Abstract
ABSTRACT
Background
A recent clinical trial suggested aspirin is a viable alternative to enoxaparin for venous thromboembolism (VTE) prophylaxis in patients after orthopedic trauma. The initial impact of these findings on VTE prophylaxis prescribing is unknown. The study aimed to evaluate stated VTE prophylaxis prescribing patterns among clinicians who treat patients after orthopedic trauma.
Methods
For this clinical vignette survey, we recruited surgeons and advanced practice providers who prescribed VTE prophylaxis to patients with orthopedic trauma across 40 states. Clinicians were shown seven clinical vignettes describing hypothetical patients with orthopedic trauma based on their fracture type, treatment, VTE risk factors, additional injuries and health insurance status. We assessed the stated VTE prophylaxis medications prescribed in-hospital and at discharge, patient factors associated with changes in medication prescribing preferences and practice variation by specialty and provider training.
Results
Among the 287 respondents, the median age was 43 years (IQR, 38–50), and 154 (weighted average, 63%) were men. For in-hospital VTE prophylaxis, enoxaparin was prescribed in 83% of the presented scenarios, and aspirin was prescribed in 13% (p<0.001). At discharge, aspirin was prescribed more frequently than enoxaparin (50% vs 41%, p<0.001). Healthcare providers with an aspirin discharge preference were 12% more likely to switch to enoxaparin if the patient had additional VTE risk factors, such as obesity (95% CI 4% to 19%, p=0.005).
Conclusions
Despite new clinical evidence, in-hospital VTE prophylaxis prescribing practices for patients with orthopedic trauma remain consistent with those reported a decade ago. However, compared with historical data, clinicians have significantly increased their preference for aspirin for thromboprophylaxis at discharge—unless the patient has additional thromboembolic risk factors.
Level of evidence
5—expert opinion.
Keywords: orthopaedics, Venous thromboembolism, treatment outcome
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
The study found that despite the new evidence, enoxaparin remains the primary choice for in-hospital VTE prophylaxis in patients with orthopedic trauma. However, aspirin is now favored by many clinicians for discharge prescriptions but used less frequently in patients with additional VTE risk factors like obesity or history of VTE.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings suggest changes in VTE prophylaxis guidelines and hospital policies may be required for substantial shifts in VTE prescribing practice, especially for in-hospital prophylaxis.
Introduction
Venous thromboembolism (VTE) is a common, potentially fatal complication after orthopedic trauma.1,4 Current clinical guidelines recommend VTE prophylaxis with enoxaparin after most types of fractures.5,8 A decade ago, a survey of orthopedic trauma surgeons suggested enoxaparin was used for VTE prophylaxis in over 80% of patients indicated for prophylaxis.6 A survey of general trauma surgeons reported similarly high utilization of enoxaparin.9
In January 2023, a clinical trial of over 12 000 patients with orthopedic trauma suggested aspirin is a viable alternative to enoxaparin for thromboprophylaxis.10 The conclusion was based on non-inferiority in all-cause mortality and similar rates of pulmonary embolism, proximal deep vein thrombosis and bleeding events. Additional research supports that the oral administration of aspirin is strongly preferred by patients and likely improves adherence compared with enoxaparin’s subcutaneous injections.11,14
In light of this new evidence, we conducted a clinical vignette survey with healthcare providers to evaluate how VTE prophylaxis prescribing patterns have changed relative to prior reports. We examined the association of patient and provider characteristics with prescribing practices and whether those factors influence healthcare providers’ confidence that their prescribing patterns are supported by the best available evidence. In addition, we assessed healthcare providers’ awareness of recent large clinical trials on the topic.10 15
Methods
Study participants
To complete this study, we recruited orthopedic surgeons, general surgeons, nurse practitioners and physician assistants who commonly prescribe VTE prophylaxis for patients with orthopedic trauma. To identify potential respondents, we emailed all members of our research consortium and former clinical fellows, asking them and their colleagues at their hospitals to respond. A follow-up reminder email was sent to these individuals 2 weeks after the initial request. We also searched the websites of all 213 level I trauma centers in the US and their academic affiliations and sent survey requests to the publicly available email addresses of surgeons and advanced practice providers in the orthopedic trauma and general trauma services. Participation in the survey was voluntary and respondents consented to participate by completing and submitting their survey responses. This study was deemed exempt by the institutional review board.
Clinical vignettes
We created seven clinical vignettes describing patients with fractures who would have met the eligibility criteria for the landmark trial.10 The vignettes were verified by two orthopedic trauma surgeons involved in the aforementioned trial’s design.10 The variation in VTE prophylaxis considerations was represented by the fracture location, operative or non-operative treatment, weight-bearing protocol, health insurance status, presence of a solid organ injury (ie, spleen) and other VTE risk factors, including previous VTE and obesity.6 9 16 17 The composition of VTE prophylaxis considerations included in each clinical vignette was determined using the Choice Design platform in JMP Pro V.17 (SAS Institute, Cary, North Carolina). The platform aims to maximize orthogonality (ie, independence) among the vignette’s components while minimizing the respondent’s cognitive burden through design efficiency.
Study design
The survey was conducted using Qualtrics (Provo, UT). The survey landing page provided a brief overview of the study objectives and the principal investigator’s email address, should any concerns arise. For each clinical vignette, the respondents indicated their in-hospital and discharge VTE prophylaxis medication (if any), the discharge prescription duration and their confidence that this prophylactic regiment was consistent with the best available evidence on a scale from 0 to 10. After completing the clinical vignettes, we asked respondents several questions regarding their demographics and scope of clinical practice. Finally, we presented brief descriptions of two recent clinical trials comparing aspirin versus enoxaparin in patients treated for orthopedic trauma (Prevention of Clots in Orthopaedic Trauma [PREVENT CLOT] trial) and patients who underwent hip or knee arthroplasty (CRISTAL trial),10 15 and assessed respondents’ awareness of these trials and their impact on respondents’ clinical practice.
Study outcomes
The primary outcome was the stated VTE medication prescribed in-hospital and at discharge. The secondary outcomes included the stated use of VTE prophylaxis in-hospital and discharge, the duration of VTE prophylaxis prescriptions at discharge, confidence that prescriptions were consistent with the best available evidence and awareness of recent clinical trials.
Statistical analysis
We calculated that 270 survey respondents would provide reliable estimates of VTE prophylaxis medication types with a margin of error of less than 6% and an alpha of 5%.
To derive sampling weights for the analysis, we drew a sample of 100 eligible providers and used publicly available sources to determine the distribution of sex, years in practice, surgeon versus advanced practice provider and orthopedic versus general trauma service. All results were weighted based on this distribution.
We described the respondent’s characteristics using medians with IQRs for continuous data and counts with percentages for categorical data. We calculated 95% CIs for the frequency of each medication option using bootstrap resampling. The association of patient factors with VTE prophylaxis administration and discharge prophylaxis duration was assessed using weighted mixed effects linear probability models. We estimated the association of patient factors with medication choice and prescriber preferences using weighted linear probability models. Finally, we calculated the association of patient factors and provider factors with provider confidence and trial awareness using weighted mixed effects linear probability models. We considered a p value less than 0.05 to be statistically significant. We did not adjust the widths of the CIs for multiple testing. The statistical analyses were performed using R V.4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Respondent demographics
The survey was distributed from 11 September 2023, through 15 December 2023. We approached 1598 healthcare providers who prescribed VTE prophylaxis to patients with orthopedic trauma. Of these, 287 providers from 40 US states responded to the survey (online supplemental appendix figure 1). The median age of the respondents was 43 years (IQR, 38–50). Of those who provided their demographic information, 63% (n=154) were men, and 34% (n=55) were women (table 1). Most respondents were surgeons (n=190), with less than 10% identifying as advanced practice providers. The median time in practice was 8 years (IQR, 4–14). The most common sources of new clinical evidence were from peer-reviewed journals (n=188, 66%), academic conferences (n=187, 65%) and peer or word of mouth (n=140, 49%). The median time to complete the survey was 6 min (IQR, 4−8).
Table 1. Respondent characteristics (n=287).
| Characteristic | Unweighted | Weighted |
| Age, years, median (IQR) | 42 (38, 48) | 43 (38, 50) |
| Gender | ||
| Male | 154 (54%) | 63% |
| Female | 55 (20%) | 34% |
| Prefer not to say | 6 (2%) | 3% |
| Other | 1 (0.5%) | 0.3% |
| Not reported | 71 (25%) | – |
| Provider type | ||
| Orthopedic surgeon | 120 (42%) | 36% |
| Trauma surgeon | 70 (23%) | 41% |
| Nurse practitioner | 10 (3%) | 9% |
| Physician assistant | 8 (3%) | 7% |
| Other | 9 (2%) | 7% |
| Not reported | 70 (24%) | – |
| Scope of practice | ||
| Orthopedic trauma | 120 (42%) | 40% |
| All patients with trauma | 85 (30%) | 54% |
| All patients with orthopedic | 9 (3%) | 3% |
| Other | 3 (1%) | 3% |
| Not reported | 70 (24%) | – |
| Years in practice, median (IQR) | 8 (4, 14) | 9 (5, 17) |
| Sources of new clinical evidence | ||
| Peer-reviewed journals | 188 (66%) | – |
| Academic conferences | 187 (65%) | – |
| Peers (word of mouth) | 140 (49%) | – |
| Professional society communications | 64 (22%) | – |
| Online evidence summaries | 37 (13%) | – |
| X (Twitter) | 31 (11%) | – |
| Podcasts | 24 (8%) | – |
| Webinars | 14 (5%) | – |
| Online discussion forums | 12 (4%) | – |
| Industry representatives | 4 (1%) | – |
Decision to prescribe VTE prophylaxis
Based on the completed patient vignettes, we estimated a 98% probability (95% CI 95% to 100%) that clinicians would prescribe in-patient VTE prophylaxis for patients with similar characteristics. The decision to prescribe VTE prophylaxis was significantly influenced by the type of fracture, with a lower probability of in-patient VTE prophylaxis for patients with operative proximal humerus fractures (difference, −10%; 95% CI −13% to −8%; p<0.001) and operative ankle fractures (difference, −4%; 95% CI −6% to −2%; p<0.001) compared with non-operative pelvis fractures (table 2). Uninsured patients were 2% less likely to receive in-patient VTE prophylaxis (95% CI −5% to 0%, p=0.04). The presence of a solid organ injury or additional VTE risk factors, such as obesity or VTE history, did not change the probability of in-patient prophylaxis.
Table 2. Patient factors that influence the decision to prescribe venous thromboembolism prophylaxis in-hospital and at discharge.
| Patient factor | In-hospital prophylaxis | Discharge prophylaxis | ||||
| Absolute percentage difference | 95% CI | P value | Absolute percentage difference | 95% CI | P value | |
| Intercept | 97.6% | 94.6% to 100% | <0.001 | 81.2% | 75.6% to 86.8% | <0.001 |
| Fracture | ||||||
| Nonoperative pelvis | Ref (0.0%) | Ref (0.0%) | ||||
| Operative ankle | −4.0% | −6.4% to −1.7% | <0.001 | −10.0% | −14.5% to −5.4% | <0.001 |
| Operative hip | −1.3% | −5.4% to 2.7% | 0.52 | 6.6% | −1.4% to 14.5% | 0.10 |
| Operative proximal humerus | −10.3% | −12.6% to −7.9% | <0.001 | −53.7% | −58.3% to −49.2% | <0.001 |
| Solid organ injury | ||||||
| No | Ref (0.0%) | Ref (0.0%) | ||||
| Yes | 1.4% | −1.0% to 43.7% | 0.26 | −0.4% | −4.9% to 4.1% | 0.86 |
| Additional VTE risk factors | ||||||
| No | Ref (0.0%) | Ref (0.0%) | ||||
| Yes | 1.5% | −0.8% to 3.8% | 0.21 | 11.9% | 7.4% to 16.4% | <0.001 |
| Health insurance status | ||||||
| Employer-based | Ref (0.0%) | Ref (0.0%) | ||||
| Uninsured | −2.4% | −4.8% to −0.1% | 0.04 | −1.2% | −5.7% to 3.4% | 0.62 |
The values presented are based on the weighted sample.
VTEvenous thromboembolism
We estimated an 81% probability (95% CI 76% to 87%) that patients with characteristics similar to those described in the vignettes would be prescribed VTE prophylaxis at discharge (table 2). Patients with an operative proximal humerus fracture (difference, −54%; 95% CI −58% to −49%; p<0.001) and an operative ankle fracture (difference, −10%; 95% CI −15% to −5%; p<0.001) were significantly less likely than patients with non-operative pelvis fractures to be prescribed VTE prophylaxis at discharge. Having additional VTE risk factors, like obesity and a VTE history, increased the probability of VTE prophylaxis at discharge by 12% (95% CI 7% to 16%, p<0.001). A solid organ injury or differences in health insurance status did not significantly change the probability of being discharged on VTE prophylaxis.
In-hospital VTE prophylaxis medication preferences
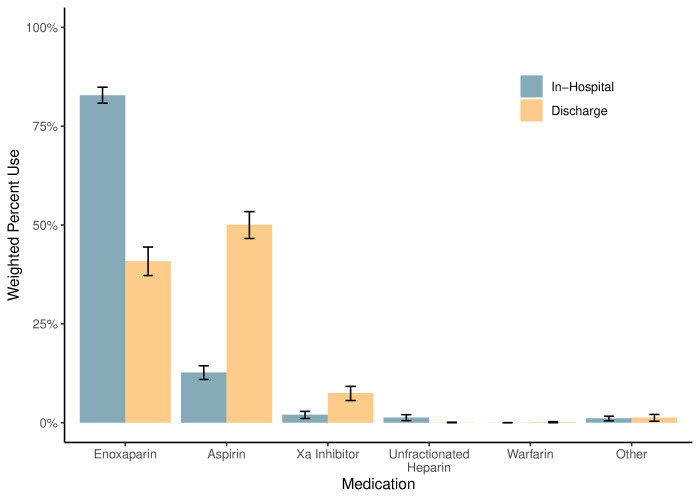
The respondents selected enoxaparin for in-hospital VTE prophylaxis in 83% (95% CI 81% to 85%) of the weighted sample (figure 1). In contrast, respondents selected aspirin for in-hospital VTE prophylaxis in 13% (95% CI 11% to 14%, p<0.001) of the weighted sample. Orthopedic providers were 24% more likely (95% CI 20% to 28%, p<0.001) to prescribe aspirin in-hospital than healthcare providers on the general trauma service (online supplemental appendix table 1).
Figure 1. Venous thromboembolism prophylaxis medications prescribed in-hospital and discharge. The weighted frequency of medications used for venous thromboembolism prophylaxis in the clinical vignettes.
Regardless of an enoxaparin or aspirin preference, we estimated healthcare providers were prescribed medications consistent with their preference 95% of the time (table 3). Providers with an enoxaparin preference increased their likelihood of an enoxaparin prescription by 5% if the patient had a solid organ injury (95% CI, 2% to 8%, p=0.004). Patients with a VTE history
Table 3. Patient factors that influence prescribers to deviate from their preferred in-hospital venous thromboembolism prophylaxis.
| Patient factor | Enoxaparin in-hospital prophylaxis prescribers | Aspirin in-hospital prophylaxis prescribers | ||||
| Absolute percentage difference | 95% CI | P value | Absolute percentage difference | 95% CI | P value | |
| Intercept | 95.2% | 91.9% to 98.5% | <0.001 | 95.2% | 84.1% to 100% | <0.001 |
| Fracture | ||||||
| Nonoperative pelvis | Ref (0.0%) | Ref (0.0%) | ||||
| Operative ankle | −4.3% | −7.5% to −1.0% | 0.01 | 4.0% | −7.2% to 15.3% | 0.48 |
| Operative hip | −2.7% | −8.3% to 3.0% | 0.36 | −2.5% | −22.0% to 17.1% | 0.80 |
| Operative proximal humerus | −2.2% | −5.5% to 1.1% | 0.18 | 9.6% | −2.1% to 21.4% | 0.11 |
| Solid organ injury | ||||||
| No | Ref (0.0%) | Ref (0.0%) | ||||
| Yes | 4.9% | 1.6% to 8.2% | 0.004 | −5.6% | −17.5% to 6.3% | 0.36 |
| Additional VTE risk factors | ||||||
| No | Ref (0.0%) | Ref (0.0%) | ||||
| Yes | 1.8% | −1.4% to 5.1% | 0.27 | −18.9% | −30.6% to −7.1% | 0.002 |
| Health insurance status | ||||||
| Employer-based | Ref (0.0%) | Ref (0.0%) | ||||
| Uninsured | −3.4% | −6.6% to −0.1% | 0.04 | 0.8% | −10.5% to 12.0% | 0.89 |
The values presented are based on the weighted sample.
VTEvenous thromboembolism
were 19% (95% CI −31% to −7%, p=0.002) less likely to be prescribed aspirin by healthcare providers who demonstrated a strong aspirin preference for in-hospital thromboprophylaxis.
Discharge VTE prophylaxis medication preferences
Aspirin was the preferred discharge VTE prophylaxis for 50% of the weighted sample (95% CI 47% to 53%) (figure 1). In comparison, enoxaparin was the preferred discharge prophylaxis for 41% of the weighted sample (95% CI 37% to 44%). Orthopedic providers were 38% more likely (32% to 44%, p<0.001) to prescribe aspirin at discharge than general trauma providers (online supplemental appendix table 2). Surgeons were 9% more likely (1% to 18%, p=0.03) to prescribe aspirin at discharge than advanced practice providers.
Providers who preferred enoxaparin VTE prophylaxis at discharge were influenced by the fracture location and the patient’s health insurance status (table 4). Specifically, an operative fracture of the proximal humerus (difference, −26%; 95% CI −42% to −10%; p=0.002) or ankle (difference, −21%; 95% CI −31% to −11%; p<0.001) increased the probability that a healthcare provider with an enoxaparin preference would prescribe aspirin at discharge relative to patients with a non-operative pelvis fracture. Similarly, uninsured patients were 13% more likely to be discharged on aspirin than insured patients (95% CI −23% to −4%, p=0.007) by a healthcare provider with an enoxaparin preference. Healthcare providers with an aspirin discharge preference were 12% more likely to prescribe enoxaparin if the patient had additional VTE risk factors (95% CI 4% to 19%, p=0.005).
Table 4. Patient factors that influence prescribers to deviate from their preferred discharge venous thromboembolism prophylaxis.
| Patient factor | Enoxaparin discharge prophylaxis prescribers | Aspirin discharge prophylaxis prescribers | ||||
| Absolute percentage difference | 95% CI | P value | Absolute percentage difference | 95% CI | P value | |
| Intercept | 94.4% | 84.5% to 100% | <0.001 | 89.2% | 82.8% to 95.6% | <0.001 |
| Fracture | ||||||
| Nonoperative pelvis | Ref (0.0%) | Ref (0.0%) | ||||
| Operative ankle | −20.6% | −30.1% to −11.1% | <0.001 | 6.0% | −0.6% to 12.7% | 0.07 |
| Operative hip | 1.0% | −19.8% to 21.8% | 0.93 | 3.0% | −9.2% to 15.1% | 0.63 |
| Operative proximal humerus | −25.6% | −41.6% to −9.7% | 0.002 | 12.3% | 4.4% to 20.2% | 0.002 |
| Solid organ injury | ||||||
| No | Ref (0.0%) | Ref (0.0%) | ||||
| Yes | −3.7% | −19.8% to 12.3% | 0.65 | −6.3% | −14.5% to 1.9% | 0.13 |
| Additional VTE risk factors | ||||||
| No | Ref (0.0%) | Ref (0.0%) | ||||
| Yes | 12.4% | −3.5% to 28.4% | 0.13 | −11.5% | −19.4% to −3.6% | 0.005 |
| Health insurance status | ||||||
| Employer-based | Ref (0.0%) | Ref (0.0%) | ||||
| Uninsured | −13.2% | −22.7% to −3.7% | 0.007 | 4.8% | −1.9% to 11.4% | 0.16 |
The values presented are based on the weighted sample.
VTEvenous thromboembolism
The weighted median VTE prophylaxis discharge prescription duration was 30 days, regardless of an enoxaparin (IQR, 28 to 30) or aspirin (IQR, 28 to 42) healthcare provider preference. Healthcare providers reduced the prescription length by 6 days (95% CI −8 to −5, p<0.001) for patients with operative proximal humerus fractures or by 3 days (95% CI −4 to −2, p<0.001) for operative ankle fractures, relative to non-operative pelvis fractures (online supplemental appendix table 3). On average, uninsured patients were prescribed an extra day of thromboprophylaxis (95% CI 0 to 2, p=0.01).
Awareness of recent VTE prophylaxis trials
Among the weighted sample, 81% were aware of the PREVENT CLOT trial, 94% thought the trial was relevant to their clinical practice, and 47% indicated they would change their VTE prophylaxis practice based on the trial. In contrast, 25% of the weighted sample were aware of the CRISTAL trial, 69% thought the trial was relevant to their clinical practice, and 30% indicated they would change their thromboprophylaxis practice based on the trial.
PREVENT CLOT trial awareness was 29% higher (95% CI 24% to 34%, p<0.001) among surgeons than among advanced practice providers and 18% higher (95% CI 14% to 22%, p<0.001) among healthcare providers working on orthopedic services than general trauma services (online supplemental appendix table 4). Awareness of the CRISTAL trial was 22% higher (95% CI 16% to 28%, p<0.001) among surgeons than advanced practice providers and also associated with a longer time in clinical practice (8% per year; 95% CI 4% to 12%; p<0.001).
Awareness of the PREVENT CLOT trial significantly increased aspirin VTE prophylaxis in-hospital by 20% (95% CI 8% to 32%, p=0.001) and at discharge by 32% (95% CI 17% to 47%, p<0.001). Awareness of the CRISTAL trial did not change the use of aspirin for in-hospital thromboprophylaxis (difference, 6%; 95% CI −4% to 16%; p=0.21) but was associated with a 15% increase in aspirin use at discharge (95% CI 3% to 27%, p=0.01).
Prescriber confidence
On average, healthcare providers rated their confidence level as 5.2 out of 10 (95% CI 4.2 to 6.2) that their VTE prophylaxis regimens are consistent with the best available evidence (online supplemental appendix table 5). Among the patient characteristics, provider confidence was 0.4 points lower when prescribing prophylaxis for patients with operative proximal humerus fractures (95% CI −0.7 to −0.2, p=0.002). Provider confidence increased by 1.1 points when prescribing aspirin instead of enoxaparin for in-hospital VTE prophylaxis (95% CI 0.8 to 1.4, p<0.001). Also, surgeons indicated more confidence than advanced practice providers (difference, 1.0; 95% CI 0.1 to 1.9; p=0.03). Awareness of the PREVENT CLOT or CRISTAL trial did not significantly change provider confidence.
Discussion
The findings suggest that enoxaparin remains the primary choice for VTE prophylaxis in patients after orthopedic trauma. However, aspirin is now favored by clinicians for VTE prophylaxis prescriptions at discharge. Interestingly, a significant proportion of healthcare providers who favor aspirin for VTE prophylaxis will prescribe enoxaparin to patients with additional VTE risk factors. Healthcare providers on orthopedic services are more likely to prescribe aspirin for VTE prophylaxis. Prescribing aspirin for in-patient VTE prophylaxis was associated with increased confidence that the practice followed the best available evidence.
Recent studies present a compelling rationale for aspirin as a leading choice for VTE prophylaxis in this patient population, both in-hospital and at discharge. Patients strongly prefer aspirin’s oral administration to enoxaparin’s subcutaneous injections.11 12 The oral administration has also been linked to a reduced risk of non-compliance.13 14 In addition, a large clinical trial demonstrated aspirin’s non-inferiority to enoxaparin for all-cause mortality and similar rates of pulmonary embolism, proximal deep vein thrombosis, and bleeding events with aspirin or enoxaparin.10 The only benefit demonstrated with enoxaparin was a 0.6% absolute decrease in the rate of distal deep vein thrombosis, which is of questionable clinical significance. For patients undergoing hip or knee arthroplasty, aspirin is strongly recommended as the primary method for VTE prophylaxis and the most commonly prescribed VTE prophylaxis medication by arthroplasty surgeons.18 19 However, the results of this clinical vignette survey highlight persuasive resistance to VTE prophylaxis with aspirin in patients after orthopedic trauma.
Healthcare providers’ skepticism of aspirin’s efficacy in preventing VTE was demonstrated in the survey responses. Specifically, the presence of other VTE risk factors increased the probability of enoxaparin prescriptions. More recent secondary analyses of the primary trial results confirm similar VTE prophylaxis effects of aspirin and enoxaparin, even in patients with a high baseline risk of VTE.20 However, we expect most respondents were unaware of these secondary findings of the trial when completing the survey. The increased prescriber confidence among those who routinely use aspirin for in-hospital VTE prophylaxis suggests this practice might become more widespread with increased dissemination of the risk-stratified results.
The substantially lower cost of aspirin addresses health equity concerns evident in our data. Respondents were less likely to prescribe any in-hospital VTE prophylaxis to uninsured patients and, specifically, less likely to prescribe enoxaparin in-hospital and at discharge. Although the lower rate of enoxaparin prescription at discharge makes intuitive sense for uninsured patients because of a much higher medication cost, there is no reason for the differential prescription rates for inpatients, suggesting an unconscious bias. Given the lower costs and ease of administration, some healthcare providers have indicated they now prescribe aspirin for 6 months after injury to increase the duration of VTE prevention.
Several possible explanations exist for why aspirin has not gained more traction for in-patient VTE prophylaxis. Some respondents indicated that they personally would prefer to prescribe aspirin for VTE prophylaxis, but their hospital policy dictates enoxaparin. Hospital policies typically lag changes in clinical practice guidelines, and the current guidelines have not yet adapted to new clinical trial evidence. Clinicians might also not be ready to change ingrained practices; the routine use of enoxaparin for VTE prophylaxis in trauma has been suggested by evidence-based guidelines since 2002.7 In addition, hospitals might have a financial incentive through programs like Medicare Part B to use new and more expensive drugs.21 While those parameters no longer apply to enoxaparin, the culture created by these regulations might explain some hospitals’ resistance to switching to aspirin for in-patient VTE prophylaxis.
Research on practice change in medicine highlights common barriers related to the organizational, social and professional contexts.22 23 Typically, multiple high-quality randomized controlled trials, meta-analyses or clinical practice guidelines from reputable organizations that transmit into hospital policy are often required to overcome many of these barriers to practice change.24 25 Evidence from the recent PREVENT CLOT and CRISTAL trials will eventually be incorporated into meta-analyses and clinical practice guidelines. However, these key steps had not occurred before the survey. While the evidence on this topic might not change 2 to 3 years from now, provider’s awareness, familiarity and attitudes toward the VTE prophylaxis option might change substantially through this diffusion of knowledge, altering how they respond to this survey.
This study had many strengths. The discrete choice modeling architecture behind the clinical vignettes creates immense design efficiencies. The respondents represent a broad sample of the target population from 40 US states. We weighted the respondents based on our target population of inference to improve the generalizability of the results. However, the weighting did not qualitatively change the findings, suggesting innate target population representability.
Despite those strengths, we acknowledge several limitations. First, the survey was conducted on a web-based interface and measured stated practices. Decisions in the clinical environment might differ. Second, we only distributed the survey to healthcare providers in the USA, and the results might not be generalizable to those practicing in other countries. Third, our clinical vignettes described elevated VTE risk with obesity and the patient’s VTE history as examples. However, clinicians might weigh VTE history and obesity differently in their VTE prophylaxis decisions. Fourth, suggesting aspirin as an outpatient option might have biased respondents who have historically discharged patients without VTE prophylaxis, thus overestimating its use. Fifth, we only described medication types in the survey and did not present dosing options. Understanding how weight-based or anti-Xa dose adjustments affect VTE prophylaxis decisions was outside the scope of this study.26 Finally, we assume those willing to respond to a survey are more likely to be aware of the latest evidence. While we weighted the responses based on the target population distributions, some degree of respondent bias likely remains.
Conclusions
Despite new clinical evidence, the proportion of healthcare providers prescribing enoxaparin for in-hospital VTE prophylaxis remains unchanged from the past decade. The findings also suggest half of the providers now commonly prescribe aspirin for VTE prophylaxis at discharge. However, there is hesitancy to prescribe aspirin for patients with additional VTE risk factors, such as obesity or a history of VTE. Most providers reported an awareness of the new clinical evidence, but substantial shifts in practice might require changes in VTE clinical practice guidelines and hospital policies.
supplementary material
Footnotes
Funding: The study was funded by the Patient-Centered Outcome Research Institute (DI-2022C3-29701). The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Provenance and peer review: Not commissioned; internally peer-reviewed.
Patient consent for publication: Not applicable.
Data availability free text: Deidentified data will be made available upon reasonable request to the corresponding author, Dr. Nathan O’Hara (nohara@som.umaryland.edu).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Contributor Information
Nathan N O'Hara, Email: nohara@som.umaryland.edu.
Deborah M Stein, Email: dstein@som.umaryland.edu.
Elliott R Haut, Email: ehaut1@jhmi.edu.
Stephen Breazeale, Email: stbreazeale@gmail.com.
Katherine P Frey, Email: kparris1@jhu.edu.
Gerard P Slobogean, Email: gslobogean@som.umaryland.edu.
Reza Firoozabadi, Email: rezaf2@uw.edu.
Renan Castillo, Email: rcastil1@jhu.edu.
Robert V O'Toole, Email: ROtoole@som.umaryland.edu.
Data availability statement
Data are available upon reasonable request.
References
- 1.Barrera LM, Perel P, Ker K, Cirocchi R, Farinella E, Morales Uribe CH. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013;2013:CD008303. doi: 10.1002/14651858.CD008303.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–6. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 3.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240:490–6. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haut ER, Chang DC, Pierce CA, Colantuoni E, Efron DT, Haider AH, Cornwell EE, 3rd, Pronovost PJ. Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors-an analysis of the National Trauma Data Bank (NTDB) J Trauma. 2009;66:994–9. doi: 10.1097/TA.0b013e3181991adc. [DOI] [PubMed] [Google Scholar]
- 5.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW., Jr Prevention of VTE in Orthopedic Surgery Patients. Chest. 2012;141:e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagi HC, Ahn J, Ciesla D, Collinge C, Molina C, Obremskey WT, Guillamondegui O, Tornetta P, Orthopaedic Trauma Association Evidence Based Quality Value and Safety Committee Venous Thromboembolism Prophylaxis in Orthopaedic Trauma Patients: A Survey of OTA Member Practice Patterns and OTA Expert Panel Recommendations. J Orthop Trauma. 2015;29:e355–62. doi: 10.1097/BOT.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 7.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice Management Guidelines for the Prevention of Venous Thromboembolism in Trauma Patients: The EAST Practice Management Guidelines Work Group. The Journal of Trauma: Injury, Infection, and Critical Care. 2002;53:142–64. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DA, Kahn SR, Rahman M, Rajasekhar A, Rogers FB, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3:3898–944. doi: 10.1182/bloodadvances.2019000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander KM, Butts CC, Lee Y-LL, Kutcher ME, Polite N, Haut ER, Spain D, Berndtson AE, Costantini TW, Simmons JD. Survey of venous thromboembolism prophylaxis in trauma patients: current prescribing practices and concordance with clinical practice guidelines. Trauma Surg Acute Care Open . 2023;8:e001070. doi: 10.1136/tsaco-2022-001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major Extremity Trauma Research Consortium (METRC) Aspirin or Low-Molecular-Weight Heparin for Thromboprophylaxis after a Fracture. N Engl J Med. 2023;388:203–13. doi: 10.1056/NEJMoa2205973. [DOI] [PubMed] [Google Scholar]
- 11.Haac BE, O’Hara NN, Mullins CD, Stein DM, Manson TT, Johal H, Castillo R, O’Toole RV, Slobogean GP. Patient preferences for venous thromboembolism prophylaxis after injury: a discrete choice experiment. BMJ Open. 2017;7:e016676. doi: 10.1136/bmjopen-2017-016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popoola VO, Tavakoli F, Lau BD, Lankiewicz M, Ross P, Kraus P, Shaffer D, Hobson DB, Aboagye JK, Farrow NA, et al. Exploring the impact of route of administration on medication acceptance in hospitalized patients: Implications for venous thromboembolism prevention. Thromb Res. 2017;160:109–13. doi: 10.1016/j.thromres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Haac BE, Van Besien R, O’Hara NN, Slobogean GP, Manson TT, O’Toole RV, Johal H, Berger PZ, Reahl GB, Marinos D, et al. Post-discharge adherence with venous thromboembolism prophylaxis after orthopedic trauma: Results from a randomized controlled trial of aspirin versus low molecular weight heparin. J Trauma Acute Care Surg. 2018;84:564–74. doi: 10.1097/TA.0000000000001771. [DOI] [PubMed] [Google Scholar]
- 14.Popoola VO, Lau BD, Tan E, Shaffer DL, Kraus PS, Farrow NE, Hobson DB, Aboagye JK, Streiff MB, Haut ER. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75:392–7. doi: 10.2146/ajhp161057. [DOI] [PubMed] [Google Scholar]
- 15.CRISTAL Study Group. Sidhu VS, Kelly T-L, Pratt N, Graves SE, Buchbinder R, Adie S, Cashman K, Ackerman I, Bastiras D, et al. Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial. JAMA. 2022;328:719–27. doi: 10.1001/jama.2022.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dashe J, Parisien RL, Pina M, De Giacomo AF, Tornetta P., 3rd Is the Caprini Score Predictive of Venothromboembolism Events in Orthopaedic Fracture Patients? J Orthop Trauma. 2019;33:269–75. doi: 10.1097/BOT.0000000000001451. [DOI] [PubMed] [Google Scholar]
- 17.Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17 Suppl 3:304–12. [PubMed] [Google Scholar]
- 18.Hip TI-V, Delegates K. Recommendations from the ICM-VTE: Hip & Knee. J Bone Joint Surg Am. 2022;104:180–231. doi: 10.2106/JBJS.21.01529. [DOI] [PubMed] [Google Scholar]
- 19.Dubin JA, Bains SS, Hameed D, Remily EA, Moore MC, Mont MA, Nace J, Delanois RE. Trends in Deep Vein Thrombosis Prophylaxis After Total Knee Arthroplasty: 2016 to 2021. J Arthroplasty. 2024;39:S328–32. doi: 10.1016/j.arth.2024.01.050. [DOI] [PubMed] [Google Scholar]
- 20.O’Hara NN, O’Toole RV, Frey KP, Castillo RC, Cuschieri J, Haut ER, Slobogean GP, Firoozabadi R, Christmas AB, Obremskey WT, et al. Risk-stratified thromboprophylaxis effects of aspirin versus low-molecular-weight heparin in orthopedic trauma patients: A secondary analysis of the PREVENT CLOT trial. J Trauma Acute Care Surg. 2024;96:573–82. doi: 10.1097/TA.0000000000004226. [DOI] [PubMed] [Google Scholar]
- 21.US Government Accountability Office Medicare Part B: Payment and use for selected new, high-cost drugs. [21-Jan-2024]. https://www.gao.gov/assets/gao-21-252.pdf Available. Accessed.
- 22.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–30. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 23.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute of Medicine (US)Committee on Standards for Developing Trustworthy Clinical Practice Guidelines . “Clinical practice guidelines we can trust.”. Washington (DC): National Academies Press (US); 2011. [Google Scholar]
- 26.Teichman AL, Cotton BA, Byrne J, Dhillon NK, Berndtson AE, Price MA, Johns TJ, Ley EJ, Costantini T, Haut ER. Approaches for optimizing venous thromboembolism prevention in injured patients: Findings from the consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg. 2023;94:469–78. doi: 10.1097/TA.0000000000003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.