Abstract
Viral infections precipitate exacerbations in many airway diseases, including asthma and cystic fibrosis. Although viral infections increase cholinergic transmission, few studies have examined how cholinergic history modifies subsequent cholinergic responses in the airway. In our previous work, we found that airway resistance in response to a second cholinergic challenge was increased in young pigs with a history of airway cholinergic stimulation. Given that mucus secretion is regulated by the cholinergic nervous system and that abnormal airway mucus contributes to exacerbations of airway disease, we hypothesized that prior cholinergic challenge would also modify subsequent mucus responses to a secondary cholinergic challenge. Using our established cholinergic challenge–rechallenge model in pigs, we atomized the cholinergic agonist bethanechol or saline control to pig airways. Forty-eight hours later, we removed tracheas and measured mucus secretion properties in response to a second cholinergic stimulation. The second cholinergic stimulation was conducted in conditions of diminished chloride and bicarbonate transport to mimic a cystic fibrosis-like environment. In pigs previously challenged with bethanechol, a second cholinergic stimulation produced a mild increase in sheet-like mucus films; these films were scarcely observed in animals originally challenged with saline control. The subtle increase in mucus films was not associated with changes in mucociliary transport. These data suggest that prior cholinergic history might modify mucus secretion characteristics with subsequent stimulation in certain environmental conditions or disease states. Such modifications and/or more repetitive stimulation might lead to retention of mucus on the airway surface, thereby potentiating exacerbations of airway disease.
Keywords: airway, cholinergic, mucin, mucus, pig
1 |. INTRODUCTION
Abnormal mucus composition and clearance are found in several chronic airway diseases, including chronic obstructive pulmonary disease, cystic fibrosis (CF) and asthma (Burgel, Montani, Danel, Dusser, & Nadel, 2007; Dunican et al., 2018; Thornton, Sheehan, & Carlstedt, 1991; Turkovic, Caudri, Rosenow, Hall, & Stick, 2017; Williams, Sharafkhaneh, Kim, Dickey, & Evans, 2006). Patients with these diseases often experience periods of stability, punctuated by acute decompensations or exacerbations. These exacerbations are thought to be triggered by noxious environmental stimuli, viral infections or allergens and frequently require hospitalization. Although the clinical definition of a pulmonary exacerbation varies, many scoring systems to characterize exacerbations use sputum volume in their symptom profile, highlighting the centrality of mucus (Goss & Burns, 2007).
Viral infections are of particular interest in understanding pulmonary exacerbations in CF (Esther, Lin, Kerr, Miller, & Gilligan, 2014; Wang, Prober, Manson, Corey, & Levison, 1984). For example, viral infections result in more outpatient visits and increased hospitalizations in people with CF. In adults with CF, influenza season contributes significantly to morbidity (Ortiz et al., 2010). Viral infections can also injure CF airway epithelia (Kong et al., 2013), and inflammation associated with viral infection can stimulate secretion of abnormal mucus (Carr, Goldie, & Henry, 1996; Zhu et al., 2009). Abnormal mucus obstructs the airways and potentially serves as a nidus for bacterial infections (Ermund et al., 2018; Knowles & Boucher, 2002). Indeed, in children with CF, respiratory syncytial virus infection is associated with Pseudomonas aeruginosa colonization (Johansen & Hoiby, 1992). Additionally, mucus transport is impaired in CF (Hoegger et al., 2014), which might increase infectivity owing to an impaired ability to clear virions after they enter airways. Although maintenance therapy targeting viruses directly is not implemented in CF, indirect virus elimination is enhanced by augmenting mucociliary transport with several techniques, including physiotherapy and inhalation of hypertonic saline (Donaldson et al., 2006).
Although many studies have demonstrated that viral infection disrupts epithelial cell integrity (Kong et al., 2013) and modifies airway host defenses (Grayson & Holtzman, 2007; Sutanto et al., 2011), fewer studies have focused on the consequences of viral infection on the nervous system. This is of crucial importance in the context of pulmonary exacerbations because nerves innervating the airway regulate mucus secretion (Canning, 2006; Jacoby, Xiao, Lee, Chan-Li, & Fryer, 1998; Rios et al., 1999; Wine, 2007). One neurotransmitter that has received limited attention in this regard is the potent secretagogue acetylcholine. With viral infections, acetylcholine release is augmented (Fryer & Jacoby, 1991; Lee, Fryer, van Rooijen, & Jacoby, 2004; Nie et al., 2011; Rynko, Fryer, & Jacoby, 2014; Sorkness, Clough, Castleman, & Lemanske, 1994), resulting in enhanced cholinergic transmission, which promotes smooth muscle contraction and the secretion of mucus and fluid.
We previously reported that cholinergic history modified subsequent airway smooth muscle function in normal physiological conditions, such that a second cholinergic challenge presented 48 h after the initial cholinergic exposure produced exaggerated airway narrowing (Reznikov et al., 2018). In that study, a second cholinergic stimulus had minimal effect on mucus secretion properties measured histologically in physiological conditions. Thus, in the present study, we tested the hypothesis that cholinergic challenge and re-exposure in an altered airway environment would modify mucus secretion properties. To test this hypothesis, we used our previously published cholinergic exposure and re-exposure protocol in pig airways (Reznikov et al., 2018). However, the second cholinergic stimulus was provided in conditions of diminished bicarbonate and chloride to mimic a CF-like environment.
2 |. METHODS
2.1 |. Ethical approval
The University of Florida Animal Care and Use Committee approved all procedures (protocol #201909462). Care was in accordance with federal policies and guidelines.
2.2 |. Animals
A total of 20 male and 20 female piglets (Yorkshire × Landrace, 2–3 days of age) were obtained from a commercial vendor and fed milk replacer (Liqui-Wean, Heritage Animal Health, Hawarden, IA, USA) ad libitum. Piglets were housed with a 12 h–12 h light–dark cycle. Piglets were allowed a 24 h acclimation period before interventions. Data were collected from five separate cohorts of piglets. At the conclusion of the experiments, animals were sedated with intramuscular ketamine (20 mg kg−1), xylazine (2.0 mg kg−1) and propofol (2 mg kg−1 I.V.; Covetrus, Portland, ME, USA), followed by euthanasia with pentobarbital sodium and phenytoin sodium solution (90 mg kg−1 I.V.; Euthasol; Covetrus, Portland, ME, USA).
2.3 |. Airway instillation
After acclimation, the piglets were anaesthetized by inhalation of 8% sevoflurane (Sevothesia; Covetrus, Portland, ME, USA). The airways were accessed with a laryngoscope, and a laryngotracheal atomizer (MADgic, Moutainside Medical Equipment, Marcy, NY, USA) was passed directly beyond the vocal folds to aerosolize either 500 μl of bethanechol chloride in 0.9% saline solution or 0.9% saline solution alone (control) to the airway (Reznikov et al., 2018). The selected dose previously resulted in an acute increase in airway resistance in piglets (Reznikov et al., 2018; Rodriguez, Bullard, Armani, Miller, & Shaffer, 2013). Airway resistance is mediated by smooth muscle in the airways that is located deep to submucosal glands, which are a key target for mucus secretion in the present study.
2.4 |. Chemicals and drugs
Bethanechol chloride (Selleckchem, Houston, TX, USA) was dissolved in 0.9% saline to a final concentration of 8 mg ml−1 and sterilized with a 0.22 μm filter (Millex GP, MilliporeSigma, Burlington, MA, USA) (Reznikov et al., 2018). Acetyl-β-methacholine chloride (Sigma, St. Louis, MO, USA) was dissolved in 0.9% saline for ex vivo application (Liao et al., 2020).
2.5 |. Mucus secretion assay ex vivo with diminished bicarbonate and chloride
We measured mucus secretion as previously described (Liao et al., 2020; Ostedgaard et al., 2017). Briefly, three or four rings of trachea were removed post mortem, and the tracheal outer surface was wrapped in gauze soaked with 5 ml of the following solution: 135 Mm NaCl, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 10 mM dextrose, 5 mM HepeS, pH 7.4 (adjusted with NaOH), 1.5 mg ml−1 of methacholine and 100 μM bumetanide. Tracheas were then placed in a temperature-controlled, humidified incubator for 3 h (Liao et al., 2020). After stimulation, tracheas were fixed overnight in 4% paraformaldehyde and permeabilized for 30 min using a Triton solution (0.15%), followed by blocking in SuperBlock PBS. Jacalin–fluorescein isothiocyanate and wheat germ agglutin (WGA)–rhodamine were used to visualize mucus (Abdullah, Wolber, Kesimer, Sheehan, & Davis, 2012; Liao et al., 2020; Ostedgaard et al., 2017) and were incubated overnight with tracheas at a dilution of 1:1000. Tracheas were then washed, cut upon the ventral surface and pinned to wax-covered Petri dishes, followed by submersion in PBS. Tracheas were imaged with an Zeiss Axio Zoom V16, dorsal to ventral, using identical microscope settings. Images were assigned scoring indices for sheet formation and strand formation by two observers blinded to conditions, using methods recently published and validated in our laboratory (Liao et al., 2020). Briefly, scoring for sheet formation was as follows: 1 = no sheet formation; 2 = 1–25% of image field shows sheet formation; 3 = 26–50% of image field shows sheet formation; 4 = 51–75% of image field shows sheet formation; and 5 = 76–100% of image field shows sheet formation. Sheet formation was defined as an area of fluorescence that did not assume the shape of a discrete cellular packet of mucus. Scoring for strand formation was similar to that for sheet formation. Scoring for strand formation was as follows: 1 = no strand formation; 2 = 1–25% of image field shows 3 = 26–50% of image field shows a strand; 4 = 51–75% of image field shows a strand; and 5 = 76–100% of image field shows a strand.
2.6 |. Ex vivo mucus transport assays
Mucociliary transport was measured using methods similar to those in our recently published manuscript (Liao et al., 2020). Briefly, three rings of tracheas were submerged in 5 ml of prewarmed solution containing the following: 138 mM NaCl, 1.5 mM KH2PO4, 0.9 mM CaCl2, 0.5 mM MgCl2, 2.67 mM KCl, 8.06 mM Na2HPO4.7H2O, 10 mM Hepes, pH 7.4 (NaOH) and 100 μM bumetanide. The solution contained 0.2 μm yellow–green carboxylated FluoSpheres (ThermoFisher Scientific) at a dilution of 1:1000. Tracheas were placed onto a heated stage and kept at 37°C. Images were acquired every 1 min for 35 min. After 5 min of baseline measurements, methacholine was administered directly into the solution covering the basolateral and apical sides of tracheas at a dose of 0.004 mg ml−1. Mucus transport was assessed for an additional 30 min. IMARIS software was used to track mucus transport throughout the time measured. Briefly, computer-assigned particles based upon signal intensity above background were automatically generated with IMARIS software (Bitplane, Zurich, Switzerland) as we described recently (Liao et al., 2020). The particles were then tracked through time using a custom IMARIS algorithm that used principles of the well-validated algorithms published by Jaqaman et al. (2008). For each trachea, the average mean speeds of the computer-assigned particles were reported. The length of the particle track was also computed automatically, and the mean track lengths of all the particles per trachea were calculated and reported.
2.7 |. Immunofluorescence in tracheal cross-sections
One or two tracheal rings were removed post mortem and embedded in Peel-A-Way embedding moulds containing Tissue-Tek OCT (Electron Microscopy Sciences, Hatfield, PA, USA). Moulds were placed in a container filled with dry ice until frozen, and subsequently stored at −80°C long term. Tissues were sectioned at a thickness of 10 μm and mounted onto SuperFrost Plus microscope slides (Thermo-Fisher Scientific, Waltham, MA, USA). We used immunofluorescence procedures and analyses similar to those previously described (Liao et al., 2020; Reznikov et al., 2016, 2019). Briefly, representative cross-sections from a single cohort of pigs were selected and fixed in 2% paraformaldehyde for 15 min. Tissues were then permeabilized in 0.15% Triton X-100, followed by blocking in PBS Superblock (Thermo-Fisher Scientific, Waltham, MA, USA) containing 2–4% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). Tissues were incubated with primary antibodies for 2 h at 37°C. Tissues were washed thoroughly in PBS and incubated in secondary antibodies for 1 h at room temperature. Tissues were washed and a Hoechst stain performed as previously described (Reznikov et al., 2019). A 1:10 glycerol:PBS solution was used to cover the sections, and a coverslip was applied.
Sections were imaged on a Zeiss Axio Zoom V16 microscope (Carl Zeiss AG, Oberkochen, Germany). Identical microscope settings were used within a single cohort of piglet tracheas. Three to five images encompassing the posterior, anterior and lateral surfaces of the trachea were obtained. Images were exported and analysed using ImageJ. The tracheal surface epithelia and entire submucosal gland regions were traced and the mean signal intensities of MUC5B and MUC5AC recorded. Background signal intensity was measured and subtracted manually. The final signal intensities were averaged to identify a mean signal intensity for MUC5B and MUC5AC per sub-compartment for each piglet. To analyse the amount of the tracheal lumen that was ciliated, three images of the trachea were assessed. The length of the epithelia that was ciliated was divided by the total length of the epithelia and reported as a percentage. The percentage of each image was averaged and reported for each piglet.
2.8 |. Antibodies and lectins
We used the following anti-mucin antibodies: rabbit anti-MUC5B (1:500; Santa Cruz Biotechnology, Dallas, TX, USA; catalogue no. 20119) and mouse anti-MUC5AC (clone 45M1, 1:1000; ThermoFisher Scientific, Waltham, MA, USA; catalogue no. MA512178), mouse anti-acetyl α-tubulin (clone 6–11B-1, 1:500; EMD Millipore, Burlington, MA, USA; catalogue no. MABT868) (Kuan et al., 2019), followed by goat anti-rabbit and goat anti-mouse secondary antibodies conjugated to Alexa-Fluor 488 or 568 (1:1000; ThermoFisher Scientific, Waltham, MA, USA). We used WGA–rhodamine (Vector Laboratories) and jacalin–fluorescein isothiocyanate (Vector Laboratories, Burlingame, CA, USA) at 1:1000 dilution. Tracheas were submerged in PBS and visualized using a Zeiss Axio Zoom V16 microscope.
2.9 |. Bronchoalveolar lavage and enzyme-linked immunosorbent assay
At the end of the experimental protocol, the caudal left lung of each piglet was excised and the main bronchus cannulated; three sequential 5 ml lavages of 0.9% sterile saline were administered as previously described (Liao et al., 2020; Reznikov et al., 2018, 2019). The lavage occurred without a second stimulation, because all secondary stimulations were accomplished ex vivo. The recovered material was pooled, centrifuged at 500g, and supernatant stored at −80°C. A porcine MUC5AC (LSBio, Seattle, WA, USA; catalogue no. LS-F45847–1) or porcine MUC5B (LSBio, Seattle, WA, USA; catalogue no. LS-F45852–1) enzyme-linked immunosorbent assay was performed according to the manufacturer’s instructions in duplicate as previously described (Liao et al., 2020). The enzyme-linked immunosorbent assay was read using a filter-based accuSkan FC micro-photometer (ThermoFisher Scientific, Waltham, MA, USA). The limits of sensitivity were >0.188 and >0.375 ng ml−1 for MUC5AC and MUC5B, respectively. The intra-assay and interassay coefficient of variability were, respectively, <6.1 and <5.2% for MUC5AC and <6.5 and <5.9% for MUC5B.
2.10 |. Statistical analysis
A two-way ANOVA (sex as one factor and treatment as the other) was performed to investigate potential sex differences. No significant interactions between sex and treatment were verified, indicating that sex did not affect the response to treatments (Table 1). Thus, separation based upon sex was not justified (Liao et al., 2020; Wang & Ware, 2013), and therefore we grouped the data to represent the population better. For parametric data that compared two groups, we used Student’s unpaired t test. Non-parametric data were examined with a Mann–Whitney U test (Liao et al., 2020; Reznikov et al., 2018, 2019). Statistical significance was determined as P < 0.05 using GraphPad Prism v.7.0a.
TABLE 1.
Results separated by sex
| Variable | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Female | Male | Two-way ANOVA P-values | |||||
| Saline | Bethanechol | Saline | Bethanechol | Sex | Treatment | Sex × treatment | |
| Bronchoalveolar lavage concentrations of MUC5AC | 0.041 ± 0.012 | 0.035 ± 0.006 | 0.043 ± 0.014 | 0.042 ± 0.011 | 0.362 | 0.441 | 0.629 |
| Bronchoalveolar lavage concentrations of MUC5B | 0.20 ± 0.091 | 0.19 ± 0.077 | 0.23 ± 0.096 | 0.22 ± 0.089 | 0.501 | 0.851 | 0.974 |
| Surface MUC5AC mean signal intensity | 87.58 ± 26.43 | 84.21 ± 40.75 | 82.91 ± 28.91 | 108.89 ± 22.02 | 0.303 | 0.246 | 0.135 |
| Surface MUC5B mean signal intensity | 40.59 ± 22.52 | 35.55 ± 21.76 | 41.61 ± 21.75 | 35.29 ± 19.24 | 0.955 | 0.406 | 0.925 |
| Gland MUC5AC mean signal intensity | 69.47 ± 22.04 | 52.59 ± 17.26 | 64.21 ± 26.44 | 67.99 ± 30.55 | 0.405 | 0.518 | 0.192 |
| Gland MUC5B mean signal intensity | 77.73 ± 33.28 | 72.52 ± 36.03 | 77.97 ± 32.10 | 73.45 ± 34.44 | 0.956 | 0.653 | 0.975 |
| Mean jacalin-labelled sheet index scores | 1.16 ± 0.23 | 1.54 ± 0.50 | 1.42 ± 0.46 | 2.15 ± 0.80 | 0.015 | 0.002 | 0.308 |
| Mean wheat germ agglutinin-labelled sheet index scores | 1.06 ± 0.14 | 1.22 ± 0.46 | 1.3 ± 0.47 | 1.57 ± 0.71 | 0.067 | 0.179 | 0.737 |
| Mean jacalin-labelled strand index scores | 1.12 ± 0.32 | 1.14 ± 0.21 | 1.28 ± 0.29 | 1.12 ± 0.19 | 0.394 | 0.394 | 0.275 |
| Mean wheat germ agglutinin-labelled strand index scores | 1.02 ± 0.06 | 1.12 ± 0.22 | 1.08 ± 0.10 | 1.08 ± 0.14 | 0.825 | 0.272 | 0.272 |
| Mean speed of mucus transport (μm s−1) | 1.28 ± 0.33 | 1.35 ± 0.28 | 1.14 ± 0.33 | 1.18 ± 0.32 | 0.124 | 0.567 | 0.864 |
| Maximal speed of mucus transport (μm s−1) | 5.20 ± 1.46 | 5.36 ± 1.35 | 4.979 ± 1.59 | 5.07 ± 1.46 | 0.580 | 0.792 | 0.939 |
| Mean length of mucus particle track (μm) | 1339.83 ± 574.96 | 1398.57 ± 424.70 | 1210.32 ± 389.03 | 1313.82 ± 445.33 | 0.470 | 0.584 | 0.880 |
| Mean intensity of fluorescently labelled mucus particles on the tracheal surface | 10.9 ± 3.44 | 15.5 ± 8.28 | 16.8 ± 8.17 | 15.0 ± 7.57 | 0.245 | 0.554 | 0.164 |
Data are shown as themean ± SD.
3 |. RESULTS
We investigated mucus morphology and secretion ex vivo in conditions of diminished bicarbonate and chloride transport 48 h after in vivo instillation of either bethanechol or saline control to pig airways. The initial bethanechol or saline challenge was in normal physiological conditions, and the subsequent ex vivo stimulation occurred with diminished anion transport to simulate CF. Similar to our previous studies, we used bethanechol for the initial in vivo delivery, owing to its stability, and methacholine for the second stimulation. The use of different drugs provided pharmacological variation to mitigate the possibility of a diminished pharmacological response to the same chemical (Gawin, Emery, Baraniuk, & Kaliner, 1991; Schöneberg, 2008). We did not include a physiological condition control because our previously published data did not reveal a significant effect of cholinergic exposure and re-exposure on mucus secretion in physiological conditions (Reznikov et al., 2018). We stained mucus with jacalin and WGA lectins (Liao et al., 2020; Ostedgaard et al., 2017). Jacalin tends to identify surface mucus more readily than submucosal gland mucus, whereas WGA tends to label both submucosal gland mucus and surface mucus. In the pig airway, MUC5AC is enriched in the surface goblet cells, whereas MUC5B is expressed both in surface goblet cells and within the submucosal glands. We assessed the airways for mucus sheet formation and mucus strand formation. These features are similar to those described in pigs with CF (Ostedgaard et al., 2017; Tang et al., 2016) and are observed in pigs challenged with intra-airway acid (Liao et al., 2020); acidification of the airways is reported in CF (Abou Alaiwa et al., 2014; Pezzulo et al., 2012).
We found that tracheal segments stimulated ex vivo with methacholine from pigs previously challenged in vivo with bethanechol exhibited a mild but statistically significant increase in film-like mucus sheets for jacalin-labelled (MUC5A) mucus (Figure 1a–c), but not for mucus labelled with WGA (MUC5B) (Figure 1d). We also assessed tracheas for mucus strands, which emanate from submucosal glands and are characteristic of CF (Ermund et al., 2018; Ostedgaard et al., 2017) and are found in pigs challenged with intra-airway acid (Liao et al., 2020). Detectable strand formation for mucus labelled with jacalin (Figure 1e) or WGA (Figure 1f) was not readily found in our static images in either saline-challenged or bethanechol-challenged piglets, and no differences in strand formation were detected between treatment groups.
FIGURE 1.
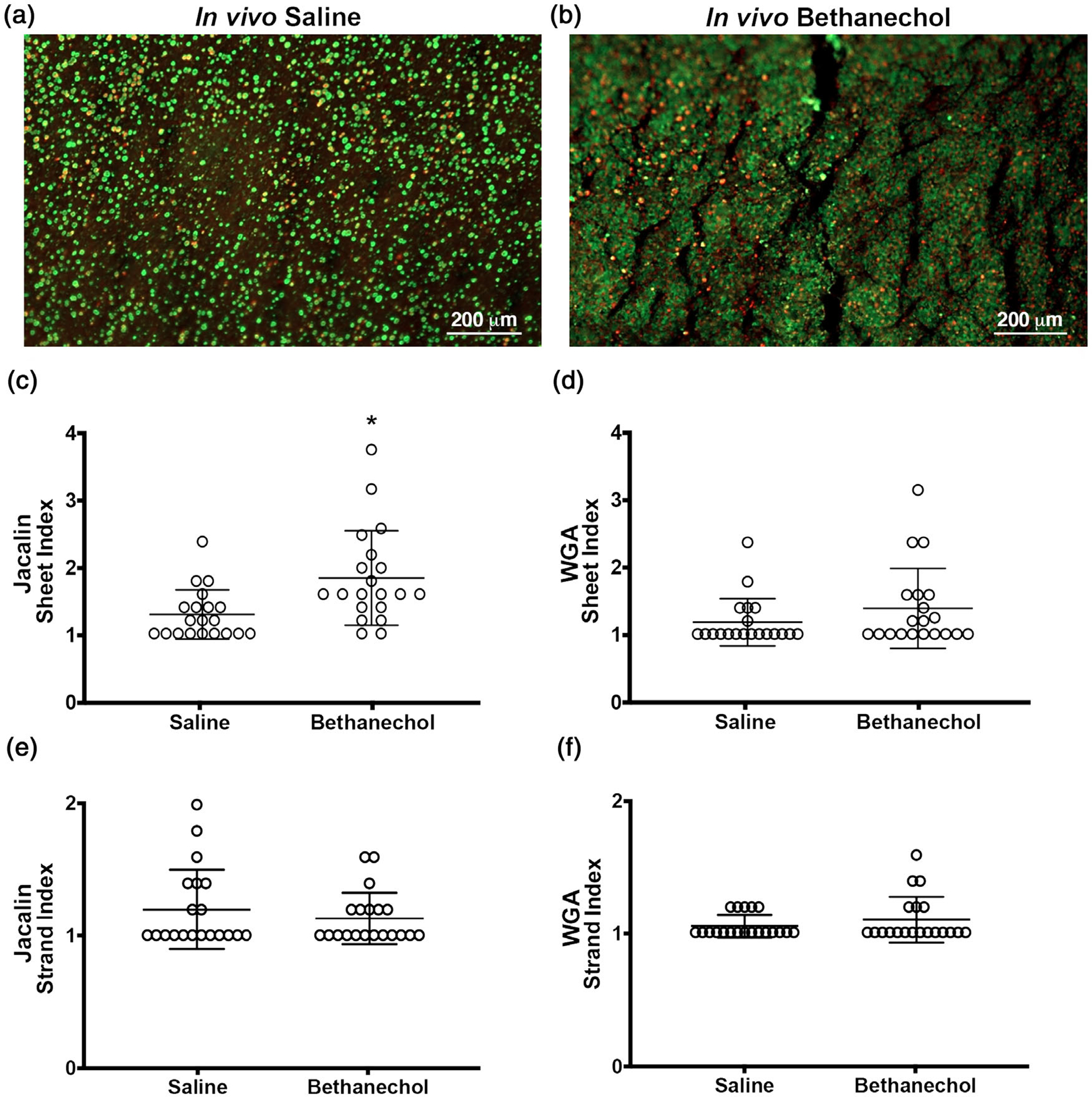
Morphology of mucus in pig airways challenged with bethanechol and exposed 48 h later to the cholinergic agonist methacholine. (a,b) Representative en face image of an ex vivo trachea from a saline (a) or bethanechol (b) challenge after secondary exposure to the secretagogue methacholine. Discrete entities of mucus were observed and visualized by jacalin lectin (green) and WGA (red) staining. (c,d) Sheet index for jacalin-labelled mucus (c) and WGA-labelled mucus (d). (e,f) Strand index for jacalin-labelled mucus (e) and WGA-labelled mucus (f). n = 20 saline-challenged piglets (10 females and 10 males) and n = 20 bethanechol-challenged pigs (10 females and 10 males). Data points represent the mean score for each piglet calculated from five to seven images analysed (encompassing the anterior, lateral and posterior regions of the trachea). A greater score indicates greater incidence of the feature measured. Abbreviation: WGA, wheat germ agglutinin. *P < 0.05 compared with saline-challenged pigs. For panels e and f, data were assessed with a non-parametric Mann–Whitney U test. All data are shown as mean values ± SD
In our previous work, mucus sheet formation was associated with defects in mucociliary transport (Liao et al., 2020). Thus, we examined mucociliary transport ex vivo in tracheal segments using live imaging and computer-assisted particle tracking in conditions of diminished bicarbonate and chloride transport (Liao et al., 2020; Figure 2). In these studies, tracheas are submerged in media containing fluorescent carboxylated nanospheres that bind to the mucus (Figure 2a,b). As expected, some of the labelled mucus forms strand-like bundles. Using this approach, we observed no differences in mean or maximal transport speed of fluorescence-labelled mucus between treatment groups (Figure 2c,d) Given that speed is equal to distance over time, we also analysed the particle track length. Our expectation was that length would not be different, because speed was not different and time was clamped. Consistent with this expectation, we observed no differences in particle track length (Figure 2e). Finally, at the conclusion of the experiment, we also measured the signal intensity of fluorosphere-labelled mucus on the airway surface as an additional metric of mucus production. However, no differences between treatment groups were observed (Figure 2f). These data suggested that cholinergic history did not affect mucociliary transport in conditions mimicking CF.
FIGURE 2.
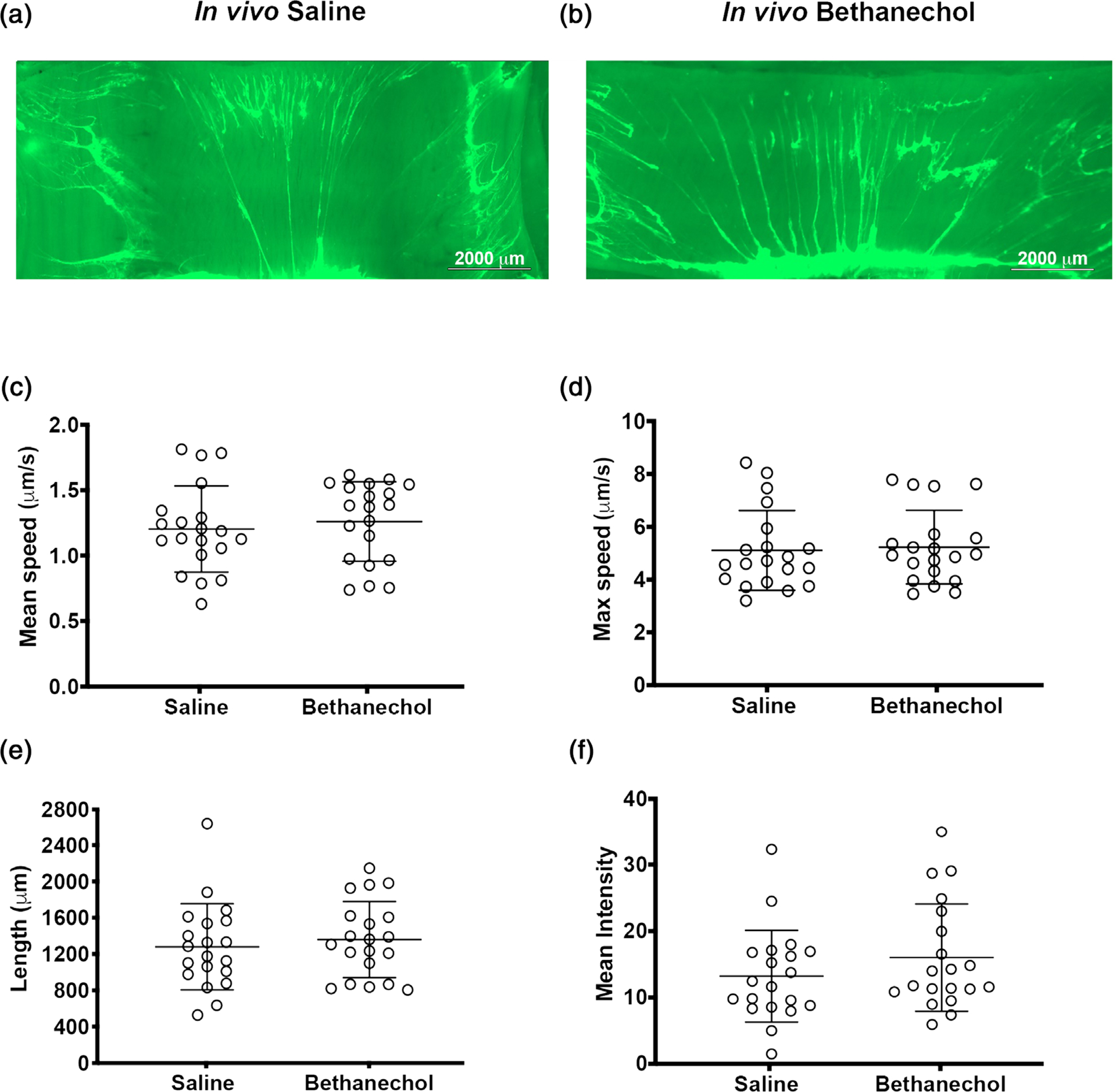
Mucus transport in pig airways challenged with bethanechol and exposed 48 h later to the cholinergic agonist methacholine. (a,b) Representative en face images of an ex vivo piglet tracheas stimulated with methacholine. Mucus is visualized in real time with fluorescent nanospheres (bright green). Mucus often forms strands. Images of tracheas are at the conclusion of the experiment, after methacholine stimulation, in saline-challenged (a) and bethanechol-challenged (b) piglet airways. (c) Mean mucus transport speed. (d) Maximal mucus transport speed. (e) Computer-assigned particle track length. (f) Quantification of the signal intensity of fluorescently labelled mucus on the airway surface at the conclusion of the experiment. n = 20 saline-challenged piglets (10 females and 10 males) and n = 20 bethanechol-challenged pigs (10 females and 10 males). Data were assessed with Student’s unpaired t test. All data are shown as mean values ± SD
Changes in mucus secretion characteristics can be attributable to changes in the biophysical properties of mucus, in addition to changes in the abundance of mucins. Thus, to determine whether bethanechol treatment impacted mucin abundance, we examined MUC5AC and MUC5B in tracheal segments of control and bethanechol-challenged piglets that were not stimulated with methacholine. Studying tracheal segments after methacholine stimulation would not have provided a reliable indicator of potential basal differences in mucins attributable to bethanechol treatment. We stained tracheal cross-sections using antibody-specific labelling and performed signal intensity analyses similar to our previous studies (Liao et al., 2020; Figure 3a,b). We found no significant differences between treatment groups in MUC5AC or MUC5B signal intensity within and on the tracheal surface (Figure 3c,d). Likewise, no differences in MUC5AC or MUC5B abundance were observed in the submucosal glands (Figure 3e,f). As a secondary approach, we also examined MUC5AC and MUC5B concentrations in the bronchoalveolar lavage fluid in basal conditions (i.e. non-methacholine stimulated). Similar to the results obtained from antibody labelling, we observed no differences between treatment groups (Figure 3g,h). These data mirrored our observations in acid-challenged pigs, in which an increase in mucus sheet formation was noted, but did not coincide with an increase or alteration in the basal production of mucins (Liao et al., 2020). They were also consistent with our previous data indicating that bethanechol challenge in pigs does not alter the mRNA expression of MUC5AC or MUC5B (Reznikov et al., 2018).
FIGURE 3.
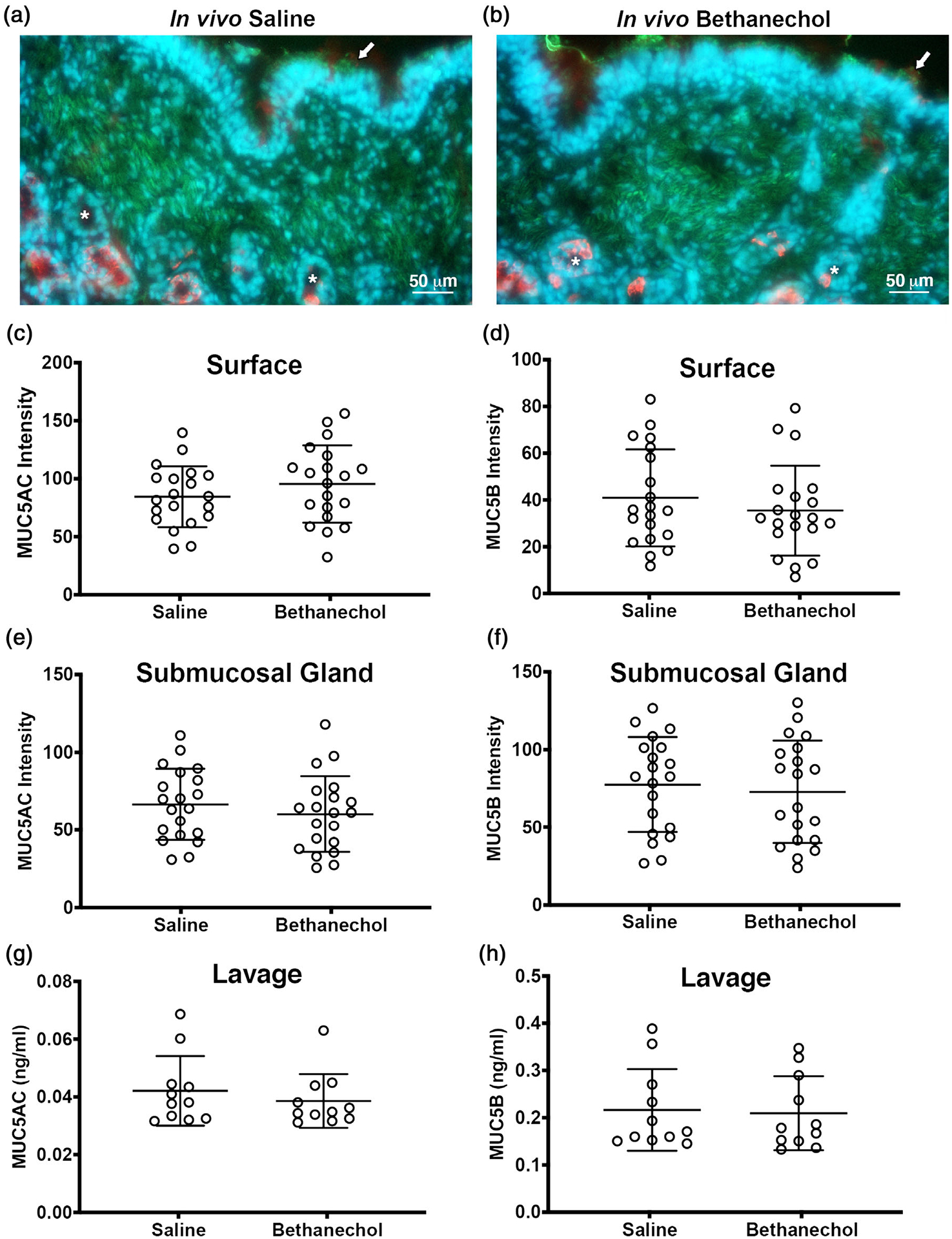
Tracheal mucin staining and bronchoalveolar concentrations in pigs challenged with bethanechol. (a,b) Representative antibody labelling of MUC5AC (green) and MUC5B (red) in 10-μm-thick tracheal cross-sections from piglets challenged with intra-airway saline (a) or intra-airway bethanechol (b). Nuclei are shown in blue (Hoechst). Arrows indicate examples of surface mucus and asterisks examples of submucosal glands. (c–f) MUC5AC and MUC5B staining signal intensity of the surface epithelia (c,d) and submucosal glands (e,f). (g,h) Bronchoalveolar lavage fluid concentrations of MUC5AC (g) and MUC5B (h). For panels c–f, n = 20 saline-challenged piglets (10 females and 10 males) and n = 20 bethanechol-challenged pigs (10 females and 10 males). For panels g and h, n = 11 saline-challenged pigs (five females and six males) and n = 11 bethanechol-challenged pigs (five females and six males). Data points represent the mean fluorescence intensity for each piglet calculated from three to five images analysed (encompassing the anterior, lateral and posterior regions of the trachea). All data are shown as mean values ± SD
4 |. DISCUSSION
Mucus abnormalities characterize several airway diseases, including CF (Abou Alaiwa et al., 2014; Birket et al., 2018; Pezzulo et al., 2012). Viral infections enhance cholinergic transmission (Fryer & Jacoby, 1991; Lee et al., 2004; Nie et al., 2011; Rynko et al., 2014; Sorkness et al., 1994), and enhanced cholinergic transmission is thought to drive mucus abnormalities in inflamed airways. However, few studies have examined how cholinergic history modifies subsequent airway mucus secretion responses. This might be especially important in CF, where viral infections are common early on. Thus, we interrogated the effect of prior cholinergic stimulation on subsequent properties of mucus in neonatal piglets challenged with intra-airway bethanechol or saline control. Airways exposed to bethanechol and subsequently exposed to another cholinergic agonist in conditions of diminished chloride and bicarbonate 48 h later exhibited abnormal airway mucus characterized by a mild increase in mucus sheets labelled with jacalin. This phenotype was independent of differences in MUC5AC or MUC5B concentrations in basal conditions. Thus, these data suggest that cholinergic history has the capacity to modify and shape subsequent airway mucus secretion responses in certain physiological states.
Previous studies have demonstrated that repeated cholinergic stimulation of the airway can increase goblet cell numbers in both humans (Grainge et al., 2011) and mice (Mailhot-Larouche et al., 2018). In the human studies, methacholine was provided three times at 48 h intervals; increased goblet cells were observed 4 days after the final methacholine challenge (Grainge et al., 2011). In mice, a longer exposure protocol was adopted, consisting of 6 weeks of exposure to methacholine every other day (Mailhot-Larouche et al., 2018). In contrast, we provided a single challenge of the cholinergic agonist bethanechol, followed by an acute exposure to methacholine 48 hlater. We previously reported that this exposure–re-exposure protocol did not increase MUC5AC or MUC5B mRNA in the trachea or lungs, nor did it increase histological scores of goblet cells or mucus (Reznikov et al., 2018). Thus, it would be interesting to determine whether studying a later time point using the present protocol would also demonstrate increased goblet cells or whether the threshold to increase goblet cells requires more than one exposure. It is also interesting to speculate that repeated cholinergic stimulation might produce more severe airway remodelling and/or functional defects in diseased conditions. In addition, without an obvious increase in goblet cells or MUC5AC or MUC5B expression, the mechanism to explain increased sheet formation in bethanechol-treated porcine airways remains unknown. It is possible that altered viscoelastic properties of mucin contribute, because cholinergic stimulation is also a potent regulator of glandular fluid secretion (Wine, 2007). Thus, a change in viscoelastic properties could change the adhesiveness of the mucus on the airway surface, resulting in a more sheet-like appearance.
Although prior bethanechol stimulation was associated with mucus sheet formation upon cholinergic re-exposure, no differences in mucociliary transport were observed. This is in contrast to our recent publication, in which prior airway acidification produced extensive mucus sheets in tracheas when exposed to the cholinergic agonist methacholine 48 h after the initial acid challenge (Liao et al., 2020). In that study, mucociliary transport was decreased. However, as mentioned, the extent of mucus sheet formation was greater, whereas in the present study the differences in mucus sheet formation were mild. Thus, these findings suggest that additional factors, such as robust inflammation and/or altered concentrations of mucins (Evans, Kim, Tuvim, & Dickey, 2009; Roy et al., 2014), are required for mucociliary transport defects (Atanasova & Reznikov, 2019). Indeed, mucociliary transport defects in CF pigs were prominent upon methacholine stimulation and involved a failure of mucus detachment from the gland (Hoegger et al., 2014).
Previous studies have demonstrated that anticholinergics reduce and delay exacerbations in chronic obstructive pulmonary disease (Casaburi et al., 2002; Vincken et al., 2002). Similar findings have been observed in asthma (Gosens & Gross, 2018; Quirce, Dominguez-Ortega, & Barranco, 2015). Although there are limited data in CF concerning the role of anticholinergics in CF exacerbations, a recent study demonstrated that anticholinergics might be beneficial in promoting mucociliary clearance early in CF (Ermund et al., 2018). Improved pulmonary function, as measured by the forced expiratory volume in 1 s, has also been reported in people with CF after the administration of the anticholinergics (Sanchez, 1995). Thus, these data, in combination with our present findings, further highlight the potential benefit of mitigating cholinergic transmission in the airway in the prevention of exacerbations in diseases such as CF.
Our study has limitations. It is challenging to predict the exact temporal dynamics of cholinergic stimulation using aerosolized bethanechol. Variation in bethanechol distribution and clearance might also generate heterogeneity in mucus phenotypes. We did not investigate mucus biophysical or rheological properties or measure ciliary beat frequency. We also did not measure mucociliary transport in non-methacholine-stimulated conditions owing to limitations in our assay. Furthermore, we do not know whether using the same drug for exposure and re-exposure (e.g. methacholine + methacholine or bethanechol + bethanechol) would modify our results or whether the combination of using two different cholinergic drugs is a prerequisite for the phenotypes observed in the present study. Additionally, given that our studies were conducted in the absence of inflammation, it is difficult to predict how the present findings might translate and map onto a background of inflammation; however, we suspect that they would be compounded or made worse. Finally, our initial stimulation occurred in wild-type animals with intact anion transport. If the initial in vivo stimulation occurred in airways with underlying anion transport abnormalities, such as in CF, this might cause more dramatic differences in mucus impairment.
In summary, prior cholinergic challenge slightly modified airway mucus characteristics in response to subsequent cholinergic stimulation in conditions of diminished bicarbonate and chloride, producing mild sheet-like mucus films on the tracheal surface. Such modifications and/or more repetitive stimulation might lead to retention of mucus on the airway surface, thereby potentiating exacerbations in airway disease.
New Findings.
-
What is the central question of this study?
What is the impact of airway cholinergic history on the properties of airway mucus secretion in a cystic fibrosis-like environment?
-
What is the main finding and its importance?
Prior cholinergic challenge slightly modifies the characteristics of mucus secretion in response to a second cholinergic challenge in a diminished bicarbonate and chloride transport environment. Such modifications might lead to retention of mucus on the airway surface, thereby potentiating exacerbations of airway disease.
ACKNOWLEDGEMENTS
We thank Joshua Dadural, Shin-Ping Kuan, Kevin Vogt and Eda Eken for excellent technical assistance. This study was funded in part by the US National Institutes of Health: R00HL119560, OT2OD026582 (Principal Investigator, L.R.R.), OT2OD023854 (Co-Investigator, L.R.R.) and 5T32EB021955 (M.J.H.).
Footnotes
COMPETING INTERESTS
None declared.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abdullah LH, Wolber C, Kesimer M, Sheehan JK, & Davis CW (2012). Studying mucin secretion from human bronchial epithelial cell primary cultures. Methods in Molecular Biology, 842, 259–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Alaiwa MH, Beer AM, Pezzulo AA, Launspach JL, Horan RA, Stoltz DA, … Zabner J (2014). Neonates with cystic fibrosis have a reduced nasal liquid pH; a small pilot study. Journal of Cystic Fibrosis, 13, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasova KR, & Reznikov LR (2019). Strategies for measuring airway mucus and mucins. Respiratory Research, 20, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birket SE, Davis JM, Fernandez CM, Tuggle KL, Oden AM, Chu KK, … Rowe SM (2018). Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight, 3, e97199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel PR, Montani D, Danel C, Dusser DJ, & Nadel JA (2007). A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax, 62, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ (2006). Reflex regulation of airway smooth muscle tone. Journal of Applied Physiology, 101, 971–985. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Goldie RG, & Henry PJ (1996). Influence of respiratory tract viral infection on endothelin-1-induced potentiation of cholinergic nerve-mediated contraction in mouse trachea. British Journal of Pharmacology, 119, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, … Witek T Jr. (2002). A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. European Respiratory Journal, 19, 217–224. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, & Boucher RC (2006). Mucus clearance and lung function in cystic fibrosis with hypertonic saline. New England Journal of Medicine, 354, 241–250. [DOI] [PubMed] [Google Scholar]
- Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, … National Heart Lung and Blood Institute Severe Asthma Research Program (SARP). (2018) Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. Journal of Clinical Investigation, 128, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermund A, Meiss LN, Dolan B, Bähr A, Klymiuk N, & Hansson GC (2018). The mucus bundles responsible for airway cleaning are retained in cystic fibrosis and by cholinergic stimulation. European Respiratory Journal, 52, 1800457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esther CR Jr., Lin FC, Kerr A, Miller MB, & Gilligan PH (2014). Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatric Pulmonology, 49, 926–931. [DOI] [PubMed] [Google Scholar]
- Evans CM, Kim K, Tuvim MJ, & Dickey BF (2009). Mucus hyper-secretion in asthma: Causes and effects. Current Opinion in Pulmonary Medicine, 15, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer AD, & Jacoby DB (1991). Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. British Journal of Pharmacology, 102, 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin AZ, Emery BE, Baraniuk JN, & Kaliner MA (1991). Nasal glandular secretory response to cholinergic stimulation in humans and guinea pigs. Journal of Applied Physiology, 71, 2460–2468. [DOI] [PubMed] [Google Scholar]
- Gosens R, & Gross N (2018). The mode of action of anticholinergics in asthma. European Respiratory Journal, 52, 1701247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss CH, & Burns JL (2007). Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax, 62, 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, … Howarth PH (2011). Effect of bronchoconstriction on airway remodeling in asthma. New England Journal of Medicine, 364, 2006–2015. [DOI] [PubMed] [Google Scholar]
- Grayson MH, & Holtzman MJ (2007). Emerging role of dendritic cells in respiratory viral infection. Journal of Molecular Medicine, 85, 1057–1068. [DOI] [PubMed] [Google Scholar]
- Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, … Welsh MJ (2014). Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science, 345, 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby DB, Xiao HQ, Lee NH, Chan-Li Y, & Fryer AD (1998). Virus- and interferon-induced loss of inhibitory M2 muscarinic receptor function and gene expression in cultured airway parasympathetic neurons. Journal of Clinical Investigation, 102, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaqaman K, Loerke D, Mettlen M, Kuwata H, Grinstein S, Schmid SL, & Danuser G (2008). Robust single-particle tracking in live-cell time-lapse sequences. Nature Methods, 5, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen HK, & Hoiby N (1992). Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax, 47, 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, & Boucher RC (2002). Mucus clearance as a primary innate defense mechanism for mammalian airways. Journal of Clinical Investigation, 109, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M, Maeng P, Hong J, Szczesniak R, Sorscher E, Sullender W, & Clancy JP (2013). Respiratory syncytial virus infection disrupts mono-layer integrity and function in cystic fibrosis airway cells. Viruses, 5, 2260–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan SP, Liao YJ, Davis KM, Messer JG, Zubcevic J, Aguirre JI, & Reznikov LR (2019). Attenuated amiloride-sensitive current and augmented calcium-activated chloride current in marsh rice rat (Oryzomys palustris) airways. iScience, 19, 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Fryer AD, van Rooijen N, & Jacoby DB (2004). Role of macrophages in virus-induced airway hyperresponsiveness and neuronal M2 muscarinic receptor dysfunction. American Journal of Physiology-Lung Cellular and Molecular Physiology, 286, L1255–L1259. [DOI] [PubMed] [Google Scholar]
- Liao YSJ, Kuan SP, Guevara MV, Collins EN, Atanasova KR, Dadural JS, … Reznikov LR (2020). Acid exposure disrupts mucus secretion and impairs mucociliary transport in neonatal piglet airways. American Journal of Physiology-Lung Cellular and Molecular Physiology, 318, L873–L887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhot-Larouche S, Deschenes L, Gazzola M, Lortie K, Henry C, Brook BS, … Bosse Y (2018). Repeated airway constrictions in mice do not alter respiratory function. Journal of Applied Physiology, 124, 1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Scott GD, Weis PD, Itakura A, Fryer AD, & Jacoby DB (2011). Role of TNF-α in virus-induced airway hyperresponsiveness and neuronal M2 muscarinic receptor dysfunction. British Journal of Pharmacology, 164, 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz JR, Neuzil KM, Victor JC, Wald A, Aitken ML, & Goss CH (2010). Influenza-associated cystic fibrosis pulmonary exacerbations. Chest, 137, 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, … Welsh MJ (2017). Gel-forming mucins form distinct morphologic structures in airways. Proceedings of the National Academy of Sciences of the United States of America, 114, 6842–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, … Zabner J (2012). Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature, 487, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirce S, Dominguez-Ortega J, & Barranco P (2015). Anticholinergics for treatment of asthma. Journal of Investigational Allergology & Clinical Immunology, 25, 84–93; quiz 94–85. [PubMed] [Google Scholar]
- Reznikov LR, Liao YSJ, Gu T, Davis KM, Kuan SP, Atanasova KR, … Vogt K (2019). Sex-specific airway hyperreactivity and sex-specific transcriptome remodeling in neonatal piglets challenged with intra-airway acid. American Journal of Physiology-Lung Cellular and Molecular Physiology, 316, L131–L143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Meyerholz DK, Adam RJ, Abou Alaiwa M, Jaffer O, Michalski AS, … Welsh MJ (2016). Acid-sensing ion channel 1a contributes to airway hyperreactivity in mice. PLoS One, 11, e0166089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Meyerholz DK, Kuan SP, Guevara MV, Atanasova KR, & Abou Alaiwa MH (2018). Solitary cholinergic stimulation induces airway hyperreactivity and transcription of distinct pro-inflammatory pathways. Lung, 196, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, & Dartt DA (1999). Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Investigative Ophthalmology & Visual Science, 40, 1102–1111. [PubMed] [Google Scholar]
- Rodriguez E, Bullard CM, Armani MH, Miller TL, & Shaffer TH (2013). Comparison study of airway reactivity outcomes due to a pharmacologic challenge test: Impulse oscillometry versus least mean squared analysis techniques. Pulmonary Medicine, 2013, 618576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, … Evans CM (2014). Muc5b is required for airway defence. Nature, 505, 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynko AE, Fryer AD, & Jacoby DB (2014). Interleukin-1β mediates virus-induced M2 muscarinic receptor dysfunction and airway hyper-reactivity. American Journal of Respiratory Cell and Molecular Biology, 51, 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I (1995). [Role of anticholinergic agents in the treatment of cystic fibrosis]. Archives De Pediatrie, 2(Suppl 2), 154S–158S. [DOI] [PubMed] [Google Scholar]
- Schöneberg T (2008). Tolerance and desensitization. In Offermanns S & Rosenthal W (Eds.), Encyclopedia of molecular pharmacology (pp. 1203–1207). Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Sorkness R, Clough JJ, Castleman WL, & Lemanske RF Jr. (1994). Virus-induced airway obstruction and parasympathetic hyper-responsiveness in adult rats. American Journal of Respiratory and Critical Care Medicine, 150, 28–34. [DOI] [PubMed] [Google Scholar]
- Sutanto EN, Kicic A, Foo CJ, Stevens PT, Mullane D, Knight DA, & Stick SM; Australian Respiratory Early Surveillance Team for Cystic Fibrosis.(2011) Innate inflammatory responses of pediatric cystic fibrosis airway epithelial cells: Effects of nonviral and viral stimulation. American Journal of Respiratory Cell and Molecular Biology, 44, 761–767. [DOI] [PubMed] [Google Scholar]
- Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, … Welsh MJ (2016). Acidic pH increases airway surface liquid viscosity in cystic fibrosis. Journal of Clinical Investigation, 126, 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton DJ, Sheehan JK, & Carlstedt I (1991). Heterogeneity of mucus glycoproteins from cystic fibrotic sputum. Are there different families of mucins? Biochemical Journal, 276, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkovic L, Caudri D, Rosenow T, Hall G, & Stick S (2017). Presence of mucus plugging is predictive of long term lung function in children with cystic fibrosis. European Respiratory Journal, 50, OA4401. [Google Scholar]
- Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, & Cornelissen PJG; Dutch/Belgian Tiotropium Study Group.(2002) Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropium. European Respiratory Journal, 19, 209–216. [DOI] [PubMed] [Google Scholar]
- Wang EE, Prober CG, Manson B, Corey M, & Levison H (1984). Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. New England Journal of Medicine, 311, 1653–1658. [DOI] [PubMed] [Google Scholar]
- Wang R, & Ware JH (2013). Detecting moderator effects using subgroup analyses. Prevention Science, 14, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams OW, Sharafkhaneh A, Kim V, Dickey BF, & Evans CM (2006). Airway mucus: From production to secretion. American Journal of Respiratory Cell and Molecular Biology, 34, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine JJ (2007). Parasympathetic control of airway submucosal glands: Central reflexes and the airway intrinsic nervous system. Autonomic Neuroscience, 133, 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, & Chen Y (2009). Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. American Journal of Respiratory Cell and Molecular Biology, 40, 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


