Abstract
The first Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma were published in 2013. Since then, new evidence on the role of endoscopy for management of malignant hilar biliary obstruction (MHBO) has emerged. To update the recommendation, we reviewed the literature using a PICO (population/intervention/comparison/outcomes) framework and created consensus statements. The expert panel voted anonymously using the modified Delphi method and all final statements were evaluated for the quality of evidence and strength of recommendation. The important points with inadequate supporting evidence were classified as key concepts. There were seven statements and five key concepts that reached consensus. The statements and key concepts dealt with multiple aspects of endoscopy-based management in MHBO starting from diagnosis, strategies and options for biliary drainage, management of recurrent biliary obstruction, management of cholecystitis after biliary stenting, and adjunctive treatment before stenting. Although the recommendations may assist physicians in planning the treatment for MHBO patients, they should not replace the decision of a multidisciplinary team in the management of individual patients.
Keywords: Pancreatobiliary (ERCP/PTCD), Strictures, ERC topics, Tissue diagnosis, Endoscopic ultrasonography, Biliary tract, Intervention EUS
Introduction
The management of malignant hilar biliary obstruction (MHBO) is challenging not only from diagnosis, due to the limited sensitivity of current available diagnostic tools 1 , but also in terms of management, due to the complexity of the biliary anatomy. Endoscopy has a major role to play in managing MHBO as it is less invasive than the other modalities. The first Asia Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma published in 2013 2 aimed to assist the clinician in managing this condition. However, the body of knowledge has evolved significantly, and new evidence has emerged, especially regarding the role of endoscopy including endoscopic ultrasonography (EUS) and cholangioscopy. Therefore, the Asia Pacific working group on hepatobiliary cancer concluded that the consensus statements needed to be updated based on the current evidence and experience.
Methods
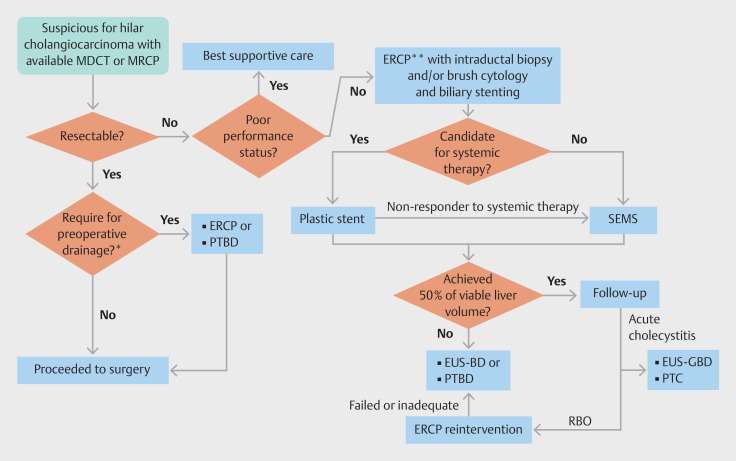
The consensus statements were developed using the GRADE framework 3 4 . The planning team (PA, SK, and AC) set clinical questions based on the population, intervention, comparison, and outcomes (PICO) outline. The evidence relevant to each question was independently searched by 2 of the planning team from 3 databases (Medline, EMBASE, and Cochrane) and additional evidence from the references was manually searched. Then, the statements were drafted. The level of evidence was graded according to the international GRADE system 4 5 . A modified Delphi method was used to establish the consensus 6 . In brief, two rounds of anonymous electronic voting system were undertaken. The expert panel was presented with the evidence and asked to rate their opinion on each statement as A: accept completely, B: accept with some reservation, C: accept with major reservation n, D: reject with some reservation, or E: reject completely. A statement would achieve consensus when over 80% of the responses were “accept completely” or “accept with minor revision”. On the other hand, a statement would be refuted when over 80% of respondents voted to “reject completely” or “reject with some reservation”. There were 24 experts in hepatobiliary endoscopy including gastroenterologists and surgeons from 14 countries in the Asia-Pacific region who participated in this consensus. All statements were edited and finally agreed at the on-line meeting session. The important points which currently have inadequate supporting evidence were classified as key concepts. Finally, 7 statements and 5 key concepts reached consensus. All statements and key concepts were finally concluded after presentation for public consultation at Asian Pacific Digestive Week 2023, Bangkok, Thailand. The roles of endoscopy in the management of MHBO were conceptualized in Fig. 1 .
Fig. 1.
Conceptualized roles of endoscopy in management of malignant hilar biliary obstruction. ERCP; endoscopic retrograde cholangiopancreatography, EUS-BD; endoscopic ultrasound-guided biliary drainage, EUS-GBD; endoscopic ultrasound-guided gallbladder drainage, MDCT; multidetector computed tomography, MRCP; magnetic resonance cholangiopancreatography, PTBD; percutaneous biliary drainage, PTC; percutaneous cholecystostomy, RBO; recurrent biliary obstruction, SEMS; self-expandable metal stent. * Preoperative biliary drainage may be indicated in patients with cholangitis, prolonged jaundice, delayed surgery (e.g. waiting for portal vein intervention, malnutrition [serum albumin less than 3 g/dL], etc.), or total bilirubin ≥15 mg/dL ** Wire-guided selection of preselected liver segment before performing cholangiogram. Followed by air/carbon dioxide cholangiogram or limited injection with contrast media. Photodynamic therapy or endo-biliary radiofrequency ablation may be used as adjunctive treatment before stenting.
Statements
Statement 1
In patients with suspected malignant hilar obstruction for which ERCP is indicated, a combination of intraductal biopsy and cytology should be performed at the index ERCP, to confirm the etiology.
Quality of evidence: high
Level of agreement: A; 100%, B; 0%, C; 0%, D; 0%, E; 0%
While the majority of biliary strictures having malignant etiology 7 , tissue diagnosis is still required and remains challenging to confirm due to limited sensitivity of standard tissue acquisition methods. Two of the most used and widely available endoscopic retrograde cholangiopancreatography (ERCP)-based diagnostic techniques are brush cytology and intraductal biopsy, however their sensitivity is low at only 21–56% and 43–67%, respectively 8 9 . Data from a meta-analysis revealed that by combining these two methods the sensitivity increases to 70% 9 . A combination of both methods at index ERCP is recommended to achieve the highest yield of malignancy.
Newer technologies have been reported to improve the diagnosis of MHBO, and these include cholangioscopy and molecular techniques. Cholangioscopy provides direct visualization and targeted biopsy of the bile duct, which have sensitivity and specificity of up to 93% and 82%, respectively 10 . The utility of cholangioscopy at the first ERCP or after negative tissue acquisition is debatable. Additional fluorescence in situ hybridization (FISH) on the cytology sample significantly improved the sensitivity for diagnosis of malignant biliary stricture; 55% vs 38%, respectively (p = 0.001) 11 . Furthermore, newer DNA-based molecular diagnostic techniques such as next generation sequencing, using tissue from either brushing or biopsy, showed promising results with an increase in the sensitivity for diagnosis of malignant biliary stricture up to 83–96% 12 13 . However, the availability and cost-effectiveness of these technologies are major limitations.
Statement 2
Preoperative biliary drainage can be performed if clinically indicated; however, this should be balanced with the risk of infection.
Quality of evidence: moderate
Level of agreement: A; 56%, B; 44%, C; 0%, D; 0%, E; 0%
In patients with resectable hilar cholangiocarcinoma, the role of pre-operative biliary drainage is debatable. Two meta-analyses showed no difference in mortality between patients with or without preoperative biliary drainage (PBD) 14 15 . However, PBD was associated with higher morbidity due to infectious complications, in unselected cases 14 15 . In patients with total bilirubin of 15 mg/dL or more though, PBD did not increase morbidity 15 . It is noteworthy that when strict criteria including cholangitis, prolonged jaundice, or delayed surgery (e.g. waiting for portal vein intervention to induce hypertrophy of future remnant liver segments, malnutrition [serum albumin less than 3 g/dL], etc.) were applied, the PBD group showed lower major morbidity when compared with patients without PBD (OR = 0.51; 95% CI; 0.18–1.42) 14 .
Statement 3
For palliation of unresectable malignant hilar biliary obstruction, the selection of segments to drain and the number of stents used is dependent on the Bismuth classification and the liver volume to be drained, with the aim of decompressing more than 50% of viable liver.
Quality of evidence: moderate
Level of agreement: A; 83%, B; 17%, C; 0%, D; 0%, E; 0%
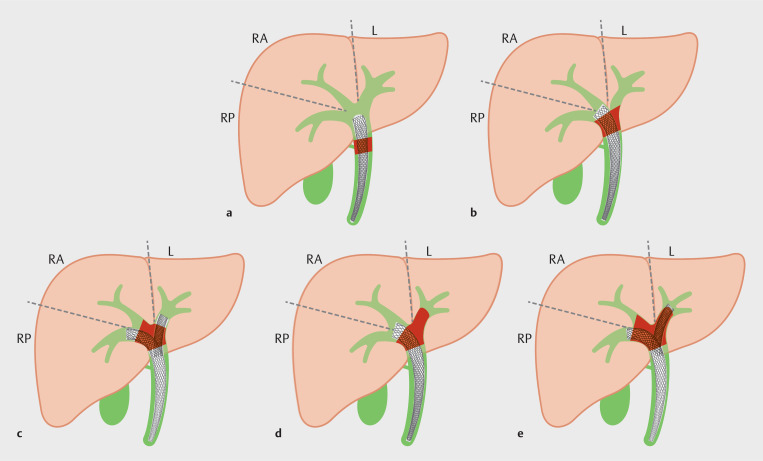
Vienne, et al. 16 reported a retrospective study in 2010 which demonstrated that drainage of >50% of liver volume was associated with better drainage effectiveness, longer survival, and less cholangitis when compared to <50% drainage. According to this study, the liver was grossly divided into 3 major sectors including the right anterior, right posterior, and left sectors. Each sector accounted for 30% of liver volume and the remaining segments I and IV accounted for 10%. An atrophic segment, if present, was considered a non-viable segment. This approach can guide segment selection and the number of stents to be placed. For example, without an atrophic segment, in Bismuth type I-II disease (with a stent inserted in the right lobe, and draining both anterior and posterior sectors), a single stent could drain >50% of viable liver volume. On the other hand, in Bismuth type III-IV, 2 or more stents may be required to obtain >50% drainage ( Fig. 2 ). In addition, the benefit of a higher percentage of liver drainage was reported in another retrospective study from Caillol, et al. in 2019 17 . Maximum drainage of greater than 80% of viable liver volume drainage was associated with longer survival when compared to less than 80% drainage (Hazard ratio [HR] = 2.46; 95% CI: 1.16–5.23, p = 0.02).
Fig. 2.
Strategy for biliary sector selection for drainage according to Bismuth classification of malignant hilar biliary obstruction. RA; right anterior sector, RP; right posterior sector, L; left sector. a Bismuth I; one stent can drain all three sectors (100%). b Bismuth II: placement of one stent in the right main hepatic duct can drain both RA and RP sectors (60%). c Bismuth IIIa: at least two stents are required to achieve at least 50% of liver volume. d Bismuth IIIb: placement of one stent in the right main hepatic duct can drain both RA and RP sectors (60%). e Bismuth IV: at least two stents are required to achieve at least 50% of liver volume.
Statement 4
To reduce the risk of post-ERCP cholangitis, hepatic segment stenting can be performed employing wire-guided intrahepatic duct selection with pre-defined MRCP and/or MDCT. Air/carbon dioxide cholangiogram may be used to confirm correct guidewire location and if contrast injection is deemed necessary, complete biliary drainage of injected ducts is recommended.
Quality of evidence: moderate
Level of agreement: A; 74%, B; 22%, C; 4%, D; 0%, E; 0%
Hilar biliary obstruction is an independent risk factor for post-ERCP cholangitis 18 19 20 . In MHBO, contrast injection without adequate drainage of the injected segments led to cholangitis and diminished patient survival 21 . Careful evaluation and planning for drainage of preselected segment(s) by magnetic resonance cholangiopancreatography (MRCP) and/or multidetector computed tomography (MDCT) is necessary before ERCP. During ERCP, guidewire selection of the desired segment under fluoroscopic guidance should be the initial step. Any injection (contrast media, air, or carbon dioxide [CO 2 ]) below the stricture may result in contamination of the unplanned segment(s). After guidewire placement into the pre-selected duct, with a good alignment of the wire course, a catheter can be passed over the guidewire into the desired segment. Bile should be aspirated to decompress the segment 22 . Two small randomized controlled trials (RCTs) have confirmed that to ensure correct location of the catheter, an air or CO 2 cholangiogram reduces the risk of cholangitis without diminishing technical and clinical success rates 22 23 . However, if an air or CO 2 cholangiogram is insufficient to provide clear anatomical delineation, an injection of the lowest possible volume of contrast media may be performed. Complete or near complete drainage of those injected ducts during the procedure is required to prevent cholangitis.
Statement 5
After failed multi-segmental drainage by metallic stenting in complex malignant hilar obstruction, either percutaneous or EUS-guided biliary drainage of undrained liver segment can be performed.
Quality of evidence: low
Level of agreement: A; 80%, B; 20%, C; 0%, D; 0%, E; 0%
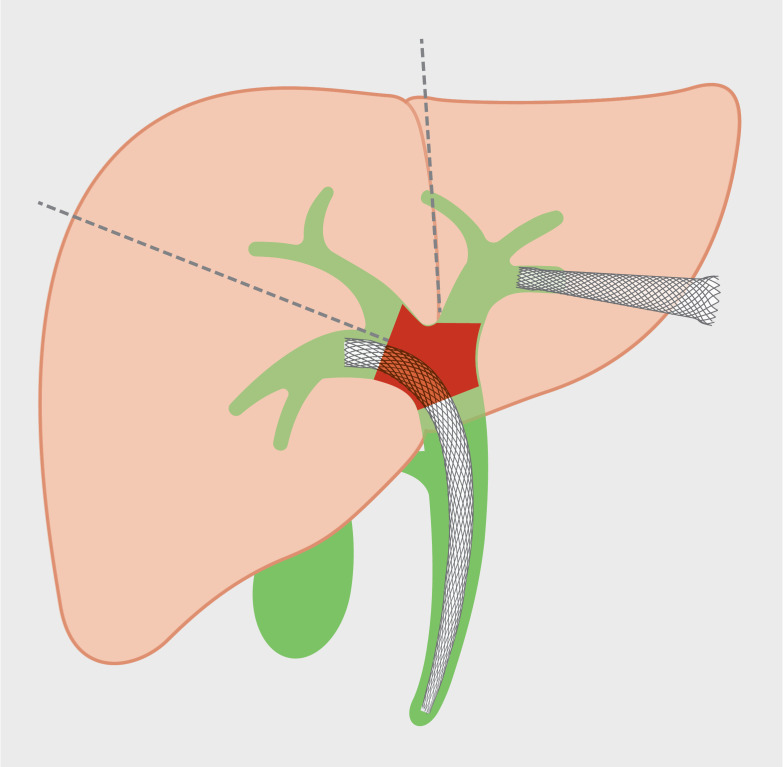
The percutaneous approach is an option for additional biliary drainage after inadequate drainage by ERCP as undrained segments on either side of the liver can be selectively targeted. However, the key drawbacks of percutaneous drainage include patient discomfort, external loss of bile content and volume, and recurrent biliary obstruction (RBO). Recent advances in therapeutic EUS make internal drainage of the undrained biliary segment possible, and this may be considered an alternative to percutaneous approach ( Fig. 3 ).
Fig. 3.
Endoscopic ultrasound-guided biliary drainage of undrained liver segment after ERCP. Endoscopic ultrasound-guided hepaticogastrostomy for drainage of the left hepatic sector after inadequate drainage of right hepatic sector by ERCP.
An open-labelled study with historical controls compared a combination of endoscopic drainage by ERCP and EUS with percutaneous drainage in patients with unresectable bismuth III or IV MHBO, who had good performance status and an expected life expectancy greater than 3 months 24 . The technical success (84% vs 100%), clinical success (79% vs 77%), and complication rates (26% vs 35%) were not different between the endoscopy and percutaneous groups, respectively. Interestingly, the endoscopy group had a significantly lower rate of recurrent RBO at 3 months (27% vs 88%) and 6 months (22% vs 100%) compared to the percutaneous group. In addition, there was a trend toward longer median time to RBO in the endoscopy group compared to the percutaneous group (92 vs 40 days, respectively; p= 0.06). Therefore, EUS-guided biliary drainage represents an alternative to percutaneous approaches after inadequate biliary drainage by ERCP.
Statement 6
For trans-papillary drainage of Bismuth II-IV, in the patient who may respond well to systemic chemotherapy multiple plastic stenting with scheduled stent exchange may be preferred over metallic stenting. However, metallic stenting by either side-by-side or stent-in-stent approach should be considered in the patient who is not a candidate for or who has failed systemic chemotherapy.
Quality of evidence: moderate
Level of agreement: A; 57%, B; 35%, C; 4%, D; 0%, E; 4%
Stent options for trans-papillary biliary drainage consist of plastic stents (PS) and self-expandable metal stents (SEMS). PS have a smaller diameter when compared to SEMS, which are associated with shorter stent patency. In the subgroup of hilar obstruction reported in a meta-analysis in 2015 (800 patients from 6 studies) 25 , SEMS had lower therapeutic failure (odds ratio [OR] 0.28; 95% CI, 0.13–0.63), 30-day occlusion rate (OR 0.16; 95% CI, 0.04–0.62), long-term occlusion rate (OR 0.28; 95% CI, 0.19–0.39), and cholangitis (OR 0.30; 95% CI, 0.12–0.74) when compared with PS. However, 30-day mortality (OR 0.62; 95% CI, 0.30–1.31) and reintervention per patient (mean difference, 0.34; 95% CI, 0.78 to 0.11) were not different between SEMS and PS. In 2021, Xia et al. 26 reported a propensity-matched, retrospective study of 356 patients comparing 4 approaches to biliary drainage in MHBO consisting of bilateral SEMS, unilateral SEMS, bilateral PS, and unilateral PS. Approximately one third of patients in all groups received adjuvant treatments (e.g. chemotherapy, radiotherapy, and immunotherapy). The bilateral SEMS group had the highest, statistically significant clinical success rate (98.9%), the lowest incidence of postprocedural cholangitis (8%), and the longest stent patency (9.6 months) when compared with the other 3 approaches. Furthermore, bilateral SEMS drainage was associated with fewer total interventions (1.2 ± 0.5 interventions) and longer overall survival (7.1 months) when compared with unilateral or bilateral PS. Kim et al. 27 retrospectively compared palliative treatment with bilateral SEMS and multiple PS in MHBO due to cholangiocarcinoma in 102 patients. Only a tenth of patients received adjuvant therapy, and the median survival was about 9 months. This study showed that multiple plastic stents had higher cholangitis risk (HR, 2.08; 95%CI, 1.21–3.58) and were associated with higher 6-month mortality (HR, 2.91; 95% CI, 1.26–6.71) than bilateral SEMS.
As a result of advances in systemic treatment of MHBO including chemotherapy, immunotherapy, and targeted therapy, patients' survival may exceed the patency of uncovered SEMS which is not removable 28 29 . Therefore, concerns of complications from uncovered SEMS have been raised in this population. In 2023, Al Nakshabandiet al. 30 reported a retrospective, 25-year tertiary cancer center experience of 333 patients with MHBO from cholangiocarcinoma. Approximately half of patients had received adjuvant treatment. The study showed that although SEMS had higher clinical success, this was countered by higher stent-specific complication rate including migration, occlusion, ingrowth, and overgrowth with OR of 4.85 (95% CI, 3.23–7.27). PS may be superior to SEMS in terms of removability and stent revision in MHBO in patients who respond to adjuvant therapy. Although a fully covered SEMS (FCSEMS) is removable, FCSEMS might occlude segmental bile ducts when placed across the liver hilum and evidence for placing bilateral FCSEMS in MHBO is awaiting.
SEMSs can be placed across the hilum either in side-by-side or stent-in-stent fashion. Lee et al. 31 reported a randomized study comparing these 2 techniques and showed no difference in technical and clinical success rates, stent patency, and survival. In 2022, Cao et al. 32 reported a meta-analysis of 315 MHBO patients from 6 studies comparing these 2 techniques of SEMSs insertion. There was no significant difference between the two groups in terms of clinical success (OR: 1.07; 95%CI: 0.46–2.49, p=0.87), complications (risk difference: 0.12; 95% CI:-0.04–0.27, p=0.15), stent dysfunction (OR: 0.68; 95% CI: 0.42–1.10, p=0.11), and overall survival (HR: 0.97; 95% CI: 0.82–1.16, p=0.74).
Statement 7
ERCP is the preferred option for revision of recurrent biliary obstruction after transpapillary placement of uncovered metallic stent(s). For inaccessible segments, or following previous stent-in-stent insertion, EUS-guided or percutaneous drainage are the preferred rescue procedures.
Quality of evidence: low
Level of agreement: A; 93%, B; 7%, C; 0%, D; 0%, E; 0%
Options for the management of recurrent biliary obstructions after placement of transpapillary metal stents include transpapillary insertion of a PS or another SEMS, and percutatneous transhepatic biliary drainage (PTBD). Ridtitid et al. 33 compared the outcomes of second interventions for occluded metallic stents. In a subgroup of 13 patients with MHBO, the reinterventions were PS (6 patients), SEMS (3 patients), and PTBD (4 patients). The median stent patency was 60, 60, and 90 days, and median survival was 130, 215, and 185 days in the PS, SEMS, and PTBD groups respectively, but the difference did not reach statistical significance. Okuno et al. 34 reported an 80.7% technical success rate of repeat transpapillary reintervention in 31 patients with MHBO who had prior stent-in-stent, dual SEMS placement. Technical failure was recorded in 6 patients as only one SEMS could be placed due to the guidewire did not traverse the mesh of the first stent, thus preventing successful second SEMS placement. All these patients were successfully drained by a percutaneous approach. Kitamura et al. 35 reported endoscopic reintervention in 49 patients who previously had 2 or more SEMSs placed across the hilum; 27 patients had side-by-side and 22 patients had stent-in-stent SEMS placement. Transpapillary re-intervention by ERCP had a technical success rate of 69.4%. In the 15 patients who failed ERCP drainage, and subsequent EUS-guided reintervention was successful in 13 patients (86.7%). Two patients who failed EUS-guided reintervention were successfully drained by a percutaneous approach.
Key concepts
Key concept 1
Palliative biliary drainage may not be beneficial in patients with poor performance status who have short life expectancy and should be avoided.
Level of agreement: A; 58%, B; 42%, C; 0%, D; 0%, E; 0%
Almost two-thirds of patients with malignant hilar biliary obstruction are not amendable to curative surgical resection at the time of diagnosis 36 . These individuals may be treated with systemic therapy including chemotherapy, immunotherapy, and radiation. Palliative biliary drainage in inoperable MHBO provides several benefits, especially in several situations, including reducing hyperbilirubinemia in patients intended for systemic therapy, alleviating pruritus, or minimizing infective adverse events such as cholangitis 37 . The route of biliary drainage mainly includes percutaneous and endoscopic approaches. These two strategies are not different in 30-day mortality rate and overall adverse events 38 39 . Adverse events associated with biliary drainage include cholangitis, RBO, cholecystitis, pancreatitis, tumor seeding, and diminished quality of life due to external drainage tubes 38 39 . Unfortunately, palliative biliary drainage in patients with poor performance status does not alter survival outcomes or quality of life. Robson et al. 40 reported a cohort study of 109 patients with unresectable malignant biliary obstruction comparing PTBD and conservative treatment. There was no difference in the quality of life in patients with post-procedure survival of fewer than five months. The negative impact of poor prognostic disease outweighed the benefit of the procedure. In addition, palliative biliary drainage in patients with high baseline American Society of Anesthesiologists grade (HR 6.47, 95% CI; 2.02–20.74), or low serum albumin (HR 1.23, 95% CI; 1.05–1.43) was associated with increased in-hospital mortality following drainage. The primary causes of mortality included biliary sepsis and renal failure. The presence of pre-procedural biliary sepsis was a major contributor to both morbidity and mortality 41 . Careful patient selection for palliative biliary drainage is therefore crucial and requires discussing the risks and benefits among the multidisciplinary team, patient, and caregivers. Risk prediction of clinical success after endoscopic biliary stenting may be a tool for making individualized decisions 42 43 .
Key concept 2
If additional transmural EUS-guided biliary drainage is required, a transgastric approach is recommended for the left hepatic lobe.
Level of agreement: A; 75%, B; 25%, C; 0%, D; 0%, E; 0%
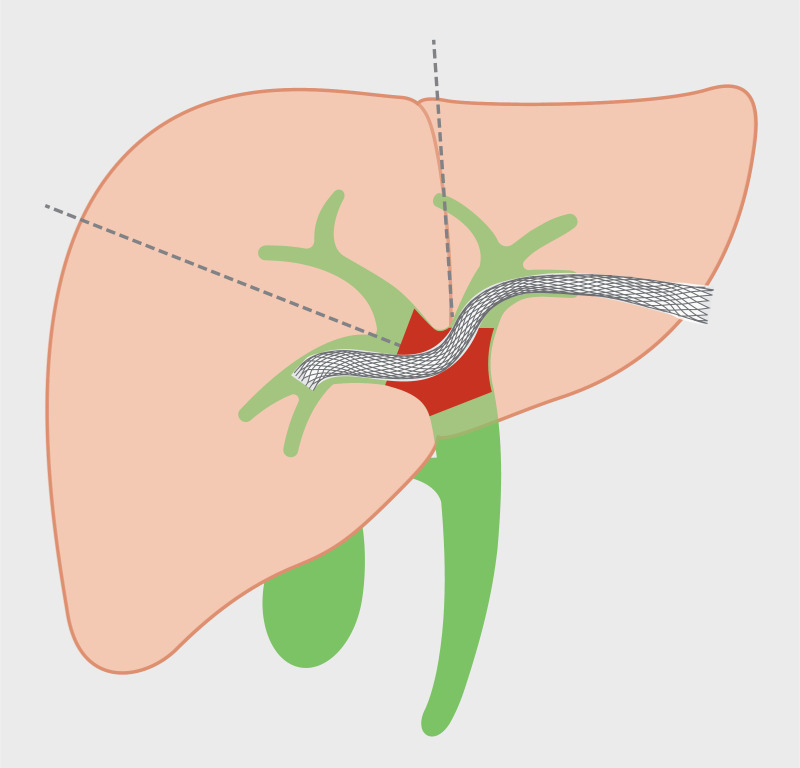
EUS-guided transmural biliary drainage has continued to evolve over the past decade. For drainage of intrahepatic bile ducts, the transgastric or transduodenal approach can be considered according to their proximity to left lateral segments (segment II and III) and right posterior segments (segment VI and VII), respectively. However, transgastric drainage is a more established technique in which specially designed accessories are available 44 . This contrasts with transduodenal drainage for which there are only a handful preliminary reports and specially designed equipment is lacking. Ogura et al. 45 reported a series of EUS-guided drainage of right hepatic duct obstruction in 11 patients with MHBO. The route of drainage was transgastric puncture of left hepatic duct and bridge to right hepatic duct in 7 patients ( Fig. 4 ), and transgastric puncture (at gastric antrum) of right hepatic duct in 3 patients, and transduodenal puncture in 1 patient. Technical and functional success was reported in all patients, without complication. Ma et al. 46 reported EUS-guided hepatoduodenostomy (HDS) with conventional FCSEMS in 35 patients with segregated right hepatic duct and failed drainage by ERCP. The technical and clinical success rates were 97% and 80%, respectively. However, adverse events were reported to be up to 20%. EUS-guided hepaticogastrostomy (HGS) may be performed via the segment II or III approach, depending on patient anatomy, selection of a puncture window without vascular interposition, guidewire manipulation skill, and endoscopists’ expertise. A recent retrospective multicenter study revealed no difference in technical success, functional success, and adverse events between puncture of segment II and III 47 . With EUS-HGS via segment II, the location of puncture site should be carefully selected such that access is made from the stomach rather than from the distal esophagus. If necessary, following left duct puncture, attempts to bridge the right intrahepatic duct via left intrahepatic duct from HGS access may be considered.
Fig. 4.
Transgastric approach of endoscopic ultrasound-guided drainage of right hepatic duct. Endoscopic ultrasound-guided biliary drainage is a viable technique to bridge right and left hepatic duct via a transgastric puncture.
Key concept 3
For EUS-guided hepaticogastrotosmy, either plastic or metallic stent can be chosen.
Level of agreement: A; 73%, B; 18%, C; 9%, D; 0%, E; 0%
Several specifically designed plastic and metallic stents have been introduced to reduce adverse events and improve outcomes of EUS-HGS. An 8-Fr single-pigtail plastic stent with a tapered proximal end, four internal flanges, and an effective length of 15 cm was reported with a 100% technical success rate and median stent patency of 4 months without serious adverse events 48 . Unfortunately, this stent is only available in Japan, and therefore a 7-Fr double-pigtail plastic stent may be an alternative option due to its widespread availability and affordability 49 . Shibuki et al. 50 retrospectively reviewed patients who underwent EUS-HGS. Their series documented 109 patients with plastic stent (7, 8, or 8.5 Fr) and 43 patients with FCSEMS. Technical success, overall survival and adverse events were not different between the two groups. However, the time to RBO was significantly longer in the FCSEMS than the PS group (646 vs 202 days, respectively). Hybrid metallic stents have been specially designed for HGS use. Their design features a combination of a proximal uncovered portion, to be placed in bile duct and hepatic parenchyma to avoid side branch obstruction, and a distal covered portion to be placed in gastric wall to prevent bile leakage. The covered portion is accountable for one-third to half of the stent which varies from 30 to 50 mm in length. The length of the covered part of the stent deployed inside the gastric cavity should be at least 20 mm to prevent stent migration into the peritoneum 51 52 53 .
Key concept 4
If acute cholecystitis develops after biliary metallic stenting, EUS-guided gallbladder decompression with or without permanent stenting of the gallbladder is an alternative to percutaneous cholecystostomy or surgery.
Level of agreement: A; 91%, B; 9%, C; 0%, D; 0%, E; 0%
Acute cholecystitis is a potential complication that may occur after metallic stent placement for MHBO 54 . Reflux of duodenal contents, pressure effect on the cystic duct orifice from tumor mass, or the stent’s expansile force potentially contribute to acute cholecystitis 55 . To manage this condition, gallbladder decompression is required to alleviate symptoms and provide source control of infection. EUS-guided gallbladder decompression is an alternative to the conventional percutaneous or surgical approaches 56 . EUS-guided gallbladder aspiration may be performed as the initial step, with the option of proceeding to transmural gallbladder drainage stent can be considered if cholecystitis persists or recurs.
Key concept 5
Photodynamic therapy or endobiliary radiofrequency ablation may be used as adjunctive treatment prior to plastic or metallic biliary stent placement to improve stent patency and patient survival.
Level of agreement: A; 50%, B; 50%, C; 0%, D; 0%, E; 0%
The aim of photodynamic therapy (PDT) and endobiliary radiofrequency ablation (RFA) is to provide an additional local control of the tumor. Lu et al. 57 reported a meta-analysis in 2015 comparing PDT plus stent versus stent alone in patients with unresectable cholangiocarcinoma. Seven studies, including two RCTs were analyzed with most patients having hilar involvement. The meta-analysis showed that the PDT plus stent group had significantly longer survival than the stent alone group (HR = 0.49, 95% CI; 0.33–0.73, p=0.0005). Dolak et al. 58 reported in 2017 that SEMS placement was associated with longer stent patency than plastic stent after PDT (269 vs 62 days, respectively, p < 0.01). In 2016, Schmidt et al. 59 reported in a retrospective study that RFA plus plastic stent was superior to PDT plus plastic stent in respect of premature stent dysfunction at 3 months (29% vs 65%, respectively, p<0.01). The main caution for patients undergoing PDT is that they need to avoid light exposure and stay in a darkened room for 3–4 days. PDT is associated with an incidence of phototoxic reaction in 11.11% 57 .
Kang et al. 60 reported a RCT in 2022 comparing RFA plus bilateral PS and bilateral PS alone in 30 patients. The study showed that RFA significantly reduced premature PS occlusion within 3 months when compared with bilateral PS alone (30.8% vs 76.9% respectively, p = 0.018), while adverse events were not different between the 2 groups. Oh et al. 61 reported a retrospective study in 2022 comparing RFA plus SEMS and SEMS alone. This study showed that there was no difference in stent patency (140 vs 192 days, p= 0.41) and survival (311 vs 311 days, p= 0.73) between RFA plus SEMS and SEMS alone groups. Multivariate cox analysis showed that “not receiving chemotherapy” was an independent risk for stent occlusion while RFA had no significant effect on stent occlusion.
Conclusions
A decade on from the first Asia–Pacific consensus, biliary endoscopy techniques have undergone significant advancements, offering new diagnostic and therapeutic approaches for MHBO. These include the improvement of diagnostic sensitivity, efficacy, and safety of drainage procedures, and particularly the growing role of therapeutic EUS. However, successful management requires careful patient selection based on several factors including, but not limited to, clinical presentation, biliary anatomy, level of obstruction, and most importantly, the availability of local expertise. A multidisciplinary approach remains crucial for optimal decision-making.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Angsuwatcharakon P, Kulpatcharapong S, Moon JH et al. Consensus guidelines on the role of cholangioscopy to diagnose indeterminate biliary stricture. HPB (Oxford) 2022;24:17–29. doi: 10.1016/j.hpb.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593–607. doi: 10.1111/jgh.12128. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Oxman AD, Kunz R et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balshem H, Helfand M, Schunemann HJ et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Dalkey N, Helmer O. An Experimental Application of the DELPHI Method to the Use of Experts. Management Science. 1963;9:458–467. doi: 10.1287/mnsc.9.3.458. [DOI] [Google Scholar]
- 7.Tummala P, Munigala S, Eloubeidi MA et al. Patients with obstructive jaundice and biliary stricture +/- mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532–537. doi: 10.1097/MCG.0b013e3182745d9f. [DOI] [PubMed] [Google Scholar]
- 8.Cadamuro M, Al-Taee A, Gonda TA. Advanced endoscopy meets molecular diagnosis of cholangiocarcinoma. J Hepatol. 2023;78:1063–1072. doi: 10.1016/j.jhep.2023.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SB, Moon SH, Ko SW et al. Brush Cytology, Forceps Biopsy, or Endoscopic Ultrasound-Guided Sampling for Diagnosis of Bile Duct Cancer: A Meta-Analysis. Dig Dis Sci. 2022;67:3284–3297. doi: 10.1007/s10620-021-07138-4. [DOI] [PubMed] [Google Scholar]
- 10.Kulpatcharapong S, Pittayanon R, Kerr SJ et al. Diagnostic performance of digital and video cholangioscopes in patients with suspected malignant biliary strictures: a systematic review and meta-analysis. Surg Endosc. 2022;36:2827–2841. doi: 10.1007/s00464-021-08571-2. [DOI] [PubMed] [Google Scholar]
- 11.Chaiteerakij R, Barr Fritcher EG, Angsuwatcharakon P et al. Fluorescence in situ hybridization compared with conventional cytology for the diagnosis of malignant biliary tract strictures in Asian patients. Gastrointest Endosc. 2016;83:1228–1235. doi: 10.1016/j.gie.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 12.Arechederra M, Rullán M, Amat I et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut. 2022;71:1141–1151. doi: 10.1136/gutjnl-2021-325178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhi AD, Nikiforova MN, Chennat J et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut. 2020;69:52–61. doi: 10.1136/gutjnl-2018-317817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehrabi A, Khajeh E, Ghamarnejad O et al. Meta-analysis of the efficacy of preoperative biliary drainage in patients undergoing liver resection for perihilar cholangiocarcinoma. Eur J Radiol. 2020;125:108897. doi: 10.1016/j.ejrad.2020.108897. [DOI] [PubMed] [Google Scholar]
- 15.Teng F, Tang YY, Dai JL et al. The effect and safety of preoperative biliary drainage in patients with hilar cholangiocarcinoma: an updated meta-analysis. World J Surg Oncol. 2020;18:174. doi: 10.1186/s12957-020-01904-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vienne A, Hobeika E, Gouya H et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 17.Caillol F, Bories E, Zemmour C et al. Palliative endoscopic drainage of malignant stenosis of biliary confluence: Efficiency of multiple drainage approach to drain a maximum of liver segments. United European Gastroenterol J. 2019;7:52–59. doi: 10.1177/2050640618803812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Wang L, Wang Y et al. Risk factor analysis of post-ERCP cholangitis: A single-center experience. Hepatobiliary Pancreat Dis Int. 2018;17:55–58. doi: 10.1016/j.hbpd.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Rerknimitr R, Kongkam P, Kullavanijaya P. Outcome of self-expandable metallic stents in low-grade versus advanced hilar obstruction. J Gastroenterol Hepatol. 2008;23:1695–1701. doi: 10.1111/j.1440-1746.2008.05562.x. [DOI] [PubMed] [Google Scholar]
- 20.Rerknimitr R, Kladcharoen N, Mahachai V et al. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J Clin Gastroenterol. 2004;38:518–523. doi: 10.1097/01.mcg.0000123204.36471.be. [DOI] [PubMed] [Google Scholar]
- 21.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 22.Sud R, Puri R, Choudhary NS et al. Air cholangiogram is not inferior to dye cholangiogram for malignant hilar biliary obstruction: a randomized study of efficacy and safety. Indian J Gastroenterol. 2014;33:537–542. doi: 10.1007/s12664-014-0516-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WH, Ding PP, Liu L et al. CO(2) or air cholangiography reduces the risk of post-ERCP cholangitis in patients with Bismuth type IV hilar biliary obstruction. BMC Gastroenterol. 2020;20:189. doi: 10.1186/s12876-020-01341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kongkam P, Orprayoon T, Boonmee C et al. ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for malignant hilar biliary obstruction: a multicenter observational open-label study. Endoscopy. 2021;53:55–62. doi: 10.1055/a-1195-8197. [DOI] [PubMed] [Google Scholar]
- 25.Sawas T, Al Halabi S, Parsi MA et al. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82:256–267 e257. doi: 10.1016/j.gie.2015.03.1980. [DOI] [PubMed] [Google Scholar]
- 26.Xia MX, Cai XB, Pan YL et al. Optimal stent placement strategy for malignant hilar biliary obstruction: a large multicenter parallel study. Gastrointest Endosc. 2020;91:1117–1128 e1119. doi: 10.1016/j.gie.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, Lee SG, Kang D et al. The Comparison of Endoscopic Biliary Drainage in Malignant Hilar Obstruction by Cholangiocarcinoma: Bilateral Metal Stents versus Multiple Plastic Stents. Gut Liver. 2021;15:922–929. doi: 10.5009/gnl20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Zeng Z, Zeng J et al. Efficacy and Safety of First-Line Chemotherapies for Patients With Advanced Biliary Tract Carcinoma: A Systematic Review and Network Meta-Analysis. Front Oncol. 2021;11:736113. doi: 10.3389/fonc.2021.736113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottaiano A, Santorsola M, Diana A et al. Treatments, prognostic factors, and genetic heterogeneity in advanced cholangiocarcinoma: A multicenter real-world study. Cancer Med. 2024;13:e6892. doi: 10.1002/cam4.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Nakshabandi A, Ali FS, Albustami I et al. Biliary Drainage in Hilar and Perihilar Cholangiocarcinoma; 25-Year Experience at a Tertiary Cancer Center. Gastrointest Endosc. 2023 doi: 10.1016/j.gie.2023.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Lee TH, Moon JH, Choi JH et al. Prospective comparison of endoscopic bilateral stent-in-stent versus stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture. Gastrointest Endosc. 2019;90:222–230. doi: 10.1016/j.gie.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Cao Q, Sun L, Li ZQ et al. Bilateral stenting for hilar biliary obstruction: a meta-analysis of side-by-side versus stent-in-stent. Minim Invasive Ther Allied Technol. 2022;31:525–530. doi: 10.1080/13645706.2020.1871371. [DOI] [PubMed] [Google Scholar]
- 33.Ridtitid W, Rerknimitr R, Janchai A et al. Outcome of second interventions for occluded metallic stents in patients with malignant biliary obstruction. Surg Endosc. 2010;24:2216–2220. doi: 10.1007/s00464-010-0931-3. [DOI] [PubMed] [Google Scholar]
- 34.Okuno M, Mukai T, Iwashita T et al. Evaluation of endoscopic reintervention for self-expandable metallic stent obstruction after stent-in-stent placement for malignant hilar biliary obstruction. J Hepatobiliary Pancreat Sci. 2019;26:211–218. doi: 10.1002/jhbp.626. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura H, Hijioka S, Nagashio Y et al. Use of endoscopic ultrasound-guided biliary drainage as a rescue of re-intervention after the placement of multiple metallic stents for malignant hilar biliary obstruction. J Hepatobiliary Pancreat Sci. 2022;29:404–414. doi: 10.1002/jhbp.1059. [DOI] [PubMed] [Google Scholar]
- 36.Launois B, Reding R, Lebeau G et al. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000;7:128–134. doi: 10.1007/s005340050166. [DOI] [PubMed] [Google Scholar]
- 37.Covey AM, Brown KT. Palliative percutaneous drainage in malignant biliary obstruction. Part 1: indications and preprocedure evaluation. J Support Oncol. 2006;4:269–273. [PubMed] [Google Scholar]
- 38.Liang XY, Li W, Liu F et al. A Retrospective Study of Biliary Drainage Strategies for Patients with Malignant Hilar Biliary Strictures. Cancer Manag Res. 2021;13:4767–4776. doi: 10.2147/cmar.S308833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Lin N, Xin F et al. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol. 2019;17:116. doi: 10.1186/s12957-019-1656-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robson PC, Heffernan N, Gonen M et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol. 2010;17:2303–2311. doi: 10.1245/s10434-010-1045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neal CP, Thomasset SC, Bools D et al. Combined percutaneous-endoscopic stenting of malignant biliary obstruction: results from 106 consecutive procedures and identification of factors associated with adverse outcome. Surg Endosc. 2010;24:423–431. doi: 10.1007/s00464-009-0586-0. [DOI] [PubMed] [Google Scholar]
- 42.Pausawasdi N, Termsinsuk P, Charatcharoenwitthaya P et al. Development and validation of a risk score for predicting clinical success after endobiliary stenting for malignant biliary obstruction. PLoS One. 2022;17:e0272918. doi: 10.1371/journal.pone.0272918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Termsinsuk P, Charatcharoenwitthaya P, Pausawasdi N. Development and validation of a 90-day mortality prediction model following endobiliary drainage in patients with unresectable malignant biliary obstruction. Front Oncol. 2022;12:922386. doi: 10.3389/fonc.2022.922386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogura T, Higuchi K. Endoscopic Ultrasound-Guided Hepaticogastrostomy: Technical Review and Tips to Prevent Adverse Events. Gut Liver. 2021;15:196–205. doi: 10.5009/gnl20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogura T, Sano T, Onda S et al. Endoscopic ultrasound-guided biliary drainage for right hepatic bile duct obstruction: novel technical tips. Endoscopy. 2015;47:72–75. doi: 10.1055/s-0034-1378111. [DOI] [PubMed] [Google Scholar]
- 46.Ma KW, So H, Cho DH et al. Durability and outcome of endoscopic ultrasound-guided hepaticoduodenostomy using a fully covered metal stent for segregated right intrahepatic duct dilatation. Journal of Gastroenterology and Hepatology. 2020;35:1753–1760. doi: 10.1111/jgh.15089. [DOI] [PubMed] [Google Scholar]
- 47.Sekine M, Hashimoto Y, Shibuki T et al. A retrospective multicenter study comparing the punctures to B2 and B3 in endoscopic ultrasound–guided hepaticogastrostomy. DEN Open. 2023;3:e201. doi: 10.1002/deo2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umeda J, Itoi T, Tsuchiya T et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: a prospective preliminary feasibility study (with videos) Gastrointest Endosc. 2015;82:390–INF. doi: 10.1016/j.gie.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 49.Park SW, Lee SS. Which Are the Most Suitable Stents for Interventional Endoscopic Ultrasound? J Clin Med. 2020;9 doi: 10.3390/jcm9113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibuki T, Okumura K, Sekine M et al. Covered self-expandable metallic stents versus plastic stents for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Clin Endosc. 2023;56:802–811. doi: 10.5946/ce.2022.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung Ki EL, Napoleon B. EUS-specific stents: Available designs and probable lacunae. Endosc Ultrasound. 2019;8:S17–s27. doi: 10.4103/eus.eus_50_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogura T, Higuchi K. Technical tips for endoscopic ultrasound-guided hepaticogastrostomy. World J Gastroenterol. 2016;22:3945–3951. doi: 10.3748/wjg.v22.i15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho DH, Lee SS, Oh D et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos) Gastrointest Endosc. 2017;85:1067–1075. doi: 10.1016/j.gie.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Han SY, Baek D et al. Risk Factors for Early- and Late-Onset Cholecystitis after Y-Configured Metal Stent Placement in Patients with Malignant Hilar Biliary Obstruction: A Single-Center Study. Journal of Clinical Medicine. 2023;12:4354. doi: 10.3390/jcm12134354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isayama H, Kawabe T, Nakai Y et al. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006;4:1148–1153. doi: 10.1016/j.cgh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Kozakai F, Kanno Y, Ito K et al. Endoscopic Ultrasonography-Guided Gallbladder Drainage as a Treatment Option for Acute Cholecystitis after Metal Stent Placement in Malignant Biliary Strictures. Clin Endosc. 2019;52:262–268. doi: 10.5946/ce.2018.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y, Liu L, Wu JC et al. Efficacy and safety of photodynamic therapy for unresectable cholangiocarcinoma: A meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:718–724. doi: 10.1016/j.clinre.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Dolak W, Schwaighofer H, Hellmich B et al. Photodynamic therapy with polyhematoporphyrin for malignant biliary obstruction: A nationwide retrospective study of 150 consecutive applications. United European Gastroenterol J. 2017;5:104–110. doi: 10.1177/2050640616654037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt A, Bloechinger M, Weber A et al. Short-term effects and adverse events of endoscopically applied radiofrequency ablation appear to be comparable with photodynamic therapy in hilar cholangiocarcinoma. United European Gastroenterol J. 2016;4:570–579. doi: 10.1177/2050640615621235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang H, Han SY, Cho JH et al. Efficacy and safety of temperature-controlled intraductal radiofrequency ablation in advanced malignant hilar biliary obstruction: A pilot multicenter randomized comparative trial. J Hepatobiliary Pancreat Sci. 2022;29:469–478. doi: 10.1002/jhbp.1082. [DOI] [PubMed] [Google Scholar]
- 61.Oh D, Chong J, Song TJ et al. The usefulness of endobiliary radiofrequency ablation before metal stent placement in unresectable malignant hilar obstruction. J Gastroenterol Hepatol. 2022;37:2083–2090. doi: 10.1111/jgh.15967. [DOI] [PubMed] [Google Scholar]