Abstract
Menopause marks the cessation of fertility and the transition to post-reproductive years. Nearly 1M US women experience menopause annually, but despite the significant impact it has on their physical and mental health, menopause has been insufficiently studied.
Oxytocin is a neurohormone that regulates emotionality, social behaviors, and fundamental physiological systems. Localization of oxytocin receptors in the brain, reproductive tissues, bone, and heart support their role in mental health and potentially sleep, along with reproductive and cardiovascular functions. While experimental data linking oxytocin to behavior and physiology in animals are largely consistent, human data are correlative and inconclusive.
As women transition into menopause, oxytocin levels decrease while their susceptibility to mood disorders, poor sleep, osteoporosis, and cardiovascular diseases increases. These concurrent changes highlight oxytocin as a potential influence on the health and mood of women along their reproductive lifespan. Here we summarize experimental rodent and non-human primate studies that link oxytocin to reproductive aging and metabolic health and highlight the inconclusive findings in studies of women. Most human studies relied on a single oxytocin assessment in plasma or on intranasal oxytocin administration. The pulsatile release and short half-life of plasma oxytocin limits the validity of these methods.
We discuss the need for oxytocin assessments in stable bio-samples, such as urine, and to use valid assays for assessment of associations between changing oxytocin levels and well-being across the reproductive lifespan. This work has the potential to guide therapeutic strategies that will one day alleviate adverse health outcomes for many women.
Keywords: Reproductive aging, menopause, sleep, cardiometabolic disease, mood disorders
INTRODUCTION
Reproductive aging in women refers to the natural and gradual decline in ovarian function that occurs naturally with aging and characterized by changes in the menstrual cycle, decline in fertility. The reproductive life of adult women has been divided into four stages – pre-menopause, peri-menopause, menopause, and post-menopause. (Soules, Sherman et al. 2001) The perimenopause phase encompasses three stages: 1) the menopausal transition; 2) the menopause timepoint, i.e., time at the final menstrual period (FMP); and 3) the twelve months after the FMP. Most women will enter menopause naturally, between ages 40 and 60y, with a median age of 50y (Zhu, Chung et al. 2018). However, 10% of women will transition naturally into menopause early, before age 45y, or prematurely, before age 40y. Alternatively, some women will enter menopause surgically, following a bilateral oophorectomy. As women transition into their post-reproductive years, they experience biological and psychological changes that influence their quality of life and health in midlife years and beyond. Hormonal fluctuations during the perimenopause stage are marked by declining levels of estrogen and progesterone and increasing levels of follicle-stimulating hormone. These fluctuations lead to irregularities in the menstrual cycle and induce vasomotor symptoms, along with changes in mood, sleep, and sexual function (El Khoudary, Greendale et al. 2019). Also common during the menopausal transition are weight gain and abdominal fat accumulation (El Khoudary, Greendale et al. 2019). These menopause-related symptoms are of neuroendocrine origin and impact brain health in late adulthood (Maki and Thurston 2020).
The process of reproductive aging has been documented across species, including monkeys, rodents, and domestic animals, although differences in their lifespan affects the onset and decline of reproductive function (Packer, Tatar et al. 1998, Lemaître, Ronget et al. 2020). For example, reproductive aging in rats is marked by declined infertility at age 8 months and by age 12 months, evidence of ovarian function is rare.(Cruz, Fernandois et al. 2017) With an average life expectancy of 36 months, (Sengupta 2013) the reproductive aging of rats occurs earlier in the lifespan relative to humans. However, chimpanzees experience a distinct and prolonged phase of reproductive cessation, as females continue to exhibit reproductive function until late in life, despite fertility decline with age.(Walker and Herndon 2008) Thus, the reproductive span of chimpanzees relative to humans is significantly longer.
Animal models for menopause have examined the physiological, hormonal, and behavioral changes associated with reproductive aging. While no animal model mimics the complexity of reproductive aging in humans, preclinical studies have extensively used rodents and non-human primates to study natural and surgical menopause transitions.(Diaz Brinton 2012) For example, ovariectomized rodents have been used to study the effect of menopause and estrogen deficiency on neurodegenerative and cardiovascular diseases and overall mortality. (Acosta, Mayer et al. 2009, Diaz Brinton 2012) Moreover, the effect of hormone replacement therapy (HRT) on postmenopausal health among ovariectomized rodents demonstrated improved cardiovascular health, cognition, as well as anxiety and depressive behaviors. (Walf, Paris et al. 2009, Ramírez-Hernández, López-Sanchez et al. 2024)
Oxytocin (OT) is a hypothalamic neuropeptide hormone and neuromodulator involved in reproductive physiology and behavior (Burbach, Young et al. 2006, Jurek and Neumann 2018). Following synthesis in the hypothalamus, OT is transported to the posterior pituitary and released into circulation (Rigney, de Vries et al. 2022). Additionally, hypothalamic OT neurons project to several brain areas regulating emotionality and behavior, including the amygdala, hippocampus, hypothalamus, and nucleus accumbens (Froemke and Young 2021, Rigney, de Vries et al. 2022). Receptors for OT have been identified in the brain, reproductive, cardiovascular, and bone systems of animals and humans (Gutkowska and Jankowski 2008, Breuil, Fontas et al. 2015, Jurek and Neumann 2018). Furthermore, peripheral oxytocin production has been reported in the cardiovascular and reproductive systems, gastrointestinal tract, adrenal gland, endothelial cells, and skin (Nicholson, Swann et al. 1984, Burbach, Young et al. 2006, Gutkowska and Jankowski 2008, Deing, Roggenkamp et al. 2013). (Figure 1) Historically, OT has been known for its role in parturition and lactation (Burbach, Young et al. 2006) and, more recently, social behavior and attachment (Bosch and Young 2018, Froemke and Young 2021, Blumenthal and Young 2023). Robust evidence from preclinical models supports the functions of central OT in social and sexual behaviors (Burbach, Young et al. 2006, Jurek and Neumann 2018, Froemke and Young 2021), bone health (Breuil, Trojani et al. 2021), appetite regulation, energy intake, and metabolic function (Morton, Thatcher et al. 2012, Blevins, Graham et al. 2015). Current clinical data also suggest influences of OT on social cognition and mental health (Jurek and Neumann 2018, Rigney, de Vries et al. 2022), cardiovascular and reproductive functions (Gutkowska and Jankowski 2008), bone health (Breuil, Trojani et al. 2021), and sleep-wake behaviors (Lancel, Krömer et al. 2003, Raymond, Rehn et al. 2021).
Figure:
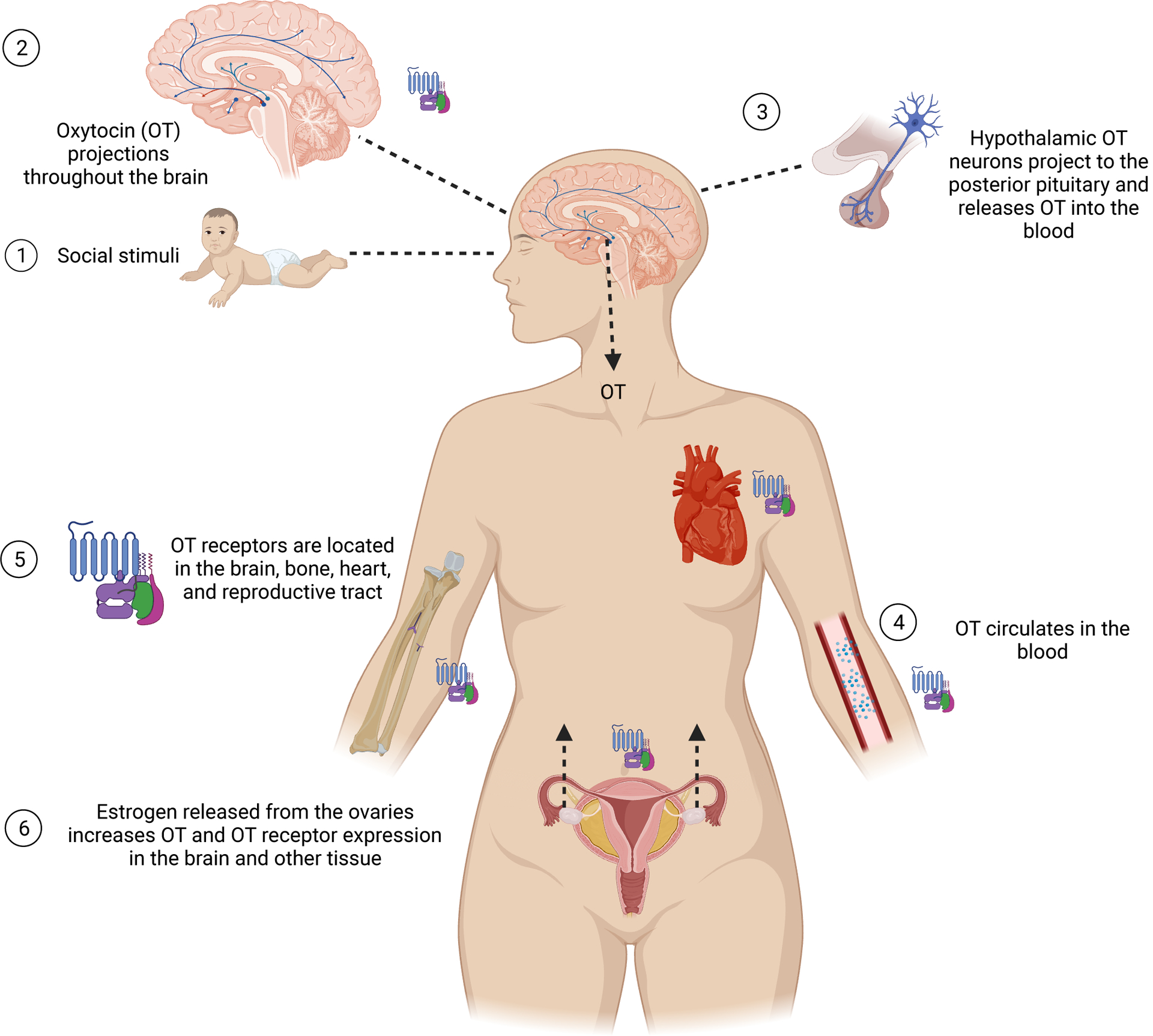
Central and peripheral release of oxytocin and its function in women’s body. (Created with BioRender.com)
1. Social experiences such interacting with loved ones causes oxytocin (OT) release in the hypothalamus.
2. OT in the brain regulates social behaviors via OT projections from the hypothalamus to emotional, reward and memory-related brain regions such as the amygdala, hippocampus, local projections to the hypothalamus, and nucleus accumbens.
3. Hypothalamic OT neurons project to the posterior pituitary gland and OT is released from the pituitary in a pulsatile manner into the blood.
4. OT circulates in the bloodstream and concentrations fluctuate due to pulsatile release and rapid degradation.
5. OT receptors are metabotropic G-protein coupled receptors (GPCRs). They are found throughout brain tissue, bone systems, the cardiovascular system, and the reproductive tract.
6. Estrogen secretion from the ovaries enhances OT activity via increasing OT and OT receptor expression throughout the brain and other tissue. Aging is accompanied by a decrease in estrogen levels and may lead to compromised OT signaling.
The role of OT in human health is plausible and promising (Carter, Kenkel et al. 2020, Ford and Young 2021, Liu, Yang et al. 2022, Rigney, de Vries et al. 2022). Reported links between OT, reproduction, cardiometabolic functions, sleep, bone, and mood. Discrepancies between animal and human studies stem in part from different approaches to assessment of OT function. Whereas investigators have directly manipulated the OT system in the central nervous system in animal experiments, in human studies, OT assessment is limited largely to quantifying endogenous OT concentrations in plasma, where OT concentrations are highly unstable (Mens, Witter et al. 1983, Leng and Sabatier 2016). Therefore, this review aimed to describe relationships between OT and the physiologic, physical, and psychological changes women endure during their reproductive aging years and provide experimental evidence from animal studies supporting those links. Specifically, we evaluated OT levels along the lifespan and during reproductive aging in women, as well as explored the role of OT on reproductive function, affective disorders and well-being, sleep health, bone health, and cardiometabolic health. Robust evidence from preclinical studies is contrasted with the lack of adequate data on associations between OT and health among humans, particularly in women. Despite the scarcity of reports on OT and health among women in general and in relation to their reproductive aging, in particular, these data suggest that OT may play a role in women’s postmenopausal health. Therefore, we hypothesize that changes in OT during the menopausal transition may contribute to menopause-related vulnerabilities.
LIMITATIONS IN ASSESSMENT
Despite the plausible and promising role of OT in human health and behavior, results from clinical studies are mixed and raise concerns that hamper confident synthesis of current findings. The inconsistency in findings across investigations of OT in human studies is likely attributable to heterogeneity in study designs and to major challenges in assessment of endogenous OT concentrations in biological samples, which several research groups have highlighted (Leng and Sabatier 2016, Tabak, Leng et al. 2023). First, the measurement of OT has been central or peripheral (plasma, urine, or saliva), which assesses different OT compartments with potentially different regulations. As OT stability varies across biological matrices, there is a disagreement on the best approach to measure OT reliably (Gnanadesikan, Hammock et al. 2022). For example, OT may vary in its clearance and integration rates in plasma, urine and saliva (AMICO, ULBRECHT et al. 1987). A systematic review and meta-analysis of human studies revealed only a low, though statistically significant, correlation between central and peripheral OT [r=0.29, 95% CI (0.14, 0.42)] (Valstad, Alvares et al. 2017). Yet, many human studies have measured OT in plasma, a less invasive method than central assessment but may not reflect brain OT activity. The pulsatile release of OT produces variability in OT levels over time, further complicated by its short half-life of <8 minutes in plasma (Leng and Sabatier 2016). Fluctuations in plasma OT complicate the timing of its collection and limit the internal validity of studies that rely on plasma OT. Additionally, there are several techniques for the measurement of OT, including immunoassays (radioimmunoassay, ELISAs), in which sample constituents may interfere with the quantification, and liquid chromatography-mass spectrometry (LC-MS) which is considered the gold standard. Finally, the processing of OT assays varies by commercial kits, as most require sample extraction prior to assay to isolate and concentrate OT molecules while removing other factors that interfere with the immunoassay. The practice of extracting or diluting OT samples to eliminate interference of matrix constituents is variable among the studies discussed above, contributing to variability and reliability of findings (Tabak, Leng et al. 2023).
OXYTOCIN LEVELS ALONG THE LIFESPAN
Preclinical Studies
The function of the OT system along the lifespan in animal studies has been infrequently examined (Audunsdottir and Quintana 2022), and reports on changes in the OT system with age are scarce. However, there is some evidence that in rodents OT function may decline with advanced age. Comparison of OT concentration assessed by OT-specific enzyme immunoassay among young (2–4 months) and old male mice (18–24 months) indicated a decline in plasma levels of OT with advanced age. Specifically, OT concentrations in older mice were 3-fold lower than younger male mice (Elabd, Cousin et al. 2014). Moreover, a reduction of brain OT receptors in 20-month-old, relative to 3-month-old rats, was observed in three brain regions: the caudate putamen, olfactory tubercle and ventromedial hypothalamic nucleus (Arsenijevic, Dreifuss et al. 1995). Similarly, age differences in OT function were also observed in an experiment that involved forced swimming among young and old male rats. This study found that during and after forced swimming, younger rats had a significant increase in intracerebral OT release within the hypothalamic paraventricular nucleus relative to older rats that had blunted stress response, despite having baseline OT levels similar to younger rats (Keck, Hatzinger et al. 2000). Moreover, evaluation of peripheral secretion of OT in response to forced swimming stress also suggested an increase in plasma OT levels in both groups of young and old rats, though OT response in older rats was attenuated and delayed relative to young rats. In contrast to these rodent data, assessment of OT concentrations in the cerebrospinal fluid (CSF) of free-range adult female rhesus macaques showed a positive correlation with age. Specifically, concentrations of CSF OT were higher in older females (15–26y) relative to younger females (age 7–15). However, more than half of the females were lactating a newborn, which could increase OT concentrations. Indeed, stratified analysis by lactating status suggested stronger positive correlations between CSF concentrations of OT and age in lactating females (r=0.68) and weak correlations with age among non-lactating females (r=0.11). These positive correlations are limited to parous, lactating females during their reproductive years and cannot be generalized to nulliparous females. As OT levels are expected to decline with age, it should be noted that the mean age of lactating and non-lactating females was 14.6±1.2 and 17.4±1.7, respectively, prior to the age at which menopause occurs in rhesus macaques, around 24–26 years. (Walker 1995). Among infant rhesus macaques aged 38–134 days, CSF OT concentrations were negatively correlated with age, but compared with adult females, infants had lower CSF OT concentrations (Parker, Hoffman et al. 2010). In short, the existing studies in animals have mixed findings, though most studies indicate a reduction in OT function with advanced age.
Clinical Studies
The function of OT throughout the lifespan of humans remains uncertain. Current human studies lack age diversity and utilize various approaches for the evaluation of OT that prevent confident conclusions. Assessment of OT receptor gene expression in the human brain has indicated an increase in both ends of the age continuum – early childhood and late adulthood – and a decrease in young adulthood (Rokicki, Kaufmann et al. 2022). Additionally, postmortem studies of human brain tissue demonstrated reduced (Calzà, Pozza et al. 1997), or unchanged (Wierda, Goudsmit et al. 1991) number and size of OT cells in the paraventricular nucleus of the hypothalamus with advanced age.
Reports on OT concentrations are inconsistent along the lifespan of women. A study with 55 healthy girls and women aged 10–45y with normal weight found an inverse relationship between age and plasma OT levels (Aulinas, Pulumo et al. 2019). Similarly, examination of plasma OT concentrations among 89 women in post-menopause found negative correlations between OT and age, r=−0.25. (Korkmaz, Deveci et al. 2023) In contrast, comparisons of plasma OT in healthy young (age 18–31y) and older (age 63–81y) women and men suggested higher levels with advanced age regardless of sex (Plasencia, Luedicke et al. 2019). However, both studies measured samples of unextracted plasma OT using immunoassay techniques (ELISA and enzyme immunoassay), an approach that can produce unreliable results due to interference with the assay (Tabak, Leng et al. 2023).
OXYTOCIN AND REPRODUCTIVE FUNCTION
Preclinical Studies
As a sex hormone, estrogen is essential for the development and regulation of the female reproductive system. The effects of estrogen on human sleep, cardiometabolic and bone health, and affective disorders have been frequently reported (Nilsson and Gustafsson 2002). The similarity in functions of estrogen and OT suggests an interactive relationship that warrants exploration. Moreover, there is significant overlap between OT neurons and estrogen receptors in the hypothalamus which further supports their potential interactions (Young, Wang et al. 1998, Patisaul, Scordalakes et al. 2003, Acevedo-Rodriguez, Mani et al. 2015). Indeed, both animal and human studies have reported relationships between OT and estrogen (Young, Wang et al. 1998, Patisaul, Scordalakes et al. 2003, Liu, Yang et al. 2022). There is robust data on the physiological functions of OT in pregnancy, during labor and parturition, and its impact on maternal behavior, mood, lactation and maternal-infant bonding in postpartum. (Grieb and Lonstein 2021) Recent studies have shown that expression of OT receptors in different brain regions is dynamic along reproductive stages and responds to changes in estrogen concentration levels. (Grieb and Lonstein 2021)
As women approach midlife, ovarian aging gives rise to hormonal fluctuations and a decline in estrogen levels that triggers physiological changes. Effects of estrogen deficiency on endogenous OT in serum and gene expression of OT in the hypothalamus of female rats demonstrated significantly lower levels in the ovariectomized group compared with control rats. Conversely, chronic estrogen administration resulted in an increase in hypothalamic OT synthesis among the ovariectomized rats (Tokui, Kawakita et al. 2021). Similarly, exposure to estrogen either through pregnancy, during an estrous cycle, or through subcutaneous injections for ovariectomized rats triggered significant increases in gene expression of OT receptors in the uterus of rats (Larcher, Neculcea et al. 1995). These data support the role of estrogen in the production, release, and function of OT through an increase in the expression of OT-producing genes and receptors in the hypothalamus (Acevedo-Rodriguez, Mani et al. 2015). The influence of estrogen on OT function and their concurrent decline during reproductive aging suggest that OT could potentially play a role in menopausal-related symptoms and health during the post-menopause years. Indeed, OT function has been linked to mood disorders, sleep disturbances, bone, and metabolic health, as will be discussed later.
Clinical studies
Along the menstrual cycle, fluctuations in estrogen suggest the potential for concurrent changes in OT function despite mixed findings. A systematic review and meta-analysis pooled evidence from 13 studies that examined endogenous OT concentrations in plasma along the menstrual cycle (Engel, Klusmann et al. 2019). Findings indicated that OT levels increase during the follicular phase, peak near ovulation, and gradually decrease toward the end of the cycle. A small study with 30 healthy and sexually active women examined OT levels among 20 women with regular ovulation and 10 on oral contraceptive pills. This study found that among women who ovulate, plasma OT levels were higher in the follicular and ovulation phases in comparison to the luteal phase, while OT levels were similar along the menstrual cycle of women on oral contraceptive pills (Salonia, Nappi et al. 2005). In contrast, a study of US graduate students (n=185) showed higher levels of OT among oral contraceptive users relative to non-users (Garforth, Degnbol et al. 2020). However, data on the duration of contraceptive use and their formulation were not available. Correlations between estrogen and OT were also evident in a study that compared OT levels among women athletes with amenorrhea and a control group of women with regular menstruation. This study found lower levels of plasma OT among the athletic group relative to women in the control group (Lawson, Ackerman et al. 2014). Whether correlation between estrogen deficiency and low OT levels could also co-occur during the menopause transition is unknown. A recent study examined plasma OT concentrations among 89 women who transitioned to menopause naturally (n=61) or surgically (n=28) and had similar age, body mass index and reproductive history, including menopause duration. This study found lower OT levels among women with surgical menopause compared to those who transitioned to menopause naturally, suggesting that abrupt rather than gradual decline in estrogen may be related to lower OT. (Korkmaz, Deveci et al. 2023) Transition to menopause, whether natural or surgical, will lead to decline in levels of sex hormones, however, HRT use in replacement of estrogen and progesterone may be protect long term health. Associations of OT, menopause and HRT have been examined in a study among 95 women aged 45–52y. (Boos, Stock et al. 1994) These women were divided into three groups: bilateral oophorectomy with HRT (n=30), bilateral oophorectomy without HRT (n=32), and hysterectomy without oophorectomy (n=33). Plasma assessment of OT showed that women in the bilateral oophorectomy who used HRT had higher OT levels than those in the other two groups, which had similar OT levels. These results indicate associations between exogeneous estrogen and OT even in the absence of ovarian function.
AFFECTIVE DISORDERS AND WELL-BEING
As women enter perimenopause, they become more vulnerable to mood disorders (El Khoudary, Greendale et al. 2019). The risk of depression, a common mood disorder, is two- to five-fold higher in perimenopause relative to late pre-menopause (El Khoudary, Greendale et al. 2019). Similarly, the risk of anxiety is increased in perimenopause and post-menopause, with prevalence estimates ranging between 10–52% during the menopausal transition (Bryant, Judd et al. 2012). The relationships between reproductive hormones and mood disorders have been noted in puberty, pregnancy, and postpartum. Thus, patterns of mood disorders along the menopause transition also suggest that hormonal shifts may drive the occurrence of depression and anxiety. However, psychological factors and life stressors could also induce poor mental health (Bryant, Judd et al. 2012).
Preclinical Studies
Experimental evidence on mood disorders post menopause have relied on ovariectomized animal models that were developed to mimic clinical phenotypes of depression and anxiety in women. (Zhang, Cao et al. 2019, Tongta, Daendee et al. 2023) For example, the impact of chronic mild stress and depressive-like behaviors have been studied in female ovariectomized rats. To assess depressive-like behaviors, these rats undergone open-field and forced swim tests at two and nine weeks after their ovariectomy. The effect of estrogen depletion on depressive-like behavior varied by duration, such that rats with longer duration of estrogen depletion, i.e., 9 weeks, had pronounced depressive-like behaviors, while rats with shorter duration of estrogen depletion did not show changes in behavior. These relationships were not affected by stress. (Khayum, Moraga-Amaro et al. 2020) Moreover, estrogen replacement therapy has been shown to have a protective effect on affective disorders. An experiment with ovariectomized female rats at midlife (14 months) and older age (19 months) assessed whether estrogen replacement therapy influences anxiety and depressive-like behaviors. These rats were divided into groups based on the timing of surgery and initiation of estrogen therapy, i.e., immediately following the ovariectomy or 5-month post-surgery. Additional group of rats did not receive estrogen therapy. At 20 months, rats that received estrogen replacement immediately post-surgery have demonstrated lower anxiety and depressive behaviors compared to rats with delayed initiation or no administration of estrogen replacement therapy.(Walf, Paris et al. 2009)
Experimental studies have examined the potential impact of OT on affective disorders using animal models with OT manipulations and assessment of anxiety and depressive-like behaviors as outcomes. The data are consistent with OT having anxiolytic and antidepressive-like effects. A review of experimental data indicated that stressful and anxiogenic events trigger rapid OT response among rodents, while administration of OT induces anxiolytic effects in both sexes and across the reproductive stages of females (Neumann and Slattery 2016). For example, exposure of female or male rats to psychosocial stress generated increased peripheral and central release of OT (Bosch, Krömer et al. 2004). The anxiolytic effect of OT was shown in another experiment with male rats subjected to mild stress through change in their living environment. Infusion of OT into the hypothalamic paraventricular nucleus of these rats had an anxiolytic effect that was mediated by local OT receptors (Blume, Bosch et al. 2008). The anxiolytic effect of OT was also observed in an experiment that administered exogenous OT to female and male mice with OT-deficiency. Following an induced stress, the treated mice had an attenuated stress response in comparison to controls (Yoshida, Takayanagi et al. 2009). The effect of centrally administered OT on anxiety-like behaviors have been examined in ovariectomized mice with or without HRT. This study found that OT reduced anxiety-like behaviors regardless of HRT use. (Nisbett, Gonzalez et al. 2023) These data imply the potential functional role of OT as a modulator for anxiety and a plausible candidate for therapeutic approaches for anxiety disorders in males and in females along their reproductive stages.
Relationships between the OT system and depression-like behavior have also been reported in experimental studies (McQuaid, McInnis et al. 2014). In addition to its anxiolytic effects, OT has been shown to reduce depressive-like behaviors. Specifically, rodents that were administered OT either acutely or daily in peripheral regions (subcutaneously or intra-peritoneally) or into the brain had improvement in their depressive symptoms in comparison to controls (Slattery and Neumann 2010, Yan, Wang et al. 2014). These animals demonstrated diminished depressive-like behaviors, i.e., increased social interaction, reduced anhedonia, and reduced behavioral despair (Yan, Wang et al. 2014).
Several animal studies found that intracerebroventricular administration of OT acted as an antidepressant (McQuaid, McInnis et al. 2014). Studies in monogamous prairie voles suggested a fundamental role of OT in promotion and maintenance of pair bonding (Walum and Young 2018). In this animal model, the loss of a bonded partner led to grieving-like depressive behavior, which was associated with reduced OT function, and which was alleviated by infusion of OT into the brain (Bosch and Young 2018). Despite these findings, the relationships between OT and depressive symptoms are complex, and their underlying mechanism is still unknown (Slattery and Neumann 2010).
Clinical Studies
The relationships between OT and human behaviors have been frequently examined, suggesting the positive influence of OT on social interactions, emotional attachment, and empathy (Campbell 2010). Most of the evidence on OT and emotional attachment has been reported in reproductive-age women after childbirth. In the postpartum period, new mothers experience significant physiological and psychological changes that increase their vulnerability to poor mental health(Thul, Corwin et al. 2020). Indeed, postpartum depression is prevalent among 17% of new mothers without pre-pregnancy depression and is influenced by biological, obstetrical, socioeconomic, and lifestyle factors(Shorey, Chee et al. 2018). Relationships between endogenous OT and postpartum depression appear mixed, though most reports suggest inverse associations, i.e., lower levels of OT in relation to a higher occurrence of postpartum depressive symptoms (Thul, Corwin et al. 2020). Inverse relationships between OT and depressive symptoms were also reported among pre- and post-menopausal women (Scantamburlo, Hansenne et al. 2007). Mechanistic pathways that link OT and mood involve other neurotransmitter systems. Experimental data have shown than OT interacts with serotonin, dopamine, and the hypothalamic-pituitary-adrenal axis, which may modulate anxiety and depressive-like behavior (Mottolese, Redouté et al. 2014). Although findings from animal and clinical studies are promising, randomized trials are necessary to establish the benefit of OT as a therapy for affective disorders and determine its optimal dosage, treatment protocols and long-term effects.
SLEEP HEALTH
Reproductive aging in women has been associated with changes in sleep patterns and sleep architecture that increase the burden of sleep disturbances. During midlife, poor sleep quality, insomnia symptoms, and particularly difficulty staying asleep, have been reported to affect 40–60% of women (Baker, Lampio et al. 2018). Moreover, the prevalence of obstructive sleep apnea, a common form of sleep-disordered breathing, is increased more than threefold in post-menopause in comparison to the premenopausal stage. This increase has been attributed to hormonal changes and weight gain during the menopausal transition (Young, Finn et al. 2003). Whether HRT is beneficial for sleep health is poorly understood. Some evidence suggests that women in peri- or post-menopause who take HRT tend to report better sleep quality and are less likely to experience sleep disordered breathing and nocturnal awakenings. (Pan, Wen et al. 2022, Haufe and Leeners 2023) The influence of estrogen on OT function and sleep, suggests that OT may be involved in sleep disturbances during reproductive aging.
Preclinical Studies
Associations between OT function and sleep-wake cycles have been hypothesized based on their adjacent neurocircuitry and observed links between OT, stress and stress-related neurocircuitry (Lancel, Krömer et al. 2003). Sleep and OT have been independently associated with mood and pain, although it is unknown whether OT and sleep jointly influence mood and pain.(Devarajan and Rusak 2004) Moreover, sleep and co-sleeping is known to promote feelings of intimacy, safety, and bonding that are strongly related to OT function (Eichhorn, Treede et al. 2018, Raymond, Rehn et al. 2021). Yet, despite their plausible relationships, investigations of sleep and OT are rare. (Raymond, Rehn et al. 2021)
As with many hormones, OT levels either in plasma or in the CSF follow a circadian rhythm pattern (Devarajan and Rusak 2004). Indeed, animal studies have demonstrated higher concentrations of plasma OT in the light phase, and lower levels during the dark phase (Devarajan and Rusak 2004). Much of the existing literature on OT and sleep has focused on intervention studies. A recent systematic review and meta-analysis examined the impact of OT intervention on sleep-wake cycles in 19 preclinical experiments and 11 human studies (Raymond, Rehn et al. 2021).
Findings from preclinical studies involving OT administrations were mixed. Continuous, 9-day intracerebroventricular administration of OT in adult male rats resulted in a longer wakefulness period during active phase in comparison to controls, 680 and 645 average minutes of wakefulness per day. (Arnauld, Bibene et al. 1989) Increased wakefulness among male rats was also shown with a 2-day administration of OT at light onset.(Lancel, Krömer et al. 2003) However, one study reported that a single OT intervention, 15 minutes prior to testing, promoted sleep time in prairie voles.(Mahalati, Okanoya et al. 1991) In summary, chronic manipulations of OT in rats through continuous administration, or release of OT from hypothalamic neural fibers activated by optogenetics have increased total wake time in 24-hour period. (Arnauld, Bibene et al. 1989, Mahoney, Kroeger et al. 2017) Similarly, acute manipulation of OT also increased wakefulness six hours after OT administration.(Lancel, Krömer et al. 2003)
Clinical Studies
Daily variation of OT levels among human participants – mainly men – observed a circadian rhythm of OT in plasma that peaked at 2am (Forsling, Montgomery et al. 1998). However, a study that examined whether salivary OT concentration changed along sleep stages in both women and men found no variation (Blagrove, Fouquet et al. 2012). Associations between OT and sleep health have been insufficiently studied, but current evidence suggest that OT supports sleep health. Assessment of salivary OT in cancer survivors found that low OT levels were associated to more self-reported sleep problems, such as insomnia symptoms daytime sleepiness, poor sleep quality, and insufficient sleep duration. (Lipschitz, Kuhn et al. 2015)
Intervention studies have shown that exogenous OT promoted wakefulness in animal studies while limited evidence implied that OT may have sleep promoting properties in humans (Raymond, Rehn et al. 2021). For example, acute nocturnal administration of OT to eight patients – women and men – with obstructive sleep apnea resulted in longer sleep time, increased subjective sleep satisfaction, and improved breathing during sleep (Jain, Marbach et al. 2017). Improved sleep was also reported in a double-blind, cross-over study with 10 healthy, young men who used a nasal OT spray three times per day for one month. Sleep was assessed before, one-week after, and at the end of the OT administration period.(Raymond, Rehn et al. 2021) Findings suggested that intranasal OT administration increased sleep efficiency, reduced sleep latency and increased the percent of time spent in rapid eye movement sleep. However, OT administration did not increase total sleep time. Similarly, a randomized controlled trial with 80 couples associated self-administration of intranasal OT with better sleep quality in both women and men, though, these relationships were more pronounced in women (Doerr, Klaus et al. 2022). As endogenous OT declines with age, concurrent with the increase in reported poor sleep, the limited data linking OT administration with better sleep suggest OT as a potential candidate for therapeutic approaches to improve sleep in aging populations.
BONE HEALTH
The aging process leads to deterioration in bone composition, structure, and function. This progressive age-related decline in bone health is associated with an increased osteoporosis risk for both women and men (Demontiero, Vidal et al. 2012). As women enter menopause, the risk of bone loss is increased and nearly 50% of women experience osteoporosis which is known to impair their mobility and function, induce pain, and degrade their quality of life (Gao and Zhao 2023).
Preclinical Studies
The presence of OT receptors in bone tissue – osteoblasts and osteoclasts – indicates the possible role of OT in regulation of bone remodeling and maintenance. Indeed, preclinical studies have suggested that OT positively impacts bone health by promotion of osteoclast genesis and bone remodeling, and inhibition of bone resorption, processes that protects the skeletal system. (Tamma, Colaianni et al. 2009) Moreover, both female and male mice with OT deficiency were profoundly vulnerable to osteoporosis (Tamma, Colaianni et al. 2009). However, while OT treatment reversed osteoporosis in ovariectomized mice, OT treatment in orchidectomized mice did not improve bone density. (Beranger, Djedaini et al. 2015) Experimental animal data have shown that intramuscular administration of OT influenced bone formation in rodents and reversed osteoporosis in ovariectomized mice (Elabd, Basillais et al. 2008). The effect of peripheral OT in the alveolar bone healing process after tooth extraction was examined in old, acyclic female rats divided into treatment and control groups that received intraperitoneal injections of OT or saline solution, respectively (Colli, Okamoto et al. 2012). Comparisons of the alveolar healing process indicated that OT-treated rats had higher markers of bone formation and decreased bone resorption during the alveolar healing process, attributing enhanced bone healing properties to peripheral OT. While mechanistic pathways between OT and bone health are mostly unknown, declines in estrogen and increases in follicle-stimulating hormone levels during the post-menopausal stage were linked to osteoporosis in ovariectomized mice (Rouach, Katzburg et al. 2011). Overall, these data suggest oxytocin affects bone cells and bone remodeling are not fully understood, these data suggest that OT plays a fundamental but differential role in skeletal health in both sexes.
Clinical Studies
Consistent with animal data, associations between OT and bone health in clinical studies suggest an influence of OT on bone health in aging women. In a study of 36 postmenopausal women aged 55–85y, those with osteoporosis compared to women with normal bone mineral density had 55% lower circulating OT concentrations (Elabd, Basillais et al. 2008). Similar associations were observed in a cohort of >1000 postmenopausal women with median age of 70.8y, suggesting increased plasma OT concentrations among women with higher bone mineral density, though plasma OT was unrelated to osteoporosis-related fractures (Breuil, Panaia-Ferrari et al. 2014). However, these associations may be sex-specific and only found in women, as no associations were apparent between OT and bone health (bone mineral density or bone fractures) in a cohort of 552 men whose median age was 64 years and interquartile age range was between 60–70 years.
(Breuil, Fontas et al. 2015) Sex-related discrepancies in these findings indicate the potential joint influence of OT and estrogen on bone health. Indeed, both intervention and epidemiologic studies have suggested HRT as a safe and an effective treatment for osteoporosis prevention in the postmenopausal years, even for women already affected by osteoporosis. (Stevenson 2023)
CARDIOMETABOLIC HEALTH
The menopausal transition is associated with physiological changes that promote abdominal weight gain, elevation in lipids and fasting glucose levels and increased blood pressure. These cardiometabolic changes have been established as determinants of type 2 diabetes and cardiovascular disease (Roa-Díaz, Raguindin et al. 2021). Several mechanisms that link menopause and cardiometabolic morbidity have been suggested, but the main pathway involves the decline in estrogen levels during the process of reproductive aging. This pathway is supported by studies that demonstrated a lower risk for cardiometabolic diseases among women who pursued HRT, though findings vary by type of therapy and timing of the therapy initiation (Roa-Díaz, Raguindin et al. 2021).
Preclinical Studies
Oxytocin has a beneficial influence on cardiometabolic health, including body weight, lipids, triglycerides, glucose, and insulin functions (McCormack, Blevins et al. 2019). Mechanistic pathways between OT, insulin and glucose are complex and poorly understood. However, the presence of OT receptors in pancreatic beta cells and adipose tissue suggests its role in regulation of insulin release and glucose metabolism.(Mohan, Khan et al. 2018, Buemann and Uvnäs-Moberg 2020) Indeed, evidence from animal studies have shown that administration of OT stimulates insulin release and leads to a reduction in blood glucose levels, which suggest its role in regulation of glucose function.(Scerbo and Gerdes 2017)
The relationships between OT, food intake, and excessive weight have been investigated among male mice. Comparisons of adiposity in male mice with and without OT receptors suggest that, despite a similar food intake, mice deficient in OT receptors as compared to those with OT receptors had excessive abdominal fat and obesity, and increased plasma triglycerides (Takayanagi, Kasahara et al. 2008). In another experimental study, OT was injected intraperitoneally for 12 days to 16 naturally peri-menopausal or post-menopausal female rats while saline was injected to female rat controls. Relative to controls, rats with daily OT administration had significantly lower body weight and visceral adiposity, lower levels of lipids, and reduced food intake, regardless of their menopause status (Erdenebayar, Kato et al. 2021). Similarly, OT was linked to reduced food intake and lower body weight among rats on low- or high-fat diet. Following a peripheral administration of OT to rats on a low-fat or high-fat diet, all rats reduced their body weight in a dose-dependent manner (Morton, Thatcher et al. 2012). These findings suggest that OT is involved in weight reduction through dietary pathways. Further evidence supporting the impact of OT on weight reduction has been shown in an experiment on male rats with high-fat diet-induced obesity that received centrally infused OT or saline. Rats in the OT group had lower weight gain relative to controls, despite similar food intake and meal patterns. Moreover, OT lowered the levels of triglycerides, but did not affect plasma leptin, glucose, or insulin levels (Deblon, Veyrat-Durebex et al. 2011). Overall, experimental studies with animal models have indicated that administered OT is protective against obesity (Blevins, Graham et al. 2015).
Clinical Studies
The relationships among OT, glucose and insulin have been examined in several small studies. Epidemiologic studies have also demonstrated relationships between OT and metabolic function highlighting OT as a marker of adiposity (Schorr, Marengi et al. 2017). A cross-sectional study of 540 older men (age range 50–85yrs) investigated serum OT levels in relation to metabolic syndrome (MetS). This study reported higher levels of serum OT among men with MetS as compared to men without MetS. Specifically, men with median OT level >0.74 pg/mL had two-fold increased odds of MetS relative to men with OT below the median (Szulc, Amri et al. 2016). Positive associations between plasma OT and cardiometabolic biomarkers have been also shown among a cohort of 721 adults (396 women and 325 men, mean age 47.7±15) (Weingarten, Scholz et al. 2019). This study reported elevated OT levels in adults with impaired glucose tolerance and a positive relationship between OT levels and BMI, waist-hip ratio, waist circumference and triglycerides, and plasma glucose and insulin. Further, adults with normal weight had significantly lower OT levels than those with overweight or obesity, 78.6 pg/mL, 98.5 pg/mL and 106.4 pg/mL, respectively (Weingarten, Scholz et al. 2019).
Women-centered investigations of OT levels and adiposity are limited. A cross-sectional study examined serum OT levels and adiposity among 59 reproductive-age women: 24 healthy controls, 19 women affected by obesity with regular menses, and 16 women with both anorexia nervosa (AN) and amenorrhea. Serum OT was measured in 20-min intervals from 8pm to 8am and pooled into mean levels while adiposity was evaluated by dual x-ray absorptiometry. This study reported higher mean overnight OT levels among women with obesity, but lower mean levels in women with AN relative to healthy controls. Positive relationships were also observed between OT levels and BMI, fat mass and lean mass, independent of weight (Schorr, Marengi et al. 2017). Conversely, inverse associations have been observed between plasma OT and adiposity in a study with 151 reproductive-age women affected by obesity and 160 women with normal weight. This study observed lower levels of fasting plasma OT among women with obesity relative to normal weight controls, suggesting OT deficiency in women with excessive weight (Fu-Man, Hong-Yu et al. 2019). Further findings indicated inverse relationships between plasma OT and HOMA-IR, LDL, and total cholesterol, but positive associations with HDL and null with fasting blood glucose, fasting insulin, or triglycerides. Similar findings were also observed in as cross-sectional study that examined associations between plasma OT levels, adiposity, and menopause status among 109 women of which 56 were affected by obesity and 53 had normal body weight. Among these women, 54 were in post-menopause. This study reported highest OT levels among women in pre-menopause with normal body weight, while the lowest levels of OT were found among women in post-menopause who are affected by obesity.(Maestrini, Mele et al. 2018) Specifically, significantly lower OT levels were observed among women in post-menopause than those in pre-menopause. Among women with obesity, OT levels were 3.5-fold lower than those with normal body weight. These data indicate that OT may be associated with ovarian and metabolic function. Associations between serum OT and cardiometabolic biomarkers were also examined among 176 adults (65% women), of which 88 were newly diagnosed with type 2 diabetes and 88 had normal glucose tolerance (Qian, Zhu et al. 2014). Adults with diabetes had significantly lower OT levels in comparison to those in the control group. Body mass index was inversely associated with OT levels, such that adults with obesity had lower OT levels than controls with normal weight.
Although observational data have been mixed, findings from intervention studies in humans have aligned with evidence from animal experiments that demonstrated the effect of administered OT on reduced caloric intake and body weight. A double-blind randomized controlled trial enrolled 21 older adults, age >60 years (71% women) with sarcopenic obesity who were randomly assigned 8-week intranasal OT or placebo. While no change in BMI emerged among adults in the intranasal OT group, they had a decrease in their fat mass and an increase in whole-body lean mass compared with those in the placebo group (Espinoza, Lee et al. 2021). An intervention crossover study examined the effect of exogenous OT on glucose homeostasis among 29 healthy, normal weight, young men (mean age 25y±0.8y). (Klement, Ott et al. 2017) Following an overnight fast, these men were administered intranasal OT or placebo and underwent an oral glucose tolerance test. In response to the glucose challenge, intranasal OT attenuated the glucose peak while increasing an early insulin secretion. However, an intervention study with similar design among men affected by obesity demonstrated no regulatory role of exogenous OT in glucose metabolism. (Brede, Fehr et al. 2019) These findings indicate that the ability of OT to regulate glucose metabolism and contribute to cardiometabolic health could depend on body weight.
In summary, few studies have examined links between OT and human adiposity and while findings from preclinical and clinical studies seem inconsistent, preclinical and intervention studies provide evidence that OT may influence cardiometabolic health through several mechanistic pathways, including reduced food intake, increased lipolysis, reduced cortisol, and improved glucose homeostasis (McCormack, Blevins et al. 2019). Discrepancies within human studies could be attributed to variability across study designs, OT assessment protocols, characteristics of study participants (sex, age, menopause status) and adjustment for confounding factors.
SUMMARY AND FUTURE DIRECTIONS
Oxytocin is a unique hormone, neuromodulator, and potential biomarker with growing evidence from animal and clinical studies for its influence on reproductive, sleep, and metabolic functions.
These reports provide a rationale for the potential role of OT in human health and behavior over the lifespan, despite mixed findings from clinical studies. Most of these studies used methods for assessment of endogenous peripheral OT with low reliability and validity, which challenge the interpretation of findings. In comparison to radioimmunoassay or ELISA methods, LC-MS is considered as the gold standard for OT assessment but is costly and complex.(Tabak, Leng et al. 2023) However, assessment of OT in plasma using either radioimmunoassay or LC-MS with an extraction procedures has generated similar, high-sensitive results. (Franke, Li et al. 2019) The presence of OT in saliva and urine provides alternate opportunities for non-invasive assessments. Although salivary OT has as low a validity as plasma OT (Martins, Gabay et al. 2020), validation studies of urine OT assays have shown promise and offer new opportunities in OT research (Schaebs, Wirobski et al. 2021). Furthermore, OT concentrations in urine are thought to reflect the integration of fluctuating plasma OT over time, reducing the variability in plasma OT that arises from pulsatile OT release and rapid clearance from plasma. Human OT can be assessed in urine, saliva, plasma, or CSF involving radioimmunoassay, ELISA, or LC-MS. While each assessment approach has unique advantages, both radioimmunoassay and LC-MS both offer high sensitivity.(Tabak, Leng et al. 2023) Thus, selection of the optimal assessment technique depends on the research question, study design, availability of resources and ethical practices.
We believe that addressing the limitations in assessing peripheral OT in humans is crucial to enhance our understanding of its function in physical health and well-being. Thus, future studies to examine the relationship between OT concentration and reproductive aging patterns should use the most reliable assays techniques, e.g. the gold-standard LC-MS, from stable and informative bio-samples (e.g., urine), using standardized procedures to measure OT function. Urinary OT assessment with CSF provides high-sensitivity, cumulative information on OT values, however, proper extraction of OT in plasma using radioimmunoassay techniques are also highly sensitive and will reflect pulsatile OT levels.
As we seek strategies for successful aging and longevity, the potential of OT to benefit mood, sleep, bone, and cardiometabolic health warrants further investigation in both observational and interventional studies. Experimental preclinical studies using animal models of human menopause, both natural and surgical, are necessary to determine the influence of OT manipulation (reduction or promotion) on physical and mental health. Large epidemiologic, longitudinal studies focusing on women along their reproductive lifespan (in pre-, peri-, and post-menopause) are needed to elucidate associations between OT and midlife health, independent of chronological aging. Finally, if OT function is strongly associated with midlife health, randomized controlled trials are necessary to evaluate whether an intervention with exogenous OT would impact women’s midlife health and well-being.
Funding:
Dr. Dunietz reports funding from the National Heart, Lung, and Blood Institute (K01 HL144914) and from the National Institute on Aging (R01AG074342).
Dr. O’Brien reports receiving funding from the National Heart, Lung, and Blood Institute (R61/33 HL151952), National Institute of Child Health and Human Development (SBIR R43HD111096 , STTR R41HD114317), National Institute of Mental Health (R01MH121531, R34MH130562), and from the Star Legacy Foundation and honoraria for attending National Institutes of Health study section meetings; advisory board role at the Star Legacy Foundation; and receiving equipment from Itamar, Ltd., Smart Human Dynamics Inc., and Arcascope Inc.
Dr. Young’s contribution was supported by NIH grants P50MH100023 to Dr. Young and P51OD11132 to Emory National Primate Research Center.
Conflict of interest:
Dr. Chervin reports research funded by the NIH; royalties as an author and editor for UpToDate; consulting for Eli Lilly & Company through a contract with the University of Michigan; and service on the board of the International Pediatric Sleep Association and advisory board for the non-profit Pajama Program.
Footnotes
In Memoriam: This review is dedicated to the memory of Dr. Larry J. Young, a world-renowned neuroendocrinologist, a devoted mentor, and an international human rights activist, for his invaluable guidance and contributions.
REFERENCES
- Acevedo-Rodriguez A, Mani SK and Handa RJ (2015). “Oxytocin and Estrogen Receptor β in the Brain: An Overview.” Front Endocrinol (Lausanne) 6: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK and Bimonte-Nelson HA (2009). “Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system.” Endocrinology 150(9): 4248–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMICO JA, ULBRECHT JS and ROBINSON AG (1987). “Clearance Studies of Oxytocin in Humans Using Radioimmunoassay Measurements of the Hormone in Plasma and Urine*.” The Journal of Clinical Endocrinology & Metabolism 64(2): 340–345. [DOI] [PubMed] [Google Scholar]
- Arnauld E, Bibene V, Meynard J, Rodriguez F and Vincent JD (1989). “Effects of chronic icv infusion of vasopressin on sleep-waking cycle of rats.” Am J Physiol 256(3 Pt 2): R674–684. [DOI] [PubMed] [Google Scholar]
- Arsenijevic Y, Dreifuss JJ, Vallet P, Marguerat A and Tribollet E (1995). “Reduced binding of oxytocin in the rat brain during aging.” Brain Res 698(1–2): 275–279. [DOI] [PubMed] [Google Scholar]
- Audunsdottir K and Quintana DS (2022). “Oxytocin’s dynamic role across the lifespan.” Aging Brain 2: 100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulinas A, Pulumo RL, Asanza E, Mancuso CJ, Slattery M, Tolley C, Plessow F, Thomas JJ, Eddy KT, Miller KK, Klibanski A, Misra M and Lawson EA (2019). “Endogenous Oxytocin Levels in Relation to Food Intake, Menstrual Phase, and Age in Females.” J Clin Endocrinol Metab 104(4): 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Lampio L, Saaresranta T and Polo-Kantola P (2018). “Sleep and Sleep Disorders in the Menopausal Transition.” Sleep Med Clin 13(3): 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranger GE, Djedaini M, Battaglia S, Roux CH, Scheideler M, Heymann D, Amri EZ and Pisani DF (2015). “Oxytocin reverses osteoporosis in a sex-dependent manner.” Front Endocrinol (Lausanne) 6: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove M, Fouquet NC, Baird AL, Pace-Schott EF, Davies AC, Neuschaffer JL, Henley-Einion JA, Weidemann CT, Thome J, McNamara P and Turnbull OH (2012). “Association of salivary-assessed oxytocin and cortisol levels with time of night and sleep stage.” J Neural Transm (Vienna) 119(10): 1223–1232. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG and Havel PJ (2015). “Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys.” Am J Physiol Regul Integr Comp Physiol 308(5): R431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M and Neumann ID (2008). “Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus.” Eur J Neurosci 27(8): 1947–1956. [DOI] [PubMed] [Google Scholar]
- Blumenthal SA and Young LJ (2023). “The Neurobiology of Love and Pair Bonding from Human and Animal Perspectives.” Biology (Basel) 12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos JN, Stock S and Schoultz B. v. (1994). “Effects of oophorectomy and estrogen treatment on basal levels and 24-h profiles of oxytocin.” Gynecological Endocrinology 8(2): 127–132. [DOI] [PubMed] [Google Scholar]
- Bosch O, Krömer S, Brunton P and Neumann I (2004). “Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence.” Neuroscience 124(2): 439–448. [DOI] [PubMed] [Google Scholar]
- Bosch OJ and Young LJ (2018). “Oxytocin and Social Relationships: From Attachment to Bond Disruption.” Curr Top Behav Neurosci 35: 97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brede S, Fehr S, Dalla-Man C, Cobelli C, Lehnert H, Hallschmid M and Klement J (2019). “Intranasal oxytocin fails to acutely improve glucose metabolism in obese men.” Diabetes Obes Metab 21(2): 424–428. [DOI] [PubMed] [Google Scholar]
- Breuil V, Fontas E, Chapurlat R, Panaia-Ferrari P, Yahia HB, Faure S, Euller-Ziegler L, Amri EZ and Szulc P (2015). “Oxytocin and bone status in men: analysis of the MINOS cohort.” Osteoporosis International 26(12): 2877–2882. [DOI] [PubMed] [Google Scholar]
- Breuil V, Panaia-Ferrari P, Fontas E, Roux C, Kolta S, Eastell R, Ben Yahia H, Faure S, Gossiel F, Benhamou CL, Euller-Ziegler L and Amri EZ (2014). “Oxytocin, a new determinant of bone mineral density in post-menopausal women: analysis of the OPUS cohort.” J Clin Endocrinol Metab 99(4): E634–641. [DOI] [PubMed] [Google Scholar]
- Breuil V, Trojani MC and Ez-Zoubir A (2021). “Oxytocin and Bone: Review and Perspectives.” Int J Mol Sci 22(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C, Judd FK and Hickey M (2012). “Anxiety during the menopausal transition: A systematic review.” Journal of Affective Disorders 139(2): 141–148. [DOI] [PubMed] [Google Scholar]
- Buemann B and Uvnäs-Moberg K (2020). “Oxytocin may have a therapeutical potential against cardiovascular disease. Possible pharmaceutical and behavioral approaches.” Medical Hypotheses 138: 109597. [DOI] [PubMed] [Google Scholar]
- Burbach J, Young LJ and Russell J (2006). “Oxytocin: synthesis, secretion, and reproductive functions.” Knobil and Neill’s physiology of reproduction 2: 3055–3128. [Google Scholar]
- Calzà L, Pozza M, Coraddu F, Farci G and Giardino L (1997). “Hormonal influences on brain ageing quality: focus on corticotropin releasing hormone-, vasopressin- and oxytocin-immunoreactive neurones in the human brain.” J Neural Transm (Vienna) 104(10): 1095–1100. [DOI] [PubMed] [Google Scholar]
- Campbell A (2010). “Oxytocin and Human Social Behavior.” 14(3): 281–295. [DOI] [PubMed] [Google Scholar]
- Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, Ferris CF, Nazarloo HP, Porges SW, Davis JM, Connelly JJ and Kingsbury MA (2020). “Is Oxytocin “Nature’s Medicine”?” Pharmacol Rev 72(4): 829–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colli VC, Okamoto R, Spritzer PM and Dornelles RC (2012). “Oxytocin promotes bone formation during the alveolar healing process in old acyclic female rats.” Arch Oral Biol 57(9): 1290–1297. [DOI] [PubMed] [Google Scholar]
- Cruz G, Fernandois D and Paredes AH (2017). “Ovarian function and reproductive senescence in the rat: role of ovarian sympathetic innervation.” Reproduction 153(2): R59–r68. [DOI] [PubMed] [Google Scholar]
- Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, Piscitelli F, Legros JJ, Geenen V, Foti M, Wahli W, Di Marzo V and Rohner-Jeanrenaud F (2011). “Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats.” PLoS One 6(9): e25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deing V, Roggenkamp D, Kühnl J, Gruschka A, Stäb F, Wenck H, Bürkle A and Neufang G (2013). “Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis.” Experimental Dermatology 22(6): 399–405. [DOI] [PubMed] [Google Scholar]
- Demontiero O, Vidal C and Duque G (2012). “Aging and bone loss: new insights for the clinician.” Ther Adv Musculoskelet Dis 4(2): 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan K and Rusak B (2004). “Oxytocin levels in the plasma and cerebrospinal fluid of male rats: effects of circadian phase, light and stress.” Neuroscience Letters 367(2): 144–147. [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R (2012). “Minireview: translational animal models of human menopause: challenges and emerging opportunities.” Endocrinology 153(8): 3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr JM, Klaus K, Troxel W, Nater UM, Bodenmann G, Heinrichs M, Ehlert U and Ditzen B (2022). “The effect of intranasal oxytocin on the association between couple interaction and sleep - a placebo-controlled study.” Psychosom Med. [DOI] [PubMed] [Google Scholar]
- Eichhorn N, Treede RD and Schuh-Hofer S (2018). “The Role of Sex in Sleep Deprivation Related Changes of Nociception and Conditioned Pain Modulation.” Neuroscience 387: 191–200. [DOI] [PubMed] [Google Scholar]
- El Khoudary SR, Greendale G, Crawford SL, Avis NE, Brooks MM, Thurston RC, Karvonen-Gutierrez C, Waetjen LE and Matthews K (2019). “The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN).” Menopause 26(10): 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, Zaragosi LE, Massiéra F, Lemichez E, Trajanoski Z, Carle G, Euller-Ziegler L, Ailhaud G, Benhamou CL, Dani C and Amri EZ (2008). “Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis.” Stem Cells 26(9): 2399–2407. [DOI] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP and Conboy IM (2014). “Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration.” Nat Commun 5: 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Klusmann H, Ditzen B, Knaevelsrud C and Schumacher S (2019). “Menstrual cycle-related fluctuations in oxytocin concentrations: A systematic review and meta-analysis.” Front Neuroendocrinol 52: 144–155. [DOI] [PubMed] [Google Scholar]
- Erdenebayar O, Kato T, Kawakita T, Kasai K, Kadota Y, Yoshida K, Iwasa T and Irahara M (2021). “Effects of peripheral oxytocin administration on body weight, food intake, adipocytes, and biochemical parameters in peri- and postmenopausal female rats.” Endocr J 68(1): 7–16. [DOI] [PubMed] [Google Scholar]
- Espinoza SE, Lee JL, Wang CP, Ganapathy V, MacCarthy D, Pascucci C, Musi N and Volpi E (2021). “Intranasal Oxytocin Improves Lean Muscle Mass and Lowers LDL Cholesterol in Older Adults with Sarcopenic Obesity: A Pilot Randomized Controlled Trial.” J Am Med Dir Assoc 22(9): 1877–1882.e1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CL and Young LJ (2021). “Harnessing the healing power of love.” Trends Mol Med 27(9): 833–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsling ML, Montgomery H, Halpin D, Windle RJ and Treacher DF (1998). “Daily patterns of secretion of neurohypophysial hormones in man: effect of age.” Exp Physiol 83(3): 409–418. [DOI] [PubMed] [Google Scholar]
- Franke AA, Li X, Menden A, Lee MR and Lai JF (2019). “Oxytocin analysis from human serum, urine, and saliva by orbitrap liquid chromatography-mass spectrometry.” Drug Test Anal 11(1): 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC and Young LJ (2021). “Oxytocin, Neural Plasticity, and Social Behavior.” Annu Rev Neurosci 44: 359–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu-Man D, Hong-Yu K, Bin-Hong D, Da-Na L and Xin-Yang Y (2019). “Associations of oxytocin with metabolic parameters in obese women of childbearing age.” Endokrynol Pol 70(5): 417–422. [DOI] [PubMed] [Google Scholar]
- Gao S and Zhao Y (2023). “Quality of life in postmenopausal women with osteoporosis: a systematic review and meta-analysis.” Quality of Life Research 32(6): 1551–1565. [DOI] [PubMed] [Google Scholar]
- Garforth B, Degnbol H, Terris ET, Zak PJ and Winterdahl M (2020). “Elevated plasma oxytocin levels and higher satisfaction with life in young oral contraceptive users.” Sci Rep 10(1): 8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanadesikan GE, Hammock EAD, Tecot SR, Lewis RJ, Hart R, Carter CS and MacLean EL (2022). “What are oxytocin assays measuring? Epitope mapping, metabolites, and comparisons of wildtype & knockout mouse urine.” Psychoneuroendocrinology 143: 105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb ZA and Lonstein JS (2021). “Oxytocin receptor expression in the midbrain dorsal raphe is dynamic across female reproduction in rats.” J Neuroendocrinol 33(2): e12926. [DOI] [PubMed] [Google Scholar]
- Gutkowska J and Jankowski M (2008). “Oxytocin revisited: It is also a cardiovascular hormone.” Journal of the American Society of Hypertension 2(5): 318–325. [DOI] [PubMed] [Google Scholar]
- Haufe A and Leeners B (2023). “Sleep Disturbances Across a Woman’s Lifespan: What Is the Role of Reproductive Hormones?” Journal of the Endocrine Society 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V, Marbach J, Kimbro S, Andrade DC, Jain A, Capozzi E, Mele K, Del Rio R, Kay MW and Mendelowitz D (2017). “Benefits of oxytocin administration in obstructive sleep apnea.” Am J Physiol Lung Cell Mol Physiol 313(5): L825–l833. [DOI] [PubMed] [Google Scholar]
- Jurek B and Neumann ID (2018). “The Oxytocin Receptor: From Intracellular Signaling to Behavior.” Physiol Rev 98(3): 1805–1908. [DOI] [PubMed] [Google Scholar]
- Keck ME, Hatzinger M, Wotjak CT, Landgraf R, Holsboer F and Neumann ID (2000). “Ageing alters intrahypothalamic release patterns of vasopressin and oxytocin in rats.” Eur J Neurosci 12(4): 1487–1494. [DOI] [PubMed] [Google Scholar]
- Khayum MA, Moraga-Amaro R, Buwalda B, Koole M, den Boer JA, Dierckx R, Doorduin J and de Vries EFJ (2020). “Ovariectomy-induced depressive-like behavior and brain glucose metabolism changes in female rats are not affected by chronic mild stress.” Psychoneuroendocrinology 115: 104610. [DOI] [PubMed] [Google Scholar]
- Klement J, Ott V, Rapp K, Brede S, Piccinini F, Cobelli C, Lehnert H and Hallschmid M (2017). “Oxytocin improves β-cell responsivity and glucose tolerance in healthy men.” Diabetes 66(2): 264–271. [DOI] [PubMed] [Google Scholar]
- Korkmaz H, Deveci CD, Üstün Y and Pehlivanoğlu B (2023). “Comparison of plasma oxytocin level in women with natural and surgical menopause.” Endocrine 82(1): 209–214. [DOI] [PubMed] [Google Scholar]
- Lancel M, Krömer S and Neumann ID (2003). “Intracerebral oxytocin modulates sleep-wake behaviour in male rats.” Regul Pept 114(2–3): 145–152. [DOI] [PubMed] [Google Scholar]
- Lancel M, Krömer S and Neumann ID (2003). “Intracerebral oxytocin modulates sleep–wake behaviour in male rats.” Regulatory Peptides 114(2): 145–152. [DOI] [PubMed] [Google Scholar]
- Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C and Zingg HH (1995). “Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment.” Endocrinology 136(12): 5350–5356. [DOI] [PubMed] [Google Scholar]
- Lawson EA, Ackerman KE, Slattery M, Marengi DA, Clarke H and Misra M (2014). “Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes.” J Clin Endocrinol Metab 99(5): E881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître J-F, Ronget V and Gaillard J-M (2020). “Female reproductive senescence across mammals: A high diversity of patterns modulated by life history and mating traits.” Mechanisms of Ageing and Development 192: 111377. [DOI] [PubMed] [Google Scholar]
- Leng G and Sabatier N (2016). “Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers.” J Neuroendocrinol 28(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschitz DL, Kuhn R, Kinney AY, Grewen K, Donaldson GW and Nakamura Y (2015). “An Exploratory Study of the Effects of Mind–Body Interventions Targeting Sleep on Salivary Oxytocin Levels in Cancer Survivors.” Integrative Cancer Therapies 14(4): 366–380. [DOI] [PubMed] [Google Scholar]
- Liu N, Yang H, Han L and Ma M (2022). “Oxytocin in Women’s Health and Disease.” Front Endocrinol (Lausanne) 13: 786271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrini S, Mele C, Mai S, Vietti R, Di Blasio A, Castello L, Surico D, Aimaretti G, Scacchi M and Marzullo P (2018). “Plasma Oxytocin Concentration in Pre- and Postmenopausal Women: Its Relationship with Obesity, Body Composition and Metabolic Variables.” Obes Facts 11(5): 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalati K, Okanoya K, Witt DM and Carter CS (1991). “Oxytocin inhibits male sexual behavior in prairie voles.” Pharmacol Biochem Behav 39(1): 219–222. [DOI] [PubMed] [Google Scholar]
- Mahoney C, Kroeger D, Grinevich V and Scammell T (2017). “0134 Oxytocin fibers in the lateral hypothalamus promote arousal in a mouse model of PWS.” Sleep 40: A50. [Google Scholar]
- Maki PM and Thurston RC (2020). “Menopause and Brain Health: Hormonal Changes Are Only Part of the Story.” Front Neurol 11: 562275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D, Gabay AS, Mehta M and Paloyelis Y (2020). “Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans.” Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack SE, Blevins JE and Lawson EA (2019). “Metabolic Effects of Oxytocin.” Endocrine Reviews 41(2): 121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Abizaid A and Anisman H (2014). “Making room for oxytocin in understanding depression.” Neuroscience & Biobehavioral Reviews 45: 305–322. [DOI] [PubMed] [Google Scholar]
- Mens WBJ, Witter A and Van Wimersma Greidanus TB (1983). “Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): Half-times of disappearance of these neuropeptides from CSF.” Brain Research 262(1): 143–149. [DOI] [PubMed] [Google Scholar]
- Mohan S, Khan D, Moffett RC, Irwin N and Flatt PR (2018). “Oxytocin is present in islets and plays a role in beta-cell function and survival.” Peptides 100: 260–268. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW and Blevins JE (2012). “Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats.” Am J Physiol Endocrinol Metab 302(1): E134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottolese R, Redouté J, Costes N, Le Bars D and Sirigu A (2014). “Switching brain serotonin with oxytocin.” Proceedings of the National Academy of Sciences 111(23): 8637–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID and Slattery DA (2016). “Oxytocin in General Anxiety and Social Fear: A Translational Approach.” Biol Psychiatry 79(3): 213–221. [DOI] [PubMed] [Google Scholar]
- Nicholson HD, Swann RW, Burford GD, Wathes DC, Porter DG and Pickering BT (1984). “Identification of oxytocin and vasopressin in the testis and in adrenal tissue.” Regul Pept 8(2): 141–146. [DOI] [PubMed] [Google Scholar]
- Nilsson S and Gustafsson J-Å (2002). “Biological role of estrogen and estrogen receptors.” Critical reviews in biochemistry and molecular biology 37(1): 1–28. [DOI] [PubMed] [Google Scholar]
- Nisbett KE, Gonzalez LA, Teruel M, Carter CS, Vendruscolo LF, Ragozzino ME and Koob GF (2023). “Sex and hormonal status influence the anxiolytic-like effect of oxytocin in mice.” Neurobiol Stress 26: 100567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer C, Tatar M and Collins A (1998). “Reproductive cessation in female mammals.” Nature 392(6678): 807–811. [DOI] [PubMed] [Google Scholar]
- Pan Z, Wen S, Qiao X, Yang M, Shen X and Xu L (2022). “Different regimens of menopausal hormone therapy for improving sleep quality: a systematic review and meta-analysis.” Menopause 29(5): 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Hoffman CL, Hyde SA, Cummings CS and Maestripieri D (2010). “Effects of age on cerebrospinal fluid oxytocin levels in free-ranging adult female and infant rhesus macaques.” Behav Neurosci 124(3): 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Scordalakes EM, Young LJ and Rissman EF (2003). “Oxytocin, but not oxytocin receptor, is rRegulated by oestrogen receptor beta in the female mouse hypothalamus.” J Neuroendocrinol 15(8): 787–793. [DOI] [PubMed] [Google Scholar]
- Plasencia G, Luedicke JM, Nazarloo HP, Carter CS and Ebner NC (2019). “Plasma oxytocin and vasopressin levels in young and older men and women: Functional relationships with attachment and cognition.” Psychoneuroendocrinology 110: 104419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Zhu T, Tang B, Yu S, Hu H, Sun W, Pan R, Wang J, Wang D, Yang L, Mao C, Zhou L and Yuan G (2014). “Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients.” J Clin Endocrinol Metab 99(12): 4683–4689. [DOI] [PubMed] [Google Scholar]
- Ramírez-Hernández D, López-Sanchez P, Lezama-Martínez D, Pérez-García E, Montoya-Hernández MFS, Aranda-Fraustro A and Flores-Monroy J (2024). “Early Estrogen Replacement Therapy Attenuates Cardiac Dysfunction Caused by Aging and Ovariectomy in Female Wistar Rats.” Front Biosci (Landmark Ed) 29(1): 46. [DOI] [PubMed] [Google Scholar]
- Raymond JS, Rehn S, Hoyos CM and Bowen MT (2021). “The influence of oxytocin-based interventions on sleep-wake and sleep-related behaviour and neurobiology: A systematic review of preclinical and clinical studies.” Neurosci Biobehav Rev 131: 1005–1026. [DOI] [PubMed] [Google Scholar]
- Rigney N, de Vries GJ, Petrulis A and Young LJ (2022). “Oxytocin, Vasopressin, and Social Behavior: From Neural Circuits to Clinical Opportunities.” Endocrinology 163(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa-Díaz ZM, Raguindin PF, Bano A, Laine JE, Muka T and Glisic M (2021). “Menopause and cardiometabolic diseases: What we (don’t) know and why it matters.” Maturitas 152: 48–56. [DOI] [PubMed] [Google Scholar]
- Rokicki J, Kaufmann T, De Lange A-MG, van der Meer D, Bahrami S, Sartorius AM, Haukvik UK, Steen NE, Schwarz E and Stein DJ (2022). “Oxytocin receptor expression patterns in the human brain across development.” Neuropsychopharmacology 47(8): 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach V, Katzburg S, Koch Y, Stern N and Somjen D (2011). “Bone loss in ovariectomized rats: dominant role for estrogen but apparently not for FSH.” J Cell Biochem 112(1): 128–137. [DOI] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P and Montorsi F (2005). “Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women.” Horm Behav 47(2): 164–169. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, Ansseau M and Legros JJ (2007). “Plasma oxytocin levels and anxiety in patients with major depression.” Psychoneuroendocrinology 32(4): 407–410. [DOI] [PubMed] [Google Scholar]
- Scerbo MJ and Gerdes JM (2017). “Bonding with β-cells—A role for oxytocin in glucose handling.” Diabetes 66(2): 256–257. [DOI] [PubMed] [Google Scholar]
- Schaebs FS, Wirobski G, Marshall-Pescini S, Range F and Deschner T (2021). “Validation of a commercial enzyme immunoassay to assess urinary oxytocin in humans.” Endocr Connect 10(3): 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr M, Marengi DA, Pulumo RL, Yu E, Eddy KT, Klibanski A, Miller KK and Lawson EA (2017). “Oxytocin and Its Relationship to Body Composition, Bone Mineral Density, and Hip Geometry Across the Weight Spectrum.” J Clin Endocrinol Metab 102(8): 2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P (2013). “The Laboratory Rat: Relating Its Age With Human’s.” Int J Prev Med 4(6): 624–630. [PMC free article] [PubMed] [Google Scholar]
- Shorey S, Chee CYI, Ng ED, Chan YH, San Tam WW and Chong YS (2018). “Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis.” Journal of psychiatric research 104: 235–248. [DOI] [PubMed] [Google Scholar]
- Slattery DA and Neumann ID (2010). “Oxytocin and Major Depressive Disorder: Experimental and Clinical Evidence for Links to Aetiology and Possible Treatment.” Pharmaceuticals (Basel) 3(3): 702–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W and Woods N (2001). “Executive summary: Stages of Reproductive Aging Workshop (STRAW).” Climacteric 4(4): 267–272. [PubMed] [Google Scholar]
- Stevenson J (2023). “Prevention and treatment of osteoporosis in women.” Post Reprod Health 29(1): 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc P, Amri EZ, Varennes A, Panaia-Ferrari P, Fontas E, Goudable J, Chapurlat R and Breuil V (2016). “High serum oxytocin is associated with metabolic syndrome in older men - The MINOS study.” Diabetes Res Clin Pract 122: 17–27. [DOI] [PubMed] [Google Scholar]
- Tabak BA, Leng G, Szeto A, Parker KJ, Verbalis JG, Ziegler TE, Lee MR, Neumann ID and Mendez AJ (2023). “Advances in human oxytocin measurement: challenges and proposed solutions.” Mol Psychiatry 28(1): 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T and Nishimori K (2008). “Oxytocin receptor-deficient mice developed late-onset obesity.” Neuroreport 19(9): 951–955. [DOI] [PubMed] [Google Scholar]
- Tamma R, Colaianni G, Zhu L.-l., DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, Colucci S, Grano M, Faccio R, Liu X, Li J, Usmani S, Bachar M, Bab I, Nishimori K, Young LJ, Buettner C, Iqbal J, Sun L, Zaidi M and Zallone A (2009). “Oxytocin is an anabolic bone hormone.” Proceedings of the National Academy of Sciences 106(17): 7149–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, Colucci S, Grano M, Faccio R, Liu X, Li J, Usmani S, Bachar M, Bab I, Nishimori K, Young LJ, Buettner C, Iqbal J, Sun L, Zaidi M and Zallone A (2009). “Oxytocin is an anabolic bone hormone.” Proc Natl Acad Sci U S A 106(17): 7149–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul TA, Corwin EJ, Carlson NS, Brennan PA and Young LJ (2020). “Oxytocin and postpartum depression: A systematic review.” Psychoneuroendocrinology 120: 104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokui T, Kawakita T, Yanagihara R, Kamada S, Minato S, Takeda A, Imaizumi J, Yamamoto Y, Yoshida K, Kato T, Irahara M and Iwasa T (2021). “Effects of gonadal status and the estrogen milieu on hypothalamic oxytocin gene expression and serum oxytocin levels in female rats.” Horm Behav 133: 105005. [DOI] [PubMed] [Google Scholar]
- Tongta S, Daendee S and Kalandakanond-Thongsong S (2023). “Anxiety-like behavior and GABAergic system in ovariectomized rats exposed to chronic mild stress.” Physiol Behav 258: 114014. [DOI] [PubMed] [Google Scholar]
- Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT and Quintana DS (2017). “The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis.” Neurosci Biobehav Rev 78: 117–124. [DOI] [PubMed] [Google Scholar]
- Walf AA, Paris JJ and Frye CA (2009). “Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance.” Psychoneuroendocrinology 34(6): 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML (1995). “Menopause in female rhesus monkeys.” Am J Primatol 35(1): 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML and Herndon JG (2008). “Menopause in Nonhuman Primates?1.” Biology of Reproduction 79(3): 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H and Young LJ (2018). “The neural mechanisms and circuitry of the pair bond.” Nat Rev Neurosci 19(11): 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MFJ, Scholz M, Wohland T, Horn K, Stumvoll M, Kovacs P and Tönjes A (2019). “Circulating Oxytocin Is Genetically Determined and Associated With Obesity and Impaired Glucose Tolerance.” The Journal of Clinical Endocrinology & Metabolism 104(11): 5621–5632. [DOI] [PubMed] [Google Scholar]
- Wierda M, Goudsmit E, Van der Woude PF, Purba JS, Hofman MA, Bogte H and Swaab DF (1991). “Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease.” Neurobiol Aging 12(5): 511–516. [DOI] [PubMed] [Google Scholar]
- Yan Y, Wang YL, Su Z, Zhang Y, Guo SX, Liu AJ, Wang CH, Sun FJ and Yang J (2014). “Effect of oxytocin on the behavioral activity in the behavioral despair depression rat model.” Neuropeptides 48(2): 83–89. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T and Nishimori K (2009). “Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice.” J Neurosci 29(7): 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donaldson R and Rissman EF (1998). “Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen.” Neuroreport 9(5): 933–936. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Austin D and Peterson A (2003). “Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study.” Am J Respir Crit Care Med 167(9): 1181–1185. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cao LL, Yang DD, Ding JH, Guo XD, Xue TF, Zhao XJ and Sun XL (2019). “Establishment and evaluation of a novel mouse model of peri/postmenopausal depression.” Heliyon 5(2): e01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Chung HF, Pandeya N, Dobson AJ, Cade JE, Greenwood DC, Crawford SL, Avis NE, Gold EB, Mitchell ES, Woods NF, Anderson D, Brown DE, Sievert LL, Brunner EJ, Kuh D, Hardy R, Hayashi K, Lee JS, Mizunuma H, Giles GG, Bruinsma F, Tillin T, Simonsen MK, Adami HO, Weiderpass E, Canonico M, Ancelin ML, Demakakos P and Mishra GD (2018). “Relationships between intensity, duration, cumulative dose, and timing of smoking with age at menopause: A pooled analysis of individual data from 17 observational studies.” PLoS Med 15(11): e1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]


