Abstract
Background & Aims
An estimated 50 million individuals have chronic hepatitis C virus (HCV) infection worldwide and people who use drugs (PWUD) are disproportionately affected. Persistent stigma and discrimination make it challenging for PWUD to access healthcare, potentially hindering HCV elimination progress in this population. To mitigate healthcare access barriers in PWUD, an HCV care model that simplified screening and linkage to care pathways was developed and rolled out in the Balearic Islands, Spain.
Methods
The prospective multicentre community model of care was implemented in 21 centres serving PWUD. This model involved: (1) participant recruitment and HCV antibody screening onsite via a point-of-care anti-HCV test, phlebotomy, or laboratory records; (2) HCV RNA, HBsAg and anti-HIV testing via a dried blood spot or phlebotomy; (3) linkage to specialist care and treatment prescription via telemedicine, when required; and (4) onsite monitoring of: (a) sustained virologic response (SVR) 4 and ≥12 weeks after treatment completion and; (b) potential new HCV infection or reinfection ∼1 year after phase 1 or SVR ≥12 monitoring. Care model acceptability was assessed.
Results
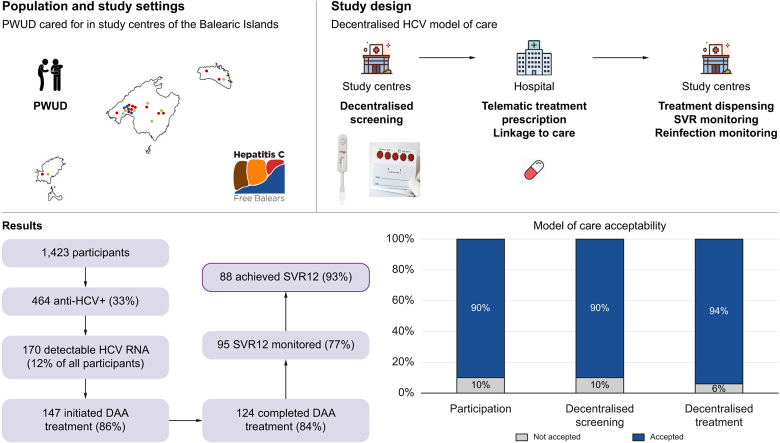
Between April 2021 and April 2023, 1,423 participants were recruited, of whom 464 (33%) were anti-HCV+ and 170 (12%) had detectable HCV RNA. Of the latter, 147 (86%) initiated therapy, of whom 124 (84%) completed it. SVR ≥12 monitoring was performed in 95 (77%) of these, of whom 88 (93%) had undetectable HCV RNA. Upon re-screening, four HCV reinfections were detected. Over 90% accepted study participation and screening and treatment decentralisation.
Conclusions
This adapted care model, which decentralised screening, diagnosis, and treatment, effectively increased healthcare access among PWUD, improving progress towards HCV elimination in this population in Spain.
Impact and implications:
People who use drugs (PWUD) are among the most affected by chronic hepatitis C virus (HCV) infection globally. A simplified model of care was implemented in 21 centres serving this population across the Balearic Islands, Spain, to offer HCV care to 1,423 PWUD in 2021-2023. This decentralised screening, diagnosis, and treatment model resulted in an HCV cure rate of 93% of those who both completed therapy and were monitored post treatment completion. The Hepatitis C Free Balears model can guide the HCV elimination efforts of regional health authorities and other stakeholders in the rest of Spain and other parts of the world.
Keywords: Hepatitis C virus, Micro-elimination, Point-of-care testing, Telemedicine, Viral hepatitis, Marginalised population, Balearic Islands, Spain
Graphical abstract
Highlights:
-
•
A decentralised HCV model of care for people who use drugs was implemented in 21 centres in the Balearic Islands, Spain.
-
•
The prevalence of active HCV infection was 12%; most were men, with a mean age of 47 years and Spanish origin.
-
•
Among those with active infection, only 14% were newly diagnosed and the rest had a prior HCV diagnosis.
-
•
Of those infected, 86% initiated treatment, of whom 84% completed it, and of those monitored for SVR, 93% were cured.
-
•
Acceptability to participate and receive decentralised screening and treatment was over 90%.
Introduction
An estimated 50 million people worldwide have chronic hepatitis C virus (HCV) infection, of which, an estimated 21% are diagnosed and 16% treated.1,2 These figures are far below the World Health Organization (WHO) elimination targets set forth in their Global Health Sector Strategy (GHSS) on viral hepatitis 2016–2021,3 renewed in 2022.2 The GHSS states that, by 2030, 90% of those with HCV should be diagnosed and 80% should be treated, and includes specific objectives to reduce the burden and distribution of HBV and HIV.2 Despite the existence of cost-effective interventions for diagnosis and treatment,2 HCV continues to be underdiagnosed and diagnosed late,4 perpetuating its onward transmission. Left untreated, chronic HCV infection can lead to fibrosis, cirrhosis, end-stage liver disease, and liver cancer,[5], [6], [7] increasing the burden for those infected and healthcare systems.
Most countries are not on track to eliminate HCV by 2030.8 To achieve the WHO elimination targets, tailored models of care are needed to identify most people living with HCV and facilitate treatment.9,10 The pragmatic micro-elimination approach, consisting of creating simplified, adapted, and optimal care models for specific subpopulations at high risk of HCV in specific geographical regions, is conducive to achieving national level elimination targets.[10], [11], [12], [13] One such population are people who use drugs (PWUD), a vulnerable group disproportionately affected by HCV.2 PWUD encounter healthcare access barriers such as stigma, discrimination,14 limited HCV knowledge, including treatment, and health-related motivation deficits associated with drug use,15,16 leading to an increased risk of infection and a lack of access to treatment.17 Tailored interventions to target this population are thus necessary.
One such model of care, proposed by the WHO to accomplish HCV elimination, involves decentralising HCV testing and treatment from higher-level health facilities, such as hospitals, to lower-level ones, such as harm reduction centres.2 Furthermore, several studies have shown that methods such as reflex testing, where a single biological sample is used to diagnose, are highly reliable and simplify HCV infection identification in PWUD.[18], [19], [20] These strategies optimise healthcare reach in PWUD by offering simplified HCV screening and therapy in settings that they are more likely to access,2 thus increasing the likelihood that infected individuals will be diagnosed and linked to care. They also improve care provision efficiency by maximising centre visit use, thus reducing the number of encounters needed for achieving HCV clearance.[21], [22], [23]
According to the Spanish Ministry of Health, of the 1.17 million people living in the Balearic Islands, an autonomous community composed of the islands of Mallorca, Menorca, Ibiza, and Formentera, approximately 1,350 have an untreated HCV infection.24 Thus, aligned with the Spanish Coalition for the Elimination of Viral Hepatitis (AEHVE),25 the Spanish Association for the Study of the Liver (AEEH),26 and the micro-elimination strategy put forward by the European Association for the Study of the Liver (EASL) International Liver Foundation,12 a new HCV care model was developed to increase the healthcare reach among PWUD living with HCV in this region. The aim of this study was to implement and evaluate this model (Hepatitis C Free Balears), which simplified HCV screening and provided easy-to-access care pathways via a micro-elimination approach.
Participants and methods
Study design and population
This was a prospective multicentre study using implementation science methods to evaluate the cascade of care and acceptability of a simplified HCV screening and linkage to care model among PWUD being cared for across 21 centres in the Balearic Islands between April 2021 and April 2023. Implementation of the model of care was done gradually. Eligible participants had to have a self-reported history of drug use (active or former), including alcohol, be ≥18 years old, and provide informed consent. Exclusion criteria included having language barriers and being on HCV treatment. This study received ethical clearance in January 2021 by the Ethics Committee of the Hospital Clínic of Barcelona, Spain (identification number: HCB/2020/1018).
Study phases and sites
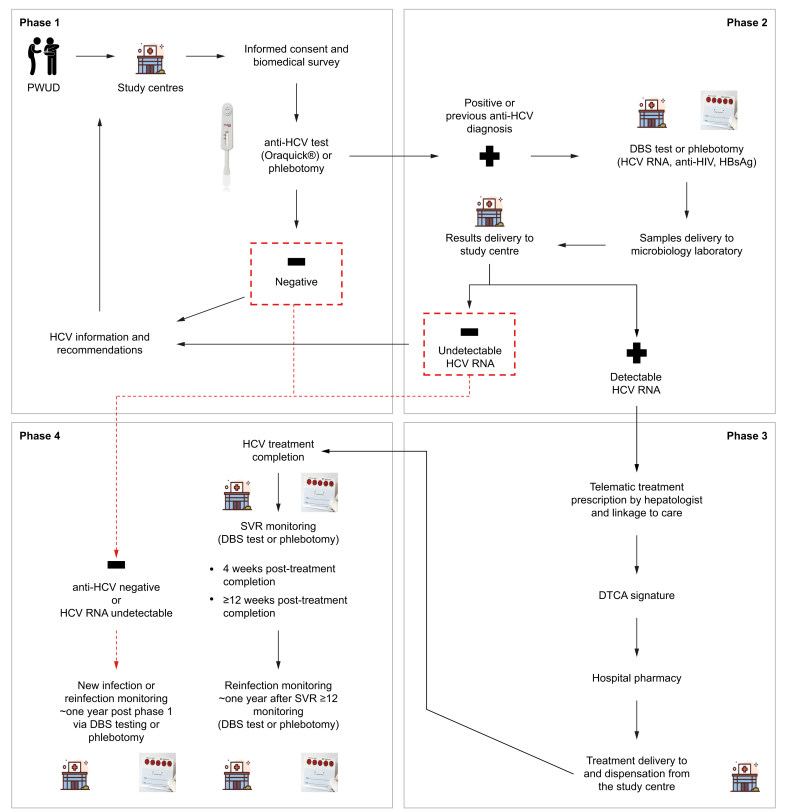
The study involved four sequential phases. First, participants were offered decentralised point-of-care (PoC) screening for HCV antibody (Ab) via an anti-HCV rapid saliva test (OraQuick®, OraSure Technologies, Bethlehem, PA, United States) or a blood sample extraction through centralised phlebotomy (the standard of care), which was performed at an external health centre or hospital. Participants who reported a past HCV infection were not offered anti-HCV rapid saliva tests, and were directly tested to determine possible presence of detectable HCV RNA.
Self-reported prior HCV diagnosis was confirmed at the central microbiology laboratories (n = 2) through validation of participants’ medical records. In cases where prior infections could not be confirmed because of a lack of evidence in medical records, anti-HCV determination was performed to confirm. Patients with recent HCV RNA results available in their laboratory records were not required to be tested to determine active infection.
Participants with a confirmed past HCV infection (of those who self-reported a past infection), and new anti-HCV+ cases (identified through anti-HCV rapid test screening) were tested for HCV RNA via dried blood spot (DBS) or phlebotomy.
DBS and phlebotomy samples, which were transported to and processed in central microbiology laboratories, and underwent HCV RNA determination, were also screened for HBsAg and anti-HIV Ab. Quantitative reverse transcription polymerase chain reaction testing (Alinitym, Abbott®, Lake Bluff, IL, United States) was used for HCV RNA molecular testing and ECLIA (Alinityi, Abbott®, Lake Bluff, IL, United States) for serological detection. DBS analyses were performed in the molecular microbiology units of two reference hospitals in the Balearic Islands (Son Llàtzer University Hospital and Son Espases University Hospital, both located in the city of Palma, Mallorca), whereas phlebotomy analyses were carried out at the Son Espases University Hospital only.
Exceptions to the screening protocol
There were two exceptions to the screening procedure: (1) among participants reporting previous diagnosis, those actively requesting anti-HCV rapid test screening underwent it and; (2) DBS were collected for some participants without previous self-reported diagnoses, either because of an error in the protocol’s performance or difficulty in linking the participants for rapid testing.
Participants with active infection who were eligible for treatment were telematically prescribed direct-acting antiviral (DAA) treatment by a hepatologist, which was delivered from a hospital pharmacy to the centre where the participant was being cared for, after agreement with the participant and signature of a delegated treatment collection authorisation (DTCA). Participants could choose to be treated via this decentralised process or to collect their medication at a hospital pharmacy. DAA dispensation scheduling was tailored according to participants’ characteristics and study centre staff recommendations, or by the hospital pharmacy in non-decentralised cases. Liver elastography was offered to some participants before HCV treatment initiation. Treatment completion was defined according to the information obtained from study centres on each participant. Eligibility for HCV treatment could be denied owing to medical reasons or participants’ personal reasons. People diagnosed with HBV and HIV were also linked to care.
Next, at Weeks 4 and ≥12 after treatment completion, participants were offered sustained virologic response (SVR) monitoring to confirm HCV clearance. SVR monitoring was done via DBS testing or phlebotomy, depending on the patient´s preference. Two SVR controls were offered and carried out, if accepted, to analyse whether the results obtained at Week 4 and at Week ≥12 were concordant in this population. DBS testing for potential reinfection was offered ∼1 year after SVR ≥12 monitoring, particularly among those who continued to engage in high-risk behaviours, such as injecting drug use. Approximately 1 year after undergoing HCV screening (phase 1), any participant could be offered HCV re-screening. Among those who were anti-HCV- during phase 1, re-screening was done via the PoC rapid saliva test (OraQuick®) to identify new infections. DBS testing or phlebotomy was offered, as per the participants preference, to participants with a previous anti-HCV+ diagnosis to screen for reinfection.
All participants were surveyed on biomedical and sociodemographic information and were provided with relevant information and recommendations on HCV. Fig. 1 details the four phases of the cascade of care, which are also described in the study’s protocol.27
Fig. 1.
Study phases.
DBS, dried blood spot; DTCA, delegated treatment collection authorisation; PWUD, people who use drugs; SVR, sustained virologic response.
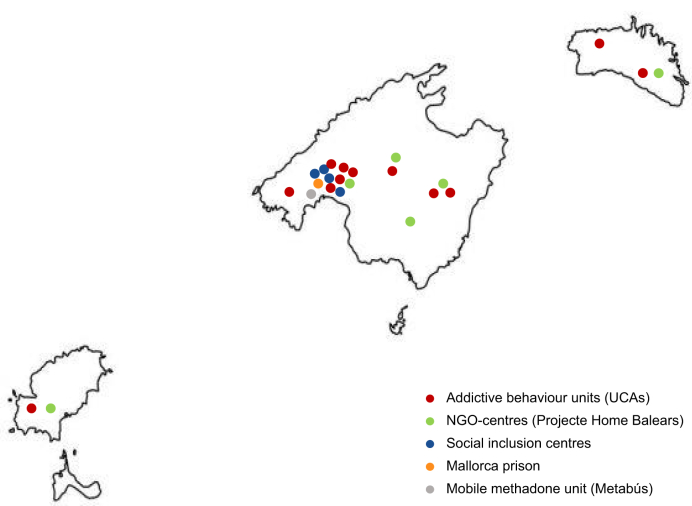
The study included a multidisciplinary team and was implemented across 21 sites in the Balearic Islands serving large PWUD populations, including: 12 addictive behaviour units, which are outpatient centres for people overcoming addictions to substances and/or addictive behaviours; a non-governmental organisation (NGO), composed of three main centres and four satellite sites whose mission is to prevent, treat, and respond to societal issues generated by addiction; a mobile unit that distributes methadone; the Mallorca prison, where HCV+ participants entering or leaving the prison initiated or were pending treatment initiation; and four social inclusion centres, included in the study as of April 2022 (Fig. 2).
Fig. 2.
Study sites.
Map of the Balearic Islands (left to right: Ibiza, Formentera, Mallorca, and Menorca) with the location of the participating study centres. NGO, non-governmental organisation; UCA, Unitat de Conductes Addictives (addictive behaviour units).
Statistical analysis
All categorical variables were summarised using frequency and proportions. Continuous variables were described using mean and standard deviation (SD) and 95% CIs were estimated for proportions and means. The main study outcomes were the number of participants involved in each step of the cascade of care and the proportion lost to follow-up; these values were used to evaluate the care cascade.
Variables collected at baseline and descriptive statistics were used to describe participant sociodemographic characteristics (i.e. age, gender, education level, country of origin, employment, housing, and drug use status) and HCV-related characteristics (i.e. previous HCV knowledge, diagnosis, treatment and prevalence). Sociodemographic characteristics were analysed and compared as two subgroups: (1) total participants and (2) participants with active HCV infection. HCV infection prevalence and the cascade of care were assessed for the total sample and later stratified according to age, gender, education level, employment, study setting, drug and alcohol use, as well as previous HCV knowledge, diagnosis, and treatment and HIV result. Loss to follow-up in each step of the care cascade was assessed for the total sample and later stratified by study setting.
The acceptability of the model of care was defined by: (1) the level of agreement to participate in the study, defined as the number of people who accepted to participate after being invited; (2) the level of preference for onsite diagnostic testing, defined as the number of participants who chose PoC tests (OraQuick® and DBS) instead of phlebotomy; and (3) among active HCV infected participants, the proportion who preferred treatment decentralisation from a hospital to a study centre.
To assess risk factors related to HCV infection as well as completion of each relevant cascade step (i.e. HCV RNA test, treatment initiation and completion, SVR ≥12 monitoring, and infection re-screening at ∼1 year post phase 1), multivariable logistic regression models to calculate odds ratios (ORs) and their 95% CIs were performed. The significance level was set to p <0.05 and data were analysed using STATA version 14 (StataCorp LLC, College Station, TX, United States).
Results
Study population
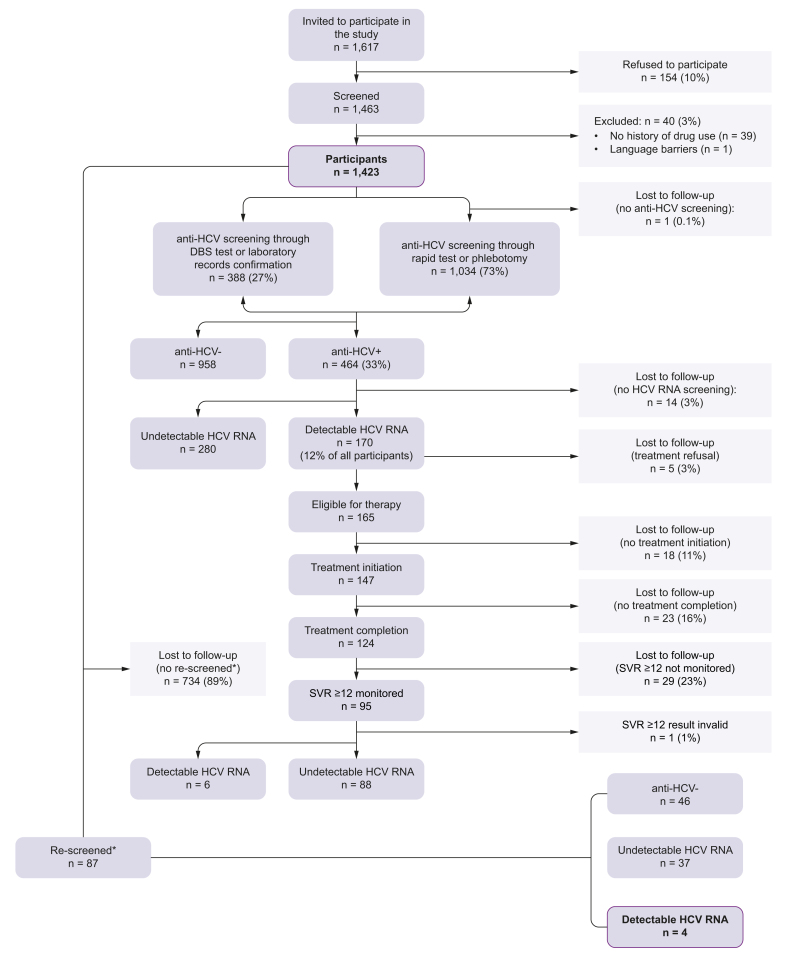
Study participation was offered to 1,617 study centre users, of whom 154 (10%) refused. The most frequent reasons for refusal were lack of time (4%), not wanting to know their HCV status (5%), and already being monitored in hospital (6%); for 72%, the refusal reason was unknown (Fig. 3). Forty potential participants (3%) were excluded after screening as they did not meet the inclusion criteria on history of drug use (98%) or had a language barrier (2%). Thus, the total number of included participants was 1,423.
Fig. 3.
Cascade of care flow-chart.
∗Only participants who were screened up until April 2022 (n = 821) were eligible for being screened again, as the study closed in April 2023 and there had to be ∼1 year between the first and second screening session. SVR12, sustained virologic response 12 weeks after treatment completion.
Baseline characteristics of participants with active HCV infection
Among participants with detectable HCV RNA (n = 170) the mean age was 47 years and most were men and Spanish born (Table 1). Based on the biomedical and sociodemographic information survey, 58% of them reported not having completed basic education (Table 1). Having psychiatric disorders was reported by 29% of participants with active HCV infection (Table 1).
Table 1.
Sociodemographic characteristics, n (%).
| All participants | Detectable HCV RNA | |
|---|---|---|
| (n = 1,423) | (n = 170) | |
| Mean age in years (SD) | 44 (11) | 47 (9) |
| Men | 1,096 (77) | 124 (73) |
| Country of birth† | ||
| Spain | 1,138 (81) | 148 (88) |
| Foreign nationality∗ | 269 (19) | 21 (12) |
| Education level‡ | ||
| No schooling | 123 (9) | 26 (15) |
| Primary school | 539 (38) | 73 (43) |
| Secondary school | 542 (38) | 59 (35) |
| University (bachelor or diploma) | 61 (4) | 5 (3) |
| Vocational training | 138 (10) | 6 (4) |
| University (≥master) | 8 (1) | 0 (0) |
| Employment status§ | ||
| Employed full-time | 425 (30) | 47 (28) |
| Employed part-time | 93 (7) | 12 (7) |
| Unemployed (<3 months) | 67 (5) | 11 (6) |
| Unemployed (3–12 months) | 183 (13) | 23 (14) |
| Unemployed (>12 months) | 454 (32) | 59 (35) |
| Freelance | 48 (3) | 7 (4) |
| Other | 144 (10) | 11 (6) |
| Housing status | ||
| House, flat | 1,132 (80) | 140 (82) |
| Hotel, hostel | 5 (0.3) | 2 (1) |
| Prison | 9 (1) | 1 (1) |
| Unstable housing | 136 (10) | 13 (8) |
| Homeless | 20 (1) | 5 (3) |
| Other | 121 (9) | 9 (5) |
| Reported health conditions | ||
| Psychiatric¶ | 257 (18) | 37 (22) |
| Dual pathology¶ | 70 (5) | 12 (7) |
| HIV∗∗ | 85 (13) | 23 (24) |
| Metabolic¶ | 60 (4) | 4 (2) |
| Cardiovascular¶ | 78 (6) | 13 (8) |
| Pulmonary¶ | 137 (10) | 18 (11) |
| Other¶ | 276 (19) | 42 (25) |
Percentages may add up to just under or over 100 because of rounding.
Foreign nationalities included (in descending order): Morocco, Argentina, Colombia, Germany, Italy, Brazil, Ecuador, the United Kingdom, France, Portugal, Russia, Chile, Nigeria, Bulgaria, India, Poland, the Dominican Republic, Romania, Bolivia, Slovakia, Ukraine, Cuba, Paraguay, Algeria, Belgium, Equatorial Guinea, Senegal, Sweden, Uruguay, Venezuela, Belarus, Bosnia & Herzegovina, Canada, Holland, Hungary, Lithuania, Mexico, Nicaragua, Norway, Serbia, Switzerland, and Thailand.
Information available for 1,407, and 169 participants.
Information available for 1,411, and 169 participants.
Information available for 1,414, and 170 participants.
Information available for 1,417, and 170 participants.
Information available for 662, and 94 participants.
Any instance of information not being available was due to participants declining to answer or answering ‘unknown’. HCV, hepatitis C virus; SD, standard deviation.
Among these 170 participants, 45% reported active drug use, and 61% were on opioid substitution therapy (OST) (Table 2). Prior HCV diagnosis was confirmed in 147 (86%) of them. Of the participants with detectable HCV RNA, prior HCV treatment was reported by 21%, and 67% reported having HCV-related knowledge before study participation (Table 2). HCV co-infection with HIV was present in 28 (16%) participants and with HBV in three (2%); one (1%) participant had an HCV/HBV/HIV co-infection.
Table 2.
Epidemiological and substance use characteristics, n (%).
| All participants | Detectable HCV RNA | |
|---|---|---|
| (n = 1,423) | (n = 170) | |
| Reported active consumption | ||
| Alcohol∗ | 460 (34) | 19 (11) |
| Other drugs† | 548 (39) | 76 (45) |
| On opioid substitution therapy | 393 (28) | 104 (61) |
| Confirmed previous HCV diagnosis‡ | 413 (30) | 147 (86) |
| Self-reported previous HCV treatment§ | ||
| Yes | 203 (14) | 35 (21) |
| Not sure | 10 (1) | 4 (2) |
| No | 1,197 (85) | 128 (77) |
| Previous HCV knowledge¶ | 637 (45) | 114 (68) |
| HIV+∗∗ | 81 (16) | 28 (16) |
| HBV+†† | 7 (1) | 3 (2) |
Information available for 1,370, and 159 participants.
Information available for 1,398, and 169 participants.
Information available for 1,422, and 170 participants.
Information available for 1,410, and 167 participants.
Information available for 1,408, and 168 participants.
Information available for 522, and 170 participants.
Information available for 520, and 170 participants.
Any instance of information not being available was because of participants declining to answer or answering ‘unknown’.
Cascade of care evaluation
Phase 1: anti-HCV screening
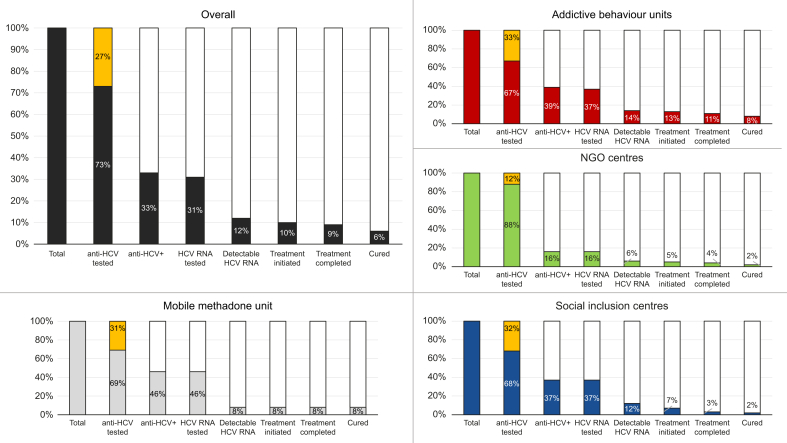
The anti-HCV prevalence was 33% (n = 464/1,423) (Fig. 4). Among those anti-HCV+, 413 (89%) had a confirmed previous diagnosis, 48 (10%) were new diagnoses, and the rest (n = 3, 1%) had no available information on previous HCV diagnosis.
Fig. 4.
Cascade of care overall and by setting.
Percentages were calculated in relation to the total number of participants (n = 1,423). For the overall chart n = 1,423, addictive behaviour units chart n = 936, NGO centres chart n = 384, social inclusion centres chart n = 90, and mobile methadone bus chart n = 13. Percentages in yellow on the ‘anti-HCV tested’ bar represent those participants with a confirmed past HCV infection after confirmation. NGO, non-governmental organisation.
Among those anti-HCV+, 79 (17%) were detected through rapid saliva test or phlebotomy, and 385 (83%) through DBS test or after medical record confirmation.
Overall (n = 1,423), 1,035 (73%) were offered screening for anti-HCV of whom 950 (92%) were performed via rapid saliva test and 84 (8%) via phlebotomy. Of these 1,035 participants, one person (0.1%) was not tested because they did not show up for the appointment (Table S1, supplementary material). Among the 1,034 participants who performed anti-HCV screening, 47 of them had reported a previous HCV infection but preferred to be screened for anti-HCV, of which 38 were anti-HCV+, and nine had their self-reported prior diagnosis ruled out because of an anti-HCV- result.
The remaining 388 (27%) participants were not screened for anti-HCV, but to confirm viraemia, because of reporting prior HCV infection (n = 375) or other reasons (n = 13) (Fig. 3). After confirmation, 1% (n = 3/388) of these participants had a negative anti-HCV result. The participant recruitment and anti-HCV testing was virtually simultaneous (0.1 days [SD = 1.6]).
Phase 2: HCV RNA screening
Of those anti-HCV+, 96% (n = 447/464) underwent HCV RNA screening and three people (n = 3/464, 1%) had recent HCV RNA results available in their laboratory records. Of those screened for HCV RNA (n = 447), 358 (80%) were tested via DBS and 89 (20%) via phlebotomy. Of all participants, 12% (n = 170) had detectable HCV RNA. Of those screened for HCV RNA, six (1%) were HBsAg+ and 81 (18%) were anti-HIV+. Of the latter, 59% (n = 48/81) were already linked to care. Among those anti-HIV+ who were not linked (n = 33/81), linkage to care with HIV monitoring and adherence to treatment was achieved in 82% (n = 27/33).
Among anti-HCV+ individuals with a new diagnosis (10%, n = 48/464), 22 were found to have an active infection (46%, n = 22/48) upon RNA HCV analysis.
Overall (n = 1,423), 422 (30%) reported a previous HCV diagnosis. After confirmation, nine (2%) participants resulted to be anti-HCV-, and 413 were confirmed. Of the remaining participants who did not report a previous HCV diagnosis (n = 1,001/1,423, 70%), 2% (n = 23) had active HCV infection. Of those participants with active infection (n = 170, 12%), 147 (86%) had a confirmed prior HCV diagnosis (Table 2).
The two most potential self-reported modes of HCV transmission were active drug use (72%) and unprotected sexual encounters (30%). The mean number of days between anti-HCV and HCV RNA screening was 4.6 (SD: 22.4) and between the latter, during which HBsAg and anti-HIV were also screened for, and results delivery was 17 (SD: 26.6).
Phase 3: HCV treatment
Of those participants with detectable HCV RNA, 97% (n = 165/170) agreed to treatment and 147 (86%) initiated it. Among those that did not start treatment despite prior acceptance (n = 18), reasons for not starting included having a pending treatment prescription (50%), being in an unstable living situation (45%), and medical contraindications (5%). In total, 23 (14%) participants with active infection did not initiate treatment (Table S1).
Among those with detectable HCV RNA, 45% reported active drug use, 11% active alcohol use, and 44% no active drug or alcohol use (Table 2). In these three subgroups, treatment initiation was 84%, 100%, and 85%, respectively. Univariate analysis showed that drug use, alcohol intake, and gender did not have a significant impact on HCV treatment initiation, whereas HIV status and study setting did (p = 0.0288, p = 0.0155, respectively). Consequently, a multivariate regression model considering participants HIV status and study settings demonstrated that those with detectable HCV RNA with HIV co-infection were significantly less likely to initiate HCV treatment when compared with those HCV mono-infected (OR = 0.34; 95% CI [0.12–0.95]; p = 0.0402). Treatment initiation differences were also observed across study settings, with participants being cared for at social inclusion centres being significantly less likely to initiate treatment in comparison with participants being cared for at addictive behaviour units (OR = 0.15; 95% CI [0.04–0.63]; p = 0.0320).
The mean number of days between HCV RNA diagnosis and treatment prescription, and treatment initiation was 22 (SD: 38.6) and 45 (SD: 66.6), respectively. The mean number of days between treatment prescription and initiation was 20 (SD = 33.3). Liver elastography was offered to 35 participants before HCV therapy initiation, of whom 25 (71%) agreed. Liver elastography was performed in 22 (88%) of the latter, and found advanced fibrosis (F3 and F4) in five (23%) cases.
Among those who initiated treatment, the participants located in Mallorca (n = 131) had the option of delegating treatment collection to the study centre where they were being treated, from where it was dispensed. A total of 95% of these participants (n = 124) signed the DTCA to enable this. Of those HCV/HIV coinfected who initiated treatment and were located in Mallorca (n = 20), seven (35%) signed the DTCA and HCV and HIV treatment was dispensed from their respective study centre. Among all treatment initiations, dispensation was decentralised to the study centre in 86% (n = 126) and centralised, from a hospital, in 14% (n = 21). In 68/104 (65%) of participants with detectable HCV RNA on OST, HCV treatment dispensation was combined with OST dispensation. HCV treatment dispensation frequency, which was recorded in 118 participants and adapted to each setting and participant, was daily in 29%, weekly in 31%, biweekly in 25%, monthly in 8%, and other in 8% of these participants.
Phase 4: HCV treatment completion, sustained virologic response monitoring and re-screening
A total of 124 participants completed treatment, corresponding to 84% of those who started it. Reasons for not having completed treatment included participants no longer being connected to the study centre and thus becoming unreachable (n = 7, 30%) and not wanting to continue in the study (n = 2, 9%); in one (4%) case the reason was unknown and in 13 (57%) treatment was ongoing. Participants actively using drugs, including alcohol, did not show significant differences in treatment completion rates compared with those with no active drug use (p = 0.7347 and p = 0.8168, respectively). The mean number of days between screening (phase 1) and completing treatment was 139 (SD: 80.5) and between HCV RNA diagnosis and completing treatment was 123 (SD: 74.1).
Post-treatment completion SVR monitoring at Week 4 was performed in 88 (71%) participants, of whom 99% (n = 87) had it done via DBS testing. Among those who underwent SVR4 monitoring, 94% (n = 83) had undetectable HCV RNA. The main reason for participants who completed treatment not undergoing SVR4 was not showing up for the appointment. A total of 95 (77%) participants underwent SVR monitoring at Week ≥12, of whom 83% (n = 79) had it done via DBS testing. Of those who underwent SVR ≥12 monitoring, 93% (n = 88) had undetectable HCV RNA. The main reason for participants who completed treatment not undergoing SVR ≥12 monitoring (n = 29, 23%) was not showing up for the appointment (n = 8, 28%); 12 participants (52%) did not undergo SVR ≥12 as they had not yet reached the required at least 12 weeks to perform the control. Both SVR controls were performed in 76 (61%) participants and the 97% who achieved SVR4 also achieved SVR≥12 (p = 0.0199).
After 1 year from phase 1, screening was offered again, especially to those who continued with risky practices and to those who had been treated. For those treated, the test was repeated 12 months after obtaining SVR12, to detect possible reinfections. In those anti-HCV-, a repetition of PoC OraQuick® was also offered, and in those who had undetectable HCV RNA, the DBS test or phlebotomy, to detect possible new infections in the first case and possible reinfections in the second case.
Of the overall participants, the re-screening was only feasible in those who were recruited one year or more previously (until April 2022), a total of 821 participants. Of these, 11% (n = 87) underwent re-screening, 46 of whom had been previously anti-HCV- and 41 had undetectable HCV RNA. A total of 91% (n = 79) of these re-screenings were decentralised, to the study centres, and the rest were done in a hospital. A total of four (5%) reinfections were detected in participants who were re-screened or achieved SVR, of whom three (75%) were women and born in Spain, two (50%) were on OST, one (25%) reported active drug use, and all were unemployed; all reinfections were linked to continued injecting drug use. The mean number of days between phase 1 and re-screening was 348 (SD: 137.4).
In the re-screening, 89% of participants were lost to follow-up and in the majority of cases the reason for this was unknown.
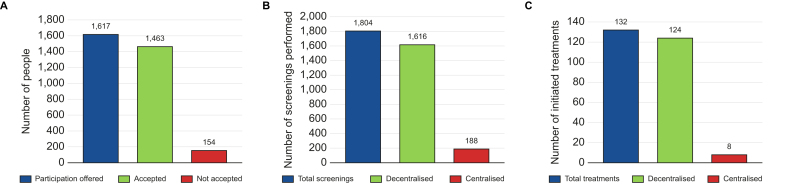
Model of care acceptability
Study participation was offered to 1,617 individuals, and 90% (n = 1,463) accepted (Fig. 5). A total of 1,081 anti-HCV tests were performed in the screening (phase 1) and the re-screening phase. Of these, 991 (92%) were done via the PoC OraQuick® rapid saliva test instead of phlebotomy. A total of 743 HCV RNA tests were performed in phase 2 and the SVR4 and SVR ≥12 monitoring and re-screening phase. Of these, 723 (97%) had both options available, DBS testing or phlebotomy. The remaining 3% were not offered both options because in two study centres in Menorca DBS testing was not available and in another in Ibiza SVR ≥12 monitoring was done via phlebotomy. This resulted in 625 (86%) HCV RNA tests done via DBS, as per participant preference. Overall, combined decentralised screening, via OraQuick® and DBS testing, was preferred in 90% (n = 1,616/1,804) of cases, when both options were available. As for HCV treatment, its decentralisation to study centres was only available for participants being cared for in Mallorca. Of those participants who initiated treatment in Mallorca (n = 132), 124 (94%) chose to decentralise it.
Fig. 5.
Model of care acceptability.
(A) Study participation acceptance; (B) HCV screening preference; (C) HCV treatment preference in Mallorca.
Discussion
This study evaluated a new model of care for HCV screening and linkage to care for PWUD in the Balearic Islands, with >90% acceptance among those offered study participation and decentralised screening and treatment. It demonstrated that adapting and decentralising healthcare pathways to meet the needs of the targeted population can lead to high intervention acceptance, and result in successfully diagnosing and treating individuals for HCV, as cascade of care retention was >76% at every step including SVR ≥12 monitoring. Among all participants (n = 1,423), prevalence of an active HCV infection was 12% and SVR was achieved in 93% of those who completed treatment and had SVR12 measured.
This model was based on the HCV micro-elimination approach, which has been used in similar initiatives worldwide to increase screening and linkage to care in vulnerable populations, accelerating progress towards the WHO HCV elimination targets.12 One example of decentralised screening was carried out among people who inject drugs (PWID) attending the largest harm reduction centre in Barcelona, Spain.28 Even though only 46% (n = 420/919) of enrolled participants accepted HCV screening, active HCV infection prevalence was found to be 56% (n = 234), of whom 53% (n = 125) were retained in care and had SVR12 measured. SVR12 revealed that 38% (n = 89) were cured. We believe that the HCV prevalence is considerably higher in this study than in ours (56% vs. 12%) because its population was exclusively PWID, which is a known risk factor for HCV infection. Like in our study, these results show that, despite the barriers in reaching and retaining such a population in care, PWUD can be treated and cured for HCV.28 Moreover, beyond viral clearance, the study performed in Barcelona demonstrated that participant involvement in the DAA therapy programme was significantly associated with a reduction in high-risk practices such as drug injection frequency. Two more Spanish HCV micro-elimination programmes were carried out in Madrid29 and Catalonia.30 These models also used DBS and PoC testing, respectively, to screen for HCV among PWUD. DBS testing was found to be an excellent tool in determining prevalence of HCV and other viral infections, such as HBV. The PoC strategy increased access to treatment and linkage to care. In our study, 90% of participants chose decentralised PoC screening (OraQuick and/or DBS) over hospital-based phlebotomy. Furthermore, we introduced DBS to regional molecular microbiology units, enabling decentralized screening with future potential beyond it. Another elimination strategy in Spain, which involved using telemedicine in a correctional facility, demonstrated that this approach was effective in eliminating HCV, as 97% of treated inmates achieved SVR.31 In our study, all treatments were prescribed through telemedicine, eliminating the necessity for specialist visits. Additionally, 94% preferred decentralized treatment dispensed at addiction centres over hospital pharmacies.
Internationally, several studies have concluded that decentred patient-centred models of care promote HCV screening, diagnosis, and treatment among PWUD.21,[32], [33], [34], [35], [36]
Our results are consistent with the above-mentioned studies and suggest that the model of care was successful in facilitating access to HCV screening and linkage to care among PWUD. Our findings encompass alternatives to the standard of care pathways and contribute to a more accessible and equitable health system with respect to HCV management among PWUD. The study team coordinated 21 addiction centres and collaborated with Gastroenterology, Pharmacy, Microbiology, and Internal Medicine services from six public hospitals in the region, resulting in a successful implementation of the model and screening over 1,400 high-risk individuals.
The prevalence of active HCV infection in this population was 12%, which is higher than the general Spanish population (0.22%)24 and, as previously mentioned, lower than reported by other studies in similar populations in Spain (56%28,30 or 29%29). The distribution of active HCV infection was heterogeneous among study centres, and higher in addiction behaviour units compared to other centres. Following the sociodemographic, epidemiological, and substance use characteristics of our population, this could be because of the profile of recruited participants, usually actively using drugs and having a reduced connection with primary healthcare, which reduces the rate of screening and treatment. Among those with an active HCV infection, 86% had been previously diagnosed. This value is close to the goal set by WHO of diagnosing 90% of HCV cases,2 suggesting that this population may not have difficulties in accessing HCV screening services, but rather, in being linked to and retained in treatment.2 This result indicates that targeting people with a previous diagnosis to directly determine their HCV viral load, is an efficient strategy to identify active HCV infections, and that this relinking strategy is more efficient than targeting people without reporting a previous infection to identify active infections. Despite the number of new diagnoses among the anti-HCV+ participants being much smaller (10%), anti-HCV screening should not be disregarded, as the proportion of active infection among this subgroup was found to be 46% in our study.
DAA cure rates exceed 95%, and are highly easy to use, tolerable, and safe, without serious side-effects.2 EASL recommends that everyone with an active HCV infection should receive timely treatment.37,38 Treatment should be considered urgent in those co-infected with HBV or HIV and at high risk of transmitting the infection, such as PWID. PWID with active HCV infection, including those on OST and with a history of past or recent injection drug use, should be treated.7 Furthermore, WHO recommends decentralising HCV testing and treatment.2 These recommendations aim to boost viral hepatitis testing, diagnosis, and treatment, particularly among high-risk marginalized populations like PWUD. They also advocate for treatment as a prevention strategy to aid in HCV elimination.
By following EASL and WHO recommendations, this study demonstrates that access to HCV treatment among PWUD can be high (86%), as well as the treatment completion rate (84%). Results also showed that actively using drugs, including alcohol, did not significantly impact treatment initiation or completion. These findings are in line with a 2018 systematic review on DAA use in PWUD, including PWID, which found that their response to HCV treatment was favourable, including among those who recently used drugs and were receiving OST.39
Moreover, the HCV cure rate (i.e. participants who completed treatment and underwent SVR≥12) of this study was 93%, which is almost equal to that of the general population, at >95%.2 Accordingly, another study in Madrid on PWUD on OST found that, despite drug use during HCV treatment potentially causing drop-outs and reduced cure rates, 89% of them achieved SVR12.40 This supports our study's findings. Similarly, a study in Italy reported an SVR12 rate of approximately 98% in HCV-infected PWUD completing DAA treatment.41
In our study, SVR monitoring rates at 4 and 12 weeks after treatment completion were 71% and 77%, respectively. Furthermore, in those who performed both controls (n = 76, 61%), 97% of those who obtained an undetectable result in the SVR4 test also obtained it in the SVR ≥12 test. Although more research is needed in this regard, this suggests that SVR monitoring at 4 weeks post-treatment completion may be a good strategy to define HCV cure in those populations in which long-term follow-up is challenging.
The results of this study are also in line with other studies that demonstrated the usefulness of decentralising HCV screening via DBS testing for diagnosis and reinfection monitoring in PWID,42 and of telemedicine, which facilitated treatment access among PWUD.43
Although Spain has a National Strategy for Addictions,44 a National Viral Hepatitis Strategy,45 which aims to promote early HCV diagnosis and access to treatment, and unrestricted access to DAA therapy since June 2017,46 addiction and HCV management is not standardised across the country, including the Balearic Islands. However, by taking person-centred approaches, health outcomes improve at an individual and a general level. Nonetheless, as demonstrated in this study, proper functioning of such decentralised models, which are time intensive, depends on proper communication and coordination among stakeholders.
Limitations and strengths
The COVID-19 pandemic hindered study implementation in some centres, resulting in slower rollout or interruptions. This impacted recruitment, potentially leading to missed HCV+ diagnosis and treatments. Another limitation relates to the re-screening proportion achieved for potential new infections or reinfections, as most participants (89%) were lost to follow-up in this cascade of care step. This likely resulted in few reinfections detected (n = 4, 5%). Although reasons for loss to follow-up are unknown for 96% of participants, we hypothesise that they may include a continued lack of awareness regarding risky practices, despite information being provided at study recruitment, and the absence of routine HCV screening every 6–12 months, despite recommendations by the National Viral Hepatitis Strategy and AEHVE.
Study strengths relate to the detection of two new HIV cases, the linking back to care of 82% (n = 27/33) of HIV patients who had been lost follow-up, and the facilitation of access to HIV treatment, through decentralisation, among 25% of those HCV/HIV co-infected. Among those with HCV/HIV co-infection, new HIV acquisitions were not frequently observed among PWUD, however, new HCV infections among people living with HIV were more frequent among PWUD.40 In such co-infection cases, treating HCV can improve linkage to HIV treatment.47 HCV/HIV co-infection detection can also help to micro-eliminate HCV in another vulnerable population.48,49 These results demonstrate that integrating health services is feasible and enabled participants to be linked to care for multiple conditions.
We consider the primary success of this study to be the demonstration of the effectiveness of simplified models tailored to specific populations and regions in achieving hepatitis C elimination. Our study has been successfully implemented across the addiction centres of the main islands of an entire autonomous community in Spain.
The Hepatitis C Free Balears model can be a reference for regional health authorities to develop an autonomous HCV elimination plan, akin to those in other Spanish regions, marking a major milestone towards its elimination.
Conclusions
In spite of having been launched during the COVID-19 pandemic, the Hepatitis C Free Balears study was able to implement a new and effective model of care for PWUD in the Balearic Islands. Decentralised diagnostic tests were found to be well received by study participants and to be a crucial tool in detecting positive HCV, HBV, and HIV cases. In the same way, telematic treatment prescription, introduced as part of the study, afforded this vulnerable population better access to disease treatment. This multidisciplinary model of care contributed to HCV elimination among PWUD in the Balearic region and can be considered in other parts of Spain and the world.
Abbreviations
Ab, antibody; AEEH, Associación Española del Estudio del Hígado (Spanish Association for the Study of the Liver); AEHVE, Alianza para la Eliminación de las Hepatitis Víricas en España; DAA, direct-acting antiviral; DBS, dried blood spot; DTCA, delegated treatment collection authorisation; EASL, European Association for the Study of the Liver; GHSS, Global Health Sector Strategy; NGO, non-governmental organisation; OST, opioid substitution therapy; PoC, point-of-care; PWID, people who inject drugs; PWUD, people who use drugs; SVR, sustained virologic response; UCA, Unitat de Conductes Addictives (addictive behaviour units); WHO, World Health Organization.
Financial support
This study was supported by Gilead Sciences, through the competitive research call HCV STAT. The funding body had no role in the study design, data collection, analysis, and interpretation, writing of the report, or decision to submit the article for publication.
Conflicts of interest
JS reports speaker fees from Gilead Sciences, ViiV Healthcare, and Merck Sharp and Dome, outside of the submitted work. MB reports advisory fees from Gilead Sciences, AbbVie, GlaxoSmithKline, and Assembly Biosciences and speaker fees from Gilead Sciences and AbbVie, outside of the submitted work. ÀV reports speaker fees from Gilead Sciences and AbbVie, outside of the submitted work. JVL reports grants and speaker fees from AbbVie, Gilead Sciences, MSD, and Roche Diagnostics to his institution, speaker fees from Echosens, Janssen, Novo Nordisk, and ViiV, and consulting fees from GSK and Novavax, outside of the submitted work. The other authors declare no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
Study concept and design: AHM, CAP, MDM, MVF-B, JS, MG-G, MB, ÀV, JVL. Acquisition of data: AHM. Analysis and interpretation of data: AHM, CAP, AN, ÀV, JVL. Drafting of the manuscript: AHM, JVL, CAP. Critical revision of the manuscript for important intellectual content: AHM, CAP, AN, MDM, MVF-B, JS, LB, MT, AS, ARR, AZ, MG-G, MB, ÀV, JVL. Statistical analysis: AHM. Administrative, technical, or material support: MDM, MVF-B, JS, LB, MT, AS, ARR, AZ, MG-G, ÀV. Study supervision: MB, ÀV, JVL. Obtained funding: JVL. Article guarantor: JVL. Approved the final version for submission: all authors.
Please refer to the accompanying ICMJE disclosure forms for further details.
Data availability statement
Individual data are not public but will be available upon reasonable request.
Acknowledgements
The authors thank all of the participants and nurses, nursing assistants, doctors, and psychological and social personnel from each of the participating centres, as well as all of the hepatologists, pharmacists, and microbiologists from the participating public hospitals in the Balearic Islands for their active engagement, expertise, and assistance in all aspects of this study. We also acknowledge the collaboration of the Health System of the Balearic Islands (IBSalut), the Councils of Mallorca (IMAS), Menorca, and Ibiza, and those involved in the Plan for Addictions and Drug Dependence of the Balearic Islands.
AHM, CAP, and JVL acknowledge the support of the ISGlobal Biostatistics and Data Management Unit and support to ISGlobal from the Spanish Ministry of Science, Innovation and Universities through the “Centro de Excelencia Severo Ochoa 2019-2023” Programme (CEX2018-000806-S), and from the Government of Catalonia through the “CERCA Programme”. CAP acknowledges support from the “Secretaria d’Universitats i Recerca de la Generalitat de Catalunya” and the European Social Fund, as an AGAUR-funded PhD fellow.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101145.
Supplementary data
The following are the supplementary data to this article:
References
- 1.World Health Organization. Hepatitis C: key facts, 2024. Available: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (Accessed 15 April 2024).
- 2.World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030, 2022. https://www.who.int/publications/i/item/9789240053779 (Accessed 19 June 2023).
- 3.World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis, 2021. https://www.who.int/publications/i/item/WHO-HIV-2016.06 (Accessed 19 June 2023).
- 4.Picchio C.A., Lens S., Hernández-Guerra M., et al. Late presentation of chronic HBV and HCV patients seeking first time specialist care in Spain: a 2-year registry review. Sci Rep. 2021;11:1–12. doi: 10.1038/s41598-021-01885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maucort-Boulch D., de Martel C., Franceschi S., et al. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471–2477. doi: 10.1002/ijc.31280. [DOI] [PubMed] [Google Scholar]
- 6.Rumgay H., Arnold M., Ferlay J., et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlotsky J.M., Negro F., Aghemo A., et al. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Gamkrelidze I., Pawlotsky J.M., Lazarus J.V., et al. Progress towards hepatitis C virus elimination in high-income countries: an updated analysis. Liver Int. 2021;41:456–463. doi: 10.1111/liv.14779. [DOI] [PubMed] [Google Scholar]
- 9.Kondili L.A., Craxì A., Aghemo A. Absolute targets for HCV elimination and national health policy paradigms: foreseeing future requirements. Liver Int. 2021;41:649–655. doi: 10.1111/liv.14796. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus J.V., Pericàs J.M., Picchio C.A., et al. We know DAAs work, so now what? Simplifying models of care to enhance the hepatitis C cascade. J Intern Med. 2019;286:503–525. doi: 10.1111/joim.12972. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus J.V., Picchio C.A., Byrne C.J., et al. A global systematic review of hepatitis C elimination efforts through micro-elimination. Semin Liver Dis. 2022;42:159–172. doi: 10.1055/a-1777-6112. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus J.V., Safreed-Harmon K., Thursz M.R., et al. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018;38:181–192. doi: 10.1055/s-0038-1666841. [DOI] [PubMed] [Google Scholar]
- 13.Lazarus J.V., Wiktor S., Colombo M., et al. Micro-elimination – a path to global elimination of hepatitis C. J Hepatol. 2017;67:665–666. doi: 10.1016/j.jhep.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Harris M., Guy D., Picchio C.A., et al. Conceptualising hepatitis C stigma: a thematic synthesis of qualitative research. Int J Drug Pol. 2021;96 doi: 10.1016/j.drugpo.2021.103320. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus J.V., Baker L., Cascio M., et al. Novel health systems service design checklist to improve healthcare access for marginalised, underserved communities in Europe. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-035621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balsom C.R., Farrell A., Kelly D.V. Barriers and enablers to testing for hepatitis C virus infection in people who inject drugs – a scoping review of the qualitative evidence. BMC Public Health. 2023;23:1038. doi: 10.1186/s12889-023-16017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joint United Nations statement on ending discrimination in health care settings. https://apps.who.int/iris/handle/10665/259622
- 18.Crespo J., Lázaro P., Blasco A.J., et al. Hepatitis C reflex testing in Spain in 2019: a story of success. Enferm Infecc Microbiol Clin. 2021;39:119–126. doi: 10.1016/j.eimc.2020.03.004. English ed. [DOI] [PubMed] [Google Scholar]
- 19.Saludes V., Antuori A., Lazarus J.V., et al. Evaluation of the Xpert HCV VL Fingerstick point-of-care assay and dried blood spot HCV-RNA testing as simplified diagnostic strategies among people who inject drugs in Catalonia, Spain. Int J Drug Pol. 2020;80 doi: 10.1016/j.drugpo.2020.102734. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Campreciós J., Rando-Segura A., Buti M., et al. Reflex viral load testing in dried blood spots generated by plasma separation card allows the screening and diagnosis of chronic viral hepatitis. J Virol Methods. 2021;289 doi: 10.1016/j.jviromet.2020.114039. [DOI] [PubMed] [Google Scholar]
- 21.Oru E., Trickey A., Shirali R., et al. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta-analysis. Lancet Glob Health. 2021;9:e431–e445. doi: 10.1016/S2214-109X(20)30505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day E., Broder T., Bruneau J., et al. Priorities and recommended actions for how researchers, practitioners, policy makers, and the affected community can work together to improve access to hepatitis C care for people who use drugs. Int J Drug Pol. 2019;66:87–93. doi: 10.1016/j.drugpo.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Day E., Hellard M., Treloar C., et al. Hepatitis C elimination among people who inject drugs: challenges and recommendations for action within a health systems framework. Liver Int. 2019;39:20. doi: 10.1111/liv.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Health Social Services and Equality (Spain) 2020. Guía de cribado de la infección por el VHC.https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/docs/GUIA_DE_CRIBADO_DE_LA_INFECCION_POR_EL_VHC_2020.pdf [Google Scholar]
- 25.Alianza para la Eliminación de las Hepatitis Víricas en España . 2019. Objetivo 2021 | AEHVE - Alianza para la Eliminación de las Hepatitis Víricas en España.http://aehve.org/objetivo-2021/ [Google Scholar]
- 26.Crespo J., Albillos A., Buti M., et al. Elimination of hepatitis C. Positioning document of the Spanish association for the study of the liver (AEEH) Rev Esp Enferm Dig. 2019;42:579–592. doi: 10.17235/reed.2019.6700/2019. [DOI] [PubMed] [Google Scholar]
- 27.Lazarus J.V., Herranz A., Picchio C.A., et al. Eliminating hepatitis C on the Balearic Islands, Spain: a protocol for an intervention study to test and link people who use drugs to treatment and care. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-053394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lens S., Miralpeix A., Gálvez M., et al. HCV microelimination in harm reduction centres has benefits beyond HCV cure but is hampered by high reinfection rates. J Hepatol. 2022;4 doi: 10.1016/j.jhepr.2022.100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan P., Valencia J., Cuevas G., et al. HCV screening based on dried blood samples and linkage to care in people who use drugs: a prospective study. Int J Drug Pol. 2021;92 doi: 10.1016/j.drugpo.2021.103134. [DOI] [PubMed] [Google Scholar]
- 30.Forns X., Colom J., García-Retortillo M., et al. Point-of-care hepatitis C testing and treatment strategy for people attending harm reduction and addiction centres for hepatitis C elimination. J Viral Hepat. 2022;29:227–230. doi: 10.1111/jvh.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez Galán G., Alia Alia C., Vegue González M., et al. The contribution of telemedicine to hepatitis C elimination in a correctional facility. Rev Esp Enferm Dig. 2019;111:550–555. doi: 10.17235/reed.2019.6152/2018. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham E.B., Wheeler A., Hajarizadeh B., et al. Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:426–445. doi: 10.1016/S2468-1253(21)00471-4. [DOI] [PubMed] [Google Scholar]
- 33.Litwin A.H., Lum P.J., Taylor L.E., et al. Patient-centred models of hepatitis C treatment for people who inject drugs: a multicentre, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2022;7:1112–1117. doi: 10.1016/S2468-1253(22)00275-8. [DOI] [PubMed] [Google Scholar]
- 34.Demant J., Krohn-Dehli L., Van der Veen J., et al. Peer-delivered point-of-care testing and linkage to treatment for hepatitis C virus infection among marginalized populations through a mobile clinic in Copenhagen, Denmark. Int J Drug Pol. 2023;121 doi: 10.1016/j.drugpo.2023.104185. [DOI] [PubMed] [Google Scholar]
- 35.Lazarus J.V., Øvrehus A., Demant J., et al. The Copenhagen test and treat hepatitis C in a mobile clinic study: a protocol for an intervention study to enhance the HCV cascade of care for people who inject drugs (T′N′HepC) BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutton J., Doyle J., Zordan R., et al. Point-of-care hepatitis C virus testing and linkage to treatment in an Australian inner-city emergency department. Int J Drug Pol. 2019;72:84–90. doi: 10.1016/j.drugpo.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Oru E., Verster A. Access to hepatitis C care for people who inject drugs and people in prisons. Lancet Gastroenterol Hepatol. 2019;4:662–663. doi: 10.1016/S2468-1253(19)30201-8. [DOI] [PubMed] [Google Scholar]
- 38.Grebely J., Hajarizadeh B., Lazarus J.V., et al. Elimination of hepatitis C virus infection among people who use drugs: ensuring equitable access to prevention, treatment, and care for all. Int J Drug Pol. 2019;72:1–10. doi: 10.1016/j.drugpo.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Hajarizadeh B., Cunningham E.B., Reid H., et al. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:754–767. doi: 10.1016/S2468-1253(18)30304-2. [DOI] [PubMed] [Google Scholar]
- 40.Macías J., Morano L.E., Téllez F., et al. Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J Hepatol. 2019;71:45–51. doi: 10.1016/j.jhep.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Messina V., Onorato L., Di Caprio G., et al. Directly acting antiviral-based treatment for HCV-infected persons who inject drugs: a multicenter real-life study. Life (Basel) 2021;11:1–9. doi: 10.3390/life11010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Not A., Saludes V., Gálvez M., et al. Usefulness of dried blood spot samples for monitoring hepatitis C treatment outcome and reinfection among people who inject drugs in a test-and-treat program. J Med Virol. 2023;95 doi: 10.1002/jmv.28544. [DOI] [PubMed] [Google Scholar]
- 43.Rosato V., Nevola R., Conturso V., et al. Telemedicine improves HCV elimination among Italian people who use drugs: an innovative therapeutic model to increase the adherence to treatment into addiction care centers evaluated before and during the COVID-19 pandemic. Biology (Basel) 2022;11:800. doi: 10.3390/biology11060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delegación del Gobierno para el Plan Nacional sobre Drogas. National Strategy on Addictions; 2017. https://pnsd.sanidad.gob.es/pnsd/Introduccion/home.htm [Google Scholar]
- 45.Ministry of Health Social services and equality. Strategic plan for tackling hepatitis C in the Spanish national health system. 2015. https://www.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/PEAHC_eng.pdf
- 46.Marshall A.D., Pawlotsky J.M., Lazarus J.V., et al. The removal of DAA restrictions in Europe – one step closer to eliminating HCV as a major public health threat. J Hepatol. 2018;69:1188–1196. doi: 10.1016/j.jhep.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Barocas J.A. 100–100–100: a new, ambitious treatment target to end the HIV and hepatitis C epidemics. Lancet Public Health. 2022;7:e98–e99. doi: 10.1016/S2468-2667(21)00276-0. [DOI] [PubMed] [Google Scholar]
- 48.Rivero-Juarez A., Téllez F., Mayorga M.I., et al. Progression to hepatitis C virus micro-elimination in people living with HIV in Spain. Clin Microbiol Infect. 2020;27:800–801. doi: 10.1016/j.cmi.2020.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Byrne C., Robinson E., Rae N., et al. Toward microelimination of hepatitis C and HIV coinfection in NHS Tayside, Scotland: real-world outcomes. Health Sci Rep. 2020;3:e191. doi: 10.1002/hsr2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual data are not public but will be available upon reasonable request.