Abstract
Migraine is a complex neurovascular pain disorder linked to the meninges, a border tissue innervated by neuropeptide-containing primary afferent fibers chiefly from the trigeminal nerve. Electrical or mechanical stimulation of this nerve surrounding large blood vessels evokes headache patterns as in migraine, and the brain, blood, and meninges are likely sources of headache triggers. Cerebrospinal fluid may play a significant role in migraine by transferring signals released from the brain to overlying pain-sensitive meningeal tissues, including dura mater. Interactions between trigeminal afferents, neuropeptides, and adjacent meningeal cells and tissues cause neurogenic inflammation, a critical target for current prophylactic and abortive migraine therapies. Here we review the importance of the cranial meninges to migraine headaches, explore the properties of trigeminal meningeal afferents, and briefly review emerging concepts, such as meningeal neuroimmune interactions, that may one day prove therapeutically relevant.
Keywords: migraine, headache, meninges, trigeminal, meningeal afferents, neurogenic inflammation, sensitization, cortical spreading depression
INTRODUCTION
Migraine pathophysiology is linked inextricably to the brain’s tripartite coverings called the cranial meninges. The meninges are the only pain source within the cranium and a potential entry portal from and into the brain. The meninges are part of a peripheral immunosurveillance network through which the brain, facilitated by circulating cerebrospinal fluid (CSF), engages with surrounding tissues. The meninges also contain resident immune cells that contribute to pain through triggers from the blood, skull, brain, meninges, and possibly the external environment. Although we are beginning to relate some of these newer immune findings to migraine pathophysiology, therapeutic advances so far predominantly target meningeal receptors, neuropeptides, and innervating afferent fibers.
This review examines the importance of the cranial meninges to migraine headache, including emerging concepts that may one day prove therapeutically relevant. The reader is referred to several excellent reviews for more details on meningeal anatomy (Coles et al. 2017), immunology and pathology (Alves De Lima et al. 2020, Ampie & McGavern 2022), and recent clinical advances (Ferrari et al. 2022).
A BRIEF INTRODUCTION TO MIGRAINE
Migraine is a recurrent syndrome, one of the most common neurological disorders, and the second leading cause of disability globally, affecting over one billion people worldwide (GBD 2019 Dis. Inj. Collab. 2020). Migraine is three times more prevalent in women than in men. Migraine is characterized by head pain that is often described as intense pulsing, pounding, or throbbing. Migraine headaches often begin on one side, are exaggerated by physical activity, and are accompanied by nausea, vomiting, and sensitivity to light and sound. Attacks last 4–72 h if untreated.
The meninges have been implicated in migraine because (a) meningeal tissues are the only pain-sensitive structures within the cranium; (b) throbbing headache, nausea, vomiting, and sensitivity to light and sound are characteristic of meningeal inflammation, as in meningitis; (c) electrical or mechanical stimulation of the meninges evokes referred head pain patterns as in migraine; (d) acute migraine drug treatments do not require transport across the blood-brain barrier for therapeutic efficacy but do pass into the dura mater from the circulation; and (e) calcitonin gene–related peptide (CGRP) is a primary therapeutic target expressed by trigeminal afferents innervating the meninges and their large blood vessels. CGRP and its receptors are essential targets for acute and chronic migraine treatment and have critical links to the immune system.
MENINGEAL ANATOMY
The cranial meninges—dura mater, arachnoid, and pia mater—physically protect the brain and spinal cord. The meninges are among the most understudied tissues, yet their relatively abundant sensory innervation makes them a key source of headaches.
The dura mater, the outermost layer, contains a dense collagen network produced by connective tissue fibroblasts. It has two layers: an outer periosteal/endosteal layer and an inner dural border cell layer that approximates the arachnoid. The two layers are tightly fused except when they separate to form and surround the dural venous sinuses. Peripheral sensory and autonomic nerves innervate the cranial dura, particularly near its blood vessels. Large dural blood vessels, including the venous sinuses, veins, and arteries, contain the densest axonal networks. The first trigeminal division primarily supplies the dura mater with contributions from the other two divisions. The tentorial nerve, a branch of the first division, supplies the supratentorial dura, the superior sagittal and transverse sinuses, and parietal branches of the middle meningeal artery. Trigeminal meningeal afferents also issue collaterals that terminate in the calvarial bone marrow or project through the skull and terminate in the external periosteal layer (Kosaras et al. 2009, Schueler et al. 2013, Zhao & Levy 2014).
Trigeminal projections to the dura and pia mater contain vasoactive neuropeptides [e.g., CGRP, substance P, and pituitary adenylate cyclase–activating polypeptide (PACAP)] that are released upon activation; CGRP has been particularly implicated in migraine headaches (see below). Trigeminal neurons express therapeutically relevant prejunctional serotonin 5-HT1B/1D/1F receptors, whose activation blocks neuropeptide release. Antimigraine drugs (especially the triptans and ditan) that block neuropeptide release are agonists at these prejunctional receptors.
Sympathetic fibers projecting mainly from the superior cervical ganglion express the vasoconstrictor norepinephrine. Parasympathetic nerves arising from sphenopalatine and otic ganglia express vasoactive molecules [e.g., acetylcholine, vasoactive intestinal polypeptide (VIP), PACAP]. Preganglionic fibers are carried by the parasympathetic division of the facial nerve VII; their activation dilates the middle meningeal artery as part of a pain-specific reflex (Bolay et al. 2002). Although autonomic dysfunction has been implicated in migraine during and between attacks, it is unclear whether dural fibers contribute significantly.
In addition to fibroblasts, the dura mater contains resident immune cells, including monocytes/macrophages, mast cells, T cells, B cells, neutrophils, natural killer cells, innate lymphoid cells, and dendritic cells (Rustenhoven et al. 2021, Van Hove et al. 2019). A dural lymphatic network, composed of small capillary-like vessels, is adjacent to the transverse and superior sagittal sinuses (Aspelund et al. 2015, Louveau et al. 2015). The venous sinus wall contains focal areas of CSF and niche cells that resemble entryways into lymphatic vessels in peripheral tissues (Louveau et al. 2018, Rustenhoven et al. 2021). Accordingly, central nervous system (CNS) antigens can enter these immune hubs, become captured by antigen-presenting cells, and present to T cells before draining into cervical lymph nodes (Alves De Lima et al. 2020). Precisely how CSF reaches these immune hubs is under active investigation, and their role in migraine remains unexplored.
Lying atop the dura mater within the inner cortical layer of bone, recently described skull channels extend from the bone marrow to the dura mater (Herisson et al. 2018). These channels contain a blood vessel surrounded by CSF that flows from the subarachnoid space to the skull bone marrow via the dura mater. Within the dura mater, CSF is localized to the perivascular space, as detected by a tracer injected into the cisterna magna. Discovered recently in mice and humans (Cai et al. 2019, Herisson et al. 2018, Mazzitelli et al. 2022, Pulous et al. 2022), skull channels appear to serve as a bidirectional migration route for myeloid cells traveling to the meninges and brain, as well as for signals released from brain via glymphatics and CSF to the dura mater and bone marrow. Their possible role in migraine is discussed below.
The arachnoid has outer and inner layers with no sensory innervation. The outer layer of arachnoid barrier cells opposes the dura mater, and the inner fibroblast layer lies above the CSF-filled subarachnoid space. Tight junctions between arachnoid barrier cells were thought to prevent dural contents from reaching the subarachnoid space. However, molecules less than 40 kDa do pass through the dura into the subarachnoid space and the brain (Roth et al. 2014, Zhao et al. 2017). While these findings are from rodent models with exposed dura or thinned calvaria, the exchange between meningeal layers may be greater than previously thought.
Arachnoid fibroblasts form collagen-containing bundles or trabeculae that extend into the subarachnoid space, which also houses large blood vessels, known as pial vessels, as well as perivascular macrophages, and dendritic cells. A recently discovered lymphatic-like barrier membrane divides the subarachnoid space into two functional compartments; an outer compartment below the arachnoid layer and an inner one that contains the pial vessels (Mollgard et al. 2023). Endothelial cells within pial vessels exhibit tight junctions separating circulatory contents from the surrounding tissues and are a key component of the blood-brain barrier. Pial blood vessels are innervated by sympathetic and parasympathetic axons (Mayberg et al. 1981, 1984), whose detailed role in meningeal physiology awaits further study.
The pia mater, the layer closest to the brain, consists of a thin sheet of connective tissue resting on the brain’s surface. The pia interfaces with the glial limitans superficialis, a layer of surface-associated astrocytes that separates the pia from the brain parenchyma. Interestingly, the pia mater lacks capillaries. Still, the pial layer itself is sufficiently fenestrated to allow large molecules to penetrate and reach glial limitans astrocytes and vice versa.
RESPONSE PROPERTIES OF MENINGEAL AFFERENTS
As noted above, the cranial meninges are a border tissue that provides a physical and immunological barrier to protect the brain. The meninges also contain a nocifensive or local chemical defense network of sensory fibers that detects and limits tissue injury by releasing its vasoactive neuropeptides and informing the brain. The following sections will examine the response properties of meningeal afferents and their role as promoters of inflammation and pain from within the meninges, with relevance to migraine headache and its pathogenesis.
Headache referred to the ipsilateral temporal region can be produced by mechanically stimulating the meninges (Fontaine et al. 2018, Ray & Wolff 1940), thereby indicating a theoretical link between activated meningeal sensory neurons and migraine headache (Moskowitz 1984, Moskowitz et al. 1979). Most meningeal afferents have slow conduction velocities in the A-delta and C-fiber range and exhibit polymodal sensitivity to chemical, mechanical, and thermal stimulation, similar to nociceptor neurons innervating other tissues (Bove & Moskowitz 1997, Levy & Strassman 2002b, Strassman & Levy 2006, Strassman et al. 1996).
Many meningeal afferents are inflammation sensors that respond to proinflammatory molecules, including protons, ATP, histamine, prostaglandins, proteases, and cytokines [e.g., tumor necrosis factor-a (TNF-α), IL-1β]. The response to such molecules points to a potential role for meningeal inflammatory mechanisms in migraine headache (Levy et al. 2007; Strassman et al. 1996; Zhang & Levy 2008; Zhang et al. 2007, 2011, 2012). A large population of meningeal afferents also exhibit increased excitability (i.e., peripheral sensitization) when exposed to algesic inflammatory molecules, either alone or in combination (i.e., inflammatory soup). Sensitized meningeal afferents discharge persistently (Strassman et al. 1996), which could drive an ongoing migraine headache. The persistent discharge of meningeal afferents sensitizes trigeminal medullary dorsal horn neurons that receive convergent inputs from the dura and cephalic skin (Burstein et al. 1998). This augmented response produces cranial cutaneous allodynia, a common sensory component of migraine attacks (Bigal et al. 2008) and a surrogate marker of migraine headache in animal models (Edelmayer et al. 2009).
The dura contains mechanosensitive high-threshold meningeal afferents that respond to compressive forces applied to their exposed depressurized dural receptive fields (Levy & Strassman 2002b). Another subset of meningeal afferents, recently observed in awake mice with intact and pressurized meninges, are sensitive to small intracranial tensile and shearing loads that deform the meninges during locomotion (Blaeser et al. 2022). Normal increases in intracranial pressure and pial vasodilation, such as during physical activity (Gao & Drew 2016), could produce the intracranial forces that drive meningeal deformations and the ensuing afferent responses. Meningeal afferents responding to physiological meningeal deformations may constitute a population of low-threshold mechanoreceptor (LTMR) afferents that possess unencapsulated Ruffini-like endings (Andres et al. 1987, von Buchholtz et al. 2020). Activation of meningeal LTMR afferents is unlikely to produce headache under steady state but may relay interoceptive signals from the meninges. During migraine, however, the sensitization of trigeminal dorsal horn circuitry could render normal responses of meningeal LTMR afferents painful and produce a state of intracranial mechanical allodynia.
Augmented mechanosensitivity of high-threshold and mechanically insensitive meningeal afferents may also contribute to migraine pain by exacerbating the headache during normally innocuous physical activities (Blau & Dexter 1981, Strassman & Levy 2006). Interestingly, the mechanisms underlying meningeal afferent mechanical hyperresponsiveness appear to be independent of those responsible for increased ongoing discharge and involve numerous signaling molecules and cascades, including cAMP-PKA, nitric oxide, prostanoids, and activation of extracellular signal–regulated kinase (ERK) and p38 MAP kinases (Levy & Strassman 2002a, 2004; Zhang et al. 2011, 2013). Fully treating migraine pain may require decreasing both ongoing discharge and mechanical hypersensitivity, perhaps by targeting signaling pathways upstream of meningeal afferent sensitization, for example, by limiting the release of proinflammatory molecules.
ION CHANNELS AND RECEPTORS UNDERLYING MENINGEAL AFFERENT RESPONSES
The response properties of meningeal afferents, including their sensitization, are governed by voltage-gated ion channels and receptors (ionic and metabotropic). In vitro, compared to nociceptive afferents innervating other trigeminal tissues, meningeal afferents exhibit different electrophysiological properties, including higher baseline excitability and a propensity to become sensitized by inflammatory stimuli (Harriott & Gold 2009). Such differences may be due to expression levels, relative numbers, and functions of voltage-gated potassium, calcium, and sodium ion channels. Meningeal afferents express both tetrodotoxin-sensitive (Nav1.7) and tetrodotoxin-resistant (Nav1.8, Nav1.9) voltage-gated sodium channels (von Buchholtz et al. 2020, Yan et al. 2012) that are critical for initiating and propagating neuronal discharges evoked by mechanical stimulation (Strassman & Raymond 1999).
Potentiation of tetrodotoxin-sensitive and tetrodotoxin-resistant voltage-gated sodium currents underlies inflammatory sensitization of meningeal afferents (Harriott & Gold 2009, Vaughn & Gold 2010). A calcium-dependent chloride current may also play a key role (Vaughn & Gold 2010). Potassium (K+) currents are also implicated in nociceptor excitability. Of potential relevance to migraine is the TWIK-related spinal cord K+ channel (TRESK, encoded by KCNK18), which is responsible, along with other two-pore domain K+ channels, for background (leak) K+ currents. Dominant-negative KCNK18 mutations were linked to familial migraine with aura (MA) (Lafreniere et al. 2010), and knockout mice lacking these channels display hyperexcitation of a meningeal afferent subset causing headache-related behaviors (Guo et al. 2019). ATP-sensitive potassium channel (KATP) opening has also been linked to migraine headache triggering in humans (Al-Karagholi et al. 2019). However, locally applying the KATP opener levcromakalim suppresses meningeal afferent discharge in a rat model (Zhao & Levy 2018b). More work is needed to clarify these apparent species differences.
Despite its significance to migraine pain, we know very little about the molecular mechanism underlying the transduction of mechanical stimuli in meningeal afferents. The mechanosensitive channel encoded by the PIEZO2 gene appears critical for mechanonociception and is expressed by a subset of medium-sized, primarily nonpeptidergic trigeminal sensory afferents (Murthy et al. 2018, Yang et al. 2022). The role of Piezo2 in meningeal nociception remains understudied. Transient receptor potential vanilloid-type 4 (TRPV4) responds to osmotic cell swelling and is another putative mechanosensitive ion channel. TRPV4 agonists evoke currents in rats’ meningeal afferents when applied to their cell bodies in vitro, whereas their dural application drives cranial cutaneous tactile allodynia (Wei et al. 2011). TRPV4, however, is minimally expressed in the trigeminal ganglia of mice and humans (Yang et al. 2022).
A large subset of meningeal afferents that contain CGRP also express transient receptor potential vanilloid-type 1 (TRPV1) channels (von Buchholtz et al. 2020). Physiologically, TRPV1 is activated by temperatures greater than 42°C and mediates cutaneous heat nociception (Patapoutian et al. 2003). However, intracranial temperatures of such magnitudes rarely occur. Inflammatory mediators [e.g., prostaglandin E2 (PGE2) and bradykinin] sensitize TRPV1 and shift the activation threshold to lower temperatures (as low as 35°C) (Patapoutian et al. 2003). The lower thermal threshold (mean 39°C) of meningeal afferents observed in vivo (Bove & Moskowitz 1997) may reflect sensitization of TRPV1 in response to inflammatory molecules released during surgical exposure (Levy et al. 2007). TRPV1-expressing cutaneous sensory neurons release CGRP when stimulated by noxious heat (Patapoutian et al. 2003). At normal body temperature, sensitized TRPV1 channels may drive meningeal afferent discharge and subsequent local CGRP (Dux et al. 2003, Reeh & Petho 2000). Additionally, TRPV1 is a proton sensor (Patapoutian et al. 2003) and may sense meningeal inflammation by detecting interstitial acidic pH (Bove & Moskowitz 1997). While animal models support the role of TRPV1 signaling in meningeal nociception (Lambert et al. 2009, Meents et al. 2015), its contribution to the headache phase of migraine in humans remains uncertain given the poor clinical efficacy of acute systemic TRPV1 targeting (Chizh et al. 2009).
A few other ionotropic receptors are important in meningeal nociception: for example, transient receptor potential ankyrin 1 (TRPA1) and acid-sensing ion channels (ASICs). TRPA1, which non-neural cells also express, is one of the most-studied receptors in preclinical models. A crucial receptor for many headache-causing environmental irritants, TRPA1 opens in response to reactive oxygen or nitrergic species. TRPA1 agonists delivered systemically, nasally, or locally to the dura cause CGRP release from dural afferents and subsequent vasodilatation (Dux et al. 2016, Nassini et al. 2012). Dural application of TRPA1 agonists produces cranial cutaneous allodynia and reduced locomotor activity, another migraine-like behavior in rats (Edelmayer et al. 2012). While these studies implicate TRPA1 in meningeal nociception and headache, its expression in meningeal afferents is low, at least in mice (von Buchholtz et al. 2020). TRPA1 activation on meningeal vascular cells and perhaps Schwann cells (see below) may also contribute (De Logu et al. 2022, Hansted et al. 2020). ASICs expressed by meningeal afferents respond to extracellular protons, and their selective activation can also drive migraine-like headache behaviors in rats (Yan et al. 2011).
Additional cellular mechanisms, receptors, and cytokines were found to be central to meningeal nociception and may be therapeutically relevant. For example, activated dural mast cells release their inflammatory mediators to produce meningeal nociception (Levy et al. 2007, Zhang et al. 2007) and cranial cutaneous allodynia (Levy et al. 2012). Released tryptases activate protease-activated receptor-2 (PAR2), expressed by meningeal afferents and fibroblasts (Hassler et al. 2019, Zhang & Levy 2008). ATP, released from damaged or activated meningeal cells and from glial limitans astrocytes, can directly stimulate meningeal afferents, presumably by activating P2X2/3 purinergic receptors (Zhao & Levy 2015). Additionally, ATP may promote meningeal nociception by stimulating the release of other algesic molecules from meningeal immune cells through P2X7 receptor activation (Van Hove et al. 2019).
The proinflammatory cytokines IL-1β, TNF-α, and IL-6 may also play a role. IL-1β sensitizes meningeal afferents (Zhang et al. 2012), most likely by acting directly on their axonal receptors and modulating voltage-gated sodium channels (Binshtok et al. 2008). Acting indirectly, TNF-α sensitizes meningeal afferents by locally activating nonneuronal TNF1 and TNF2 receptors as well as downstream nonneuronal cyclooxygenase (COX) and the p38 MAP kinase (Zhang et al. 2011). Local meningeal IL-6 action sensitizes meningeal afferent somata in vitro (Yan et al. 2012) but not when applied to their dural axonal terminals in vivo (Zhang et al. 2012).
MENINGEAL RESPONSES IN A MIGRAINE WITH AURA MODEL
Animal models studying attack triggers may reveal endogenous processes that drive the meningeal sensory system during migraine. For example, cortical spreading depolarization (CSD) is a key pathophysiological event that underlies visual and sensory auras in migraine and activates the meningeal sensory system (Carneiro-Nascimento & Levy 2022). CSD is a slowly propagating depolarization of neurons and astrocytes that drastically disrupts transmembrane gradients and cortical synaptic activity. The evidence implicating CSD in migraine aura dates back almost a century to the clinical observation that slowly propagating visual auras may relate retinotopically to a slowly propagating depolarization along the primary visual cortex (Lashley 1941). A study using blood oxygenation level–dependent (BOLD) imaging (to measure tissue oxygenation and blood flow) documented signal changes in a patient who could trigger his own attacks of MA (Hadjikhani et al. 2001). When recording from consecutive voxels in this study, the BOLD signal change marched across the primary visual cortex with spatial and temporal characteristics matching CSD in experimental animals. The signal changes were congruent with the retinotopy of the visual percept and exhibited at least eight characteristics shared with CSD-induced blood flow changes in anesthetized rats, including unique propagation velocity (approximately 2–5 mm/min). Despite the strong experimental CSD data in the injured human brain (e.g., stroke, trauma, subarachnoid hemorrhage) (Dreier 2011), there is still an unmet need to record and study propagating depolarizations electrophysiologically using noninvasive tools in migraineurs.
Consistent with the links between CSD and migraine, as detailed above, brain-penetrant drugs used to prevent migraine attacks raise the CSD evocation threshold when administered chronically (Ayata et al. 2006). These pharmacologically promiscuous drugs, including topiramate, valproic acid, amitriptyline, propranolol, and methysergide, likely target glutamate cycling or K+ clearance mechanisms, both critical to CSD initiation and propagation (Pietrobon & Moskowitz 2014). That CSD plays a role in migraine is also supported by work with genetically engineered mice expressing human mutations causing severe migraine auras (e.g., familial hemiplegic migraine types 1, 2, and 3), as these animals also have reduced CSD evocation threshold. [For more detailed information offering invaluable insights into MA, see Pietrobon & Brennan (2019).]
Animal experiments have led to the theory that CSD is the main driver of inflammatory nociceptive signaling and the ensuing headache in MA. Firstly, within minutes of CSD provocation, numerous algesic mediators are released into the cortical interstitium, including K+, H+, ATP, arachidonic acid, glutamate, serotonin, and nitric oxide (Csiba et al. 1985, Enger et al. 2015, Lauritzen et al. 1990, Schock et al. 2007, Zhou et al. 2013). IL-1β has also been measured acutely, followed by delayed production of C-C motif chemokine ligand 2 and TNF-α (Karatas et al. 2013, Takizawa et al. 2020). According to one formulation (Figure 1), a fraction of released algesic molecules migrate outward via bulk diffusion or glymphatic CSF flow and reach afferent nerve endings near pial vessels and arachnoid trabeculae within the subarachnoid space (Fricke et al. 1997, Liu-Chen et al. 1983, Mayberg et al. 1981). Branching trigeminal axons that innervate the dura and leptomeningeal tissues (O’Connor & van der Kooy 1986) also allow dural afferent collaterals to be activated via an axon reflex. A pain-specific trigeminal-parasympathetic reflex follows (Bolay et al. 2002, Karatas et al. 2013). In agreement, electrophysiological recordings document a brief discharge of meningeal afferents immediately after CSD in rats (Zhang et al. 2010, Zhao & Levy 2015), as predicted (Moskowitz 1984). About 15 min later, CSD also produces a prolonged afferent discharge that lasts for at least 1 h (Zhang et al. 2010, Zhao & Levy 2015). Importantly, the delayed activation correlates with headache latency; in 90% of cases, the headache begins within 30 min of aura (Viana et al. 2016).
Figure 1.
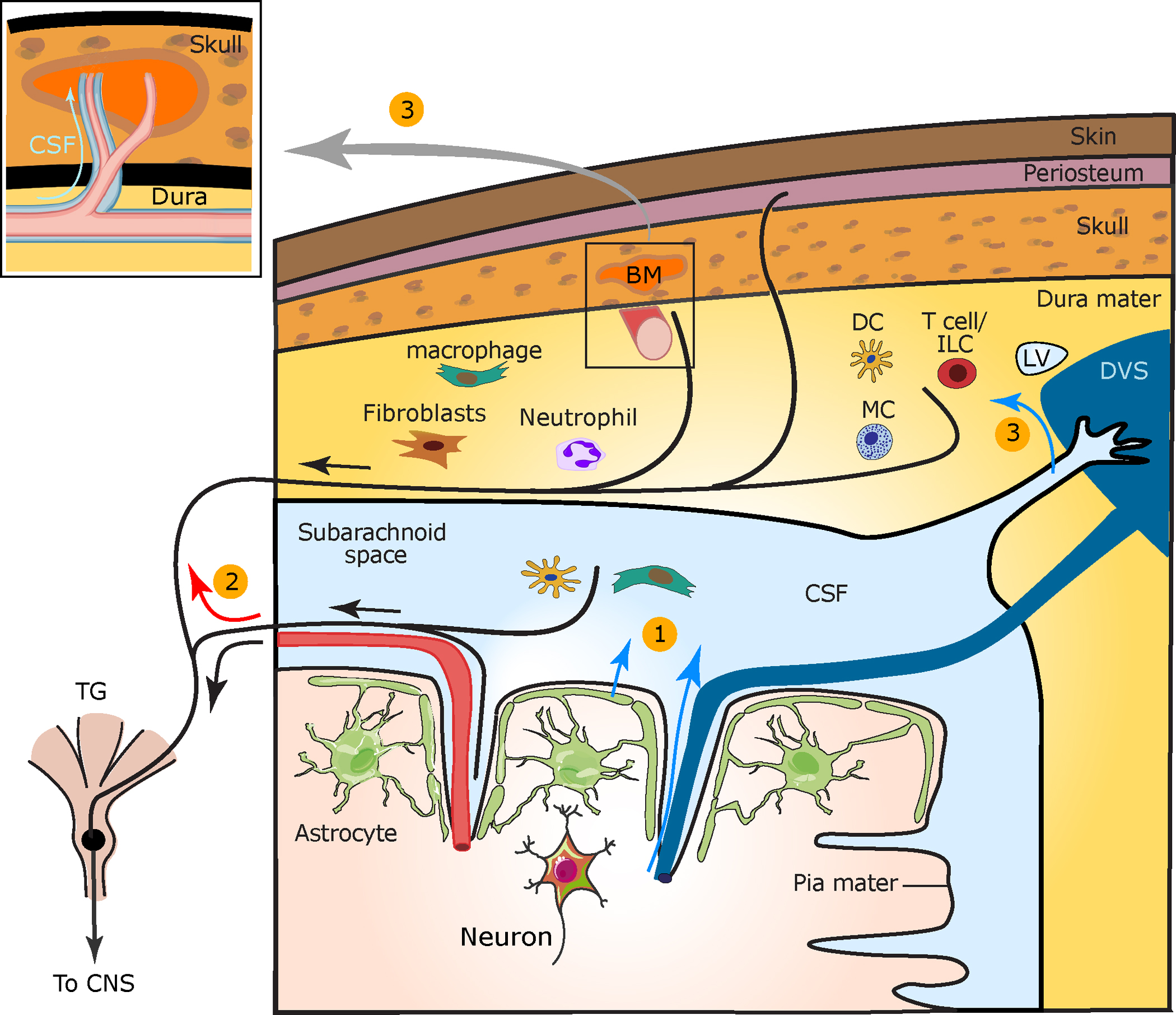
Cortical to meningeal signaling underlying CSD-evoked meningeal nociception. CSD is a key event in migraine with aura. (①) In the wake of CSD, algesic mediators are released from cortical neurons and astrocytes and diffuse across the pial membrane or are transported via glymphatic flow into the CSF (blue arrows). These mediators may directly activate meningeal afferents surrounding pial vessels. Released cortical mediators may also activate subdural immune cells to release pronociceptive factors and excite pial afferents. (②) Antidromic axon reflex (red arrow) is posited to cause the release of neuropeptides from collateral dural afferent fibers that produce neurogenic inflammation, which involves vascular and immune responses with subsequent sensitization of dural afferents. (③) CSF algesic mediators are additionally transported into the dura mater near venous sinuses, lymphatic vessels, and perivascular spaces surrounding dural vessels (box) and may further sensitize dural afferents. CSF flow into calvarial bone marrow via channels may influence meningeal immune cell production and transport and locally trigger collateral afferents. Abbreviations: BM, bone marrow; CNS, central nervous system; CSD, cortical spreading depolarization; CSF, cerebrospinal fluid; DC, dendritic cell; DVS, dural venous sinus; LV, lymphatic vessel; MC, mast cell; TG, trigeminal ganglion. Figure adapted from Carneiro-Nascimento & Levy (2022) (CC BY 4.0).
Delayed and prolonged meningeal afferent discharge following CSD may be due to delayed production and sustained release of additional algesic mediators (e.g., cytokines) from cortical neurons, astrocytes, and perhaps other cortical cells. Shrinkage of the paravascular space surrounding penetrating arteries and pial vessels (Schain et al. 2017), possibly via astrocytic endfeet swelling (Rosic et al. 2019), and related slowing of CSF outflow along glymphatic clearance routes could explain delayed delivery of inflammatory mediators to the meninges and the responses of dural afferents.
Furthermore, CSF may carry nociceptive signals directly to the dura mater, where CSF surrounds dural blood vessels in proximity to dural afferent axons (Pulous et al. 2022). Outflow of CSF into the dura mater adjacent to the superior sagittal sinus (Ringstad & Eide 2020) and transverse sinus (Louveau et al. 2018) may also affect dural afferents that densely innervate these areas (Strassman et al. 2004). CSF also exits locally into the overlying skull bone marrow via small bony channels (Mazzitelli et al. 2022, Pulous et al. 2022) that PET/MRI imaging studies suggest may also be involved in MA (Hadjikhani et al. 2020). CSF outflow into local bone and CSF drainage via dural lymphatic vessels to deep cervical lymph nodes (Louveau et al. 2018, Rustenhoven et al. 2021) could explain why cytokine levels in remote lumbar CSF remain unchanged in migraine (Cowan et al. 2021).
Electrophysiological studies of meningeal afferents in rats revealed that CSD also evokes persistent mechanical sensitization with an initial latency similar to the delayed discharge of meningeal afferents (Zhao & Levy 2016). CSD-evoked mechanical sensitization of meningeal afferents depends on local production of COX-driven prostanoids and not prostanoid-induced cortical vasoconstriction or related metabolic perturbations (Gariepy et al. 2017, Zhao & Levy 2018b). Cortical astrocytes may also participate via Ca2+-independent signaling (Zhao et al. 2021) involving nuclear factor κB (NF-κB) translocation followed by COX-2 and inducible nitric oxide synthase (iNOS) induction (Karatas et al. 2013).
MENINGEAL NEUROGENIC INFLAMMATION: MECHANISM AND THERAPEUTIC TARGET
Activated nociceptors release CGRP and substance P (and other vasoactive mediators) from their peripheral axons. These neuropeptides act on vascular endothelial and smooth muscle cells to promote vasodilatation and increased capillary permeability, a process known as neurogenic inflammation. Animal experiments suggest that dural neurogenic inflammation may play an important role in migraine pathophysiology (Bolay et al. 2002; Moskowitz 1984, 1993). Neurogenically mediated elevated dural capillary permeability occurs in response to antidromic electrical trigeminal ganglion (TG) stimulation, consistent with local vasoactive neuropeptide release (Markowitz et al. 1987). In MA, the formulation proposes that cortical factors released in response to CSD activate TG pial sensory afferents, instigating axon reflex and antidromic release of vasoactive neuropeptides from collateral dural afferent nerve endings that drive dural neurogenic inflammation (Figures 1 and 2). In migraine without aura, other endogenous or exogenous triggers may produce the initial discharge of dural afferents that drive antidromic neuropeptide release and dural neurogenic inflammation.
Figure 2.
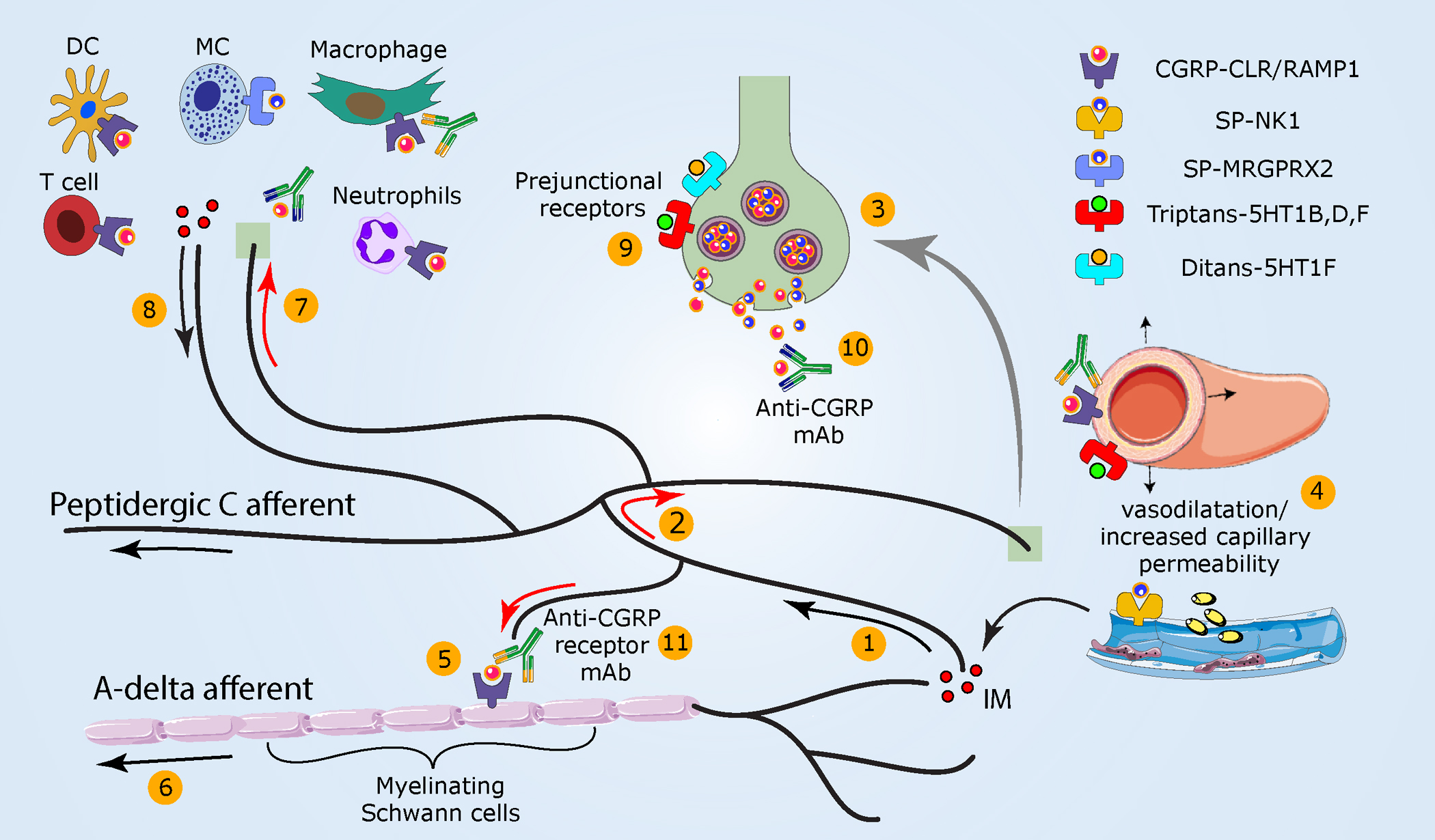
Meningeal neurogenic inflammation as a potential migraine headache mechanism. (①) As noted in Figure 1, the activation of peptidergic meningeal afferents (②) generates an antidromic axonal reflex (red arrows) and (③) neuropeptide release. (④) Subsequently, vasodilatation, increased capillary permeability, and IM release ensue. (⑤) CGRP, locally released, can also engage cell surface receptors that internalize on Schwann cells ensheathing myelinated A-delta meningeal afferents. Hypothesized downstream mechanisms include persistent CGRP receptor signaling within endosomes that (⑥) enhances A-delta afferent activity via TRPA1-dependent mechanisms. (⑦) Released sensory neuropeptides may also drive a local meningeal neurogenic immune response (⑧) that could further sensitize meningeal afferents. (⑨) Triptans used to abort migraine headache activate prejunctional 5-HT1B/1D/1F receptors on meningeal afferent terminals to inhibit the release of neuropeptides and related development of meningeal neurogenic inflammation. Triptans additionally act on vascular receptors to produce arterial vasoconstriction. Activation of prejunctional 5-HT1F receptors inhibits meningeal afferent neuropeptide release and aborts migraine pain without causing vasoconstriction. CGRP receptor antagonists block meningeal neurogenic vasodilatation and abort migraine headaches. (⑩) mAbs that bind CGRP or its receptor are used preventatively in the clinic and act selectively in the periphery. Abbreviations: CGRP, calcitonin gene–related peptide; CLR, calcitonin receptor–like receptor, IM, inflammatory mediator; mAb, monoclonal antibody; MRGPRX2, Mas-related G protein–coupled receptor-X2; NK1, neurokinin 1 receptor; RAMP1, receptor activity–modifying protein-1; SP, substance P.
Abortive migraine drugs such as ergot alkaloids and triptans (5-HT1B/1D/1F agonists) block neuropeptide release from stimulated dural afferent axons through their prejunctional receptor sites and inhibit subsequent neurogenic inflammation (Buzzi & Moskowitz 1990, Buzzi et al. 1991, Moskowitz & Cutrer 1993) (Figure 2). CGRP receptor antagonists that abort migraine headaches also limit dural neurogenic vasodilatation (Petersen et al. 2004). Importantly, however, if these antimigraine agents penetrated the blood-brain barrier, they could also curtail neuropeptide release from the central terminals of dural afferents, thereby disrupting their communication with second-order dorsal horn neurons (Levy et al. 2004, Storer & Goadsby 1997, Storer et al. 2004) to abort migraine pain. Monoclonal antibodies against CGRP, which likely act selectively in the periphery, hinder dural neurogenic inflammation and prevent migraine (Zeller et al. 2008).
A 5-HT1F receptor agonist, the first-in-class ditan, was recently launched as an abortive migraine treatment (Ashina et al. 2019). This receptor is expressed by TG neurons (Yang et al. 2022) but, unlike the 5-HT1B/1D receptors, not by vascular smooth muscle cells (Bouchelet et al. 1996). Functional receptors were found in rat meninges (Phebus et al. 1997), and, as with triptans, receptor activation blocks dural neuropeptide release and related neurogenic inflammation (Labastida-Ramirez et al. 2020, Phebus et al. 1997). Further suggesting a central action site, the superficial lamina of the medullary dorsal horn contains 5-HT1F-like binding sites (Waeber & Moskowitz 1995), plus a 5-HT1F agonist reduced medullary dorsal horn neuronal activation in response to meningeal noxious stimulation (Mitsikostas et al. 1999). Importantly, ditan is safe for patients at risk for stroke or coronary artery disease and is, therefore, a significant clinical advance.
Despite the therapeutic success and preclinical data indicating that meningeal neurogenic inflammation plays a role in migraine, a key yet unanswered question is whether such a response impacts meningeal nociception and drives the persistent headache in migraine. Theoretically, increased vascular permeability recruits additional nociceptive molecules that further sensitize dural afferents. Meningeal vasodilatation may also drive activity in sensitized mechanosensitive perivascular meningeal afferents. However, ablating a meningeal vasodilating pathway (TG afferents→parasympathetic efferents) did not affect the persistent meningeal afferent activation following CSD (Zhang et al. 2010).
CGRP is a key mediator of meningeal neurogenic vasodilatation and a migraine headache trigger, but its role in meningeal nociception in experimental animal models remains unclear. For example, CGRP drives potent dural vasodilatation, but dural application neither activates nor sensitizes meningeal afferents in male rats (Levy et al. 2005). Yet, brief local stimulation of meningeal afferents (as expected in CSD) using hyperosmolar K+ leads to a CGRP-dependent prolonged rise in afferent activity but not mechanical sensitization (Zhao & Levy 2018a). Dural stimulation by CGRP produces cranial cutaneous allodynia selectively in female rats and mice (Avona et al. 2019) through a mechanism involving local prolactin release and activation of its cognate receptors on dural afferents (Avona et al. 2021). Clinical data show, however, that migraine headache triggered via systemic CGRP administration is not female specific (Hansen et al. 2010). Moreover, the therapeutic actions of CGRP receptor antagonists and blocking monoclonal antibodies are also sex independent (Connor et al. 2009, Dodick et al. 2014). Additional studies are needed to clarify this.
The therapeutic effects of blocking CGRP signaling are clear in migraineurs but more complicated in animal models. For example, intravenously administered anti-CGRP monoclonal antibody reduced the propensity of meningeal A-delta (but not C) afferents to become activated by CSD (Melo-Carrillo et al. 2017) but had no effect on the related dural vasodilatation (Schain et al. 2019). On the other hand, similar administration of a CGRP receptor antagonist abrogated CSD-evoked meningeal vasodilation but did not prevent the accompanying activation and mechano-sensitization of meningeal afferents (Zhao & Levy 2018a). However, oral administration of a different CGRP receptor antagonist attenuated the activation of both A-delta and C-meningeal afferents by CSD (Strassman et al. 2022).
Recent experimental evidence implicates Schwann cells in CGRP receptor signaling (De Logu et al. 2022). According to this model, internalized CGRP receptor signals from Schwann cell endosomes increase cyclic AMP/PKA and nitric oxide synthesis, directly opening Schwann cell TRPA1 channels. Subsequent reactive oxygen species (ROS) release leads to further recruitment and opening of TRPA1 on adjacent nociceptive axons, resulting in their activation and sensitization. In this animal model, TRPA1 channel activation, ROS elaboration, and adjacent sensory fiber recruitment have been suggested as a neurogenic inflammation-associated mechanism mediating cutaneous mechanical allodynia in migraine, potentially independent of meningeal nociception. The CGRP receptor complex is also expressed in myelinating Schwann cell–ensheathing meningeal afferent axons and satellite ganglion cells in the TG (Lennerz et al. 2008), but their specific link to neurogenic inflammation and meningeal nociception remains to be established. Regardless, this new formulation is significant because it ties together molecules, receptors, and neurogenic mechanisms previously found to be relevant in animal and human migraine research. This work complements another cranial allodynia model implicating central sensitization of trigeminal dorsal horn neurons that receive convergent dural and cutaneous afferent inputs.
Other neuropeptides released from activated dural afferents may drive dural neurogenic inflammation and meningeal nociception. Initial studies identified substance P as a mediator following antidromic TG stimulation and CSD (Bolay et al. 2002, Lee et al. 1994). However, clinical trials blocking substance P neurokinin 1 (NK1) receptors did not reach statistical significance (May & Goadsby 2001). New strategies targeting the internalized SP-receptor complex improve drug efficacy in animal pain models (Jensen et al. 2017) and may be clinically useful in migraine. Substance P could also signal via Mas-related G protein–coupled receptors expressed on nociceptive afferents and mast cells, independent of NK1 and NK2 receptor signaling (Azimi et al. 2017, Green et al. 2019). PACAP’s role is also being actively pursued, although a recent clinical trial showed that blocking pituitary adenylate cyclase–activating polypeptide type I receptor (PAC1) had no benefit for migraine prevention (Ashina et al. 2021).
In addition to the vascular response, a growing body of evidence also points to an immune component of neurogenic inflammation, including immune cell recruitment and amplification of their functional responses (Chu et al. 2020). Recent findings that several meningeal immune cells, including macrophages, dendritic cells, mast cells, and T cells, express the CGRP receptor (Van Hove et al. 2019) suggest that CGRP may function as an immunomodulator in meningeal nociception. Notably, one study reported that human mast cells lack CGRP receptors, whereas another demonstrated CGRP receptor expression in primate mast cells (Miller et al. 2016). Whether species differences also occur for CGRP receptor expression in other immune cells remains unclear.
Consistent with a possible meningeal neuroimmune interaction in migraine, CSD activates dural and subdural macrophages and dural dendritic cells (Schain et al. 2018). Systemically administering the migraine trigger nitroglycerin (NTG) also activates dural immune cells (see below). Hematopoietic stem cells express CGRP receptors; their activation by CGRP-expressing sensory nerves in femur bone marrow leads to myeloid mobilization and egress from the bone marrow (Gao et al. 2021). A similar activation in the calvarium may be relevant to migraine, given the presence of skull channels connecting the skull bone marrow to the dura. Furthermore, the skull bone marrow serves as a reservoir for myeloid cells that can migrate through skull channels to enter the meninges and brain (Herisson et al. 2018), potentially affecting meningeal nociception. The recent finding of enhanced parameningeal 18-kDa translocator protein (TSPO) PET signal in MA patients may reflect activation, or perhaps inflammation, within the cortex and overlying peripheral tissues (Hadjikhani et al. 2020). Furthermore, the TSPO PET signal reveals a cortex-meninges-skull interaction that likely results from CSF transport between tissues rather than from blood (i.e., the meninges and bone marrow receive a blood supply distinct from that of the brain).
VASODILATORS, THE MENINGES, AND MIGRAINE
While the contribution of meningeal vasodilation to migraine pain remains uncertain, systemic administration of several vasodilating agents, in addition to CGRP, can trigger migraine-like attacks in migraineurs. These agents include nitric oxide donors, PACAP, the ATP-sensitive potassium channel opener levcromakalim, the phosphodiesterase type 3 inhibitor cilostazol, the cGMP-specific phosphodiesterase type 5 inhibitor sildenafil, VIP, and amylin (Al-Karagholi et al. 2019, Ashina et al. 2017, Ghanizada et al. 2021). Among those, the nitric oxide donor NTG is the most widely studied, and the mechanisms by which it provokes migraine pain have been addressed in animal models.
In humans, low-dose NTG infusion evokes a brief, minor headache in almost all subjects. Administering the same dose to migraine sufferers, however, produces premonitory symptoms, headaches, and cranial cutaneous allodynia after several hours that mimic spontaneous migraine attacks (Akerman et al. 2019, Karsan et al. 2020). While the antimigraine drug sumatriptan relieves the NTG-evoked delayed migraine-like headache (Afridi et al. 2005), neither CGRP signaling nor meningeal vasodilatation appears to mediate the headache (Schoonman et al. 2008, Tvedskov et al. 2010). NTG susceptibility in migraineurs may reflect an inflammatory mechanism that potentiates meningeal afferent responses to NTG and perhaps to other exogenous or endogenous triggers. In previously naive animals, exposure to inflammatory mediators facilitates cranial allodynia in response to low-dose NTG or a related nitric oxide donor (Burgos-Vega et al. 2016, Oshinsky & Gomonchareonsiri 2007).
In clinically relevant doses, NTG causes delayed meningeal inflammation such as mast cell degranulation, macrophage activation, NF-κB-dependent upregulation of iNOS, and the appearance of IL-1β and IL-6 (Reuter et al. 2001, 2002). Low-dose NTG also provoked a delayed and robust increase in the mechanosensitivity of meningeal afferents involving ERK phosphorylation in dural arteries, with a time course resembling the delayed onset of migraine headache (Zhang et al. 2013). Hence, studying the effects of low-dose NTG infusion might be useful for harmonizing responses in animal models and humans.
CONCLUDING REMARKS
This review emphasizes the cranial meninges as a critical tissue for studying migraine headaches and a major source of headache from within the cranium. So far, animal models have served us reasonably well, as the human meninges are challenging to study in vivo. These animal models have helped to establish serotonin receptor–driven inhibition of neuropeptide release from meningeal afferent fibers as a relevant triptan and ergot alkaloid action that relieves acute migraine headaches; the results thereby underscored vasoactive neuropeptides as potential therapeutic targets. As one consequence, animal models have contributed to the development of a new class of drugs that block neuropeptide release without constricting vascular smooth muscle (the ditans). Models have also helped to establish CGRP as a major target of acute and chronic migraine therapy and to identify its multiplicity of targets (e.g., Schwann cells) in addition to vascular smooth muscle. Which of these targets is most therapeutically relevant in migraine needs further study. Animal models have also established CSD as the first endogenous headache trigger that links intense brain activity (CSD in MA) to augmented meningeal afferent responses. In an earlier stage of discovery, recent data suggests CSF as the conveyor of pain and inflammatory signaling between the cortex and meninges. Similarly, there is much to learn about migraine triggering, such as in migraine without aura. In this subtype, the brain, blood vessels, blood, and meningeal processes (e.g., inflammation) are candidates, although presently, data are lacking. Many of these discoveries in animal models were built on research characterizing the trigeminal afferents innervating the meninges, including their response properties and the constellation of ion channels, receptors, and second messenger cascades critical to their sensitization. The ultimate goal here is to achieve a clearer understanding of migraine pathogenesis and, by so doing, expand the repertoire of therapeutic targets and treatments for migraine patients.
ACKNOWLEDGMENTS
We would like to thank Simone Carneiro-Nascimento for helping with the figures and Kaley Joyes for editing the manuscript. We apologize to scientists whose work we could not include due to space and citation limitations. The writing of this review was supported by grants from the National Institutes of Health [R01NS078263, R01NS115972, R21NS130561 (to D.L.) and R01NS108419 (to M.A.M)].
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, et al. 2005. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 128:932–39 [DOI] [PubMed] [Google Scholar]
- Akerman S, Karsan N, Bose P, Hoffmann JR, Holland PR, et al. 2019. Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity. Brain 142:103–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Karagholi MA, Hansen JM, Guo S, Olesen J, Ashina M. 2019. Opening of ATP-sensitive potassium channels causes migraine attacks: a new target for the treatment of migraine. Brain 142:2644–54 [DOI] [PubMed] [Google Scholar]
- Alves De Lima K, Rustenhoven J, Kipnis J. 2020. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol. 38:597–620 [DOI] [PubMed] [Google Scholar]
- Ampie L, McGavern DB. 2022. Immunological defense of CNS barriers against infections. Immunity 55:781–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres KH, von During M, Muszynski K, Schmidt RF. 1987. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat. Embryol. 175:289–301 [DOI] [PubMed] [Google Scholar]
- Ashina M, Dolezil D, Bonner JH, Zhou L, Klatt J, et al. 2021. A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia 41:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashina M, Møller Hansen J, Oladóttir Á Dunga B, Olesen J. 2017. Human models of migraine—short-term pain for long-term gain. Nat. Rev. Neurol. 13:713–24 [DOI] [PubMed] [Google Scholar]
- Ashina M, Vasudeva R, Jin L, Lombard L, Gray E, et al. 2019. Onset of efficacy following oral treatment with lasmiditan for the acute treatment of migraine: integrated results from 2 randomized double-blind placebo-controlled phase 3 clinical studies. Headache 59:1788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, et al. 2015. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212:991–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. 2019. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J. Neurosci. 39:4323–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avona A, Mason BN, Burgos-Vega C, Hovhannisyan AH, Belugin SN, et al. 2021. Meningeal CGRP-prolactin interaction evokes female-specific migraine behavior. Ann. Neurol. 89:1129–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. 2006. Suppression of cortical spreading depression in migraine prophylaxis. Ann. Neurol. 59:652–61 [DOI] [PubMed] [Google Scholar]
- Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. 2017. Substance P activates Mas-related G protein-coupled receptors to induce itch. J. Allergy Clin. Immunol. 140:447–53.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, et al. 2008. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology 70:1525–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, et al. 2008. Nociceptors are interleukin-1β sensors. J. Neurosci. 28:14062–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaeser AS, Sugden A, Zhao J, Carneiro-Nascimento S, Shipley FB, et al. 2022. Trigeminal afferents sense locomotion-related meningeal deformations. Cell Rep. 41:111648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau JN, Dexter SL. 1981. The site of pain origin during migraine attacks. Cephalalgia 1:143–47 [DOI] [PubMed] [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. 2002. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 8:136–42 [DOI] [PubMed] [Google Scholar]
- Bouchelet I, Cohen Z, Case B, Seguela P, Hamel E. 1996. Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral blood vessels. Mol. Pharmacol. 50:219–23 [PubMed] [Google Scholar]
- Bove GM, Moskowitz MA. 1997. Primary afferent neurons innervating guinea pig dura. J. Neurophysiol. 77:299–308 [DOI] [PubMed] [Google Scholar]
- Burgos-Vega CC, Quigley LD, Avona A, Price T, Dussor G. 2016. Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain 157:2722–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. 1998. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 79:964–82 [DOI] [PubMed] [Google Scholar]
- Buzzi MG, Carter WB, Shimizu T, Heath H 3rd, Moskowitz MA. 1991. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology 30:1193–200 [DOI] [PubMed] [Google Scholar]
- Buzzi MG, Moskowitz MA. 1990. The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br. J. Pharmacol. 99:202–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Forstera B, et al. 2019. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat. Neurosci. 22:317–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro-Nascimento S, Levy D. 2022. Cortical spreading depression and meningeal nociception. Neurobiol. Pain 11:100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh B, Palmer J, Lai R, Guillard F, Bullman J, et al. 2009. A randomised, two-period cross-over study to investigate the efficacy of the Trpv1 antagonist SB-705498 in acute migraine. Eur. J. Pain 13:S202a [Google Scholar]
- Chu C, Artis D, Chiu IM. 2020. Neuro-immune interactions in the tissues. Immunity 52:464–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Myburgh E, Brewer JM, McMenamin PG. 2017. Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog. Neurobiol. 156:107–48 [DOI] [PubMed] [Google Scholar]
- Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, et al. 2009. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 73:970–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RP, Gross NB, Sweeney MD, Sagare AP, Montagne A, et al. 2021. Evidence that blood-CSF barrier transport, but not inflammatory biomarkers, change in migraine, while CSF sVCAM1 associates with migraine frequency and CSF fibrinogen. Headache 61:536–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiba L, Paschen W, Mies G. 1985. Regional changes in tissue pH and glucose content during cortical spreading depression in rat brain. Brain Res. 336:167–70 [DOI] [PubMed] [Google Scholar]
- De Logu F, Nassini R, Hegron A, Landini L, Jensen DD, et al. 2022. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun. 13:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, et al. 2014. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 13:1100–7 [DOI] [PubMed] [Google Scholar]
- Dreier JP. 2011. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 17:439–47 [DOI] [PubMed] [Google Scholar]
- Dux M, Santha P, Jancso G. 2003. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J. Physiol. 552:859–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux M, Will C, Vogler B, Filipovic MR, Messlinger K. 2016. Meningeal blood flow is controlled by H2S-NO crosstalk activating a HNO-TRPA1-CGRP signalling pathway. Br. J. Pharmacol. 173:431–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, et al. 2012. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain 153:1949–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, et al. 2009. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann. Neurol. 65:184–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enger R, Tang W, Vindedal GF, Jensen V, Helm PJ, et al. 2015. Dynamics of ionic shifts in cortical spreading depression. Cereb. Cortex 25:4469–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, et al. 2022. Migraine. Nat. Rev. Dis. Primers 8:2. [DOI] [PubMed] [Google Scholar]
- Fontaine D, Almairac F, Santucci S, Fernandez C, Dallel R, et al. 2018. Dural and pial pain-sensitive structures in humans: new inputs from awake craniotomies. Brain 141:1040–48 [DOI] [PubMed] [Google Scholar]
- Fricke B, von Düring M, Andres KH. 1997. Topography and immunocytochemical characterization of nerve fibers in the leptomeningeal compartments of the rat. A light- and electron-microscopical study. Cell Tissue Res. 287:11–22 [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang D, Xu C, Li H, Caron KM, Frenette PS. 2021. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589:591–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YR, Drew PJ. 2016. Effects of voluntary locomotion and calcitonin gene-related peptide on the dynamics of single dural vessels in awake mice. J. Neurosci. 36:2503–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy H, Zhao J, Levy D. 2017. Differential contribution of COX-1 and COX-2 derived prostanoids to cortical spreading depression—evoked cerebral oligemia. J. Cereb. Blood Flow. Metab. 37:1060–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Dis. Inj. Collab. 2020. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396:1204–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizada H, Al-Karagholi MA, Walker CS, Arngrim N, Rees T, et al. 2021. Amylin analog pramlintide induces migraine-like attacks in patients. Ann. Neurol. 89:1157–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. 2019. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101:412–20.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Qiu CS, Jiang X, Zhang J, Li F, et al. 2019. TRESK K+ channel activity regulates trigeminal nociception and headache. eNeuro 6:ENEURO.0236–19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Albrecht DS, Mainero C, Ichijo E, Ward N, et al. 2020. Extra-axial inflammatory signal in parameninges in migraine with visual aura. Ann. Neurol. 87:939–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, et al. 2001. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. PNAS 98:4687–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Hauge AW, Olesen J, Ashina M. 2010. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 30:1179–86 [DOI] [PubMed] [Google Scholar]
- Hansted AK, Jensen LJ, Olesen J, Jansen-Olesen I. 2020. Localization of TRPA1 channels and characterization of TRPA1 mediated responses in dural and pial arteries in vivo after intracarotid infusion of Na2S. Cephalalgia 40:1310–20 [DOI] [PubMed] [Google Scholar]
- Harriott AM, Gold MS. 2009. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. J. Neurophysiol. 101:3126–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler SN, Ahmad FB, Burgos-Vega CC, Boitano S, Vagner J, et al. 2019. Protease activated receptor 2 (PAR2) activation causes migraine-like pain behaviors in mice. Cephalalgia 39:111–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, et al. 2018. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21:1209–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DD, Lieu T, Halls ML, Veldhuis NA, Imlach WL, et al. 2017. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci. Transl. Med. 9:eaal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, et al. 2013. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339:1092–95 [DOI] [PubMed] [Google Scholar]
- Karsan N, Bose PR, Thompson C, Newman J, Goadsby PJ. 2020. Headache and non-headache symptoms provoked by nitroglycerin in migraineurs: a human pharmacological triggering study. Cephalalgia 40:828–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaras B, Jakubowski M, Kainz V, Burstein R. 2009. Sensory innervation of the calvarial bones of the mouse. J. Comp. Neurol. 515:331–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labastida-Ramirez A, Rubio-Beltran E, Haanes KA, Chan KY, Garrelds IM, et al. 2020. Lasmiditan inhibits calcitonin gene-related peptide release in the rodent trigeminovascular system. Pain 161:1092–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere RG, Cader MZ, Poulin JF, Andres-Enguix I, Simoneau M, et al. 2010. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 16:1157–60 [DOI] [PubMed] [Google Scholar]
- Lambert GA, Davis JB, Appleby JM, Chizh BA, Hoskin KL, Zagami AS. 2009. The effects of the TRPV1 receptor antagonist SB-705498 on trigeminovascular sensitisation and neurotransmission. Naunyn-Schmiedeberg’s Arch. Pharmacol. 380:311–25 [DOI] [PubMed] [Google Scholar]
- Lashley KS. 1941. Patterns of cerebral integration indicated by the scotoma of migraine. Arch. Neurol. Psychiatry 46:331–39 [Google Scholar]
- Lauritzen M, Hansen AJ, Kronborg D, Wieloch T. 1990. Cortical spreading depression is associated with arachidonic acid accumulation and preservation of energy charge. J. Cereb. Blood Flow Metab. 10:115–22 [DOI] [PubMed] [Google Scholar]
- Lee WS, Moussaoui SM, Moskowitz MA. 1994. Blockade by oral or parenteral RPR 100893 (a non-peptide NK1 receptor antagonist) of neurogenic plasma protein extravasation within guinea-pig dura mater and conjunctiva. Br. J. Pharmacol. 112:920–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, et al. 2008. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J. Comp. Neurol. 507:1277–99 [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. 2007. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 130:166–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Burstein R, Strassman AM. 2005. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann. Neurol. 58:698–705 [DOI] [PubMed] [Google Scholar]
- Levy D, Jakubowski M, Burstein R. 2004. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT1B/1D receptor agonists. PNAS 101:4274–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Kainz V, Burstein R, Strassman AM. 2012. Mast cell degranulation distinctly activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity. Brain Behav. Immun. 26:311–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. 2002a. Distinct sensitizing effects of the cAMP-PKA second messenger cascade on rat dural mechanonociceptors. J. Physiol. 538:483–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. 2002b. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J. Neurophysiol. 88:3021–31 [DOI] [PubMed] [Google Scholar]
- Levy D, Strassman AM. 2004. Modulation of dural nociceptor mechanosensitivity by the nitric oxide-cyclic GMP signaling cascade. J. Neurophysiol. 92:766–72 [DOI] [PubMed] [Google Scholar]
- Liu-Chen LY, Mayberg MR, Moskowitz MA. 1983. Immunohistochemical evidence for a substance P-containing trigeminovascular pathway to pial arteries in cats. Brain Res. 268:162–66 [DOI] [PubMed] [Google Scholar]
- Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, et al. 2018. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21:1380–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, et al. 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Saito K, Moskowitz MA. 1987. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J. Neurosci. 7:4129–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Goadsby PJ. 2001. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Investig. Drugs 10:673–78 [DOI] [PubMed] [Google Scholar]
- Mayberg M, Langer RS, Zervas NT, Moskowitz MA. 1981. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science 213:228–30 [DOI] [PubMed] [Google Scholar]
- Mayberg M, Zervas NT, Moskowitz MA. 1984. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J. Comp. Neurol. 223:46–56 [DOI] [PubMed] [Google Scholar]
- Mazzitelli JA, Smyth LCD, Cross KA, Dykstra T, Sun J, et al. 2022. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat. Neurosci. 25:555–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents JE, Hoffmann J, Chaplan SR, Neeb L, Schuh-Hofer S, et al. 2015. Two TRPV1 receptor antagonists are effective in two different experimental models of migraine. J. Headache Pain 16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Carrillo A, Strassman AM, Nir RR, Schain AJ, Noseda R, et al. 2017. Fremanezumab—a humanized monoclonal anti-CGRP antibody-inhibits thinly myelinated (Aδ) but not unmyelinated (C) meningeal nociceptors. J. Neurosci. 37:10587–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Liu H, Warfvinge K, Shi L, Dovlatyan M, et al. 2016. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 328:165–83 [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M, Moskowitz MA, Waeber C. 1999. Both 5-HT1B and 5-HT1F receptors modulate c-fos expression within rat trigeminal nucleus caudalis. Eur. J. Pharmacol. 369:271–77 [DOI] [PubMed] [Google Scholar]
- Mollgard K, Beinlich FRM, Kusk P, Miyakoshi LM, Delle C, et al. 2023. A mesothelium divides the subarachnoid space into functional compartments. Science 379: 84–88 [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. 1984. The neurobiology of vascular head pain. Ann. Neurol. 16:157–68 [DOI] [PubMed] [Google Scholar]
- Moskowitz MA. 1993. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology 43:S16–20 [PubMed] [Google Scholar]
- Moskowitz MA, Cutrer FM. 1993. SUMATRIPTAN: a receptor-targeted treatment for migraine. Annu. Rev. Med. 44:145–54 [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Reinhard JF Jr., Romero J, Melamed E, Pettibone DJ. 1979. Neurotransmitters and the fifth cranial nerve: Is there a relation to the headache phase of migraine? Lancet 2:883–85 [DOI] [PubMed] [Google Scholar]
- Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, et al. 2018. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 10:eaat9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, et al. 2012. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 135:376–90 [DOI] [PubMed] [Google Scholar]
- O’Connor TP, van der Kooy D. 1986. Pattern of intracranial and extracranial projections of trigeminal ganglion cells. J. Neurosci. 6:2200–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshinsky ML, Gomonchareonsiri S. 2007. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 47:1026–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. 2003. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat. Rev. Neurosci. 4:529–39 [DOI] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Doods H, Edvinsson L, Olesen J. 2004. Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br. J. Pharmacol. 143:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, et al. 1997. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci. 61:2117–26 [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Brennan KC. 2019. Genetic mouse models of migraine. J. Headache Pain 20:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. 2014. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat. Rev. Neurosci. 15:379–93 [DOI] [PubMed] [Google Scholar]
- Pulous FE, Cruz-Hernandez JC, Yang C, Kaya Z, Paccalet A, et al. 2022. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat. Neurosci. 25:567–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray BS, Wolff HG. 1940. Experimental studies on headache: pain sensitive structures of the head and their significance in headache. Arch. Surg. 41:813–56 [Google Scholar]
- Reeh PW, Petho G. 2000. Nociceptor excitation by thermal sensitization—a hypothesis. Prog. Brain Res. 129:39–50 [DOI] [PubMed] [Google Scholar]
- Reuter U, Bolay H, Jansen-Olesen I, Chiarugi A, Sanchez del Rio M, et al. 2001. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain 124:2490–502 [DOI] [PubMed] [Google Scholar]
- Reuter U, Chiarugi A, Bolay H, Moskowitz MA. 2002. Nuclear factor-κB as a molecular target for migraine therapy. Ann. Neurol. 51:507–16 [DOI] [PubMed] [Google Scholar]
- Ringstad G, Eide PK. 2020. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat. Commun. 11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosic B, Dukefoss DB, Abjorsbraten KS, Tang W, Jensen V, et al. 2019. Aquaporin-4-independent volume dynamics of astroglial endfeet during cortical spreading depression. Glia 67:1113–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. 2014. Transcranial amelioration of inflammation and cell death after brain injury. Nature 505:223–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, et al. 2021. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184:1000–16.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain AJ, Melo-Carrillo A, Stratton J, Strassman AM, Burstein R. 2019. CSD-induced arterial dilatation and plasma protein extravasation are unaffected by fremanezumab: implications for CGRP’s role in migraine with aura. J. Neurosci 39:6001–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain AJ, Melo-Carrillo A, Borsook D, Grutzendler J, Strassman AM, Burstein R. 2018. Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann. Neurol. 83:508–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R. 2017. Cortical spreading depression closes paravascular space and impairs glymphatic flow: implications for migraine headache. J. Neurosci. 37:2904–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock SC, Munyao N, Yakubchyk Y, Sabourin LA, Hakim AM, et al. 2007. Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res. 1168:129–38 [DOI] [PubMed] [Google Scholar]
- Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. 2008. Migraine headache is not associated with cerebral or meningeal vasodilatation—a 3T magnetic resonance angiography study. Brain 131:2192–200 [DOI] [PubMed] [Google Scholar]
- Schueler M, Messlinger K, Dux M, Neuhuber WL, De Col R. 2013. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain 154:1622–31 [DOI] [PubMed] [Google Scholar]
- Storer RJ, Akerman S, Goadsby PJ. 2004. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br. J. Pharmacol. 142:1171–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer RJ, Goadsby PJ. 1997. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain 120:2171–77 [DOI] [PubMed] [Google Scholar]
- Strassman AM, Levy D. 2006. Response properties of dural nociceptors in relation to headache. J. Neurophysiol. 95:1298–306 [DOI] [PubMed] [Google Scholar]
- Strassman AM, Melo-Carrillo A, Houle TT, Adams A, Brin MF, Burstein R. 2022. Atogepant—an orally-administered CGRP antagonist—attenuates activation of meningeal nociceptors by CSD. Cephalalgia 42:933–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA. 1999. Electrophysiological evidence for tetrodotoxin-resistant sodium channels in slowly conducting dural sensory fibers. J. Neurophysiol. 81:413–24 [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. 1996. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384:560–64 [DOI] [PubMed] [Google Scholar]
- Strassman AM, Weissner W, Williams M, Ali S, Levy D. 2004. Axon diameters and intradural trajectories of the dural innervation in the rat. J. Comp. Neurol. 473:364–76 [DOI] [PubMed] [Google Scholar]
- Takizawa T, Qin T, Lopes de Morais A, Sugimoto K, Chung JY, et al. 2020. Non-invasively triggered spreading depolarizations induce a rapid pro-inflammatory response in cerebral cortex. J. Cereb. Blood Flow Metab. 40:1117–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvedskov JF, Tfelt-Hansen P, Petersen KA, Jensen LT, Olesen J. 2010. CGRP receptor antagonist olcegepant (BIBN4096BS) does not prevent glyceryl trinitrate-induced migraine. Cephalalgia 30:1346–53 [DOI] [PubMed] [Google Scholar]
- Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, et al. 2019. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22:1021–35 [DOI] [PubMed] [Google Scholar]
- Vaughn AH, Gold MS. 2010. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J. Neurosci. 30:7878–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana M, Linde M, Sances G, Ghiotto N, Guaschino E, et al. 2016. Migraine aura symptoms: duration, succession and temporal relationship to headache. Cephalalgia 36:413–21 [DOI] [PubMed] [Google Scholar]
- von Buchholtz LJ, Lam RM, Emrick JJ, Chesler AT, Ryba NJP. 2020. Assigning transcriptomic class in the trigeminal ganglion using multiplex in situ hybridization and machine learning. Pain 161:2212–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. 1995. [3H]sumatriptan labels both 5-HT1D and 5-HT1F receptor binding sites in the guinea pig brain: an autoradiographic study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 352:263–75 [DOI] [PubMed] [Google Scholar]
- Wei X, Edelmayer RM, Yan J, Dussor G. 2011. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 31:1595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G. 2011. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain 152:106–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Melemedjian OK, Price TJ, Dussor G. 2012. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol Pain 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Xu M, Bhuiyan SA, Li J, Zhao J, et al. 2022. Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron 110:1806–21.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller J, Poulsen KT, Sutton JE, Abdiche YN, Collier S, et al. 2008. CGRP function-blocking antibodies inhibit neurogenic vasodilatation without affecting heart rate or arterial blood pressure in the rat. Br. J. Pharmacol. 155:1093–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Burstein R, Levy D. 2012. Local action of the proinflammatory cytokines IL-1β and IL-6 on intracranial meningeal nociceptors. Cephalalgia 32:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kainz V, Burstein R, Levy D. 2011. Tumor necrosis factor-α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain 152:140–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kainz V, Zhao J, Strassman AM, Levy D. 2013. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Ann. Neurol. 73:741–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D. 2008. Modulation of meningeal nociceptors mechanosensitivity by peripheral proteinase-activated receptor-2: the role of mast cells. Cephalalgia 28:276–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. 2010. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J. Neurosci. 30:8807–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Strassman AM, Burstein R, Levy D. 2007. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J. Pharmacol. Exp. Ther. 322:806–12 [DOI] [PubMed] [Google Scholar]
- Zhao J, Blaeser AS, Levy D. 2021. Astrocytes mediate migraine-related intracranial meningeal mechanical hypersensitivity. Pain 162:2386–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bree D, Harrington MG, Strassman AM, Levy D. 2017. Cranial dural permeability of inflammatory nociceptive mediators: potential implications for animal models of migraine. Cephalalgia 37:1017–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Levy D. 2014. The sensory innervation of the calvarial periosteum is nociceptive and contributes to headache-like behavior. Pain 155:1392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Levy D. 2015. Modulation of intracranial meningeal nociceptor activity by cortical spreading depression: a reassessment. J. Neurophysiol. 113:2778–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Levy D. 2016. Cortical spreading depression promotes persistent mechanical sensitization of intracranial meningeal afferents: implications for the intracranial mechanosensitivity of migraine. eNeuro 3:ENEURO.0287–16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Levy D. 2018a. The CGRP receptor antagonist BIBN4096 inhibits prolonged meningeal afferent activation evoked by brief local K+ stimulation but not cortical spreading depression-induced afferent sensitization. Pain Rep 3:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Levy D. 2018b. Dissociation between CSD-evoked metabolic perturbations and meningeal afferent activation and sensitization: implications for mechanisms of migraine headache onset. J. Neurosci. 38:5053–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Rungta RL, Malik A, Han H, Wu DC, MacVicar BA. 2013. Regenerative glutamate release by presynaptic NMDA receptors contributes to spreading depression. J. Cereb. Blood Flow Metab. 33:1582–94 [DOI] [PMC free article] [PubMed] [Google Scholar]


