Abstract
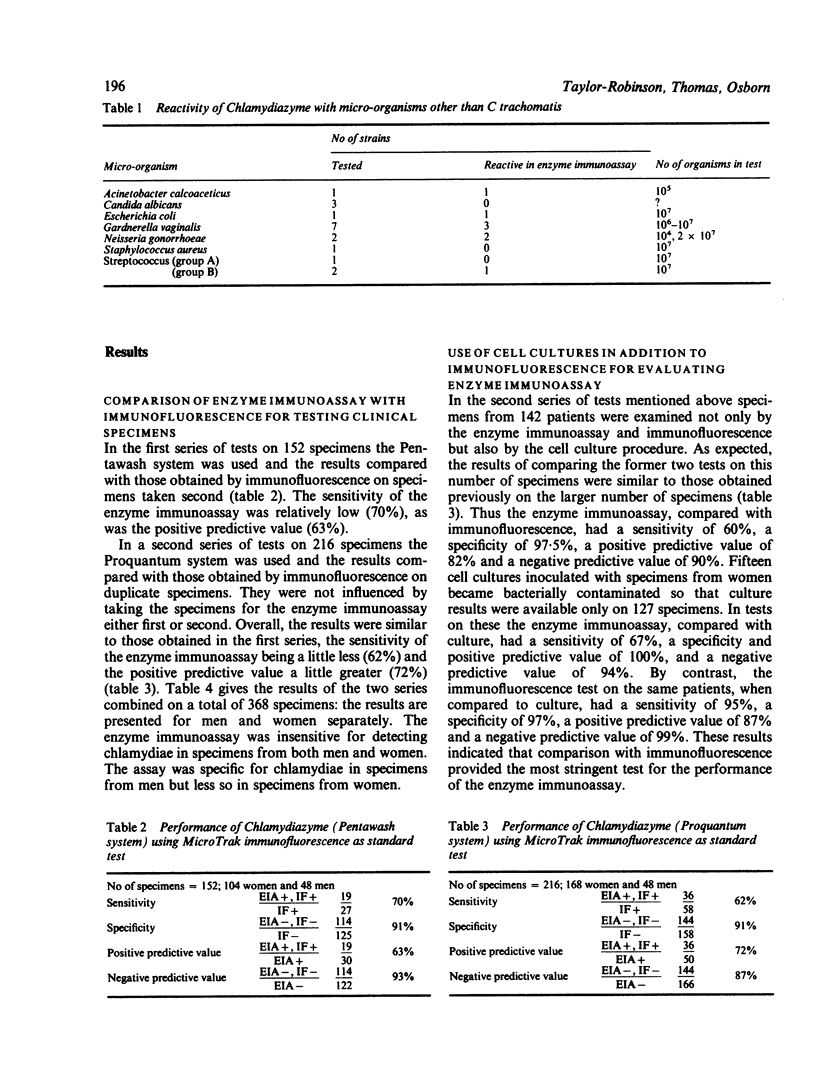
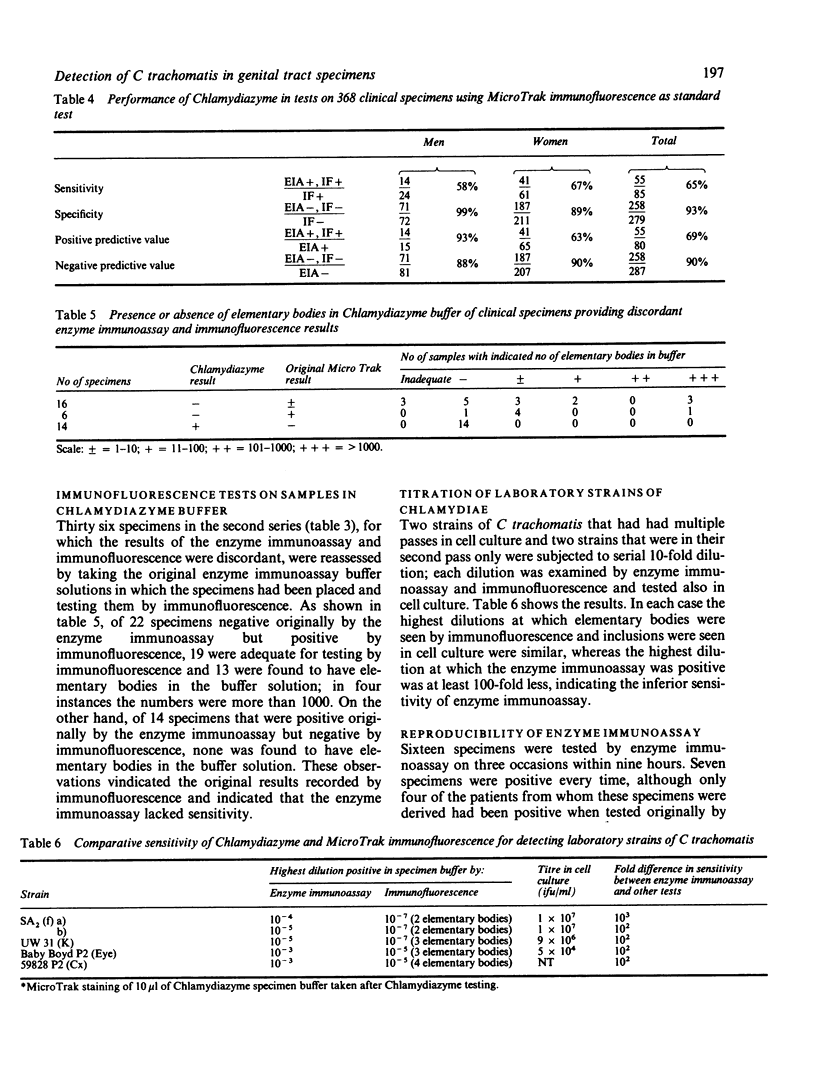
An enzyme immunoassay (Chlamydiazyme) for detecting Chlamydia trachomatis was evaluated on genital specimens from 96 men and 272 women attending a clinic for sexually transmitted diseases (STD clinic). Compared with a direct immunofluorescence test for chlamydial elementary bodies, the enzyme immunoassay had a sensitivity of 58% on specimens from men, a specificity of 90%, a positive predictive value of 93%, and a negative predictive value of 88%; the assay had a sensitivity of 67% on specimens from women, a specificity of 89%, a positive predictive value of 63% and a negative predictive value of 90%. Immunofluorescence provided the most stringent test for the performance of the enzyme immunoassay as values were improved a little when a cell culture procedure was used for comparison. Further evidence for the lack of sensitivity was the detection of elementary bodies, sometimes in large numbers, in the enzyme immunoassay buffer of 13 of 19 specimens that had given a negative enzyme immunoassay result and the finding in comparative titrations of four laboratory strains that the enzyme immunoassay was at least 100-fold less able to detect chlamydiae than either immunofluorescence or the cell culture procedure. Lack of specificity may be associated with the finding that the enzyme immunoassay antibody reacted with strains of Acinetobacter calcoaceticus, Escherichia coli, Gardnerella vaginalis, Neisseria gonorrhoeae and group B streptococci. The enzyme immunoassay was not considered to be sufficiently sensitive, specific, or reproducible for routine use.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amortegui A. J., Meyer M. P. Enzyme immunoassay for detection of Chlamydia trachomatis from the cervix. Obstet Gynecol. 1985 Apr;65(4):523–526. [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Immunoassay for detecting Chlamydia trachomatis major outer membrane protein. J Clin Microbiol. 1983 Sep;18(3):539–545. doi: 10.1128/jcm.18.3.539-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caul E. O., Paul I. D. Monoclonal antibody based ELISA for detecting Chlamydia trachomatis. Lancet. 1985 Feb 2;1(8423):279–279. doi: 10.1016/s0140-6736(85)91056-6. [DOI] [PubMed] [Google Scholar]
- Chernesky M. A., Mahony J. B., Castriciano S., Mores M., Stewart I. O., Landis S. J., Seidelman W., Sargeant E. J., Leman C. Detection of Chlamydia trachomatis antigens by enzyme immunoassay and immunofluorescence in genital specimens from symptomatic and asymptomatic men and women. J Infect Dis. 1986 Jul;154(1):141–148. doi: 10.1093/infdis/154.1.141. [DOI] [PubMed] [Google Scholar]
- Hambling M. H., Kurtz J. B. Preliminary evaluation of an enzyme immunoassay test for the detection of Chlamydia trachomatis. Lancet. 1985 Jan 5;1(8419):53–53. doi: 10.1016/s0140-6736(85)91007-4. [DOI] [PubMed] [Google Scholar]
- Hawkins D. A., Wilson R. S., Thomas B. J., Evans R. T. Rapid, reliable diagnosis of chlamydial ophthalmia by means of monoclonal antibodies. Br J Ophthalmol. 1985 Sep;69(9):640–644. doi: 10.1136/bjo.69.9.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L. V., Coleman P. F., England B. J., Herrmann J. E. Evaluation of chlamydiazyme for the detection of genital infections caused by Chlamydia trachomatis. J Clin Microbiol. 1986 Feb;23(2):329–332. doi: 10.1128/jcm.23.2.329-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. F., Smith T. F., Houglum A. J., Herrmann J. E. Detection of Chlamydia trachomatis in genital specimens by the Chlamydiazyme test. J Clin Microbiol. 1984 Sep;20(3):465–467. doi: 10.1128/jcm.20.3.465-467.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen M. T., Lehtonen O. P. Relation between avidity and specificity of monoclonal anti-chlamydial antibodies in culture supernatants and ascitic fluids determined by enzyme immunoassay. J Immunol Methods. 1984 Sep 4;72(2):341–347. doi: 10.1016/0022-1759(84)90002-4. [DOI] [PubMed] [Google Scholar]
- Mohanty K. C., O'Neill J. J., Hambling M. H. Comparison of enzyme immunoassays and cell culture for detecting Chlamydia trachomatis. Genitourin Med. 1986 Jun;62(3):175–176. doi: 10.1136/sti.62.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz G., Mellars B. J., Ridgway G. L., Oriel J. D. Enzyme immunoassay for the detection of Chlamydia trachomatis antigen in urethral and endocervical swabs. J Clin Pathol. 1985 Jul;38(7):740–742. doi: 10.1136/jcp.38.7.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh S. F., Slack R. C., Caul E. O., Paul I. D., Appleton P. N., Gatley S. Enzyme amplified immunoassay: a novel technique applied to direct detection of Chlamydia trachomatis in clinical specimens. J Clin Pathol. 1985 Oct;38(10):1139–1141. doi: 10.1136/jcp.38.10.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Puolakkainen M., Leinonen M., Nurminen M., Nissinen A. Cross-reactivity between Chlamydiazyme and Acinetobacter strains. N Engl J Med. 1986 Apr 3;314(14):922–923. doi: 10.1056/NEJM198604033141413. [DOI] [PubMed] [Google Scholar]
- Stokes G. V., Khan M. W. Enzyme-linked immunosorbent assay for the detection of chlamydial antigens from human specimens. Microbios. 1984;40(159):15–23. [PubMed] [Google Scholar]
- Terho P., Matikainen M. T. Detection of Chlamydia trachomatis antigen by radioimmunoassay. J Immunoassay. 1981;2(3-4):239–262. doi: 10.1080/15321818108056980. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Evans R. T., Hawkins D. A., Taylor-Robinson D. Sensitivity of detecting Chlamydia trachomatis elementary bodies in smears by use of a fluorescein labelled monoclonal antibody: comparison with conventional chlamydial isolation. J Clin Pathol. 1984 Jul;37(7):812–816. doi: 10.1136/jcp.37.7.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Evans R. T., Hutchinson G. R., Taylor-Robinson D. Early detection of chlamydial inclusions combining the use of cycloheximide-treated McCoy cells and immunofluorescence staining. J Clin Microbiol. 1977 Sep;6(3):285–292. doi: 10.1128/jcm.6.3.285-292.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffrey M., Falder P., Gale J., Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol. 1986 Aug;67(4):605–616. [PMC free article] [PubMed] [Google Scholar]


