Abstract
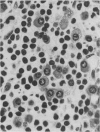
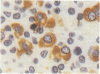
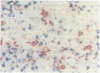
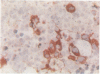
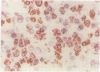
A routine method allows bone marrow biopsy specimens to be embedded in glycol methacrylate (GMA), a water miscible plastic, and to benefit from the advantages of good morphology with immunoperoxidase detection of a wide range of cellular antigens useful in diagnosing and classifying various haematopoietic disorders. Marrow cores were fixed in cold Bouin's solution, rinsed in cold phosphate buffer, dehydrated in cold methanol, infiltrated and embedded in cold GMA, then polymerised at 4 degrees C. Sections were cut at 2 micron thickness with a Tungsten carbide knife in a Jung's high performance microtome (Autocut). Antigenecity was preserved when drying slides at room temperature but pronase digestion was necessary to re-expose the antigens in bone marrow biopsy sections embedded in GMA. Histostik, a new adhesive, was used to coat the glass slides to prevent section loss during enzyme digestion and immunostaining procedures. This method of adapting plastic embedding to undecalcified marrow cores preserves marrow architecture and cellular details and it can serve as a useful adjunct to analyse the bone marrow from patients with myeloproliferative and lymphoproliferative disorders. This technique may also be applicable in non-haematological malignant conditions which affect the marrow.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archimbaud E., Islam A., Preisler H. D. Alcian blue method for attaching glycol methacrylate bone marrow sections to glass slides. Stain Technol. 1986 Mar;61(2):121–122. doi: 10.3109/10520298609110719. [DOI] [PubMed] [Google Scholar]
- Archimbaud E., Islam A., Preisler H. D. Immunoperoxidase detection of myeloid antigens in glycolmethacrylate-embedded human bone marrow. J Histochem Cytochem. 1987 May;35(5):595–599. doi: 10.1177/35.5.2435785. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Pizzolo G., Fiore-Donati L., Bofill M., Janossy G. Routine immunofluorescent and histochemical analysis of bone marrow involvement of lymphoma/leukaemia: the use of cryostat sections. Br J Cancer. 1983 Dec;48(6):763–775. doi: 10.1038/bjc.1983.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falini B., Martelli M. F., Tarallo F., Moir D. J., Cordell J. L., Gatter K. C., Loreti G., Stein H., Mason D. Y. Immunohistological analysis of human bone marrow trephine biopsies using monoclonal antibodies. Br J Haematol. 1984 Mar;56(3):365–386. doi: 10.1111/j.1365-2141.1984.tb03968.x. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Todd R. F., 3rd Immunologic classification of leukemia and lymphoma. Blood. 1986 Jul;68(1):1–31. [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hokland P. The use of monoclonal antibodies in clinical haematology--the art of limitation. Scand J Haematol. 1984 Jul;33(1):5–8. doi: 10.1111/j.1600-0609.1984.tb02202.x. [DOI] [PubMed] [Google Scholar]
- Islam A. A new bone marrow biopsy needle with core securing device. J Clin Pathol. 1982 Mar;35(3):359–364. doi: 10.1136/jcp.35.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A., Henderson E. S. Glycol methacrylate embedding for light microscopy. I. enzyme histochemistry on semithin sections of undecalcified marrow cores. J Clin Pathol. 1987 Oct;40(10):1194–1200. doi: 10.1136/jcp.40.10.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J. P., Vernant J. P., Dreyfus B., Breton-Gorius J. Ultrastructural localization of peroxidases in 'undifferentiated' blasts during the blast crisis of chronic granulocytic leukaemia. Br J Haematol. 1979 Dec;43(4):549–558. doi: 10.1111/j.1365-2141.1979.tb03787.x. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Biberfeld P. Technical aspects of lymphoma immunohistology. J Histochem Cytochem. 1980 Aug;28(8):731–745. doi: 10.1177/28.8.6160179. [DOI] [PubMed] [Google Scholar]
- Rywlin A. M., Ortega R. S., Dominguez C. J. Lymphoid nodules of bone marrow: normal and abnormal. Blood. 1974 Mar;43(3):389–400. [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Takamiya H., Bodemer W., Vogt A. Masking of protein antigen by modification of amino groups with carbobenzoxychloride (benzyl chloroformate) and demasking by treatment with nonspecific protease. J Histochem Cytochem. 1978 Nov;26(11):914–920. doi: 10.1177/26.11.82572. [DOI] [PubMed] [Google Scholar]
- Taylor C. R. Immunocytochemical methods in the study of lymphoma and related conditions. J Histochem Cytochem. 1978 Jul;26(7):496–512. doi: 10.1177/26.7.357639. [DOI] [PubMed] [Google Scholar]
- Vykoupil K. F., Thiele J., Georgii A. Hairy cell leukemia. Bone marrow findings in 24 patients. Virchows Arch A Pathol Anat Histol. 1976 Jul 21;370(4):273–289. doi: 10.1007/BF00445773. [DOI] [PubMed] [Google Scholar]