Abstract
Predation risk is one of the most important factors generating behavioral differences among populations. In addition, recent attention focusses on predation as a potential driver of patterns of individual behavioral variation within prey populations. Previous studies provide mixed results, reporting either increased or decreased among-individual variation in response to risk. Here, we take an explicit developmental approach to documenting how among-individual variation develops over time in response to predator exposure, controlling for both genetic and experiential differences among individuals. We reared juveniles of naturally clonal Amazon mollies, Poecilia formosa, either with or without a predator visible during feedings over 4 weeks and analyzed activity during feedings, time spent feeding and number of visits to the feeding spot. (I) Predator-exposed fish did not differ from control fish in average feeding behavior, but they were less active during feeding trials. (II) In the absence of the predator, substantial changes in among-individual variation over time were detected: among-individual differences in feeding duration increased whereas differences in activity decreased, but there were no changes in feeder visits. In contrast, in the presence of a predator, among-individual variation in all three behaviors was stable over time and often lower compared to control conditions. Our work suggests that predation risk may have an overall stabilizing effect on the development of individual variation and that differences in predation risk may well lead to population-wide differences in among-individual behavioral variation.
Subject terms: Ecology, Behavioural ecology, Freshwater ecology
Introduction
Predation risk is one of the major forces of natural selection shaping virtually every aspect of prey behavior, including, for example, grouping, activity, collective decision-making, mating, and foraging1–7. Many classic studies have focused on how predation risk influences average behavior at the population level. For example, poeciliid fish from high predation sites are on average bolder, more explorative, and more active than their conspecifics from low-predation sites8–11. And, with increasing predation risk, reef fish (several species, including parrotfish, Sparisoma aurofrenatum and surgeonfish, Acanthurus bahianus) consume drastically less food but fed at a faster rate12.
More recently, studies looking at consistent among-individual behavioral variation (aka individuality or animal personality), have highlighted that not all individuals within a population respond to a stimuli or cue in the same way13–19. This means that individuals consistently differ in their response to predation risk, producing patterns of consistent among-individual variation20–24 and co-variation among behaviors25–28. For example, when perceived predation risk was highest, Western mosquitofish, Gambusia affinis, exhibited the greatest consistent among-individual behavioral variation22. Similarly, wild-caught mud crabs, Panopeus herbstii, expressed more pronounced consistent among-individual variation in refuge use when presented with predator cues compared to a control condition were no such cues were presented21. Predation risk has thus been implicated as an important factor driving the emergence and maintenance of consistent individual behavioral variation29. However, not all studies found predation risk to lead to greater consistent among-individual variation in behavior within populations; several studies report a reverse pattern30,31. For example, wild-caught guppies, Poecilia reticulata, have been shown to exhibit reduced among-individual variation in their risk-taking behavior when presented with a computer-animated predator30.
This discrepancy in results (higher vs. lower degree of consistent among-individual behavioral variation in response to risk) may be rooted in several contributing factors. First, and perhaps most importantly, individual experience can play a vital role. That means, among-individual differences in the timeline, duration, intensity, or number of exposures can determine an individual’s risk-perception and, thus, its reaction towards predator cues32–34. In particular, exposure to predator cues early in life can have substantial and long-lasting effects35–37. Predicting such experiential effects may not be straightforward, for example, repeated exposures to threats can have diverse influences on behavior, alternatively leading to habituation or sensitization to the threat34. Second, populations can have diverse evolutionary histories (predator absence vs. predator presence, or spatial–temporal fluctuations in predation risk) leading to differences in genetic backgrounds (heterogenic populations where different genotypes may or may not react differently to predation risk vs. homogenic populations where individuals may behave more uniformly)29,38,39. Therefore, for a comprehensive understanding of how predation risk shapes behavioral variation at the individual level, we need studies that carefully control for both experiential and evolutionary backgrounds. Specifically, studies that take an explicit developmental approach to documenting components of behavioral variation in response to predation risk over time can foster our understanding of how and when predation risk generates patterns of behavioral divergence versus convergence40–42.
In the current study, we used a controlled experimental set-up to isolate the effects of predator exposure early in life on the development of individual behavioral variation. We do so by leveraging the naturally clonal Amazon molly, P. formosa. This clonal fish allows for the unique opportunity to genetically standardize test individuals and therefore isolate the effects of salient ecological experiences on patterns of individual behavioral development43. Two weeks after birth, juveniles were separated into virtually identical experimental environments. For the following 4 weeks, individuals were either reared in a predator treatment, where they were exposed to a natural piscine predator while foraging (i.e., visual contact with a convict cichlid during feedings), or a control treatment, where there was no predator present while foraging. In both treatments, we recorded individual feeding behavior (time spent feeding and number of visits to the feeding spot) and activity (average swimming speed) during periods where food was available; allowing us to estimate patterns of both within- and among-individual behavioral variation and hence estimate each behavior’s repeatability. Repeatability is a common metric used to quantify the degree of individuality within a population; it describes the extent to which differences among individuals explain the overall observed variation (including both among- and within-individual variation) in a given trait18,44,45. Amazon mollies are known to show strong individuality in both activity and feeding behavior when reared under benign control conditions46–48.
We predicted that (I) the predator exposure would influence average behavior at a population level, with fish in the predator treatment avoiding the predator, and therefore spending less time feeding, visiting the feeding spot less frequently, and being less active, compared to the control group. We further predicted that (II) individual behavioral variation in the three observed behaviors (time spent feeding, visits to the feeding spot, activity) would be altered by the predator exposure. Given that the predator may induce a trade-off between the relative risk of approaching and the reward of foraging, we predicted that fish in the predator treatment develop greater among-individual differences in behavior if they resolve this trade-off differently, leading to divergence (e.g., because individuals differ in their perception of risk or vulnerability)32,33. Alternatively, given the salience of predation risk on small schooling fish and the lack of genetic variation among our fish, we could instead expect individuals to conform on a behavioral response: avoid the predator or learn to ignore it30.
Results
(I) Reduced activity in the presence of the predator but no effect on feeding behavior
We found neither a difference in feeding duration (Fig. 1a) nor in the number of visits to the feeding spot (Fig. 1b) between the predator and control treatment (Supplementary Table S1). And in both treatments, fish showed similar changes in feeding behavior over time (i.e., there was no evidence of a treatment x week interaction, Supplementary Table S1): fish significantly increased their time spent feeding (χ2 = 111.47, df = 1, p < 0.001; Supplementary Table S1, Fig. 1a), and visited the feeding spot significantly more often (χ2 = 11.14, df = 1, p = 0.001; Supplementary Table S1, Fig. 1b) as they got older. Fish in the predator treatment were generally less active than fish in the control treatment (χ2 = 4.305, df = 1, p = 0.038; Supplementary Table S1, Fig. 1c). There were no differences in how fish in the two treatments changed their activity over time (i.e., no treatment x week interaction, Supplementary Table S1) but rather individuals in both treatments decreased their activity over the course of the experiment (χ2 = 135.17, df = 1, p < 0.001; Supplementary Table S1, Fig. 1c). For all three behaviors, the main effects of treatment and week explained a modest amount of variation (marginal R2 < 0.19 for all three behaviors; Supplementary Table S1).
Fig. 1.
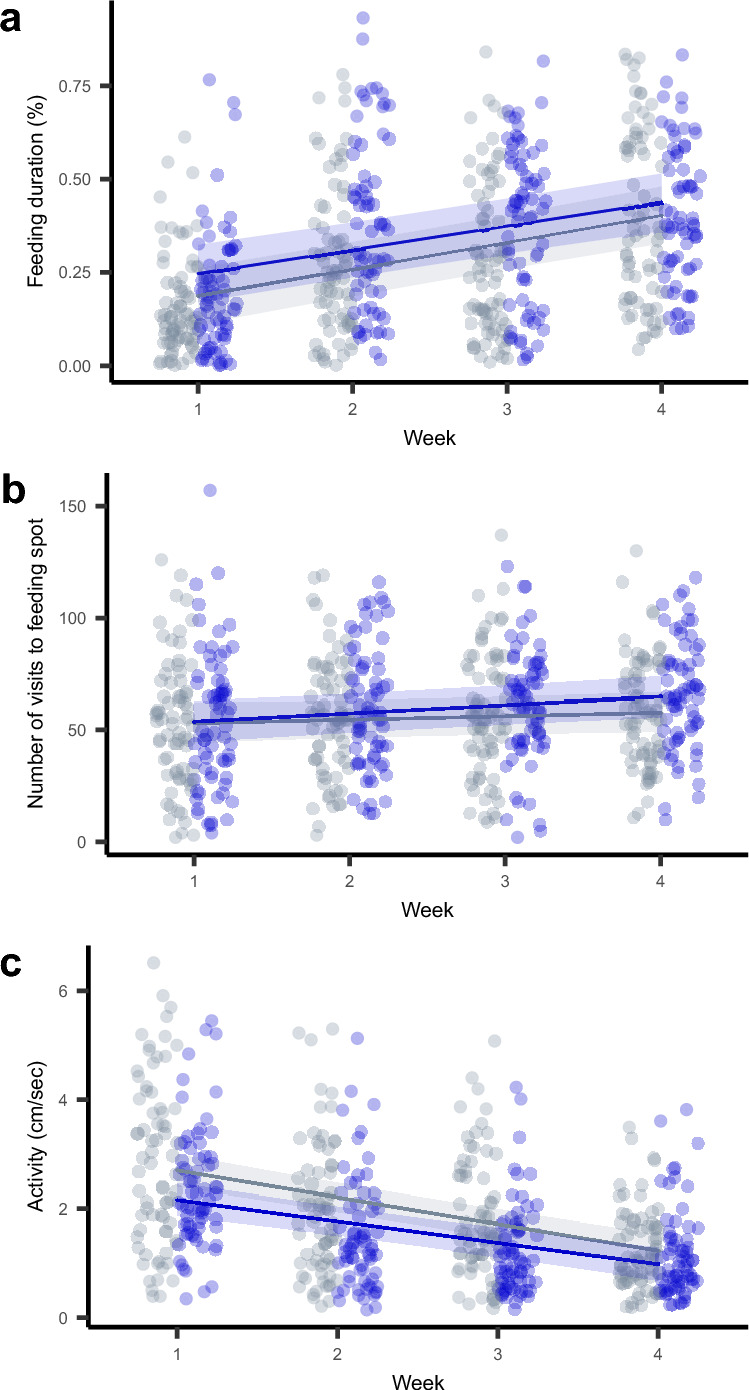
Effects of predator exposure on average behavior over time. (a–c) Presented are raw data (points, jittered horizontally for illustration purposes) and regression lines (gray = predator absence, blue = predator presence) with 95% confidence intervals, estimated from models including a non-significant treatment-week interaction term. (a, b) In both treatments, individuals increased their feeding duration and the number of visits to the feeding spot over time, but no difference between treatments was detected. (c) In the presence of the predator, individuals were on average less active than in the control, and in both treatments, individuals became less active over time.
(II) Stable and low among-individual variation in response to predator exposure
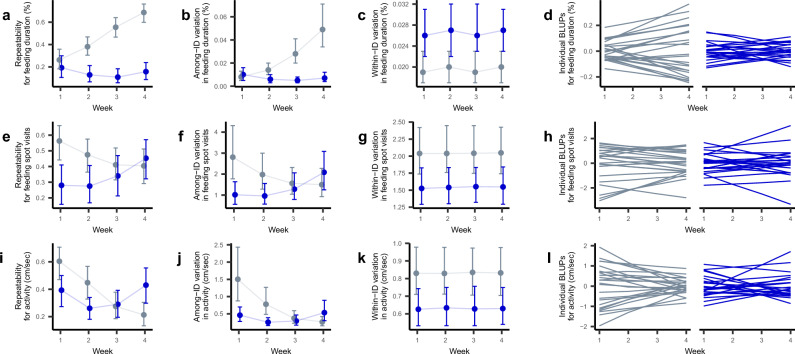
In general, we found that fish reared under predator exposure exhibited comparatively lower magnitudes of among-individual variation, and these levels of individual variation were very stable over time. In contrast, fish reared in the absence of the predator showed greater changes in the magnitude of among-individual variation over the 4-week experiment. The exact patterns of individual behavioral variation in our three different behaviors (feeding duration, visits to feeding spot, activity) differed from each other, both across the two treatment conditions and over time (Fig. 2).
Fig. 2.
Effects of predator exposure on variance components. Shown are (a, e, i) repeatabilities, (b, f, j) among-individual variation, (c, g, k) within-individual variation, and (d, h, l) individual BLUPs (best linear unbiased predictors, i.e., random intercepts for individuals) for individuals in the predator (blue) vs. control (gray) treatment.
For time spent feeding, we found that repeatability significantly increased over the course of the experiment when the predator was absent (i.e., in the control treatment) (Supplementary Table S2, Fig. 2a). The pattern was mainly driven by increases in among-individual variation, as within-individual behavioral variation remained stable throughout the course of the experiment (Supplementary Table S2, Fig. 2b,c). This resulted in a fanning-out pattern of individual BLUPs (best linear unbiased predictors, i.e., random intercepts for individuals) over the 4 experimental weeks (Fig. 2d). In contrast, in the predator treatment, both among-individual variation and repeatability remained stable and significantly lower than in the control treatment (Fig. 2a,b, Supplementary Table S2).
For the two other behaviors, number of visits to the feeding spot and activity, we found similar differences in the patterns of among-individual behavioral variation over time between the control and predators treatments, in that, among-individual variation was initially higher in the control treatment fish compared to the predator-exposed fish and decreased over the course of the experiment (the latter is statistically significant for activity only) (Fig. 2f and j; Supplementary Tables S3-S4), consequently, we observed a fanning-in pattern of individual BLUPs for activity in the control fish (Fig. 2l). In contrast, predator treatment fish exhibited more stable patterns of among-individual variation over the 4 weeks (Supplementary Tables S3-S4, Fig. 2e-l). For both behaviors, within-individual variation remained stable over time and similar in both treatments (Supplementary Tables S3-S4, Fig. 2g and k).
Interestingly, we found significant brood differences in feeding behavior (time spent feeding as well as number of visits to the feeder) among predator-exposed fish; but not among control-fish (Supplementary Tables S2-S3). Conversely, we found repeatable brood differences in activity among control- but not predator-fish, although, here, the repeatabilities are negligibly low, i.e., these repeatabilities explained only approx. 1–2% of the total variation in activity (Supplementary Tables S4).
Discussion
We tested how exposure to a natural predator early in life may alter the development of consistent among-individual behavioral variation. To do so, we used a clonal fish, the Amazon molly, allowing us to isolate the effects of this salient experience on behavioral development, while controlling for genetic variation43. We found that (I) fish exposed to predators were on average less active than fish reared under control conditions, but there was no change in average feeding behavior (time spent feeding and visits to feeding spot) in response to the predator. (II) In the control, among-individual variation changed substantially over time, with contrasting patterns depending on the behavior of interest: we observed a strong divergence, i.e., increase in among-individual variation, in the time fish spent feeding, and a convergence, i.e., decrease in among-individual variation, in activity; but no changes in feeder visits. Predator-exposed fish, on the other hand, showed no signs of change in among-individual variation over time and showed an overall tendency to behave more similarly to each other compared to control fish.
In line with much previous work, individuals were on average less active in the presence of the predator6,49, though there was no decrease in their average feeding behavior (feeding duration and number of visits to the feeding spot) compared to control individuals. The predator’s lack of influence on feeding behavior may be explained by the predation risk allocation hypothesis, which postulates that as exposure to predation risk increases, individuals reduce their avoidance of predators because the associated loss of energy intake becomes too significant50. Our fish showing predator avoidance by reducing their activity may indicate that the two behaviors differ in their cost–benefit trade-offs. That is, decreasing both activity and feeding can help minimize overall exposure to danger51–53, however, feeding is crucial for energy intake and cannot be entirely avoided, whereas reducing activity may be less costly.
The presence vs. absence of the predator had profound consequences on the development of behavioral variation in our Amazon mollies with two general trends. First, predator-exposed fish exhibited generally lower among-individual variation compared to fish in the predator-free control scenario, indicating that individuals may agree in their perception of the predator as a potential threat and align in their risk assessment, leading to individuals conforming to a behavioral strategy that might reflect an ‘optimal response’30,54. Second, while we observed substantial changes in among-individual variation over time in control fish (see below for developmental processes potentially contributing to the pattern observed), among-individual variation among predator-exposed fish remained stable. The lack of change suggests that individual decisions about how to behave in the presence of the predator were persistent. These results likely indicate that the predator was perceived as a potential threat throughout the experimental period. Previous studies demonstrate innate predator recognition of predator-naïve poeciliid fishes when confronted with piscivorous and omnivorous cichlids3,55 (see56–60 for further studies demonstrating innate predator recognition in fishes). Thus, it is highly likely that behavioral responses of Amazon mollies to the cichlids used in the present study, which are a natural predator for mollies, represent anti-predator responses to perceived predation risk and not just general fear responses towards a new stimulus in our test fish.
Individuals aligning their behavior under potential risk could have significant ecological consequences. In particular, the suppression of the development of among-individual diversity in feeding behavior in response to predator exposure might lead to reduced differences in growth and body size among individuals. This, in turn, could impact a wide range of fitness-relevant intraspecific interactions that are dependent on body size, including competition and hierarchies61,62, and reproductive behaviors63. Additionally, it may affect interspecific interactions, such as prey oddity and consequently predator hunting success64. Reduced variation in activity among individuals may impair processes related to the acquisition of environmental information and therefore adaptation if individuals behaving more alike translates into individuals acquiring similar or fewer information. While we can here only speculate about the consequences of predator-induced homogenization of prey behavior, future studies may address these questions more specifically.
Our finding of behavioral alignment under risk contrasts with several other studies indicating that predation risk promotes among-individual variation20–23. In our study, we strongly reduced variation in both genetic and experiential background by testing genetically identical individuals with no prior predator exposure in a highly standardized procedure. We thereby demonstrate that genes and/or experiential differences may drive the development of among-individual variation under risk as observed elsewhere20–23. Future experimental investigations could build upon our results by manipulating either of these two factors independently (e.g., by using the sexually reproducing parental species of the Amazon molly or different clonal lines), allowing for a more detailed examination of the distinct roles played by genes and experience.
We observed strong among-individual diversification in the time spent feeding when the predator was absent (while no such development was observed in the presence of the predator). Potentially, this pattern could be caused by individuals differing in their perception of whether the feeding apparatus (bottle with feeder) introduced into the tank during feeding trials posed a threat. That means, under control conditions, there might have been higher environmental uncertainty (not clear if the bottle is dangerous) compared to the predator treatment (where the predator itself represents an accurate cue about potential risk) leading to more pronounced differences in how individuals perceive and assess risk65,66. Initial differences in risk assessment may be enhanced via positive behavior-state feedback loops leading to pattern of behavioral divergence over time41,67. This way, even small initial differences in the perception of potential risk could become substantial over time. Future studies may investigate the role of environmental uncertainty and feedback loops in the development on among-individual behavioral variation in more detail.
Regarding activity, we observed high among-individual variation under control conditions to begin with, which then strongly decreased over the experimental period. This pattern of behavioral convergence may potentially be a by-product of individuals becoming on average less active (fewer differences among individuals are possible when the total behavioral range gets smaller), which in turn may reflect a response to the environment, specifically adaptation or habituation. Individual environments were stable and simple throughout the experiment and while being active is energetically costly the expected benefits of exploring this specific experimental environment were rather low. Declining activity could potentially also be caused by illness, however, all fish appeared healthy during trials as well as during the weeks following our experiment.
In conclusion, we observed that predator-exposed fish were on average less active but did not reduce feeding behavior compared to control fish. These population-level responses towards the predator indicate that individuals make strategic decisions about how to avoid a potential threat depending on the specific trade-offs (i.e., no predator avoidance when avoidance is too costly), thereby supporting the risk allocation hypothesis. Importantly, the presence of the predator led to behavioral conformance (potentially reflecting an optimal response) characterized by lower variation among individuals, which remained stable over the course of the experiment, compared to higher and more variable among-individual differences under control conditions. This suggests that when confronted with a predator, individuals align their risk evaluation, whereas in the absence of an immediate threat, variation in environmental perception among individuals may become more pronounced. Thus, next to the effects predators have on average population level behavior, there are also important—and largely overlooked—effects on the development of variation among individuals, with potentially important ecological and evolutionary consequences.
Methods
Study species and animal care
The Amazon molly is a gynogenetically reproducing freshwater fish from the subtropics of North America68–72 and the first discovered clonal vertebrate73,74, stemming from a single hybridization event between a female Atlantic molly, Poecilia mexicana, and a male sailfin molly, Poecilia latipinna, about 100,000 years ago71,72; but see75 for a population genomics study suggesting a history of backcrossing with the parental species before the onset of gynogenesis. Gynogenetic reproduction means that Amazon mollies require sperm from one of their parental species to trigger embryonic development, but paternal genetic material is not incorporated into the egg69,71,72 except in rare cases of male DNA fragment introgression76–78. Resulting offspring are therefore genetically identical to their mother and each other (except minute genetic differences created, e.g., by mutation).
Our experimental fish were lab-reared descendants of wild-caught fish originating from waters around the Mexican city of Tampico. Regular molecular checks confirmed that all P. formosa individuals used were clones79. Fish were maintained in large, uni-clonal tanks (200 L) with several P. mexicana males as sperm donors and were fed twice a day (7 days a week) with flake food (TetraMin) and once a week with live or frozen Chironomid larvae. Temperature was maintained at 25 °C with a 12/12 light/dark circle provided.
Experimental procedure
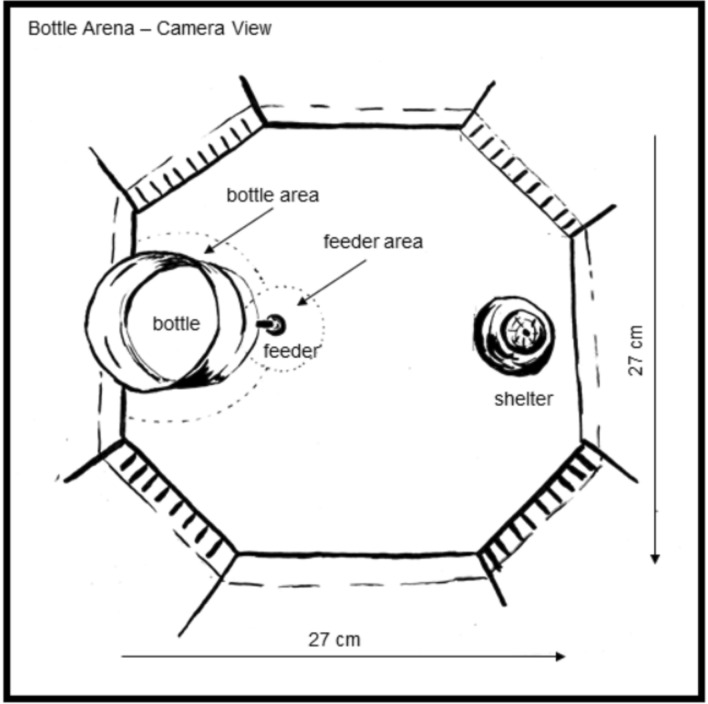
Before this study, we isolated several gravid females from one clonal line into smaller tanks (5 L) equipped with artificial plants and the same water conditions and feeding regime as outlined above. After parturition, we immediately removed the mothers (N = 6) and raised their broods for 2 weeks on Artemia nauplii and dusted flake food (TetraMin). We then placed offspring individually in one of 24 experimental tanks (Fig. 3), which we used in succession as broods were born at different times. We employed a split-brood design, i.e., for each brood, half of the individuals were allocated to the control treatment and the other half were allocated to the predator treatment (in total, N (control) = 24 fish and N (predator) = 23 fish). After 2 days of habituation during which the feeding of the first 2 weeks was continued, we started our experimental feeding trials: fish in the control treatment were presented with a water-filled bottle at which we clamped a food tablet (Fig. 3). Fish in the predator treatment were presented with the same bottle and food tablet but this time, the bottle held a convict cichlid (Amatitlania nigrofasciata, total length: 3.96 cm ± 0.62 cm (mean ± SD)). Predators were allowed to acclimate for 3–5 min inside the bottle, before the bottle was added to the experimental tank. For each trial, predators were chosen randomly from a pool of 100 juvenile cichlids (200 L tank, holding conditions as above). Bottles were put into the tanks every Monday, Wednesday, and Friday for 4 weeks so that each molly was presented 12 times with either an empty or a predator bottle. After the bottle with the food tablet was inserted, we gave our fish 30 min to feed and recorded at 2 frames per second from above47. Test individuals were not habituated to the feeder prior to starting experimental feeding trials. On days without feeding trials (i.e. Tuesdays, Thursdays, and Sundays; no feeding on Saturdays), fish were fed in the morning with a food tablet and the remaining food was removed after 3 h. Videos from the feeding trials were tracked using software Ethovision XT (Noldus Inc.). We assessed the time spent feeding as time spent in the feeder area (‘feeding duration’, in %), how many times the fish visited the feeder area (‘number of visits to feeding area’) and average velocity (‘activity’, in cm/sec) for each trial (Fig. 3). In addition to these behavioral variables, we used the detected blob sizes during the tracking as approximation of the fish’s body sizes at the first day of testing (day 1) and at the last day (day 12). Individuals allocated to the two treatments did not differ in body size from each other; either at the beginning or at the end of feeding trials (see Supplementary Figure S1 and Supplementary Table S6 for more details). The experimental set-up, including camera and feeding cylinder position was optimized to capture all facets of the molly behavior, which, however, circumvented the analysis of the cichlids’ behavior. Nevertheless, we observed cichlids swimming regularly in their cylinders during the trials (pers. observation).
Fig. 3.
Experimental set-up of feeding trials. Illustrated is a single experimental tank (tank size: 27 cm × 27 cm, corners (banded) serve as water in- and outflow to maintain water quality and connect the tank to the filter system) with a permanent shelter (up-side-down flower pot, 4.5 cm lower diameter). The bottle (without predator, bottle: 7.6 cm diameter, bottle area: 11.2 cm), including the feeder (food tablet (Tetra WaferMix) and clamp, feeder area: 4.5 cm diameter), was introduced at a standardized position into the tank during feeding trials.
The predator fish used in this experiment, the convict cichlid, is a natural predator for poeciliid fishes stemming from central America and is introduced worldwide due to releases from the ornamental trade80. Previous studies on closely related poeciliid fishes (P. reticulata and P. mexicana) demonstrate that predator-naïve individuals show typical anti-predator responses (altered mating preferences and avoidance behavior) when presented with predatory and omnivorous cichlids (Cichlasoma salvini, Crenicichla alta, Andinoacara pulcher, Petenia splendida), indicating that the predators are perceived as such3,30,55. These studies further show that behavior expressed in the presence of these predators is distinctly different compared to behavior expressed in the presence of a related, non-predatory species (Xiphophorus hellerii)3,55. See, e.g.,56–60 for further studies demonstrating innate predator recognition in fishes.
Statistical analyses
General details
Statistical analyses were performed in R 4.2.181. LMMs (linear mixed-effect models) were fitted using the lmer-package82. Variance components (repeatabilities, among-, and within-individual variation) with 95% confidence intervals (CIs) were estimated from LMMs following83. We tested whether variance components differed significantly between treatments or weeks by comparing the 95% Cis of estimates (significant difference when CIs do not overlap). Significance for behavioral repeatabilities was derived from the 95% CIs being distinctly different to zero. Model assumptions were verified using q-q plots and residual plots. We included random intercepts for both individuals and broods in all models, however, in cases where brood ID had no explanatory power, the term was removed from the model. Complete model structures, including random terms, are provided as Supplementary Table S1 and S5.
Our three target variables (feeding duration, visits to feeding spot, activity) were not (feeding duration and visits to feeding spot), moderately (activity and feeding duration), or weakly (activity and visits to feeding spot) predictive of each other (for more information and R2 see Supplementary Table S5), we therefore did not consider these variables redundant.
(I) Does predator exposure affect average behavior?
We tested if average behavior differed between the predator and control treatment by building LMMs with the behavior of interest as the response (time spent feeding, visits to the feeding spot, activity) and treatment (predator vs. control) as fixed effects. We further included week (1–4) as a continuous fixed effect to test if average behavior changed over development (e.g., fish getting older or habituating over the course of the experiment), as well as the week x treatment interaction term to test for potential differences between the two treatments in how behavior may have changed over time. The interaction term was removed from the models when non-significant. We included random intercepts for both individuals and broods (N (total, i.e., predator and control) = 559 observations from 47 individuals and 6 broods per model).
(II) Does predator exposure affect individual behavioral variation?
To test if predator exposure affected behavioral variation, we estimated the among- and within-individual variation, and hence repeatability, for each of our three target behaviors (time spent feeding, visits to the feeding spot, activity) in the two treatments (predator vs. control) separately. That is, for each behavior and treatment, we ran an LMM with the behavior of interest as response and experimental week (1–4) as continuous fixed effect (in total 6 models, for each behavior: N (control) = 285 observations from 24 individuals and N (predator) = 274 observations from 23 individuals). As random terms, we included individual intercepts and individual slopes as well intercepts for brood. To assess how variance components may change over the course of the experiment, we ran each of the above 6 models 4 times, where we centered the model to a different week in each run. That is, we ‘sliced’ the model at different time points allowing us to estimate the variance components for each week (because random intercepts are estimated where fixed effects, in this case, ‘week’, are equal to 0)47.
Supplementary Information
Acknowledgements
We gratefully acknowledge funding by the Deutsche Forschungsgemeinschaft through overheads on grants BI 1828/2-1 and BI 1828/3-1 and under Germany’s Excellence Strategy EXC 2002/1, “Science of Intelligence” project number 390523135 and by the U.S. National Science Foundation (IOS-2100625). Members of the lab at the Leibniz Institute of Freshwater Ecology and Inland Fisheries provided invaluable help with animal husbandry, experimental design and logistics; particular thanks are extended to D. Lewis and M. Ebert.
Author contributions
D.B., K.L., M.W., and M.M.K. conceived the project idea and designed the experiment. D.B., K.L., and M.W. secured funding for the experiment. D.B. and K.L. designed the housing tanks and tracking system. M.M.K. conducted the experiment. U.S. and S.M.E. conducted analyses. U.S. wrote the first draft of the manuscript. U.S., K.L., M.W., M.K., S.M.E. and D.B. edited manuscript drafts.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data generated in this study are deposited in Figshare.
Competing interests
The authors declare no competing interests.
Ethics declarations and approval for animal experiments
All animal care and experimental protocols complied with local and federal laws and guidelines and were approved by the appropriate governing body in Berlin, Germany, the ‘Landesamt für Gesundheit und Soziales’ (LaGeSo G-0224/20). Our study is in accordance with the ARRIVE guidelines. After experimental trials, fish remained in the husbandry.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72550-5.
References
- 1.Sosna, M. M. G. et al. Individual and collective encoding of risk in animal groups. Proc. Natl. Acad. Sci.116, 20556–20561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doran, C. et al. Fish waves as emergent collective antipredator behavior. Curr. Biol.32, 708-714.e4 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Bierbach, D. et al. Predator-induced changes of female mating preferences: Innate and experiential effects. BMC Evol. Biol11, 190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sih, A. Predation risk and the evolutionary ecology of reproductive behaviour. J. Fish Biol.45, 111–130 (1994). [Google Scholar]
- 5.Walls, M., Kortelainen, I. & Sarvala, J. Prey responses to fish predation in freshwater communities. Ann. Zool. Fennici.27, 183–199 (1990). [Google Scholar]
- 6.Lima, S. L. & Dill, L. M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool.68, 619–640 (1990). [Google Scholar]
- 7.Gabor, C., Coyle, J. & Aspbury, A. Effect of predation on male mating behaviour in a unisexual-bisexual mating system. Behaviour147, 53–63 (2010). [Google Scholar]
- 8.Brown, C., Burgess, F. & Braithwaite, V. A. Heritable and experiential effects on boldness in a tropical poeciliid. Behav. Ecol. Sociobiol.62, 237–243 (2007). [Google Scholar]
- 9.Harris, S. et al. Picking personalities apart: estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata. Oikos119, 1711–1718 (2010). [Google Scholar]
- 10.Archard, G. A. & Braithwaite, V. A. Increased exposure to predators increases both exploration and activity level in Brachyrhaphis episcopi. J. Fish Biol.78, 593–601 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Brown, C., Jones, F. & Braithwaite, V. In situ examination of boldness–shyness traits in the tropical poeciliid Brachyraphis episcopi. Anim. Behav.70, 1003–1009 (2005). [Google Scholar]
- 12.Catano, L. B. et al. Reefscapes of fear: predation risk and reef heterogeneity interact to shape herbivore foraging behaviour. J. Anim. Ecol.85, 146–156 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Bolnick, D. I. et al. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol.26, 183–192 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf, M. & Weissing, F. J. Animal personalities: Consequences for ecology and evolution. Trends Ecol. Evol.27, 452–461 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Carere, C. & Gherardi, F. Animal personalities matter for biological invasions. Trends Ecol. Evol.28, 5–6 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Ingley, S. J. & Johnson, J. B. Animal personality as a driver of reproductive isolation. Trends Ecol. Evol.29, 369–371 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Réale, D. et al. Integrating animal temperament within ecology and evolution. Biol. Rev.82, 291–318 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Bell, A. M., Hankison, S. J. & Laskowski, K. L. The repeatability of behaviour: a meta-analysis. Anim. Behav.77, 771–783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake, C. A. & Gabor, C. R. Effect of prey personality depends on predator species. Behav. Ecol.25, 871–877 (2014). [Google Scholar]
- 20.Dammhahn, M. & Almeling, L. Is risk taking during foraging a personality trait? A field test for cross-context consistency in boldness. Anim. Behav.84, 1131–1139 (2012). [Google Scholar]
- 21.Toscano, B. J., Gatto, J. & Griffen, B. D. Effect of predation threat on repeatability of individual crab behavior revealed by mark-recapture. Behav. Ecol. Sociobiol.68, 519–527 (2014). [Google Scholar]
- 22.Ehlman, S. M. et al. Intermediate turbidity elicits the greatest antipredator response and generates repeatable behaviour in mosquitofish. Anim. Behav.158, 101–108 (2019). [Google Scholar]
- 23.Mitchell, D. J., Beckmann, C. & Biro, P. Maintenance of behavioral variation under predation risk: effects on personality, plasticity, and predictability. Am. Nat.10.1086/728421 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Urszán, T. J. et al. No personality without experience? A test on Rana dalmatina tadpoles. Ecol. Evol.5, 5847–5856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell, A. M. & Sih, A. Exposure to predation generates personality in three-spined sticklebacks (Gasterosteus aculeatus). Ecol. Lett.10, 828–834 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Dingemanse, N. J. et al. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol.76, 1128–1138 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Muraco, J. J. et al. Do females in a unisexual-bisexual species complex differ in their behavioral syndromes and cortisol production?. Biology (Basel)10, 186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muraco, J. J., Aspbury, A. S. & Gabor, C. R. Does male behavioral type correlate with species recognition and stress?. Behav. Ecol.25, 200–205 (2014). [Google Scholar]
- 29.Dingemanse, N. J. et al. Individual experience and evolutionary history of predation affect expression of heritable variation in fish personality and morphology. Proc. R. Soc. B: Biol. Sci.276, 1285–1293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommer-Trembo, C. et al. Predator experience homogenizes consistent individual differences in predator avoidance. J. Ethol.34, 155–165 (2016). [Google Scholar]
- 31.Castellano, S. & Friard, O. Environmental effects on the ontogenesis of tadpole personality. Anim. Behav.175, 153–161 (2021). [Google Scholar]
- 32.Kelley, J. L. & Magurran, A. E. Learned predator recognition and antipredator responses in fishes. Fish Fish.4, 216–226 (2003). [Google Scholar]
- 33.Tulley, J. J. & Huntingford, F. A. Age, experience and the development of adaptive variation in anti-predator responses in three-spined sticklebacks (Gasterosteus aculeatus). Ethology75, 285–290 (1987). [Google Scholar]
- 34.Toscano, B. J. et al. Among-individual behavioral responses to predation risk are invariant within two species of freshwater snails. Ethology129, 269–279 (2023). [Google Scholar]
- 35.Carlson, B. A. Early life experiences have complex and long-lasting effects on behavior. Proc. Natl. Acad. Sci.114, 11571–11573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crane, A. L. et al. Early-life and parental predation risk shape fear acquisition in adult minnows. Anim. Cogn.24, 471–481 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Jonsson, B. & Jonsson, N. Early environment influences later performance in fishes. J. Fish Biol.85, 151–188 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Barbosa, M. et al. Individual variation in reproductive behaviour is linked to temporal heterogeneity in predation risk. Proc. R. Soc. B: Biol. Sci.285, 20171499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polverino, G. et al. Ecological conditions drive pace-of-life syndromes by shaping relationships between life history, physiology and behaviour in two populations of Eastern mosquitofish. Sci. Rep.8, 14673 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamps, J. A. & Biro, P. A. Time-specific convergence and divergence in individual differences in behavior: Theory, protocols and analyzes. Ecol. Evol.10.1002/ece3.10615 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehlman, S., Scherer, U. & Wolf, M. Developmental feedbacks and the emergence of individuality. R. Soc. Open Sci.9, 221189 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlman, S. M. et al. Leveraging big data to uncover the eco-evolutionary factors shaping behavioural development. Proc. R. Soc. B290, 20222115 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laskowski, K. L. et al. Naturally clonal vertebrates are an untapped resource in ecology and evolution research. Nat. Ecol. Evol.3, 161–169 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Biro, P. A. & Stamps, J. A. Using repeatability to study physiological and behavioural traits: ignore time-related change at your peril. Anim. Behav.105, 223–230 (2015). [Google Scholar]
- 45.Dingemanse, N. J. & Dochtermann, N. A. Quantifying individual variation in behaviour: Mixed-effect modelling approaches. J. Anim. Ecol.82, 39–54 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Bierbach, D., Laskowski, K. L. & Wolf, M. Behavioural individuality in clonal fish arises despite near-identical rearing conditions. Nat. Commun.8, 15361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laskowski, K. L. et al. The emergence and development of behavioral individuality in clonal fish. Nat. Commun.13, 6419 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scherer, U. et al. Reproductive individuality of clonal fish raised in near-identical environments and its link to early-life behavioral individuality. Nat. Commun.14, 7652 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wooster, D. & Sih, A. A review of the drift and activity responses of stream prey to predator presence. Oikos73, 3 (1995). [Google Scholar]
- 50.Lima, S. L. & Bednekoff, P. A. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat.153, 249–259 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Scherer, U., Godin, J.-G.J. & Schuett, W. Validation of 2D-animated pictures as an investigative tool in the behavioural sciences: A case study with a West African cichlid fish, Pelvicachromis pulcher. Ethology10.1111/eth.12630 (2017). [Google Scholar]
- 52.O’Connor, C. M. et al. Social cichlid fish change behaviour in response to a visual predator stimulus, but not the odour of damaged conspecifics. Behav. Process.121, 21–29 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Milinski M. Constraints placed by predators on feeding behaviour. In: The Behaviour of Teleost Fishes. Boston, MA: Springer US, 1986, pp. 236–252.
- 54.Cooper, W. E. & Blumstein, D. T. Escaping from predators (Cambridge University Press, 2015). [Google Scholar]
- 55.Bierbach, D. et al. Predator avoidance in extremophile fish. Life3, 161–180 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawkins, Magurran, Armstrong. Innate predator recognition in newly-hatched Atlantic salmon. Behaviour 141, 1249–1262 (2004).
- 57.Culumber, Z. W. Early recognition and response to predator, heterospecific, and conspecific visual cues by multiple species of poeciliid fry. Behaviour152, 1463–1479 (2015). [Google Scholar]
- 58.Plath, M. et al. Predator-induced changes of male and female mating preferences: innate and learned components. Curr. Zool.65, 305–316 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karplus, I. & Algom, D. Visual cues for predator face recognition by reef fishes. Z. Tierpsychol.55, 343–364 (1981). [Google Scholar]
- 60.Kelley, J. L. & Magurran, A. E. Effects of relaxed predation pressure on visual predator recognition in the guppy. Behav. Ecol. Sociobiol.54, 225–232 (2003). [Google Scholar]
- 61.Smith, J. M. & Brown, R. L. W. Competition and body size. Theor. Popul. Biol.30, 166–179 (1986). [DOI] [PubMed] [Google Scholar]
- 62.Buston, P. M. & Cant, M. A. A new perspective on size hierarchies in nature: Patterns, causes, and consequences. Oecologia149, 362–372 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Bisazza, A. & Marconato, A. Female mate choice, male-male competition and parental care in the river bullhead, Cottus gobio L. (Pisces, Cottidae). Anim. Behav.36, 1352–1360 (1988). [Google Scholar]
- 64.Landeau, L. & Terborgh, J. Oddity and the ‘confusion effect’ in predation. Anim. Behav.34, 1372–1380 (1986). [Google Scholar]
- 65.Crane, A. L. et al. Uncertainty about predation risk: a conceptual review. Biol. Rev.10.1111/brv.13019 (2023). [DOI] [PubMed] [Google Scholar]
- 66.Sih, A. Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat.139, 1052–1062 (1992). [Google Scholar]
- 67.Sih, A. et al. Animal personality and state–behaviour feedbacks: A review and guide for empiricists. Trends Ecol. Evol.30, 50–60 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Lamatsch, D. K., Schmid, M. & Schartl, M. A somatic mosaic of the gynogenetic Amazon molly. J. Fish Biol.60, 1417–1422 (2005). [Google Scholar]
- 69.Lampert, K. P. & Schartl, M. The origin and evolution of a unisexual hybrid: Poecilia formosa. Phil. Trans. R. Soc. B: Biol. Sci.363, 2901–2909 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlupp, I. The evolutionary ecology of gynogenesis. Annu. Rev. Ecol. Evol. Syst.36, 399–417 (2005). [Google Scholar]
- 71.Stöck, M. et al. Monophyletic origin of multiple clonal lineages in an asexual fish (Poecilia formosa). Mol. Ecol.19, 5204–5215 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Warren, W. C. et al. Clonal polymorphism and high heterozygosity in the celibate genome of the Amazon molly. Nat. Ecol. Evol.2, 669–679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hubbs, C. L. & Hubbs, L. C. Apparent parthenogenesis in nature, in a form of fish of hybrid origin. Science1932(76), 628–630 (1979). [DOI] [PubMed] [Google Scholar]
- 74.Schultz, R. J. Origin and synthesis of a unisexual fish. In Genetics and Mutagenesis of Fish (ed. Schröder, J. H.) 207–211 (Springer, Berlin, Heidelberg, 1973). [Google Scholar]
- 75.Alberici da Barbiano, L. et al. Population genomics reveals a possible history of backcrossing and recombination in the gynogenetic fish Poecilia formosa. Proc. Natl. Acad. Sci.110, 13797–13802 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kallman, K. D. Gynogenesis in the teleost, Mollienesia formosa (Girard), with a discussion of the detection of parthenogenesis in vertebrates by tissue transplantation. J. Genet.58(1), 7–24 (1962). [Google Scholar]
- 77.Rasch, E. M. et al. Cytophotometric evidence for triploidy in hybrids of the gynogenetic fish, Poecilia formosa. J. Exp. Zool.160, 155–169 (1965). [DOI] [PubMed] [Google Scholar]
- 78.Turner, B. J. et al. Evolutionary genetics of a gynogenetic fish, Poecilia formosa, the Amazon molly. Evolution (NY)34, 246 (1980). [DOI] [PubMed] [Google Scholar]
- 79.Lu, Y. et al. Fixation of allelic gene expression landscapes and expression bias pattern shape the transcriptome of the clonal Amazon molly. Genome Res.31, 372–379 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lukas, J. A. Y. et al. On the occurrence of three non-native cichlid species including the first record of a feral population of Pelmatolapia (Tilapia) mariae (Boulenger, 1899) in Europe. R. Soc. Open Sci.4, 170160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.R Core Team. R: A language and environment for statistical computing.
- 82.Bates, D., Mächler, M. & Bolker, B. Fitting linear mixed-effects models using the lme4 package in R. J. Stat. Softw.67, 1–48 (2015). [Google Scholar]
- 83.Hertel, A. G. et al. A guide for studying among-individual behavioral variation from movement data in the wild. Mov. Ecol.8, 30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are deposited in Figshare.