Abstract
Human herpesvirus 8 (HHV-8) (also known as Kaposi's sarcoma-associated herpesvirus) encodes a novel noncoding polyadenylated nuclear (PAN) RNA (also known as T1.1 or nut-1) during the early phase of lytic replication. PAN RNA is the most abundant transcript of HHV-8, comprising 80% of total poly(A)-selected transcripts in HHV-8-infected cells during lytic replication. We directly measured the abundance of PAN RNA by visualizing 1.1- to 1.2- kb PAN RNA in an ethidium bromide-stained gel from poly(A)-selected RNA. We further pursued the mechanisms by which PAN RNA expression is induced to such high levels. rta, an immediate-early gene of HHV-8, is a transactivator that is sufficient and necessary to activate lytic gene expression in latently infected cells. Ectopic expression of Rta was previously shown to induce PAN RNA expression from the endogenous viral genome and activate the PAN promoter in a reporter system. Here, we have identified the Rta-responsive element (RRE) in the PAN promoter. Deletion analysis revealed that the RRE is present in a region between nucleotides −69 and −38 of the PAN promoter. A promoter construct containing the 69 nucleotides upstream of the transcription start site of the PAN promoter was activated by Rta in the absence or presence of the HHV-8 genome. Rta activated the PAN promoter up to 7,000-fold in 293T cells and 2,000-fold in B cells. Electrophoretic mobility shift assays demonstrated that Rta formed a highly stable complex with the RRE of the PAN promoter. Our study suggests that Rta can induce PAN RNA expression by direct binding of Rta to the RRE of the PAN promoter. This study has highlighted an important mechanism controlling PAN RNA expression and also provides a model system for investigating how Rta transactivates gene expression during lytic replication.
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma (KS)-associated herpesvirus, is a member of the gammaherpesvirus subfamily, which also includes herpesvirus saimiri, murine gammaherpesvirus 68 (MHV-68) and Epstein-Barr virus (EBV) (5, 29, 35). Gammaherpesviruses have been shown to be associated with tumor formation in infected hosts. HHV-8 is commonly found in all types of KS, including AIDS-associated KS, classic KS, endemic forms of KS, and renal transplant-related KS (1, 2, 14, 16, 25, 28, 32, 40, 43). HHV-8 is also associated with primary effusion lymphoma (PEL) (4) and multicentric Castleman's disease (38). While most tumor cells are latently infected with HHV-8, in a small number of cells in tumor lesions, the virus undergoes lytic replication (8, 33, 39, 40, 43, 51). Recent data have demonstrated the expression of viral macrophage inflammatory proteins and viral interleukin-6 in a subset of tumor cells (3, 40, 43). In these cells, while undergoing lytic replication, the virus expresses viral cytokines, which may play an important role in the pathogenesis of HHV-8 (9, 26), for example, by creating a favorable environment for supporting growth of latently infected neighboring cells.
Studies of HHV-8 gene expression have revealed a novel transcript, polyadenylated nuclear (PAN) RNA (also referred as T1.1 or nut-1) (41, 51). PAN RNA was initially identified in KS tumor tissue (51), as well as in PEL cells latently infected with HHV-8 (41). The expression of PAN RNA in PEL cells was induced by chemicals. More than 80% of cDNA clones from induced BC-1 cells represented a single transcript, PAN RNA, indicating the abundance of PAN RNA during lytic replication. PAN RNA is the most abundant viral transcript of HHV-8. Its copy number per cell has been estimated by a Northern analysis to be up to 2.5 × 105 to 5.0 × 105 copies in PEL cells (41) and 10,000 to 25,000 copies in KS tumor cells (41, 51). PAN RNA is expressed with early kinetics, and high levels of expression are maintained until late stages of lytic replication (43).
PAN RNA is unique in that it contains features of both mRNAs and small nuclear RNAs (snRNAs). In common with mRNA, PAN RNA has a canonical poly(A) signal and is polyadenylated. PAN RNA is also transcribed by RNA polymerase II, based on its sensitivity to α-amanitin (5 μg/ml), an inhibitor of RNA polymerase II (41). However, similar to snRNAs, PAN RNA appears to be a noncoding transcript, as deduced from the following (41). First, 57 stop codons are distributed randomly in all three reading frames throughout the transcript, making the longest possible open reading frame (ORF) of 61 amino acids. Second, this ORF is located in a region where codon usage is not typical of that most commonly found in eukaryotes. In addition, PAN RNA was not associated with ribosomes, according to sucrose gradient sedimentation. Instead, PAN RNA was found by fluorescence in situ hybridization to be in the nuclear speckles (41) and is known to form a ribonucleoprotein complex in the nucleoplasm (41, 50). Although it has been speculated that PAN RNA is involved in RNA processing in the nucleus, its function has yet to be defined. However, elucidating the mechanism controlling the expression of PAN RNA, the most abundant transcript of HHV-8, may help us understand how the virus maximizes its expression during lytic replication.
Upon reactivation from latency, viral lytic genes of HHV-8 (immediate-early, early, and late genes) are expressed in a highly regulated manner. rta, an immediate-early gene, is conserved among all gammaherpesviruses, including EBV (23), herpesvirus saimiri (45), bovine herpesvirus 4 (44), and MHV-68 (20, 48), and plays a central role in the switch of the viral life cycle from latency to lytic replication. Rta acts as a transcriptional activator, and its expression has been demonstrated to be sufficient for viral reactivation, since ectopic expression of Rta has been found to reactivate the latent HHV-8 genome to lytic replication in PEL cell lines (22, 42). In addition, a dominant-negative form of Rta was shown to suppress viral reactivation, indicating that Rta is necessary for the switch of the viral life cycle (21).
It has been recently reported that in MHV-68, a gamma-2 herpesvirus closely related to HHV-8, Rta alone is sufficient to disrupt latency and activate viral lytic replication, indicating a conserved role of Rta in the reactivation of gamma-2 herpesviruses (48). Two immediate-early genes of EBV (a gamma-1 herpesvirus), ZEBRA (also referred to as BZLF1, Zta, or Z) and rta, play essential roles in disruption of viral latency, activating downstream genes in a cooperative fashion (6, 7, 31, 49). However, there is no evidence that K-bZIP (known as K8), the ZEBRA homologue of HHV-8, can function as a switch gene for reactivation of HHV-8 (12, 19, 42, 52). The mechanism by which Rta controls lytic gene expression of gammaherpesviruses is currently being investigated.
Consistent with the facts that PAN RNA is an early lytic gene transcript of HHV-8 and that Rta is a potent transactivator of early genes, Rta has been shown to induce the expression of PAN RNA from the endogenous viral genome (42) and activate the PAN promoter linked to a heterologous gene (22). Promoters of other early genes of HHV-8 are also responsive to Rta but are expressed at lower levels than PAN RNA. Therefore, it is intriguing how HHV-8 produces PAN RNA at such high levels. Here, we address this mechanism by measuring the abundance of PAN RNA, mapping the Rta-responsive element (RRE) in the PAN promoter and demonstrating the direct binding of Rta to this RRE.
MATERIALS AND METHODS
Cell culture.
All cells were cultured at 37°C in the presence of 5% CO2. BC-1 cells (kindly provided by Yuan Chang, Columbia University, New York, N.Y.) were dually infected with HHV-8 and EBV, and BCBL-1 cells (obtained from the AIDS Research and Reagent Reference Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health) were infected with HHV-8 alone. These cells were derived from patients with PEL cells. BC-1 and BCBL-1 cells were maintained in RPMI 1640 medium supplemented with fetal bovine serum (FBS) (10% for BC-1 and 15% for BCBL-1) and antibiotics (penicillin [50 U/ml] and streptomycin [50 μg/ml]). DG75 cells (kindly provided by Samuel H. Speck, Washington University, St. Louis, Mo.) were grown in RPMI 1640 containing 10% FBS. COS-1 (an African green monkey kidney cell line transfected with simian virus 40 [SV40] T antigen), 293T (a human embryonic fibroblast cell line transfected with the E1 region of adenovirus and the SV40 T antigen), and 293 cells were cultured in Dulbecco's modified Eagle's medium containing 10% FBS.
Plasmid construction.
The PAN RNA expression plasmid pPAN/A contains 1,136 bp of PAN RNA sequences as well as its 2,974-bp upstream sequence (nucleotides [nt] 25693 to 29803, according to the HHV-8 genomic sequence) (35) in pBluescript II KS(−) (Stratagene, La Jolla, Calif.). The insert was amplified from total genomic DNA prepared from BC-1 cells, using primers pan1a/HindIII (5′-tcctagaagcttCTGTGCACCCAAGTGGT-3′) and pan2/EcoRI (5′-gtacatgaattCCACACCCCCATCCCACA-3′). In all sequences, the underlined nucleotides represent restriction enzyme sites for cloning the PCR products, and the uppercase letters represent viral sequences. pPAN/B, -C, and -D were cloned with fragments from restriction digestions of the pPAN/A with XhoI, NarI/ClaI and AccI, respectively. The inserts for pPAN/E through pPAN/I were prepared by PCR with genomic DNA from BC-1 cells, using a common primer, pan2/EcoRI, and a unique primer for each clone as follows: pan6/KpnI (5′-tagttaggtaccGCAGCTTGGCTACTCTG-3′) for pPAN/E, pan8b/KpnI (5′-cattgaggtaccAGGGTCAGCTTGAAGGATG-3′) for pPAN/F, pan9/KpnI (5′-atgttaggtacCATGGGTGGCTAACCTGTC-3′) for pPAN/G, pan9b/KpnI (5′-cacttaggtaccACTGGAGATAAAAGGGGCCAG-3′) for pPAN/H, and pan10/KpnI (5′-catttaggtaccAGTTTAGCACTGGGACTGCCC-3′) for pPAN/I. Sizes of the promoters in the constructs are indicated in the figures.
The luciferase reporter construct pLUC/−1241 contains the PAN promoter region spanning bp −1241 to +14 (nt 27426 to 28680). The promoter region was amplified from total genomic DNA from BC-1 cells with primers pan3/SacI (5′-tatgaagagctcTGGAGGTGCCAAGTTCGC-3′) and pan4/NheI (5′-tagatagctagcTGGGCAGTCCCAGTGCTAAAC-3′) and cloned into the pGL3-basic vector (Promega, Madison, Wis.) with SacI and NheI cloning sites. pLUC/−470, pLUC/−261, and pLUC/−200 were cloned with fragments from restriction digestions of pLUC/1241 with AccI, BstEII, and AseI, respectively. The inserts for pLUC/−122 to pLUC/−8 were amplified from BC-1 total genomic DNA, using a common primer, pan4/NheI, and a specific primer for each construct as follows: pan8b/KpnI for pLUC/−122, pan9/KpnI for pLUC/−69, pan9b/KpnI for pLUC/−38, and pan10/KpnI for pLUC/−8. Double-stranded oligonucleotides were used to clone the RRE (nt −69 to −38) or the RRE-L (nt −78 to −30) into the pGL3-promoter vector (Promega) containing the SV40 promoter sequence. pan7/BamHI_BglII (5′-cgggatccAAATGGGTGGCTAACCTGTCCAAAATATGGGAACagatcttcg-3′) was used for the pRRE constructs, and pan1/BamHI_BglII (pan1) (5′-cgggatccGCTTCCAAAATGGGTGGCTAACCTGTCCAAAATATGGGAACagatcttcg-3′) was used for the RRE-L constructs. The number and orientation of the inserts were verified by sequencing.
Induction of viral lytic replication.
BC-1 or BCBL-1 cells harboring the latent HHV-8 genome were resuspended in complete medium at a density of 106 cells/ml and induced with 3 mM sodium butyrate to reactivate viral lytic replication. After induction, cells were harvested for total RNA extraction at various time points.
Transfections.
For Northern analysis of PAN RNA expression in 293T cells, 3.5 × 105 cells were transfected with 0.25 μg of pcDNA3 (Invitrogen, Carlsbad, Calif.) or pcDNA3/Rta and 0.75 μg of a PAN RNA expression plasmid in six-well plates, using LipofectaminePlus (Gibco BRL, Grand Island, N.Y.) according to the manufacturer's instructions. For luciferase reporter assays, 1.25 × 105 293T cells were transfected with 40 ng of pcDNA3 or pcDNA3/Rta and 5 ng of a PAN promoter reporter plasmid into 24-well plates, using a calcium phosphate transfection method (47). For GAL4-VP16 transfection, 40 ng of a GAL4-VP16 expression plasmid and 5 ng of a reporter construct (pGAL4-M2-Luc) containing five GAL4 binding sites were used. 293 cells were transfected in the same manner as 293T cells except that 0.8 × 105 cells were used per well. Each transfection for reporter assays included 4 ng of pRLCMV (Promega), as well as 350 ng of a carrier DNA (plasmid DNA lacking any mammalian promoter/enhancer sequences), to control transfection efficiencies. At 48 h posttransfection, cells were washed with 1× phosphate-buffered saline (PBS) and subjected to RNA extraction or reporter assays. To test PAN promoter activity in B cells, 4 μg of pcDNA3/Rta and 0.5 μg of a series of luciferase reporter constructs were introduced by electroporation (960 μF, 240 V) with a Genepulser II (Bio-Rad, Hercules, Calif.) into 107 B cells in incomplete medium in the presence of 0.4 μg of pRLCMV. Electroporated cells were transferred into complete medium and harvested at 48 h postelectroporation.
RNA preparation and Northern analysis.
Total RNA was extracted from BC-1, BCBL-1, and 293T cells, using TriReagent (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer's instructions. One round of poly(A) selection was done using oligo(dT)-cellulose (type 3) (BioCoat, Bedford, Mass.). To calculate the copy number of PAN RNA, signals of an agarose gel stained with ethidium bromide were quantitated with ImageQuant Version 1.1 (Molecular Dynamics, Sunnyvale, Calif.). For Northern blotting, total RNA was treated with a mixture of 1 M glyoxal and 50% (vol/vol) dimethyl sulfoxide at 50°C for 30 min (47). Glyoxalated RNA was then separated on 1% agarose gels in circulating 10 mM sodium phosphate buffer (pH 6.8). The gel was vacuum transferred onto a nylon membrane (Amersham Pharmacia Biotech, Arlington Heights, Ill.) by using a vacuum blotter (Bio-Rad). The membrane was UV cross-linked and deglyoxalated at 80°C in 20 mM Tris-HCl (pH 8). Prehybridization and hybridization were carried out at 65°C in 500 mM potassium phosphate buffer (pH 6.8) containing 7% sodium dodecyl sulfate (SDS), 1% bovine serum albumin (BSA), and 1 mM EDTA. A probe for PAN RNA was labeled by a random priming method, using [α-32P]dCTP, as described previously (41). After hybridization, the membrane was washed with 40 mM sodium phosphate (pH 6.8) containing 5% SDS and 0.5% BSA, followed by washing with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.5% SDS. The membrane was then exposed on a phosphorimager screen. Quantitative analysis of signals was performed with a STORM imaging system (Molecular Dynamics). The same membrane was stripped at 80°C in 10 mM Tris-HCl (pH 8) containing 1% SDS and rehybridized with another probe for an internal loading control, such as human glyceraldehyde 3-phosphate dehydrogenase (G3PDH) mRNA or U1 RNA.
Primer extension.
Total RNA (10 μg per sample) was hybridized with a PAN-specific probe, pan199 (nt 28879 to 28895) or pan046 (nt 28726 to 28748). The hybridized mixtures then underwent primer extension at 42°C for 1 h, using 200 U of SuperScriptII (Gibco BRL) in the presence of Anti-RNase (Ambion, Austin, Tex). The extended products were run on an 8% sequencing gel with a set of sequencing reactions. The sequencing reactions were performed with the same probe as that used in the primer extension procedures, according to the manufacturer's instructions for version 2.0 of the T7 Sequenase DNA sequencing kit (Amersham Pharmacia).
Dual luciferase assay.
The dual luciferase reporter assay system (Promega) was used to test promoter activity. Transfected 293T and 293 cells in a 24-well plate were washed with 1× PBS and incubated with 200 μl of 1× passive lysis buffer provided by the manufacturer. B cells were resuspended in 200 μl of 1× passive lysis buffer after washing with PBS. Lysates were frozen, thawed once, and centrifuged at top speed in a microcentrifuge for 5 min. Supernatants of 293T cells were diluted to 1/100 to obtain a reading in a linear range and assayed using an Optocomp I Luminometer (MGM Instruments, Hamden, Calif.). The reporter assays were carried out according to manufacturer's protocol for the dual luciferase reporter assay system (Promega).
EMSAs.
End-labeled double-stranded oligonucleotides, pan1∗ to pan6∗ (asterisks indicate labeled oligonucleotides) spanning different regions of the PAN promoter with flanking sequences were incubated with purified FLAG-tagged Rta protein on ice for 30 min in binding buffer [10 mM Tris-HCl (pH 7.5), 7.5 mM MgCl2, 1 mM EDTA, 0.1 μg of poly(dI-dC), 5μg of BSA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 50 mM β-mercaptoethanol, 5% glycerol] with salt concentrations ranging from 60 to 300 mM KCl. Recombinant FLAG-tagged Rta was expressed in bacteria and affinity purified from bacterial sonicate as previously described (18). The binding mixture was loaded onto a 4.5% polyacrylamide gel in 1× TGE buffer (50 mM Tris, 1.9 M glycine, and 10 mM EDTA [pH 8.3]) in the presence of 50 mM β-mercaptoethanol. After being run at 130 V at 4°C, the gel was dried and autoradiographed. For the competition assay, excess (5- 10-, or 50-fold) unlabeled oligonucleotides (pan1 to pan6) or a negative control oligonucleotide containing unrelated sequences (NS) was mixed with a labeled oligonucleotide prior to the addition of protein. The sequences of the oligonucleotides used in electrophoretic mobility shift assays (EMSAs) were as follows: pan2, 5′-cgggatccAAATGGGTGGCTAACCTGTCCAAAATATGagatcttcg-3′; pan3, 5′-cgggatccAAATGGGTGGCTAACagatcttcg-3′; pan4, 5′-cgggatccTGGCTAACCTGTCCAAAATATGagatcttcg-3′; pan5, 5′-cgggatccGCTTCCAAAAATGGGgtgagatcttcg-3′; pan6, 5′-cgggatccgtTCCAAAATATGGGAACagatcttcg-3′; and NS 5′-cgagatcggggtgaggcatgggggatcccg-3′. The uppercase letters represent PAN promoter sequences.
RESULTS
Abundance of PAN RNA induced during lytic replication of HHV-8.
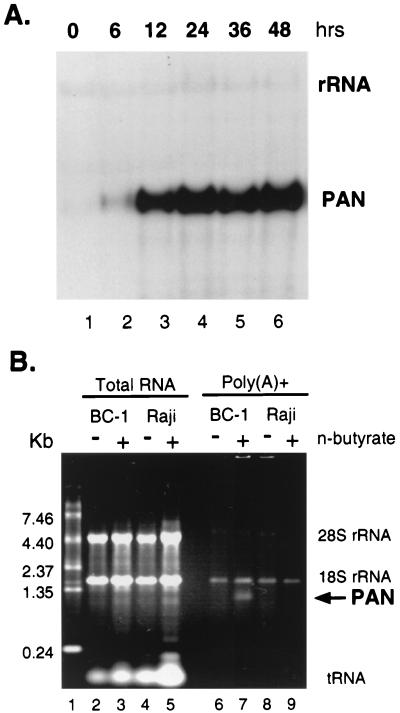
HHV-8 in latently infected PEL cell lines can undergo lytic replication upon induction with chemicals such as sodium butyrate or 12-O-tetradecanoylphorbol 13-acetate (27, 30, 34, 37). To confirm levels of PAN RNA expression during the lytic cycle, BC-1 cells latently infected with both EBV and HHV-8 were induced with sodium butyrate (3 mM), and total RNA was isolated at 0, 6, 12, 24, 36, and 48 h postinduction. Expression of PAN RNA was detected by Northern blotting using a PAN-specific probe (Fig. 1A). Without induction, PAN RNA was detected at limited levels (Fig. 1A, lane 1) representing spontaneous lytic replication in a small subset of cells. Upon induction, PAN RNA levels then increased rapidly, with peak expression at 24 h. This level was sustained until at least 48 h postinduction. This result is consistent with previously shown kinetics of PAN RNA expression (43).
FIG. 1.
Abundance of PAN RNA induced during lytic replication. (A) Kinetics of PAN RNA expression. At 0, 6, 12, 24, 36, and 48 h (lanes 1 to 6) after BC-1 cells were induced by sodium butyrate (3 mM), total RNA was extracted and analyzed by Northern blotting with a PAN RNA-specific probe. rRNA served as a loading control, which is more apparent in longer exposure. (B) Visualization of PAN RNA in an ethidium bromide-stained agarose gel. BC-1 (HHV-8- and EBV-positive) and Raji (HHV-8-negative and EBV-positive) cells were treated with sodium butyrate for 18 h. Total RNA was isolated from untreated (−) or treated (+) cells (lanes 2 to 5) and subjected to one round of poly(A) selection, using type 3 oligo(dT)-cellulose (lanes 6 to 9). Total RNA from 106 cells and poly(A)-selected fractions from 8 × 106 cells were run on an agarose gel and stained with ethidium bromide. The RNA ladder (2 μg) was run in lane 1. An arrow indicates a 1.2-kb polyadenylated transcript in lane 7.
To quantitate PAN RNA expression by agarose gel electrophoresis, BC-1 and Raji cells were incubated with and without sodium butyrate and harvested 18 h later; the 18-h period of induction was chosen as optimal, based on peak PAN RNA expression and cell viability. Raji cells are latently infected with EBV but not HHV-8 and therefore served as a negative control. Total RNA was extracted from cells and subjected to one round of poly(A) selection. We measured the amount of PAN RNA directly on an agarose gel stained with ethidium bromide (Fig. 1B). While residual rRNAs were detected in each sample, poly(A)-selected RNA from induced BC-1 cells presented a distinct band corresponding to the size of PAN RNA, 1.1 to 1.2 kb (Fig. 1B, lane 7). No other polyadenylated mRNAs were detected in these samples. Based on the previous result that more than 80% of total poly(A) RNA in induced BC-1 cells was PAN RNA (41), we concluded that this 1.1- to 1.2-kb band represented PAN RNA. Compared with the amount of the RNA ladder loaded (2 μg) in Fig. 1B, lane 1, the band in lane 7 was estimated to be 55 to 110 ng from 8 × 106 cells. When the recovery rates of poly(A) selection (30 to 50%) and the percentage of PEL cells undergoing lytic replication upon chemical induction (∼20%) were taken into account, the copy number of PAN RNA was calculated to be approximately 1.0 × 105 to 3.0 × 105 copies per cell, consistent with that previously shown in induced PEL cells by Northern analysis. Next, we addressed the mechanism by which PAN RNA is expressed at such high levels during lytic replication.
Rta activated PAN RNA expression in the absence of viral infection.
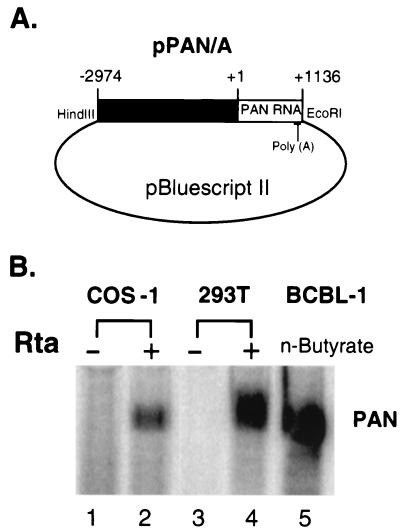
To study the regulation of PAN RNA expression, we constructed a PAN expression plasmid. This construct contains 1,136 bp of the PAN RNA sequences, as well as its 2,974-bp upstream sequence (nt 25693 to 29803), in pBluescript II (pPAN/A) (Fig. 2A). pPAN/A was cotransfected with an Rta expression plasmid (pcDNA3/Rta) or pcDNA3 vector into two HHV-8-negative cell lines, COS-1 and 293T. Total RNA was isolated at 48 h posttransfection, and PAN RNA expression was assayed by Northern blotting (Fig. 2B). PAN RNA was highly expressed in the presence of Rta in both COS-1 and 293T cells but barely detected in the absence of Rta in either cell line. The expression level of PAN RNA was higher in 293T cells than in COS-1 cells, possibly due to higher transfection efficiencies in 293T cells (data not shown). The extent of PAN RNA expression from 106 293T cells (Fig. 2B, lane 4) was comparable to that of 106 BCBL-1 cells harboring the latent HHV-8 genome induced by sodium butyrate for 24 h (Fig. 2B, lane 5) that were used as a positive control. This result shows that Rta is a potent activator of PAN RNA expression, suggesting that Rta is sufficient to mediate high levels of PAN RNA expression during lytic replication.
FIG. 2.
Rta activates PAN RNA expression in the absence of other viral proteins. (A) Diagram of PAN expression plasmid pPAN/A. The PAN gene, including the transcribed region (1,136 bp) with the poly(A) signal and its upstream sequences (2,974 bp), was cloned into pBluescript II. HindIII and EcoRI indicate the cloning sites in pPAN/A. (B) PAN RNA expression was activated by Rta in the absence of other viral factors. pPAN/A was cotransfected with pcDNA3 (−) or pcDNA3/Rta (+) into COS-1 and 293T cells. At 48 h posttransfection, total RNA from 106 COS-1 and 293T cells was isolated and subjected to Northern analysis (lanes 1 to 4). Total RNA from 3 × 106 BCBL-1 cells induced with sodium butyrate for 24 h served as a positive control (lane 5).
Determination of the transcription initiation site(s) of PAN RNA.
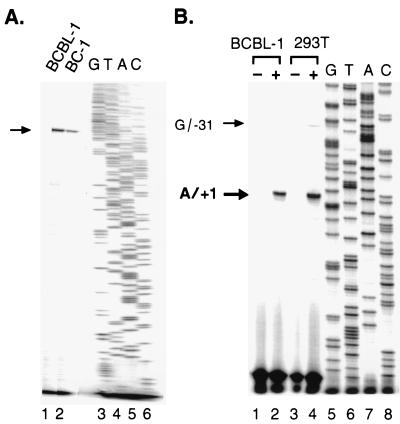
We noticed that the mobility of the transcript expressed from pPAN/A in COS-1 and 293T cells was lower than that from induced BCBL-1 cells (Fig. 2B). To investigate a possible discrepancy in the 5′ end of PAN RNA, primer extension of PAN RNA was performed. First, to identify the transcription initiation site (+1) of PAN RNA expressed from the viral genome, we used total RNA from two cell lines BCBL-1 and BC-1 induced with sodium butyrate. BCBL-1 is a PEL cell line latently infected with HHV-8 alone, while BC-1 is a cell line dually infected with HHV-8 and EBV. Total RNA was isolated and hybridized with a PAN-specific antisense oligonucleotide, pan199, spanning nt 28879 to 28894. Following primer extension, a single extended product was detected, using RNA from both BC-1 and BCBL-1 (Fig. 3A, lanes 1 and 2). To precisely map the transcription initiation site, we used another oligonucleotide, pan046, spanning a different region (nt 28726 to 28748) (Fig. 3B). Consistent with the result with pan199, a single primer extension product was detected with pan046 from induced (Fig. 3B, lane 2) but not uninduced BCBL-1 cells (lane 1). Based on the sequencing ladder run next to the samples, the transcription initiation site of PAN RNA was mapped to nt 28667 (A/+1).
FIG. 3.
Determination of transcription initiation site of PAN RNA. (A) Transcription initiation site of PAN RNA from the endogenous viral genome. Total RNA was isolated from BC-1 and BCBL-1 cells induced with sodium butyrate (3 mM) for 24 h and annealed with an end-labeled oligonucleotide (pan199∗) spanning nt 28879 to 28895. The primer extension products were resolved on a sequencing gel. The arrow indicates the extended products in lanes 1 and 2. A sequencing ladder generated by the same oligonucleotide (pan199∗) is shown in lanes 3 to 6. (B) Transcription initiation site of PAN RNA expressed in 293T cells. An end-labeled oligonucleotide (pan046∗) spanning nt 28726 to 28748 was used to fine map the 5′ end of PAN RNA in BCBL-1 cells and transfected 293T cells. The products from primer extension with RNAs from uninduced (−) and induced (+) BCBL-1 cells, as well as vector-transfected (−) and pPAN/A-transfected (+) 293T cells with pcDNA3/Rta, are shown in lanes 1 to 4. Arrows indicate extended products. A sequencing ladder generated by the same oligonucleotide (pan046∗) was run in lanes 5 to 8.
Next, we determined the transcription start site of PAN RNA transcribed from pPAN/A in 293T cells. When RNA from 293T cells cotransfected with pcDNA3/Rta plus pPAN/A was used for primer extension, two extended products were detected (Fig. 3B, lane 4). The major product indicated that the transcription of PAN RNA in 293T cells was primarily initiated at nt 28667 (A/+1), the same site as that in induced BCBL-1 cells (Fig. 3B, lanes 2 and 4). The minor product was 31 nt longer than the major one, indicating a minor transcription initiation site at G/−31. No primer extension product was generated with RNA from 293T cells cotransfected with pcDNA3/Rta plus pBluescript II (Fig. 3B, lane 3).
These results confirm that Rta plays a critical role in activation of PAN RNA expression and demonstrate that transcription of PAN RNA from the expression construct mainly starts at the same site as that from the endogenous viral genome. Therefore, the regulation of PAN RNA transcription by Rta can be studied using PAN RNA expression constructs in the absence of other viral gene products. The discrepancy in the length of PAN RNA between 293T cells and BCBL-1 cells is likely due to the different 3′ end of PAN RNA, i.e., a different length of the poly(A) tail.
Deletion analysis of the PAN gene to identify a transcription regulatory element(s) responsive to Rta.
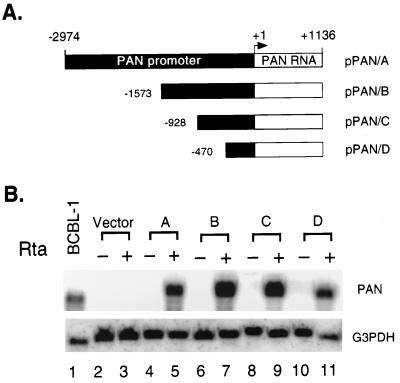
To define cis-acting DNA elements mediating the response to Rta in the PAN promoter, we made a series of 5′ deletion constructs. (Fig. 4A) The 5′ deletion constructs of the PAN promoter in pBluescript II were cotransfected into 293T cells with pcDNA3 or pcDNA3/Rta. Total RNA was extracted at 48 h posttransfection, and PAN RNA expression was measured by Northern blotting. Total RNA from induced BCBL-1 cells was used as a positive control (Fig. 4B, lane 1); an empty vector, pBluescript II, was also transfected in the absence or presence of Rta to confirm PAN-specific hybridization (Fig. 4B, lanes 2 and 3). Hybridization with G3PDH served as a loading control. Although the expression levels of PAN RNA varied, deletion of approximately 2.5 kb did not result in a dramatic loss of Rta induction. This suggests than an element(s) mediating the response to Rta is contained within the remaining 470 bp of the promoter sequence and the transcribed region of PAN RNA.
FIG. 4.
Deletion analysis of the PAN promoter in 293T cells. (A) Schematic diagram of 5′ deletions of the PAN promoter. The size of the PAN promoter sequences in each construct is indicated relative to the transcription initiation site (+1). (B) Northern analysis of 5′ deletions of the PAN promoter in 293T cells. pPAN/A to -D were transfected with pcDNA3 (−) or pcDNA3/Rta (+) into 293T cells (lanes 4 to 11). Northern analysis of total RNA isolated at 48 h posttransfection was carried out with the PAN probe (upper panel). Total RNA from induced BCBL-1 cells was loaded as a positive control (lane 1). The vector, pBluescript II, was also transfected in the absence and presence of pcDNA3/Rta to confirm PAN-specific hybridization (lanes 2 and 3). Hybridization with the G3PDH probe served as a loading control (lower panel).
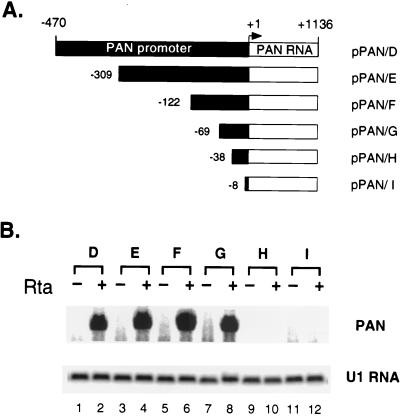
To further map the RRE within this 470 bp of the PAN promoter, we generated an additional series of 5′ deletion constructs (Fig. 5A). Transfection and Northern analysis of these deletion constructs were carried out as described previously. Deletion of the promoter down to nt −69 of the PAN promoter did not significantly reduce responsiveness to Rta (Fig. 5B, lanes 1 to 8). However, an additional 31-bp deletion from nt −69 to −38 of the PAN promoter abolished Rta responsiveness to almost background levels (Fig. 5B, lanes 9 and 10). A further deletion to nt −8 of the promoter also rendered the PAN promoter incapable of responding to Rta (Fig. 5B, lanes 11 and 12). Thus, the element mediating the Rta response is most likely to be present between nt −69 and −38 of the PAN promoter.
FIG. 5.
Localization of the RRE of the PAN promoter in 293T cells. (A) Schematic diagram of 5′ deletion constructs of the PAN promoter. The size of the PAN promoter sequences in each construct is indicated relative to the transcription initiation site (+1). (B) Northern analysis of PAN RNA expression from pPAN/D to I in 293T cells. Transfection of PAN expression plasmids with pcDNA3 (−) or pcDNA3/Rta (+) and Northern analysis were performed as described for Fig. 4. PAN-specific hybridization is shown in the upper panel. U1 RNA (lower panel) served as a loading control.
Analysis of the PAN promoter, using the luciferase reporter.
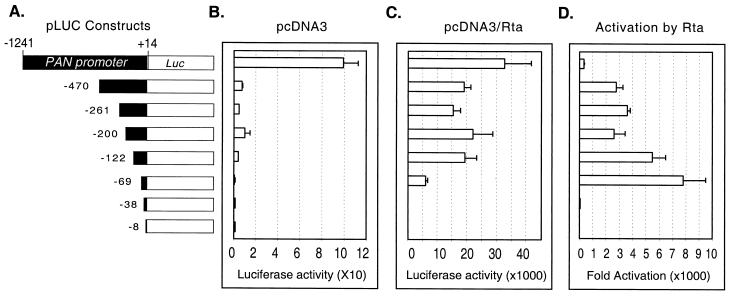
The PAN RNA transcribed sequence may have a cis-acting transcription regulatory element (10) or contain an internal promoter, similar to other noncoding transcripts (13). We sought to determine whether any cis elements within the PAN RNA sequence might play a role in PAN RNA expression. Reporter gene expression driven by the PAN promoter was analyzed to examine whether changing the transcribed sequence could alter the responsiveness to Rta, i.e., whether there are important regulatory elements within the transcribed region. We constructed a series of reporter plasmids (pLUC series) by inserting various regions of the PAN promoter from bp 1241 to 8 into the pGL3-basic plasmid (Promega) containing the firefly luciferase coding sequence (Fig. 6A). All of the reporter constructs contained the first 14 nt of the PAN RNA sequence. The pLUC reporter plasmids were cotransfected into 293T cells with either pcDNA3/Rta or pcDNA3. pRLCMV, which contains the coding sequence for Renilla luciferase under the control of a constitutively active human cytomegalovirus immediate-early enhancer/promoter, was included in each transfection and served as an internal control for transfection efficiency. At 48 h posttransfection, cell lysates were assayed for both firefly and Renilla luciferase activity. Fold activation was calculated by comparing the normalized firefly luciferase activity of pcDNA3/Rta-transfected cells to that of pcDNA3-transfected cells (Fig. 6). The highest level of luciferase activity in the absence and presence of Rta was obtained for pLUC/−1241, which contains the longest PAN promoter. To compare the strength of the PAN promoter, the pGL3-control vector (expressing firefly luciferase driven by SV40 enhancer/promoter sequences; Promega) was cotransfected with pcDNA3/Rta into 293T cells. The PAN promoter (pLUC/−1241) in the presence of Rta was approximately 30-fold stronger than the SV40 promoter/enhancer, one of the strongest known promoters, in the presence of the large T antigen and Rta (data not shown). With the sequential deletion of 5′ promoter sequences to nt −69, total luciferase activity was reduced (Fig. 6B and C). However, activation by Rta remained high, up to approximately 7,000-fold (Fig. 6D), suggesting that cellular transcriptional factors might be acting on sequences upstream of nt −69 of the PAN promoter. Fold activation of pLUC/−69 by Rta was higher than that of other constructs. This seems to be mainly due to an extremely low background level of pLUC/−69 in the absence of Rta. When the promoter was deleted to nt −38, there was a remarkable drop in fold activation by Rta. These results were consistent with those from 5′ deletion analysis of the PAN RNA expression constructs, indicating that the RRE of the PAN promoter is present between nt −69 and −38.
FIG. 6.
Analysis of the PAN promoter, using reporter assays in 293T cells. (A) Schematic representation of the PAN promoter sequences used in the reporter system. Various regions of the PAN promoter, including the first 14 bp of the PAN RNA sequence from the transcription initiation site, were cloned into a pGL3-basic vector to drive the expression of firefly luciferase as a reporter. The PAN promoters are drawn to scale. (B and C) Luciferase activities of reporters cotransfected with pcDNA3 (B) and pcDNA3/Rta (C) in 293T cells. The PAN promoter constructs shown in panel A were cotransfected into 293T cells with pcDNA3 or pcDNA3/Rta in the presence of a control vector, pRLCMV, that constitutively expresses Renilla luciferase driven by the cytomegalovirus immediate-early enhancer/promoter. At 48 h posttransfection, cells were harvested, and dual luciferase assays were performed. Firefly luciferase activity in pLUC constructs was normalized to the corresponding Renilla luciferase activity. The normalized luciferase activity from the smallest fragment of the promoter in the presence of pcDNA3 vector alone was set as 1 arbitrary unit to standardize luciferase activity for subsequent data analyses. The values represent averages of two transfections in triplicate, with the standard deviation shown. (D) Activation of the PAN promoters by Rta in 293T cells. Fold activation of the reporter by Rta was obtained by comparing the normalized firefly luciferase activity of pcDNA3/Rta-transfected cells to that of pcDNA3-transfected cells.
To precisely examine the role of cis-acting elements within the transcribed region of PAN RNA, we performed Northern analysis to compare transcript levels from a reporter construct with those of a PAN RNA expression construct. pLUC/−69, expressing firefly luciferase, or pPAN/G, expressing PAN RNA containing the same size of the PAN promoter, was cotransfected with pcDNA3/Rta into 293T cells. The luciferase transcript was expressed at a level comparable to that of PAN RNA (data not shown). Northern and reporter analyses demonstrated that the PAN promoter (nt −69 to +14) was able to drive the expression of heterologous mRNA to high levels.
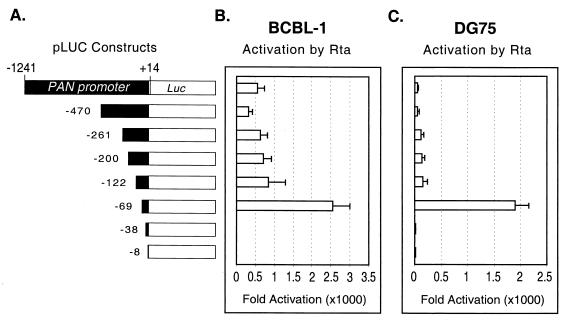
Although Rta can activate the PAN promoter in an epithelial cell line (293T) in the absence of the viral genome, we had not excluded the possibility that any other B-cell-specific or viral factors might play an additional role in the transcription regulation of PAN RNA. It was recently reported that another viral factor, ORF57 (also referred to M or Mta), might be involved in regulation of PAN RNA expression (17). To address this issue, we used two cell lines, DG75 (an HHV-8-negative B-cell line) and BCBL-1 (an HHV-8-positive PEL cell line). DG75 and BCBL-1 cells were electroporated with each reporter construct and either pcDNA3 or pcDNA3/Rta. The overall trend among the constructs remained similar in DG75 and BCBL-1 cells; i.e., fold activation drastically dropped upon the loss of 31 bp, from nt −69 to −38 (Fig. 7B and C), supporting previous data obtained for the epithelial cell line (293T). Although the fold activation of each construct varied in the absence and presence of the viral genome, pLUC/−69, which contains the RRE, manifested the highest fold activation in both DG75 and BCBL-1 cells, consistent with results for 293T cells. Therefore, Rta alone was able to direct high levels of gene expression from the PAN promoter, and the region responsible for mediating Rta response is contained in the region between nt −69 and −38, regardless of the cell line used or whether the viral genome was present.
FIG. 7.
Activation of the PAN promoter deletions in B cells. (A) Schematic representation of the PAN promoter sequences used in the reporter system shown in Fig. 6. The promoters are drawn to scale. (B and C) Activation of the PAN promoters by Rta in BCBL-1 (B) and DG75 (C) cells. Promoter constructs were electroporated into BCBL-1 and DG75 cells with pcDNA3 or pcDNA3/Rta, along with pRLCMV. At 48 h posttransfection, dual luciferase assays were performed, results were normalized, and fold activation was calculated as described for Fig. 6. The values represent averages of four transfections, with the standard deviation shown.
Direct binding of Rta to the PAN promoter.
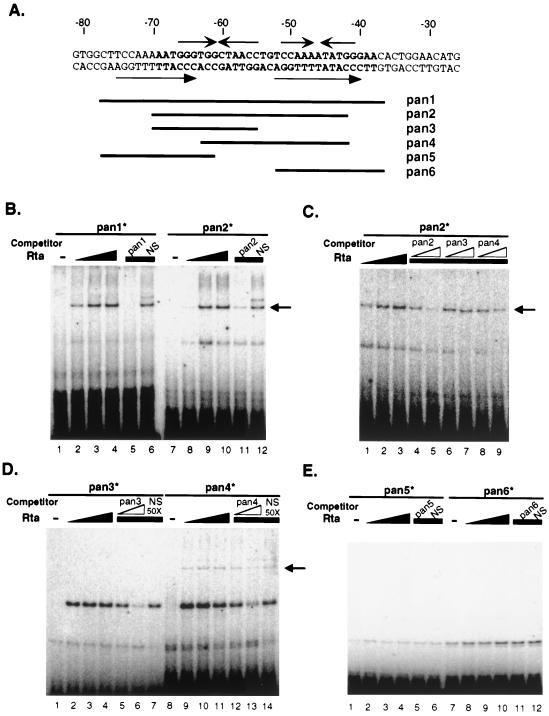
Rta of other gammaherpesviruses has been shown to activate transcription with or without directly binding to DNA (11, 24, 46). To determine whether activation of the PAN promoter is mediated by direct binding of Rta, EMSAs were performed. The labeled double-stranded oligonucleotide, pan1∗, spanning from nt −78 to −37 of the PAN promoter with flanking sequences, was used (Fig. 8A). Purified recombinant FLAG-tagged Rta protein was incubated with end-labeled pan1∗, and the binding mixtures were resolved on a native polyacrylamide gel. Rta-specific binding was detected, as indicated by the arrow in Fig. 8B. With increasing amounts of the Rta protein, the amount of Rta-pan1∗ complex increased (Fig. 8B, lanes 1 to 4 for pan1∗), demonstrating the direct binding of Rta to the PAN promoter in a dose-dependent manner. To narrow down the region responsible for Rta binding, a second labeled oligonucleotide, pan2∗ (nt −70 to −42) was used (Fig. 8B, lanes 7 to 10). The PAN promoter region from nt −70 to −42 was sufficient for specific binding by Rta. Thus, additional sequences adjacent to the RRE were not required for Rta binding. A competition assay was used to confirm the sequence specific binding of Rta to the PAN promoter. A 50-fold excess of an unlabeled oligonucleotide (pan1 or pan2) or the nonspecific oligonucleotide NS was incubated with binding mixtures. Introduction of a specific competitor to the binding mixtures significantly decreased Rta binding to the labeled oligonucleotide, pan1∗ or pan2∗ (Fig. 8B, lanes 5 and 11), while the nonspecific competitor did not (Fig. 8B, lanes 6 and 12). Furthermore, unlabeled pan2 abrogated Rta binding to pan2∗ in a dose-dependent manner (Fig. 8C, lanes 5 and 6; Fig. 9, lanes 5 to 7), while increased NS did not (Fig. 9, lanes 8 and 9). These results indicate that Rta directly binds to this region of the PAN promoter in a dose-dependent and sequence-specific fashion. Rta lacking the DNA binding domain could not activate the PAN promoter (data not shown), consistent with our hypothesis that direct binding of Rta to DNA is required for activation of the PAN promoter.
FIG. 8.
Rta binding to the RRE of the PAN promoter. (A) The sequences of the RRE in the PAN promoter. RRE sequences are in bold; flanking sequences are in italics. Virus sequences in the synthetic oligonucleotides used for EMSA (pan1 to pan6) are indicated under the PAN promoter sequences. Arrows indicate inverted repeats or direct repeats. (B) EMSA of the RRE in the PAN promoter with purified Rta protein. Purified recombinant FLAG-Rta protein was incubated with labeled double-stranded oligonucleotides, pan1∗ (lanes 1 to 6) and pan2∗ (lanes 7 to 12). Increasing amounts of Rta (0, 30, 60, and 90 ng) were incubated with pan1∗ and pan2∗, shown in lanes 1 to 4 and lanes 7 to 10, respectively. A 50-fold excess of unlabeled specific competitors (pan1 in lane 5; pan2 in lane 11) and a nonspecific competitor (NS; lanes 6 and 12) was incubated in the presence of purified FLAG-Rta (60 ng) for the competition assay. The arrow indicates the specific binding of Rta to DNA. (C) EMSA of pan2∗ in the absence and presence of different oligonucleotide competitors. Increasing amounts of Rta (30, 60, and 90 ng) were mixed with pan2∗ (lanes 1 to 3). Excess (5- and 50-fold) unlabeled oligonucleotides (pan2 in lanes 4 and 5; pan3 in lanes 6 to 7; pan4 in lanes 8 to 9) were incubated with pan2∗ in the presence of Rta protein (60 ng) for competition assays. The arrow indicates the specific binding of Rta to DNA. (D) EMSA of pan3∗ and pan4∗. Increasing amounts of Rta (0, 30, 60, and 90 ng) were incubated with labeled pan3∗ and pan4∗ (lanes 1 to 4 and lanes 8 to 11, respectively). For the competition assay, 5- and 50-fold excess specific competitor (pan3 in lanes 5 and 6; pan4 in lanes 12 and 13) and 50-fold-excess nonspecific competitor (NS; lanes 7 and 14) were used. The arrow indicates the specific binding of Rta to DNA. (E) EMSA of pan5∗ and pan6∗. Increasing amounts of Rta (0, 30, 60, and 90 ng) were incubated with pan5∗ and pan6∗, shown in lanes 1 to 4 and lanes 7 to 10, respectively. A 50-fold excess of unlabeled specific competitors (pan5 in lane 5; pan6 in lane 11) and a nonspecific competitor (NS; lanes 6 and 12) was incubated in the presence of purified FLAG-Rta (60 ng) for the competition assays.
FIG. 9.
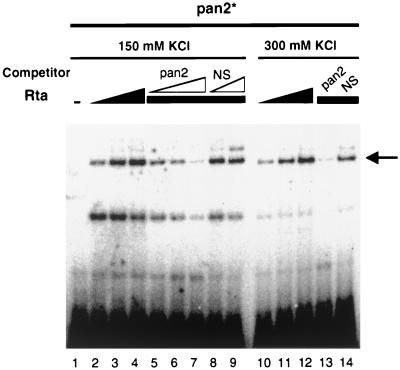
Rta forms a stable complex with the PAN promoter. Purified FLAG-Rta protein was incubated with a labeled double-stranded oligonucleotide, pan2∗. Increasing amounts of Rta (0, 30, 60, and 90 ng) were incubated with pan2∗ in the presence of 150 or 300 mM KCl. For competition assays, excess unlabeled pan2 (5-, 10-, and 50-fold; lanes 5 to 7) and unlabeled NS (5- and 50-fold; lanes 8 and 9) were mixed with pan2∗ prior to the addition of purified FLAG-Rta (60 ng) in the presence of 150 mM KCl. Another competition assay was performed with the specific competitor (50× pan2) and the nonspecific competitor (50× NS) in the presence of 300 mM KCl (lanes 13 and 14). The arrow indicates the specific binding of Rta to DNA.
Nevertheless, we noticed two shifted bands in the presence of Rta with varied intensities. The upper band represents the specific Rta binding to the PAN promoter, since its intensity was increased with increasing amounts of Rta and decreased in the presence of sequence-specific competitors but not in the presence of a nonspecific competitor (Fig. 8B and 9). The identity of the lower band is not known.
To further narrow down the Rta binding site, we used different oligonucleotides spanning different regions of the PAN promoter (Fig. 8A). Competition assays with unlabeled oligonucleotides, pan3 and pan4, containing a deletion of either end of the PAN promoter between nt −70 and −42, was carried out in the presence of pan2∗ and purified FLAG-Rta (Fig. 8C). EMSAs of Rta with the labeled oligonucleotides, pan3∗ and pan4∗, were also performed (Fig. 8D). pan3 contained a region from nt −70 to −55; pan4 contained a region from nt −63 to −42. Results from the competition assay with unlabeled pan3 and pan4 indicated that pan3 failed to inhibit Rta binding to the PAN promoter (Fig. 8C, lanes 6 and 7), whereas pan4 reduced it to a lesser extent than pan2 (Fig. 8C, lanes 8 and 9). Consistent with the results from the competition assays, EMSA of labeled pan3∗ did not show any specific Rta binding (Fig. 8D, lanes 1 to 7), but labeled pan4∗ showed weaker Rta binding to DNA than did labeled pan2∗ (Fig. 8D, lanes 8 to 14). These results showed that deletions at either end of the RRE affected Rta binding to the PAN promoter. In addition, labeled pan5∗ (from nt −78 to −61) and pan6∗ (from nt −52 to −37) resulted in no Rta-specific binding (Fig. 8E). Since the band shown in Fig. 8E (lanes 1 and 7) was also detected in the absence of Rta, it is likely to represent the Rta-DNA complex. Collectively, these results suggest that the region from nt −70 to −42 is required for Rta binding to the PAN promoter. Thus, Rta binds directly to DNA within the region highlighted as the RRE in functional assays.
To examine the stability of the Rta-PAN promoter complex, we tested the effects of different salt concentrations (60 to 300 mM) in binding buffers. Rta bound to pan2∗ in the presence of 150 and 300 mM KCl (Fig. 9). The ability of Rta to form a stable complex with the PAN promoter under highly stringent conditions (300 mM KCl) may contribute to the unusual strength of the PAN promoter.
Promoter-independent and specific activation of the RRE.
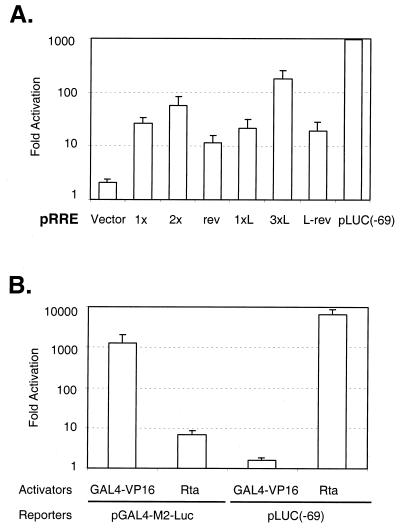
To determine the functionality of the Rta binding site, we fused a region from nt −69 to −38 (RRE) or from nt −78 to −36 (RRE-L) to a heterologous promoter such as SV40 promoter (Fig. 10A). The basal activity of the pGL3-promoter vector alone was high in 293T cells, due to the presence of the SV40 large T antigen in 293T cells and the SV40 origin in the vector (data not shown). Thus, 293 cells were used for the transfection of these constructs. The SV40 promoter backbone was minimally activated by Rta, but the RRE with the SV40 promoter was activated 25-fold in the presence of Rta (Fig. 10A, 1x and 1xL). Little difference was observed between pRRE and pRRE-L, consistent with EMSA results using pan1∗ and pan2∗. In addition, this activity was orientation independent (Fig. 10A, rev and L-rev). The activity of the promoter was enhanced when the RRE was multimerized (Fig. 10A, 2x and 3xL). These results demonstrate that the RRE mapped from the deletion analysis and shown to contain the Rta binding site is sufficient to confer Rta responsiveness to a heterologous promoter, suggesting that it act as an enhancer.
FIG. 10.
Promoter-independent and specific activation of the RRE. (A) The RRE of the PAN promoter confers Rta responsiveness to a heterologous promoter. A fragment containing nt −69 to −38 (RRE) or nt −78 to −36 (RRE-L) of the PAN promoter was cloned in both orientations into a pGL3-promoter vector containing the SV40 promoter. The number (1×, 2×, 1×L, and 3×L) and reverse orientation (rev and L-rev) of the RRE fragment are indicated below the columns. The RRE constructs were cotransfected into 293 cells with pcDNA3 or pcDNA3/Rta in the presence of pRLCMV for dual luciferase assays. At 48 h posttransfection, cells were harvested and activation fold was calculated as described for Fig. 6. A pGL3-promoter vector alone served as a negative control, and pLUC (−69) was used as a positive control. Note that fold activation is presented on a logarithmic scale. (B) Specificity of activation of the PAN promoter by Rta. pcDNA3/Rta (Rta) or a GAL4-VP16 expression construct (pGAL4-VP16) was transfected into 293T cells in the presence of pLUC(−69) or a reporter containing five copies of the GAL4 binding site (pGAL4-M2-Luc). At 48 h posttransfection, dual luciferase assays were performed, and fold activation was calculated as described for Fig. 6. The values represent averages of three transfections, with the standard deviation shown. Note that fold activation is presented on a logarithmic scale.
To test the specific activation of the PAN promoter by Rta, we used another potent transactivator, GAL4-VP16 (Fig. 10B). In the GAL4-VP16 system, the activation domain of VP16, a transcription activator of human herpes simplex virus, is fused to the DNA binding domain of GAL4, a yeast transcription activator (36). GAL4-VP16 strongly activates a reporter construct, pGAL4-M2-Luc that contains five GAL4 binding sites upstream of luciferase (15). Although GAL4-VP16 was highly active on its own promoter, it was unable to activate the PAN promoter by GAL4-VP16: activation of pGAL4-M2-Luc was up to 1,200-fold, and that of pLUC(−69) less than 2-fold (Fig. 10B). In contrast, Rta was able to highly activate the PAN promoter, but activation of pGAL4-M2-Luc by Rta was minimal. These results indicate that activation of the PAN promoter is highly specific to Rta.
DISCUSSION
Since PAN RNA is the most abundant transcript of HHV-8, study of the regulation of PAN RNA expression will give us insights into how the virus efficiently regulates its own gene expression during lytic replication. To understand the mechanisms controlling the regulation of PAN RNA expression, we determined the copy number of PAN RNA. Using deletion analyses of the PAN promoter, we found that Rta alone was able to direct high levels of PAN RNA expression. We located the RRE of PAN RNA expression in the 31-bp region (nt −69 to −38) of the PAN promoter. The identified transcription regulatory element of the PAN promoter was consistently mapped to the same region in 293T, DG75, and BCBL-1 cells. We showed that purified FLAG-tagged Rta bound to the region of the PAN promoter responsive to Rta in a dose-dependent and sequence-specific manner, which suggests that activation of the PAN promoter may be mediated through direct binding of Rta to DNA.
There was some inconsistency in the reported transcription start site(s) of PAN RNA from KS tumor tissue and the BC-1 cell line (51). Primer extension data obtained from RNA from KS tumor tissue showed multiple bands, but the signal from the longest product was not the strongest one (51). A shorter 5′ end of PAN RNA was reported, based on the cDNA clones obtained from induced BC-1 cells (41). We mapped the transcription start site of PAN RNA expressed from the viral genome of BC-1 and BCBL-1 cells at nt 28667 (A/+1), consistent with the longest primer extension product from KS tumor tissue. PAN RNA transcribed from the plasmid construct was also mainly initiated at the same site. There is a putative TATA binding protein recognition sequence (GATAAAA) at nt −31 relative to the transcription initiation site. A PAN RNA expression construct retaining 38 bp of the promoter (pPAN/H) contains this GATA box but lacks the transcription regulatory element responsive to Rta and fails to support PAN RNA expression in the presence of Rta, suggesting that PAN RNA expression is highly dependent on Rta expression.
We studied the regulation of PAN RNA expression using two different methods, Northern analysis of PAN RNA transcribed from the expression vectors in 293T cells and reporter assays of the PAN promoter linked to the firefly luciferase coding sequence. These two independent series of experiments showed a consistent result that the expression of PAN RNA was highly induced by Rta and the RRE was contained in the 31-bp region of the PAN promoter (nt −69 to −38).
Activation of the PAN promoter by Rta increased up to 7,000-fold as the deletion of the PAN promoter progressed. Since other cellular factors might activate PAN RNA expression via upstream sequences, progressive deletions of the PAN promoter likely decreased luciferase activity in the absence of Rta. This resulted in the highest fold activation of the minimal promoter (69 bp) responsive to Rta, although its absolute activity was lower than any other Rta-responsive reporter constructs. We checked the transcript levels by transfecting 293T cells with equal amounts of a PAN expression plasmid or a luciferase expression plasmid containing the same size of the PAN promoter without or with Rta. In Northern analysis of total RNA from transfected cells, we found that the transcript levels were similar for both PAN RNA and luciferase. Our preliminary data suggest that PAN RNA does not seem to be unusually more stable than the luciferase transcript (data not shown). This supports the hypothesis that the strong inducibility of the PAN promoter by Rta mainly accounts for high levels of PAN RNA during lytic replication.
Fold activation of the PAN promoter varied among cell lines used. Luciferase activity driven by the PAN promoter in the presence of Rta was higher in 293T cells than in B cells, possibly due to enhanced expression and amplification of plasmid pcDNA3/Rta by the T antigen. Although both B-cell lines have similar transfection efficiencies, luciferase activity was higher in BCBL-1 cells than in DG75 cells. Although a similar amount of Rta was expressed initially, more Rta might be present in BCBL-1 cells at the time of cell harvest, due to the ability of transfected Rta to induce expression of the rta gene in the viral genome (7a). However, regardless of the cell line used, the PAN promoter remains silent in the absence of Rta but acts as a strong promoter in the presence of Rta, representing the efficient induction of PAN RNA expression during viral reactivation, i.e., a tightly controlled program of lytic gene expression.
Studying the mechanisms controlling Rta activation of the PAN promoter may provide a better understanding of how Rta interacts with DNA, as well as with cellular factors or other viral factors during viral reactivation. Results from EMSAs, using purified recombinant Rta, demonstrated that the 31-bp region (nt −60 to −38) mapped by 5′ deletion analysis of the PAN promoter contained sequences able to bind to Rta. Sequence analysis of the nt −69 to −38 region of the PAN promoter has suggested that there may be inverted repeats as well as a direct repeat, which possibly serves as a putative Rta binding site(s), shown by arrows in Fig. 8A. Extensive mutagenesis is required to characterize cis DNA sequence requirements for Rta binding and activation of the PAN promoter. All of the data presented here support the hypothesis that PAN RNA is expressed at high levels due to the strong activation capacity of the PAN promoter responsive to Rta that binds to the RRE of the PAN promoter. It will serve as a model system to investigate the molecular interactions between Rta and its DNA binding site and those between Rta and other cellular or viral factors.
ACKNOWLEDGMENTS
We thank members of R. Sun's and M. Carey's laboratories for helpful discussion, A. Berk for providing pGAL4-VP16 and pGAL4-M2-Luc constructs, and Wendy Aft for editing the manuscript.
This work was supported by NIH grant CA 83525 (R.S.), the University of California Cancer Research Coordinating Committee (R.S.), and the Universitywide AIDS Research Program (H.J.B.).
REFERENCES
- 1.Alkan S, Karcher D S, Ortiz A, Khalil S, Akhtar M, Ali M A. Human herpesvirus−8/Kaposi's sarcoma-associated herpesvirus in organ transplant patients with immunosuppression. Br J Haematol. 1997;96:412–414. doi: 10.1046/j.1365-2141.1997.d01-2040.x. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O'Leary J J. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 3.Cannon J S, Nicholas J, Orenstein J M, Mann R B, Murray P G, Browning P J, DiGiuseppe J A, Cesarman E, Hayward G S, Ambinder R F. Heterogeneity of viral IL−6 expression in HHV-8-associated diseases. J Infect Dis. 1999;180:824–828. doi: 10.1086/314956. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox M A, Leahy J, Hardwick J M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990;64:313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Deng H, Young A, Sun R. Autoactivation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J Gen Virol. 2000;12:3043–3048. doi: 10.1099/0022-1317-81-12-3043. [DOI] [PubMed] [Google Scholar]
- 8.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande J P, Weiss R A, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc Natl Acad Sci USA. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ensoli B, Barillari G, Gallo R C. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi's sarcoma. Immunol Rev. 1992;127:147–155. doi: 10.1111/j.1600-065x.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 10.Ernst P, Smale S T. Combinatorial regulation of transcription II: the immunoglobulin mu heavy chain gene. Immunity. 1995;2:427–438. doi: 10.1016/1074-7613(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 11.Gruffat H, Manet E, Rigolet A, Sergeant A. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence- specific DNA binding protein. Nucleic Acids Res. 1990;18:6835–6843. doi: 10.1093/nar/18.23.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruffat H, Portes-Sentis S, Sergeant A, Manet E. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) encodes a homologue of the Epstein-Barr virus bZip protein EB1. J Gen Virol. 1999;80:557–561. doi: 10.1099/0022-1317-80-3-557. [DOI] [PubMed] [Google Scholar]
- 13.Hannon G J, Chubb A, Maroney P A, Hannon G, Altman S, Nilsen T W. Multiple cis-acting elements are required for RNA polymerase III transcription of the gene encoding H1 RNA, the RNA component of human RNase P. J Biol Chem. 1991;266:22796–22799. [PubMed] [Google Scholar]
- 14.Huang Y Q, Li J J, Kaplan M H, Poiesz B, Katabira E, Zhang W C, Feiner D, Friedman-Kien A E. Human herpesvirus-like nucleic acid in various forms of Kaposi's sarcoma. Lancet. 1995;345:759–761. doi: 10.1016/s0140-6736(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 15.Keaveney M, Berkenstam A, Feigenbutz M, Vriend G, Stunnenberg H G. Residues in the TATA-binding protein required to mediate a transcriptional response to retinoic acid in EC cells. Nature. 1993;365:562–566. doi: 10.1038/365562a0. [DOI] [PubMed] [Google Scholar]
- 16.Kedda M A, Margolius L, Kew M C, Swanepoel C, Pearson D. Kaposi's sarcoma-associated herpesvirus in Kaposi's sarcoma occurring in immunosuppressed renal transplant recipients. Clin Transplant. 1996;10:429–431. [PubMed] [Google Scholar]
- 17.Kirshner J R, Lukac D M, Chang J, Ganem D. Kaposi's sarcoma-associated herpesvirus open reading frame 57 encodes a posttranscriptional regulator with multiple distinct activities. J Virol. 2000;74:3586–3597. doi: 10.1128/jvi.74.8.3586-3597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh S S, Hengartner C S, Young R A. Baculoviral transfer vectors for expression of FLAG fusion proteins in insect cells. BioTechniques. 1997;23:627–630. doi: 10.2144/97234bm14. [DOI] [PubMed] [Google Scholar]
- 19.Lin S F, Robinson D R, Miller G, Kung H J. Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein-Barr virus. J Virol. 1999;73:1909–1917. doi: 10.1128/jvi.73.3.1909-1917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Pavlova I V, Virgin H W T, Speck S H. Characterization of gammaherpesvirus 68 gene 50 transcription. J Virol. 2000;74:2029–2037. doi: 10.1128/jvi.74.4.2029-2037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukac D M, Kirshner J R, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 23.Manet E, Gruffat H, Trescol B M C, Moreno N, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manet E, Rigolet A, Gruffat H, Giot J F, Sergeant A. Domains of the Epstein-Barr virus (EBV) transcription factor R required for dimerization, DNA binding and activation. Nucleic Acids Res. 1991;19:2661–2667. doi: 10.1093/nar/19.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memar O M, Rady P L, Tyring S K. Human herpesvirus-8: detection of novel herpesvirus-like DNA sequences in Kaposi's sarcoma and other lesions. J Mol Med. 1995;73:603–609. doi: 10.1007/BF00196354. [DOI] [PubMed] [Google Scholar]
- 26.Miles S A, Martinez-Maza O, Rezai A, Magpantay L, Kishimoto T, Nakamura S, Radka S F, Linsley P S. Oncostatin M as a potent mitogen for AIDS-Kaposi's sarcoma-derived cells. Science. 1992;255:1432–1434. doi: 10.1126/science.1542793. [DOI] [PubMed] [Google Scholar]
- 27.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov V M, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 29.Moore P S, Kingsley L A, Holmberg S D, Spira T, Gupta P, Hoover D R, Parry J P, Conley L J, Jaffe H W, Chang Y. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS. 1996;10:175–180. doi: 10.1097/00002030-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Nicholas J, Ruvolo V, Zong J, Ciufo D, Guo H G, Reitz M S, Hayward G S. A single 13-kilobase divergent locus in the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J Virol. 1997;71:1963–1974. doi: 10.1128/jvi.71.3.1963-1974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinlivan E B, Holley-Guthrie E A, Norris M, Gutsch D, Bachenheimer S L, Kenney S C. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 1993;21:1999–2007. doi: 10.1093/nar/21.8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rady P L, Yen A, Rollefson J L, Orengo I, Bruce S, Hughes T K, Tyring S K. Herpesvirus-like DNA sequences in non-Kaposi's sarcoma skin lesions of transplant patients. Lancet. 1995;345:1339–1340. doi: 10.1016/s0140-6736(95)92538-4. [DOI] [PubMed] [Google Scholar]
- 33.Reed J A, Nador R G, Spaulding D, Tani Y, Cesarman E, Knowles D M. Demonstration of Kaposi's sarcoma-associated herpes virus cyclin D homolog in cutaneous Kaposi's sarcoma by colorimetric in situ hybridization using a catalyzed signal amplification system. Blood. 1998;91:3825–3832. [PubMed] [Google Scholar]
- 34.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 35.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi's sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 37.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M F, Clauvel J P, Raphael M, Degos L, Sigaux F. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 39.Staskus K A, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Brett-Smith H, Haase A T. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J Virol. 1999;73:4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun R, Lin S F, Gradoville L, Miller G. Polyadenylylated nuclear RNA encoded by Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1996;93:11883–11888. doi: 10.1073/pnas.93.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun R, Lin S F, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Santen V L. Characterization of a bovine herpesvirus 4 immediate-early RNA encoding a homolog of the Epstein-Barr virus R transactivator. J Virol. 1993;67:773–784. doi: 10.1128/jvi.67.2.773-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehouse A, Carr I M, Griffiths J C, Meredith D M. The herpesvirus saimiri ORF50 gene, encoding a transcriptional activator homologous to the Epstein-Barr virus R protein, is transcribed from two distinct promoters of different temporal phases. J Virol. 1997;71:2550–2554. doi: 10.1128/jvi.71.3.2550-2554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehouse A, Stevenson A J, Cooper M, Meredith D M. Identification of a cis-acting element within the herpesvirus saimiri ORF 6 promoter that is responsive to the HVS.R transactivator. J Gen Virol. 1997;78:1411–1415. doi: 10.1099/0022-1317-78-6-1411. [DOI] [PubMed] [Google Scholar]
- 47.Wigler M, Pellicer A, Silverstein S, Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978;14:725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- 48.Wu T T, Usherwood E J, Stewart J P, Nash A A, Sun R. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J Virol. 2000;74:3659–3667. doi: 10.1128/jvi.74.8.3659-3667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong W, Ganem D. Characterization of ribonucleoprotein complexes containing an abundant polyadenylated nuclear RNA encoded by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) J Virol. 1997;71:1207–1212. doi: 10.1128/jvi.71.2.1207-1212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu F X, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]