Abstract
Clostridial gas gangrene (CGG) is among the most rapidly spreading infections in humans, with mortality rates approaching 100 % if not treated promptly. Most cases follow traumatic inoculation, although spontaneous infections occur in a minority of patients with immunodeficiency. Spontaneous CGG is primarily caused by Clostridium septicum, whereas traumatic infection is associated with Clostridium perfringens. Patients with CGG present abruptly with rapidly progressive symptoms, underscoring the importance of early recognition, prompt surgical intervention, and appropriate antimicrobial therapy. We describe an illustrative case of spontaneous CGG caused by C. perfringens in a polymorbid 73-year-old female patient. Despite aggressive medical and surgical management, she succumbed to metastatic infection within 48 h of presentation.
Keywords: Subcutaneous emphysema, Pyogenic ventriculitis, Anaerobic bacteremia, Emphysematous cholecystitis, Clostridial myonecrosis, Anaerobic infections
Highlights
-
•
Rare case of clostridial gas gangrene involving at least four organs.
-
•
Clostridium perfringens can cause gas gangrene in the absence of traumatic injury.
-
•
NSTIs may present atypically at unsuspecting sites of infection in polymorbid patients.
-
•
Surrogate markers may alert clinicians to NSTI diagnosis before surgical exploration.
-
•
Delayed surgical intervention increases patient morbidity and mortality.
Introduction
Clostridial gas gangrene (CGG) is a rare but life-threatening infection commonly caused by Clostridium perfringens. Historically, gas gangrene was seen following war wounds, such as the traumatic inoculation of soil-dwelling microorganisms [1]. Spontaneous CGG caused by Clostridium septicum often arises in the setting of immunodeficiency, such as diabetes or malignancy [2].
C. perfringens (formerly Clostridium welchii) is a Gram-positive, spore-forming, rod-shaped, anaerobic bacterium with many virulence factors, including alpha toxin [3]. First isolated in 1891 by William H. Welch, C. perfringens is ubiquitous in nature and has been recovered from soil, aquatic sediments, and the human gastrointestinal tract [4], [5]. It inhabits the genitourinary tract in 1–10 % of healthy women [6].
Approximately 1000 to 3000 cases of CGG are reported annually in the United States; 50 % of these occur after traumatic injuries, 30 % are due to postoperative complications, and 20 % arise spontaneously [4], [7]. The mortality rate of spontaneous CGG has been reported to be 70 to 100 %, typically occurring 2 to 4 days after hospitalization [8].
Diagnosis relies on a physician’s acute clinical awareness of CGG from the patient’s history and physical exam. Patients with necrotizing soft tissue infections (NSTI) may present with fever alongside soft-tissue edema, erythema, and pain [9]. Skin blebs, bullae, or necrosis are also commonly reported [9]. Necrosis of superficial tissues produces a musty fluid with a dishwater appearance [10]. These cardinal signs of infection should prompt immediate surgical debridement and antimicrobial therapy [11]. Untreated, overwhelming infections may result in sepsis, shock, and multisystem organ failure [12].
We report a case of spontaneous CGG caused by C. perfringens in a 73-year-old female patient with gas production in the myocardium, pericardium, cerebral ventricles, gallbladder, biliary tract, and soft tissues. Despite appropriate antibiotic therapy and urgent glenohumeral disarticulation, the patient rapidly decompensated and succumbed to metastatic infection.
Clinical features
A 73-year-old female with a history of end stage renal disease, hypertension, type II diabetes mellitus, heart failure, and atrial fibrillation collapsed following hemodialysis. According to the patient’s caretaker, she developed worsening confusion, agitation, and difficulty remaining upright two days before presentation with a sharp decline after hemodialysis.
On her initial physical exam, she appeared lethargic and toxic appearing, tachycardic, and noted to have right eye deviation with nystagmus. Her tachycardia was noted to be atrial fibrillation with rapid ventricular response. A computed tomography (CT) scan of the head showed a hypodensity in the left temporoparietooccipital region. Initial labs were notable for leukocytosis (36.8 cells x 103/μL), lactic acidosis, and elevated 5th generation high-sensitivity cardiac troponin T (4352 ng/L). Given her significant leukocytosis, she was started on empiric vancomycin, clindamycin, and piperacillin-tazobactam. Due to her altered mental status and suspected ischemic stroke, she was transferred to the neuroscience intensive care unit at an academic medical institution for further care and evaluation.
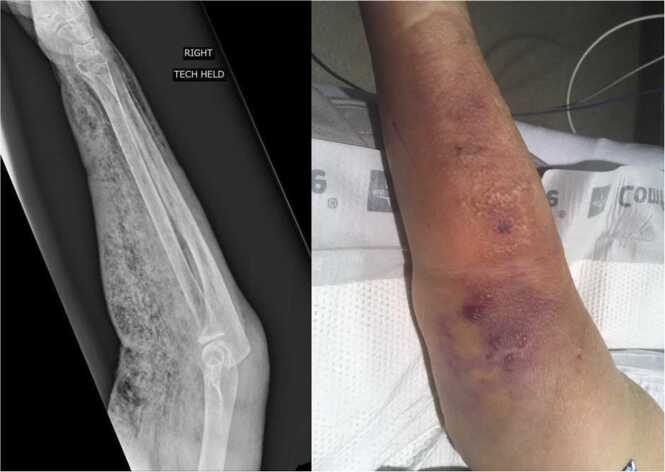
Upon arrival, she was noted to be hypotensive and in respiratory failure. Her neurologic exam was concerning for a comatose state (did not follow commands, withdraw to pain, or demonstrate purposeful movement). She was immediately intubated for airway protection and placed on vasopressin and norepinephrine for hemodynamic support. Upon further examination, she was noted to have warmth in the right upper extremity with subcutaneous emphysema, concerning for NSTI (Fig. 1). Further laboratory values were notable for leukocytosis (28.5 cells x 103/μL), elevated lactate (6 mmol/L) without acidosis, and elevated C-reactive protein (203.7 mg/L) with anemia (hemoglobin 8.9 g/dL). She also had hypokalemia (3.3 mmol/L), hypoalbuminemia (2.7 g/dL), and hypocalcemia (8.5 mg/dL) with elevated aspartate aminotransferase (148 units/L), alkaline phosphatase (181 units/L), creatinine (4.37 mg/dL), and blood urea nitrogen (57 mg/dL).
Fig. 1.
Radiographic and clinical images of the patient’s right upper extremity on the night of admission showing erythema, bullae, and subcutaneous emphysema over the forearm and midarm. During glenohumeral disarticulation, surgeons discovered copious dishwater-type fluid and nonviable biceps brachii and brachialis muscles, consistent with clostridial myonecrosis.
Given her septic shock in the setting of a suspected NSTI, she underwent emergent debridement of her right upper extremity. During the procedure, large amounts of dishwater-type malodorous fluid were noted alongside grey, noncontractile brachialis and biceps brachii muscles. Ischemic tissue and infected fluid extended into the proximal axilla via the neurovascular sheath. Given these findings, the patient underwent glenohumeral disarticulation. Surgical biopsy revealed fibroadipose tissue and skeletal muscle with extensive necrosis and abundant Gram-positive, rod-shaped bacteria.
Blood cultures were collected in aerobic (BacT/Alert FA plus) and anaerobic (BacT/Alert FN plus) blood culture bottles and incubated and monitored using the BacT/Alert Virtuo Microbial Detection System. After three days of incubation, the anerobic bottle signaled positive, and a Gram stain showed Gram-positive rods. Nucleic acid amplification testing (GenMark ePlex BCID-GP Panel) on the positive blood culture bottle did not detect any of the 20 Gram-positive targets on the panel; the pan-Gram negative and pan-Candida targets were also not detected. Anaerobic cultures of the wound were also collected. The specimen was plated to brucella blood agar and a kanamycin-vancomycin/phenylethyl alcohol agar biplate. Plates were incubated anaerobically at 37 °C in a Bactron anaerobic chamber. Both the blood culture and wound culture grew C. perfringens, which was identified using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS) (VITEK MS, bioMérieux, Inc., Durham, NC). Before these cultures resulted, the patient’s empiric antimicrobial therapy was transitioned to one intravenous dose of cefepime (1 g), metronidazole (500 mg), vancomycin (500 mg), clindamycin (900 mg), and fluconazole (400 mg).
Her troponin increased to 22,407 ng/L and creatine kinase was elevated (1281 units/L). An electrocardiogram showed ST changes with possible left ventricular hypertrophy, concerning for a ST elevation myocardial infarction. Further evaluation with bedside point of care ultrasound as well as radiologically performed echocardiography revealed a severely reduced left ventricular ejection fraction in the range of 20–25 %, mitral valve vegetation concerning for endocarditis, and clot in transit within the inferior vena cava. Given concern for mixed septic and cardiogenic shock, epinephrine was added for additional ionotropic support.
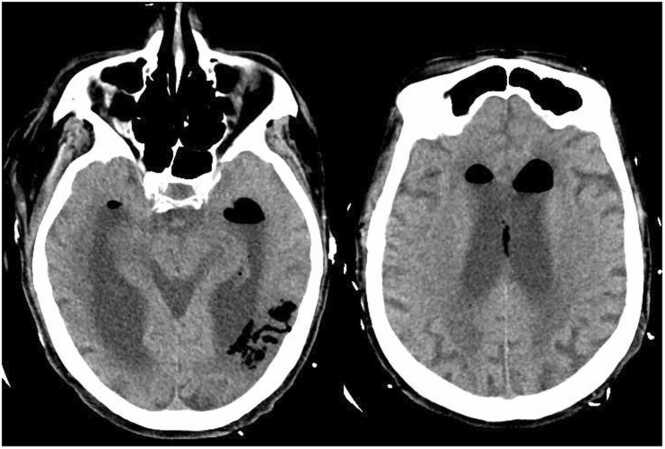
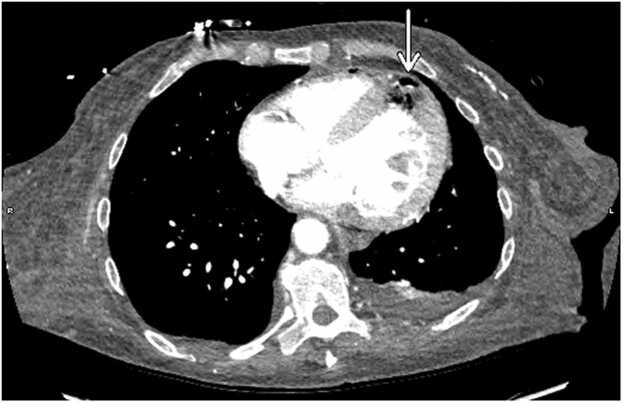
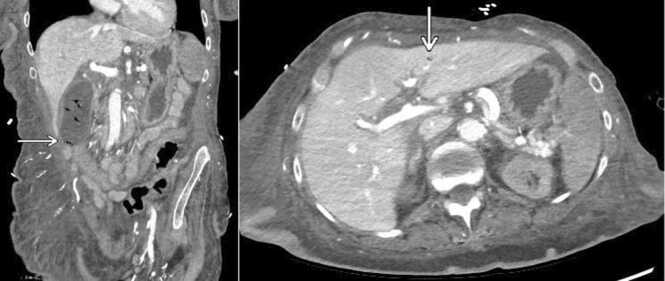
Given her global clinical state, she underwent CT imaging of multiple body regions to determine if source control had been obtained or if there were other areas of infection. A CT scan of the head showed intraparenchymal gas foci in the left temporoparietal region, likely the result of septic emboli. Additionally, new complex appearing air fluid levels were seen in the lateral ventricles, most consistent with pyogenic ventriculitis due to a gas-producing infection (Fig. 2). A chest CT demonstrated gas involving the full thickness of the myocardium at the cardiac apex with extension into the pericardium, consistent with a necrotizing myocardial infection (Fig. 3). Abdomen and pelvis CT showed gas within the lumen and wall of the gallbladder as well as scattered pneumobilia in the left hepatic lobe. These findings were most consistent with emphysematous cholecystitis (Fig. 4).
Fig. 2.
CT head without contrast demonstrating intraparenchymal gas foci within the left temporoparietal region in area of prior infarct and probable communication with the adjacent left ventricle. Additional complex appearing air fluid levels within the lateral ventricles are most consistent with pyogenic ventriculitis due to a gas forming organism.
Fig. 3.
CT chest showing soft tissue gas foci within the cardiac apex myocardium and nondependent pericardium, consistent with a necrotizing myocardial infection. The gas collection appears to extend the full thickness of the myocardial wall.
Fig. 4.
Coronal CT abdomen and pelvis showing gas within the lumen and fundal wall of the gallbladder, consistent with emphysematous cholecystitis (left). Tiny focus of gas favored to be within bile ducts of the left hepatic lobe (right).
Considering the above findings and despite aggressive surgical intervention, it was determined that the infection would have a 100 % mortality and source control would not be attainable due to the extent of inoperable necrotizing infection. Her family elected to proceed with comfort care, and the patient passed away peacefully with family at the bedside. The elapsed time between clinical presentation and demise was less than 48 h. Our patient’s death was reported to the county coroner, who declined to perform an autopsy. Her family was offered but also declined an autopsy.
Discussion
Our patient was a 73-year-old woman who presented with atraumatic CGG following hemodialysis. She developed progressive neurologic symptoms and right upper extremity erythema, edema, and crepitus. On radiographic imaging, she was found to have gas production in the myocardium, pericardium, gallbladder, biliary tract, cerebral ventricles, and subcutaneous tissue. She succumbed to metastatic infection within two days of hospital admission.
This case highlights several atypical features of spontaneous CGG. For example, C. septicum is responsible for most cases of atraumatic CGG and is associated with colonic malignancy and neutropenia [13]. The organism often enters through a defect in the bowel wall due to ischemia, bowel perforation, diverticulitis, or malignancy [13]. However, our patient was infected with C. perfringens, the most common cause of CGG related to penetrating trauma or crush injuries [8]. Without known antecedent injury, febrile neutropenia, or gastrointestinal barrier disruption, this patient had a dubious route of infection.
Reviewing the literature, we found 13 cases of spontaneous CGG secondary to C. perfringens (Table 1) [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. All but two patients had known risk factors, including atherosclerosis, diabetes, immunosuppression, or malignancy. All patients < 50 years had an underlying hematologic malignancy, while patients > 50 years had various cardiovascular, metabolic, and neoplastic comorbidities. C. perfringens was isolated from blood, muscle, or wound cultures in every patient. Most patients underwent surgical intervention (77 %) and antibiotic treatment (92 %), although antibiotic selection varied markedly. Excluding our case, we calculated a mortality rate of 85 %.
Table 1.
Clinical and microbiological characteristics of patients with spontaneous or atraumatic gas gangrene secondary to Clostridium perfringens.
| Soscia et al.[14] | Marty et al.[15] | Gazzaniga et al. 16] | Whyland et al.[17] | Minutti et al. [18] | Ito et al. [19] | Jendrzejewski et al. [20] | Garcia-Suarez et al. [21] | Temple et al. [22] | Niimi et al. [23] | Lee et al. [24] | Yildiz et al. [25] | This Case | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, Sex | 52, M | 74, M | 81, F | 70, M | 7, M | 72, M | 43, M | 43, M | 18, M | 16, M | 54, M | 57, M | 52, M | 73, F |
| Comorbidities | No known PMHx | Myocardial infarction T2DM |
Cecal adenocarcinoma Cardiomegaly HTN T2DM |
Rectal adenocarcinoma | ALL | Alcoholism T2DM |
AML | NHL (Diffuse Large B-cell Lymphoma) |
NHL (Lymphoblastic Lymphoma) |
ALL | Atherosclerosis obliterans AFib Mitral stenosis T2DM |
ALL | No known PMHx | AFib ESRD HFpEF HTN T2DM |
| Presenting Symptoms | Hematemesis, RUQ abdominal pain | Chills and pain with crepitation in right forearm | Right arm pain | Left thigh pain | Emesis, fever, malaise, nausea | Fever, abdominal pain | Left leg pain | Anxiety, diaphoresis, dyspnea, fever | Fever, right calf pain | Left thigh pain | Chills, fever, left thigh pain | Pain of left chest wall, buttock, thigh | Fever, RLQ abdominal pain | Agitation, confusion, syncope |
| Antibiotics | Penicillin | None but received HBO therapy | Tetracycline | Penicillin Sulfadiazine Topical nitrofurazone |
Ceftazidime Cefazolin Clindamycin Gentamicin Piperacillin |
Cefoperazone- Sulbactam Penicillin |
Cefapirin Clindamycin Gentamicin |
Amikacin Ceftriaxone Clindamycin Penicillin |
Cefepime Clindamycin Gentamicin Penicillin |
Cefepime Clindamycin Gentamicin |
Imipenem/Cilastatin Piperacillin |
Piperacillin-Tazobactam Vancomycin |
Amikacin Ceftazidime Metronidazole |
Cefepime Clindamycin Fluconazole Metronidazole Vancomycin |
| Surgery | None | Debridement | Glenohumeral disarticulation | Debridement | Debridement | None | Debridement | Debridement and excision of necrotic tumor mass | Transfemoral amputation | Hip disarticulation | Transfemoral amputation | None | Exploratory fasciotomy without debridement | Glenohumeral disarticulation |
| Outcome | Deceased | Deceased | Deceased | Survived | Deceased | Deceased | Deceased | Deceased | Survived | Deceased | Deceased | Deceased | Deceased | Deceased |
| Comments | Wound cx grew C. perfringens | Wound cx grew C. perfringens and Proteus mirabilis. Died from Gram-negative sepsis | Wound cx grew C. perfringens. Died from necrotizing endocarditis and myocarditis |
Muscle and stool cx grew C. perfringens and anaerobic streptococci | Wound cx grew C. perfringens. Died in operating room | Bile and wound cx grew C. perfringens | Muscle cx grew C. perfringens. Died in operating room | Peritoneal cx grew C. perfringens. Massive hemolysis and DIC | Wound cx grew C. perfringens. | Wound cx grew C. perfringens | Blood cx grew C. perfringens and Enterococcus faecalis | Wound aspirate grew C. perfringens | Wound cx grew C. perfringens | Wound cx grew C. perfringens. Gas in four organs. |
Abbreviations. AFib: atrial fibrillation; AML: acute myeloid leukemia; ALL: acute lymphocytic leukemia; CNS: central nervous system; Cx: culture; DIC: disseminated intravascular coagulopathy; ESRD: end-stage renal disease; HBO: hyperbaric oxygen; HFpEF: heart failure with preserved ejection fraction; HLD: hyperlipidemia; HTN: hypertension; NHL: non-Hodgkin lymphoma; PMHx: past medical history; RLQ: right lower quadrant; RUQ: right upper quadrant; T2DM: type II diabetes mellitus.
Literature on multiorgan CGG is sparse, although several case reports have detailed multiorgan infections following liver transplantation and C. septicum bacteremia [26], [27]. Separate reports describing isolated gas production in the brain and heart have also been published [28], [29]. However, cases of disseminated infection with documented gas production in four or more organs were not discovered in our literature review.
Spontaneous CGG may present atypically in immunocompromised states, such as neutropenia, colon cancer, leukemia, and diabetes [2]. As seen in this case, spontaneous CGG may also present with anaerobic bacteremia, a rarity among bloodstream infections as anaerobes are responsible for 1–17 % of positive blood cultures [30]. In patients with mysterious infections, an autopsy may help clarify the disease course [28].
Early diagnosis with timely surgical intervention and appropriate antibiotic management reduces the morbidity and mortality of NSTI. Surrogate laboratory markers for the early diagnosis of infections with significant disfigurement and mortality are valuable and should be further researched. For example, the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score is an objective scoring system based on six laboratory values – white blood cells, hemoglobin, C-reactive protein, sodium, creatinine, and glucose – that distinguishes necrotizing fasciitis from cellulitis, abscesses, and other skin and soft tissue infections [31], [32]. Scored out of 13 points, patients with scores ≥ 6 or ≥ 8 have positive predictive values of 92 % and 93.4 %, respectively [32]. When used in appropriate settings, surrogate markers may alert clinicians to the possibility of NSTI prior to surgical exploration.
Prompt surgical exploration and source control are the most important components of NSTI therapy because antibiotics are unable to penetrate necrotic tissue. Surgical exploration facilitates specimen collection for microbiology and pathology and may result in debridement or amputation [8]. Given the rapid progression of NSTI, diagnostic imaging should not delay surgical intervention [1]. In fact, two studies found that inadequate, delayed (>24 h after admission), or lack of surgical debridement was linked to 7.5-fold and 9.4-fold increases in mortality, respectively [33], [34]. Standardizing and validating the definition of “early” surgical intervention, proposed by some as <6 h following diagnosis or admission, may further refine NSTI treatment algorithms and improve hospital outcomes [35], [36].
With few clinicians ordering and fewer labs performing susceptibility testing for anaerobic organisms, antibiotic recommendations for CGG are based on multicenter surveys and literature reviews [37]. Most Clostridium spp., including C. perfringens, are susceptible to penicillin and ampicillin, although resistance has been reported [38]. Definitive CGG is currently treated with penicillin and clindamycin for 10–14 days [39], [40]. Experimental studies have shown that combination therapy with clindamycin and penicillin or monotherapy with clindamycin or metronidazole treated fulminant CGG in murine models efficaciously [41], [42]. Without knowing the causative agent, NSTI is treated empirically with vancomycin plus piperacillin-tazobactam, ampicillin-sulbactam, or a carbapenem [40].
Conclusion
CGG is a rare but life-threatening infection often incited by traumatic injury, although spontaneous cases occur secondarily in immunocompromised patients. Given its rapid progression and high mortality rate, physicians should maintain a high index of suspicion for CGG in patients presenting with soft tissue discoloration, crepitus, or blisters containing dishwater fluid. Prompt diagnosis, surgical debridement, and intravenous antibiotics are necessary to prevent amputation or death. Our patient illustrates how CGG may present atypically at unsuspecting sites of infection in immunocompromised and polymorbid patients. A computerized literature review found no similar cases.
Ethical approval
We sincerely thank our institution’s faculty ethics committee and chief privacy officer for reviewing this manuscript.
CRediT authorship contribution statement
Ashton D. Hall: Original draft and revisions, Conceptualization. Joshua M. Ferreri: Writing – original draft. Jennifer E. Baker: Writing – original draft. Eleanor A. Powell: Writing – review & editing. Imran Ahmed: Writing – review & editing. Timothy T. Klostermeier: Writing – review & editing. Keith M. Luckett: Writing - reviewing & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the patient and the multidisciplinary care team that contributed to her care.
Consent
HIPAA lists 18 information identifiers that, when paired with health information, become protected/personal health information. We have removed all such identifying details. The patient’s next-of-kin consented to the use of medical photographs for educational purposes upon hospital admission.
Authorship statement
All authors made substantial contributions to the following:
-
1.
The conception and design of the study, or acquisition of data, or analysis and interpretation of data.
-
2.
Drafting the article or revising it critically for important intellectual content.
-
3.
Final approval of the version to be submitted.
All authors agree to be accountable for all aspects of the work to ensure that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Leiblein M., Wagner N., Adam E.H., Frank J., Marzi I., Nau C. Clostridial gas gangrene ‐ a rare but deadly infection: case series and comparison to other necrotizing soft tissue infections. Orthop Surg. 2020;12:1733–1747. doi: 10.1111/os.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens D.L., Musher D.M., Watson D.A., Eddy H., Hamill R.J., Gyorkey F., et al. Spontaneous, nontraumatic gangrene due to Clostridium septicum. Rev Infect Dis. 1990;12:286–296. doi: 10.1093/clinids/12.2.286. [DOI] [PubMed] [Google Scholar]
- 3.Hassan K.A., Elbourne L.D.H., Tetu S.G., Melville S.B., Rood J.I., Paulsen I.T. Genomic analyses of Clostridium perfringens isolates from five toxinotypes. Res Microbiol. 2015;166:255–263. doi: 10.1016/j.resmic.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Fu Y., Alenezi T., Sun X. Clostridium perfringens-induced necrotic diseases: an overview. Immuno. 2022;2:387–407. [Google Scholar]
- 5.Lucey B.P., Hutchins G.M. William H. Welch, MD, and the Discovery of Bacillus welchii. Arch Pathol Lab Med. 2004;128:1193–1195. doi: 10.5858/2004-128-1193-WHWMAT. [DOI] [PubMed] [Google Scholar]
- 6.Adams B.N., Lekovic J.P., Robinson S. Clostridium perfringens sepsis following a molar pregnancy. Am J Obstet Gynecol. 2014;210:e13–e14. doi: 10.1016/j.ajog.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 7.Gerding D.N., Johnson S. Clostridial Infections. In: Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine. 24th ed., Philadelphia: Saunders Elsevier; 2011, p. e304:1–7.
- 8.Stevens D.L., Bryant A.E. Necrotizing soft-tissue infections. NEJM. 2017;377:2253–2265. doi: 10.1056/NEJMra1600673. [DOI] [PubMed] [Google Scholar]
- 9.McHenry C.R., Piotrowski J.J., Petrinic D., Malangoni M.A. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–565. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkerson R., Paull W., Coville F.V. Necrotizing fasciitis: review of the literature and case report. Clin Orthop Relat Res. 1987;216:187–192. [PubMed] [Google Scholar]
- 11.Giri B., Kole L. In: Biological Toxins and Bioterrorism. Gopalakrishnakone P., editor. Springer; Dordrecht: 2014. Combating the Perilous Consequence of Clostridial Gas Gangrene: An Overview; pp. 1–21. [Google Scholar]
- 12.Elliott D.C., Kufera J.A., Myers R.A.M. Necrotizing soft tissue infections: risk factors for mortality and strategies for management. Ann Surg. 1996;224:672–683. doi: 10.1097/00000658-199611000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava I., Aldape M.J., Bryant A.E., Stevens D.L. Spontaneous C. septicum gas gangrene: a literature review. Anaerobe. 2017;48:165–171. doi: 10.1016/j.anaerobe.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Soscia J., Grace W.J. Gas Bacillus Infections: Two Unusual Cases. Am J Dig Dis. 1965;10:625–630. doi: 10.1007/BF02237636. [DOI] [PubMed] [Google Scholar]
- 15.Marty A.T., Filler R.M. Recovery from non-traumatic localised gas gangrene and Clostridial septicaemia. Lancet. 1969;2:79–81. doi: 10.1016/s0140-6736(69)92391-5. [DOI] [PubMed] [Google Scholar]
- 16.Gazzaniga A.B. Nontraumatic, clostridial, gas gangrene of the right arm and Adenocarcinoma of the cecum: report of a case. Dis Colon Rectum. 1967;10:298–300. doi: 10.1007/BF02617143. [DOI] [PubMed] [Google Scholar]
- 17.Whyland W.A., Levin M.N. Gas gangrene without visible portal of entry. Am J Surg. 1960;99:77–79. doi: 10.1016/0002-9610(60)90254-3. [DOI] [PubMed] [Google Scholar]
- 18.Minutti C.Z., Immergluck L.C., Schmidt M.Lou. Spontaneous gas gangrene due to Clostridium perfringens. Clin Infect Dis. 1999;28:159–160. doi: 10.1086/517192. [DOI] [PubMed] [Google Scholar]
- 19.Ito T., Shiraki K., Sekoguchi K., Hamada M., Yamanaka T., Takase K., et al. Metastatic gas gangrene of the leg due to acute emphysematous cholecystitis. Dig Dis Sci. 2001;46:2480–2483. doi: 10.1023/a:1012336222483. [DOI] [PubMed] [Google Scholar]
- 20.Jendrzejewski J.W., Jones S.R., Newcombe R.L., Gilbert D.N. Nontraumatic clostridial myonecrosis. Am J Med. 1978;65:542–546. doi: 10.1016/0002-9343(78)90782-9. [DOI] [PubMed] [Google Scholar]
- 21.García-Suárez J., de Miguel D., Krsnik I., Barr-Alí M., Hernanz N., Burgaleta C. Spontaneous gas gangrene in malignant lymphoma: an underreported complication? Am J Hematol. 2002;70:145–148. doi: 10.1002/ajh.10106. [DOI] [PubMed] [Google Scholar]
- 22.Temple A.M.M., Thomas N.J. Gas gangrene secondary to Clostridium perfringens in pediatric oncology patients. Pedia Emerg Care. 2004;20:457–459. doi: 10.1097/01.pec.0000132218.42729.97. [DOI] [PubMed] [Google Scholar]
- 23.Niimi M., Ikeda Y., Kan S., Takami H. Gas gangrene in patient with atherosclerosis obliterans. Asian Cardiovasc Thorac Ann. 2002;10:178–180. doi: 10.1177/021849230201000223. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.L., Cho S.Y., Lee D.G., Ko Y., Hyun J.I., Kim B.K., et al. A fatal spontaneous gas gangrene due to Clostridium perfringens during neutropenia of allogeneic stem cell transplantation: case report and literature review. Infect Chemother. 2014;46:199–203. doi: 10.3947/ic.2014.46.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yildiz T., Gündeş S., Willke A., Solak M., Toker K. Spontaneous, nontraumatic gas gangrene due to Clostridium perfringens. Int J Infect Dis. 2006;10:83–85. doi: 10.1016/j.ijid.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Kousa O., Essa A., Ramadan B., Aly A., Awad D., Zhao X., et al. Multiorgan fatal gas gangrene in the setting of Clostridium septicum bacteremia: a case report. J Emerg Crit Care Med. 2020;4:1–7. [Google Scholar]
- 27.Kitterer D., Braun N., Jehs M.C., Schulte B., Dominik Alscher M., Latus J. Gas gangrene caused by Clostridium perfringens involving the liver, spleen, and heart in a man 20 years after an orthotopic liver transplant: a case report. Exp Clin Transplant. 2014;12:165–168. doi: 10.6002/ect.2013.0034. [DOI] [PubMed] [Google Scholar]
- 28.Khan H.M.W., Yousaf A., Ahmad M., Changezi H.U. Gas in the myocardium: a fatal presentation of Clostridium perfringens: a case report. Eur Heart J Case Rep. 2023;7:1–5. doi: 10.1093/ehjcr/ytac488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke P.R. Gas gangrene abscess of the brain. J Neurol Neurosurg Psychiatry. 1968;31:391–392. doi: 10.1136/jnnp.31.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16:183–189. doi: 10.1016/j.anaerobe.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Bechar J., Sepehripour S., Hardwicke J., Filobbos G. Laboratory risk indicator for necrotising fasciitis (LRINEC) Score for the assessment of early necrotising fasciitis: a systematic review of the literature. Ann R Coll Surg Engl. 2017;99:341–346. doi: 10.1308/rcsann.2017.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong C.H., Khin L.W., Heng K.S., Tan K.C., Low C.O. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) Score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32:1535–1541. doi: 10.1097/01.ccm.0000129486.35458.7d. [DOI] [PubMed] [Google Scholar]
- 33.Mok M.Y., Wong S.Y., Chan T.M., Tang W.M., Wong W.S., Lau C.S. Necrotizing fasciitis in rheumatic diseases. Lupus. 2006;15:380–383. doi: 10.1191/0961203306lu2314cr. [DOI] [PubMed] [Google Scholar]
- 34.Wong C.H., Chang H.C., Pasupathy S., Khin L.W., Tan J.L., Low C.O. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Jt Surg. 2003;85:1454–1460. [PubMed] [Google Scholar]
- 35.Bandyopadhyay D., Jacobs J., Panchabhai T. What’s new in emergencies, trauma and shock? The tortuous path in the management of necrotizing fasciitis: is early surgical intervention critical? J Emerg Trauma Shock. 2016;9:1–2. doi: 10.4103/0974-2700.173862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadeed G., Smith J., O’Keeffe T., Kulvatunyou N., Wynne J., Joseph B., et al. Early surgical intervention and its impact on patients presenting with necrotizing soft tissue infections: a single academic center experience. J Emerg Trauma Shock. 2016;9:22–27. doi: 10.4103/0974-2700.173868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein E.J.C., Citron D.M., Goldman P.J., Goldman R.J. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14:68–72. doi: 10.1016/j.anaerobe.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhong J. xin, Zheng H. ran, Wang Y. yuan, Bai L. lu, Du X. li, Wu Y., et al. Molecular characteristics and phylogenetic analysis of Clostridium perfringens from different regions in China, from 2013 to 2021. Front Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1195083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens D.L., Bryant A.E., Goldstein E.J. Necrotizing soft tissue infections. Infect Dis Clin North Am. 2021;35:135–155. doi: 10.1016/j.idc.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Stevens D.L., Bisno A.L., Chambers H.F., Dellinger E.P., Goldstein E.J.C., Gorbach S.L., et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10–e52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 41.Stevens D.L., Laine B.M., Mitten J.E. Comparison of single and combination antimicrobial agents for prevention of experimental gas gangrene caused by Clostridium perfringens. Antimicrob Agents Chemother. 1987;31:312–316. doi: 10.1128/aac.31.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevens D.L., Maier K.A., Laine B.M., Mitten J.E. Comparison of Clindamycin, Rifampin, Tetracycline, Metronidazole, and Penicillin for efficacy in prevention of experimental gas gangrene due to Clostridium perfringens. J Infect Dis. 1987;155:220–228. doi: 10.1093/infdis/155.2.220. [DOI] [PubMed] [Google Scholar]