Abstract
The ion exchange function of zeolite is useful for such a purpose as the removal of radioactive Cs species from water. In the very early days of zeolite science, the affinity of zeolites for metal cations was explained based on geometry. After the explanation presented above was proposed, many new zeolites and related materials were discovered or synthesized. Furthermore, it has become clear that the chemical nature of the ion exchange sites is strongly dependent on the framework topology. In this study, the ion exchange behavior between Na-form zeolites with different framework topologies and Cs-containing aqueous solutions was analyzed, and the equilibrium constant was calculated based on the Langmuir type equation to investigate the influence of the chemical nature of the zeolite framework on ion selectivity. The equilibrium constants at room temperature were in the order FAU < LTA < MFI < YFI < MOR. This order is the same as the order of Bro̷nsted acid strengths in the corresponding proton form zeolites. It is suggested that the delocalization of charge around the ion exchange site stabilizes a large cation whose charge is delocalized. In contrast, the equilibrium constant was not related to the pore and cavity size. This opens new insight into the influence of the chemical nature of zeolite on the affinity to cation.
Introduction
Separation of Cs+ from aqueous solutions containing Na+ is necessary for the removal of radioactive Cs species in effluents from nuclear facilities1−36 and for the recovery of Cs as a resource from seawater.37−40 Na-form zeotypes (zeolites and related materials with zeolitic frameworks) have been utilized in many studies for the separation of Cs+ as ion exchangers or promoters.5−7,9,10,13,14,17,19,20,22,23,26,29,30,35,36,41,42 Leaching of Cs+ from zeolites has also been investigated for the purpose of immobilizing radioactive Cs species.41,43−48
Zeolites are a group of crystalline materials that have a three-dimensional framework consisting of Si–O covalent bonds and micropores resulting from the framework structure, and some of the Si atoms in the framework are replaced with Al atoms. Various types of framework topologies have been discovered and categorized by three-letter framework type codes (FTCs).49 On the other hand, replacing Si with an oxidation number of +4 with Al with an oxidation number of +3 creates a negative charge that is shared by the Al and the four surrounding O atoms, or the AlO4 unit. In most cases, zeolites are synthesized in basic media containing alkaline cations where one cation is weakly bound to the AlO4 unit. Cations can be exchanged in a variety of media, usually aqueous solutions, and this is the origin of the ion exchange function of zeolite.50
The behavior of ion exchange between Na+ and Cs+ on zeolites is classified as Langmuir type,19,31,51 if it is based on the simplifying assumption that the thermodynamic properties of the ion exchange sites are homogeneous. The ion exchange between Na form zeolite and Cs-containing solution can be depicted as 1
| 1 |
where the equilibrium constant K is shown by 2.
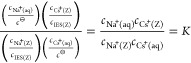 |
2 |
where cJ(aq) and cJ(Z) denote the molarity
of chemical species J in the aqueous solution normalized by the volume
of the solution and the molarity of species J in the zeolite normalized
by the weight of the zeolite, respectively. The symbol 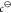 indicates the molar concentration
under
standard conditions (1 kmol m–3).
indicates the molar concentration
under
standard conditions (1 kmol m–3).
Ion exchange capacity cIES(Z) and equilibrium constant K characterize the ion exchange properties of a particular zeolite. The ion exchange capacity cIES(Z) = cNa+(Z) + cCs+(Z), i.e., the concentration of exchangeable cations per unit weight of the zeolite, depends primarily on the Al content (cAl(Z)) but can be influenced by structural properties such as steric hindrance. The equilibrium constant K should reflect the chemical nature of the zeolite.
Affinities to Na+ and Cs+, reflected by the above parameters, are different among zeolites with different compositions and framework topologies. It is known that, among zeolites with a relatively high aluminum concentration, A-type zeolite with LTA framework topology has a high Cs+ removal efficiency,6,8,19 and among zeolites with a moderate Al concentration, mordenite with MOR framework topology has a high Cs+ removal efficiency.17,20 In the very early days of zeolite science, around the 1960s, the affinity of zeolites for metal cations was explained based on geometry, that is, the fitting of the cations to the zeolite cavities.50,52,53 Differences in the affinity for Na+ and Cs+ between different zeolite species are mainly explained by the sizes of cations and micropores.23,42,54 In other words, the influences of geometry on the differences in the affinity for Na+ and Cs+, i.e., selectivity in the ion exchange, have been discussed, but the influence of a chemical nature such as acid strength of the zeolite has not been studied.
After the above explanation was proposed, many types of zeotypes with different framework topologies and chemical compositions were discovered or synthesized. Furthermore, over the past three decades, it has become clear that the chemical nature of the ion exchange sites is strongly dependent on the framework topology, as indicated by the striking differences in the Bro̷nsted acid strengths of the H-form.55,56 It is speculated that the acid strength of H-form zeolite depends on the local geometry, such as the Al–O–Si angle and Al–O distance,57 and we propose that compression of the Al–OH–Si group from both ends enhances the Bro̷nsted acidity of the proton.58−60 It is considered necessary to reflect these new findings in the interpretation of the ion exchange properties of the zeolites.
In this study, we analyze the influence of the chemical properties of ion exchange sites on Na–Cs exchange. The equilibrium constant K was analyzed based on the Langmuir model and compared with the Bro̷nsted acid strength of H-form zeolite with the same framework topology.
Experimental Section
The Na-form zeolites shown in Table 1 were used as parent samples. All of them did not contain binders. They are named as shown in Table 1. For example, FAU 6.1 is a zeolite with FAU framework topology and 6.1 mol kg–1 Al. The structural properties were analyzed as follows: Powder X-ray diffraction (XRD) was recorded using an Ultima IV diffractometer (Rigaku) with a Cu Kα X-ray source operated at a voltage of 40 kV and a current of 40 mA, and the reference XRD patterns were obtained from the International Zeolite Association (IZA) Structure Database.49 The nitrogen adsorption capacity at 77 K was measured using a BEL-Sorp Max (Microtrac BEL) or a BEL-Sorp Mini (Microtrac BEL) after pretreatment at 573 K in a vacuum. The micropore volume and external surface area were estimated from the t-plot method.61 In addition, the micropore volume was estimated also from the amount of liquid nitrogen that filled the micropores when the capillary condensation was completed at p/p0 = 0.005 (p and p0 are the observed pressure and equilibrium vapor pressure at 77 K, respectively, of nitrogen).62
Table 1. Origin, Chemical Composition of Employed Zeolite Samples and Measured Ion Exchange Parameters.
| sample name | origin | framework type | Si/Al molar ratio | cAl(z) (mol kg–1) | cIEZ(z) (mol kg–1) | K |
|---|---|---|---|---|---|---|
| FAU 6.1 | Na-X type zeolite, Reference Catalyst JRC-Z-NaX2.5(1) supplied by Reference Committee, Catalysis Society of Japan65 | FAU | 1.37 | 6.08 | 0.55 | 3.41 |
| LTA 6.7 | Na-A type zeolite, Zeolum A-4, powder, > 100 mesh, purchased from Tosoh Corp. | LTA | 1.12 | 6.69 | 0.18 | 20.4 |
| MFI 1.2 | Na-ZSM-5 type zeolite, International Reference Zeolite IRZ-MFI002 (NH4-MFI, supplied by Catalysis Commission, International Zeolite Association66), ion-exchanged with excess of NaNO3. | MFI | 12.3 | 1.22 | 1.00 | 50.9 |
| YFI 1.3 | Na-YNU-5 type zeolite, synthesized according to literature,79 and ion-exchanged with excess of NaNO3. | YFI | 11.1 | 1.33 | 0.83 | 73.1 |
| MOR 1.5 | Na-mordenite, HSZ-642NAA supplied by Tosoh Corp. | MOR | 9.88 | 1.48 | 1.44 | 177 |
| MOR 1.7 | Na-mordenite, Reference Catalyst JRC-Z-M15(1) supplied by Reference Committee, Catalysis Society of Japan65 | MOR | 8.37 | 1.71 | 1.59 | 187 |
Ion exchange between Na form zeolite and Cs-containing solution was examined as follows. An aqueous solution containing 60–360 g m–3 of Cs and 1000 g m–3 of Na was prepared from ion-exchanged water, cesium nitrate, and sodium nitrate (FUJIFILM Wako Pure Chemicals, Inc.). At room temperature, about 298 K, Na-form zeolite powder (0.1 g) was added to the solution (100 cm3), stirred for 5 min with a magnetic stirrer at about 400 rpm in a conical flask (inner volume 300 cm3), and filtered with a filter paper (No. 101, Advantec, retention particle size 5 μm). The Cs content of the solution was analyzed by an inductively coupled plasma (ICP) optical emission spectrometer (Agilent 5110). In some cases, the Cs content of the solid was confirmed by ICP analysis after dissolving the solid using hydrofluoric acid.
The analysis was performed from the measured relationship between the Cs content in the zeolite and the solution after ion exchange (Figure S1) as follows. The ion exchange between Na zeolite and a Cs-containing solution can be expressed as 2 where the equilibrium constant is shown by 2. It is assumed that the ion exchange site is covered by Na+ or Cs+. Therefore,
where cIES(Z) denotes the concentration of ion exchange sites responsible for the ion exchange reaction under discussion. Since the substitution of one Si atom (oxidation number +4) by one Al atom (oxidation number +3) in the zeolite framework generates one ion exchange site for charge compensation, the number of ion exchange sites should be equal to cAl(Z), but some ion exchange sites may not be responsible due to steric hindrance or other reasons. Therefore, only functional exchange sites are considered, and in the following analysis, cIES(Z) is used.
From these, 3 is derived.
| 3 |
Equation 3 shows that 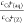 is a linear function of cCs+(aq), when measured with varying the Cs+/zeolite ratio and maintaining cNa+(aq) a constant and high value on one zeolite. Therefore, cIES(Z) and K were determined
from the slope and intercept of the plot of
is a linear function of cCs+(aq), when measured with varying the Cs+/zeolite ratio and maintaining cNa+(aq) a constant and high value on one zeolite. Therefore, cIES(Z) and K were determined
from the slope and intercept of the plot of 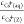 against cCs+(aq). From K, the standard Gibbs energy
against cCs+(aq). From K, the standard Gibbs energy 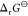 of reaction 1 was calculated by 4.
of reaction 1 was calculated by 4.
| 4 |
where R = 8.3145 J K–1 mol–1 and T was the temperature (298 K).
Results and Discussion
Quality of Parent Zeolite
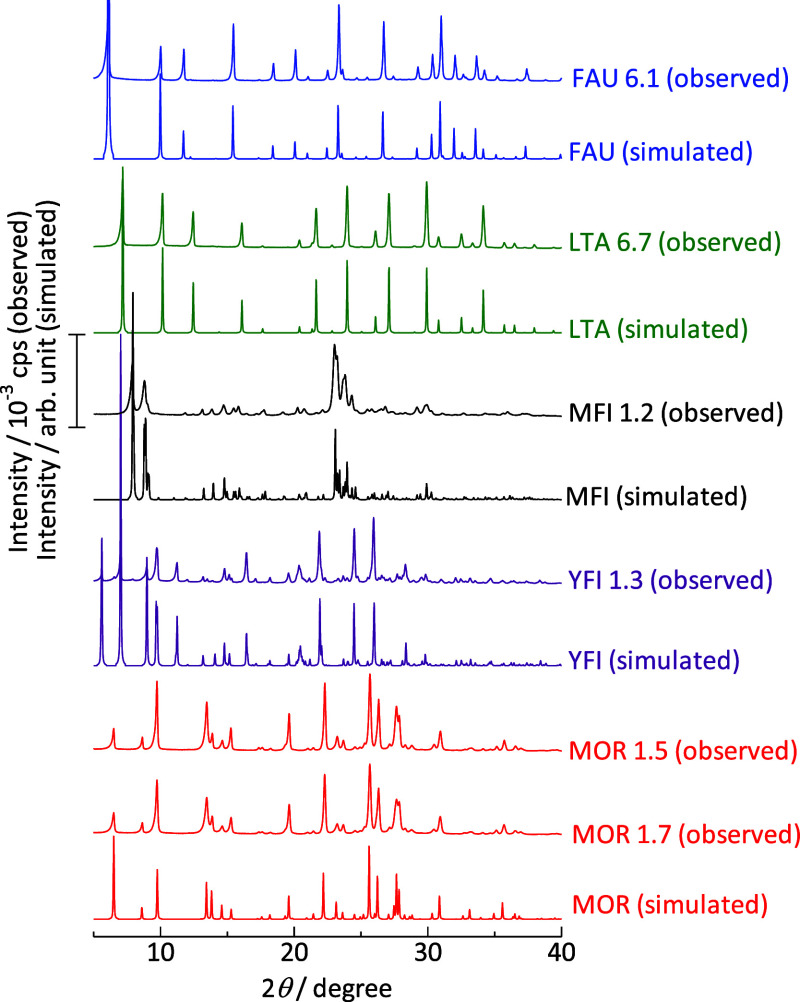
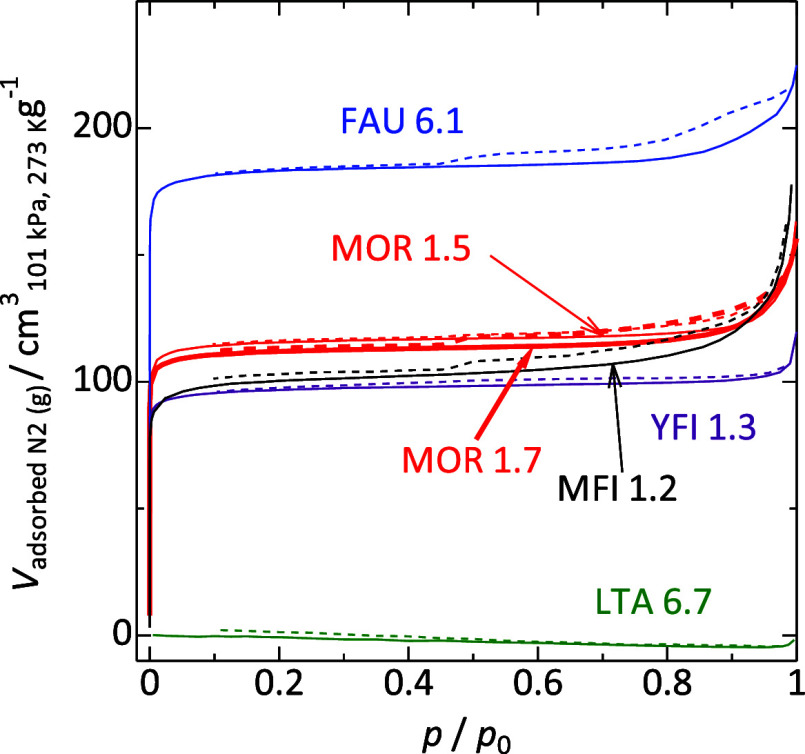
XRD measurements of the parent samples showed diffraction patterns consistent with those simulated based on the corresponding crystal types (Figure 1).49 Nitrogen adsorption on FAU, MFI, YFI, and MOR type samples at 77 K showed isotherms classified as type I (Figure 2).63 The micropore volumes are estimated from the t-plot method61 and also from the micropore condensation capacity.62 The values estimated by the two methods are similar and are higher than 0.14 cm3 g–1 for the parent samples of FAU, MFI, YFI, and MOR types (Table 2). These observations demonstrate high crystallinity. In addition, MFI 1.2 is an International Reference Zeolite supplied by IZA (IRZ-MFI002). FAU 6.1 and MOR 1.7 are Reference catalysts from the Catalysis Society of Japan [JRC-Z-NaX2.5 (1) and JRC-Z-M15 (1), respectively]. The physicochemical properties of these samples analyzed by multiple research groups have been opened public.64−66 On the other hand, LTA 6.7 (Na-A zeolite) showed a quite low nitrogen adsorption capacity at 77 K due to the blocking of the pore opening by Na+ cation.67−69 This is characteristic of the LTA-type zeolite. These results confirm the high crystallinity of the parent zeolites.
Figure 1.
X-ray diffraction of parent (Na-form) zeolites. The sample name should be referred to Table 1. Simulated patterns were obtained from the International Zeolite Association (IZA) structure database.49
Figure 2.
Nitrogen adsorption isotherms at 77 K of parent (Na-form) zeolites. Solid and dotted lines show the adsorption and desorption branches, respectively. p and p0 represent the observed pressure and equilibrium vapor pressure at 77 K, respectively, of nitrogen.
Table 2. Textural Properties Estimated from Nitrogen Adsorption Behavior at 77 K.
The external surface areas of the FAU-, MFI-, YFI-, and MOR-type samples are estimated from the nitrogen adsorption isotherms. In general, the external surface area was small (<23 m2 g–1), indicating relatively large crystallite sizes (Table 2).
Acidic Properties
Infrared/mass spectroscopy temperature-programmed desorption (IRMS-TPD) of ammonia quantifies the number and strength of each of Bro̷nsted and Lewis acid sites.70,71 This method has shown that the ammonia desorption enthalpy from the Bro̷nsted acid sites on H-form zeolite, an index of Bro̷nsted acid strength, depends mainly on the type of framework but not on the aluminum concentration.55,72−75 This is because the Al–O distance mainly controls the acid strength of the Si–OH–Al bridge.58,60,76 Therefore, it is speculated that an aluminosilicate framework has its own inherent acidity, or electron-withdrawing property, due to its framework topology, and in this study, we compare the Bro̷nsted acid strength of the H-form zeolite and the ion exchange property of the Na-form zeolite.
The ammonia desorption enthalpy from the Bro̷nsted acid sites of H-form zeolite has been found to be 130–140 kJ mol–1 for MFI with 0.3–1.3 mol kg–1 of Al,55,77 averagely 143 kJ mol–1 for YFI with 0.3–1.4 mol kg–1 of Al76 and averagely 148 kJ mol–1 for MOR with 0.4–2.4 mol kg–1 of Al.55,70,72 The Al contents of the parent Na-zeolites (MFI: 1.2 mol kg–1, YFI: 1.3 mol kg–1 and MOR: 1.5–1.7 mol kg–1 as shown in Table 1) were within the above ranges for which the acid strengths of the corresponding H-zeolites have been revealed.
Furthermore, we studied the properties of YFI 1.3 in detail. Although the synthesis lot was different from that in the present study, the composition and synthesis methods were the same. Infrared (IR) spectra of adsorbed d3-acetonitrile, pyridine, and ammonia, the conventional ammonia TPD profile, the TPD profile from Bro̷nsted acid site, and the ammonia desorption enthalpy distribution can be referred to our paper.76 The applied techniques showed that the Lewis acidity was negligible (0.1 mol kg–1), and the number of Bro̷nsted acid sites (1.3 mol kg–1) was similar to the number of Al atoms (1.4 mol kg–1). These results indicate that most of the Al is incorporated into the framework. The ammonia desorption enthalpy from the Bro̷nsted acid sites was 143 kJ mol–1. Therefore, it can be concluded that the Bro̷nsted acid strength of H-form YFI, which corresponds to the presently used Na-form sample, is indicated by the ammonia desorption enthalpy of 143 kJ mol–1.
MFI 1.2 is an international reference zeolite, and it is opened public66 that the Bro̷nsted acid amount (1.3 mol kg–1) was close to the Al content (1.3 mol kg–1), whereas the Lewis acid amount was negligible (0.002 mol kg–1), indicating that most of the Al is incorporated into the framework. The ammonia desorption enthalpy of the H-form is reported to be 136 kJ mol–1. The conventional ammonia TPD profile, the TPD profile from the Bro̷nsted acid site, and the ammonia desorption enthalpy distribution can be found in public databases, as well as other analytical results.66
MOR 1.7 was the subject of group study, and various characterization techniques such as nuclear magnetic resonance (NMR), ammonia TPD, and IR of CO and pyridine showed that most of Al atoms were incorporated into the framework.64
The above zeolites (MFI, YFI and MOR) allow comparison of the acidic property of the H-form and the ion exchange property of the Na-form in the same Al concentration range.
On the other hand, in this study, we used Na-form samples of FAU and LTA with high Al concentrations; FAU: 6.1 mol kg–1 (X type zeolite), LTA: 6.7 mol kg–1 (A type zeolite). Zeolites with such high Al concentrations are destroyed in acidic conditions, so conversion into the NH4- or H-form is not possible. However, as mentioned in the previous paragraphs, we speculate that the inherent acidity of the aluminosilicate framework is mainly controlled by the framework topology, allowing us to compare the ion exchange behavior of the Na-form and the acidic property of the H-form even though they have different the aluminum concentrations. The ammonia desorption enthalpy from the Bro̷nsted acid sites was observed to be ca. 110 kJ mol–1 for FAU (Y type zeolite) with 4.4–5.5 mol kg–1 of Al,72,74,78 and 132 kJ mol–1 for LTA (UZM-9 type zeolite) with 4.8 mol kg–1 of Al,60 and will be compared with the ion exchange properties in the next section.
Ion Exchange Properties
Throughout this study, the ion exchange properties were evaluated by the Cs concentration in the aqueous solution after the ion exchange procedure and the analysis of the Cs contents in the solid was omitted. There are two reasons for this. (1) The final objective of the study is to understand the ion exchange function for the removal of Cs species from water. (2) The dissolution of a small amount of zeolite containing a low concentration of Cs after filtration would result in a large experimental error. However, the analysis of the Cs content was performed on LTA 6.7 under equilibrium with an aqueous solution containing 0.851 mol m–3 Cs+ (final composition), followed by dissolution in hydrofluoric acid for ICP analysis. The Cs content in the solid was 0.0579 mol kg–1, in agreement with 0.0582 mol kg–1 estimated from the solution composition, supporting its validity.
Then, from the analysis of the solutions, the relationship between
the compositions of zeolite and solution was estimated in 0–3
mmol m–3 region for the zeolite samples used, as
shown in Figure S1. From these results,
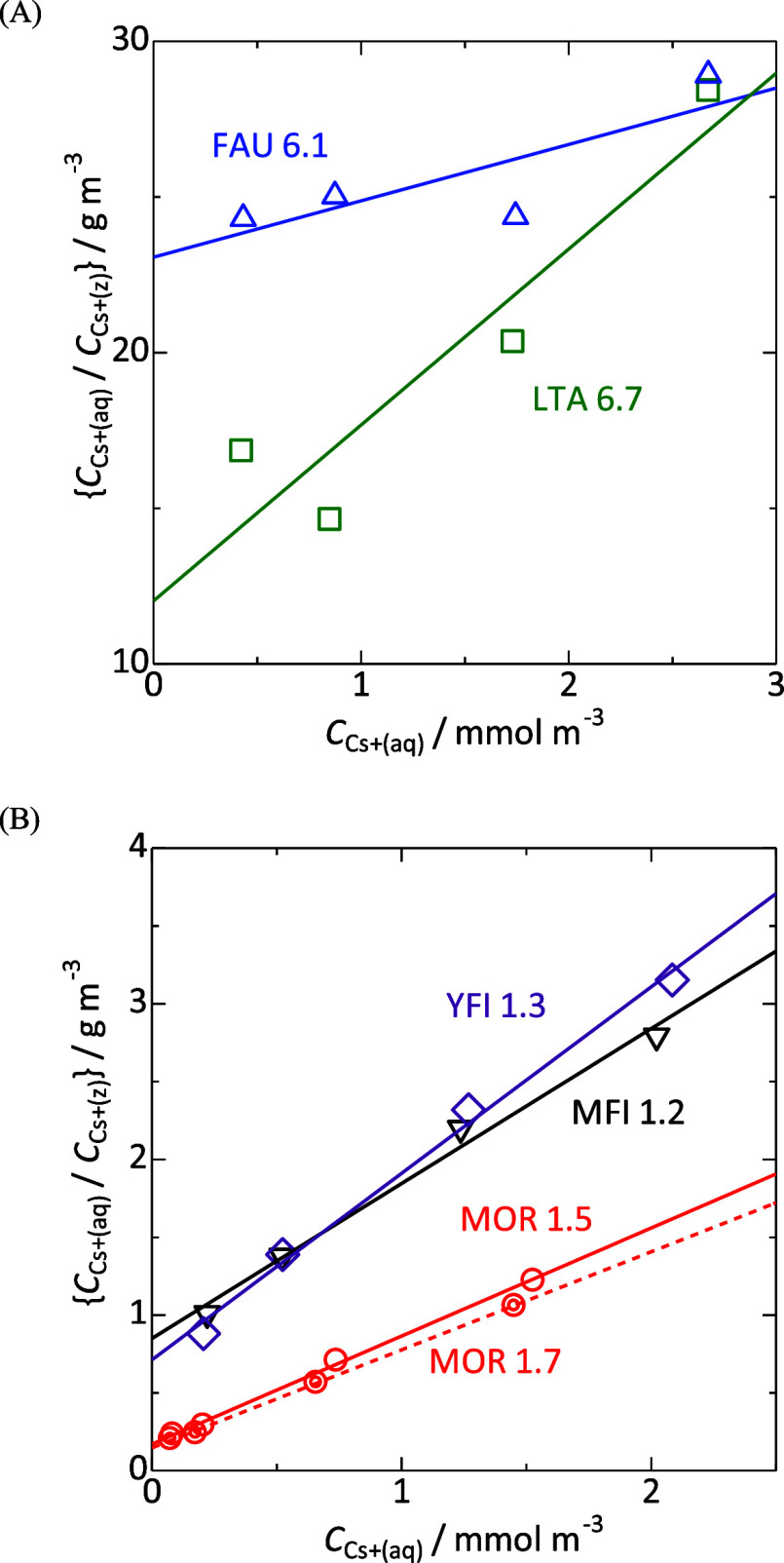
plots of 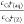 against cCs+(aq) are obtained as shown in Figure 3. Nearly straight lines were
obtained, showing
the availability of Langmuir-type analysis using eq 3. From the slope and intercept, the ion exchange
capacity cIEZ(Z) and the equilibrium constant K were estimated as shown in Table 1. For reference, the ion-exchange isotherms
calculated based on the determined cIEZ(Z) are shown in Figure S2.
against cCs+(aq) are obtained as shown in Figure 3. Nearly straight lines were
obtained, showing
the availability of Langmuir-type analysis using eq 3. From the slope and intercept, the ion exchange
capacity cIEZ(Z) and the equilibrium constant K were estimated as shown in Table 1. For reference, the ion-exchange isotherms
calculated based on the determined cIEZ(Z) are shown in Figure S2.
Figure 3.
Plots of cCs+(aq)/cCs+(Z) against cCs+(aq) on (A) FAU and LTA, and (B) MFI, YFI and MOR type zeolites.
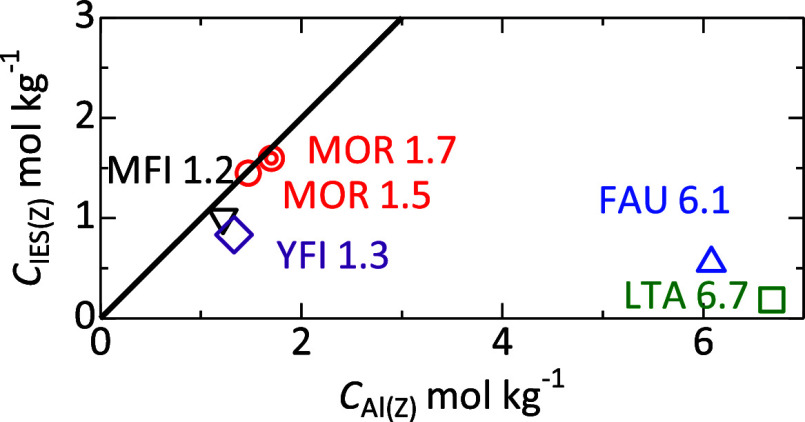
For MFI-, YFI-, and MOR-type zeolites, the estimated cIEZ(Z) (the number of sites playing a role of ion exchange sites under the present experimental conditions) was approximately in agreement with cAl(Z) (Figure 4), confirming that one ion exchange site was generated by substitution of one Si (+4) atom by one Al (+3) atom in the framework. However, FAU and LTA showed far lower cIEZ(Z) values than cAl(Z). Presumably, some of the cations in the sodalite cage, which is separated from the outside by the 6-ring windows, which require high temperature for passage of cations,50 were not exchanged under these conditions (room temperature). It is noteworthy that wastewater treatment is performed at ambient temperature, and the present experiments demonstrate the ion exchange functions applicable to practical treatment processes.
Figure 4.
Plots of estimated amount of ion exchange sites cIES(Z) against aluminum content cAl(Z). The thick line shows cIES(Z) = cAl(Z).
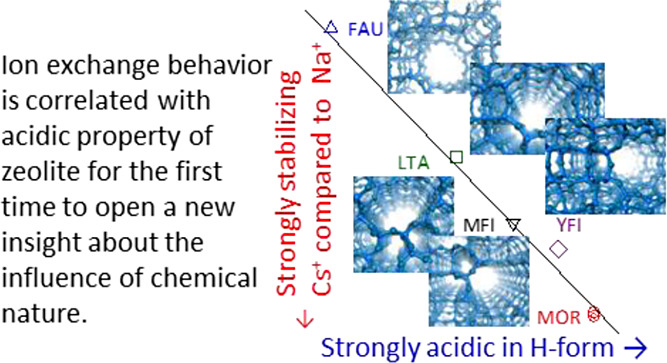
On the other hand, the calculated K was clearly different among zeolite species as FAU < LTA < MFI < YFI < MOR, while the difference in cAl(Z) between the two MOR samples did not change K. The larger K indicates the high ability of Na-form zeolite to remove Cs+ from aqueous solutions in the coexistence of Na+. The high exchange ability of MOR is consistent with the preceding literature.20
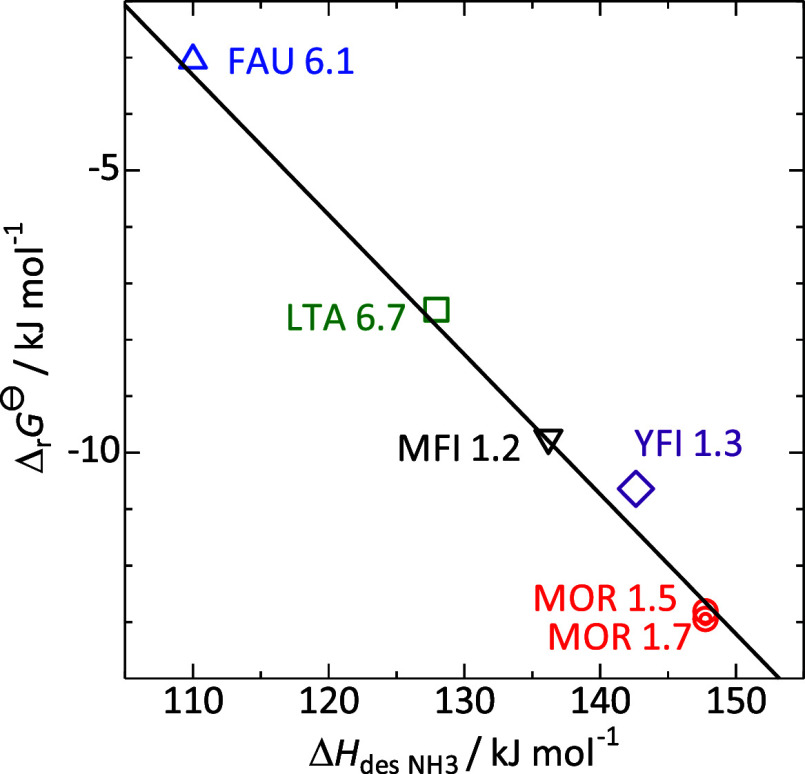
As shown in Figure 5, the standard Gibbs energy 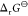 of the ion exchange (1) decreases
with
the increase in ammonia desorption enthalpy from the Bro̷nsted
acid sites of the corresponding H-form zeolite. Since ammonia desorption
enthalpy (heat of ammonia adsorption) is an indicator of acid strength,
this indicates that the zeolite framework, which generates strong
Bro̷nsted acidity in the H-form, strongly stabilizes Cs+.
of the ion exchange (1) decreases
with
the increase in ammonia desorption enthalpy from the Bro̷nsted
acid sites of the corresponding H-form zeolite. Since ammonia desorption
enthalpy (heat of ammonia adsorption) is an indicator of acid strength,
this indicates that the zeolite framework, which generates strong
Bro̷nsted acidity in the H-form, strongly stabilizes Cs+.
Figure 5.
Plots of standard Gibbs energy of reaction 1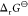 of zeolites against ammonia desorption
enthalpy ΔHdes NH3 of corresponding
H-form zeolites.
of zeolites against ammonia desorption
enthalpy ΔHdes NH3 of corresponding
H-form zeolites.
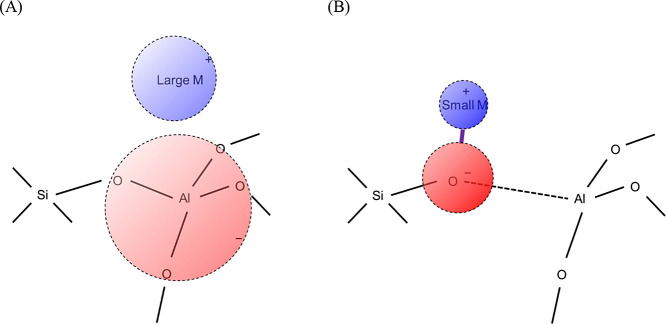
As mentioned above, we propose that the Bro̷nsted acid strength of the Si–OH–Al bridge is controlled by the Al–O distance, suggesting that the acid strength (electron withdrawing property) is due to structural stress. An H-form zeolite has Al(−O–Si)4 units where H+ is bonded to one of the four O atoms. Theoretical calculations reveal that the O–H bond is a covalent bond, not an ionic bond, and the Al–O distance in Al–OH–Si becomes longer than the other three Al–O distances. In the NH4 form, the NH4+ cation does not have a covalent bond with the framework atoms and is usually coordinated to two oxygen atoms. The four Al–O distances are similar and shorter than the Al–O distance in the Al–OH–Si of the H-form.58 Meanwhile, there are complex mechanical forces around the Al–O–Si bridge. Theoretical calculations reveal that the stress and compression from both ends of Al–O–Si change the distance between Al–O, and the shorter the Al–O, the higher the desorption energy (enthalpy) of ammonia.58
In the case of Al–O–Si compressed from both ends, the geometry in which the distance between all four Al–Os is the same is relatively stable (Scheme 1A), whereas in the case of Al–O–Si stretched from both ends, the geometry in which one Al–O distance is longer is relatively stable (B). In the former, the negative charge generated by the isomorphous substitution of Si4+ by Al3+ is delocalized, and the charge is shared by four oxygen atoms. In contrast, in the latter, the negative charge is localized on one oxygen atom. A cation with a positive charge delocalized over a wide space, i.e., a soft Lewis acid, prefers the former Al–O–Si (soft Lewis base), while a hard Lewis acid prefers the latter hard Lewis base.
Scheme 1. Schematic Drawings of Influence of Local Geometry on Charge Localization around Ion Exchange Sites.
(A) shows a Si–O–Al bridge compressed from both ends, whose negative charge is delocalized, strongly acidic (prefers NH4+ than H+), and prefers Cs+ than Na+. (B) shows a Si–O–Al bridge stretched from both ends, whose negative charge is localized to an O atom, weakly acidic (prefers H+ than NH4+), and prefers Na+ than Cs+.
NH4+ is a larger and therefore softer Lewis acid than H+. The strong Bro̷nsted acidity means instability of H+, and more exactly, high ammonia desorption energy means the stability of NH4+ compared to H+ on the discussed ion exchange site. It reflects the softness of the Lewis base property of the site.
On the other hand, Cs+ is larger and therefore softer Lewis acid than Na+. Cs+ should prefer a soft Lewis base, i.e., the Al(−O–Si)4 group with equal Al–O distances and a shared negative charge on four oxygen atoms. This tendency should be common for the cases of ammonia adsorption (H+ → NH4+) and Na–Cs exchange (Na+ → Cs+). Thus, the influence of the chemistry of the silicate framework on the selectivity of ions can be explained.
In contrast, the equilibrium constant K or the
Gibbs energy 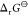 was not related to the pore and cavity
size of these zeolites (representative parameters are shown in Table S1). At least for the four framework types
treated in this study, it can be said that chemical properties have
a stronger influence than porosity.
was not related to the pore and cavity
size of these zeolites (representative parameters are shown in Table S1). At least for the four framework types
treated in this study, it can be said that chemical properties have
a stronger influence than porosity.
Conclusions
The analysis of ion exchange behavior between Na-type zeolite and Cs-containing aqueous solution showed that the equilibrium constants at room temperature were in the order FAU < LTA < MFI < YFI < MOR, as the order of Bro̷nsted acid strengths in the corresponding proton form zeolites. The Gibbs energy of ion exchange, which reflects the above equilibrium constant, showed a linear relationship with the ammonia desorption enthalpy from the Bro̷nsted acid sites on the proton form zeolites, an indicator of the Bro̷nsted acid strength. In contrast, the equilibrium constant was not related to the pore and cavity size. At least for the four framework types treated in the present study, it can be said that chemical properties have a stronger influence than porosity.
Acknowledgments
This research was carried out as part of the Tottori University Junior Doctor Training School, Environmental Research Program with support from the JST Next Generation Human Resources Development Project. We also received support from JSPS KAKENHI (21H01717, 23H05454).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.langmuir.4c00801.
Microporous properties of frameworks, compositions of solvent and zeolite after the ion exchange, and ion exchange isotherms (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Andreeva N. R.; Shubaeva M. A. Ion-exchange properties of geolite k-g relative to strontium and cesium. Sov. Radiochem. 1989, 31 (4), 469–473. [Google Scholar]
- Solache-Rios M.; Garcia-Sosa I.; Sosa-Reyes S. Ethylenediamine effect on the sorption of cesium on zeolites ZSM-5 and Y. J. Radioanal. Nucl. Chem. 1998, 237 (1–2), 151–153. 10.1007/BF02386680. [DOI] [Google Scholar]
- Sylvester P.; Clearfield A.; Diaz R. J. Pillared montmorillonites: Cesium-selective ion-exchange materials. Sep. Sci. Technol. 1999, 34 (12), 2293–2305. 10.1081/SS-100100773. [DOI] [Google Scholar]
- Qian G. R.; Li Y. X.; Yi F. C.; Shi R. M. Improvement of metakaolin on radioactive Sr and Cs immobilization of alkali-activated slag matrix. J. Hazard. Mater. 2002, 92 (3), 289–300. 10.1016/S0304-3894(02)00022-5. [DOI] [PubMed] [Google Scholar]
- Goni S.; Guerrero A.; Lorenzo M. P. Efficiency of fly ash belite cement and zeolite matrices for immobilizing cesium. J. Hazard. Mater. 2006, 137 (3), 1608–1617. 10.1016/j.jhazmat.2006.04.059. [DOI] [PubMed] [Google Scholar]
- El-Naggar M. R.; El-Kamash A. M.; El-Dessouky M. I.; Ghonaim A. K. Two-step method for preparation of NaA-X zeolite blend from fly ash for removal of cesium ions. J. Hazard. Mater. 2008, 154 (1–3), 963–972. 10.1016/j.jhazmat.2007.10.115. [DOI] [PubMed] [Google Scholar]
- Rajec P.; Domianovia K. Cesium exchange reaction on natural and modified clinoptilolite zeolites. J. Radioanal. Nucl. Chem. 2008, 275 (3), 503–508. 10.1007/s10967-007-7105-3. [DOI] [Google Scholar]
- Iucolano F.; Liguori B.; Sabova L.; Chmielewska E.; Caputo D.; Colella C.. Safe trapping of Cs in heat-treated zeolite matrices. In Part 2, 4th Conference of the Federation of European Zeolite Associations (FEZA), Univ Pierra Marie Curie, Paris, France, Sep 02–06; Univ Pierra Marie Curie: Paris, France, 2008; pp 537–540. [Google Scholar]
- El-Kamash A. M. Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater. 2008, 151 (2–3), 432–445. 10.1016/j.jhazmat.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Borai E. H.; Harjula R.; Malinen L.; Paajanen A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172 (1), 416–422. 10.1016/j.jhazmat.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Bayulken S.; Bascetin E.; Guclu K.; Apak R. Investigation and Modeling of Cesium(I) Adsorption by Turkish Clays: Bentonite, Zeolite, Sepiolite, and Kaolinite. Environ. Prog. Sustain. Energy 2011, 30 (1), 70–80. 10.1002/ep.10452. [DOI] [Google Scholar]
- Sekizawa H.; Yamashita S.; Tanji K.; Okoshi S.; Yoshioka K. Reduction of Radioactive Cesium in Apple Juice by Zeolite. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60 (5), 212–217. 10.3136/nskkk.60.212. [DOI] [Google Scholar]
- Johan E.; Yamada T.; Munthali M. W.; Kabwadza-Corner P.; Aono H.; Matsue N.. Natural zeolites as potential materials for decontamination of radioactive cesium. In 5th Sustainable Future for Human Security (SustaiN), Bali, Indonesia, Oct 19–21; Bali, Indonesia, 2014; pp 52–56. [Google Scholar]
- Torad N. L.; Naito M.; Tatami J.; Endo A.; Leo S. Y.; Ishihara S.; Wu K. C. W.; Wakihara T.; Yamauchi Y. Highly Crystallized Nanometer-Sized Zeolite A with Large Cs Adsorption Capability for the Decontamination of Water. Chem. – Asian J. 2014, 9 (3), 759–763. 10.1002/asia.201301132. [DOI] [PubMed] [Google Scholar]
- Lihareva N.; Kostov-Kytin V. Sorption of Cs+ by nano-sized microporous titanium silicates with pharmacosiderite structure. Bulg. Chem. Commun. 2014, 46 (3), 569–575. [Google Scholar]
- Sano M.; Nakagawa K.; Oda H.; Obata H.; Kitani S.; Yamazaki K. Fundamental Study for the Development of Cs Removal Technique from Low Level Radioactive Liquid Wastes of Decontamination Processes. Kagaku Kogaku Ronbun 2014, 40 (3), 261–265. 10.1252/kakoronbunshu.40.261. [DOI] [Google Scholar]
- Yamada K.; Johan E.; Matsue N.; Itagaki Y.; Aono H. Preparation of mordenite and its composite material with nano-sized magnetite from diatomites for radioactive Cs decontamination. J. Ceram. Soc. Jpn. 2015, 123 (1435), 129–135. 10.2109/jcersj2.123.129. [DOI] [Google Scholar]
- Ozeki K.; Aoki H. Evaluation of the adsorptive behavior of cesium and strontium on hydroxyapatite and zeolite for decontamination of radioactive substances. Biomed. Mater. Eng. 2016, 27 (2–3), 227–236. 10.3233/BME-161584. [DOI] [PubMed] [Google Scholar]
- Vipin A. K.; Ling S.; Fugetsu B. Removal of Cs+ and Sr2+ from water using MWCNT reinforced Zeolite-A beads. Microporous Mesoporous Mater. 2016, 224, 84–88. 10.1016/j.micromeso.2015.11.024. [DOI] [Google Scholar]
- Aono H.; Kaji N.; Itagaki Y.; Johan E.; Matsue N. Synthesis of mordenite and its composite material using chemical reagents for Cs decontamination. J. Ceram. Soc. Jpn. 2016, 124 (5), 617–623. 10.2109/jcersj2.15317. [DOI] [Google Scholar]
- Ishikawa N. K.; Kuwata M.; Ito A.; Umita T. Effect of pH and Chemical Composition of Solution on Sorption and Retention of Cesium by Feldspar, Illite, and Zeolite as Cesium Sorbent From Landfill Leachate. Soil Sci. 2017, 182 (2), 63–68. 10.1097/SS.0000000000000194. [DOI] [Google Scholar]
- Palamarchuk M.; Egorin A.; Tokar E.; Tutov M.; Marinin D.; Avramenko V. Decontamination of spent ion-exchangers contaminated with cesium radionuclides using resorcinol-formaldehyde resins. J. Hazard. Mater. 2017, 321, 326–334. 10.1016/j.jhazmat.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Lee H. Y.; Kim H. S.; Jeong H.-K.; Park M.; Chung D.-Y.; Lee K.-Y.; Lee E.-H.; Lim W. T. Selective Removal of Radioactive Cesium from Nuclear Waste by Zeolites: On the Origin of Cesium Selectivity Revealed by Systematic Crystallographic Studies. J. Phys. Chem. C 2017, 121 (19), 10594–10608. 10.1021/acs.jpcc.7b02432. [DOI] [Google Scholar]
- Wu Y.; Zhang X. X.; Wei Y. Z.; Mimura H. Development of adsorption and solidification process for decontamination of Cs-contaminated radioactive water in Fukushima through silica-based AMP hybrid adsorbent. Sep. Purif. Technol. 2017, 181, 76–84. 10.1016/j.seppur.2017.03.019. [DOI] [Google Scholar]
- Kim G. N.; Kim S. S.; Choi J. W. Development of an agent suited for adsorbing Cs-137 from ash and soil waste solutions. Sep. Purif. Technol. 2017, 173, 193–199. 10.1016/j.seppur.2016.09.025. [DOI] [Google Scholar]
- Sterba J. H.; Sperrer H.; Wallenko F.; Welch J. M. Adsorption characteristics of a clinoptilolite-rich zeolite compound for Sr and Cs. J. Radioanal. Nucl. Chem. 2018, 318 (1), 267–270. 10.1007/s10967-018-6096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov P.; Semenkova A.; Egorova T.; Romanchuk A.; Zakusin S.; Dorzhieva O.; Tyupina E.; Izosimova Y.; Tolpeshta I.; Chernov M.; Krupskaya V. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9 (10), 625. 10.3390/min9100625. [DOI] [Google Scholar]
- Eljamal O.; Shubair T.; Tahara A.; Sugihara Y.; Matsunaga N. Iron based nanoparticles-zeolite composites for the removal of cesium from aqueous solutions. J. Mol. Liq. 2019, 277, 613–623. 10.1016/j.molliq.2018.12.115. [DOI] [Google Scholar]
- Ding D.; Li K. X.; Fang D. Z.; Ye X. S.; Hu Y. Q.; Tan X. L.; Liu H. N.; Wu Z. J. Novel Biomass-Derived Adsorbents Grafted Sodium Titanium Silicate with High Adsorption Capacity for Rb+ and Cs+ in the Brine. Chemistryselect 2019, 4 (46), 13630–13637. 10.1002/slct.201903828. [DOI] [Google Scholar]
- Tuaimah S. K.; Al-Nasri S. K.; Al-Rahmani A. A.; Abbas T. K. Using Dates Leaves Midribs to Prepare Hierarchical Structures Incorporating Porous Carbon and Zeolite A Composites for Cesium Cs-137 Ion Exchange. Baghdad Sci. J. 2020, 17 (3), 818–825. 10.21123/bsj.2020.17.3.0818. [DOI] [Google Scholar]
- Liao H. Y.; Li Y.; Li H. R.; Li B. L.; Zhou Y. Z.; Liu D. B.; Wang X. L. Efficiency and mechanism of amidoxime-modified X-type zeolite (AO-XZ) for Cs+ adsorption. Chem. Phys. Lett. 2020, 741, 137084 10.1016/j.cplett.2019.137084. [DOI] [Google Scholar]
- Lee Y.; Park C. W.; Kim H. J.; Kim S. J.; Lee T. S.; Yang H. M. Sulfur-encapsulated zeolite micromotors for the selective removal of cesium from high-salt water with accelerated cleanup times. Chemosphere 2021, 276, 130190 10.1016/j.chemosphere.2021.130190. [DOI] [PubMed] [Google Scholar]
- Yanaka A.; Shibata K.; Matsumoto N.; Yoshida H. STUDY ON REMOVAL OF CESIUM FROM CONTAMINATED SOIL BY ELECTROPHORESIS USING POTASSIUM ACETATE AS ELECTROLYTE. Int. J. Geomate 2021, 20 (81), 16–21. 10.21660/2021.81.6222. [DOI] [Google Scholar]
- Ferreira D. R.; Phillips G. D.; Baruah B. A COMPARISON OF THE ADSORPTION OF CESIUM ON ZEOLITE MINERALS VS VERMICULITE. Clays Clay Miner. 2021, 69 (6), 663–671. 10.1007/s42860-021-00150-9. [DOI] [Google Scholar]
- Abbas T. K.; Rashid K. T.; Alsalhy Q. F. NaY zeolite-polyethersulfone-modified membranes for the removal of cesium-137 from liquid radioactive waste. Chem. Eng. Res. Des. 2022, 179, 535–548. 10.1016/j.cherd.2022.02.001. [DOI] [Google Scholar]
- Arbel-Haddad M.; Harnik Y.; Schlosser Y.; Goldbourt A. Cesium immobilization in metakaolin-based geopolymers elucidated by Cs-133 solid state NMR spectroscopy. J. Nucl. Mater. 2022, 562, 153570 10.1016/j.jnucmat.2022.153570. [DOI] [Google Scholar]
- Zhang N.; Gao D. L.; Liu M. M.; Deng T. L. Rubidium and Cesium Recovery from Brine Resources. Adv. Mater. Res. 2014, 1015, 417–420. 10.4028/www.scientific.net/AMR.1015.417. [DOI] [Google Scholar]
- Ali A.; Quist-Jensen C. A., Chapter 18 - Membrane Operations for Minerals’ Recovery From Seawater. In Current Trends and Future Developments on (Bio-) Membranes; Basile A.; Curcio E.; Inamuddin, Eds.; Elsevier: 2019; pp 449–471. [Google Scholar]
- Zhang X.; Zhao W.; Zhang Y.; Jegatheesan V. A review of resource recovery from seawater desalination brine. Rev. Environ. Sci. Biotechnol. 2021, 20 (2), 333–361. 10.1007/s11157-021-09570-4. [DOI] [Google Scholar]
- Chen S.; Dong Y.; Wang H.; Sun J.; Wang J.; Zhang S.; Dong H. Highly efficient and selective cesium recovery from natural brine resources using mesoporous Prussian blue analogs synthesized by ionic liquid-assisted strategy. Resour. Conserv. Recycl. 2022, 186, 106542 10.1016/j.resconrec.2022.106542. [DOI] [Google Scholar]
- Belkhiri S.; Guerza M.; Chouikh S.; Boucheffa Y.; Mekhalif Z.; Delhalle J.; Colella C. Textural and structural effects of heat treatment and γ-irradiation on Cs-exchanged NaX zeolite, bentonite and their mixtures. Microporous Mesoporous Mater. 2012, 161, 115–122. 10.1016/j.micromeso.2012.05.027. [DOI] [Google Scholar]
- Malek A.; Ozin G. A.; Macdonald P. M. Probing cation sites in cesium-exchanged zeolite Y via Cs-133 MAS NMR. J. Phys. Chem. 1996, 100 (41), 16662–16666. 10.1021/jp961265w. [DOI] [Google Scholar]
- Lima E. J.; Ibarra I. A.; Vera M. A.; Lara V. H.; Bosch P.; Bulbulian S. I. Cesium leaching in CsA and CsX zeolites. J. Phys. Chem. B 2004, 108 (32), 12103–12110. 10.1021/jp048683i. [DOI] [Google Scholar]
- Ibarra I. A.; Lima E.; Loera S.; Bosch P.; Bulbulian S.; Lara V. II. Cesium leaching in CsA and CsX zeolites: Use of blocking agents to inhibit the cesium cation mobility. J. Phys. Chem. B 2006, 110 (42), 21086–21091. 10.1021/jp061926h. [DOI] [PubMed] [Google Scholar]
- Lima E.; Ibarra I. A.; Lara V.; Bosch P.; Bulbulian S. Cesium leaching from gamma-irradiated CsA and CsX zeolites. J. Hazard. Mater. 2008, 160 (2–3), 614–620. 10.1016/j.jhazmat.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Tansho M.; Tamura K.; Shimizu T. Identification of Multiple Cs+ Adsorption Sites in a Hydroxy-interlayered Vermiculite-like Layered Silicate through Cs-133 MAS NMR Analysis. Chem. Lett. 2016, 45 (12), 1385–1387. 10.1246/cl.160850. [DOI] [Google Scholar]
- Yabiki K.; Eba H.; Sakurai K. Behavior and Mechanism of Cesium Adsorption on Layered Sodium Silicate Ilerite. Kagaku Kogaku Ronbun 2016, 42 (1), 1–7. 10.1252/kakoronbunshu.42.1. [DOI] [Google Scholar]
- Kuroda K.; Toda K.; Kobayashi Y.; Sato T.; Otake T.. Cs Leaching Behavior During Alteration Process of Calcium Silicate Hydrate and Potassium Alumino Silicate Hydrate. In 14th International Congress for Applied Mineralogy (ICAM), Belgorod, RUSSIABelgorod, Russia, Belgorod Technol UnivBelgorod Technol Univ: Sep 23–27; Belgorod Technol Univ: Belgorod, Russia, 2019; pp 451–452. [Google Scholar]
- Baerlocher C.; McCusker L. B.. Database of Zeolite Structures. http://www.iza-structure.org/databases/ (accessed 2023/12/11).
- Breck D. W., Ion Exchange Reactions in Zeolites. In Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley: 1974; pp 529–592. [Google Scholar]
- Lihareva N.; Petrov O.; Tzvetanova Y. Modelling of Cs+ uptake by natural clinoptilolite from water media. Bulg. Chem. Commun. 2017, 49 (3), 577–582. [Google Scholar]
- Sherry H. S.; Walton H. F. The ion-exchange properties of zeolites. II. Ion exchange in the synthetic zeolite Linde 4A. J. Phys. Chem. 1967, 71 (5), 1457–1465. 10.1021/j100864a042. [DOI] [Google Scholar]
- Sherry H. S. The Ion-Exchange Properties of Zeolites. I. Univalent Ion Exchange in Synthetic Faujasite. J. Phys. Chem. 1966, 70 (4), 1158–1168. 10.1021/j100876a031. [DOI] [Google Scholar]
- Liu H. M.; Grey C. P. Probing Cs+ cation accessibility with O-2 and Cs-133 MAS NMR spectroscopy. Microporous Mesoporous Mater. 2002, 53 (1–3), 109–120. 10.1016/S1387-1811(02)00330-X. [DOI] [Google Scholar]
- Katada N.; Igi H.; Kim J. H.; Niwa M. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium. J. Phys. Chem. B 1997, 101 (31), 5969–5977. 10.1021/jp9639152. [DOI] [Google Scholar]
- Niwa M.; Katada N.; Okumura K. Solid Acidity of Zeolites. In Springer Ser. Mater. S 2010, 141, 9–27. 10.1007/978-3-642-12620-8_2. [DOI] [Google Scholar]
- Senchenya I. N.; Kazansky V. B.; Beran S. Quantum Chemical Study of the Effect of the Structural Characteristics of Zeolites on the Properties of Their Bridging Oh Groups. Part 2. J. Phys. Chem. 1986, 90 (20), 4857–4859. 10.1021/j100411a028. [DOI] [Google Scholar]
- Katada N.; Suzuki K.; Noda T.; Sastre G.; Niwa M. Correlation between Bronsted Acid Strength and Local Structure in Zeolites. J. Phys. Chem. C 2009, 113 (44), 19208–19217. 10.1021/jp903788n. [DOI] [Google Scholar]
- Suzuki K.; Katada N.; Niwa M. Measurements of Acidity of H-SSZ-35 by a Combined Method of IRMS-TPD Experiment and DFT Calculation. Catal. Lett. 2010, 140 (3–4), 134–139. 10.1007/s10562-010-0440-y. [DOI] [Google Scholar]
- Katada N.; Nouno K.; Lee J. K.; Shin J.; Hong S. B.; Niwa M. Acidic Properties of Cage-Based, Small-Pore Zeolites with Different Framework Topologies and Their Silicoaluminophosphate Analogues. J. Phys. Chem. C 2011, 115 (45), 22505–22513. 10.1021/jp207894n. [DOI] [Google Scholar]
- Voogd P.; Scholten J. J. F.; van Bekkum H. Use of the t-plot—De Boer method in pore volume determinations of ZSM-5 type zeolites. Colloids Surf. 1991, 55, 163–171. 10.1016/0166-6622(91)80090-B. [DOI] [Google Scholar]
- Nakajima K.; Suganuma S.; Tsuji E.; Katada N. Mechanism of tetralin conversion on zeolites for the production of benzene derivatives. React. Chem. Eng. 2020, 5 (7), 1272–1280. 10.1039/D0RE00128G. [DOI] [Google Scholar]
- Sing K. S. W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57 (4), 603–619. 10.1351/pac198557040603. [DOI] [Google Scholar]
- Katada N.; Takeguchi T.; Suzuki T.; Fukushima T.; Inagaki K.; Tokunaga S.; Shimada H.; Sato K.; Oumi Y.; Sano T.; Segawa K.; Nakai K.; Shoji H.; Wu P.; Tatsumi T.; Komatsu T.; Masuda T.; Domen K.; Yoda E.; Kondo J. N.; Okuhara T.; Kageyama Y.; Niwa M.; Ogura M.; Matsukata M.; Kikuchi E.; Okazaki N.; Takahashi M.; Tada A.; Tawada S.; Kubota Y.; Sugi Y.; Higashio Y.; Kamada M.; Kioka Y.; Yamamoto K.; Shouji T.; Arima Y.; Okamoto Y.; Matsumoto H. Standardization of catalyst preparation using reference catalyst: ion exchange of mordenite type zeolite - 1. Remarkable dealumination accompanying ion exchange. Appl. Catal., A 2005, 283 (1–2), 63–74. 10.1016/j.apcata.2004.12.036. [DOI] [Google Scholar]
- Reference Catalyst Committee . Catalysis Society of Japan, Reference Catalyst Portal. https://jrc.catsj.jp/JRCpublic.html (accessed 2023/12/11).
- Catalysis Commission , International Zeolite Association, International Reference Zeolite. http://www.iza-online.org/catalysis/referencezeolite.htm (accessed 2023/12/11).
- Sebastian J.; Pillai R. S.; Peter S. A.; Jasra R. V. Sorption of N2, O2, and Ar in Mn(II)-Exchanged Zeolites A and X Using Volumetric Measurements and Grand Canonical Monte Carlo Simulation. Ind. Eng. Chem. Res. 2007, 46 (19), 6293–6302. 10.1021/ie070067w. [DOI] [Google Scholar]
- Shang Y.; Wu J.; Zhu J.; Wang Y.; Meng C. Study on adsorption of N2 and O2 by magnesium (II)-exchanged zeolite A. J. Alloys Compd. 2009, 478 (1), L5–L7. 10.1016/j.jallcom.2008.11.082. [DOI] [Google Scholar]
- Palomino M.; Corma A.; Rey F.; Valencia S. New Insights on CO2–Methane Separation Using LTA Zeolites with Different Si/Al Ratios and a First Comparison with MOFs. Langmuir 2010, 26 (3), 1910–1917. 10.1021/la9026656. [DOI] [PubMed] [Google Scholar]
- Niwa M.; Suzuki K.; Katada N.; Kanougi T.; Atoguchi T. Ammonia IRMS-TPD study on the distribution of acid sites in mordenite. J. Phys. Chem. B 2005, 109 (40), 18749–18757. 10.1021/jp051304g. [DOI] [PubMed] [Google Scholar]
- Niwa M.; Katada N. New Method for the Temperature-Programmed Desorption (TPD) of Ammonia Experiment for Characterization of Zeolite Acidity: A Review. Chem. Rec. 2013, 13 (5), 432–455. 10.1002/tcr.201300009. [DOI] [PubMed] [Google Scholar]
- Katada N.; Kageyama Y.; Niwa M. Acidic property of Y- and mordenite-type zeolites with high aluminum concentration under dry conditions. J. Phys. Chem. B 2000, 104 (31), 7561–7564. 10.1021/jp000906r. [DOI] [Google Scholar]
- Miyamoto Y.; Katada N.; Niwa M. Acidity of beta zeolite with different Si/Al-2 ratio as measured by temperature programmed desorption of ammonia. Microporous Mesoporous Mater. 2000, 40 (1–3), 271–281. 10.1016/S1387-1811(00)00264-X. [DOI] [Google Scholar]
- Katada N.; Kageyama Y.; Takahara K.; Kanai T.; Begum H. A.; Niwa M. Acidic property of modified ultra stable Y zeolite: increase in catalytic activity for alkane cracking by treatment with ethylenediaminetetraacetic acid salt. J. Mol. Catal. A: Chem. 2004, 211 (1–2), 119–130. 10.1016/j.molcata.2003.10.001. [DOI] [Google Scholar]
- Katada N.; Niwa M. Analysis of acidic properties of zeolitic and non-zeolitic solid acid catalysts using temperature-programmed desorption of ammonia. Catal. Surv. Asia 2004, 8 (3), 161–170. 10.1023/B:CATS.0000038534.37849.16. [DOI] [Google Scholar]
- Katada N.; Yamamoto K.; Fukui M.; Asanuma K.; Inagaki S.; Nakajima K.; Suganuma S.; Tsuji E.; Palcic A.; Valtchev V.; Petkov P. S.; Simeonova K.; Vayssilov G. N.; Kubota Y. Acidic property of YNU-5 zeolite influenced by its unique micropore system. Microporous Mesoporous Mater. 2022, 330, 111592 10.1016/j.micromeso.2021.111592. [DOI] [Google Scholar]
- Suganuma S.; Nakamura K.; Okuda A.; Katada N. Enhancement of catalytic activity for toluene disproportionation by loading Lewis acidic nickel species on ZSM-5 zeolite. Mol. Catal. 2017, 435, 110–117. 10.1016/j.mcat.2017.03.029. [DOI] [Google Scholar]
- Suzuki K.; Katada N.; Niwa M. Detection and quantitative measurements of four kinds of OH in HY zeolite. J. Phys. Chem. C 2007, 111 (2), 894–900. 10.1021/jp065054v. [DOI] [Google Scholar]
- Nakazawa N.; Ikeda T.; Hiyoshi N.; Yoshida Y.; Han Q.; Inagaki S.; Kubota Y. A Microporous Aluminosilicate with 12-, 12-, and 8-Ring Pores and Isolated 8-Ring Channels. J. Am. Chem. Soc. 2017, 139 (23), 7989–7997. 10.1021/jacs.7b03308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.