Abstract
The B1 gene of vaccinia virus encodes a serine/threonine protein kinase that is expressed early after infection. Under nonpermissive conditions, temperature-sensitive mutants (ts2 and ts25) that map to B1 fail to efficiently replicate viral DNA. Our goal was to extend studies on the function of B1 by determining if the kinase is required for intermediate or late gene expression, two events that ordinarily depend on viral DNA replication. First, we established that early viral gene expression occurred at the nonpermissive temperature. By using a transfection procedure that circumvents the viral DNA replication requirement, we found that reporter genes regulated by an intermediate promoter were transcribed only under conditions permissive for expression of active B1. To assay late gene expression, the T7 RNA polymerase gene was inserted into the genome of ts25 to form ts25/T7. A DNA replication-independent late gene transcription system was established by cotransfecting plasmids containing T7 promoter-driven late gene transcription factors and a late promoter reporter gene into ts25/T7-infected cells. Late genes, unlike intermediate genes, were expressed at the nonpermissive temperature. Last, we showed that overexpression of B1 stimulated intermediate but inhibited late gene expression in cells infected with wild-type virus.
DNA viruses have evolved regulatory systems in which early and late stage genes are transcribed from parental and replicated genomes, respectively. Poxviruses have added some unique features to this general strategy. The enzymes needed for transcription of early genes are expressed late in infection, packaged within progeny virions, and activated when the core is released into the cytoplasm following infection (26). In addition, there are two postreplicative stages of poxvirus gene expression, intermediate and late, rather than just one. All of the viral factors required for DNA replication and expression of intermediate genes are products of early genes, whereas the viral factors needed for late gene expression are products of both early and intermediate genes. Thus, the poxvirus life cycle occurs in a temporally ordered sequence: early gene expression → viral DNA replication → intermediate gene expression → late gene expression. The extent to which vaccinia virus proteins interact with one another and/or with cellular proteins to link viral DNA replication with intermediate and late gene expression is not understood.
Studies on the regulation of intracellular processes involved in eukaryotic nucleic acid metabolism have pointed to protein phosphorylation as playing a key role. Poxviruses have acquired analogs of a number of host cell genes to maintain their autonomy from the cell, including two serine/threonine protein kinases (B1 and F10) and a dual-specificity protein phosphatase (H1). The F10 kinase (22) and H1 phosphatase (15) are expressed late in infection, are incorporated into virions, and play major roles in virion morphogenesis (11, 24, 38). Interestingly, the H1 phosphatase has been implicated in early gene transcription (24). Mutant virus particles devoid of H1 phosphatase were unable to transcribe early genes either in vivo or in vitro. This finding led us to investigate the role that phosphorylation might play in the transcription of the other gene classes. The B1 kinase is expressed exclusively early in infection; it localizes to viral replication factories and appears to be a minor component of the virion (3, 23, 34). The B1 kinase associates with (25) and phosphorylates (5, 6) viral late gene transcription factor 4 (VLTF-4; viral protein H5) and two ribosomal proteins in vitro. Temperature-sensitive (ts) vaccinia virus mutants (ts2 and ts25) that map to the B1 gene have a defect in viral DNA replication (9, 28) and consequently would not be expected to express intermediate or late genes. To analyze intermediate and late gene expression, we used a transfection-based assay that mimics the requirement for viral DNA replication (18, 37). Our results show that B1 is essential for the transcription of viral intermediate but not late genes.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (BS-C-1) and murine fibroblast (L929) cells were maintained in minimal essential medium (MEM; Life Technologies) supplemented with 10% fetal bovine serum (Life Technologies). The ts mutants ts2 and ts25 were obtained from Richard Condit (8, 9) and grown and titered on BS-C-1 cells. For virological assays, incubations were at permissive (32°C) and nonpermissive (39.5°C) temperatures. Cytosine β-d-arabinosidase (AraC; Sigma) was used at 44 μg/ml to block viral DNA replication in certain experiments.
Plasmid constructions and reporter gene assays.
Three reporter genes were used in this study: p30/300, pG8R-CAT, and p11KCAT. The construction of p30/300, a plasmid that contains 300 bp of DNA upstream of the vaccinia virus intermediate G8R gene open reading frame (ORF) fused to the lacZ ORF, has been described elsewhere (1). pG8R-CAT was constructed by digesting p30/300 with BamHI and HindIII, completely removing the lacZ ORF, and subsequently inserting a compatible fragment containing the chloramphenicol acetyltransferase (CAT) ORF in its place. The resultant plasmid (pG8R-CAT) retains the same 300 bp of G8R upstream sequences as p30/300. A late gene reporter plasmid, p11K-CAT, was constructed in two steps. First, pG8R-CAT was digested with BamHI, and a synthetic DNA duplex containing 65 bp of sequence upstream of the 11K ORF was inserted so that only the upstream BamHI site was regenerated. Second, the resultant plasmid was digested with BamHI and SalI to completely remove the G8R regulatory sequences. The final plasmid (p11K-CAT) contains the CAT ORF under the regulation of the 11K late promoter. A T7 promoter-B1 expression plasmid (pT7-B1) was constructed by PCR amplification of the wild-type B1 ORF from vaccinia virus strain WR DNA and insertion of this fragment into the EcoRI and BamHI sites of pSG5 (14). The same vector expressing a nonsense mutant (pT7ΔB1) of the B1 gene was engineered by inserting a synthetic adapter (5′-AATTCTAGCTAGCTAG-3′) into the MfeI site in the B1R ORF. Expression of pT7-ΔB1 would result in a truncated B1 gene product terminating at the 11th amino acid.
Transfections were carried out with Qiagen column-purified DNAs using the DOTAP transfection reagent (Boehringer Mannheim) and OptiMEM (Life Technologies). Typically, 5 μg of covalently closed, circular, supercoiled DNA was mixed with 15 μl of DOTAP per transfection. In transfection-infection experiments, cells (approximately 2 × 106 to 3 × 106 per dish) were transfected 3 h prior to infection. The temperature of transfections was kept constant at 37°C. Viral inocula were prepared by trypsinizing stocks at 30°C for 30 min. Extracts were prepared by washing cells in ice-cold phosphate-buffered saline twice and freeze-thawing three times in 500 μl of 1× reporter lysis buffer (Promega). Typically, 1 to 10 μl of cell extract was analyzed to remain within the linear range of enzyme assays. β-Galactosidase assays were conducted as specified by the manufacturer (Promega) and quantitated using an EL 312E Bio-Kinetics Reader (Bio-Tek Instruments). CAT assays were done using a fluorescently labeled chloramphenicol substrate (FAST CAT yellow; Molecular Probes) (17, 39). Reaction products were separated by thin-layer chromatography, analyzed on a FluorImager (Molecular Dynamics), and quantitated with ImageQuant software (Molecular Dynamics).
Protein analyses.
Radioimmunoprecipitations were conducted by labeling cells for 30 min with 50 μCi of [35S]methionine (Amersham) per ml in MEM lacking methionine. Extracts were prepared by washing cells with ice-cold phosphate-buffered saline, suspending the cells in 1× radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.2], 150 mM NaCl, 1% NP-40), and clarifying the lysate by microcentrifugation for 5 min. Subsequently, anti-vaccinia virus antibody (BioGenesis) was added at 1:500 to the clarified supernatant, and mixtures were rocked for 4 to 6 h at 4°C. Immune complexes were captured with 50 μl of 20% protein A-Sepharose CL-4B (Pharmacia), and the beads were washed three times with 1× RIPA buffer containing 0.1% sodium dodecyl sulfate (SDS). Immune complexes were boiled in SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer (0.05 M Tris, 4% SDS, 4% β-mercaptoethanol, 10% glycerol, 0.1% bromophenol blue) for 5 min and microcentrifuged for 30 s. The solubilized proteins were then resolved on 5 to 20% polyacrylamide gels (21). Gels were dried and analyzed by autoradiography.
Western blotting was conducted by transferring proteins onto polyvinylidene difluoride membranes (Millipore), incubating in blocking buffer (0.89 M Tris, 0.89 M borate, 0.02 M EDTA, 1% NP-40, 1% nonfat dry milk), and probing with either anti-vaccinia virus (1:1,000; BioGenesis), anti-β-galactosidase (1:500; 5′-3′, Inc.), anti-TrpE-B1 (1:500), or anti-T7 polymerase (1:1,500) in blocking buffer. Immune complexes were detected with an alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody (1:1,500; Life Technologies) and the Western Blue reagent (Promega).
RNase protection assay.
Total RNA was purified from approximately 4 × 106 to 5 × 106 cells using the TRlzol total RNA isolation reagent (Life Technologies), treated with DNase I (5 U for 15 min at 37°C; Ambion), precipitated with LiCl, and resuspended in H2O. A T7 promoter-containing template for riboprobe synthesis was made by PCR using two oligonucleotides (5′-GTCAAAAATTGTAGAACGAC-3′ and 5′-TAATACGACTCACTATAGGGGTACATTGAGCAAC-3′; T7 promoter is underlined) and pG8R-CAT as the DNA template. The 161-nucleotide (nt) antisense riboprobe derived from T7 RNA polymerase transcription of this template should protect the first 96 nt of the G8RCAT mRNA. Approximately, 600 pg of uniformly radiolabeled riboprobe was mixed with 10 μg of total RNA and assayed using an RPA II kit essentially as described by the manufacturer (Ambion). Protected fragments were separated on 5% polyacrylamide–1× Tris-borate EDTA TBE–8 M urea gels and visualized by autoradiography.
RESULTS
Synthesis of viral early proteins under nonpermissive conditions.
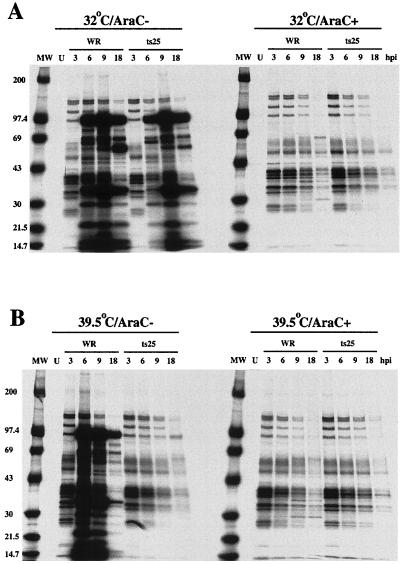
Vaccinia virus ts B1 mutants exhibit a defect in viral DNA replication at the nonpermissive temperature (8, 9, 28) and consequently should not express intermediate or late gene products. To confirm this phenotype and establish whether viral early protein synthesis was preserved, cells were infected with wild-type virus (WR) or ts25 at 32°C (permissive for ts25) or 39.5°C (nonpermissive for ts25). L929 cells were used because the mutants exhibit a stringent ts phenotype in them (28). The infected cells were metabolically labeled with [35S]methionine at various times after infection. Lysates were prepared and incubated with a polyclonal antiserum that was raised by repeatedly injecting a rabbit with live vaccinia virus Lister strain and therefore recognizes early as well as late viral proteins. Some cells were treated with AraC to block viral DNA synthesis in order to have a bona fide early protein pattern for comparison. At 32°C in the absence of AraC, we detected viral early proteins in 3 h and intermediate or late proteins at subsequent times, regardless of whether the cells were infected with WR or ts25 (Fig. 1A). In the presence of AraC, the 3-h early pattern persisted and similar patterns were obtained with WR and ts25 at 32°C (Fig. 1A). Virtually identical results were also obtained with the two viruses at 39.5°C in the presence of AraC, indicating that ts25 is fully competent to express early proteins at the nonpermissive temperature. In the absence of AraC, however, only early viral proteins were made by cells infected with ts25 at 39.5°C, whereas a typical early/late pattern was made by WR under these conditions. We also demonstrated by Western blotting that the B1 gene, which is regulated by an early promoter, is expressed under nonpermissive conditions (Fig. 2B). Although the results were fully compatible with a primary defect in DNA replication, they did not preclude the possibility of additional specific defects in intermediate or late gene expression.
FIG. 1.
SDS-PAGE analysis of viral proteins synthesized in ts25-infected cells. L929 cells were infected with either the WR or ts25 strain of vaccinia virus and incubated at the permissive (A) or nonpermissive (B) temperature in the presence or absence of AraC. Cells were labeled with [35S]methionine for 30 min at the indicated times (hours postinfection [hpi]). Lysates were prepared, immunoprecipitated with anti-vaccinia virus antiserum, and analyzed by SDS-PAGE. Masses of protein standards in kilodaltons are indicated to the left. U, uninfected; MW, molecular weight standards.
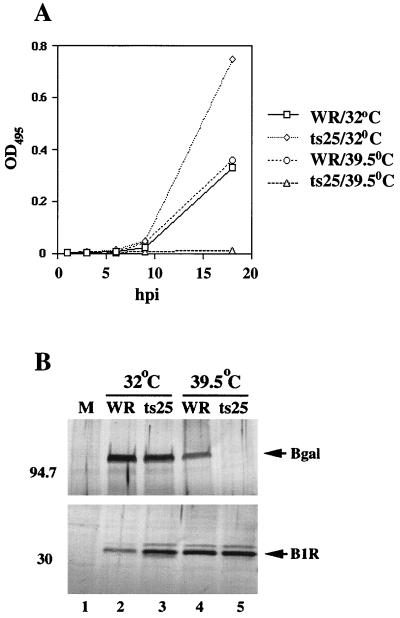
FIG. 2.
Temperature dependence of intermediate gene expression in ts25-infected L929 cells. (A) L929 cells were transfected with p30/300 and subsequently infected with WR or ts25 at 32 or 39.5°C. Extracts were prepared at 0, 3, 6, 9, and 18 h postinfection (hpi) and assayed for β-galactosidase activity. Absorbances (optical densities) at 495 nm (OD495) were plotted on the ordinate. (B) The lysates prepared at 18 h after infection were also assayed by Western blotting using antiserum specific for β-galactosidase (Bgal) or the B1R protein. The masses of protein standards are indicated in kilodaltons on the left. Arrows denote the location of the proteins of interest. M, mock infected.
Specific inhibition of intermediate gene expression under nonpermissive conditions.
To determine if B1 had an additional role in intermediate gene expression, we needed to bypass the requirement for viral DNA synthesis which is blocked in ts25-infected cells at the nonpermissive temperature. Previous studies had shown that genes regulated by intermediate promoters are expressed from transfected plasmids, which functionally mimic replicated DNA when DNA synthesis is inhibited (37). When L929 cells were transfected with a plasmid (p30/300) containing the lacZ gene regulated by an intermediate (G8R) promoter (1) and then infected with WR, synthesis of β-galactosidase was detected between 8 and 18 h at both 32 and 39.5°C (Fig. 2A). At 32°C, β-galactosidase activity was even higher in cells infected with ts25 than in cells infected with WR (Fig. 2A). However, at 39.5°C, little or no activity was detected in cells infected with ts25 (Fig. 2A). Corresponding results were obtained by Western blotting with antibody to β-galactosidase (Fig. 2B). Furthermore, results similar to these were obtained using another B1 mutant virus (ts2) (data not shown).
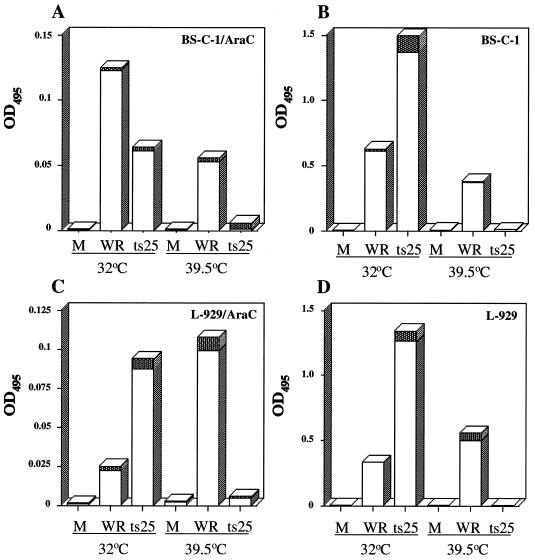
Although previous reports demonstrated the expression of intermediate genes from transfected plasmids in the absence of DNA replication, we needed to confirm that this occurs using L929 cells. In addition, we decided to carry out similar studies with BS-C-1 cells, which are used more commonly than L929 cells for studies with vaccinia virus. Plasmid p30/300, containing an intermediate gene promoter fused to the lacZ gene, was transfected into cells that were then infected with WR or ts25. As in L929 cells, β-galactosidase synthesis was higher in BS-C-1 cells infected with ts25 than with WR at 32°C but was nearly undetectable at 39.5°C (Fig. 3B and C). We carried out parallel experiments with L929 and BS-C-1 cells in which AraC was added to the medium at the start of infection. In this way, there would be a DNA replication block under all conditions and with both wild-type and mutant viruses. We found that reporter gene expression occurred in the presence of AraC at 32°C (Fig. 3A and C), although the levels were reduced in cells infected with either WR or ts25. At 39.5°C, however, reporter gene expression was specifically blocked in cells infected with ts25 (Fig. 3A and C). We concluded, therefore, that there was a ts defect in intermediate gene expression independent of effects on viral DNA replication.
FIG. 3.
The block in intermediate gene expression is independent of DNA replication. BS-C-1 or L929 cells were transfected with p30/300 and subsequently infected with 5 PFU of WR or ts25 per cell in the presence of absence of AraC at the indicated temperature. Lysates were prepared at 24 h after infection and assayed for β-galactosidase activity (optical density at 495 nm [OD495]). Experiments were done in triplicate, and standard deviations are indicated by the shaded areas at the tops of the bars. M, mock infected.
Inhibition of intermediate gene expression occurred at the transcriptional level.
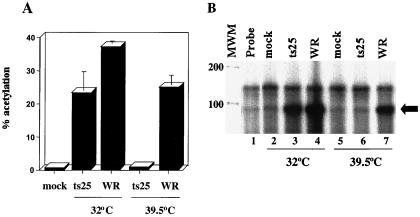
Because the synthesis of early proteins appeared to be unaffected by the ts mutation of B1, i.e., there was no global effect on translation, we suspected that the inhibition of intermediate gene expression occurred at the transcriptional level. To investigate this, we selected CAT as the reporter gene because its mRNA is only 600 nt, compared to 3,000 nt for lacZ mRNA. We considered that if the defect were in RNA elongation or degradation, then synthesis of CAT might be less severely affected than that of β-galactosidase. Such experiments, however, indicated that CAT synthesis was severely inhibited at 39.5°C in ts25-infected L929 cells (Fig. 4A).
FIG. 4.
Intermediate gene expression is inhibited at the level of transcription. (A) BS-C-1 cells were transfected with pG8R-CAT and then mock infected at 39°C or infected with 0.5 PFU of WR or ts25 per cell at 32 or 39.5°C. Lysates were prepared at 24 h after infection and assayed for CAT activity, expressed as percent acetylation. Experiments were done in triplicate, and standard deviations are denoted over the bars. (B) BS-C-1 cells were transfected with pG8R-CAT and mock infected or infected with WR or ts25 at 32 or 39.5°C as indicated above. Approximately 18 h later, total RNA was purified and analyzed by an RNase protection assay. Reaction products were separated by denaturing electrophoresis. The arrow denotes the size of the protected G8R-CAT RNA fragment. Sizes of RNA markers (MWM; RNA Century Marker; Ambion) in nucleotides are shown at the left.
An RNase protection assay was performed to determine more directly whether the block in intermediate gene expression occurred at the level of transcription. RNA was purified from BS-C-1 cells that had been transfected with pG8R-CAT and infected with either ts25 or WR. We used BS-C-1 instead of L929 cells in this experiment because of the better integrity of RNAs isolated from the former cell type. The riboprobe was designed to quantify steady-state levels of CAT mRNA and also to determine if the mRNAs initiated at the correct position relative to the G8R promoter. A discrete RNA product of predicted size was detected in cells infected with WR or ts25 at 32°C or with WR at 39°C (Fig. 4B). Little or no G8RCAT mRNA was detected in cells infected with ts25 at the nonpermissive temperature, suggesting that in the absence of B1, intermediate mRNAs are either not transcribed or have very short half-lives (Fig. 4B).
Construction of a ts25 mutant that expresses bacteriophage T7 RNA polymerase.
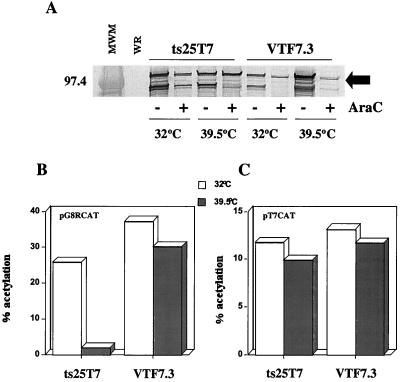
We decided to make recombinant virus ts25T7, which retains the ts25 mutation in the B1 gene and expresses the T7 RNA polymerase gene regulated by an early promoter. This virus would allow us to further evaluate the block in intermediate transcription by determining (i) whether a reporter gene regulated by a T7 promoter would be expressed at 39.5°C, (ii) if coexpression of the wild-type B1 gene would reverse the ts phenotype, and (iii) whether expression of late genes was affected at the nonpermissive temperature. Since the assays would be done at 39.5°C and in the presence of AraC, we first needed to determine whether sufficient T7 RNA polymerase would be expressed by ts25T7 under these conditions. A Western blot analysis (Fig. 5A) indicated that at least as much T7 RNA polymerase was synthesized by ts25T7 as by VTF7.3, a well-characterized vaccinia virus WR recombinant containing the T7 RNA polymerase gene (13). At least two cross-reactive species of lower molecular weight were detected; because they are not present in the wild-type virus-infected lysate, we posit that they are cleavage and/or breakdown products of T7 polymerase. As expected, since the T7 gene is under an early/late promoter, blocking late transcription with AraC resulted in less synthesis of full-length as well as truncated T7 proteins. We also needed to establish that ts25T7 could function as a T7 RNA polymerase expression vector in transient assays and retained the ts defect. Cells were transfected with either pG8RCAT or pT7CAT and infected with ts25T7 or VTF7.3. The transfection experiment shown in Fig. 5B indicated that ts25T7 allowed the expression of CAT regulated by an intermediate promoter at 32°C but not at 39.5°C. In contrast, vTF7.3 allowed the expression of CAT at either temperature (Fig. 5B). Significantly, the CAT gene regulated by a T7 promoter was expressed to similar levels at either temperature by ts25T7 or VTF7.3 (Fig. 5C). Thus, the block in transcription was specific for an intermediate gene promoter.
FIG. 5.
Expression of reporter genes in cells infected with ts25T7. (A) L929 cells were infected with ts25T7 at the indicated temperatures in the presence or absence of AraC. Lysates were prepared at 24 h after infection and analyzed by Western blotting using an anti-T7 RNA polymerase antibody. A 97.4-kDa protein standard (MWM) and the expected position of T7 RNA polymerase (denoted by the arrow) are indicated on the left and right, respectively. (B and C) L929 cells were transfected with pG8R-CAT or pT7-CAT and infected with either ts25T7 or VTF7.3 at the indicated temperatures. Extracts were prepared at 18 h after infection and assayed for CAT activity (expressed as percent acetylation).
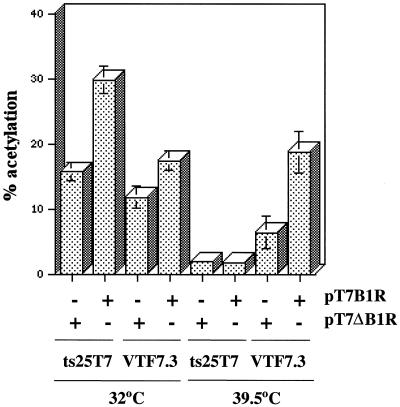
The next series of experiments were carried out to determine whether the ts25 defect could be overcome by coexpression of a wild-type copy of the B1 gene. Plasmid pT7-B1, containing the B1 ORF adjacent to a T7 promoter, was constructed and shown to program the synthesis of a 34-kDa protein that bound to anti-B1 serum in a coupled reticulocyte transcription-translation system (data not shown). Cells were then transfected with pG8R-CAT and pT7-B1 (containing an intact B1 ORF under T7 promoter control) or pT7-ΔB1 (containing a truncated B1 ORF under T7 promoter control) and then infected with ts25T7 or vTF7.3. Transfection of pT7-B1 enhanced intermediate gene expression in cells infected with vTF7.3 at 32 or 39.5°C, whereas transfection with pT7-ΔB1 did not (Fig. 6). In contrast, intermediate gene expression was enhanced by transfection of pT7-B1 in cells that were infected with ts25T7 at 32°C but not at 39.5°C (Fig. 6). Apparently, trans expression of B1 enhanced intermediate gene expression in the presence of limiting amounts of active B1 kinase but not in the presence of mutated B1 kinase at the nonpermissive temperature. Either the mutated B1 protein had a dominant negative effect or active kinase was not expressed early enough from the transfected plasmid to prevent an irreversible inhibition of intermediate gene transcription.
FIG. 6.
Expression of B1 in trans enhances intermediate gene expression under permissive but not nonpermissive conditions. L929 cells were transfected with either pG8R-CAT alone or pG8R-CAT and pT7-B1 or pT7-ΔB1. After transfection, cells were infected with ts25T7 or VTF7.3 at the indicated temperatures. Lysates were prepared at 24 h after infection and assayed for CAT activity (expressed as percent acetylation). This experiment was conducted five separate times, and standard deviations are denoted over the bars.
Analysis of late gene expression.
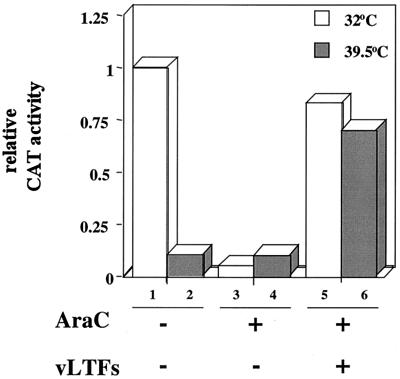
To extend our analysis to the role of the B1 kinase in late gene expression, we adapted a transfection protocol devised by Keck et al. (18). Late promoters, like intermediate promoters, can regulate transcription of a reporter gene in a transfected plasmid during an AraC block. An additional requirement, however, is that the genes encoding VLTF-1, -2, and -3 (viral genes A1, A2, and G8, respectively) must be cotransfected with the late promoter reporter plasmid. However, because A1, A2, and G8 are of the intermediate gene class, they would be silent during nonpermissive infection of cells with ts25 or ts25T7, and consequently the late transcription factors would not be made. To overcome this problem, we transfected plasmids containing the A1, A2, and G8 ORFs under the control of T7 promoters (18). Late transcription was monitored by cotransfecting a reporter plasmid (p11K-CAT) containing the CAT gene under the control of the viral 11K late gene promoter. The cells were subsequently infected with ts25T7 to provide the T7 RNA polymerase to drive the expression of the three VLTF genes. Under these conditions, CAT synthesis occurred either at 32 or 39.5°C in the presence of AraC, indicating that B1 was not specifically required for transcription of late genes (Fig. 7). Control experiments verified that there was low CAT expression in the absence of transfected VLTF plasmids when cells were infected with ts25T7 at either temperature in the presence of AraC or at 39.5°C in the absence of AraC (Fig. 7).
FIG. 7.
B1 is not required for late gene expression. L929 cells were transfected with p11K-CAT alone or with three plasmids (pT7-A1L, pT7-A2L, and pT7-G8R) that express VLTFs regulated by T7 promoters. The transfected cells were infected with ts25T7 at 32 or 39.5°C to provide T7 RNA polymerase. CAT activity was assayed at 24 h after infection and normalized to the value obtained for sample 1.
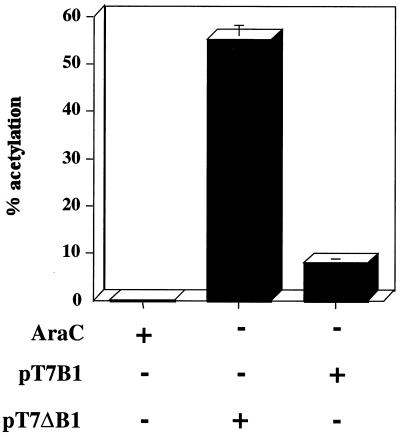
Knowing that B1 was not essential for late gene expression, we determined the effects of overexpression. Cells were cotransfected with pT7-B1 or pT7Δ-B1 and p11K-CAT and subsequently infected with VTF7.3 containing a wild-type B1 gene and T7 RNA polymerase (Fig. 8). The results indicate that trans expression of B1 inhibited late gene expression by nearly 85%.
FIG. 8.
Expression of B1 in trans inhibits late gene expression. L929 cells were transfected with p11K-CAT and pT7-B1 or pT7-ΔB1 and subsequently infected with VTF7.3 at 37°C. CAT activity (expressed as percent acetylation) was assayed at 24 h after infection. Standard deviations are shown above the bars.
DISCUSSION
Several studies demonstrated that the B1 kinase plays an important role in viral DNA replication temperature (8, 9, 28). However, in some cell lines this effect seemed insufficient to entirely account for the reduction in virus titer and plaque formation. For example, at 39.5°C, ts25 directed the synthesis of up to 60% of control levels of DNA but only 15% of control levels of viable progeny and no distinct plaques in BS-C-40 cells (28). This is in contrast to other studies conducted on ts vaccinia virus mutants, where a decrease in viral DNA synthesis resulted in a concomitant reduction of comparable magnitude in viral titers (33). Therefore, we considered that B1 might have another role downstream of viral DNA replication. In this report, we provide evidence that intermediate gene transcription is blocked at the nonpermissive temperature and, unlike the defect in DNA replication, is similarly restricted in mouse and monkey cells.
We first showed that early gene expression was unaltered in cells infected with ts25 at the nonpermissive temperature, whereas late gene expression was completely blocked. Because this defect would result from inhibition of viral DNA synthesis, we relied on transfection experiments to specifically analyze the expression of intermediate and late genes at the nonpermissive temperature. Although a reporter gene regulated by an intermediate promoter was expressed in cells infected with wild-type vaccinia virus even in the presence of a potent inhibitor of DNA synthesis, expression did not occur at 39.5°C in cells infected with ts25. This result provided evidence that B1 was required for intermediate gene expression independent of any effect on DNA replication. Furthermore, the block occurred at the level of transcription. By constructing a recombinant virus that contained the ts25 defect and a T7 RNA polymerase gene, we demonstrated that a reporter gene regulated by a T7 promoter was expressed under nonpermissive conditions, ruling out a global defect in plasmid-based transcription. Moreover, by cotransfecting the intermediate genes encoding late transcription factors under T7 promoters, we could demonstrate that B1 was not required for transcription of a reporter plasmid with a late promoter. Transfection experiments further showed that overexpression of wild-type B1 stimulated intermediate gene expression from 1.5- to 3.5-fold but severely inhibited late gene expression in cells expressing functional B1. Failed attempts to reverse the block in intermediate transcription by expression of wild-type B1 at 39.5°C suggested that the ts B1 protein may be trans-dominant to the wild-type protein. Alternatively, if the timing of B1 synthesis is crucial, then expression from the T7 promoter, which depends on prior synthesis of T7 RNA polymerase, may occur too late.
We are uncertain as to how the B1 kinase is involved in intermediate gene expression. In vivo studies have shown that intermediate genes are transiently expressed from 1 to 4 h after infection (2). Biochemical analyses have indicated that at least six proteins (viral intermediate transcription factors [VITFs]) are required for transcription; these include the multisubunit viral RNA polymerase (35), viral capping enzyme (16, 36), VITF-1 (viral protein E4 [29]), VITF-3 (a complex of the viral A8 and A23 proteins [31]), and an unidentified cellular factor termed VITF-2 (30). An in vitro transcription system consisting solely of recombinant proteins has not been derived; thus, the possibility of additional factors or posttranslational modification of known ones remains. For example, the B1 kinase may be associated with a partially purified component of the transcription system or may have phosphorylated a viral or cellular factor prior to purification. Another possibility is that the viral H5 protein is involved in intermediate transcription. This protein, identified as VLTF-4 (19), is the only VLTF synthesized prior to DNA replication (20). The fact that H5 is phosphorylated in vitro by the B1 protein kinase (5) and is underphosphorylated by a B1 ts mutant at the nonpermissive temperature (4) makes H5 an attractive candidate for further investigation.
Additional studies indicate that the H5 protein interacts with viral proteins encoded by the A18 and G2 genes, which are involved in transcription elongation (7), and the A20 gene (25), involved in DNA replication (12). The situation is even more complex because a recent report of charge-to-alanine mutagenesis of the H5 ORF produced a ts mutant that has a block in viral morphogenesis with no apparent defect in either viral DNA or protein synthesis (10). Whether the B1 kinase is directly involved in this additional role of the H5 protein is unknown.
The B1 gene is conserved among many but not all poxviruses. Molluscum contagiosum virus encodes homologs of the F10 protein kinase and the H1 phosphatase but does not contain a B1 homolog (32). One explanation for its absence from the molluscum contagiosum virus genome is that it may be compensated by a host cell protein kinase. In this regard, there are two human putative serine/threonine protein kinases, VRK1 and VRK2, with a high degree of sequence similarity to the B1 kinase (27).
Taking into consideration the complexity of the cellular pathways that are regulated by protein phosphorylation and dephosphorylation, it is not surprising that the B1 protein kinase has multiple effects on the vaccinia virus life cycle. Clearly, more work is required to identify all of the viral and cellular factors that are regulated by the B1 protein kinase.
ACKNOWLEDGMENTS
We thank Richard Condit for ts2, ts25, and helpful discussions, Paula Traktman for sharing unpublished results and ideas, Ehud Katz for advice on virological assays, Cristina Cassetti and Stephen Udem for reviewing the manuscript, Steve Broyles for anti-TrpE-B1 antiserum, Sally Lee for anti-T7 polymerase antiserum, and Norman Cooper for invaluable assistance with cell culture.
G.R.K. was supported by a National Research Council fellowship while working at the NIH.
Footnotes
This work is dedicated to the memory of Roskey Jennings, whose character was an inspiration to the many people who passed through the Laboratory of Viral Diseases.
REFERENCES
- 1.Baldick C J, Keck J G, Moss B. Mutational analysis of the core, spacer, and initiator regions of vaccinia virus intermediate class promoters. J Virol. 1992;66:4710–4719. doi: 10.1128/jvi.66.8.4710-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldick C J, Jr, Moss B. Characterization and temporal regulation of mRNAs encoded by vaccinia virus intermediate stage genes. J Virol. 1993;67:3515–3527. doi: 10.1128/jvi.67.6.3515-3527.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banham A, Smith G L. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992;191:803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- 4.Beaud G, Beaud R. Temperature-dependent phosphorylation state of the H5R protein sythesized at the early stage of infection in cells infected with vaccinia virus ts mutants of the B1R and F10L protein kinases. Intervirology. 2000;43:67–70. doi: 10.1159/000025025. [DOI] [PubMed] [Google Scholar]
- 5.Beaud G, Beaud R, Leader D P. Vaccinia virus gene H5R encodes a protein that is phosphorylated by the multisubstrate vaccinia virus B1R protein kinase. J Virol. 1995;69:1819–1826. doi: 10.1128/jvi.69.3.1819-1826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaud G, Sharif A, Topamass A, Leader D P. Ribosomal-protein S2/SA kinase purified from HeLa cells infected with vaccinia virus corresponds to the B1R protein-kinase and phosphorylates in-vitro the viral ssDNA-bindng protein. J Gen Virol. 1994;75:283–293. doi: 10.1099/0022-1317-75-2-283. [DOI] [PubMed] [Google Scholar]
- 7.Black E P, Moussatche N, Condit R C. Characterization of the interactions among vaccinia virus transcription factors G2R, A18R, and H5R. Virology. 1998;245:313–322. doi: 10.1006/viro.1998.9166. [DOI] [PubMed] [Google Scholar]
- 8.Condit R C, Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981;113:224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- 9.Condit R C, Motyczka A, Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983;128:429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- 10.DeMasi J, Traktman P. Clustered charge-to-alanine mutagenesis of the vaccinia virus H5 gene: isolation of a dominant, temperature-sensitive mutant with a profound defect in morphogenesis. J Virol. 2000;74:2393–2405. doi: 10.1128/jvi.74.5.2393-2405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derrien M, Punjabi A, Khanna M, Grubisha O, Traktman P. Tyrosine phosphorylation of A17 during vaccinia virus infection: involvement of the H1 phosphatase and the F10 kinase. J Virol. 1999;73:7287–7296. doi: 10.1128/jvi.73.9.7287-7296.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du S, Traktman P. Vaccinia virus DNA replication: two hundred base pairs of telomeric sequence confer optimal replication efficiency on minichromosome templates. Proc Natl Aca Sci USA. 1996;93:9693–9698. doi: 10.1073/pnas.93.18.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green S, Issemann I, Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988;16:369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan K, Broyles S S, Dixon J E. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature. 1991;350:359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- 16.Harris N, Rosales R, Moss B. Transcription initiation factor activity of vaccinia virus capping enzyme is independent of mRNA guanylylation. Proc Natl Acad Sci USA. 1993;90:2860–2864. doi: 10.1073/pnas.90.7.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hruby D E, Wilson E M. Use of fluorescent chloramphenicol derivative as a substrate for chloramphenicol acetyltransferase assays. Methods Enzymol. 1992;216:369–376. doi: 10.1016/0076-6879(92)16034-h. [DOI] [PubMed] [Google Scholar]
- 18.Keck J G, Baldick C J, Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late transactivator genes. Cell. 1990;61:801–809. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs G R, Moss B. The vaccinia virus H5R gene encodes viral late gene transcription factor 4: purification, cloning, and overexpression. J Virol. 1996;70:6796–6802. doi: 10.1128/jvi.70.10.6796-6802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs G R, Rosales R, Keck J G, Moss B. Modulation of the cascade model for regulation of vaccinia virus gene expression: purification of a prereplicative, late-stage-specific transcription factor. J Virol. 1994;68:3443–3447. doi: 10.1128/jvi.68.5.3443-3447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Broyles S S. Vaccinia protein kinase 2: a second essential serine/threonine protein kinase encoded by vaccinia virus. Proc Natl Acad Sci USA. 1994;91:7653–7657. doi: 10.1073/pnas.91.16.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S, Chen W, Broyles S S. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J Virol. 1992;66:2717–2723. doi: 10.1128/jvi.66.5.2717-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K, Lemon B, Traktman P. The dual-specificity phosphatase encoded by vaccinia virus, VH1, is essential for viral transcription in vivo and in vitro. J Virol. 1995;69:7823–7834. doi: 10.1128/jvi.69.12.7823-7834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCraith S, Holtzman T, Moss B, Fields S. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:4879–4884. doi: 10.1073/pnas.080078197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2637–2671. [Google Scholar]
- 27.Nezu J, Oku A, Jones M H, Shimane M. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics. 1997;45:327–331. doi: 10.1006/geno.1997.4938. [DOI] [PubMed] [Google Scholar]
- 28.Rempel R E, Anderson M K, Evans E, Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990;64:574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosales R, Harris N, Ahn B-Y, Moss B. Purification and identification of a vaccinia virus-encoded intermediate stage promoter-specific transcription factor that has homology to eukaryotic transcription factor SII (TFIIS) and an additional role as a viral RNA polymerase subunit. J Biol Chem. 1994;269:14260–14267. [PubMed] [Google Scholar]
- 30.Rosales R, Sutter G, Moss B. A cellular factor is required for transcription of vaccinia viral intermediate stage genes. Proc Natl Acad Sci USA. 1994;91:3794–3798. doi: 10.1073/pnas.91.9.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz P, Moss B. Identification of a transcription factor, encoded by two vaccinia virus early genes, that regulates the intermediate stage of viral gene expression. Proc Natl Acad Sci USA. 1999;96:2692–2697. doi: 10.1073/pnas.96.6.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senkevich T G, Koonin E V, Bugert J, Darai G, Moss B. The genome of molluscum contagiosum virus: analysis and comparison with other poxiviruses. Virology. 1997;233:19–42. doi: 10.1006/viro.1997.8607. [DOI] [PubMed] [Google Scholar]
- 33.Taddie J A, Traktman P. Genetic characterization of the vaccinia virus DNA polymerase: identification of point mutations conferring altered drug sensitivities and reduced fidelity. J Virol. 1991;65:869–879. doi: 10.1128/jvi.65.2.869-879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traktman P, Anderson M K, Rempel R E. Vaccinia virus encodes an essential gene with strong homology to protein kinases. J Biol Chem. 1989;264:21458–21461. [PubMed] [Google Scholar]
- 35.Vos J C, Sasker M, Stunnenberg H G. Promoter melting by a stage-specific vaccinia virus transcription factor is independent of the presence of RNA polymerase. Cell. 1991;65:105–114. doi: 10.1016/0092-8674(91)90412-r. [DOI] [PubMed] [Google Scholar]
- 36.Vos J C, Sasker M, Stunnenberg H G. Vaccinia virus capping enzyme is a transcription initiation factor. EMBO J. 1991;10:2553–2558. doi: 10.1002/j.1460-2075.1991.tb07795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vos J C, Stunnenberg H G. Derepression of a novel class of vaccinia virus genes upon DNA replication. EMBO J. 1988;7:3487–3492. doi: 10.1002/j.1460-2075.1988.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Shuman S. Vaccinia virus morphogenesis is blocked by temperature-sensitive mutations in the F10 gene, which encodes protein kinase 2. J Virol. 1995;69:6376–6388. doi: 10.1128/jvi.69.10.6376-6388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young S L, Barbera L, Kaynard A H, Haugland R P, Kang H C, Brinkley M, Melner M H. A nonradioactive assay for transfected chloramphenicol acetyltransferase activity using fluorescent substrates. Anal Biochem. 1991;197:401–407. doi: 10.1016/0003-2697(91)90411-l. [DOI] [PubMed] [Google Scholar]