan, Kanagawa) and female Wistar rats weighing 120–140 g (CLEA Japan, Tokyo) were used. All animal experiments were approved by the Animal Care and Experimentation Committee of Kyoto University. Animals were housed in a constant temperature room with a 12-h dark/12-h light cycle. The general condition and body weight of the rats were observed over the course of the experiments.
Anti-Glomerular Basement Membrane (GBM) GN. GBM antigen for the rats was prepared as described (13). Five albino rabbits were immunized s.c. with GBM antigen emulsified with Freund's complete adjuvant (Difco). A booster was given three times every 2 weeks using the same antigen. Four days after the final booster, the rabbits were bled from the carotid artery under anesthesia. Anti-GBM sera were heat-decomplemented for 30 min at 56°C and absorbed with freshly harvested rat erythrocytes. Wistar–Kyoto rats were divided into several groups, each of which consisted of four to eight rats. The rats assigned to the GN groups were injected in the dorsal tail vein with 3 ml/kg anti-GBM serum diluted 10-fold with saline under ether anesthesia. The day of the anti-GBM serum injection was defined as day 0. The rats assigned to the control groups were injected intravenously with the same volume of nonimmune rabbit normal serum for comparison with the anti-GBM GN rats.
Anti-Thy1 GN. Wistar rats were divided into several groups, each of which consisted of four rats. The rats assigned to the GN groups were injected in the dorsal tail vein with 1 mg/kg monoclonal anti-Thy1 antibody OX-7 (BD Pharmingen) in saline under ether anesthesia. The day of the anti-Thy1 antibody injection was defined as day 0. The rats assigned to the control groups were injected intravenously with the same volume of saline for comparison with the anti-Thy1 GN rats.
Drug Treatment. Prednisolone (Shionogi Pharmaceutical) was administered orally at 1 mg/kg body weight twice a day from day 14 of anti-GBM serum injection until they died. CK2 inhibitors 3-methyl-1,6,8-trihydroxyanthraquinone (emodin; Sigma) and 4′,5,7-trihydroxyflavone (apigenin; Sigma) were administered i.p. at 20 mg/kg of body weight once a day after an injection of anti-GBM serum or anti-Thy1 antibody until they died.
AS-ODN. The sequences of the AS-ODN were selected to target rat CK2α/α′ (Antisense, 5′-GTAATCATCTTGATTACCCCA-3′; Sense, 5′-TGGGGTAATCAAGATGATTAC-3′). Phosphorothioate-modified ODNs were purified by high-pressure liquid chromatography before use. ODNs were mixed with cationic transfection reagent [in vivo jetPEI, PolyPlus transfection (Illkrich, France) and Avanti Polar Lipids] according to the manufacturer's instructions. The ODN–liposome complexes were infused into the rat renal cortex by using a catheter attached to an i.p. osmotic minipump (model 1002, Alza). The tubing was connected to an osmotic minipump, which delivered 100 μg of ODNs continuously into the renal cortex at a rate of 0.25 μl/h for 14 days.
Renal Function Tests. The 24-h urine samples were obtained at the indicated time points after the induction of GN, with each rat being kept in an individual metabolic cage with free access to water and food. The amount of urinary protein was determined by the Pyrogallol red method and expressed as mg/day of urine. At the end of urine collection, 0.5 ml of blood was drawn from the dorsal tail vein of each rat. The levels of serum creatinine were determined by the creatinine amidohydrolase-N-ethyl-N-(2-hydroxy-3-sulfopropyl)-m-toluidine method (Wako Pure Chemical, Osaka) and expressed as milligrams per 100 ml of serum. The blood urea nitrogen levels in the serum samples were determined by the urease-indophenol method (Wako Pure Chemical) and expressed as milligrams per 100 ml of serum.
Histological Analysis. Kidneys were fixed in 10% buffered formalin and embedded in paraffin. Thin sections (4 μm) were stained with periodic acid-Schiff (PAS) or hematoxylin/eosin reagents and evaluated by using light microscopy. The percentage of area occupied by crescents in each glomerulus was calculated by using an ocular micrometer (10 × 10, 1 mm per grid) for a total of 30 glomeruli, which were randomly selected with use of a modification of the system described by Oseto et al. (14) and Koo et al. (15). GBM thickening and tubular dilatation were graded as follows: normal, slight, moderate, or marked. All histological analyses were performed in a blinded fashion. Experiments using human tissues derived from Lupus nephritis and IgA nephropathy patients were approved by the Ethical Committee of Tokyo Women's Medical University.
cDNA Microarray Analysis. cDNA microarray experiments were performed as described (16, 17). We selected genes with average residuals that were more than +1 or less than –1, i.e., that represented a 2-fold difference in expression level. The microarray data are available at the National Center for Biotechnology Information's Gene Expression Omnibus site (which can be accessed at www.ncbi.nlm.nih.gov/geo) accession no. GSE1262.
RT-PCR. One microgram of total RNA was reverse-transcribed, and cDNA samples were amplified by using PCR. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase was used to standardize the mRNA levels of the target genes. Real-time PCR analysis was performed by using the DNA Engine Opticon2 System and the DyNAmo HS SYBR green qPCR kit (Bio-Rad). Sequences of PCR primers are shown in Table 1, which is published as supporting information on the PNAS web site.
Western Blotting. Protein was extracted from the renal cortex, and 20 μg of the total protein was denatured and resolved by SDS/PAGE on a 12.5% (wt/vol) polyacrylamide gel. The proteins were electroblotted onto polyvinylidene difluoride membranes (Millipore). The blocked membranes were incubated with a primary polyclonal goat anti-CK2α antibody at 1:100 dilution (Santa Cruz Biotechnology) and with a secondary horseradish peroxidase-conjugated donkey anti-rabbit IgG antibody (Santa Cruz Biotechnology) diluted at 1:1,000. Detection was achieved by using the enhanced chemiluminescence method (Amersham Biosciences).
Immunohistochemical Staining. Kidneys were removed, rolled in Tissue-Tek 22 OCT compound (Miles), and snap-frozen in liquid nitrogen. Frozen sections were cut at a thickness of 4 μm and fixed in acetone. The endogenous peroxidase in the frozen sections was quenched by hydrogen peroxide, and sections were incubated with polyclonal goat anti-CK2α antibody (Santa Cruz Biotechnology), anti-Ki67 (Santa Cruz Biotechnology), and anti-phospho ERK (Cell Signaling Technology, Beverly, MA). The sections were then processed by using an avidin-biotinylated peroxidase complex method (Vectastain Elite ABC kit, Vector Laboratories).
In Vitro CK2 Kinase Assay. CK2 activity was assayed by using a CK2 assay kit (Upstate Biotechnology) according to the manufacturer's instructions. Kinase activity was calculated by subtracting the mean of the background control samples without enzyme from the mean of samples with enzyme.
Endogenous CK2 Activity in Kidney. Renal cortex was removed, homogenized, and centrifuged at 1000 × g for 5 min at 4°C. Fifty micrograms of proteins from the supernatant was used to assay the CK2 activity. CK2 activity was assayed by using a CK2 assay kit (Upstate Biotechnology) according to the manufacturer's instructions.
TUNEL Staining. TUNEL analysis was performed as described (18).
Statistical Analysis. Results are shown as mean ± SEM. Statistical significance of differences in mean values was assessed by using a Student t test or ANOVA (One-way ANOVA followed by the Dunnet's multiple comparison test) with use of sas software (SAS Institute, Cary, NC). Differences among means were considered significant at P values of < 0.05.
Results and Discussion
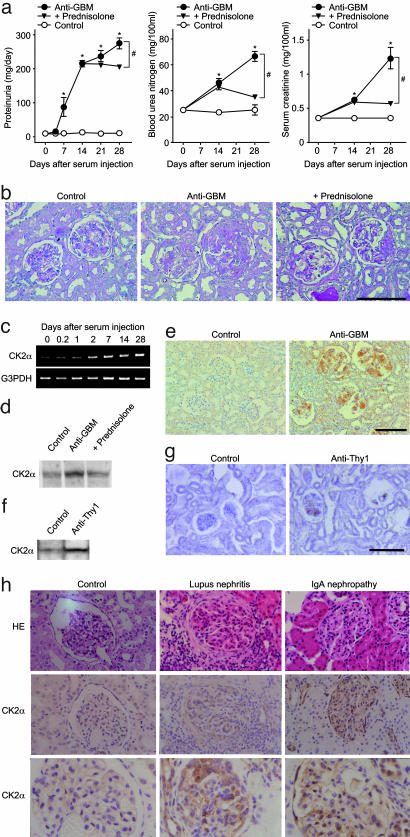
As an initial effort to gain insight into the underlying molecular basis of GN, we have used cDNA microarrays to assess changes in gene expression in the kidneys of anti-GBM serum-induced GN rats. The anti-GBM GN rat is a model of human crescentic GN that rapidly progresses to renal failure. These rats are characterized by prominent inflammatory cell infiltration into the stroma, mesangial cell proliferation, crescent formation in the glomerulus, GBM thickening, and tubular dilatation (13, 14). The renal function of these rats deteriorated progressively after the injection of anti-GBM serum, as reported (13, 14). All anti-GBM serum-injected rats showed a severe proteinuria on day 7, which reached a peak on day 28, whereas the rate of urinary protein excretion was very low (≈20 mg/day) throughout the experiment in normal serum-injected rats (Fig. 1a). Also, two serum markers of renal damage, blood urea nitrogen, and serum creatinine levels, significantly (P < 0.05) increased on day 14 in anti-GBM serum-injected rats compared with controls. Thereafter, the levels increased further until day 28 (Fig. 1a). The kidneys of anti-GBM serum-injected rats showed histopathological changes characteristic of GN, including marked crescent formation in the glomerulus, GBM thickening, and tubular dilatation (Fig. 1b). Glucocorticoid prednisolone (1 mg/kg twice a day) was administered orally beginning on day 14 of anti-GBM serum injections. This significantly (P < 0.05) alleviated the damage according to all parameters examined (proteinuria, blood urea nitrogen, and serum creatinine levels; Fig. 1a). Also, the kidneys of anti-GBM GN rats that were treated with prednisolone showed considerably less severe crescent formation in the glomeruli (anti-GBM: 26.7 ± 2.6%, +prednisolone: 17.3 ± 2.2%, P < 0.05; Fig. 1b). However, GBM thickening (anti-GBM: marked 33%, moderate 67%; +prednisolone: marked 50%, moderate 50%) and tubular dilatation (anti-GBM: moderate 33%, slight 67%; +prednisolone: moderate 50%, slight 40%) were not alleviated remarkably by the treatment with prednisolone.
Fig. 1.
Expression of CK2α during progression of GN. (a) Urinary protein excretion, blood urea nitrogen levels, and serum creatinine levels in anti-GBM GN rats. Data are shown as mean ± SEM.; n = 6 animals. *, P < 0.05 compared with the control group. #, P < 0.05 compared with the anti-GBM group. (b) PAS staining of the renal tissue in anti-GBM GN rats. (Original magnification: ×200. Bar, 100 μm.) (c) Time-related changes in CK2α mRNA expression in the renal cortex of anti-GBM GN rats by RT-PCR analysis. G3PDH, glyceraldehyde 3-phosphate dehydrogenase. (d) Western blot analysis of CK2α protein expression in the renal cortex of anti-GBM GN rats on day 28. (e) Immunohistochemical staining for CK2α on the renal tissue of anti-GBM GN rats. (Original magnification: ×200. Bar, 100 μm.) (f) Western blot analysis of CK2α protein expression in the renal cortex of anti-Thy1 GN rats on day 3. (g) Immunohistochemical staining for CK2α on the renal tissue of anti-Thy1 GN rats. (Original magnification: ×200. Bar, 100 μm.) Control, rats injected with normal serum or saline. Anti-GBM, rats injected with anti-GBM serum. Anti-Thy1, rats injected with anti-Thy1 antibody. +prednisolone, rats administered prednisolone orally (1 mg/kg twice a day) from day 14 of anti-GBM serum injection. (h) Hematoxylin/eosin staining (Top) and immunohistochemical staining for CK2α (Middle and Bottom) on human renal biopsy specimens of lupus nephritis (Middle) and IgA nephropathy (Right). Both pictures are representatives of at least four to five different patients. Lupus nephritis shown is class IV-G of the World Health Organization classification (31). (Original magnification: ×400.)
Expression profiling was carried out by using mRNA from the renal cortex of anti-GBM GN or control rats on day 28 and cDNA microarrays enriched for clones representing rat kidney genes (16). We selected 43 of 3,000 cDNAs that were examined, in which the expression levels differed by >2-fold intensity from controls (Table 2, which is published as supporting information on the PNAS web site). The expression of 29 genes, including CK2α, TGFβ1, osteopontin, and collagen IVα1 were up-regulated, whereas the expression of 14 genes, including pendrin and organic anion transporter 1, were down-regulated. Expression profiling performed in the renal cortex of prednisolone-treated anti-GBM GN rats showed that 18 up-regulated and 7 down-regulated GN-related genes, respectively, were repressed by prednisolone treatment (Table 2). TGFβ1 (19), osteopontin (20), collagen IVα1 (21), pendrin (22), and organic anion transporter 1 (23) were previously reported as genes for which expression levels change during the development of renal disease. Real-time RT-PCR analysis on these genes further verified that the microarray data accurately represented gene expression in anti-GBM GN rats (Table 2). Among the differentially expressed genes, we focused on one gene, CK2α, that was overexpressed in the anti-GBM GN rats.
CK2 has been reported to phosphorylate a variety of protein substrates involved in diverse cellular functions such as signal transduction, cell proliferation, malignant transformation, and apoptosis. However, the role of CK2 in GN is unknown. We confirmed ubiquitous expression of CK2α, e.g., in the heart, lung, liver, thymus, spleen, and intestine by RT-PCR analysis of both anti-GBM GN and control rats and recorded similar expression levels; however, expression of CK2α was markedly enhanced only in the kidneys of GN model rats (data not shown). RT-PCR monitoring showed a time-dependent increase of CK2α in the renal cortex of anti-GBM model rats during progression of GN (Fig. 1c). Corresponding well with the RT-PCR analysis (Table 2), Western blots verified the enhanced expression of CK2α in renal cortex from anti-GBM GN rats on day 28 (Fig. 1d). Immunohistochemical staining showed that expression of CK2α was markedly enhanced in the affected area of glomeruli in anti-GBM GN rats (Fig. 1e). Enhanced expression of CK2α was suppressed by treatment with prednisolone (Table 2 and Fig. 1d). Also, the endogenous CK2 activity was markedly increased in the kidneys of anti-GBM GN rats (Control: 33.0 ± 19.3 pmol per min per mg of tissue; Anti-GBM: 91.3 ± 21.8 pmol per min per mg of tissue; P < 0.05). This enhanced CK2 activity in GN rats was partially suppressed by treatment with prednisolone (64.6 ± 6.3 pmol per min per mg of tissue). Also, the expression of CK2α in the kidneys was examined in anti-Thy1 GN rats, another model with many features mimicking human mesangial proliferative GN, such as IgA nephropathy (24). The rats injected with anti-Thy1 antibody showed a severe proteinuria on day 3 (Control: 11.1 ± 1.0 mg/day; anti-Thy1: 155.5 ± 4.8 mg/day; P < 0.05). Real-time RT-PCR analysis (Control: 1.0 ± 0.1; Anti-Thy1: 1.7 ± 0.2; P < 0.05) and Western blots (Fig. 1f) showed enhanced CK2α expression in the renal cortex of the anti-Thy1 GN rats on day 3. Immunohistochemical staining showed that CK2α expression was markedly enhanced in the glomeruli of anti-Thy1 GN rats (Fig. 1g). Furthermore, the histologic evaluation was conducted on human renal biopsy specimens obtained from untreated lupus nephritis (Fig. 1h Middle) and IgA nephropathy (Fig. 1h Right) patients. In all specimens examined, CK2α was overexpressed in the glomeruli, and in some cases, in the peritubular interstitium (Fig. 1h). Hence, overexpression of CK2α appeared to be closely associated with glomerular injury not only in the GN animal models but also in GN patients.
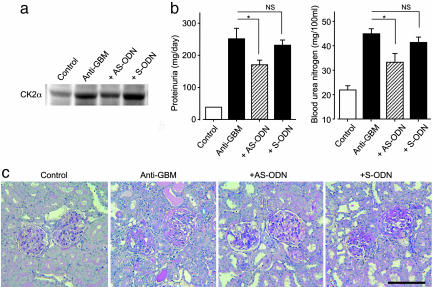
To elucidate the causal relationship between GN progression and enhanced CK2α expression, we examined the effects of an AS-ODN against CK2α in anti-GBM GN rats. By using an osmotic minipump, 100 μg of either specific AS-ODN or sense oligodeoxynucleotide (S-ODN) was continuously administered into the renal cortex for 14 days, starting 1 day before the induction of anti-GBM GN. The enhanced CK2α protein expression in the renal cortex of anti-GBM GN rats was suppressed by AS-ODN treatment, whereas S-ODN treatment showed no inhibitory effect (Fig. 2a). Also, the AS-ODN treatment significantly (P < 0.05) abrogated both the anti-GBM GN-induced increase in proteinuria (166 ± 19 vs. 253 ± 29 mg/day; P < 0.05) and blood urea nitrogen levels (21.5 ± 1.6 vs. 44.3 ± 2.4 mg per 100 ml; P < 0.05) on day 14, whereas S-ODN treatment showed no inhibitory effect (Fig. 2b). Also, the renal histopathologic alterations, GBM thickening, and tubular dilatation were improved by the AS-ODN treatment (Fig. 2c).
Fig. 2.
CK2α AS-ODN blocks the progression of GN in an anti-GBM serum-induced rat model. (a) Western blot analysis of CK2α protein expression in the renal cortex. (b) Urinary protein excretion and blood urea nitrogen levels. Data are shown as mean ± SEM.; n = 4 or 8 animals. *, P < 0.05 compared with Anti-GBM group. NS, not significant. (c) PAS staining of the renal tissue in anti-GBM GN rats. (Original magnification: ×200. Bar, 100 μm.) Control, rats injected with normal serum (n = 8). Anti-GBM, rats injected with anti-GBM serum (n = 8). +AS-ODN, rats continuously administered a total of 100 μg of specific AS-ODN against CK2α into the renal cortex for 14 days, starting 1 day before the injection of anti-GBM serum (n = 4). +S-ODN, rats continuously administered a total of 100 μg of specific S-ODN against CK2α into the renal cortex for 14 days, starting 1 day before the injection of anti-GBM serum (n = 4).
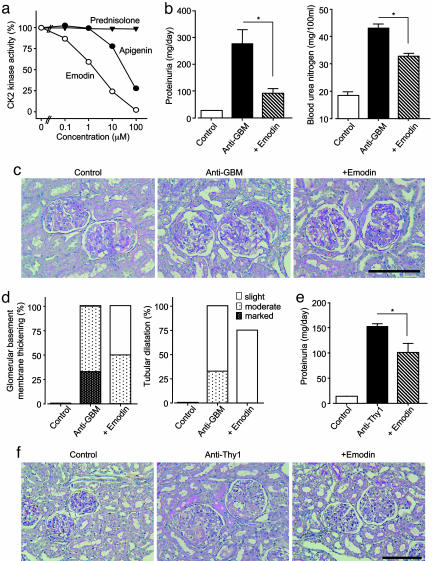
We further examined the effects of low molecular weight CK2 inhibitors on the pathology of GN. The anthraquinone derivative emodin and the flavonoid compound apigenin, both extracted from natural products, have been recently reported to be specific ATP-competitive inhibitors of CK2 (25, 26). First, we examined the specificity of these compounds against a panel of seven protein kinases in vitro. In the presence of 10 μM emodin, only CK2 was drastically inhibited, whereas the six other kinases (ERK, p38-regulated/activated kinase, S6 kinase, cAMP-dependent protein kinase, protein kinase C, and c-Src kinase) underwent little inhibition (Fig. 5, which is published as supporting information on the PNAS web site). Similar specificity was observed for apigenin as well (data not shown). Emodin and apigenin inhibited the CK2 kinase activity in a concentration-dependent manner, with an IC50 value of 2 and 30 μM, respectively, whereas prednisolone (up to 100 μM) did not have any effect on CK2 kinase activity in vitro (Fig. 3a). Emodin (20 mg/kg), when administrated i.p. once a day from day 1, effectively inhibited the increase in endogenous CK2 kinase activity in the renal cortex of GN rats (anti-GBM: 93.1 ± 19.1 pmol per min per mg of tissue; +emodin: 26.6 ± 3.0 pmol per min per mg of tissue; P < 0.05). Also, pharmacokinetic analysis showed that the maximum plasma concentration after 20 mg/kg i.p. (18 μM) was in the same range of the concentration (10 μM) we used for in vitro kinase assay.
Fig. 3.
CK2 inhibitors block the progression of GN in an anti-GBM serum-induced rat model and an anti-Thy1 antibody induced rat model. (a) Inhibitory activity of CK2 inhibitors against CK2 kinase in vitro. The value for total enzyme activity was 1.1 μmol per min per mg of protein. IC50 values of emodin and apigenin were 2 and 30 μM, respectively. (b) Urinary protein excretion and blood urea nitrogen levels in anti-GBM GN rats. Data are shown as mean ± SEM.; n = 5 animals. *, P < 0.05 compared with the anti-GBM group. (c) PAS staining of the renal tissue in anti-GBM GN rats. (Original magnification: ×200. Bar, 100 μm.) (d) GBM thickening and tubular dilatation in anti-GBM GN rats. Control, rats injected with normal serum. Anti-GBM, rats injected with anti-GBM serum. Emodin, rats administered emodin (20 mg/kg once a day) i.p. after injection of anti-GBM serum. (e) Urinary protein excretion in anti-Thy1 GN rats. Data are shown as mean ± SEM.; n = 5 animals. *, P < 0.05 compared withthe anti-Thy1 group. (f) PAS staining of the renal tissue in anti-Thy1 GN rats. (Original magnification: ×200. Bar, 100 μm.)
Next, we examined the in vivo effects of the CK2 inhibitors on GN progression. Emodin treatment significantly (P < 0.05) improved the anti-GBM GN-induced renal dysfunction (proteinuria: Fig. 3b Right; blood urea nitrogen: Fig. 3b Left; serum creatinine; anti-GBM: 0.57 ± 0.08 mg per 100 ml; +emodin: 0.41 ± 0.06 mg per 100 ml; and creatinine clearance; anti-GBM: 1.25 ± 0.05 ml/min; +emodin: 1.64 ± 0.37 ml/min). Also, treatment with emodin significantly modulated the histological alterations observed in anti-GBM GN rats (Fig. 3c); thus, the crescent formation area of glomeruli in anti-GBM GN rats was significantly alleviated (anti-GBM: 27.2 ± 1.5%; +emodin: 15.3 ± 0.4%; P < 0.05). Unlike prednisolone, the emodin treatment effectively prevented GBM thickening and tubular dilatation (Fig. 3d). Similar therapeutic effects were also observed upon treatment with apigenin (20 mg/kg i.p. once a day; data not shown). Additionally, we further examined the therapeutic activity of emodin by administering later, but not at the onset. The emodin treatment (20 mg/kg i.p.) started on the day 7 also significantly inhibited the aggravation of proteinuria (221 ± 17 vs. 331 ± 42 mg/day; P < 0.05) on day 28.
The effects of CK2 inhibitors appear to be different from those of prednisolone, which effectively decreases the expression of CK2. In fact, the treatment with prednisolone moderately inhibited the enhanced CK2 activity in the kidneys of anti-GBM GN rats. This in vivo inhibition of CK2 activity by prednisolone may be mainly due to its reducing effect on CK2 expression, because in vitro kinase assay showed that prednisolone has little effect on CK2 kinase activity. Prednisolone, hence, may have CK2-specific as well as other effects. This different mode of action between prednisolone and emodin may be reflected in the different histological features caused by the two agents.
The in vivo effects of emodin on anti-Thy1 GN progression were also assessed. Emodin treatment significantly (P < 0.05) reduced anti-Thy1 GN-induced proteinuria (Fig. 3e). Also, treatment with emodin reduced the histological alterations observed in anti-Thy1 GN rats (Fig. 3f). The emodin treatment effectively prevented mesangiolysis and glomerulosclerosis. These results show that suppression of CK2α activity by specific inhibitors significantly inhibited the progression of glomerular injury, and thereby renal pathology. However, when considering CK2 inhibitors as therapeutic agents against GN, potential toxicity problems with the CK2 inhibitors should be taken into account. In fact, emodin has been reported to have genotoxicity in in vitro experiments (27), although it is not fully understood whether its genotoxicity is due to CK2 inhibitory effect.
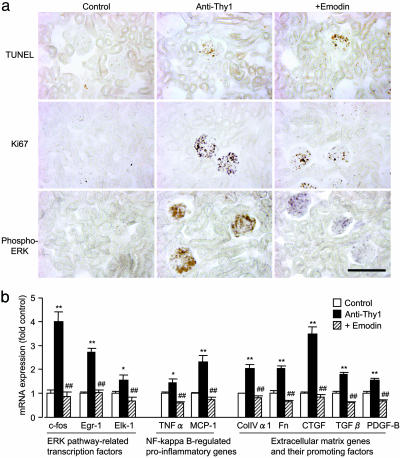
To provide mechanistic insight into the role of CK2 in GN, we examined in vivo the effect of CK2 inhibition on apoptosis, proliferation, inflammation, and fibrosis, all processes that are relevant to resolution and/or progression of GN. First, we confirmed that the number of TUNEL-positive glomerular cells increased in anti-Thy 1 GN (Fig. 4a Top); however, this increase in apoptotic activity was not enhanced significantly by treatment with emodin (Fig. 4a Top), indicating that CK2 inhibition may not be related to increased apoptotic activity. On the other hand, increased cell proliferation (as measured by the proliferative index Ki67) in GN was markedly suppressed by emodin treatment (Fig. 4a Middle). Concomitant with cell proliferation, immunohistochemical observation revealed increased glomerular staining for phospho-ERK in GN, and this activation of ERK was markedly suppressed by emodin (Fig. 4a Bottom). In good agreement with changes in ERK activation (28), real-time RT-PCR analysis showed that expression of ERK pathway related transcription factors [c-fos, early growth response-1 (Egr-1), and Ets-like protein 1 (Elk-1)], was enhanced in GN, and was significantly suppressed by emodin in all cases (Fig. 4b). Furthermore, the NF-κB pathway, which promotes expression of a wide range of proinflammatory genes, is activated in GN (29). Real-time RT-PCR analysis confirmed that expression of NF-κB-regulated proinflammatory genes such as TNF-α and monocyte chemoattractant protein 1 (MCP-1) was increased in GN, and this enhanced inflammatory response was significantly reduced by emodin treatment (Fig. 4b). Moreover, we found that emodin treatment markedly suppressed the enhanced expression of both extracellular matrix genes (collagen IVα1 and fibronectin) and their promoting factors (such as connective tissue growth factor, TGFβ, and platelet-derived growth factor B; Fig. 4b). Changes in the expression of these genes corresponded well with changes in fibrotic response, as assessed by PAS staining (Fig. 3f), indicating that CK2 inhibition is closely associated with the reduced production of extracellular matrix proteins. This observation is in good agreement with a recent study showing that CK2 activation mediates TGFβ-promoted collagen IV gene expression (30). Taken together, the protective effects of CK2 inhibition in GN may result from its suppression of ERK-mediated cell proliferation, and its suppression of inflammatory, as well as fibrotic processes that are enhanced in GN; however, CK2 inhibition apparently does not result in increased apoptotic activity.
Fig. 4.
The resolution of GN by CK2 inhibition is closely associated with its antiproliferative, antiinflammatory, and antifibrotic effects. (a) TUNEL staining (Top) and immunohistochemical staining for Ki67 (Middle) and phospho-ERK (Bottom) of the renal tissue in anti-Thy1 GN rats. (Original magnification: ×120. Bar, 100 μm.) (b) Real-time PCR analysis of expression of ERK pathway related transcription factors (c-fos, Egr-1, and Elk-1), NF-κB-regulated proinflammatory genes (TNFα and MCP-1), extracellular matrix genes (collagen IVα1 and fibronectin), and their promoting factors (connective tissue growth factor, TGFβ1, and platelet-derived growth factor B) in the renal cortex of anti-Thy1 GN rats. Data are shown as mean ± SEM.; n = 5 animals. *, P < 0.05; **, P < 0.01 compared with the control group; ##, P < 0.01 compared with the anti-Thy1 group. ColIVα1, collagen IVα1; Fn, fibronectin; CTGF, connective tissue growth factor; PDGF-B, platelet-derived growth factor B.
In conclusion, we have isolated a GN-related gene, CK2, by microarray analysis performed on kidney cDNA from experimental GN model rats, and demonstrated that in vivo inhibition of the kinase ameliorates the renal dysfunction and histological progression. Because diverse insults can induce similar clinicopathologic presentations in GN, a marked overlap among downstream molecular and cellular responses has been suggested (31). Hence, pharmacologic agents that inhibit common underlying cellular mechanisms are expected to prove effective in treating glomerular diseases of diverse etiologies. Our present study indicates that CK2 could be an ideal therapeutic target for treating immunogenic GN.
Supplementary Material
Acknowledgments
We thank H. Kurumatani, T. Sudo, K. Takeda, M. Shimamura, and T. Yamada for scientific discussion. We also thank R. Misumi, S. Akegami, and H. Motegi for their excellent technical assistance. This work was supported in part by research grants from the Scientific Fund of the Ministry of Education, Science, and Culture of Japan (to G.T.).
Author contributions: M.Y. and G.T. designed research; M.Y., S.K., T.A., A.H., T.-a.K., S.F., Y.S., Y.M., M.T., W.Y., and M.K. performed research; S.F., Y.S., Y.M., M.T., W.Y., and H.N. contributed new reagents/analytic tools; S.K., T.A., S.S., T.K., Y.O., H.N., M.K., and G.T. analyzed data; and M.Y., S.K., and G.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GN, glomerulonephritis; ERK, extracellular signal-regulated kinase; GBM, glomerular basement membrane; emodin, 3-methyl-1,6,8-trihydroxyanthraquinone; apigenin, 4′,5,7-trihydroxyflavone; PAS, periodic acid-Schiff; AS-ODN, antisense oligodeoxynucleotide; S-ODN, sense ODN; Egr-1, early growth response 1; Elk-1, Ets-like protein 1; MCP-1, monocyte chemoattractant protein 1.
Data deposition: The microarray data reported in this paper have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus database (accession no. GSE1262).
References
- 1.Madaio, M. P. & Harrington, J. T. (2001) Arch. Intern. Med. (Moscow) 161, 25–34. [DOI] [PubMed] [Google Scholar]
- 2.Donadio, J. V. & Grande, J. P. (2002) N. Engl. J. Med. 347, 738–748. [DOI] [PubMed] [Google Scholar]
- 3.Couser, W. G. (1999) Lancet 353, 1509–1515. [DOI] [PubMed] [Google Scholar]
- 4.Johnson, R. J. (1994) Kidney Int. 45, 1769–1782. [DOI] [PubMed] [Google Scholar]
- 5.Schocklmann, H. O., Lang, S. & Sterzel, R. B. (1999) Kidney Int. 56, 1199–1207. [DOI] [PubMed] [Google Scholar]
- 6.Bokemeyer, D., Sorokin, A. & Dunn, M. J. (1996) Kidney Int. 49, 1187–1198. [DOI] [PubMed] [Google Scholar]
- 7.Meggio, F. & Pinna, L. A. (2003) FASEB J. 17, 349–368. [DOI] [PubMed] [Google Scholar]
- 8.Litchfield, D. W. (2003) Biochem. J. 369, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinna, L. A. (2002) J. Cell Sci. 115, 3873–3878. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed, K., Gerber, D. A. & Cochet, C. (2002) Trends Cell Biol. 12, 226–230. [DOI] [PubMed] [Google Scholar]
- 11.Landesman-Bollag, E., Song, D. H., Romieu-Mourez, R., Sussman, D. J., Cardiff, R. D., Sonenshein, G. E. & Seldin, D. C. (2001) Mol. Cell. Biochem. 227, 153–165. [PubMed] [Google Scholar]
- 12.Lebrin, F., Chambaz, E. M. & Bianchini, L. (2001) Oncogene 20, 2010–2022. [DOI] [PubMed] [Google Scholar]
- 13.Yamada, M., Sasaki, R., Sato, N., Suzuki, M., Tamura, M., Matsushita, T. & Kurumatani, H. (2002) Eur. J. Pharmacol. 449, 167–176. [DOI] [PubMed] [Google Scholar]
- 14.Oseto, S., Moriyama, T., Kawada, N., Nagatoya, K., Takeji, M., Ando, A., Yamamoto, T., Imai, E. & Hori, M. (2003) Kidney Int. 64, 1241–1252. [DOI] [PubMed] [Google Scholar]
- 15.Koo, J. W., Kim, Y., Rozen, S. & Mauer, M. (2003) J. Nephrol. 16, 203–209. [PubMed] [Google Scholar]
- 16.Katsuma, S., Shiojima, S., Hirasawa, A., Suzuki, Y., Takagaki, K., Murai, M., Kaminishi, Y., Hada, Y., Koba, M., Muso, E., et al. (2001) Pharmacogenomics J. 1, 211–217. [DOI] [PubMed] [Google Scholar]
- 17.Katsuma, S., Shiojima, S., Hirasawa, A., Suzuki, Y., Ikawa, H., Takagaki, K., Kaminishi, Y., Murai, M., Ohgi, T., Yano, J., et al. (2002) Methods Enzymol. 345, 585–600. [DOI] [PubMed] [Google Scholar]
- 18.Gavrieli, Y., Sherman, Y. & Ben-Sasson, S.A. (1992) J. Cell Biol. 119, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou, A., Ueno, H., Shimomura, M., Tanaka, R., Shirakawa, T., Nakamura, H., Matsuo, M. & Iijima, K. (2003) Kidney Int. 64, 92–101. [DOI] [PubMed] [Google Scholar]
- 20.Lan, H. Y., Yu, X. Q., Yang, N., Nikolic-Paterson, D. J., Mu, W., Pichler, R., Johnson, R. J. & Atkins, R. C. (1998) Kidney Int. 53, 136–145. [DOI] [PubMed] [Google Scholar]
- 21.Bergijk, E. C., Van Alderwegen, I. E., Baelde, H. J., de Heer, E., Funabiki, K., Miyai, H., Killen, P. D., Kalluri, R. K. & Bruijn, J. A. (1998) J. Pathol. 184, 307–315. [DOI] [PubMed] [Google Scholar]
- 22.Petrovic, S., Wang, Z., Ma, L. & Soleimani, M. (2003) Am. J. Physiol. 284, F103–F112. [DOI] [PubMed] [Google Scholar]
- 23.Motojima, M., Hosokawa, A., Yamato, H., Muraki, T. & Yoshioka, T. (2002) Br. J. Pharmacol. 135, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jefferson, J. A. & Johnson, R. J. (1999) J. Nephrol. 12, 297–307. [PubMed] [Google Scholar]
- 25.Battistutta, R., Sarno, S., De Moliner, E., Papinutto, E., Zanotti, G. & Pinna, L.A. (2000) J. Biol. Chem. 275, 29618–29622. [DOI] [PubMed] [Google Scholar]
- 26.Sarno, S., de Moliner, E., Ruzzene, M., Pagano, M. A., Battistutta, R., Bain, J., Fabbro, D., Schoepfer, J., Elliott, M., Furet, P., et al. (2003) Biochem. J. 374, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, S. O., Eckert, I., Lutz, W. K. & Stopper, H. (1996) Mutat. Res. 371, 165–173. [DOI] [PubMed] [Google Scholar]
- 28.Bokemeyer, D., Panek, D., Kramer, H. J., Lindemann, M., Kitahara, M., Boor, P., Kerjaschki, D., Trzaskos, J. M., Floege, J. & Ostendorf, T. (2002) J. Am. Soc. Nephrol. 13, 1473–1480. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Franco, O., Suzuki, Y., Sanjuan, G., Blanco, J., Hernandez-Vargas, P., Yo, Y., Kopp, J., Egido, J. & Gomez-Guerrero, C. (2002) Am. J. Pathol. 161, 1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zdunek, M., Silbiger, S., Lei, J. & Neugarten, J. (2001) Kidney Int. 60, 2097–2108. [DOI] [PubMed] [Google Scholar]
- 31.Mittal, B., Hurwitz, S., Rennke, H. & Singh, A. K. (2004) Am. J. Kidney Dis. 44, 1050–1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.