Abstract
Atherosclerotic cardiovascular disease (ASCVD) is one of the leading causes of death worldwide and in Taiwan. It is highly prevalent and has a tremendous impact on global health. Therefore, the Taiwan Society of Cardiology developed these best-evidence preventive guidelines for decision-making in clinical practice involving aspects of primordial prevention including national policies, promotion of health education, primary prevention of clinical risk factors, and management and control of clinical risk factors. These guidelines cover the full spectrum of ASCVD, including chronic coronary syndrome, acute coronary syndrome, cerebrovascular disease, peripheral artery disease, and aortic aneurysm. In order to enhance medical education and health promotion not only for physicians but also for the general public, we propose a slogan (2H2L) for the primary prevention of ASCVD on the basis of the essential role of healthy dietary pattern and lifestyles: "Healthy Diet and Healthy Lifestyles to Help Your Life and Save Your Lives". We also propose an acronym of the modifiable risk factors/enhancers and relevant strategies to facilitate memory: " ABC2D2EFG-I’M2 ACE": Adiposity, Blood pressure, Cholesterol and Cigarette smoking, Diabetes mellitus and Dietary pattern, Exercise, Frailty, Gout/hyperuricemia, Inflammation/infection, Metabolic syndrome and Metabolic dysfunction-associated fatty liver disease, Atmosphere (environment), Chronic kidney disease, and Easy life (sleep well and no stress). Some imaging studies can be risk enhancers. Some risk factors/clinical conditions are deemed to be preventable, and healthy dietary pattern, physical activity, and body weight control remain the cornerstone of the preventive strategy.
Keywords: Atherosclerotic cardiovascular disease, Guidelines, Primary prevention
Abbreviations
ABI, Ankle-brachial index
ACC, American College of Cardiology
ACS, Acute coronary syndrome
ADH, Alcohol dehydrogenase
AHA, American Heart Association
ALDH, Aldehyde dehydrogenase
AMI, Acute myocardial infarction
apoB, Apolipoprotein B
ArtS, Arterial stiffness
ASCVD, Atherosclerotic cardiovascular disease
baPWV, Brachial-ankle pulse wave velocity
BMI, Body mass index
BP, Blood pressure
CAC, Coronary artery calcium
CAD, Coronary artery disease
CCS, Chronic coronary syndrome
CCTA, Coronary computed tomographic angiography
cfPWV, Carotid-femoral pulse wave velocity
CI, Confidence interval
CIMT, Carotid intima-media thickness
CKD, Chronic kidney disease
COPD, Chronic obstructive pulmonary disease
COR, Class of recommendation
CVD, Cardiovascular disease
DASH, Dietary Approaches to Stop Hypertension
DBP, Diastolic blood pressure
DHA, Docosahexaenoic acid
DM, Diabetes mellitus
eGFR, Estimated glomerular filtration rate
EPA, Eicosapentaenoic acid
ESC, European Society of Cardiology
FH, Familial hypercholesterolemia
HbA1C, Hemoglobin A1C
HDL-C, High-density lipoprotein cholesterol
HR, Hazard ratio
hsCRP, High-sensitivity C-reactive protein
LDL-C, Low-density lipoprotein cholesterol
LOE, Level of evidence
Lp(a) , Lipoprotein (a)
MAFLD, Metabolic dysfunction-associated fatty liver disease
MeDiet, Mediterranean diet
MetS, Metabolic syndrome
MI, Myocardial infarction
NRT, Nicotine replacement therapy
OSA, Obstructive sleep apnea
PAD, Peripheral artery disease
PCE, Pooled Cohort Equation
PCSK9, Proprotein convertase subtilisin/kexin 9
PM, Particulate matter
RCT, Randomized controlled trial
SBP, Systolic blood pressure
SCORE, Systemic COronary Risk Evaluation
SGLT2, Sodium-dependent glucose cotransporter-2
TC, Total cholesterol
TEA, Taiwanese Eating Approaches
TG, Triglyceride
TNHIRD, Taiwan National Health Insurance Research Database
TSOC, Taiwan Society of Cardiology
TwCCCC, Taiwan Chin-Shan Community Cardiovascular Cohort
UACR, Urine albumin clearance ratio
Table of Contents
Part I
1. Introduction
1.1. Scope of the guidelines
1.2. Risk assessment
2. Impact of risk factors/clinical conditions and preventive strategies for modifiable risk factors/clinical conditions
2.1. Diabetes mellitus
2.2. Hypertension
2.3. Dyslipidemia
2.4. Cigarette smoking
2.5. Obesity
2.6. Hyperuricemia
2.7. Metabolic syndrome
2.8. Gender
2.9. Genetics and family history
2.10. Chronic kidney disease
2.11. Sleep disorders/obstructive sleep apnea
2.12. Inflammation/infection
2.13. Mental disorders and socioeconomic stress
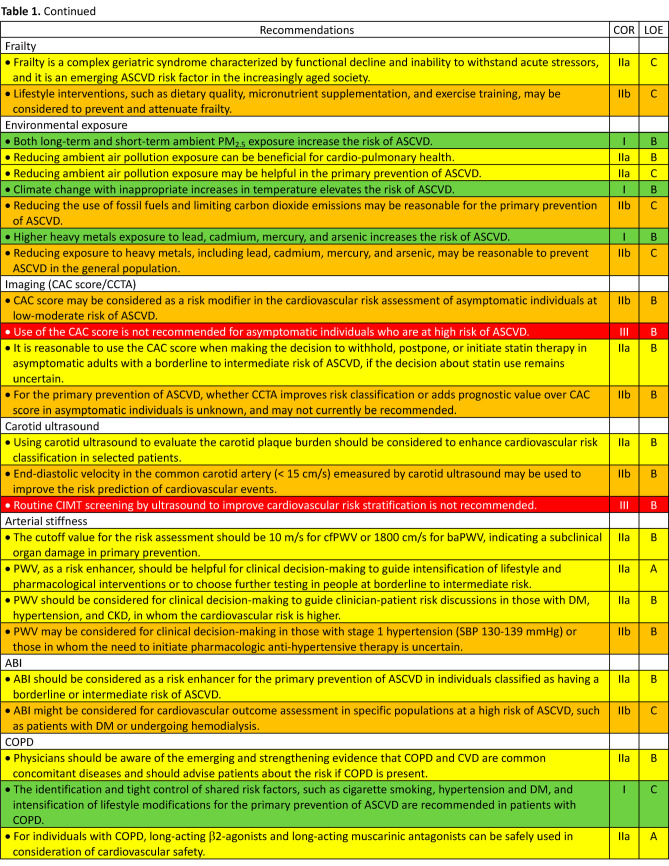
2.14. Frailty
2.15. Environmental exposure
3. Surrogate markers as risk enhancers
3.1. Imaging
3.1.1. Coronary artery calcium
3.1.2. Coronary computed tomographic angiography
3.1.3. Carotid ultrasound
3.2. Arterial stiffness
3.3. Ankle-brachial index
3.4. Biomarkers
Part II
4. Other common concomitant disorders: non-atherosclerotic cardiovascular disease associated with an increased risk of atherosclerotic cardiovascular disease
4.1. Chronic obstructive pulmonary disease
4.2. Metabolic dysfunction-associated fatty liver disease
4.3. Colorectal polyps
4.4. Nephrolithiasis
4.5. Erectile dysfunction
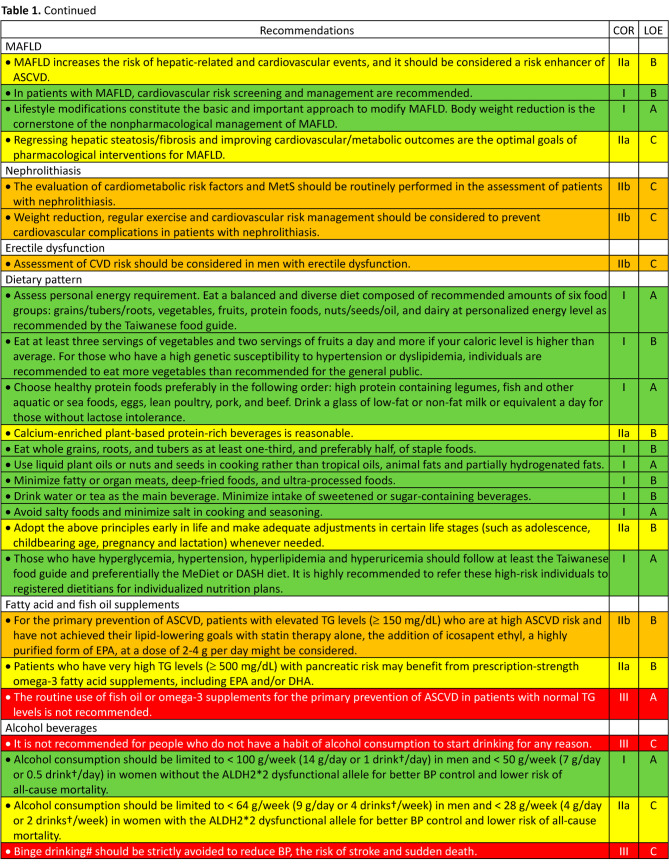
5. Interventions for primary prevention of atherosclerotic cardiovascular disease
5.1. Nutrition and beverage
5.1.1. Dietary pattern for general population
5.1.2. Dietary pattern for specific population: diabetes, hyperlipidemia, dyslipidemia, and hyperuricemia
5.1.3. Fatty acid and fish oil supplements
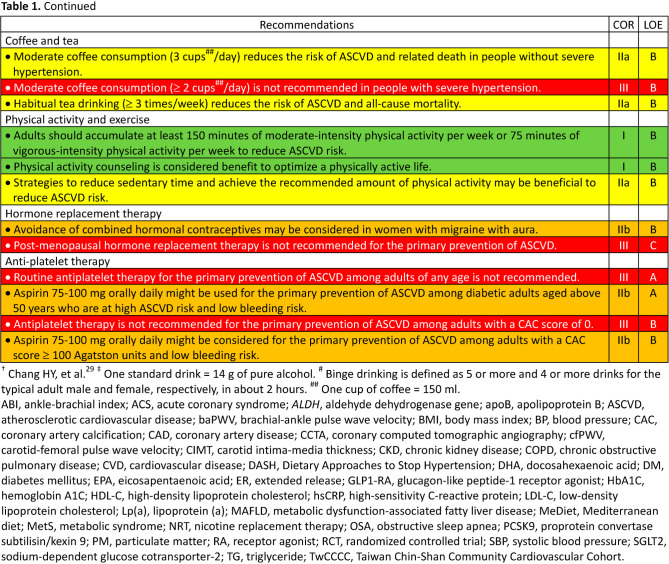
5.1.4. Alcohol beverages
5.1.5. Coffee and tea
5.1.6. Miscellaneous
5.2. Physical activity and exercise
5.3. Body weight reduction
5.4. Smoking intervention
5.5. Interventions for blood sugar
5.6. Interventions for blood pressure
5.7. Pharmacological interventions for lipids
5.7.1 Cholesterol
5.7.2 Triglyceride
5.7.3 Other lipoproteins
5.8. Pharmacological interventions for uric acid
5.9. Hormone replacement therapy
5.10. Pharmacological interventions for non-dialysis chronic kidney disease
5.11. Anti-platelet therapy
5.12. Anti-inflammation therapy
5.13. Interventions for mental disorders and socioeconomic stress
1. INTRODUCTION
This guideline is divided into 2 parts. Part I (section 1.1-3.4) includes introduction, impact of risk factors and clinical conditions, preventive strategies, and surrogate markers. Part II (section 4.1-5.13) includes other concomitant disorders and interventions.
1.1. Scope of the guidelines
1.1.1. Definition of atherosclerotic cardiovascular disease (ASCVD)
The initiation and progression of atherosclerosis involve complex mechanisms, including atherogenic lipid deposition and oxidation, pro-inflammation, vascular endothelial dysfunction, smooth muscle cell proliferation and migration, extracellular matrix degradation, and immune cell recruitment, thereby leading to plaque formation and growth.1 Clinically manifested ASCVD occurs when atherosclerotic plaque obstructs vascular lumen due to blood flow limitation or thrombosis formation from rupture of vulnerable plaque.1 The initiation of atherosclerosis usually occurs early in life.2 Some studies have demonstrated that fatty streak, an early pathological sign of atherosclerosis, can be found in relatively younger individuals.2-4 The scope of the current primary prevention guidelines focus on the prevention of ASCVD involving aspects of primordial prevention.5 The definition of ASCVD in these guidelinesis in line with the recently published Taiwan lipid guidelines for primary prevention6 and chronic coronary syndrome (CCS),7 and includes:
1. CCS, such as stable angina with positive stress test and/or major coronary artery diameter stenosis ≥ 50% by imaging studies, stable symptoms after acute coronary syndrome (ACS)/percutaneous coronary intervention, vasospastic angina, micro-vascular angina, and silent coronary artery disease (CAD) on screening.
2. ACS, such as unstable angina and acute myocardial infarction (AMI).
3. Cerebrovascular disease, such as transient ischemic attack, ischemic stroke, and carotid artery stenosis ≥ 50% by imaging studies.
4. Peripheral artery disease (PAD), such as symptoms suggestive of PAD with ankle-brachial index (ABI) < 0.9 or ≥ 1.3, and major extremity artery diameter stenosis ≥ 50% by imaging studies.
5. Aortic atherosclerotic disease, such as thoracic or abdominal aortic aneurysm by imaging studies.
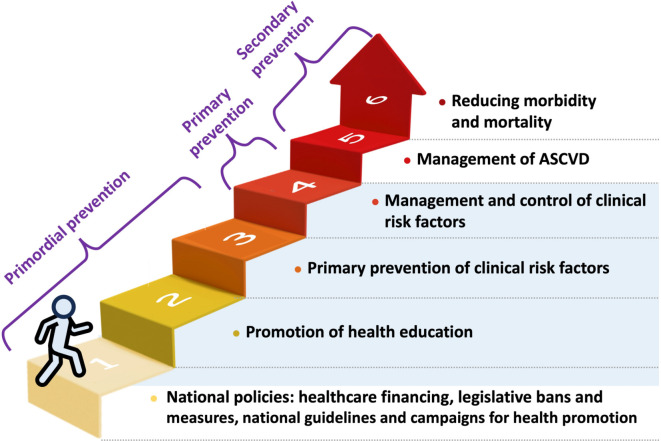
The full spectrum of ASCVD is covered in these guidelines (Figure 1), and the scope is demonstrated (blue area) in the hierarchy of ASCVD prevention shown in Figure 2.
Figure 1.
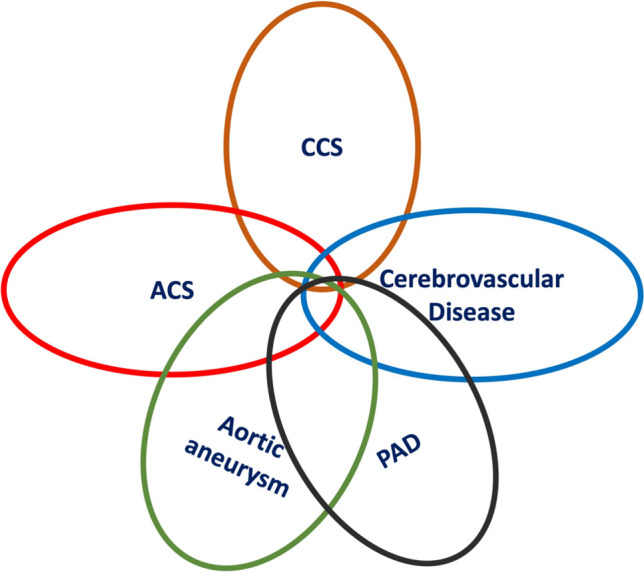
Full spectrum of ASCVD. ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CCS, chronic coronary syndrome; PAD, peripheral artery disease.
Figure 2.
The scope of the guidelines: ASCVD prevention hierarchy. The scope of these guidelines is demonstrated in blue area. ASCVD, atherosclerotic cardiovascular disease.
1.1.2. Epidemiology and prognosis
ASCVD is one of the leading causes of death worldwide and in Taiwan.8,9 In 2022, cardiovascular disease (CVD) accounted for approximately 24,000 deaths in Taiwan.9 More than 17,000 people die of CAD annually in Taiwan.7
According to analyses of the Taiwan National Health Insurance Research Database (TNHIRD), the incidence rate of AMI gradually increased from 1997-2011, mainly driven by an increase in non-ST segment elevation myocardial infarction (MI),10-12 and the in-hospital mortality rate of AMI was 6.5% in 2011.11 The incidence rate of ischemic stroke has declined worldwide, whereas the mean global life-time risk of stroke has increased.8,13 In agreement with global data, the incidence rate of ischemic stroke declined from 2000-2011 in Taiwan, and the 30-day in-hospital mortality rate was 1.9% from 2008-2011.14 The reported prevalence rate of aortic aneurysm in Western countries is around 1.3% to 8%.15 A population-based nationwide analysis showed an increasing trend in the incidence and prevalence of aortic aneurysm from 2005 to 2011, with average annual incidence and prevalence rates of 7.35 per 100,000 persons and 29.04 per 100,000 persons, respectively.15 In addition, the mortality rate increased from 1.41 per 100,000 persons to 4.70 per 100,000 persons during this period.15 Owing to discrepancies in the definitions and study populations of CCS and PAD in the literature, it is difficult to clearly identify the prevalence and incidence of both diseases. A systematic review estimated that the global prevalence of PAD was 5.6% in 2015.16,17 Taken together, ASCVD is highly prevalent and has a tremendous impact on global health, and this was the rationale for developing these primary prevention guidelines.
1.1.3. Guideline development process
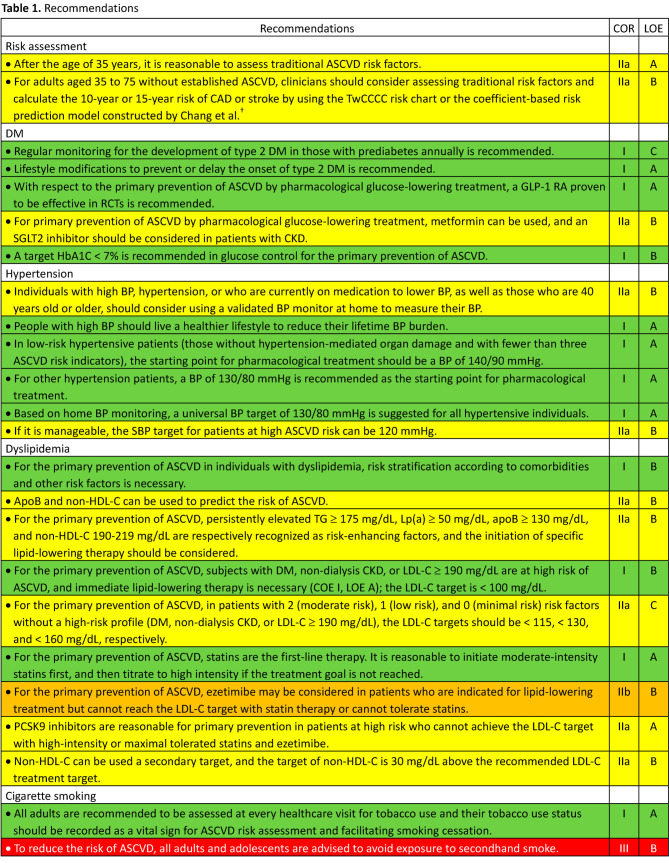
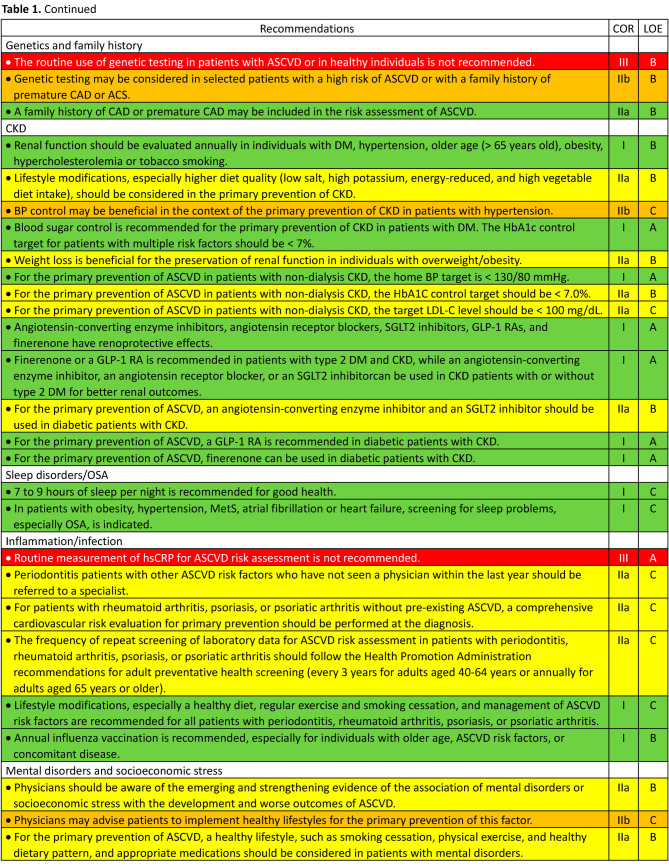
In October 2022, the development of guidelines for the primary prevention of ASCVD was proposed by the Preventive Medicine Committee of the Taiwan Society of Cardiology (TSOC) and approved by the Executive Board of the TSOC thereafter. The members of the writing group were selected by the chairperson of the Preventive Medicine Committee of the TSOC and some of them were also recommended by the Hypertension Committee of the TSOC, the Taiwan Hypertension Society, and the Taiwan Society of Lipid and Atherosclerosis. To establish consensus among the writers by reviewing clinical evidence, three virtual meetings were held on February 11, 12 and 13 in 2023. Some issues were further discussed and clarified during subsequent symposiums held by the TSOC to reach final consensus. All recommendations are listed in Table 1, Table 1 Continued, Table 1 Continued, Table 1 Continued, Table 1 Continued, Table 1 Continued, and the top ten features/key messages/highlights are summarized in Table 2.
Table 1.
Table 1 Continued.
Table 1 Continued.
Table 1 Continued.
Table 1 Continued.
Table 1 Continued.
Table 2. Top 10 features/key messages/highlights of the recommendations.
| 1. We clearly define the definitions of ASCVD and cover the full spectrum of ASCVD, not only MI or stroke, elsewhere appropriate in this guideline. |
| 2. The scope of the current primary prevention guideline focuses on primary prevention of ASCVD involving parts of primordial prevention, including national policies, promotion of health education, primary prevention of clinical risk factors, management and control of clinical risk factors. |
| 3. In order to enhance medical education and health promotion not only for physicians but also for the general public, we propose a slogan for primary prevention of ASCVD on the basis of the essential role of healthy dietary pattern and lifestyles: “Healthy Diet and Healthy Lifestyles to Help Your Life and Save Your Lives”. |
| 4. In addition to the TwCCCC point-based risk chart, we also recommend the coefficient-based risk prediction model constructed by Chang et al. for risk assessment. |
| 5. We design an acronym of the modifiable risk factors/enhancers and relevant strategies to facilitate memories: “ABC2D2EFG-I’M2 ACE”: Adiposity, BP, Cholesterol and Cigarette smoking, DM and Dietary pattern, Exercise, Frailty, Gout/hyperuricemia, Inflammation/infection, MetS and MAFLD, Atmosphere (environment), CKD, and Easy life (sleep well and no stress). |
| 6. Among common concomitant disorders, we particularly focus on the MAFLD, sleep disorders and psoriasis arthritis based on the consensus statement jointly published by the TSOC and other relevant Societies/Associations in Taiwan. |
| 7. For primary prevention of ASCVD, the treatment target for each modifiable risk factor is shown as below: reducing body weight by 5-10%; BP < 130/80 mmHg (SBP < 120 mmHg in high risk); LDL-C < 100 mg/dL in high risk, LDL-C < 115 mg/dL in moderate risk, LDL-C < 130 mg/dL in low risk, and LDL-C < 160 mg/dL in minimal risk; complete and persistent abstinence from cigarette smoking; HbA1C < 7.0%; fulfilling recommended amounts of six food groups by the Taiwan food guide; and moderate-intensity physical activity 150 min/wk or vigorous physical activity 75 min/wk. |
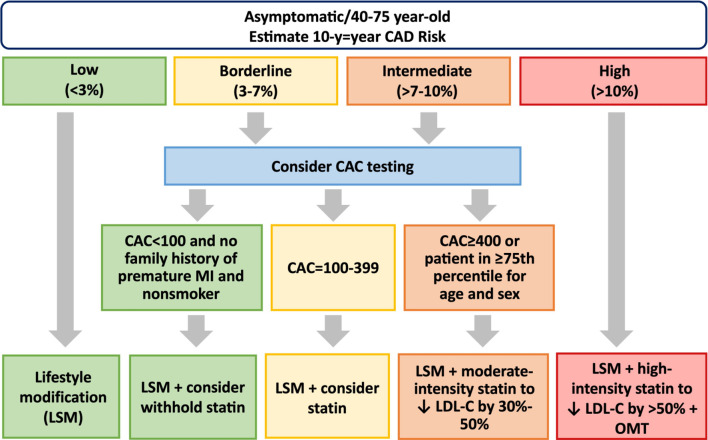
| 8. With regard to the role of images on ASCVD risk assessment, a CAC score might be useful in low-to-intermediate risk; carotid plaque burden evaluated by carotid ultrasound should be considered and measurement of end-diastolic velocity in the common carotid artery may be used to enhance cardiovascular risk classification; cfPWV (> 10 m/s) or baPWV (> 1800 cm/s) should be helpful to make the clinical decision making in selected individuals; ABI should be considered as a risk enhancer in borderline or intermediate risk. |
| 9. Some risk factors/clinical conditions, such as hypertension, DM, cigarette smoking, obesity, MetS, CKD, and mental disorders, are deemed to be preventable. Healthy dietary pattern, physical activity, and body weight control remain the cornerstone of the preventive strategy. |
| 10. For primary prevention of ASCVD, pharmacological approach for management of risk factors/clinical conditions includes: Statin is the first-line therapy for reducing LDL-C levels and ezetimibe may be considered if LDL-C not on target under statin therapy or statin intolerance. A PCSK9 inhibitor is reasonable in high risk in the context of high-intensity statin or maximally tolerated statin + ezetimibe therapy; a GLP-1 RA is recommended and metformin can be used in patients with type 2 DM and an SGLT2 inhibitor should be considered in patients with type 2 DM and CKD; GLP-1 RAs (liraglutide or semaglutide), orlistat, or naltrexone/bupropion ER is recommended to assist in weight management for obese patients with a BMI ≥ 30 Kg/m2 (or 27 kg/m2 who also have at least one ASCVD risk factor or obesity-related comorbidity) despite no RCT in terms of primary prevention of ASCVD; an angiotensin converting enzyme inhibitor should be and finerenone can be used in diabetic patients with CKD for primary prevention of ASCVD; aspirin 75-100 mg orally daily might be used for primary prevention of ASCVD among adults who are at high ASCVD risk or with a CAC score ≥ 100 and low bleeding risk. |
ABI, ankle-brachial index; ASCVD, atherosclerotic cardiovascular disease; baPWV, brachial-ankle pulse wave velocity; BMI, body mass index; BP, blood pressure; CAC, coronary artery calcium; cfPWV, carotid-femoral pulse wave velocity; CKD, chronic kidney disease; DM, diabetes mellitus; ER, extended release; GLP-1 RA, Glucagon-like peptide-1 receptor agonist; HbA1C, hemoglobin A1C; LDL-C, low-density lipoprotein cholesterol; MAFLD, metabolic dysfunction-associated fatty liver disease; MetS, metabolic syndrome; MI, myocardial infarction; PCSK9, proprotein convertase subtilisin/kexin 9; RCT, randomized controlled trial; SGLT2, sodium-dependent glucose cotransporter-2; TSOC, Taiwan Society of Cardiology; TwCCCC, Taiwan Chin-Shan Community Cardiovascular Cohort.
1.1.4. The purpose and thinking map of the guidelines
The purpose of the guidelines is to provide best-evidence guidance for decision-making in clinical practice rather than exclusive regulation. Healthcare providers are at full discretion in clinical practice, owing to the diversity of individuals and practice, and the availability of resources and facilities.
A review of the evidence with respect to risk factors and concomitant diseases was arranged in the following order if appropriate:
1. Epidemiology of this risk factor or concomitant disease.
2. Clinical impact of this risk factor or concomitant disease on the development of ASCVD.
3. Potential role of this risk factor or concomitant disease for risk assessment.
4. Strategies to prevent this risk factor or concomitant disease.
5. Strategies to prevent ASCVD by modifying this risk factor or concomitant disease.
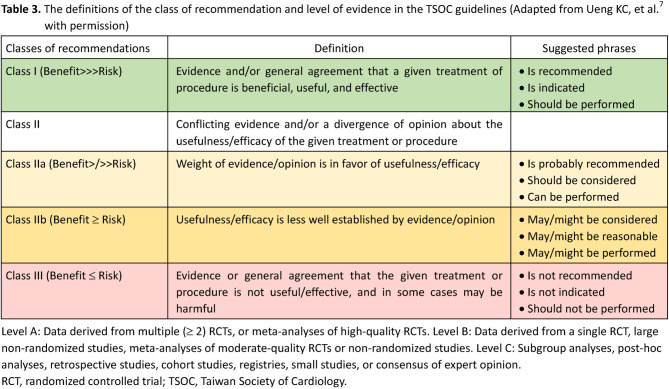
1.1.5. Class of recommendation (COR) and level of evidence (LOE)
We adopted COR and LOE for each recommendation in these guidelines for a better understanding of how strong our recommendations are with the available evidence. The definitions of COR and LOE, in line with previous TSOC guidelines,7,18 are shown as follows and listed in Table 3. CORs are used to denote whether a recommendation is beneficial/useful/effective or not useful/effective and may be harmful in some cases.7,18 Class I recommendations indicate that they are beneficial/useful/effective and should be performed. Class II recommendations indicate that there is conflicting evidence and/or divergent opinions about the benefit/usefulness/efficacy and should be weighed with benefit and risk if they are applied in clinical practice.7,18 Class IIa indicates that the weight of benefit and risk is in favor of the recommendations, whereas class IIb indicates that the recommendations are less well established by evidence/opinions.7,18 Class III recommendations indicate that they are not useful/effective and may be harmful in some cases, and should not be performed.7,18 LOEs are used to denote the strength of evidence supporting the recommendations.7,18 LOE A indicates that the recommendations are based on multiple randomized controlled trials (RCTs) or meta-analyses of high-quality RCTs.7,18 LOE B indicates that the recommendations are derived from one RCT only or large non-randomized studies, meta-analyses of moderate-quality RCTs or non-randomized studies.7,18 LOE C indicates that the recommendations are established by only small studies, expert consensus, or observational studies, that is, subgroup analyses, post-hoc analyses, retrospective studies, cohort studies, and registries.7,18
Table 3.
1.1.6. The TSOC slogan for the primordial and primary prevention of ASCVD and the acronym of the modifiable risk factors/enhancers and strategies
In order to enhance medical education and health promotion not only for physicians but also for all people in the world, we propose a slogan (2H2L) for the primordial and primary prevention of ASCVD on the basis of the essential role of healthy dietary pattern and lifestyles: "Healthy Diet and Healthy Lifestyles to Help Your Life and Save Your Lives " (Figure 3). Furthermore, we propose an acronym of the modifiable risk factors/ enhancers and relevant strategies to facilitate memory: "ABC2D2EFG-I’M2 ACE" (Figure 4). A denotes adiposity, B indicates blood pressure (BP), C2 denotes cholesterol and cigarette smoking, D2 represents diabetes mellitus (DM) and dietary pattern, E indicates exercise, F denotes frailty, G denotes gout/hyperuricemia, I represents inflammation/infection, M indicates metabolic syndrome (MetS) and metabolic dysfunction-associated fatty liver disease (MAFLD), A denotes atmosphere (environment), C denotes chronic kidney disease (CKD), and the last E represents easy life (sleep well and no stress).
Figure 3.
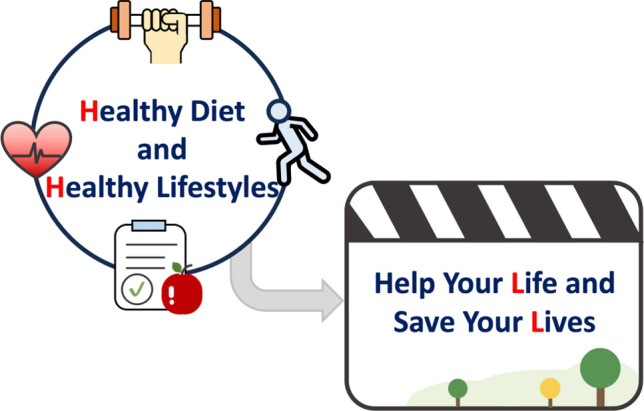
A slogan for the primordial and primary prevention of ASCVD. ASCVD, atherosclerotic cardiovascular disease.
Figure 4.
An acronym of the modifiable risk factors/enhancers and strategies. BP, blood pressure; CKD, chronic kidney disease; DM, diabetes mellitus; MAFLD, metabolic dysfunction-associated fatty liver disease; MetS, metabolic syndrome.
1.2. Risk assessment
1.2.1. Introduction of risk assessment
ASCVD causes a great disease burden in Taiwan and globally. Risk assessment for primary prevention in the general population is helpful to identify individuals at risk of ASCVD and further consultation and treatment of high-risk groups. The purpose of risk assessment is to identify individuals who are at high risk for ASCVD and to implement preventive strategies to reduce their risk. Risk assessment involves collecting and analyzing data on various risk factors such as demographic information, lifestyle factors, clinical disease status, and biological markers, and the collected information is then used to construct a prediction model for the absolute risk for ASCVD.
1.2.2. Steps for constructing the prediction model and evaluation of the prediction model performance
A score chart can be applied to calculate a person’s overall risk score, summarizing the weight of specific risk factors. First, a researcher applied a multivariate Cox proportional hazards model to establish a parsimonious model to predict the risk of ASCVD. This model was comprised of several significant predictors, including age, gender and significant lifestyle factors and clinical disease status. The researcher then constructed a categorization point system according to the concise model,19 and the predictive performance of the model was evaluated against available models in the literature.
1.2.3. Major risk assessment models for ASCVD globally and in Taiwan
The Pooled Cohort Equation (PCE) model,20 Systemic COronary Risk Evaluation (SCORE) algorithm,21 and Framingham Heart Study Risk Score22 have been applied to assess the risk of ASCVD in the USA, Europe, and globally. The PCE modelis currently recommended by the 2019 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on the primary prevention of CVD.23 The SCORE 2 algorithm was adopted in the 2021 European Society of Cardiology (ESC) guidelines on CVD prevention in clinical practice.24 However, due to ethnic differences in ASCVD risk, the risk assessment models developed and adopted in Caucasians cannot precisely predict the overall ASCVD risks in Asians25 and Chinese,26 although validation of the Framingham General Cardiovascular Risk Score and PCE showed good calibration and modest discrimination in a recent prospective cohort study in Taiwan, except for PCE in females.27 Therefore, various prediction models have been developed in specific countries. In Taiwan, there are two major risk assessment models: 1) a point-based Taiwan Chin-Shan Community Cardiovascular Cohort (TwCCCC) prediction model for CAD (Table 4)26 and stroke,28 and 2) a coefficient-based major cardiovascular event (CAD and stroke) prediction model derived from national survey data linked with national insurance records for validation (Table 5).29
Table 4. The point-based TwCCCC risk prediction model for CAD (adapted from Chien KL, et al.26 and Ueng KC, et al.7 with permission).
| Risk factor | Category | Points |
| Age | 35-39 | 0 |
| 40-44 | 1 | |
| 45-49 | 2 | |
| 50-54 | 3 | |
| 55-59 | 4 | |
| 60-64 | 5 | |
| 65-69 | 6 | |
| 70-74 | 7 | |
| ≥ 75 | 8 | |
| Sex | Men | 3 |
| Women | 0 | |
| BMI | < 22 | 0 |
| 22-25.9 | 1 | |
| ≥ 26 | 2 | |
| SBP | < 110 | 0 |
| 110-129 | 1 | |
| 130-149 | 2 | |
| 150-159 | 3 | |
| ≥ 160 | 4 | |
| LDL | < 90 | 0 |
| 90-149 | 1 | |
| ≥ 150 | 2 | |
| HDL | < 30 | 5 |
| 30-39 | 4 | |
| 40-59 | 3 | |
| 60-69 | 2 | |
| 70-79 | 1 | |
| ≥ 80 | 0 |
| Total point | Estimated risk |
| 3 | 0.001 |
| 4 | 0.001 |
| 5 | 0.002 |
| 6 | 0.003 |
| 7 | 0.003 |
| 8 | 0.005 |
| 9 | 0.006 |
| 10 | 0.008 |
| 11 | 0.011 |
| 12 | 0.014 |
| 13 | 0.019 |
| 14 | 0.025 |
| 15 | 0.033 |
| 16 | 0.044 |
| 17 | 0.058 |
| 18 | 0.076 |
| 19 | 0.099 |
| 20 | 0.129 |
| 21 | 0.168 |
| 22 | 0.216 |
| 23 | 0.276 |
| 24 | 0.349 |
An on-line TwCCCC risk calculator is available at website (http://140.112.117.151/klchien/).
BMI, body mass index; CAD, coronary artery disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; TwCCCC, Taiwan Chin-Shan Community Cardiovascular Cohort.
Table 5. The Chang’s et al. risk prediction model (from Chang HY, et al.)29.
| Disease | |||
| Men | MACE | CHD | Stroke |
| Incidence | 13.77/1000 | 7.14/1000 | 9.53/1000 |
| Variable | Coefficient | Coefficient | Coefficient |
| Age (years) | 7.2782 | 8.3007 | 8.9606 |
| SBP (mmHg) | 0.0257 | - | 0.0231 |
| Glucose (mg/dL) | - | 0.005 | |
| Triglycerides (mg/dL) | - | 0.0013 | |
| HDL | -0.0039 | - | |
| LDL | -0.0081 | ||
| Uric acid (mg/dL) | 0.0214 | - | 0.0603 |
| Cholesterol/HDL | - | ||
| Waist-hip ratio | - | ||
| Waist (cm) | 0.0163 | ||
| Smoke (yes) | - | ||
| Diabetes (yes) | - | ||
| Hypertension (yes) | 0.6715 | ||
| C statistic | 0.76 | 0.73 | 0.80 |
| Women | MACE | CHD | Stroke |
| Incidence | 7.76/1000 | 4.63/1000 | 6.98/1000 |
| Variable | Coefficient | Coefficient | Coefficient |
| Age (years) | 6.8833 | 9.3891 | 4.3538 |
| SBP (mmHg) | 0.0128 | 0.0015 | 0.0138 |
| Glucose (mg/dL) | |||
| Triglycerides (mg/dL) | 0.0001 | ||
| HDL | |||
| LDL | |||
| Uric acid (mg/dL) | |||
| CHOL/HDL | 0.3054 | 0.3581 | |
| Waist-hip ratio | 3.9712 | ||
| Waist (cm) | 0.0257 | 0.0425 | |
| Smoke (yes) | 0.1923 | 0.7821 | |
| Diabetes (yes) | 0.5348 | ||
| Hypertension (yes) | 0.5572 | ||
| C statistic | 0.7 | 0.8 | 0.7529 |
An on-line risk calculator is available at website (https://cdrc.hpa.gov.tw/index.jsp).
CHD, coronary heart disease; CHOL, cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MACE, major adverse cardiovascular events; SBP, systolic blood pressure.
The risk estimators in the TwCCCC prediction model were derived from a community based TwCCCC cohort study, comprising 3,430 adults aged 35 years old and above without pre-existing ASCVD, conducted in Northern Taiwan in the 1990s. The risk estimators for predicting CAD risk include age, sex, body mass index (BMI), systolic blood pressure (SBP), serum levels of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). However, the risk estimators for predicting stroke risk include age, sex, SBP, diastolic blood pressure (DBP), family history of stroke, atrial fibrillation, and DM. The TwCCCC risk prediction models for CAD and stroke were externally validated.26,28 Based on this model, a 10-year risk of future CAD is calculated and categorized into those at low (1-14 points; < 3%/10 years), borderline (15-17 points; 3-7%/10 years), intermediate (18-19 points; > 7%-10%/10 years), and high (20-24 points; > 10%/10 years) risk.26 A web-based calculator has been established and is available at http://140.112.117.151/klchien/.26 Furthermore, a 15-year risk of future stroke is categorized into those at lowest (1-3 points; < 1%/15 years), low (4-8 points; 1-4.9%/15 years), medium (9-12 points; 5-20%/15 years), and high (≥ 13 points; > 20%/15 years) risk. Therefore, the 2023 TSOC CCS guidelines recommended the TwCCCC prediction mo- del for risk assessment in the primary prevention of CCS.7
The coefficient-based prediction model constructed by Chang et al.29 was derived from a nationwide survey, the 1993-1996 Nutrition and Health Survey in Taiwan, comprising 3,310 adults (1,658 men and 1,652 women) aged between 35-70 years without disease at baseline. The model was externally validated using Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia data linked to TNHIRD.29 The risk estimators include age, SBP, plasma or serum levels of glucose, triglyceride (TG), HDL-C, LDL-C, uric acid, total cholesterol (TC)/HDL-C ratio, waist-hip ratio, waist circumference, and duration of tobacco smoking, DM or hypertension. The variables included are different in the models for each disease by sex.29 The predictive performance has been shown to be good compared to previous prediction models.26,28 In addition, the samples in this model were selected using a probability sampling scheme and covered the entire population of Taiwan, implying that it is more representative.29 Furthermore, a web-based calculator for this model is currently available (https://cdrc.hpa.gov.tw/index.jsp).
1.2.4. Some issues in further developments
Novel biomarkers, such as genomic information using the polygenic risk score,30 and subclinical disease status such as coronary artery calcification,31 can be incorporated into the risk assessment tools to improve risk stratification. In addition, some artificial intelligence-based technologies using machine learning and deep learning have been implemented in risk assessment from big data.32 In addition to clinically related information, geographic area, air pollutants, neighborhood, socioeconomic status and psychosocial factors have also been implemented in risk assessment.33 Besides, risk assessment is not a one-time event, but rather a continuous process that should be revisited periodically to monitor changes in risk and adjust preventive strategies accordingly. Moreover, in Taiwan, the Aboriginal population and new incomers have a high ASCVD burden. Therefore, specific populations should be taken into consideration for risk assessment. However, evidence regarding these issues is not strong enough to make recommendations for clinical practice and incorporate these factors into current guidelines.
In conclusion, the results of a risk assessment can inform the development of personalized preventive strategies, such as lifestyle changes, medical treatments, and monitoring for early signs of ASCVD.
Key Recommendations
• After the age of 35 years, it is reasonable to assess traditional ASCVD risk factors (COR IIa, LOE A).
• For adults aged 35 to 75 years without established ASCVD, clinicians should consider assessing traditional risk factors and calculate the 10-year or 15-year risk of CAD or stroke by using the TwCCCC risk chartor the coefficient-based risk prediction model constructed by Chang et al. (COR IIa, LOE B).
2. IMPACT OF RISK FACTORS/CLINICAL CONDITIONS AND PREVENTIVE STRATEGIES FOR MODIFIABLE RISK FACTORS/CLINICAL CONDITIONS
Currently, the well-documented risk factors for ASCVD include hypertension, DM, hypercholesterolemia, cigarette smoking, obesity, age, family history, and gender.
2.1. DM
2.1.1. Prevalence and incidence of DM
The prevalence of DM in Taiwan continues to increase. From 2005 to 2014, the total population with DM increased by 66%, and the age-standardized prevalence in adults aged 20-79 years increased by 41%.34 The prevalence of DM was generally higher in men; however, the prevalence was higher in women aged ≥ 65 years.34 The prevalence of DM was approximately 50% in those aged > 80 years.34 In the 10th edition of the International Diabetes Federation Diabetes Atlas, the age-adjusted comparative prevalence of DM, impaired glucose tolerance and impaired fasting glucose were 9.7%, 11.5%, and 4.5%, respectively, in Taiwanese aged 20-79 years in 2021.35 The age-adjusted prevalence of DM is expected to increase to 11.5% in 2030 and 12.6% in 2045 in Taiwan.35 The incidence also increased by 19% from 0.621% to 0.741% between 2005 and 2014, and this increase was most obvious in patients aged 20-39 years.34 The higher incidence of DM in men is consistent with the pandemic of overweight and obesity in men in Taiwan.34
2.1.2. Impact of diabetes on the development of ASCVD
Patients with type 2 DM and impaired glucose tolerance tend to have advanced CAD and systemic atherosclerosis. The pathophysiology of hyperglycemic vascular disease is complex, and includes endothelial dysfunction, inflammation and thrombosis.36 Studies have also suggested that type 2 DM and impaired glucose tolerance are significant causes of coronary plaque progression and affect plaque vulnerability.37 Furthermore, patients with hyperglycemia also have additional risk factors which can promote atherosclerosis including obesity, dyslipidemia, hypertension, and insulin resistance.
2.1.3. Role of diabetes in cardiovascular risk
There is a close relationship between abnormal glucose tolerance/type 2 DM and CVD. An updated meta-analysis of 10,069,955 individuals showed that prediabetes was associated with an increased 13% risk of all-cause mortality, 15% risk of composite CVD, 16% risk of CAD, and 14% risk of stroke in a median follow-up duration of 9.8 years.38 Patients with DM are at a 2-fold increased risk of CVD compared to non-diabetic individuals.39,40 Diabetic patients without any history of MI have an equivalent risk of a future acute coronary event as non-diabetic individuals with a prior history of MI, i.e., a "coronary risk equivalent".41 In the Multiple Risk Factor Intervention Trial, men who reported taking medications for diabetes were three times as likely to develop a stroke.42 Epidemiological studies have also identified a 2- to 4-fold higher incidence of PAD in patients with diabetes.36 Although DM is a major cardiovascular risk factor, most epidemiological studies have revealed a paradoxically inverse relationship between DM and the incidence, growth rate, and rupture risk of an aortic aneurysm.43 Mechanisms of action underlying the negative relationship have been reported, involving a number of biological pathways and glucose-lowering agents.44 Nevertheless, clinicians should always keep in mind that the surgical risk of an aortic aneurysm remains higher in patients with DM than in those without.43
2.1.4. Strategies to prevent the occurrence of DM
Screening and appropriate management of prediabetes might contribute to primary and secondary prevention of CVD.45 It is suggested to monitor for the development of type 2 DM in those with prediabetes at least annually.45 Several major RCTs, including the Da Qing Diabetes Prevention Study, the Diabetes Prevention Program trial and the Finnish Diabetes Prevention Study, have shown that lifestyle modifications and behavioral interventions can prevent or delay the onset of type 2 DM.46-48 Based on findings from the Diabetes Prevention Program trial, metformin can be recommended as a diabetes prevention medication option for high-risk individuals, such as those with a BMI ≥ 35 kg/m2, in younger participants aged 25-44 years, or individuals with a history of gestational diabetes.45,46 However, official labeling of metformin in Taiwan is not indicated for the prevention of DM. Glucagon-like peptide-1 receptor agonists, α-glucosidase inhibitors, thiazolidinediones, insulin, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have also been shown to lower the incidence of diabetes in specific populations, however long-term data are lacking and the cost-effectiveness is unclear.45
Key Recommendations
• Regular monitoring for the development of type 2 DM in those with prediabetes annually is recommended (COR I, LOE C).
• Lifestyle modifications to prevent or delay the onset of type 2 DM is recommended (COR I, LOE A).
2.2. Hypertension
2.2.1. Prevalence and incidence of hypertension
The prevalence and incidence of hypertension vary according to the definition of hypertension. According to analysis of national statistics in Taiwan in 2018 using 140/90 mmHg as the cutoff for hypertension, the prevalence, awareness and treatment rates of hypertension were 24.1%, 72.8%, and 57.2%, respectively.49 However, the age- and sex-standardized median of the relative increase in the rate of hypertension with a change in thresholds from ≥ 140/≥ 90 to ≥ 130/≥ 80 mmHg was reported to be 72.3% globally.50 When 130/80 mmHg was used as the threshold, 52% of females and 60.7% of males were classified as having hypertension according to an international registry, the May Measurement Month initiative conducted by the International Society of Hypertension between 2017-2019.50 The lifetime risk of having hypertension is approximately 90%.51,52
2.2.2. Impact of hypertension on the development of ASCVD and cardiovascular risk
High BP is the most important risk factor for global disease burden, especially ASCVD.52-54 In 2017, high BP was the leading risk factor globally, accounting for 10.4 million deaths.53 The largest numbers of SBP-related deaths are caused by CAD (4.9 million), hemorrhagic stroke (2.0 million), and ischemic stroke (1.5 million),54 and approximately 47% of cases of CAD and 54% of stroke are attributable to high BP worldwide.52,55 In addition, every 10 mmHg increase in SBP has been associated with a 45% higher risk of CAD and approximately 65% higher risk of stroke in individuals aged 55-64 years.56-58 Large-scale epidemiological studies have demonstrated a strong connection between eventual cardiovascular events and BP levels even as low as 90/60 mmHg.59,60 Many epidemiological studies and pharmacological intervention trials have shown that lowering BP can lower the risk of cardiovascular events and mortality.59,61 Hypertension is one of the major risk factors for PAD16 and abdominal aortic aneurysm.62 Taken together, hypertension is always included in the risk assessment of ASCVD.
2.2.3. Strategies to prevent the occurrence of hypertension
A healthy lifestyle and diet pattern is strongly advised for the general population since it can effectively modify and avoid cardiovascular risk factors such as hypertension.63-65 Healthy diet pattern and exercise are especially effective in the prevention of hypertension in prehypertensive individuals.66,67 Furthermore, the risk of elevated BP has been strongly associated with pediatric obesity in elementary and high school students both in Taiwan and Japan.68,69 Therefore, it is essential to educate young people to be aware of their health status and learn about healthy lifestyles beginning in childhood to modify this factor for the lifetime prevention of hypertension.69
Furthermore, it may be advisable to have a validated BP monitor at home for measuring BP, not only for individuals with high BP, hypertension, or who are taking medication, but also for those who are over the age of 40.
Key Recommendations
• Individuals with high BP, hypertension, or who are currently on medication to lower BP, as well as those who are 40 years old or older, should consider using a validated BP monitor at home to measure their BP (COR IIa, LOE B).
• People with high BP should live a healthier lifestyle to reduce their lifetime BP burden (COR I, LOE A).
2.3. Dyslipidemia
2.3.1. Prevalence and incidence of dyslipidemia
According to a report of the Nutrition and Health Surveys 2017-2020 in Taiwan, the prevalence rate of dyslipidemia was 34.4% in male and 25.5% in female adults (≥ 19 years old), which were higher than that (22.9% and 18.0%, respectively) in the first surveys conducted between 1993-1996.70 The definition of dyslipidemia used in these surveys was TC ≥ 240 mg/dL, TG ≥ 200 mg/dL, LDL-C ≥ 160 mg/dL, or receiving lipid-lowering treatment.
2.3.2. Impact of dyslipidemia on the development of ASCVD
LDL-C and other apolipoprotein B (apoB)-rich lipoproteins, including very low-density lipoprotein, their remnants, and lipoprotein(a) (Lp(a)), efficiently enter and accumulate in the arterial intima at sites of predilection for plaque formation to initiate the development and progression of ASCVD.71 When the plasma LDL-C concentration is above the physiological level (20-40 mg/dL), the probability of intimal retention of LDL leading to the initiation and progression of atherosclerotic plaque increases in a dose-dependent manner.71,72
2.3.3. Role of dyslipidemia in cardiovascular risk
Evidence regarding the association of LDL-C levels and ASCVD risk is strongly based on observations from inherited disorders of lipid metabolism, prospective epidemiologic studies, Mendelian randomization studies, and RCTs of interventions.71 Therefore, LDL-C is considered as a causal factor for ASCVD and is traditionally included in risk assessment.
The effect of accumulative exposure to LDL-C has attracted increasing attention recently. This effect refers to the association of the cumulative amount of LDL-C exposure over time and the development of atherosclerosis.71 A recent study proposed a statistical model estimating the effect of accumulative exposure to LDL-C by calculating the area under the curve derived from the product of an individual’s LDL-C levels and time.73 The results from this study highlighted that cumulative LDL-C exposure before the age of 40, as assessed by the area under the curve for the 18- to 40-year-old period, was significantly associated with the future risk of ASCVD. In addition, the same area accumulated earlier in life, compared with later in life, conferred a higher risk of ASCVD. Similar outcomes were also observed in Japanese patients with familial hypercholesterolemia (FH),74 in which the effect of accumulative exposure to LDL-C was significantly associated with ASCVD events independently of other traditional risk factors, including age, male sex, hypertension, diabetes, smoking, and serum LDL-C levels.
Severe hypercholesterolemia, defined as serum LDL-C ≥ 190 mg/dL, is a complex disorder that may result from a combination of genetic and environmental factors. Severe hypercholesterolemia has been associated with a 5-fold higher risk of developing ASCVD and a 10-20-year acceleration in ASCVD progression in males and 20-30 years in females when compared to the general population with normal LDL-C levels.75 It is worth noting that about 7.2% of patients with LDL-C ≥ 190 mg/dL meet the diagnosis of FH,76 which is an inherited genetic disorder caused by mutations in genes encoding the LDL receptor, apoB, or proprotein convertase subtilisin/kexin type 9.77
The association and causal effects of serum TG levels and the risk of ASCVD are supported by observation from epidemiological and Mendelian randomization studies;78 however, the relationships are further complicated by interindividual heterogeneity in the fate of TG-rich lipoproteins due to variability in the function of key enzymes.79 Furthermore, few RCTs have clearly demonstrated a specific effect of TG-targeting therapy on ASCVD risk, especially when statins have already been used.79 Finally, the correlation of TG levels and ASCVD risk in epidemiological studies has been attenuated or even lost after adjusting for non-HDL-C or apoB.78 Therefore, ASCVD risk is probably determined by the atherogenic component of TG-rich lipoproteins, such as apoB, rather than TG per se.78,79 Collectively, the current ACC/ AHA guidelines have recommended that persistently elevated TG ≥ 175 mg/dL is considered as a risk-enhancing factor.80
Although an inverse relationship between serum HDL-C concentrations and ASCVD risk has been found in population studies,81,82 evidence from genetic studies and Mendelian randomized trials has questioned whether the inverse association is causal.82 HDL-C can be atherogenic.81 Furthermore, RCTs regarding HDL-elevating therapy have mostly failed to demonstrate the expected outcome.81,82 With regards to the effect of HDL-C on ASCVD, the focus of recent research has shifted to the function of HDL particles, especially in the context of macrophage cholesterol efflux, from serum HDL-C levels alone.81 Nevertheless, the concentration of HDL-C remains a useful tool in the risk stratification of ASCVD.
In the context of primary prevention, other emerging lipoprotein biomarkers have been proposed when assessing an individual’s risk of ASCVD, including Lp(a), apoB, and remnant cholesterol. Lp(a) is a lipoprotein subclass which contains an additional protein called apolipoprotein(a), and it is of emerging importance in atherosclerosis formation. Multiple studies have confirmed the association between elevated levels of Lp(a) and the risk of ASCVD.83-85 There may be a myriad of mechanisms by which Lp(a) contributes to ASCVD, including proatherogenic, proinflammatory, and prothrombotic pathways.85 In addition, Lp(a) also potentiates vascular inflammation through its content of oxidized phospholipids, and interrupts antifibrinolytic effects by inhibiting plasminogen. The current ACC/AHA guidelines have identified that Lp(a) ≥ 50 mg/dL is a risk-enhancing factor for ASCVD.80
ApoB is the primary protein constituent of atherogenic lipoproteins, including chylomicrons, LDL, intermediate-density lipoprotein, and very low-density lipoprotein particles. Its unique one-to-one ratio with each lipoprotein particle makes it a direct biomarker of the amount of circulating atherogenic lipoproteins. Furthermore, a robust correlation has been observed between apoB and non-HDL-C, suggesting that apoB and non-HDL-C are potentially better predictors of ASCVD than LDL-C.86-88 Currently, the serum levels of apoB ≥ 130 mg/dL and non-HDL-C 190-219 mg/dL are both considered as risk-enhancing factors of ASCVD by the ACC/AHA guidelines.80
Remnant cholesterol, which can be simply calculated as TC minus HDL-C and LDL-C, is an emerging biomarker for assessing the risk of ASCVD. It represents cholesterol in chylomicrons, very low-density lipoprotein, and intermediate-density lipoprotein, and is formed during the process of TG-rich lipoprotein metabolism.89 In a post-hoc analysis of a large RCT, it was found that remnant cholesterol levels were an independent risk factor for ASCVD in high-risk patients, with a better predictive power than LDL-C levels.90 These findings suggest that measuring remnant cholesterol levels in addition to LDL-C and HDL-C may provide additional value to the evaluation of ASCVD risk. However, current evidence focusing on this topic and the optimal serum levels of remnant cholesterol are still limited, and further research is required before it can be used clinically.
2.3.4. Strategies to prevent the occurrence of dyslipidemia
Genetic variants partially account for the occurrence of dyslipidemia. Secondary causes of hypertriglyceridemia include diet with high positive energy-intake balance and high fat or high glycemic index, increased alcohol consumption, obesity, MetS, insulin resistance, type 2 DM, hypothyroidism, renal disease, and some medications, etc.78 Various clinical conditions such as obesity,91 DM,92 and CKD93 are frequently associated with variable types of dyslipidemia, and strategies to modify relevant risk factors and avoid the occurrence of these clinical conditions can also reduce the risk of dyslipidemia.
Patients with genetically confirmed FH have a significantly higher risk of ASCVD compared to individuals with LDL-C ≥ 190 mg/dL but no identified genetic mutation.94 Therefore, it is advisable to raise clinical suspicion and conduct comprehensive family screening with genetic testing among patients with LDL-C ≥ 190 mg/dL, especially in those who have typical characteristics of tendinous xanthoma, corneal arcus, and family history of premature ASCVD.
Key Recommendations
• For the primary prevention of ASCVD in individuals with dyslipidemia, risk stratification according to comorbidities and other risk factors is necessary (COR I, LOE B).
• ApoB and non-HDL-C can be used to predict the risk of ASCVD (COR IIa, LOE B).
• For the primary prevention of ASCVD, persistently elevated TG ≥ 175 mg/dL, Lp(a) ≥ 50 mg/dL, apoB ≥ 130 mg/dL, and non-HDL-C 190-219 mg/dL are respectively recognized as risk-enhancing factors, and the initiation of specific lipid-lowering therapy should be considered (COR IIa, LOE B).
2.4. Tobacco smoking
2.4.1. Prevalence and incidence of tobacco smoking
According to the Adult Smoking Behavior Survey reported by the Taiwan Health Promotion Administration in 2023, the prevalence of smoking among people aged over 18 years was 21.9% in 2008 but it decreased to 14.0% in 2022. Of these people, 24.4% were male and 3.7% were female with a peak age ranging from 40-49 years.95 However, the prevalence of secondhand smoke exposure at home increased from 27.2% in 2008 to 28.9% in 2022. Of note, the prevalence of e-cigarette smoking among people aged over 18 years was 0.6% in 2018 but it increased to 1.4% in 2022. The main age of people consuming e-cigarettes was younger than 40 years.
2.4.2. Impact of tobacco smoking on the development of ASCVD
Smoking induces ASCVD via endothelial dysfunction, atherosclerosis, stimulation of inflammatory cytokines and activated prothrombotic state. These factors are mediated through three principal constituents: nicotine, carbon monoxide, and oxidant gases.96 In the brain, nicotine binds to α4β2 nicotinic cholinergic receptors and acts as a sympathomimetic agent. This stimulates the release of catecholamines, resulting in tachycardia, hypertension and myocardial stress, which induce the imbalance of myocardial work and oxygen demand.97 Carbon monoxide can cause relative hypoxemia that precipitates ischemic events. The high levels of nitrogen oxides and free radicals in cigarette smoke induce inflammation, decrease the cellular production of nitric oxide, cause dysfunction of the endothelial system, and activate prothrombotic state and lipid oxidation, which are associated with the pathogenesis of ASCVD.
Even though e-cigarettes have previously been considered to be less harmful than smoking combustible tobacco products in the short term, their long-term safety is uncertain due to other constituent chemicals, that is, nicotine, propylene glycol, and glycerin.98 Electronic cigarette or vaping product use-associated lung injury is a described organizing pneumonia in patients with the past 90 days of vaping history.99 Su et al. found that vegetable glycerin enhances neutrophil chemotaxis and fibrosis and amplifies the inflammatory response associated with lipopolysaccharide-induced lung injury by enhancing p38 MAPK activity.100
Secondhand smoke is the combination of smoke from the burning end of a cigarette and smoke breathed out by smokers. Secondhand smoke contains more than 7,000 chemicals and causes almost 34,000 premature deaths from heart disease every year in the United States.101
2.4.3. Role of tobacco smoking in cardiovascular risk
Based on the previous epidemiology, tobacco use is a major health concern worldwide, and it is responsible for over 6 million deaths annually — almost 12% of all deaths.102 Tobacco-attributable mortality accounts for 10% to 30% of all deaths from ASCVD based on the Global Report of the World Health Organization.101 In the United States, almost a third of all deaths associated with smoking are related to ASCVD.103 According to the 2019 report of the Taiwan Health Promotion Administration, nearly 25,000 people die of smoking-related heart disease every year in Taiwan, with 1 person dying of smoking-induced harm every 20 min.104 Smoking has been shown to increase the risk of CAD [hazard ratio (HR): 3.2-3.5], CVD (HR: 1.7-3.2), PAD, and abdominal aortic aneurysm.105 The sex-specific relative risk of smoking mortality in Taiwan is the same as that in international reports. Mortality from all causes and CVD is significantly higher in women than in men.106 Observational epidemiological research and clinical studies have provided evidence of a non-linear dose effect of exposure to cigarette smoke on ASCVD.107,108 In the INTERHEART study,109 the odds of MI were 9-fold higher in those who smoked over 40 cigarettes per day [HR: 9.16, 95% confidence interval (CI): 6.79-12.36] than in never smokers, and the risk increased by 5.6% for every additional cigarette smoked. The influence of smoking on younger individuals (HR: 3.53, 95% CI: 3.23-3.86) was higher when compared to older individuals (HR: 2.55, 95% CI: 2.35-2.76; p < 0.0001 for interaction).
The INTERHEART study found that secondhand smoke was associated with a graded increase in exposure-related AMI risk; the HR was 1.24 (1.17-1.32) in individuals with a lower exposure (1-7 h per week) and 1.62 (1.45-1.81) in those with higher exposure (> 21 h per week).109 A systematic review and meta-analysis110 reported that pooled relative risks for never smokers exposed to secondhand smoke compared with those unexposed were 1.23 (95% CI: 1.16-1.31) for CVD and 1.18 (95% CI: 1.10-1.27) for all-cause mortality.
In the American Health eHeart Study regarding cigarette and e-cigarette users,111 dual users had a higher risk of CAD than single cigarette users due to the two different sources of poison. In the National Health Interview Surveys of 2014 (n = 36,697) and 2016 (n = 33,028),112 daily e-cigarette use was independently associated with an increased odds of MI (HR: 1.79, 95% CI: 1.20-2.66), as was daily conventional cigarette smoking (HR: 2.72, 95% CI: 2.29-3.24).
Heat-not-burn tobacco is heated with an electric blade at 350 °C, lower than a conventional cigarette (684 °C). Volatile organic compounds, polycyclic aromatic hydrocarbons, and carbon monoxide are present in heat-not-burn tobacco. Additionally, heat-not-burn tobacco has 84% of the nicotine found in conventional cigarette smoke.113 In a systematic review,114 heat-not-burn tobacco exposed users and bystanders to potentially harmful toxicants, although at substantially lower levels than cigarettes.
Waterpipe smoking or hookah is an emerging trend in the United States, especially among youths.115 Waterpipe smoking affects heart rate, BP regulation, baroreflex sensitivity, tissue oxygenation, and vascular function over the short term. Long-term water pipe use is associated with an increased risk of CAD due to several harmful or potentially harmful substances.116
2.4.4. Strategies to avoid cigarette smoking in non-smokers and secondhand smoke exposure
Several guidelines state that all patients should avoid secondhand smoke exposure to reduce ASCVD risk as primary prevention.7,23 Laws regarding tobacco hazard prevention including avoiding cigarette smoke and secondhand smoke for non-smokers based on national administration policy would be useful. Robust evidence supports many measures to avoid cigarette smoking and secondhand smoke exposure, and they can be included in such laws to enhance the prohibition/restriction power via government authorities. This can include standardized packs with larger health warnings,117 targeted mass media interventions promoting healthy behaviors,118 legislative smoking bans to reduce harm from secondhand smoke exposure and smoking prevalence,119 and tobacco taxation to reduce the initiation of tobacco smoking.120 The recently updated Taiwan Tobacco Hazards Prevention Act has implemented a complete ban on e-cigarette and strict control of heat-not-burn tobacco products.104,121 After the introduction of the first version of the Taiwan Tobacco Hazards Prevention Act in 1997 and following revisions, the prevalence of smoking among people aged over 18 years declined from 29.2% in 1996 to 14.0% in 2022.95
Key Recommendations
• All adults are recommended to be assessed at every healthcare visit for tobacco use, and their tobacco use status should be recorded as a vital sign for ASCVD risk assessment and facilitating smoking cessation (COR I, LOE A).
• To reduce the risk of ASCVD, all adults and adolescents are advised to avoid exposure to secondhand smoke (COR III, LOE B).
• Legislative bans and measures to avoid cigarette smoking and secondhand smoke exposure are recommended to enhance the prohibition/restriction power via government authorities (COR I, LOE B).
2.5. Obesity
The National Health Promotion Administration of Taiwan has been utilizing specific diagnostic cut points for overweight and obesity since 2013, considering the degree of comorbidity, overall mortality rate, and public health epidemic screening. These cut points are based on BMI measurements, with a BMI of ≥ 24 kg/m2 being considered overweight, and a BMI of ≥ 27 kg/m2 indicating obesity.122 This approach ensures a standardized and consistent method for identifying individuals who fall within these weight categories, allowing for effective monitoring and intervention strategies to promote better health outcomes. In addition, the Ministry of Health and Welfare has recommended using waist circumference measurements as another indicator for obesity and as one of the diagnostic criteria for MetS. According to these guidelines, a waist circumference above 90 cm for men and 80 cm for women is considered indicative of obesity. However, there is currently no definitive consensus on whether to employ waist-hip ratio or percentage of body fat as alternative diagnostic criteria. Table 6 presents the established cutoff point standards for overweight and obesity.
Table 6. Classification of body weight according to BMI.
| BMI | Waist circumference | |
| Underweight | BMI < 18.5 kg/m2 | |
| Normal | 18.5 ≤ BMI < 24 kg/m2 | |
| Overweight | 24 ≤ BMI < 27 kg/m2 | |
| Mild obesity | 27 ≤ BMI < 30 kg/m2 | Men ≥ 90 cm |
| Moderate obesity | 30 ≤ BMI < 35 kg/m2 | Women ≥ 80 cm |
| Severe obesity | BMI ≥ 35 kg/m2 |
BMI, body mass index.
2.5.1. Prevalence and incidence of obesity
Over the past 3 decades, the prevalence of obesity has steadily increased throughout the world.123 Based on data provided by the World Health Organization, it is estimated that the global prevalence of overweight, defined as a BMI of ≥ 25 kg/m2, was approximately 1.9 billion people in 2014. This accounted for approximately 39% of the total population, with 39% of females and 38% of males falling into this category. Among the individuals classified as overweight or obese, around 13% had a BMI of ≥ 30 kg/m2, indicating obesity. The prevalence of obesity was higher in women, with 15% classified as obese, compared to 11% of men. Furthermore, the statistics revealed that severe obesity, defined as a BMI of ≥ 40 kg/m2, affected approximately 1.6% of women and 0.64% of men. These figures highlight the significant global burden of overweight and obesity, emphasizing the need for effective strategies to address this public health issue.124 According to the World Obesity Federation, the global adult population affected by overweight or obesity is projected to increase from 2 billion in 2014 to 2.7 billion by 2025. In Taiwan, data from the three waves of the Taiwan National Nutrition and Health Change Survey (conducted during the periods of 1993-1996, 2005-2008, and 2013-2016) indicate notable trends in weight categories. The proportion of individuals with a normal BMI (18.5 ≤ BMI < 24 kg/m2) gradually declined over time, from 58.1% in the first wave to 51.5% in the second wave, and further decreasing to 49.2% in the third wave. On the other hand, the prevalence of overweight and obesity (defined as a BMI of ≥ 24 kg/m2) steadily increased, rising from 33.2% in the first wave to 43.4% in the second wave, and further reaching 45.9% in the third wave. Within this category, the prevalence of overweight (defined as a BMI ranging from 24 to < 27 kg/m2) decreased slightly from 21.5% in the first wave to 25.5% in the second wave, and then stabilized at 22.8% in the third wave. In contrast, the prevalence of obesity (BMI ≥ 27 kg/m2) showed a significant rise, increasing from 11.8% in the first wave to 17.9% in the second wave, and further to 23.0% in the third wave. Of particular concern is the substantial increase in severe obesity (BMI ≥ 35 kg/m2) over a 20-year period. The prevalence of severe obesity more than tripled, escalating from 0.4% in the first wave to 0.6% in the second wave, and ultimately reaching 1.3% in the third wave. These findings underscore the growing prevalence of overweight and obesity, particularly severe obesity, and highlight the urgent need for effective measures to address this escalating public health issue.125,126
2.5.2. Impact of obesity on the development of ASCVD
Obesity is strongly linked to an increased risk of various CVDs, including hypertension, diabetes, dyslipidemia, MetS, and sleep apnea. It is recognized as a major risk factor for the development of ASCVD, and associated with other specific cardiovascular conditions such as atrial fibrillation and heart failure.127 Findings from the 2013-2016 National Nutrition and Health Survey in Taiwan highlighted the association between obesity, central obesity in particular, and the development of isolated systolic hypertension, and a strong association between higher levels of the atherogenic index of plasma (log TG/HDL-C) and the presence of obesity, indicating the importance of managing weight and addressing obesity-related factors in the prevention and treatment of ASCVD.128,129 Furthermore, the Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia study revealed that the metabolically healthy obesity group (BMI ≥ 24 kg/m2) had a significantly higher risk of CVD (adjusted HR: 1.74, 95% CI: 1.02-2.99) when compared to the normal weight group.130 These findings indicate that even in the absence of metabolic abnormalities, obesity itself remains a significant risk factor for CVD. Based on data of the National Health Interview Survey collected in 2013, it has been estimated that an 18% reduction in ASCVD could be achieved if obesity/overweight can be prevented.131
2.5.3. Role of obesity in CVD risk assessment
The 2019 ACC/AHA Guidelines on the Primary Prevention of Cardiovascular Disease recommends calculating BMI on an annual basis or even more frequently for individuals identified as obese.23 This emphasizes the importance of ongoing monitoring of weight status in order to assess and manage CVD risk. Additionally, the guidelines suggest that measuring waist circumference is a reasonable approach to identify individuals who may be at higher risk of cardiometabolic complications.23 By incorporating these measurements into routine clinical practice, healthcare professionals can effectively identify individuals who may require closer monitoring and appropriate interventions to mitigate their cardiovascular risk.23 It is recommended to conduct screening for other concomitant risks of ASCVD, including non-alcoholic fatty liver disease and obstructive sleep apnea (OSA).132,133 In obese patients with hypertension and those with clinically evident CVD, it is recommended to perform an electrocardiogram to detect left ventricular hypertrophy. Furthermore, it is crucial to screen for the occurrence of atrial fibrillation in individuals suspected of having this condition. In cases where sleep apnea is suspected, it is recommended to conduct nocturnal polysomnography. By incorporating these screening measures, healthcare providers can effectively identify and address underlying cardiovascular conditions and associated risks in obese patients. This enables appropriate interventions and management strategies to reduce the burden of CVD in this population.134
2.5.4. Strategies to prevent obesity
The pathogenesis of obesity is complex and multifactorial, involving energy imbalance, hormone disorders, genetic diseases, gut microbiota and medications.135,136 While adopting a population health approach within an obesogenic environment is crucial to tackle the complexity of obesity, it is equally important to expand the scope of health services beyond medical treatment to encompass obesity prevention.137,138 It is of utmost importance to initiate obesity prevention efforts early, beginning within the settings of early care and education as well as schools. Research has shown that interventions targeting obesity prevention in these settings yield significant benefits, with particularly noteworthy effects observed in children between the ages of 6 and 12 years.139 Meta-analyses have consistently demonstrated the positive impact of school-based interventions and worksite health promotion programs on improving physical activity and/or nutrition, leading to reductions in body weight and BMI.140,141 Favorable outcomes regarding reducing weight have still been found in adults at risk of obesity.142
Key Recommendations
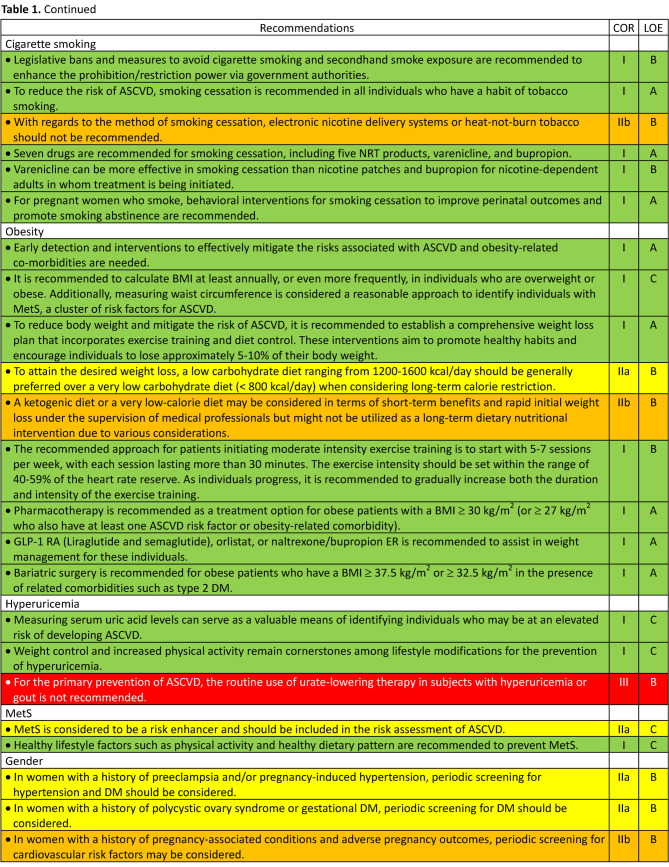
• Early detection and interventions to effectively mitigate the risks associated with ASCVD and obesity-related co-morbidities are needed (COR I, LOE A).
• It is recommended to calculate BMI at least annually, or even more frequently, in individuals who are overweight or obese. Additionally, measuring waist circumference is considered a reasonable approach to identify individuals with MetS, a cluster of risk factors for ASCVD (COR I, LOE C).
2.6. Hyperuricemia
Hyperuricemia can be defined as: (1) serum uric acid levels 7.0 mg/dL or higher in males and 6.0 mg/dL or higher in females; (2) uric acid levels 7.7 mg/dL or higher in males, 6.6 mg/dL or higher in females; or (3) those already taking uric acid lowering agents.70
2.6.1. Prevalence and incidence of hyperuricemia
Hyperuricemia is a common condition with a prevalence of 17.4% to 21.2% worldwide.143,144 According to the first wave of the Taiwan National Nutrition and Health Change Survey (conducted from 1993-1996), the prevalence rate of hyperuricemia was approximately 26.1% in male adults and 17.0% in female adults (≥ 19 years old) by using definition 2 in Taiwan.145 According to the report of the Nutrition and Health Surveys 2017-2020 in Taiwan, the prevalence rate of hyperuricemia was 17.9% in male and 9.9% in female adults (≥ 19 years old),70 both of which were lower than the first survey conducted between 1993-1996. The definition of hyperuricemia used in the Nutrition and Health Surveys 2017-2020 was a combination of definitions 2 and 3.
2.6.2. Impact of hyperuricemia on the development of ASCVD
Hyperuricemia is associated with an increased risk of developing ASCVD, including mortality (HR: 1.209), CAD (HR: 1.206),146 and stroke (HR: 1.47).147 An analysis of a health check-up database from Taiwan showed that hyperuricemia, defined as definition 1, was associated with an increased risk of CAD (HR: 1.25, 95% CI: 1.11-1.40 in males and HR: 1.19, 95% CI: 1.02-1.38 in females, respectively),148 and it was also associated with an increased risk of total mortality (HR: 1.16) and ischemic stroke (HR: 1.35).149 An analysis of the TNHIRD further demonstrated that gout was associated with an increased risk of CAD and stroke 3 years after diagnosis.150 The underlying mechanisms may include endothelial dysfunction, oxidative stress, and inflammation, among others. Furthermore, hyperuricemia can also contribute to the development of cardiovascular risk factors including hypertension,151 MetS,152 and CKD,153 thereby further increasing the risk of ASCVD.
2.6.3. Role of hyperuricemia in ASCVD risk assessment
Hyperuricemia has been associated with the occurrence of traditional cardiovascular risk factors such as hypertension, dyslipidemia, DM, and MetS.154,155 Furthermore, it was significantly associated with the clustering of cardiovascular risk factors, and a higher 10-year ASCVD risk score in those with higher quintiles of serum uric acid levels.154 In addition, hyperuricemia has been independently associated with a higher risk of ASCVD after adjusting for traditional risk factors.148,149 Therefore, measuring serum uric acid levels can serve as a valuable means of identifying individuals who may be at an elevated risk of developing ASCVD.
2.6.4. Strategies to prevent hyperuricemia
In addition to genetic factors and tumor lysis, causes of hyperuricemia include obesity, increased purine consumption from meat, alcohol, and high fructose corn syrup, as well as medications such as cyclosporine, low-dose aspirin, and diuretics.156,157 While diet may contribute to hyperuricemia, the direct effect of diet on hyperuricemia is weak,158 and genetic contributions and obesity itself appear to be larger drivers of hyperuricemia in the general population.157 Therefore, weight control and increased physical activity remain cornerstones among lifestyle modifications for the prevention of hyperuricemia,158 whereas limited evidence suggests that avoidance of certain foods and beverages may decrease the frequency of gout flares.157
Key Recommendations
• Measuring serum uric acid levels can serve as a valuable means of identifying individuals who may be at an elevated risk of developing ASCVD (COR I, LOE C).
• Weight control and increased physical activity remain cornerstones among lifestyle modifications for the prevention of hyperuricemia (COR I, LOE C).
2.7. MetS
The Asian-modified definition of MetS is widely used in Taiwan and is shown as follows: ≥ 3 of the following: waist size ≥ 90 cm in men or ≥ 80 cm in women; TG ≥ 150 mg/dL or taking a TG-lowering agent; HDL-C < 40 mg/dL in men or < 50 mg/dL in women; SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or taking a BP-lowering agent; fasting plasma glucose ≥ 100 mg/dL or taking a glucose-lowering agent.70,159,160
2.7.1. Prevalence and incidence of MetS
According to the report of the Nutrition and Health Surveys 2017-2020 in Taiwan, the prevalence rate of MetS was 39.3% in male and 30.3% in female adults (≥ 19 years old), which were higher than 9.8% and 13.9%, respectively, reported in the first surveys conducted between 1993-1996.70 The prevalence rate reached a peak of 59.2% at 65-74 years of age in males, whereas it increased sharply after 45-64 years and reached up to 67.0% in female adults aged ≥ 75 years old.70
2.7.2. Impact of MetS on the development of ASCVD
Individuals with MetS have 6-, 4- and 3-fold higher risks of developing DM, hypertension, and hyperlipidemia, respectively.160 MetS has been found to be more prevalent in non-diabetic patients with CAD than in those without CAD.161 An analysis of TwCCCC showed that as the number of MetS components increased, the HR increased significantly, up to 5.5 (95% CI: 2.2-13.7) for CAD and 3.5 (95% CI: 1.9-6.5) for stroke.162 Another cohort study in Taiwan revealed that the HR for the risk of stroke of subjects with 1 to 2 and ≥ 3 MetS components were 3.16 and 5.15, respectively, according to the 2005 definition from the National Cholesterol Education Program Adult Treatment Panel III.163 A follow-up study of a Chinese cohort involving 10,292 individuals in Taiwan showed that MetS with hypertension as a component was associated with an increased risk of ischemic and hemorrhagic stroke [adjusted HR: 2.96 (95% CI: 1.94-4.50) and 2.93 (95% CI: 1.25-6.90), respectively] compared with those who had neither hypertension nor MetS.164
2.7.3. Role of MetS in ASCVD risk assessment
Debate on the definition of the diagnosis of MetS has continued since its introduction.165,166 Areas of debate include whether 1) insulin resistance, obesity, or inflammation is the core mechanism of action; 2) only the cutoff values of each component should be present rather than continuous values or the degree of the severity of the syndrome with multiple cutoff values; 3) to incorporate all the risk factors known for CVD, such as physical activity; 4) MetS should be considered a cluster of risk factors or an additional independent risk factor to assess CVD risk; and 5) MetS is a better predictor than obesity to prevent CVD.165,166 Nevertheless, there is no doubt that MetS is associated with a higher risk of developing traditional metabolic risk factors as well as ASCVD.160-164 In addition, it is considered to be a risk enhancer and is often included in the risk assessment of ASCVD in lipid guidelines.6,167
2.7.4. Strategies to prevent MetS
Healthy lifestyle factors such as physical activity and healthy dietary pattern are associated with a lower risk of MetS and are recommended as the best strategy to prevent MetS.166,168,169 Physical fitness training among 1,720 soldiers was shown to improve the components of MetS, with running performance proving to be most relevant to MetS, in Taiwan.170 In addition, an RCT involving 136 metabolically abnormal obese individuals in Taiwan showed that a 6-month community-based exercise intervention program, including providing exercise environments, exercise skills and reminding from volunteers, could significantly improve the components of MetS including HDL-C, BMI, waist circumference, BP and fasting blood glucose levels.171 The Health Promotion Administration in Taiwan provides free checks for the components of MetS every 3 years for individuals aged ≥ 40 and < 65 years and every year for those aged ≥ 65 years.172
Key Recommendations
• MetS is considered to be a risk enhancer and should be included in the risk assessment of ASCVD (COR IIa, LOE C).
• Healthy lifestyle factors such as physical activity and healthy dietary pattern are recommended to prevent MetS (COR I, LOE C).
2.8. Gender
2.8.1. Sex and gender and their impact on the development of ASCVD
CVD is the leading cause of death among both men and women in Taiwan, while the burden is greater for women than for men, especially with the aging population. Compared with men, women are less likely to be diagnosed appropriately, receive preventive care, or be treated aggressively for CVD. The current prevention guidelines recognize the importance of integrating sex, gender, and gender identity considerations into the risk assessment and clinical management of individuals and populations. With these differing risk profiles, risk assessment, risk stratification, and primary preventive measures for ASCVD are different in women and men.173 Evidence exists for the risk modifying effects of sex or sex-specific clinical conditions on socioeconomic status, determinants of health access, healthcare utilization, clinical management strategies, and even therapeutic responses in the field of CVD and ASCVD prevention.174-176 Research is ongoing, however gaps in the evidence remain. Sex differences between men and women have allowed for the identification of CVD risk factors and risk markers that are unique to women, including pregnancy-related cardiovascular health (cardio-obstetrics), premenopausal vs. postmenopausal status, sex hormone-related issues, cancer-related therapies (cardio-oncology), all of which can facilitate appropriate prevention strategies and might improve long-term outcomes.
2.8.2. Role of sex and gender in risk assessment
For the primary prevention of ASCVD, risk scores and models have been developed to improve the ability to detect atherosclerosis at an earlier stage. In Taiwan, the TwCCCC risk model revealed that the risk of CVD/sudden death was higher for men than for women during follow-up (HR: 1.9, 95% CI: 1.3-2.9).177 Another model built using data from the Nutrition and Health Survey in Taiwan from 1993-1996 and linked with 10-year events from the TNHIRD showed that the incidence rates of major adverse cardiovascular events per 1000 person-years were 13.77 for men and 7.76 for women.28 Furthermore, the incidence rates of major adverse cardiovascular events in the Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia conducted in 2002 were 7.27 for men and 3.58 for women.28
Recent US population estimates from the National Health and Nutrition Examination Survey found that significantly fewer women had high-risk (< 1% vs. 5%) and intermediate-risk (4% vs. 29%) scores than men, respectively, and that the lifetime risk of developing CVD for men was higher than for women (approximately 1 in 2 for men and 1 in 3 for women).25,178-183 Similar trends have also been noted in Chinese populations.7,184,185 A study in Sweden comprising a large, random sample of the general population aged 50 to 64 years (50.6% women) without established disease using coronary computed tomography angiography (CCTA), found that silent coronary atherosclerosis was not uncommon (42.1%), and that the onset of atherosclerosis was delayed on average by 10 years in women.183 Some reports have suggested that women are more likely to develop plaque erosions with non-calcified plaques, more diffuse lesions and multivessel disease, resulting in higher mortality.186
Some cardiovascular risk factors are unique to women,173,187-189 including pregnancy-related conditions (eclampsia, pre-eclampsia, gestational hypertension or/diabetes), polycystic ovary syndrome, early menopause, premature ovarian insufficiency, functional hypothalamic amenorrhea, failure of fertility therapy, infertility, exogenic hormone use, etc. Early and late onset of menarche is also associated with an increased risk of CVD, while breast-feeding has been demonstrated to have a cardioprotective effect. A history of inflammatory diseases, especially rheumatoid arthritis and psoriasis,190 enhances the risk of ASCVD, and their prevalence is higher in women. Furthermore, clinical cardio-oncology considerations specific to women span many areas and are particularly relevant for the management of patients with sex-specific cancers, such as breast cancer.191,192 These factors, if present, would favor more intensified lifestyle interventions and consideration of initiating or intensifying statin therapy for primary prevention to mitigate the increased risk. In patients receiving cardio-toxic agents, it is recommended to periodically monitor cardiac function and screen for CVD risk factors.
2.8.2.1. Obstetric and adverse pregnancy outcome-related conditions
Pre-eclampsia, defined as pregnancy-related hypertension accompanied by proteinuria, occurs in 1-2% of all pregnancies and is associated with a 1.5-2.7-fold increased risk of CVD compared with all women,193,194 while the relative risk of developing hypertension is 3-fold and DM is 2-fold.193 A retrospective longitudinal study using the TNHIRD from 1996 to 2010 revealed that women with a history of pre-eclampsia/eclampsia were at increased risks of a subsequent diagnosis of DM, dyslipidemia, hypertension, congestive heart failure and cerebrovascular disease (HR: 3.84 and 5.42, HR: 2.75 and 3.40, HR: 6.52 and 7.31, HR: 9.07 and 7.39, and HR: 10.71 and 3.47, all p < 0.05).195
Pregnancy-related hypertension affects 10-15% of all pregnancies. The associated risk of later CVD is lower than for preeclampsia, but is still elevated (HR: 1.7-2.5).196,197 The risks of sustained or future hypertension (HR from 2.0 to 7.2 or even higher)198,199 and developing DM (HR: 1.6-2.0) are also elevated in these women.200 Both preterm (HR: 1.6) and still-birth (HR: 1.5) have been associated with a moderate increase in the risk of CVD.196
Gestational diabetes confers a sharply elevated risk of future DM, with up to 50% of affected women developing DM within 5 years after pregnancy, and up to a 2-fold increased risk of future CVD.201 Screening by fasting glucose or HbA1c may be preferable to oral glucose tolerance testing.202
Adverse pregnancy outcomes such as hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age delivery, placental abruption, and pregnancy loss increase a woman’s risk of developing CVD risk factors and of developing subsequent CVD (including fatal and nonfatal CAD, stroke, PAD, and heart failure).203,204 This is particularly relevant for younger women in whom global risk scores are in a low-risk range. Black and Asian women have a higher proportion of adverse pregnancy outcomes, with more severe clinical presentations and worse outcomes than Caucasian women. This highlights the importance of recognizing adverse pregnancy outcomes when evaluating the CVD risk in women, especially in Asia.
We stress the importance of eliciting a thorough obstetrical and gynecological history during cardiovascular risk assessment and providing a health care system framework for how to initiate appropriate preventive measures, periodic screening for hypertension, DM, and common cardiovascular risk factors. This can facilitate the transition of care for women with adverse pregnancy outcomes, and the implementation of strategies to reduce their long-term CVD risk when sex-specific risk factors are present.205
2.8.2.2. Non-obstetric conditions
The presence of components of MetS should prompt clinicians to look for polycystic ovarian syndrome, hormone contraceptive use, and gestational DM.
Polycystic ovary syndrome affects 5% of all women in their fertile years,206,207 and it is associated with a 2- to 4-fold increase in the risk of developing DM. The risk of developing hypertension may also be increased, however conflicting data exist.208 It has been associated with an increased risk of CVD, probably through common risk factors, suggesting that periodic screening for cardiovascular risk factors is appropriate.
Premature menopause occurs in approximately 1% of women under 40 years of age. Up to 10% of women experience an early menopause, defined as that occurring by 45 years of age.209 Early menopause is associated with an increased risk of CVD (HR: 1.5).210-212 A linear inverse relationship between earlier menopause and CAD risk has been found, whereby each 1-year decrease in age at menopause is associated with a 2% increased risk of CAD.213
Key Recommendations
• In women with a history of preeclampsia and/or pregnancy-induced hypertension, periodic screening for hypertension and DM should be considered (Class IIa, LOE B).
• In women with a history of polycystic ovary syndrome or gestational DM, periodic screening for DM should be considered (Class IIa, LOE B).
• In women with a history of pregnancy-associated conditions and adverse pregnancy outcomes, periodic screening for CV risk factors may be considered (Class IIb, LOE B).
2.9. Genetics and family history
Several previous studies have shown that the etiology and mechanisms of ASCVD have a genetic component, but these findings are complex,214 and it is not currently used in personal and clinical preventive management. In the past few years, polygenic risk scoring has shown some potential to improve ASCVD risk prediction for primary prevention.215-217 In addition, improving reporting standards and reproducibility of polygenic risk scores for risk stratification could open a window for clinical utility in ASCVD, and may increase the use of genetics in the primary prevention of ASCVD.218-220 However, there are some limitations. First, the incremental prediction accuracy is relatively modest and needs further evaluation.221,222 Second, consensus regarding which genes and corresponding single nucleotide polymorphisms should be included is currently lacking. Third, whether to use risk factor-specific or outcome-specific polygenic risk scores is under debate.223 Fourth, most of these studies were mainly conducted in Caucasian populations with a large number of individuals. In East Asians, only one large-scale prospective Chinese cohort study using 41,271 individuals as a validation cohort showed that polygenic risk scores could stratify individuals into different trajectories of CAD risk, and further refine risk stratification for CAD within each clinical risk strata.224 In South Asians, a validation study with 7,244 individuals in the UK Biobank also showed that compared to the middle quintile, the risk of CAD was most pronounced in those in the top 5% of the polygenic risk score distribution with odds ratios of 4.16, 2.46, and 3.22 in the South Asian UK Biobank, Bangladeshi, and Indian studies, respectively. On the other hand, more evidence is also needed to assess the clinical utility of polygenic risk scores in other clinical settings, such as in patients with pre-existing ASCVD.225 Taken together, the routine use of genetic testing in patients with ASCVD or in healthy individuals is not recommended, although it could be considered in select patients with a high risk of CAD or with a family history of premature CAD or ACS.
A family history of premature CAD is a simple and traditional risk indicator of ASCVD, with an interplay of genetic and environmental effects.24,226 There are varying definitions of family history.24 A family history of CAD is usually defined as the onset of CAD [in males ≤ (or <) 55 years of age and females ≤ (or <) 65 years of age] in first degree relatives,6,227 and a family history of premature CAD is usually defined as the onset of CAD in first degree relatives aged < 50 years.18,227 The predicted value of family history varies. Some studies have shown incremental risk prediction of CAD or CAD death in addition to traditional risk factors,226,228-230 whereas others have shown marginal incremental predicted value and risk reclassification effect on the basis of conventional and novel CAD risk factors.231,232 This discrepancy may be due to the varying definitions of family history applied,233 and because conventional ASCVD risk factors largely explain the impact of family history.24
Key Recommendations
• The routine use of genetic testing in patients with ASCVD or in healthy individuals is not recommended (Class III, LOE B).
• Genetic testing may be considered in select patients with a high risk of ASCVD or with a family history of premature CAD or ACS (Class IIb, LOE B).
• A family history of CAD or premature CAD may be included in the risk assessment of ASCVD (Class IIa, LOE B).
2.10. CKD
2.10.1. Prevalence and incidence of CKD
The current prevalence of CKD worldwide is 11-13%.234 Reasons for the high prevalence include population aging, DM, and high BP. Because the management of other chronic diseases such as malignant tumors, stroke and MI has improved, the mortality rate of these patients has been reduced. Therefore, in these patients, the chance of kidney failure has increased. An analysis of data from the TNHIRD estimated that the national prevalence of CKD was 11.9%.235 About 6.9% of people over the age of 20 years have stage 3-5 CKD, and the prevalence and incidence of end-stage renal disease in Taiwan have been the highest globally for years.236
2.10.2. Impact of CKD on the development of ASCVD
The relationship between CKD and ASCVD is complex and multifactorial. In general, two main mechanisms are thought to contribute to the development of ASCVD in patients with CKD.234,237 First, CKD is associated with several traditional risk factors for ASCVD, including hypertension, DM, dyslipidemia, and obesity. Second, CKD is also associated with non-traditional risk factors for ASCVD, including chronic inflammation, oxidative stress, endothelial dysfunction, and mineral disorders. In addition to traditional and non-traditional risk factors, the kidneys can release hormones, enzymes and cytokines in response to kidney injury, resulting in characteristic changes in the vasculature.238 On the other hand, CKD-related mediators and hemodynamic changes contribute to heart damage. Patients with CKD are at an increased risk of developing ASCVD, and the risk increases as kidney function declines.
2.10.3. Role of CKD in ASCVD risk assessment
Individuals with CKD are at an increased risk of developing ASCVD and having poor ASCVD outcomes, including CAD,239 stroke,240,241 PAD,242,243 and aortic aneurysm.244,245 Assessing the risk of ASCVD in patients with CKD is important for identifying those who may benefit from early interventions to prevent or delay the onset of ASCVD.246 Several factors should be taken into account when assessing the ASCVD risk in patients with CKD, including age, gender, BP, lipid levels, smoking status, and presence of DM.247 In addition, kidney function itself can be used as a marker for CVD risk.239,242,248 A decrease in estimated glomerular filtration rate (eGFR) and an increase in urine albumin clearance ratio (UACR) are associated with an increased risk of ASCVD events. Therefore, CKD is considered to be a high-risk factor for ASCVD, and modification of relevant risk factors should be more aggressive in the context of CKD, even in the primary prevention of ASCVD.6,167 The Taiwan Society of Nephrology recommend that eGFR and UACR should be checked annually to identify CKD in individuals with type 2 DM, hypertension, older age (> 65 years), and obesity, while UACR should be checked every year in individuals with hypercholesterolemia or tobacco smoking.249
2.10.4. Strategies to prevent the occurrence of CKD
Several strategies can be applied for the primary prevention of CKD, including lifestyle modifications and the control of risk factors modulating both CKD and ASCVD. Several modifiable lifestyle factors have been identified for the primary prevention of CKD. A large meta-analysis250 involving 104 studies of 2,755,719 participants with generally a low risk of bias showed that higher dietary potassium intake (HR: 0.78, 95% CI: 0.65-0.94), higher vegetable intake (HR: 0.79, 95% CI: 0.70-0.90), physically active versus sedentary (HR: 0.82, 95% CI: 0.69-0.98), and moderate consumption of alcohol versus no consumption (HR: 0.86, 95% CI: 0.79-0.93) were associated with a lower risk of CKD, whereas higher salt intake (HR: 1.21, 95% CI: 1.06-1.38), and current and former smokers were associated with a higher risk of CKD. These associations were consistent, but evidence was predominantly of low to very low certainty.250 Higher diet quality was most important among these factors in the Framingham Heart Study.251 Alcohol consumption can be a "double-edged sword" for CKD patients.252 Drinking alcohol can cause adverse events, and non-drinkers do not need to start drinking, but moderate drinking may be beneficial for CKD. The definition of moderate consumption of alcohol here equal to less than 2 drinks/day (28 g/day alcohol) for men and less than 1 drink/day (14 g/day alcohol) for women. However, concerns about alcohol consumption are fully discussed in the Part II of the current guidelines (section 5.1.4). The following risk factors which modulate both CKD and ASCVD should be well controlled for the primary prevention of CKD. First, BP control: high BP can damage the kidneys and increase the risk of CKD and ASCVD.253 BP control for the primary prevention of ASCVD is evident and recommended by guidelines.18 Population-based cohort studies have shown that the primary prevention of CKD can be achieved with BP control.254 Optimal BP control according to guidelines is essential. Second, blood sugar control: high blood sugar levels can damage the blood vessels and increase the risk of CKD and ASCVD.36,255 Keeping blood sugar levels and HbA1C under control is crucial for preventing CKD and ASCVD.256,257 Third, LDL-lowering treatment: high cholesterol levels lead to the initiation and progression of atherosclerosis, which remain the major causes of morbidity and mortality in patients with CKD and ASCVD.258 A post hoc analysis of six RCTs regarding intensive LDL-lowering treatment with high-dose atorvastatin showed that atorvastatin improved kidney function over time in a dose-dependent manner, and that kidney function improvement was strongly associated with lower cardiovascular risk in patients at risk of or with CVD but no baseline kidney disease.259 Fourth, weight loss: potential factors underlying the increased risks of CKD and end-stage renal disease include obesity-mediated hypertension, insulin resistance, obesity-related glomerulonephropathy, renin–angiotensin–aldosterone system activation, inflammation, and dysregulated adipocytokine production.260 An RCT including 6,719 overweight/obese adults without ASCVD who were randomized to receive an intensive weight-loss lifestyle intervention with an energy-reduced Mediterranean diet, physical activity promotion, and behavioral support (intervention group) or usual care advice to adhere to an energy-unrestricted Mediterranean diet (control group), showed that an intensive weight-loss lifestyle intervention approach may preserve renal function and delay CKD progression in overweight/obese adults.260 A recently published RCT, the SELECT study, involving 17,604 participants (aged ≥ 45 years and BMI ≥ 27 kg/m2) who were overweight or obese with established ASCVD and no history of DM demonstrated that weekly semaglutide injections (2.4 mg) provided a significant reduction in the risk of composite renal events accompanied with body weight reduction, although it was not an ASCVD primary prevention trial.261 A cohort study in Taiwan showed that weight loss with bariatric surgery was associated with eGFR preservation even in individuals with baseline eGFR ≥ 90 mL/min/1.73 m2 and UACR < 30 mg/g.262
Key Recommendations
• Renal function should be evaluated annually in individuals with DM, hypertension, older age (> 65 years), obesity, hypercholesterolemia or tobacco smoking (COR I, LOE B).
• Lifestyle modifications, especially higher diet quality (low salt, high potassium, energy-reduced, and high vegetable intake), should be considered for the primary prevention of CKD (COR IIa, LOE B).
• BP control may be beneficial in the context of the primary prevention of CKD in patients with hypertension (COR IIb, LOE C).
• Blood sugar control is recommended for the primary prevention of CKD in patients with DM. The HbA1c control target for patients with multiple risk factors should be < 7% (COR I, LOE A).
• Weight loss is beneficial for the preservation of renal function in individuals with overweight/obesity (COR IIa, LOE B).
2.11. Sleep disorders/OSA
2.11.1. Prevalence and incidence of sleep disorders/OSA
Sleep disturbances are common and underdiagnosed among middle-aged and older adults, and the prevalence varies by race/ethnicity, sex, and obesity status. In the general population, the prevalence of general sleep disturbances is around 32.1%, and 7.1% for sleep-related breathing disorders such as OSA.263
Approximately 34% and 17% of middle-aged men and women, respectively, meet the diagnostic criteria for OSA. The prevalence is as high as 40% to 80% in patients with hypertension, heart failure, CAD, pulmonary hypertension, atrial fibrillation, and stroke.264-267 It is highly prevalent and associated with adverse outcomes in patients with heart failure.264-267 The prevalence of sleep-disordered breathing among patients with symptomatic heart failure ranges from 40% to 60%, with OSA accounting for approximately one-third of cases.264-267
2.11.2. Impact of sleep disorders/OSA on the development of ASCVD
Sleep disturbance or abnormal sleep duration is associated with increased MI and severe CAD risks in Taiwan,268 and optimal sleep duration (7 to 8 h) is associated with a lower risk of CVD.269,270 A higher wakeup frequency has been associated with atherogenic dyslipidemia in Taiwanese adults, particularly in women.271 OSA is considered as a risk factor for the development of arterial hypertension, DM, MetS,272,273 CAD, MI, stroke,274 PAD,275 and progression of aortic aneurysm.276,277 Longitudinal observational studies in clinical and community cohorts278,279 have demonstrated that compared with mild and moderate OSA, untreated severe OSA was associated with a 2- to 3-fold higher rate of all-cause mortality and major adverse cardiac and cerebrovascular events during 5-10 years of follow-up. OSA may be linked to CVD in a bidirectional manner. In addition to shared risk factors, all sleep disturbances are strongly associated with mental disorders and share hyperarousal as an underlying mechanism.280,281 The mechanisms of action underlying OSA-associated CVD include elevated sympathetic activity, cardiovascular variability, intrathoracic pressure changes, inflammation, oxidative stress, endothelial dysfunction, insulin resistance and thrombosis provoked by OSA.274
Well-established risk factors for OSA include older age, male sex, obesity, ethnicity, anomalies of craniofacial features, and menopause in women. Chinese patients have more craniofacial bony restrictions. Less well-established risk factors include smoking and family history, while alcohol and drug use may aggravate preexisting OSA but not cause it.282-284
2.11.3. Role of sleep disorders/OSA in risk assessment
Screening for OSA is important for ASCVD risk assessment in individuals at high risk of OSA. It is characterized by repeated partial or total collapse of the upper airway during sleep resulting in airflow cessation or reduction, and is associated with either desaturation or cortical arousal.264,265,285,286 Symptoms suggestive of OSA include habitual snoring, witnessed breathing pause, choking or gasping during sleep, frequent awakening, nocturia, nocturnal gastroesophageal reflux, unrefreshed sleep, morning headache, fatigue, tiredness, and excessive daytime sleepiness. The assessment of OSA includes sleep history taking, physical examination, and questionnaires. Diagnostic testing, including in-lab or home polysomnography, or home sleep apnea testing, should be performed in conjunction with a comprehensive sleep evaluation and follow-up.
2.11.4. Strategies to prevent sleep disorders/OSA
There is no evidence regarding strategies to prevent OSA. Healthy sleeping habits is a complex balance between behavior, environment and circadian rhythm.287 Appropriate behavior may be beneficial for the quality of sleep, such as eating tryptophan- and carbohydrate-rich foods, physical exercise in the afternoon, a cold shower just before going to bed, and thermoneutrality during sleep.287 Caffeine intake 30 to 60 minutes before sleeping shortens total sleep time, and alcohol intake can also lead to night-time awakening due to sympathetic activation despite better initiation of sleep.287 Therefore, both should be avoided before sleeping.
Key Recommendations
• 7 to 9 hours of sleep per night is recommended for good health (Class I, LOE C).
• In patients with obesity, hypertension, MetS, atrial fibrillation or heart failure, screening for sleep problems, especially OSA, is indicated (Class I, LOE C).
2.12. Inflammation/infection
2.12.1. Prevalence and incidence of inflammation/infection
Chronic low-grade inflammation/infection plays an important role in the pathogenesis of atherosclerosis and provocation of acute vascular events.1,288 An analysis of the Atherosclerosis Risk in Communities Study, a large multicenter cohort study, involving 8,947 participants without CAD revealed that elevation of high-sensitivity C-reactive protein (hsCRP) level (≥ 2 mg/L) was observed in 5,018 participants (56.1%).289 Furthermore, the Framingham Heart Study, a prospective community cohort study involving 4,446 participants without ASCVD, revealed that 2,831 participants (63.7%) had a higher CRP level (≥ 1 mg/L) and that 1,706 participants (38.4%) had a CRP level ≥ 3 mg/L.290 Taken together, these findings imply that the prevalence of chronic low-grade inflammation/infection in relatively healthy populations is high.
2.12.2. Impact of inflammation/infection on the development of ASCVD
Inflammation is a critical driver in the multistep process of ASCVD development.1,288 Inflammatory cascade also plays a key role in the architectural stability of fibrous caps. Destabilization of collagen in fibrous caps leads to plaque rupture and overlying thrombus formation, resulting in ACS.291 Patients with ACS have a higher hsCRP level than those without.
2.12.2.1. The role of periodontitis
The relationship between inflammation/infection and the development of ASCVD has been reported in several association studies. The role of periodontitis has received more attention, and it probably plays a causative role rather than a coincidental association.292 Periodontitis has been shown to contribute to elevated CRP levels in many studies.292-295 CRP levels progressively increase from periodontal health to disease.294 Non-surgical periodontal therapy can effectively reduce serum CRP levels either in individuals without or with ASCVD.294-297 Relapse of periodontitis after surgery is reflected by changes in proinflammatory biomarkers, including hsCRP, during follow-up.298 A genome-wide association study revealed a novel shared risk locus for CAD and periodontitis.299 Associations between periodontitis and CAD,300-304 ACS,304,305 ischemic stroke,303,304,306 PAD,307,308 the development and progression of aortic aneurysm,309 cardiovascular death,304 and total mortality304 have been reported in both cross-sectional and longitudinal follow-up studies. Furthermore, an analysis of the TNHIRD showed that dental prophylaxis and periodontal treatment in patients with periodontitis was associated with a lower risk of ischemic stroke than in those without periodontitis (HR: 0.78, 95% CI: 0.75-0.81, and 0.95, 95% CI: 0.91-0.99, respectively) after adjusting for confounders, whereas those with periodontitis but no treatment had a significantly higher HR for ischemic stroke (1.15, 95% CI: 1.07-1.24).310 The same study group further demonstrated that dental prophylaxis was associated with a lower risk of AMI (HR: 0.90, 95% CI: 0.86-0.95).311 A nationwide database analysis from South Korea showed that performing one more tooth brushing a day was associated with a 9% significantly lower risk of cardiovascular events after multivariable adjustment, and that regular dental visits (once a year or more) for professional cleaning also reduced cardiovascular risk by 14%.312 However, no well-designed RCT has evaluated the effects of dental prophylaxis/periodontal treatment on the primary prevention of ASCVD.24,313
2.12.2.2. Hepatitis C
Associations of hepatitis C infection and the occurrence of CAD,314 ACS,315,316 ischemic stroke,316 PAD,317 major arterial events (ACS, acute ischemic stroke, and PAD),316 and all-cause mortality316 have been reported. However, hepatitis B is not associated with ASCVD316 or ASCVD-related death.318 Furthermore, an analysis of the TNHIRD showed that antiviral treatment for hepatitis C infection resulted in a lower risk of ischemic stroke (HR: 0.53, 95% CI: 0.30-0.93) and a trend of a lower risk of ACS (HR: 0.64, 95% CI: 0.39-1.06).319
2.12.2.3. Helicobacter pylori
Associations of Helicobacter pylori infection and the occurrence of CAD,320 ACS,321 ischemic stroke,322,323 and PAD324 have been reported. However, no observational study or prospective RCT has evaluated the effect of eradicating this micro-organism infection on the occurrence of ASCVD.
2.12.2.4. Mycoplasma pneumoniae
Some observational studies have demonstrated an association between Mycoplasma pneumoniae infection and the occurrence of ASCVD.325-328 However, clarithromycin treatment was shown to increase cardiovascular risks in several population-based studies,329,330 and did not show outcome benefits in an ACS RCT.331
2.12.2.5. Rheumatoid arthritis
Rheumatoid arthritis has been reported to be associated with ASCVD.332-334 A recently published study revealed that genetic liability to rheumatoid arthritis was associated with an increased risk of CAD, probably being mediated by CRP.335 Patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs have been shown to have an improved cardiovascular profile.336 Treatment with disease-modifying anti-rheumatic drugs as well as Chinese herbal medicine for rheumatoid arthritis has been associated with a lower risk of CVD,337-340 whereas an inadequate response to disease-modifying anti-rheumatic drug therapy has been associated with a higher risk of ACS in Taiwan.341 However, no RCT has evaluated the effects of anti-rheumatic treatment on the primary prevention of ASCVD in individuals with rheumatoid arthritis.
2.12.2.6. Other inflammation/infections
Other inflammation/infection-related diseases including systemic lupus erythematosus,342,343 systemic sclerosis,344 psoriasis,345,346 inflammatory bowel disease,347-349 human immunodeficiency virus infection,350 gall stone disease,351,352 influenza infection,353 and tuberculosis infection,354-356 have been reported to be associated with the occurrence of ASCVD. An analysis of the TNHIRD showed that long-term hydroxychloroquine treatment for systemic lupus erythematosus was associated with a lower risk of vascular events,357 while patients with gall stone disease undergoing cholecystectomy were associated with a lower risk of ischemic stroke.358 Taken together, chronic low-grade inflammation plays an important role in the pathogenesis of ASCVD.
2.12.3. Role of inflammation/infection in risk assessment
There are two approaches in terms of the role of inflammation/infection in ASCVD risk assessment. The first is a biomarker-oriented approach, and the second is a concomitant disease-oriented approach.
2.12.3.1. Biomarker-oriented approach
The role of hsCRP, a canonical biomarker of inflammation/infection, has been thoroughly investigated in ASCVD risk assessment. Its predicted value has been reported to be independent from, and even superior to, traditional ASCVD risk factors.359,360 Therefore, hsCRP was included in a risk-prediction model, which was deemed superior to other risk scoring models.361,362 However, its role in ASCVD risk assessment is arguable,363,364 and thus routine measurement of hsCRP is not recommended in the guidelines.7,24
2.12.3.2. Concomitant disease-oriented approach
In 2013, the European Federation of Periodontology and the American Academy of Periodontology recommended that313 practitioners should be aware of the emerging and strengthening evidence that periodontitis is a risk factor for developing ASCVD and periodontitis patients with other risk factors for ASCVD who have not seen a physician within the last year, should be referred to a specialist.
In 2022, the Taiwanese Dermatological Association, the Taiwanese Association for Psoriasis and Skin Immunology, and the TSOC jointly published a consensus on the management of psoriatic disease with attention to cardiovascular comorbidities and recommended cardiovascular risk assessment and management.365 Of note, for patients without pre-existing CVD, a comprehensive cardiovascular risk evaluation for primary prevention should be performed at the diagnosis of psoriasis. In addition, patients with severe psoriasis or at moderate or high CVD risk, or those who exhibit cardiovascular signs and symptoms should be referred to a cardiologist for further evaluation and management.
In 2017, the European League Against Rheumatism Task Force published update recommendations for CVD risk assessment and management in patients with rheumatoid arthritis and other inflammatory joint disorders, including ankylosing spondylitis and psoriatic arthritis.366 CVD risk assessment is recommended for all patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis at least once every 5 years and should be reconsidered following major changes in antirheumatic therapy, and lifestyle recommendations should emphasize the benefits of a healthy diet, regular exercise and smoking cessation for all patients.
The CV risk assessment and management in patients with systemic lupus erythematosus, gout, and other vasculitis were also discussed by the European League Against Rheumatism Task Force in 2022.367
2.12.4. Strategies to prevent inflammation/infection
Some ASCVD-relevant inflammatory and infectious diseases are preventable. Hyperuricemia with gouty arthritis, periodontitis, hepatitis C and human immunodeficiency virus infections should be prevented and managed according to the guidelines from related societies.
Influenza virus infection can be effectively prevented by vaccination. Most influenza vaccines can protect against three (trivalent) or four (quadrivalent) different influenza viruses.7,368
Key Recommendations
• Routine measurement of hsCRP for ASCVD risk assessment is not recommended (Class III, LOE A).
• Periodontitis patients with other ASCVD risk factors who have not seen a physician within the last year should be referred to a specialist (Class IIa, LOE C).
• For patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis without pre-existing ASCVD, a comprehensive cardiovascular risk evaluation for primary prevention should be performed at the diagnosis (Class IIa, LOE C).
• The frequency of repeat screening of laboratory data for ASCVD risk assessment in patients with periodontitis, rheumatoid arthritis, psoriasis, or psoriatic arthritis should follow the Health Promotion Administration recommendations for adult preventative health screening (every 3 years for adults aged 40-64 years or annually for adults aged 65 years or older) (Class IIa, LOE C).
• Lifestyle modifications, especially a healthy diet, regular exercise and smoking cessation, and management of ASCVD risk factors are recommended for all patients with periodontitis, rheumatoid arthritis, psoriasis, or psoriatic arthritis (Class I, LOE C).
• Annual influenza vaccination is recommended, especially for individuals with older age, ASCVD risk factors, or concomitant disease (COR I, LOE B).
2.13. Mental disorders and socioeconomic stress
2.13.1. The prevalence and incidence of mental disorders
The prevalence of probable common mental disorders in Taiwan doubled from 11.5% in 1990 to 23.8% in 2010.369 These increases paralleled rises in national rates of unemployment, divorce, and suicide.369 According to a report from the Taiwan National Health Insurance Administration, approximately 2.8 million patients with psychological disorders sought medical aid in 2019.370 Therefore, it is an important issue in the current era.
2.13.2. Impact of mental disorders and socioeconomic stress on the development of ASCVD
Mental disorders (e.g. anxiety, mood, psychotic, personality, eating, sleep, sexuality-related or other disorders) mainly develop due to psychosocial stress, and are directly associated with socioeconomic and behavior risk factors.371,372 The impact of mental disorders is independent of traditional CVD risk factors, and is associated with an increased risk of the development and progression of CVD and worse CV outcomes.373,374 Severe mental illness (schizophrenia, bipolar disorder, and major depressive disorder) is associated with an increased risk of developing CAD (adjusted HR: 1.54, 95% CI: 1.30-1.82).373 Anxiety symptoms/disorders (HR: 1.41, 95% CI: 1.23-1.61) as well as experiences of persistent or intense stress or posttraumatic stress disorder (adjusted HR: 1.27, 95% CI: 1.08-1.49) are also associated with a higher risk of CAD.373 On the other hand, ASCVD patients are at an approximately 2- to 3-fold increased risk of mental disorders compared to healthy general populations.375,376 A cross-sectional study involving 11,956 permanent residents in China aged ≥ 35 years revealed that depressive symptoms were associated with 10-year ASCVD risk after adjusting for confounding risk factors.377
Psychosocial, biological, and behavioral risk factors are prevalent in disadvantaged individuals, accentuating the link between socioeconomic status and ASCVD. Socioeconomic status has a measurable and significant effect on cardiovascular health. Considering the impact of socioeconomic stress, the INTERHEART study,378 a large-scale case-control study in 52 countries, showed that all four psychosocial stressors (stress at work and at home, financial stress, major life events in the past year and locus of control and presence of depression) were associated with an increased risk of AMI events. Income level, educational attainment, employment status, and environmental factors are four dominant markers of socioeconomic status. An association of socioeconomic status with ASCVD in high income countries has been demonstrated.379-383 A prospective observational cohort study in the United States showed that participants with both depressive symptoms and stress had the greatest elevation in the risk of developing total CVD (HR: 1.48, 95% CI: 1.21-1.81) and all-cause mortality (HR: 1.33, 95% CI: 1.13-1.56), but only in those with a low income.384 Studies in Taiwan have shown that hospitalized AMI patients with low individual socioeconomic status had lower rates of diagnostic angiography and subsequent percutaneous coronary interventions and a higher mortality rate,385 and that low-income patients undergoing coronary artery bypass grafting received lower-quality care and had higher mortality than high-income patients.386
2.13.3. Role of mental disorders and socioeconomic stress in risk assessment
Preliminary evidence suggests that taking mental disorders and low socioeconomic status into account improves classical ASCVD risk models and is thus recommended.387,388 A cohort study reported that CVD risk assessment tools which did not include severe mental illness as a predictor underestimated CVD risk by about one-third in men and two-thirds in women.387
2.13.4. Strategies to prevent mental disorders and socioeconomic stress
Strategies which have been shown to be beneficial, and some of them cost-effective, in the primary prevention of common mental disorders include selective primary preventive interventions (interventions in the offspring of patients with severe mental disorders) and indicated primary preventive interventions (interventions in people at clinical high risk of mental disorders or psychosis).389 Clinical and social preventive measures both seem to be important in the prevention of mental disorders by reducing socioeconomic stress.369 It would be an important issue to survey and refer patients with low socioeconomic status to mental health services. Some lifestyle factors, such as exercise, physical activity, yoga, and qigong, may be effective in the primary prevention of common mental disorders.390-391
Key Recommendations
• Physicians should be aware of the emerging and strengthening evidence of the association of mental disorders or socioeconomic stress with the development and worse outcomes of ASCVD (Class IIa, LOE B). In addition, physicians may advise patients to implement healthy lifestyles for the primary prevention of this factor (Class IIb, LOE C).
2.14. Frailty
2.14.1. Prevalence and incidence of frailty
Frailty is the health status of decreased resistance to stressors characterized by increased vulnerability and decline in multidirectional physiologic reserve. According to extensive cohort studies, the prevalence of frailty is varied, ranging from 5.0% to 6.4%, in residents over 50 years of age in Taiwan.392,393 The discrepancy between both studies is due to different assessment tools of frailty and the various timing and areas of study enrollment.
2.14.2. Impact of frailty on the development of ASCVD
Taiwan became an aged society in 2018 due to a decline in fertility rate and increased life expectancy. The accelerating pace of aging is expected to make Taiwan a super-aged community in 2026. The prevalence of frailty and CVD increase with age, with shared common risk factors including activated inflammation.
The association between CVD and frailty is strong and bidirectional. Lifestyle risk factors such as smoking, low physical activity, economic stress, and aging link pathways between CVD and frailty.394 A meta-analysis study found that frail individuals had an increased CVD risk compared to pre-frail or robust individuals.395 Pre-frail status, defined as the presence of one or two Fried criteria, is also an independent risk factor for the development of CVD,396 and frail individuals without previous CAD have significantly higher risks of major adverse cardiovascular events, AMI, stroke, PAD, and CAD.397,398
2.14.3. Role of frailty in ASCVD risk assessment
There are currently various definitions of frailty owing to a lack of consensus agreement.399 The most commonly used approaches are to define phenotypic frailty and deficit accumulation index. Other domains including cognitive dysfunction and psychological and social frailty form the conceptual frailty framework.400 A single tool is needed to define a comprehensive model of frailty, and some domains are less applicable in the clinical practice of CVD.
Various instruments have been developed to provide deficit scores based on at least 30 variables. The frailty index is based on comprehensive geriatric assessments, and scores the number of deficits in the domains of impairment, disability, and comorbidity burden.401,402 The Canadian Study of Health and Aging clinical frailty score encompasses ten standard territories and 70 deficits from clinical assessments.403
The Fried frailty phenotype, developed using data from the Cardiovascular Health Study, is the most frequently cited instrument in previous studies.404 The Fried frailty phenotype encompasses five measurable components, namely slow walking speed, weakness of grip strength, low physical activity, self-reported exhaustion, and unintentional weight loss. Participants who meet three or more of these criteria are defined as being frail, and those who meet one or two criteria as prefrail. Other single-item measures of frailty, such as questionnaires, gait speed, handgrip strength, or Short Physical Performance Battery405,406 are used as surrogates for predictability because of the time-consuming and effort required for comprehensive assessments. To conclude, further research is required to develop a globally accepted tool to assess frailty and its specific application for CVD prevention. In addition, the ability of frailty measures to improve CVD risk prediction should be further evaluated.407
2.14.4. Strategies to prevent frailty
Lifestyle interventions such as dietary quality, micronutrient supplementation, and exercise training have been shown to be effective to prevent and attenuate frailty in observational studies and small-scale interventional trials.24,408-410
Key Recommendations
• Frailty is a complex geriatric syndrome characterized by functional decline and inability to withstand acute stressors, and it is an emerging ASCVD risk factor in the increasingly aged society (COR IIa, LOE C).
• Lifestyle interventions such as dietary quality, micronutrient supplementation, and exercise training may be considered to prevent and attenuate frailty (COR IIb, LOE C).
2.15. Environmental exposure
2.15.1. Environmental exposure and CVD
The rapid development of current civilization has brought enormous improvements in our daily lives. However, these improvements have also resulted in byproducts that affect our health and the environment. The effects of environmental pollutants can have serious impacts on human health, particularly on populations living in industrialized societies. Diseases of civilization, such as CVD, asthma, and DM, are tightly linked to environmental pollutants. These pollutants, which come from sources such as industrial and agricultural activities, vehicle emissions, and waste disposal, can cause inflammation, atherothrombosis, endothelial dysfunction, and oxidative stress, leading to a range of adverse health outcomes.411 Among them, air pollutants, and especially particulate matter (PM) with variable size, can penetrate the respiratory system via inhalation, causing respiratory disease and CVD, central nervous system dysfunction, reproductive abnormalities, and even an increase in cancer incidence.412
There are some emergent environmental exposures, such as air pollution, climate change, and heavy metal pollution that have been established closely related to ASCVD and mitigation measures should be taken in action for ASCVD prevention.
2.15.2. Ambient air pollution
2.15.2.1. Exposure to fine PM (PM2.5) and gaseous pollutants increases the risk of CVD
Numerous scientific studies have demonstrated the detrimental effects of ambient PM2.5 exposure and gaseous pollutants on cardiovascular health and an increased risk of CVD.413 PM2.5 refers to fine particulate matter with a diameter of 2.5 micrometers or less, which can easily penetrate deep into the respiratory system and subsequently enter the blood circulation leading to systemic health effects.411-414 Exposure to PM2.5 has been consistently associated with adverse cardiovascular outcomes, including increased risks of heart attack, stroke, and heart failure.415,416 Gaseous pollutants such as nitrogen dioxide, sulfur dioxide, and ozone have also been linked to increased inflammation, thrombosis, and autonomic imbalance,417 and the incidence of CVD.418,419 These pollutants can induce oxidative stress, inflammation, and endothelial dysfunction, contributing to the development and progression of cardiovascular pathologies.420 One study of adolescents and young adults in Taiwan suggested that traffic and industrial sources of PM2.5 and elements were associated with higher carotid intima-media thickness (CIMT).421 Recent evidence indicates that even at low pollution levels below the current European and North American standards and World Health Organization guideline values, outdoor air pollution is associated with mortality.422 These findings are supported by high-quality scientific evidence, including systematic reviews, cohort studies, and experimental studies.411-414,423,424
2.15.2.2. Strategies to reduce ambient air pollution for the primary prevention of CVD in the general population
Mounting evidence suggests that reducing ambient air pollution has the potential to prevent CVD in the general population. Epidemiological studies have consistently demonstrated an association between higher levels of air pollution and increased cardiovascular morbidity and mortality.411-414 Implementing interventions to decrease air pollution levels, such as reducing the use of fossil fuels, adopting cleaner energy sources, and implementing stricter emission standards, has been shown to have positive impacts on cardiovascular health.411,425 A previous study reported that a decrease of 10 μg/m3 in PM2.5 across 51 U.S. metropolitan areas with matching data for the late 1970s and early 1980s versus the late 1990s and early 2000s was associated with an estimated increase in mean life expectancy of 0.61 ± 0.20 years.426 These findings are supported by a systematic review and meta-analysis of observational studies and intervention trials.427-429 Therefore, reducing ambient air pollution has emerged as an important strategy for preventing CVD and promoting public health.
Key Recommendations
• Both long-term and short-term ambient PM2.5 exposure increase the risk of ASCVD (COR I, LOE B).
• Reducing ambient air pollution exposure can be beneficial for cardio-pulmonary health (COR IIa, LOE B).
• Reducing ambient air pollution exposure may be helpful in the primary prevention of ASCVD (COR IIa, LOE C).
2.15.3. Climate change
Scientific research indicates that climate change, and particularly inappropriate temperature increases, can elevate the risk of CVD. Rising temperatures, heatwaves, and extreme weather events associated with climate change have been linked to adverse cardiovascular outcomes.430,431 The physiological stress imposed by high temperatures can lead to increased cardiovascular strain, exacerbation of pre-existing conditions, and higher rates of hospitalizations and deaths due to cardiovascular events.432,433 Moreover, climate change-related factors such as air pollution, changes in infectious disease patterns, and alterations in food availability and quality can further contribute to the burden of CVD.430,434,435 Rajagopalan et al. supported the assertion that inappropriate temperature increases associated with climate change can elevate the risk of CVD.436 Extreme temperature plays a crucial role in increasing the effects of air pollution and exacerbating cardiovascular risks.437,438 A higher temperatures can intensify the harmful effects of air pollution on the cardiovascular system, leading to an increased incidence of heart attack, stroke, and other cardiovascular events.436-438 Thus, climate warming can exacerbate the hazardous effects of other pre-existing environmental toxicants on the cardiovascular system, leading to adverse cardiovascular outcomes.
To reduce CVD morbidity and mortality, it is recommended to mitigate climate change by reducing the use of fossil fuels and limiting carbon dioxide emissions.436,439 These measures can help to mitigate temperature increases and associated health risks, thereby safeguarding cardiovascular health. This recommendation is supported by scientific studies and expert consensus.440-442 Implementing sustainable practices and transitioning to cleaner energy sources are crucial steps towards protecting cardiovascular health in the face of climate change.
Key Recommendations
• Climate change with inappropriate increases in temperature elevates the risk of ASCVD (COR I, LOE B).
• Reducing the use of fossil fuels and limiting carbon dioxide emissions may be reasonable for the primary prevention of ASCVD (COR IIb, LOE C).
2.15.4. Heavy metals
Previous studies have consistently demonstrated that higher exposure to heavy metals, including lead, cadmium, mercury, and arsenic, increases the risk of CVD.443,444 Heavy metals, which can be found in various environmental sources such as air, water, soil, and food, have been implicated in the development and progression of ASCVD. These metals can induce oxidative stress, inflammation, endothelial dysfunction, DNA methylation, and disruption of vascular homeostasis, ultimately contributing to the pathogenesis of cardiovascular disorders.436,445-448 Epidemiological studies have shown associations between heavy metal exposure and an increased incidence of CVDs, including CAD, hypertension, MetS, and heart failure.436,443,444,448-450 To prevent CVD in the general population, reducing exposure to heavy metals is recommended. Strategies such as minimizing occupational and environmental exposure, implementing regulations on industrial emissions, and ensuring safe drinking water sources can help mitigate the risk of heavy metal-related cardiovascular health effects.436,444,445 This recommendation is supported by scientific evidence, including epidemiological studies, experimental research, and systematic reviews.451-456 Considering the widespread exposure of heavy metals, even modest contributions to CVD risk can have a substantial effect on the general population. Evidence-based clinical and public-health strategies aimed at reducing environmental exposure and mitigating the toxic effects could substantially lower the disease burden of CVD worldwide.436,448,457 Arsenic is a naturally occurring element that an individual typically encounters every day in daily life, including food, water, soil, and air. In some areas of the world, high levels of arsenic are naturally present in the groundwater in some areas of the world, including Argentina, Bangladesh, Cambodia, Chile, China, Taiwan, India, Mexico, Pakistan, the United States of America and Vietnam, and are a toxicological concern.458 The relative risks were found with dose-response relationship for those who had a cumulative arsenic exposure compared with those without the arsenic exposure after covariates adjustment from the earlier studies in Taiwan.459,460 Reducing arsenic exposure may contribute to a lower risk of ASCVD.461 Taking measures to reduce heavy metals exposure, particularly lead, cadmium, mercury, and arsenic, are essential for promoting cardiovascular health and preventing associated diseases.
Key Recommendations
• Higher heavy metal exposure to lead, cadmium, mercury, and arsenic increases the risk of ASCVD (COR I, LOE B).
• Reducing exposure to heavy metals, including lead, cadmium, mercury, and arsenic, may be reasonable to prevent ASCVD in the general population (COR IIb, LOE C).
3. SURROGATE MARKERS AS RISK ENHANCERS
3.1. Imaging
3.1.1. Coronary artery calcium (CAC)
Vascular calcification is a hallmark of atherosclerosis. Factors including inflammatory processes and metabolic risks such as hyperlipidemia and DM all influence the process of coronary calcification.462 The coronary calcium area is highly correlated with coronary plaque area both in individual arteries and the heart, indicating that CAC quantification can be used as an index of disease severity.463,464 The current prognostic value of CAC in clinical practice is mainly derived from major prospective cohort studies which enrolled subjects aged between 45 and 75 years.465-468 The population-based Rotterdam Study enrolled 1,795 asymptomatic participants with a mean follow-up of 3.3 years, and showed that the risk of CAD was highest in participants with a CAC score > 1000, followed by those with a score from 401 to 1000 and then 101 to 400 compared to those with a score < 100 (adjusted HR: 8.3, 4.6, and 3.1, respectively).469 An analysis of pooled US population-based studies (the Framingham Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study) involving 4,778 participants over 11 years of follow-up revealed that CAC scores had a greater association with the occurrence of CAD and stroke than age.470 In addition, adding CAC score to risk prediction models with traditional risk factors and only age being removed improved the discrimination for incident CAD but not for stroke.470 Compared with other serum biomarkers, CAC score appears to be superior in the risk prediction of future ASCVD events, suggesting that CAC may be useful in the risk assessment and primary prevention of ASCVD.471,472
Considering cost-effectiveness, the primary prevention strategies for CAD mainly focus on eligibility for statin therapy. CAC is useful in asymptomatic people for planning primary prevention intervention strategies such as the use of statins and aspirin. Use of the CAC score in primary prevention is recommended by the ACC/AHA guidelines23,80 for adults at intermediate risk or selected adults at borderline risk. If the decision on the use of statins remains uncertain, it is reasonable to use the CAC score in the decision to withhold, postpone, or initiate statin therapy (Class IIa, LOE B), and the CAC score may be considered as a risk modifier in the cardiovascular risk assessment of asymptomatic individuals at low or moderate risk. Therefore, after the assessment of future cardiovascular risk based on risk score analysis, the addition of CAC distribution measures can improve the discriminatory capacity of models for major CAD events. No prospective cohort study has investigated the use of CAC score in Taiwan. The TSOC recently published CCS guidelines recommending the use of CAC score to improve risk assessment in those with a borderline (10-year CAD risk 3-7%) or intermediate (10-year CAD risk 7-10%) risk.7 CAC can reclassify the risk of CVD upwards or downwards in addition to conventional risk factors to guide the initiation or intensification of preventive pharmacotherapies (Figure 5).7 In addition to statin use, CAC may also have value in the decision to recommend prophylactic daily aspirin. The Multi-Ethnic Study of Atherosclerosis cohort included 4,229 subjects without diabetes, and reported net harm with the use of aspirin in those with a CAC score of 0, and a net benefit in those with a CAC score > 100.473 Therefore, the Society of Cardiovascular Computed Tomography recommend considering aspirin therapy for all patients with a CAC score > 100.474
Figure 5.
The role of CAC scoring in ASCVD risk stratification and management (Adapted from Ueng KC, et al. with permission).7 Risk stratification is based on the TwCCCC risk prediction model.26 ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcification; CAD, coronary artery disease; LDL-C, low-density lipoprotein cholesterol; LSM, life-style modification; MI, myocardial infarction; OMT, optimal medical treatment; TwCCCC, Taiwan Chin-Shan Community Cardiovascular Cohort.
Although the coronary calcification identified in the chest computed tomography is nongated, it predicts mortality in a manner similar to ECG-gated CAC scoring.475-477 Therefore, notification of patients and their clinicians about the presence of significant CAC will result in risk category re-evaluation and initiation of statin therapy along with additional preventive interventions to reduce the risk of ASCVD.
CAC score has emerged as the most predictive single cardiovascular risk marker in asymptomatic people, capable of adding predictive information beyond the traditional cardiovascular risk factors. However, the CAC score does not provide direct information on total plaque burden or stenosis severity, and it can be low or even zero in middle-aged patients with soft non-calcified plaque.24 Furthermore, although CAC measurements are considered to be useful for enhancing risk assessment in primary prevention, trial evidence is limited in younger and older adults, and there is currently no clinical trial evidence showing that better outcomes are achieved merely with a CAC testing approach.478 The results of future clinical trials and observations are needed before recommendations for the use of CAC in young and elderly populations can be made.
3.1.2. CCTA
CCTA provides a comprehensive assessment of the entire coronary tree, including the presence of plaque, its morphology, and its extent.479 In patients without significant epicardial obstruction, CCTA can rule out atherosclerosis or detect subclinical plaque that should be monitored for progression/regression following preventive therapy and provide risk classification.480 In patients with significant epicardial obstruction, CCTA can assist in planning revascularization by determining the disease complexity, vessel size, lesion length and tissue composition of the atherosclerotic plaque, as well as the best fluoroscopic viewing angle. It may also help in selecting adjunctive percutaneous devices and in determining the best landing zone for stents or bypass grafts.480
Silent coronary atherosclerosis in the general population is common and is correlated with CAC. The Swedish Cardiopulmonary Bioimage Study recruited 30,154 randomly invited individuals aged 50 to 64 years who were not known to have CAD and had high-quality CCTA data, and the results showed that silent coronary atherosclerosis was common (42.1%), significant stenosis (≥ 50%) was less common (5.2%), and more severe forms such as left main, proximal left anterior descending artery or 3-vessel disease, were rare (1.9%) in the middle-aged population.481 In addition, CCTA-detected atherosclerosis was found to increase in those with a higher CAC score, and all of the participants with a CAC score > 400 had atherosclerosis, of whom 45.7% had significant stenosis. In addition, 5.5% of those with a CAC score of 0 had atherosclerosis, of whom 0.4% had significant stenosis, with an increasing prevalence at higher baseline risk.481 High CAC scores convey a significant probability of substantial stenosis, and a CAC score of 0 does not exclude atherosclerosis, particularly in those at higher baseline risk.481 Among 23,759 symptomatic patients from the Western Denmark Heart Registry who underwent diagnostic CCTA, the event rate increased stepwise with CAC scores, and patients with a comparable calcified atherosclerosis burden generally had a similar risk of CVD events regardless of whether they had nonobstructive or obstructive CAD.482 This indicates that plaque burden, not stenosis per se, is the main predictor of the risk of CVD events and death.482
Although CCTA can be used to identify subclinical atherosclerosis and severity/stability of the atheroma, whether it can improve risk classification or add prognostic value for the purpose of primary prevention over CAC score is unknown.483 In addition, increased cost, radiation exposure, and the use of downstream invasive coronary angiography and preventive treatment are other concerns. The CONFIRM study investigated statin or aspirin use in examining the risk of mortality associated with nonobstructive CAD by CCTA and evaluated the impact of baseline statin and aspirin use on mortality.484 After analyzing 10,418 patients (5,712 with normal CCTA and 4,706 with nonobstructive CAD), a relationship between increasing burden of nonobstructive CAD and higher mortality rates was identified and a reduction in mortality risk associated with baseline statin therapy but not aspirin was observed. Like CAC score used in risk reclassification in intermediate risk group, baseline statin use is significantly beneficial in patients with the presence of nonobstructive coronary plaque. In CONFIRM study, there is no comparison investigating different impacts of CAC score or nonobsctructive plaque identified by CCTA on long term mortality associated with baseline statin use. Furthermore, it is only an observational study. An RCT involving 900 people with type 1 or type 2 DM without symptoms of CAD with a mean follow-up time of 4.0 years demonstrated that CCTA-directed treatment versus standard treatment did not result in a lower composite rate of all-cause mortality, nonfatal MI, and unstable angina requiring hospitalization.485 An ongoing RCT planning to enroll at least 6,000 middle-aged participants at risk of CVD but without CAD at baseline to test the prognostic role of CCTA will provide more conclusive answers in terms of the role of CCTA in the primary prevention of ASCVD.486 In the 2019 ESC guidelines for the diagnosis and management of CCS, CCTA with non-invasive functional imaging was recommended as the initial test to diagnose CAD in symptomatic patients in whom obstructive CAD could not be excluded by clinical assessments alone.483
Key Recommendations
• CAC score may be considered as a risk modifier in the cardiovascular risk assessment of asymptomatic individuals at low-moderate risk of ASCVD (COR IIb, LOE B).
• Use of the CAC score is not recommended for asymptomatic individuals who are at high risk of ASCVD (COR III, LOE B).
• It is reasonable to use the CAC score when making the decision to withhold, postpone, or initiate statin therapy in asymptomatic adults with a borderline to intermediate risk of ASCVD, if the decision about statin use remains uncertain (Class IIa, LOE B).
• For the primary prevention of ASCVD, whether CCTA improves risk classification or adds prognostic value over CAC score in asymptomatic individuals is unknown, and may not currently be recommended (Class IIb, LOE B).
3.1.3. Carotid ultrasound
Carotid ultrasound serves as a non-invasive technique for the early detection of subclinical or clinical atherosclerosis. This procedure primarily measures CIMT and checks for the presence of carotid plaques. However, the routine use of carotid ultrasound for screening in the general population is subject to debate and controversy.
CIMT is assessed at the far wall of the carotid arteries using ultrasound imaging. This measure is taken between the lumen-intima interface and the media-adventitia interface, clearly marked by a distinct double-line sign on ultrasound images. However, discrepancies across various studies have been noted and can be attributed to five primary factors: (1) the specific sites where CIMT is measured; (2) the specific CIMT parameters selected for statistical analysis; (3) the defined endpoints for each study; (4) the inclusion or exclusion of carotid plaques in the analysis; and (5) the predetermined cutoff values utilized to predict the CVD risk.487 A meta-analysis of individual patient-level data which involved 16 prospective studies demonstrated a direct correlation between the mean CIMT and a 16% increase in cardiovascular risk.488 However, no link was found between CIMT progression and cardiovascular event occurrence.488 In another meta-analysis,489 the added value of CIMT was questioned. This study revealed a minimal net reclassification improvement of only 0.8% when CIMT was incorporated into the 10-year Framingham risk score-based predictive model for cardiovascular risk.489 The questionable predictive ability of CIMT progression along with the debatable additional value of integrating CIMT into traditional risk prediction models, diminishes the priority of including CIMT measurements in cardiovascular risk evaluations.24,64,490
Conversely, accumulating evidence from prospective studies has identified the presence of carotid plaques as a robust independent ASCVD risk factor, with significant additive value for risk prediction models.491,492 A meta-analysis of 11 population-based studies with 54,336 patients showed that the presence of carotid plaque assessed by carotid ultrasound, compared with CIMT, had a higher diagnostic accuracy for the prediction of future CAD events.492 Carotid plaques are characterized as having a local thickness that exceeds the surrounding wall thickness by at least 50%, or a distinct local thickness exceeding 1.5 mm, often protruding into the lumen, particularly at carotid bulbs.493 According to the most recent guidelines from the American Society of Echocardiography494 for evaluating carotid arterial plaques via ultrasound, carotid plaques can be divided into three categories. Grade I refers to protruding plaques with a CIMT of less than 1.5 mm. Grade II comprises either protruding or diffuse plaques with a CIMT of at least 1.5 mm but less than 2.5 mm. Grade III includes either protruding or diffuse plaques with a CIMT of 2.5 mm or more.494 Overall, the presence of carotid plaques has been shown to be more potent than CIMT for predicting cardiovascular risks.
In addition to CIMT and the presence of carotid plaque, a low end-diastolic velocity in the common carotid artery has been independently associated with future cerebro-cardiovascular events in Taiwanese and Korean populations.495,496 Moreover, the Two-Township Study in Taiwan demonstrated that end-diastolic velocity (< 15 cm/s) improved the prognostic power of future vascular events (combined ischemic heart disease and stroke) when added to traditional risk prediction models.497 Therefore, end-diastolic velocity measured by carotid ultrasound may provide additional predictive value for cardiovascular events in selected patients.
Key Recommendations
• Using carotid ultrasound to evaluate the carotid plaque burden should be considered to enhance cardiovascular risk classification in selected patients (COR IIa, LOE B).
• End-diastolic velocity in the common carotid artery (< 15 cm/s) measured by carotid ultrasound may be used to improve the risk prediction of cardiovascular events (COR IIb, LOE B).
• Routine CIMT screening by ultrasound to improve cardiovascular risk stratification is not recommended (COR III, LOE B).
3.2. Arterial stiffness (ArtS)
3.2.1. ArtS and ASCVD
ArtS results mainly from arteriosclerosis of central arteries (principally a disease of the media) rather than from atherosclerosis of peripheral arteries (mainly a disease of the intima). ArtS largely reflects gradual fragmentation and loss of elastin fibers and the accumulation of stiffer collagen fibers in elastic arterial walls.498 Elastic-type large arteries such as the aorta, carotid, and iliac arteries are primarily affected, as opposed to large muscular arteries such as the brachial, radial, and femoral arteries.499 A large number of studies have reported that various physiological and pathophysiological conditions are associated with increased ArtS.500 In a longitudinal study involving 1,518 community-dwelling persons in Taiwan, MetS affected the progression to ArtS in 3 years follow-up.501 In this study, ArtS progressed as the number of MetS components increased.501 Apart from the dominant effects of BP and aging, other important factors affecting ArtS include genetic background, cardiovascular risk factors, end-stage renal disease502 and also chronic inflammatory diseases such as inflammatory bowel disease.503
3.2.2. ArtS as a risk enhancer
ArtS can be present even in the absence of traditional cardiovascular risk factors, and the loss of arterial elasticity has been linked to the full spectrum of ASCVD. Research indicates that ArtS precedes the typical and overt symptoms of diseases by many years. The predictive value of ArtS has been largely demonstrated. Recently, numerous studies in patients with uncomplicated essential hypertension504,505 and the general population506 have prospectively validated the predictive value of ArtS. Of note, central BP has been shown to be better than conventional brachial BP to assess target organ damage and long-term cardiovascular outcomes.507,508 The major determinant of central BP is increased ArtS, which is also the dominant hemodynamic manifestation of arterial aging. Furthermore, ArtS is a robust predictor of all-cause and cardiovascular mortality, fatal and non-fatal coronary events and fatal strokes,509 and thus it can serve as a surrogate marker for ASCVD events. Risk scores are used in clinical decision-making but can under- or overestimate the risk. Of note, ArtS has been shown to be a predictor of ASCVD events and mortality independently of traditional risk factors. In addition, ArtS has been shown to retain its predictive value for cardiovascular events after adjusting for the Framingham Risk Score510 or SCORE,511 supporting that it can add value to a combination of traditional risk factors. Recent studies and an individual data meta-analysis showed that patients at intermediate risk could be reclassified into a different risk category when ArtS was measured.506,511,512 In another recently published individual data meta-analysis (n = 17,635 participants), the improvements in the 5-year overall net reclassification for CAD and stroke that have some clinical relevance, especially for those at intermediate risk.509 These studies indicate that ArtS, an early biomarker for ASCVD, is associated with the full spectrum of ASCVD, and that it can serve as a valuable risk modifier/enhancer in the setting of primary prevention. In terms of risk reclassification and risk stratification, ArtS has been even reported to be superior to carotid ultrasound or ABI.500 In the setting of primary prevention, ArtS reflects early morphological changes well before overt disease manifests. This identification of subclinical disease may open a window of opportunity to prevent the occurrence of clinical ASCVD disease with timely interventions.
3.2.3. Methodology and standardization of ArtS measurements
Many non-invasive methods to measure ArtS have been reported.513 The most widely used and validated techniques involve the assessment of pulse waves as they travel over a significant portion of the arterial tree, including carotid-femoral pulse wave velocity (cfPWV) and brachial-ankle PWV (baPWV). The main differences between methods are the arterial measurement sites that vary from the central elastic aorta (cfPWV) to the aorta and peripheral muscular arteries (baPWV). Europeans favor cfPWV, whereas baPWV is favored in Asia. In 3,034 Atherosclerosis Risk in Communities study participants without CVD, cfPWV showed the strongest association with CVD among different PWV measures over a median follow-up of 4.4 years.514 cfPWV is usually obtained using surface tonometry probes at the right common carotid artery and the right femoral artery, and the time delay (transit time) is measured between the two waveforms. By directly reflecting central ArtS and having the best predictive value for cardiovascular outcomes, cfPWV is now considered the gold standard for assessment in daily practice.498 Reference values for cfPWV have been established in 1,455 healthy subjects and a larger population of 11,092 subjects with cardiovascular risk factors.515 Due to the fact that age and BP are major determinants of PWV, a nomogram describing the correlation of the variables is needed.516 For ease of use in daily practice, a threshold of 12 m/s was initially suggested as a conservative estimate of significant alterations in aortic function in middle-aged hypertensives,517 however this value was subsequently revised in a recent consensus document to 10 m/s.518 An increase in cfPWV by 1 m/s has been associated with age-, sex-, and risk factor-adjusted risk increases of 15% in cardiovascular mortality and all-cause mortality, respectively.498 In Taiwan, baPWV, a composite of central and peripheral ArtS, is primarily used to measure a longer arterial length. The measurement of baPWV is easier than cfPWV, as it only involves wrapping a pressure cuff around each of the four exposed extremities without exposing the groin region. baPWV was shown to be a useful predictor of ASCVD in 4,881 Taiwanese subjects during voluntary health examinations.519 In addition, the J-BAVEL trial, which included a total of 14,673 Japanese participants without a history of ASCVD, reported that a higher baPWV was significantly associated with a higher risk of CVD and mortality, even after adjustments for conventional risk factors.520 Furthermore, the addition of baPWV to a model incorporating the clinical risk score significantly improved the accuracy of the risk assessment for ASCVD.520 Considering the results of published prospective studies, a baPWV value of 1800 cm/s is used as a cutoff value in the assessment of the ASCVD risk.521 It has been reported that each 100 cm/s increase in baPWV is associated with a 13% increase in lethal cardiovascular events.522 Of note, the standardization of PWV measures must consider how these procedures should be recorded in clinical practice. Most importantly, we recommend that the measurements are taken in a quiet and stable temperature environment after 10 min of rest, avoiding smoking, caffeine, alcohol, and eating in the hours preceding the measurement, and not speaking during the measurement.523 Of note, each technique may have specific contraindications (e.g., significant carotid stenosis for cfPWV and PAD for baPWV).
3.2.4. Strategies to improve ArtS
Lifestyle modifications, and in particular weight reduction,524 smoking cessation,525 aerobic exercise526 and sodium restriction,527 appear to be clinically efficacious therapeutic interventions for preventing and treating ArtS. Pharmacological studies have shown that it is feasible to improve ArtS or to slow its progression with a few medications. Recent studies and a meta-analysis of RCTs have shown that statins can reduce ArtS by multiple mechanisms.528,529 In a Chinese cohort study involving 5,105 participants, statin use was associated with slower progression of baPWV (-23.3 cm/s per year) compared with non-statin using adults with high ASCVD risk.530 As such, statin therapy should be considered as a pharmacological strategy to improve arterial elasticity. ArtS is closely and bidirectionally related to BP, and is considered to be both a cause and a consequence of hypertension. Importantly, different antihypertensive drug classes may have different effects on ArtS. Despite the decrease in BP, substantial studies with diuretics and non-selective beta-blockers did not show a decrease in ArtS.531,532 A recent meta-analysis of RCTs explored the effects of antihypertensive agents on both central and peripheral systolic BP and central augmentation index, and the results showed that compared with older agents (diuretics, beta-blockers, or a-blockers), the newer agents (renin-angiotensin system inhibitors and calcium channel blockers) more efficaciously reduced both central SBP and augmentation index.533 An angiotensin-converting enzyme inhibitor tended to be the most effective anti-hypertensive agent in a comparative study, suggesting a direct BP independent effect of this drug class on arterial wall remodeling.534 Similar anti-stiffening effects have been reported after long-term treatment with olmesartan, suggesting that renin-angiotensin system blockade exerts beneficial effects on ArtS.535,536 Selected antihypertensive drugs (renin-angiotensin system inhibitors and calcium channel blockers) can reduce PWV beyond the BP-lowering effect, and have pleiotropic beneficial effects on ArtS.537
3.2.5. ArtS: from surrogate marker to therapeutic target?
Given the evidence supporting ArtS as an independent risk factor for ASCVD and total mortality, some studies including RCTs have assessed the potential of PWV as a target for therapy and whether such a strategy would result in better clinical outcomes. One study of end-stage renal disease patients showed a better outcome in those with decreased ArtS.538 Recently, a multicenter open-label RCT demonstrated that PWV-driven treatment for hypertension enabled better BP control and could prevent vascular aging in patients with hypertension at medium to very high risk, compared with the strict application of guidelines.539 However, this study lacked sufficient statistical power to demonstrate its primary outcome. More evidence is needed to determine whether a PWV-guided therapeutic approach will be beneficial to the prevention of ASCVD beyond current strategies.
3.2.6. Clinical applications of ArtS in primary prevention
In 2013, the European Society of Hypertension and ESC Guidelines for the Management of Hypertension included cfPWV as an indicator of subclinical organ damage.540 The last published 2021 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice recognized that ArtS can predict future ASCVD risk and improve risk classification, but that its systematic use in the general population is not recommended.24 Asian hypertension guidelines include recommendations about the use of PWV for assessing ArtS. The 2018 Korean Society Hypertension Guidelines recommend that PWV can be a useful test at diagnosis, and that a cfPWV > 10 m/s or a baPWV > 1800 cm/s can be considered as a subclinical marker of organ damage.541 Moreover, the 2019 Japanese Society of Hypertension Guidelines for the Management of Hypertension state that cfPWV > 10 m/s is an indicator of organ damage, and that it could also be used as needed for further evaluation of risk assessment.542 In summary, as ArtS predicts ASCVD risk independently of traditional risk factors, it has a significant impact on decision-making in various clinical scenarios. As such, PWV could be used effectively to improve referral decisions to more centralized cardiac evaluations. This proposed scenario is even more pertinent to patients with DM, hypertension, and kidney dysfunction, in whom the cardiovascular risk is higher.
Key Recommendations
• The cutoff value for risk assessment might be 10 m/s for cfPWV or 1800 cm/s for baPWV, indicating subclinical organ damage in primary prevention (COR IIa, LOE B).
• PWV, as a risk enhancer, should be helpful for clinical decision-making to guide intensification of lifestyle and pharmacological interventions or to choose further testing in people at borderline to intermediate risk (COR IIa, LOE A).
• PWV should be considered for clinical decision-making to guide clinician-patient risk discussions in those with DM, hypertension, and CKD, in whom the cardiovascular risk is higher (COR IIa, LOE B).
• PWV may be considered for clinical decision-making in those with stage 1 hypertension (SBP 130-139 mmHg) or those in whom the need to initiate pharmacologic anti-hypertensive therapy is uncertain (COR IIb, LOE B).
3.3. ABI
ABI is a quick, simple and non-invasive test that measures the ratio of SBP at the ankle and the brachial artery in both arms and legs. A value of < 0.9 or ≥ 1.3 is indicative of PAD, which is caused by the narrowing or blockage of the arteries that supply blood to the legs and feet.543 While ABI is primarily used for diagnosing PAD, it has also been studied as a potential marker and tool for screening and risk assessment of ASCVD.544
Several studies have demonstrated that a low ABI is related to an increased risk of total and cardiovascular mortality, MI, and stroke. It is well established that PAD is a risk factor for other ASCVDs, and that the risk is greater with more advanced PAD. From a systematic review perspective, the specificity of a low ABI to predict future cardiovascular outcomes is high, but its sensitivity is low. ABI should be part of the vascular risk assessment strategy in select individuals.545,546
While ABI is categorized as having insufficient evidence for screening and risk stratification of suspected PAD without symptoms, numerous studies in diverse populations have demonstrated an association between low ABI and increased cardiovascular risk.547 An analysis of data from the 1999-2004 National Health and Nutrition Examination Survey using the PCE for risk estimation, two-thirds of the participants with a low ABI had an intermediate or high risk of ASCVD.548 In addition, a low ABI was linked to higher all-cause mortality in the overall cohort, and specifically among those with a borderline/intermediate or high risk of ASCVD but not in those with a low risk of ASCVD.548 These studies suggest that ABI should be considered as a risk enhancer in the primary prevention of ASCVD in individuals classified as having a borderline or intermediate risk of ASCVD.80,548 Recent studies have shown that the incorporation of ABI into the Framingham Risk Score is a cost-effective strategy, particularly for women classified as having a low or intermediate risk based on Framingham Risk Score alone.549
A wealth of local data support the use of ABI for ASCVD risk assessment, with many studies demonstrating its predictive power across different specific groups. For instance, one study found that either ABI alone or a combination of ABI < 0.9 and ABI difference (calculated as right ABI-left ABI) ≥ 0.17 was a significant predictor of overall and cardiovascular mortality.550 The use of mean arterial pressure instead of SBP in ABI calculations has also been suggested to potentially enhance the accuracy of mortality prediction.551 In another study of type 2 DM patients mostly without ASCVD, the use of the percentage of mean arterial pressure in combination with ABI was shown to significantly improve the prediction of all-cause mortality.552 In addition, calculating ABI using DBP and mean arterial pressure may provide additional benefits in predicting survival in patients without known ASCVD undergoing hemodialysis, especially those with normal ABI by SBP.553 In summary, local data support the usefulness of ABI measurements in predicting ASCVD risk and mortality in specific populations. The combination of ABI with other measures, such as mean arterial pressure, may enhance its predictive power and provide additional benefits in risk assessment. These findings highlight the importance of ABI screening as a tool for ASCVD risk assessment and the need for continued research to explore its potential benefits in different patient populations.
Key Recommendations
• ABI should be considered as a risk enhancer for the primary prevention of ASCVD in individuals classified as having a borderline or intermediate risk of ASCVD (COR IIa, LOE B).
• ABI might be considered for cardiovascular outcome assessment in specific populations at a high risk of ASCVD, such as patients with DM or undergoing hemodialysis (COR IIb, LOE C).
3.4. Biomarkers
3.4.1. Inflammatory biomarkers
Although elevated hsCRP levels are strongly associated with CVD events, the US Preventive Services Task Force suggested that the available evidence is not sufficient to evaluate the overall benefits and risks of incorporating hsCRP testing into the conventional assessment of ASCVD in asymptomatic adults for preventing further events.364
Several inflammatory biomarkers have been investigated. GlycA, a quantitative measurement of glycan N-acetylglucosamine residues on enzymatically glycosylated acute-phase proteins using nuclear magnetic resonance, has been correlated with inflammation markers including hsCRP and interleukin-6.554 It exhibits better precision than hsCRP in predicting ASCVD risk.554 Large-scale studies have shown that GlycA could independently predict future CVD events,555-557 and that it was associated with subclinical atherosclerosis.558,559 However, further investigations are needed before recommending it clinically, including establishing reference ranges.554 In addition, the accessibility of GlycA is limited compared to CRP. The interpretation of dynamic inflammatory biomarkers should consider the context and serial measurements for identifying concerning trends.554 Furthermore, myeloperoxidase, lipoprotein-associated phospholipase A2, myeloid-related protein, lectin-like oxidized low-density lipoprotein receptor-1, growth differentiation factor-15, osteoprotegerin and osteopontin have been studied as other potential inflammatory biomarkers.560,561 Currently, there is insufficient evidence to include these inflammation-relevant biomarkers as risk enhancers in the primary prevention of ASCVD,560 even though they may be helpful in identifying those at increased ASCVD risk warranting greater efforts for risk reduction using existing therapies.560
3.4.2. Metabolic biomarkers
Several metabolic biomarkers have been studied. Adiponectin is reduced in obesity, and it improves insulin sensitivity and reduces inflammation. In contrast, resistin is elevated in obesity, and it promotes dysfunction and inflammation. Adipokine dysregulation is linked to DM and ASCVD independently of BMI.562 Homocysteine, ceramides, dimethylglycine, and choline have also been investigated as other potential metabolic biomarkers.561 However, measuring these biomarkers is mainly done in research, not clinical practice. Further studies are needed to understand their role in ASCVD risk prediction and treatment monitoring, considering their interplay with lifestyle modifications and other factors.
3.4.3. Biomarkers of myocardial injury and wall stress
Previous studies have demonstrated the strong predictive ability of B-type natriuretic peptide level for CVD outcomes. The Framingham Heart Study revealed a significant association between plasma B-type natriuretic peptide levels and first major CVD events.563 Furthermore, a meta-analysis of 40 long-term prospective studies involving over 87,000 patients indicated that individuals in the highest B-type natriuretic peptide tertile had a 2.8-fold greater risk of CVD events compared to those in the lowest tertile.564 Similar results have been observed in the general population.565 However, the improvement in risk discrimination was only modest.564
Cardiac troponin level has emerged as a critical component of risk stratification for ACS. With the advent of high-sensitivity assays, precise measurement of cardiac troponin levels in healthy individuals has become possible.566 Several recent studies have demonstrated a growing association between cardiovascular risk and elevated cardiac troponin concentrations even within the normal range.567-569 However, the role of cardiac troponins in assessing cardiovascular risk in asymptomatic populations remains a topic of active discussion among experts.24 There is an urgent need to improve cardiovascular risk assessment in the general population by using cardiac troponins, validate the threshold of these biomarkers in those at high risk, and prove the utility of targeting these high-risk individuals for primary preventive strategies.567
3.4.4. Renal-related biomarkers
The presence of albuminuria has been linked to long-term all-cause mortality, regardless of Framingham risk.570 While the addition of a panel of renal biomarkers has been explored for predicting risk, the benefits appear to be limited when compared to using the Framingham Risk Score alone.570 Furthermore, in patients with CKD, high levels of albuminuria, serum cystatin C, and fibroblast growth factor-23, as well as low eGFR, have been shown to increase CAC progression. These novel risk factors have been shown to be independent of traditional CVD risk factors.571
3.4.5. Novel biomarkers
Novel biomarkers, including exosomes, microRNAs, and genetic risk scores, have been investigated in the primary prevention of ASCVD.572,573 By integrating our current understanding of the molecular signatures in high-dimensional molecular data acquisition platforms (such as genome sequencing, epigenomic profiling, transcriptomic analysis, proteomic studies, metabolomic investigations, and gut microbiome characterization), it has become possible to capture snapshots of every phase in the development of ASCVD.574,575 The future of cardiovascular medicine envisions precision medicine as a promising approach that has the potential to revolutionize the management of CVD.
Numerous biomarkers have been proposed to enhance risk stratification, each with its own potential benefits. Promising advancements have been made in the field of cardiac biomarkers, yet further research is required to fully evaluate their efficacy.
DECLARATION OF CONFLICT OF INTEREST
Ting-Hsing Chao has been on the speaker bureau for Astrazeneca, Bayer, Eli Lilly, Boehringer Ingelheim, Daiichi-Sankyo, MSD, Novartis, Pfizer, Sanofi, Tanabe, and TSH biopharm. All other authors report no potential conflicts of interest in relation to these guidelines.
Acknowledgments
This work is supported by the grant from the National Science and Technology Council under the grant number: NSC 111-2314-B-006 -019 -MY3. We thank for the assistance from Dr. Kuan-Ting Chen in preparation of this manuscript.
REFERENCES
- 1.Nayor M, Brown KJ, Vasan RS. The molecular basis of predicting atherosclerotic cardiovascular disease risk. Circ Res. 2021;128:287–303. doi: 10.1161/CIRCRESAHA.120.315890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA. 1990;264:3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 3.Strong JP, Malcom GT, Newman WP, 3rd, et al. Early lesions of atherosclerosis in childhood and youth: natural history and risk factors. J Am Coll Nutr. 1992;11 Suppl:51S–54S. doi: 10.1080/07315724.1992.10737984. [DOI] [PubMed] [Google Scholar]
- 4.Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Wood DA. Primary prevention of ischaemic heart disease: populations, individuals, and health professionals. Lancet. 2019;394:685–696. doi: 10.1016/S0140-6736(19)31893-8. [DOI] [PubMed] [Google Scholar]
- 6.Huang PH, Lu YW, Tsai YL, et al. 2022 Taiwan lipid guidelines for primary prevention. J Formos Med Assoc. 2022;121:2393–2407. doi: 10.1016/j.jfma.2022.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Ueng KC, Chiang CE, Chao TH, et al. 2023 Guidelines of the Taiwan Society of Cardiology on the diagnosis and management of chronic coronary syndrome. Acta Cardiol Sin. 2023;39:4–96. doi: 10.6515/ACS.202301_39(1).20221103A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health and Welfare. Causes of death statistics. Accessed October 16, 2023;https://www.mohw.gov.tw/cp-16-74869-1.html [Google Scholar]
- 10.Lee CH, Cheng CL, Yang YH, et al. Trends in the incidence and management of acute myocardial infarction from 1999 to 2008: get with the guidelines performance measures in Taiwan. J Am Heart Assoc. 2014;3:e001066. doi: 10.1161/JAHA.114.001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin WH, Lu TH, Chen KC, et al. The temporal trends of incidence, treatment, and in-hospital mortality of acute myocardial infarction over 15 years in a Taiwanese population. Int J Cardiol. 2016;209:103–113. doi: 10.1016/j.ijcard.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Li YH, Wang YC, Wang YC, et al. 2018 Guidelines of the Taiwan Society of Cardiology, Taiwan Society of Emergency Medicine, and Taiwan Society of Cardiovascular Interventions for the management of non ST-segment elevation acute coronary syndrome. J Formos Med Assoc. 2018;117:766–790. doi: 10.1016/j.jfma.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Wu YL, Ovbiagele B. Trends in incident and recurrent rates of first-ever ischemic stroke in Taiwan between 2000 and 2011. J Stroke. 2016;18:60–65. doi: 10.5853/jos.2015.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SW, Huang YB, Huang JW, et al. Epidemiology, clinical features, and prescribing patterns of aortic aneurysm in Asian population from 2005 to 2011. Medicine (Baltimore) 2015;94:e1716. doi: 10.1097/MD.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 17.Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128:1818–1832. doi: 10.1161/CIRCRESAHA.121.318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang TD, Chiang CE, Chao TH, et al. 2022 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. Acta Cardiol Sin. 2022;38:225–325. doi: 10.6515/ACS.202205_38(3).20220321A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 20.Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 21.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 23.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 25.DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J. 2017;38:598–608. doi: 10.1093/eurheartj/ehw301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chien KL, Hsu HC, Su TC, et al. Constructing a point-based prediction model for the risk of coronary artery disease in a Chinese community: a report from a cohort study in Taiwan. Int J Cardiol. 2012;157:263–268. doi: 10.1016/j.ijcard.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Jian JZ, Tzeng IS, Hsieh CF, et al. Validation of the Framingham General Cardiovascular Risk Score and Pooled Cohort Equations in a community-based population: a prospective cohort study analysis 2006-2017. Acta Cardiol Sin. 2023;39:879–887. doi: 10.6515/ACS.202311_39(6).20230405A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chien KL, Su TC, Hsu HC, et al. Constructing the prediction model for the risk of stroke in a Chinese population: report from a cohort study in Taiwan. Stroke. 2010;41:1858–1864. doi: 10.1161/STROKEAHA.110.586222. [DOI] [PubMed] [Google Scholar]
- 29.Chang HY, Fang HL, Huang CY, et al. Developing and validating risk scores for predicting major cardiovascular events using population surveys linked with electronic health insurance records. Int J Environ Res Public Health. 2022;19:1319. doi: 10.3390/ijerph19031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marston NA, Pirruccello JP, Melloni GEM, et al. Predictive utility of a coronary artery disease polygenic risk score in primary prevention. JAMA Cardiol. 2023;8:130–137. doi: 10.1001/jamacardio.2022.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue K, Seeman TE, Horwich T, et al. Heterogeneity in the association between the presence of coronary artery calcium and cardiovascular events: a machine-learning approach in the MESA Study. Circulation. 2023;147:132–141. doi: 10.1161/CIRCULATIONAHA.122.062626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siva Kumar S, Al-Kindi S, Tashtish N, et al. Machine learning derived ECG risk score improves cardiovascular risk assessment in conjunction with coronary artery calcium scoring. Front Cardiovasc Med. 2022;9:976769. doi: 10.3389/fcvm.2022.976769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson K, Kaufman B, Rotter JS, et al. Socioeconomic status and modification of atherosclerotic cardiovascular disease risk prediction: epidemiological analysis using data from the atherosclerosis risk in communities study. BMJ Open. 2022;12:e058777. doi: 10.1136/bmjopen-2021-058777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheen YJ, Hsu CC, Jiang YD, et al. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J Formos Med Assoc. 2019;118 Suppl 2:S66–S73. doi: 10.1016/j.jfma.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 35.IDF Diabetes Atlas, 10th edition. Taiwan diabetes report 2000-2045. Accessed December 19, 2023;https://www.diabetesatlas.org/data/en/country/194/tw.html [Google Scholar]
- 36.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 37.Nasu K, Tsuchikane E, Katoh O, et al. Plaque characterisation by virtual histology intravascular ultrasound analysis in patients with type 2 diabetes. Heart. 2008;94:429–433. doi: 10.1136/hrt.2007.118950. [DOI] [PubMed] [Google Scholar]
- 38.Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emerging Risk Factors Collaboration. Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 42.Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 43.Raffort J, Lareyre F, Clément M, et al. Diabetes and aortic aneurysm: current state of the art. Cardiovasc Res. 2018;114:1702–1713. doi: 10.1093/cvr/cvy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dattani N, Sayers RD, Bown MJ. Diabetes mellitus and abdominal aortic aneurysms: a review of the mechanisms underlying the negative relationship. Diab Vasc Dis Res. 2018;15:367–374. doi: 10.1177/1479164118780799. [DOI] [PubMed] [Google Scholar]
- 45.American Diabetes Association Professional Practice Committee. 3. Prevention or delay of type 2 diabetes and associated comorbidities: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S39–S45. doi: 10.2337/dc22-S003. [DOI] [PubMed] [Google Scholar]
- 46.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 48.Gong Q, Zhang P, Wang J, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019;7:452–461. doi: 10.1016/S2213-8587(19)30093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng HM, Lin HJ, Wang TD, et al. Asian management of hypertension: current status, home blood pressure, and specific concerns in Taiwan. J Clin Hypertens (Greenwich) 2020;22:511–514. doi: 10.1111/jch.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nolde JM, Beaney T, Carnagarin R, et al. Global impact of different blood pressure thresholds in 4,021,690 participants of the May measurement month initiative. Hypertension. 2022;79:1497–1505. doi: 10.1161/HYPERTENSIONAHA.122.19144. [DOI] [PubMed] [Google Scholar]
- 51.Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 52.Chiang CE, Wang TD, Ueng KC, et al. 2015 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 53.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990-2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 55.Lawes CM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 56.Zhou B, Perel P, Mensah GA, et al. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802. doi: 10.1038/s41569-021-00559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 58.Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi YJ, Kim SH, Kang SH, et al. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: a nationwide population-based study from Korea. Eur Heart J. 2019;40:724–731. doi: 10.1093/eurheartj/ehy801. [DOI] [PubMed] [Google Scholar]
- 61.Blood Pressure Lowering Treatment Trialists’ Collaboration. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altobelli E, Rapacchietta L, Profeta VF, et al. Risk factors for abdominal aortic aneurysm in population-based studies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2018;15:2805. doi: 10.3390/ijerph15122805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kokubo Y. Prevention of hypertension and cardiovascular diseases: a comparison of lifestyle factors in Westerners and East Asians. Hypertension. 2014;63:655–660. doi: 10.1161/HYPERTENSIONAHA.113.00543. [DOI] [PubMed] [Google Scholar]
- 64.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filippou C, Tatakis F, Polyzos D, et al. Overview of salt restriction in the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet for blood pressure reduction. Rev Cardiovasc Med. 2022;23:36. doi: 10.31083/j.rcm2301036. [DOI] [PubMed] [Google Scholar]
- 66.Li W, Liu H, Wang X, et al. Interventions for reducing blood pressure in prehypertension: a meta-analysis. Front Public Health. 2023;11:1139617. doi: 10.3389/fpubh.2023.1139617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu J, Liu Y, Zhang L, et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J Am Heart Assoc. 2020;9:e016804. doi: 10.1161/JAHA.120.016804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choy CS, Chan WY, Chen TL, et al. Waist circumference and risk of elevated blood pressure in children: a cross-sectional study. BMC Public Health. 2011;11:613. doi: 10.1186/1471-2458-11-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawabe H, Azegami T, Takeda A, et al. Features of and preventive measures against hypertension in the young. Hypertens Res. 2019;42:935–948. doi: 10.1038/s41440-019-0229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan WH. Nutrition and Health Surveys 2017-2020. Accessed September 20, 2023;https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=3999&pid=15562 [Google Scholar]
- 71.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Domanski MJ, Tian X, Wu CO, et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76:1507–1516. doi: 10.1016/j.jacc.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 74.Tada H, Okada H, Nohara A, et al. Effect of cumulative exposure to low-density lipoprotein-cholesterol on cardiovascular events in patients with familial hypercholesterolemia. Circ J. 2021;85:2073–2078. doi: 10.1253/circj.CJ-21-0193. [DOI] [PubMed] [Google Scholar]
- 75.Perak AM, Ning H, de Ferranti SD, et al. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9–19. doi: 10.1161/CIRCULATIONAHA.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bucholz EM, Rodday AM, Kolor K, et al. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999–2014). Circulation. 2018;137:2218–2230. doi: 10.1161/CIRCULATIONAHA.117.032321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iacocca MA, Chora JR, Carrié A, et al. ClinVar database of global familial hypercholesterolemia-associated DNA variants. Hum Mutat. 2018;39:1631–1640. doi: 10.1002/humu.23634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laufs U, Parhofer KG, Ginsberg HN, et al. Clinical review on triglycerides. Eur Heart J. 2020;41:99–109. doi: 10.1093/eurheartj/ehz785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duell PB. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2023;81:153–155. doi: 10.1016/j.jacc.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 80.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pownall HJ, Rosales C, Gillard BK, et al. High-density lipoproteins, reverse cholesterol transport and atherogenesis. Nat Rev Cardiol. 2021;18:712–723. doi: 10.1038/s41569-021-00538-z. [DOI] [PubMed] [Google Scholar]
- 82.von Eckardstein A, Nordestgaard BG, Remaley AT, et al. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. 2023;44:1394–1407. doi: 10.1093/eurheartj/ehac605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61:1146–1156. doi: 10.1016/j.jacc.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 85.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 86.Behbodikhah J, Ahmed S, Elyasi A, et al. Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Metabolites. 2021;11:690. doi: 10.3390/metabo11100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balling M, Afzal S, Varbo A, et al. VLDL cholesterol accounts for one-half of the risk of myocardial infarction associated with apoB-containing lipoproteins. J Am Coll Cardiol. 2020;76:2725–2735. doi: 10.1016/j.jacc.2020.09.610. [DOI] [PubMed] [Google Scholar]
- 88.Carr SS, Hooper AJ, Sullivan DR, et al. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. 2019;51:148–154. doi: 10.1016/j.pathol.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Burnett JR, Hooper AJ, Hegele RA. Remnant cholesterol and atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2020;76:2736–2739. doi: 10.1016/j.jacc.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 90.Castañer O, Pintó X, Subirana I, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76:2712–2724. doi: 10.1016/j.jacc.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 91.Vekic J, Zeljkovic A, Stefanovic A, et al. Obesity and dyslipidemia. Metabolism. 2019;92:71–81. doi: 10.1016/j.metabol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 92.Athyros VG, Doumas M, Imprialos KP, et al. Diabetes and lipid metabolism. Hormones (Athens) 2018;17:61–67. doi: 10.1007/s42000-018-0014-8. [DOI] [PubMed] [Google Scholar]
- 93.Theofilis P, Vordoni A, Koukoulaki M, et al. Dyslipidemia in chronic kidney disease: contemporary concepts and future therapeutic perspectives. Am J Nephrol. 2021;52:693–701. doi: 10.1159/000518456. [DOI] [PubMed] [Google Scholar]
- 94.Trinder M, Francis GA, Brunham LR. Association of monogenic vs polygenic hypercholesterolemia with risk of atherosclerotic cardiovascular disease. JAMA Cardiol. 2020;5:390. doi: 10.1001/jamacardio.2019.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Health Promotion Administration Ministry of Health and Welfare. Adult Smoking Behavior Survey 2021-2022. Accessed September 25, 2023;https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=1718&pid=9913 [Google Scholar]
- 96.Rigotti NA, Clair C. Managing tobacco use: the neglected cardiovascular disease risk factor. Eur Heart J. 2013;34:3259–3267. doi: 10.1093/eurheartj/eht352. [DOI] [PubMed] [Google Scholar]
- 97.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2018;72:3332–3365. doi: 10.1016/j.jacc.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 99.Winnicka L, Shenoy MA. EVALI and the pulmonary toxicity of electronic cigarettes: a review. J Gen Intern Med. 2020;35:2130–2135. doi: 10.1007/s11606-020-05813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Su VY, Chen WC, Yu WK, et al. The main e-cigarette component vegetable glycerin enhances neutrophil migration and fibrosis in endotoxin-induced lung injury via p38 MAPK activation. Respir Res. 2023;24:9. doi: 10.1186/s12931-022-02307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.CDC 2014 Surgeon General’s Report: The Health Consequences of Smoking—50 Years of Progress. Accessed September 25, 2023;https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm [Google Scholar]
- 102.GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.World Health Organization. WHO Global Report: Mortality Attributable to Tobacco. ed. 2012. Accessed November 4, 2018. [Google Scholar]
- 104.Health Promotion Administration Ministry of Health and Welfare Adult Smoking Behavior Surveillance System and tobacco control. Accessed September 25, 2023;https://www.hpa.gov.tw/Pages/List.aspx?nodeid=41 [Google Scholar]
- 105.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 106.Wen CP, Tsai SP, Chen CJ, et al. The mortality risks of smokers in Taiwan: part I: cause-specific mortality. Prev Med. 2004;39:528–535. doi: 10.1016/j.ypmed.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 107.Barua RS, Ambrose JA, Eales-Reynolds LJ, et al. Heavy and light cigarette smokers have similar dysfunction of endothelial vasoregulatory activity: an in vivo and in vitro correlation. J Am Coll Cardiol. 2002;39:1758–1763. doi: 10.1016/s0735-1097(02)01859-4. [DOI] [PubMed] [Google Scholar]
- 108.Hackshaw A, Morris JK, Boniface S, et al. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360:j5855. doi: 10.1136/bmj.j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647–658. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 110.Lv X, Sun J, Bi Y, et al. Risk of all-cause mortality and cardiovascular disease associated with secondhand smoke exposure: a systematic review and meta-analysis. Int J Cardiol. 2015;199:106–115. doi: 10.1016/j.ijcard.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 111.Wang JB, Olgin JE, Nah G, et al. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One. 2018;13:e0198681. doi: 10.1371/journal.pone.0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alzahrani T, Pena I, Temesgen N, et al. Association between electronic cigarette use and myocardial infarction. Am J Prev Med. 2018;55:455–461. doi: 10.1016/j.amepre.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050–1052. doi: 10.1001/jamainternmed.2017.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simonavicius E, McNeill A, Shahab L, et al. Heat-not-burn tobacco products: a systematic literature review. Tob Control. 2019;28:582–594. doi: 10.1136/tobaccocontrol-2018-054419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qasim H, Alarabi AB, Alzoubi KH, et al. The effects of hookah/waterpipe smoking on general health and the cardiovascular system. Environ Health Prev Med. 2019;24:58. doi: 10.1186/s12199-019-0811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhatnagar A, Maziak W, Eissenberg T, et al. Water pipe (hookah) smoking and cardiovascular disease risk: a scientific statement from the American Heart Association. Circulation. 2019;139:e917–e936. doi: 10.1161/CIR.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McNeill A, Gravely S, Hitchman SC, et al. Tobacco packaging design for reducing tobacco use. Cochrane Database Syst Rev. 2017;4:CD011244. doi: 10.1002/14651858.CD011244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mosdøl A, Lidal IB, Straumann GH, et al. Targeted mass media interventions promoting healthy behaviours to reduce risk of non-communicable diseases in adult, ethnic minorities. Cochrane Database Syst Rev. 2017;2:CD011683. doi: 10.1002/14651858.CD011683.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frazer K, Callinan JE, McHugh J, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev. 2016;2:CD005992. doi: 10.1002/14651858.CD005992.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parks MJ, Patrick ME, Levy DT, et al. Tobacco taxation and its prospective impact on disparities in smoking initiation and progression among young adults. J Adolesc Health. 2021;68:765–772. doi: 10.1016/j.jadohealth.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Health Promotion Administration Ministry of Health and Welfare. Adult Smoking Behavior Surveillance System and tobacco control: Taiwan Tobacco Hazards Prevention Act. Accessed September 26, 2023;https://www.hpa.gov.tw/Pages/List.aspx?nodeid=4721 [Google Scholar]
- 122.Health Promotion Administration Ministry of Health and Welfare. Definitions of overweight and obesity. Accessed September 26, 2023;https://www.hpa.gov.tw/Pages/List.aspx?nodeid=1757 [Google Scholar]
- 123.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang HC, Yang HC, Chang HY, et al. Morbid obesity in Taiwan: prevalence, trends, associated social demographics, and lifestyle factors. PLoS One. 2017;12:e0169577. doi: 10.1371/journal.pone.0169577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yeh CJ, Chang HY, Pan WH. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993-1996 to NAHSIT 2005-2008. Asia Pac J Clin Nutr. 2011;20:292–300. [PubMed] [Google Scholar]
- 127.Bianchettin RG, Lavie CJ, Lopez-Jimenez F. Challenges in cardiovascular evaluation and management of obese patients: JACC state-of-the-art review. J Am Coll Cardiol. 2023;81:490–504. doi: 10.1016/j.jacc.2022.11.031. [DOI] [PubMed] [Google Scholar]
- 128.Chuang SY, Chang HY, Tsai TY, et al. Isolated systolic hypertension and central blood pressure: implications from the national nutrition and health survey in Taiwan. J Clin Hypertens (Greenwich) 2021;23:656–664. doi: 10.1111/jch.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang JS, Yeh WC, Tsai YW, et al. The relationship between atherogenic index of plasma and obesity among adults in Taiwan. Int J Environ Res Public Health. 2022;19:14864. doi: 10.3390/ijerph192214864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yeh TL, Hsu HY, Tsai MC, et al. Association between metabolically healthy obesity/overweight and cardiovascular disease risk: a representative cohort study in Taiwan. PLoS One. 2021;16:e0246378. doi: 10.1371/journal.pone.0246378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yeh TL, Chen YR, Hsu HY, et al. Cardiovascular disease burden attributable to high body mass index in Taiwan. Acta Cardiol Sin. 2023;39:628–642. doi: 10.6515/ACS.202307_39(4).20221219C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arab JP, Candia R, Zapata R, et al. Management of nonalcoholic fatty liver disease: an evidence-based clinical practice review. World J Gastroenterol. 2014;20:12182–12201. doi: 10.3748/wjg.v20.i34.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The Rational Clinical Examination systematic review. JAMA. 2013;310:731–741. doi: 10.1001/jama.2013.276185. [DOI] [PubMed] [Google Scholar]
- 134.McNellis RJ, Thomas S. Screening for obstructive sleep apnea in adults. Am Fam Physician. 2017;96:123–124. [PubMed] [Google Scholar]
- 135.Pigeyre M, Yazdi FT, Kaur Y, et al. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond) 2016;130:943–986. doi: 10.1042/CS20160136. [DOI] [PubMed] [Google Scholar]
- 136.Motevalli M, Drenowatz C, Tanous DR, et al. Management of childhood obesity-time to shift from generalized to personalized intervention strategies. Nutrients. 2021;13:1200. doi: 10.3390/nu13041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stoner L, Rowlands D, Morrison A, et al. Efficacy of exercise intervention for weight loss in overweight and obese adolescents: meta-analysis and implications. Sports Med. 2016;46:1737–1751. doi: 10.1007/s40279-016-0537-6. [DOI] [PubMed] [Google Scholar]
- 139.Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39:79–132. doi: 10.1210/er.2017-00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brown T, Moore TH, Hooper L, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2019;7:CD001871. doi: 10.1002/14651858.CD001871.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anderson LM, Quinn TA, Glanz K, et al. The effectiveness of worksite nutrition and physical activity interventions for controlling employee overweight and obesity: a systematic review. Am J Prev Med. 2009;37:340–357. doi: 10.1016/j.amepre.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 142.Muntefering C, Fitzpatrick M, Johnson K, et al. Primary prevention interventions for adults at-risk of obesity: an international scoping review. Prev Med. 2023;171:107498. doi: 10.1016/j.ypmed.2023.107498. [DOI] [PubMed] [Google Scholar]
- 143.Huang J, Ma ZF, Zhang Y, et al. Geographical distribution of hyperuricemia in mainland China: a comprehensive systematic review and meta-analysis. Glob Health Res Policy. 2020;5:52. doi: 10.1186/s41256-020-00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 145.Chang HY, Pan WH, Yeh WT, et al. Hyperuricemia and gout in Taiwan: results from the Nutritional and Health Survey in Taiwan (1993-96). J Rheumatol. 2001;28:1640–1646. [PubMed] [Google Scholar]
- 146.Braga F, Pasqualetti S, Ferraro S, et al. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. 2016;54:7–15. doi: 10.1515/cclm-2015-0523. [DOI] [PubMed] [Google Scholar]
- 147.Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:885–892. doi: 10.1002/art.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chuang SY, Chen JH, Yeh WT, et al. Hyperuricemia and increased risk of ischemic heart disease in a large Chinese cohort. Int J Cardiol. 2012;154:316–321. doi: 10.1016/j.ijcard.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 149.Chen JH, Chuang SY, Chen HJ, et al. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 2009;61:225–232. doi: 10.1002/art.24164. [DOI] [PubMed] [Google Scholar]
- 150.Tsai PH, Kuo CF, See LC, et al. Stroke risk in patients with gout: a nationwide retrospective cohort study in Taiwan. J Clin Med. 2022;11:3779. doi: 10.3390/jcm11133779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grayson PC, Kim SY, LaValley M, et al. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goli P, Riahi R, Daniali SS, et al. Association of serum uric acid concentration with components of pediatric metabolic syndrome: a systematic review and meta-analysis. J Res Med Sci. 2020;25:43. doi: 10.4103/jrms.JRMS_733_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li L, Yang C, Zhao Y, et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tian T, Wang Y, Xie W, et al. Associations of serum uric acid with clustering of cardiovascular risk factors and a 10-year atherosclerotic cardiovascular disease risk score in Jiangsu adults, China. Diabetes Metab Syndr Obes. 2021;14:3447–3460. doi: 10.2147/DMSO.S323917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen F, Yuan L, Xu T, et al. Association of hyperuricemia with 10-year atherosclerotic cardiovascular disease risk among Chinese adults and elders. Int J Environ Res Public Health. 2022;19:6713. doi: 10.3390/ijerph19116713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Helget LN, Mikuls TR. Environmental triggers of hyperuricemia and gout. Rheum Dis Clin North Am. 2022;48:891–906. doi: 10.1016/j.rdc.2022.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Danve A, Sehra ST, Neogi T. Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol. 2021;35:101723. doi: 10.1016/j.berh.2021.101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang J, Chen S, Zhao J, et al. Association between nutrient patterns and hyperuricemia: mediation analysis involving obesity indicators in the NHANES. BMC Public Health. 2022;22:1981. doi: 10.1186/s12889-022-14357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen IC, Chao TH, Tsai WC, et al. Rosiglitazone reduces plasma levels of inflammatory and hemostatic biomarkers and improves global endothelial function in habitual heavy smokers without diabetes mellitus or metabolic syndrome. J Formos Med Assoc. 2010;109:113–119. doi: 10.1016/S0929-6646(10)60031-9. [DOI] [PubMed] [Google Scholar]
- 160.Health Promotion Administration. Metabolic syndrome. Accessed October 16, 2023;https://www.hpa.gov.tw/pages/list.aspx?nodeid=221 [Google Scholar]
- 161.Lin RT, Lee WJ, Jeng CY, et al. Metabolic syndrome and its contribution to coronary artery disease in non-diabetic subjects. J Formos Med Assoc. 2004;103:317–320. [PubMed] [Google Scholar]
- 162.Chien KL, Hsu HC, Sung FC, et al. Metabolic syndrome as a risk factor for coronary heart disease and stroke: an 11-year prospective cohort in Taiwan community. Atherosclerosis. 2007;194:214–221. doi: 10.1016/j.atherosclerosis.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 163.Chen HJ, Bai CH, Yeh WT, et al. Influence of metabolic syndrome and general obesity on the risk of ischemic stroke. Stroke. 2006;37:1060–1064. doi: 10.1161/01.STR.0000206458.58142.f3. [DOI] [PubMed] [Google Scholar]
- 164.Chen YC, Sun CA, Yang T, et al. Impact of metabolic syndrome components on incident stroke subtypes: a Chinese cohort study. J Hum Hypertens. 2014;28:689–693. doi: 10.1038/jhh.2013.152. [DOI] [PubMed] [Google Scholar]
- 165.Ma X, Zhu S. Metabolic syndrome in the prevention of cardiovascular diseases and diabetes--still a matter of debate? Eur J Clin Nutr. 2013;67:518–521. doi: 10.1038/ejcn.2013.24. [DOI] [PubMed] [Google Scholar]
- 166.Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3:1–58. doi: 10.1002/cphy.c110062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 168.Gallardo-Alfaro L, Bibiloni MDM, Mascaró CM, et al. Leisure-time physical activity, sedentary behaviour and diet quality are associated with metabolic syndrome severity: the PREDIMED-Plus study. Nutrients. 2020;12:1013. doi: 10.3390/nu12041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cano-Ibáñez N, Gea A, Martínez-González MA, et al. Dietary diversity and nutritional adequacy among an older Spanish population with metabolic syndrome in the PREDIMED-Plus study: a cross-sectional analysis. Nutrients. 2019;11:958. doi: 10.3390/nu11050958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chang CF, Wu YC, Lai CH, et al. Effects of physical fitness training on metabolic syndrome among military personnel in Taiwan. BMJ Mil Health. 2023;169:e15–e19. doi: 10.1136/bmjmilitary-2020-001761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chang SH, Chen MC, Chien NH, et al. Effectiveness of community-based exercise intervention programme in obese adults with metabolic syndrome. J Clin Nurs. 2016;25:2579–2589. doi: 10.1111/jocn.13301. [DOI] [PubMed] [Google Scholar]
- 172.Health Promotion Administration. Metabolic Syndrome Study Manual. Accessed October 16, 2023;https://www.hpa.gov.tw/File/Attach/6681/File_6242.pdf [Google Scholar]
- 173.Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40:832–838. doi: 10.2337/dc16-1769. [DOI] [PubMed] [Google Scholar]
- 174.Metser G, Bradley C, Moise N, et al. Gaps and disparities in primary prevention statin prescription during outpatient care. Am J Cardiol. 2021;161:36–41. doi: 10.1016/j.amjcard.2021.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lindley KJ, Aggarwal NR, Briller JE, et al. Socioeconomic determinants of health and cardiovascular outcomes in women: JACC review topic of the week. J Am Coll Cardiol. 2021;78:1919–1929. doi: 10.1016/j.jacc.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 176.Jain V, Al Rifai M, Turpin R, et al. Evaluation of factors underlying sex-based disparities in cardiovascular care in adults with self-reported premature atherosclerotic cardiovascular disease. JAMA Cardiol. 2022;7:341–345. doi: 10.1001/jamacardio.2021.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee Y, Lin RS, Sung FC, et al. Chin-Shan Community Cardiovascular Cohort in Taiwan-baseline data and five-year follow-up morbidity and mortality. J Clin Epidemiol. 2000;53:838–846. doi: 10.1016/s0895-4356(00)00198-0. [DOI] [PubMed] [Google Scholar]
- 178.Ford ES, Giles WH, Mokdad AH. The distribution of 10-year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43:1791–1796. doi: 10.1016/j.jacc.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 179.Lloyd-Jones DM, Wilson PW, Larson MG, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 180.Cooney MT, Dudina AL, Graham IM. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009;54:1209–1227. doi: 10.1016/j.jacc.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 181.Cooney MT, Dudina A, D'agostino R, et al. Cardiovascular risk-estimation systems in primary prevention: do they differ? Do they make a difference? Can we see the future? Circulation. 2010;122:300–310. doi: 10.1161/CIRCULATIONAHA.109.852756. [DOI] [PubMed] [Google Scholar]
- 182.Freaney PM, Khan SS, Lloyd-Jones DM, et al. The role of sex-specific risk factors in the risk assessment of atherosclerotic cardiovascular disease for primary prevention in women. Curr Atheroscler Rep. 2020;22:46. doi: 10.1007/s11883-020-00864-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bergström G, Persson M, Adiels M, et al. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation. 2021;144:916–929. doi: 10.1161/CIRCULATIONAHA.121.055340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang X, Li J, Hu D, et al. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: the China-PAR project (Prediction for ASCVD Risk in China). Circulation. 2016;134:1430–1440. doi: 10.1161/CIRCULATIONAHA.116.022367. [DOI] [PubMed] [Google Scholar]
- 185.Chien KL. Mini-review of the Chin-Shan Community Cardiovascular Cohort Study in population health research in Taiwan. Acta Cardiol Sin. 2017;33:226–232. doi: 10.6515/ACS20161021A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bigeh A, Shekar C, Gulati M. Sex differences in coronary artery calcium and long-term CV mortality. Curr Cardiol Rep. 2020;22:21. doi: 10.1007/s11886-020-1267-9. [DOI] [PubMed] [Google Scholar]
- 187.Young L, Cho L. Unique cardiovascular risk factors in women. Heart. 2019;105:1656–1660. doi: 10.1136/heartjnl-2018-314268. [DOI] [PubMed] [Google Scholar]
- 188.Wong ND, Budoff MJ, Ferdinand K, et al. Atherosclerotic cardiovascular disease risk assessment: an American Society for Preventive Cardiology clinical practice statement. Am J Prev Cardiol. 2022;10:100335. doi: 10.1016/j.ajpc.2022.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.German CA, Baum SJ, Ferdinand KC, et al. Defining preventive cardiology: a clinical practice statement from the American Society for Preventive Cardiology. Am J Prev Cardiol. 2022;12:100432. doi: 10.1016/j.ajpc.2022.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li WQ, Han JL, Manson JE, et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. 2012;166:811–818. doi: 10.1111/j.1365-2133.2011.10774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barish R, Lynce F, Unger K, et al. Management of cardiovascular disease in women with breast cancer. Circulation. 2019;139:1110–1120. doi: 10.1161/CIRCULATIONAHA.118.039371. [DOI] [PubMed] [Google Scholar]
- 192.Kassaian SE, Gandhi B, Barac A. Cardio-oncology: implications for clinical practice for women. Curr Cardiol Rep. 2022;24:1685–1698. doi: 10.1007/s11886-022-01779-1. [DOI] [PubMed] [Google Scholar]
- 193.Lykke JA, Langhoff-Roos J, Sibai BM, et al. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 194.Skjaerven R, Wilcox AJ, Klungsøyr K, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677. doi: 10.1136/bmj.e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kuo YL, Chan TF, Wu CY, et al. Preeclampsia-eclampsia and future cardiovascular risk among women in Taiwan. Taiwan J Obstet Gynecol. 2018;57:364–369. doi: 10.1016/j.tjog.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 196.Grandi SM, Filion KB, Yoon S, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748. [DOI] [PubMed] [Google Scholar]
- 197.Riise HKR, Sulo G, Tell GS, et al. Association between gestational hypertension and risk of cardiovascular disease among 617,589 Norwegian women. J Am Heart Assoc. 2018;7:e008337. doi: 10.1161/JAHA.117.008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Grandi SM, Reynier P, Platt RW, et al. The timing of onset of hypertensive disorders in pregnancy and the risk of incident hypertension and cardiovascular disease. Int J Cardiol. 2018;270:273–275. doi: 10.1016/j.ijcard.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 200.Timpka S, Markovitz A, Schyman T, et al. Midlife development of type 2 diabetes and hypertension in women by history of hypertensive disorders of pregnancy. Cardiovasc Diabetol. 2018;17:124. doi: 10.1186/s12933-018-0764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–914. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 202.Claesson R, Ignell C, Shaat N, et al. HbA1c as a predictor of diabetes after gestational diabetes mellitus. Prim Care Diabetes. 2017;11:46–51. doi: 10.1016/j.pcd.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 203.Parikh NI, Gonzalez JM, Anderson CaM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–e916. doi: 10.1161/CIR.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 204.Hansen AL, Søndergaard MM, Hlatky MA, et al. Adverse pregnancy outcomes and incident heart failure in the women’s health initiative. JAMA Netw Open. 2021;4:e2138071. doi: 10.1001/jamanetworkopen.2021.38071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Agarwala A, Michos ED, Samad Z, et al. The use of sex-specific factors in the assessment of women’s cardiovascular risk. Circulation. 2020;41:592–599. doi: 10.1161/CIRCULATIONAHA.119.043429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ding T, Hardiman PJ, Petersen I, et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8:96351–96358. doi: 10.18632/oncotarget.19180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu J, Wu Q, Hao Y, et al. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum Reprod. 2021;36:1108–1119. doi: 10.1093/humrep/deaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shaw LJ, Bairey Merz CN, Azziz R, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93:1276–1284. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 209.Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 210.Ding DC, Tsai IJ, Wang JH, et al. Coronary artery disease risk in young women with polycystic ovary syndrome. Oncotarget. 2018;9:8756–8764. doi: 10.18632/oncotarget.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hong JS, Yi SW, Kang HC, et al. Age at menopause and cause-specific mortality in South Korean women: Kangwha Cohort Study. Maturitas. 2007;56:411–419. doi: 10.1016/j.maturitas.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 212.Zhao L, Zhu Z, Lou H, et al. Polycystic ovary syndrome (PCOS) and the risk of coronary heart disease (CHD): a meta-analysis. Oncotarget. 2016;7:33715–33721. doi: 10.18632/oncotarget.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wellons M, Ouyang P, Schreiner PJ, et al. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Musunuru K, Kathiresan S. Genetics of common, complex coronary artery disease. Cell. 2019;177:132–145. doi: 10.1016/j.cell.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 215.Inouye M, Abraham G, Nelson CP, et al. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72:1883–1893. doi: 10.1016/j.jacc.2018.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sun L, Pennells L, Kaptoge S, et al. Polygenic risk scores in cardiovascular risk prediction: a cohort study and modelling analyses. PLoS Med. 2021;18:e1003498. doi: 10.1371/journal.pmed.1003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018;19:581–590. doi: 10.1038/s41576-018-0018-x. [DOI] [PubMed] [Google Scholar]
- 219.Lambert SA, Gil L, Jupp S, et al. The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat Genet. 2021;53:420–425. doi: 10.1038/s41588-021-00783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wand H, Lambert SA, Tamburro C, et al. Improving reporting standards for polygenic scores in risk prediction studies. Nature. 2021;591:211–219. doi: 10.1038/s41586-021-03243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Elliott J, Bodinier B, Bond TA, et al. Predictive accuracy of a polygenic risk score-enhanced prediction model vs a clinical risk score for coronary artery disease. JAMA. 2020;323:636–645. doi: 10.1001/jama.2019.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mosley JD, Gupta DK, Tan J, et al. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA. 2020;323:627–635. doi: 10.1001/jama.2019.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28:R133–R142. doi: 10.1093/hmg/ddz187. [DOI] [PubMed] [Google Scholar]
- 224.Lu X, Liu Z, Cui Q, et al. A polygenic risk score improves risk stratification of coronary artery disease: a large-scale prospective Chinese cohort study. Eur Heart J. 2022;43:1702–1711. doi: 10.1093/eurheartj/ehac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Levin MG, Rader DJ. Polygenic risk scores and coronary artery disease: ready for prime time? Circulation. 2020;141:637–640. doi: 10.1161/CIRCULATIONAHA.119.044770. [DOI] [PubMed] [Google Scholar]
- 226.Bachmann JM, Willis BL, Ayers CR, et al. Association between family history and coronary heart disease death across long-term follow-up in men: the Cooper Center Longitudinal Study. Circulation. 2012;125:3092–3098. doi: 10.1161/CIRCULATIONAHA.111.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sadeghi R, Adnani N, Erfanifar A, et al. Premature coronary heart disease and traditional risk factors-can we do better? Int Cardiovasc Res J. 2013;7:46–50. [PMC free article] [PubMed] [Google Scholar]
- 228.Tikkanen E, Havulinna AS, Palotie A, et al. Genetic risk prediction and a 2-stage risk screening strategy for coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:2261–2266. doi: 10.1161/ATVBAHA.112.301120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tamosiunas A, Radisauskas R, Klumbiene J, et al. The prognostic value of family history for the estimation of cardiovascular mortality risk in men: results from a long-term cohort study in Lithuania. PLoS One. 2015;10:e0143839. doi: 10.1371/journal.pone.0143839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sivapalaratnam S, Boekholdt SM, Trip MD, et al. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010;96:1985–1989. doi: 10.1136/hrt.2010.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.van Dis I, Geleijnse JM, Kromhout D, et al. Do obesity and parental history of myocardial infarction improve cardiovascular risk prediction? Eur J Prev Cardiol. 2013;20:793–799. doi: 10.1177/2047487312444233. [DOI] [PubMed] [Google Scholar]
- 233.Weijmans M, van der Graaf Y, Reitsma JB, et al. Paternal or maternal history of cardiovascular disease and the risk of cardiovascular disease in offspring. A systematic review and meta-analysis. Int J Cardiol. 2015;179:409–416. doi: 10.1016/j.ijcard.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 234.Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;16;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kuo HW, Tsai SS, Tiao MM, et al. Epidemiological features of CKD in Taiwan. Am J Kidney Dis. 2007;49:46–55. doi: 10.1053/j.ajkd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 236.NIH NIDDK USRDS. 2023 Annual Data Report. Accessed December 19, 2023;https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/11-international-comparisons [Google Scholar]
- 237.Cai Q, Mukku VK, Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. 2013;9:331–339. doi: 10.2174/1573403X10666140214122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1823–1838. doi: 10.1016/j.jacc.2019.08.1017. [DOI] [PubMed] [Google Scholar]
- 239.Hsieh MC, Hsiao JY, Tien KJ, et al. Chronic kidney disease as a risk factor for coronary artery disease in Chinese with type 2 diabetes. Am J Nephrol. 2008;28:317–323. doi: 10.1159/000111388. [DOI] [PubMed] [Google Scholar]
- 240.Chen YC, Su YC, Lee CC, et al. Chronic kidney disease itself is a causal risk factor for stroke beyond traditional cardiovascular risk factors: a nationwide cohort study in Taiwan. PLoS One. 2012;7:e36332. doi: 10.1371/journal.pone.0036332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cherng YG, Lin CS, Shih CC, et al. Stroke risk and outcomes in patients with chronic kidney disease or end-stage renal disease: two nationwide studies. PLoS One. 2018;13:e0191155. doi: 10.1371/journal.pone.0191155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hsieh MC, Tien KJ, Perng DS, et al. Diabetic nephropathy and risk factors for peripheral artery disease in Chinese with type 2 diabetes mellitus. Metabolism. 2009;58:504–509. doi: 10.1016/j.metabol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 243.Chen IC, Yu CC, Wu YH, et al. Elevated neutrophil-to-lymphocyte ratio predicts intermediate-term outcomes in patients who have advanced chronic kidney disease with peripheral artery disease receiving percutaneous transluminal angioplasty. Acta Cardiol Sin. 2016;32:532–541. doi: 10.6515/ACS20150731D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matsushita K, Kwak L, Ballew SH, et al. Chronic kidney disease measures and the risk of abdominal aortic aneurysm. Atherosclerosis. 2018;279:107–113. doi: 10.1016/j.atherosclerosis.2018.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wu CC, Chou AH, Lin YS, et al. Late outcomes of endovascular aortic stent graft therapy in patients with chronic kidney disease. Medicine (Baltimore) 2020;11:e22157. doi: 10.1097/MD.0000000000022157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99:34–47. doi: 10.1016/j.kint.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 247.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vallianou NG, Mitesh S, Gkogkou A, et al. Chronic kidney disease and cardiovascular disease: is there any relationship? Curr Cardiol Rev. 2019;15:55–63. doi: 10.2174/1573403X14666180711124825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.The Taiwan Society of Nephrology. Accessed October 20, 2023;https://www.tsn.org.tw/guide.html [Google Scholar]
- 250.Kelly JT, Su G, Zhang L, et al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol. 2021;32:239–253. doi: 10.1681/ASN.2020030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Foster MC, Hwang SJ, Massaro JM, et al. Lifestyle factors and indices of kidney function in the Framingham Heart Study. Am J Nephrol. 2015;41:267–274. doi: 10.1159/000430868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fan Z, Yun J, Yu S, et al. Alcohol consumption can be a "double-edged sword" for chronic kidney disease patients. Med Sci Monit. 2019;25:7059–7072. doi: 10.12659/MSM.916121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lee HH, Lee H, Townsend RR, et al. Cardiovascular implications of the 2021 KDIGO blood pressure guideline for adults with chronic kidney disease. J Am Coll Cardiol. 2022;79:1675–1686. doi: 10.1016/j.jacc.2022.02.040. [DOI] [PubMed] [Google Scholar]
- 254.Hardy ST, Zeng D, Kshirsagar AV, et al. Primary prevention of chronic kidney disease through population-based strategies for blood pressure control: The ARIC study. J Clin Hypertens (Greenwich) 2018;20:1018–1026. doi: 10.1111/jch.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chiang CE, Ueng KC, Chao TH, et al. 2020 consensus of Taiwan Society of Cardiology on the pharmacological management of patients with type 2 diabetes and cardiovascular diseases. J Chin Med Assoc. 2020;83:587–621. doi: 10.1097/JCMA.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 257.Chiang CE, Ueng KC, Chao TH, et al. 2021 consensus pathway of the Taiwan Society of Cardiology on novel therapy for type 2 diabetes. JACC Asia. 2021;1:129–146. doi: 10.1016/j.jacasi.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Harper CR, Jacobson TA. Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol. 2008;51:2375–2384. doi: 10.1016/j.jacc.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 259.Vogt L, Bangalore S, Fayyad R, et al. Atorvastatin has a dose-dependent beneficial effect on kidney function and associated cardiovascular outcomes: post hoc analysis of 6 double-blind randomized controlled trials. J Am Heart Assoc. 2019;8:e010827. doi: 10.1161/JAHA.118.010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Díaz-López A, Becerra-Tomás N, Ruiz V, et al. Effect of an intensive weight-loss lifestyle intervention on kidney function: a randomized controlled trial. Am J Nephrol. 2021;52:45–58. doi: 10.1159/000513664. [DOI] [PubMed] [Google Scholar]
- 261.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 262.Lin YC, Lai YJ, Lin YC, et al. Effect of weight loss on the estimated glomerular filtration rates of obese patients at risk of chronic kidney disease: the RIGOR-TMU study. J Cachexia Sarcopenia Muscle. 2019;10:756–766. doi: 10.1002/jcsm.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kerkhof GA. Epidemiology of sleep and sleep disorders in The Netherlands. Sleep Med. 2017;30:229–239. doi: 10.1016/j.sleep.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 264.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 265.Jordan AS, Mcsharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144:e56–e67. doi: 10.1161/CIR.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 267.Lee PL, Wu YW, Cheng HM, et al. Recommended assessment and management of sleep disordered breathing in patients with atrial fibrillation, hypertension and heart failure: Taiwan Society of Cardiology/Taiwan Society of Sleep Medicine/Taiwan Society of Pulmonary and Critical Care Medicine joint consensus statement. J Formos Med Assoc. 2024;123:159–178. doi: 10.1016/j.jfma.2023.08.024. [DOI] [PubMed] [Google Scholar]
- 268.Cheng Y, Du CL, Hwang JJ, et al. Working hours, sleep duration and the risk of acute coronary heart disease: a case-control study of middle-aged men in Taiwan. Int J Cardiol. 2014;171:419–422. doi: 10.1016/j.ijcard.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 269.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.German CA, Baum SJ, Ferdinand KC, et al. Defining preventive cardiology: a clinical practice statement from the American Society for Preventive Cardiology. Am J Prev Cardiol. 2022;12:100432. doi: 10.1016/j.ajpc.2022.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yao CA, Chen IL, Chen CY, et al. Association between wakeup frequency at night and atherogenic dyslipidemia: evidence for sex differences. J Atheroscler Thromb. 2023;30:87–99. doi: 10.5551/jat.63254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 274.Vrints H, Shivalkar B, Hilde H, et al. Cardiovascular mechanisms and consequences of obstructive sleep apnoea. Acta Clin Belg. 2013;68:169–178. doi: 10.2143/ACB.2981. [DOI] [PubMed] [Google Scholar]
- 275.AlSheikh S. Relationship between peripheral arterial diseases and obstructive sleep apnea: a systematic review. Cureus. 2023;15:e35550. doi: 10.7759/cureus.35550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mason RH, Ruegg G, Perkins J, et al. Obstructive sleep apnea in patients with abdominal aortic aneurysms: highly prevalent and associated with aneurysm expansion. Am J Respir Crit Care Med. 2011;183:668–674. doi: 10.1164/rccm.201001-0051OC. [DOI] [PubMed] [Google Scholar]
- 277.Gaisl T, Rejmer P, Roeder M, et al. Obstructive sleep apnoea and the progression of thoracic aortic aneurysm: a prospective cohort study. Eur Respir J. 2021;57:2003322. doi: 10.1183/13993003.03322-2020. [DOI] [PubMed] [Google Scholar]
- 278.Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis. 2011;219:963–968. doi: 10.1016/j.atherosclerosis.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 280.Rémi J, Pollmächer T, Spiegelhalder K, et al. Sleep-related disorders in neurology and psychiatry. Dtsch Arztebl Int. 2019;116:681–688. doi: 10.3238/arztebl.2019.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kalmbach DA, Cuamatzi-Castelan AS, Tonnu CV, et al. Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep. 2018;10:193–201. doi: 10.2147/NSS.S138823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Marti-Soler H, Hirotsu C, Marques-Vidal P, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4:742–748. doi: 10.1016/S2213-2600(16)30075-3. [DOI] [PubMed] [Google Scholar]
- 283.Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33:1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chi L, Comyn FL, Keenan BT, et al. Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep. 2014;37:1689–1698. doi: 10.5665/sleep.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Onen SH, Onen F, Bailly D, et al. Prevention and treatment of sleep disorders through regulation of sleeping habits. Presse Med. 1994;23:485–489. [PubMed] [Google Scholar]
- 288.Gusev E, Sarapultsev A. Atherosclerosis and inflammation: insights from the theory of general pathological processes. Int J Mol Sci. 2023;24:7910. doi: 10.3390/ijms24097910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Whelton SP, Roy P, Astor BC, et al. Elevated high-sensitivity C-reactive protein as a risk marker of the attenuated relationship between serum cholesterol and cardiovascular events at older age. The ARIC Study. Am J Epidemiol. 2013;178:1076–1084. doi: 10.1093/aje/kwt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wilson PW, Nam BH, Pencina M, et al. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165:2473–2478. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- 291.Libby P, Ridker PM, Hansson GK, et al. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and atherogenesis: causal association or simple coincidence? J Clin Periodontol. 2004;31:402–411. doi: 10.1111/j.1600-051X.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 293.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 294.Patil VA, Desai MH. Effect of periodontal therapy on serum C-reactive protein levels in patients with gingivitis and chronic periodontitis: a clinicobiochemical study. J Contemp Dent Pract. 2013;14:233–237. doi: 10.5005/jp-journals-10024-1305. [DOI] [PubMed] [Google Scholar]
- 295.Hussain Bokhari SA, Khan AA, Tatakis DN, et al. Non-surgical periodontal therapy lowers serum inflammatory markers: a pilot study. J Periodontol. 2009;80:1574–1580. doi: 10.1902/jop.2009.090001. [DOI] [PubMed] [Google Scholar]
- 296.Bokhari SA, Khan AA, Butt AK, et al. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. 2012;39:1065–1074. doi: 10.1111/j.1600-051X.2012.01942.x. [DOI] [PubMed] [Google Scholar]
- 297.Liu C, Shi F, Li W, et al. Efficacy of non-surgical periodontal treatment on patients with coronary artery disease: a meta-analysis of randomized controlled trials. Med Oral Patol Oral Cir Bucal. 2022;27:e578–e587. doi: 10.4317/medoral.25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Esberg A, Isehed C, Holmlund A, et al. Serum proteins associated with periodontitis relapse post-surgery: a pilot study. J Periodontol. 2021;92:1805–1814. doi: 10.1002/JPER.21-0089. [DOI] [PubMed] [Google Scholar]
- 299.Munz M, Richter GM, Loos BG, et al. Genome-wide association meta-analysis of coronary artery disease and periodontitis reveals a novel shared risk locus. Sci Rep. 2018;8:13678. doi: 10.1038/s41598-018-31980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Bahekar AA, Singh S, Saha S, et al. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 301.Humphrey LL, Fu R, Buckley DI, et al. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gao S, Tian J, Li Y, et al. Periodontitis and number of teeth in the risk of coronary heart disease: an updated meta-analysis. Med Sci Monit. 2021;27:e930112. doi: 10.12659/MSM.930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Leng Y, Hu Q, Ling Q, et al. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: a meta-analysis. Front Cardiovasc Med. 2023;10:1114927. doi: 10.3389/fcvm.2023.1114927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Guo X, Li X, Liao C, et al. Periodontal disease and subsequent risk of cardiovascular outcome and all-cause mortality: a meta-analysis of prospective studies. PLoS One. 2023;18:e0290545. doi: 10.1371/journal.pone.0290545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Meregildo-Rodriguez ED, Robles-Arce LG, Chunga-Chévez EV, et al. Periodontal disease as a non-traditional risk factor for acute coronary syndrome: a systematic review and meta-analysis. Infez Med. 2022;30:501–515. doi: 10.53854/liim-3004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lee YT, Tsai CF, Yen YC, et al. Periodontitis is a potential risk factor for transient ischemic attack and minor ischemic stroke in young adults: a nationwide population-based cohort study. J Periodontol. 2022;93:1848–1856. doi: 10.1002/JPER.21-0528. [DOI] [PubMed] [Google Scholar]
- 307.Yeh YT, Tseng YS, Wu YL, et al. Risk of peripheral arterial occlusive disease with periodontitis and dental scaling: a nationwide population-based cohort study. Int J Environ Res Public Health. 2022;19:10057. doi: 10.3390/ijerph191610057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Yang S, Zhao LS, Cai C, et al. Association between periodontitis and peripheral artery disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2018;18:141. doi: 10.1186/s12872-018-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Salhi L, Rompen E, Sakalihasan N, et al. Can periodontitis influence the progression of abdominal aortic aneurysm? A systematic review. Angiology. 2019;70:479–491. doi: 10.1177/0003319718821243. [DOI] [PubMed] [Google Scholar]
- 310.Lee YL, Hu HY, Huang N, et al. Dental prophylaxis and periodontal treatment are protective factors to ischemic stroke. Stroke. 2013;44:1026–1030. doi: 10.1161/STROKEAHA.111.000076. [DOI] [PubMed] [Google Scholar]
- 311.Lee YL, Hu HY, Chou P, et al. Dental prophylaxis decreases the risk of acute myocardial infarction: a nationwide population-based study in Taiwan. Clin Interv Aging. 2015;10:175–182. doi: 10.2147/CIA.S67854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Park SY, Kim SH, Kang SH, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J. 2019;40:1138–1145. doi: 10.1093/eurheartj/ehy836. [DOI] [PubMed] [Google Scholar]
- 313.Tonetti MS, Van Dyke TE Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84:S24–S29. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 314.Butt AA, Xiaoqiang W, Budoff M, et al. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tsai MS, Hsu YC, Yu PC, et al. Long-term risk of acute coronary syndrome in hepatitis C virus infected patients without antiviral treatment: a cohort study from an endemic area. Int J Cardiol. 2015;181:27–29. doi: 10.1016/j.ijcard.2014.11.200. [DOI] [PubMed] [Google Scholar]
- 316.Wu VC, Chen TH, Wu M, et al. Comparison of cardiovascular outcomes and all-cause mortality in patients with chronic hepatitis B and C: a 13-year nationwide population-based study in Asia. Atherosclerosis. 2018;269:178–184. doi: 10.1016/j.atherosclerosis.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 317.Hsu YH, Muo CH, Liu CY, et al. Hepatitis C virus infection increases the risk of developing peripheral arterial disease: a 9-year population-based cohort study. J Hepatol. 2015;62:519–525. doi: 10.1016/j.jhep.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 318.Wang CH, Chen CJ, Lee MH, et al. Chronic hepatitis B infection and risk of atherosclerosis-related mortality: a 17-year follow-up study based on 22,472 residents in Taiwan. Atherosclerosis. 2010;211:624–629. doi: 10.1016/j.atherosclerosis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 319.Hsu YC, Lin JT, Ho HJ, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 320.Yu XJ, Yang X, Feng L, et al. Association between Helicobacter pylori infection and angiographically demonstrated coronary artery disease: a meta-analysis. Exp Ther Med. 2017;13:787–793. doi: 10.3892/etm.2017.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lai CY, Yang TY, Lin CL, et al. Helicobacter pylori infection and the risk of acute coronary syndrome: a nationwide retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2015;34:69–74. doi: 10.1007/s10096-014-2207-7. [DOI] [PubMed] [Google Scholar]
- 322.Huang WS, Tseng CH, Lin CL, et al. Helicobacter pylori infection increases subsequent ischemic stroke risk: a nationwide population-based retrospective cohort study. QJM. 2014;107:969–975. doi: 10.1093/qjmed/hcu117. [DOI] [PubMed] [Google Scholar]
- 323.Jiang J, Chen Y, Shi J, et al. Population attributable burden of Helicobacter pylori-related gastric cancer, coronary heart disease, and ischemic stroke in China. Eur J Clin Microbiol Infect Dis. 2017;36:199–212. doi: 10.1007/s10096-016-2810-x. [DOI] [PubMed] [Google Scholar]
- 324.Sawayama Y, Hamada M, Otaguro S, et al. Chronic Helicobacter pylori infection is associated with peripheral arterial disease. J Infect Chemother. 2008;14:250–254. doi: 10.1007/s10156-008-0613-4. [DOI] [PubMed] [Google Scholar]
- 325.Momiyama Y, Ohmori R, Taniguchi H, et al. Association of mycoplasma pneumoniae infection with coronary artery disease and its interaction with chlamydial infection. Atherosclerosis. 2004;176:139–144. doi: 10.1016/j.atherosclerosis.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 326.Yang J, Zhao H, Yuan H, et al. Prevalence and association of mycoplasma infection in the development of coronary artery disease. Braz J Biol. 2021;83:e246385. doi: 10.1590/1519-6984.246385. [DOI] [PubMed] [Google Scholar]
- 327.Chung WS, Hsu WH, Lin CL, et al. Mycoplasma pneumonia increases the risk of acute coronary syndrome: a nationwide population-based cohort study. QJM. 2015;108:697–703. doi: 10.1093/qjmed/hcv015. [DOI] [PubMed] [Google Scholar]
- 328.Chiang CH, Huang CC, Chan WL, et al. Association between mycoplasma pneumonia and increased risk of ischemic stroke: a nationwide study. Stroke. 2011;42:2940–2943. doi: 10.1161/STROKEAHA.110.608075. [DOI] [PubMed] [Google Scholar]
- 329.Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. doi: 10.1136/bmj.h6926. [DOI] [PubMed] [Google Scholar]
- 330.Mosholder AD, Lee JY, Zhou EH, et al. Long-term risk of acute myocardial infarction, stroke, and death with outpatient use of clarithromycin: a retrospective cohort study. Am J Epidemiol. 2018;187:786–792. doi: 10.1093/aje/kwx319. [DOI] [PubMed] [Google Scholar]
- 331.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 332.Hansen PR, Feineis M, Abdulla J. Rheumatoid arthritis patients have higher prevalence and burden of asymptomatic coronary artery disease assessed by coronary computed tomography: a systematic literature review and meta-analysis. Eur J Intern Med. 2019;62:72–79. doi: 10.1016/j.ejim.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 333.Chen YR, Hsieh FI, Chang CC, et al. The effect of rheumatoid arthritis on the risk of cerebrovascular disease and coronary artery disease in young adults. J Chin Med Assoc. 2018;81:772–780. doi: 10.1016/j.jcma.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 334.Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439–445. doi: 10.1160/TH15-07-0600. [DOI] [PubMed] [Google Scholar]
- 335.Yuan S, Carter P, Mason AM, et al. Genetic liability to rheumatoid arthritis in relation to coronary artery disease and stroke risk. Arthritis Rheumatol. 2022;74:1638–1647. doi: 10.1002/art.42239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Giachi A, Cugno M, Gualtierotti R. Disease-modifying anti-rheumatic drugs improve the cardiovascular profile in patients with rheumatoid arthritis. Front Cardiovasc Med. 2022;9:1012661. doi: 10.3389/fcvm.2022.1012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hung YM, Lin L, Wang YH, et al. Combination effect of anti-rheumatic medications for coronary artery diseases risk in rheumatoid arthritis: a nationwide population-based cohort study. Curr Med Res Opin. 2019;35:313–320. doi: 10.1080/03007995.2018.1492910. [DOI] [PubMed] [Google Scholar]
- 338.Johnson TM, Sayles HR, Baker JF, et al. Investigating changes in disease activity as a mediator of cardiovascular risk reduction with methotrexate use in rheumatoid arthritis. Ann Rheum Dis. 2021;80:1385–1392. doi: 10.1136/annrheumdis-2021-220125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Yu HH, Hsiung NH, Chiang JH, et al. The risk of coronary artery disease in patients with rheumatoid arthritis using Chinese herbal products and conventional medicine in parallel: a population-based cohort study. BMC Complement Med Ther. 2020;20:100. doi: 10.1186/s12906-020-02894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hung YM, Wang YH, Lin L, et al. Hydroxychloroquine may be associated with reduced risk of coronary artery diseases in patients with rheumatoid arthritis: a nationwide population-based cohort study. Int J Clin Pract. 2018;72:e13095. doi: 10.1111/ijcp.13095. [DOI] [PubMed] [Google Scholar]
- 341.Hsu CY, Su YJ, Chen JF, et al. Patients with rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs at a higher risk of acute coronary syndrome. J Am Heart Assoc. 2021;10:e018290. doi: 10.1161/JAHA.120.018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Chiu CC, Huang CC, Chan WL, et al. Increased risk of ischemic stroke in patients with systemic lupus erythematosus: a nationwide population-based study. Intern Med. 2012;51:17–21. doi: 10.2169/internalmedicine.51.6154. [DOI] [PubMed] [Google Scholar]
- 343.Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with systemic lupus erythematosus: a nationwide population-based cohort study. Medicine (Baltimore) 2015;94:e2121. doi: 10.1097/MD.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Hsieh MC, Chen HH, Chou TY, et al. Association between systemic sclerosis and peripheral arterial disease: a nationwide observation retrospective claim records cohort study in Taiwan. BMJ Open. 2021;11:e048149. doi: 10.1136/bmjopen-2020-048149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Chiang CH, Huang CC, Chan WL, et al. Psoriasis and increased risk of ischemic stroke in Taiwan: a nationwide study. J Dermatol. 2012;39:279–281. doi: 10.1111/j.1346-8138.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- 346.Yeh CB, Yeh LT, Yang SF, et al. Association between psoriasis and peripheral artery occlusive disease: a population-based retrospective cohort study. Front Cardiovasc Med. 2023;10:1136540. doi: 10.3389/fcvm.2023.1136540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Huang WS, Tseng CH, Chen PC, et al. Inflammatory bowel diseases increase future ischemic stroke risk: a Taiwanese population-based retrospective cohort study. Eur J Intern Med. 2014;25:561–565. doi: 10.1016/j.ejim.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 348.Lin TY, Chen YG, Lin CL, et al. Inflammatory bowel disease increases the risk of peripheral arterial disease: a nationwide cohort study. Medicine (Baltimore) 2015;94:e2381. doi: 10.1097/MD.0000000000002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kang JH, Keller JJ, Lin HC. Ischemic bowel disease and risk of stroke: a one-year follow-up study. Int J Stroke. 2014;9:1083–1089. doi: 10.1111/j.1747-4949.2012.00905.x. [DOI] [PubMed] [Google Scholar]
- 350.Lin HL, Muo CH, Lin CY, et al. Incidence of stroke in patients with HIV infection: a population-based study in Taiwan. PLoS One. 2019;14:e0217147. doi: 10.1371/journal.pone.0217147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tsai MS, Li YF, Lin CL, et al. Long-term risk of acute coronary syndrome in patients with cholangitis: a 13-year nationwide cohort study. Eur J Intern Med. 2014;25:444–448. doi: 10.1016/j.ejim.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 352.Wei CY, Chung TC, Chen CH, et al. Gallstone disease and the risk of stroke: a nationwide population-based study. J Stroke Cerebrovasc Dis. 2014;23:1813–1820. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 353.Liao KM, Chen YJ, Shen CW, et al. The influence of influenza virus infections in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022;17:2253–2261. doi: 10.2147/COPD.S378034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sheu JJ, Chiou HY, Kang JH, et al. Tuberculosis and the risk of ischemic stroke: a 3-year follow-up study. Stroke. 2010;41:244–249. doi: 10.1161/STROKEAHA.109.567735. [DOI] [PubMed] [Google Scholar]
- 355.Lee HR, Yoo JE, Choi H, et al. Tuberculosis and risk of ischemic stroke: a nationwide cohort study. Stroke. 2022;53:3401–3409. doi: 10.1161/STROKEAHA.122.039484. [DOI] [PubMed] [Google Scholar]
- 356.Wang SH, Chien WC, Chung CH, et al. Tuberculosis increases the risk of peripheral arterial disease: a nationwide population-based study. Respirology. 2017;22:1670–1676. doi: 10.1111/resp.13117. [DOI] [PubMed] [Google Scholar]
- 357.Hsu CY, Lin YS, Su YJ, et al. Effect of long-term hydroxychloroquine on vascular events in patients with systemic lupus erythematosus: a database prospective cohort study. Rheumatology (Oxford) 2017;56:2212–2221. doi: 10.1093/rheumatology/kex357. [DOI] [PubMed] [Google Scholar]
- 358.Wei CY, Chuang SH, Lin CL, et al. Reduced risk of stroke following cholecystectomy: a nationwide population-based study. J Gastroenterol Hepatol. 2019;34:1992–1998. doi: 10.1111/jgh.14678. [DOI] [PubMed] [Google Scholar]
- 359.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 360.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 361.Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 362.Ridker PM, Paynter NP, Rifai N, et al. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hemingway H, Philipson P, Chen R, et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7:e1000286. doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Lin JS, Evans CV, Johnson E, et al. Nontraditional risk factors in cardiovascular disease risk assessment: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:281–297. doi: 10.1001/jama.2018.4242. [DOI] [PubMed] [Google Scholar]
- 365.Chi CC, Wu YW, Chao TH, et al. 2022 Taiwanese Dermatological Association (TDA), Taiwanese Association for Psoriasis and Skin Immunology (TAPSI), and Taiwan Society of Cardiology (TSOC) joint consensus recommendations for the management of psoriatic disease with attention to cardiovascular comorbidities. J Formos Med Assoc. 2023;122:442–457. doi: 10.1016/j.jfma.2022.10.010. [DOI] [PubMed] [Google Scholar]
- 366.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 367.Drosos GC, Vedder D, Houben E, et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. 2022;81:768–779. doi: 10.1136/annrheumdis-2021-221733. [DOI] [PubMed] [Google Scholar]
- 368.Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69:1–24. doi: 10.15585/mmwr.rr6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Fu TS, Lee CS, Gunnell D, et al. Changing trends in the prevalence of common mental disorders in Taiwan: a 20-year repeated cross-sectional survey. Lancet. 2013;381:235–241. doi: 10.1016/S0140-6736(12)61264-1. [DOI] [PubMed] [Google Scholar]
- 370.https://dep.mohw.gov.tw/DOMHAOH/mp-107.html. Accessed October 23, 2023. [Google Scholar]
- 371.Albus C, Waller C, Fritzsche K, et al. Significance of psycho-social factors in cardiology: update 2018: position paper of the German Cardiac Society. Clin Res Cardiol. 2019;108:1175–1196. doi: 10.1007/s00392-019-01488-w. [DOI] [PubMed] [Google Scholar]
- 372.Rozanski A. Behavioral cardiology: current advances and future directions. J Am Coll Cardiol. 2014;64:100–110. doi: 10.1016/j.jacc.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 373.De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci. 2018;20:31–40. doi: 10.31887/DCNS.2018.20.1/mdehert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–1302. doi: 10.1093/ajh/hpv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Harter M, Baumeister H, Reuter K, et al. Increased 12-month prevalence rates of mental disorders in patients with chronic somatic diseases. Psychother Psychosom. 2007;76:354–360. doi: 10.1159/000107563. [DOI] [PubMed] [Google Scholar]
- 376.Jha MK, Qamar A, Vaduganathan M, et al. Screening and management of depression in patients with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1827–1845. doi: 10.1016/j.jacc.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sun GZ, Ye N, Wu SJ, et al. 10-year ASCVD risk is positively correlated with depressive symptoms in a large general population. BMC Psychiatry. 2019;19:1–6. doi: 10.1186/s12888-019-2114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 379.Mosquera PA, San Sebastian M, Waenerlund AK, et al. Income-related inequalities in cardiovascular disease from mid-life to old age in a Northern Swedish cohort: a decomposition analysis. Soc Sci Med. 2016;149:135–144. doi: 10.1016/j.socscimed.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 380.Liu K, Cedres LB, Stamler J, et al. Relationship of education to major risk factors and death from coronary heart disease, cardiovascular diseases and all causes, findings of three Chicago epidemiologic studies. Circulation. 1982;66:1308–1314. doi: 10.1161/01.cir.66.6.1308. [DOI] [PubMed] [Google Scholar]
- 381.Meneton P, Kesse-Guyot E, Méjean C, et al. Unemployment is associated with high cardiovascular event rate and increased all-cause mortality in middle-aged socially privileged individuals. Int Arch Occup Environ Health. 2015;88:707–716. doi: 10.1007/s00420-014-0997-7. [DOI] [PubMed] [Google Scholar]
- 382.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 383.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sumner JA, Khodneva Y, Muntner P, et al. Effects of concurrent depressive symptoms and perceived stress on cardiovascular risk in low- and high-income participants: findings from the Reasons for Geographical and Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc. 2016;5:1–14. doi: 10.1161/JAHA.116.003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wang JY, Wang CY, Juang SY, et al. Low socioeconomic status increases short-term mortality of acute myocardial infarction despite universal health coverage. Int J Cardiol. 2014;172:82–87. doi: 10.1016/j.ijcard.2013.12.082. [DOI] [PubMed] [Google Scholar]
- 386.Yu TH, Hou YC, Chung KP. Do low-income coronary artery bypass surgery patients have equal opportunity to access excellent quality of care and enjoy good outcome in Taiwan. Int J Equity Health. 2014;13:64. doi: 10.1186/s12939-014-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Cunningham R, Poppe K, Peterson D, et al. Prediction of cardiovascular disease risk among people with severe mental illness: a cohort study. PLoS One. 2019;14:e0221521. doi: 10.1371/journal.pone.0221521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Osborn D, Burton A, Walters K, et al. Primary care management of cardiovascular risk for people with severe mental illnesses: the Primrose research programme including cluster RCT. Southampton (UK): NIHR Journals Library; 2019 Apr. [PubMed] [Google Scholar]
- 389.Arango C, Díaz-Caneja CM, McGorry PD, et al. Preventive strategies for mental health. Lancet Psychiatry. 2018;5:591–604. doi: 10.1016/S2215-0366(18)30057-9. [DOI] [PubMed] [Google Scholar]
- 390.Zschucke E, Gaudlitz K, Ströhle A. Exercise and physical activity in mental disorders: clinical and experimental evidence. J Prev Med Public Health. 2013;46 Suppl 1:S12–S21. doi: 10.3961/jpmph.2013.46.S.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Posadzki P, Parekh S, Glass N. Yoga and qigong in the psychological prevention of mental health disorders: a conceptual synthesis. Chin J Integr Med. 2010;16:80–86. doi: 10.1007/s11655-009-9002-2. [DOI] [PubMed] [Google Scholar]
- 392.Chen CY, Wu SC, Chen LJ, et al. The prevalence of subjective frailty and factors associated with frailty in Taiwan. Arch Gerontol Geriatr. 2010;50 Suppl 1:S43–S47. doi: 10.1016/S0167-4943(10)70012-1. [DOI] [PubMed] [Google Scholar]
- 393.Hwang AC, Chen LY, Tang TC, et al. Transitions in frailty and 4-year mortality risk in Taiwan longitudinal study on aging. J Am Med Dir Assoc. 2023;24:48–56. doi: 10.1016/j.jamda.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 394.Stewart R. Cardiovascular disease and frailty: what are the mechanistic links? Clinical Chemistry. 2019;65:80–86. doi: 10.1373/clinchem.2018.287318. [DOI] [PubMed] [Google Scholar]
- 395.Veronese N, Cereda E, Stubbs B, et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev. 2017;35:63–73. doi: 10.1016/j.arr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol. 2015;65:976–983. doi: 10.1016/j.jacc.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 397.Damluji AA, Chung SE, Xue QL, et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur Heart J. 2021;42:3856–3865. doi: 10.1093/eurheartj/ehab468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Damluji AA, Chung SE, Xue QL, et al. Physical frailty phenotype and the development of geriatric syndromes in older adults with coronary heart disease. Am J Med. 2021;134:662–671. doi: 10.1016/j.amjmed.2020.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Richter D, Guasti L, Walker D, et al. Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur J Prev Cardiol. 2022;29:216–227. doi: 10.1093/eurjpc/zwaa167. [DOI] [PubMed] [Google Scholar]
- 400.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 401.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 402.Jones D, Song X, Mitnitski A, et al. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. 2005;17:465–471. doi: 10.1007/BF03327413. [DOI] [PubMed] [Google Scholar]
- 403.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 405.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 406.Pavasini R, Guralnik J, Brown JC, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Medicine. 2016;14:215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Vaes B, Depoortere D, Van Pottelbergh G, et al. Association between traditional cardiovascular risk factors and mortality in the oldest old: untangling the role of frailty. BMC Geriatr. 2017;17:234. doi: 10.1186/s12877-017-0626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Nascimento CM, Ingles M, Salvador-Pascual A, et al. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019;132:42–49. doi: 10.1016/j.freeradbiomed.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 409.Ni Lochlainn M, Cox NJ, Wilson T, et al. Nutrition and frailty: opportunities for prevention and treatment. Nutrients. 2021;13:2349. doi: 10.3390/nu13072349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Coelho-Junior HJ, Marzetti E, Picca A, et al. Protein intake and frailty: a matter of quantity, quality, and timing. Nutrients. 2020;12:2915. doi: 10.3390/nu12102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Chin MT. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart. 2015;101:253–256. doi: 10.1136/heartjnl-2014-306379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Manisalidis I, Stavropoulou E, Stavropoulos A, et al. Environmental and health impacts of air pollution:a review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. 2021. [PubMed] [Google Scholar]
- 414.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 415.Alexeeff SE, Liao NS, Liu X, et al. Long-term PM2.5 exposure and risks of ischemic heart disease and stroke events: review and meta-analysis. J Am Heart Assoc. 2021;10:e016890. doi: 10.1161/JAHA.120.016890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Atkinson RW, Kang S, Anderson HR, et al. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69:660–665. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Chen SY, Chan CC, Su TC. Particulate and gaseous pollutants on inflammation, thrombosis, and autonomic imbalance in subjects at risk for cardiovascular disease. Environ Pollut. 2017;223:403–408. doi: 10.1016/j.envpol.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 418.Chen TM, Gokhale J, Shofer S, et al. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci. 2007;333:249–256. doi: 10.1097/MAJ.0b013e31803b900f. [DOI] [PubMed] [Google Scholar]
- 419.Chen Z, Liu N, Tang H, et al. Health effects of exposure to sulfur dioxide, nitrogen dioxide, ozone, and carbon monoxide between 1980 and 2019: a systematic review and meta-analysis. Indoor Air. 2022;32:e13170. doi: 10.1111/ina.13170. [DOI] [PubMed] [Google Scholar]
- 420.Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 421.Chen SY, Wu CF, Wu C, et al. Urban fine particulate matter and elements associated with subclinical atherosclerosis in adolescents and young adults. Environ Sci Technol. 2022;56:7266–7274. doi: 10.1021/acs.est.1c06347. [DOI] [PubMed] [Google Scholar]
- 422.Strak M, Weinmayr G, Rodopoulou S, et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ. 2021;374:1904. doi: 10.1136/bmj.n1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Pope CA, 3rd, Turner MC, Burnett RT, et al. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–115. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 424.de Bont J, Jaganathan S, Dahlquist M, et al. Ambient air pollution and cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses. J Intern Med. 2022;291:779–800. doi: 10.1111/joim.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Lelieveld J, Klingmüller K, Pozzer A, et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 2019;40:1590–1596. doi: 10.1093/eurheartj/ehz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Cai S, Wang Y, Zhao B, et al. The impact of the "Air Pollution Prevention and Control Action Plan" on PM2.5 concentrations in Jing-Jin-Ji region during 2012-2020. Sci Total Environ. 2017;580:197–209. doi: 10.1016/j.scitotenv.2016.11.188. [DOI] [PubMed] [Google Scholar]
- 428.Rajagopalan S, Al-Kindi S, Brook R, et al. Air pollution and cardiovascular disease. J Am Coll Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 429.Al-Kindi SG, Brook RD, Biswal S, et al. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. 2020;17:656–672. doi: 10.1038/s41569-020-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Khraishah H, Alahmad B, Ostergard RL, Jr., et al. Climate change and cardiovascular disease: implications for global health. Nat Rev Cardiol. 2022;19:798–812. doi: 10.1038/s41569-022-00720-x. [DOI] [PubMed] [Google Scholar]
- 431.Liu J, Varghese BM, Hansen A, et al. Heat exposure and cardiovascular health outcomes: a systematic review and meta-analysis. Lancet Planet Health. 2022;6:e484–e495. doi: 10.1016/S2542-5196(22)00117-6. [DOI] [PubMed] [Google Scholar]
- 432.Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121:162–180. doi: 10.1161/CIRCRESAHA.117.306458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Meade RD, Akerman AP, Notley SR, et al. Physiological factors characterizing heat-vulnerable older adults: a narrative review. Environ Int. 2020;144:105909. doi: 10.1016/j.envint.2020.105909. [DOI] [PubMed] [Google Scholar]
- 434.Münzel T, Hahad O, Sørensen M, et al. Environmental risk factors and cardiovascular diseases: a comprehensive expert review. Cardiovasc Res. 2022;118:2880–2902. doi: 10.1093/cvr/cvab316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Vaduganathan M, Mensah GA, Turco JV, et al. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 436.Rajagopalan S, Landrigan PJ. Pollution and the heart. N Engl J Med. 2021;385:1881–1892. doi: 10.1056/NEJMra2030281. [DOI] [PubMed] [Google Scholar]
- 437.Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev. 2002;24:190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- 438.Qian Z, He Q, Lin HM, et al. High temperatures enhanced acute mortality effects of ambient particle pollution in the "oven" city of Wuhan, China. Environ Health Perspect. 2008;116:1172–1178. doi: 10.1289/ehp.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Haines A, Kovats RS, Campbell-Lendrum D, et al. Climate change and human health: impacts, vulnerability, and mitigation. Lancet. 2006;367:2101–2109. doi: 10.1016/S0140-6736(06)68933-2. [DOI] [PubMed] [Google Scholar]
- 440.Watts N, Adger WN, Agnolucci P, et al. Health and climate change: policy responses to protect public health. Lancet. 2015;386:1861–1914. doi: 10.1016/S0140-6736(15)60854-6. [DOI] [PubMed] [Google Scholar]
- 441.Franchini M, Mannucci PM. Impact on human health of climate changes. Eur J Intern Med. 2015;26:1–5. doi: 10.1016/j.ejim.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 442.Woodward G, Perkins DM, Brown LE. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos Trans R Soc Lond B Biol Sci. 2010;365:2093–2106. doi: 10.1098/rstb.2010.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Chowdhury R, Ramond A, O'Keeffe LM, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310. doi: 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J Toxicol. 2011;2011:870125. doi: 10.1155/2011/870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wu X, Cobbina SJ, Mao G, et al. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. 2016;23:8244–8259. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- 446.Lee CK, Wu C, Lin CY, et al. Positive association between endothelium-platelet microparticles and urinary concentration of lead and cadmium in adolescents and young adults. Nutrients. 2021;13:2913. doi: 10.3390/nu13092913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Balali-Mood M, Naseri K, Tahergorabi Z, et al. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. 2021;12:643972. doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Lamas GA, Bhatnagar A, Jones MR, et al. Contaminant metals as cardiovascular risk factors: a scientific statement from the American Heart Association. J Am Heart Assoc. 2023;12:e029852. doi: 10.1161/JAHA.123.029852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Lin CY, Lee HL, Hwang YT, et al. Urinary heavy metals, DNA methylation, and subclinical atherosclerosis. Ecotoxicol Environ Saf. 2020;204:111039. doi: 10.1016/j.ecoenv.2020.111039. [DOI] [PubMed] [Google Scholar]
- 450.Lin CY, Huang PC, Wu C, et al. Association between urine lead levels and cardiovascular disease risk factors, carotid intima-media thickness and metabolic syndrome in adolescents and young adults. Int J Hyg Environ Health. 2020;223:248–255. doi: 10.1016/j.ijheh.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 451.Kuo CC, Balakrishnan P, Gribble MO, et al. The association of arsenic exposure and arsenic metabolism with all-cause, cardiovascular and cancer mortality in the Strong Heart Study. Environ Int. 2022;159:107029. doi: 10.1016/j.envint.2021.107029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Tellez-Plaza M, Navas-Acien A, Menke A, et al. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect. 2012;120:1017–1022. doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Navas-Acien A, Selvin E, Sharrett AR, et al. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 454.Navas-Acien A, Guallar E, Silbergeld EK, et al. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Yang AM, Lo K, Zheng TZ, et al. Environmental heavy metals and cardiovascular diseases: status and future direction. Chronic Dis Transl Med. 2020;6:251–259. doi: 10.1016/j.cdtm.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Guo X, Su W, Li N, et al. Association of urinary or blood heavy metals and mortality from all causes, cardiovascular disease, and cancer in the general population: a systematic review and meta-analysis of cohort studies. Environ Sci Pollut Res Int. 2022;29:67483–67503. doi: 10.1007/s11356-022-22353-w. [DOI] [PubMed] [Google Scholar]
- 457.Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015;12:627–642. doi: 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- 458.Podgorski J, Berg M. Global threat of arsenic in groundwater. Science. 2020;368:845–850. doi: 10.1126/science.aba1510. [DOI] [PubMed] [Google Scholar]
- 459.Wu MM, Kuo TL, Hwang YH, et al. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 460.Chen CJ, Chiou HY, Chiang MH, et al. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16:504–510. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 461.Rahaman MS, Rahman MM, Mise N, et al. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ Pollut. 2021;289:117940. doi: 10.1016/j.envpol.2021.117940. [DOI] [PubMed] [Google Scholar]
- 462.Nakahara T, Dweck MR, Narula N, et al. Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imaging. 2017;10:582–593. doi: 10.1016/j.jcmg.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 463.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 464.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 465.Schmermund A, Möhlenkamp S, Stang A, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J. 2002;144:212–218. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 466.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 467.Lehmann N, Erbel R, Mahabadi AA, et al. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR Study (Heinz Nixdorf Recall). Circulation. 2018;137:665–679. doi: 10.1161/CIRCULATIONAHA.116.027034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Ferencik M, Pencina KM, Liu T, et al. Coronary artery calcium distribution is an independent predictor of incident major coronary heart disease events: results from the Framingham Heart Study. Circ Cardiovasc Imaging. 2017;10:e006592. doi: 10.1161/CIRCIMAGING.117.006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 470.Yano Y, O'Donnell CJ, Kuller L, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2:986–994. doi: 10.1001/jamacardio.2017.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Ho JS, Cannaday JJ, Barlow CE, et al. Utility of high-sensitivity C-reactive protein versus coronary artery calcium for the detection of obstructive stenoses in stable patients. Am J Cardiol. 2013;111:328–332. doi: 10.1016/j.amjcard.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 472.Qureshi WT, Rana JS, Yeboah J, et al. Risk stratification for primary prevention of coronary artery disease: roles of C-reactive protein and coronary artery calcium. Curr Cardiol Rep. 2015;17:110. doi: 10.1007/s11886-015-0666-9. [DOI] [PubMed] [Google Scholar]
- 473.Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11:157–168. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 475.Budoff MJ, Lutz SM, Kinney GL, et al. Coronary artery calcium on noncontrast thoracic computerized tomography scans and all-cause mortality. Circulation. 2018;138:2437–2438. doi: 10.1161/CIRCULATIONAHA.118.036835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Hughes-Austin JM, Dominguez A, 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9:152–159. doi: 10.1016/j.jcmg.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198:505–511. doi: 10.2214/AJR.10.5577. [DOI] [PubMed] [Google Scholar]
- 478.Greenland P, Michos ED, Redmond N, et al. Primary prevention trial designs using coronary imaging: a National Heart, Lung, and Blood Institute Workshop. JACC Cardiovasc Imaging. 2021;14:1454–1465. doi: 10.1016/j.jcmg.2020.06.042. [DOI] [PubMed] [Google Scholar]
- 479.Dawson LP, Lum M, Nerleker N, et al. Coronary atherosclerotic plaque regression: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:66–82. doi: 10.1016/j.jacc.2021.10.035. [DOI] [PubMed] [Google Scholar]
- 480.Serruys PW, Kotoku N, Nørgaard BL, et al. Computed tomographic angiography in coronary artery disease. EuroIntervention. 2023;18:e1307–e1327. doi: 10.4244/EIJ-D-22-00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Bergström G, Persson M, Adiels M, et al. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation. 2021;144:916–929. doi: 10.1161/CIRCULATIONAHA.121.055340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Mortensen MB, Dzaye O, Steffensen FH, et al. Impact of plaque burden versus stenosis on ischemic events in patients with coronary atherosclerosis. J Am Coll Cardiol. 2020;76:2803–2813. doi: 10.1016/j.jacc.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 483.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 484.Chow BJ, Small G, Yam Y, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter registry) registry. Arterioscler Thromb Vasc Biol. 2015;35:981–989. doi: 10.1161/ATVBAHA.114.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Muhlestein JB, Lappé DL, Lima JA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312:2234–2243. doi: 10.1001/jama.2014.15825. [DOI] [PubMed] [Google Scholar]
- 486.https://classic.clinicaltrials.gov/ct2/show/ NCT03920176. Accessed October 26, 2023. [Google Scholar]
- 487.Li H, Xu X, Luo B, et al. The predictive value of carotid ultrasonography with cardiovascular risk factors-a "spider" promoting atherosclerosis. Front Cardiovasc Med. 2021;8:706490. doi: 10.3389/fcvm.2021.706490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053–2062. doi: 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Den Ruijter HM, Peters SAE, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 490.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 491.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 493.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 494.Johri AM, Nambi V, Naqvi TZ, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:917–933. doi: 10.1016/j.echo.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 495.Chuang SY, Bai CH, Chen JR, et al. Common carotid end-diastolic velocity and intima-media thickness jointly predict ischemic stroke in Taiwan. Stroke. 2011;42:1338–1344. doi: 10.1161/STROKEAHA.110.605477. [DOI] [PubMed] [Google Scholar]
- 496.Chung H, Jung YH, Kim KH, et al. Carotid artery end-diastolic velocity and future cerebro-cardiovascular events in asymptomatic high risk patients. Korean Circ J. 2016;46:72–78. doi: 10.4070/kcj.2016.46.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Chuang SY, Bai CH, Cheng HM, et al. Common carotid artery end-diastolic velocity is independently associated with future cardiovascular events. Eur J Prev Cardiol. 2016;23:116–124. doi: 10.1177/2047487315571888. [DOI] [PubMed] [Google Scholar]
- 498.Chirinos JA, Segers P, Hughes T, et al. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Lacolley P, Regnault V, Segers P, et al. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev. 2017;97:1555–1617. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 500.Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 501.Li CI, Kardia SL, Liu CS, et al. Metabolic syndrome is associated with change in subclinical arterial stiffness: a community-based Taichung community health study. BMC Public Health. 2011;11:808. doi: 10.1186/1471-2458-11-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.London GM, Blacher J, Pannier B, et al. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 503.Zanoli L, Cannavo M, Rastelli S, et al. Arterial stiffness is increased in patients with inflammatory bowel disease. J Hypertens. 2012;30:1775–1781. doi: 10.1097/HJH.0b013e3283568abd. [DOI] [PubMed] [Google Scholar]
- 504.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 505.Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 506.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 507.Cheng HM, Chuang SY, Sung SH, et al. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol. 2013;62:1780–1787. doi: 10.1016/j.jacc.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Cheng HM, Chuang SY, Sung SH, et al. 2019 consensus of the Taiwan Hypertension Society and Taiwan Society of Cardiology on the clinical application of central blood pressure in the management of hypertension. Acta Cardiol Sin. 2019;35:234–243. doi: 10.6515/ACS.201905_35(3).20190415B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 511.Sehestedt T, Jeppesen J, Hansen TW, et al. Risk stratification with the risk chart from the European Society of Hypertension compared with SCORE in the general population. J Hypertens. 2009;27:2351–2357. doi: 10.1097/HJH.0b013e328330e90a. [DOI] [PubMed] [Google Scholar]
- 512.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Charlton PH, Paliakaitė B, Pilt K, et al. Assessing hemodynamics from the photoplethysmogram to gain insights into vascular age: a review from VascAgeNet. Am J Physiol Heart Circ Physiol. 2022;322:H493–H522. doi: 10.1152/ajpheart.00392.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Kim ED, Ballew SH, Tanaka H, et al. Short-term prognostic impact of arterial stiffness in older adults without prevalent cardiovascular disease. Hypertension. 2019;74:1373–1382. doi: 10.1161/HYPERTENSIONAHA.119.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: establishing normal and reference values. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Vallée A. Arterial stiffness nomogram identification by cluster analysis: a new approach of vascular phenotype modeling. J Clin Hypertens (Greenwich) 2022;24:1415–1426. doi: 10.1111/jch.14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 518.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 519.Ko HJ, Liu CC, Hsu PJ, et al. Risk assessment indicators and brachial-ankle pulse wave velocity to predict atherosclerotic cardiovascular disease. Medicine. 2022;101:e29609. doi: 10.1097/MD.0000000000029609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69:1045–1052. doi: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 521.Ninomiya T, Kojima I, Doi Y, et al. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: the Hisayama Study. J Hypertens. 2013;31:477–483. doi: 10.1097/HJH.0b013e32835c5c23. [DOI] [PubMed] [Google Scholar]
- 522.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012;60:556–562. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 523.Wilkinson IB, Mäki-Petäjä KM, Mitchell GF. Uses of arterial stiffness in clinical practice. Arterioscler Thromb Vasc Biol. 2020;40:1063–1067. doi: 10.1161/ATVBAHA.120.313130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Cooper JN, Buchanich JM, Youk A, et al. Reductions in arterial stiffness with weight loss in overweight and obese young adults: potential mechanisms. Atherosclerosis. 2012;223:485–490. doi: 10.1016/j.atherosclerosis.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Saz-Lara A, Martínez-Vizcaíno V, Sequí-Domínguez I, et al. The effect of smoking and smoking cessation on arterial stiffness: a systematic review and meta-analysis. Eur J Cardiovasc Nurs. 2022;21:297–306. doi: 10.1093/eurjcn/zvab102. [DOI] [PubMed] [Google Scholar]
- 526.Kresnajati S, Lin YY, Mündel T, et al. Changes in arterial stiffness in response to various types of exercise modalities: a narrative review on physiological and endothelial senescence perspectives. Cells. 2022;11:3544. doi: 10.3390/cells11223544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.D'Elia L, Galletti F, La Fata E, et al. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta-analysis of the randomized controlled trials. J Hypertens. 2018;36:734–743. doi: 10.1097/HJH.0000000000001604. [DOI] [PubMed] [Google Scholar]
- 528.Upala S, Wirunsawanya K, Jaruvongvanich V, et al. Effects of statin therapy on arterial stiffness: a systematic review and meta-analysis of randomized controlled trial. Int J Cardiol. 2017;227:338–341. doi: 10.1016/j.ijcard.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 529.Alidadi M, Montecucco F, Jamialahmadi T, et al. Beneficial effect of statin therapy on arterial stiffness. Biomed Res Int. 2021;2021:5548310. doi: 10.1155/2021/5548310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Zhou YF, Wang Y, Wang G, et al. Association between statin use and progression of arterial stiffness among adults with high atherosclerotic risk. JAMA Netw Open. 2022;5:e2218323. doi: 10.1001/jamanetworkopen.2022.18323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Dudenbostel T, Glasser SP. Effects of antihypertensive drugs on arterial stiffness. Cardiol Rev. 2012;20:259–263. doi: 10.1097/CRD.0b013e31825d0a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Budoff MJ, Alpert B, Chirinos JA, et al. Clinical applications measuring arterial stiffness: an expert consensus for the application of cardio-ankle vascular index. Am J Hypertens. 2022;35:441–453. doi: 10.1093/ajh/hpab178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Lin HH, Wang CS, Lin JL, et al. Effects of anti-hypertensive monotherapy with either calcium channel blocker or angiotensin receptor blocker on arterial stiffness, central hemodynamics, and ventriculo-arterial coupling in uncomplicated hypertension patients. Acta Cardiol Sin. 2013;29:19–27. [PMC free article] [PubMed] [Google Scholar]
- 534.Boutouyrie P, Chowienczyk P, Humphrey JD, et al. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–886. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 535.Mediavilla García JD, Fernández-Torres C, Jaén Aguila F, et al. Effect of olmesartan medoxomil on arterial stiffness in patients with essential hypertension. Med Clin (Barc) 2007;128:726–729. doi: 10.1157/13106127. [DOI] [PubMed] [Google Scholar]
- 536.Miyoshi T, Doi M, Hirohata S, et al. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart Vessels. 2011;26:408–413. doi: 10.1007/s00380-010-0060-x. [DOI] [PubMed] [Google Scholar]
- 537.Cheng YB, Xia JH, Li Y, et al. Antihypertensive treatment and central arterial hemodynamics: a meta-analysis of randomized controlled trials. Front Physiol. 2021;12:762586. doi: 10.3389/fphys.2021.762586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Guerin AP, Blacher J, Pannier B, et al. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 539.Laurent S, Chatellier G, Azizi M, et al. SPARTE Study: normalization of arterial stiffness and cardiovascular events in patients with hypertension at medium to very high risk. Hypertension. 2021;78:983–995. doi: 10.1161/HYPERTENSIONAHA.121.17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 541.Lee HY, Shin J, Kim GH, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20. doi: 10.1186/s40885-019-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 543.Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral,mesenteric, renal, upper and lower extremity arteries endorsed by:the European Stroke Organization (ESO) The Task Force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 544.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Criqui MH, Ninomiya JK, Wingard DL, et al. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52:1736–1742. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Doobay AV, Anand SS. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol. 2005;25:1463–1469. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]
- 547.Unkart JT, Allison MA, Araneta MRG, et al. Burden of peripheral artery disease on mortality and incident cardiovascular events. Am J Epidemiol. 2020;189:951–962. doi: 10.1093/aje/kwaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Singla A, Chobufo MD, Meesum A, et al. Prevalence and outcomes of low ankle brachial index by atherosclerotic cardiovascular disease risk level: insights from the National Health and Nutrition Examination Survey (NHANES). Am J Med Sci. 2023;365:121–129. doi: 10.1016/j.amjms.2022.08.022. [DOI] [PubMed] [Google Scholar]
- 549.Cortesi PA, Maloberti A, Micale M, et al. Costs and effects of cardiovascular risk reclassification using the ankle-brachial index (ABI) in addition to the Framingham risk scoring in women. Atherosclerosis. 2021;317:59–66. doi: 10.1016/j.atherosclerosis.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 550.Tsai WC, Lee WH, Chen YC, et al. Combination of low ankle-brachial index and high ankle-brachial index difference for mortality prediction. Hypertens Res. 2021;44:850–857. doi: 10.1038/s41440-021-00636-y. [DOI] [PubMed] [Google Scholar]
- 551.Hsu PC, Lee WH, Chen YC, et al. Comparison of different ankle-brachial indices in the prediction of overall and cardiovascular mortality. Atherosclerosis. 2020;304:57–63. doi: 10.1016/j.atherosclerosis.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 552.Li YH, Sheu WH, Lee IT. Use of the ankle-brachial index combined with the percentage of mean arterial pressure at the ankle to improve prediction of all-cause mortality in type 2 diabetes mellitus:an observational study. Cardiovasc Diabetol. 2020;19:173. doi: 10.1186/s12933-020-01149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Hsu PC, Huang JC, Lee WH, et al. Usefulness of ankle-brachial index calculated using diastolic blood pressure and mean arterial pressure in predicting overall and cardiovascular mortality in hemodialysis patients. Int J Med Sci. 2021;18:65–72. doi: 10.7150/ijms.50831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Sweeney T, Quispe R, Das T, et al. The use of blood biomarkers in precision medicine for the primary prevention of atherosclerotic cardiovascular disease: a review. Expert Rev Precis Med Drug Dev. 2021;6:247–258. doi: 10.1080/23808993.2021.1930531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Akinkuolie AO, Buring JE, Ridker PM, et al. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014;3:e001221. doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Lawler PR, Akinkuolie AO, Chandler PD, et al. Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res. 2016;118:1106–1115. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Duprez DA, Otvos J, Sanchez OA, et al. Comparison of the predictive value of glyca and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin Chem. 2016;62:1020–1031. doi: 10.1373/clinchem.2016.255828. [DOI] [PubMed] [Google Scholar]
- 558.Fashanu OE, Oyenuga AO, Zhao D, et al. GlycA, a novel inflammatory marker and its association with peripheral arterial disease and carotid plaque: the multi-ethnic study of atherosclerosis. Angiology. 2019;70:737–746. doi: 10.1177/0003319719845185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Tibuakuu M, Fashanu OE, Zhao D, et al. GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS. 2019;33:547–557. doi: 10.1097/QAD.0000000000002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Wong ND, Budoff MJ, Ferdinand K, et al. Atherosclerotic cardiovascular disease risk assessment: an American Society for Preventive Cardiology clinical practice statement. Am J Prev Cardiol. 2022;10:100335. doi: 10.1016/j.ajpc.2022.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Choi S. The potential role of biomarkers associated with ASCVD risk: risk-enhancing biomarkers. J Lipid Atheroscler. 2019;8:173–182. doi: 10.12997/jla.2019.8.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Zorena K, Jachimowicz-Duda O, Ślęzak D, et al. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int J Mol Sci. 2020;21:3570. doi: 10.3390/ijms21103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 564.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 565.Linssen GC, Bakker SJ, Voors AA, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J. 2010;31:120–127. doi: 10.1093/eurheartj/ehp420. [DOI] [PubMed] [Google Scholar]
- 566.Passino C, Aimo A, Masotti S, et al. Cardiac troponins as biomarkers for cardiac disease. Biomark Med. 2019;13:325–330. doi: 10.2217/bmm-2019-0039. [DOI] [PubMed] [Google Scholar]
- 567.Lam CSP, Castillo R, Ho DT, et al. High-sensitivity troponin I for cardiovascular risk stratification in the general asymptomatic population: perspectives from Asia-Pacific. Int J Cardiol. 2019;282:93–98. doi: 10.1016/j.ijcard.2019.01.107. [DOI] [PubMed] [Google Scholar]
- 568.Willeit P, Welsh P, Evans JDW, et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J Am Coll Cardiol. 2017;70:558–568. doi: 10.1016/j.jacc.2017.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Hoff J, Wehner W, Nambi V. Troponin in cardiovascular disease prevention: updates and future direction. Curr Atheroscler Rep. 2016;18:12. doi: 10.1007/s11883-016-0566-5. [DOI] [PubMed] [Google Scholar]
- 570.Wu CK, Chang MH, Lin JW, et al. Renal-related biomarkers and long-term mortality in the US subjects with different coronary risks. Atherosclerosis. 2011;216:226–236. doi: 10.1016/j.atherosclerosis.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 571.Bundy JD, Chen J, Yang W, et al. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis. 2018;271:53–60. doi: 10.1016/j.atherosclerosis.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Zhou F, Li K, Yang K. Adipose-derived stem cell exosomes and related micrornas in atherosclerotic cardiovascular disease. J Cardiovasc Transl Res. 2023;16:453–462. doi: 10.1007/s12265-022-10329-7. [DOI] [PubMed] [Google Scholar]
- 573.Henning RJ. Cardiovascular exosomes and microRNAs in cardiovascular physiology and pathophysiology. J Cardiovasc Transl Res. 2021;14:195–212. doi: 10.1007/s12265-020-10040-5. [DOI] [PubMed] [Google Scholar]
- 574.Nayor M, Brown KJ, Vasan RS. The molecular basis of predicting atherosclerotic cardiovascular disease risk. Circ Res. 2021;128:287–303. doi: 10.1161/CIRCRESAHA.120.315890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Sethi Y, Patel N, Kaka N, et al. Precision medicine and the future of cardiovascular diseases: a clinically oriented comprehensive review. J Clin Med. 2023;12:1799. doi: 10.3390/jcm12051799. [DOI] [PMC free article] [PubMed] [Google Scholar]