Abstract
Clinical trials of active surveillance (AS) for Ductal Carcinoma in Situ (DCIS) are underway. We sought to understand the historical management of biologically favorable DCIS and to determine the outcomes of patients who did not have immediate surgery. Using data from the NCDB from 2004 to 2017, the selected cohort included women >40 years of age, with low or intermediate grade and hormone receptor (HR) positive DCIS. AS was defined as either no surgery or surgery >12 months from diagnosis. Women in the AS group were compared to women who had immediate surgery. A Cochran-Armitage test was used to assess the trend of AS over year of diagnosis. Kaplan-Meier curves were estimated to compare overall survival (OS), stratified by age (<50, 50–64, ≥65), and Cox proportional hazard models were used to determine the effects of prognostic factors on survival distributions. 74,367 women met study inclusion criteria; 2384 (3.2%) were treated with AS. The proportion of patients in the AS cohort increased yearly, peaking in 2017 at 4.2% (p < 0.01). On multivariable analysis, increasing age (OR 1.02, p < 0.01), black race (OR 1.7, p < 0.001), and being uninsured (OR 2.2, p < 0.001) were associated with increased likelihood of AS. In women <50 years of age, OS outcomes were similar, with 10-year OS of 97.4% in the immediate surgery cohort versus 99.1% in AS cohort (p = 0.43). The proportion of patients with DCIS treated with AS has remained small but is increasing over time. AS of biologically favorable DCIS in younger, healthier women is not associated with adverse survival.
Subject terms: Surgical oncology, Breast cancer
Introduction
In the United States, the diagnosis of ductal carcinoma in situ (DCIS) has significantly increased since screening mammography was introduced in the 1970s, and DCIS now comprises about 20–25% of new breast cancer diagnoses1–3. However, this increase in diagnosis of DCIS has not led to a decrease in the incidence of invasive breast cancer, and models have suggested the rate of progression of DCIS to clinically significant invasive cancer is low4. In addition, the breast cancer specific survival from studies using large population cohorts with DCIS has been shown to be excellent at 10 and 20 years1,5. Therefore, some have suggested that clinicians are over-diagnosing and overtreating some patients with DCIS that may be inconsequential3,6,7.
Despite these concerns, surgery currently remains the cornerstone of guideline concordant care for DCIS, with radiation therapy and endocrine therapy utilized where appropriate. While randomized clinical trials of active surveillance (AS) in patients with low risk DCIS, typically defined as low or intermediate grade and hormone receptor positive, are ongoing, data from those trials will not be readily available for several years (NCT02926911, LORIS trial UK, LORD trial Netherlands, LORETTA trial Japan)8–11. The aim of our study was to use a large national cancer database to retrospectively analyze the trends over time of treating patients with biologically favorable DCIS without surgery, and to determine the impact of omission of surgery on OS.
Results
Trend in AS over time and characteristics of patients treated with AS
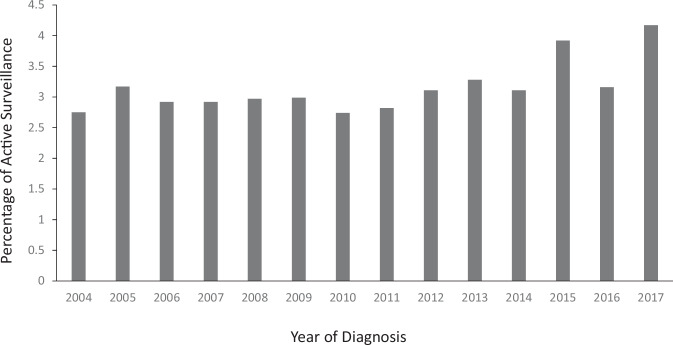
There were 74,367 patients included in the biologically favorable DCIS cohort, and 2,384 (3.2%) of those were not treated with upfront surgery. The proportion of patients in this cohort treated with AS was trended over each year from 2004 to 2017 and is shown in Fig. 1. A statistically significant increase in utilization of AS was noted over time, peaking in 2017, with 4.2% of patients diagnosed in this final year of the study period treated with AS (p < 0.001, Cochran-Armitage test). These trends in increased utilization of AS were seen in both cohorts of women less than 50 years as well as greater than 50 years of age, with highest levels of AS in these groups again seen in 2017 (3.9% in <50 years; 4.2% in >50 years, data not shown).
Fig. 1. Trend of active surveillance over time.
Results of Cochran-Armitage trend test to assess the changes in the proportion of surgery omitted for biologically favorable DCIS over year. There was a statistically significant increase in the proportion over time, peaking in 2017 with 4.2%, p < 0.001.
The characteristics for the patients in the biologically favorable cohort treated with surgery or AS are listed in Supplementary Table 2. Patients in the AS group were older compared to patients treated with upfront surgery (mean age 62.2 ± 13.3 [standard deviation] years vs 59.8 ± 11.3 years, p < 0.01), however they were more likely to be reported as having a Charlson Comorbidity Index score of 0 (92.0% in the AS group vs. 86.0% in the surgery group, p < 0.01). Race based differences were also noted, with a greater proportion of white patients undergoing surgery as compared to black or other race cohorts (81.5%, 12.7%, 5.8%, respectively for surgery vs. 72.9%, 19.8%, 7.3%, respectively for AS, p < 0.01). Type of insurance coverage also significantly varied between surgery and AS patients, with surgical patients more likely to have private insurance (60.5% vs 51.6%) and AS patients more likely to have Medicare coverage (39.2% vs 32.4%) or be uninsured (2.9% vs. 1.4%) (overall p < 0.01). A significant difference was also noted in DCIS size between surgery and AS cohorts, with a higher percentage of smaller lesions (<2 cm) in the surgery group (p < 0.01). However, there was a large proportion of unknown data for this variable, making it difficult to interpret a trend. There was also a significant difference in utilization of endocrine therapy between the groups. In the surgery group, 48.1% of patients used endocrine therapy, compared to only 12.9% of the patients in the AS group (<0.01). Of note, utilization of endocrine therapy in the AS group varied by age, with lowest rate of use noted in younger patient (7.1%, 10.7%, and 17.6% in patients <50 years, 50–64 years and ≥65 years respectively, p < 0.01, Supplementary Table 3).
Factors associated with AS
The results of the multivariable logistic regression model of factors associated with AS are demonstrated in Table 1. Compared to white patients, black patients with biologically favorable DCIS had a 1.66 (95% CI: 1.48–1.86, p < 0.01) odds likelihood of being managed non-operatively. When compared to patients with private insurance, patients who were not insured were more likely to be managed without surgery (OR 2.18, 95% CI: 1.67–2.84, p < 0.01). Patients treated at a comprehensive community cancer program were less likely to be treated with AS compared to an academic center (OR 0.84, 95% CI: 0.76–0.93, p < 0.01), and patients in the South were more likely to be treated with AS compared to patients in the Northeast (OR 1.33, 95% CI: 1.19–1.49, p < 0.01). Compared to patients with a Charlson Comorbidity Score of 0, patients with a score of 1 and 2 were less likely to be treated with AS (OR 0.44, 95% CI: 0.37–0.52, p < 0.01; OR 0.59, 95% CI: 0.42–0.83, p < 0.01 respectively). When looking at tumor size, tumors 5 cm or larger were more likely to be treated without surgery compared to tumors <1 cm (OR 2.12, 95% CI: 1.63–2.77, p < 0.01). Patients treated with endocrine therapy were also less likely to be treated non-operatively, compared to patients who were not treated with endocrine therapy (OR 0.16, 95% CI: 0.14–0.18, p < 0.01).
Table 1.
Multivariable logistic regression model of characteristics associated with non-operative management of biologically favorable DCIS
| Covariate | Odds ratio | 95% CI | P value | ||
|---|---|---|---|---|---|
| Age (every 5-year increase) | 1.10 | 1.07 | 1.13 | <0.01 | |
| Race (ref = White) | Black | 1.66 | 1.48 | 1.86 | <0.01 |
| Other | 1.43 | 1.21 | 1.70 | <0.01 | |
| Unknown | 3.08 | 2.39 | 3.97 | <0.01 | |
| Year of Diagnosis (ref = 2004–2009) | 2010–2013 | 1.21 | 1.08 | 1.35 | <0.01 |
| 2014–2017 | 1.08 | 0.97 | 1.20 | 0.17 | |
| Insurance Status (ref: private) | Medicaid | 1.19 | 0.97 | 1.46 | 0.09 |
| Medicare | 1.00 | 0.89 | 1.13 | 0.95 | |
| Other Government | 1.43 | 1.00 | 2.05 | 0.05 | |
| Not Insured | 2.18 | 1.67 | 2.84 | <0.01 | |
| Unknown | 2.24 | 1.74 | 2.90 | <0.01 | |
| Facility Type (ref = Academic/Research Program) | Community Cancer Program | 0.91 | 0.81 | 1.02 | 0.11 |
| Comprehensive Community Cancer Program | 0.84 | 0.76 | 0.93 | <0.01 | |
| Facility Location (ref = Northeast) | South | 1.33 | 1.19 | 1.49 | <.001 |
| Midwest | 0.89 | 0.79 | 1.02 | 0.10 | |
| West | 1.08 | 0.93 | 1.26 | 0.30 | |
| Charlson Comorbidity Score (ref = 0) | 1 | 0.44 | 0.37 | 0.52 | <.001 |
| 2 | 0.59 | 0.42 | 0.83 | <0.01 | |
| 3 | 0.91 | 0.57 | 1.44 | 0.68 | |
| Tumor Size (ref = <1 cm) | ≥1 cm, <2 cm | 0.85 | 0.71 | 1.01 | 0.07 |
| ≥2 cm, <3 cm | 1.00 | 0.77 | 1.29 | 0.99 | |
| ≥3 cm, <4 cm | 1.35 | 0.96 | 1.90 | 0.09 | |
| ≥4 cm, <5 cm | 1.15 | 0.73 | 1.80 | 0.54 | |
| ≥5 cm | 2.12 | 1.63 | 2.77 | <0.01 | |
| Unknown | 3.06 | 2.73 | 3.41 | <0.01 | |
| Endocrine Therapy | Yes | 0.16 | 0.14 | 0.18 | <0.01 |
| (ref = No) | Unknown | 1.09 | 0.95 | 1.25 | 0.23 |
Overall survival analysis
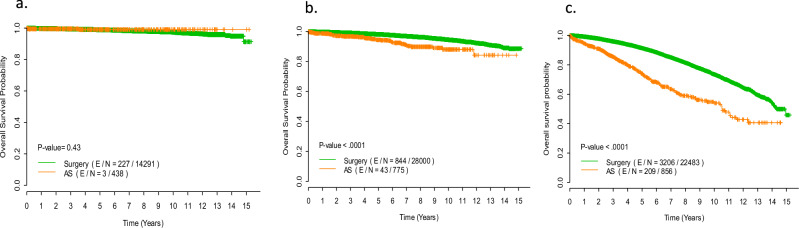
Analysis of the overall cohort for survival outcomes showed significantly improved overall survival (OS) for women undergoing surgical treatment for DCIS with 10-year OS of 88.1% as compared to 75.8% for women in the AS group (p < 0.01 log-rank test) (Supplementary Fig. 1). In multivariable Cox proportional hazards regression model, after adjustment for age and co-morbidity, women who received surgical care at the time of diagnosis remained significantly more likely to have improved OS (hazard ratio 0.47 [95%CI: 0.41–0.53], p < 0.01)(Table 2). In order to further assess the potential impact of surgery while controlling for the confounding effects of age and co-morbidity, the biologically-favorable cohort was then divided by age into three groups-those less than 50 years of age, those between 50 and 64 years of age, and those 65 years of age and older. The Kaplan Meier survival curves stratified by treatment type (surgery vs AS) for each age group are seen in Fig. 2. In women less than 50 years of age, the 10-year OS was 97.4% in the surgery group vs 99.1% in the AS group (p = 0.43). In women between 50 and 64 years of age, the 10-year OS was 94.6% in the surgery group vs 88.0% in the AS group (p < 0.01). In women 65 years and older, the 10-year OS was 73.6% in the surgery group vs 54.0% in the AS group (p < 0.01). This association between improved OS and surgery for biologically favorable DCIS in the 50 to 64 year old age group and 65 years and older age group was maintained even after adjusting for age, race, insurance status, facility type, comorbidity score, tumor size, and endocrine therapy use (for 50 to 64, hazard ratio for surgery: 0.43 [95% CI: 0.31–0.58], p < 0.01; for ≥65 years of age, the hazard ratio for surgery: 0.49 [95% CI: 0.42–0.56], p < 0.01). (Table 3).
Table 2.
Multivariable Cox proportional hazards model assessing the association between overall survival outcome and receipt of surgery after adjusting for comorbidity and age
| Covariate | Hazard Ratio | 95% CI | P value | ||
|---|---|---|---|---|---|
| Age (per 5-year increase) | 1.61 | 1.59 | 1.63 | <0.01 | |
| Surgery (ref: No) | Yes | 0.47 | 0.41 | 0.53 | <0.01 |
| Charlson Comorbidity Score | 0 vs. 3 | 0.26 | 0.21 | 0.33 | <0.01 |
| 1 vs. 3 | 0.41 | 0.33 | 0.52 | <0.01 | |
| 2 vs. 3 | 0.71 | 0.55 | 0.91 | <0.01 | |
Fig. 2. Survival outcomes by treatment.
Kaplan Meier Survival Curves of Immediate Surgery vs Active Surveillance (AS) in the Biologically Favorable DCIS Cohort, per Age Group. Survival outcomes were comparable between surgery and AS in the cohort of women under age 50 at diagnosis of DCIS (a). Surgery appeared to confer survival benefit over AS in women 50–64 years (b) and those ≥65 years (c). A log-rank test was employed to compare survival curves between two groups.
Table 3.
Multivariable Cox proportional hazards model assessing the association between overall survival outcome and receipt of surgery after adjusting for other covariates among patients in the subgroup age 50–64 years (n = 28,775, # of events = 887) and the subgroup age ≥65 years (n = 23,339, # of events = 3415)
| Subgroup age 50–64 years | Subgroup age ≥ 65 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |||
| Surgery (ref = No) | Yes | 0.43 | 0.31 | 0.58 | <0.01 | 0.49 | 0.42 | 0.56 | <0.01 |
| Age | per 5-year increase | 1.31 | 1.21 | 1.42 | <0.01 | 1.68 | 1.63 | 1.72 | <0.01 |
| Year of diagnosis (ref = 2004–2009) | 2010–2013 | 1.05 | 0.89 | 1.24 | 0.56 | 0.97 | 0.89 | 1.05 | 0.41 |
| 2014–2017 | 1.01 | 0.78 | 1.32 | 0.94 | 0.90 | 0.78 | 1.03 | 0.13 | |
| Race (ref=White) | Black | 1.34 | 1.13 | 1.60 | <0.01 | 1.13 | 1.02 | 1.25 | 0.02 |
| Other | 0.72 | 0.48 | 1.07 | 0.10 | 0.72 | 0.57 | 0.91 | 0.01 | |
| Unknown | 0.32 | 0.10 | 1.01 | 0.05 | 0.94 | 0.65 | 1.36 | 0.74 | |
| Insurance Status (ref = Private Insurance) | Medicaid | 2.07 | 1.61 | 2.66 | <0.01 | 1.12 | 0.81 | 1.53 | 0.49 |
| Medicare | 3.49 | 2.93 | 4.14 | <0.01 | 1.11 | 1.00 | 1.24 | 0.06 | |
| Other Government | 1.71 | 1.04 | 2.82 | 0.04 | 1.50 | 0.86 | 2.61 | 0.15 | |
| Not Insured | 1.35 | 0.84 | 2.17 | 0.21 | 0.97 | 0.59 | 1.830 | 0.93 | |
| Unknown | 1.65 | 1.00 | 2.72 | 0.05 | 0.77 | 0.54 | 1.08 | 0.13 | |
| Facility Type (ref = Academic/Research Program) | Community Cancer Program | 1.36 | 1.13 | 1.65 | <0.01 | 1.22 | 1.11 | 1.35 | <0.01 |
| Comprehensive Community Cancer Program | 1.34 | 1.14 | 1.59 | <0.01 | 1.16 | 1.06 | 1.27 | <0.01 | |
| Facility location (ref = Northeast) | South | 1.14 | 0.94 | 1.38 | 0.20 | 1.07 | 0.97 | 1.17 | 0.18 |
| Midwest | 1.26 | 1.04 | 1.54 | 0.02 | 1.10 | 1.00 | 1.21 | 0.06 | |
| West | 1.01 | 0.79 | 1.30 | 0.92 | 0.87 | 0.77 | 0.99 | 0.03 | |
| Rural-Urban Continuum | Urban | 1.16 | 0.95 | 1.42 | 0.14 | 1.11 | 1.01 | 1.23 | 0.04 |
| (ref = Metro) | Rural | 0.59 | 0.28 | 1.25 | 0.17 | 0.96 | 0.73 | 1.27 | 0.79 |
| Unknown | 0.89 | 0.55 | 1.44 | 0.64 | 0.99 | 0.79 | 1.24 | 0.93 | |
| Charlson Deyo Comorbidity Score (ref = 0) | 1 | 1.67 | 1.39 | 1.99 | <0.01 | 1.48 | 1.36 | 1.61 | <0.01 |
| 2 | 2.72 | 1.99 | 3.70 | <0.01 | 2.34 | 1.99 | 2.75 | <0.01 | |
| 3 | 5.27 | 3.42 | 8.11 | <0.01 | 2.85 | 2.17 | 3.74 | <0.01 | |
| Tumor Size (ref = <1 cm) | ≥1 cm, <2 cm | 1.02 | 0.84 | 1.24 | 0.87 | 1.03 | 0.93 | 1.13 | 0.55 |
| ≥2 cm, <3 cm | 1.10 | 0.82 | 1.48 | 0.53 | 1.07 | 0.92 | 1.24 | 0.38 | |
| ≥3 cm, <4 cm | 1.66 | 1.13 | 2.44 | 0.01 | 1.31 | 1.06 | 1.61 | 0.01 | |
| ≥4 cm, <5 cm | 1.52 | 0.94 | 2.48 | 0.09 | 1.06 | 0.83 | 1.37 | 0.64 | |
| ≥5 cm | 1.21 | 0.81 | 1.82 | 0.36 | 1.07 | 0.85 | 1.35 | 0.56 | |
| Unknown | 0.92 | 0.78 | 1.08 | 0.29 | 1.02 | 0.94 | 1.11 | 0.60 | |
| Endocrine Therapy (ref = No) | Yes | 0.80 | 0.70 | 0.92 | <0.01 | 0.83 | 0.77 | 0.90 | <0.01 |
| Unknown | 1.00 | 0.76 | 1.31 | 0.98 | 0.93 | 0.81 | 1.07 | 0.30 | |
Discussion
We sought to understand the utilization of AS for DCIS over the past two decades and to evaluate the survival outcomes for patients with biologically favorable DCIS who do not undergo surgery. The proportion of women with biologically favorable DCIS who are not undergoing surgery at time of diagnosis has increased overtime, peaking at 4.2% in 2017, the final year of our study. These trends were noted across the age continuum. As with other components of breast cancer care, treatment with AS differed by race, insurance status, geographic location and practice type. Interestingly, although increasing age was associated with higher odds of AS, increasing comorbidity was largely associated with lower odds of AS and the association between extent of DCIS and likelihood of AS was inconsistent. These data suggest a lack of a systematic framework for treatment decision making in this population, consistent with the lack of guidelines for AS at present.
Our data also suggested that patients with biologically favorable DCIS and less than 50 years of age treated with AS have favorable long-term OS, comparable to those seen with surgical intervention. Interestingly, in older populations, survival appeared improved with surgical intervention, with the absolute benefit in favor of surgery increasing with increasing age cohorts. While the low overall rate of endocrine therapy in the AS cohort may also be contributory to this difference in survival outcomes, it is unlikely to fully explain the improvements seen in favor of surgery, as older women were more likely than younger ones to take endocrine therapy if opting for AS. These age-based observations of the benefit of surgery over AS most likely reflect underlying health conditions in older populations that cannot be fully accounted for with adjustment for comorbidity scores. These findings mirror a recent report by Akushevich et al, who similarly reported a survival benefit to surgery in patients with DCIS over 65 years of age at diagnosis12. Importantly, they reported that the magnitude of survival benefit was markedly reduced after accounting for baseline comorbidities, underscoring both the challenge of fully accounting for this bias in such analyses, as well as the complexity of treatment decision making in this population of older patients.
As the possibility of AS for DCIS patients has increasingly been explored in recent years, a number of studies have reported on historical outcomes of patients who have not completed guideline concordant care. In a meta-analysis of over 9000 patients with DCIS drawn largely from retrospective studies and treated with a range of approaches, Stuart et al reported a local regional recurrence rate of 27.8% (both DCIS and invasive) in the subset of patients that were treated with biopsy (frequently excision biopsy with positive margins) alone13. Although the 10 year breast cancer death rate was not significantly higher in this group as compared to other treatment groups, the breast cancer death rate with at least 15 years of follow up increased to 17.9% in DCIS patients who underwent a biopsy alone13. Importantly in this study, the analysis was done by treatment which was aggregated at study level; patient and tumor characteristics that could have confounded results were not considered. These data therefore do not preclude the possibility of patient subsets who may be appropriate for AS.
A number of studies have considered tumor and patient characteristics in identifying subsets who may be appropriate for AS. Ryser et al reported that in a cohort of 1286 DCIS patients who did not undergo locoregional therapy, the 10-year cumulative incidence of ipsilateral invasive cancer was 10.5%. This risk was influenced by disease grade, with grade I/II patients reported to have a 10 year cumulative incidence of ipsilateral invasive cancer of 12.2% as compared to a 17.6% risk in grade III disease14. Grade was unknown in almost 40% of the cohort, likely influencing these estimates. Sun et al. utilized the SEER database to compare the risk of invasive disease in three groups of women with grade I/II, HR + DICS: those who had no treatment, those treated with breast conserving surgery (BCS) alone, and those treated with surgery and radiation15. When stratifying the cohort by age, the authors found that in patients above the age of 70, there was no difference in the risk of development of invasive cancer in patients who had no treatment versus BCS only (adjusted HR 0.86, 95% confidence interval 0.34–2.16) or BCS plus radiation (adjusted HR 0.51, 95% confidence interval 0.20–1.31). Lastly, using the SEER registry, Sagara et al. reported a 10 year breast cancer specific survival of 93.4% in the AS group compared to 98.5% in the surgery group5. This difference in survival however was variable by grade of disease, with no difference in survival observed between AS and surgical groups with low grade DCIS. Similar patterns were observed for OS. Collectively these data, together with observations from our study, underscore that there are subsets for whom AS may be appropriate without compromise of long-term survival, although which subsets of DCIS can be considered low risk and thus appropriate for AS remain to be conclusively defined.
To definitively address the appropriateness of AS, a number of large, randomized control trials have recently been undertaken that are examining the outcomes of omitting surgery for biologically favorable DCIS. The LORIS (Low Risk DCIS) trial in the United Kingdom and the Comparison of Operative versus Monitoring and Endocrine Therapy (COMET) in the United States have recently closed8,16,17. The LORD (Management of Low-Risk DCIS) trial from the Netherlands, continues to be open to accrual, but now utilizes patient preference when assigning to surgery or active surveillance arms18. While these trials did not all meet their accrual targets, data from these studies will nonetheless provide important insights into the role of AS in selected populations of DCIS patients.
This study has limitations that are inherent to using a large national database to retrospectively analyze outcomes. We could not control for unmeasured variables not captured in the database, which may have confounded the survival results. Although our analyses adjusted for available comorbidities, unmeasured variables captured in clinical geriatric assessments such as functional status or cognitive function which impact life expectancy could not be captured. In large databases, it is also difficult to control for non-standardization of practices, and how the treatment decision was derived. In our study, only 13% of the AS cohort received endocrine therapy, despite all of the patients having estrogen receptor positive DCIS, when ideally this therapy is offered to all women with hormone receptor positive DCIS wishing to undergo active surveillance. In addition, the NCDB does not include data on DCIS or invasive breast cancer recurrence, so we can only analyze OS outcomes using this dataset. Lastly, our methodology also largely excluded patients that were upstaged to invasive cancer at the time of surgery, whereas some in the AS group would have been expected to harbor occult cancer. This may have affected the survival analysis in favor of surgery, particularly in older women.
Methods
Data source
The National Cancer Data Base (NCDB) is a clinical cancer database maintained by the Commission on Cancer (COC) and the American Cancer Society. The NCDB collects hospital registry data from more than 1500 COC-accredited facilities in the United States and Puerto Rico, captures ~75% of new cancer diagnoses, and includes information on patient demographics, tumor staging and histology, treatment, and OS. The data is available to members of the American College of Surgeons. Patient level identifiers are not available to users of the database; therefore, this study was exempt from IRB review and approval.
Selection of cohort
The stepwise inclusion criteria to develop a cohort of patients with biologically favorable DCIS from the NCDB that would meet inclusion criteria for AS trials in the United States is listed in Supplementary Table 1. Exclusion criteria included all men, women under the age of 40 or older than 99, patients with high grade DCIS, and patients with ER and PR negative DCIS. Patients with DCIS were included if they were coded as having in situ disease based on the clinical TNM variable and if they also were coded as having a Behavior Code of “2” consistent with in situ disease; in the patients treated with surgery, there was no additional exclusion based on pathologic TNM coding showing upstaging to invasive cancer. Nonetheless, this approach substantially eliminated patients who were upstaged at surgery, with less than 1% of our cohort recorded as definitively having >pTIS disease (data not shown). Patients were also excluded if their International Classification of Diseases for Oncology histology codes were not 8500, 8501, 8010, 8050, 8522, 8504, 8201, or 8230, and if their surgery variable was listed as unknown. We classified patients as being in the AS cohort if they did not have surgery, or their surgery date from day of diagnosis was >1 year.
Statistical analysis
A Cochran-Armitage test was used to assess the trend of AS over year of diagnosis. Descriptive statistics were used to summarize patient demographic and clinical/pathologic characteristics. The chi-square tests, Fisher exact tests and Wilcoxon rank-sum tests were used to test the differences in baseline covariates (including age, year of diagnosis, race, insurance status, facility type, facility location, Charlson comorbidity index, tumor size, and endocrine therapy) between those treated with surgery and those in the AS group. Multivariable logistic regression was used to assess association between baseline factors and AS. Kaplan-Meier curves were estimated to show OS in this biologically favorable DCIS group that underwent AS, stratified by age (<50, 50–64, >65 years). The log rank test was used to test the difference in OS between the surgery group and the AS group. Univariate and multivariable Cox proportional hazard models were used to determine the effects of prognostic factors on survival distributions. All tests were two-sided. P values of <0.05 were considered statistically significant. All analyses were conducted using the SAS (version 9.4; SAS Institute, Cary, NC) and S-plus (version 8.04, TIBCO Software Inc., Palo Alto, CA) statistical software.
Supplementary information
Acknowledgements
This study was supported in part by the Cancer Center Support Grant P30 CA016672 and R01 CA269696 from the National Cancer Institute of the National Institutes of Health (Dr Shen). The funder played no role in the study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Author contributions
Conception and Design: E.C.P., Y.S., I.B. Collection and assembly of the data: all authors. Data analysis and interpretation: All authors. Manuscript writing: all authors. All authors reviewed and approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available through the NCDB but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of NCDB.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-024-00689-5.
References
- 1.Narod, S. A., Iqbal, J., Giannakeas, V., Sopik, V. & Sun, P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol.1, 888–896 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Feinberg J., Wetstone R., Greenstein D. & Borgen P. Is DCIS overrated? In: Gradishar W. J., editor. optimizing breast cancer management. Springer International Publishing. Cancer Treat. Res. 173, 53–72 (2018). [DOI] [PubMed]
- 3.Esserman, L. & Yau, C. Rethinking the standard for ductal carcinoma in situ treatment. JAMA Oncol.1, 881–883 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Ozanne, E. M. et al. Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res. Treat.129, 165–173 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Sagara, Y. et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ a population-based cohort study. JAMA Surg.150, 739–745 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Esserman, L. J. et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol.15, 234–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groen, E. J. et al. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast31, 274–283 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Hwang, E. S. et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open9, e026797 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheelwright, S. et al. Recruiting women with ductal carcinoma in situ to a randomised controlled trial: lessons from the LORIS study. Trials24, 1–12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz, R. S. J. M. et al. Active surveillance versus treatment in low-risk DCIS: women’s preferences in the LORD-trial. Eur. J. Cancer192, 113276 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanbayashi, C. & Iwata, H. Current approach and future perspective for ductal carcinoma in situ of the breast. Jpn J. Clin. Oncol.47, 671–677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akushevich, I., Yashkin, A. P., Greenup, R. A. & Hwang, E. S. A medicare-based comparative mortality analysis of active surveillance in older women with DCIS. npj Breast Cancer6, 57 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuart, K. E., Houssami, N., Taylor, R., Hayen, A. & Boyages, J. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer15, 890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryser, M. D. et al. Cancer outcomes in DCIS patients without locoregional treatment. J. Natl. Cancer Inst.111, 952–960 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun, S. X. et al. No treatment versus partial mastectomy plus radiation for ductal carcinoma in situ. Ann. Surg. Oncol.29, 39–41 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Francis, A. et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur. J. Cancer51, 2296–2303 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Francis, A., Fallowfield, L. & Rea, D. The LORIS trial: addressing overtreatment of ductal carcinoma in situ. Clin. Oncol.27, 6–8 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Elshof, L. E. et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—the LORD study. Eur. J. Cancer51, 1497–1510 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available through the NCDB but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of NCDB.