Abstract
The rise of Multi-Drug Resistant (MDR) bacterial pathogens to most, if not all, currently available antibacterial agents has become a global threat. As a consequence of the antibiotic resistance epidemic, phage therapy has emerged as a potential alternative to conventional antibiotics. Despite the high therapeutic advantages of phage therapy, they have not yet been successfully used in the clinic due to various limitations of narrow host specificity compared to antibiotics, poor adhesion on biofilm surface, and susceptibility to both human and bacterial defences. This review focuses on the antibacterial effect of bacteriophage and their recent clinical trials with a special emphasis on the underlying mechanism of lytic phage action with the help of endolysin and holin. Furthermore, recent clinical trials of natural and modified endolysins and some marketed products have also been emphasized with future prospective.
Keywords: Multi-drug resistant, Pathogens, Endolysin, Phage-therapy, Clinical products
1. Introduction
The invention of antibiotics has become the solution for pathogenic bacterial infectious diseases since the discovery of penicillin, results revolutionizing modern medical therapy. However, widespread use or misuse of antibiotics has become the cause for the persistence of antibiotic-resistant (ABR) pathogenic bacteria that may result in their ever-escalating prevalence posing a great threat to the world [[1], [2], [3]]. A group of Multi-Drug Resistant (MDR) pathogens including, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumannii, and Enterobacter species, have become a global threat according to WHO, causing a majority of nosocomial infections developing resistant to common antibiotics used in the clinics. It is also estimated that by 2050 around 10 million deaths willoccur due to the growing MDR pathogens per year [[4], [5], [6], [7], [8], [9], [10]]. Currently, MDR pathogens are the reason for many life-threatening diseases and pose a global economic burden. A recent study by Data Bridge Market Research estimated that the global MDR market size will increase to USD 16.02 billion by 2029 from USD 10.359 billion in 2021, with a Compound Annual Growth Rate (CAGR) of 5.60 % between the period 2022 to 2029. The growing rate of drug resistance pulls back the big pharmaceutical companies from developing new antibiotics due to their non-effectiveness and high cost of production [11,12]. Because the present slow pace of developing new antibiotics cannot keep up with the life-threatening infections caused by MDR, hence it is essential need for the development of novel techniques or alternatives to antibiotics [13,14]. In the recent years, researchers have used computational screening such as Artificial Intelligence (AI) to ease the process of screening new antibiotics. Although computational screening provides a better platform for designing novel antibiotics to address the unmet needs of the exponentially increasing global MDR threat. However, there is a lack of detailed understanding of the pathogenic strain resistance mechanism to antibiotics [15].
In recent years, many strategies have been adopted such as cationic biomaterials, phytocompounds, nanoparticles loaded antibiotics, phage therapy etc. as an alternative to antibiotics against the increased MDR pathogens. Among all the strategies, phage therapy is a century-old concept that has reignited interest in the past 20 years and is now being considered as one of the most promising approaches that can tackle the MDR challenges [[16], [17], [18], [19], [20], [21], [22], [23], [24]]. Bacteriophages are widely available ubiquitous microorganisms found on earth (1030-1032) with self-replicating potential, harmless and nontoxic to animals, plants, and humans [25,26]. It poses great threat to pathogenic bacteria to maintain the ecological balance [27,28]. They lyse bacterial cells by infecting and replicating their proteins and genomic material using the host machinery system [29], which was long back reported by Ernest Hankin, in the year 1896. The accidently observed an unidentified substance that limited the spread of cholera epidemics against Vibrio cholera in the Ganges and Yamuna rivers of India [30]. Later many researchers such as Gamaleya (Russian bacteriologist), Frederick Twort (England bacteriologist), and Felix d'Herelle (French-Canadian microbiologist) also reported the same phenomena. Finally, Felix d'Herelle officially discovered and named them “bacteriophage” in the year 1916 after he observed small, clear plaques, in the agar plate cultures of Shigella strains incubated with bacterium-free filtrates of the patients' fecal samples during the outbreak of severe hemorrhagic dysentery in 1915 [[31], [32], [33], [34]].
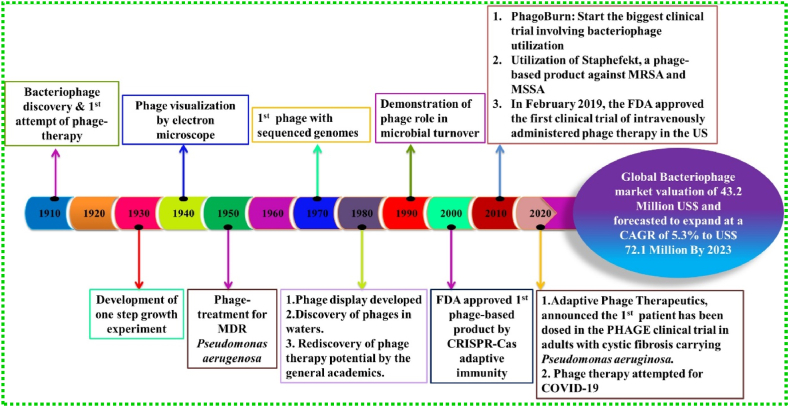
Furthermore, elucidating the underlying mechanism of phage action can be a key point in exploiting phage as a potential antibacterial agent. There are two types of replication mechanisms followed by phages namely, lytic and lysogenic. The bacterial cells are killed when the phage infects and replicates inside the host cell which is otherwise called as lytic cycle [35,36]. The lysis of bacterial cells was once thought to be due to the accumulation of sufficient lysosomal activity during the replication cycle which has recently unfolded the mechanism to be controlled [37]. However, in the lysogenic cycle, the phage inserts its genome into the host genome that remains in a dormant phase and this stage is called a prophage. Later on, the prophage either continues to be in the same state or can re-enter the lytic cycle. This adds to the limitation of phage therapy because it may help the bacterial cell to be resistant to the phage which was formerly sensitive to the virus. The phages that are obligately lytic and do not display lysogeny are the ones that can be exploited for therapeutical use [36,[38], [39], [40], [41]]. In brief, from the starting of phage discovery in the year 1910, till phage therapy attempted for COVID-19 pandemic, the global bacteriophage market is expected to achieve US$ 72.1 million by the end of 2023 [42,43]. Therefore, the milestones and success of phage therapy have been represented in the timeline (Fig. 1).
Fig. 1.
Timeline and milestones of phage therapy.
Phage therapy has many advantages over antibiotics and can be an alternative to the serious problem of MDR, however, several biological limitations such as bacterial resistance, nonspecific immobilization, dosage of administration, the reaction of the immune system, difficulty of finding the specific phage for the treatment and translation of phage therapy into animal studies restrict the use of phage therapy in the biomedical field [41,[44], [45], [46], [47], [48]]. Therefore, currently one of the promising strategies is the use of peptidoglycan hydrolases (PGH) in specific, bacteriophage-consisting endolysins as a new therapeutic antimicrobial agent.
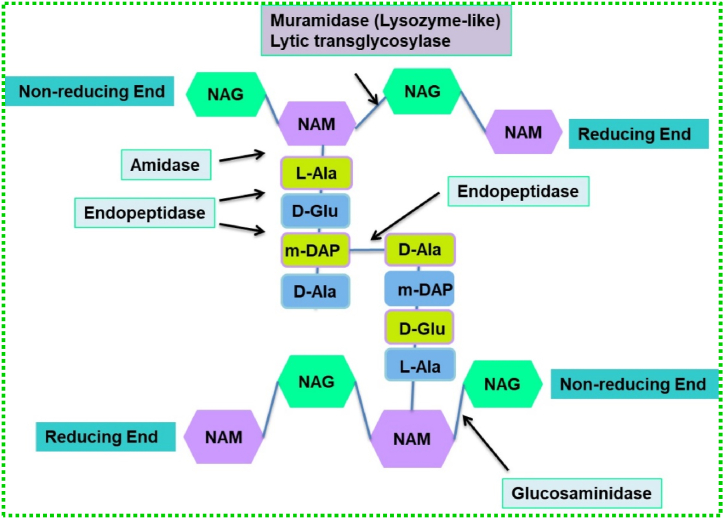
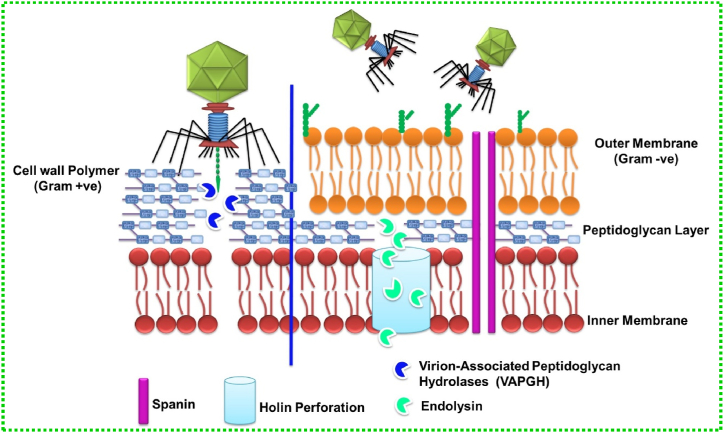
Endolysins are a class of enzymes that show bactericidal activity with their capacity to degrade the peptidoglycan (PG) layer of the bacterial cell wall without any damage to the surrounding cells [49]. These enzymes are produced at the end of the lytic replication cycle which results in osmotic lysis and release of virion particles. In addition, another small protein holin, which is the second element of the lysis cassette of tailed phages, is used to precisely regulate host cell lysis. Endolysins are divided into five classes namely acetylmuramidases, transglycosylases, glucosaminidases, amidases, and endopeptidases [50]. The schematic diagram (Fig. 2) explains the different classes of endolysins and their respective cleaving sites in the bacterial cell wall. Briefly, the bacteriophage injects its genetic material by hydrolysing the outer membrane (OM) with the help of Virion-associated PG hydrolases (VAPGH). Then the endolysins with the help of holin cleave specific sites present in the PG layer of the cell wall membrane.
Fig. 2.
Catalytic activities of endolysins indicated as acetylmuramidases, transglycosylases, glucosaminidases, amidases, and endopeptidases. A sub-class of Glycosidases, N-acetyl-β-D-muramidases cleave the β-1,4 bonds between NAM (N-acetylmuramic acid) and NAG (N-acetylglucosamines), and N-acetyl-β-D-glucosidases cleave the β-1,4 bonds between NAG and NAM residues. N-acetylmuramoyl-L-alanine amidases which are amidases cleave the amide bonds between NAM and L-alanine. Endopeptidases cleave interpeptide and stem peptide–interpeptide bridges.
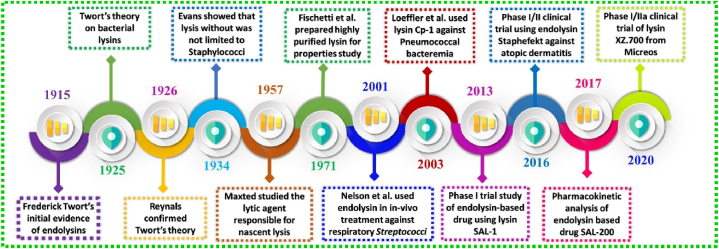
When a threshold concentration of holins is reached during the late stages of infection, they oligomerize to generate holes in the cytoplasmic membrane, allowing the endolysins that have collected in the cytoplasm to access their PG substrate layer. The major advantage of phage-derived endolysin is its efficacy against MDR pathogens, biofilms and persister cells, with extremely low risk for development of bacterial resistance as compared to conventional antibiotics. In recent decades, there has been a dramatic increase in studies related to phage-derived endolysins and their derivatives which has shown successful results in in vivo studies [18,[51], [52], [53]]. The story of phage-derived endolysin started in the year 1915 when Frederick Twort gave the initial evidence of endolysins which was later confirmed by Reynals in the year 1926. Subsequently, highly purified lysine was prepared by the Fischetti team in 1971 and another team used in vivo endolysin to treat respiratory Streptococci pathogen in 2001. First clinical trial of endolysin based drug SAL-1 entered into Phase I trial in the year 2013 after which there were massive discoveries and achievements all of which are represented in Fig. 3 [51,54]. This review focuses on the antibacterial effect of bacteriophage and their recent clinical trials with a special emphasis on the underlying mechanism of lytic phage action with the help of endolysin and holin. Furthermore, recent clinical trials of phage-derived endolysins and some marketed products using endolysins have also been emphasized with future prospectives.
Fig. 3.
Timeline of endolysin development in the biological timescale.
2. Phage-therapy
Phage therapy only employs lytic phages that have the potential to infect and kill the pathogenic host cells with high efficacy. Before the discovery of antibiotics, d’Herelle utilized phage to treat three brothers suffering from dysentery and noticed quick recovery within 24 h of treatment [4]. Similarly, in the year 1925, d’Herelle directly injected bacteriophages to treat bubonic plague in the year of 1925 which resulted in full recovery of the patients in less than a month reducing the mortality in the phage-treated group rather than the untreated group [55]. The use of phages by d’Herelle kick-started the global demands for testing the efficacy of phage therapy against treating typhoid, cholera etc. largely by the former Soviet Union especially Georgia, where it is still in practice. Although discovery of phage is an undisputable merit of English bacteriologist and Canadian-French microbiologist, but history also cites the phage study in Polish countries back to those years when phage was discovered. Unlike the Western world, Poland has never been restricted to using the developing phage therapy despite the situations of the Second World War (WWII) and the communist era. Phage therapy is still widely practiced in Georgia, Poland and Russia [20].
After the discovery of penicillin in the year 1928, phage therapy was overshadowed due to the lack of understanding of its detailed mechanism and unsuccessful controlled clinical trials [19,56]. Later on the emergence of resistance among the bacterial isolates to antibiotics, their non-specificity towards the host cells showing secondary infections and intestinal problems fuelled the use of bacteriophages in the past decades [57]. Additionally, the 2005 establishment of the Phage Therapy Unit at the Hirszfeld Institute of Immunology and Experimental Therapy in Wroclaw, the first facility of its kind in Europe, has become a model for other nations dealing with the spread of MDR infections [58]. Therefore, Poland has been marked as a prominent and successful country for phage therapy research in the current global scenario. According to Global Phage Therapy Market, Europe dominates the share market with 57 % followed by Russia and Germany [59].
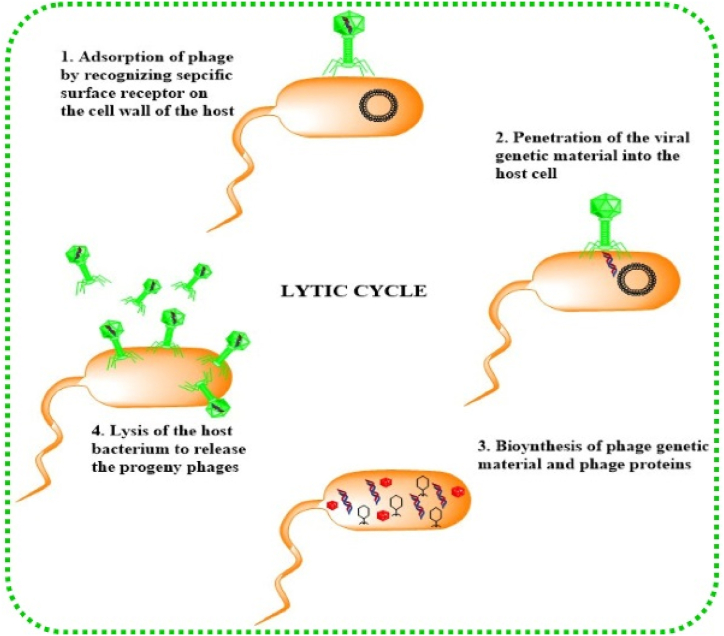
The general mechanism of lytic bacteriophage which binds and adsorbs to the bacterial cell wall (specific receptors) injecting its genome into the host and using host machinery to undergo propagation. After a load of viral particles exceeds, the phage lyses the bacterial cell and releases its progeny into the environment [60,61] as shown in Fig. 4. The adsorption starts with the recognition of a specific host cell receptor with which the phage receptor-binding protein (RBD) present on the tip of the bacteriophage tail interacts. After specific interaction, the phage genetic material is injected into the host cytoplasm which is affected by the localization, density, and volume of the cell receptors. Protein receptors like OmpA & OmpC, lipopolysaccharide receptors (LPS), Vi-antigen located in the capsular polysaccharide, pilli, and flagella are some of the surface receptors recognized by the bacteriophage. Sf6, SfMu, KSF-1, ICP1, PP01, JG004 are some phages that recognize OmpA, OmpC of Shigella flexneri found in contaminated water and food, O-antigen of the LPS of Shigella flexneri, Mannose-sensitive hemagglutinin type IV pilus of Vibrio cholera found in contaminated food and water, O1 antigen of Vibrio cholera, OmpC of Escherichia coli O157:H7 carried by some amphibians, fish, and invertebrates, O157 antigen of Escherichia coli O157:H7 and flagellum respectively [[62], [63], [64], [65], [66], [67]]. Many other phages like Gamma phage, AP50c, ᶲ11, ᶲSLT, A118, and P35 recognize the receptors (GamR, CsaB, wall teichoic acids, lipoteichoic acids, rhamnose residues in wall teichoic acids, rhamnose and N-acetylglucosamine respectively) present on gram-negative bacteria [[68], [69], [70], [71], [72]]. After specific binding, the phage genome is injected into the host cell through sheath contraction due to alteration in base plate conformation. Before the replication in the host cell begins, the phage has to pass through the carbohydrate boundaries present on the cellular surface of the host. These capsular carbohydrate moieties can mask the cell surface receptors which are recognized by the phage and also can be helpful at the time of biofilm formation. To counter this, phages have evolved depolymerases (hydrolases and lyases) that recognize and degrade the carbohydrate components to soluble oligosaccharides, making their path clear for replication. Finally, the newly produced phages must be released to the surroundings which can be achieved by lysis of the bacterial host cell. This step is accomplished by holin-mediated phage-encoded enzymes called endolysins. They lyse the bacterial cell “from within”, degrading the PG layer during the last phase of replication in the lytic cycle.
Fig. 4.
Pictorial representation of lytic cycle of bacteriophage.
There are several commercially available phage products on the market, including Pyofag®, which kills pathogens (Streptococcus pyogenes, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Proteus mirabilis) causing dysbacteriosis, wounds, burns, ulcers, and acute enteric infection [73,74]. The Eliava Institute in Tbilisi Georgia has been a leading world leader in bacteriophage research since the 1930s with active products like Pyo, Ferssisi, Intesti, Enko, Ses, and Staphylococcal bacteriophage [75,76]. Sextaphage is another composition from the company Microgen, Russia that targets 6 specific pathogens residing in the urinary tract of pregnant women in case of urinary tract infections (UTI). Moreover, several clinical trials of phage therapy have been recently worked out successfully [31,77,78]. Phagoburn was, the first French-led European clinical trial in 2013 that used phage therapy on Escherichia coli and Pseudomonas aeruginosa-infected burn wounds utilizing good manufacturing practices (GMP) [79]. However, it was terminated in the year 2017 due to failure in reducing bacterial burden in some patients, lack of test subjects, and phage stability issue [80]. Table 1 summarises some of the recent clinical trials based on phage therapy. Intravenous phage therapy was first attempted in the United States to treat a severe systemic infection patient infected by MDR pathogens. The patient was saved from an end-stage comatose condition by utilizing a specially curated phage therapeutic isolated from environmental samples. Some researchers have reported the re-sensitization of antibiotic sensitivity in MDR Pseudomonas aeruginosa. Recently, phage therapy has reached milestones in the treatment of intestinal infections by the use of phage cocktails. Some are in the pre-clinical stages and some have completed phase-I/II clinical trials [81]. Phage therapy has been presented as a clinical option for restoring gut microbiota in the absence of an effective treatment. This occurs due to its immunomodulatory and bactericidal properties against its target bacteria. Phage therapy has been studied mainly as a potential approach in the treatment of infectious disorders such as cholera and diarrhea.
Table 1.
| Infection | Status of trial | Type of contents in the therapy | Country | Trial Title and characteristics of the study |
|---|---|---|---|---|
| Diabetic Foot, Staphylococcal Infections therapy | Not Yet Recruiting | PhagoPied: Topical anti-Staphylococcus bacteriophage Trial no.- NCT02664740 |
France |
|
| MDR Staphylococcus aureus infections | In Progress | AB-SA01 (3- phage cocktail) | USA | Individual Patient Expanded Access for ABSA01, an Investigational Anti- S. aureus Bacteriophage Therapeutic |
| Pseudomonas aeruginosa infections (incl. MDR stains) | In Progress | AB-PA01 (4-phage cocktail) | USA | Individual Patient Expanded Access for ABPA01, an Investigational Anti-Pseudomonas aeruginosa Bacteriophage Therapeutic |
| Crohn's Disease | Preclinical | EcoActive (collection of bacteriophages) | USA | Intestinal Adherent Invasive E. coli and the Safety and Effectiveness of EcoActive in Patients With Inactive Crohn's Disease |
| Postoperative infections of the bone, upper respiratory tract, genital tract, or urinary tract that are widespread, nonhealing, and resistant to intensive antibiotic therapy | Completed | bacteriophage lysates, pure phage formulations, and/or phage cocktails administered orally, rectal, and/or topically | Poland | Examination of inflammatory marker alterations in patients receiving bacterial viruses |
| Wound infection | Completed | PhagoBurn: 15 E. coli phages cocktail, 13 Pseudomonas aeruginosa phages cocktail Trial no.- NCT02116010 |
Switzerland, Belgium, France |
|
| Cystic Fibrosis (CF) | Completed | Mucophages (10-phage cocktail) Trial no.- NCT01818206 |
France |
|
| Antibiotic-resistant Pseudomonas aeruginosa in chronic otitis | Completed | Biophage-PA | United Kingdom | Therapeutic bacteriophage preparation in chronic otits due to antibiotic-resistant Pseudomonas aeruginosa |
| ETEC and EPEC Diarrhea | Completed | The oral T4 phage cocktail Trial no.- NCT00937274 |
Bangladesh |
|
| Urinary Tract Infections (UTI) | Completed | Intravesical instillation PYO phage | Georgia | Treatment with bacteriophages for UTI in patients having transurethral prostate resection |
| Venous Leg Ulcers | Completed | WPP-201 (8-phage cocktail) Trial no.- NCT00663091 |
USA |
|
| Gastrointestinal distress E.coli |
Phase-II clinical trial completed | PreforPro (4 phages) | Georgia (U.S patent) |
|
| Inflammatory bowel disease (IBD) K.pneumoniae |
Phase-I completed | Combination of 5 phages given orally BX002-A Trial no.- NCT04737876 |
Israel |
|
Moreover, genetically modified and personalized phage treatment can help overcome the limitations of wild-type phages that have narrow host specificity [84]. Although it has been reported that phage therapy is successful in many diseases and infections like cystic fibrosis, chronic wound infections, pulmonary infection, metabolic syndrome, joint infections, gut infections etc. Unfortunately, no clinical evidence is found for oral diseases. The presence of high number of bacteriophages in the mouth shows that they have a strong association with the bacteria causing oral diseases. Their presence can potentiate or regress multiple oral diseases. Many lytic and lysogenic phages target various periodontal diseases, caries, and endodontic infections [85,86]. Research focusing on phage therapy reports that the MS2 bacteriophage acts as an outstanding agent for targeted vaccines hence, can be used as a potential tool to prevent oral diseases. Some other methods such as apical negative pressure root canal irrigation systems, antimicrobial peptides (AMPs), polymeric or inorganic nanoscopic fillers etc. in synergy with phage therapy can yield great success [[87], [88], [89]]. More research on oral phages can lead to its broad applicability in diagnosing, preventing, and treating infections and diseases.
The advantages of using novel phages against the pathogenic isolates may be attributed to their host specificity which is otherwise taken as its limitation. However, preparing a cocktail of narrow-range phages targeting the specific bacterial strains in an infection can be alternatively used to overcome the limitation of being host-specific without harming the surrounding microbiota. Auto dosing is another unique advantage that allows bacteriophage to increase their number according to the high availability of their host. The property of auto-dosing also results in the single-dose potential of phage therapy.
2.1. Disadvantages of phage therapy
Despite the advantages that make phages a potential antibacterial agent, the limitations of narrow host range and potential immunogenicity cannot be ignored. Although phage cocktails are much more advantageous than single specific one, but it costs money and time. It is much more challenging to prepare a therapeutic cocktail of phages than to design and use antibiotic regime due to the vast diversity of phages in the environment. It is quite hard to isolate and analyse their effectiveness against various bacteria-causing diseases. The isolation of phages from sewage, wastewater and medical waste is a easy process for some bacterial pathogens and difficult for many others. This step then leads to check the effectiveness of the phage against the particular strain as they are highly strain specific. The potential therapeutic application can be studied after confirming the lytic capacity of the phage which can change according to the load of bacteria and the dosage given with time. Finally, formulation and stabilization come into play to be clinically safe [90]. This process becomes highly time-consuming and deciding whether to use the conventional method or personalized cocktails for a specific disease becomes difficult. Therefore, phage makeup needs to be carefully studied to know their strain specificity for successful therapeutic effects. It is also reported that bacterial strains often develop resistance by mutations, passive adaptation, restriction-modification, receptor modification, releasing decoy molecules, CRISPR-Cas, and pseudo lysogeny to the phages used [91]. In addition, epigenetic modifications and changes in reversible gene expression by the host play a crucial role in decreased availability of the cell surface receptors for attachment. There is an “arm-race” that continues between the host bacteria and the phage which is still not understood properly till date. These limitations may be further circumvented by the use of purified endolysin from the phage and their recombinants as there is no report of resistance against the endolysins [92]. The next section describes the structure and mechanism of phage-derived endolysin against emerging resistant bacterial pathogens.
3. Phage-derived Endolysins
3.1. Endolysin Structure
Endolysins are proteins that are produced in the late stages of the lytic cycle. By hydrolysing the PG, they aid the virion particles in rupturing the host bacterium's cell wall. In general, there is a structural difference in the gram-negative (simple globular) and gram-positive endolysin (modular). However, few gram-negative targeting endolysin that pose a modular structure have been identified and categorized from phages with large genomes also called as jumbo phages. Lys68, KZ144, PVP-SE1gp146, EL188, etc. are some of the endolysins with modular architecture from various gram-negative infecting phages [93].
Endolysins against gram-negative bacteria mostly possess a single domain for digesting the PG layer called an enzymatically active domain (EAD). But in the case of modular endolysins, they comprise of another domain called cell wall binding domain (CBD) along with one or more EADs having different activities. Most of the modular types of endolysin have N-terminus EADs and C-terminus bound CBD, although exceptions exist. In an in silico study, it was found that AP3gp15 endolysin from AP3 phage has a modular structure with N-terminus bound CBD and C-terminus DUF3380 domain [93]. Similarly in PlySK1249 endolysin, there exists 2 EADs between which lies the central CBD [94]. The N-terminal EAD and a CBD, which are joined by a brief linker, make up the modular endolysins. Whereas the gram-negative endolysins only have EADs but the few gram-negative endolysin that have modular structures show the inverted presence of EADs and CBDs. Gram-negative endolysins have EADs at the C-terminal end and CBDs at the N-terminal end, in contrast to gram-positive infecting endolysin. According to reports, the Pseudomonas putida phage OBP's endolysin OBPgp279 has two CBDs [95]. There are similar cases observed where the endolysin contains two C-terminal EADs. Scientists have identified 723 endolysins with high diversity observed in their EAD and CBD arrangement [96].
Endolysins have proved to be a promising class of anti-bacterial agents to treat various infections and diseases in the clinics. The first use of purified endolysin as an anti-bacterial agent was reported in the year 1959 [60]. The in vivo efficacy of an endolysin was first published by Nelson et al., in 2001 after which evaluation of purified and recombinant endolysins in animal models of bacterial infection hasn't stopped [97]. Since then many endolysins have been characterized and have proved to be potential biocontrol agents against various MDR pathogens. The section below describes how endolysin works in both gram-positive and gram-negative bacteria.
3.2. Mechanism of holin-endolysin action to disrupt the bacterial cell wall
Endolysin, as previously mentioned, targets the PG layer to rupture the host bacterium's cell wall, but this is a well-synchronized process. In general, holin mediates the endolysin rupture mechanism seen in gram-positive bacteria, where holins make perforations in the cytoplasm accumulated with endolysins. These holin proteins mediate the endolysins to their target, PG through the hole they have created in the cytoplasmic membrane. The holins aggregate into oligomers changing the membrane permeability to lose its polarization forming pores. The PG layer provides rigidity and structural integrity to the bacterial cell, slight rupture (internal osmotic pressure) in the wall leads to cell instability eventually leading to cell rupture and release of progeny. This mechanism applies to gram-positive bacteria because they lack OM, unlike gram-negative bacteria. Hence, endolysin when applied from the outside can have direct access to the PG layer and carbohydrate moieties acting as an antibacterial agent. Moreover, a small amount of endolysin can rupture the host cell within 20 min of its application. The complete mechanism of action of phage endolysin is schematically represented in Fig. 5.
Fig. 5.
Mechanism of action of bacteriophage endolysin on gram-positive and gram-negative bacterial cell lysis.
Gram-negative bacteria's PG layer is shielded by an outer layer, making it difficult for endolysins to attack and penetrate the cell wall from the outside. However, several approaches have been implemented to enable the endolysins to penetrate the PG layer which has been extensively described in the next section [98].
The endolysins being used to overcome the phage therapy has advantage of having broad spectrum antibacterial activity acting both on dormant and growing bacteria. They act against bacterial biofilms with no reported resistance and with better pharmacokinetics than antibiotics and bacteriophage. It also has the advantage of showing the lower degree of antibody neutralization and can be well combined with various agents for its action.
3.3. Approaches of Endolysins access to the PG layer of Gram-negative Bacteria
The limitation of endolysin to access the PG layer of the gram-negative bacteria has made researchers employ combination therapies using endolysins and various agents such as the use of membrane destabilizing agents, encapsulation system, utilization of physical stressors, OM permeabilizing peptides, liposome, receptor-mediated uptake, and AI-based approaches.
3.3.1. Membrane Destabilizing Agents
These are broadly divided into two categories which include chelators and polycationic agents that help in crossing the barrier of OM (phospholipids and LPS) present in gram-negative bacteria. Several studies have employed the combination of endolysins with various membrane destabilizing agents such as chelating agents and organic acids. The most used chelating agent is Ethylenediamine Tetra-acetic Acid (EDTA) which weakens the OM by removing the divalent cations (Mg2+ and Ca2+) from the binding sites of the bacteriophage and finally leading to its disruption.
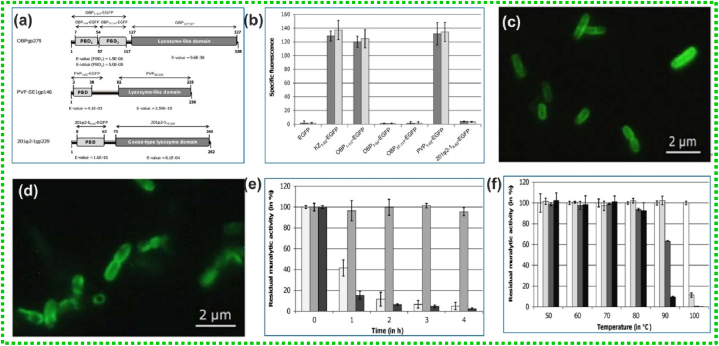
Walmagh et al., have isolated endolysin OBPgp279, PVP-SE1gp146 and 201Q2-1gp229 from Pseudomonas fluorescens phage OBP, Salmonella enterica serovar Enteritidis phage PVP-SE1 and Pseudomonas chlororaphis phage 201Q2-1 to disrupt the bacterial membrane respectively[99]. All isolated endolysin exhibiting N-terminal cell wall binding domain and a C-terminal catalytic domain. The three isolated modular endolysins unveiled potent muralytic activity against the PG layer owing to the to the inclusion of cell wall binding domain (Fig. 6a). To explore the PG binding capacity, the team generated fusion proteins with the binding domains of OBPgp279, PVP-SE1gp146 and 201Ψ2-1gp229, N-terminally fused to EGFP. The cell wall of the targeted S. Typhimurium LT2 and P. aeruginosa PAO1 cells became fluorescent in just 5 min after incubating with PVP1–63-EGFP and OBP1–117-EGFP. It was observed, a single subdomain of OBPgp279 (either OBP57–117 or, OBP7–54) was insufficient for binding cell wall as no fluorescence was retained with both fusion proteins (Fig. 6b). For 201Ψ 2-18–63-EGFP, no fluorescence was observed, due to improper protein folding during expression. Binding capacity of OBP1–117-EGFP and PVP1–63-EGFP was confirmed from epifluorescence microscopy (Fig. 6c and d). The residual muralytic activity is depicted in Fig. 6e and f.
Fig. 6.
(a) Domain organization of endolysin, (b) The fusion protein specific fluorescence plot measured after incubation with OM permeabilized P. aeruginosa PAO1 (dark grey bars) or S. Typhimurium LT2 cells (light grey bars) for different EGFP fusion constructs, (c and d) Epifluorescence microscopy of OM permeabilized P. aeruginosa PAO1 cells treated with OBP1–117-EGFP and PVP1–63-EGFP respectively, (e) The residual muralytic activity of OBPgp279 (1 mM, light grey bars), PVP-SE1gp146 (5 mM, intermediate grey bars) and 201Q2-1gp229 (3 mM, dark grey bars) on OM permeabilized P. aeruginosa PAO1 cell substrate after 1, 2, 3 and 4 h heat treatment, (f) For PVP-SE1gp146 (5 mM), the residual activity on OM permeabilized P. aeruginosa PAO1 after incubation for 0 (white bars), 20 (light grey bars), 40 (dark grey bars) and 60 (black bars) min on different temperatures between 50 and 100μC was determined. Adapted with permission from Ref. [99] Copyright 2012 PLOS.
Similar activity is shown by weak organic acids such as citric acid, lactic acid, malic acid, acetic acid, and benzoic acid which can be linked to its low pH causing harm to the OM. Unfortunately, EDTA possess significant toxicity to mammalian cells by affecting proliferation as well as influences apoptosis. In addition, endolysins being enzymes mostly become inactivated in pH less than 4. This can limit the potential effect of the endolysin. The other group consists of polycationic agents that displace the divalent cations by competing with them which makes interaction between the cations and the LPS weak. Studies have shown the eradication of the Vibrio parahaemolyticus and its biofilms on various surfaces by the synergistic effect of endolysin Lysqdvp001 and ε-poly-lysine (ε-PL) [100]. Some of the other agents include polymyxin, aminoglycosides, colistin, polymyxin B, etc.
3.3.2. Endolysin encapsulation system
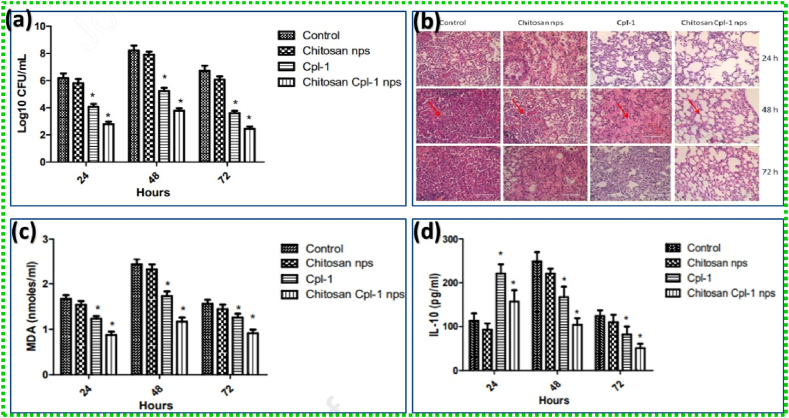
Scientists have developed ways to enhance the penetrating effects of the endolysin without the use of permeabilizers. It has been observed that encapsulation plays an important role in maintaining the efficiency with controlled release of the endolysin as well as protecting it from degradation. Bai et al., have developed a liposome-encapsulated endolysin system for easy penetration of the endolysin into the host through the OM. A cationic liposome made of dipalmitoyl-phosphatidylcholine (DPPC), cholesterol, and hexadecyl amine filled with the BSP16Lys endolysin that targeted the negatively charged OM was shown to reduce the viable cell counts of Salmonella typhimurium and Escherichia coli by 2.2 logCFU/mL and 1.6 logCFU/mL, respectively [101,102]. Gondil et al. explored the potential role of endolysin (Cpl-1) loaded chitosan nanoparticles (NPs) for treating pneumococcal pneumonia. The mean log bactreial count is depicted in Fig. 7a. The histopathology study also demonstrated the reduced levels of pneumococcal infection in the lungs of Cpl-1 loaded chitosan NPs treatment group (Fig. 7b). The inflammatory analysis displayed a lower inflammation level in the lungs of animal treated with chitosan-Cpl-1 NPs compared to free Cpl-1 (Fig. 7c). Cytokine studies also revealed the decreasing level of pro-inflammatory/anti-inflammatory cytokines chitosan-Cpl-1 NPs compared to free Cpl-1 at 48 and 72 h (Fig. 7d) [103].
Fig. 7.
(a) Mean log bacterial count, (b) histopathology study, (c) Malondialdehyde levels, (d) IL-10 levels in lung homogenates of S.pneumoniae infected control, chitosan nanoparticles, Cpl-1 and Cpl-1 loaded chitosan nanoparticles treatment groups at different time (24, 48 and 72 h) intervals. Adapted with permission from Ref. [103] Copyright 2020 Elsevier.
Some other encapsulating agents include cationic guar gum, alginate, cellulose nanocrystals, dendrimers, etc. which have been used in the recent past to help endolysins breach the OM of gram-negative bacterial host [[104], [105], [106]].
3.3.3. Physical stressors
Mechanical ways of penetrating the OM have also been studied widely including utilization of physical stressors like high hydrostatic pressure and pre-treatment of bacterial cells with chloroform/heat are also reported [95,107,108]. Optimization of the required factors such as pressure and temperature has been shown to enhance the penetrating ability of the endolysins, bacteriocins, and AMPs. These AMPs can have great potential in combating carries due to their inherent antibacterial properties against oral pathogens and their biofilms [88].
3.3.4. Advanced methods
Nowadays genetic engineering is a topic of discussion worldwide and used for various applications like gene therapy, genetically modified organisms, vaccines, gene editing, gene targeting, gene silencing, etc. This has also made some impact in modifying and editing the amino acids of the endolysins to overcome its limitation of not being able to penetrate the OM. Amino acid mutations using site-directed mutagenesis and recombination are also widely used approaches to facilitate the activity of novel recombinant and chimeric endolysins [109]. However, some endolysins have a strong antibacterial peptide with an inherent ability to cross the OM known as AMPs. These can be very helpful in the genetic engineering of the penetrating system framework. These can be categorized as polycationic, hydrophobic, or amphipathic. An amphipathic AMP fused with LysCo2 endolysin from phage ΦC02 has significantly reduced (∼3-log reduction within 2 h) C. sakazakii in infected Galleria mellonella larvae by disrupting the OM with its intracellular turgor pressure [110]. Similarly, Islam et al., observed a 2- to 8-fold decrease in bactericidal activity of various MDR A. baumannii when the N-terminus of endolysin was fused with AMP cecropin A than the control groups [111]. These are natural AMPs, but some can also be engineered to have the charges and hydrophobic properties necessary for the system [112]. It was observed that the extracellular action of endolysin Lysep3 increases proportionally against gram-negative bacteria upon the addition of 5–15 positively charged/hydrophobic amino acids to each end of the endolysin [113]. Another method of enhancing the activity of endolysin includes a fusion of endolysin with proteins that help for better attachment with the host cell surface and finally disrupt it. These are lysocins, pore-forming bacteriocins, and innolysins (endolysin fused to a phage receptor binding protein) [114].
Despite all these methods, artificial intelligence (AI) in screening and designing of novel endolysins is also making a significant impact on the scientific community. Scientists have used bioinformatics for pipelining endolysins from large group of uncultured phage genomes. AI with the help of various other protein structure predicting techniques can help overcome the hectic module of recombination and mutation process till the engineering of a novel endolysin [115]. This can reduce the time, energy, and cost of its complete development. However, there still remains a vast area of uncovered mechanisms and studies are required regarding the same. In addition, the lack of experimental validations and clinical studies poses a significant hurdle in the emerging strategies.
Clinical trials of phage-derived endolysin have been done with phase I and phase II for the safety and efficacy of P128 (administered through the intranasal route) by GangaGen (ClinicalTrials.gov Identifier NCT01746654). P128 has been effective in both in vitro and in vivo is a recombinant chimeric protein that targets coagulase-negative and positive Staphylococci. GangaGen has received a patent for the “Lysin-deficient bacteriophages having reduced immunogenicity”, which led the scientific community for further studies. Another advanced lysin PlySs2 (CF-301) also called exebacase (ClinicalTrials.gov Identifier: NCT04160468) administered intravenously has completed the clinical trial of phase III [116]. The study was sponsored by ContraFect which aimed to use the exebacase in treating methicillin-resistant S. aureus (MRSA)-induced sepsis, and right-heart endocarditis along with persistent knee prosthetic joint infections. However, due to lack of statistical power, the trials were discontinued [117]. It is also reported that in addition to the above activity of exebacase, it also works against both planktonic and biofilm S. epidermidis in vitro and shows elevated effects in the presence of albumin [118]. N-Rephasin® SAL200 by iNtRON Biotechnology, Inc. was also in the Phase IIa clinical trials (ClinicalTrials.gov Identifier: NCT03089697) but was terminated before completion due to strategic reasons. It also targets S.aureus by a single dose of intravenous administration along with conventional antibiotics [119]. One more enzyme, LMN-201 has also completed the phase I trial which is a mixture of 2 broader classes of therapeutic proteins that target gram-positive Clostridioides difficile infection (CDI). Three antibody-like proteins make the first class that binds and neutralize the bacterial toxin responsible for diarrhea and other severe CDI symptoms. An enzyme protein that targets and breaks down the cell wall of the C. difficile bacteria makes up the second class. However, the phase II trials are still not recruiting [120]. The FDAgranted Fast Track Designation to Lumen Bioscience for its oral biologic medicine LMN-201 in 2023 [121]. The first endolysin-containing product has hit the market Staphefekt™, developed by Micreos. It specifically targets S.aureus including MRSA on the human skin with no harmful effects. Micreos have also developed series of cosmeceuticals (creams, and gels) containing Staphfekt sold under the brand Gladskin [122]. The drugs targeting gram-positive bacteria have advanced a lot to cross the clinical trials and enter the market. But lysin's efficiency in the case of gram-negative bacteria (due to the presence of OM) has shown various concerns regarding formulation, safety, dosage, routes of administration, etc. Despite having promising results yet to reach the clinical studies. Further research and sustained R&D efforts can pave the way for clinical advancements in lysins as therapeutics against gram-negative bacteria [123]. The in vivo application of phage-derived and recombinant endolysins in various animal models has been summarized in Table 2.
Table 2.
Recent studies showing the in vivo efficacy of phage-derived and modified endolysins.
| Target pathogens and model used | Endolysin/derivatives | Route of administration | Outcomes | Clinical trials | Ref. |
|---|---|---|---|---|---|
| Staphylococus aureus (MRSA) Mouse | P128 (chimeric lysin) | Intraperitoneal | The combination of P128 and oxacillin resulted in the inhibition of 4 MRSA strains and could kill biofilm-embedded bacteria. | Phase I/II completed (NCT01746654) | [124] |
|
Staphylococcus epidermidis Mouse |
LysGH15 | Intraperitoneal | Bacteria in blood and organs were reduced by 4 and 3 logs, respectively after treatment when compared with untreated control mice. | - | [125] |
|
Streptococcus pneumonia Mouse |
ClyJ-3 (chimeric lysin with an improved linker) | Intraperitoneal | Superior to the parental enzyme ClyJ, demonstrating 20 % more efficacy, with the linker sequence also having a significant impact on the chimeric lysin's activity. | Pre-clinical | [126] |
|
Streptococcus pneumonia Mouse |
ClyJ (chimeric lysin) | Intraperitoneal | 100 % and 20 % survival when treated 1 h and 3 h post-infection, respectively; | Pre-clinical | [127] |
| No resistance to the chimeric lysine was observed even after doubling the concentration of ClyJ for 8 consecutive days. | |||||
|
Streptococcus pneumonia Zebrafish |
Cpl-711 and PL3 (chimeric lysins) | Intraperitoneal | 77.8 % survival was observed with the combination treatment; 50 % survival for PL3 alone; 44.4 % survival for Cpl-711 alone; compared to 27.8 % survival for the control group. | Pre-clinical | [128] |
|
Streptococcus pneumonia Mouse and zebrafish |
Cpl-711 (chimeric lysin) | subcutaneous | 58 % of mice survived as compared to 53 % for the control group; | Pre-clinical | [129] |
| 100 % survival was observed when the Cpl-711 was combined with cefotaxime (67 % for cefotaxime alone). | |||||
| In the case of zebrafish, 100 % survival was achieved compared to 23 % in the control group. | |||||
|
Streptococcus agalactiae Mouse |
ClyV (chimeric lysin) | Intraperitoneal | 100 % survival was observed in the mouse model as compared to the 29%survaival rate in the case of control models. No adverse effects were observed even after administration of higher dose of ClyV. |
– | [130] |
|
Streptococcus suis Mouse |
Ply5218 | Intraperitoneal | 80–90 % survival rate after immediate treatment; 70–80 % survival rate with delayed triple treatment as compared to 10–20 % survival in the case of the control group after 7 days of infection. | – | [131] |
| Bacterial burden was found to be less in the case of both triple and immediate treatment when compared to the group treated after 1 and 2 h post-infection. | |||||
|
Streptococcus suis Piglet |
Ply5218 | Intramuscular | Bacterial burden in the blood was significantly reduced than in the control untreated group; reduced body temperature, clinical scores, and pro-inflammatory cytokines were observed in the treated group. | – | |
|
Acinetobacter baumannii Mouse |
LysSS | Intraperitoneal | 40 % survival after treatment with 125 μg of LysSS; a high mortality rate was seen after treatment with 500 μg when compared with the control group. | – | [132] |
| Ply6A3 | 70 % survival (0 % for the control); reduced white blood cell counts, IL-10, and procalcitonin levels were observed after treatment. | Pre-clinical (ALM01856) | [133] | ||
|
Pseudomonas aeruginosa Mouse |
PyS2-GN4 (pyocin-endolysin fusion protein) | Intraperitoneal | 73 %, 80 %, 93 %, and 100 % survival rate was observed in infected mouse treated with 2.5, 5, 12.5, and 25 mg/kg lysocin respectively as compared to the control group with 37 % survival rate, organs of the surviving lysocin injected mice had no sign of bacterial infection. | – | [134] |
|
Staphylococus aureus (MRSA and MSSA) Mouse and Galleria mellonella larvae |
SAL200 (N-Rephasin) | Intravenous and intraperitoneal | ∼1.2 log reduction of CFU/ml in blood and up to 1.8 log reduction in bacteremia when combined with antibiotics. | Phase IIa terminated (NCT03089697) | [135] |
| After 96 h post-infection, the combination also improved the Galleria mellonella larvae's survival rate. | |||||
|
Staphylococcus aureus Mouse |
ABD_M23 (chimeric lysin fused to albumin-binding domain) | Intravenous | ∼2 log reduction of S. aureus in blood post 48 h when administered with ABD-M23 (featuring extended serum half-life) as compared to ∼1 log reduction of bacteremia in case of parental M23. | – | [136] |
|
Bacillus anthracis Mouse |
PlyB | Intravenous | Control murine models administered with only buffer after infection had a survival rate of 14 % that increased to 28 % and 100 % at 0.625 mg/kg and 5 mg/kg of PlyB respectively with no significant side effects. | PlyG is in pre-clinical stage (PFW40491) | [137] |
| The synergistic effect of single doses of PlyB and PlyG increased the survival rate to 71 % (28 % survival rate in case of either PlyB or PlyG administered alone at 0.625 mg/kg). | |||||
|
Staphylococus aureus (MRSA) Rat and rabbit |
CF-301 (Exebacase) | Intravenous | ∼6 log drop in bacterial densities was observed in the case of both rat and rabbit at 10 mg/kg (∼2600 μg/animal) & 0.09–0.18 mg/kg (∼210–420 μg/animal) of CF-301 respectively when compared to 3 log reduction in control having administered with only daptomycin. | Phase III completed (NCT04160468) | [138] |
| Staphylococus aureus (MRSA) Rabbit | CF-301 (Exebacase) | Intravenous | 3 log reduction of MRSA vegetation in the control group that increased to >8 log reduction when daptomycin was combined with the exebacase. | Phase III completed (NCT04160468) | [139] |
| Staphylococus aureus (MRSA and MSSA) Mouse | SAL200 (N-Rephasin) | Intranasal | ∼10-fold reduction of bacterial density in the lungs of the mouse when treated with SAL200 when compared to the control group (90–95 % survival rate in the treated group compared to 10–40 % in the control group), recovery from pneumonia was observed in histopathological studies after treatment. | Phase IIa terminated (NCT03089697) | [140] |
| Bacillus anthracis, Mouse | LysB4 | Intranasal | 100 % survival was observed in a high dose LysB4-treated group with 100μg/head at 6, 24, and 48h post-infection whereas a low dose of 10μg/head extended the onset of death improving the survival rate. Reduced bacterial numbers in lungs (<1 log) and other organs (2–3 log) compared to control. |
– | [141] |
|
Acinetobacter baumannii, Galleria mellonella and mouse |
ElyA1 | Intranasal | When treated with combination of colistin (1/4 MIC) and 25 g/ml ElyA, infected wax moth larvae demonstrated a higher survival rate than those treated with colistin alone. | – | [142] |
| <1 log reduction of A. baumannii when treated with ElyA1 and colistin on the skin of infected mouse compared to <0.5 log reduction when treated with colistin alone. | |||||
|
Pseudomonas aeruginosa Mouse |
PlyPa91 | 2 intranasal or 1 each intranasal and intratracheal | 70 % survival after intranasal plus intratracheal treatment whereas 20 % after 2 intranasal treatments (having the same amount of lysin) indicating that the mice's survival rate was significantly influenced by the delivery method. | – | [143] |
|
Streptococcus pneumonia Mouse |
Cpl-711 (chimeric lysin) | Intranasal | ∼2 log reduction of nasopharyngeal carriage, independent of strain and treatment regime; superior to parental endolysin Cpl-1 | Pre-clinical | [144] |
|
Clostridioides difficile Mouse |
LHD (phage lysin–human defensin fusion protein) | Oral (gavage) | 100 % survival rate observed in C. difficile infected mice as that of 60 % survival for the control, reduced percentage of diarrhea, and significantly reduced concentration of C. difficile spores and toxins in the feces of infected mice. | – | [145] |
|
Staphylococus aureus (MRSA) Rat |
CF-301 (Exebacase) | Intravenous | 0.48 log reduction of MRSA in the bone compared to control (1.56 log reduction when combined with daptomycin) | Phase III completed (NCT04160468) | [146] |
| The treated group of rats (with daptomycin, exebase, and a combination of both) had a mean bacterial density of 4.09 (±0.37), 4.65 (±0.65), and 3.57 (±0.48) log10 CFU/g of bone as compared to control group having a bacterial density of 5.13 (±0.34) log10 CFU/g of bone. | |||||
|
Klebsiella pneumonia Rat |
LysECD | Intraperitoneal | >1 log reduction of viable bacteria in biofilms within the implant; significantly reduced biofilm mass observed in LysECD treated rats. | – | [147] |
|
Staphylococcus aureus Mouse |
SEP_TAT and LST_TAT (lysins fused to cell-penetrating peptides, CPPs) | Subcutaneous (peripheral) | >2.2 log reduction of bacteria within abscesses treated with lysin-CPP cocktail when compared to control ones with 1 log reduction, significant reduction of intracellular bacteria in the pus | – | [148] |
|
Staphylococus aureus Mouse |
S25-3LYS-his | Topical | A significant decrease (1–2 logs) in intraepidermal Staphylococci numbers and the size of pustules in impetigo mice with increased skin microbiota diversity. | – | [149] |
|
Staphylococus aureus (MRSA) Mouse |
LysGH15 | Topical (ointment) | The mean bacterial count of S. aureus on the skin of infected mice was ∼102 CFU/mg after 18 h of treatment which became undetectable after 96 h (105 CFU/ml bacterial count in control groups); accelerated wound healing in the mouse model by reducing the levels of pro-inflammatory cytokines. | Pre-clinical (ADG26756) | [150] |
|
Staphylococcus aureus Mouse |
TSPphg | Topical | ∼3 log reduction of S. aureus on the skin of infected mice with accelerated wound closure. | – | [151] |
|
Pseudomonas aeruginosa Mouse |
PlyPa03, PlyPa91 | Topical | P. aeruginosa was reduced by > 2 logs (PlyPa03) and 1 log (PlyPa91) on infected mouse skin when compared to control, and 20 % and 70 % of mice treated with PlyPa91 in two intranasal instillations and mice treated with one intranasal and one intratracheal instillation, respectively, survived lung infection. These results suggest that the route of delivery is important for increased efficacy. | – | [143] |
|
Mycobacterium ulcerans Mouse |
LysB | Subcutaneous | ∼1 log reduction of bacteria in footpads compared to the control group along with the production of IFN-γ and TNF in the draining lymph node. | – | [152] |
|
Staphylococus aureus, S. epidermidis Zebrafish and mouse |
LysRODI | Intramammary (preventive treatment) | In protein-treated groups, zebrafish embryos had a survival rate of >92 % survival in presence of LysRODI and CHAPSH3b, indicating a non-toxic effect. | – | [153] |
| 3-4 log units' reduction in bacterial burden when compared with the control group, improved mammary gland health. | |||||
|
Streptococcus mutans and S. sobrinus Rat |
ClyR (chimeric lysin) | Oral | Continuous administration of ClyR showed a significant reduction in the severity of caries (56 %) in rat models. | – | [154] |
|
Fusobacterium necrophorum Rabbit |
LysAm24, LysAp22, and LysECD7 (gel) | Topical | The lifetime of the infected rabbits was enhanced by approximately two times compared to the placebo-treated rabbits after the topical gel was administered twice daily for five days. Less acute infection and a delay in the course of infection were also noted in the gel-treated rabbit model. | – | [155] |
|
Staphylococcus aureus (MRSA) Mouse |
LysP108 | – | In vivo tests showed that compared to monotherapy, the subcutaneous abscess that was produced in the mice was greatly diminished when treated with LysP108 plus vancomycin. | Preclinical (YP_009099525) | [156] |
| This was further supported by H&E (hematoxylin and eosin) staining, which demonstrated that combined therapy did not result in the persistence of the abscess and inflammatory response as compared to the control groups. | |||||
|
Streptococcus pneumonia Mouse |
Cpl-1 loaded chitosan nanoparticles | – | Histopathological and inflammatory analysis showed that treatment with Cpl-1 loaded chitosan nanoparticles resulted in the lowest bacterial load in the lungs of infected animals when compared to the control group treated with Cpl-1 and chitosan nanoparticles alone, and that treatment groups had lower concentrations of pro-inflammatory and anti-inflammatory cytokines than other groups at 48 and 72 h. | – | [103] |
|
Streptococcus suis and Streptococcus agalactiae Mouse |
Ply0643 | – | 80 % survival rate from lethal bacteremia was observed in Streptococcus suis infected mice when treated with Ply0643 (total 0.8 mg/mouse) and Streptococcus agalactiae infected mice showed a significant reduction in bacterial infection in mammary glands. | – | [157] |
|
Klebsiella pneumoniae Mouse |
LysCA and LysG24 | Intranasal | The infected mice treated with LysCA at the onset of symptoms showed full recovery after 48 h with no signs of abnormality in the lung tissue, but their mental condition and mobility were affected as compared to untreated ones. | – | [158] |
| The mice treated with LysG24 showed partial recovery of mental status and mobility after 48 h which was not good as that of mice treated with LysCA, slight congestion and edema were also observed in the lungs of this group of mice. | |||||
|
Staphylococcus aureus (MRSA) Mouse |
XZ.700 (Chimeric endolysin) | Topical cream and gel | In vivo bioluminescence experiment showed that male mice were 2 times more efficient in eliminating the bacterial load than the female when treated with XZ.700. | Pre-clinical | [159] |
| In vivo bioluminescence analysis also revealed that both cream and gel were capable of reducing a significant amount of bacterial numbers in the skin-infected mice as compared to untreated control. |
- Means no information available on clinical trials.
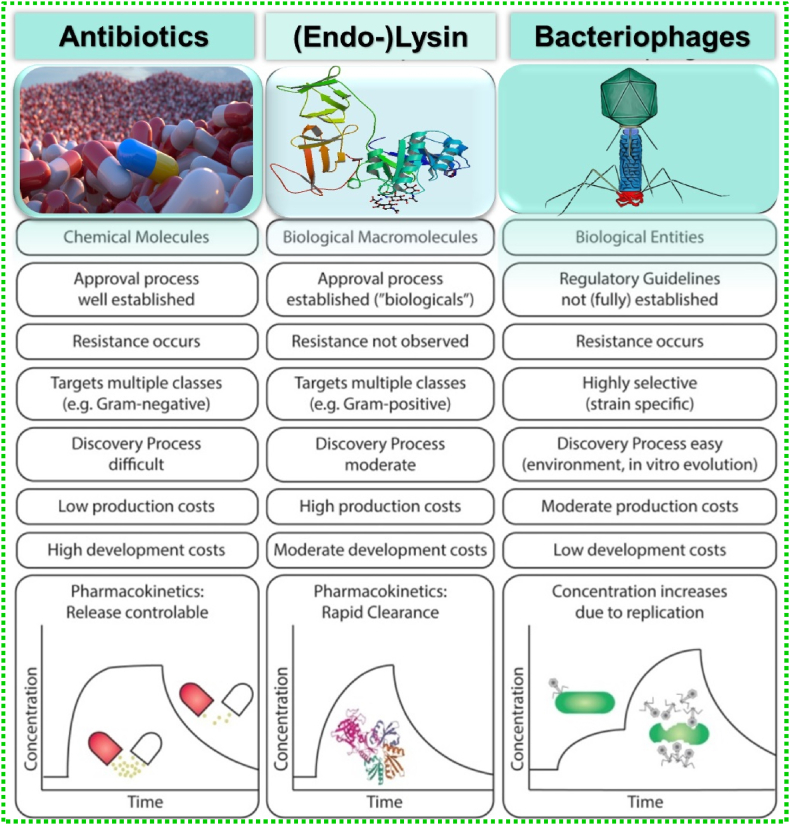
With increasing MDR, and depletion of antibiotic resources, phage therapy, and phage-endolysin-based therapy have become a potential alternative for the scientific society with various advantages over antibiotics. Antibiotics are chemical molecules whose discovery process is difficult and possesses high development costs. In this regard, bacteriophages are easily isolated from the environment with low processing costs. However, both strategies can develop resistance among pathogens which is not observed in the case of endolysins. The comparative advantages and limitations of all three strategies are shown in Fig. 8 [84].
Fig. 8.
Comparison of scientific, clinical, and pharmaceutical characteristics of chemical antibiotics, endolysins, and bacteriophages for the treatment of bacterial infections [84].
3.4. Current limitations of endolysin therapy
Despite the significant advancement of endolysin application, their practical use against bacteria, especially in gram-negative bacteria, has significant limitations [160]. The limitations can be summarized in four major groups; pharmacokinetics and immunomodulatory aspects, variety of drug delivery methods, specificity for general treatments, and regulatory issues. The structural complexity of the endolysin may lead to significant differences in the antibacterial activity which can further affect the formulation design and pharmacokinetics of the system. Moreover, endolysins are mostly used in the form of topical products which may not be suitable for body parts other than the skin. This is mainly because of the proteinaceous nature of the endolysins that may possess hindrances in the way through the gastrointestinal tract by oral route [161]. In addition, other routes may trigger the immune system highlighting the need for safer ways to utilize endolysins for the benefit of society. Nowadays recombinant endolysins are being used for personalized treatments which is not reliable for general treatments. In general, diseases are still being prescribed to use antibiotics. Determining if such customised care is an economically feasible alternative is the primary challenge. Recombinant-endolysin manufacturing and usage in human therapy are complicated by certain guidelines and restrictions associated with the technology. Additionally, the legal framework around endolysin applications is still non-standardized and is costlier than the conventional use of antibiotics [53]. However, to overcome this machine learning, AI can be adopted. However, screening of endolysins from the existing databases is a long process and needs validation. De novo synthesis of endolysins by use of AI also needs validation for its antibacterial activity in vitro. Some reports also state the variation in the action of artificially synthesized endolysins in laboratory assays using suspended cells and in complex environments such as milk or serum. Hence, there is a need for careful assessment of the safety, stability, and cost-effectiveness of engineered endolysins before using them [115].
4. Conclusion and future prospective
The development of resistant mechanisms in the bacteria is way too old as the bacteria itself which has now become a global threat of the overuse and misuse of various antibiotics. This current situation of antibiotic resistance can be countered by using a lytic phage as an antibacterial agent. Researchers have proved that phage therapy is a potential alternative to ineffective antibiotics. Phage therapy has several benefits over the use of antibiotics, including their "auto-dosing effect," low inherent toxicity, minimal disruption of normal flora, narrow potentials for inducing resistance, lack of cross-resistance with antibiotics, biofilm clearance, single-dose potential, potential for phage transfer between subjects, capacity for low-dosage use, single-hit kinetics, and the low environmental impact. The use of phage as an antibacterial agent may not be a new concept but can yield promising results with the proper understanding of its pharmacokinetics (PK) and pharmacodynamics (PD) in vivo. However, a new strategy of CRISPR-Cas3 technology has been used by Locus Biosciences, a clinical-stage pharmaceutical company to develop phage therapeutics named crPhage™ [162,163]. In recent years endolysins and their recombinants have emerged as a possible replacement for the problem of MDR [164]. The antibacterial activity makes it a potential candidate for multiple pathogens in the field of biomedical sciences, food industry, agriculture, etc. The use of conventional antibiotics has been established long before that makes it virtually impossible for phage therapy to replace it. However, several disadvantages like narrow range host specificity, poor understanding of underlying mechanisms, and weak push from the scientific community limit the translation of phage therapy into clinical trials. More clinical trials showing higher efficacy and potential than conventional antibiotics can be a breakthrough in persisting antibiotic resistance. However, endolysin-based products have already entered the commercial market and more developments are anticipated shortly with ongoing clinical trials. The advancement in biotechnology and biomedicine will be able to circumvent the current problems of phage and phage-derived endolysin therapy, which can be expected to be available as commercial products in hospitals and markets soon.
CRediT authorship contribution statement
Ananya Pattnaik: Writing – review & editing, Writing – original draft, Conceptualization. Sanghamitra Pati: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Sangram Keshari Samal: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ms. Ananya Patnaik acknowledges the Indian Council of Medical Research for awarding the fellowship of ICMR-SRF (Letter No. 45/01/2022-Nano/BMS). Dr. Sangram Keshari Samal highly acknowledges the Ramanujan fellowship (SB/S2/RJN-038/2016) of the Department of Science and Technology, and Ramalingaswami Re-entry fellowship (Ref: D.O. No. BT/HRD/35/02/2006) of Department of Biotechnology, Government of India. The authors also thank the Indian Council of Medical Research-Regional Medical Research Centre, Bhubaneswar for providing a scientific platform.
References
- 1.Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats., P T. 2015;40:277–283. http://www.ncbi.nlm.nih.gov/pubmed/25859123 [PMC free article] [PubMed] [Google Scholar]
- 2.Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy S.B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 4.Ganeshan Hosseinidoust. Phage therapy with a focus on the human microbiota. Antibiotics. 2019;8 doi: 10.3390/antibiotics8030131. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance. A Review, Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00539. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y., Wang C., Li Y., Li J., Wan Q., Chen J., Tay F.R., Niu L. Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci. 2020;7 doi: 10.1002/advs.201901872. 1901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen X., Lundborg C.S., Sun X., Hu X., Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob. Resist. Infect. Control. 2019;8 doi: 10.1186/s13756-019-0590-7. 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borin J.M., Avrani S., Barrick J.E., Petrie K.L., Meyer J.R. Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2104592118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakasis A., Panitsa G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review, Int. J. Antimicrob. Agents. 2019;53:16–21. doi: 10.1016/j.ijantimicag.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Dutescu I.A., Hillier S.A. Encouraging the development of new antibiotics: are financial incentives the right way forward? A systematic review and case study., infect. Drug Res. 2021;14:415–434. doi: 10.2147/IDR.S287792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beeton M.L., Alves D.R., Enright M.C., Jenkins A.T.A. Assessing phage therapy against Pseudomonas aeruginosa using a Galleria mellonella infection model. Int. J. Antimicrob. Agents. 2015;46:196–200. doi: 10.1016/j.ijantimicag.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Miethke M., Pieroni M., Weber T., Brönstrup M., Hammann P., Halby L., Arimondo P.B., Glaser P., Aigle B., Bode H.B., Moreira R., Li Y., Luzhetskyy A., Medema M.H., Pernodet J.-L., Stadler M., Tormo J.R., Genilloud O., Truman A.W., Weissman K.J., Takano E., Sabatini S., Stegmann E., Brötz-Oesterhelt H., Wohlleben W., Seemann M., Empting M., Hirsch A.K.H., Loretz B., Lehr C.-M., Titz A., Herrmann J., Jaeger T., Alt S., Hesterkamp T., Winterhalter M., Schiefer A., Pfarr K., Hoerauf A., Graz H., Graz M., Lindvall M., Ramurthy S., Karlén A., van Dongen M., Petkovic H., Keller A., Peyrane F., Donadio S., Fraisse L., Piddock L.J.V., Gilbert I.H., Moser H.E., Müller R. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021;5:726–749. doi: 10.1038/s41570-021-00313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu T.K., Collins J.J. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc. Natl. Acad. Sci. USA. 2009;106:4629–4634. doi: 10.1073/pnas.0800442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesudason T. Maintaining a robust pipeline of antibiotics. Lancet Infect. Dis. 2023;23 doi: 10.1016/S1473-3099(23)00384-5. 791. [DOI] [PubMed] [Google Scholar]
- 16.Housby J.N., Mann N.H. Phage therapy, drug discov. Today Off. 2009;14:536–540. doi: 10.1016/j.drudis.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Chan B.K., Abedon S.T., Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013;8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- 18.Gordillo Altamirano F.L., Barr J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019;32 doi: 10.1093/femsre/fuaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pires D.P., Costa A.R., Pinto G., Meneses L., Azeredo J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020;44:684–700. doi: 10.1093/femsre/fuaa017. [DOI] [PubMed] [Google Scholar]
- 20.Brives C., Pourraz J. Phage therapy as a potential solution in the fight against AMR. obstacles and possible futures, Palgrave Commun. 2020;6 doi: 10.1057/s41599-020-0478-4. 100. [DOI] [Google Scholar]
- 21.Durbas I., Machnik G. Phage therapy: an old concept with new perspectives. J. Appl. Pharmaceut. Sci. 2022 doi: 10.7324/JAPS.2022.120502. [DOI] [Google Scholar]
- 22.Rohde C., Wittmann J., Kutter E. Bacteriophages: a therapy concept against multi-drug–resistant bacteria, surg. Infect. (Larchmt) 2018;19:737–744. doi: 10.1089/sur.2018.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uyttebroek S., Chen B., Onsea J., Ruythooren F., Debaveye Y., Devolder D., Spriet I., Depypere M., Wagemans J., Lavigne R., Pirnay J.-P., Merabishvili M., De Munter P., Peetermans W.E., Dupont L., Van Gerven L., Metsemakers W.-J. Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review, Lancet Infect. Dis. 2022;22:e208–e220. doi: 10.1016/S1473-3099(21)00612-5. [DOI] [PubMed] [Google Scholar]
- 24.Hanlon G.W. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agents. 2007;30:118–128. doi: 10.1016/j.ijantimicag.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Rogovski P., Cadamuro R.D., da Silva R., de Souza E.B., Bonatto C., Viancelli A., Michelon W., Elmahdy E.M., Treichel H., Rodríguez-Lázaro D., Fongaro G. Uses of bacteriophages as bacterial control tools and environmental safety indicators. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.793135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavignon M., Kolenda C., Medina M., Bonhomme M., Blazere L., Legendre T., Tristan A., Laurent F., Butin M. Bacteriophage-based decontamination to control environmental colonization by Staphylococcus capitis in neonatal intensive care units: an in vitro proof-of-concept. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1060825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moye Z., Woolston J., Sulakvelidze A. Bacteriophage applications for food production and processing. v10040205Viruses. 2018;10 doi: 10.3390/v10040205. 10.3390/205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letarov A.V. History of early bacteriophage research and emergence of key concepts in virology. Biochem. 2020;85:1093–1112. doi: 10.1134/S0006297920090096. [DOI] [PubMed] [Google Scholar]
- 29.Dang V.T., Sullivan M.B. Emerging methods to study bacteriophage infection at the single-cell level. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abedon S.T., Thomas-Abedon C., Thomas A., Mazure H. Bacteriophage prehistory: is or is not Hankin, 1896, a phage reference? Bacteriophage. 2011;1:174–178. doi: 10.4161/bact.1.3.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulakvelidze A., Alavidze Z., Morris J.G. Bacteriophage therapy, antimicrob. Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summers W.C. The strange history of phage therapy, Bacteriophage. 2012;2:130–133. doi: 10.4161/bact.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell A. The future of bacteriophage biology, Nat. Rev. Genet. 2003;4:471–477. doi: 10.1038/nrg1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golkar Z., Bagasra O., Pace D.G. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014;8:129–136. doi: 10.3855/jidc.3573. [DOI] [PubMed] [Google Scholar]
- 35.Maciejewska B., Olszak T., Drulis-Kawa Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 2018;102:2563–2581. doi: 10.1007/s00253-018-8811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada L.K., Silva E.C., Campos W.F., Del Fiol F.S., Vila M., Dąbrowska K., Krylov V.N., Balcão V.M. Biotechnological applications of bacteriophages: state of the art. Microbiol. Res. 212–213. 2018:38–58. doi: 10.1016/j.micres.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Young R. Phage lysis: three steps, three choices, one outcome. J. Microbiol. 2014;52:243–258. doi: 10.1007/s12275-014-4087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard-Varona C., Hargreaves K.R., Abedon S.T., Sullivan M.B. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J. 2017;11:1511–1520. doi: 10.1038/ismej.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramisetty B.C.M., Sudhakari P.A. Bacterial ‘grounded’ prophages: hotspots for genetic renovation and innovation. Front. Genet. 2019;10 doi: 10.3389/fgene.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moradpour Z., Ghasemian A. Modified phages: novel antimicrobial agents to combat infectious diseases. Biotechnol. Adv. 2011;29:732–738. doi: 10.1016/j.biotechadv.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Mitsunaka S., Yamazaki K., Pramono A.K., Ikeuchi M., Kitao T., Ohara N., Kubori T., Nagai H., Ando H. Synthetic engineering and biological containment of bacteriophages. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2206739119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zurabov F.M., Chernevskaya E.A., Beloborodova N.V., Zurabov A.Y., Petrova M.V., Yadgarov M.Y., Popova V.M., Fatuev O.E., Zakharchenko V.E., Gurkova M.M., Sorokina E.A., Glazunov E.A., Kochetova T.A., Uskevich V.V., Kuzovlev A.N., Grechko A.V. Bacteriophage cocktails in the post-COVID rehabilitation. v14122614Viruses. 2022;14 doi: 10.3390/v14122614. 10.3390/2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J., Jain S., Sha J., Batra H., Ananthaswamy N., Kilgore P.B., Hendrix E.K., Hosakote Y.M., Wu X., Olano J.P., Kayode A., Galindo C.L., Banga S., Drelich A., Tat V., Tseng C.-T.K., Chopra A.K., Rao V.B. A bacteriophage-based, highly efficacious, needle- and adjuvant-free, mucosal COVID-19 vaccine. mBio. 2022;13 doi: 10.1128/mbio.01822-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan X., Huang Z., Zhu Z., Zhang J., Wu Q., Xue L., Wang J., Ding Y. Recent advances in phage defense systems and potential overcoming strategies. Biotechnol. Adv. 2023;65 doi: 10.1016/j.biotechadv.2023.108152. 108152. [DOI] [PubMed] [Google Scholar]
- 45.João J., Lampreia J., Prazeres D.M.F., Azevedo A.M. Manufacturing of bacteriophages for therapeutic applications. Biotechnol. Adv. 2021;49 doi: 10.1016/j.biotechadv.2021.107758. 107758. [DOI] [PubMed] [Google Scholar]
- 46.Magalhães C., Lima M., Trieu-Cuot P., Ferreira P. To give or not to give antibiotics is not the only question, Lancet Infect. Dis. 2021;21:e191–e201. doi: 10.1016/S1473-3099(20)30602-2. [DOI] [PubMed] [Google Scholar]
- 47.Abedon S. Bringing phage therapy soon to a clinic near you. Lancet Infect. Dis. 2020;20 doi: 10.1016/S1473-3099(20)30307-8. 551. [DOI] [Google Scholar]
- 48.Kosznik-Kwaśnicka K., Podlacha M. Ł. Grabowski, M. Stasiłojć, A. Nowak-Zaleska, K. Ciemińska, Z. Cyske, A. Dydecka, L. Gaffke, J. Mantej, D. Myślińska, A. Necel, K. Pierzynowska, E. Piotrowska, E. Radzanowska-Alenowicz, E. Rintz, K. Sitko, G. Topka-Bielecka, G. Węgrzyn, A. Węgrzyn, Biological aspects of phage therapy versus antibiotics against Salmonella enterica serovar Typhimurium infection of chickens. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.941867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukacik P., Barnard T.J., Keller P.W., Chaturvedi K.S., Seddiki N., Fairman J.W., Noinaj N., Kirby T.L., Henderson J.P., Steven A.C., Hinnebusch B.J., Buchanan S.K. Structural engineering of a phage lysin that targets Gram-negative pathogens. Proc. Natl. Acad. Sci. USA. 2012;109:9857–9862. doi: 10.1073/pnas.1203472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson D., Schuch R., Chahales P., Zhu S., Fischetti V.A. PlyC: a multimeric bacteriophage lysin, Proc. Natl. Acad. Sci. USA. 2006;103:10765–10770. doi: 10.1073/pnas.0604521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdelrahman F., Easwaran M., Daramola O.I., Ragab S., Lynch S., Oduselu T.J., Khan F.M., Ayobami A., Adnan F., Torrents E., Sanmukh S., El-Shibiny A. Phage-encoded endolysins. Antibiotics. 2021;10 doi: 10.3390/antibiotics10020124. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmelcher M., Donovan D.M., Loessner M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012;7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray E., Draper L.A., Ross R.P., Hill C. The advantages and challenges of using endolysins in a clinical setting, v13040680Viruses. 2021;13 doi: 10.3390/v13040680. 10.3390/680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jun S.Y., Jung G.M., Yoon S.J., Oh M.-D., Choi Y.-J., Lee W.J., Kong J.-C., Seol J.G., Kang S.H. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int. J. Antimicrob. Agents. 2013;41:156–161. doi: 10.1016/j.ijantimicag.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Filippov A.A., Sergueev K.V., Nikolich M.P. Can phage effectively treat multidrug-resistant plague? Bacteriophage. 2012;2:186–189. doi: 10.4161/bact.22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganeshan S.D., Hosseinidoust Z. Phage therapy with a focus on the human microbiota. Antibiotics. 2019;8 doi: 10.3390/ANTIBIOTICS8030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin D.M., Koskella B., Lin H.C. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017;8 doi: 10.4292/wjgpt.v8.i3.162. 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Żaczek M., Weber-Dąbrowska B., Międzybrodzki R., Łusiak-Szelachowska M., Górski A. Phage therapy in Poland – a centennial journey to the first ethically approved treatment facility in Europe. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Insight ace analytic com/report/global-phage. Global Phage Therapy Market. 2022 https://www.insightaceanalytic.com/report/global-phage-therapy-market-/1094 [Google Scholar]
- 60.Roach D.R., Donovan D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage. 2015;5 doi: 10.1080/21597081.2015.1062590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone E., Campbell K., Grant I., McAuliffe O., Understanding, Interactions Exploiting Phage-Host. 567Viruses. 2019;11 doi: 10.3390/v11060567. 10.3390/v11060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parent K.N., Erb M.L., Cardone G., Nguyen K., Gilcrease E.B., Porcek N.B., Pogliano J., Baker T.S., Casjens S.R. OmpA and OmpC are critical host factors for bacteriophage Sf6 entry in S higella. Mol. Microbiol. 2014;92:47–60. doi: 10.1111/mmi.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jakhetia R., Verma N.K., Identification, Molecular Characterisation of a novel mu-like bacteriophage, SfMu, of Shigella flexneri. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faruque S.M., Bin Naser I., Fujihara K., Diraphat P., Chowdhury N., Kamruzzaman M., Qadri F., Yamasaki S., Ghosh A.N., Mekalanos J.J. Genomic sequence and receptor for the Vibrio cholerae phage KSF-1Φ: evolutionary divergence among filamentous vibriophages mediating lateral gene transfer. J. Bacteriol. 2005;187:4095–4103. doi: 10.1128/JB.187.12.4095-4103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seed K.D., Faruque S.M., Mekalanos J.J., Calderwood S.B., Qadri F., Camilli A. Phase variable O antigen biosynthetic genes control expression of the major protective antigen and bacteriophage receptor in Vibrio cholerae O1. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morita M., Tanji Y., Mizoguchi K., Akitsu T., Kijima N., Unno H. Characterization of a virulent bacteriophage specific for Escherichia coli O157:H7 and analysis of its cellular receptor and two tail fiber genes. FEMS Microbiol. Lett. 2002;211:77–83. doi: 10.1111/j.1574-6968.2002.tb11206.x. [DOI] [PubMed] [Google Scholar]
- 67.Le S., He X., Tan Y., Huang G., Zhang L., Lux R., Shi W., Hu F. Mapping the tail fiber as the receptor binding protein responsible for differential host specificity of Pseudomonas aeruginosa bacteriophages PaP1 and JG004. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gillis A., Mahillon J. Phages preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: past, present and future. v6072623Viruses. 2014;6:2623–2672. doi: 10.3390/v6072623. 10.3390/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bishop-Lilly K.A., Plaut R.D., Chen P.E., Akmal A., Willner K.M., Butani A., Dorsey S., Mokashi V., Mateczun A.J., Chapman C., George M., Luu T., Read T.D., Calendar R., Stibitz S., Sozhamannan S. Whole genome sequencing of phage resistant Bacillus anthracismutants reveals an essential role for cell surface anchoring protein CsaB in phage AP50c adsorption. 246Virol. J. 2012;9 doi: 10.1186/1743-422X-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia G., Corrigan R.M., Winstel V., Goerke C., Gründling A., Peschel A. Wall teichoic acid-dependent adsorption of staphylococcal siphovirus and myovirus. J. Bacteriol. 2011;193:4006–4009. doi: 10.1128/JB.01412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaneko J., Narita-Yamada S., Wakabayashi Y., Kamio Y. Identification of ORF636 in phage φSLT carrying panton-valentine leukocidin genes, acting as an adhesion protein for a poly(Glycerophosphate) chain of lipoteichoic acid on the cell surface of Staphylococcus aureus. J. Bacteriol. 2009;191:4674–4680. doi: 10.1128/JB.01793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bielmann R., Habann M., Eugster M.R., Lurz R., Calendar R., Klumpp J., Loessner M.J. Receptor binding proteins of Listeria monocytogenes bacteriophages A118 and P35 recognize serovar-specific teichoic acids. Virology. 2015;477:110–118. doi: 10.1016/j.virol.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 73.phagex, Pyofag® 2021. https://bacteriophages.info/en/
- 74.Leitner L., Ujmajuridze A., Chanishvili N., Goderdzishvili M., Chkonia I., Rigvava S., Chkhotua A., Changashvili G., McCallin S., Schneider M.P., Liechti M.D., Mehnert U., Bachmann L.M., Sybesma W., Kessler T.M. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial, Lancet Infect. Dis. 2021;21:427–436. doi: 10.1016/S1473-3099(20)30330-3. [DOI] [PubMed] [Google Scholar]
- 75.Villarroel J., Larsen M., Kilstrup M., Nielsen M. Metagenomic analysis of therapeutic PYO phage cocktails from 1997 to 2014. Viruses. 2017;9:328. doi: 10.3390/v9110328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirby T. Nina Chanishvili—keeping bacteriophages in the limelight. Lancet Infect. Dis. 2021;21 doi: 10.1016/S1473-3099(21)00348-0. 926. [DOI] [PubMed] [Google Scholar]
- 77.K N.A. (RU), R.V.I. (RU), F.E.V. (RU), E.M.G. (RU), S.O.I. (RU), Sextaphage (pyobacteriophage polyvalent) or bacteriophage salmonellous pharmaceutical composition and method for producing thereof. 2009. https://patents.google.com/patent/RU2410084C1/en
- 78.Furfaro L.L., Payne M.S., Chang B.J. Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 2018;8 doi: 10.3389/fcimb.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jault P., Leclerc T., Jennes S., Pirnay J.P., Que Y.-A., Resch G., Rousseau A.F., Ravat F., Carsin H., Le Floch R., Schaal J.V., Soler C., Fevre C., Arnaud I., Bretaudeau L., Gabard J. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial, Lancet Infect. Dis. 2019;19:35–45. doi: 10.1016/S1473-3099(18)30482-1. [DOI] [PubMed] [Google Scholar]
- 80.Patrick Jault G.R., Thomas Leclerc Jérôme Gabard. Guy-Charles Fanneau de la Horie, Olivier Boisteau, Marc Meichenin, Jean-Paul Pirnay, Serge Jennes, Yok Ai Que, Evaluation of phage therapy for the treatment of Escherichia coli and Pseudomonas aeruginosa burn wound infections (Phase I-II clinical trial) 2017. https://cordis.europa.eu/project/id/601857/reporting
- 81.Fujiki J., Schnabl B. Phage therapy. Targeting intestinal bacterial microbiota for the treatment of liver diseases, JHEP Reports. 2023;5 doi: 10.1016/j.jhepr.2023.100909. 100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooper C.J., Khan Mirzaei M., Nilsson A.S. Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Federici S., Kredo-Russo S., Valdés-Mas R., Kviatcovsky D., Weinstock E., Matiuhin Y., Silberberg Y., Atarashi K., Furuichi M., Oka A., Liu B., Fibelman M., Weiner I.N., Khabra E., Cullin N., Ben-Yishai N., Inbar D., Ben-David H., Nicenboim J., Kowalsman N., Lieb W., Kario E., Cohen T., Geffen Y.F., Zelcbuch L., Cohen A., Rappo U., Gahali-Sass I., Golembo M., Lev V., Dori-Bachash M., Shapiro H., Moresi C., Cuevas-Sierra A., Mohapatra G., Kern L., Zheng D., Nobs S.P., Suez J., Stettner N., Harmelin A., Zak N., Puttagunta S., Bassan M., Honda K., Sokol H., Bang C., Franke A., Schramm C., Maharshak N., Sartor R.B., Sorek R., Elinav E. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185:2879–2898. doi: 10.1016/j.cell.2022.07.003. e24. [DOI] [PubMed] [Google Scholar]
- 84.Manohar P., Loh B., Athira S., Nachimuthu R., Hua X., Welburn S.C., Leptihn S. Secondary bacterial infections during pulmonary viral disease: phage therapeutics as alternatives to antibiotics? Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo X., Wang X., Shi J., Ren J., Zeng J., Li J., Li Y. A review and new perspective on oral bacteriophages: manifestations in the ecology of oral diseases, J. Oral Microbiol. 2024;16 doi: 10.1080/20002297.2024.2344272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiao Y., Tay F.R., Niu L., Chen J. Advancing antimicrobial strategies for managing oral biofilm infections, Int. J. Oral Sci. 2019;11 doi: 10.1038/s41368-019-0062-1. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng C., Meghil M.M., Miller M., Gou Y., Cutler C.W., Bergeron B.E., Niu L., Ma J., Tay F.R. Antimicrobial efficacy of an apical negative pressure root canal irrigation system against intracanal microorganisms. J. Dent. 2018;72:71–75. doi: 10.1016/j.jdent.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Mai S., Mauger M.T., Niu L., Barnes J.B., Kao S., Bergeron B.E., Ling J., Tay F.R. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater. 2017;49:16–35. doi: 10.1016/j.actbio.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 89.Makvandi P., Gu J.T., Zare E.N., Ashtari B., Moeini A., Tay F.R., Niu L. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020;101:69–101. doi: 10.1016/j.actbio.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 90.Principi N., Silvestri E., Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections, front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olszak T., Latka A., Roszniowski B., Valvano M.A., Drulis-Kawa Z. Phage life cycles behind bacterial biodiversity. Curr. Med. Chem. 2017;24 doi: 10.2174/0929867324666170413100136. [DOI] [PubMed] [Google Scholar]
- 92.ur Rahman M., Wang W., Sun Q., Shah J.A., Li C., Sun Y., Li Y., Zhang B., Chen W., Wang S. Endolysin, a promising solution against antimicrobial resistance. Antibiotics. 2021;10 doi: 10.3390/antibiotics10111277. 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maciejewska B., Źrubek K., Espaillat A., Wiśniewska M., Rembacz K.P., Cava F., Dubin G., Drulis-Kawa Z. Modular endolysin of Burkholderia AP3 phage has the largest lysozyme-like catalytic subunit discovered to date and no catalytic aspartate residue. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14797-9. 14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oechslin F., Daraspe J., Giddey M., Moreillon P., Resch G. In Vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia, antimicrob. Agents Chemother. 2013;57:6276–6283. doi: 10.1128/AAC.01701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang Y. Bacteriophage-derived endolysins applied as potent biocontrol agents to enhance food safety. Microorganisms. 2020;8 doi: 10.3390/microorganisms8050724. 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliveira H., Melo L.D.R., Santos S.B., Nóbrega F.L., Ferreira E.C., Cerca N., Azeredo J., Kluskens L.D., Aspects Molecular, Genomics Comparative. Of bacteriophage endolysins. J. Virol. 2013;87:4558–4570. doi: 10.1128/JVI.03277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmelcher M., Loessner M.J. Bacteriophage endolysins — extending their application to tissues and the bloodstream. Curr. Opin. Biotechnol. 2021;68:51–59. doi: 10.1016/j.copbio.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 98.Gerstmans H., Criel B., Briers Y. Synthetic biology of modular endolysins. Biotechnol. Adv. 2018;36:624–640. doi: 10.1016/j.biotechadv.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 99.Walmagh M., Briers Y. S.B. Dos santos, J. Azeredo, R. Lavigne, characterization of modular bacteriophage endolysins from myoviridae phages OBP. 201φ2–1 and PVP-SE1, PLoS One. 2012;7 doi: 10.1371/journal.pone.0036991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ning H.-Q., Lin H., Wang J.-X. Synergistic effects of endolysin Lysqdvp001 and ε-poly-lysine in controlling Vibrio parahaemolyticus and its biofilms, Int. J. Food Microbiol. 343. 2021;109112 doi: 10.1016/j.ijfoodmicro.2021.109112. [DOI] [PubMed] [Google Scholar]
- 101.Bai J., Yang E., Chang P.-S., Ryu S. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria, Enzyme Microb. Technol. 2019;128:40–48. doi: 10.1016/j.enzmictec.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 102.Gontijo M.T.P., Jorge G.P., Brocchi M. Current status of endolysin-based treatments against gram-negative bacteria. Antibiotics. 2021;10 doi: 10.3390/antibiotics10101143. 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gondil V.S., Harjai K., Chhibber S. Investigating the potential of endolysin loaded chitosan nanoparticles in the treatment of pneumococcal pneumonia. J. Drug Deliv. Sci. Technol. 2021;61 doi: 10.1016/j.jddst.2020.102142. 102142. [DOI] [Google Scholar]
- 104.Ning H., Zhang J., Zhao Q., Lin H., Wang J. Development of the phage lysin-loaded liposomes as preservatives for live clams, Int. J. Food Microbiol. 2023;387 doi: 10.1016/j.ijfoodmicro.2022.110059. 110059. [DOI] [PubMed] [Google Scholar]
- 105.Abouhmad A., Dishisha T., Amin M.A., Hatti-Kaul R. Immobilization to positively charged cellulose nanocrystals enhances the antibacterial activity and stability of hen egg white and T4 lysozyme. Biomacromolecules. 2017;18:1600–1608. doi: 10.1021/acs.biomac.7b00219. [DOI] [PubMed] [Google Scholar]
- 106.Ciepluch K., Maciejewska B., Gałczyńska K., Kuc-Ciepluch D., Bryszewska M., Appelhans D., Drulis-Kawa Z., Arabski M. The influence of cationic dendrimers on antibacterial activity of phage endolysin against P. aeruginosa cells. Bioorg. Chem. 2019;91 doi: 10.1016/j.bioorg.2019.103121. 103121. [DOI] [PubMed] [Google Scholar]
- 107.Briers Y., Lavigne R. Breaking barriers: expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol. 2015;10:377–390. doi: 10.2217/fmb.15.8. [DOI] [PubMed] [Google Scholar]
- 108.Endersen L., Guinane C.M., Johnston C., Neve H., Coffey A., Ross R.P., McAuliffe O., O'Mahony J. Genome analysis of Cronobacter phage vB_CsaP_Ss1 reveals an endolysin with potential for biocontrol of Gram-negative bacterial pathogens. J. Gen. Virol. 2015;96:463–477. doi: 10.1099/vir.0.068494-0. [DOI] [PubMed] [Google Scholar]
- 109.Zhang C., Wang Y., Sun H., Ren H. Multiple-site mutations of phage Bp7 endolysin improves its activities against target bacteria. Virol. Sin. 2015;30:386–395. doi: 10.1007/s12250-015-3618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim D., Kim J., Kim M. Potential of antimicrobial peptide-fused endolysin LysC02 as therapeutics for infections and disinfectants for food contact surfaces to control Cronobacter sakazakii, Food Control. 157. 2024;110190 doi: 10.1016/j.foodcont.2023.110190. [DOI] [Google Scholar]
- 111.Islam M.M., Kim D., Kim K., Park S.-J., Akter S., Kim J., Bang S., Kim S., Kim J., Lee J.C., Hong C.-W., Shin M. Engineering of lysin by fusion of antimicrobial peptide (cecropin A) enhances its antibacterial properties against multidrug-resistant Acinetobacter baumannii. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.988522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ghose C., Euler C.W. Gram-negative bacterial lysins. Antibiotics. 2020;9 doi: 10.3390/antibiotics9020074. 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma Q., Guo Z., Gao C., Zhu R., Wang S., Yu L., Qin W., Xia X., Gu J., Yan G., Lei L. Enhancement of the direct antimicrobial activity of Lysep3 against Escherichia coli by inserting cationic peptides into its C terminus. Antonie Leeuwenhoek. 2017;110:347–355. doi: 10.1007/s10482-016-0806-2. [DOI] [PubMed] [Google Scholar]
- 114.Zampara A., Sørensen M.C.H., Gencay Y.E., Grimon D., Kristiansen S.H., Jørgensen L.S., Kristensen J.R., Briers Y., Elsser-Gravesen A., Brøndsted L. Developing innolysins against Campylobacter jejuni using a novel prophage receptor-binding protein. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.619028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nazir A., Xu X., Liu Y., Chen Y. Phage endolysins. Advances in the World of Food Safety, Cells. 2023;12 doi: 10.3390/cells12172169. 2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Traczewski M.M., Ambler J.E., Schuch R. Determination of MIC quality control parameters for exebacase, a novel lysin with antistaphylococcal activity. J. Clin. Microbiol. 2021;59 doi: 10.1128/JCM.03117-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.ContraFect Direct. Lysis of staph aureus resistant pathogen trial of exebacase (DISRUPT) 2023. www.clinicaltrials.gov/study
- 118.Souche A., Kolenda C., Teoli J., Schuch R., Ferry T., Laurent F., Josse J. Activity of exebacase (CF-301) against biofilms formed by Staphylococcus epidermidis strains isolated from prosthetic joint infections, antimicrob. Agents Chemother. 2022;66 doi: 10.1128/aac.00588-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.I. Intron Biotechnology Phase IIa clinical study of N-Rephasin® SAL200. 2021. https://clinicaltrials.gov/study/NCT03089697
- 120.I. Lumen Bioscience LMN-201 for prevention of C. Difficile infection recurrence, NCT05330182. 2024. https://clinicaltrials.gov/study/NCT05330182
- 121.No Title, (n.d.) https://www.lumen.bio/news/lumen-bioscience-receives-fast-track-designation-us-fda-lmn-201.
- 122.Haddad Kashani H., Schmelcher M., Sabzalipoor H., Seyed Hosseini E., Moniri R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin. Microbiol. Rev. 2018;31 doi: 10.1128/CMR.00071-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shah S., Das R., Chavan B., Bajpai U., Hanif S., Ahmed S. Beyond antibiotics: phage-encoded lysins against Gram-negative pathogens. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nair S., Poonacha N., Desai S., Hiremath D., Tuppad D., Mohan T. Restoration of sensitivity of a diverse set of drug-resistant Staphylococcus clinical strains by bactericidal protein P128 Restoration of sensitivity of a diverse set of drug-resistant Staphylococcus clinical strains by bactericidal protein P128. 2018. [DOI] [PubMed]
- 125.Zhang Y., Cheng M., Zhang H., Dai J., Guo Z., Li X., Ji Y., Cai R., Xi H., Wang X., Xue Y., Sun C., Feng X., Lei L., Han W., Gu J. Antibacterial effects of phage lysin LysGH15 on planktonic cells and biofilms of diverse Staphylococci. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00886-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang H., Luo D., Etobayeva I., Li X., Gong Y., Wang S., Li Q., Xu P., Yin W., He J., Nelson D.C., Wei H. Linker editing of pneumococcal lysin ClyJ conveys improved bactericidal activity, antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01610-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang H., Gong Y., Zhang H., Etobayeva I., Miernikiewicz P., Luo D., Li X., Zhang X., Dąbrowska K., Nelson D.C., He J., Wei H. ClyJ is a novel pneumococcal chimeric lysin with a cysteine- and histidine-dependent amidohydrolase/peptidase catalytic domain, antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vázquez R., García P. Synergy Between Two Chimeric Lysins to Kill Streptococcus pneumoniae. 2019;10:1–10. doi: 10.3389/fmicb.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Letrado P., Corsini B., Díez-Martínez R., Bustamante N., Yuste J.E., García P. Bactericidal synergism between antibiotics and phage endolysin Cpl-711 to kill multidrug-resistant pneumococcus. Future Microbiol. 2018;13:1215–1223. doi: 10.2217/fmb-2018-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang L., Luo D., Gondil V.S., Gong Y., Jia M., Yan D., He J., Hu S. 2020. Construction and Characterization of a Chimeric Lysin ClyV with Improved Bactericidal Activity against Streptococcus Agalactiae in Vitro and in Vivo. [DOI] [PubMed] [Google Scholar]
- 131.Wang Z., Ma J., Wang J., Yang D., Kong L., Fu Q., Cheng Y., Wang H., Yan Y., Sun J. 2019. Application of the Phage Lysin Ply5218 in the Treatment of Streptococcus Suis Infection in Piglets; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim S., Lee D.-W., Jin J.-S., Kim J. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2020;22:32–39. doi: 10.1016/j.jgar.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 133.Wu M., Hu K., Xie Y., Liu Y., Mu D., Guo H., Zhang Z., Zhang Y., Chang D., Shi Y. A novel phage PD-6A3, and its endolysin Ply6A3, with extended lytic activity against acinetobacter baumannii, front. Microbiol. 2019;9 doi: 10.3389/fmicb.2018.03302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heselpoth R.D., Euler C.W., Schuch R., Fischetti V.A. Lysocins: bioengineered antimicrobials that deliver lysins across the outer membrane of gram-negative bacteria, antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00342-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim N., Park W.B., Cho J.E., Choi Y.J., Choi S.J., Jun S.Y., Kang C.K., Song K., Choe P.G., Kim E.S., Park S.W., Kim N., Oh M., Bin H. Effects of phage endolysin SAL200 combined with antibiotics on Staphylococcus aureus infection. 2018. [DOI] [PMC free article] [PubMed]
- 136.Sobieraj A.M., Huemer M., Zinsli L.V., Meile S., Keller A.P., Röhrig C., Eichenseher F., Shen Y., Zinkernagel A.S., Loessner M.J., Schmelcher M. Engineering of long-circulating peptidoglycan hydrolases enables efficient treatment of systemic Staphylococcus aureus infection. mBio. 2020;11 doi: 10.1128/mBio.01781-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schuch R., Pelzek A.J., Nelson D.C., Fischetti V.A. The PlyB endolysin of bacteriophage vB_BanS_Bcp1 exhibits broad-spectrum bactericidal activity against Bacillus cereus sensu lato isolates, Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.00003-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Indiani C., Sauve K., Raz A., Abdelhady W., Xiong Y.Q., Cassino C., Bayer A.S., Schuch R. The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis, antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shah S.U., Xiong Y.Q., Abdelhady W., Iwaz J., Pak Y., Schuch R., Cassino C., Lehoux D., Bayer A.S. Effect of the lysin exebacase on cardiac vegetation progression in a rabbit model of methicillin-resistant Staphylococcus aureus endocarditis as determined by echocardiography, antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00482-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bae J.Y., Il Jun K., Kang C.K., Song K.-H., Choe P.G., Bang J.-H., Kim E.S., Park S.W., Bin Kim H., Kim N.-J., Park W.B., Oh M. Efficacy of intranasal administration of the recombinant endolysin SAL200 in a lethal murine Staphylococcus aureus pneumonia model, antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park S., Jun S.Y., Kim C.-H., Jung G.M., Son J.S., Jeong S.T., Yoon S.J., Lee S.Y., Kang S.H. Characterisation of the antibacterial properties of the recombinant phage endolysins AP50-31 and LysB4 as potent bactericidal agents against Bacillus anthracis. Sci. Rep. 2018;8 doi: 10.1038/s41598-017-18535-z. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blasco L., Ambroa A., Trastoy R., Bleriot I., Moscoso M., Fernández-Garcia L., Perez-Nadales E., Fernández-Cuenca F., Torre-Cisneros J., Oteo-Iglesias J., Oliver A., Canton R., Kidd T., Navarro F., Miró E., Pascual A., Bou G., Martínez-Martínez L., Tomas M. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-64145-7. 7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Raz A., Serrano A., Hernandez A., Euler C.W., Fischetti V.A. Isolation of phage lysins that effectively kill Pseudomonas aeruginosa in mouse models of lung and skin infection, antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00024-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Corsini B., Díez-Martínez R., Aguinagalde L., González-Camacho F., García-Fernández E., Letrado P., García P., Yuste J. Chemotherapy with phage lysins reduces pneumococcal colonization of the respiratory tract, antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.02212-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peng Z., Wang S., Gide M., Zhu D. H.M. Lamabadu Warnakulasuriya Patabendige, C. Li, J. Cai, X. Sun, A novel bacteriophage lysin-human Defensin fusion protein is effective in treatment of Clostridioides difficile infection in Mice, Front. Microbiol. 2019;9 doi: 10.3389/fmicb.2018.03234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karau M.J., Schmidt-Malan S.M., Yan Q., Greenwood-Quaintance K.E., Mandrekar J., Lehoux D., Schuch R., Cassino C., Patel R. Exebacase in addition to Daptomycin is more active than Daptomycin or exebacase Alone in methicillin-resistant Staphylococcus aureus Osteomyelitis in Rats, Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.01235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fursov M.V., Abdrakhmanova R.O., Antonova N.P., Vasina D.V., Kolchanova A.D., Bashkina O.A., Rubalsky O.V., Samotrueva M.A., Potapov V.D., Makarov V.V., Yudin S.M., Gintsburg A.L., Tkachuk A.P., Gushchin V.A., Rubalskii E.O. Antibiofilm activity of a broad-range recombinant endolysin LysECD7. In Vitro and In Vivo Study, Viruses. 2020;12 doi: 10.3390/v12050545. 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Röhrig C., Huemer M., Lorgé D., Luterbacher S., Phothaworn P., Schefer C., Sobieraj A.M., Zinsli L.V., Mairpady Shambat S., Leimer N., Keller A.P., Eichenseher F., Shen Y., Korbsrisate S., Zinkernagel A.S., Loessner M.J., Schmelcher M. Targeting Hidden pathogens: cell-penetrating Enzybiotics eradicate intracellular drug-resistant Staphylococcus aureus. mBio. 2020;11 doi: 10.1128/mBio.00209-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Imanishi I., Uchiyama J., Tsukui T., Hisatsune J., Ide K., Matsuzaki S., Sugai M., Nishifuji K. Therapeutic potential of an endolysin derived from Kayvirus S25-3 for Staphylococcal Impetigo. Viruses. 2019;11:769. doi: 10.3390/v11090769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng M., Zhang L., Zhang H., Li X., Wang Y., Xia F., Wang B., Cai R., Guo Z., Zhang Y., Ji Y., Sun C., Feng X., Lei L., Yang Y., Han W., Gu J. An Ointment consisting of the phage lysin LysGH15 and Apigenin for Decolonization of methicillin-resistant Staphylococcus aureus from skin wounds. Viruses. 2018;10 doi: 10.3390/v10050244. 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang F., Ji X., Li Q., Zhang G., Peng J., Hai J., Zhang Y. TSPphg lysin from the Extremophilic Thermus bacteriophage TSP4 as a potential antimicrobial agent against both gram-negative and gram- positive pathogenic bacteria. 2020. https://doi.org/10.3390/ [DOI] [PMC free article] [PubMed]
- 152.Fraga A.G., Trigo G., Murthy R.K., Akhtar S., Hebbur M., Pacheco A.R., Dominguez J., Silva-Gomes R., Gonçalves C.M., Oliveira H., Castro A.G., Sharma U., Azeredo J., Pedrosa J. Antimicrobial activity of Mycobacteriophage D29 lysin B during Mycobacterium ulcerans infection, PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.García P. Phage Lytic Protein LysRODI Prevents Staphylococcal Mastitis in Mice. 2020;11 doi: 10.3389/fmicb.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu J., Yang H., Bi Y., Li W., Wei H., Li Y. Activity of the chimeric lysin ClyR against common gram-positive oral Microbes and its Anticaries efficacy in Rat models. Viruses. 2018;10 doi: 10.3390/v10070380. 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vasina D.V., Antonova N.P., Vorobev A.M., Laishevtsev A.I., Kapustin A.V., Zulkarneev E.R., Bochkareva S.S., Kiseleva I.A., Anurova M.N., Aleshkin A.V., Tkachuk A.P., Gushchin V.A. Efficacy of the endolysin-based antibacterial gel for treatment of Anaerobic infection caused by Fusobacterium necrophorum. Antibiotics. 2021;10 doi: 10.3390/antibiotics10101260. 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu Y., Wang Y., Wang J., Zhao Y., Zhong Q., Li G., Fu Z. Phage Endolysin LysP108 Showed Promising Antibacterial Potential Against Methicillin-resistant Staphylococcus aureus. 2021;11:1–12. doi: 10.3389/fcimb.2021.668430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu G., Zhang S., Gao T., Mao Z., Shen Y., Pan Z., Guo C., Yu Y., Yao H. Identification of a novel broad-spectrum endolysin, Ply0643, with high antibacterial activity in mouse models of streptococcal bacteriaemia and mastitis. Res. Vet. Sci. 2022;143:41–49. doi: 10.1016/j.rvsc.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 158.Lu B., Yao X., Han G., Luo Z., Zhang J., Yong K., Wang Y., Luo Y., Yang Z., Ren M., Cao S. Isolation of Klebsiella pneumoniae phage vB_KpnS_MK54 and Pathological assessment of endolysin in the treatment of pneumonia Mice model. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.854908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Eichenseher F., Herpers B.L., Badoux P., Leyva-Castillo J.M., Geha R.S., van der Zwart M., McKellar J., Janssen F., de Rooij B., Selvakumar L., Röhrig C., Frieling J., Offerhaus M., Loessner M.J., Schmelcher M. Linker-improved chimeric endolysin Selectively kills Staphylococcus aureus in vitro, on Reconstituted human Epidermis, and in a murine model of skin infection, Antimicrob. Agents Chemother. 2022;66 doi: 10.1128/aac.02273-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wong K.Y. M.H. Megat Mazhar Khair, A.A.-L. Song, M.J. Masarudin, C.M. Chong, L.L.A. In, M.Y.M. Teo, endolysins against Streptococci as an antibiotic alternative. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.935145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.de Souto Barreto P., Vellas B., Rolland Y. Physical activity and exercise in the context of SARS-Cov-2: a perspective from geroscience field. Ageing Res. Rev. 2021;66 doi: 10.1016/j.arr.2021.101258. 101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barnard A.M.L., Fairhead H.I.M. A commentary on the development of engineered phage as therapeutics, Drug Discov. Today Off. 2021;26:2095–2098. doi: 10.1016/j.drudis.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 163.Yeh T.-K., Jean S.-S., Lee Y.-L., Lu M.-C., Ko W.-C., Lin H.-J., Liu P.-Y., Hsueh P.-R. Bacteriophages and phage-delivered CRISPR-Cas system as antibacterial therapy, Int. J. Antimicrob. Agents. 2022;59 doi: 10.1016/j.ijantimicag.2021.106475. 106475. [DOI] [PubMed] [Google Scholar]
- 164.Gondil V.S., Harjai K., Chhibber S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections, Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2019.11.001. 105844. [DOI] [PubMed] [Google Scholar]