Abstract
Proper attachment to the extracellular matrix is essential for cell survival. Detachment from the extracellular matrix results in an apoptotic process termed anoikis. Anoikis induction in MCF-10A mammary epithelial cells is due not only to loss of survival signals following integrin disengagement, but also to consequent downregulation of epidermal growth factor (EGFR) and loss of EGFR-induced survival signals. Here we demonstrate that G1/S arrest by overexpression of the cyclin-dependent kinase inhibitors p16INK4a, p21Cip1, or p27Kip1 or by treatment with mimosine or aphidicolin confers anoikis resistance in MCF-10A cells. G1/S arrest-mediated anoikis resistance involves suppression of the BH3-only protein Bim. Furthermore, in G1/S-arrested cells, Erk phosphorylation is maintained in suspension and is necessary for Bim suppression. Following G1/S arrest, known proteins upstream of Erk, including Raf and Mek, are not activated. However, retained Erk activation under conditions in which Raf and Mek activation is lost is observed, suggesting that G1/S arrest acts at the level of Erk dephosphorylation. Thus, anoikis resistance by G1/S arrest is mediated by a mechanism involving Bim suppression through maintenance of Erk activation. These results provide a novel link between cell cycle arrest and survival, and this mechanism could contribute to the survival of nonreplicating, dormant tumor cells that avert apoptosis during early stages of metastasis.
The ECM is a complex meshwork of macromolecules, such as fibronectin, vitronectin, laminin, and collagen, that provides adhesive support for tissues and transduces a variety of signals that regulate cell behavior (26). Epithelial cells require attachment to the ECM through adhesion receptors, called integrins, for cellular survival (18, 38). Disruption of cell-matrix attachment results in a loss of prosurvival signals and culminates in programmed cell death, referred to as anoikis (19). Anoikis was first observed for endothelial and epithelial cells, for which loss of matrix attachment, even in the presence of serum, results in cell death (17, 37). Expression of certain oncogenes can provide protection from anoikis (17), and this protection is thought to be critical during tumorigenesis to allow cell survival outside of the natural cell microenvironment and during metastasis to secondary sites (14, 19, 53, 58).
Mechanisms governing the cell cycle and apoptosis are closely linked, as several proteins that regulate cell cycle progression can also induce apoptosis under conditions in which cell cycle progression is not properly orchestrated (15, 23, 28, 39). Furthermore, sensitivity to apoptotic stress can vary depending on the stage of the cell cycle, and certain cyclin/CDK complexes can influence the cellular outcome following apoptotic stress (10, 44). Induction of apoptosis under certain stress conditions, such as γ irradiation or treatment with taxol, staurosporine, or tumor necrosis factor alpha, have been reported to be dependent on cyclin B1/CDK1 (Cdc2 or p34Cdc2) activity, as CDK1 activity is required for apoptosis induced by these stress conditions (10, 55, 65). Cyclin B1/CDK1 is active only during the G2/M transition, suggesting that progression through G2/M is required for sensitivity to these stress conditions (10).
In addition, several lines of evidence suggest that arrest in the G1 stage of the cell cycle can provide resistance to different forms of apoptotic stress. For example, G1 arrest induced following the overexpression or upregulation of CKIs has been shown to provide resistance to several apoptotic stimuli including γ or UV irradiation, adriamycin treatment, oxidative stress, tumor necrosis factor alpha, or gamma interferon, while CKI ablation can sensitize cells to apoptosis (21, 22, 24, 44, 64).
Furthermore, studies on apoptosis following growth factor withdrawal have suggested that the G1/S stages of the cell cycle may be apoptosis-insensitive stages while other stages do not provide such resistance (16, 41). For example, arresting PC12 cells or primary postmitotic sympathetic neurons with the one of the G1/S blockers mimosine, ciclopirox, or deferoxamine suppresses apoptosis of these cells following serum or growth factor withdrawal, whereas arresting in other stages of the cell cycle does not suppress apoptosis (16).
Previous studies have also shown a correlation between cell cycle arrest and anoikis resistance (7, 8, 27). In one study, the population of human keratinocyte cells that underwent apoptosis in suspension was found to be selectively derived from the proliferating cell population; furthermore, surviving cells underwent a G0/G1 cell cycle arrest in suspension (8). Upon the detachment of epithelial cells from matrix, G1 growth arrest is observed for a population of cells because integrin engagement is essential for productive cell cycle progression (52). In addition, protection from detachment-induced apoptosis in breast epithelial cells expressing ectopic galectin-3 is dependent on cell cycle arrest at a late G1, anoikis-insensitive stage of the cell cycle (27). While there is evidence supporting G1/S protection from anoikis, little is known about the mechanisms mediating this effect.
In this study, we investigated the molecular mechanism of anoikis resistance provided by cell cycle arrest in an immortalized MEC line, MCF-10A (56). Apoptosis induction in MCF-10A cells can be distinguished from that reported for primary MECs. In the latter, 50% of the cell population undergoes apoptosis within 6 to 8 h after detachment. However, in MCF-10A cells, apoptosis is significantly delayed, occurring after 24 h in suspension (47, 51, 61). We have previously shown that the loss of integrin engagement in MCF-10A cells causes a downregulation of EGFR, leading to an induction of the proapoptotic, BH3-only protein Bim through loss of signaling via the Ras-Erk MAPK pathway (47). Bim induction is required for apoptosis following detachment of MCF-10A cells (47). While Bim is not required for early apoptosis following detachment in mouse primary MECs (61), it is induced at later periods (8 to 12 h) in primary MECs that survive early apoptosis and in other epithelial cells such as IEC-18 intestinal epithelial cells and MDCK (Madin-Darby canine kidney) cells (47). As in MCF-10A cells, EGFR is also downregulated in suspended primary MECs, IEC-18 cells, and MDCK cells. Taken together, these results suggest that Bim upregulation significantly contributes to apoptosis in epithelial cells and that its induction is secondary to loss of EGFR following loss of integrin engagement.
We report here that G1- or early-S-phase arrest by various methods provides resistance to anoikis in MCF-10A cells by suppression of Bim expression (42, 57). Bim suppression by G1/S arrest occurs posttranscriptionally, as the induction of Bim mRNA following detachment in G1/S-arrested cells is not inhibited. Furthermore, we show that G1/S-arrested cells maintain Erk phosphorylation in suspension and that this sustained Erk phosphorylation is necessary to suppress Bim in suspended cells. However, G1/S-arrested cells do not exhibit activation of proteins upstream of Erk and display elevated Erk phosphorylation under conditions in which Raf and Mek are not active, suggesting that G1/S arrest acts at the level of Erk dephosphorylation.
MATERIALS AND METHODS
Abbreviations.
Abbreviations used in this paper are as follows: CDK, cyclin-dependent kinase; CKI, cyclin-dependent kinase inhibitor; DAPI, 4′6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorter; HRP, horseradish peroxidase; JNK, Jun N-terminal protein kinase; MAPK, mitogen-activated protein kinase; MEC, mammary epithelial cell; PBS, phosphate-buffered saline; PI, propidium iodide; Poly-HEMA, poly(2-hydroxyethylmethacrylate); SEM, standard errors of the means.
cDNA constructs.
pBABE-puro was obtained from Jay Morgenstern (Millennium Pharmaceuticals, Cambridge, MA), and pLXSN was purchased from Clontech (Palo Alto, CA). pBABE-p16INK4a (CDKN2B) was obtained from the Breast Cancer 1000 library (Joshua LaBaer, Harvard Institute of Proteomics [www.hip.harvard.edu]). pLXSN-CDK4 R24C was a gift from Phil Hinds (Tufts Medical School, Boston, MA). pCDNA3-p21Cip1 (CDKN1A) and pCDNA3-p21Cip1 T145D were generous gifts from Mien-Chie Hung (University of Texas, M. D. Anderson Cancer Center, Houston, TX). pET21a-hp27Kip1 (CDKN1B) was a gift from Kornelia Polyak (Harvard Medical School, Boston, MA). pBABE-p21Cip1 and pBABE-p27Kip1 were created by subcloning p21Cip1 and p27Kip1 PCR products into the EcoRI and BamHI sites of the pBABE-puro vector by using pCDNA3-p21Cip1 and p-ET21a-hp27Kip1, respectively, as PCR templates. All constructs were sequence verified at the Harvard Institute of Proteomics sequencing facility.
Cell culture and materials.
MCF-10A MECs (56) and IEC-18 intestinal epithelial cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured as described previously (13, 47). Poly-HEMA, methocellulose, mimosine, aphidicolin, DAPI, puromycin, and G418 were purchased from Sigma. UO126 was purchased from Calbiochem (San Diego, CA). PI and RNase A were purchased from Roche (Basel, Switzerland). The SuperScript double-stranded cDNA synthesis kit was purchased from Invitrogen (Carlsbad, CA). Primary antibodies used for immunoblotting include the following: anti-Bim from Stressgen (Victoria, British Columbia, Canada); antiactin (C-11), anti-Erk2, anti-p16INK4a (C-20), anti-Mek1 (C-18), anti-CDK4 (C-22), and anti-phospho-c-Jun (KM-1) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-phospho-Erk1/2 T185/Y187 from Biosource (Camarillo, CA); anti-phospho-Akt S473, anti-phospho-Mek1/2 S217/S221, and anti-phospho-Raf S338 from Cell Signaling (Beverly, MA); anti-p16INK4a (Ab-1) and anti-p21Cip1 (Ab-3) from Neomarkers (Fremont, CA); anti-p21Cip1 and anti-p27Kip1 from BD Biosciences Pharmingen (San Diego, CA); anti-p21Cip1 (EA10) from Calbiochem; and anti-phospho-tyrosine (4G10) from Upstate (Lake Placid, NY). Secondary antibodies include goat anti-rabbit-HRP, rabbit anti-goat-HRP, and goat anti-mouse-HRP from Bio-Rad (Hercules, CA). Cell lines were generated by retroviral infection of MCF-10A cells, followed by treatment with selectable marker (puromycin or G418). For puromycin-selected lines, the cells were used immediately following 4 days of selection, and fresh infections were performed prior to each experiment involving CKIs. Fresh infections with CKI viruses were performed because prolonged culture of these selected cells resulted in a loss of the G1-arrested phenotype, presumably by overgrowth of puromycin-resistant cells not expressing CKIs. Vesicular stomatitis virus-pseudotyped retroviruses were produced by transfection of the VSV-GPG producer cell line as previously described (43) (provided by R. Mulligan, Children's Hospital, Harvard Medical School) with 15 μg of DNA by using Lipofectamine 2000 (Invitrogen).
Detachment-induced apoptosis assay.
Tissue culture six-well plates were coated with 6 mg/ml Poly-HEMA in 95% ethanol and incubated at 37°C for several days until dry. MCF-10A cells were plated in Poly-HEMA-coated plates in complete growth medium containing 0.5% methocellulose at a density of 400,000 cells/well for 48 h. Cells were collected, washed with PBS, and counted by using a hemocytometer. Apoptosis was measured using a cell death detection ELISA kit (Roche Diagnostics, Mannheim, Germany), which determines cytoplasmic histone content, according to the manufacturer's specifications and using 25,000 cells per sample. In a single experiment, each cell type/condition was performed in duplicate. These duplicates were averaged to obtain a value for each cell type/condition from one experiment, and each independent experiment was performed at least three times. For comparisons of several cell types/conditions across multiple experiments, values of each cell type/condition were normalized relative to those of the vector control and averaged over at least three independent experiments. Error bars were used to represent SEM determined for the normalized values of each cell type/condition from at least three independent experiments.
Immunoblotting.
Cell lysates from attached or suspended MCF-10A cells were prepared in either NP-40 buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 1% NP-40, and 10% glycerol) or radioimmunoprecipitation assay buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 50 mM Tris HCl, pH 8.0, 0.1% sodium dodecyl sulfate, 10% glycerol, and 5 mM EDTA) supplemented with pepstatin (1 μg/ml), leupeptin (1 μg/ml), aprotinin (1 μg/ml), phenylmethylsulfonyl fluoride (200 μg/ml), NaF (20 to 50 mM), and Na3VO4 (1 mM). Following a 15-minute lysis on ice, lysates were cleared by centrifugation at 16,000 × g for 15 min at 4°C, flash frozen in liquid nitrogen, and stored at −80°C. Protein concentration was normalized by use of a bicinchoninic acid assay (Pierce, Rockford, IL) according to the manufacturer's instructions. Following protein normalization, lysates were prepared by use of 10× sample buffer and were boiled for 5 min. Lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting using primary antibodies overnight at 4°C and secondary antibodies for 45 min at room temperature. The HRP signal was detected by using enhanced chemiluminescence and exposed by using X-OMAT-Blue film (Kodak, Rochester, NY).
PI staining and FACS analysis.
Attached MCF-10A cells were harvested for FACS analysis by incubating the cells at 37°C in PBS with 2.5 mM EDTA for 30 min. Following EDTA treatment, the cells were fixed in 80% ethanol for 15 min at 4°C. After fixation, the cells were washed with PBS containing 1% goat serum and stained at 37°C for 1 h in PI/RNase A solution (PBS with 2.5 mM EDTA, 10 μg/ml PI, and 0.25 mg/ml RNase A). Flow cytometry was performed using a Becton Dickinson FACSCalibur to determine DNA content, and data were analyzed by using CELLQuest (Becton Dickinson).
EGF withdrawal.
Following the plating of MCF-10A cells at a density of 500,000 cells per 10-cm2 plate and attachment overnight in full medium, cells were rinsed once with 10 ml of growth medium without EGF. Then, the cells were subject to EGF withdrawal in growth medium without EGF for 24 h. Control cells were grown in the presence of EGF throughout the entire time course of the experiment.
Real-time quantitative PCR.
Total RNA was extracted from control or mimosine-treated MCF-10A cells under attached or suspended conditions by use of RNA STAT-60 (Tel-Test) according to the manufacturer's protocol. First- and second-strand syntheses were performed using 5 μg of total RNA with oligo(dT) primers to generate double-stranded cDNA (SuperScript double-stranded cDNA synthesis kit; Invitrogen), which was diluted and used as a template for real-time quantitative PCR analysis. Analysis of human Bim mRNA expression was performed as described previously (47). RNA samples were harvested, and cDNA synthesis reactions from three independent experiments were performed. Triplicate quantitative PCRs were performed on each double-stranded cDNA triplicate. Error bars were used to represent SEM determined for the normalized values of each condition from nine quantitative PCRs from three independent experiments.
Mimosine or aphidicolin treatment.
MCF-10A cells were plated at a density of 500,000 cells per 10-cm2 plate and allowed to attach overnight. Cells were then treated with either 10 μl vehicle control (DMSO), 200 μM mimosine, or 10 μM aphidicolin for 24 h prior to manipulation in any of the assays described above. Inhibitors or vehicle control were maintained throughout the time course of each assay.
RESULTS
G1/S arrest provides anoikis resistance.
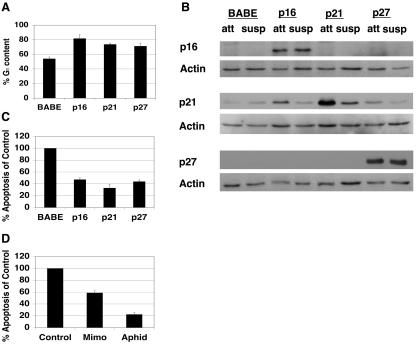
To examine the mechanism of anoikis resistance following cell cycle arrest, we utilized MCF-10A cells, an immortalized human MEC line (36, 47, 50, 56). To determine if G1 arrest confers resistance to anoikis in MCF-10A cells, we first examined the effect of overexpressing CKIs (54, 59), namely, the CIP/KIP family members p21Cip1 and p27Kip1 and the INK4 family member p16INK4a, on sensitivity to anoikis. The ectopic expressions of p16INK4a, p21Cip1, and p27Kip1 each caused an expected increase in the percentage of cells in G1 (81%, 74%, and 71% G1 content, respectively, relative to 54% G1 content in vector control cells) based on FACS analysis to determine DNA content (Fig. 1A). Immunoblotting with antisera recognizing p16INK4a, p21Cip1, or p27Kip1 confirmed the ectopic expression of each protein under attached and detached conditions (Fig. 1B). Using a DNA fragmentation ELISA to assess anoikis, we found that the expression of p16INK4a, p21Cip1, or p27Kip1 caused 55%, 70%, or 50% inhibition of apoptosis, respectively (Fig. 1C), suggesting that G1 arrest provides anoikis resistance.
FIG. 1.
G1/S arrest protects from anoikis. (A) Expression of p16INK4a, p21Cip1, or p27Kip1 induces G1 arrest. MCF-10A cells were infected with retroviruses encoding control vector (BABE), p16INK4a, p21Cip1, or p27Kip1. Immediately following puromycin selection for stably infected lines, PI staining and FACS analysis were performed to measure DNA content. Percent G1 content for each condition is shown. These results represent the averages ± SEM from at least three separate experiments. (B) Confirmation of p16INK4a, p21Cip1, or p27Kip1 overexpression under attached and suspended conditions. Following puromycin selection, cells described for panel A were plated on either tissue culture plastic or Poly-HEMA-coated plates in full growth medium for 24 h. Attached (att) and suspended (susp) lysates were harvested and immunoblotted with antisera against p16INK4a, p21Cip1, or p27Kip1 to examine overexpression or with antiserum against actin as a loading control. (C) Expression of p16INK4a, p21Cip1, or p27Kip1 provides anoikis resistance. Cells expressing p16INK4a, p21Cip1, p27Kip1, or control vector (BABE) were plated in Poly-HEMA-coated plates in growth medium containing 0.5% methocellulose for 48 h. Apoptosis was measured by use of a colorimetric DNA fragmentation ELISA. Apoptosis of each sample was normalized compared to that of control vectors. The values shown represent averages of normalized values ± SEM from at least three separate experiments as described in Materials and Methods. (D) G1/S arrest by mimosine or aphidicolin treatment confers anoikis resistance. MCF-10A cells were treated with vehicle control (Control), mimosine (Mimo), or aphidicolin (Aphid) as described in Materials and Methods. Following treatment, cells were plated on Poly-HEMA-coated plates in growth medium containing 0.5% methocellulose with the appropriate drug or vehicle control, and DNA fragmentation ELISAs were performed as described for panel C.
To further examine whether G1- or early S-phase arrest provides protection from anoikis, MCF-10A cells were arrested by use of either the plant amino acid mimosine or the DNA polymerase alpha inhibitor aphidicolin to arrest cells near the G1/S boundary (25, 31, 32, 62). PI staining and FACS analysis confirmed cell cycle arrest at this stage of the cell cycle (data not shown). Mimosine and aphidicolin treatments protected MCF-10A cells from anoikis by 40% and 80%, respectively, compared to vector controls (Fig. 1D), supporting the possibility that cell cycle arrest in late G1 or in early S phase provides anoikis resistance.
G1/S arrest inhibits Bim expression following detachment.
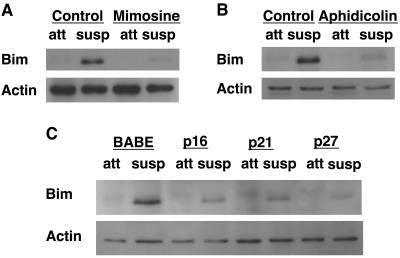
To elucidate the mechanism by which G1/S arrest provides anoikis resistance, we examined whether G1/S arrest suppresses the induction of Bim in cells detached from matrix. We initially focused on Bim because it is strongly induced following the detachment of MCF-10A cells from matrix and downregulation of Bim expression by RNA interference inhibits anoikis (47). Interestingly, all of the treatments employed to block cell cycle progression in G1 or G1/S significantly inhibited the expression of Bim in suspension (Fig. 2). Treatment with mimosine or aphidicolin to arrest cells in G1/S caused a significant inhibition of detachment-induced Bim upregulation (Fig. 2A and B). In addition, the expression of p16INK4a, p21Cip1, or p27Kip1 also reduced detachment-induced Bim expression (Fig. 2C). The reduction in Bim expression observed for G1/S-arrested cells is similar to the level of reduction we observed previously with Bim small inhibitory RNA transfection of control MCF-10A cells (47), which resulted in a similar twofold anoikis resistance. Therefore, the level of Bim reduction observed here would be sufficient to result in anoikis resistance.
FIG. 2.
G1/S arrest inhibits Bim expression following detachment. (A) Mimosine treatment inhibits detachment-induced Bim upregulation. MCF-10A cells were treated with vehicle (Control) or mimosine as described in Materials and Methods. Following vehicle control or mimosine treatment for 24 h, cells were plated on either tissue culture plastic or Poly-HEMA-coated plates in full growth medium for 24 h. Attached (att) and suspended (susp) lysates were harvested and immunoblotted with anti-Bim antiserum to examine Bim expression. (B) Aphidicolin treatment inhibits detachment-induced Bim upregulation. Cells were treated with vehicle (Control) or aphidicolin as described in Materials and Methods. Bim expression was determined 24 h following detachment as described for panel A. (C) CKI expression inhibits detachment-induced Bim upregulation. MCF-10A cells transduced with vectors encoding p16INK4a, p21Cip1, p27Kip1, or empty control vector (BABE) were plated as described for panels A and B. Bim expression was determined following detachment as described for panel A.
CKI-mediated Bim suppression is dependent on G1/S arrest.
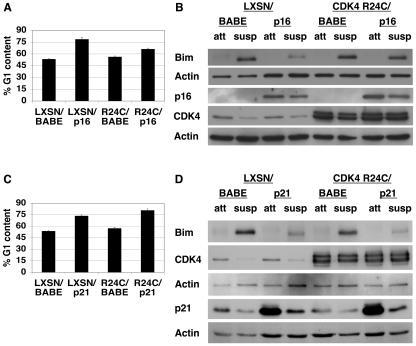
The suppression of Bim induction by the CKI p16INK4a was confirmed to be dependent on G1 arrest by utilizing a CDK4 mutant, CDK4 R24C, which cannot be inhibited by p16INK4a (46). Coexpression of CDK4 R24C and p16INK4a in MCF-10A cells significantly reduced p16INK4a-induced G1 arrest (Fig. 3A) and suppression of Bim in detached cells (Fig. 3B). To confirm the specificity of CDK4 R24C, CDK4 R24C was coexpressed with p21Cip1 in MCF-10A cells. Coexpression of CDK4 R24C and p21Cip1 had no effect on p21Cip1-induced G1 arrest (Fig. 3C) or p21Cip1-induced Bim suppression (Fig. 3D). These results indicate that CDK4 R24C acts specifically to bypass a p16INK4a-induced but not p21Cip1-induced G1 arrest. Therefore, circumventing the cell cycle inhibitory activity of p16INK4a abrogated its ability to suppress Bim upregulation in suspension. Together with the results in Fig. 2, these data indicate that cell cycle arrest in G1 or near the G1/S boundary prevents detachment-induced Bim upregulation. Furthermore, this observation is not specific to MCF-10A cells, as following 6 h of detachment, a similar inhibition of Bim expression in IEC-18 cells arrested by either mimosine or aphidicolin treatment was seen (data not shown).
FIG. 3.
p16INK4a-mediated Bim suppression is dependent on G1/S arrest. (A) CDK4 R24C expression precludes p16INK4a-mediated growth arrest. MCF-10A cells transduced with empty LXSN vector or LXSN encoding CDK4 R24C (denoted R24C) were superinfected with empty BABE vector or BABE encoding p16INK4a. Following double selection with G418 and puromycin, subconfluent, attached cells were harvested for FACS analysis. The percentage of G1 content for each cell type is displayed as a measure of G1 arrest. These results represent averages ± SEM from at least three separate experiments. (B) CDK4 R24C expression allows detachment-induced Bim upregulation in p16INK4a-expressing cells. Following selection, cells described for panel A were plated under attached (att) or suspended (susp) conditions for 24 h, at which point lysates were harvested. The lysates were immunoblotted with antiserum to Bim, p16INK4a, CDK4, or actin (the last as a loading control) under attached and suspended conditions following the coexpression of CDK4 R24C and p16INK4a. These images are of two immunoblots of the same cell lysates. The first blot was probed with antiserum against Bim and then reprobed for actin as a loading control. The second blot was probed with antiserum against p16INK4a and then reprobed for both CDK4 and actin (as a loading control). (C) CDK4 R24C specifically prevents p16INK4a-mediated growth arrest and has no effect on growth arrest mediated by p21Cip1. As described for panel A, MCF-10A cells were transduced with empty LXSN vector or LXSN encoding CDK4 R24C but were superinfected with empty BABE vector or BABE encoding p21Cip1. Following double selection, subconfluent, attached cells were harvested and FACS analysis was performed as described for panel A. (D) CDK4 R24C expression has no effect on detachment-induced Bim upregulation in p21Cip1-expressing cells. Following selection, cells described for panel C were plated under attached or suspended conditions for 24 h and lysates were harvested as described for panel B. Lysates were immunoblotted with antiserum to Bim, CDK4, p21Cip1, or actin. These images are of two separate immunoblots. The first blot was probed with antiserum against Bim and then reprobed for CDK4 and actin (as a loading control). The second blot was probed with antiserum against p21Cip1 and then reprobed for actin as a loading control.
G1/S arrest does not suppress detachment-induced Bim mRNA expression.
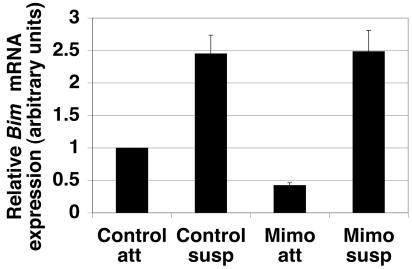
To determine if G1/S arrest inhibits Bim mRNA expression following detachment, real-time quantitative PCR was performed on control and mimosine-treated MCF-10A cells under attached and suspended conditions (Fig. 4A). As previously described (47), an increase in Bim mRNA expression in control cells following detachment was observed. Mimosine treatment resulted in a reduction in Bim mRNA expression under attached conditions; however, Bim mRNA was induced in mimosine-treated cells under suspended conditions. These data indicate that G1/S arrest does not inhibit Bim mRNA induction following detachment.
FIG. 4.
G1/S arrest does not inhibit detachment-induced Bim mRNA expression. Control and mimosine-treated MCF-10A cells were plated at 400,000 cells/well in either tissue culture-treated (attached) or Poly-HEMA-coated (suspended) six-well plates for 24 h. RNA was harvested, and double-stranded cDNA using oligo(dT) primers was synthesized from 5 μg total RNA. Real-time quantitative PCR was performed on equal amounts of total RNA as described previously (47). The relative Bim mRNA expression for each condition was plotted. Error bars represent the SEM of nine quantitative PCRs from three independent experiments.
G1/S arrest enhances Erk phosphorylation that is maintained following detachment.
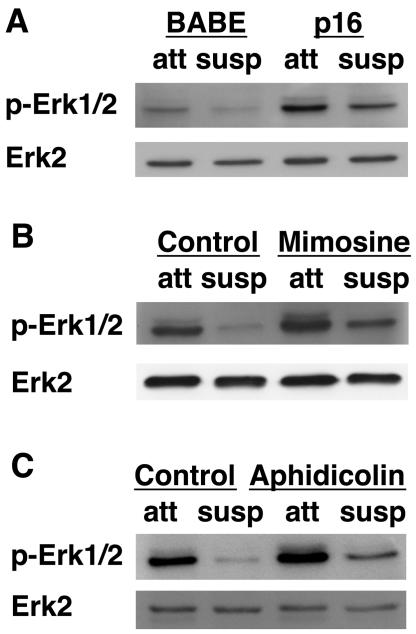
To establish how G1/S cell cycle arrest inhibits Bim upregulation in suspension, we examined the survival pathways that have been implicated in Bim regulation. Detachment-induced Bim expression is associated with inhibition of Erk signaling (47). Erk has been shown to regulate Bim through both transcriptional and posttranscriptional controls, affecting either the level of mRNA expression or ubiquitin-mediated degradation triggered by direct Erk phosphorylation (33-35, 47). To investigate the role of Erk in G1/S control of Bim expression, we examined Erk phosphorylation and activity in growth-arrested MCF-10A cells. As we have previously shown (47), the detachment of cells from matrix causes a loss of Erk phosphorylation (Fig. 5). Cells expressing p16INK4a or p21Cip1 displayed enhanced Erk phosphorylation when attached to matrix and retained Erk phosphorylation following detachment (Fig. 5A and data not shown). Similarly, cells treated with mimosine or aphidicolin also displayed an enhancement of Erk phosphorylation in both attached and suspended cells (Fig. 5B and C).
FIG. 5.
G1/S arrest increases Erk phosphorylation under attached and suspended conditions. (A) Expression of p16INK4a enhances Erk phosphorylation in attached cells and following detachment. MCF-10A cells transduced with control vector (BABE) or BABE encoding p16INK4a were placed under attached or suspended conditions following selection. After 24 h in suspension, cell lysates were harvested and immunoblotted with antiserum against total Erk2 or against phosphorylated Erk1/2 (p-Erk1/2). (B) Mimosine treatment enhances Erk phosphorylation under attached and suspended conditions. MCF-10A cells were treated as described for Fig. 2A, except the lysates were immunoblotted with the Erk antibodies described for Fig. 5A. (C) Aphidicolin treatment enhances Erk phosphorylation in attached and suspended cells. MCF-10A cells were treated as described for Fig. 2B, except the lysates were immunoblotted with the Erk antibodies described for panel A.
Increased Erk phosphorylation in G1/S-arrested cells is necessary for Bim inhibition.
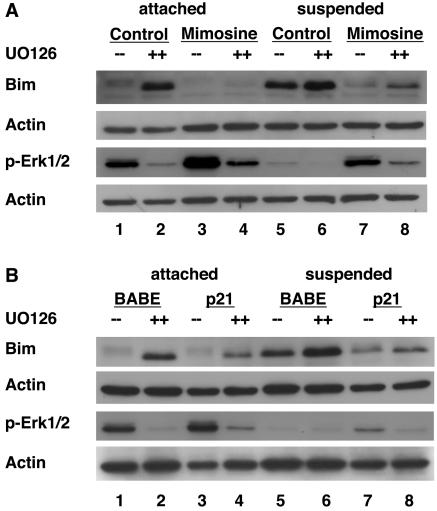
Since Erk has been shown to be an important regulator of Bim during anoikis (47), we examined whether the enhanced Erk phosphorylation observed following G1/S arrest is necessary for Bim suppression during anoikis by using the Mek inhibitors UO126 (Fig. 6) and PD98059 (data not shown). Similar results for both Mek inhibitors were observed. Treatment of control cells with UO126 induced Bim upregulation in attached cells concomitant with a decrease in Erk phosphorylation (Fig. 6A, lanes 1 and 2) consistent with the known role of Erk in regulating Bim expression. Mimosine treatment prevented induction of Bim by UO126, and interestingly, this correlated with a significant retention of Erk phosphorylation, despite Mek inhibition (lanes 3 and 4).
FIG. 6.
Increased Erk phosphorylation is necessary for G1/S-mediated Bim suppression. (A) Increased Erk activity is necessary for mimosine-mediated Bim suppression. MCF-10A cells were treated with DMSO (control) or 200 μM mimosine as described in Materials and Methods. Following 24 h of DMSO or mimosine treatment, cells were then treated with either DMSO (−−) or the MEK inhibitor UO126 (++) and incubated for another 24 h. Cells were then either left attached or placed in suspension in the presence of all drugs or vehicle for 24 h. At this point, cell lysates were prepared and immunoblotted with antiserum to Bim, actin (as a loading control), or phosphorylated Erk (p-Erk1/2). These images are of two immunoblots of the same cell lysates with corresponding actin loading controls beneath each. (B) Increased Erk phosphorylation is required for p21Cip1-mediated Bim suppression. MCF-10A cells expressing either control vector (BABE) or BABE encoding p21Cip1 were treated with DMSO or UO126, placed under attached or suspended conditions, and cell lysates were prepared. These lysates were immunoblotted with antiserum to Bim, phosphorylated Erk1/2 (p-Erk1/2), or actin (loading control). These images are of two immunoblots of the same cell lysates with corresponding actin loading controls beneath each.
We also examined the effects of Mek inhibition on suppression of Bim under anoikis conditions (Fig. 6A, lanes 5 to 8). As shown in Fig. 2 and 5, control cells in suspension upregulate Bim and downregulate Erk phosphorylation (lane 5). Under suspended conditions, treatment of control cells with UO126 caused slight further upregulation of Bim (Fig. 6, lanes 5 and 6). As with the cases shown in Fig. 2 and 5, mimosine-arrested cells in suspension retained Erk phosphorylation and failed to upregulate Bim (lane 7). However, mimosine-arrested, suspended cells treated with UO126 exhibited a greater reduction in Erk phosphorylation than that observed for cells treated with mimosine alone, and there was a concomitant increase in Bim levels (lane 8). These data indicate that downregulation of Erk phosphorylation correlates with Bim upregulation. Furthermore, these results suggest that the retained Erk phosphorylation observed in mimosine-arrested, suspended cells is necessary for Bim suppression.
Similar results were observed for attached or suspended UO126-treated cells that were growth arrested by the overexpression of p21Cip1 (Fig. 6B). Bim upregulation in p21Cip1-expressing cells treated with UO126 under attached and suspended conditions correlated with the corresponding downregulation of Erk phosphorylation (Fig. 6B, lanes 4 and 8). These results support the conclusion that the retention of Erk phosphorylation in suspended, G1/S-arrested cells is required for Bim suppression.
G1/S arrest does not activate the Ras/MAPK pathway upstream of Erk.
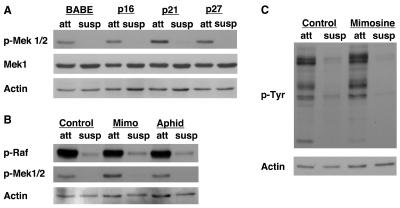
To further investigate the mechanism by which G1/S arrest leads to enhanced Erk activation and subsequent anoikis resistance, we examined the activation status of the components of the pathway upstream of Erk by using phospho-specific antibodies (Fig. 7). Surprisingly, Mek activation, unlike Erk activation, was not retained in suspended G1/S-arrested cells. Indeed, we observed no difference in Mek phosphorylation between detached cells arrested by CKI expression, mimosine treatment, or aphidicolin treatment (Fig. 7A and B) and control cells. We did observe a slight increase in Mek phosphorylation in attached cells arrested with p21Cip1 expression or mimosine treatment, but this difference was not observed for detached cells. In addition, we observed no difference in Raf phosphorylation between control cells and cells arrested by mimosine or aphidicolin treatment (Fig. 7B). Furthermore, the profile of tyrosine-phosphorylated proteins does not change following mimosine treatment under either attached or suspended conditions (Fig. 7C). These data suggest that sustained Erk activation by G1/S arrest following detachment is not mediated by activation of upstream kinases or by maintenance of Raf or Mek activation in suspension.
FIG. 7.
G1/S arrest does not activate the Ras/MAPK pathway upstream of Erk. (A) CKI expression does not enhance Mek phosphorylation. Attached (att) and suspended (susp) lysates harvested from MCF-10A cells treated similarly to those in Fig. 1B were immunoblotted with antisera against phosphorylated Mek1/2 (p-Mek 1/2), Mek1, and actin (as a loading control). (B) G1/S arrest by mimosine or aphidicolin treatment does not enhance Raf or Mek phosphorylation. Attached (att) and suspended (susp) lysates from cells treated with vehicle control, mimosine (Mimo), or aphidicolin (Aphid) as described for Fig. 2A and B were immunoblotted with antisera against phosphorylated Raf (p-Raf), phosphorylated Mek1/2 (p-Mek1/2), and actin (as a loading control). (C) Overall tyrosine phosphorylation is not enhanced following mimosine treatment. Lysates treated similarly to those in Fig. 2A were immunoblotted with antisera against phosphorylated tyrosine (p-Tyr) and actin (as a loading control).
The PI3K/Akt and JNK/MAPK pathways do not contribute to G1/S arrest-mediated anoikis resistance.
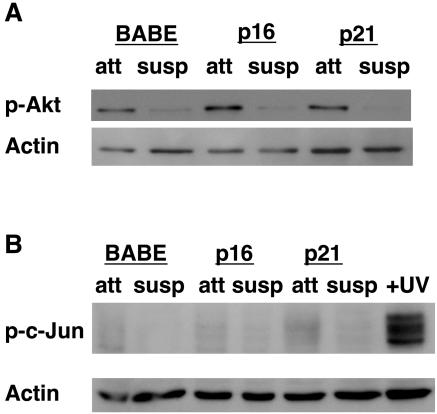
While our data indicate that Erk plays a critical role in anoikis resistance in G1/S-arrested MCF-10A cells through Bim, several other signaling pathways have been reported to regulate anoikis, such as the PI3K/Akt pathway and the JNK/MAPK pathway (19). We examined both of these pathways in G1/S-arrested cells to determine if they contributed to G1/S arrest-mediated anoikis resistance (Fig. 8). However, no changes in either of these pathways following detachment of G1/S-arrested cells compared to that of control cells were observed (Fig. 8), suggesting that anoikis resistance in G1/S-arrested cells is not due to perturbation of the PI3K/Akt or JNK/MAPK pathways.
FIG. 8.
The PI3K/Akt and JNK/MAPK pathways do not contribute to G1/S arrest-mediated anoikis resistance. (A) Akt phosphorylation does not change following G1 arrest. MCF-10A cells overexpressing p16INK4a, p21Cip1, or control vector (BABE) were plated under attached (att) or suspended (susp) conditions for 24 h. Lysates were harvested as described for Fig. 1B and immunoblotted with antiserum against phosphorylated Akt (p-Akt) or actin (as a loading control). (B) c-Jun is not phosphorylated following detachment or G1 arrest in MCF-10A cells. Lysates from attached (att) or suspended (susp) cells treated as described for panel A, along with lysates from UV-irradiated MCF-10A cells (as a positive control), were harvested as described for Fig. 1B. Lysates were immunoblotted with antiserum against phosphorylated c-Jun (p-c-Jun) as a measure of the activity of the JNK/MAPK pathway. The immunoblot was then reprobed for actin as a loading control.
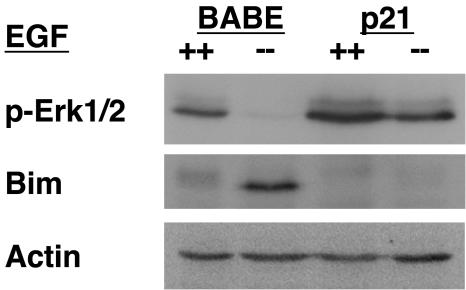
G1/S arrest suppresses Bim upregulation and maintains Erk phosphorylation in response to growth factor withdrawal. Since the processes of growth factor withdrawal and anoikis are thought to be intimately coupled, we examined the effects of EGF withdrawal on p21Cip1-arrested cells (Fig. 9). EGF withdrawal for 24 h caused downregulation of Erk phosphorylation and upregulation of Bim in control vector cells. However, when EGF was withdrawn from p21Cip1-expressing cells, Erk phosphorylation was maintained, and Bim was not upregulated. These data indicate that G1/S-arrested cells retain Erk phosphorylation and suppress Bim upregulation in response to growth factor withdrawal, and thus display a response similar to that observed in G1/S-arrested cells detached from matrix.
FIG. 9.
G1/S arrest inhibits Bim upregulation and maintains Erk phosphorylation following growth factor withdrawal. MCF-10A cells transduced with control vector (BABE) or BABE encoding p21Cip1 were cultured with (++) or without (−−) EGF for 24 h and analyzed for Bim expression and Erk phosphorylation (p-Erk1/2) as described for Fig. 6.
DISCUSSION
The results presented in this report reveal novel molecular cross talk between cell cycle arrest and protection from detachment-induced apoptosis. We show that G1/S-arrested cells are protected from anoikis and exhibit sustained Erk phosphorylation in suspension, thus preventing the induction of the proapoptotic, BH3-only protein Bim. We have previously shown that the induction of apoptosis in suspended MCF-10A cells is at least partially mediated by upregulation of Bim and that the downregulation of Bim by use of small inhibitory RNA oligonucleotides provides partial anoikis resistance (47). Furthermore, Bim expression has been shown to be regulated by the Erk pathway, being induced by a reduction in Erk activation and suppressed during anoikis when the Erk pathway is maintained by ErbB2 activation or constitutively active mutants of Ras, Raf, or Mek (47). Thus, G1/S arrest provides protection from anoikis by a mechanism similar to that of certain oncogenes.
A recent study using Calbiochem Bim antibody no. 202000 has suggested that the antibody used in our study to detect Bim protein levels (Stressgen no. AAP-330) does not recognize phosphorylated Bim (61). Furthermore, this report has suggested that increased Bim protein expression following detachment of MCF-10A cells that we have previously described (47) may represent dephosphorylation (61). However, we have not detected any difference between the abilities of the Stressgen antibody to recognize phosphorylated and unphosphorylated Bim (see Fig. S1 in the supplemental material). In addition, we observed the same ratios of Bim expression in control cells and arrested cells following detachment (i.e., reduced Bim expression in arrested cells compared to control cells) by use of either the Stressgen or Calbiochem Bim antibody (data not shown). Furthermore, the Stressgen Bim antibody has been previously shown to recognize both the phosphorylated and unphosphorylated forms of Bim (4). Lastly, using a Calbiochem Bim antibody in MCF-10A cells, Marani et al. have also shown that Bim protein levels are upregulated following anoikis (36). These results, taken together, rule out differential recognition of phosphorylated and unphosphorylated Bim by the Stressgen antibody and confirm that Bim protein expression does increase in MCF-10A cells following detachment.
The Erk pathway has been identified as a key regulator of Bim (33-36, 47, 63). Bim is regulated by Erk at both the transcriptional (36, 47) and posttranslational levels (33-36). While the mechanism by which Erk inhibits Bim transcription is unclear, the posttranslational prosurvival effects of Erk are thought to be dependent on direct phosphorylation of Bim by Erk, leading to the proteasomal degradation of Bim (33-35). We have shown previously that, in MCF-10A cells, Bim mRNA increases sevenfold following detachment for 24 h and that this increase can be inhibited by the expression of active MEK2 (MEK2-DD) (47). Additionally, we have observed phosphorylation of BimEL by activation of the MEK/Erk pathway in MCF-10A cells (see Fig. S1 in the supplemental material) and enhanced apoptosis in a nonphosphorylatable variant of BimEL (Bim S69A) (M. Reginato and J. Brugge, unpublished observations). Since no difference between the Bim mRNA levels of control and mimosine-treated detached cells was observed, these data suggest that posttranslational regulation by Erk mediates the Bim suppression observed following G1/S arrest.
While we have elucidated one pathway by which maintenance of Erk activation during G1/S arrest can provide anoikis resistance, sustained Erk activity is likely to provide other prosurvival signals in addition to Bim suppression. For example, the Ras-Erk MAPK pathway has been shown to promote phosphorylation and inhibition of Bad, which could be involved G1/S-mediated anoikis resistance, as well (48, 49). Furthermore, Erk activation can provide survival signals through inhibitory phosphorylation of caspase 9 (1) as well as through activating phosphorylation of IEX-1 (20), either of which may play a role in G1/S-mediated anoikis resistance. Sustained Erk activation in G1/S-arrested cells could also provide other survival signals through Erk-dependent transcription of survival proteins, such as Bcl-2, Bcl-xL, and Mcl-1 (6, 29, 45); however, Bcl-2 and Bcl-xL protein levels do not change upon G1/S arrest or detachment (data not shown).
The mechanism by which G1/S arrest allows maintenance of Erk activation in suspension is unclear. We did not observe any increase in Raf or Mek activation or tyrosine phosphorylation of any proteins in whole-cell lysates from G1/S-arrested cells. Our experiments show that Erk activation is maintained under conditions in which activation of the upstream kinases Raf and Mek is lost. These results indicate that G1/S arrest acts to enhance Erk activation at the level of Erk dephosphorylation rather than through upstream activation, possibly through inhibition of an Erk phosphatase.
In addition to Bim, another proapoptotic BH3-only protein, Bad, has also been shown to be regulated during the cell cycle (30). Following apoptotic stimuli in rat neurons, CDK1 can phosphorylate Bad on S128, resulting in Bad-mediated apoptosis. Proapoptotic Bad phosphorylation on S128 opposes growth factor-induced, prosurvival Bad phosphorylation on S136 by inhibiting the interaction between Bad and 14-3-3 proteins. If CDK1 activation is required for anoikis through a similar mechanism, then G1/S arrest could also provide anoikis resistance by preventing CDK1 activation, which occurs only during the G2/M transition.
Recent reports have suggested that p21Cip1 or p27Kip1 can undergo phosphorylation-induced cytoplasmic relocalization, allowing for cell cycle/CDK-independent functions, including inhibition of various proapoptotic signaling pathways (5, 12). We used several approaches to address whether cytoplasmic activities of p21Cip1 or p27Kip1 are involved in anoikis resistance. For example, we expressed two p21Cip1 mutants in MCF-10A cells that have been previously shown to localize to the cytoplasm (see Fig. S2A in the supplemental material) (3, 66). In MCF-10A cells, the expression of these mutants did not abrogate G1 arrest as described for other cell types (see Fig. S2B in the supplemental material). In addition, we did not observe any cytoplasmic localization of overexpressed wild-type or mutant p21Cip1 or p27Kip1 (see Fig. S3 in the supplemental material). Thus, while we cannot rule out the involvement of p21Cip1 and p27Kip1 activities that are independent of growth arrest, we have no evidence that supports such a role in MCF-10A cells. Although mimosine treatment has been shown to increase p21Cip1 or p27Kip1 expression (2, 60), p21Cip1 and p27Kip1 levels remained unchanged following mimosine treatment (data not shown). These data, together with our data from the CDK4 R24C experiment (Fig. 3) and evidence that mimosine or aphidicolin treatment induces anoikis protection (Fig. 1), are consistent with the conclusion that cell cycle arrest provides anoikis resistance in our model.
Acquisition of the ability to survive in the absence of normal matrix components is believed to represent a critical property of metastatic cells, since tumor cells that intravasate into the blood and extravasate into the secondary tissue sites are either deprived of matrix or exposed to foreign matrix components (11, 17, 19). Evidence that manipulations, such as the downregulation of FAK, which sensitize tumor cells to anoikis can suppress metastasis of tumor cells in vivo supports this possibility (14). Recent reports involving studies of spontaneous metastasis or intravenous injection of tumor cells have provided evidence that dormant, solitary, nonreplicating cells are able to survive in the circulation and at secondary sites until they initiate cell proliferation (9, 40). This event triggers apoptosis in cells that lack metastatic activity, whereas metastatic tumor cells are able to survive and expand at the secondary site. These studies suggest that cell cycle arrest is an important mechanism of apoptotic resistance for metastatic cells. The sensitivity of nonmetastatic proliferating cells to apoptosis in unnatural microenvironments likely represents a natural homeostatic control mechanism to prevent aberrant expansion of cells (11). Our studies indicate that the ability of nonreplicating cells to maintain Erk activity and suppress Bim induction under conditions of matrix deprivation represents one mechanism by which dormant, nonmetastatic cells can survive detachment-induced apoptosis. In addition, our data from studies of EGF withdrawal suggest that the sustained Erk activity would also allow nonproliferating cells to survive in environments where cells are deprived of natural growth factors, and one could extrapolate that sustained Erk activity could contribute to resistance to other apoptotic stimuli.
Our studies reveal a novel molecular mechanism by which G1/S arrest provides anoikis resistance, that is, through sustained Erk phosphorylation resulting in Bim suppression and protection from anoikis. It is hypothesized that this mechanism of arrest-mediated anoikis resistance could be responsible, in part, for the ability of arrested, dormant cells to survive detachment-induced apoptosis during the early stages of metastasis.
Supplementary Material
Acknowledgments
We thank D. Livingston, R. Weinberg, M. Ewen, S. Muthuswamy, J. Ruderman, P. Hinds, W. Sellers, and members of the Brugge lab for stimulating scientific discussions and feedback. We also thank A. Witt for preparation of some of the viral vectors, H. Irie and M. Overholtzer for scientific and technical advice, S. Isakoff for critical reading of the manuscript, P. Hinds, M.-C. Hung, and K. Polyak for plasmids, and R. Mulligan for VSV-GPG cells.
This work was supported by grants from the National Cancer Institute (CA89393 and CA80111) to J.S.B., a National Science Foundation predoctoral fellowship to N.L.C., a Susan Komen Breast Cancer Postdoctoral Fellowship to M.J.R., and support from the Breast Cancer Research Foundation to J.L. and J.S.B. J.S.B. was an American Cancer Society Research Professor during the period when this research was performed.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Allan, L. A., N. Morrice, S. Brady, G. Magee, S. Pathak, and P. R. Clarke. 2003. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat. Cell Biol. 5:647-654. [DOI] [PubMed] [Google Scholar]
- 2.Alpan, R. S., and A. B. Pardee. 1996. p21WAF1/CIP1/SDI1 is elevated through a p53-independent pathway by mimosine. Cell Growth Differ. 7:893-901. [PubMed] [Google Scholar]
- 3.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas, S. C., and L. A. Greene. 2002. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem. 277:49511-49516. [DOI] [PubMed] [Google Scholar]
- 5.Blagosklonny, M. V. 2002. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle 1:391-393. [DOI] [PubMed] [Google Scholar]
- 6.Boucher, M. J., J. Morisset, P. H. Vachon, J. C. Reed, J. Laine, and N. Rivard. 2000. MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J. Cell. Biochem. 79:355-369. [PubMed] [Google Scholar]
- 7.Boudreau, N., Z. Werb, and M. J. Bissell. 1996. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl. Acad. Sci. USA 93:3509-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bretland, A. J., J. Lawry, and R. M. Sharrard. 2001. A study of death by anoikis in cultured epithelial cells. Cell Prolif. 34:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron, M. D., E. E. Schmidt, N. Kerkvliet, K. V. Nadkarni, V. L. Morris, A. C. Groom, A. F. Chambers, and I. C. MacDonald. 2000. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 60:2541-2546. [PubMed] [Google Scholar]
- 10.Castedo, M., J. L. Perfettini, T. Roumier, and G. Kroemer. 2002. Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 9:1287-1293. [DOI] [PubMed] [Google Scholar]
- 11.Chambers, A. F., A. C. Groom, and I. C. MacDonald. 2002. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2:563-572. [DOI] [PubMed] [Google Scholar]
- 12.Coqueret, O. 2003. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 13:65-70. [DOI] [PubMed] [Google Scholar]
- 13.Debnath, J., S. K. Muthuswamy, and J. S. Brugge. 2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30:256-268. [DOI] [PubMed] [Google Scholar]
- 14.Duxbury, M. S., H. Ito, M. J. Zinner, S. W. Ashley, and E. E. Whang. 2004. Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery 135:555-562. [DOI] [PubMed] [Google Scholar]
- 15.Evan, G. I., L. Brown, M. Whyte, and E. Harrington. 1995. Apoptosis and the cell cycle. Curr. Opin. Cell Biol. 7:825-834. [DOI] [PubMed] [Google Scholar]
- 16.Farinelli, S. E., and L. A. Greene. 1996. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J. Neurosci. 16:1150-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisch, S. M., and H. Francis. 1994. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisch, S. M., and E. Ruoslahti. 1997. Integrins and anoikis. Curr. Opin. Cell Biol. 9:701-706. [DOI] [PubMed] [Google Scholar]
- 19.Frisch, S. M., and R. A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13:555-562. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, J., Y. Ye, V. Arranz, C. Letourneux, G. Pezeron, and F. Porteu. 2002. IEX-1: a new ERK substrate involved in both ERK survival activity and ERK activation. EMBO J. 21:5151-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gartel, A. L., and A. L. Tyner. 2002. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol. Cancer Ther. 1:639-649. [PubMed] [Google Scholar]
- 22.Hama, S., Y. Heike, I. Naruse, M. Takahashi, H. Yoshioka, K. Arita, K. Kurisu, C. K. Goldman, D. T. Curiel, and N. Saijo. 1998. Adenovirus-mediated p16 gene transfer prevents drug-induced cell death through G1 arrest in human glioma cells. Int. J. Cancer 77:47-54. [DOI] [PubMed] [Google Scholar]
- 23.Harbour, J. W., and D. C. Dean. 2000. Rb function in cell-cycle regulation and apoptosis. Nat. Cell Biol. 2:E65-E67. [DOI] [PubMed] [Google Scholar]
- 24.Hiromura, K., J. W. Pippin, M. L. Fero, J. M. Roberts, and S. J. Shankland. 1999. Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27(Kip1). J. Clin. Investig. 103:597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes, T. A., and P. R. Cook. 1996. Mimosine arrests the cell cycle after cells enter S-phase. Exp. Cell Res. 222:275-280. [DOI] [PubMed] [Google Scholar]
- 26.Hynes, R. O. 1999. Cell adhesion: old and new questions. Trends Cell Biol. 9:M33-M37. [PubMed] [Google Scholar]
- 27.Kim, H. R., H. M. Lin, H. Biliran, and A. Raz. 1999. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 59:4148-4154. [PubMed] [Google Scholar]
- 28.King, K. L., and J. A. Cidlowski. 1995. Cell cycle and apoptosis: common pathways to life and death. J. Cell Biochem. 58:175-180. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita, T., T. Yokota, K. Arai, and A. Miyajima. 1995. Regulation of Bcl-2 expression by oncogenic Ras protein in hematopoietic cells. Oncogene 10:2207-2212. [PubMed] [Google Scholar]
- 30.Konishi, Y., M. Lehtinen, N. Donovan, and A. Bonni. 2002. Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol. Cell 9:1005-1016. [DOI] [PubMed] [Google Scholar]
- 31.Krude, T. 1999. Mimosine arrests proliferating human cells before onset of DNA replication in a dose-dependent manner. Exp. Cell Res. 247:148-159. [DOI] [PubMed] [Google Scholar]
- 32.Lalande, M. 1990. A reversible arrest point in the late G1 phase of the mammalian cell cycle. Exp. Cell Res. 186:332-339. [DOI] [PubMed] [Google Scholar]
- 33.Ley, R., K. Balmanno, K. Hadfield, C. Weston, and S. J. Cook. 2003. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278:18811-18816. [DOI] [PubMed] [Google Scholar]
- 34.Ley, R., K. E. Ewings, K. Hadfield, E. Howes, K. Balmanno, and S. J. Cook. 2004. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J. Biol. Chem. 279:8837-8847. [DOI] [PubMed] [Google Scholar]
- 35.Luciano, F., A. Jacquel, P. Colosetti, M. Herrant, S. Cagnol, G. Pages, and P. Auberger. 2003. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22:6785-6793. [DOI] [PubMed] [Google Scholar]
- 36.Marani, M., D. Hancock, R. Lopes, T. Tenev, J. Downward, and N. R. Lemoine. 2004. Role of Bim in the survival pathway induced by Raf in epithelial cells. Oncogene 23:2431-2441. [DOI] [PubMed] [Google Scholar]
- 37.Meredith, J. E., Jr., B. Fazeli, and M. A. Schwartz. 1993. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 4:953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miranti, C. K., and J. S. Brugge. 2002. Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4:E83-E90. [DOI] [PubMed] [Google Scholar]
- 39.Nahle, Z., J. Polakoff, R. V. Davuluri, M. E. McCurrach, M. D. Jacobson, M. Narita, M. Q. Zhang, Y. Lazebnik, D. Bar-Sagi, and S. W. Lowe. 2002. Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 4:859-864. [DOI] [PubMed] [Google Scholar]
- 40.Naumov, G. N., I. C. MacDonald, P. M. Weinmeister, N. Kerkvliet, K. V. Nadkarni, S. M. Wilson, V. L. Morris, A. C. Groom, and A. F. Chambers. 2002. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 62:2162-2168. [PubMed] [Google Scholar]
- 41.Nguyen, L., B. Malgrange, V. Rocher, G. Hans, G. Moonen, J. M. Rigo, and S. Belachew. 2003. Chemical inhibitors of cyclin-dependent kinases control proliferation, apoptosis and differentiation of oligodendroglial cells. Int. J. Dev. Neurosci. 21:321-326. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor, L., A. Strasser, L. A. O'Reilly, G. Hausmann, J. M. Adams, S. Cory, and D. C. Huang. 1998. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17:384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ory, D. S., B. A. Neugeboren, and R. C. Mulligan. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93:11400-11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pucci, B., M. Kasten, and A. Giordano. 2000. Cell cycle and apoptosis. Neoplasia 2:291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramljak, D., C. M. Coticchia, T. G. Nishanian, M. Saji, M. D. Ringel, S. D. Conzen, and R. B. Dickson. 2003. Epidermal growth factor inhibition of c-Myc-mediated apoptosis through Akt and Erk involves Bcl-xL upregulation in mammary epithelial cells. Exp. Cell Res. 287:397-410. [DOI] [PubMed] [Google Scholar]
- 46.Rane, S. G., S. C. Cosenza, R. V. Mettus, and E. P. Reddy. 2002. Germ line transmission of the Cdk4(R24C) mutation facilitates tumorigenesis and escape from cellular senescence. Mol. Cell. Biol. 22:644-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reginato, M. J., K. R. Mills, J. K. Paulus, D. K. Lynch, D. C. Sgroi, J. Debnath, S. K. Muthuswamy, and J. S. Brugge. 2003. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat. Cell Biol. 5:733-740. [DOI] [PubMed] [Google Scholar]
- 48.Scheid, M. P., and V. Duronio. 1998. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc. Natl. Acad. Sci. USA 95:7439-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheid, M. P., K. M. Schubert, and V. Duronio. 1999. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 274:31108-31113. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt, M., S. Hovelmann, and T. L. Beckers. 2002. A novel form of constitutively active farnesylated Akt1 prevents mammary epithelial cells from anoikis and suppresses chemotherapy-induced apoptosis. Br. J. Cancer 87:924-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulze, A., K. Lehmann, H. B. Jefferies, M. McMahon, and J. Downward. 2001. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 15:981-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz, M. A., and R. K. Assoian. 2001. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114:2553-2560. [DOI] [PubMed] [Google Scholar]
- 53.Shanmugathasan, M., and S. Jothy. 2000. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol. Int. 50:273-279. [DOI] [PubMed] [Google Scholar]
- 54.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 55.Shi, L., W. K. Nishioka, J. Th'ng, E. M. Bradbury, D. W. Litchfield, and A. H. Greenberg. 1994. Premature p34cdc2 activation required for apoptosis. Science 263:1143-1145. [DOI] [PubMed] [Google Scholar]
- 56.Soule, H. D., T. M. Maloney, S. R. Wolman, W. D. Peterson, Jr., R. Brenz, C. M. McGrath, J. Russo, R. J. Pauley, R. F. Jones, and S. C. Brooks. 1990. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 50:6075-6086. [PubMed] [Google Scholar]
- 57.Strasser, A., H. Puthalakath, P. Bouillet, D. C. Huang, L. O'Connor, L. A. O'Reilly, L. Cullen, S. Cory, and J. M. Adams. 2000. The role of bim, a proapoptotic BH3-only member of the Bcl-2 family in cell-death control. Ann. N. Y. Acad. Sci. 917:541-548. [DOI] [PubMed] [Google Scholar]
- 58.Streuli, C. H., and A. P. Gilmore. 1999. Adhesion-mediated signaling in the regulation of mammary epithelial cell survival. J. Mammary Gland Biol. Neoplasia 4:183-191. [DOI] [PubMed] [Google Scholar]
- 59.Vidal, A., and A. Koff. 2000. Cell-cycle inhibitors: three families united by a common cause. Gene 247:1-15. [DOI] [PubMed] [Google Scholar]
- 60.Wang, G., R. Miskimins, and W. K. Miskimins. 2000. Mimosine arrests cells in G1 by enhancing the levels of p27(Kip1). Exp. Cell Res. 254:64-71. [DOI] [PubMed] [Google Scholar]
- 61.Wang, P., A. P. Gilmore, and C. H. Streuli. 2004. Bim is an apoptosis sensor that responds to loss of survival signals delivered by epidermal growth factor but not those provided by integrins. J. Biol. Chem. 279:41280-41285. [DOI] [PubMed] [Google Scholar]
- 62.Watson, P. A., H. H. Hanauske-Abel, A. Flint, and M. Lalande. 1991. Mimosine reversibly arrests cell cycle progression at the G1-S phase border. Cytometry 12:242-246. [DOI] [PubMed] [Google Scholar]
- 63.Weston, C. R., K. Balmanno, C. Chalmers, K. Hadfield, S. A. Molton, R. Ley, E. F. Wagner, and S. J. Cook. 2003. Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 22:1281-1293. [DOI] [PubMed] [Google Scholar]
- 64.Yan, Y., J. Frisen, M. H. Lee, J. Massague, and M. Barbacid. 1997. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 11:973-983. [DOI] [PubMed] [Google Scholar]
- 65.Yu, D., T. Jing, B. Liu, J. Yao, M. Tan, T. J. McDonnell, and M. C. Hung. 1998. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol. Cell 2:581-591. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.