Abstract
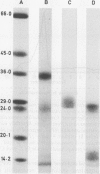
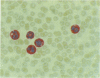
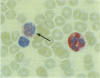
A monoclonal antibody, NP57, was produced and used against the neutrophil granule protein elastase, which selectively stain neutrophils in cryostat and paraffin wax sections. The antibody stains neutrophils and a subpopulation of monocytes in blood smears and neutrophil precursors in bone marrow smears, and gives positive reactions with the cell lines HL60 and U-937. It labelled the blast cells in 68% of cases of acute myeloid leukaemia (M1-M5) but was unreactive with all cases of lymphoid leukaemias. Most of the elastase negative myeloid leukaemias were labelled by monoclonal anti-myeloperoxidase (antibody MPO-7) as were cells from the promyelocytic line HL60. No cases of myeloid leukaemia showed the opposite pattern--that is elastase positive, myeloperoxidase negative, suggesting that the production of myeloperoxidase precedes the onset of elastase synthesis during myeloid maturation. The anti-elastase antibody NP57 is a useful addition to the range of monoclonal antibodies available for the differential diagnosis of acute leukaemia by alkaline phosphatase-antialkaline phosphatase (APAAP) labelling of cell smears; it may also be of value for the histopathological diagnosis of tumour deposits in myeloid leukaemia and for the detection of neutrophils in paraffin sections.
Full text
PDF

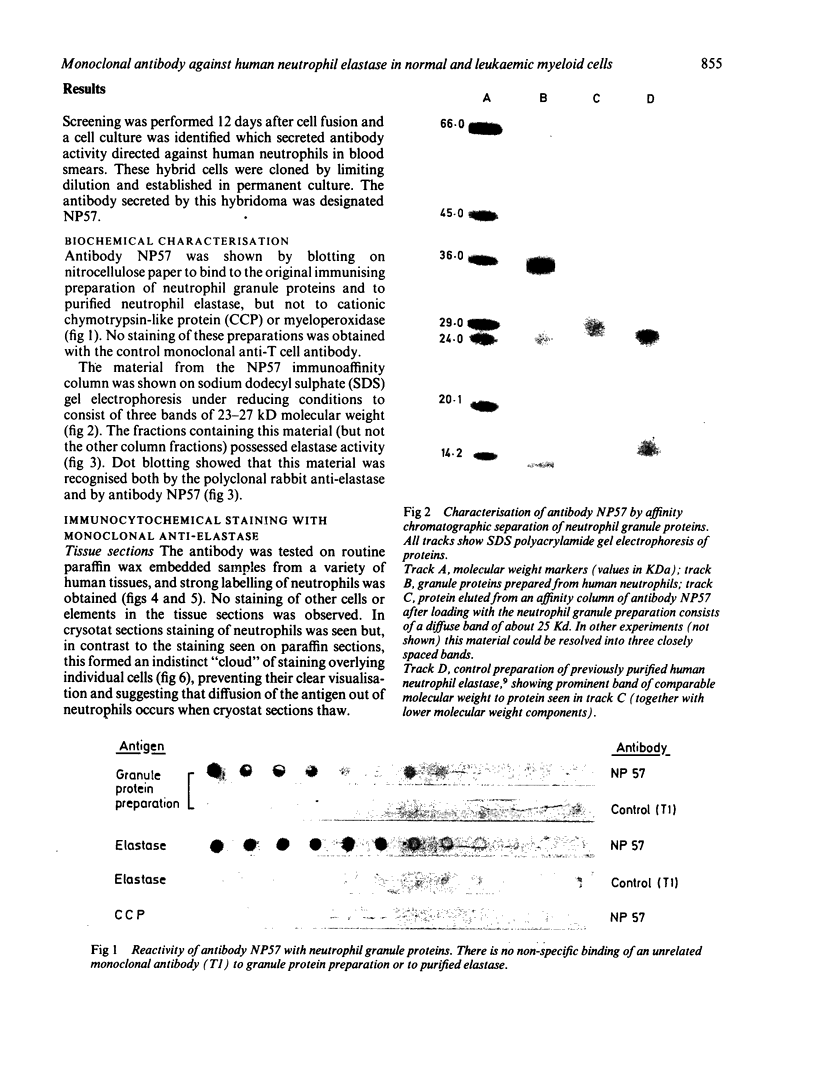
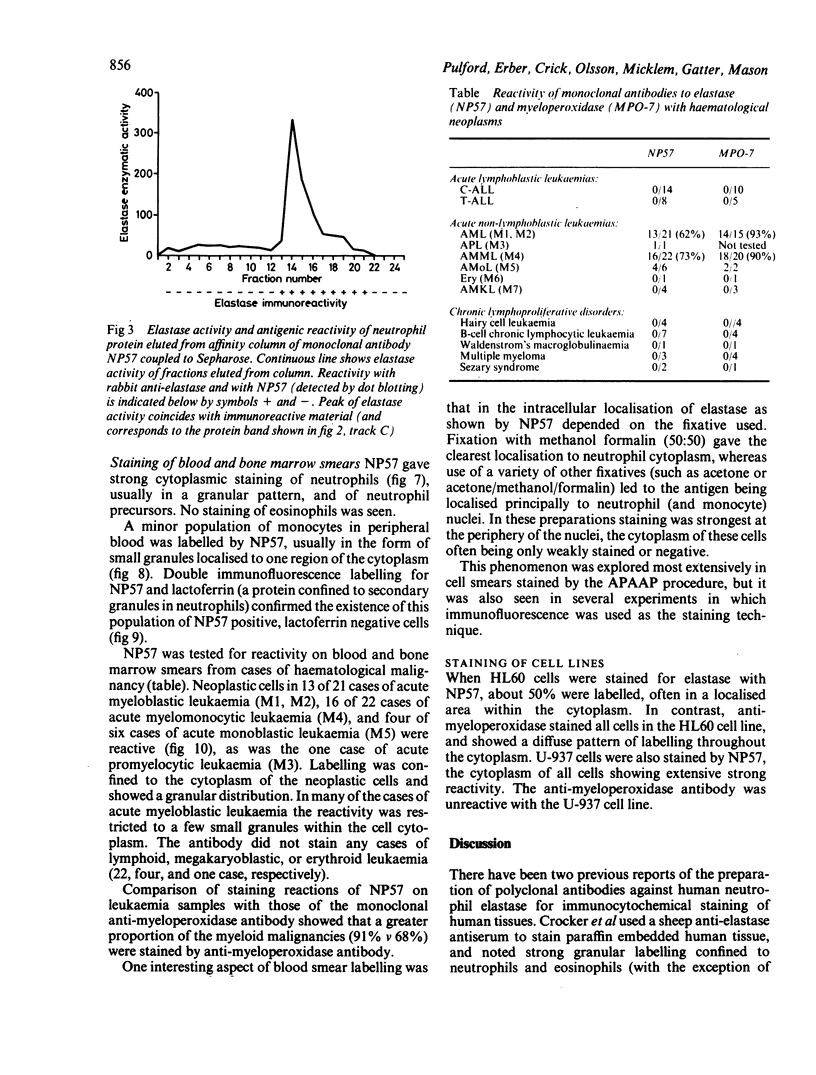
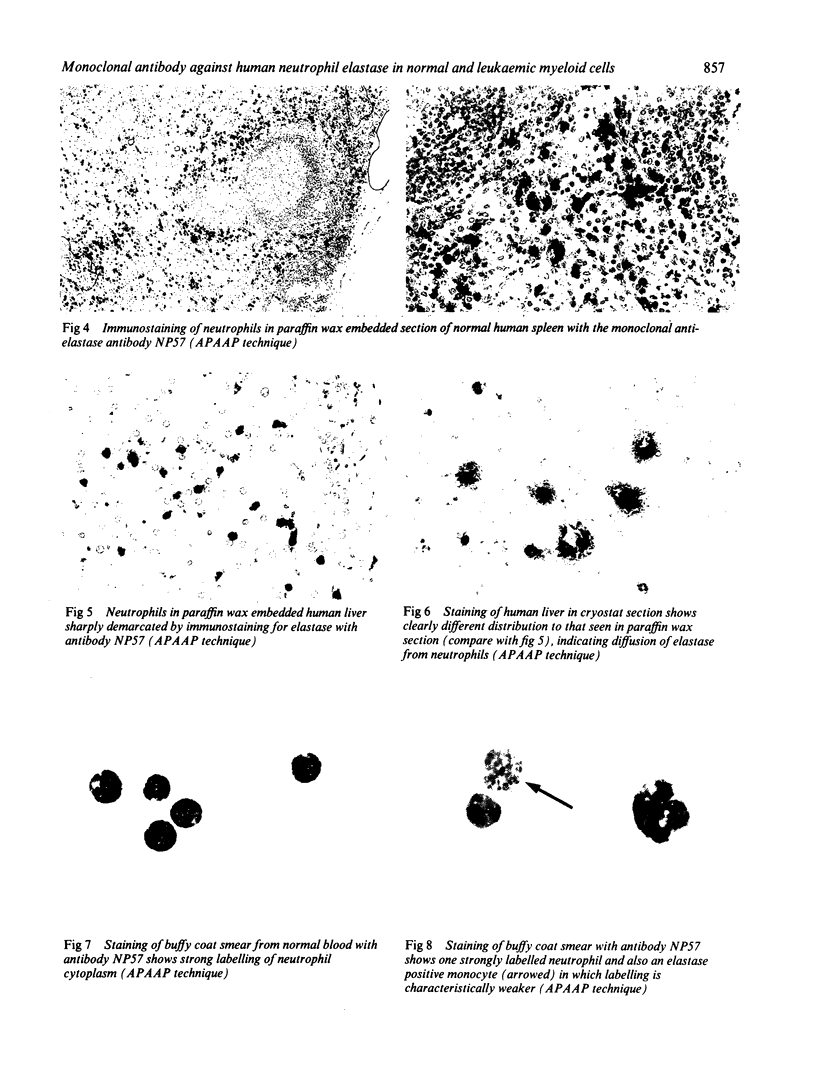



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Briggs R. C., Glass W. F., 2nd, Montiel M. M., Hnilica L. S. Lactoferrin: nuclear localization in the human neutrophilic granulocyte? J Histochem Cytochem. 1981 Oct;29(10):1128–1136. doi: 10.1177/29.10.7028857. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber W. N., Mynheer L. C., Mason D. Y. APAAP labelling of blood and bone-marrow samples for phenotyping leukaemia. Lancet. 1986 Apr 5;1(8484):761–765. doi: 10.1016/s0140-6736(86)91781-2. [DOI] [PubMed] [Google Scholar]
- Erber W. N., Pinching A. J., Mason D. Y. Immunocytochemical detection of T and B cell populations in routine blood smears. Lancet. 1984 May 12;1(8385):1042–1046. doi: 10.1016/s0140-6736(84)91451-x. [DOI] [PubMed] [Google Scholar]
- Green I., Kirkpatrick C. H., Dale D. C. Lactoferrin--specific localization in the nuclei of human polymorphonuclear neutrophilic leukocytes. Proc Soc Exp Biol Med. 1971 Sep;137(4):1311–1317. doi: 10.3181/00379727-137-35779. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Davis R., Nelson D. A., Davey F. R., Mayer R. J., Schiffer C., McIntyre O. R., Bloomfield C. D. Use of surface marker analysis to predict outcome of adult acute myeloblastic leukemia. Blood. 1986 Dec;68(6):1232–1241. [PubMed] [Google Scholar]
- Janoff A., Sandhaus R. A., Hospelhorn V. D., Rosenberg R. Digestion of lung proteins by human leukocyte granules in vitro. Proc Soc Exp Biol Med. 1972 Jun;140(2):516–519. doi: 10.3181/00379727-140-36493. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Kramps J. A., van der Valk P., van der Sandt M. M., Lindeman J., Meijer C. J. Elastase as a marker for neutrophilic myeloid cells. J Histochem Cytochem. 1984 Apr;32(4):389–394. doi: 10.1177/32.4.6561228. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Farrell C., Taylor C. R. The detection of intracellular antigens in human leucocytes by immunoperoxidase staining. Br J Haematol. 1975 Nov;31(3):361–370. doi: 10.1111/j.1365-2141.1975.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. I. Isolation of the cationic proteins from the granules of leukaemic myeloid cells. Scand J Haematol. 1972;9(3):204–214. doi: 10.1111/j.1600-0609.1972.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Paradinas F. J., Boxer G., Bagshawe K. D. Distribution of the Ca (Oxford) antigen in lung neoplasms and non-neoplastic lung tissues. J Clin Pathol. 1984 Jan;37(1):1–5. doi: 10.1136/jcp.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J., Landis J. A., Cox F. R., Kuhn C., Koren H. S. Elastase of U-937 monocytelike cells. Comparisons with elastases derived from human monocytes and neutrophils and murine macrophagelike cells. J Clin Invest. 1982 Feb;69(2):384–393. doi: 10.1172/JCI110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P. Retardation of immunofluorescence fading during microscopy. J Histochem Cytochem. 1985 Aug;33(8):755–761. doi: 10.1177/33.8.3926864. [DOI] [PubMed] [Google Scholar]