Abstract
Immunotherapy is an important cancer treatment method that offers hope for curing cancer patients. While immunotherapy has achieved initial success, a major obstacle to its widespread adoption is the inability to benefit the majority of patients. The success or failure of immunotherapy is closely linked to the tumor's immune microenvironment. Recently, there has been significant attention on strategies to regulate the tumor immune microenvironment in order to stimulate anti-tumor immune responses in cancer immunotherapy. The distinctive physical properties and design flexibility of nanomedicines have been extensively utilized to target immune cells (including tumor-associated macrophages (TAMs), T cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated fibroblasts (TAFs)), offering promising advancements in cancer immunotherapy. In this article, we have reviewed treatment strategies aimed at targeting various immune cells to regulate the tumor immune microenvironment. The focus is on cancer immunotherapy models that are based on nanomedicines, with the goal of inducing or enhancing anti-tumor immune responses to improve immunotherapy. It is worth noting that combining cancer immunotherapy with other treatments, such as chemotherapy, radiotherapy, and photodynamic therapy, can maximize the therapeutic effects. Finally, we have identified the challenges that nanotechnology-mediated immunotherapy needs to overcome in order to design more effective nanosystems.
Key words: Immunotherapy, Nanomedicine, Tumor immunosuppressive microenvironment, Cancer, T cells, Immunogenic cell death, Immune checkpoint inhibitors, Extracellular matrix
Graphical abstract
Nanomedicine activates anti-tumor immunity by regulating immune cells, thereby remodeling the immunosuppressive microenvironment and enhancing anti-tumor efficiency.
1. Introduction
Immunotherapy has become an effective approach to cancer treatment, aiming to inhibit tumor development by enhancing anti-tumor immune responses1, 2, 3. The clinical success of impeding antibodies targeting cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed death-1 (PD-1) suggests the advantages of immunotherapy in cancer treatment4, 5, 6. While immunotherapy has made significant progress in cancer treatment over the past two decades, it is only effective for a small number of people7. The formation of a tumor immunosuppressive microenvironment (TIME) is considered to be the primary cause of treatment failure8, 9, 10. Therefore, improving the TIME is an elegant strategy to facilitate the clinical implementation of cancer immunotherapy, capable of promoting antitumor immune responses to eradicate tumors.
Nanotechnology is an emerging cancer treatment that mainly relies on the “enhanced permeability and retention (EPR)” effect to deliver chemical drugs into tumors while minimizing accumulation in other tissues and reducing side effects11,12. EPR effects are not universal because of tumor heterogeneity13, 14, 15. The utilization of nanocarriers as delivery systems for chemical drugs was limited in cancer therapy. However, nanotechnology-mediated remodeling of the tumor microenvironment (TME) holds great promise in immunotherapy and is considered to be very useful in improving TIME16, 17, 18. Nanotechnologies participate in the remodeling of TIME by targeting immune cells, thereby enhancing their anticancer effect19,20. Nanotechnologies can also stimulate the immune system by promoting immunogenic cell death (ICD)21. Moreover, nanotechnologies can also be used in combination with other methods to simultaneously apply immunotherapy and combat cancer progression and metastasis.
In this review, we described the composition of TIME and the challenges it presents, as well as how nanotechnology can be utilized to overcome these challenges and improve immunotherapy. Our goal was to demonstrate that nanotechnology can not only be used to deliver chemotherapeutic drugs but also to inhibit tumor development by acting on TIME.
2. What is TIME?
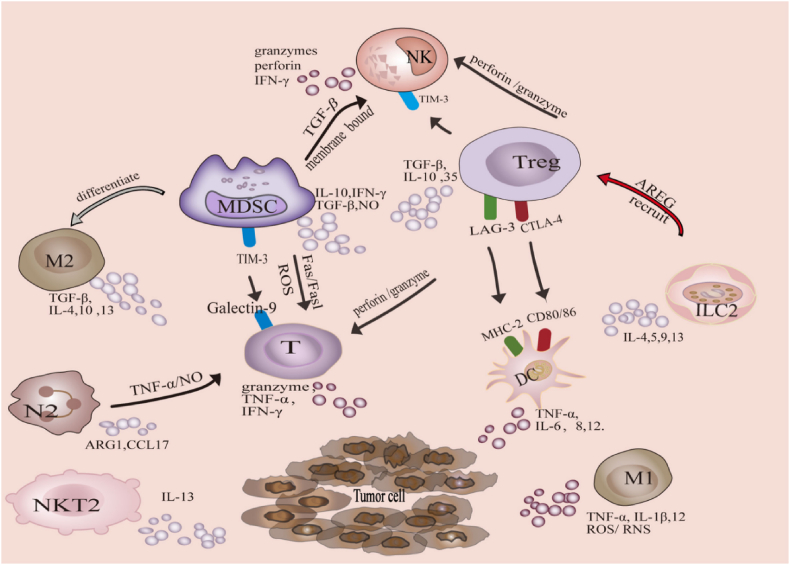
A variety of immune and non-immune cells are present in the TME, contributing to immunosuppression, matrix deposition, and angiogenesis22, 23, 24 (Fig. 1 and Table 1). Among them, immune cells such as natural killer (NK) cells, lymphocytes, and dendritic cells (DCs) participate in suppressing tumor growth25, 26, 27. For example, NK cells kill tumor cells by recognizing their surface molecules28. The process of generating an immune response begins with immature DCs recognizing pathogens and maturing. Mature DCs then present antigens with the assistance of major histocompatibility complex (MHC) class I and II molecules to activate CD4+ and CD8+ T cells. However, the congenital immune system is suppressed, and systemic adaptive immune activation is disrupted in the TME29,30. The main reason for this phenomenon is the infiltration of immunosuppressive cells in the TME31. Cytokines secreted by common immunosuppressive cells, such as MDSCs, regulatory T cells (Tregs), and M2-type macrophages, interfere with the immune response. This result allows tumor cells to evade immune recognition and ultimately form TIME32, 33, 34. For example, tumor-associated macrophages (TAMs) promote T-cell failure by expressing the transcription factor interferon regulatory factor-8 (IRF8)35. The extracellular matrix (ECM) secreted by TAFs hinders T cells from entering the tumor36. Tregs maintain the M2 phenotype of TAMs by inhibiting the secretion of interferon-γ by CD8+ T cells37. The retinoic acid and transforming growth factors (TGF-β) secreted by MDSCs can convert CD4+ T cells into Tregs38. The current challenge for immunotherapy lies in the presence of TIME.
Figure 1.
The TIME composed of immune cells. Reprinted with the permission from Ref. 22. Copyright © 2022 FRONTIERS MEDIA SA.
Table 1.
The composition and characteristics of TIEM.
| Classification | Constituent | Characteristic |
|---|---|---|
| Immune cells | DCs | Present antigens to T cells to initiate anti-tumor immunity |
| T cells | Responsible for killing tumor cells | |
| NK cells | Responsible for killing tumor cells | |
| B cells | Directly or indirectly presenting antigens to T cells | |
| Non-immune cells | MDSCs | Inhibition of T cell activity |
| Tregs | Prevent the anti-tumor response of immune cells | |
| TAFs | Generating ECM to inhibit T cell function | |
| TAMs | Including classically activated M1 macrophages and alternative activated M2 macrophages, M1 macrophages have anti-tumor effects, while M2 macrophages have pro-tumor effects |
DCs, dendritic cells; NK cells, natural killer cells; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; TAFs, tumor-associated fibroblasts; TAMs, tumor-associated macrophage.
3. Tumor immunotherapy enhanced via nanotechnology
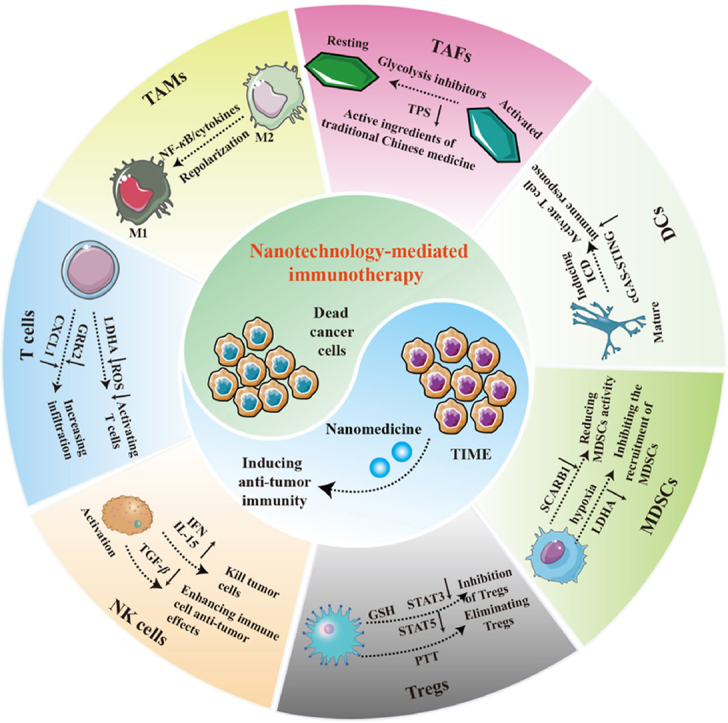
Currently, numerous nanomedicines are being utilized for cancer immunotherapy. Nanosystems can deliver immune modulators or genes to specific immune cells to stimulate the immune system. Importantly, some nanomaterials have the ability to regulate immune cells. In addition, the designability of nanosystems provides the possibility for the development of nanomedicines with specific functions. In summary, nanomedicines-mediated immunotherapy has great application prospects. Herein, we have summarized the research on the targeted regulation of immune cells by nanomedicines in Supporting Information Table S1.
3.1. Nanomedicine for targeting TAMs
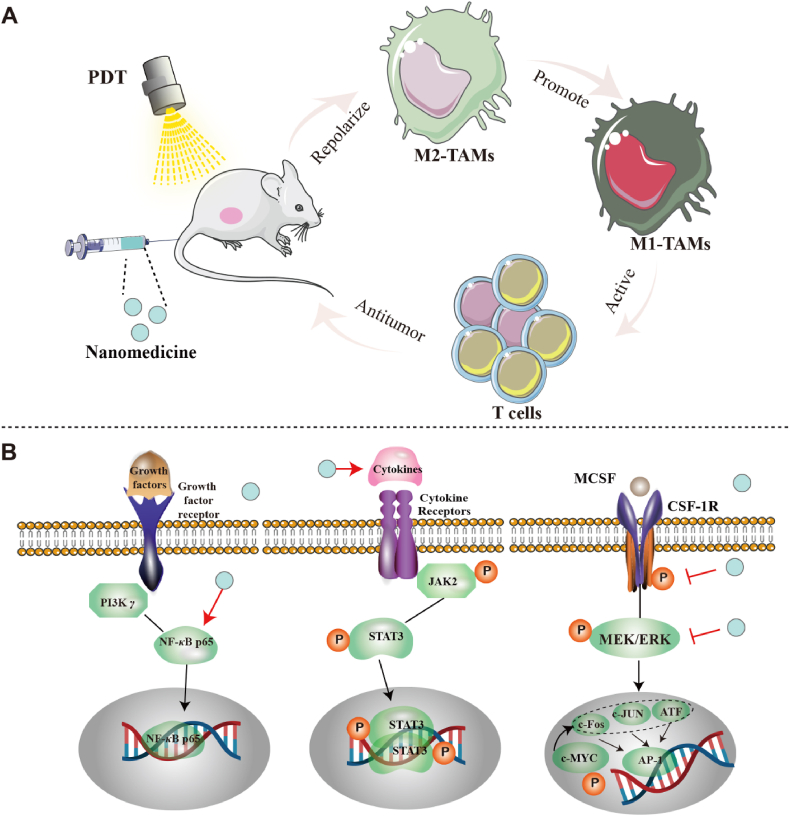
TAMs are the primary cells responsible for regulating the immune microenvironment of tumors. They can be polarized into a classically activated M1 phenotype and an alternatively activated M2 phenotype. Macrophages are generally considered a “double-edged sword” in cancer treatment. M1 macrophages have tumor growth-suppressing effects, while M2-type macrophages have tumor growth-promoting effects39. Notably, TAMs are predominantly of the M2-type in the TME, contributing to the development of immunosuppression40,41. Therefore, repolarization of TAMs may be an effective method to inhibit M2 macrophage infiltration in tumors and improve TIME42. Recently, there has been significant interest in nanomedicine for regulating the repolarization of TAMs (Fig. 2A and B).
Figure 2.
Schematic illustration of the repolarization mechanism of TAMs mediated by nanotechnology. (A) Nanotechnology triggers the repolarization of M2 TAMs to M1 TAMs, thereby activating the anti-tumor immunity of T cells and providing opportunities for remodeling TIME. (B) The mechanism by which nanotechnology promotes the repolarization of M2 TAMs mainly involves the activation of NF-κB p65, promotes cytokine release and inhibits CSF1R signaling.
3.1.1. Nanomedicine modulates the nuclear factor-kB (NF-κB) pathway involved in repolarizing TAMs
NF-κB is a dimeric transcription factor composed of two of the five protein family members. It can play a pivotal role in immunity by regulating the expression of chemokines, cytokines, proteins, etc43. In tumor progression, the NF-κB pathway is considered a key regulator of TAMs polarization44,45. The activation of NF-κB in macrophages is a critical factor for tumor occurrence and development. Regulating the NF-κB pathway can lead to the repolarization of M2 TAMs46. Therefore, utilizing nanotechnology to target the NF-κB pathway is an effective approach for reducing M2 macrophages in TME and enhancing the response to tumors.
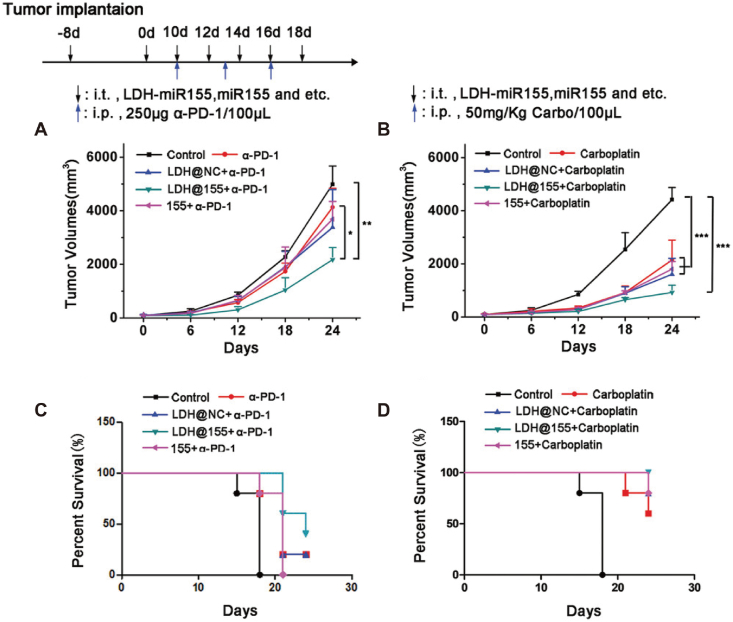
Methotrexate (MTX) is widely used for its effective anti-tumor and anti-inflammatory effects47,48. To enhance the delivery efficiency of MTX, researchers developed poly (lactic acid)-glycolic acid (PLGA) nanoparticles encapsulated with polyethyleneimine (Pei) and hyaluronic acid (HA) loaded with MTX, known as HA-PeiPLGA-MTX NPs49. After the administration of HA-PeiPLGA-MTX NPs, the NF-κB signaling pathway was inhibited, resulting in a transition of M2 TAMs to the M1 phenotype. Ultimately, the nanoparticles enhanced the immune response and inhibited the growth of breast cancer. PI3 kinase γ (PI3-kinase γ, PI3K γ) is a crucial molecule found in macrophages involved in the immune response to tumors. PI3K γ can participate in regulating tumor immune suppression by inhibiting NF-κB p65. Therefore, selectively suppressing the expression of PI3K γ using PI3K γ inhibitors is a method to enhance the tumor immune response. Li et al.50 prepared porous hollow iron oxide nanoparticles modified with mannose for loading PI3K γ small molecule inhibitors (3-methyladenine, 3-MA). The prepared nanoparticles can target TAMs and activate NF-κB p65, promoting the polarization of TAMs into M1-type macrophages. IMD-0354 is a specific inhibitor of the NF-κB pathway, which effectively suppresses the NF-κB pathway to achieve repolarization of M2 TAMs. Thus, Liu et al.51 first prepared mannose-modified lipid nanoparticles loaded with IMD-0354 (M-IMD-LNP). Next, nanogels responsive to matrix metalloproteinase 2 (MMP2) (P/ML-NNG) were used to encapsulate the PD-1 antibody and M-IMD-LNP. P/ML-NNG can release drugs to different targets and simultaneously inhibit the PD-1/PD-L1 and NF-κB pathways, thereby repolarizing M2 macrophages to M1 macrophages. The assessment of anti-tumor immunity confirmed that P/ML-NNG can effectively enhance the immune response and improve the effectiveness of immunotherapy. MicroRNA-155 (miR155) is a potent regulatory factor for immune cell function, effectively influencing TAMs polarization and enhancing tumor immunotherapy. In order to achieve effective delivery of miR155, researchers have developed layered double hydroxides (LDHs) nanoparticles52. LDH@155 nanoparticles effectively activated NF-κB expression in TAMs, induced polarization of TAMs towards the M1 phenotype, and enhanced the efficacy of anti-PD-1 antibody immunotherapy (Fig. 3).
Figure 3.
The anti-tumor efficacy of LDH@155 combined with chemotherapy and immunotherapy. (A) Tumor growth curves of LDH@155 combined with α-PD-1. (B) Tumor growth curves of LDH@155 combined with Carboplatin. (C) Kaplan–Meier survival curves of LDH@155 combined with α-PD-1. (D) Kaplan–Meier survival curves of LDH@155 combined with Carboplatin. Reprinted with the permission from Ref. 52. Copyright © 2019 John Wiley and Sons. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
The primary method for nanomedicine to regulate NF-κB is by encapsulating NF-κB inhibitors into nanocarriers, thus effectively delivering the inhibitors to the tumor sites. Compared to using inhibitors alone, the intervention of nanomedicines increases the accumulation of inhibitors at the tumor site, leading to more TAMs being repolarized to the M1 phenotype.
3.1.2. Nanomedicine regulation of cytokines involved in TAMs repolarization
Cytokines are classified as small signaling proteins and comprise a family of proteins, including interleukins, lymphokines, chemokines, single factors, and interferons53. Cytokines are crucial components of the immune system and serve as biomarkers for various diseases54,55. Therefore, regulating cytokine expression can help improve TIME.
IL-12 is a heterodimeric cytokine that regulates the transformation of M2 macrophages into M1 macrophages, resulting in anti-tumor responses56. The microenvironment-responsive nanocarriers containing IL-12 were engulfed by macrophages, leading to the release of IL-12. This promoted the repolarization of TAMs into the M1 phenotype and exhibited significant anti-tumor effects57. The combination of IL-12 and chemotherapy drugs in cancer treatment has garnered increasing attention. Li et al.58 developed therapeutic nanoparticles (TNPs) for the co-delivery of IL-12 and doxorubicin. Doxorubicin and IL-12 were co-released in TME. The released IL-12 promoted the polarization of TAMs into the M1 phenotype, enhancing the anti-tumor effectiveness of doxorubicin.
Macrophage colony-stimulating factor (MCSF) participates in macrophage recruitment and facilitates polarization into M2 macrophages by binding to the CSF1R and transmembrane type III protein tyrosine kinase receptor found on macrophages and monocytes59, 60, 61. Overall, targeted inhibition of CSF1R signaling is a promising immunotherapy strategy that promotes the repolarization of M2 macrophages into an anti-tumor M1 phenotype.
BLZ945 is a small molecule CSF1R inhibitor that can effectively repolarize M2 macrophages into M1 macrophages62. Simultaneously loading BLZ945 and selumetinib (a MAPK signaling inhibitor) into supramolecular nanoparticles can continuously inhibit both CSF1R and MAPK signaling pathways, promoting the repolarization of M2 macrophages to the M1 phenotype63. Therefore, the simultaneous inhibition of CSF1R and MAPK signals is a valid tactic for macrophage-mediated immunotherapy. The PDL1/PD1 signaling pathway is also involved in the polarization of TAMs, in addition to the CSF1R axis. Studies have found that anti-PDL1 can increase iNOS and reduce arginase levels, thereby affecting the phenotype of TAMs64. The overexpression of PDL1 in TAMs is regulated by the activation of the CSF1/CSF1R pathway. Therefore, the PDL1/PD1 signaling pathway is considered a key player in assisting the CSF1R signaling pathway in regulating the polarization of TAMs. In response to this phenomenon, a system of self-assembled lipid nanoparticles loaded with small molecule inhibitors of the CSF1R signaling axis (BLZ945) and surface-modified by an anti-PDL1 monoclonal antibody (α-PDL1-CSF-LNPs) has been developed for anti-tumor research65. This system can target and inhibit the PDL1 and CSF1R pathways simultaneously, thereby promoting the transformation of the M2 phenotype into the M1 phenotype and enhancing the anti-tumor immune response.
Cytokines can participate in regulating TAMs polarization through various pathways. Loading cytokines or their inhibitors into nanosystems can simultaneously regulate multiple pathways to induce the polarization of TAMs into the M1 phenotype. Therefore, immunotherapy based on nanomedicines tends to incorporate cytokines and chemotherapy drugs into the nanosystem to exert synergistic anti-tumor effects.
3.1.3. Others
TMP195 is a specific class II HDAC small molecule inhibitor that repolarizes M2 macrophages into M1 macrophages by altering their epigenetic properties66. However, TMP195 may have potential side effects when applied in vivo. To tackle this issue, macrophage membrane-coated biomimetic nanoparticles were developed as delivery vehicles for TMP19567. The biomimetic nanoparticles repolarize M2 macrophages to M1 macrophages and synergize with photothermal therapy (PTT) to enhance anti-tumor effects. As a dual agonist of Toll-like receptor TLR7/8, R848 can promote the polarization of M2 macrophages towards M1 macrophages. Lignin biopolymers were used as carriers to prepare nanoparticles containing R848 (R848@LNPs) and labeled with 5 (6)-carboxyfluorescein (FAM)68. R848@LNPs can target CD206-positive M2-like TAMs and convert them into anti-tumor M1 macrophages. Therefore, when combined with vinblastine, R848@LNPs can stimulate immune cells in the TME to transition to an anti-tumor immune state, thereby enhancing the anticancer effect of vinblastine. Iron can promote the conversion of anti-inflammatory M2 macrophages into pro-inflammatory M1 macrophages. Research has shown that iron oxide nanoparticles can induce the differentiation of M2-like TAMs into M1 phenotypes, which can inhibit tumor growth69. Mitochondria not only produce energy for cells but also regulate cell survival and death. In the TME, mitochondria are involved in the regulation and activation of immune cells. It is worth noting that mitochondria are involved in regulating macrophage polarization. To this end, Zhao et al.70 developed twin-like charge-switchable nanoparticles that target mitochondria. The nanoparticles selectively silence mitofusin 1 (MFN1), thereby inhibiting mitochondrial fusion in TAMs and promoting the repolarization of TAMs from M2 to M1 phenotype. This drug delivery strategy effectively reversed TIME and provided an efficient solution for tumor immunotherapy. MicroRNA-125a (miR-125a), a non-coding RNA, participates in regulating immune cell function by binding to the 3′-untranslated region of targeted mRNA. Furthermore, miR-125a exhibits ideal tumor growth inhibitory effects. Accordingly, miR-125a may function as a genetic drug with dual effects, reversing TAMs polarization and inhibiting tumor growth. Researchers have developed polyethyleneimine-modified dendritic mesoporous silica nanoparticles loaded with miR-125a (DMSN-PEI@125a) to synergistically reverse immune suppression and kill tumor cells71. In TC-1 mouse models, DMSN-PEI@125a can repolarize TAMs to stimulate anti-tumor immune responses and synergistically inhibit tumor growth. The Cu-doped polypyrrole nanozyme modified with polyethylene glycol (CuPP), developed by Zeng et al.72, can increase oxygen levels in the TME and reverse the hypoxic conditions. More importantly, CuPP promotes the transformation of M2 macrophages into M1 macrophages, inducing a strong immune response. OX40L-overexpressed M1 macrophage exosomes (OX40L M1-exos) promoted the reprogramming of TAMs into M1 macrophages by activating the OX40/OX40L pathway73. This strategy effectively inhibited the growth and metastasis of breast cancer.
3.2. Nanomedicine for targeting T cells
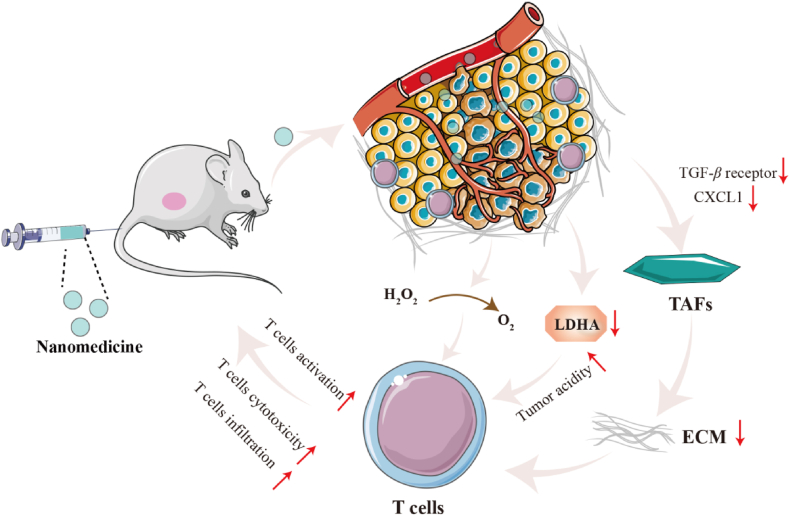
T cells eliminate tumor cells based on their antigenic specificity and play a role in anti-tumor immune responses74,75. The function of T cells in regulating immune responses is mediated by CD4+ and CD8+ T cells, respectively76, 77, 78. Among them, CD8+ T cells kill tumor cells by recognizing the MHC I molecules expressed by tumor cells. CD4+ T cells can directly kill tumor cells through MHC II, or indirectly kill tumor cells by releasing cytokines to activate other immune cells with anti-tumor functions79. However, T cells encounter challenges in penetrating tumor tissue and unleashing their anti-tumor immune response because of various immunosuppressive mechanisms80. Currently, research on T cell therapy primarily focuses on increasing T cell infiltration into tumor sites, activating T cells, and enhancing their activity (Fig. 4)81, 82, 83.
Figure 4.
Schematic illustration presenting brief mechanism of nanomedicine targeting T cells. (1) After entering tumor tissues, nanomedicines silence the expression of TGF-β and CXCL1, reducing ECM deposition. Subsequently, the permeation of T cells at the tumor site increases, thereby regulating TIME. (2) The production of lactate decreases after the silencing of LDHA expression by nanomedicines. The reduction of lactate production induces the reversal of the acidic microenvironment of tumors, thereby restoring T cells immune function. (3) Nanomedicines reduce ROS level and promote T cells survival by catalyzing the decomposition of H2O2 within tumors.
3.2.1. Nanomedicine increases T-cell infiltration
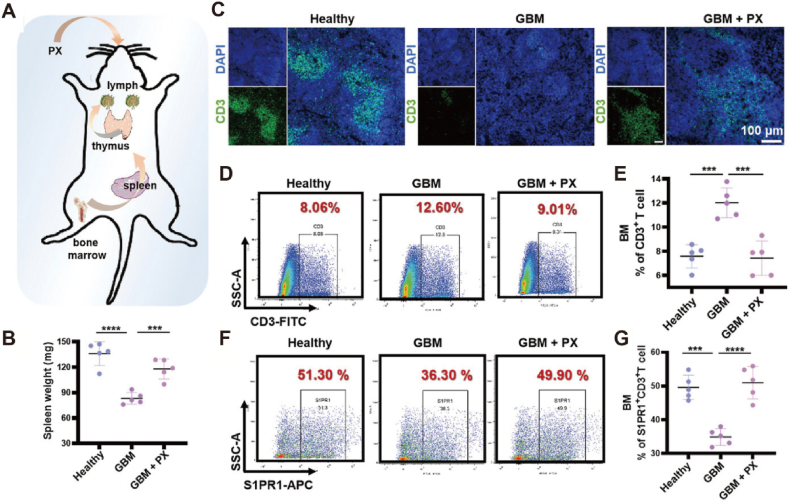
The absence or mutation of the gene encoding the phosphatase and tensin homolog (PTEN), which is located on chromosome 10, is associated with reduced T-cell infiltration at tumor sites. Restoring PTEN function is expected to enhance T-cell infiltration at tumor sites. To accomplish this objective, nanoparticles loaded with PTEN messenger RNA (mRNA) have been developed to enhance PTEN expression in tumors84. There is no doubt that PTEN mRNA nanoparticles promote the infiltration of CD8+ T cells into tumor tissues and reverse TIME. In addition to the obstruction of T cell infiltration in tumors, T cell depletion is also a factor leading to the failure of immunotherapy85. Therefore, increasing T cell infiltration while preventing their depletion in tumors has become an important strategy for enhancing immunotherapy. The absence of sphingosine 1-phosphate receptor 1 (S1PR1) on the surface of T cells results in a considerable number of T cells being unable to infiltrate tumor tissue. Due to the internalization of S1PR1 dependent on G protein-coupled receptor kinase 2 (GRK2). Inhibiting GRK2 can stabilize the expression of S1PR1 on the outside of T cells. Meanwhile, blocking PD-1, T-cell immunoglobulin, and mucindomain-containing molecule-3 (TIM-3), cytotoxic T lymphocyte antigen-4 (CTLA-4) can reduce T cell depletion. Therefore, the combination of biomimetic nanoparticles and paroxetine (PX, a GRK2 inhibitor) reduced PD-1 and TIM-3 on T cells by downregulating TET2 levels and inhibited GRK2 to stabilize S1PR1 expression on the outside of T cells86. The combination of biomimetic nanoparticles and PX increased T cell infiltration at the tumor site and reversed their dysfunction (Fig. 5). The FeS-GOx nanodots synthesized by glucose oxidase self-assemble into FeS-GOx@PTX through hydrophilic interactions with paclitaxel87. FeS-GOx@PTX can promote T-cell infiltration and completely eliminate primary tumors by inducing ICD. The permeation efficiency of chimeric antigen receptor (CAR)-redirected T lymphocytes (CAR T cells) in solid tumors is low. PLGA nanoparticles loaded with indocyanine green (PLGA-ICG) can damage ECM by generating heat88. Subsequently, the infiltration of CAR T cells into the tumor increased, indicating a significant therapeutic effect on melanoma.
Figure 5.
(A) Schematic illustration of biomimetic nanoparticles and PX increase T cell infiltration in tumor. (B) The spleen weights of each group. (C) Images of CD3+ T cells in the spleens of mice from different groups (scale bar = 100 μm). (D, E) Bone marrow CD3+ T cells and their percentages in different groups of mice. (F, G) Bone marrow S1PR1+CD3+ T cells and their percentage in different groups of mice. Reprinted with the permission from Ref. 86. Copyright © 2023 John Wiley and Sons. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 (n = 5).
Ensuring that a sufficient number of T cells initiate an immune response at the tumor site requires not only increasing T cell infiltration but also reducing T cell depletion. Therefore, when designing nano-delivery systems, it is important to consider both increasing T cell infiltration and avoiding T cell depletion.
3.2.2. Nanomedicine activation of T cells
The acidic microenvironment resulting from the production of lactic acid in solid tumors hinders the immune response of T cells89, 90, 91. Specifically, the increase in lactate levels can lead to the inactivation and apoptosis of CD8+ T cells92. Therefore, reversing the acidic microenvironment of tumors caused by lactic acid can help restore the immune function of T cells93. A scheme for cancer treatment has been developed based on regulating tumor acidity using nanomedicine94. Lactate dehydrogenase A (LDHA) is primarily responsible for converting pyruvate to lactate, which contributes to tumor acidity. Silencing LDHA with small interfering RNA (siRNA) can help reverse tumor acidity and restore T cell anti-tumor function. Therefore, Zhang et al.95 developed vascular CLAN nanoparticles as a siRNA delivery system (VNPsiLdha) to silence LDHA in tumor cells. This treatment significantly reduced lactate production and neutralized tumor acidity. Ultimately, the infiltration of CD8+ T cells increased, and their functionality was restored, resulting in improved immunotherapy with anti-PD-1. Moreover, ROS secreted by immunosuppressive cells in TME can induce apoptosis and functional inhibition in T cells, leading to suboptimal T cell-mediated immunotherapy. Simultaneously stimulating T cell activation and regulating ROS levels in TME is a promising treatment approach. To achieve this goal, Lu et al.96 developed antibodies (anti-CD3/CD28 mAbs, CD) functionalized MnOx nanoparticles camouflaged with tumor cell membranes (CD-MnOx@CM) to regulate TME and activate T cells. As envisioned, CD-MnOx@CM not only effectively activated CD8+ T cells but also regulated ROS levels in TME by catalyzing the decomposition of H2O2 into O2, thereby promoting T cell survival within the tumor.
T cells need to be activated before they can function in immunotherapy. However, the function of T cells is often inhibited in TIME. The development of nanomedicines should not neglect the activation of T cells while considering increasing T cell infiltration.
3.3. Nanomedicine for targeting Tregs
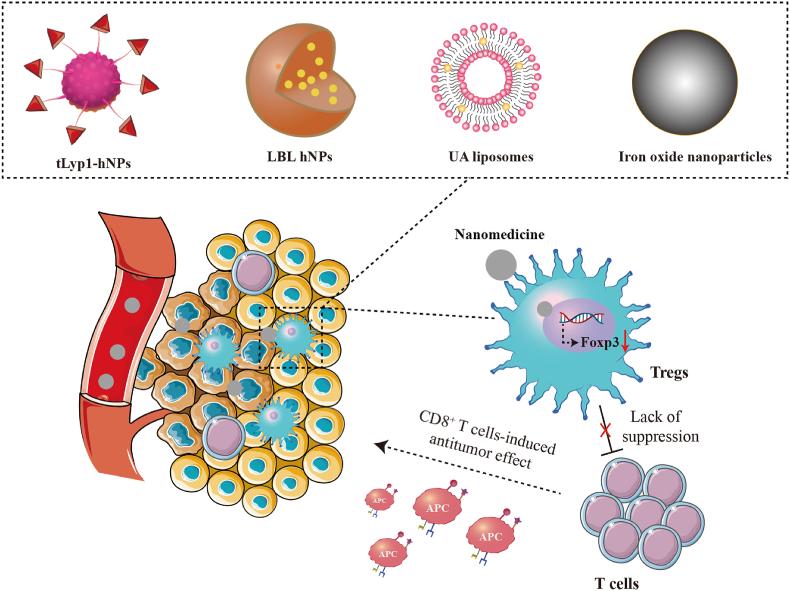
Tregs, an important subgroup of CD4+ T cells, maintain immune homeostasis by suppressing immune responses97,98. The abnormal number and function of Tregs can lead to autoimmune diseases99. In tumor immunity, Tregs are involved in suppressing anti-tumor immune responses100. Tregs can induce tumor immune escape by blocking effector T cells and by inducing monocytes to differentiate into the M2 subtype, which promotes tumor growth and metastasis101,102. In addition, TGF-β secreted by Tregs restricts immune cells from reaching TME, ultimately leading to TIME. Inhibiting or eliminating Tregs has become a key focus in promoting an anti-tumor immune response (Fig. 6).
Figure 6.
Schematic illustration of nanomedicines targeting Tregs to increase T cells immune response. Regulation of different nanomedicines (tLyp1-hNPs, LBL hNPs, UA liposomes, and iron oxide nanoparticles).
3.3.1. Nanomedicine inhibition of Tregs
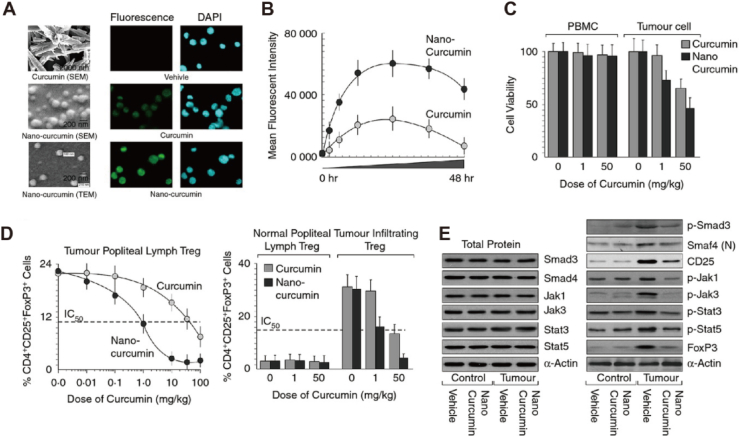
Glutathione (GSH) is a key participant in maintaining the expression of Foxp3 in Tregs and their immunosuppressive function103. Furthermore, a hypoxic TME can not only recruit Tregs infiltration but also promote Tregs proliferation104,105. Consequently, some researchers have proposed overcoming Tregs-mediated immunosuppression by regulating the redox metabolism of the TME. Fluorine-assembled nanoparticles have been developed to overcome Tregs-mediated immunosuppression106. Herein, the oxygen contained in perfluorocarbon within fluorine-assembled nanoparticles can effectively alleviate hypoxia in the TME, thereby inhibiting the proliferation and infiltration of Tregs. On the other hand, the chemical prodrugs released by fluorine-assembled nanoparticles under laser irradiation reduce the level of GSH, thereby decreasing the expression of Foxp3 in Tregs, and accomplishing the objective of reversing tumor immune suppression. Imatinib (IMT) can decrease the quantity of Tregs and hinder their function by disrupting STAT3 and STAT5 signals. In order to accurately deliver IMT to Tregs, hybrid nanoparticles coupled with tLyp1 peptide were prepared for the delivery of IMT107. As expected, the tLyp1 peptide-coupled hybrid nanoparticles blocked the phosphorylation of STAT3 and STAT5, resulting in a decrease in Tregs within the tumors. Mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling is a prospective target for inducing the expansion of Tregs. Demethylcantharidin (DMC) can interfere with FOXP3 expression by inhibiting protein phosphatase 2A (PP2A), thereby reducing Treg levels in TME. Researchers have utilized DMC-conjugated β-cyclodextrin to create supramolecular photodynamic nanoparticles for the smart delivery of photosensitizers and immunomodulators108. The results showed that supramolecular photodynamic nanoparticles reduced Treg levels in TME and increased infiltration of CD8+ T cells, thereby reversing TIME. Of course, some researchers have combined the anti-tumor efficacy of PDT with the downregulation of Tregs in tumors by IMT. In this strategy, layer-by-layer hybrid nanoparticles (LBL-hNPs) were used to encapsulate IR-780 and IMT, thereby achieving dual effects of downregulating Tregs and exerting anti-tumor activity109. The results showed that LBL-hNPs reduced the inhibitory function of Tregs and improved the anti-tumor efficacy of PDT. Curcumin is an inhibitor of the MEK/ERK signaling pathway and can effectively reduce the increase of Tregs in tumors when loaded into nanoparticles (Fig. 7)110. In addition, ursolic acid (UA) is a lead compound with immune regulatory properties derived from plants and fruits. UA liposomes can reduce tumor-infiltrating Tregs by inhibiting STAT5 phosphorylation and IL-10 secretion111.
Figure 7.
Curcumin nanoparticles inhibit Tregs expansion. (A) Scanning and transmission electron microscopic images of curcumin and curcumin nanoparticles (left panel). Fluorescence images of carrier, curcumin, and curcumin nanoparticles in 4T1 cells (right panels), green fluorescent: curcumin. (B) Diagram of the relationship between average fluorescence intensity and time. (C) In vitro cell viability of normal peripheral blood monocytes and 4T1 cells. (D) Percentage of CD4+ CD25+ FoxP3+ Tregs induced in tumour-draining lymph nodes (left panel) and tumour-infiltrating lymphocytes of curcumin/nano-curcumin-treated (right panel) (n = 5). (E) The expression level of proteins. Reprinted with the permission from Ref. 110. Copyright © 2015 John Wiley and Sons.
Tregs expressing Foxp3 are abundant in the TME and are often associated with a poor prognosis. Interfering with Foxp3 expression is an effective approach to suppress Tregs and boost immune response. Therefore, the current nanomedicine design focuses on interfering with Foxp3 expression.
3.3.2. Nanomedicine elimination of Tregs
Considering that Tregs in tumor tissue are more closely associated with tumor immunosuppression, it is more effective to eliminate Tregs only at the tumor site than throughout the entire body. Nanoparticle-mediated PTT is widely used for local anticancer treatment, but its ability to eliminate Tregs has been neglected112, 113, 114. Therefore, nanoparticle-mediated PTT may be an effective method for locally eliminating Tregs and promoting immunotherapy. To verify this hypothesis, iron oxide nanoparticle-mediated PTT was used in mouse breast tumor models to investigate its combined anti-tumor effect with CTLA-4115. The results showed that iron oxide nanoparticle-mediated PTT can selectively eliminate Tregs at the tumor sites and enhance the inhibition of CTLA-4 on tumor growth.
It is worth noting that Tregs are responsible not only for suppressing tumor immunity but also for maintaining immune balance in the body116. Eliminating systemic Tregs may disrupt the body's immune balance and trigger certain autoimmune diseases117. In addition, eliminated Tregs rapidly release adenosine triphosphate (ATP) and convert it into adenosine near death. Afterward, adenosine binds to the surface receptors of T cells to inhibit their activity118. Hence, the treatment strategy of eliminating systemic Tregs to reverse immune suppression may not yield the anticipated results.
3.4. Nanomedicine for targeting MDSCs
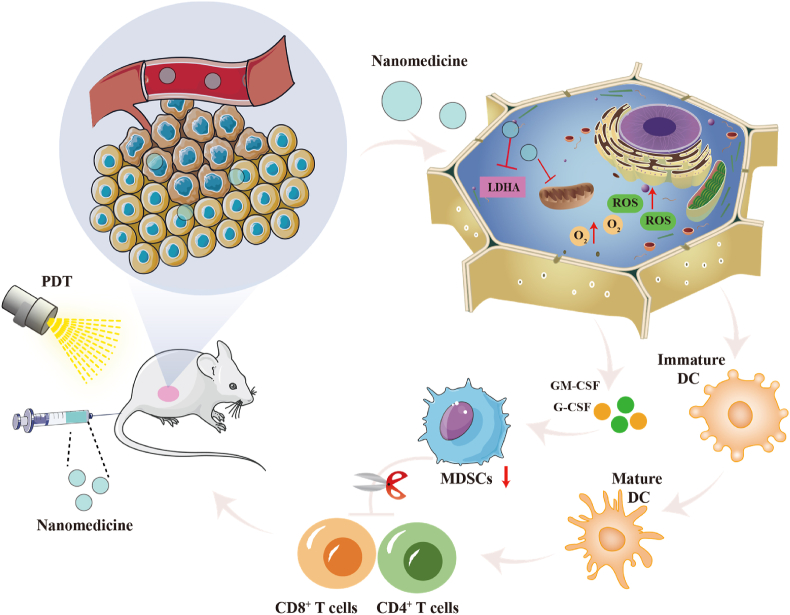
MDSCs are immature bone marrow cells with immunosuppressive effects and play an essential role in TIME119,120. MDSCs primarily play a role in immune suppression by depleting metabolites, upregulating ROS, and secreting various cytokines121. MDSCs also play a role in inhibiting the immune function of T cells122. In recent years, nano-immunotherapy has primarily improved anti-tumor effectiveness by decreasing the activity of MDSCs or inhibiting their recruitment (Fig. 8).
Figure 8.
Schematic illustration of the mechanisms of nanotechnology regulating MDSCs.
3.4.1. Nanomedicine reduces the activity of MDSCs
Scavenger receptor type B-1 (SCARB1) is a high-affinity receptor for globular high-density lipoproteins (HDL) and is expressed in MDSCs. Focusing on this feature, researchers synthesized high-density lipoprotein nanoparticles (HDL NPs) for the specific targeting of SCARB1123. The results suggested that HDL NPs significantly inhibited the activity of MDSCs and suppressed tumor growth. Tadalafil is expected to regulate anti-tumor immunity by inhibiting the function of MDSCs. Therefore, Zhang et al.124 used nanotechnology to co-deliver tadalafil and indocyanine green, referred to as FIT NPs. The released tadalafil can effectively inhibit the activity of MDSCs at the tumor sites and enhance the therapeutic efficiency of PTT.
The immunosuppressive network composed of MDSCs inhibits the anti-tumor response of T cells, thereby promoting tumor immune escape. The main treatment method for MDSCs is to leverage the benefits of nanosystems to precisely deliver drugs into TME and suppress MDSC activity.
3.4.2. Nanomedicine inhibits the recruitment of MDSCs
Tumor hypoxia, as a characteristic marker of TME, activates TIME by recruiting MDSCs125. Researchers attempted to overcome the challenge caused by tumor hypoxia by interfering with mitochondrial respiration. Specifically, IR780 (a photosensitizer) and metformin (a mitochondrial respiratory inhibitor) were loaded onto the nanoplatforms simultaneously. The nanoplatforms released IR780 and metformin upon reaching the tumor site. The released metformin overcame tumor hypoxia by inhibiting mitochondrial respiration, thereby inhibiting the immunosuppressive function induced by MDSCs. Additionally, the accumulated O2 enhanced the anti-tumor therapy mediated by IR780126. Three negative breast cancer cells primarily depend on glycolysis (known as the “Warburg” effect) to acquire the energy required for growth. LDHA is highly expressed in glycolysis to facilitate the conversion of pyruvate to lactate. In addition, high LDHA expression promotes the recruitment of MDSCs by inducing the secretion of granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF). Based on this, researchers developed a redox reaction nanoassembly system (R-mPDV/PDV/DOX/siL) to silence LDHA and induce ICD127. R-mPDV/PDV/DOX/siL respond to the release of siRNA in tumors, enabling efficient silencing of LDHA. After silencing the expression of LDHA, the secretion of G-CSF and GM-CSF cytokines decreased, and the recruitment of MDSCs was inhibited. In summary, R-mPDV/PDV/DOX/siL have significant anti-tumor effects on 4T1 tumors.
Preventing MDSCs from entering TME is another strategy for initiating immunotherapy. Nanomedicines primarily inhibit the recruitment of MDSCs by impeding the key process of attracting MDSCs into the TME.
3.5. Nanomedicine for targeting TAFs
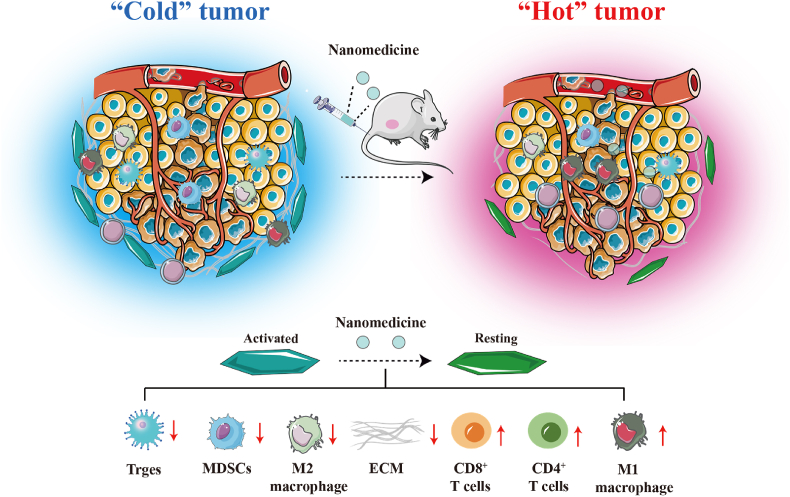
TAFs are one of the most abundant cells in the tumor matrix and play a crucial role in the development and metastasis of tumors128,129. Additionally, TAFs are the primary factor affecting TIME130,131. Specifically, the cytokines, chemokines, and cell surface proteins secreted by TAFs directly limit the infiltration of immune cells. TAFs can indirectly impede the infiltration of immune cells by producing ECM deposition. The direct elimination of TAFs may cause the spread of cancer cells, facilitating the invasion and metastasis of cancers. Therefore, reprogramming active TAFs into a quiescent state for fine-tuning therapy is an optimal approach to stimulate tumor immune responses. Nanodelivery systems are commonly used to regulate TAFs and enhance immune response due to their capability to efficiently penetrate tumor lesion areas (Fig. 9)132,133.
Figure 9.
Schematic illustration of nanomedicine regulation of TAFs. Nanomedicine reverse activated TAFs to resting state, thereby reducing ECM deposition in tumor tissue. Then, the “cold” tumor transforms into a “hot” tumor, increasing the efficacy of immunotherapy.
Relaxin (RLN) has the effect of inhibiting TAFs activation and improving TIME. Researchers synthesized a PolyMet-pRLN complex using the plasmid RLN (pRLN) and the polymer metformin (PolyMet) to overcome the short half-life of RLN134. To enhance the stability of the complex, lipid poly-γ-glutamic acid (PGA)/PolyMet-pRLN nanoparticles (LPPR) were further prepared. LPPR can effectively penetrate 4T1 luc/TAFs tumor spheres and inhibit the proliferation of TAFs. Furthermore, LPPR can increase the permeation of cytotoxic T cells and reduce the recruitment of immunosuppressive cells by suppressing the activation of TAFs in vivo. In brief, LPPR can remodel the immune microenvironment of triple negative breast cancer and elevate the anti-tumor efficacy of PD-L1 antibody. The release of trypsin (TPS) by mast cells can elevate the expression of α-smooth muscle actin (α-SMA) to activate TAFs. Based on this, He et al.135 developed tryptase-imprinted nanoparticles (DMSN@MIPs) using dendritic mesoporous silica as a carrier and TPS as the template molecule to neutralize TPS and inhibit TAFs activation. In addition, DMSN@MIPs suppressed the activation of TAFs and enhanced the penetration of doxorubicin liposomes (DOX/LIP) in tumors. As expected, the combination therapy of DMSN@MIPs and DOX/LIP significantly increased the levels of immune cells (including DCs, CD8+ T cells, and NK cells) and remodeled TIME.
In recent years, the active ingredients of traditional Chinese medicine have frequently been utilized as regulators of the tumor immune microenvironment in combination with chemotherapy drugs for anti-tumor treatment136,137. However, challenges such as poor water solubility and a short half-life in vivo limit the advancement of active ingredients in traditional Chinese medicine138. Therefore, many researchers have incorporated nano-delivery systems into the development of active ingredients139. For example, baicalin extracted from the roots of Scutellaria altissima L., has anti-fibrotic effects in various organs. Therefore, Zheng et al.140 constructed mPEG-modified poly (lactide-co-glycolide) (PLGA) nanoparticles loaded with baicalin (PMs-Ba). PMs-Ba can enhance the infiltration of cytotoxic T cells and stimulate the tumor immune microenvironment by inhibiting the activation of TAFs. Furthermore, PMs-Ba can significantly elevate the capacity of doxorubicin nanoparticles. Danshen-derived salvianolic acid B (SAB) also exhibits anti-fibrotic effects in various organs. In order to prolong the half-life of SAB in vivo, PEG-modified liposomes were used to encapsulate SAB (PEG-SAB-Lip)141. The research results indicate that PEG-SAB-Lip can inhibit the activation of TAFs by suppressing TGF-β1, thereby reducing collagen deposition at the tumor sites. After improving the tumor fibrosis microenvironment, CD4+ and CD8+ T cells, along with M1 macrophages, were recruited to the tumor sites, resulting in modulation of the TIME. In summary, the active components of traditional Chinese medicine are effective remedies for stimulating tumor immunity and enhancing the anti-tumor effects of chemotherapy drugs.
The occurrence of dynamic immune evasion can lead to a poor response to immune therapy. The induction of fibrosis by TAFs and the failure of T cells mediated by tumor cells are key factors contributing to dynamic immune evasion. In response to this discovery, Pan et al.142 developed a self-adaptive nanoregulator based on peptide-drug conjugates to alleviate dynamic immune evasion in pancreatic ductal adenocarcinoma (PDAC). This self-adaptive nanoregulator can achieve morphological transformation from spherical micelles to nanofibers to spherical nanoparticles. The self-adaptive nanoregulator disrupted tumor fibrosis by delivering an inhibitor of TAFs. In addition, this self-adaptive nanoregulator can also deliver an indoleamine 2,3-dioxygenase 1 inhibitor to alleviate the dysfunction of T cells induced by the IDO1-kynurenine axis. In summary, the self-adaptive nanoregulator can stimulate sustained anti-tumor immunity and enhance anti-tumor effectiveness. TAFs are compelled to act as “energy factories” for cancer cells, supplying energy for their rapid growth and proliferation. The glycolytic metabolite lactate produced by TAFs leads to impaired immune cell function, ultimately resulting in TIME. Therefore, simultaneous inhibition of glycolysis in cancer cells and TAFs may be crucial for the functioning of immune cells. Zang et al.143 modified the hybrid membrane of cancer cells and TAFs on solid lipid nanoparticles encapsulated with paclitaxel (a chemotherapy drug) and PFK15 (a glycolytic inhibitor). They aimed to target cancer cells and TAFs, respectively, and to block the metabolic network between them. Biomimetic nanoparticles simultaneously blocked the glycolysis of cancer cells and TAFs decreased lactate production in the TME, and stimulated immune responses. Furthermore, biomimetic nanoparticles have been demonstrated to enhance tumor growth inhibition. This suggests that disrupting the glycolysis of cancer cells and TAFs is a promising strategy for enhancing the anti-tumor immune response and improving the effectiveness of chemotherapy when combined with immunotherapy.
Notably, recent studies have also reported that TAFs may inhibit tumor growth. Completely eliminating TAFs may promote tumor growth and metastasis. Therefore, the goal of nanomedicine is to inhibit the activity of TAFs or partially eliminating eliminate them.
3.6. Nanomedicine for targeting NK cells
NK cells are a type of innate lymphocytes that have the ability to eliminate tumor cells and inhibit tumor development144. NK cells can not only kill tumor cells but also enhance the anti-tumor effects of other immune cells, such as DCs, CD8+ T cells, and macrophages145. Therefore, its role in cancer immunotherapy has gradually attracted the attention of researchers. It is worth noting that the cytotoxicity of NK cells in TME is the main factor determining its therapeutic effect146. However, NK cell toxicity is easily suppressed in TIME. Recent studies have shown that nanotechnology-mediated immunotherapy can activate NK cells to enhance cancer immunotherapy.
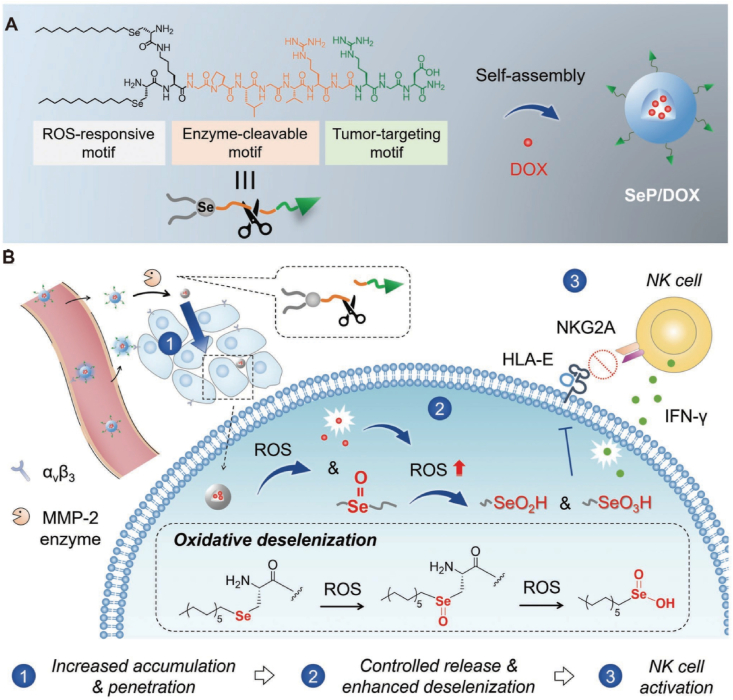
The anti-tumor activity of NK cells is inhibited by the immunosuppressive molecule TGF-β in TME. Galunisertib can counteract the inhibitory effect of TGF-β on NK cells by blocking TGF-β receptor type I kinase. Interleukin-15 (IL-15) can activate NK cells, thereby improving anti-tumor efficiency. Therefore, it is necessary to develop a nanosystem that can simultaneously deliver galunisertib and IL-15. Herein, Sun et al.147 prepared nanoparticles loaded with galunisertib and IL-15 using calcium carbonate as the core and sodium alginate as the outer layer (Gal/IL-15@CaLN). Gal/IL-15@CaLN can promote drug retention in tumors and activate NK cells, ultimately enhancing anti-tumor immune responses. In addition to directly targeting TGF-β, downstream signaling pathways such as transcription factors Smad2 and Smad3 can also be targeted to activate NK cells. SIS3 is a small molecule inhibitor of Smad3 that can be used to interfere with TGF-β-induced immunosuppression. A self-carried nanodrug-SIS3 (SCND-SIS3) enhances NK cell-mediated immune response by inhibiting Smad3-mediated Ndrg1 transcription148. The STING pathway activates NK cells by inducing the production of type I interferon (IFN), thereby inhibiting metastatic renal cell carcinoma. STING-loaded lipid nanoparticles can significantly activate NK cells and kill circulating metastatic cancer cells149. The cellular activity of NK cells is influenced by Src homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1), Cb1-b, and c-Cb1. In response to this phenomenon, lipid-based nanocarriers have been developed for encapsulating siRNA to target interference with SHP-1, Cb1-b, and c-Cb1150. The research results showed that nanoparticles safely and effectively silenced the expression of SHP-1, Cb1-b, and c-Cb1 in NK cells, thereby enhancing the ability of NK cells to kill cancer cells. In addition, the oxidative metabolites of self-assembled selenopeptide nanoparticles (SeNPs) can activate NK cells and enhance the anti-tumor efficacy of chemotherapy drugs (Fig. 10)151. The selenite produced through the self-assembly of β-selenate ester and Pem nanoparticles can inhibit the activation of NK cells by human leukocyte antigen E152. Overall, activating NK cells is an effective strategy for nanomedicines to stimulate anti-tumor immune responses.
Figure 10.
(A) Schematic illustration of the Sep/DOX formation. (B) Sep/DOX improve NK cells mediated immunotherapy. Reprinted with the permission from Ref. 151. Copyright © 2022 John Wiley and Sons.
The anti-tumor activity of NK cells is regulated by multiple pathways. Currently, the developed nanomedicines only focus on one pathway. In the future, we should develop a strategy to simultaneously target multiple pathways to activate NK cells and induce anti-tumor immunity.
3.7. Nanomedicine for targeting DCs
DCs are functionally specific antigen-presenting cells responsible for coordinating innate and adaptive immune responses153. The anti-tumor response of DCs mainly relies on transporting tumor-related antigens to draining lymph nodes to activate T-cell immune responses. Maturation of DCs is a necessary condition for presenting immune response signals to T cells. In tumors, the maturation process of DCs is often inhibited, preventing T cells from initiating anti-tumor immunity. Promoting the maturation of DCs is an effective strategy for enhancing T-cell immune responses and improving the clinical efficacy of cancer immunotherapy.
The initiation of anti-tumor immunity by DCs involves multiple steps, including ICD, recruitment of DCs, and presentation of antigen signals. Designing a type of nanoplat that simultaneously regulates these steps is an urgent need to enhance anti-tumor immune responses. The nanosystem (CC-6td NP) composed of chlorin e6 (Ce6), celecoxib, and 6-thio-2ʹ-deoxyguanosine (6-thio-dG) can achieve the ideal of simultaneously regulating the DCs anti-tumor immune response cascade step154. Among them, Ce6 is responsible for stimulating ICD, celecoxib recruits DCs by inducing the production of chemokine CCL5, and 6-thio-dG is responsible for enhancing the antigen cross-presentation ability of DCs. The designed nanosystem enhances the immune response of tumors and inhibits the growth and postoperative recurrence of colon cancer. Manganese (Mn) is crucial for human health as a nutritional trace element. Mn can promote the maturation of DCs and trigger the anti-tumor immune responses of T cells by activating the cGAS–STING pathway. Therefore, Wang et al.155 developed cancer cell membrane-modified Mn hydroxide nanoparticles for loading doxorubicin (mHMnO-Dox) to exert synergistic anti-tumor effects. The Mn2+ released by mHMnO-Dox promotes the maturation of DCs by activating cGAS-STING, which in turn increases the homing of T and NK cells, ultimately inducing anti-tumor immune responses. The mature DCs membrane activated by tumor antigens (αDCM) retains the ability to present antigens and can enhance the anti-tumor immune response of T cells. Therefore, encapsulating αDCM on the surface of poly (lactic-co-glycolic acid) loaded with rapamycin (aDCM@PLGA/RAPA) is a simple and efficient strategy for improving immunotherapy156. aDCM@PLGA/RAPA can stimulate the maturation of DCs and activate T-cell infiltration, ultimately inhibiting the progression of glioma in situ. In addition, polymer nanoparticles (PAG/BTZ) prepared by combining bortezomib (BTZ) and aminoguanidine (AG) modified polymer nanocarriers through electrostatic adsorption can respond to the release of BTZ and AG in vivo157. The released BTZ is responsible for inducing ICD, while AG is responsible for promoting the maturation of DCs, thereby enhancing the induction of anti-tumor immune responses. Certain chemotherapy drugs can activate anti-tumor immune responses. Therefore, RNA extracted from cancer cells treated with chemotherapy drugs can stimulate the maturation of DCs and induce anti-cancer immune responses. Su et al.158 prepared composite nanoparticles (RNA NPs) by electrostatically binding induced RNA with protamine to promote the maturation of DCs and enhance the efficacy of immune checkpoint inhibitors (ICIs).
Although activating DCs can induce anti-cancer immune responses, relying solely on DCs has limited inhibitory effects on cancer. Therefore, currently developed nanomedicines mostly combine immunotherapy with chemotherapy.
3.8. Nanomedicine for targeting tumor-associated B cells (TABs)
Compared to other immune cells, the role of TABs in anti-tumor immunity is often overlooked159. In the tertiary lymphoid structure, TABs are activated and present tumor-associated antigens to T cells to kill tumor cells160. In addition, TABs can indirectly regulate the anti-tumor response of T cells by producing antibodies. However, almost no nanomedicine specifically targeting TABs has been developed. Similar to TAMs, TABs can promote and inhibit tumor growth. At present, it is unclear how to distinguish between different types of TABs. Therefore, the future development of nanomedicines can focus on targeting different types of TABs.
3.9. Non-specific targeted nanomedicine
In addition to targeting a specific immune cell, some nanomedicines can non-specifically target different immune cells to initiate anti-tumor immunity. For example, the combination of nanomedicine-mediated PDT and immunotherapy enhances anti-tumor efficiency. Nanomedicine enhances the homing of anti-tumor immune cells by remodeling ECM.
3.9.1. Nanomedicine-mediated PDT
Reactive oxygen species (ROS), which are byproducts of oxygen consumption and cellular metabolism, are important factors in cancer growth and progression. ROS not only affects the polarization of macrophages in TME but also influences the maturation of DCs161. Under illumination, PDT can activate the immune response and promote tumor cell apoptosis by producing ROS through photosensitizers162. Therefore, PDT combined with immunotherapy is receiving increasing attention. Two-dimensional sheet-like nanocarriers exhibit unique optical properties and are ideal for drug loading. Therefore, Liu et al.163 prepared ruthenium nanoparticles to load BLZ-945 (a small molecule inhibitor of the CSF-1/CSF-1R pathway) and indocyanine green (Ru@ICG BLZ NPs). Ru@ICG-BLZ NPs facilitated the repolarization of TAMs to the M1 phenotype and eliminated tumor cells through phototherapy. PDT can lead to the upregulation of hypoxia-inducible factor 1α (HIF-1α), thereby promoting TIME and immune escape. HIF-1α, as a crucial transcriptional activator, can regulate the polarization of TAMs towards the M2 phenotype, which promotes tumor growth and limits the effectiveness of PDT. Therefore, the combination of PDT and anti–HIF–1α is an effective strategy for promoting tumor immune response. Curcumin is a bioactive compound extracted from the rhizome of Curcuma longa. It can significantly reduce the level of HIF-1α in tumor cells. It is worth noting that curcumin can also act as a photosensitizer to yield ROS under laser irradiation in vitro. Therefore, curcumin was embedded into NIR-triggered core-satellite upconverting nanoparticles (Cur-CSNPs) to achieve the dual effects of PDT and HIF-1α reversal164. The results showed that Cur-CSNPs mediated PDT repolarized M2 macrophages to M1 macrophages, initiating synergistic immunotherapy and inhibiting 4T1 tumor growth. The hydrophilic molecule zoledronic acid (Zol) is frequently utilized to enhance immune suppression caused by TAMs. The combination of Zol and PDT can achieve dual effects by promoting tumor immune response and inhibiting tumor growth. In order to accurately deliver Zol and photosensitizer (IR780) to TAMs and tumor cells, Jian et al.165 designed Mn dioxide (MnO2)-embedded and LyP-1 peptide-labeled liposomal nanoparticles for encapsulation of Zol and IR780 (Lipo Zol/IR NPs). Lipo Zol/IR NPs release Zol in TEM and are selectively engulfed by TAMs, causing them to repolarize from the immunosuppressed M2 macrophages to the immunostimulated M1 macrophages. Simultaneously, IR780 entered tumor cells under the influence of the LyP-1 peptide, and subsequently produced a substantial amount of ROS upon laser irradiation, leading to the death of tumor cells. Copper sulfide nanoparticles (CuS NPs), a commonly used photothermal material, can induce the polarization of TAMs towards M1 macrophages by increasing intracellular ROS levels. Therefore, Tong et al.166 developed an active targeted delivery system based on mesoporous sulfide CuS (IL@H-PP). The copper ions released by IL@H-PP induce an increase in ROS in TAMs, leading to their repolarization into an M1 phenotype. The light-triggered prodrug nanoparticles (LT-NPs) assembled from photosensitizers (Vitipofen), cathepsin B-specific clear peptide (FRRG), and doxorubicin can effectively stimulate the maturation of DCs and initiate anti-tumor immune responses167. MnO2@Ce6 nanoprobes-loaded-iPS cells (iPS-MnO2@Ce6) have been developed to enhance immunotherapy for cancer. Under illumination, iPS-MnO2@Ce6 promotes the maturation of DCs, thereby activating T cells and NK cells, and inducing immune responses168.
3.9.2. Nanomedicine-mediated ECM remodeling
ECM is a key factor in inducing tumor immune suppression. Targeting ECM can enhance immune cell infiltration and immune response. The abundant ECM in the TME acts as a physical barrier that prevents T cell infiltration169. In addition, ECM also participates in the polarization of TAMs. Reconstructing the ECM is crucial for promoting T-cell infiltration and polarizing TAMs toward an anti-tumor phenotype. Therefore, an increasing number of researchers are focusing on how to reconstruct the ECM170.
TGF-β can restrict T cell infiltration by inducing ECM deposition, which leads to the formation of TIME171. Therefore, Wang et al.172 utilized stimuli-responsive clustered nanoparticles (iCluster) for the hierarchical delivery of TGF-β receptor inhibitors (TGF-β Ri, LY2157299) and siRNA targeting the PD-L1 gene (LYiClustersiRNA). LYiClustersiRNA significantly increased the infiltration of CD8+ T cells and inhibited tumor growth. In recent years, studies have found that ECM is involved in regulating the phenotype of immune cells. For example, ECM can induce macrophages to exhibit an anti-tumor phenotype173. Therefore, future nanomedicines targeting ECM can be utilized to regulate the polarization of TAMs and contribute to anti-tumor immunity.
Compared to nanomedicines that specifically target one immune cell, nanomedicines that do not specifically target multiple immune cells may be a hot topic of future attention. Non-specific targeting of various immune cells can simultaneously regulate the anti-tumor response of multiple immune cells, as well as inhibit tumor growth and metastasis.
4. Conclusions, challenges, and future directions
As of now, chemotherapy remains the standard treatment for primary and metastatic cancer in clinical practice. It primarily induces cancer cell apoptosis or necrosis through the use of chemotherapy drugs174, 175, 176, 177. However, the occurrence of various adverse reactions and multidrug resistance poses a significant challenge for chemotherapy178, 179, 180. The emergence of cancer immunotherapy has opened up new avenues for chemotherapy. Unlike traditional chemotherapy, immunotherapy primarily stimulates the immune system to fight malignant tumors and prevent tumor recurrence181. Compared to chemotherapy drugs that directly kill cancer cells, immunotherapy has a lower rate of off-target effects. Immunotherapy is primarily regulated by a complex array of stimulating and inhibitory cell surface interactions. To ensure the effectiveness of immunotherapy, a series of immune cascade reactions must occur, including the expression or release of cancer antigens, antigen presentation, activation of immune cells, transport of immune cells, and their infiltration into the tumor, ultimately leading to the killing of cancer cells182. Conventional immunotherapy includes adoptive T cell therapy183,184, anticancer vaccination185,186, oncolytic viruses187,188, and ICIs189. Among these, ICIs represent a significant breakthrough in immunotherapy and are currently the primary focus of scientists. In cancer treatment, the two primary targets of ICIs are CTLA-4190,191 and PD-1/PD-L1192,193. CTLA-4, which is expressed in immune cells, was the first ICI approved by the U.S. Food and Drug Administration (FDA). It participates in the negative regulation of immune responses by binding to CD80 and CD86 ligands. The second-generation ICIs target PD-1 or its ligand PD-L1. PD-1 mainly regulates the function of T cells by binding to PD-L1 and PD-L2. Although ICIs have brought significant survival benefits to patients and have become a promising clinical treatment method. However, there are significant differences in tumor invasiveness, immune response, and prognosis among different types of cancers194. ICIs are not effective for most patients. The main reason for the limited clinical benefits is the inadequate or challenging infiltration of immune cells in the TME195,196. According to the type, quantity, and location of immune cells in the TME, tumors can be categorized as hot, altered-excluded, altered-immunosuppressed, and cold tumors197. The limited clinical benefits of chemotherapy and immunotherapy are attributed to the minimal infiltration of immune cells in cold tumors and the reduced infiltration of immune cells in altered-excluded and altered-immunosuppressed tumors198,199. Therefore, reprogramming these tumors to become “hot” tumors is a clear strategy for enhancing the clinical benefits of chemotherapy and immunotherapy200,201.
With the rapid advancement of nanotechnology, significant progress has been made in the field of cancer research. Research in the field of nanotechnology for cancer encompasses several directions: a) diagnosing and treating cancer202, 203, 204, b) regulating the TIME205, 206, 207, c) controlling drug release in the TME208,209, and d) reversing multidrug resistance210,211. The FDA has approved numerous nanoparticles, such as paclitaxel albumin-bound nanoparticles (Abraxane) and Onivydes, for clinical cancer treatment212,213. Nanotechnology has been proven to be an effective strategy for stimulating anti-tumor immunity214. The primary methods for nanotechnology to activate anti-tumor immunity involve regulating the function or infiltration of immune cells. Specifically, it mainly includes a) nanotechnology used to deliver immune cell function activators or inhibitors to regulate immune cell function215, and b) nanotechnology used as a drug delivery system to regulate TME and increase immune cell infiltration216, 217, 218. Due to the unsatisfactory efficacy of cancer treatment solely through the activation of anti-tumor immunity, nanotechnology is frequently employed in combination with chemotherapy drugs, ICIs, or PDTs219, 220, 221, 222. Nano drugs can penetrate more tumor sites and remain within tumors due to their unique size, allowing them to exert their effects more effectively223. In addition, because of the specific conditions in the TME (including hypoxia, weak acidity, and high GSH levels, etc.), nanomedicines designed to respond to the TME can accumulate more effectively at the intended sites, reducing potential damage to healthy organs224,225. Traditional immunotherapy can lead to the occurrence of immune-related adverse events (irAEs) during clinical translation due to nonspecific accumulation226. Compared to traditional immunotherapy, drugs or genes encapsulated in nanosystems can protect them from the influence of the internal environment. This strategy addresses the issue of short half-life and can extend the onset time of immunotherapy. Modifying the surface of nanomedicines can enable them to target immune cells more accurately and efficiently. As a result, the non-targeted issue of traditional immunotherapy has been resolved. Therefore, the introduction of nanotechnology represents a significant breakthrough in chemotherapy and immunotherapy.
While the integration of nanotechnology with chemotherapy and immunotherapy has yielded significant results, there are still unresolved issues that must be addressed before clinical application. a) The safety issues of nanomedicine227,228. In the field of cancer treatment, nanomedicines are primarily administered through intravenous or subcutaneous injection into the body. Understanding the distribution, metabolism, and excretion behavior of nanodrugs in vivo is essential for their development. After entering the body, 30%–99% of nanodrugs accumulate in the liver, leading to increased liver toxicity229, 230, 231. Polyethylene glycol-modified liposomes can induce immune responses and accelerate their clearance in the body232. Inorganic nanoparticles are difficult to degrade in the body, and long-term accumulation can affect bodily safety233. b) The side effects of immunotherapy. Immunotherapy may result in permanent neurological deficits and/or mortality234. Some immunotherapies can trigger irAEs, such as CTLA4 inhibition, which increases the incidence of pituitary inflammation235, and PD-L1 blockade, which increases the incidence of thyroid dysfunction236. Overcoming these challenges is crucial for the clinical advancement of nanotechnology-mediated immunotherapies. Therefore, future nanotechnology-mediated immunotherapy should focus on addressing its safety and side effects.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82304853), University Natural Science Research Project of Anhui Province (2022AH050533, KJ2021A0586, China), Chinese Society of Traditional Chinese Medicine Young Talent Support Project Program (CACM-2022-QNRC2-B03), and Anhui University of Traditional Chinese Medicine Talent Support Program (2022rczd004).
Author contributions
Yunna Chen: Writing – review & editing, Writing – original draft, Visualization. Qianqian Zhou: Writing – review & editing, Visualization. Zongfang Jia: Writing – review & editing. Nuo Cheng: Visualization. Sheng Zhang: Visualization. Weidong Chen: Writing – review & editing, Project administration. Lei Wang: Writing – review & editing, Project administration.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.05.032.
Contributor Information
Weidong Chen, Email: wdchen@ahtcm.edu.cn.
Lei Wang, Email: wanglei@ahtcm.edu.cn.
Appendix A. Supporting information
The following is the Supporting information to this article:
References
- 1.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y.Y., Zhang Z.M. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagher O.K., Schwab R.D., Brookens S.K., Posey A.D. Advances in cancer immunotherapies. Cell. 2023;186:1814–1815. doi: 10.1016/j.cell.2023.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Han Y.Y., Liu D.D., Li L.H. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727–742. [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Y.X., Xue J.H., Zhao Y.Y., Zhang Y., Huang Y., Yang Y.P., et al. Phase I trial of KN046, a novel bispecific antibody targeting PD-L1 and CTLA-4 in patients with advanced solid tumors. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2022-006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X.Y., Xu N., Li Z.Y., Shen L., Ji K., Zheng Z., et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 2023;24:1134–1146. doi: 10.1016/S1470-2045(23)00411-4. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., et al. Nivolumab plus Ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura K., Smyth M.J. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cel Mol Immunol. 2020;17:1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L.H., Yu R., Cai T.G., Chen Z., Lan M., Zou T.T., et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106939. [DOI] [PubMed] [Google Scholar]
- 10.Shafqat A., Omer M.H., Ahmed E.N., Mushtaq A., Ijaz E., Ahmed Z., et al. Reprogramming the immunosuppressive tumor microenvironment: exploiting angiogenesis and thrombosis to enhance immunotherapy. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1200941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan D.H., Cao Y.K., Cao M.Q., Wang Y.J., Cao Y.L., Gong T. Nanomedicine in cancer therapy. Signal Transduct Tar. 2023;8:293. doi: 10.1038/s41392-023-01536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M.M., Han X.P., Xiong H.T., Gao Y.J., Xu B.W., Zhu G.H., et al. Cancer nanomedicine: emerging strategies and therapeutic potentials. Molecules. 2023;28:5145. doi: 10.3390/molecules28135145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gawali P., Saraswat A., Bhide S., Gupta S., Patel K. Human solid and clinical relevance of the enhanced permeation and retention effect: a 'golden gate' for nanomedicine in preclinical studies? Nanomedicine-Uk. 2023;18:169–190. doi: 10.2217/nnm-2022-0257. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M., Ma H., Wang X.J., Yu B., Cong H.L., Shen Y.Q. Polysaccharide-based nanocarriers for efficient transvascular drug delivery. J Control Release. 2023;354:167–187. doi: 10.1016/j.jconrel.2022.12.051. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y., Van der Meel R., Chen X.Y., Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921–7924. doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng R.Y., Santos H.A. Smart nanoparticle-based platforms for regulating tumor microenvironment and cancer immunotherapy. Adv Healthc Mater. 2022;12 doi: 10.1002/adhm.202202063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia M., Zhang D., Zhang C.X., Li C.H. Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy. J Nanobiotechnol. 2021;19:384. doi: 10.1186/s12951-021-01134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y.Y., Xiong J.Y., Sun X.Y., Gao H.L. Targeted nanomedicines remodeling immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Acta Pharm Sin B. 2022;12:4327–4347. doi: 10.1016/j.apsb.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Z.Y., Liu T.Q., Liu C., Yang Y.N., Tang J., Song H., et al. Ferroptosis-strengthened metabolic and inflammatory regulation of tumor-associated macrophages provokes potent tumoricidal activities. Nano Lett. 2021;21:6471–6479. doi: 10.1021/acs.nanolett.1c01401. [DOI] [PubMed] [Google Scholar]
- 21.Ma X., Yang S., Zhang T., Wang S., Yang Q., Xiao Y., et al. Bioresponsive immune-booster-based prodrug nanogel for cancer immunotherapy. Acta Pharm Sin B. 2022;12:451–466. doi: 10.1016/j.apsb.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv B.Z., Wang Y.P., Ma D.J., Cheng W., Liu J., Yong T., et al. Immunotherapy: reshape the tumor immune microenvironment. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.844142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grout J.A., Sirven P., Leader A.M., Maskey S., Hector E., Puisieux I., et al. Spatial positioning and matrix programs of cancer-associated fibroblasts promote T-cell exclusion in human lung tumor. Cancer Discov. 2022;12:2606–2625. doi: 10.1158/2159-8290.CD-21-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ugel S., Canè S., De Sanctis F., Bronte V. Monocytes in the tumor microenvironment. Annu Rev Pathol-mech. 2021;16:93–122. doi: 10.1146/annurev-pathmechdis-012418-013058. [DOI] [PubMed] [Google Scholar]
- 25.Elanany M.M., Mostafa D., Hamdy N.M. Remodeled tumor immune microenvironment (TIME) parade natural killer cells reprogramming in breast cancer. Life Sci. 2023;330 doi: 10.1016/j.lfs.2023.121997. [DOI] [PubMed] [Google Scholar]
- 26.Wang X.X., Zha H.R., Wu W., Yuan T., Xie S.L., Jin Z., et al. CD200+ cytotoxic T lymphocytes in the tumor microenvironment are crucial for efficacious anti-PD-1/PD-L1 therapy. Sci Transl Med. 2023;15 doi: 10.1126/scitranslmed.abn5029. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich M., Hahn M., Michel J., Sankowski R., Kilian M., Kehl N., et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. 2023;25:263–276. doi: 10.1093/neuonc/noac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terrén I., Orrantia A., Vitallé J., Zenarruzabeitia O., Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol. 2019;10:2278. doi: 10.3389/fimmu.2019.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C., Wang Z.H., Ding Y., Qin Y.R. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sathe A., Mason K., Grimes S.M., Zhou Z.L., Lau B.T., Bai X.Q., et al. Colorectal cancer metastases in the liver establish immunosuppressive spatial networking between tumor-associated macrophages and fibroblasts. Clin Cancer Res. 2023;29:244–260. doi: 10.1158/1078-0432.CCR-22-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonaventura P., Shekarian T., Alcazer V., Valladeau-Guilemond J., Valsesia-Wittmann S., Amigorena S., et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B.X., Hu M., Ma Q.Z., Li K., Li X., He X., et al. Optimized CAR-T therapy based on spatiotemporal changes and chemotactic mechanisms of MDSCs induced by hypofractionated radiotherapy. Mol Ther. 2023;31:2105–2119. doi: 10.1016/j.ymthe.2023.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B.Y., Zhang Z.B., Liu W.B., Tan B.B. Targeting regulatory T cells in gastric cancer: pathogenesis, immunotherapy, and prognosis. Biomed Pharmacother. 2023;158 doi: 10.1016/j.biopha.2022.114180. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y.J., Li G.N., Li X.J., Wei L.X., Fu M.J., Cheng Z.L., et al. Targeting IRG1 reverses the immunosuppressive function of tumor-associated macrophages and enhances cancer immunotherapy. Sci Adv. 2023;9 doi: 10.1126/sciadv.adg0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nixon B.G., Kuo F.S., Ji L.L., Liu M., Capistrano K., Do M., et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity. 2022;55:2044–2058. doi: 10.1016/j.immuni.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao X.Q., Xu J., Wang W., Liang C., Hua J., Liu J., et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C., Chikina M., Deshpande R., Menk A.V., Wang T., Tabib T., et al. Treg cells promote the SREBP1-dependent metabolic fitness of tumor-promoting macrophages via repression of CD8+ T cell-derived interferon-γ. Immunity. 2019;51:381–397. doi: 10.1016/j.immuni.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zalfa C., Paust S. Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.633205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L., Zhou X.L., Cheng S., Ge Y.Y., Chen B.M., Shi J., et al. RNA-binding protein DHX9 promotes glioma growth and tumor-associated macrophages infiltration via TCF12. Cns Neurosci Ther. 2023;29:988–999. doi: 10.1111/cns.14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawaguchi Y., Ohshio Y., Watanabe A., Shiratori T., Okamoto K., Ueda K., et al. Depletion of tumor-associated macrophages inhibits lung cancer growth and enhances the antitumor effect of cisplatin. Cancer Sci. 2023;114:750–763. doi: 10.1111/cas.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohila D., Park I.H., Pham T.V., Weitz J., de Mendoza T.H., Madheswaran S., et al. Syk inhibition reprograms tumor-associated macrophages and overcomes gemcitabine-induced immunosuppression in pancreatic ductal adenocarcinoma. Cancer Res. 2023;83:2675–2689. doi: 10.1158/0008-5472.CAN-22-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J.F., Xiang J.X., Jin C.Y., Ye L.S., Wang L., Gao Y.A., et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnol. 2023;21:78. doi: 10.1186/s12951-023-01835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fathi N., Mojtahedi H., Nasiri M., Abolhassani H., Marzbali M.Y., Esmaeili M., et al. How do nuclear factor kappa B (NF-κB)1 and NF-κB2 defects lead to the incidence of clinical and immunological manifestations of inborn errors of immunity? Expert Rev Clin Immu. 2023;19:329–339. doi: 10.1080/1744666X.2023.2174105. [DOI] [PubMed] [Google Scholar]
- 44.He Z.W., Wang J., Zhu C.H., Xu J., Chen P., Jiang X.Y., et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022;548 doi: 10.1016/j.canlet.2022.215751. [DOI] [PubMed] [Google Scholar]
- 45.Zhao T., Zeng J.M., Xu Y.J., Su Z.P., Chong Y.L., Ling T., et al. Chitinase-3 like-protein-1 promotes glioma progression via the NF-κB signaling pathway and tumor microenvironment reprogramming. Theranostics. 2022;12:6989–7008. doi: 10.7150/thno.75069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li G.L., Tang J.F., Tan W.L., Zhang T., Zeng D., Zhao S., et al. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-κB signaling pathway. Food Funct. 2023;14:3155–3168. doi: 10.1039/d2fo02191a. [DOI] [PubMed] [Google Scholar]
- 47.Mishima K., Nishikawa R., Narita Y., Mizusawa J., Sumi M., Koga T., et al. Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro Oncol. 2023;25:687–698. doi: 10.1093/neuonc/noac246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bharadwaj R., Lusi C.F., Mashayekh S., Nagar A., Subbarao M., Kane G.I., et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998–1012. doi: 10.1016/j.immuni.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavalcante R.M.S., Ishikawa U., Silva E.S., Silva A.A., Araújo A.A., Cruz L.J., et al. STAT3/NF-κB signalling disruption in M2 tumour-associated macrophages is a major target of PLGA nanocarriers/PD-L1 antibody immunomodulatory therapy in breast cancer. Br J Pharmacol. 2021;178:2284–2304. doi: 10.1111/bph.15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li K., Lu L., Xue C.C., Liu J., He Y., Zhou J., et al. Polarization of tumor-associated macrophage phenotype porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;12:130–144. doi: 10.1039/c9nr06505a. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y., Liang S., Jiang D.D., Gao T., Fang Y.X., Fu S.L., et al. Manipulation of TAMs functions to facilitate the immune therapy effects of immune checkpoint antibodies. J Control Release. 2021;336:621–634. doi: 10.1016/j.jconrel.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Yang L.N., Sun J., Liu Q., Zhu R.R., Yang Q.N., Hua J.H., et al. Synergetic functional nanocomposites enhance immunotherapy in solid tumors by remodeling the immunoenvironment. Adv Sci. 2019;6 doi: 10.1002/advs.201802012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saxton R.A., Glassman C.R., Garcia K.C. Emerging principles of cytokine pharmacology and therapeutics. Nat Rev Drug Discov. 2023;22:21–37. doi: 10.1038/s41573-022-00557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X.Y., Liu M., Fu Y.J., Jiang Y.J., Zhang Z.N. Alterations in levels of cytokine following treatment to predict outcome of sepsis: a meta-analysis. Cytokine. 2023;161 doi: 10.1016/j.cyto.2022.156056. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia V., Kamat N.V., Pariva T.E., Wu L.T., Tsao A., Sasaki K., et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun. 2023;14:2041. doi: 10.1038/s41467-023-37874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu N.S., Wang G.W., Wang J.Q., Zhou Q., Guo M.Y., Wang Y.L., et al. Tumor-associated macrophage and tumor-cell dually transfecting polyplexes for efficient interleukin-12 cancer gene therapy. Adv Mater. 2021;33 doi: 10.1002/adma.202006189. [DOI] [PubMed] [Google Scholar]
- 57.Wan Y.F., Yu W.J., Li J.M., Peng N., Ding X., Wang Y.L., et al. Multi-functional carboxymethyl chitin-based nanoparticles for modulation of tumor-associated macrophage polarity. Carbohyd Polym. 2021;267 doi: 10.1016/j.carbpol.2021.118245. [DOI] [PubMed] [Google Scholar]
- 58.Li T.S., Liu Z.H., Fu X., Chen Y.Q., Zhu S.L., Zhang J. Co-delivery of interleukin-12 and doxorubicin loaded nano-delivery system for enhanced immunotherapy with polarization toward M1-type macrophages. Eur J Pharm Biopharm. 2022;177:175–183. doi: 10.1016/j.ejpb.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y., Cronin M.F., Mendonça M.C.P., Guo J.F., O'Driscoll C.M. Sialic acid-targeted cyclodextrin-based nanoparticles deliver siRNA and reprogram tumour-associated macrophages for immunotherapy of prostate cancer. Eur J Pharm Sci. 2023;185 doi: 10.1016/j.ejps.2023.106427. [DOI] [PubMed] [Google Scholar]
- 60.Li Y., Yang B., Miao H.J., Liu L.G., Wang Z.Y., Jiang C.K., et al. Nicotinamide-methyltransferase promotes M2 macrophage polarization by IL6 and MDSC conversion by GM-CSF in gallbladder carcinoma. Hepatology. 2023;78:1352–1367. doi: 10.1097/HEP.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y., Cronin M.F., Mendonça M.C.P., Guo J.F., O'Driscoll C.M. M2pep-modified cyclodextrin-siRNA nanoparticles modulate the immunosuppressive tumor microenvironment for prostate cancer therapy. Mol Pharmaceut. 2023;20:5921–5936. doi: 10.1021/acs.molpharmaceut.3c00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magkouta S.F., Vaitsi P.C., Pappas A.G., Iliopoulou M., Kosti C.N., Psarra K., et al. CSF1/CSF1R axis blockade limits mesothelioma and enhances efficiency of anti-PDL1 immunotherapy. Cancers. 2021;13:2546. doi: 10.3390/cancers13112546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramesh A., Brouillard A., Kumar S., Nandi D., Kulkarni A. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong H.Z., Mittman S., Rodriguez R., Moskalenko M., Pacheco-Sanchez P., Yang Y.G., et al. Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79:1493–1506. doi: 10.1158/0008-5472.CAN-18-3208. [DOI] [PubMed] [Google Scholar]
- 65.Ramesh A., Malik V., Ranjani H.A., Smith H., Kulkarni A.A. Rational combination of an immune checkpoint inhibitor with CSF1R inhibitor-loaded nanoparticle enhances anticancer efficacy. Drug Deliv Transl Re. 2021;11:2317–2327. doi: 10.1007/s13346-021-01040-2. [DOI] [PubMed] [Google Scholar]
- 66.Han Y.C., Sun J.C., Yang Y.Y., Liu Y.L., Lou J., Pan H.M., et al. TMP195 exerts antitumor effects on colorectal cancer by promoting M1 macrophages polarization. Int J Biol Sci. 2022;18:5653–5666. doi: 10.7150/ijbs.73264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue Y.L., Li F.F., Li Y., Wang Y.Z., Guo X.J., Cheng Z.X., et al. Biomimetic nanoparticles carrying a repolarization agent of tumor-associated macrophages for remodeling of the inflammatory microenvironment following photothermal therapy. Acs Nano. 2021;15:15166–15179. doi: 10.1021/acsnano.1c05618. [DOI] [PubMed] [Google Scholar]
- 68.Figueiredo P., Lepland A., Scodeller P., Fontana F., Torrieri G., Tiboni M., et al. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2021;133:231–243. doi: 10.1016/j.actbio.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 69.Costa da Silva M., Breckwoldt M.O., Vinchi F., Correia M.P., Stojanovic A., Thielmann C.M., et al. Iron induces anti-tumor activity in tumor-associcated macrophages. Front Immunol. 2017;8:1479. doi: 10.3389/fimmu.2017.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao M., Li J., Liu J.W., Xu M.X., Ji H.R., Wu S.W., et al. Charge-switchable nanoparticles enhance cancer immunotherapy based on mitochondrial dynamic regulation and immunogenic cell death induction. J Control Release. 2021;335:320–332. doi: 10.1016/j.jconrel.2021.05.036. [DOI] [PubMed] [Google Scholar]
- 71.Yang L.N., Li F., Cao Y.S., Liu Q., Jing G.X., Niu J.T., et al. Multifunctional silica nanocomposites prime tumoricidal immunity for efficient cancer immunotherapy. J Nanobiotechnol. 2021;19:328. doi: 10.1186/s12951-021-01073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeng W.W., Yu M., Chen T., Liu Y.Q., Yi Y.F., Huang C.Y., et al. Polypyrrole nanoenzymes as tumor microenvironment modulators to reprogram macrophage and potentiate immunotherapy. Adv Sci. 2022;9 doi: 10.1002/advs.202201703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu Y.K., Li T.X., Ou M.T., Luo R., Chen H.Z., Ren H., et al. OX40L-expressing M1-like macrophage exosomes for cancer immunotherapy. J Control Release. 2024;365:469–479. doi: 10.1016/j.jconrel.2023.11.051. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Q.N., Hu J.M., Kong L.Y., Jiang S., Tian X.J., Wang J., et al. FGL2-targeting T cells exhibit antitumor effects on glioblastoma and recruit tumor-specific brain-resident memory T cells. Nat Commun. 2023;14:735. doi: 10.1038/s41467-023-36430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui Y.B., Yuan T.J., Wang Y., Zheng D.W., Qin L., Li S.L., et al. T lymphocytes expressing the switchable chimeric Fc receptor CD64 exhibit augmented persistence and antitumor activity. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112797. [DOI] [PubMed] [Google Scholar]
- 76.Park J., Hsueh P.C., Li Z.Y., Ho P.C. Microenvironment-driven metabolic adaptations guiding CD8+ T cell anti-tumor immunity. Immunity. 2023;56:32–42. doi: 10.1016/j.immuni.2022.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y.X., Wang W.R., Gu R., Chen J., Chen Q., Lin T.S., et al. In situ vaccination with mitochondria-targeting immunogenic death inducer elicits CD8 T cell-dependent antitumor immunity to boost tumor immunotherapy. Adv Sci. 2023;10 doi: 10.1002/advs.202300286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brightman S.E., Becker A., Thota R.R., Naradikian M.S., Chihab L., Zavala K.S., et al. Neoantigen-specific stem cell memory-like CD4 T cells mediate CD8 T cell-dependent immunotherapy of MHC class II-negative solid tumors. Nat Immunol. 2023;24:1345–1357. doi: 10.1038/s41590-023-01543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Protti M.P., Monte L.D., Lullo G.D. Tumor antigen-specific CD4+ T cells in cancer immunity: from antigen identification to tumor prognosis and development of therapeutic strategies. Tissue Antigens. 2014;83:237–246. doi: 10.1111/tan.12329. [DOI] [PubMed] [Google Scholar]
- 80.Cheng J., Yan J.X., Liu Y., Shi J.Z., Wang H.Y., Zhou H.Y., et al. Cancer-cell-derived fumarate suppresses the anti-tumor capacity of CD8+ T cells in the tumor microenvironment. Cell Metab. 2023;35:961–978. doi: 10.1016/j.cmet.2023.04.017. [DOI] [PubMed] [Google Scholar]
- 81.Hu C., Song Y.J., Zhang Y.W., He S.Q., Liu X.Y., Yang X.T., et al. Sequential delivery of PD-1/PD-L1 blockade peptide and IDO inhibitor for immunosuppressive microenvironment remodeling via an MMP-2 responsive dual-targeting liposome. Acta Pharm Sin B. 2023;13:2176–2187. doi: 10.1016/j.apsb.2023.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lester D.K., Burton C., Gardner A., Innamarato P., Kodumudi K., Liu Q., et al. Fucosylation of HLA-DRB1 regulates CD4+ T cell-mediated anti-melanoma immunity and enhances immunotherapy efficacy. Nat Cancer. 2023;4:222–239. doi: 10.1038/s43018-022-00506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trefny M.P., Kirchhammer N., Auf der Maur P., Natoli M., Schmid D., Germann M., et al. Deletion of alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy. Nat Commun. 2023;14:86. doi: 10.1038/s41467-022-35583-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin Y.X., Wang Y., Ding J.X., Jiang A.P., Wang J., Yu M.A., et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.aba9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lukhele S., Abd Rabbo D., Guo M.D., Shen J., Elsaesser H.J., Quevedo R., et al. The transcription factor IRF2 drives interferon-mediated CD8+ T cell exhaustion to restrict anti-tumor immunity. Immunity. 2022;55:2369–2385. doi: 10.1016/j.immuni.2022.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang T.T., Zhang H., Han Y.B., Zheng Q., Liu H.H., Han M.X., et al. Reversing T cell dysfunction to boost glioblastoma immunotherapy by paroxetine-mediated GRK2 inhibition and blockade of multiple checkpoints through biomimetic nanoparticles. Adv Sci. 2023;10 doi: 10.1002/advs.202204961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren H., Yong J.H., Yang Q.Q., Yang Z., Liu Z.Y., Xu Y., et al. Self-assembled FeS-based cascade bioreactor with enhanced tumor penetration and synergistic treatments to trigger robust cancer immunotherapy. Acta Pharm Sin B. 2021;11:3244–3261. doi: 10.1016/j.apsb.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Q., Hu Q.Y., Dukhovlinova E., Chen G.J., Ahn S., Wang C., et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31 doi: 10.1002/adma.201900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanmarco L.M., Rone J.M., Polonio C.M., Lahore G.F., Giovannoni F., Ferrara K., et al. Lactate limits CNS autoimmunity by stabilizing HIF-1α in dendritic cells. Nature. 2023;620:881–889. doi: 10.1038/s41586-023-06409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao J.L., Tian Z.M., Zhao S.J., Feng D.Y., Guo Z.X., Wen L.Z., et al. Insights into the effect of catalytic intratumoral lactate depletion on metabolic reprogramming and immune activation for antitumoral activity. Adv Sci. 2023;10 doi: 10.1002/advs.202204808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J.X., Choi S.Y.C., Niu X.J., Kang N., Xue H., Killam J., et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. 2020;21:8363. doi: 10.3390/ijms21218363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaggero S., Martinez-Fabregas J., Cozzani A., Fyfe P.K., Leprohon M., Yang J., et al. IL-2 is inactivated by the acidic pH environment of tumors enabling engineering of a pH-selective mutein. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.ade5686. [DOI] [PubMed] [Google Scholar]
- 93.Fang Y., Liu W.R., Tang Z., Ji X., Zhou Y.F., Song S.S., et al. Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack. Hepatology. 2023;77:109–123. doi: 10.1002/hep.32348. [DOI] [PubMed] [Google Scholar]
- 94.Shi P.F., Sun X.R., Yuan H.M., Chen K.X., Bi S., Zhang S.S. Nanoscale metal-organic frameworks combined with metal nanoparticles and metal oxide/peroxide to relieve tumor hypoxia for enhanced photodynamic therapy. Acs Biomater Sci Eng. 2023;9:5441–5456. doi: 10.1021/acsbiomaterials.3c00509. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y.X., Zhao Y.Y., Shen J.Z., Sun X., Liu Y., Liu H., et al. Nanoenabled modulation of acidic tumor microenvironment reverses anergy of infiltrating T cells and potentiates anti-PD-1 therapy. Nano Lett. 2019;19:2774–2783. doi: 10.1021/acs.nanolett.8b04296. [DOI] [PubMed] [Google Scholar]
- 96.Lu Y., Li L.H., Du J.W., Chen J.L., Xu X.Y., Yang X.F., et al. Immunotherapy for tumor metastasis by artificial antigen-presenting cells via targeted microenvironment regulation and T-cell activation. Acs Appl Mater Inter. 2021;13:55890–55901. doi: 10.1021/acsami.1c17498. [DOI] [PubMed] [Google Scholar]
- 97.Tay C., Tanaka A., Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41:450–465. doi: 10.1016/j.ccell.2023.02.014. [DOI] [PubMed] [Google Scholar]
- 98.Kumar P., Bhattacharya P., Prabhakar B.S. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 2018;95:77–99. doi: 10.1016/j.jaut.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eggenhuizen P.J., Ng B.H., Ooi J.D. Treg enhancing therapies to treat autoimmune diseases. Int J Mol Sci. 2020;21:7015. doi: 10.3390/ijms21197015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Hooren L., Handgraaf S.M., Kloosterman D.J., Karimi E., van Mil L.W.H.G., Gassama A.A., et al. CD103 regulatory T cells underlie resistance to radio-immunotherapy and impair CD8 T cell activation in glioblastoma. Nat Cancer. 2023;4:665–681. doi: 10.1038/s43018-023-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.So L., Obata-Ninomiya K., Hu A., Muir V.S., Takamori A., Song J., et al. Regulatory T cells suppress CD4 effector T cell activation by controlling protein synthesis. J Exp Med. 2023;220 doi: 10.1084/jem.20221676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Domvri K., Petanidis S., Zarogoulidis P., Anestakis D., Tsavlis D., Bai C., et al. Treg-dependent immunosuppression triggers effector T cell dysfunction via the STING/ILC2 axis. Clin Immunol. 2021;222 doi: 10.1016/j.clim.2020.108620. [DOI] [PubMed] [Google Scholar]
- 103.Kurniawan H., Franchina D.G., Guerra L., Bonetti L., Soriano-Baguet L., Grusdat M., et al. Glutathione restricts serine metabolism to preserve regulatory T cell function. Cel Metab. 2020;31:920–936. doi: 10.1016/j.cmet.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suthen S., Lim C.J., Nguyen P.H.D., Dutertre C.A., Lai H.L.H., Wasser M., et al. Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology. 2022;76:1329–1344. doi: 10.1002/hep.32419. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y.L., Chai N., Wei Z.Z., Li Z., Zhang L., Zhang M.J., et al. YYFZBJS inhibits colorectal tumorigenesis by enhancing Tregs-induced immunosuppression through HIF-1α mediated hypoxia in vivo and in vitro. Phytomedicine. 2022;98 doi: 10.1016/j.phymed.2021.153917. [DOI] [PubMed] [Google Scholar]
- 106.Li Z.T., Deng Y.Y., Sun H.H., Tan C.X., Li H.M., Mo F.Y., et al. Redox modulation with a perfluorocarbon nanoparticle to reverse Treg-mediated immunosuppression and enhance anti-tumor immunity. J Control Release. 2023;358:579–590. doi: 10.1016/j.jconrel.2023.05.013. [DOI] [PubMed] [Google Scholar]
- 107.Ou W., Thapa R.K., Jiang L.Y., Soe Z.C., Gautam M., Chang J.H., et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J Control Release. 2018;281:84–96. doi: 10.1016/j.jconrel.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 108.He S.Q., Wang L.L., Wu D.X., Tong F., Zhao H., Li H.M., et al. Dual-responsive supramolecular photodynamic nanomedicine with activatable immunomodulation for enhanced antitumor therapy. Acta Pharm Sin B. 2024;14:765–780. doi: 10.1016/j.apsb.2023.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ou W., Jiang L., Thapa R.K., Soe Z.C., Poudel K., Chang J.H., et al. Combination of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles for effective cancer photoimmunotherapy. Theranostics. 2018;8:4574–4590. doi: 10.7150/thno.26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hossain D.M.S., Panda A.K., Chakrabarty S., Bhattacharjee P., Kajal K., Mohanty S., et al. MEK inhibition prevents tumour-shed transforming growth factor-β-induced T-regulatory cell augmentation in tumour milieu. Immunology. 2015;144:561–573. doi: 10.1111/imm.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang N., Liu S.N., Shi S.Y., Chen Y.T., Xu F.W., Wei X.H., et al. Solubilization and delivery of Ursolic-acid for modulating tumor microenvironment and regulatory T cell activities in cancer immunotherapy. J Control Release. 2020;320:168–178. doi: 10.1016/j.jconrel.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 112.Das A., Tsai H.C., Sen T., Moirangthem R.S. Plasmonic nanoparticle-based surface-enhanced Raman spectroscopy-guided photothermal therapy: emerging cancer theranostics. Nanomedicine-Uk. 2023;18:555–576. doi: 10.2217/nnm-2023-0010. [DOI] [PubMed] [Google Scholar]
- 113.Chen Z.Y., Chen Y.C., Xu Y.J., Shi X.C., Han Z., Bai Y., et al. BODIPY-based multifunctional nanoparticles for dual mode imaging-guided tumor photothermal and photodynamic therapy. ACS Appl Bio Mater. 2023;18:3406–3413. doi: 10.1021/acsabm.3c00083. [DOI] [PubMed] [Google Scholar]
- 114.Deng C.T., Zheng M.C., Xin J.Q., An F.F. A nanoparticle composed of totally hospital-available drugs and isotope for fluorescence/SPECT dual-modal imaging-guided photothermal therapy to inhibit tumor metastasis. J Colloid Interf Sci. 2023;651:384–393. doi: 10.1016/j.jcis.2023.07.163. [DOI] [PubMed] [Google Scholar]
- 115.Chen H.W., Luan X., Paholak H.J., Burnett J.P., Stevers N.O., Sansanaphongpricha K., et al. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine-Uk. 2020;15:77–92. doi: 10.2217/nnm-2019-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bednar K.J., Lee J.H., Ort T. Tregs in autoimmunity: insights into intrinsic brake mechanism driving pathogenesis and immune homeostasis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.932485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 118.Maj T., Wang W., Crespo J., Zhang H.J., Wang W.M., Wei S., et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan R., Li J.J., Xiao Z.R., Fan X.A., Liu H.S., Xu Y., et al. DCLK1 suppresses tumor-specific cytotoxic T lymphocyte function through recruitment of MDSCs via the CXCL1-CXCR2 axis. Cell Mol Gastroenter. 2023;15:463–485. doi: 10.1016/j.jcmgh.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giannotta C., Autino F., Massaia M. The immune suppressive tumor microenvironment in multiple myeloma: the contribution of myeloid-derived suppressor cells. Front Immunol. 2023;13 doi: 10.3389/fimmu.2022.1102471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu H.Y., Wang Z.C., Zhou Y.T., Yang Y.M. MDSCs in breast cancer: an important enabler of tumor progression and an emerging therapeutic target. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1199273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dai X.H., Ren L., Liu M.X., Cai H., Zhang H., Gong Q.Y., et al. Nanomedicines modulating myeloid-derived suppressor cells for improving cancer immunotherapy. Nano Today. 2021;39 [Google Scholar]
- 123.Plebanek M.P., Bhaumik D., Bryce P.J., Thaxton C.S. Scavenger receptor type B1 and lipoprotein nanoparticle inhibit myeloid-derived suppressor cells. Mol Cancer Ther. 2018;17:686–697. doi: 10.1158/1535-7163.MCT-17-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang T., Xiong H.G., Ma X.B., Gao Y., Xue P., Kang Y.J., et al. Supramolecular tadalafil nanovaccine for cancer immunotherapy by alleviating myeloid-derived suppressor cells and heightening immunogenicity. Small Methods. 2021;5 doi: 10.1002/smtd.202100115. [DOI] [PubMed] [Google Scholar]
- 125.Chiu D.K.C., Xu I.M.J., Lai R.K.H., Tse A.P.W., Wei L.L., Koh H.Y., et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology. 2016;64:797–813. doi: 10.1002/hep.28655. [DOI] [PubMed] [Google Scholar]
- 126.Zuo H.Q., Hou Y.C., Yu Y.J., Li Z.Q., Liu H.X., Liu C., et al. Circumventing myeloid-derived suppressor cell-mediated immunosuppression using an oxygen-generated and -economized nanoplatform. Acs Appl Mater Inter. 2020;12:55723–55736. doi: 10.1021/acsami.0c18180. [DOI] [PubMed] [Google Scholar]
- 127.Xia C.Y., Li M., Ran G.Y., Wang X.H., Lu Z.Z., Li T., et al. Redox-responsive nanoassembly restrained myeloid-derived suppressor cells recruitment through autophagy-involved lactate dehydrogenase a silencing for enhanced cancer immunochemotherapy. J Control Release. 2021;335:557–574. doi: 10.1016/j.jconrel.2021.05.034. [DOI] [PubMed] [Google Scholar]
- 128.Lu G.F., Du R., Dong J.Q., Sun Y., Zhou F.L., Feng F., et al. Cancer associated fibroblast derived SLIT2 drives gastric cancer cell metastasis by activating NEK9. Cell Death Dis. 2023;14:421. doi: 10.1038/s41419-023-05965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Z.X., Zhang Y.Q., Guo E.R., Zhang Y., Wang Y.N. Periostin secreted from podoplanin-positive cancer-associated fibroblasts promotes metastasis of gastric cancer by regulating cancer stem cells via AKT and YAP signaling pathway. Mol Carcinogen. 2023;62:685–699. doi: 10.1002/mc.23517. [DOI] [PubMed] [Google Scholar]
- 130.Jenkins L., Jungwirth U., Avgustinova A., Iravani M., Mills A., Haider S., et al. Cancer-associated fibroblasts suppress CD8+ T-cell infiltration and confer resistance to immune-checkpoint blockade. Cancer Res. 2022;82:2904–2917. doi: 10.1158/0008-5472.CAN-21-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu G.Q., Tang Z., Huang R., Qu W.F., Fang Y., Yang R., et al. CD36 cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell Discov. 2023;9:25. doi: 10.1038/s41421-023-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ai W., Liu T.H., Lv C.S., Feng X.R., Wang Q.S. Modulation of cancer-associated fibroblasts by nanodelivery system to enhance efficacy of tumor therapy. Nanomedicine-Uk. 2023;18:1025–1039. doi: 10.2217/nnm-2023-0088. [DOI] [PubMed] [Google Scholar]
- 133.Fei B.Y., Mo Z.H., Yang J.H., Wang Z., Li S. Nanodrugs reprogram cancer-associated fibroblasts and normalize tumor vasculatures for sequentially enhancing photodynamic therapy of hepatocellular carcinoma. Int J Nanomed. 2023;18:6379–6391. doi: 10.2147/IJN.S429884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang H.Y., Chen L.Y., Zhao Y., Luo N.C., Shi J.B., Xu S.J., et al. Relaxin-encapsulated polymeric metformin nanoparticles remodel tumor immune microenvironment by reducing CAFs for efficient triple-negative breast cancer immunotherapy. Asian J Pharm Sci. 2023;18 doi: 10.1016/j.ajps.2023.100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He Y., Wu S.Y., Yuan Y.B., Sun Y.C., Ai Q.J., Zhou R.Q., et al. Remodeling tumor immunosuppression with molecularly imprinted nanoparticles to enhance immunogenic cell death for cancer immunotherapy. J Control Release. 2023;362:44–57. doi: 10.1016/j.jconrel.2023.08.026. [DOI] [PubMed] [Google Scholar]
- 136.Huang M.Y., Chen Y.C., Lyu W.Y., He X.Y., Ye Z.H., Huang C.Y., et al. Ginsenoside Rh2 augmented anti-PD-L1 immunotherapy by reinvigorating CD8+ T cells via increasing intratumoral CXCL10. Pharmacol Res. 2023;198 doi: 10.1016/j.phrs.2023.106988. [DOI] [PubMed] [Google Scholar]
- 137.Man S.L., Yao J.W., Lv P.P., Liu Y., Yang L., Ma L. Curcumin-enhanced antitumor effects of sorafenib regulating the metabolism and tumor microenvironment. Food Funct. 2020;11:6422–6432. doi: 10.1039/c9fo01901d. [DOI] [PubMed] [Google Scholar]
- 138.Racz L.Z., Racz C.P., Pop L.C., Tomoaia G., Mocanu A., Barbu I., et al. Strategies for improving bioavailability, bioactivity, and physical-chemical behavior of curcumin. Molecules. 2022;27:6854. doi: 10.3390/molecules27206854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang R.X., Chen L.R., Qamar M., Wen Y.J., Li L.Z., Zhang J.Y., et al. The bioavailability, metabolism and microbial modulation of curcumin-loaded nanodelivery systems. Adv Colloid Interfac. 2023;318 doi: 10.1016/j.cis.2023.102933. [DOI] [PubMed] [Google Scholar]
- 140.Zheng F., Luo Y.J., Liu Y.Q., Gao Y.Y., Chen W.Y., Wei K. Nano-baicalein facilitates chemotherapy in breast cancer by targeting tumor microenvironment. Int J Pharmaceut. 2023;635 doi: 10.1016/j.ijpharm.2023.122778. [DOI] [PubMed] [Google Scholar]
- 141.Chen Y.N., Hu M.R., Wang S.W., Wang Q., Lu H.Y., Wang F., et al. Nano-delivery of salvianolic acid B induces the quiescence of tumor-associated fibroblasts via interfering with TGF-β1/Smad signaling to facilitate chemo- and immunotherapy in desmoplastic tumor. Int J Pharm. 2022;623 doi: 10.1016/j.ijpharm.2022.121953. [DOI] [PubMed] [Google Scholar]
- 142.Pan J.X., Lai Y., Zhang S.A., Zhang H.J., Shan Y.M., Huang L.J., et al. Self-adaptive nanoregulator to mitigate dynamic immune evasion of pancreatic cancer. Adv Mater. 2023;35 doi: 10.1002/adma.202305798. [DOI] [PubMed] [Google Scholar]
- 143.Zang S.Y., Huang K.X., Li J.X., Ren K.B., Li T., He X., et al. Metabolic reprogramming by dual-targeting biomimetic nanoparticles for enhanced tumor chemo-immunotherapy. Acta Biomater. 2022;148:181–193. doi: 10.1016/j.actbio.2022.05.045. [DOI] [PubMed] [Google Scholar]
- 144.Vivier E., Rebuffet L., Narni-Mancinelli E., Cornen S., Igarashi R.Y., Fantin V.R. Natural killer cell therapies. Nature. 2024;626:727–736. doi: 10.1038/s41586-023-06945-1. [DOI] [PubMed] [Google Scholar]
- 145.Li L.F., Li A., Jin H., Li M.Y., Jia Q.G. Inhibitory receptors and checkpoints on NK cells: implications for cancer immunotherapy. Pathol Res Pract. 2024;253 doi: 10.1016/j.prp.2023.155003. [DOI] [PubMed] [Google Scholar]
- 146.Zheng W., Ling S.K., Cao Y.D., Shao C.L., Sun X.C. Combined use of NK cells and radiotherapy in the treatment of solid tumors. Front Immunol. 2024;14 doi: 10.3389/fimmu.2023.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun X.S., Xu X.X., Wang J., Zhang X.Y., Zhao Z.T., Liu X.C., et al. Acid-switchable nanoparticles induce self-adaptive aggregation for enhancing antitumor immunity of natural killer cells. Acta Pharm Sin B. 2023;13:3093–3105. doi: 10.1016/j.apsb.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lian G.Y., Wan Y., Mak T.S., Wang Q.M., Zhang J., Chen J., et al. Self-carried nanodrug (SCND-SIS3): a targeted therapy for lung cancer with superior biocompatibility and immune boosting effects. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121730. [DOI] [PubMed] [Google Scholar]
- 149.Nakamura T., Sasaki S., Sato Y., Harashima H. Cancer immunotherapy with lipid nanoparticles loaded with a stimulator of interferon genes agonist against renal tumor lung metastasis. Pharmaceutics. 2023;16:31. doi: 10.3390/pharmaceutics16010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Biber G., Sabag B., Raiff A., Ben-Shmuel A., Puthenveetil A., Benichou J.I.C., et al. Modulation of intrinsic inhibitory checkpoints using nano-carriers to unleash NK cell activity. EMBO Mol Med. 2022;14 doi: 10.15252/emmm.202114073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wei Z.Y., Yi Y., Luo Z., Gong X.Y., Jiang Y.X., Hou D.Y., et al. Selenopeptide nanomedicine activates natural killer cells for enhanced tumor chemoimmunotherapy. Adv Mater. 2022;34 doi: 10.1002/adma.202108167. [DOI] [PubMed] [Google Scholar]
- 152.Pan S.J., Li T.Y., Tan Y.Z., Xu H.P. Selenium-containing nanoparticles synergistically enhance Pemetrexed&NK cell-based chemoimmunotherapy. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121321. [DOI] [PubMed] [Google Scholar]
- 153.Marciscano A.E., Anandasabapathy N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol. 2021;52 doi: 10.1016/j.smim.2021.101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qin X.H., Zhang M.Z., Zhao Z.P., Du Q., Li Q., Jiang Y., et al. A carrier-free photodynamic nanodrug to enable regulation of dendritic cells for boosting cancer immunotherapy. Acta Biomater. 2022;147:366–376. doi: 10.1016/j.actbio.2022.05.022. [DOI] [PubMed] [Google Scholar]
- 155.Wang P., Wang Y.F., Li H.M., Wang M.M., Wang Y., Wang X.F., et al. A homologous-targeting cGAS-STING agonist multimodally activates dendritic cells for enhanced cancer immunotherapy. Acta Biomater. 2024;177:400–413. doi: 10.1016/j.actbio.2024.02.003. [DOI] [PubMed] [Google Scholar]
- 156.Ma X.Y., Kuang L., Yin Y., Tang L., Zhang Y., Fan Q., et al. Tumor-antigen activated dendritic cell membrane-coated biomimetic nanoparticles with orchestrating immune responses promote therapeutic efficacy against glioma. ACS Nano. 2023;17:2341–2355. doi: 10.1021/acsnano.2c09033. [DOI] [PubMed] [Google Scholar]
- 157.Xu X.D., Wang R., Li D.D., Xiang J.J., Zhang W., Shi X.Y., et al. Guanidine-modified nanoparticles as robust BTZ delivery carriers and activators of immune responses. J Control Release. 2023;357:310–318. doi: 10.1016/j.jconrel.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 158.Su L.H., Pan W., Li X.X., Zhou X.Y., Ma X.P., Min Y.Z. Utilizing chemotherapy-induced tumor RNA nanoparticles to improve cancer chemoimmunotherapy. Acta Biomater. 2023;158:698–707. doi: 10.1016/j.actbio.2022.12.039. [DOI] [PubMed] [Google Scholar]
- 159.Sharonov G.V., Serebrovskaya E.O., Yuzhakova D.V., Britanova O.V., Chudakov D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20:294–307. doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 160.Fridman W.H., Meylan M., Petitprez F., Sun C.M., Italiano A., Sautès-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol. 2022;19:441–457. doi: 10.1038/s41571-022-00619-z. [DOI] [PubMed] [Google Scholar]
- 161.He M.Y., Wang M.Y., Xu T., Zhang M.Y., Dai H.X., Wang C., et al. Reactive oxygen species-powered cancer immunotherapy: current status and challenges. J Control Release. 2023;356:623–648. doi: 10.1016/j.jconrel.2023.02.040. [DOI] [PubMed] [Google Scholar]
- 162.Yu J.T., Li Q., Wei Z.X., Fan G.L., Wan F.Y., Tian L.L. Ultra-stable MOF@MOF nanoplatform for photodynamic therapy sensitized by relieved hypoxia due to mitochondrial respiration inhibition. Acta Biomater. 2023;170:330–343. doi: 10.1016/j.actbio.2023.08.025. [DOI] [PubMed] [Google Scholar]
- 163.Liu Y.N., Wen Y.Y., Chen X., Zhu X.F., Yu Q.Q., Gong Y.C., et al. Inflammation-responsive functional Ru nanoparticles combining a tumor-associated macrophage repolarization strategy with phototherapy for colorectal cancer therapy. J Mater Chem B. 2019;7:6210–6223. doi: 10.1039/c9tb01613a. [DOI] [PubMed] [Google Scholar]
- 164.Zhang L.J., Huang R., Shen Y.W., Liu J., Wu Y., Jin J.M., et al. Enhanced anti-tumor efficacy by inhibiting HIF-1α to reprogram TAMs core-satellite upconverting nanoparticles with curcumin mediated photodynamic therapy. Biomater Sci-Uk. 2021;9:6403–6415. doi: 10.1039/d1bm00675d. [DOI] [PubMed] [Google Scholar]
- 165.Jian H., Wang X.B., Song P.P., Wu X.Q., Zheng R.X., Wang Y.J., et al. Tumor microcalcification-mediated relay drug delivery for photodynamic immunotherapy of breast cancer. Acta Biomater. 2022;140:518–529. doi: 10.1016/j.actbio.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 166.Tong F., Hu H.L., Xu Y.Y., Zhou Y., Xie R., Lei T., et al. Hollow copper sulfide nanoparticles carrying ISRIB for the sensitized photothermal therapy of breast cancer and brain metastases through inhibiting stress granule formation and reprogramming tumor-associated macrophages. Acta Pharm Sin B. 2023;13:3471–3488. doi: 10.1016/j.apsb.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Choi J., Shim M.K., Yang S., Hwang H.S., Cho H., Kim J., et al. Visible-light-triggered prodrug nanoparticles combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. ACS Nano. 2021;15:12086–12098. doi: 10.1021/acsnano.1c03416. [DOI] [PubMed] [Google Scholar]
- 168.Liu Y.L., Yang J.X., Liu B., Cao W., Zhang J.P., Yang Y.M., et al. Human iPS cells loaded with MnO2-based nanoprobes for photodynamic and simultaneous enhanced immunotherapy against cancer. Nanomicro Lett. 2020;12:127. doi: 10.1007/s40820-020-00452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu Y., Xun Z.Z., Ma K., Liang S.H., Li X.Y., Zhou S., et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78:770–782. doi: 10.1016/j.jhep.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 170.Horn L.A., Chariou P.L., Gameiro S.R., Qin H.Y., Iida M., Fousek K., et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-β signaling enables PD-L1-mediated tumor eradication. J Clin Invest. 2022;132 doi: 10.1172/JCI155148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peng D.D., Fu M.Y., Wang M.N., Wei Y.Q., Wei X.W. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104. doi: 10.1186/s12943-022-01569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang Y., Gao Z.X., Du X.J., Chen S.B., Zhang W.C., Wang J.L., et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci-Uk. 2020;8:5121–5132. doi: 10.1039/d0bm00916d. [DOI] [PubMed] [Google Scholar]
- 173.Puttock E.H., Tyler E.J., Manni M., Maniati E., Butterworth C., Burger Ramos M., et al. Extracellular matrix educates an immunoregulatory tumor macrophage phenotype found in ovarian cancer metastasis. Nat Commun. 2023;14:2514. doi: 10.1038/s41467-023-38093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eide S., Feng Z.P. Doxorubicin chemotherapy-induced "chemo-brain": meta-analysis. Eur J Pharmacol. 2020;881 doi: 10.1016/j.ejphar.2020.173078. [DOI] [PubMed] [Google Scholar]
- 175.Yang J., Liang X., Zhai Y.F., Hu X.L., Jia Z.W., Cheng X.L. Considering gemcitabine-based combination chemotherapy as a potential treatment for advanced oesophageal cancer: a meta-analysis of randomised trials. Int J Clin Pract. 2020;74 doi: 10.1111/ijcp.13510. [DOI] [PubMed] [Google Scholar]
- 176.Amonkar M.M., Abderhalden L.A., Frederickson A.M., Aksomaityte A., Lang B.M., Leconte P., et al. Clinical outcomes of chemotherapy-based therapies for previously treated advanced colorectal cancer: a systematic literature review and meta-analysis. Int J Colorectal Dis. 2023;38:10. doi: 10.1007/s00384-022-04301-9. [DOI] [PubMed] [Google Scholar]
- 177.Wysocki P.J., Lobacz M., Potocki P., Kwinta L., Michalowska-Kaczmarczyk A., Slowik A., et al. Metronomic chemotherapy based on topotecan or topotecan and cyclophosphamide combination (CyTo) in advanced, pretreated ovarian cancer. Cancers. 2023;15:1067. doi: 10.3390/cancers15041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hoshitsuki K., Zhou Y.M., Miller A.M., Choi J.K., Swanson H.D., Bhakta N.H., et al. Rituximab administration in pediatric patients with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2023;37:1782–1791. doi: 10.1038/s41375-023-01992-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fan J.Y., To K.K.W., Chen Z.S., Fu L.W. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist Update. 2023;66 doi: 10.1016/j.drup.2022.100905. [DOI] [PubMed] [Google Scholar]
- 180.Tezcan O., Elshafei A.S., Benderski K., Rama E., Wagner M., Moeckel D., et al. Effect of cellular and microenvironmental multidrug resistance on tumor-targeted drug delivery in triple-negative breast cancer. J Control Release. 2023;354:784–793. doi: 10.1016/j.jconrel.2022.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yasinjan F., Xing Y., Geng H.Y., Guo R., Yang L., Liu Z.L., et al. Immunotherapy: a promising approach for glioma treatment. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1255611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu H., Fu X.L., Zhai Y.J., Gao S., Yang X.Y., Zhai G.X. Development of effective tumor vaccine strategies based on immune response cascade reactions. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202100299. [DOI] [PubMed] [Google Scholar]
- 183.Jeon E.Y., Choi D.S., Choi S., Won J.Y., Jo Y., Kim H.B., et al. Enhancing adoptive T-cell therapy with fucoidan-based IL-2 delivery microcapsules. Bioeng Transl Med. 2023;8 doi: 10.1002/btm2.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lu D., Chen Y., Jiang M., Wang J., Li Y.T., Ma K.K., et al. KRAS G12V neoantigen specific T cell receptor for adoptive T cell therapy against tumors. Nat Commun. 2023;14:6389. doi: 10.1038/s41467-023-42010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lecoq I., Kopp K.L., Chapellier M., Mantas P., Martinenaite E., Perez-Penco M., et al. CCL22-based peptide vaccines induce anti-cancer immunity by modulating tumor microenvironment. Oncoimmunology. 2022;11 doi: 10.1080/2162402X.2022.2115655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vajari M.K., Sanaei M.J., Salari S., Rezvani A., Ravari M.S., Bashash D. Breast cancer vaccination: latest advances with an analytical focus on clinical trials. Int Immunopharmacol. 2023;123 doi: 10.1016/j.intimp.2023.110696. [DOI] [PubMed] [Google Scholar]
- 187.Wang G.Q., Zhang Z.L., Zhong K.H., Wang Z., Yang N., Tang X., et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31:134–153. doi: 10.1016/j.ymthe.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin J., Sun S.H., Zhao K., Gao F., Wang R.L., Li Q., et al. Oncolytic parapoxvirus induces gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat Commun. 2023;14:224. doi: 10.1038/s41467-023-35917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xiong Z.W., Chan S.L., Zhou J.Y., Vong J.S.L., Kwong T.T., Zeng X.Z., et al. Targeting PPAR-gamma counteracts tumour adaptation to immune-checkpoint blockade in hepatocellular carcinoma. Gut. 2023;72:1758–1773. doi: 10.1136/gutjnl-2022-328364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen D., Varanasi S.K., Hara T., Traina K., Sun M., Mcdonald B., et al. CTLA-4 blockade induces a microglia-Th1 cell partnership that stimulates microglia phagocytosis and anti-tumor function in glioblastoma. Immunity. 2023;56:2086–2104. doi: 10.1016/j.immuni.2023.07.015. [DOI] [PubMed] [Google Scholar]
- 191.Yang W.L., Pang Y.Y., Wang X., Lai Z.H., Lu Y.D., Zheng S.J., et al. A novel CTLA-4 blocking strategy based on nanobody enhances the activity of dendritic cell vaccine-stimulated antitumor cytotoxic T lymphocytes. Cel Death Dis. 2023;14:406. doi: 10.1038/s41419-023-05914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Arance A., de la Cruz-Merino L., Petrella T.M., Jamal R., Ny L., Carneiro A., et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J Clin Oncol. 2023;41:75–85. doi: 10.1200/JCO.22.00221. [DOI] [PubMed] [Google Scholar]
- 193.Xiao X.L., Shi J., He C., Bu X., Sun Y.S., Gao M.L., et al. ERK and USP5 govern PD-1 homeostasis via deubiquitination to modulate tumor immunotherapy. Nat Commun. 2023;14:2859. doi: 10.1038/s41467-023-38605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wu H.B., Guo C.C., Wang C.Y., Xu J., Zheng S.Y., Duan J., et al. Single-cell RNA sequencing reveals tumor heterogeneity, microenvironment, and drug-resistance mechanisms of recurrent glioblastoma. Cancer Sci. 2023;114:2609–2621. doi: 10.1111/cas.15773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yuan Z.N., Li Y.P., Zhang S.F., Wang X.Y., Dou H., Yu X., et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. 2023;22:48. doi: 10.1186/s12943-023-01744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tajaldini M., Poorkhani A., Amiriani T., Amiriani A., Javid H., Aref P., et al. Strategy of targeting the tumor microenvironment via inhibition of fibroblast/fibrosis remodeling new era to cancer chemo-immunotherapy resistance. Eur J Pharmacol. 2023;957 doi: 10.1016/j.ejphar.2023.175991. [DOI] [PubMed] [Google Scholar]
- 197.Davern M., Donlon N.E., Power R., Hayes C., King R., Dunne M.R., et al. The tumour immune microenvironment in oesophageal cancer. Br J Cancer. 2021;125:479–494. doi: 10.1038/s41416-021-01331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jia Y.B., Hu J., Zhu C.J., Li Z.J., Yang X.Y., Liu R.T., et al. Engineered NanoAlum from aluminum turns cold tumor hot for potentiating cancer metalloimmunotherapy. J Control Release. 2023;354:770–783. doi: 10.1016/j.jconrel.2023.01.043. [DOI] [PubMed] [Google Scholar]
- 199.Zhou F., Li X.J.Y., Xue X.Y., Li S., Fan G.F., Cai Y.J., et al. A novel tri-functional liposome re-educates "cold tumor" and abrogates tumor growth by synergizing autophagy inhibition and PD-L1 blockade. Adv Healthc Mater. 2023;12 doi: 10.1002/adhm.202202757. [DOI] [PubMed] [Google Scholar]
- 200.Duan Q.Q., Zhang H.L., Zheng J.N., Zhang L.J. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 201.Ni J.J., Zhang Z.Z., Ge M.J., Chen J.Y., Zhuo W. Immune-based combination therapy to convert immunologically cold tumors into hot tumors: an update and new insights. Acta Pharmacol Sin. 2023;44:288–307. doi: 10.1038/s41401-022-00953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chaturvedi V.K., Singh A., Singh V.K., Singh M.P. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Curr Drug Metab. 2019;20:416–429. doi: 10.2174/1389200219666180918111528. [DOI] [PubMed] [Google Scholar]
- 203.Nasrollahpour H., Khalilzadeh B., Hasanzadeh M., Rahbarghazi R., Estrela P., Naseri A., et al. Nanotechnology-based electrochemical biosensors for monitoring breast cancer biomarkers. Med Res Rev. 2023;43:464–569. doi: 10.1002/med.21931. [DOI] [PubMed] [Google Scholar]
- 204.Hegde M., Naliyadhara N., Unnikrishnan J., Alqahtani M.S., Abbas M., Girisa S., et al. Nanoparticles in the diagnosis and treatment of cancer metastases: current and future perspectives. Cancer Lett. 2023;556 doi: 10.1016/j.canlet.2023.216066. [DOI] [PubMed] [Google Scholar]
- 205.Huang C., Xie T., Liu Y.F., Yan S., OuYang F.J., Zhang H.T., et al. A sodium alginate-based multifunctional nanoplatform for synergistic chemo-immunotherapy of hepatocellular carcinoma. Adv Mater. 2023;35 doi: 10.1002/adma.202301352. [DOI] [PubMed] [Google Scholar]
- 206.Wang H., Chen Y., Wei R., Zhang J.L., Zhu J.H., Wang W.B., et al. Synergistic chemoimmunotherapy augmentation via sequential nanocomposite hydrogel-mediated reprogramming of cancer-associated fibroblasts in osteosarcoma. Adv Mater. 2024;36 doi: 10.1002/adma.202309591. [DOI] [PubMed] [Google Scholar]
- 207.Barnwal A., Ganguly S., Bhattacharyya J. Multifaceted Nano-DEV-IL for sustained release of IL-12 to avert the immunosuppressive tumor microenvironment and IL-12-associated toxicities. Acs Appl Mater Inter. 2023;15:20012–20026. doi: 10.1021/acsami.3c02934. [DOI] [PubMed] [Google Scholar]
- 208.Hemmatpour H., Haddadi-Asl V., Burgers T.C.Q., Yan F., Stuart M.C.A., Reker-Smit C., et al. Temperature-responsive and biocompatible nanocarriers based on clay nanotubes for controlled anti-cancer drug release. Nanoscale. 2023;15:2402–2416. doi: 10.1039/d2nr06801j. [DOI] [PubMed] [Google Scholar]
- 209.Zhong H.P., Li X.W., Yu N., Zhang X., Mu J.Q., Liu T., et al. Fine-tuning the sequential drug release of nano-formulated mutual prodrugs dictates the combination effects. Chem Sci. 2023;14:3789–3799. doi: 10.1039/d3sc00550j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang C., Li Q.S., Xiao J., Liu Y. Nanomedicine-based combination therapies for overcoming temozolomide resistance in glioblastomas. Cancer Biol Med. 2023;20:325–343. doi: 10.20892/j.issn.2095-3941.2022.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lu Y., Pan Q.Q., Gao W.X., Pu Y.J., He B. Reversal of cisplatin chemotherapy resistance by glutathione-resistant copper-based nanomedicine cuproptosis. J Mater Chem B. 2022;10:6296–6306. doi: 10.1039/d2tb01150f. [DOI] [PubMed] [Google Scholar]
- 212.Huffman B.M., Mallick A.B., Horick N.K., Wang-Gillam A., Hosein P.J., Morse M.A., et al. Effect of a MUC5AC antibody (NPC-1C) administered with second-line gemcitabine and nab-paclitaxel on the survival of patients with advanced pancreatic ductal adenocarcinoma: a randomized clinical trial. Jama Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.49720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Milano G., Innocenti F., Minami H. Liposomal irinotecan (Onivyde): exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022;113:2224–2231. doi: 10.1111/cas.15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li Q.Q., Shi Z.Q., Zhang F., Zeng W.W., Zhu D.W., Mei L. Symphony of nanomaterials and immunotherapy based on the cancer-immunity cycle. Acta Pharm Sin B. 2022;12:107–134. doi: 10.1016/j.apsb.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yan J., Li W.X., Tian H., Li B., Yu X.Y., Wang G.H., et al. Metal-phenolic nanomedicines regulate T-cell antitumor function for sono-metabolic cancer therapy. Acs Nano. 2023;17:14667–14677. doi: 10.1021/acsnano.3c02428. [DOI] [PubMed] [Google Scholar]
- 216.Zhan M.S., Qiu J.R., Fan Y., Chen L., Guo Y.Q., Wang Z.Q., et al. Phosphorous dendron micelles as a nanomedicine platform for cooperative tumor chemoimmunotherapy via synergistic modulation of immune cells. Adv Mater. 2023;35 doi: 10.1002/adma.202208277. [DOI] [PubMed] [Google Scholar]
- 217.Li L.G., Yang X.X., Xu H.Z., Yu T.T., Li Q.R., Hu J., et al. A dihydroartemisinin-loaded nanoreactor motivates anti-cancer immunotherapy by synergy-induced ferroptosis to activate cgas/STING for reprogramming of macrophage. Adv Healthc Mater. 2023;12 doi: 10.1002/adhm.202301561. [DOI] [PubMed] [Google Scholar]
- 218.Wu Y.P., Chang X.W., Yang G.Z., Chen L., Wu Q., Gao J.M., et al. A physiologically responsive nanocomposite hydrogel for treatment of head and neck squamous cell carcinoma via proteolysis-targeting chimeras enhanced immunotherapy. Adv Mater. 2023;35 doi: 10.1002/adma.202210787. [DOI] [PubMed] [Google Scholar]
- 219.Hu L.J., Huang S.C., Chen G.J., Li B., Li T., Lin M.Z., et al. Nanodrugs incorporating LDHA siRNA inhibit M2-like polarization of TAMs and amplify autophagy to assist oxaliplatin chemotherapy against colorectal cancer. Acs Appl Mater Inter. 2022;14:31625–31633. doi: 10.1021/acsami.2c05841. [DOI] [PubMed] [Google Scholar]
- 220.Han X., Wei Q., Lv Y., Weng L., Huang H.Y., Wei Q.Y., et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30:327–340. doi: 10.1016/j.ymthe.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Xu H.Z., Li T.F., Wang C., Ma Y., Liu Y., Zheng M.Y., et al. Synergy of nanodiamond-doxorubicin conjugates and PD-L1 blockade effectively turns tumor-associated macrophages against tumor cells. J Nanobiotechnol. 2021;19:268. doi: 10.1186/s12951-021-01017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huis In 't Veld R.V., Da Silva C.G., Jager M.J., Cruz L.J., Ossendorp F. Combining photodynamic therapy with immunostimulatory nanoparticles elicits effective anti-tumor immune responses in preclinical murine models. Pharmaceutics. 2021;13:1470. doi: 10.3390/pharmaceutics13091470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim J., Cho H., Lim D.K., Joo M.K., Kim K. Perspectives for improving the tumor targeting of nanomedicine via the EPR effect in clinical tumors. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241210082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lim E.K., Chung B.H., Chung S.J. Recent advances in pH-sensitive polymeric nanoparticles for smart drug delivery in cancer therapy. Curr Drug Targets. 2018;19:300–317. doi: 10.2174/1389450117666160602202339. [DOI] [PubMed] [Google Scholar]
- 225.Guo B.D., Yang F.Y., Zhang L.P., Zhao Q.X., Wang W.K., Yin L., et al. Cuproptosis induced by ROS responsive nanoparticles with elesclomol and copper combined with αPD-L1 for enhanced cancer immunotherapy. Adv Mater. 2023;35 doi: 10.1002/adma.202212267. [DOI] [PubMed] [Google Scholar]
- 226.Yu N.Y., Ding M.B., Li J.C. Near-infrared photoactivatable immunomodulatory nanoparticles for combinational immunotherapy of cancer. Front Chem. 2021;9 doi: 10.3389/fchem.2021.701427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ali M. What function of nanoparticles is the primary factor for their hyper-toxicity? Adv Colloid Interfac. 2023;314 doi: 10.1016/j.cis.2023.102881. [DOI] [PubMed] [Google Scholar]
- 228.Abulikemu A., Zhao X.Y., Xu H.L., Li Y., Ma R., Yao Q., et al. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol. 2023;59 doi: 10.1016/j.redox.2022.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sun M.Q., Zhang J.Y., Liang S., Du Z., Liu J.Y., Sun Z.W., et al. Metabolomic characteristics of hepatotoxicity in rats induced by silica nanoparticles. Ecotox Environ Safe. 2021;208 doi: 10.1016/j.ecoenv.2020.111496. [DOI] [PubMed] [Google Scholar]
- 230.Pei X.Y., Tang S.S., Jiang H.Y., Zhang W.J., Xu G., Zuo Z.H., et al. Paeoniflorin recued hepatotoxicity under zinc oxide nanoparticles exposure via regulation on gut-liver axis and reversal of pyroptosis. Sci Total Environ. 2023;904 doi: 10.1016/j.scitotenv.2023.166885. [DOI] [PubMed] [Google Scholar]
- 231.Ali A., Saeed S., Hussain R., Afzal G., Siddique A., Parveen G., et al. Synthesis and characterization of silica, silver-silica, and zinc oxide-silica nanoparticles for evaluation of blood biochemistry, oxidative stress, and hepatotoxicity in albino rats. Acs Omega. 2023;8:20900–20911. doi: 10.1021/acsomega.3c01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.He K.Y., Tang M. Safety of novel liposomal drugs for cancer treatment: advances and prospects. Chem Biol Interact. 2018;295:13–19. doi: 10.1016/j.cbi.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 233.Paris J.L., Baeza A., Vallet-Regí M. Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expert Opin Drug Deliv. 2019;16:1095–1112. doi: 10.1080/17425247.2019.1662786. [DOI] [PubMed] [Google Scholar]
- 234.Haugh A.M., Probasco J.C., Johnson D.B. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf. 2020;19:479–488. doi: 10.1080/14740338.2020.1738382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wright J.J., Powers A.C., Johnson D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17:389–399. doi: 10.1038/s41574-021-00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Iwama S., Kobayashi T., Yasuda Y., Arima H. Immune checkpoint inhibitor-related thyroid dysfunction. Best Pract Res Clin Endocrinol Metab. 2022;36 doi: 10.1016/j.beem.2022.101660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.