Abstract
The use of a faecal preservative and several staining methods, together with formalin ether concentration, were evaluated for the improved diagnosis of intestinal amoebiasis and giardiasis in 1285 patients with diarrhoea or dysentery and from asymptomatic controls. All samples were screened by three wet mount techniques. Thirty eight specimens of diarrhoeal or dysenteric stool were preserved in polyvinyl alcohol (PVA) and stained by trichrome and Spencer and Monroe short iron haematoxylin stain. Thirty nine preserved faecal samples submitted for routine screening were subjected to formalin ether concentration, wet mount examination, and permanent staining. Saline and buffered methylene blue (BMB) mounts were equally good for detection of trophozoite Entamoebae while Giardia trophozoites were detected only by the saline mount. The iodine mount was superior to the other mounts for protozoan cyst detection. The concentration procedure enhanced cyst recovery. Faecal preservation and subsequent staining was superior to wet mount examination for detection of the trophozoite stage and avoided the need for fresh specimens. Both the trichrome and the iron haematoxylin stains were comparable for the detection of cysts and trophozoites of the Entomoebae. Giardia lamblia trophozoites stained better with iron haematoxylin than with the trichrome. Preservation and permanent staining is recommended as the most productive means for the accurate identification of the various protozoan parasites.
Full text
PDF
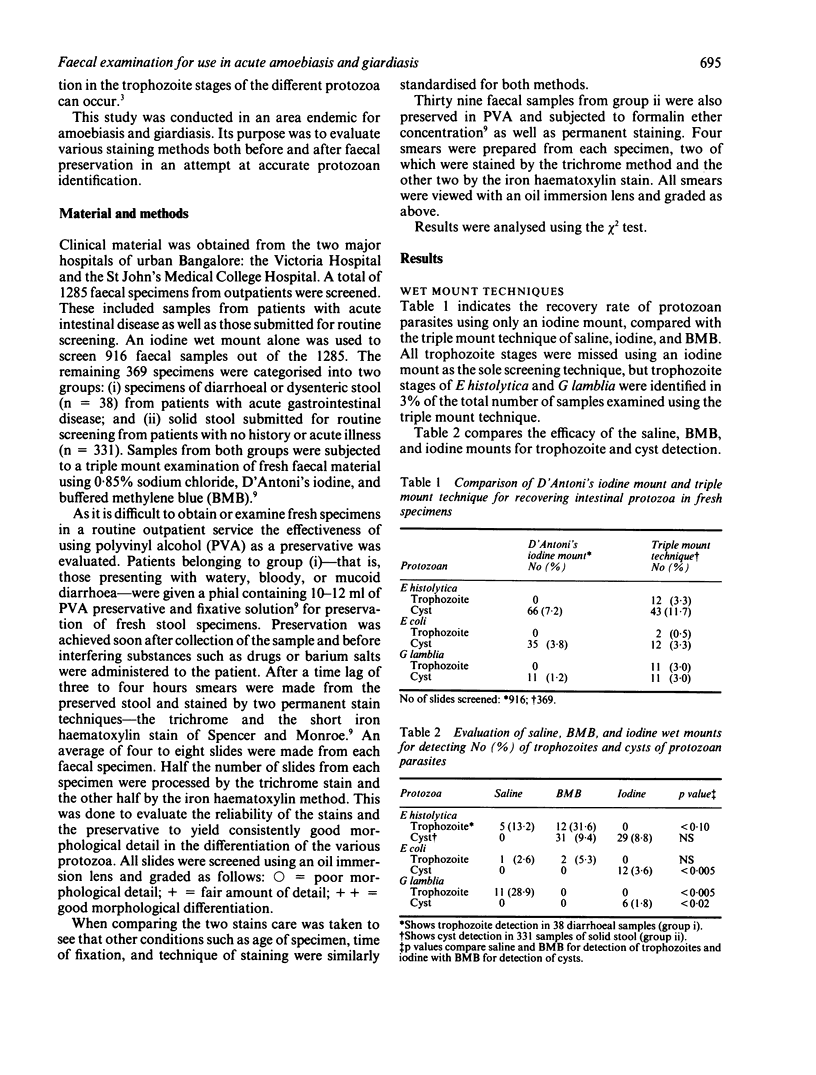
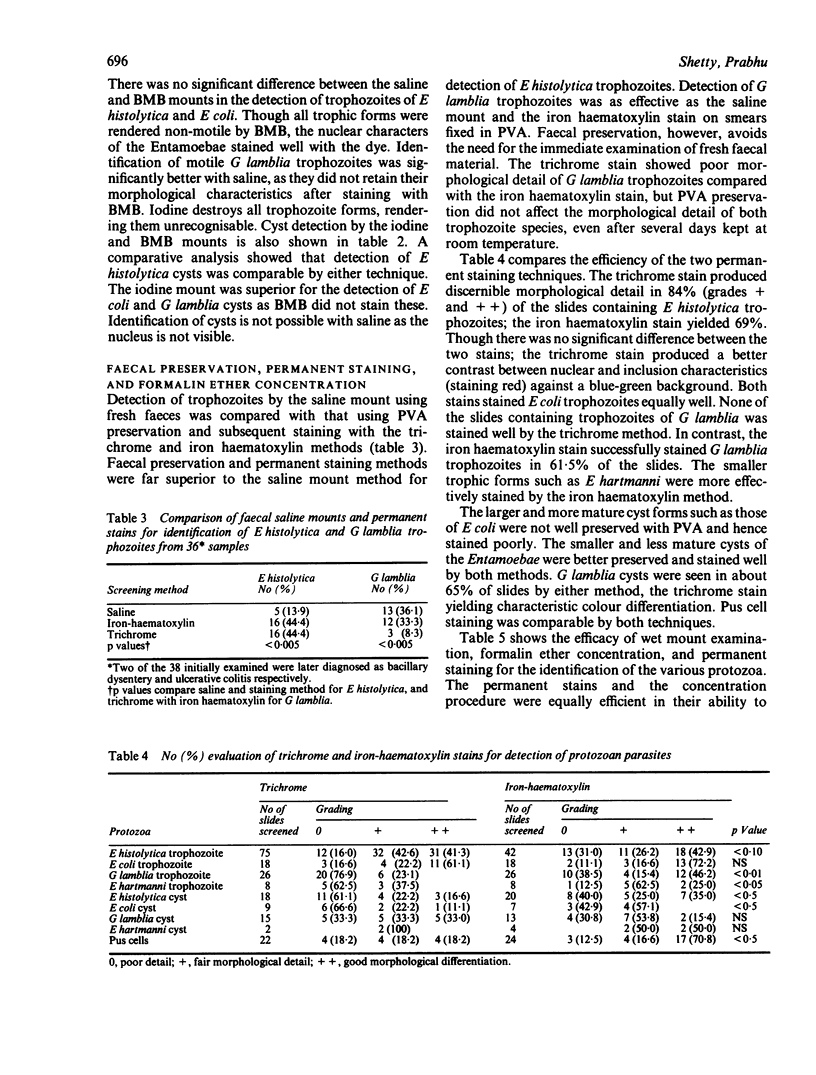
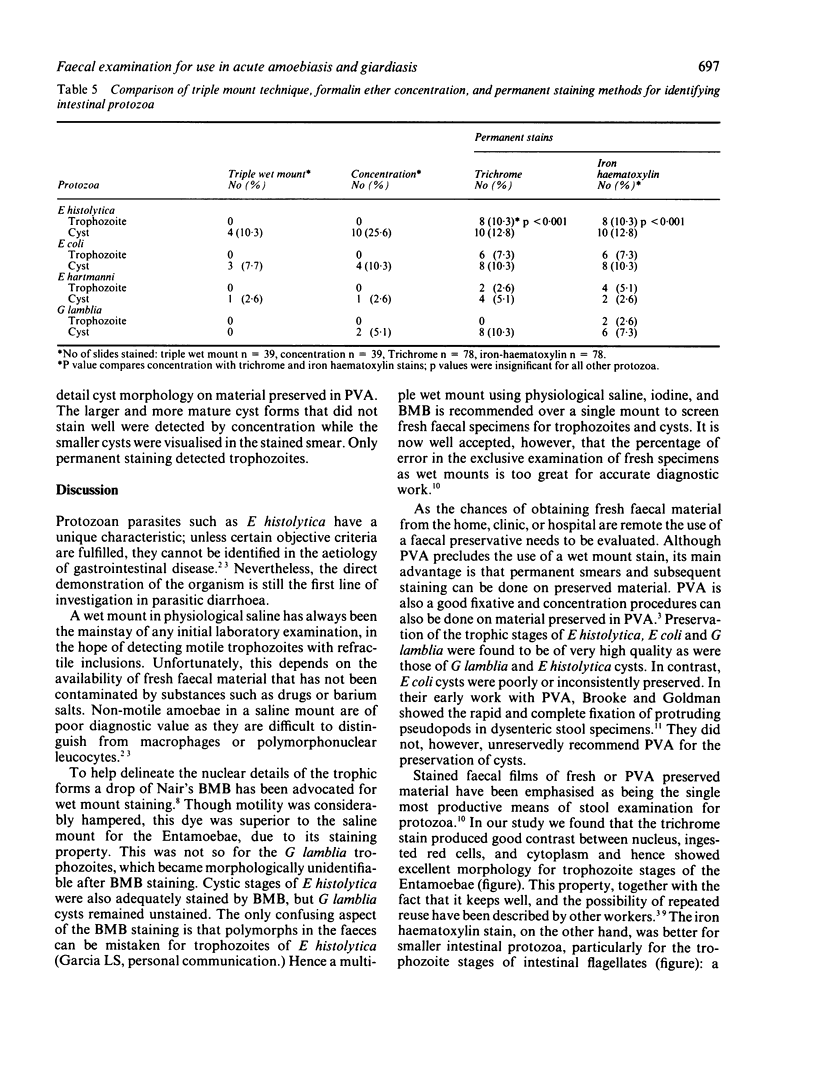
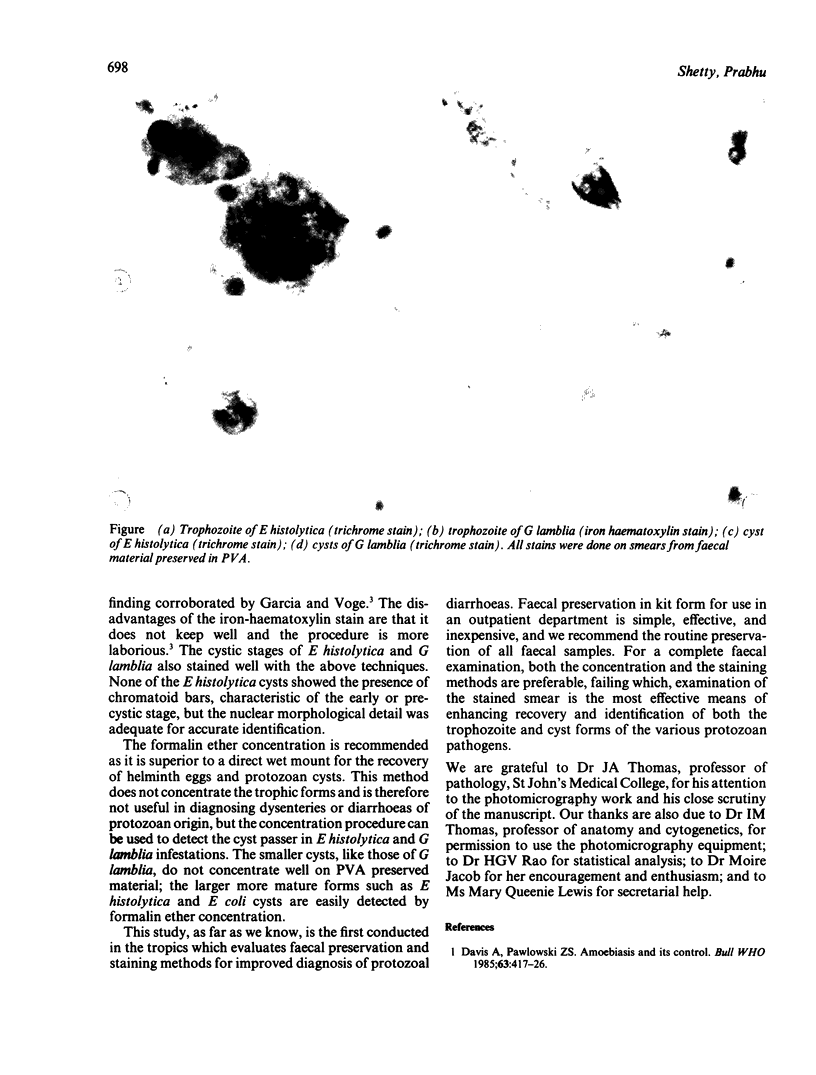

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis A., Pawlowski Z. S. Amoebiasis and its control. Bull World Health Organ. 1985;63(3):417–426. [PMC free article] [PubMed] [Google Scholar]
- Garcia L. S., Brewer T. C., Bruckner D. A. A comparison of the formalin-ether concentration and trichrome-stained smear methods for the recovery and identification of intestinal protozoa. Am J Med Technol. 1979 Nov;45(11):932–935. [PubMed] [Google Scholar]
- Garcia L. S., Voge M. Diagnostic clinical parasitology: I. Proper specimen collection and processing. Am J Med Technol. 1980 Jun;46(6):459–466. [PubMed] [Google Scholar]
- Green E. L., Miles M. A., Warhurst D. C. Immunodiagnostic detection of Giardia antigen in faeces by a rapid visual enzyme-linked immunosorbent assay. Lancet. 1985 Sep 28;2(8457):691–693. doi: 10.1016/s0140-6736(85)92932-0. [DOI] [PubMed] [Google Scholar]
- Grundy M. S., Voller A., Warhurst D. An enzyme-linked immunosorbent assay for the detection of Entamoeba histolytica antigens in faecal material. Trans R Soc Trop Med Hyg. 1987;81(4):627–632. doi: 10.1016/0035-9203(87)90436-6. [DOI] [PubMed] [Google Scholar]
- Healy G. R. Laboratory diagnosis of amebiasis. Bull N Y Acad Med. 1971 May;47(5):478–493. [PMC free article] [PubMed] [Google Scholar]
- Healy G. R. Use of and limitations to the indirect hemagglutination test in the diagnosis of intestinal amebiasis. Health Lab Sci. 1968 Jul;5(3):174–179. [PubMed] [Google Scholar]
- Kovacs J. A., Gill V., Swan J. C., Ognibene F., Shelhamer J., Parrillo J. E., Masur H. Prospective evaluation of a monoclonal antibody in diagnosis of Pneumocystis carinii pneumonia. Lancet. 1986 Jul 5;2(8497):1–3. doi: 10.1016/s0140-6736(86)92555-9. [DOI] [PubMed] [Google Scholar]