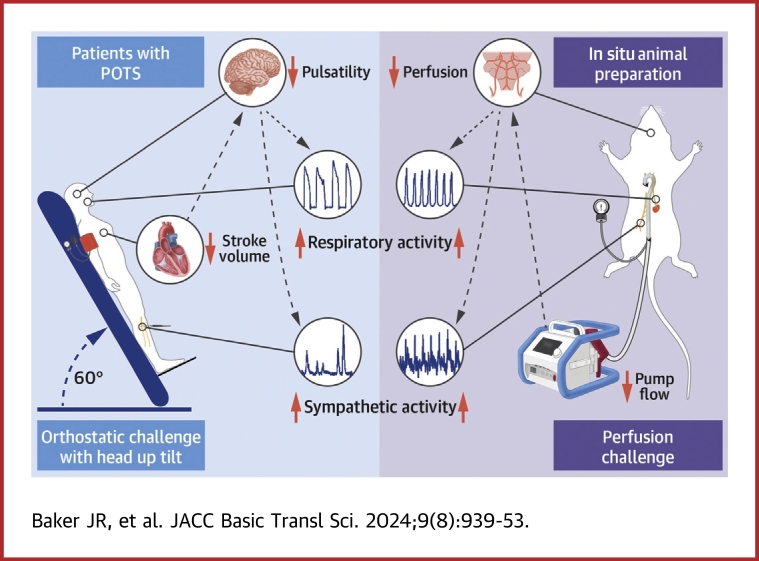
Visual Abstract
Key Words: brain perfusion, chemoreceptor activity, postural hyperventilation, postural orthostatic tachycardia syndrome, stroke volume
Highlights
-
•
POTS causes cardiovascular instability and severe symptoms.
-
•
Postural hyperventilation is thought to cause POTS, but the mechanisms are unclear.
-
•
Reduced stroke volume and brain perfusion drive postural hyperventilation in POTS.
-
•
Stroke volume and brain perfusion should be targeted as management strategies.
Summary
Postural hyperventilation has been implicated as a cause of postural orthostatic tachycardia syndrome (POTS), yet the precise mechanisms underlying the heightened breathing response remain unclear. This study challenges current hypotheses by revealing that exaggerated peripheral chemoreceptor activity is not the primary driver of postural hyperventilation. Instead, significant contributions from reduced stroke volume and compromised brain perfusion during orthostatic stress were identified. These findings shed light on our understanding of POTS pathophysiology, emphasizing the critical roles of systemic hemodynamic status. Further research should explore interventions targeting stroke volume and brain perfusion for more effective clinical management of POTS.
Postural orthostatic tachycardia syndrome (POTS) is a chronic, debilitating disorder of the autonomic nervous system that predominantly affects premenopausal women.1, 2, 3 Adult patients with POTS present with cardiovascular dysregulation and crippling cardiac and neurologic symptoms (eg, light-headedness, blurred vision, shortness of breath, presyncope).2,4 While upright, patients can also experience exaggerated sympathetic nervous system activity, paralleled by exaggerated increases in ventilation,5, 6, 7, 8, 9 which decreases carbon dioxide levels in the blood (ie, hypocapnia). Hypocapnia reduces cerebral blood velocity10,11 and exacerbates symptoms.10, 11, 12 As postural hyperventilation has been implicated as a cause of POTS, identifying the underlying mechanisms is crucial to the development of targeted therapies.
Studies have proposed that heightened peripheral chemoreceptor activity underlies postural ventilation and sympathetic activation in patients with POTS.5,9,13,14 Peripheral chemoreceptors, mainly the carotid bodies, respond to hypoxia (low O2), hypercapnia (high CO2), and hypoperfusion,15,16 and they increase ventilation and sympathetic activity when activated.15, 16, 17 In patients with POTS, reduced cardiac venous return and stroke volume in the upright position5,18, 19, 20 are thought to reduce blood flow to the carotid bodies, leading to hypoperfusion, and subsequent activation.5,9
Alternatively, reduced cerebral blood velocity at the onset of orthostasis can precede increases in ventilation and sympathetic activity in patients with POTS.6,21 These findings suggest that brain hypoperfusion may underlie increased ventilation and sympathetic activity when patients are upright. The roles of peripheral chemoreceptor activity and changes in systemic and cerebrovascular hemodynamic status as drivers of postural ventilation and sympathetic activation have not been examined concurrently in patients with POTS.
In this study, we explored potential mechanisms driving postural ventilation in patients with POTS. Initially, we assessed exaggerated peripheral chemoreceptor activity driving postural ventilation and increased sympathetic activity. Next, we examined the relationship among postural hemodynamic status, ventilation, and sympathetic activity in both humans and an in situ preparation.
Methods
Ethics
Ethical approval for this study was obtained from the Conjoint Health Research Ethics Board (REB21-1409) and Animal Care Committee (AC19-0037) at the University of Calgary. Written informed consent was obtained from all human participants prior to other study procedures.
Human experiments
Participants
All studies were performed in Calgary (altitude 1,045 m above sea level). A total of 25 female patients with POTS were recruited from the Calgary Autonomic Investigation and Management Clinic, the autonomic research laboratory, and through our local study database. All patients had physician diagnoses of POTS according to the consensus statement criteria.1,3 Diagnostic orthostatic vital signs are presented in Table 1. Eleven sex- and age-matched (±3 years) control subjects were recruited from the community via local advertising. There were no baseline differences between female patients with POTS and control subjects (Table 2). Study participants were excluded if they were smokers, pregnant or breastfeeding, could not tolerate wearing an oxygen mask, required portable oxygen at rest or with exercise, or had chronic heart failure or severe pulmonary disease. Additional exclusion criteria included dementia, alcohol and/or drug abuse, cerebrovascular disease, kidney or liver disease, and sympathectomy. All participants were asked to abstain from alcohol, caffeine, and exercise for 12 hours prior to testing. Patients were asked to hold medications that modulate heart rate and blood pressure, if possible. Fourteen patients (56%) were not taking any heart rate–modulating or blood pressure–modulating medications during the study, including beta-blockers, midodrine, fludrocortisone, pyridostigmine, ivabradine, clonidine, and methyldopa (Table 2). Stratified analyses were done to compare outcomes between patients who were off all relevant medications and those who remained on some of these medications. Only baseline heart rate was different between patients on vs off medications. There were no other differences in baseline or upright ventilatory, sympathetic, or hemodynamic parameters between patients on vs off medications (Supplemental Table 1). Additionally, there were no differences in the supine or upright ventilatory responses to hyperoxia between patients on vs off their treatment medications (Supplemental Figure 1).
Table 1.
Orthostatic Vital Signs During Head-Up Tilt in Female Patients With Postural Orthostatic Tachycardia Syndrome (n = 25)
| Supine heart rate, beats/min | 76 ± 13 |
| Supine systolic blood pressure, mm Hg | 121 ± 17 |
| Upright heart rate, beats/min | 124 ± 17 |
| Upright systolic blood pressure, mm Hg | 116 ± 20 |
| Δ Heart rate, beats/min | 47 ± 13 |
| Δ Systolic blood pressure, mm Hg | −5 ± 17 |
Values are mean ± SD.
Table 2.
Baseline Characteristics in Female Patients With POTS and Control Subjects
| Control Subjects (n = 11) |
Patients With POTS (n = 25) |
SMD | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 31 ± 7 | 31 ± 8 | 0.11 | 0.76 |
| Height, cm | 163 ± 4 | 167 ± 9 | 0.62 | 0.10 |
| Weight, kg | 62.0 ± 5.2 | 67.5 ± 8.7 | 0.71 | 0.059 |
| Body mass index, kg/m2 | 23.5 ± 2.3 | 24.3 ± 3.9 | 0.23 | 0.53 |
| Medications (held/prescribed) | ||||
| Beta-blockers | NA | 11/17 | NA | |
| Midodrine | NA | 3/9 | NA | |
| Fludrocortisone | NA | 0/2 | NA | |
| Ivabradine | NA | 1/2 | NA | |
| Sympatholytic | NA | 5/6 | NA | |
| Pyridostigmine | NA | 1/2 | NA | |
| Ventilatory | ||||
| Ventilation, L/min | 10.1 ± 2.0 | 9.2 ± 2.6 | 0.35 | 0.34 |
| End-tidal CO2, mm Hg | 39.5 ± 2.9 | 38.0 ± 3.8 | 0.42 | 0.26 |
| End-tidal O2, mm Hg | 90.4 ± 5.0 | 92.5 ± 7.0 | 0.32 | 0.39 |
| Peripheral hemodynamics | ||||
| Heart rate, beats/min | 69 ± 9 | 71 ± 13 | 0.13 | 0.72 |
| Stroke volume, mL | 78 ± 12 | 79 ± 15 | 0.05 | 0.89 |
| Cardiac output, L/min | 5.4 ± 0.7 | 5.6 ± 1.1 | 0.18 | 0.62 |
| Mean arterial pressure, mm Hg | 91 ± 12 | 91 ± 13 | 0.01 | 0.97 |
| Systemic vascular resistance, dynes/cm5 | 1,920 ± 695 | 1,671 ± 557 | 0.41 | 0.26 |
| Pulse pressure, mm Hg | 66 ± 9 | 63 ± 11 | 0.32 | 0.38 |
| Arterial pulsatility index | 0.73 ± 0.1 | 0.69 ± 0.1 | 0.39 | 0.29 |
| Cerebral hemodynamics | ||||
| MCAvmean, cm/s | 85 ± 10 | 78 ± 13 | 0.62 | 0.094 |
| MCAvpeak, cm/s | 123 ± 14 | 114 ± 18 | 0.57 | 0.12 |
| MCAvmin, cm/s | 66 ± 8 | 60 ± 11 | 0.63 | 0.093 |
| MCAv pulsatility index | 0.68 ± 0.1 | 0.70 ± 0.1 | 0.26 | 0.48 |
| Sympathetic | (n = 9) | (n = 12) | ||
| Burst frequency, bursts/min | 15 ± 6 | 13 ± 5 | 0.51 | 0.26 |
| Burst amplitude, % of peak | 40 ± 9 | 41 ± 14 | 0.12 | 0.78 |
| Total MSNA, AU/min | 645 ± 312 | 566 ± 320 | 0.25 | 0.58 |
Values are mean ± SD. Group differences were compared using an independent-samples 2-tailed Student’s t-test.
AU = arbitrary units; MCAv = middle cerebral artery velocity; MSNA = muscle sympathetic nerve activity; NA = not applicable; POTS = postural orthostatic tachycardia syndrome; SMD = standardized mean difference.
Human instrumentation
Hemodynamic status
Participants were instrumented with a 3-lead electrocardiograph to record heart rate and a noninvasive beat-to-beat finger blood pressure cuff (Finapres NOVA, FMS). Noninvasive brachial blood pressure measurements were performed throughout the study for intermittent finger cuff calibration. The beat-to-beat blood pressure waveform was used to calculate mean, systolic, and diastolic blood pressures and was analyzed to obtain estimates of stroke volume, cardiac output, and systemic vascular resistance using Modelflow waveform analysis,22 which has been previously validated to provide estimates of advanced hemodynamic during orthostatic challenges.23 The beat-to-beat systemic arterial pulsatility index was calculated as: (systolic blood pressure − diastolic blood pressure) / mean blood pressure. A 2-MHz transcranial Doppler ultrasound system (Doppler Box, Compumedics DWL USA) was used to measure cerebral blood velocity in the middle cerebral artery velocity (MCAv), including beat-to-beat peak, minimum, and mean MCAv. The beat-to-beat MCAv pulsatility index was calculated as: (peak MCAv − minimum MCAv)/mean MCAv. For quantifying responses to early head-up tilt, stable MCAv recordings were obtained in 21 patients (84%) with POTS and 11 (100%) control subjects.
Muscle sympathetic nerve activity
Muscle sympathetic nerve activity (MSNA) recordings were obtained using tungsten microelectrodes with 2-MΩ impedance (Frederick Haer). The raw signal was amplified (75,000-fold), band-pass filtered (0.3-2.0 kHz), rectified, and integrated using a 0.1-second time constant to obtain the mean voltage neurogram (Nerve Traffic Analyzer model 662C-4, University of Iowa). The microelectrode was inserted percutaneously into the common fibular nerve, and a reference electrode was placed about 2 cm adjacent to the insertion point at a similar depth. The microelectrode was advanced into nerve bundles directed toward skeletal muscle, as indicated by afferent feedback during tapping or palpation of the tibialis anterior and peroneal muscles and absent feedback from light stroking of the skin on the dorsal foot and lower leg. The presence of MSNA was confirmed by the occurrence of spontaneous pulse synchronous bursts (ie, burst widths no greater than the R-R interval) and reflexive burst increases to a Valsalva maneuver strain and/or a voluntary end-expiration apnea but not to a startle stimulus (eg, unexpected clapping). The neural signal was continuously monitored and any changes to microelectrode position were documented. Total MSNA was quantified as the product of burst frequency (per minute) and mean burst amplitude (quantified as the burst peak relative to the noise immediately preceding; the mean burst peak was normalized to the largest burst within each prestimulus period24,25). All hemodynamic and nerve recording data were sampled at 10 kHz (WinDaq, DATAQ) for off-line analysis (LabChart Pro 8, AD Instruments). Baseline MSNA recordings in the supine position were obtained in 12 patients with POTS and 9 control subjects. Only recordings with repeated measures in both the supine and upright positions were used to quantify responses to hyperoxia (6 patients with POTS, 8 control subjects) and early head-up tilt (9 patients with POTS, 5 control subjects).
End-tidal sequential gas delivery
Participants were fitted with a facemask sealed with medical-grade skin adhesive tape (Tegaderm, 3M Health Care). Respired gases were sampled at the mouth, analyzed for end-tidal O2 and CO2, and controlled by an automated, noninvasive programmable sequential gas delivery system (RespirAct, Thornhill Medical).26,27 Breath-by-breath end-tidal O2, end-tidal CO2, tidal volume, respiratory rate, and ventilation were measured and calculated using the RespirAct software. All respiratory data were sampled at 50 Hz.
Human experimental protocol
We tested hyperoxia vs normoxia in a random sequence in the supine and head-up tilt positions in patients with POTS and control subjects. Following a minimum 10-minute baseline, participants were passively tilted to 60° from supine. Head-up tilt was performed with one leg supported in a slightly bent position for the microneurographic recording. To improve microelectrode stability, the transition from supine to 60° was slowly performed over 30 to 45 seconds.
To inhibit the peripheral chemoreceptors, participants breathed 5 30-second bouts of normocapnic hyperoxia (400-450 mm Hg O2) with 90 seconds of normocapnic normoxia between each bout.28,29 Administration of short, repetitive bouts of hyperoxia was used to ensure cardiovascular and ventilatory parameters were not confounded by the production of CO2 and to avoid the confounding effects of extended periods of hyperoxia acting as a central stimulant.30 A minimum 3-minute baseline preceded each hyperoxia protocol, with a 10-minute rest between supine and head-up tilt protocols to ensure that ventilatory and hemodynamic parameters returned to baseline. All participants completed testing in the supine position. Two patients with POTS could not complete the full duration of head-up tilt. Therefore, data from 23 patients with POTS during the head-up tilt hyperoxia assessment were included.
Human data analysis
For each participant, average baseline hemodynamic and ventilatory data were calculated over the final 2 minutes of baseline prior to supine and head-up tilt testing. Hemodynamic and ventilatory responses to head-up tilt were calculated as the average change from baseline during the first 90 seconds of 60° head-up tilt (ie, early head-up tilt). To assess responses to hyperoxia, hemodynamic and ventilatory parameters were averaged over the 30-second normoxic period preceding each hyperoxic bout and as 5-second averages during hyperoxia. The ventilatory response to hyperoxia were determined as the average 5-second nadir during each hyperoxic bout. For each participant, ventilatory and hemodynamic data during the final 3 hyperoxic trials were averaged to obtain a steady-state average response.
MSNA was quantified as burst frequency (bursts per minute), normalized burst amplitude (percentage of tallest burst in a prestimulus period), and total MSNA (product of burst frequency and normalized burst height, expressed as arbitrary units [AU] per minute). The variable nature of spontaneous MSNA can compromise the validity of sampling durations <60 seconds.24 To circumvent this limitation while still capturing transient hyperoxic inhibition, MSNA values were first calculated in 5-second bins and then averaged across 3 prehyperoxic periods and respective hyperoxic bouts. These bins were then used to calculate 20-second averages that continually moved ahead by one 5-second bin for the duration of the prehyperoxic period and hyperoxic bout. These procedures achieved 60 seconds of data to represent each 5-second window before and during hyperoxia delivery. The hyperoxic response was determined as the 5-second nadir relative to the prehyperoxic period. Because of unpredictable shifts in the MSNA signal during head-up tilt, only trials in which the MSNA signal was stable (ie, no audible nerve sheath crossings or obvious visual or auditory change to the signal-to-noise ratio) for the duration of the hyperoxic bouts (ie, 30 seconds preceding hyperoxia and 30 seconds during hyperoxia delivery) were used. Thus, the last 3 trials were not used for all participants. For each hyperoxic bout, MSNA burst amplitudes were normalized to the largest burst in each 30-second prehyperoxic period.
Semi-intact animal experiments
The perfused working heart–brain stem preparation
To investigate the influence of brain perfusion pressure on ventilatory and sympathetic control, we used a an iteration of semi-intact preparation of the rat neurovascular system (the working heart–brain stem preparation).31,32 The working heart–brain stem preparation is an established research tool designed to assess the neural mechanisms regulating the respiratory and cardiovascular systems and their coupling in health and disease.31 In this reduced in situ model, there is no heart, lungs, abdominal viscera, or input from other brain areas (ie, decerebrate), and the brain stem is perfused with artificial blood by a peristaltic pump. This semi-intact model was chosen because: 1) the preparation is eviscerated and exsanguinated, which allows us to assess the effects of brain perfusion on respiratory and sympathetic nerve activity independent of humoral, blood-borne factors, and visceral afferents; 2) respiratory and autonomic nerve activity can be monitored in the absence of peripheral respiratory chemosensory and baroreceptors feedback; 3) the perfusate composition, gases, flow rate, and pressure are under precise experimental control;31 and 4) the use of a decerebrate preparation eliminates the need for anesthesia, which in turn eliminates its influence on cardiovascular hemodynamic status.
Animals
For the working heart–brain stem preparation, prepubescent male Sprague-Dawley rats (4-6 weeks of age, weight 80-150 g) were used. Male rats were used because they are larger at younger, prepubescent ages, which allows higher flow rates and better perfusion pressure control. Data from preparations lacking rhythmic eupneic phrenic bursts (n = 1) or in which phrenic or sympathetic nerve recordings were lost before protocol completion (n = 4) were excluded.32 Data presented were obtained from 10 complete preparations.
In situ animal instrumentation
Pressure recordings
Perfusion pressure was measured in the lumen of the descending aorta using a dual-lumen cannula connected to a pressure transducer (DTX Blood Pressure Transducer, BPM-382 Pressure Monitor, CWE).
Nerve recordings
Silver hook electrodes were used to record from the phrenic and greater splanchnic nerves to provide indexes of respiratory and sympathetic nerve activity, respectively. Neural activity was amplified, filtered (phrenic, 300- to 1,000-Hz band-pass filter; splanchnic, 100- to 1,000-Hz band-pass filter), rectified, and integrated (200-ms time constant decay; MA-821/RSP Moving Averager, CWE). All data were digitized (Digidata 1322A, Molecular Devices) and sampled at 5 kHz (AxoScope 9.0, Molecular Devices) for off-line analysis (LabChart Pro 8).
In situ animal experimental protocol
The rats were deeply anesthetized only at the onset of the experiment protocol (absent response to noxious tail or hind paw pinch) with 5% isoflurane in air. While under a deep surgical anesthetic plane, rats were bathed in ice-chilled physiological saline solution (115 mM NaCl, 4 mM KCl, 1 mM MgSO4, 24 mM NaHCO3, 1.25 mM NaH2PO4, 2 mM CaCl2, 10 mM D-glucose, and 12 mM sucrose), spinally transected below the diaphragm near the thoracic-lumbar junction, and decerebrated at the midbrain (ie, the midcollicular level). Rats were then carefully eviscerated with preservation of the phrenic and greater splanchnic nerves before being transferred to an artificial perfusion rig. Once decerebrated, anesthesia is no longer necessary, and the preparation is considered unanesthetized during the subsequently described experimental protocol.
The descending aorta was perfused with physiological saline solution equilibrated with 40 torr CO2 and balance O2. The preparation was first perfused with cool (∼15 °C) perfusate and then gradually warmed until the preparation reached 32 to 33 °C. Mean arterial pressure was measured using a dual-lumen cannula and gradually raised to 90 mm Hg with a servo-controlled rotary pump.
To determine whether the brain can modulate respiratory and sympathetic nerve activity during acute changes in perfusion (ie, flow and pressure) independent of peripheral chemo- and baroreceptor input, 2 groups of animals were tested, those with intact and bilateral denervated carotid sinus and common vagus nerves. In both groups, after a 10-minute baseline at constant temperature and pressure, the servo-controlled pump speed was randomized to either decrease pressure to about 60 mm Hg or increase pressure to about 120 mm Hg. Each pressure challenge lasted 30 to 60 seconds. Baseline respiratory and sympathetic nerve activity parameters in preparations with intact and denervated peripheral chemoreceptor and baroreceptor afferents were not different (Supplemental Table 6).
In situ animal data analysis
Phrenic bursts were used to indicate the inspiratory phase of the respiratory cycle. The product of phrenic burst rate and burst amplitude (an index of inspiratory tidal volume33) was used as an index of respiratory nerve activity.32 For analysis of sympathetic nerve activity, all activity was first subtracted by the noise level determined after a 10- to 20-minute period after ceasing arterial perfusion to the preparation.34 Mean sympathetic activity was then signal averaged relative to each respiratory cycle (ie, whole respiratory cycle, from phrenic burst onset to next burst onset; inspiration, from phrenic burst onset to burst peak; expiration, from phrenic burst peak to the next phrenic burst onset).34
Respiratory frequency, inspiratory and expiratory times, ratio of inspiratory to expiratory time, eupneic index (inspiratory ramp time divided by the total inspiratory time32), and normalized sympathetic nerve activity were assessed at baseline. Respiration frequency, neural tidal volume, total respiratory nerve activity, and sympathetic nerve activity were normalized to the mean of a 1-minute baseline period. During perfusion pressure challenges, the peak and nadir activity across a 15-second moving average (averaging window moved ahead 1 phrenic burst at a time) was determined.
Statistical analysis
Human experiments
All data are presented as mean ± SD, unless otherwise specified. There are no prior data assessing acute hyperoxia on ventilation and sympathetic nerve responses in patients with POTS. The sample size was therefore estimated on the basis of previous studies using acute hyperoxia to assess the contribution of the peripheral chemoreceptors to ventilation in various clinical populations.35, 36, 37, 38, 39, 40 Normality of continuous variables was determined using the Shapiro-Wilk test. Participant demographics, baseline hemodynamic status, orthostatic hemodynamic status, and ventilatory changes (expressed relative to baseline) were compared between patients with POTS and control subjects using an independent-samples, 2-tailed Student’s t-test. Continuous cardiorespiratory responses to hyperoxia were compared using a 2-factor mixed-effects model: [group: control vs patient] × [gas (repeated measure): normoxia vs hyperoxia] with Bonferroni post hoc testing correction. Group differences in ventilation and MSNA inhibition during hyperoxia were compared using a 2-factor mixed-effects model: [group: POTS vs control] × [position (repeated measure): supine vs head-up tilt] with Bonferroni post hoc testing correction. Linear regressions were used to investigate the relationship of changes in ventilation and sympathetic nerve activity with hemodynamic status using Pearson’s correlation coefficient (r).
In situ animal experiments
Changes from baseline in neural activity for the denervated group were assessed using a nonparametric, 2-tailed Mann-Whitney U test. A 2-factor mixed-effects model—[group: intact vs denervated] × [pressure (repeated measure): baseline vs low pressure vs high pressure]—with a Bonferroni post hoc analysis was used to compare pressure and neural activity in the denervated and intact groups.
Statistical significance was set at P < 0.05. Statistical analyses were performed using Prism version 9.4.1 (GraphPad Software). Figures were created using Prism and BioRender (biorender.com).
Results
Cardiorespiratory responses to postural stress are impaired in patients with POTS
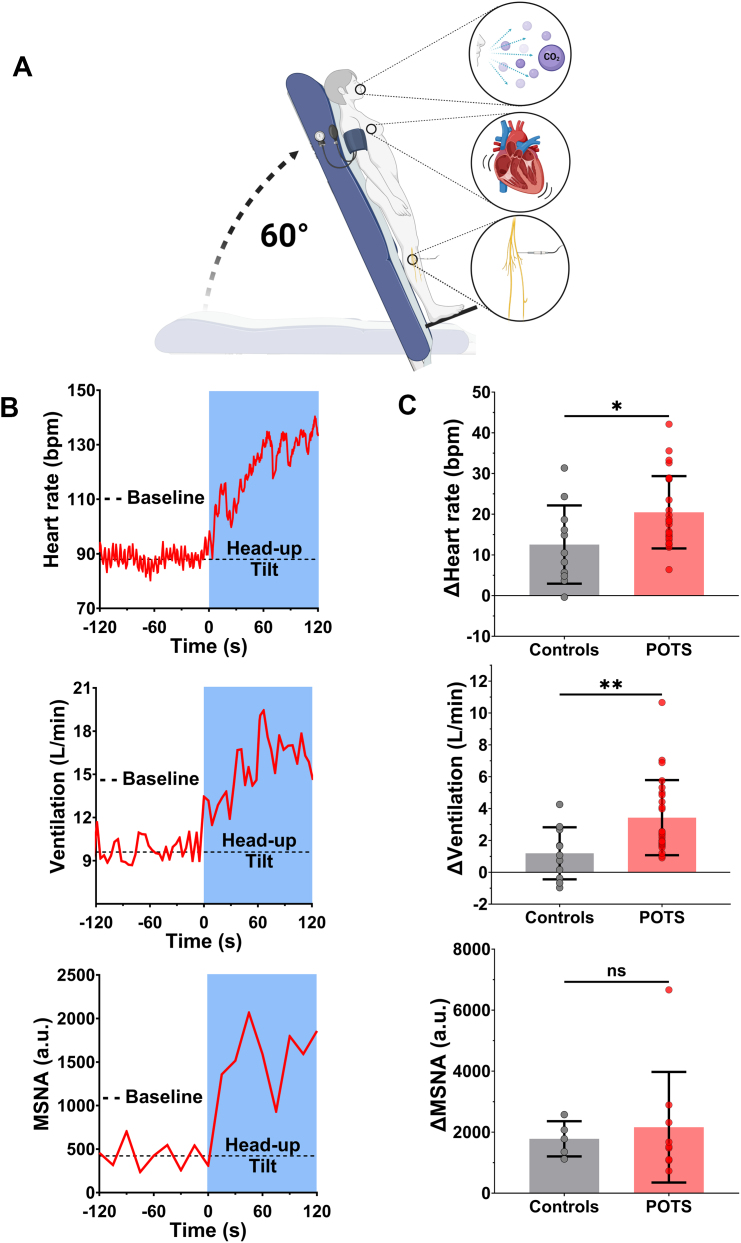
Cardiorespiratory responses to postural stress were characterized in patients with POTS and control subjects (Figure 1A). In response to head-up tilt, patients had larger increases in heart rate (+21 ± 9 beats/min vs +13 ± 10 beats/min; P = 0.022) (Figures 1B and 1C, top; Table 3) and ventilation (+3.4 ± 2.4 L/min vs +1.2 ± 1.6 L/min; P = 0.007) (Figures 1B and 1C, middle) compared with control subjects, despite no differences in systolic (POTS vs control subjects: Δ6 ± 12 mm Hg vs Δ10 ± 9 mm Hg; P = 0.29), diastolic (POTS vs control subjects: Δ10 ± 9 mm Hg vs Δ9 ± 7 mm Hg; P = 0.87), or mean blood pressure responses (POTS vs control subjects: Δ9 ± 10 mm Hg vs Δ10 ± 8 mm Hg; P = 0.74) (Table 3). Unexpectedly, our POTS cohort did not demonstrate exaggerated increases in MSNA compared with control subjects (Figures 1F and 1G, Table 3).
Figure 1.
Neurocardiorespiratory Responses to Head-Up Tilt
(A) Orthostatic challenges were performed in patients with postural orthostatic tachycardia syndrome (POTS) and control subjects using a 60° head-up tilt test with continuous measurements of ventilation, heart rate, and muscle sympathetic nerve activity (MSNA). (B) Neurocardiovascular responses to head-up tilt. Line plots illustrate representative patient traces of heart rate (top), ventilation (middle), and MSNA (bottom). (C) Bar charts show changes in heart rate (top), ventilation (middle), and MSNA (bottom). Data are presented as mean ± SD. Cardiorespiratory responses were compared between patients with POTS and control subjects using independent-samples 2-tailed Student’s t-tests. a.u. = arbitrary units.
Table 3.
Cardiorespiratory and Sympathetic Changes During Head-Up Tilt
| Control Subjects (n = 11) |
Patients With POTS (n = 25) |
P Value | |
|---|---|---|---|
| Peripheral hemodynamics | |||
| Δ Heart rate, beats/min | 12 ± 10 | 21 ± 9 | 0.022 |
| Δ Stroke volume, mL | −19 ± 10 | −26 ± 7 | 0.019 |
| Δ Cardiac output, L/min | −0.5 ± 0.5 | −0.8 ± 0.7 | 0.29 |
| Δ Mean arterial pressure, mm Hg | 10 ± 8 | 9 ± 10 | 0.74 |
| Δ Systemic vascular resistance, dynes/cm5 | 439 ± 412 | 425 ± 382 | 0.92 |
| Δ Pulse pressure, mm Hg | 0.6 ± 5.0 | −4.3 ± 5.6 | 0.016 |
| Δ Arterial pulsatility index | −0.06 ± 0.06 | −0.1 ± 0.04 | 0.026 |
| Cerebral hemodynamics | |||
| Δ MCAvmean, cm/s | −1.3 ± 3.6 | 0.7 ± 5.7 | 0.31 |
| Δ MCAvpeak, cm/s | −4.5 ± 5.1 | −4.1 ± 8.0 | 0.88 |
| Δ MCAvmin, cm/s | 0.3 ± 4.1 | 3.1 ± 5.0 | 0.13 |
| Δ MCAv pulsatility index | −0.05 ± 0.07 | −0.1 ± 0.05 | 0.022 |
| Sympathetic | (n = 5) | (n = 9) | |
| Δ Burst frequency, bursts/min | 20 ± 5 | 20 ± 11 | 0.87 |
| Δ Burst amplitude, % of peak | 32 ± 9 | 37 ± 14 | 0.43 |
| Δ Total MSNA, AU/min | 1,782 ± 576 | 2,162 ± 1,813 | 0.66 |
| End-tidal gases | |||
| Δ End tidal CO2, mm Hg | 0.01 ± 2.4 | −0.1 ± 0.3 | 0.35 |
| Δ End tidal O2, mm Hg | 0.2 ± 1.3 | 0.1 ± 1.2 | 0.76 |
Values are mean ± SD. P values in bold denote statistical significance.
Abbreviations as in Table 2.
To explore mechanisms underlying exaggerated postural ventilation without the confounding influence of changing arterial blood gases, we incorporated end-tidal gas clamping, which allowed us to precisely control end-tidal CO2 and O2 throughout the study. As a result, neither supine end-tidal CO2 or O2 (Table 2) nor the change in end-tidal CO2 and O2 (Table 3) during head-up tilt was different between patients and control subjects. Importantly, clamping end-tidal CO2 and controlling the confounding effects of arterial blood gases on cerebral blood flow helped maintain MCAv (peak, minimum, and mean) at similar levels during supine and head-up tilt (Table 3).
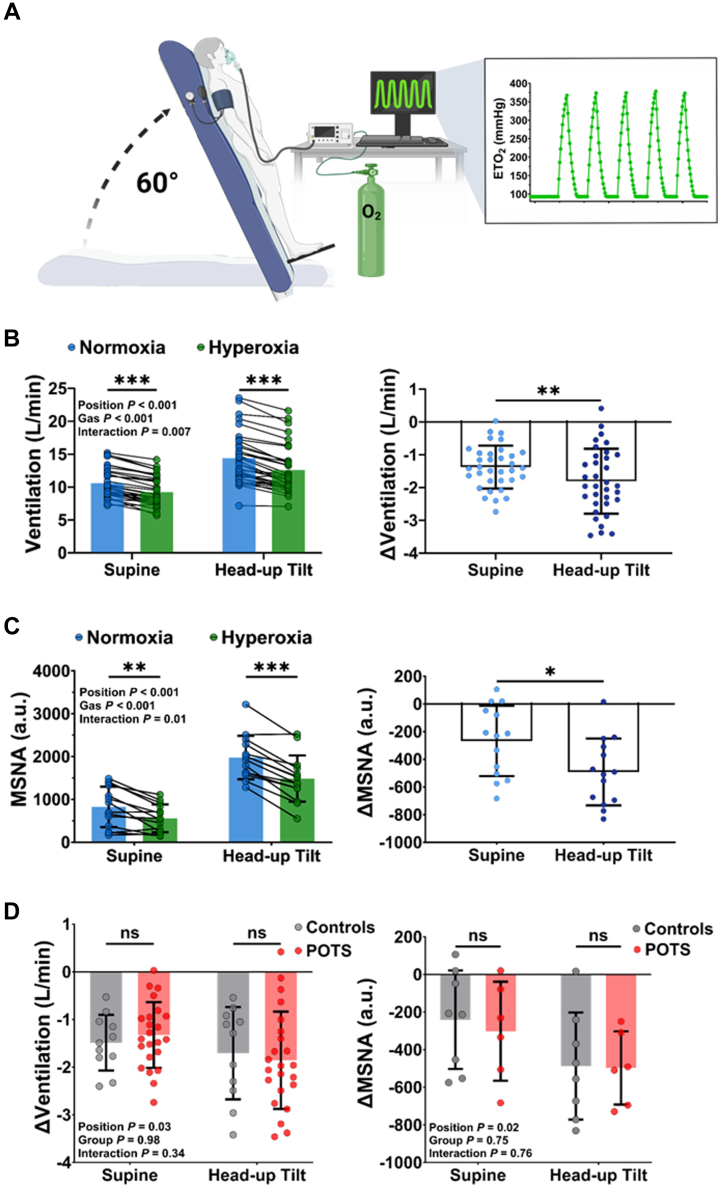
Peripheral chemoreceptor activity is not exaggerated in patients with POTS
To determine whether exaggerated peripheral chemoreceptor activity drives postural ventilation and sympathetic activity in patients, activity was inhibited using acute, transient exposures to hyperoxia in the supine and upright position (Figure 2A). During supine and upright hyperoxia, end-tidal O2 was significantly increased compared with normoxia in both groups (Supplemental Tables 2 to 5). The change in end-tidal O2 was not different between patients with POTS and control subjects in the either the supine (POTS vs control subjects: Δ269 ± 37 mm Hg vs Δ287 ± 21 mm Hg; P = 0.15) or upright (POTS vs control subjects: Δ280 ± 42 mm Hg vs Δ280 ± 16 mm Hg; P = 0.99) position. Across the cohort (patients with POTS and control subjects), hyperoxia significantly reduced ventilation in both the supine (Figure 2B) and upright (Figure 2B) positions, as well as total MSNA in the supine (Figure 2C) and upright (Figure 2C) positions. Compared with supine hyperoxia, during head-up tilt there was a greater reduction in ventilation (supine −1.4 ± 0.7 L/min vs head-up tilt −1.8 ± 1.0 L/min; P = 0.007) (Figure 2B) and total MSNA (supine −267 ± 254 AU vs head-up tilt −492 ± 241 AU; P = 0.013) (Figure 2C), but neither the magnitude of ventilatory inhibition nor total MSNA inhibition was different between patients with POTS and control subjects in either position (Figure 2D). These findings suggest that peripheral chemoreceptor activity is not exaggerated in patients with POTS relative to control subjects and thus is not a significant mechanism driving postural ventilation. A full summary of the cardiorespiratory responses to hyperoxia in patients with POTS and control subjects in the supine and upright position is shown in Supplemental Tables 2 to 5.
Figure 2.
Sympathorespiratory Responses to Peripheral Chemoreceptor Inhibition
(A) Hyperoxia was supplied using sequential gas delivery to precisely control acute, transient bouts of oxygen while participants were supine and upright. (B) Across the cohort, hyperoxia significantly reduced ventilation in the supine and upright positions (left), and the ventilatory reduction was greater during head-up tilt (right). (C) Pooled MSNA responses to hyperoxia were also reduced in the supine and upright position (left) and were similar reduced to a greater magnitude during head-up tilt (right). (D) Between-group comparisons showed that neither the magnitude of ventilation inhibition (left) nor sympathetic inhibition (right) was different between patients with POTS and control subjects in either position. Data are presented as mean ± SD. Pooled ventilatory and MSNA responses to hyperoxia and group differences in ventilation and MSNA inhibition during hyperoxia were compared using a 2-factor mixed-effects model with Bonferroni post hoc testing correction. Abbreviations as in Figure 1.
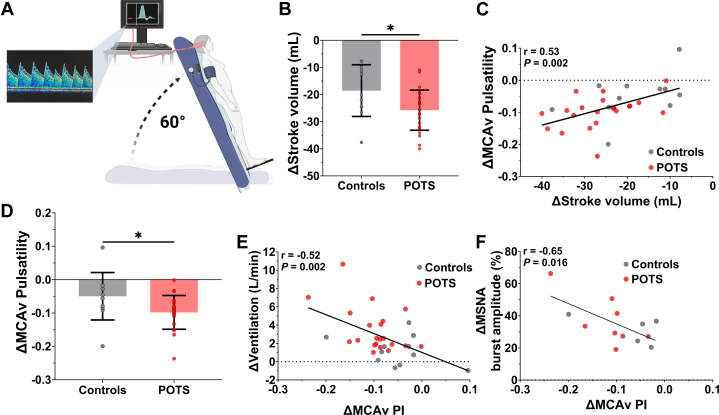
Stroke volume and cerebrovascular pulsatility as determinants of postural ventilation and sympathetic activity in patients with POTS
Next, we examined the relationship between postural hemodynamic status and ventilation and sympathetic activity using beat-to-beat transcranial Doppler ultrasound of the middle cerebral arteries (Figure 3A). During head-up tilt, patients with POTS had larger reductions in stroke volume compared with control subjects (Figure 3B, Table 3). Across the cohort (patients with POTS and control subjects), larger reductions in stroke volume were significantly correlated with increases in ventilation (r = −0.48; P = 0.003), heart rate (r = −0.53; P < 0.001), and systemic arterial pulsatility (r = 0.64; P < 0.001) but not with changes in systolic (r = −0.15; P = 0.64) or mean blood pressure (r = −0.30; P = 0.13) and only weakly with change in diastolic blood pressure (r = −0.39; P = 0.019). Importantly, stroke volume remained significantly related to ventilation (r = −0.54; P = 0.005), heart rate (r = −0.45; P = 0.023), and systemic arterial pulsatility (r = 0.48; P = 0.015) in only patients with POTS, suggesting that the results were not related to an artifact introduced by including control subjects. During head-up tilt, systemic arterial pulsatility was also significantly reduced in patients with POTS (Table 3). This reduction was only weakly correlated with increases in heart rate (r = −0.38; P = 0.021) and had no correlation with changes in ventilation (r = −0.23; P = 0.15), suggesting that the associations between reduced stroke volume and increased ventilation and heart rate may not be through mechanisms related to changes in peripheral arterial hemodynamic status, per se.
Figure 3.
Reduced Stroke Volume and Cerebrovascular Pulsatility Drive Postural Ventilation
(A) Middle cerebral artery velocity (MCAv) pulsatility was continuously measured during 60° head-up tilt using beat-to-beat transcranial Doppler ultrasound. (B) Postural reductions in stroke volume were greater in patients with POTS. (C) Changes in stroke volume correlated with changes in MCAv pulsatility. (D) Postural reductions in MCAv pulsatility were also greater in patients with POTS and were strongly predictive of postural changes in ventilation (E) and MSNA burst amplitude (F). Data are presented as mean ± SD. Hemodynamic response to head-up tilt was compared between patients with POTS and control subjects using an independent-samples, 2-tailed Student’s t-test. Linear regressions were used to investigate the relationship of changes in ventilation and sympathetic nerve activity and hemodynamic status. PI = pulsatility index; other abbreviations as in Figure 1.
Indeed, reductions in stroke volume were also predictive of reductions in MCAv pulsatility (Figure 3C), and we found that the reduction in MCAv pulsatility during head-up tilt was larger in patients with POTS compared with control subjects (Figure 3D, Table 3). Importantly, across the cohort (patients with POTS and control subjects), we found that reductions in MCAv pulsatility were strongly correlated with increases in ventilation (Figure 3E) and heart rate (r = −0.47; P = 0.007). Similar to stroke volume, the relationship between MCAv pulsatility and ventilation remained significant in only patients with POTS (r = −0.51; P = 0.018). Finally, although MSNA was not different between groups during head-up tilt, and no MSNA variables were correlated with changes in stroke volume (|r| < 0.40 for all; P > 0.16) or systemic arterial pulsatility (|r| < 0.36 for all; P > 0.21), reductions in MCAv pulsatility strongly correlated with increases in MSNA burst amplitude (Figure 3F) but not with changes in MSNA burst frequency (r = −0.37; P = 0.21) or total MSNA (r = −0.46; P = 0.12). Together, these data suggest a role for cerebrovascular pulsatility in the control of ventilation and sympathetic activity and strongly implicate reduced brain perfusion41,42 as a key mechanism underlying exaggerated postural ventilation in patients with POTS.
Reduced brain perfusion increases sympathorespiratory nerve activity in an in situ preparation
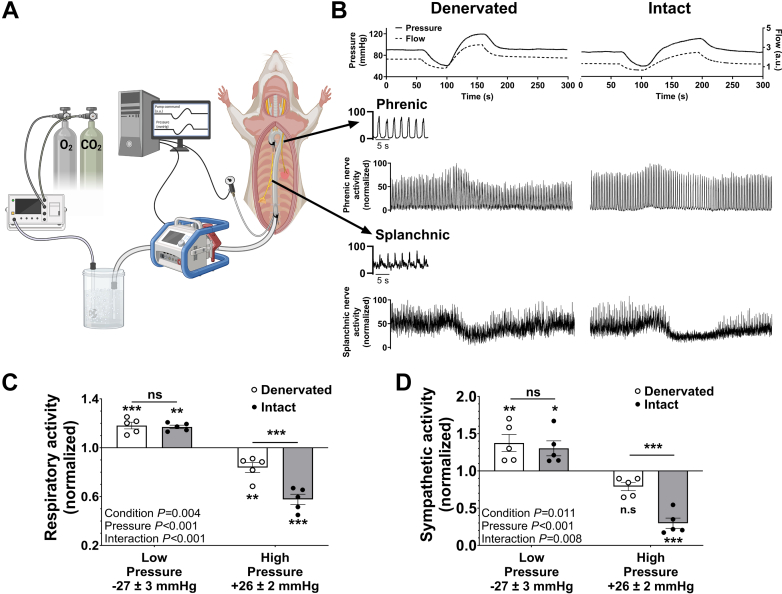
To test whether changes in brain perfusion modulate respiratory and sympathetic nerve activity independent of peripheral chemoreceptor and baroreceptor input, we used a rotary pump system to manipulate perfusion pressure in an arterially perfused in situ preparation with bilateral denervation of carotid sinus and common vagus nerves (n = 5) (Figure 4A). Perfusion pressure was randomly increased and decreased (Figure 4B, top), while phrenic (Figure 4B, middle) and splanchnic (Figure 4B, bottom) nerve activities were continuously recorded. Here, we observed that a reduction in brain perfusion pressure increased respiratory (Figure 4C) and sympathetic (Figure 4D) nerve activity relative to baseline. When perfusion pressure was raised, respiratory nerve activity decreased relative to baseline (Figure 4C), whereas sympathetic nerve activity was not significantly changed (Figure 4D).
Figure 4.
Sympathorespiratory Responses in a Semi-Intact Perfused Brain Preparation Due to Perfusion Changes
(A) The decerebrate rat preparation is perfused via the cannulated descending aorta. Spontaneous phrenic nerve (respiratory efferent) and greater splanchnic nerve (sympathetic efferent) activity are recorded in parallel with perfusion pressure changes. (B) Perfusion pressure was randomly increased and decreased (top) in preparations with denervated (top left) (n = 5) and intact (top right) (n = 5) peripheral chemoreceptor and baroreceptor afferents while phrenic (middle) and splanchnic (bottom) nerve activity was continuously measured. (C) Respiratory nerve activity and (D) sympathetic nerve activity were both reflexively inhibited and activated in response to high and low perfusion pressure, respectively. This pattern was observed in both denervated and intact rats. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 represent values significantly different from the normalized baseline of 1.0. Data are presented as mean ± SD. Respiratory and sympathetic nerve activity during pressure challenges in intact vs denervated animals were compared using a 2-factor mixed-effects model, Bonferroni post hoc analysis.
Importantly, respiratory and sympathetic responses to drops in perfusion pressure in denervated preparations were comparable with preparations with intact peripheral chemoreceptor and baroreceptor afferents (ie, no differences in total respiratory nerve activity [Figure 4C] or sympathetic nerve activity [Figure 4D]), suggesting that lower perfusion pressure is sensed primarily at the level of the central nervous system. Conversely, in response to increased perfusion pressure, we found that preparations with intact peripheral chemoreceptor and baroreceptor afferents had a greater decrease in respiratory (Figure 4C) and sympathetic nerve activity (Figure 4D). Together, these data show that reductions in brain perfusion can trigger increases in respiratory (ie, phrenic nerve) and sympathetic (ie, splanchnic nerve) nerve activity, signifying a key mechanism underlying postural ventilation in patients with POTS. A complete summary of the respiratory and sympathetic nerve responses to low and high perfusion in preparations with intact and denervated peripheral chemoreceptor and baroreceptor afferents is provided in Supplemental Table 7.
Discussion
Postural hyperventilation has been implicated as a cause of POTS; however, the mechanisms driving this clinical presentation are unclear. In this study, we examined potential mechanisms modulating postural ventilation in patients with POTS. First, we explored the possibility of exaggerated peripheral chemoreceptor activity and found that activity is not exaggerated in patients with POTS relative to control subjects and thus not a likely mechanism driving increased postural ventilation. We then examined the relationship between postural hemodynamic status, ventilation, and sympathetic nerve activity and showed that postural reductions in stroke volume and cerebrovascular pulsatility, a marker of brain perfusion, were significant determinants of postural ventilation and sympathetic activity. Finally, to isolate the effects of brain perfusion on sympathorespiratory control, we demonstrated in an in situ rodent preparation that reductions in brain perfusion increased respiratory (ie, phrenic) and sympathetic (ie, splanchnic) nerve activity. Overall, this set of translational studies have uncovered key mechanisms underlying postural ventilation and sympathetic activity in patients with POTS.
Cardiorespiratory and sympathetic responses to peripheral chemoreceptor inhibition
Previous studies have postulated exaggerated peripheral chemoreceptor activity drives postural ventilation and sympathetic activity in POTS.5,9,13 We tested this hypothesis using inspired hyperoxia to inhibit the peripheral chemoreceptors. In response to peripheral chemoreceptor inhibition, we observed transient reductions in ventilation and sympathetic nerve activity in both control subjects and patients with POTS in both the supine and upright position. However, contrary to previous hypotheses,9,13 there was no significant difference in the magnitude of either the ventilatory or sympathetic inhibition between patients and control subjects. These findings suggest that peripheral chemoreceptor activity is not exaggerated in patients with POTS and thus likely not a significant factor underlying exaggerated postural hyperventilation and sympathetic nerve activity in patients.
Impaired systemic hemodynamic status in POTS
During head-up tilt, patients had larger reductions in stroke volume compared with control subjects, which was strongly correlated with the change in ventilation. Reduced stroke volume in patients with POTS is likely due to reductions in cardiac preload from either impaired cardiac venous return or from reduced diastolic filling time secondary to tachycardia. Although splanchnic and lower limb venous distension was not assessed in the present study, prior studies have shown persistent splanchnic hyperemia during head-up tilt in patients with POTS,20,43 which may indicate splanchnic venous distension with subsequent reduced cardiac venous return. Moreover, many patients with POTS have low blood volumes,2 which could also contribute to reduced stroke volume and, in turn, reduced brain perfusion. Further investigations into the role of peripheral venous hemodynamic status and impaired blood volume regulation as a mechanism driving postural ventilation are warranted.
Reduced brain perfusion increased respiratory and sympathetic nerve activity
In addition to stroke volume differences, patients with POTS also had larger postural reductions in cerebrovascular pulsatility compared with control subjects. Reductions in cerebral blood velocity during orthostatic stress have been shown to precede ventilatory changes,6,21 suggesting that brain blood flow and perfusion may influence ventilatory and sympathetic activity during orthostatic stress. Accordingly, in response to upright posture, we observed a 2-fold reduction in cerebrovascular pulsatility in patients with POTS, which was largely explained by the reduction in stroke volume (Figure 3C). Using an arterially perfused in situ preparation, we confirmed that changes in brain perfusion modulate respiratory and sympathetic activity independent of peripheral chemoreceptor and baroreceptor input. Together our findings suggest that in patients with POTS, reduced cerebrovascular pulsatility, likely driven by a reduction in stroke volume, may compromise brain perfusion resulting in increased ventilation and sympathetic activity.
Clinical implications
The findings of this study have potential clinical implications for managing patients with POTS. Contrary to previous hypotheses, our results indicate that exaggerated peripheral chemoreceptor activity does not significantly contribute to postural ventilation and sympathetic activation in patients with POTS. This challenges previous assumptions and suggests that targeting peripheral chemoreceptor activity may not be an effective avenue for therapeutic interventions. Instead, our study highlights the importance of understanding and addressing peripheral and central hemodynamic factors in patients with POTS. Specifically, the larger reductions in stroke volume during orthostatic stress in patients with POTS, accompanied by significant correlations with increased ventilation and heart rate, highlight the relevance of impaired systemic hemodynamic status in contributing to the disorder. Therefore, interventions aimed at optimizing cardiac preload and addressing factors influencing stroke volume may be crucial in the management of patients with POTS, potentially alleviating the debilitating symptoms associated with cardiovascular dysregulation.
Furthermore, these studies introduce a novel perspective by demonstrating that reduced cerebrovascular pulsatility may be a key factor underlying postural ventilation and sympathetic activity. This insight emphasizes the importance of maintaining adequate brain perfusion in patients with POTS, as compromised perfusion appears to play a significant role in triggering respiratory and sympathetic responses. Clinicians managing patients with POTS may consider interventions focused on improving brain perfusion to alleviate symptoms and enhance overall patient well-being.
Study limitations
First, we were unable to make biological sex comparisons. POTS affects primarily women (>90%), so although attempts were made to recruit across all sexes, only female patients were successfully recruited. Second, not all patients were willing or able to stop all medications related to their treatment prior to the study visit. Although we did not observe physiological differences between patients on vs off medications (other than baseline heart rate), medication use could still have introduced variability within the POTS data set.
Third, to maintain baseline end-tidal CO2, the sequential gas delivery system maintains CO2 about 2 to 3 mm Hg above resting levels. This likely contributed to a small degree of tonic chemoreflex activation in both the supine and upright positions, which likely contributed to the observed reduction in ventilation in both groups even in the supine position.
Fourth, we did not assess for baseline differences in blood volume or venous distribution, which could contribute to the differences in stroke volume in POTS. Additionally, estimates of stroke volume were obtained using a Modelflow waveform analysis,22 which may not always be accurate in all experimental and clinical scenarios. Despite its limitations, this metric offers the most accessible and cost-effective means to obtain estimates of stroke volume and has been previously validated to provide estimates of stroke volume specifically during orthostatic challenges,23 which was pertinent to the present study.
Fifth, because of the highly specialized nature of the MSNA technique, signal loss can be common in the supine position, especially when attempting to capture repeated measures in both the supine and upright positions. Low numbers could be the reason for not seeing differences between patients with POTS and control subjects, as previous reported.44, 45, 46 Although there was a small sample size, the MSNA results mirror those observed for ventilation such that the peripheral chemoreflex contributed to increasing both ventilation and MSNA during upright posture, and that both the change in ventilation and MSNA burst amplitude during head-up tilt were significantly correlated to changes in MCA pulsatility, 2 unique observations that have never been shown before.
Finally, although we recognize that POTS affects predominantly women, only male rats were used for the reduced in situ preparation. Male animals were chosen for the preparation; however, all animals were prepubescent and exsanguinated, which removes any potential sex-based influences on our observed responses. Male rats were chosen because they are larger at younger, prepubescent ages, which allows higher flow rates and better perfusion pressure control. Moreover, it is important to clarify that our study was not designed to address the underlying reasons for sex differences in POTS. On the contrary, we assessed differences between 2 groups of female participants (ie, healthy vs POTS). Ventilation and MSNA increased in both groups with head-up tilt, although the ventilatory responses were greater in POTS. Hence, both groups appear to share a common physiological principle that fluid redistribution toward the extremities leads to increased ventilation and sympathetic activity. The working heart–brain stem preparation was chosen to test if these responses were mediated by reductions in brain perfusion. This unique preparation allows us to manipulate brain perfusion free from humoral and peripheral feedback mechanisms. Thus, the working heart–brain stem preparation was chosen not as a model of POTS but rather to further study the basic physiology underlying the effects of reduced brain perfusion leading to increased ventilatory and sympathetic drive, which appears exaggerated in POTS.
Conclusions
This study uncovers novel mechanisms driving postural ventilation in female patients with POTS. Contrary to previous assumptions, exaggerated peripheral chemoreceptor activity is not a significant contributor to postural hyperventilation in POTS. Instead, reductions in stroke volume and cerebrovascular pulsatility during orthostatic stress emerge as critical factors associated with increased ventilation and sympathetic activity in patients with POTS. These findings have important clinical implications, emphasizing the need to target systemic hemodynamic status and preserve adequate brain perfusion in the management of patients with POTS.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The present study carries significant clinical implications. Contrary to prior assumptions, the research challenges the notion that exaggerated peripheral chemoreceptor activity is a key contributor to postural hyperventilation in patients with POTS. Instead, the study highlights the importance of addressing systemic hemodynamic factors, particularly reductions in stroke volume and brain perfusion, as primary drivers of postural ventilation and sympathetic activity. Clinicians might consider therapeutic interventions focused on optimizing cardiac preload and preserving brain perfusion to alleviate symptoms associated with POTS, paving the way for more targeted and effective management strategies for this debilitating disorder.
TRANSLATIONAL IMPLICATIONS: Although the findings highlight the importance of systemic hemodynamic status in POTS, future work focused on postural hyperventilation in POTS may consider targeting cerebrovascular pulsatility potentially through mechanisms that can help preserve stroke volume. Furthermore, exploring interventions targeting stroke volume and brain perfusion in a clinical setting would provide valuable insight on the feasibility and efficacy of these approaches, guiding the development of novel therapies for patients with POTS.
Funding Support and Author Disclosures
This work was supported by Standing Up to POTS, the National Center for Advancing Translational Sciences (grant UL1 TR000445 to Dr Raj), and the Canadian Institutes of Health Research (grant RN291964-366421 to Dr Wilson). Drs Baker and Incognito are supported by a Canadian Institutes of Health Research Fellowship. Drs Baker and Ranada are supported by the Natural Sciences and Engineering Research Council of Canada Brain CREATE Program. Dr Baker is supported by the Libin Cardiovascular Institute Post-Doctoral Fellowship in Women’s Cardiovascular Health. Dr Incognito is supported by an Achievers in Medical Science Postdoctoral Fellowship. Dr Ranada is supported by an Alberta Graduate Excellence Scholarship, Alberta Strategy for Patient Oriented SUPPORT Unit scholarship. Dr Raj is a consultant to Lundbeck, Theravance Biopharma, and Amneal Pharmaceuticals (related to neurogenic orthostatic hypotension); is a consultant to Servier Affaires Medicales, Regeneron, STAT Health, and argenx (related to POTS). Dr Phillips is a consultant for and shareholder in ONWARD Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the patients who took the time to participate in this study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Appendix
References
- 1.Freeman R., Wieling W., Axelrod F.B., et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 2.Raj S.R. Postural tachycardia syndrome (POTS) Circulation. 2013;127:2336–2342. doi: 10.1161/CIRCULATIONAHA.112.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj S.R., Guzman J.C., Harvey P., et al. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Arnold A.C., Ng J., Raj S.R. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci Basic Clin. 2018;215:3–11. doi: 10.1016/j.autneu.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart J.M., Pianosi P., Shaban M.A., et al. Postural hyperventilation as a cause of postural tachycardia syndrome: increased systemic vascular resistance and decreased cardiac output when upright in all postural tachycardia syndrome variants. J Am Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.118.008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Pozzi AT., Schwartz C.E., Tewari D., Medow M.S., Stewart J.M. Reduced cerebral blood flow with orthostasis precedes hypocapnic hyperpnea, sympathetic activation, and postural tachycardia syndrome. Hypertension. 2014;63:1302–1308. doi: 10.1161/HYPERTENSIONAHA.113.02824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart J.M., Medow M.S., Cherniack N.S., Natelson B.H. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol. 2006;291:H904–H913. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart J.M., Pianosi P., Shaban M.A., et al. Hemodynamic characteristics of postural hyperventilation: POTS with hyperventilation versus panic versus voluntary hyperventilation. J Appl Physiol (1985) 2018;125:1396–1403. doi: 10.1152/japplphysiol.00377.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart J.M., Pianosi P.T. Postural orthostatic tachycardia syndrome: a respiratory disorder? Curr Res Physiol. 2021;4:1–6. doi: 10.1016/j.crphys.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak P. Hypocapnic cerebral hypoperfusion: a biomarker of orthostatic intolerance. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0204419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak V., Spies J.M., Novak P., McPhee B.R., Rummans T.A., Low P.A. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29:1876–1881. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 12.Laffey J.G., Kavanagh B.P. Hypocapnia. N Engl J Med. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- 13.Stewart J.M., Medow M.S. Anticipatory central command on standing decreases cerebral blood velocity causing hypocapnia in hyperpneic postural tachycardia syndrome. J Appl Physiol (1985) 2023;135(1):26–34. doi: 10.1152/japplphysiol.00016.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nardone M., Guzman J., Harvey P.J., Floras J.S., Edgell H. Effect of a neck compression collar on cardiorespiratory and cerebrovascular function in postural orthostatic tachycardia syndrome (POTS) J Appl Physiol (1985) 2020;128:907–913. doi: 10.1152/japplphysiol.00040.2020. [DOI] [PubMed] [Google Scholar]
- 15.Iturriaga R. Translating carotid body function into clinical medicine. J Physiol. 2018;596:3067–3077. doi: 10.1113/JP275335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey J.A., Smith C.A. Pathophysiology of human ventilatory control. Eur Respir J. 2014;44:495–512. doi: 10.1183/09031936.00048514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machhada A., Trapp S., Marina N., et al. Vagal determinants of exercise capacity. Nat Commun. 2017;8 doi: 10.1038/ncomms15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuki S., Eisenach J.H., Schrage W.G., et al. Reduced stroke volume during exercise in postural tachycardia syndrome. J Appl Physiol (1985) 2007;103:1128–1135. doi: 10.1152/japplphysiol.00175.2007. [DOI] [PubMed] [Google Scholar]
- 19.Masuki S., Eisenach J.H., Johnson C.P., et al. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol (1985) 2007;102:896–903. doi: 10.1152/japplphysiol.00927.2006. [DOI] [PubMed] [Google Scholar]
- 20.Bourne K.M., Sheldon R.S., Hall J., et al. Compression garment reduces orthostatic tachycardia and symptoms in patients with postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2021;77:285–296. doi: 10.1016/j.jacc.2020.11.040. [DOI] [PubMed] [Google Scholar]
- 21.González-Hermosillo J.A., Rubio-Vega A., González-Olvera K.A.F. Early cerebral hypoperfusion in patients with orthostatic intolerance without tachycardia during head-up tilt test is independent of vasovagal response. Rev Invest Clin. 2021;73:388–398. doi: 10.24875/RIC.21000199. [DOI] [PubMed] [Google Scholar]
- 22.Wesseling K.H., Jansen J.R.C., Settels J.J., Schreuder J.J. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985) 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 23.Lucci V.E.M., Parsons I.T., Hockin B.C.D., Claydon V.E. Evaluation of stroke volume estimation during orthostatic stress: the utility of Modelflow. Blood Press Monit. 2023;28:330–337. doi: 10.1097/MBP.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 24.Notay K., Seed J.D., Incognito A.V., et al. Validity and reliability of measuring resting muscle sympathetic nerve activity using short sampling durations in healthy humans. J Appl Physiol (1985) 2016;121:1065–1073. doi: 10.1152/japplphysiol.00736.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sverrisdóttir Y.B., Rundqvist B., Elam M. Relative burst amplitude in human muscle sympathetic nerve activity: a sensitive indicator of altered sympathetic traffic. Clin Auton Res. 1998;8:95–100. doi: 10.1007/BF02267819. [DOI] [PubMed] [Google Scholar]
- 26.Fisher J.A., Iscoe S., Duffin J. Sequential gas delivery provides precise control of alveolar gas exchange. Respir Physiol Neurobiol. 2016;225:60–69. doi: 10.1016/j.resp.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Ito S., Mardimae A., Han J., et al. Non-invasive prospective targeting of arterial Pco2 in subjects at rest. J Physiol. 2008;586:3675–3682. doi: 10.1113/jphysiol.2008.154716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejours P. Chemoreflex in breathing. Physiol Rev. 1962;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- 29.Lahiri S., Mulligan E., Andronikou S., Shirahata M., Mokashi A. Carotid body chemosensory function in prolonged normobaric hyperoxia in the cat. J Appl Physiol (1985) 1987;62:1924–1931. doi: 10.1152/jappl.1987.62.5.1924. [DOI] [PubMed] [Google Scholar]
- 30.Dean J.B., Mulkey D.K., Henderson R.A., Potter S.J., Putnam R.W. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol (1985) 2004;96:784–791. doi: 10.1152/japplphysiol.00892.2003. [DOI] [PubMed] [Google Scholar]
- 31.Paton J.F.R., Machado B.H., Moraes D.J.A., et al. Advancing respiratory–cardiovascular physiology with the working heart–brainstem preparation over 25 years. J Physiol. 2022;600:2049–2075. doi: 10.1113/JP281953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day T.A., Wilson R.J.A. Specific carotid body chemostimulation is sufficient to elicit phrenic poststimulus frequency decline in a novel in situ dual-perfused rat preparation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R532–R544. doi: 10.1152/ajpregu.00812.2004. [DOI] [PubMed] [Google Scholar]
- 33.Eldridge F.L. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971;221:535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- 34.Zoccal D.B., Simms A.E., Bonagamba L.G.H., et al. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byers B.W., Fuhr D.P., Moore L.E., Bhutani M., Wong E.Y.L., Stickland M.K. The effect of pulmonary rehabilitation on carotid chemoreceptor activity and sensitivity in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2019;127:1278–1287. doi: 10.1152/japplphysiol.00799.2018. [DOI] [PubMed] [Google Scholar]
- 36.Phillips D.B., Steinback C.D., Collins S., et al. The carotid chemoreceptor contributes to the elevated arterial stiffness and vasoconstrictor outflow in chronic obstructive pulmonary disease. J Physiol. 2018;596:3233–3244. doi: 10.1113/JP275762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chua T.P., Ponikowski P.P., Harrington D., Chambers J., Coats A.J.S. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart. 1996;76:483–489. doi: 10.1136/hrt.76.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahiri S., Edelman N. Peripheral chemoreflexes in the regulation of breathing of high altitude natives. Respir Physiol. 1969;6:375–385. doi: 10.1016/0034-5687(69)90035-8. [DOI] [PubMed] [Google Scholar]
- 39.Leitch A.G., McLennan J.E., Balkenhol S., McLaurin R.L., Loudon R.G. Ventilatory response to transient hyperoxia in head injury hyperventilation. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:52–58. doi: 10.1152/jappl.1980.49.1.52. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed M., Serrette C., Kryger M.H., Anthonisen N.R. Ventilatory instability in patients with congestive heart failure and nocturnal Cheyne-Stokes breathing. Sleep. 1994;17:527–534. doi: 10.1093/sleep/17.6.527. [DOI] [PubMed] [Google Scholar]
- 41.Su X.W., Guan Y., Barnes M., Clark J.B., Myers J.L., Ündar A. Improved cerebral oxygen saturation and blood flow pulsatility with pulsatile perfusion during pediatric cardiopulmonary bypass. Pediatr Res. 2011;70:181–185. doi: 10.1203/PDR.0b013e3182226b75. [DOI] [PubMed] [Google Scholar]
- 42.Xu H., Lu S., Ding L., Lyu L., Ma Z., Lu Q. Pulsatility index as a novel parameter for perfusion in mouse model of hindlimb ischemia. Cell Physiol Biochem. 2018;48:2114–2122. doi: 10.1159/000492553. [DOI] [PubMed] [Google Scholar]
- 43.Stewart J.M., Medow M.S., Glover J., Montgomery L.D. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2006;290:H665–H673. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swift N.M., Charkoudian N., Dotson R.M., Suarez G.A., Low P.A. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2005;289:1226–1233. doi: 10.1152/ajpheart.01243.2004. [DOI] [PubMed] [Google Scholar]
- 45.Fu Q., VanGundy T., Galbreath M., et al. Cardiac origins of the postural tachycardia syndrome. J Am Coll Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert E., Eikelis N., Esler M., et al. Altered sympathetic nervous reactivity and norepinephrine transporter expression in patients with postural tachycardia syndrome. Circ Arrhythm Electrophysiol. 2008;1:103–109. doi: 10.1161/CIRCEP.107.750471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.