Abstract
Background.
We previously optimized several reconstruction strategies in SPECT myocardial perfusion imaging (MPI) with low dose for perfusion-defect detection. Here we investigate whether reducing the administered activity can also maintain the diagnostic accuracy in evaluating cardiac function.
Methods.
We quantified the myocardial motion in cardiac-gated stress 99m-Tc-sestamibi SPECT studies from 163 subjects acquired with full dose (29.8 ± 3.6 mCi), and evaluated the agreement of the obtained motion/thickening and ejection fraction (EF) measures at various reduced dose levels (uniform reduction or personalized dose) with that at full dose. We also quantified the detectability of abnormal motion via a receiver-operating characteristics (ROC) study. For reconstruction we considered both filtered backprojection (FBP) without correction for degradations, and iterative ordered-subsets expectation-maximization (OS-EM) with resolution, attenuation and scatter corrections.
Results.
With dose level lowered to 25% of full dose, the obtained results on motion/thickening, EF and abnormal motion detection were statistically comparable to full dose in both reconstruction strategies, with Pearson’s r > 0.9 for global motion measures between low dose and full dose.
Conclusions.
The administered activity could be reduced to 25% of full dose without degrading the function assessment performance. Low dose reconstruction optimized for perfusion-defect detection can be reasonable for function assessment in gated SPECT.
Keywords: SPECT, MPI, gated SPECT, image reconstruction, CAD
INTRODUCTION
Myocardial perfusion imaging (MPI) with single-photon emission computed tomography (SPECT) is typically combined with assessment of ventricular function in gated studies. Functional assessment involves quantitative evaluation of the left ventricle (LV), including wall motion, thickening, and ejection fraction (EF). Together, perfusion and ventricular function assessment can provide improved diagnostic accuracy1 and detection sensitivity of CAD.2–5 Lowering the radiation exposure in SPECT-MPI is important for reducing its associated potential cancer risk.6–8 There are also ASNC guidelines which mandate lower dose.8 However, lowered injected dose may potentially degrade the diagnostic accuracy due to increased noise in the resulting images.
We previously investigated9,10 the extent to which the injected dose can be reduced without sacrificing the diagnostic performance in perfusion studies, where the diagnostic accuracy was quantified according to the detectability of perfusion-defects in the myocardium. We optimized the parameters of several reconstruction strategies over a range of simulated dose levels, including the filtered backprojection (FBP) and the iterative ordered-subsets expectation-maximization (OS-EM) with different combinations of attenuation, scatter and resolution corrections (AC-SC-RC). It was demonstrated that with optimized parameters, OS-EM reconstruction with AC-SC-RC could reduce the average dose down to 25% (uniformly for all patients9) or 18.5% (personalized dose10) of the standard administered dose without causing the detection performance to decrease below that of FBP at standard dose.
In this study, we further investigate how reduced dose in SPECT-MPI may affect the diagnostic accuracy in evaluating cardiac function, as characterized by myocardial motion in cardiac-gated studies. Because each frame has fewer counts in a gated study, the noise level is much higher in gated images than in an ungated study. Thus, lowering the dose will further exacerbate the noise problem in gated studies. Therefore, it is important to investigate whether a reduced dose level which is adequate for perfusion-defect detection is also adequate for functional analysis. Using gated acquisitions from 163 patients, we performed an analysis of the regional and global wall motion and thickening for the standard count level, and then evaluated statistically whether the obtained results from reduced dose levels would agree with that obtained at standard clinical dose. For each study, both regional and global wall motion/thickening as well as ejection fraction (EF) were quantified using motion parameters from the extensively validated Quantitative Gated SPECT (QGS) software package.11 We also performed a receiver-operating characteristic (ROC) study of detecting abnormal wall motion in these patients based on global motion/thickening scores from QGS for reconstruction with different dose levels.
MATERIALS AND METHODS
Clinical Data Acquisition
Under institutional review board approval, cardiac-gated SPECT-MPI data were acquired from a total of 163 patients (83/80, male/female) between 2013 and 2016 at the Department of Radiology, University of Massachusetts Medical School, Worcester, MA. These were studies with no technical issues from patients who also gave written consent to participate in our investigations. Data were acquired by a Philips BrightView SPECT/CT system as cardiac-gated list-mode studies. The list-mode studies were binned into eight cardiac gates (frames) according to the ECG signal. All these patients underwent a one-day rest/stress SPECT-MPI protocol with Tc-99m sestamibi as described in.9 The rest activity was ranging from 10 to 12 mCi depending on BMI, and stress activity level that was three times higher. The stress data were used for this study. Attenuation maps were estimated from cone-beam CT,12 which were not cardiac-gated. It was previously observed that as an approximation correction for attenuation changes during cardiac contraction was not needed,13 as the volume and overall shape of the heart do not change significantly with contraction.14
The clinical characteristics of the 163 subjects are summarized in Table 1. Among them, 96 were read as having normal wall motion and thickening clinically by expert nuclear cardiology and nuclear medicine physicians; these patients were also interpreted as having normal perfusion in the myocardium. The other 67 patients were read as having various degrees of motion and thickening abnormalities; among them, 10 had normal perfusion in the myocardium, and the rest had abnormal perfusion.
Table 1.
Clinical characteristics for patient population used for this study
| Normal studies (n = 96) | Abnormal studies (n = 67) | |
|---|---|---|
|
| ||
| Female gender (% n) | 64.6% (62) | 12.1% (18) |
| Body mass index (kg m−2) (female) | 31.0 ± 5.2 | 34.5 ± 6.3 |
| Age (years) (female) | 62.0 ± 11.0 | 59.4 ± 10.2 |
| Effective injected dose (MBq) (female) | 1195 ± 132 (32.3 ± 3.6 mCi) | 1296 ± 227 (35.0 ± 6.1 mCi) |
| Body mass index (kg m−2) (male) | 29.7 ± 5.6 | 31.9 ± 6.3 |
| Age (years) (male) | 61.0 ± 12.4 | 59.4 ± 11.5 |
| Effective injected dose (MBq) (male) | 1184 ±111 (32.0 ± 3.0 mCi) | 1189 ± 114(32.1 ± 3.1 mCi) |
The effective injected activity was corrected for the post-injection activity left in the syringe and radioactive decay
Low Dose Data (Half, Quarter and 1/8 Dose)
As detailed in9 we retrospectively simulated dose reduction by applying statistical sub-sampling of the list-mode data acquired at the clinical dose, from which each recorded photon event was randomly accepted or rejected according to a binomial distribution with probability equal to the proportion of desired dose level reduction.15 We considered dose levels lowered to 50% (one half), 25% (one quarter), and 12.5% (one eighth) with respect to the full clinical dose (i.e., 100%). The reduced dose data were simulated for each cardiac gate individually.
Personalized Dose Data
We also simulated personalized dose reduction based on a predictive model developed in our previous study.10 This model uses a set of patient-specific features (BMI, body size measurements, blood panel) to determine the minimum dose required for each patient such that the perfusion-defect detection performance is statistically no different from full dose for a given reconstructed strategy.10 For a given patient we first calculated the desired fraction of the full dose counts to be acquired in the personalized dose, and then applied statistical sub-sampling using this fraction to obtain the reduced count data.9,10,15
Optimized Reconstruction Strategies at Different Dose Levels
For this study we considered two reconstruction strategies which were optimized previously for maximum perfusion-defect detection at different dose levels.9 They are: (1) filtered-back projection (FBP) without correction for degradations, for which the cut-off frequency of the 2D pre-reconstruction Butterworth filter16 was optimized for each dose level; and (2) ordered-subsets expectation-maximization (OS-EM) with corrections for attenuation (AC), scatter (SC) and resolution (RC), for which the number of iterations and the 3D-Gaussian post-reconstruction filter were both optimized at each dose.9
Specifically, for FBP, the optimized cut-off frequency of the Butterworth filter was 0.22, 0.2, 0.19, and 0.18 cycles/pixel for 100%, 50%, 25%, and 12.5% of standard dose, respectively.9 For OS-EM, the number of subsets was fixed at 16, and the optimized (standard deviation of the filter [voxels; 0.466 cm], number of iterations) were (1.2, 12), (1.2, 8), (1.2, 4), (1.4, 4) for 100%, 50%, 25%, and 12.5% of standard dose, respectively.9 For the personalized dose the parameters were set according to the fraction of the dose level relative to the full dose.10 The SC was implemented using the triple energy window (TEW) method.17 The primary energy window was centered at 140.5 keV with a 15% width and the scatter window was centered at 121 keV with a 4% width. The scatter data were processed with a 2D Gaussian filter (sigma = 1.5 pixels). The scatter estimate was incorporated into the projection step of the OS-EM algorithm.18 RC was performed using a distance-dependent Gaussian diffusion model in the projection-backprojection step. For brevity, “OS-EM with AC-SC-RC” is simply referred to as “OS-EM reconstruction” in the rest of this manuscript.
QGS Software: Clinical Model Observer for Function Assessment
For the assessment of cardiac function in the reconstruction slices at different dose levels, we used the commercial package Quantitative Gated SPECT (QGS) from Cedars-Sinai, which is clinically validated as a surrogate for human readers.11,19 We considered two sets of quantification scores for wall motion and thickening: regional scores (aka segment scores) and global scores. Regional scores were computed based on a 17-segment LV model (Figure 1). In QGS the wall motion is measured for each segment (in mm) from the distance of the mid-myocardial surface between end-diastolic (ED) and end-systolic (ES) phases. Similarly, wall thickening for each segment is measured as the relative percentage (%) increase of the myocardial thickness, (defined as the distance between the endocardial and epicardial surfaces), at the ES phase relative to the ED phase.19 Besides regional scores, QGS also provides a set of global scores for quantifying the global motion and thickening for all segments. These are the summed motion score/percent (SMS/SM%) and the summed thickening score/percent (STS/ST%). To compute these global scores, QGS first derives the individual motion scores (scale 0–5) and thickening scores (scale 0–3) for the 17 segments.19 The SMS and STS scores are obtained as the sum of all 17 segmental scores; SM% and ST% are SMS and STS normalized by their respective maximal numerical value obtainable.19
Figure 1.
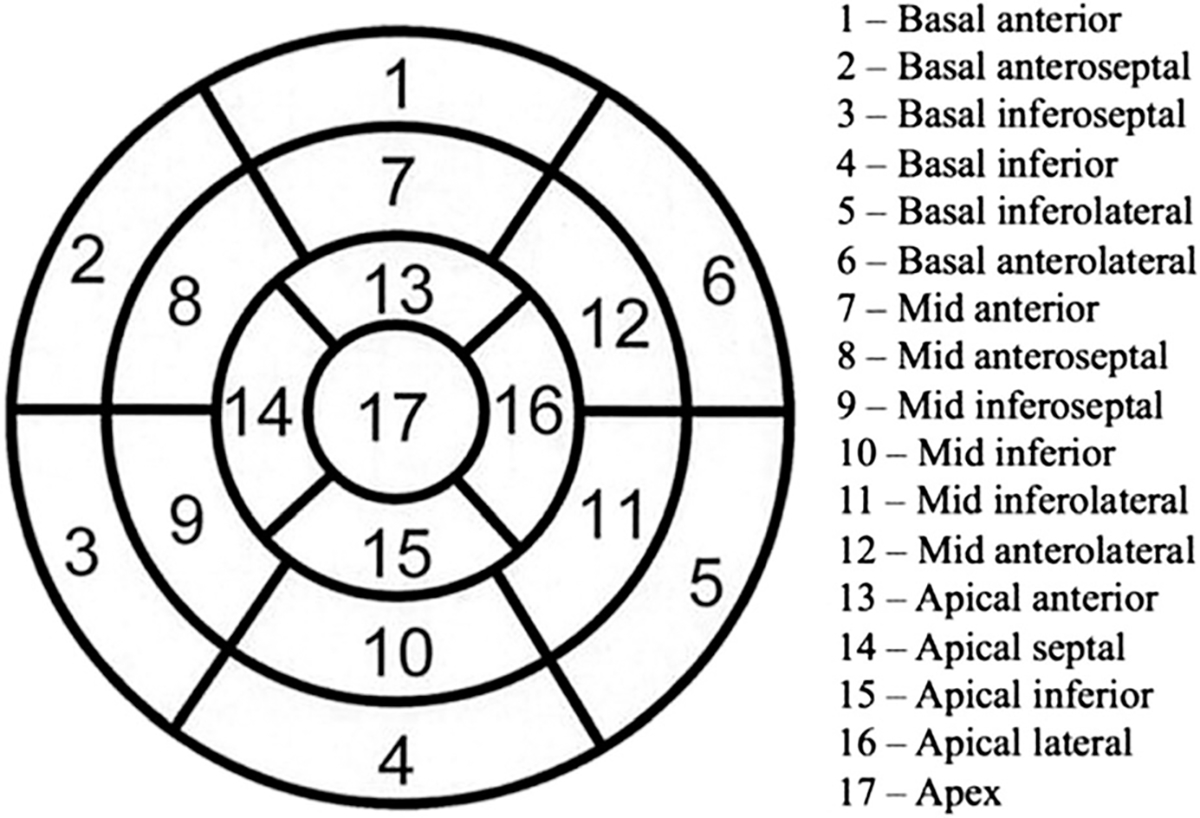
Segment definitions used for regional scores (from QGS software), which correspond to the 17-segment model of the American Heart Association.
We quantified the motion and thickening of reconstruction from different reduced dose levels and evaluated their agreement with standard clinical dose (i.e., 100% dose) in terms of regional and global scores, as well as detectability of abnormal wall motion, as described in detail below.
Evaluation of Regional Motion and Thickening
We first evaluated whether a given reduced dose level would yield comparable regional motion and thickening scores to standard clinical dose. The evaluation strategy was as follows. For each dose level and reconstruction algorithm (FBP or OS-EM), we computed the regional motion (in mm) and thickening (in %) for all the patients using QGS. We then performed one-way repeated measures multivariate analysis of variance analysis (MANOVA)20 on the obtained regional scores, where the motion/thickening scores of all 17 segments were treated collectively as a vector (i.e., dependent variables). The MANOVA test was used to compare the scores of a reduced dose (i.e., 50%, 25%, 12.5%, or personalized dose) with 100% dose, where the dose level was treated as the independent variable (factor). If a main effect (significant P value) was found for a given dose level, then the null hypothesis (H0) representing equal vector of means (17-segment scores) between the reduced dose and 100% dose was rejected. In such a case, we further performed repeated measures analysis of variance (ANOVA) with multiple comparison (Bonferroni) correction21 on the individual segment scores to identify which of the 17 segments were the main sources of variability. The analysis was performed using a two-sided type I error rate of 0.05 using R Studio.22
Furthermore, we performed a linear regression analysis on the regional motion/thickening scores to quantify the level of agreement between a reduced dose and standard dose. The Pearson correlation-coefficient (r) was computed for each of the 17 segments between their regional scores obtained at the reduced dose and full dose.
Evaluation of Global Motion and Thickening
We next evaluated whether a given reduced dose level would yield comparable global motion (SMS) and thickening scores (STS) and ejection fraction (EF) values to standard clinical dose. For each dose level and reconstruction algorithm, we computed SMS, STS, and EF for each patient using QGS. We then applied paired t test to determine whether there was a statistical difference on the global scores between a reduced dose (i.e., 50%, 25%, 12.5%, or personalized dose) and 100% dose. We also performed a linear regression analysis on the global scores between the reduced dose and standard dose, and computed their Pearson correlation-coefficient.
Evaluation of Abnormal Motion Detectability
Finally, we evaluated the detection performance of abnormal wall motion from reconstruction with both standard dose and reduced dose levels. For each dose level and reconstruction strategy, we computed the global motion percent (SM%) and thickening (ST%) scores of each patient using QGS. We then conducted a receiver-operating characteristic (ROC) study to quantify the detection performance based on these global scores. The ground truth for the ROC study was the expert readings of the patients as having normal vs abnormal motion. We used Metz’s ROCkit software to obtain the area under the ROC curve (AUC).23 Note that a higher AUC value represents a better separation between normal and abnormal wall motion in patients. The ROC study was performed for both FBP and OS-EM reconstruction at the different dose levels.
RESULTS
Regional Function Assessment
Tables 2 and 3 show the summary MANOVA results on quantifying the motion scores and thickening scores of all the 17 segments of the LV for FBP and OS-EM reconstruction, respectively. In each table, the MANOVA statistics are given on comparing a reduced dose (i.e., 50%, 25%, 12.5%, or personalized dose) with the 100% dose. No significant difference was found between 50% and 100% dose, or between 25% and 100% dose (P-values given in Tables 2, 3). Thus, for both FBP and OS-EM reconstruction, reducing the dose down to 25% of full dose did not show a significant impact on the regional scores. However, at 12.5% dose, there was a significant difference (P value < 0.05) found from the 100% dose on the segment scores (both motion and thickening) for both FBP and OS-EM.
Table 2.
MANOVA results from comparing reduced dose with full dose for motion and thickening scores of all 17 LV segments with FBP reconstruction
| Motion scores |
Thickening scores |
|||
|---|---|---|---|---|
| F statistic | P value | F statistic | P value | |
|
| ||||
| 50% vs 100% | 0.37 | 0.99 | 0.39 | 0.98 |
| 25% vs 100% | 0.44 | 0.97 | 0.52 | 0.93 |
| 12.5% vs 100% | 2.11 | < 0.01 | 1.02 | 0.43 |
| Personalized vs 100% | 0.35 | 0.99 | 0.39 | 0.99 |
Statistical P values are given in italics
The summary statistic values are given for each of the following reduced dose levels: 50%, 25%, and 12.5% of full dose, and personalized dose. The results are listed separately for motion scores and thickening scores. These results were obtained from 163 subjects. The null hypothesis represents an equal mean vector (17-segment scores) between reduced dose and 100% dose. The F statistic was approximated from the multivariate Wilks’ λ statistic, which is the percent variance in dependent variables not explained by differences in levels of the independent variable
Table 3.
MANOVA results from comparing reduced dose with full dose on motion and thickening scores of all 17 LV segments with OS-EM reconstruction, as in Table 2 for FBP reconstruction
| Motion scores |
Thickening scores |
|||
|---|---|---|---|---|
| F statistic | P value | F statistic | P value | |
|
| ||||
| 50% vs 100% | 0.34 | 0.99 | 0.78 | 0.71 |
| 25% vs 100% | 0.92 | 0.55 | 1.41 | 0.12 |
| 12.5% vs 100% | 2.44 | < 0.001 | 6.85 | < 0.001 |
| Personalized vs 100% | 0.77 | 0.72 | 0.80 | 0.69 |
Statistical P values are given in italics
In addition, Tables 4 and 5 show the quantified motion scores for the 17 individual segments at different dose levels, wherein the mean and standard-deviation values among the patients in the data set are given for each segment. Given the statistical difference found between 12.5% and 100% dose in the MANOVA results above, a further ANOVA comparison showed that segments 4–6 (i.e., basal inferior, inferolateral, and anterolateral) were the main sources of difference between the two both in FBP and OS-EM. These segments are noted to all correspond to the LV base (Figure 1). Furthermore, similar results were also obtained for the thickening scores of individual segments; they were omitted here in the interest of space.
Table 4.
The mean and standard-deviation values of motion scores (in mm) obtained for 17 individual segments with FBP reconstruction
| LV segment | 100% Dose | 50% Dose | 25% Dose | 12.5% Dose |
|---|---|---|---|---|
|
| ||||
| 1 | 11.05 ± 2.32 | 10.94 ± 2.47 | 10.78 ± 2.52 | 10.72 ± 2.72 |
| 2 | 7.12 ± 2.40 | 7.29 ± 2.40 | 7.20 ± 2.38 | 6.91 ± 2.72 |
| 3 | 5.21 ± 2.35 | 5.38 ± 2.45 | 5.14 ± 2.53 | 4.87 ± 2.66 |
| 4 | 7.19 ± 2.49 | 7.19 ± 2.54 | 6.95 ± 2.61 | 6.33 ± 2.68 (P < 0.01) |
| 5 | 10.32 ± 2.41 | 10.36 ± 2.46 | 10.15 ± 2.75 | 9.25 ± 2.67 (P < 0.01) |
| 6 | 11.60 ± 2.16 | 11.59 ± 2.23 | 11.37 ± 2.52 | 10.22 ± 2.58 (P < 0.01) |
| 7 | 9.36 ± 2.32 | 9.19 ± 2.44 | 9.05 ± 2.47 | 8.98 ± 2.77 |
| 8 | 6.77 ± 2.60 | 6.80 ± 2.54 | 6.85 ± 2.60 | 6.75 ± 2.92 |
| 9 | 5.40 ± 2.48 | 5.45 ± 2.68 | 5.45 ± 2.64 | 5.12 ± 2.83 |
| 10 | 6.98 ± 2.45 | 6.98 ± 2.51 | 6.88 ± 2.51 | 6.57 ± 2.75 |
| 11 | 9.27 ± 2.26 | 9.38 ± 2.34 | 9.17 ± 2.43 | 8.89 ± 2.67 |
| 12 | 9.33 ± 2.10 | 9.38 ± 2.19 | 9.16 ± 2.51 | 9.12 ± 2.56 |
| 13 | 7.22 ± 2.46 | 7.18 ± 2.47 | 7.25 ± 2.57 | 7.36 ± 2.87 |
| 14 | 5.60 ± 3.02 | 5.42 ± 2.99 | 5.58 ± 3.06 | 5.48 ± 3.06 |
| 15 | 7.98 ± 2.97 | 7.94 ± 2.92 | 8.02 ± 3.12 | 7.74 ± 3.18 |
| 16 | 9.03 ± 2.70 | 9.06 ± 2.66 | 9.16 ± 2.80 | 9.23 ± 2.95 |
| 17 | 7.70 ± 3.06 | 7.74 ± 3.05 | 7.93 ± 3.34 | 7.78 ± 3.61 |
Statistical P values are given in italics
The results are given for 100% dose, 50%, 25%, and 12.5% of full dose. These results were obtained from 163 subjects. Post hoc ANOVA with correction for multiple comparisons indicates statistically significant differences between 12.5% dose and 100% dose in segments #4, #5, and #6, but not other segments
Table 5.
The mean and standard-deviation values of motion scores (in mm) obtained for 17 individual segments with OS-EM reconstruction, as in Table 4 for FBP reconstruction
| LV segment | 100% Dose | 50% Dose | 25% Dose | 12.5% Dose |
|---|---|---|---|---|
|
| ||||
| 1 | 10.28 ± 2.26 | 10.15 ± 2.27 | 10.19 ± 2.26 | 9.83 ± 2.61 |
| 2 | 6.12 ± 2.45 | 6.18 ± 2.50 | 6.22 ± 2.48 | 6.38 ± 2.82 |
| 3 | 4.99 ± 2.55 | 5.01 ± 2.54 | 4.98 ± 2.52 | 4.78 ± 2.75 |
| 4 | 7.23 ± 2.50 | 7.18 ± 2.69 | 7.11 ± 2.77 | 6.84 ± 2.96 (P < 0.01) |
| 5 | 9.99 ± 2.48 | 9.90 ± 2.61 | 9.86 ± 2.68 | 9.38 ± 2.82 (P < 0.01) |
| 6 | 11.24 ± 2.27 | 11.20 ± 2.33 | 11.18 ± 2.27 | 10.56 ± 2.63 (P < 0.01) |
| 7 | 9.12 ± 2.20 | 8.99 ± 2.31 | 9.06 ± 2.46 | 8.77 ± 2.79 |
| 8 | 6.40 ± 2.51 | 6.38 ± 2.56 | 6.42 ± 2.66 | 6.40 ± 2.95 |
| 9 | 5.04 ± 2.55 | 5.07 ± 2.68 | 5.10 ± 2.75 | 5.01 ± 2.84 |
| 10 | 6.65 ± 2.50 | 6.66 ± 2.56 | 6.71 ± 2.63 | 6.57 ± 2.93 |
| 11 | 9.07 ± 2.13 | 9.02 ± 2.29 | 8.99 ± 2.24 | 8.74 ± 2.47 |
| 12 | 9.24 ± 2.10 | 9.22 ± 2.22 | 9.23 ± 2.38 | 9.22 ± 2.64 |
| 13 | 7.36 ± 2.42 | 7.32 ± 2.42 | 7.38 ± 2.47 | 7.55 ± 2.94 |
| 14 | 5.50 ± 2.84 | 5.49 ± 2.74 | 5.52 ± 2.83 | 5.59 ± 3.01 |
| 15 | 7.11 ± 2.63 | 7.14 ± 2.64 | 7.20 ± 2.70 | 7.07 ± 2.96 |
| 16 | 8.44 ± 2.48 | 8.44 ± 2.50 | 8.48 ± 2.58 | 8.67 ± 2.79 |
| 17 | 7.31 ± 2.84 | 7.33 ± 2.91 | 7.35 ± 3.02 | 7.57 ± 3.41 |
Statistical P values are given in italics
Post hoc ANOVA with correction for multiple comparisons indicates statistically significant differences between 12.5% dose and 100% dose in segments #4, #5, and #6, but not other segments
We also analyzed the correlation of the motion scores between a reduced dose level and full dose. For FBP, the average value of the Pearson correlation-coefficient among the 17 segments was r = 0.91 ± 0.02, 0.83 ± 0.04 and 0.73 ± 0.07 for 50%, 25%, and 12.5% dose, respectively; correspondingly, the average correlation value for thickening scores was r = 0.92 ± 0.03, 0.84 ± 0.06 and 0.75 ± 0.08, respectively. For OS-EM, the average correlation value for motion scores was r = 0.93 ± 0.02, 0.84 ± 0.04 and 0.75 ± 0.07 for 50%, 25% and 12.5% dose, respectively; correspondingly, the average correlation value for thickening scores was 0.94 ± 0.05, 0.89 ± 0.08 and 0.80 ± 0.19 for 50%, 25% and 12.5% dose, respectively. The P-values were below 0.05 for all cases (null hypothesis H0: r = 0).
Similarly, for personalized dose, the average correlation value of the motion scores with full dose was r = 0.94 ± 0.02 and 0.95 ± 0.04 for FBP and OS-EM, respectively. The average correlation value for thickening scores was r = 0.95 ± 0.02 and 0.94 ± 0.06 for FBP and OS-EM, respectively. These correlation values are noted to be slightly higher than those obtained with 50% dose. This was to be expected, as the average personalized dose level is above 50% for the 163 subjects (62.7 ± 10.54% for FBP and 58.2 ± 12.10% for OS-EM).
Figures 2 and 3 show the scatterplots of the motion scores of an example mid-ventricular segment (#12) obtained with reduced dose vs with full dose. The scores from reduced dose levels generally show a good agreement with scores from full dose; however, at 12.5% dose, the correlation deteriorates.
Figure 2.
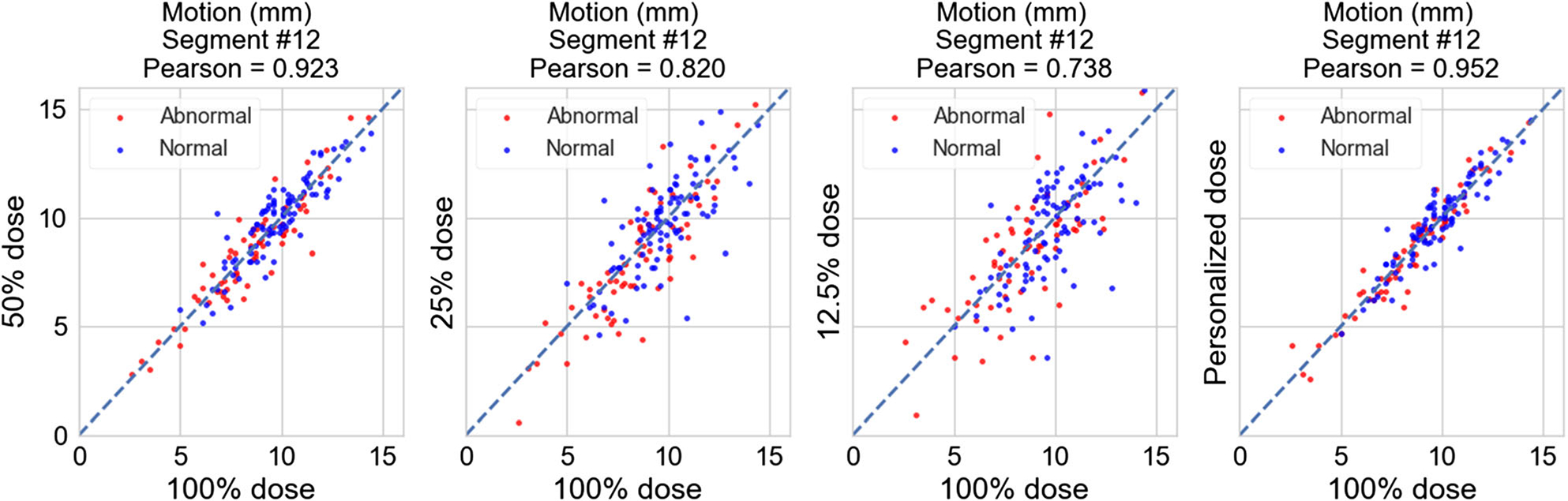
Scatterplots of the motion scores (in mm) of segment #12 obtained with reduced dose vs with full dose for FBP reconstruction. From left to right: 50%, 25%, 12.5% dose, and personalized dose. Red dots: abnormal motion cases (67 total), blue dots: normal motion cases (96 total).
Figure 3.
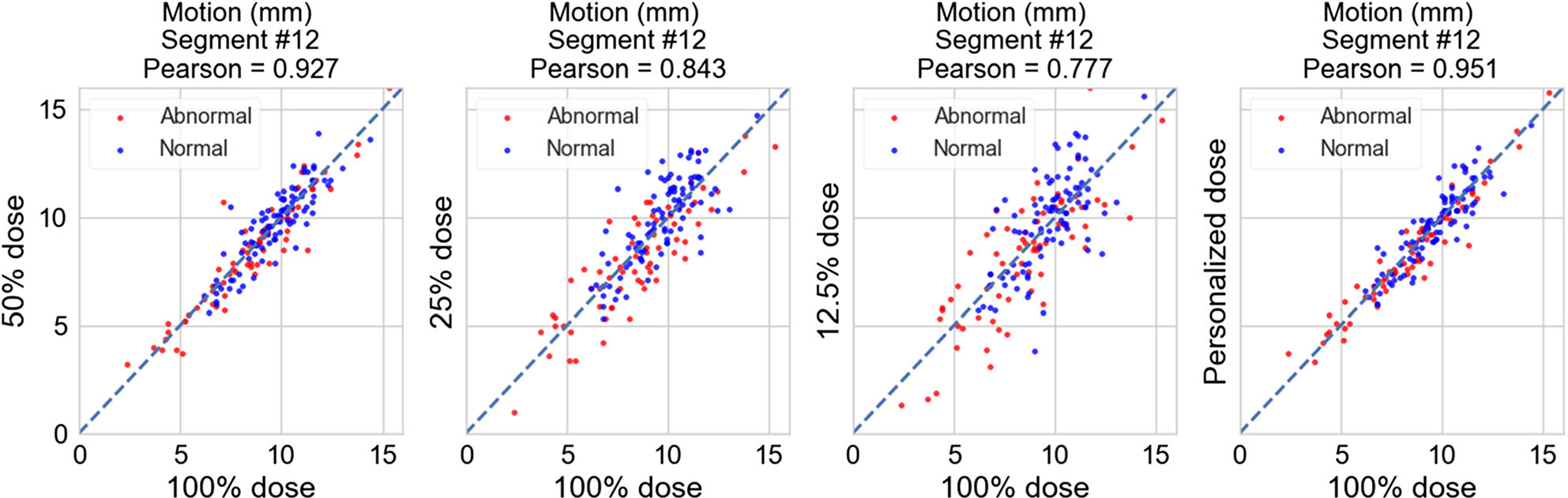
Scatterplots of the motion scores (in mm) of segment #12 with reduced dose vs with full dose for OS-EM reconstruction. From left to right: 50%, 25%, 12.5% dose, and personalized dose. Red dots: abnormal motion cases (67 total), blue dots: normal motion cases (96 total).
Global Function Assessment
Table 6 shows the results on quantifying the global motion scores (SMS), global thickening scores (STS), and ejection fraction (EF) values for FBP and OS-EM reconstruction, respectively. The mean and standard-deviation values of these global measures (SMS, STS, EF) obtained with FBP and OS-EM are shown for each dose level. As in the regional assessment results above, no statistically significant difference was found between 50% and 100% dose, nor between 25% and 100% dose for SMS, STS, and EF. Thus, for both FBP and OS-EM, reducing the dose down to 25% of full dose did not show a significant impact on the global measures either. However, at 12.5% dose, there was a significant difference found from the 100% dose on STS, SMS, and EF measures for both FBP and OS-EM.
Table 6.
The mean and standard-deviation values of global motion scores (SMS), global thickening scores (STS), and ejection fraction (EF) obtained with FBP and OS-EM reconstruction
| 100% Dose | 50% Dose | 25% Dose | 12.5% Dose | Personalized | |
|---|---|---|---|---|---|
|
| |||||
| FBP | |||||
| SMS | 5.42 ± 8.63 | 5.55 ± 8.59 | 5.81 ± 8.48 | 7.66 ± 9.27 * | 5.44 ± 8.56 |
| STS | 3.50 ±6.16 | 3.68 ± 6.19 | 4.26 ± 6.54 | 5.69 ± 7.16 * | 3.51 ± 6.13 |
| EF | 51.22 ± 12.26 | 51.35 ± 12.27 | 51.54 ± 12.24 | 50.32 ± 12.39 * | 51.30 ± 12.30 |
| OS-EM | |||||
| SMS | 5.89 ± 9.39 | 6.15 ± 9.59 | 6.51 ± 9.69 | 7.93 ± 10.59 * | 5.92 ± 9.44 |
| STS | 3.92 ± 6.24 | 4.37 ± 6.57 | 4.79 ± 6.66 | 6.04 ± 7.64 * | 4.12 ± 6.48 |
| EF | 49.31 ± 11.88 | 49.39 ± 11.97 | 49.95 ± 12.10 | 50.31 ± 13.68 * | 49.35 ± 11.95 |
The asterisk (*) signifies a statistically significant difference from full dose at level < 0.001 (paired t test)
We also analyzed the correlation of the global measures (SMS, STS, EF) between a reduced dose and full dose. For FBP, the correlation value for SMS was r = 0.96, 0.94, 0.85 for 50%, 25% and 12.5% dose, respectively; correspondingly, the correlation value for STS was r = 0.97, 0.93 and 0.85, respectively; and the correlation value for EF was r = 0.98, 0.97 and 0.94, respectively. For OS-EM, the correlation value for SMS was r = 0.97, 0.94, 0.88 for 50%, 25% and 12.5% dose, respectively; correspondingly, the correlation value for STS was 0.97, 0.94, and 0.86, respectively; and the correlation value for EF was r = 0.99, 0.97 and 0.93, respectively.
Similarly, for the personalized dose, the correlation value of SMS with full dose was r = 0.98 and 0.97 for FBP and OS-EM, respectively; correspondingly, the correlation value for STS was r = 0.97 and 0.98, respectively; and the correlation value for EF was r = 0.99 for both. These correlation values are also noted to be slightly higher than those obtained with 50% dose. The p-values were below 0.05 for all cases (H0: r = 0).
Abnormal Motion Detectability
Figure 4 shows the ROC study results on wall motion based on the summed motion percentage (SM%) score (Left) and the summed thickening percentage (ST%) score (Right). In each plot, the abnormal motion detection performance (AUC) was given for both FBP and OS-EM reconstruction at both 100% dose and reduced dose (i.e., 50%, 25%, and 12.5% dose). The AUC value obtained with personalized dose was also shown (average 62.7% and 58.2% of full dose for FBP and OS-EM, respectively).
Figure 4.
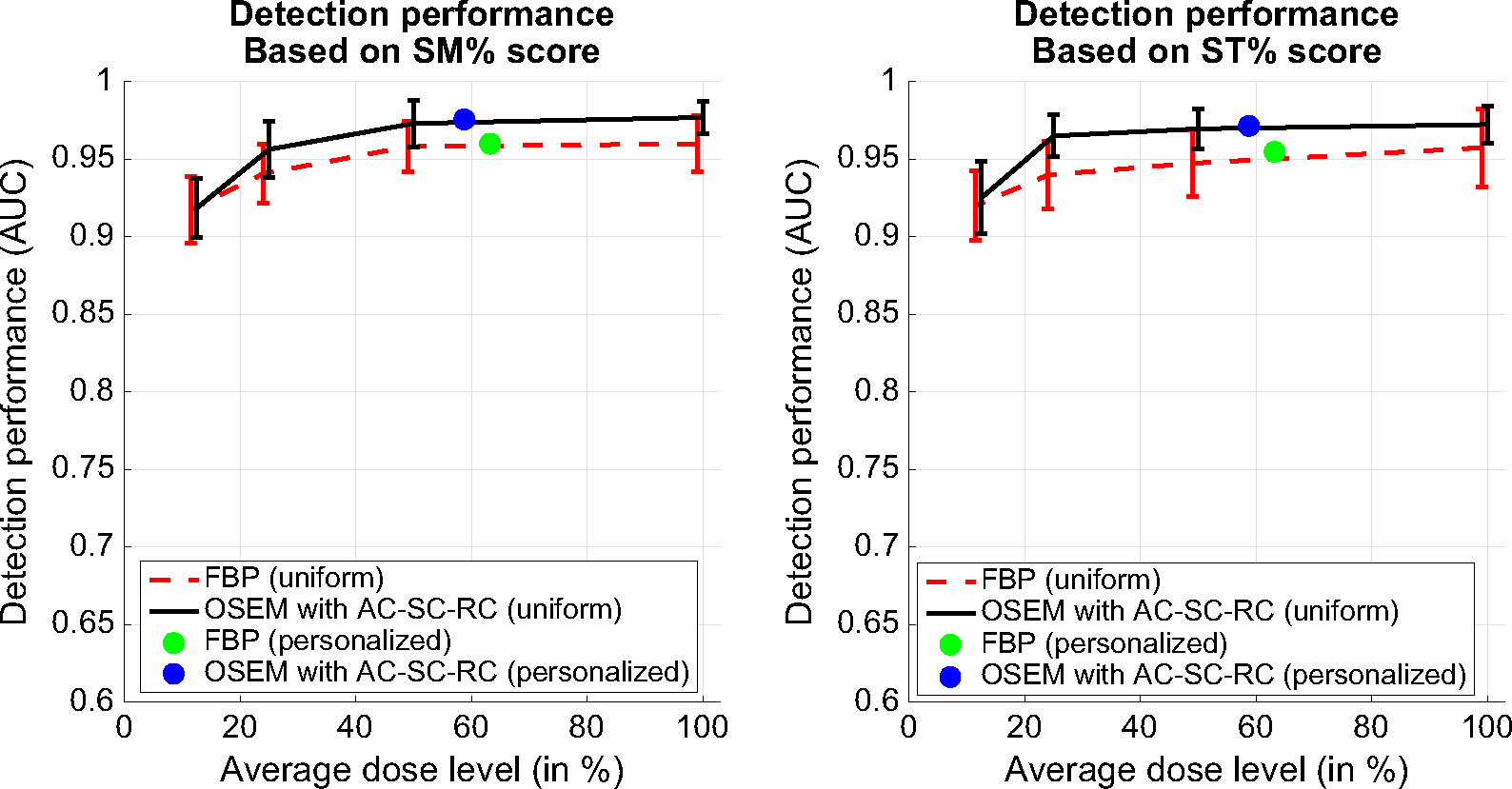
ROC study results on abnormal motion detection based on the summed motion percentage score (SM%) (Left) and summed thickening percentage score (ST%) (Right). Results are shown for both FBP and OS-EM reconstruction at different dose levels (i.e., 100%, 50%, 25%, 12.5% dose, and personalized dose).
For both FBP and OS-EM, the AUC values based on both SM% and ST% are noted to decrease slightly at 50% and 25% dose levels from 100% dose, though no statistical difference was found in each case (i.e., P value > 0.05). However, at 12.5% dose, the AUC values for both SM% and ST% were statistically lower than 100% dose (P value < 0.05). Interestingly, this also agrees with the comparison results on regional and global scores above.
It is also noted that the AUCs obtained with personalized dose are almost identical to that obtained with 100% dose for both FBP (P value = 0.92) and OS-EM (P value = 0.95). Thus, the personalized dose could maintain the detection performance of full dose on abnormal wall motion with both FBP and OS-EM reconstruction.
DISCUSSION
In this study, we systematically evaluate the effect of reduced dose reconstruction on assessment of function in cardiac-gated SPECT-MPI using simulated data derived from standard dose clinical acquisitions. We conducted for the first time an ROC study on how reducing the dose may affect diagnostic accuracy in evaluating cardiac function. We considered two reconstruction strategies previously optimized for perfusion-defect detection with lowered dose levels in SPECT-MPI: FBP and OS-EM with AC-SC-RC. Our quantitative results indicate that with the dose level reduced to 25% of full dose, comparable EF and wall motion/thickening assessment performance to the full dose was maintained. For abnormal motion detection, OS-EM consistently achieved a higher AUC than FBP at all dose levels, and the AUC obtained by OS-EM with 25% dose did not fall below that obtained by FBP with full dose. Interestingly, such relative ordering between FBP and OS-EM in performance at different dose levels is similar to that observed previously for perfusion-defect detection.9 This suggests that reducing the dose level in SPECT-MPI can have similar effect on function assessment as on perfusion detect detection.
The quantitative results for regional and global motion/thickening also indicated a statistical difference between 12.5% dose and 100% dose. A further analysis on the regional scores revealed that the difference was attributed to three basal segments. These segments of variability were also found to be consistent in FBP and OS-EM reconstruction. We believe that this was likely caused by the variations in LV segmentation when the imaging noise was much elevated at 12.5% dose (as seen in the images in Figures 5, 6).
Figure 5.
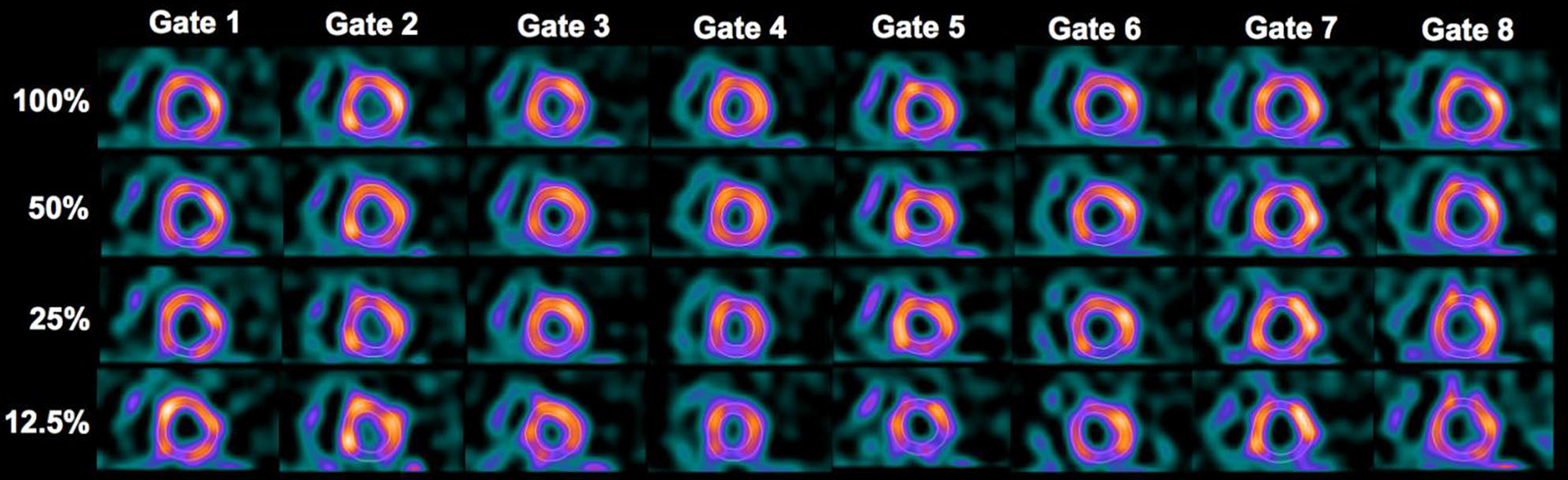
Reconstructed images by FBP at different dose levels for a 66-year-old male patient (BMI = 27.8) interpreted to have normal motion. The images in each row represent the eight gates of a short-axis slice at a given dose level.
Figure 6.
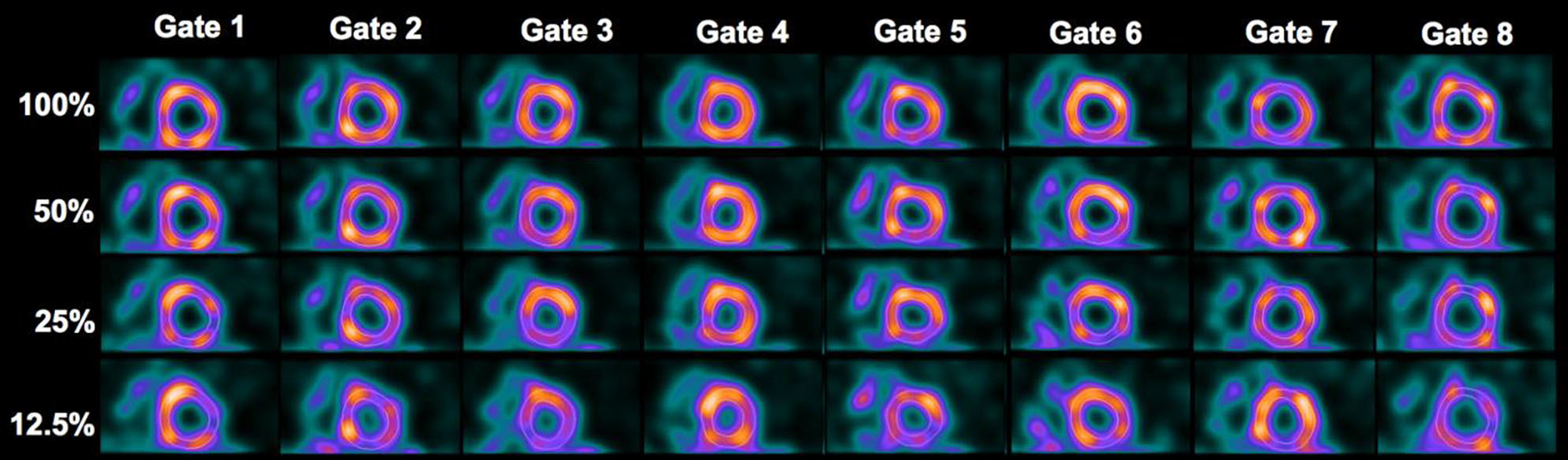
Reconstructed images by OS-EM at different dose levels for the same patient is shown in Fig. 5. The images in each row represent the eight gates of a short-axis slice at a given dose level.
Function assessment with reduced dose/acquisition time was also investigated recently in,24,25 where dose reduction was simulated by reducing acquisition time. In,24 a separate function assessment was performed on normal-weight (40 cases) and obese patients (40 cases), in which the ED volume (EDV) and ES volume (ESV) were found to be statistically different between 100% and 25% dose on stress images in overweight patients. In,25 motion/thickening and phase analysis were performed using QGS on 24 patients. The motion/thickening and EF measurements were found to be not affected down to 20% dose; however, phase values were affected after 50% dose. In both studies, the reconstruction parameters were the same for different dose levels. In our study, we used the optimized parameters for maximum perfusion-defect detection at each dose level, and showed that reducing the dose down to 25% does not have a significant impact on the function assessment in both FBP and OS-EM reconstruction. In addition, we also evaluated the performance on detecting abnormal motion with reduced dose.
In the ROC analysis for abnormal motion detection, we used the expert readings of the patients as ground truth for having normal vs abnormal motion. It would be desirable to have further validated this ground truth by an independent of nuclear imaging gold standard (e.g., 3D US, MRI, or CT). Nevertheless, the AUC value achieved by OS-EM with 100% dose was as high as 0.97 using ST%, which indicates a strong agreement between the expert readings and the QGS model observer. Thus, it is reasonable to believe that the clinical readings of these patients were fairly accurate.
The reconstruction software used for this manuscript, as also used in our previous work,9,10 is not the same as vendor’s implementation. However, the algorithms used share a common theoretical foundation with those employed by manufacturers. Despite potential minor implementation differences, the findings of the study are expected to be consistent with what would be obtained with commercial software.
CONCLUSIONS
Similar levels of reducing the dose for perfusion-defect detection in SPECT-MPI9,10 were found to be also suitable for function assessment. The quantitative results indicate that reducing the dose down to 25% of full dose does not have a statistically significant impact on the regional or global motion/thickening measures. At 12.5% of full dose, three LV basal segments were found to be sources of difference in motion/thickening scores from the 100% dose. The ROC study for abnormal motion detection showed that OS-EM achieved better performance than FBP at all dose levels except 12.5% dose, and that the performance of OS-EM with 25% dose did not fall below that of FBP at full dose. The detection performance with personalized dose was equivalent to that with full dose. In future work, we plan to further validate the reduced dose results from this study using expert human readers.
NEW KNOWLEDGE GAINED
This paper provides a comprehensive study of wall motion and thickening using reduced administered activity in gated SPECT-MPI. The study shows that the reduced dose levels optimized for perfusion-defect detection can also be reasonable to achieve accurate assessment of regional and global function as well as accurate detection of abnormal motion.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Grant R01-HL122484. P.S. was also supported by NIH Grant R01-HL089765. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- SPECT
Single-photon emission computed tomography
- MPI
Myocardial perfusion imaging
- FBP
Filtered backprojection
- OS-EM
Ordered-subsets expectation-maximization
- AC
Attenuation correction
- SC
Scatter correction
- RC
Resolution correction
- AUC
Area under the ROC curve
- CAD
Coronary-artery disease
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12350-018-01505-x) contains supplementary material, which is available to authorized users.
Disclosure
The University of Massachusetts had a research agreement with Philips Healthcare at the time some of this work was performed.
References
- 1.Choi JY, Lee KH, Kim SJ, Kim SE, Kim B-T, Lee SH, et al. Gating provides improved accuracy for differentiating artifacts from true lesions in equivocal fixed defects on technetium 99m tetrofosmin perfusion SPECT. J Nucl Cardiol. 1998;5:395–401. [DOI] [PubMed] [Google Scholar]
- 2.Stratmann HG, Williams GA, Wittry MD, Chaitman BR, Miller DD. Exercise technetium-99m sestamibi tomography for cardiac risk stratification of patients with stable chest pain. Circulation. 1994;89:615–22. [DOI] [PubMed] [Google Scholar]
- 3.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: Incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–14. [DOI] [PubMed] [Google Scholar]
- 4.Piccini JP, Horton JR, Shaw LK, Al-Khatib SM, Lee KL, Iskandrian AE, et al. SPECT myocardial perfusion defects are associated with an increased risk of all-cause death, cardiovascular death and sudden cardiac death. Circulation. 2008;1:180–8. [DOI] [PubMed] [Google Scholar]
- 5.Gimelli A, Rossi G, Landi P, Marzullo P, Iervasi G, L’Abbate A, et al. Stress/rest myocardial perfusion abnormalities by gated SPECT: Still the best predictor of cardiac events in stable ischemic heart disease. J Nucl Med. 2009;50:546. [DOI] [PubMed] [Google Scholar]
- 6.Administration UFaD (2010) Initiative to reduce unnecessary radiation exposure from medical imaging. US Food and Drug Administration, p. 9. [Google Scholar]
- 7.Jerome SD, Tilkemeier PL, Farrell MB, Shaw LJ. Nationwide laboratory adherence to myocardial perfusion imaging radiation dose reduction practices: A report from the intersocietal accreditation commission data repository. JACC. 2015;8:1170–6. [DOI] [PubMed] [Google Scholar]
- 8.Cerqueira MD, Allman KC, Ficaro EP, Hansen CL, Nichols KJ, Thompson RC, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol. 2010;17:709–18. [DOI] [PubMed] [Google Scholar]
- 9.Juan Ramon A, Yang Y, Pretorius PH, Slomka PJ, Johnson KL, King MA, et al. Investigation of dose reduction in cardiac perfusion SPECT via optimization and choice of the image reconstruction strategy. J Nucl Cardiol. 2017. 10.1007/s12350-017-0920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juan Ramon A, Yongyi Y, Pretorius PH, King MA, Wernick MN (2018) Personalized models for injected activity levels in SPECT myocardial perfusion imaging Trans Med Imaging [DOI] [PMC free article] [PubMed]
- 11.Germano G, Kavanagh PB, Slomka PJ, Van Kriekinge SD, Pollard G, Berman DS. Quantitation in gated perfusion SPECT imaging: the Cedars-Sinai approach. J Nucl Cardiol. 2007;14:433–54. [DOI] [PubMed] [Google Scholar]
- 12.Bai C, Shao L, Da Silva AJ, Zhao Z. A generalized model for the conversion from CT numbers to linear attenuation coefficients. IEEE Trans Nucl Sci. 2003;50:1510–5. [Google Scholar]
- 13.Klein G, Reutter B, Huesman R, Budinger T (1998) Cardiac gating of transmission data is unnecessary for attenuation compensation of double-gated emission scans. Nuclear Science Symposium, 1998 Conference Record 1998, IEEE. pp. 2019–2022 [Google Scholar]
- 14.Hoffman EA, Ritman EL. Invariant total heart volume in the intact thorax. Am J Physiol-Heart Circ Physiol. 1985;249:H883–90. [DOI] [PubMed] [Google Scholar]
- 15.Jin M, Niu X, Qi W, Yang Y, Dey J, King MA, et al. 4D reconstruction for low-dose cardiac gated SPECT. Med Phys. 2013;40(2):022501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayanan MV, King MA, Pretorius PH, Dahlberg ST, Spencer F, Simon E, et al. Human-observer receiver-operating-characteristic evaluation of attenuation, scatter, and resolution compensation strategies for 99mTc myocardial perfusion imaging. J Nucl Med. 2003;44:1725–34. [PubMed] [Google Scholar]
- 17.Ogawa K, Harata Y, Ichihara T, Kubo A, Hashimoto S. A practical method for position-dependent Compton-scatter correction in single photon emission CT. IEEE Trans Med Imaging. 1991;10:408–12. [DOI] [PubMed] [Google Scholar]
- 18.King M, Pan T-S, Pretorius P, Case J. An investigation of the filtering of TEW scatter estimates used to compensate for scatter with ordered subset reconstructions. IEEE Trans Nucl Sci. 1997;44:1140–5. [Google Scholar]
- 19.Slomka PJ, Berman DS, Xu Y, Kavanagh P, Hayes SW, Dorbala S, et al. Fully automated wall motion and thickening scoring system for myocardial perfusion SPECT: Method development and validation in large population. J Nucl Cardiol. 2012;19:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison DF. Multivariate analysis of variance. Hoboken: Wiley; 2005. [Google Scholar]
- 21.Keppel G, Wickens T. Simultaneous comparisons and the control of type I errors. Design and analysis: A researcher’s handbook. 4th ed. Pearson Prentice Hall: Wiley; 2004. p. 111–30. [Google Scholar]
- 22.Team R (2015) RStudio: integrated development for R. RStudio, Inc, Boston, MA. http://www.rstudio.com [Google Scholar]
- 23.Metz C, Lorenzo LP, Papaioannu J (2011) ROC-kit software. http://metz-roc.uchicago.edu/MetzROC/software [Google Scholar]
- 24.Lecchi M, Martinelli I, Zoccarato O, Maioli C, Lucignani G, Del Sole A. Comparative analysis of full-time, half-time, and quarter-time myocardial ECG-gated SPECT quantification in normal-weight and overweight patients. J Nucl Cardiol. 2017;24(3):876–87. [DOI] [PubMed] [Google Scholar]
- 25.Kortelainen MJ, Koivumäki TM, Vauhkonen MJ, Hakulinen MA. Dependence of left ventricular functional parameters on image acquisition time in cardiac-gated myocardial perfusion SPECT. J Nucl Cardiol. 2015;22:643–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


