Abstract
The rise of antimicrobial resistance poses a critical public health threat worldwide. While antimicrobial photodynamic therapy (aPDT) has demonstrated efficacy against multidrug-resistant (MDR) bacteria, its effectiveness can be limited by several factors, including the delivery of the photosensitizer (PS) to the site of interest and the development of bacterial resistance to PS uptake. There is a need for alternative methods, one of which is superhydrophobic antimicrobial photodynamic therapy (SH-aPDT), which we report here. SH-aPDT is a technique that isolates the PS on a superhydrophobic (SH) membrane, generating airborne singlet oxygen (1O2) that can diffuse up to 1 mm away from the membrane. In this study, we developed a SH polydimethylsiloxane dressing coated with PS verteporfin. These dressings contain air channels called a plastron for supplying oxygen for aPDT and are designed so that there is no direct contact of the PS with the tissue. Our investigation focuses on the efficacy of SH-aPDT on biofilms formed by drug-sensitive and MDR strains of Gram-positive (Staphylococcus aureus and S. aureus methicillin-resistant) and Gram-negative bacteria (Pseudomonas aeruginosa and P. aeruginosa carbapenem-resistant). SH-aPDT reduces bacterial biofilms by approximately 3 log with a concomitant decrease in their metabolism as measured by MTT. Additionally, the treatment disrupted extracellular polymeric substances, leading to a decrease in biomass and biofilm thickness. This innovative SH-aPDT approach holds great potential for combating antimicrobial resistance, offering an effective strategy to address the challenges posed by drug-resistant wound infections.
Keywords: drug-resistant biofilms, verteporfin, Pseudomonas aeruginosa, Staphylococcus aureus, wound healing
Introduction
Antimicrobial resistance poses a significant global health threat, impacting not only individual patients but also having broader consequences for public health.1 It has led to substantial economic impacts as treating resistant infections becomes more challenging and expensive, resulting in increased healthcare costs and elevated mortality rates.1,2
The emergence of multidrug-resistant (MDR) bacteria has gained significant attention in recent years. It has been attributed to factors such as misuse/overuse of antibiotics, inappropriate prescribing, and the lack of development of new drugs.1−3 These factors enable the bacteria to adapt and develop resistance mechanisms, including the overexpression of efflux pumps and reduction in drug internalization.3
Moreover, bacteria can form biofilms, presenting an additional challenge to treatment.4 Biofilms, existing in an attached and sessile state, differ from planktonic cells due to their numerous upregulated genes, degradation enzymes, and the presence of extracellular polymeric substances (EPS) that contribute to their protection, proliferation, and dissemination.4,5 In the context of wound infections, biofilms play a major role and can delay the healing process.5 Pathogenic biofilms pose a threat as they can be resistant to antimicrobial drugs and more tolerant to immune responses, hindering wound healing and leading to chronic inflammation, sepsis, and death.4,5
Since antibiotics are the primary treatment for bacterial-related infections, there is an urgent need for alternative strategies to tackle the problem of drug resistance. Antimicrobial photodynamic therapy (aPDT) has emerged as an attractive light-based technology to treat localized infections, as it can kill a wide range of pathogens through oxidative stress.6 aPDT produces reactive oxygen species, including the highly reactive singlet oxygen (1O2), which is a potent oxidant within microorganisms.6 However, 1O2 has a very short 3.5 μs lifetime and diffuses short distances of ∼100–200 nm in H2O, making its interaction with pathogens dependent on photosensitizer (PS) localization and accumulation.6−12
Moreover, bacteria can develop resistance to PSs via mechanisms such as efflux pumps.13,14 aPDT may also be limited by the conditions of the infected tissue as the PS is likely to bind the biological components present in inflammatory exudates rather than only bacterial membranes, thus decreasing the availability of PS to bind and kill pathogens.15
To address these limitations, we developed a new aPDT technique that delivers airborne 1O2 to the infection site while retaining the PS on superhydrophobic (SH) surfaces. This superhydrophobic antimicrobial photodynamic therapy (SH-aPDT) method was used to kill various bacterial biofilms with promising results.16−18 These surfaces can work as dressings and contain air channels that supply sufficient oxygen to react with the PS upon light illumination, as shown schematically in Figure 1.9,16−18 The airborne 1O2 diffuses approximately 1 mm in the air without the PS directly contacting the tissue, thus offering a new strategy to overcome some of the aPDT limitations.17−19
Figure 1.
Schematic of an SH-aPDT dressing used to disinfect a wound by generating airborne 1O2 when illuminated with visible light of the appropriate wavelength. The airborne 1O2 diffuses from the bandage to the wound surface, where it reacts with the biofilm to kill both Gram-positive and Gram-negative bacteria as well as destroy the surrounding EPS.
We recently demonstrated the potential of this innovative treatment in vivo. By using Wistar rat models with periodontitis, it was observed that SH-aPDT not only significantly decreased Pseudomonas gingivalis biofilms but also reduced inflammation, resulting in an increase in fibroblast cells, thus allowing complete tissue healing.17 Further investigation is required to explore the effectiveness of SH-aPDT on the MDR biofilms.
In this work, we developed freestanding SH polydimethylsiloxane (PDMS) films coated with PS verteporfin (VP) at loadings of 12, 60, and 300 μg/cm2. We note that VP is a constituent of the FDA-approved PS emulsion visudyne.9 Our investigation focuses on the efficacy of SH-aPDT on biofilms formed by drug-sensitive and MDR strains of Gram-negative (Pseudomonas aeruginosa PAO1, P. aeruginosa carbapenem-resistant, CRPA) and Gram-positive bacteria (Staphylococcus aureus ATCC and S. aureusmethicillin-resistant, MRSA).
SH-aPDT was performed using a red laser emitting at a peak of 690 ± 3 nm to match the absorbance of the PS. To evaluate the antimicrobial potential of SH-aPDT, we used the colony-forming unit (CFU) method to estimate the number of viable cells and confocal microscopy using LIVE/DEAD staining. We also aimed to investigate the efficacy of SH-aPDT on biofilm metabolic activity using an MTT assay. Biofilm biomass was evaluated using the crystal violet (CV) method and confocal microscopy for EPS staining. Additionally, a quenching study was conducted to demonstrate that singlet oxygen is the initial species involved in bacterial killing, followed by oxygen radicals from bioperoxide decomposition based on studies with HPF.
Methods
Bacteria Culture and Biofilm Formation
The bacteria species P. aeruginosa (PAO1), P. aeruginosa carbapenem-resistant (CRPA), and S. aureus (ATCC25923 and MRSA-USA300) were used. Biofilms were grown and cultured in brain heart infusion broth (BHI) or BHI supplemented with 15 g/L agar at 37 °C. Cell densities of the bacterial suspensions were adjusted to 1 × 106 CFU/mL and seeded in 96-well plates. After 24 h of incubation, the media were refreshed, and biofilms were incubated for another 24 h, thus resulting in 48 h of incubation time. Then, biofilms were gently washed twice with PBS, and SH membranes were placed on the top of the biofilms to be further irradiated.
Susceptibility Tests
Antimicrobial susceptibility tests were conducted to assess the minimum inhibitory concentration (MIC) values of various clinically relevant antibiotics. The MIC values were determined using the broth microdilution method, according to the guidelines provided by the Clinical and Laboratory Standards Institute.20
Efficacy of SH-aPDT against Bacterial Biofilms
The laser emitting at a peak of 690 ± 3 nm was set to deliver 3 different incident fluences (100, 200, and 300 J/cm2) at an incident irradiance of 160 mW/cm2. These fluences resulted in an illumination time of 625, 1250, and 1875 s, respectively. Five control groups were evaluated: nontreated control (here defined as NT), SH PDMS surfaces without PS and without light (SH+PS–L−), SH PDMS surfaces without PS but with light at a transmitted fluence (TF) of 155.7 J/cm2 (SH+PS–L+), SH surfaces coated with three different VP loading levels (12, 60, and 300 μg/cm2) without light exposure (SH+PS+L−), and light only at 300 J/cm2. Because of the PS coating, PDMS dressings can diminish the amount of light transmitted through the SH surface. Different concentrations resulted in different TFs. Therefore, the TF was calculated and described in Table 2. The experimental groups and exposure time corresponding to each light dose are described in Tables 1 and 2, respectively.
Table 2. Transmitted Irradiance and Fluence through SH Dressings Coated with Different Concentrations of VP.
| SH dressing type | verteporfin concentration (μg/cm2) | transmitted irradiance (mW/cm2) | incident fluence (J/cm2) | transmitted fluence (J/cm2) |
|---|---|---|---|---|
| SH+PS–L+ | none | 83 | 100 | 51.9 |
| 200 | 103.7 | |||
| 300 | 155.7 | |||
| SH+PS+L+ | 12 | 78.5 | 100 | 49.0 |
| 200 | 98.1 | |||
| 300 | 147.2 | |||
| SH+PS+L+ | 60 | 70.4 | 100 | 44.0 |
| 200 | 88.0 | |||
| 300 | 132.0 | |||
| SH+PS+L+ | 300 | 53.1 | 100 | 33.2 |
| 200 | 66.4 | |||
| 300 | 99.6 |
Table 1. Experimental Control and SH-aPDT Groups.
| groups | comment |
|---|---|
| NT | no treatment |
| SH–PS–L+ | light at fluence 300 J/cm2 |
| SH+PS–L– | SH PDMS without PS and no light |
| SH+PS–L+ | SH PDMS without PS but with light |
| SH+PS+L– | SH PDMS with PS (12, 60, 300 VP) without light |
| SH+PS+L+ | SH PDMS with PS (12, 60, 300 VP) and with light at different transmitted fluences (Table 2) |
After SH-aPDT, wells were scraped with a sterile pipet tip, resuspended in PBS, and sonicated for 5 min. Then, 10-fold serial dilutions were conducted for each group for colony counting, determined as colony-forming units per mL (CFU/mL).
MTT Assay
To evaluate the bacterial metabolic activity, an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-dyphenyltetrazolium bromide] assay was performed. MTT is a common method used to evaluate the cell viability and metabolic activity in various biological systems, including biofilms. The reduction of MTT in bacterial specimens involves various metabolic pathways and enzymes. For this, biofilms of P. aeruginosa PAO1, CRPA, S. aureus ATCC, and MRSA were exposed to SH-aPDT at different VP concentrations and light doses, as described above. After treatment, biofilms were carefully washed with PBS, and 0.5 mg/mL MTT was added to each well for 4 h at 37 °C. Then, 100 μL of DMSO was added for 30 min, and absorbance was measured with a plate reader at 570 nm.
Confocal Microscopy
Confocal microscopy was used to assess the biofilm viability and thickness. Biofilms of all 4 strains were treated with SH-aPDT at 132 J/cm2 and 60 μg/cm2 VP. Then, biofilms were washed twice with PBS to remove nonadherent cells and 200 μL of distilled water containing LIVE/DEAD dye BacLight bacterial viability assay (Molecular Probes, Inc.) containing SYTO9, and propidium iodide (PI) was added to the wells. After 15 min, specimens were rinsed with PBS and evaluated under confocal microscopy (Olympus FV1000) at wavelengths of 488 nm excitation/500 nm emission for SYTO9 and 535 nm excitation/635 nm emission for PI. Biofilm thickness was assessed by performing 3D confocal imaging using 1 μm stacks.
To analyze the structure of the EPS matrix, biofilms were exposed to the same conditions as described above. Then, they were washed twice with PBS to remove nonadherent cells, and 200 μL of distilled water containing FilmTracer SYPRO Ruby biofilm matrix stain (Invitrogen, Inc.) was added to the wells. After 30 min, specimens were rinsed with PBS and evaluated under confocal microscopy (Olympus FV1000) at 450 nm excitation/610 ± 30 nm emission.
CV Staining
Biofilm biomass was evaluated by the addition of 100 μL of CV at 0.5% to each well immediately after SH-aPDT. The plate was incubated for 10 min at 25 °C. CV was removed, and the sample was washed three times with PBS. Then 100 μL of 33% acetic acid was added per well to dissolve the biofilm. Absorbance was measured at 595 nm.
Singlet Oxygen Quenching Studies
For the quenching studies, 48 h biofilms were grown in BHI at 37 °C. Prior to irradiation, the media were replaced with media containing sodium azide, selecting a concentration of 2.0 mM,21,22 followed by placement of the SH membrane on top of the biofilm.21 Thereafter, they were exposed to the best SH-aPDT conditions (132 J/cm2 and 60 μg/cm2 VP). Wells were then scraped, resuspended in PBS, and sonicated for 5 min. Then, 10-fold serial dilutions were performed for colony counting (CFU/mL).
Assessing Bioperoxide Decomposition by HPF
Biofilms were grown and washed as previously described to seek evidence for downstream oxygen radicals formed by the decomposition of the initially photogenerated bioperoxides after airborne 1O2 exposure. Here, the fluorescence dye 2-[6-(4′-hydroxy)phenoxy-3H-xanthen-3-on-9-yl]benzoic acid (HPF) (Invitrogen) (10 μM) was used by adding to the wells containing the biofilms and incubating at 37 °C for 30 min. Biofilm specimens were then illuminated with a red laser at three SH-aPDT (4.2, 44, and 88 J/cm2) conditions along with controls without SH surfaces. The incident fluence for the lowest dose was 20 J/cm2, which corresponds to a TF of 4.2 J/cm2. Evidence for radicals was detected by using a plate reader.23 The excitation/emission wavelengths were 490 nm/515 nm, respectively.8
Statistical Analysis
Statistical analysis was evaluated by using GraphPad Prism 10 software by one-way analysis of variance (ANOVA), followed by the Tukey post-test for CFU counting, biofilm thickness, quenching studies, and peroxide radicals. For metabolic activity and biofilm biomass, statistical analysis was assessed by two-way ANOVA, followed by the Tukey post-test. Differences were considered statistically significant when p < 0.05.
Results
Antibiotic Susceptibility of Bacterial Strains
Table 3 shows the MIC of various antibiotics against different bacterial strains, highlighting their susceptibility or resistance.
Table 3. MIC of Various Antibiotics for All Four Bacterial Strainsa.
| antibiotic | MIC
in μg/mL |
|||
|---|---|---|---|---|
| S. aureus ATTC | PAO1 | MRSA | CRPA | |
| amoxicillin | >0.5, S | 512, R | >0.5, S | 512, R |
| nitrofurantoin | 8, S | N/A | 32, R | N/A |
| ciprofloxacin | >0.5, S | >0.5, S | 16, R | 32, R |
| levofloxacin | >0.5, S | >0.5, S | 4, R | 64, R |
| cefazolin | >0.5, S | >1024, R | >0.5, S | >1024, R |
| ceftazidime | 16, R | 1, S | 64, R | 16, R |
| chloramphenicol | 8, S | 32, R | 8, S | 512, R |
| gentamicin | 2, S | 1, S | >0.5, S | >0.5, S |
| doxycycline | >0.5, S | N/A | >0.5, S | N/A |
| imipenem | >0.5, S | 32, R | >0.5, S | 256, R |
| meropenem | N/A | 2, S | N/A | 16, R |
| doripenem | N/A | 2, S | N/A | 16, R |
R = resistant, S = sensitive, N/A = not available.
The strains examined include S. aureus ATTC, P. aeruginosa PAO1, methicillin-resistant S. aureus (MRSA), and carbapenem-resistant P. aeruginosa (CRPA). While S. aureus ATTC demonstrates susceptibility to several antibiotics, including amoxicillin, nitrofurantoin, gentamicin, and chloramphenicol, MRSA shows resistance to most antibiotics tested.
PAO1 exhibits susceptibility to gentamicin and other carbapenems such as meropenem and doripenem. However, it is resistant to amoxicillin, imipenem, cefazolin, and chloramphenicol. In contrast, the carbapenem-resistant strains (CRPA) display resistance to a wide range of antibiotics, including ciprofloxacin, levofloxacin, cefazolin, ceftazidime, chloramphenicol, imipenem, meropenem, and doripenem. It is susceptible to only gentamicin among the antibiotics tested.
SH-aPDT Effectively Inactivated Gram-Positive and Gram-Negative MDR Biofilms
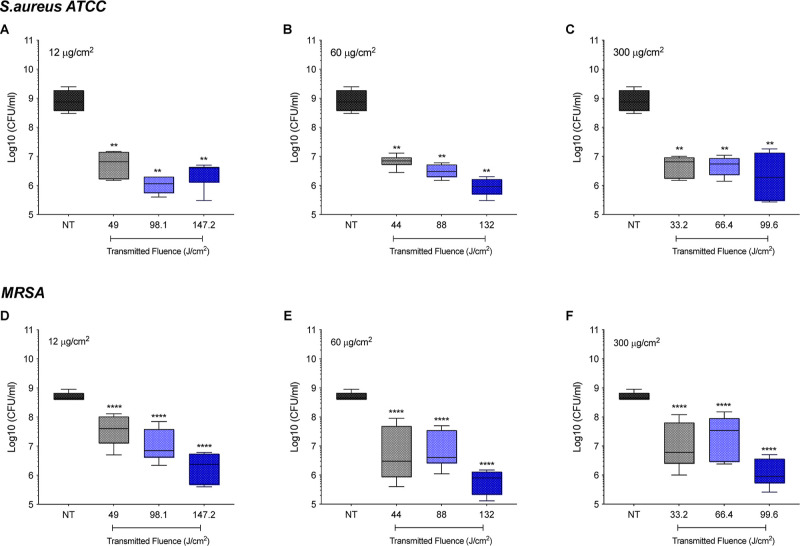
Our results show that SH-aPDT successfully inactivated biofilms of Gram-positive and Gram-negative MDR strains. All controls, including SH surfaces alone (SH+PS–L−) and coated with different loadings of PS and without light (SH+PS+L−), did not induce any significant effect on the bacterial specimens (Supporting Information, Figure S1). The efficacy of treatment was dependent on the VP loading and fluence, as shown in Figure 2. The lowest loading (12 μg/cm2) and TF 49 J/cm2 caused a 2.1 and 2.0 log10 reduction of S. aureus and MRSA, respectively, relative to NT (p < 0.01, p < 0.0001, respectively). The effect was more pronounced at the highest light dose (147.2 J/cm2), achieving nearly 2.5 and 2.3 log10 inactivation in both Gram-positive strains (ATCC p < 0.01, MRSA p < 0.0001) (Figure 2A,D). Remarkably, we observed that the VP loading of 60 μg/cm2 was more effective at the highest TF (132 J/cm2), leading to a 3 log reduction (ATCC p < 0.01, MRSA p < 0.0001) (Figure 2B,E). In contrast, no improvement was noticed under a higher PS loading (300 μg/cm2) (Figure 2C,F). MRSA killing showed a greater dependency on fluence for all PS loadings in comparison to S. aureus.
Figure 2.
Efficacy of SH+PS+L+ surfaces coated with (A,D) 12, (B,E) 60, and (C,F) 300 μg/cm2 of VP against biofilms of S. aureus and MRSA at different fluences (J/cm2). Statistically significant differences: **p < 0.01 and ****p < 0.0001 between SH-aPDT and NT.
We also demonstrate that SH-aPDT effectively inactivated both strains of P. aeruginosa regardless of the PS loading (Figure 3), whereas no significant impact was observed with controls (SH–PS–L+, SH+PS–L+, SH+PS–L–, and SH+PS+L−) on either strain, as shown in Supporting Information, Figure S1.
Figure 3.
Efficacy of SH+PS+L+ surfaces coated with (A,D) 12, (B,E) 60, and (C,F) 300 μg/cm2 of VP against biofilms of P. aeruginosa PAO1 and CRPA at different fluences (J/cm2). Statistically significant differences: *p < 0.05 and **p < 0.01 between SH-aPDT and NT.
For PAO1 at the lowest loading (12 μg/cm2, Figure 3A), no significant differences were observed between NT and biofilms exposed to 49 J/cm2. However, by increasing the light dose, biofilms were reduced by approximately 2 log10 when compared to NT or SH+PS–L+ (p < 0.05) (Figure 3A). SH-aPDT was more effective when PAO1 biofilms were exposed to SH surfaces coated with VP at higher loading levels. The increase in PS loading resulted in significant bacterial inactivation by 1.6 log10 at the lowest fluence (44 and 33.2 J/cm2) (p < 0.01), while at the highest light dose (132 and 99.6 J/cm2), more than 2.7 and 2.5 log10 reduction was achieved at loadings of 60 and 300 μg/cm2, respectively (p < 0.05 and p < 0.01, respectively) (Figure 3B,C).
SH-aPDT also showed high bacterial killing against the CRPA strain, which was significantly reduced by 1.7 log10 at the lowest transmitted light dose (49 J/cm2) and VP loading (12 μg/cm2) (p < 0.05) (Figure 3D). The killing rate was greatly enhanced by increasing the light dose and PS loading to 60 and 300 μg/cm2, thus showing 2.6 and 2.7 log10 reduction under a higher TF of 132 and 99.6 J/cm2, respectively (p < 0.05 and p < 0.01, respectively) (Figure 3E,F).
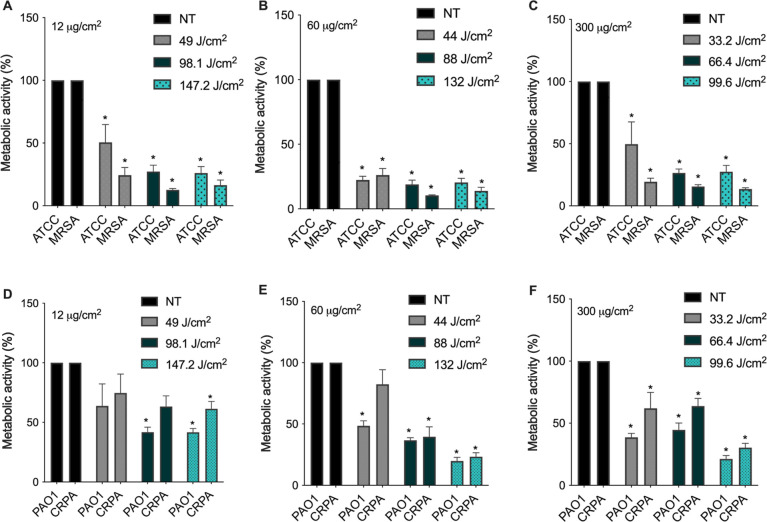
SH-aPDT Reduced Metabolic Activity of Gram-Positive and Gram-Negative MDR Biofilms
SH-aPDT effectively reduced biofilm metabolism, as shown in Figure 4. At the lowest (12 μg/cm2) PS loading, significant decreases were observed for both Gram-positive strains (S. aureus and MRSA, Figure 4A), whereas the decreases were not significant for the Gram-negative strains (PAO1 and CRPA) (Figure 4D), which is consistent with the CFU results (Figures 2 and 3). Nevertheless, higher VP loadings resulted in a significant decrease in the measured metabolism for all strains. The best condition was for the loading of 60 μg/cm2, in which both S. aureus ATCC and MRSA showed a considerable decrease in their metabolism regardless of the light dose (Figure 4B). At 132 J/cm2, S. aureus ATCC activity was reduced by 81.7%, while MRSA showed 86% decrease (Figure 4B). Under the same conditions, the viability of PAO1 and CRPA was diminished by about 80.2 and 73.7% (Figure 4E). No additional improvement was noticed when the PS loading was increased to 300 μg/cm2 (Figure 4C,F). The other controls SH+PS–L–, SH+PS–L+, SH–PS–L–, and SH coated with different loading (SH+PS+12,60,300L−) did not promote any significant killing (Supporting Information, Figure S2).
Figure 4.
Metabolic activity after SH-aPDT killing of biofilms of drug-sensitive and drug-resistant S. aureus and P. aeruginosa at different TFs (J/cm2) with PDMS coated with (A,D) 12, (B,E) 60, and (C,F) 300 μg/cm2. * denotes statistically significant differences compared to the respective NT (p < 0.05).
SH-aPDT Reduces Biofilm Thickness and Promotes Disruption in Their Structure
Biofilm viability was also assessed via confocal microscopy. Bacterial specimens were stained with fluorescent dyes SYTO 9 and PI. While SYTO 9 stains live bacteria and emits green fluorescence, and dead bacteria are marked with PI, thus emitting red fluorescence. Images are consistent with the metabolic activity and CFU counting described above, in which most of the biofilms were dead (indicated by red fluorescence) under the highest fluence and 60 μg/cm2 PS. In addition, SH-aPDT induced disruption in biofilms, leading to irregularities, increased roughness, and greater porosity of the surface compared to untreated control (Figure 5).
Figure 5.
Confocal microscopy images of S. aureus ATCC, MRSA, PAO1, and CRPA biofilms stained with LIVE/DEAD after SH-aPDT 60 μg/cm2 of VP and a TF of 132 J/cm2 compared to NT. Scale bar = 30 μm.
Biofilm thickness was measured by capturing 3D (z-stack) images and then examining them to determine the thickness. Figure 6 shows that biofilm thickness was substantially reduced after SH-aPDT, resulting in 54.4 and 39.6% reduction for S. aureus ATCC and MRSA, respectively. Surprisingly, the impact was more pronounced in Gram-negative strains, with a 68 and 68.8% reduction in biofilm thickness of PAO1 and CRPA, respectively.
Figure 6.
Biofilm thickness of drug-sensitive and drug-resistant biofilms of Gram-positive and Gram-negative bacteria after SH-aPDT treatment using 60 μg/cm2 of VP and a TF of 132 J/cm2. Statistically significant differences: *p < 0.05, **p < 0.01, and ****p < 0.0001 between SH-aPDT and NT.
SH-aPDT Significantly Decreases Biofilm Biomass
We observed that SH-aPDT promoted a significant decrease in the biomass of both drug-sensitive and drug-resistant biofilms, as shown in Figure 7. Controls (SH+PS–L–, SH+PS–L+, SH–PS–L–, and SH) coated with different VP concentrations (12, 60, and 300 μg/cm2) did not result in any significant biofilm mass (Supporting Information, Figure S3). We found that S. aureus and MRSA biomass decreased by >64% at all PS loading levels and all fluence values. Gram-negative P. aeruginosa strains followed the same trend, but the magnitudes of the biomass decrease were smaller <41%. At 300 μg/cm2 and 132 J/cm2, a 56.7% decrease in biomass was observed in the drug-sensitive strain, while for the drug-resistant line, a 53.1% decrease was achieved.
Figure 7.
Efficacy of SH PDMS surfaces coated with (A,D) 12, (B,E) 60, and (C,F) 300 μg/cm2 of VP on biofilms biomass of S. aureus ATCC, MRSA, P. aeruginosa PAO1, and CRPA at different TFs (J/cm2). * denotes statistically significant differences compared to the respective control. ** denotes statistically significant differences between different strains (p < 0.05).
SH-aPDT Promotes Disruption of the EPS Matrix of Biofilms
SH-aPDT also had a pronounced impact on the structure of the EPS matrix of all four strains (Figure 8). Exposure of bacterial specimens to a fluence of 132 J/cm2 and 60 μg/cm2 of VP resulted in significant damage to the EPS. Untreated controls exhibited mature biofilms with preserved architecture, while treated ones displayed loss of their structure and smaller aggregates. Microscopy findings are consistent with reductions in the biofilm thickness (Figure 6) and biomass (Figure 7).
Figure 8.
Confocal microscopy images of drug-sensitive and drug-resistant biofilms stained with FilmTracer SYPRO RUBY biofilm matrix stain after SH-aPDT 60 μg/cm2 of VP and 132 J/cm2. Scale bar = 30 μm.
EPS plays an important role in physical stability, resistance to antimicrobials, mechanical removal, and host immunity, thus having significant implications for wound healing. Therefore, disrupting the biofilm structure would make the pathogens more susceptible to drugs, facilitate mechanical removal, and, thus, enhance treatment efficacy.
Singlet Oxygen Quenching by Sodium Azide
To evaluate whether the killing effect originated from airborne 1O2 delivery, we added sodium azide (a 1O2 quencher) to the biofilms before illumination. Consequently, we observed (Figure 9) that no killing occurred compared to the controls, which is consistent with the quenching of 1O2 and the prevention of biofilm death. Although we observed a slight decrease in the average bacterial load (≤0.8 log10) for MRSA and CRPA, this reduction was not statistically significant.
Figure 9.

Activity of SH PDMS surfaces coated with 60 μg/cm2 of VP against biofilms of drug-sensitive and drug-resistant bacteria at a TF of 132 J/cm2 in the presence of the 1O2 quencher sodium azide (2 mM) (A) S. aureus, (B) MRSA, (C) PA01, and (D) CRPA.
HPF Detection of Downstream Oxygen Radicals
Figure 10 shows the HPF fluorescence results from biofilms treated with SH-aPDT. At 132 J/cm2, there was an 8.2-fold and 7.4-fold increase in these radicals after SH-aPDT treatment of S. aureus ATCC and MRSA biofilms (Figure 10). The effect was more pronounced in Gram-negative strains, in which the levels of radicals were nearly 26-fold higher compared with the untreated control group. The significant increase suggests that 1O2 first reacts with lipids or some amino acid sites/proteins within the biofilm to form bioperoxides that subsequently decay to form oxygen radicals detected by HPF.
Figure 10.
HPF fluorescence on biofilms resulting from SH-aPDT (60 μg/cm2 of VP) and different light doses for (A) S. aureus and (B) P. aeruginosa. Mean values not sharing any letters indicate a significant statistical difference.
Discussion
In the present work, we demonstrate that SH-aPDT is a highly effective antimicrobial method for killing MDR biofilms of S. aureus and P. aeruginosa, which are among the most common drug-resistant pathogens that cause high mortality rates in patients. MRSA and CRPA are considered significant public health threats and are included in the high and critical priority list of the World Health Organization (WHO) for drug discovery and research, respectively.24 This highlights the urgent need for developing alternative strategies to combat these antibiotic-resistant pathogens.
While aPDT has demonstrated promising results in multiple MDR strains, the efficacy of aPDT is dependent upon the PS’s uptake by bacteria, which could lead to the development of resistance. Our SH-aPDT system eliminates the need for direct PS contact with the tissue, where we established its efficacy in a dose-dependent manner, with a TF of 132 J/cm2 at a VP loading of 60 μg/cm2, resulting in a >3 log reduction.
SH-aPDT also led to a substantial reduction in biofilm thickness and a significant decrease in metabolic activity against all of the tested strains. Biofilm-associated bacteria are difficult to treat and inherently more tolerant to antimicrobials, including antibiotics.4,25
Reducing biofilm thickness is one of the primary goals of treatment strategies because thicker biofilms act as a physical barrier, limiting drug penetration and thus contributing to increased resistance. Thicker biofilms can provide a protective environment due to the EPS matrix that prevents antimicrobials and the host immune cells from effectively targeting and eradicating bacteria, thereby resulting in greater survival and resistance to therapy.4,25
The efficacy of antimicrobial treatments depends on the biofilm’s age, where a number of studies indicate that 48 h biofilm is considered to be mature for the strains S. aureus and P. aeruginosa.26−29 Not only does SH-aPDT cause a significant reduction in biofilm thickness but it also results in a disruption of the biofilm structure and a significant reduction in the biomass regardless of the PS or light dose applied. The efficacy of antimicrobial treatments depends on the biofilm’s age, where a number of studies indicate that 48 h biofilm is considered to be mature for the strains S. aureus and P. aeruginosa. This highlights the effectiveness of SH-aPDT not only in inactivating bacteria but also in disrupting the structure of the biofilms, including the EPS matrix. The outcome was consistent with confocal microscopy, in which obvious EPS damage was observed following treatment. This means that SH-aPDT should facilitate the detachment of biofilms from the wound surfaces. Furthermore, the disruption of EPS provides opportunities for the penetration of antimicrobials, thereby enhancing the efficacy of adjuvant treatments.
SH surfaces are reported to emit airborne 1O2.18,30 A mechanistic assessment of SH-aPDT to account for the above results is the initial contact between airborne 1O2 and the biofilm. In a previous report,18 we measured the 1270 nm luminescence intensity within a SH system, confirming the presence of airborne 1O2. We found that this airborne 1O2 could be transported from the PS-coated SH surface and chemically trapped in an aqueous droplet positioned up to 0.6 mm away, which is consistent with airborne 1O2 with a lifetime of ∼0.7 ms instead of much shorter-lived oxygen radicals.
Airborne 1O2 can react with multiple sites within the EPS matrix. The significant reduction in phototoxicity in the presence of sodium azide supports 1O2 as the main species responsible for bacterial killing (Figure 9), which is consistent with prior VP studies.31,32
Sodium azide deactivates 1O2 by converting it to ground-state 3O2 by physical quenching.33 The results also suggest the formation of bioperoxides, as well as their subsequent decomposition. The HPF studies point to bioperoxide decomposition after 1O2 exposure with downstream detection of oxygen radicals that arise from peroxidized bacterial membranes within the EPS. Such peroxides can decompose over time by radical chain and other processes.16,34−41 Thus, we suggest that bioperoxides diffusing through the biofilms and subsequently forming oxygen radicals can contribute to bacterial killing.
The airborne 1O2 produced from the SH surface has a limited diffusion distance of approximately 1 mm in air but is about 104 times shorter in an aqueous solution (100–200 nm)9,19 While SH-aPDT offers the advantage of introducing the key species thought to underlie PDT, it does so without the PS directly contacting the tissue. Uptake of the PS by the bacteria is avoided; thus, this aPDT limitation is overcome.17,18
Conclusions
SH-aPDT is emerging as an attractive strategy to overcome some of the limitations of aPDT for the treatment of drug-resistant infectious diseases. The innovative SH-aPDT system offers a promising approach to treat wounds infected by medically relevant MDR bacteria by delivering 1O2 directly into the target while minimizing contact with surrounding tissues. Future studies will be aimed at assessing the potential of SH-aPDT for in vivo (animal model) applications. The SH membrane is made of medical-grade PDMS, thereby ensuring safety and facilitating acceptance. The present work is similar to our previous report on the use of VP for the treatment of the Wistar rat periodontal disease model.17 This PS is a component of an FDA-approved treatment of age-related macular degeneration.42 Notably, the quantity of VP applied to the SH membrane is <0.1% of the FDA-approved dose for intravenous use for age-related macular degeneration,43 indicating a safe and low-risk treatment method for patients.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsabm.4c00733.
Details on SH and light controls on colony-forming units, metabolic activity of Gram-positive and Gram-negative drug-resistant biofilms, and biofilm biomass reduction (PDF)
Author Contributions
Fernanda Viana Cabral: conceptualization, investigation, methodology, writing—original draft. QianFeng Xu: conceptualization, development of SH dressing, review and editing. Alexander Greer and Alan Lyons: conceptualization, data interpretation, drafting the manuscript, review and editing. Tayyaba Hasan: conceptualization, funding acquisition, supervision, writing—review and editing. All authors have given final approval for the version to be published.
This work was supported by the Department of Defense/Air Force Office of Scientific Research (FA9550-20-1-0063). F.V.C. also thanks her Thomas F. Deutsch Fellowship supported by The Optica Foundation and MGH. We also acknowledge support from the National Institute of Dental and Craniofacial Research (SBIR Phase-II 2R44DE026083-03).
The authors declare the following competing financial interest(s): QianFeng Xu, Alexander Greer, and Alan Lyons are inventors of patents issued to the City University of New York and licensed to SingletO2 Therapeutics LLC. Dr. Xu is Chief Scientist at SingletO2 Therapeutics, and Drs. Greer and Lyons are co-founders and co-owners of SingletO2 Therapeutics LLC. Drs. Cabral and Hasan report no conflicts of interest related to this study.
Supplementary Material
References
- Wagenlehner F. M. E.; Dittmar F. Re: Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Eur. Urol. 2022, 82, 658. 10.1016/j.eururo.2022.08.023. [DOI] [PubMed] [Google Scholar]
- Prestinaci F.; Pezzotti P.; Pantosti A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C. L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. P T 2015, 40, 277–283. [PMC free article] [PubMed] [Google Scholar]
- Ciofu O.; Moser C.; Jensen P. Ø.; Høiby N. Tolerance and Resistance of Microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. 10.1038/s41579-022-00682-4. [DOI] [PubMed] [Google Scholar]
- Percival S. L.; McCarty S. M.; Lipsky B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. 10.1089/wound.2014.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. R.; Hasan T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease?. Photochem. Photobiol. Sci. 2004, 3, 436–450. 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano A. P.; Demidova T. N.; Hamblin M. R. Mechanisms in Photodynamic Therapy: Part 1-Photosensitizers, Photochemistry and Cellular Localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinagreiro C. S.; Zangirolami A.; Schaberle F. A.; Nunes S. C. C.; Blanco K. C.; Inada N. M.; da Silva G. J.; Pais A. A. C.; Bagnato V. S.; Arnaut L. G.; Pereira M. M. Antibacterial Photodynamic Inactivation of Antibiotic-Resistant Bacteria and Biofilms with Nanomolar Photosensitizer Concentrations. ACS Infect. Dis. 2020, 6, 1517–1526. 10.1021/acsinfecdis.9b00379. [DOI] [PubMed] [Google Scholar]
- Tonon C. C.; Ashraf S.; Alburquerque J. Q.; de Souza Rastelli A. N.; Hasan T.; Lyons A. M.; Greer A. Antimicrobial Photodynamic Inactivation Using Topical and Superhydrophobic Sensitizer Techniques: A Perspective from Diffusion in Biofilms. Photochem. Photobiol. 2021, 97, 1266–1277. 10.1111/php.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza T. H.; Sarmento-Neto J. F.; Souza S. O.; Raposo B. L.; Silva B. P.; Borges C. P.; Santos B. S.; Cabral Filho P. E.; Rebouças J. S.; Fontes A. Advances on Antimicrobial Photodynamic Inactivation Mediated by Zn(II) Porphyrins. J. Photochem. Photobiol., C 2021, 49, 100454. 10.1016/j.jphotochemrev.2021.100454. [DOI] [Google Scholar]
- Scanone A. C.; Gsponer N. S.; Alvarez M. G.; Heredia D. A.; Durantini A. M.; Durantini E. N. Magnetic Nanoplatforms for in Situ Modification of Macromolecules: Synthesis, Characterization, and Photoinactivating Power of Cationic Nanoiman-Porphyrin Conjugates. ACS Appl. Bio Mater. 2020, 3, 5930–5940. 10.1021/acsabm.0c00625. [DOI] [PubMed] [Google Scholar]
- Heredia D. A.; Martínez S. R.; Durantini A. M.; Pérez M. E.; Mangione M. I.; Durantini J. E.; Gervaldo M. A.; Otero L. A.; Durantini E. N. Antimicrobial Photodynamic Polymeric Films Bearing Biscarbazol Triphenylamine End-Capped Dendrimeric Zn(II) Porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. 10.1021/acsami.9b09119. [DOI] [PubMed] [Google Scholar]
- Tegos G. P.; Masago K.; Aziz F.; Higginbotham A.; Stermitz F. R.; Hamblin M. R. Inhibitors of Bacterial Multidrug Efflux Pumps Potentiate Antimicrobial Photoinactivation. Antimicrob. Agents Chemother. 2008, 52, 3202–3209. 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas A.; Di Venosa G.; Hasan T.; Batlle A. l. Mechanisms of Resistance to Photodynamic Therapy. Curr. Med. Chem. 2011, 18, 2486–2515. 10.2174/092986711795843272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. R.; Dai T. Can Surgical Site Infections be Treated by Photodynamic Therapy?. Photodiagn. Photodyn. Ther. 2010, 7, 134–136. 10.1016/j.pdpdt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon C. C.; Ashraf S.; de Souza Rastelli A. N.; Ghosh G.; Hasan T.; Xu Q.; Greer A.; Lyons A. M. Evaluation of Photosensitizer-containing Superhydrophobic Surfaces for the Antibacterial Treatment of Periodontal Biofilms. J. Photochem. Photobiol., B 2022, 233, 112458. 10.1016/j.jphotobiol.2022.112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon C. C.; de Souza Rastelli A. N.; Bodahandi C.; Ghosh G.; Hasan T.; Xu Q.; Greer A.; Lyons A. M. Superhydrophobic Tipped Antimicrobial Photodynamic Therapy Device for the in vivo Treatment of Periodontitis Using a Wistar Rat Model. ACS Appl. Mater. Interfaces 2023, 15, 50083–50094. 10.1021/acsami.3c12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebisher D.; Bartusik-Aebisher D.; Belh S.; Ghosh G.; Durantini A. M.; Liu Y.; Xu Q.; Lyons A. M.; Greer A. Superhydrophobic Surfaces as a Source of Airborne Singlet Oxygen Through Free Space for Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 2370–2377. 10.1021/acsabm.0c00114. [DOI] [PubMed] [Google Scholar]
- Durantini A. M.; Greer A. Interparticle Delivery and Detection of Volatile Singlet Oxygen at Air/Solid Interfaces. Environ. Sci. Technol. 2021, 55, 3559–3567. 10.1021/acs.est.0c07922. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Tonon C. C.; Hasan T. Dramatic Destruction of Methicillin-resistant Staphylococcus aureus Infections with a Simple Combination of Amoxicillin and Light-activated Methylene Blue. J. Photochem. Photobiol., B 2022, 235, 112563. 10.1016/j.jphotobiol.2022.112563. [DOI] [PubMed] [Google Scholar]
- Baptista M. S.; Cadet J.; Greer A.; Thomas A. H. Practical Aspects in the Study of Biological Photosensitization Including Reaction Mechanisms and Product Analyses: A Do’s and Don’ts Guide. Photochem. Photobiol. 2023, 99, 313–334. 10.1111/php.13774. [DOI] [PubMed] [Google Scholar]
- Kimani S.; Ghosh G.; Ghogare A.; Rudshteyn B.; Bartusik D.; Hasan T.; Greer A. Synthesis and Characterization of Mono-Di-and Tri-poly(ethylene glycol) Chlorin e6 Conjugates for the Photokilling of Human Ovarian Cancer Cells. J. Org. Chem. 2012, 77, 10638–10647. 10.1021/jo301889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabeen S.; Farag M.; Malek B.; Choudhury R.; Greer A. A Singlet Oxygen Priming Mechanism: Disentangling of Photooxidative and Downstream Dark Effects. J. Org. Chem. 2020, 85, 12505–12513. 10.1021/acs.joc.0c01712. [DOI] [PubMed] [Google Scholar]
- Tacconelli E.; Carrara E.; Savoldi A.; Harbarth S.; Mendelson M.; Monnet D. L.; Pulcini C.; Kahlmeter G.; Kluytmans J.; Carmeli Y.; Ouellette M.; Outterson K.; Patel J.; Cavaleri M.; Cox E. M.; Houchens C. R.; Grayson M. L.; Hansen P.; Singh N.; Theuretzbacher U.; Magrini N.; et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. 10.1016/s1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- Costerton J. W.; Stewart P. S.; Greenberg E. P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Bowler L. L.; Zhanel G. G.; Ball T. B.; Saward L. L. Mature Pseudomonas aeruginosa Biofilms Prevail Compared to Young Biofilms in the Presence of Ceftazidime. Antimicrob. Agents Chemother. 2012, 56, 4976–4979. 10.1128/AAC.00650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Thomsen T. R.; Winkler H.; Xu Y. Influence of Biofilm Growth Age, Media, Antibiotic Concentration and Exposure Time on Staphylococcus aureus and Pseudomonas aeruginosa biofilm removal in vitro. BMC Microbiol. 2020, 20, 264. 10.1186/s12866-020-01947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; Yang T.; Luo Y.; Li X.; Zhang X.; Tang J.; Ma X.; Wang Z. Efficacy of the Novel Oxazolidinone Compound FYL-67 for Preventing Biofilm Formation by Staphylococcus aureus. J. Antimicrob. Chemother. 2014, 69, 3011–3019. 10.1093/jac/dku240. [DOI] [PubMed] [Google Scholar]
- Beaudoin T.; Yau Y. C. W.; Stapleton P. J.; Gong Y.; Wang P. W.; Guttman D. S.; Waters V. Staphylococcus aureus Interaction with Pseudomonas aeruginosa Biofilm Enhances Tobramycin Resistance. npj Biofilms Microbiomes 2017, 3, 25. 10.1038/s41522-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebisher D.; Bartusik D.; Liu Y.; Zhao Y.; Barahman M.; Xu Q.; Lyons A. M.; Greer A. Superhydrophobic Photosensitizers. Mechanistic studies of 1O2 generation in the Plastron and Solid/liquid Droplet Interface. J. Am. Chem. Soc. 2013, 135, 18990–18998. 10.1021/ja410529q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Busch T.; Zhu T. C. Singlet Oxygen Dosimetry Modeling for Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2011, 8, 142. 10.1016/j.pdpdt.2011.03.063. [DOI] [Google Scholar]
- Dinache A.; Smarandache A.; Simon A.; Nastasa V.; Tozar T.; Pascu A.; Enescu M.; Khatyr A.; Sima F.; Pascu M. L.; Staicu A. Photosensitized Cleavage of Some Olefins as Potential Linkers to be Used in Drug Delivery. Appl. Surf. Sci. 2017, 417, 136–142. 10.1016/j.apsusc.2017.03.177. [DOI] [Google Scholar]
- Baptista M. S.; Cadet J.; Di Mascio P.; Ghogare A. A.; Greer A.; Hamblin M. R.; Lorente C.; Nunez S. C.; Ribeiro M. S.; Thomas A. H.; Vignoni M.; Yoshimura T. M. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. 10.1111/php.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti A. W.; Korytowski W. Cholesterol Peroxidation as a Special Type of Lipid Oxidation in Photodynamic Systems. Photochem. Photobiol. 2019, 95, 73–82. 10.1111/php.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacellar I. O. L.; Baptista M. S. Mechanisms of Photosensitized Lipid Oxidation and Membrane Permeabilization. ACS Omega 2019, 4, 21636–21646. 10.1021/acsomega.9b03244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S.; Martinez G. R.; Medeiros M. H.; Di Mascio P. Singlet Molecular Oxygen Generated by Biological Hydroperoxides. J. Photochem. Photobiol., B 2014, 139, 24–33. 10.1016/j.jphotobiol.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Kanofsky J. R. Singlet Oxygen Production from the Reactions of Alkylperoxy Radicals. Evidence from 1268-nm Chemiluminescence. J. Org. Chem. 1986, 51, 3386–3388. 10.1021/jo00367a032. [DOI] [Google Scholar]
- Belh S. J.; Ghosh G.; Greer A. Surface-Radical Mobility Test by Self-Sorted Recombination: Symmetrical Product upon Recombination (SPR). J. Phys. Chem. B 2021, 125, 4212–4220. 10.1021/acs.jpcb.1c01099. [DOI] [PubMed] [Google Scholar]
- Chang L.; An Q.; Duan L.; Feng K.; Zuo Z. Alkoxy Radicals See the Light: New Paradigms of Photochemical Synthesis. Chem. Rev. 2022, 122, 2429–2486. 10.1021/acs.chemrev.1c00256. [DOI] [PubMed] [Google Scholar]
- Orlando J. J.; Tyndall G. S.; Wallington T. J. The Atmospheric Chemistry of Alkoxy Radicals. Chem. Rev. 2003, 103, 4657–4690. 10.1021/cr020527p. [DOI] [PubMed] [Google Scholar]
- Di Mascio P.; Martinez G. R.; Miyamoto S.; Ronsein G. E.; Medeiros M. H. G.; Cadet J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. 10.1021/acs.chemrev.8b00554. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U.; Hasan T. Mechanisms of Action of Photodynamic Therapy with Verteporfin for the Treatment of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 195–214. 10.1016/S0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- Visudyne (Verteporfin); 400 Somerset Corporate Blvd: Bridgewater, NJ, last updated Sept 2020. https://www.bauschretinarx.com/visudyne/ecp/about/ (accessed online May 8, 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.