Abstract
We describe here the development of a reverse genetics system for the phlebovirus Uukuniemi virus, a member of the Bunyaviridae family, by using RNA polymerase I (pol I)-mediated transcription. Complementary DNAs containing the coding sequence for either chloramphenicol acetyltransferase (CAT) or green fluorescent protein (GFP) (both in antisense orientation) were flanked by the 5′- and 3′-terminal untranslated regions of the Uukuniemi virus sense or complementary RNA derived from the medium-sized (M) RNA segment. This chimeric cDNA (pol I expression cassette) was cloned between the murine pol I promoter and terminator and the plasmid transfected into BHK-21 cells. When such cells were either superinfected with Uukuniemi virus or cotransfected with expression plasmids encoding the L (RNA polymerase), N (nucleoprotein), and NSs (nonstructural protein) viral proteins, strong CAT activity or GFP expression was observed. CAT activity was consistently stronger in cells expressing L plus N than following superinfection. No activity was seen without superinfection, nor was activity detected when either the L or N expression plasmid was omitted. Omitting NSs expression had no effect on CAT activity or GFP expression, indicating that this protein is not needed for viral RNA replication or transcription. CAT activity could be serially passaged to fresh cultures by transferring medium from CAT-expressing cells, indicating that recombinant virus containing the reporter construct had been produced. In summary, we demonstrate that the RNA pol I system, originally developed for influenza virus, which replicates in the nucleus, has strong potential for the development of an efficient reverse genetics system also for Bunyaviridae members, which replicate in the cytoplasm.
The procedures developed during the 1990s to genetically manipulate the genomes of negative-strand viruses and to rescue infectious viruses entirely from cloned cDNAs, commonly referred to as reverse genetics, have revolutionized the analyses of viral gene expression and the dissection of cis-acting regulatory sequences important for replication and transcription. They have also paved the road for engineering these viruses for vaccine and gene therapy purposes (6, 36). The ability to rescue infectious viruses from cloned cDNAs has by now been well established for nonsegmented, negative-strand viruses (Mononegavirales), such as members of the Rhabdoviridae (22, 41, 48) and Paramyxoviridae (1, 5, 18, 19, 35) families. The development of similar protocols for manipulating the genomes and creating viruses from cloned cDNAs of segmented, negative-strand viruses, i.e., members of the Orthomyxoviridae, Bunyaviridae, and Arenaviridae families, have turned out to be much more difficult. Although the ability to manipulate RNA segments of influenza A viruses was developed more than a decade ago (10, 25), it was not until last year that the first reports on the rescue of infectious influenza A virus entirely from cloned cDNAs were published (15, 20, 31, 32).
Members of the Bunyaviridae family, which comprises more than 300 viruses (28) grouped into the five genera Bunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus, are enveloped viruses with a tripartite, single-stranded RNA genome of negative polarity. The L segment encodes the RNA-dependent RNA polymerase (L), the M segment encodes the two spike proteins (G1 and G2) and in some viruses a nonstructural protein (NSm), while the S segment encodes the nucleoprotein (N) and in some viruses a nonstructural protein (NSs) (8, 40). Initiation of transcription of the viral mRNAs is primed by short sequences derived from the 5′ end of host mRNAs (2, 40, 43). This cap-snatching mechanism is reminiscent of that first described for influenza virus (21), with the important difference that cap snatching occurs in the cytoplasm of Bunyaviridae-infected cells, as opposed to the nucleus in influenza virus-infected cells. This is due to the fact that Bunyaviridae members replicate exclusively in the cytoplasm. As is the case for all negative-strand RNA viruses, the templates for L polymerase-catalyzed replication and transcription of Bunyaviridae members are the ribonucleoproteins (RNPs) consisting of the full-length positive- or negative-strand RNA segments associated with the N protein.
To date, methods to study the role of cis-acting sequences at the 5′ and 3′ termini of viral RNA (vRNA) segments have been developed for Bunyamwera (BUN) virus (Bunyavirus) (7) and Rift Valley fever (RVF) virus (Phlebovirus) (24, 34), using the now classical T7-vaccinia virus (T7-VV) system (16) to express a chloramphenicol acetyltransferase (CAT) reporter cDNA flanked by 5′ and 3′ vRNA ends. An important step was taken when Bridgen and Elliott in 1996 (4) were able to rescue infectious BUN virus entirely from cloned cDNAs, although the procedure was cumbersome and the efficiency of generating infectious virus was rather low.
To look for an alternative approach for developing a reverse genetics system for Bunyaviridae, we have turned to the RNA polymerase I (pol I) expression system, which was recently successfully used to rescue infectious influenza virus (15, 31, 32). This system, originally developed by Hobom and coworkers (30, 49), has been used to study cis-acting sequences important for transcription and replication (14) and to develop a procedure for indirect selection of recombinant influenza viruses (12). An ambisense strategy to further simplify the procedure was recently reported (20). In the pol I system, cDNAs coding for viral RNA segments, or reporter genes flanked by viral sequences, are cloned between the RNA pol I promoter and terminator to generate transcripts that have correct 5′ and 3′ ends without modifications such as a cap structure and a poly(A) tail (12, 49). In the case of influenza virus, these pol I transcripts are then replicated and transcribed in the nucleus by the necessary viral proteins. Following transport of the RNPs to the cytoplasm, infectious particles are assembled by budding at the plasma membrane.
We have adopted the pol I system to express reporter genes flanked by the 5′ and 3′ noncoding sequences of the M RNA segment of Uukuniemi (UUK) virus, a member of the Phlebovirus genus (28). We have previously characterized extensively the molecular and cell biology of UUK virus. Full-length cDNAs corresponding to the L (6,423 nucleotides [nt]) (9), M (3,229 nt) (38), and S (1,720 nt) (42) segments have been constructed, and cDNAs encoding the open reading frames (ORFs) for the L, G1, G2, N, and NSs proteins have been derived (9, 26, 37, 44). As the first step toward the generation of infectious virus from cloned cDNAs, we show here that the pol I system can be used to synthesize chimeric RNA templates, which, despite lacking a cap structure and poly(A) tail, are transported to the cytoplasm, where they are amplified and transcribed by the UUK virus replicase components supplied either by superinfection with UUK virus or by expression of viral proteins from separate plasmids. The L and N proteins were found to be necessary and sufficient for transcription and replication, while NSs was completely dispensable. We also show that CAT activity could be transferred serially from culture to culture by passaging supernatants from transfected and superinfected cells. This indicates that the chimeric reporter RNA could be packaged into virus particles. Thus, the pol I system holds great potential as an effective alternative approach for a versatile reverse genetics system for members of the Bunyaviridae family.
MATERIALS AND METHODS
Cells and Virus.
BHK-21 cells (American Type Culture Collection) were grown on plastic dishes in Eagle's minimal essential medium (EMEM) supplemented with 10% fetal calf serum (FCS; Life Technologies, Gibco-BRL), 5% tryptose phosphate broth, 2 mM l-glutamine, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml. The origin and the preparation of stock virus from the prototype strain S23 of UUK virus have been described elsewhere (33). The stock virus had a titer of 2 × 108 PFU/ml. Cells were infected with a multiplicity of infection (MOI) of about 5 to 10 PFU/cell.
Construction of plasmids.
The PCR primers used for plasmid constructions are shown in Table 1. Plasmids designed for expression of UUK virus RNA molecules by RNA pol I in vivo carried the rRNA gene (rDNA) promoter region (−251 to −1 relative to the 45S pre-rRNA start point) and the rDNA terminator sequence (+571 to +745 relative to the 3′ end of the 28S rDNA) derived from murine rDNA (49). Between these two elements, UUK virus cDNA constructs from the M segment were exactly inserted in antisense orientation for vRNA expression (primer RF2/RF9) or in sense orientation for viral complementary (cRNA) expression (primer RF33/RF34). For efficient detection of protein expression following transcription and replication, the G1/G2 (p110) ORF in plasmid pRF7, encoding the full-length UUK virus M RNA (UUK M vRNA) segment, was replaced by reporter genes encoding CAT or a modified (enhanced) green fluorescent protein (GFP) (R. Flick and G. Hobom, unpublished data), using two PCR fragments (primers RF4B/RF10 and RF5/RF6, respectively) (Fig. 1; Table 1), without changing any of the nucleotides in the 5′ and 3′ untranslated regions (UTRs) of the UUK M segment. This resulted in pRF33 (UUK-M-CAT-vRNA), pRF31 (UUK-M-GFP-vRNA), or pRF19 (UUK-M-CAT-cRNA), respectively.
TABLE 1.
Oligonucleotide primers used to construct plasmids
FIG. 1.
Schematic diagram of the cloning strategy for constructing chimeric UUK virus reporter plasmids. Two PCR fragments, one containing the murine pol I promoter and the UUK M vRNA 5′ UTR (RF10/4B) and the other containing the CAT (RF5/6) or GFP (RF7B/8) ORF, were ligated with the large ApaI-NcoI fragment from plasmid pRF7 containing the UUK M vRNA 3′ UTR and the pol I terminator. This gave plasmids pRF33 (UUK M-CAT) and pRF31 (UUK M-GFP). Plasmid pRF20 was constructed by inserting multiple cloning sites immediately downstream of the UUK M vRNA 5′ UTR, using the two PCR fragments RF10/30 and RF5/6, respectively (see also Materials and Methods).
RNA pol I transcription plasmids were constructed using pHL1261 (12) or pRF42 (Fig. 2) as the basic vector system. After BsmBI or BbsI digestion, the vector plasmid can incorporate PCR fragments (BsmBI or BbsI restricted) in an oriented way. After transfection into different eukaryotic cell lines, the resulting constructs can be transcribed by RNA pol I, generating transcripts without any additional nucleotides or with modifications at the 5′ or 3′ end [e.g., cap structure or poly(A) tail].
FIG. 2.
Schematic diagram of the RNA polymerase I transcription plasmid (pRF42) and the generation of chimeric UUK virus-reporter RNA segments. To construct reporter plasmids, PCR-amplified expression cassettes are inserted between the BbsI sites (shaded boxes) in the RNA pol I-driven expression plasmid pRF42. The cassettes are flanked by the murine RNA pol I promoter (pM1) and terminator (tM1) (shown in bold). The system allows for the transcription of any expression cassette by RNA pol I in rodent cell lines. The transcript starts exactly at the first position of the expression cassette and terminates at the last position before tM1, generating the correct 5′ and 3′ termini of the insert. BbsI recognition sites are shaded; primers I and II used to amplify the expression cassette are shown in bold; UUK virus-specific 5′ and 3′ sequences are in italics. TAC, complementary to the ATG initiation codon in the reporter cDNA, is underlined.
Cloning constructs were verified either by dideoxy sequencing using an ABI PRISM 310 sequenator across the flanking regions and exchanging the central segment of the inserted PCR fragment by authentic (i.e., non-PCR-derived) DNA, using internally located unique restriction sites, or by sequencing across the entire PCR insert.
To insert the Mazon-Pfizer monkey virus (MPMV) constitutive transport element (CTE) sequence, additional cleavage sites (XhoI-BamHI-SmaI/XmaI-SacI-XbaI) were introduced exactly after the translation termination codon of the CAT gene in pRF33 using two PCR fragments (fragment 1, primer RF10/RF30; fragment 2, primer RF5/RF6) and the large ApaI/NcoI fragment from pRF7 (Fig. 1). The resulting construct pRF20 was used to insert two different forms of CTE sequences (MPMV 250 and MPMV 566; kindly provided by M.-L. Hammarskjöld, Charlottesville, Va.) in sense and antisense orientations (plasmids pRF35 to pRF38; see also Fig. 8).
FIG. 8.
The CTE from MPMV RNA does not increase CAT activity expressed from reporter plasmids. (A) Schematic diagram showing the insertion of one of two forms (MPMV 566 or MPMV 250) of the CTE (in either sense or antisense orientation) between the 5′ UTR of the UUK M vRNA and the CAT ORF. (B) CAT activity in lysates from BHK-21 cells cotransfected with the CTE-containing reporter plasmids and the UUK L and N expression plasmids. Lane 1, CAT activity in cells transfected without viral expression plasmids. CAT activity in cells cotransfected with pRF33 (CAT-M vRNA) and UUK L and N expression plasmids was arbitrarily set at 100 (lane 2). Plasmid pRF20, containing an additional multiple cloning site downstream of the UUK 5′ UTR, displayed the same CAT activity as the parental pRF33 plasmid. CAT activity measured from the other lysates is expressed as percentage of that obtained in this control.
For UUK virus protein expression, cDNAs from the UUK S segment encoding the N and NSs proteins, were inserted into the pcDNA3(+) vector (Invitrogen) using EcoRV cleavage. This gave rise to plasmids pCMV-UUK-N and pCMV-UUK-NSs. For UUK L expression, a NotI fragment from pBSK-UUK-L (kindly provided by R. Elliott, Glasgow, United Kingdom) was inserted into the NotI site of pcDNA3(+), giving rise to pCMV-UUK-L.
Transfection and superinfection with UUK virus.
Plasmid DNA was transfected into subconfluent BHK-21 cells (3 × 106 to 6 × 106) using 2 to 4 μg of the respective plasmid and 8 to 20 μl of liposome plus buffer (LipofectAMINE PLUS; Life Technologies, Gibco-BRL) mixed in serum-free EMEM and incubated for 15 min at room temperature. After addition of 12 to 30 μl of liposome reagent, incubation was continued for a further 15 min. The cells were incubated at 37°C with the DNA-Lipofectamine mixture for 3 to 5 h. To determine the efficiency of transfection, plasmid pHL2823, expressing enhanced GFP (EGFP) under the cytomegalovirus (CMV) promoter (Flick and Hobom, unpublished), was transfected similarly. After further incubation for 20 h in EMEM containing 10% FCS and 5% tryptose phosphate broth, the transfected cells were washed with adsorption medium (EMEM supplemented with 20 mM HEPES and 0.2% bovine serum albumin) and superinfected with UUK virus at an MOI of 5 to 10 PFU/cell. After a 60-min adsorption period, the cells were washed once and incubated with adsorption medium for 15 to 30 h. A complete replication cycle takes place during this period.
CAT assay.
Cell extracts were prepared as described by Gorman et al. (17). In an initial series, 50 μl of each cell lysate (prepared from 106 cells in the case of cotransfection experiments or from 3 × 106 cells in the case of superinfection experiments), and depending on the results, serially diluted samples of the various cell lysates were mixed with 10 μl of acetyl coenzyme A (4 mM) and 10 μl of fluorescence-labeled chloramphenicol (boron dipyrromethane difluoride fluorophore substrate; Flash Cat kit; Stratagene) and incubated at 37°C for 2 h. For extraction of reaction products, 0.5 ml of ethylacetate was added; after centrifugation for 1 min at 15,000 × g, the upper phase containing the reaction products was isolated and the solvent was evaporated. The resulting pellet was resuspended in 20 μl of ethylacetate, and the reaction products were separated by thin-layer chromatography (TLC plates, 20 by 20 cm; Silica Gel 60; Merck) using a solvent mixture (mobile phase) of chloroform and methanol (87:13). Finally, the reaction products were visualized by UV illumination, documented by photography, and evaluated using WinCam software (Cybertech, Berlin, Germany) or Quantity One (Bio-Rad). Ratios of activities were calculated based on at least three independent sets of serial dilutions of cell lysates down to a level of 30 to 50% product formation.
Indirect immunofluorescence microscopy.
BHK-21 cells grown on coverslips were transiently transfected as described above. Five to eight hours after transfection, the cells were washed with phosphate-buffered saline (PBS), fixed with 3% paraformaldehyde in PBS for 15 min at room temperature, washed with PBS again, quenched with 10 mM glycine for 20 min at room temperature, and finally washed with PBS. Depending on the antibodies used, the cells were permeabilized either with 0.1% Triton X-100 for 30 min or with methanol for 2 min at room temperature. Cells were incubated with PBS containing 0.1% bovine serum albumin and incubated for 30 min with a monoclonal antibody against the N protein (J. Veijola, A. Bergström, and R. F. Pettersson, unpublished data) and a rabbit polyclonal antiserum against NSs (44). Primary antibodies were visualized with tetramethyl rhodamine isothiocyanate-conjugated anti-mouse immunoglobulin G or fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G secondary antibody, washed, and mounted in 50% glycerol containing 50 mM Tris-HCl (pH 8.0) and 9.2 mM p-phenylenediamine.
To visualize GFP expression, coverslips were immersed in PBS instead of the standard mounting solution, and fluorescence was monitored at 48 h after transfection with expression plasmids pHL2823 (CMV-GFP) and pRF31 (UUK M-GFP). Immunofluorescence micrographs were obtained either with an Axiophot (Zeiss) or an Eclipse E1000M (Nikon) fluorescence microscope. The latter was equipped with a Spot charge-coupled device camera (Diagnostic Instruments, Inc.).
Serial passaging of virus-containing supernatants.
BHK-21 cells were cotransfected with plasmid pRF33 (UUK M-CAT vRNA), together with the L (pCMV-UUK-L) and N (pCMV-UUK-N) expression plasmids, followed by superinfection with UUK virus at an MOI of 5 to 10 PFU/cell 20 h later. Cells were analyzed for CAT activity 30 h postinfection, and the corresponding supernatants were used for virus passaging. Cell debris was removed by centrifugation at 10,000 rpm (13,000 × g) for 5 min; a 2-ml sample of undiluted supernatant was used to infect a dish containing 6 × 106 BHK-21 cells and incubated for 60 min. After a change of medium (EMEM, 10% FCS) and incubation for 24 to 30 h, cells and supernatants were treated and passaged another round as described above.
RESULTS
General strategy of the pol I-driven expression system.
Our strategy to develop a reverse genetics system for UUK virus by using the RNA pol I expression system basically followed the one established for influenza virus (12, 13, 14, 30, 31, 32, 49). Reporter cDNAs containing the exact ORF for either CAT or GFP (in antisense orientation) were flanked either by the 5′ (185-nt) and 3′ (17-nt) ends of the M vRNA segment or by the cRNA ends (17 and 185 residues, respectively; only for CAT) (Fig. 1 and 2) (38). The CAT and GFP ORFs exactly replaced that of p110, the precursor of G1 and G2 (38, 46), such that no extra nucleotides or any other changes were introduced in the 5′ or 3′ UTR. These chimeric cDNAs were then cloned into plasmid pRF42 (Fig. 2) or pHL1261 (12) between the promoter and terminator of the murine rDNA gene to generate plasmids pRF33 (expressing CAT-M vRNA), pRF31 (GFP-M vRNA) (Fig. 1), and pRF19 (CAT-M cRNA).
In initial control experiments using the pol I CAT-M vRNA construct, we observed a weak CAT activity in the absence of any viral proteins provided from expression plasmids or superinfection. Analysis of the nucleotide sequence upstream of the CAT ORF (i.e., within and beyond of the pol I terminator [Fig. 1]) revealed an AUG codon in frame with the CAT initiator AUG with no intervening in-frame stop codon. We assumed that a weak cryptic promoter upstream of the AUG was generating a transcript that could be translated into an active CAT enzyme. We therefore introduced an oligonucleotide encoding translational stop codons in all three ORFs in the NheI site (Fig. 1; Table 1) between the two AUGs. This completely abolished the background CAT activity. This modification was introduced in the three plasmids pRF19, pRF31, and pRF33.
Based on the results from the influenza system, pol I-driven transcription of the reporter RNAs will initiate and terminate exactly at the 5′ and 3′ of the inserted cDNAs, thus giving rise to transcripts with the correct vRNA or cRNA ends (12, 49). The nucleotide sequences and the strategy for the synthesis of the vRNA sense transcript are shown in Fig. 1 and 2. Following transport to the cytoplasm, these pol I transcripts would be transcribed and replicated by the necessary viral proteins either expressed from plasmids encoding the individual proteins or provided by superinfection with UUK virus. For the synthesis of viral proteins, plasmids expressing mRNAs for L (pCMV-UUK-L), N (pCMV-UUK-N), or NSs (pCMV-UUK-NSs) under the CMV promoter were constructed from previously cloned cDNAs containing the corresponding ORFs. Immunofluorescence analyses of transfected cells showed that N and NSs were efficiently synthesized (data not shown).
Expression of CAT and GFP from chimeric cDNAs flanked by the UUK M vRNA or cRNA 5′ and 3′ ends.
The reporter plasmid pRF33 (CAT-M vRNA) or pRF19 (CAT-M cRNA) was introduced into BHK-21 cells by liposome-mediated transfection. Control experiments using a CMV-EGFP expression plasmid pHL2823 (Flick and Hobom, unpublished) indicated a transfection efficiency of about 20 to 25% (see Fig. 4A). To drive replication and transcription of the chimeric RNA, cells were either infected with UUK virus 20 to 24 h after transfection or cotransfected with expression plasmids encoding L, N, and NSs. Some cultures were both cotransfected with the expression plasmids and superinfected with virus. As shown in Fig. 3, CAT activity was readily detected in lysates from cells transfected with pRF33 and superinfected with UUK virus (lane 2). Interestingly, CAT activity was about 2.4-fold stronger in the lysate from pRF33-transfected cells expressing L, N, and NSs from the plasmids (lane 3) than in that from superinfected cells (activity arbitrarily set at 100%). Superinfection combined with the expression plasmids did not further enhance CAT activity (lane 4).
FIG. 4.
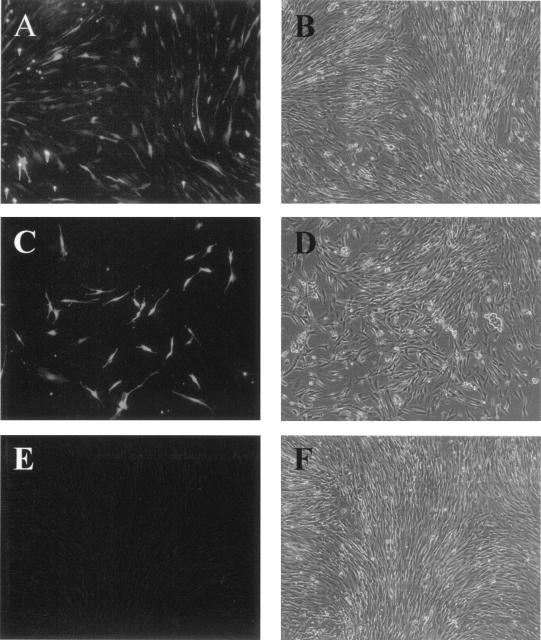
Immunofluorescence analysis showing that expression of GFP from pRF31 is dependent on UUK virus proteins. (A) BHK-21 cells transfected with only pHL2823 encoding GFP under the control of the CMV promoter; (B) same cells as in panel A, viewed by regular light microscopy; (C) cells cotransfected with the reporter plasmid pRF31 (UUK M-GFP vRNA) and the expression plasmids pCMV-UUK-L and pCMV-UUK-N; (D) same cells as in panel C, viewed by light microscopy; (E) cells transfected with pRF31 alone, with GFP expression visualized 48 h later by fluorescence microscopy; (F) same cells as in panel E, viewed by light miccropscopy.
FIG. 3.
CAT activity in BHK-21 cells transfected with pRF33 or pRF19 expressing UUK M-CAT RNA chimeras is dependent on UUK virus proteins. BHK-21 cells were transfected with plasmids pRF33, producing vRNA sense transcripts (lanes 1 to 4), or pRF19, producing cRNA sense transcripts (lanes 5 to 8). Viral helper proteins were supplied either by cotransfection with plasmids expressing the L (pCMV-UUK-L), N (pCMV-UUK-N), and NSs (pCMV-UUK-NSs) proteins (lanes 3 and 7), by superinfection with UUK virus 24 h posttransfection (lanes 2 and 6), or by the combination of superinfection and protein expression (lanes 4 and 8). CAT activity was assayed 20 h postinfection (lanes 2 and 6), 20 h posttransfection (lanes 3 and 7), or 44 h posttransfection (lanes 4 and 8). As shown by transfection with the reporter plasmids alone (lanes 1 and 5), CAT activity was found to be completely dependent on viral protein expression. CAT activity measured from each lysate is expressed as percentage of the activity obtained from superinfected cells, which was arbitrarily set at 100 (lane 2).
Very weak CAT activity was observed in cells transfected with pRF19 (CAT-M cRNA) and infected with UUK virus (Fig. 3, lane 6), while strong activity was evident in cells cotransfected with the plasmids expressing the three viral proteins (lane 7). Again, no enhanced effect was seen if infection was combined with transfection of expression plasmids (lane 8). In the absence of viral superinfection or expression plasmids, no CAT activity was observed in either case (lanes 1 and 5), showing that regardless of whether synthesis is in sense or antisense orientation, the pol I transcript cannot be directly translated into CAT (see also Materials and Methods).
About 5 to 10% of BHK-21 cells transfected with pRF31 (GFP-M vRNA) and cotransfected with expression plasmids were GFP positive (Fig. 4C). The intensity varied between cells, ranging from weak to intense. No GFP-positive cells were observed in the absence of expression plasmids (Fig. 4E). As mentioned above, about 20 to 25% of the cells were positive when GFP was expressed from a plasmid under the CMV promoter (Fig. 4A).
Taken together, these results indicated that pol I-transcribed reporter RNAs are transported to the cytoplasm, where they are transcribed and most likely also replicated by the viral polymerase components to yield the desired reporter protein.
Optimization of reporter gene expression.
To find the optimal conditions for reporter expression, cells were transfected with different ratios of the L, N, and NSs expression plasmids. As shown in Fig. 5A, the NSs protein was found to be completely dispensable for CAT expression, since the same level of activity was observed without NSs and in the presence of increasing amounts of NSs plasmids. The importance of the relative levels of L and N expression was also analyzed. No activity was observed in the absence of the L (Fig. 5B, control lane) or N (Fig. 5C, control lane) plasmid. As shown in Fig. 5B and C, the molar ratio between the L and N expression plasmids was not critical. Molar N:L plasmid ratios of 8:1 (Fig. 5B) or molar L:N plasmid ratio of 8:1 (Fig. 5C) yielded similar levels of CAT activity. Since NSs was not required for CAT expression, plasmid pCMV-UUK-NSs was omitted from the expression studies described below.
FIG. 5.
Optimization of reporter gene expression by titration of UUK L, N, and NSs expression plasmids. (A) Titration of pCMV-UUK-NSs. BHK-21 cells (3 × 106) were cotransfected with constant amounts of the reporter plasmid pRF33 (UUK M-CAT vRNA) (1 μg), expression plasmids pCMV-UUK-L and pCMV-UUK-N in a molar ratio of 2:1, and various amounts of pCMV-UUK-NSs as indicated. A sample corresponding to 1/50 of the cell lysate prepared at 20 h posttransfection was used for CAT reactions. In the control experiment (leftmost lane), BHK-21 cells were transfected only with pRF33 (1 μg). (B) Titration of pCMV-UUK-N. BHK-21 cells were transfected with pRF33 (1 μg) and a constant amount of pCMV-UUK-L (2.5 μg) together with various molar amounts of pCMV-UUK-N as indicated. CAT activity was determined as for panel A. (C) Titration of pCMV-UUK-L. BHK-21 cells were transfected with pRF33 (1 μg) and a constant amount of pCMV-UUK-N (0.3 μg) together with various molar amounts of CMV-UUK-L as indicated. To achieve equal transfection conditions in each experiment, the total transfected DNA was adjusted to the same level by adding plasmid pHL2823 (CMV-GFP).
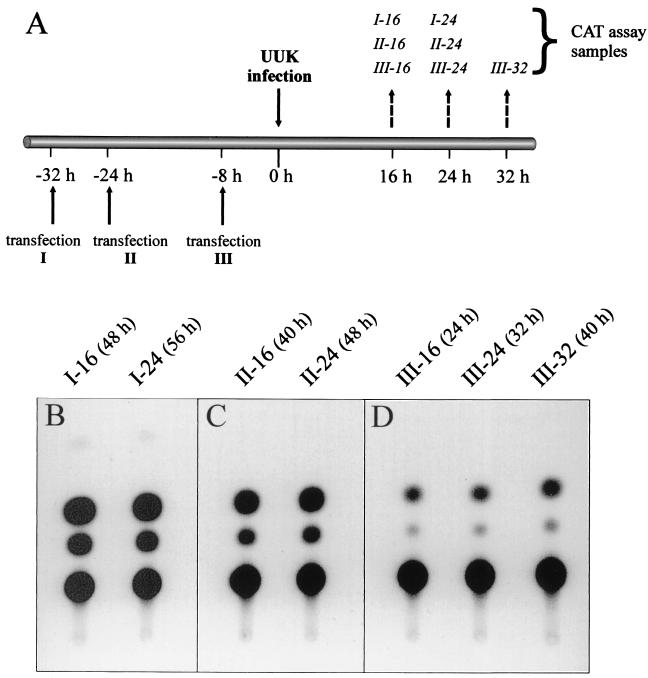
We next analyzed whether the timing of the transfection of the reporter plasmid relative to transfection of the L and N expression plasmids (Fig. 6), or virus superinfection (Fig. 7), was critical for optimal expression. The protocols used for the plasmid transfections are outlined in Fig. 6A. Cells were transfected simultaneously with the reporter and expression plasmids (transfection I), or the expression plasmids were transfected 6 h before the reporter plasmid (transfections IIa and IIb), or vice versa (transfections IIIa and IIIb). Cell lysates were then analyzed for CAT activity at various time points after transfection (Fig. 6B to D). The highest CAT activity was recorded in cells at 24 to 48 h following cotransfection with all three plasmids (Fig. 6B). Somewhat lower activity was also observed at 48 h if the two transfections were separated by 6 h (Fig. 6C and D). It should be noted that the time points above the CAT signals in Fig. 6C and D indicate the time after the second transfection (see also the legend to Fig. 6).
FIG. 6.
Optimization of time intervals between transfection with reporter plasmid and viral protein-expressing plasmids, and analysis of CAT activity. (A) Schematic diagram depicting the timetable for transfection with the RNA pol I expression cassette plasmid (pRF33) and the viral expression plasmids (pCMV-UUK-L and pCMV-UUK-N) (solid arrows), as well as the time points for assaying CAT activity (broken arrows). Plasmids were either cotransfected (I) or transfected in either order separated by 6 h (II and III). CAT activity was assayed at indicated times after the last transfection (set as time zero). (B to D) Analysis of CAT activity from the transfection protocols shown in panel A. To be able to determine minor differences in expression levels, cell lysates were diluted 1:50. The number of the experiment (I to III) and time points (hours) when cell lysates were harvested are shown above the CAT signals.
FIG. 7.
Optimization of time intervals between plasmid transfections, superinfection with UUK virus, and analysis of CAT activity. (A) Schematic diagram depicting the timetable for (i) cotransfection with the RNA pol I expression cassette plasmid (pRF33) and the two plasmids expressing L and N, (ii) superinfection with UUK virus, and (iii) CAT assay. Plasmids were cotransfected either 32 (I), 24 (II), or 8 (III) h before superinfection (set as time zero). (B to D) Analysis of CAT activity from the transfection/superinfection protocols shown in panel A. To be able to determine minor differences in expression levels, cell lysates were diluted 1:50. The number of the experiment (I to III) and time points when cell lysates were harvested are shown above the CAT signals. The time period between transfection and CAT assay is shown in brackets.
Figure 7A shows the protocol used for reporter plasmid transfection followed by superinfection with UUK virus. The reporter plasmid was transfected into cells 32 (transfection I), 24 (II), or 8 (III) h before superinfection, followed by analysis of CAT activity from cell lysates prepared at 16, 24, and 32 h postinfection. The highest activity was observed if transfection preceded superinfection by 32 h (Fig. 7B), and the weakest was seen if the superinfection was done 8 h after transfection (Fig. 7D). Intermediate activity was observed for the transfection II protocol (Fig. 7C).
Based on these analyses, we have adopted the following standard conditions for maximum reporter expression: (i) a molar ratio of L and N plasmids of 2:1, (ii) cotransfection of the pol I cassette (reporter) plasmid and the L and N expression plasmids followed by CAT assay 24 h later, and (iii) superinfection with UUK virus 20 to 24 h after transfection with the pol I cassette plasmid followed by CAT assay 24 h later.
Effect of CTE sequences on CAT expression.
Pol I transcripts lack a cap structure and a poly(A) tail. This is also true for the chimeric reporter transcripts (12, 49). We initially worried that these RNA species would be inefficiently exported from the nucleus to the cytoplasm. To analyze if nuclear export could be further enhanced, we first inserted additional restriction enzyme cleavage sites (Table 1) into pRF33 (UUK M-CAT) exactly between the CAT translation termination codon and the UUK M segment 5′ UTR, generating plasmid pRF20 (Fig. 1). We then inserted a longer (MPMV 566) and a shorter (MPMV 250) version of the CTE sequence from MPMV into pRF20 (Fig. 8). This CTE sequence has been shown to greatly enhance export of the intron-containing MPMV RNA from the nucleus (3, 11). The CTE sequences were inserted in either the sense or the antisense orientation (Fig. 8A). The pol I expression cassette and the L and N expression plasmids were cotransfected into BHK-21 cells, and cell lysates were analyzed for CAT activity 24 h later. As seen in Fig. 8B, the CTE sequence in either orientation had no enhancing effect on the reporter gene activity (lanes 4 to 7). The CTE in the antisense orientation had a slight inhibitory effect on CAT activity (lanes 6 and 7), possibly because the stem-loop structure in this orientation constitutes a relative physical barrier for the RNA polymerase; alternatively, the sequence context in the antisense orientation could interfere with replication or transcription signals in the noncoding region of the UUK virus segment. The results also showed that the additional restriction enzyme cleavage sites introduced into pRF20 had no disturbing effect on CAT expression compared to pRF33 (lanes 2 and 3).
Generation of recombinant UUK virus.
Finally, we analyzed whether the chimeric reporter RNA could be packaged into infectious virus particles that could be serially passaged to fresh cultures. BHK-21 cells were either cotransfected with the reporter plasmid pRF33 (CAT-M vRNA) and the L and N expression plasmids or transfected with the reporter plasmid alone, followed in both cases by superinfection with UUK virus 20 h later. The medium was collected 30 h later (i.e., 50 h posttransfection) and used to infect new BHK-21 cultures. This was repeated for another cycle. From each passage, cell lysates were analyzed for CAT activity. As shown in Fig. 9, CAT activity was strong in cells transfected with the combination of the three plasmids and infected with UUK virus (lane 1). CAT activity could be passaged at least twice, although the activity was reduced between each passage (lanes 1 to 3). If superinfection was omitted, no CAT activity could be detected upon passaging (data not shown), nor could CAT activity be passaged if cells were transfected only with the reporter plasmid followed by superinfection (lanes 4 to 6).
FIG. 9.
Analysis of CAT activity in BHK-21 cells after serial passages of supernatants containing recombinant UUK virus. BHK-21 cells were cotransfected either with pRF33 and UUK L and N expression plasmids (lane 1) or only with pRF33 (lane 4) 24 h prior to superinfection with UUK virus (MOI of 10). Cell lysates were prepared 30 h later and assayed for CAT activity, while the media were collected and undiluted samples were transferred to new BHK-21 cells. This was repeated for another cycle, and CAT activity was assayed after each passage. CAT activities are expressed as percentage of the activity obtained after the first transfection/superinfection (lane 1).
DISCUSSION
In this study, we have successfully adopted the RNA pol I transcription system (30, 31, 49) for the development of a reverse genetics protocol for members of the Bunyaviridae family. As a model virus, we used UUK virus, a member of the Phlebovirus genus. Reporter cDNAs encoding CAT or GFP flanked by the terminal sequences of the UUK M RNA segment were transcribed in the cell nucleus by pol I, transported to the cytoplasm, and transcribed and amplified by the RNA polymerase L in the presence of the nucleoprotein N provided either from expression plasmids or by superinfection with UUK virus. Our results indicate that expression of the L and N proteins alone yielded more efficient reporter expression than could be achieved by superinfection and that the nonstructural NSs protein was completely dispensable for CAT activity. Finally, CAT activity could be serially passaged from culture to culture, indicating that the chimeric reporter RNA was packaged into virions.
We decided to use the 5′ and 3′ untranslated sequences from the M RNA segment to flank the reporter ORFs, rather than those from the L or S segment. In virus-infected BHK-21 cells, the M RNA segment is synthesized in a twofold molar excess over the L RNA and a fourfold excess over the S RNA (33), suggesting that the M RNA may possess the strongest promoter. In addition, the S RNA is transcribed into two subsegmental mRNAs encoding N and NSs using an ambisense strategy (42), which might complicate the use of the terminal sequences from this RNA segment. Our previous results have indicated that the mRNA transcribed from the M segment and translated into p110, the G1/G2 precursor, is about 100 nt shorter than the M vRNA template (R. Rönnholm and R. Pettersson, unpublished data) due to an as yet uncharacterized transcriptional termination signal. To ensure proper transcription termination of the reporter mRNAs, we therefore included the whole 185-residue-long UTR from the 5′ end of the vRNA (or 3′ end of the cRNA, depending on the polarity of the construct). Previous work with BUN (7), and RVF (34) viruses has shown that the extreme 3′ end contains sequence elements critical for transcription initiation.
The fact that pol I reporter transcripts are noncapped and nonpolyadenylated raised the concern that these RNAs would not be efficiently transported out of the nucleus. In the case of influenza virus, the pol I transcripts do not have to exit the nucleus, since transcription and replication of vRNAs take place in the nucleus. In contrast, Bunyaviridae members replicate solely in the cytoplasm and the pol I transcripts therefore have to be exported from the nucleus. Our results showed that these concerns were unfounded, since CAT and GFP activities were readily detected. Newly synthesized nuclear RNA species rapidly associate with a set of proteins to form RNP structures. Some of these proteins contain an export signal and serve as export factors that guide the RNPs to and through the nuclear pore complex (29). We can only speculate that such an export factor(s) binds to our chimeric reporter RNA and facilitates its export to the cytoplasm. The CTE of MPMV has been shown to stimulate the export of unspliced RNAs from the nucleus (3, 11). To analyze whether the CTE could further enhance the export of pol I transcripts as assayed by increased reporter activity, we introduced two CTE variants in the sense or antisense orientation downstream of the 5′ UTR of the CAT-M vRNA. These constructs did not enhance CAT expression, suggesting either that the CTE does not work in this sequence context or that the RNA export is already high enough to allow amplification of the reporter RNA by the viral proteins.
The finding that expression of the L and N proteins was more efficient compared to superinfection in stimulating CAT activity is interesting. One can only speculate as to the reason for this result. For all negative-strand RNA viruses, the template for transcription and replication is an RNP made up of the vRNA and the nucleocapsid protein. The formation of such RNPs is thus a prerequisite for the polymerase to transcribe and replicate template RNAs. It is conceivable that in virus-infected cells, transcription, replication, and viral protein synthesis are compartmentalized in some kind of “virus factories” to which the pol I transcripts might not readily have access. Thus, the pol I transcripts would have a rather low probability of being encapsidated by the N protein. In contrast, newly synthesized virion-derived RNAs would immediately associate with newly synthesized N protein and thus serve as templates for further replication and transcription. In L- and N-expressing cells, such competition and spatial constraints would not exist, resulting in efficient transcription and replication of the pol I transcripts. In addition, it is also likely that rather few pol I transcripts reach the cytoplasm and that they are simply outcompeted by the much more efficient vRNA synthesis.
In the experiments described here, we used the murine pol I promoter/terminator sequences to express the reporter constructs in BHK-21 cells. Although a transfection rate of 20 to 25% was regularly achieved, the use of the human pol I promoter and highly transfectable human embryonic kidney cells (293T) (20, 31, 32) has the potential to further increase the efficiency of reporter expression.
The function of the S RNA segment-derived NSs protein has remained elusive. We found that NSs was not required for CAT activity. Previous work with a natural mutant of RVF virus (clone 13), which has a large internal in-frame deletion in the NSs gene, has shown that it replicates normally in some cell lines while establishing abortive infections in others, and that it is avirulent in mice and hamsters (27, 47). Using an in vitro transcription-replication system, it was recently shown that NSs of RVF virus had neither a stimulatory nor an inhibitory effect on transcription (24, 34). Finally, NSs has similarly been shown not to be required for the transcription of BUN virus RNAs (7). Recently, an NSs deletion mutant of BUN virus was made by using a reverse genetics system (A. Bridgen, J. K. Fazakerley, and R. M. Elliott, Abstr. XIth Int. Congr. Virol., abstr. VW47.06, 1999). This mutant grew to somewhat lower titers than wild-type virus, had a small-plaque phenotype, displayed a reduced shutoff of host cell protein synthesis, and had an attenuated pathogenicity when inoculated into mice. Our results reported here are thus in conformity with these results showing that NSs is not essential for transcription or replication in tissue culture cells.
One important question in regard to our results is whether the pol I transcript is amplified by replication. Although we have not directly quantified RNA synthesis, we argue that the observed high expression level of CAT and GFP could not have been achieved unless replication had occurred. Immunofluorescence analysis showed that individual cells displayed a very strong GFP signal. Based on our previous experience (13, 14), the overall level of CAT activity was much higher than that obtained in the influenza virus pol I system, even compared to the most effective influenza virus up-regulation mutant. Finally, the fact that extracellular medium from transfected and UUK virus-superinfected cells could be used to serially passage CAT activity strongly suggests that the pol I transcript must have been amplified and packaged.
A reverse genetics system was developed some years ago for BUN virus (4, 7). Using the T7-VV RNA polymerase-driven system, full-length L, M, and S antigenome RNA segments were expressed from plasmids under the T7 RNA polymerase promoter. Correct 3′ ends were generated with the hepatitis δ ribozyme. All viral proteins were expressed under the T7 promoter from plasmids encoding L, G1, G2, NSm, N, and NSs. This is to date the only system that has allowed the rescue of an infectious Bunyaviridae member entirely from cloned cDNAs without the use of the homologous helper virus. Although constituting a major advance in Bunyaviridae research, this system still suffers from low efficiency and the need to use the VV helper to drive expression.
A reverse genetics system has also been developed for RVF virus, which like UUK virus is a phlebovirus (24, 34). In this system, the antisense CAT reporter cDNA was expressed using the T7-VV system, while the L and N proteins were supplied from VV recombinants. No recombinant RVF virus has been reported to have been produced using this system.
Arenaviruses also contain a segmented (bipartite), negative-strand genome (39). Recently, the first report on the development of a reverse genetics system for lymphocytic choriomeningitis virus was published (23). Although no recombinant virus was rescued, it was shown that only the RNA polymerase (L) and nucleoprotein (NP) proteins were sufficient to support transcription and replication of a CAT reporter flanked by 5′- and 3′-terminal viral sequences. Both the reporter transcript and the viral proteins were expressed by using the T7-VV system.
The pol I system offers clear advantages over the VV-based reverse genetics systems used for many other negative-strand viruses. VV has been used either to direct the synthesis of the T7 RNA polymerase (16), which then drives the expression of the reporter construct and the viral proteins (1, 5, 18, 19, 22, 23, 41, 48), or to express the viral helper proteins directly (7, 24, 34). VV introduces into the cell a number of unwanted enzymatic activities, which are avoided by using the pol I system. In addition, there is no need to remove the VV, by physical or biochemical means (22, 41, 48), by passaging the virus through cells not permissive to VV (4) or by using a variant VV (MVA-T7) which does not replicate in mammalian cells (45). The pol I system also has the advantage of generating the exact 5′ and 3′ ends of the RNA transcripts, thus avoiding the need for expressing runoff transcripts from restriction enzyme-cleaved plasmids or the use of a ribozyme to produce the correct 3′ end.
The pol I system has recently been successfully developed to reconstitute infectious influenza virus entirely from cloned cDNAs (15, 20, 31, 32). Our present results suggest that this could also be possible for Bunyaviridae members. In analogy to the influenza virus protocol, all three full-length RNA segments would be expressed from the pol I promoter, preferably as antigenomes (positive strands) (4, 22, 41), while the L, G1, G2, N, and possibly also the NSs proteins would be expressed from plasmids using the CMV promoter. Such a system would allow further characterization of cis- and trans-acting determinants important for the regulation of transcription and replication, as well as for virus maturation and packaging. It might also allow for the generation of genetically altered recombinant viruses to study structure-function relationships and molecular aspects of viral pathogenicity and the engineering of effective attenuated vaccines. It also remains to be investigated whether this approach is generally applicable to Bunyaviridae members other than UUK virus, such as the medically important hanta- and nairoviruses.
ACKNOWLEDGMENTS
We thank Anita Bergström and Elisabeth Raschperger for excellent technical assistance. We are grateful to Gerd Hobom for the pol I plasmids, Marie-Louise Hammarskjöld for the MPMV CTE fragment, and Richard Elliott for providing the full-length UUK L cDNA.
REFERENCES
- 1.Baron M D, Barrett T. Rescue of rinderpest virus from cloned cDNA. J Virol. 1997;71:1265–1271. doi: 10.1128/jvi.71.2.1265-1271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop D H L, Gay M E, Matsuoko Y. Nonviral heterogeneous sequences are present at the 5′ ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983;11:6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray M, Prasad S, Dubay J W, Hunter E, Jeang K T, Rekosh D, Hammarskjöld M-L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type I expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgen A, Elliott R M. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc Natl Acad Sci USA. 1996;93:15400–15404. doi: 10.1073/pnas.93.26.15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conzelmann K-K. Nonsegmented negative-strand RNA viruses: genetics and manipulation of viral genomes. Annu Rev Genet. 1998;32:123–162. doi: 10.1146/annurev.genet.32.1.123. [DOI] [PubMed] [Google Scholar]
- 7.Dunn E F, Pritlove D C, Jin H, Elliott R M. Transcription of a recombinant Bunyavirus RNA template by transiently expressed Bunyavirus proteins. Virology. 1995;211:133–143. doi: 10.1006/viro.1995.1386. [DOI] [PubMed] [Google Scholar]
- 8.Elliott R M, Schmaljohn C S, Collett M S. Bunyaviridae genome structure and gene expression. Curr Top Microbiol Immunol. 1991;169:91–141. doi: 10.1007/978-3-642-76018-1_4. [DOI] [PubMed] [Google Scholar]
- 9.Elliott R M, Dunn E, Simons J F, Pettersson R F. Nucleotide sequence and coding strategy of the Uukuniemi virus L RNA. J Gen Virol. 1992;73:1745–1752. doi: 10.1099/0022-1317-73-7-1745. [DOI] [PubMed] [Google Scholar]
- 10.Enami M, Luytjes W, Krystal M, Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci USA. 1990;87:3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst R K, Bray M, Rekosh D, Hammarskjöld M-L. A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol Cell Biol. 1997;17:135–144. doi: 10.1128/mcb.17.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flick R, Hobom G. Transient bicistronic vRNA segments for indirect selection of recombinant influenza viruses. Virology. 1999;262:93–103. doi: 10.1006/viro.1999.9895. [DOI] [PubMed] [Google Scholar]
- 13.Flick R, Hobom G. Interaction of influenza virus polymerase with viral RNA in the ‘corkscrew’ conformation. J Gen Virol. 1999;80:2565–2572. doi: 10.1099/0022-1317-80-10-2565. [DOI] [PubMed] [Google Scholar]
- 14.Flick R, Neumann G, Hofmann E, Neumeier E, Hobom G. Promoter elements in the influenza vRNA terminal structure. RNA. 1996;2:1046–1057. [PMC free article] [PubMed] [Google Scholar]
- 15.Fodor E, Devenish L, Engelhardt O G, Palese P, Brownlee G G, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He B, Paterson R G, Ward C D, Lamb R A. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology. 1997;237:249–260. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman M A, Banerjee A K. An infectious clone of parainfluenza virus type 3. J Virol. 1997;71:4272–4277. doi: 10.1128/jvi.71.6.4272-4277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann E, Neumann G, Hobom G, Webster R G, Kawaoka Y. Ambisense approach for the generation of influenza A virus: vRNA and mRNA synthesis from one template. Virology. 2000;267:310–317. doi: 10.1006/viro.1999.0140. [DOI] [PubMed] [Google Scholar]
- 21.Krug R M, Alonso-Caplen F V, Julkunen I, Katze M G. Expression and replication of influenza virus genome. In: Krug R M, editor. The influenza viruses. New York, N.Y: Plenum Press; 1989. pp. 89–152. [Google Scholar]
- 22.Lawson N D, Stillman E A, Whitt M A, Rose J K. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K J, Novella I S, Teng M N, Oldstone M B A, de la Torre J C. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J Virol. 2000;74:3470–3477. doi: 10.1128/jvi.74.8.3470-3477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez N, Muller R, Prehaud C, Bouloy M. The L protein of Rift Valley fever virus can rescue viral ribonucleoproteins and transcribe synthetic genome-like RNA molecules. J Virol. 1995;69:3972–3979. doi: 10.1128/jvi.69.7.3972-3979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luytjes W, Krystal M, Enami M, Pavin J D, Palese P. Amplification, expression, and packaging of a foreign gene by influenza virus. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- 26.Melin L, Persson R, Andersson A, Bergström A, Rönnholm R, Pettersson R F. The membrane glycoprotein G1 of Uukuniemi virus contains a signal for localization to the Golgi complex. Virus Res. 1995;36:49–66. doi: 10.1016/0168-1702(95)00006-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller R, Saluzzo J-F, Lopez N, Dreier T, Turell M, Smith J, Bouloy M. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg. 1995;53:405–411. doi: 10.4269/ajtmh.1995.53.405. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, F. A., C. M. Fauquet, D. H. L. Bishop, S. A. Ghabrial, A. W. Jarvis, G. P. Martelli, M. A. Mayo, and M. D. Summers. 1995. Virus taxonomy, 6th report of the International Committee of Taxonomy of Viruses. Arch. Virol. (Suppl. 10):300–315. [PubMed]
- 29.Nakielny S, Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- 30.Neumann G, Zobel A, Hobom G. RNA polymerase I-mediated expression of influenza viral RNA molecules. Virology. 1994;202:477–479. doi: 10.1006/viro.1994.1365. [DOI] [PubMed] [Google Scholar]
- 31.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez D R, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann G, Watanabe T, Kawaoka Y. Plasmid-driven formation of influenza virus-like particles. J Virol. 2000;74:547–551. doi: 10.1128/jvi.74.1.547-551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettersson R, Kääriäinen L. The ribonucleic acids of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1973;56:608–619. doi: 10.1016/0042-6822(73)90062-7. [DOI] [PubMed] [Google Scholar]
- 34.Prehaud C, Lopez N, Blok M J, Obry V, Bouloy M. Analysis of the 3′ terminal sequence recognized by the Rift Valley fever virus transcription complex in its ambisense S segment. Virology. 1997;227:189–197. doi: 10.1006/viro.1996.8324. [DOI] [PubMed] [Google Scholar]
- 35.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C, Christiansen G, Billeter M A. Rescue of measles virus from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts A, Rose J. Recovery of negative-strand RNA viruses from plasmid DNAs: A positive approach to a negative field. Virology. 1998;247:1–6. doi: 10.1006/viro.1998.9250. [DOI] [PubMed] [Google Scholar]
- 37.Rönnholm R. Localization to the Golgi complex of Uukuniemi virus glycoproteins G1 and G2 expressed from cloned cDNAs. J Virol. 1992;66:4525–4531. doi: 10.1128/jvi.66.7.4525-4531.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rönnholm R, Pettersson R F. Complete nucleotide sequence of the M RNA segment of Uukuniemi virus encoding the membrane glycoproteins G1 and G2. Virology. 1987;160:191–202. doi: 10.1016/0042-6822(87)90060-2. [DOI] [PubMed] [Google Scholar]
- 39.Salvato M S, Shimomaye E M. The complete sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989;173:1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- 40.Schmaljohn C S. Bunyaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1447–1471. [Google Scholar]
- 41.Schnell M J, Mebatsion T, Conzelmann K-K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simons J F, Hellman U, Pettersson R F. Uukuniemi virus S RNA: ambisense coding strategy, packaging of complementary strands into virions, and homology to members of the genus Phlebovirus. J Virol. 1990;64:247–255. doi: 10.1128/jvi.64.1.247-255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons J F, Pettersson R F. Host-derived 5′ ends and overlapping complementary 3′ ends of the two mRNAs transcribed from the ambisense S segment of Uukuniemi virus. J Virol. 1991;65:4741–4748. doi: 10.1128/jvi.65.9.4741-4748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simons J F, Persson R, Pettersson R F. Association of the nonstructural protein NSs of Uukuniemi virus with the 40S ribosomal subunit. J Virol. 1992;66:4233–4241. doi: 10.1128/jvi.66.7.4233-4241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutter G, Ohlmann M, Erfle V. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 1995;371:9–12. doi: 10.1016/0014-5793(95)00843-x. [DOI] [PubMed] [Google Scholar]
- 46.Ulmanen I, Seppälä P, Pettersson R F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981;37:72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vialat P, Billecocq A, Kohl A, Bouloy M. The S segment of Rift Valley fever phlebovirus (Bunyaviridae) carries determinants for attenuation and virulence in mice. J Virol. 2000;74:1538–1543. doi: 10.1128/jvi.74.3.1538-1543.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whelan S P J, Ball L A, Barr J N, Wertz G T W. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zobel A, Neumann G, Hobom G. RNA polymerase I catalysed transcription of insert viral cDNA. Nucleic Acids Res. 1993;21:3607–3614. doi: 10.1093/nar/21.16.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]