Abstract
Background
We aimed to analyse the differences in the risk of geriatric syndromes between older adults with and without coronavirus disease 2019 (COVID-19).
Methods
We conducted a retrospective cohort study of patients from the US Collaborative Network in the TriNetX between January 1, 2020, and December 31, 2022. We included individuals aged older than 65 years with at least 2 health care visits who underwent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) tests during the study period. We excluded those with SARS-CoV-2 vaccination, diagnosis with neoplasm and geriatric syndromes before the index date, and death within 30 days after the index date. The index date was defined as the first date of the PCR test for SARS-CoV-2 during the study period. Hazard ratios (HRs) and 95% confidence intervals (CIs) for eight geriatric syndromes were estimated for propensity score-matched older adults with and without COVID-19. Subgroup analyses of sex and age were also performed.
Results
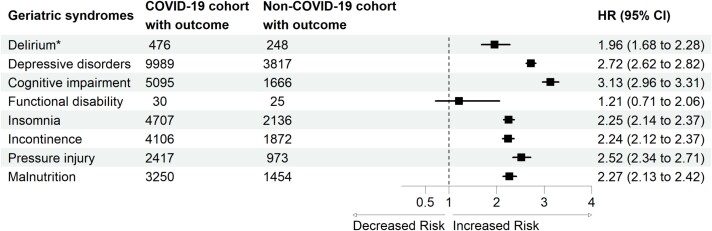
After propensity score matching, 315 826 patients were included (mean [standard deviation] age, 73.5 [6.4] years; 46.7% males and 51.7% females). The three greatest relative increases in the risk of geriatric syndromes in the COVID-19 cohort were cognitive impairment (HR: 3.13; 95% CI: 2.96–3.31), depressive disorder (HR: 2.72; 95% CI: 2.62–2.82) and pressure injury (HR: 2.52; 95% CI: 2.34–2.71).
Conclusions
The risk of developing geriatric syndromes is much higher in the COVID-19 cohort. It is imperative that clinicians endeavour to prevent or minimise the development of these syndromes in the post-COVID-19 era.
Keywords: older adults, COVID-19, geriatric syndromes, TriNetX
Key Points
Recognising geriatric syndromes in older COVID-19 survivors is crucial for geriatric syndromes prevention.
We aimed to analyse the differences in the risk of geriatric syndromes between older adults with and without COVID-19.
Cognitive impairment, depressive disorder and pressure injury were the three greatest relative increases in the risk.
It is imperative that clinicians endeavour to prevent or minimise the development of these syndromes in the post-COVID-19 era.
Introduction
Coronavirus disease 2019 (COVID-19) has brought unprecedented severe challenges to many countries [1]. It is associated with damage to respiratory systems [2], cardiovascular/cerebrovascular diseases [3], and neurological/psychiatric-related complications [4, 5].
However, as the population ages, post-COVID-19-related issues for older adults have become increasingly prevalent, but these issues have rarely been discussed [6, 7]. Previous reports regarding older adults in the post-COVID-19 period have focused mainly on symptoms, such as fatigue, dyspnea, joint pain and cough [8]. COVID-19-related mortality was mainly attributed to delayed diagnosis, immune system problems, comorbidities, etc. [6, 9]. Older adults are at increased risk for severe COVID-19, but they are not simply an age-defined entity [10]. One meta-analysis of 118 373 older individuals with COVID-19 demonstrated the importance of frailty in predicting mortality [11], but not older age alone. In addition to older age, geriatric syndromes have been identified as major determinants for poor outcomes of older adults with various clinical conditions, and COVID-19 was no exception [12].
Geriatric syndromes [13] are defined as ‘conditions experienced by the older adults, especially the frail older adults, that occur intermittently rather than continuously or in single episodes, may be triggered by acute problems and are often associated with subsequent functional decline.’ The occurrence of geriatric syndromes is associated with poor life satisfaction [14] and increased mortality in older adults [15]. Although there are reports regarding geriatric syndromes in older adults with COVID-19, these reports are relatively sporadic and scarce. Studies on geriatric syndromes related to COVID-19 in older adults have included sleep difficulties [16], depression [16, 17], cognition [4, 5], fatigue [16] and falls [18]. Some of these reports [5, 17, 18] focused on a single geriatric syndrome, and some of these reports [4, 16] did not focus on older adults. Of interest, one study [19] explored the impact of geriatric syndromes on function in the older adults with COVID-19 at discharge, and another study [20] investigated the occurrence of geriatric syndromes in the older adults with COVID-19 at admission and during hospitalisation. However, these two studies [19, 20] had relatively small sample size, and there were no control groups. The objective of the current study was to evaluate the impact of COVID-19 on the risk of geriatric syndromes. Therefore, we conducted a propensity score-matched cohort using data from the US Collaborative Network in TriNetX to compare the differences in the risk of geriatric syndromes between the older adults with and without COVID-19.
Methods
Data source
We used the U.S. Collaborative Network of 48 health care organisations (HCOs) in the TriNetX research network. The TriNetX database, comprising electronic medical records (EMRs), provides a living ecosystem of real-world data and evidence for life science and health care. With more than 250 million participants from more than 120 HCOs, the TriNetX offers a comprehensive representation of patient populations across various regions and health care settings. TriNetX is a useful database for large epidemiological studies [3, 4, 21], and it includes a wide range of information on demographics; diagnoses (based on the International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]); procedures (International Classification of Diseases, Tenth Revision, Procedure Coding System, ICD-10-PCS or Current Procedural Terminology); medications (based on the Anatomical Therapeutic Chemical Classification System or Veterans Affairs National Formulary); laboratory measurements (based on Logical Observation Identifiers Names and Codes); and health care utilisation. Details about TriNetX are available on its website (http://trinetx.com/). The use of TriNetX in the present study was approved by the Institutional Review Board of the National Cheng Kung University Hospital (A-EX-113-003).
Design, setting and study cohort
We conducted a retrospective cohort study of 48 HCOs in a subset of the TriNetX network from the U.S. Collaborative Network between 1 January 2020, and 31 December 2022. We included individuals aged older than 65 years who had at least 2 health care visits and who underwent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) tests during the study period in the TriNetX database. We included the individuals with at least 2 health care visits to make sure that we can capture the individuals’ health information, because discontinuity may lead to information bias. The detailed codes for the PCR tests are shown in Supplementary Table 1. The index date was defined as the first date of the PCR test for SARS-CoV-2 during the study period. The COVID-19 cohort was identified based on the positive result of a PCR test at the index date. The non-COVID-19 cohort was defined as patients with negative PCR results and who had never had ICD-10 codes for COVID-19 prior to the index date. A flow diagram of the cohort establishment involving 1 834 797 older adults recruited between 1 January 2020, and 31 December 2022 is provided in Supplementary Figure 1. Individuals who had (i) received SARS-CoV-2 vaccination before the index date, (ii) were diagnosed with neoplasms before the index date, (iii) were diagnosed with geriatric syndromes prior to the index date or (iv) died within 30 days after the index date were excluded. The geriatric syndromes in this study included delirium, depressive disorder, cognitive impairment, functional disability, insomnia, incontinence, pressure injury and malnutrition.
Baseline characteristics
The baseline characteristics were constructed from the TriNetX database during the 1-year period prior to the index date. The demographic information included age (age was calculated at the index date), sex, race and problems related to housing and economic circumstances. The comorbidities included hypertension, diabetes mellitus (type 1 and type 2), heart disease (ischemic heart disease and other forms of heart disease), chronic lower respiratory disease, chronic kidney disease (chronic kidney disease and hypertensive chronic kidney disease), cerebrovascular disease and liver disease. The laboratory measurements included glucose (mg/dL) and haemoglobin (mg/dL). The number of individuals with missing data is reported in Table 1. The detailed covariate codes are shown in Supplementary Table 2.
Table 1.
Baseline characteristics of the study subjects before and after propensity score matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| COVID-19 cohort (n = 161 767) |
Non-COVID-19 cohort (n = 496 306) |
Std diff. | COVID-19 cohort (n = 157 913) |
Non-COVID-19 cohort (n = 157 913) |
Std diff. | |
| Age at the index date | ||||||
| Mean ± SD | 73.5 ± 6.4 | 73 ± 6.2 | 0.083 | 73.5 ± 6.4 | 73.3 ± 6.3 | 0.018 |
| Sex | ||||||
| Female | 83 347 (51.5) | 254 806 (51.3) | 0.004 | 81 574 (51.7) | 81 141 (51.4) | 0.005 |
| Male | 74 421 (46) | 235 782 (47.5) | 0.030 | 73 008 (46.2) | 73 712 (46.7) | 0.009 |
| Missing | 3999 (2.5) | 5718 (1.2) | 0.097 | 3331 (2.1) | 3060 (1.9) | 0.014 |
| Race | ||||||
| White | 118 298 (73.1) | 372 396 (75.0) | 0.043 | 116 127 (73.5) | 116 056 (73.5) | 0.001 |
| African American | 24 534 (15.2) | 67 680 (13.6) | 0.044 | 23 732 (15.0) | 24 103 (15.3) | 0.007 |
| Asian | 2900 (1.8) | 9863 (2.0) | 0.014 | 2872 (1.8) | 2857 (1.8) | 0.001 |
| American Indian or Alaska Native | 373 (0.2) | 1319 (0.3) | 0.007 | 369 (0.2) | 309 (0.2) | 0.008 |
| Native Hawaiian or other Pacific Islander | 182 (0.1) | 637 (0.1) | 0.005 | 182 (0.1) | 161 (0.1) | 0.004 |
| Other race | 4454 (2.8) | 15,565 (3.1) | 0.018 | 4402 (2.8) | 4656 (2.9) | 0.006 |
| Unknown race | 11 026 (6.8) | 28 846 (5.8) | 0.041 | 10 229 (6.5) | 9771 (6.2) | 0.012 |
| Social economic status | ||||||
| Problems related to housing and economic circumstances | 697 (0.4) | 418 (0.1) | 0.068 | 337 (0.2) | 369 (0.2) | 0.004 |
| Comorbidities (present) | ||||||
| Hypertensive diseases | 74 852 (46.3) | 135 190 (27.2) | 0.403 | 71 029 (45) | 71 516 (45.3) | 0.006 |
| Type 1 diabetes mellitus | 1549 (1.0) | 1696 (0.3) | 0.077 | 1148 (0.7) | 1073 (0.7) | 0.006 |
| Type 2 diabetes mellitus | 35 185 (21.8) | 53 665 (10.8) | 0.300 | 32 202 (20.4) | 33 087 (21) | 0.014 |
| Ischemic heart diseases | 31 057 (19.2) | 54 240 (10.9) | 0.233 | 28 484 (18.0) | 27 460 (17.4) | 0.017 |
| Other forms of heart disease | 47 301 (29.2) | 90 033 (18.1) | 0.263 | 44 066 (27.9) | 42 958 (27.2) | 0.016 |
| Chronic lower respiratory diseases | 25 011 (15.5) | 35 298 (7.1) | 0.266 | 22 184 (14.0) | 22 429 (14.2) | 0.004 |
| Chronic kidney disease | 22 549 (13.9) | 29 040 (5.9) | 0.273 | 19 492 (12.3) | 19 655 (12.4) | 0.003 |
| Hypertensive chronic kidney disease | 9863 (6.1) | 9031 (1.8) | 0.221 | 7656 (4.8) | 7963 (5) | 0.009 |
| Cerebrovascular diseases | 14 013 (8.7) | 25 212 (5.1) | 0.142 | 12 683 (8.0) | 11 857 (7.5) | 0.020 |
| Diseases of liver | 7022 (4.3) | 9684 (2.0) | 0.137 | 5893 (3.7) | 5627 (3.6) | 0.009 |
| Laboratory | ||||||
| Glucose | ||||||
| n (%) | 119 832 (74.1) | 309 585 (62.4) | 0.253 | 119 832 (74.1) | 110 285 (69.8) | 0.096 |
| Mean ± SD, mg/dL | 124.9 ± 52 | 121.4 ± 48.1 | 0.069 | 124.9 ± 52 | 124.4 ± 51.3 | 0.001 |
| Missing | 41 935 (25.9) | 186 721 (37.6) | 0.253 | 41 935 (25.9) | 47 628 (30.2) | 0.096 |
| Haemoglobin | ||||||
| n (%) | 110 998 (68.6) | 286 504 (57.7) | 0.227 | 107 297 (67.9) | 102 561 (64.9) | |
| Mean ± SD, g/dL | 12.7 ± 2.1 | 12.9 ± 2.2 | 0.092 | 12.8 ± 2.1 | 12.8 ± 2.2 | 0.015 |
| Missing | 50 769 (31.4) | 209,802 (42.3) | 0.227 | 50 616 (32.1) | 55 352 (35.1) | 0.064 |
Outcome and follow-up
The outcomes were geriatric syndromes, including delirium, depressive disorder, cognitive impairment, functional disability, insomnia, incontinence, pressure injury and malnutrition. The ICD-10-CM for the geriatric syndromes are shown in Supplementary Table 3. To reduce protopathic bias, we used a 30-day observation window within 30 days of the index date as a washout period for the outcome measures to avoid increased vigilance and earlier diagnosis of symptoms after SARS-CoV-2 infections. Incident delirium in the post-acute phase of COVID-19 was evaluated during the follow-up period between 1 day after the index date and the end of the 30-day follow-up. Except for delirium, other incident geriatric syndromes in the post-acute phase of COVID-19 were evaluated during the follow-up period between 30 days after the index date and the end of the 1-year follow-up. The follow-up was also censored at death or diagnosis of the specific geriatric syndrome of interest.
Statistical analysis
Descriptive statistics were used to summarise the baseline characteristics of the study cohort. Continuous variables are presented as the mean and standard deviation (SD). Categorical variables are described using frequencies and proportions. To reduce the effect of confounding, we used the TriNetX built-in function to perform propensity score matching without replacement and matched the two groups at a 1:1 ratio by greedy nearest neighbour matching for baseline characteristics. The standardised mean difference (SMD) was used to compare the difference in baseline characteristics between the COVID-19 and non-COVID-19 cohorts before and after propensity score matching. Any variable with an SMD < 0.1 was considered well-matched. Baseline characteristics were used for propensity score matching, including age (age was calculated at the index date), sex, race, socioeconomic status, comorbidities and laboratory data. To avoid time-varying issue or survival bias, we used age at the index date for following analysis. We used a conditional Cox hazard model to estimate hazard ratios (HRs) after propensity score matching to quantify the relative risk of geriatric syndromes in the COVID-19 cohort compared to the non-COVID-19 cohort. Kaplan–Meier plots and forest plots were generated based on the results of the TriNetX platform and subsequently redrawn using R (version 4.2.1) with the ggplot2 v3.3.5 and forestploter v1.1.1 packages. All analyses provided a 95% confidence interval (95% CI), which was used to indicate statistical significance. The proportional hazards assumption was tested using the Schoenfeld residual test. We found that the outcomes of depression and cognitive impairment violated the assumption, which could overestimate the relative risk between two groups. Therefore, we also used nonparametric estimation to compare the relative risk between two groups for all analyses. The risk ratio (RR) was calculated as the cumulative incidence in the COVID-19 group divided by the cumulative incidence in the non-COVID-19 group, and its corresponding 95% CI was estimated according to the natural logarithm of the RR, which is approximately normally distributed.
Subgroup analysis
Subgroup analysis was used to assess whether the risk of geriatric syndromes differed between the COVID-19 and non-COVID-19 cohorts according to sex and age. The age was calculated at the index date. Age was classified as 65–74 years for the young old, 75–84 years for the middle old, and 85 years and older for the oldest old [22].
For detailed subgroup analysis, please refer to the Appendix S1 (Methods-subgroup analysis).
Results
The demographic characteristics, comorbidities and laboratory measurements between the COVID-19 and non-COVID-19 cohorts before and after propensity score matching are shown in Table 1. The mean age at the index date in the COVID-19 cohort was 73.5 (SD 6.4) years. The proportion of females (51.7%) was greater than that of males (46.7%). The main race was Caucasian (73.5%). After propensity score matching, the differences in demographic characteristics, comorbidities and laboratory measurements between the COVID-19 and non-COVID-19 cohorts were small and well-matched.
The risk of geriatric syndromes was significantly greater in the COVID-19 cohort than in the non-COVID-19 cohort (Figure 1). We estimated the risk of developing geriatric syndromes in the COVID-19 cohort compared to the non-COVID-19 cohort. The COVID-19 cohort exhibited significantly greater risks of delirium (HR: 1.96; 95% CI: 1.68–2.28), depressive disorder (HR: 2.72; 95% CI: 2.62–2.82), cognitive impairment (HR: 3.13; 95% CI: 2.96–3.31), insomnia (HR: 2.25; 95% CI: 2.14–2.37), incontinence (HR: 2.24; 95% CI: 2.12–2.37), pressure injury (HR: 2.52; 95% CI: 2.34–2.71) and malnutrition (HR: 2.27; 95% CI: 2.13–2.42). Among these outcomes, cognitive impairment was the most common in the COVID-19 cohort compared to the non-COVID-19 cohort. Kaplan–Meier curves of patients with different geriatric syndromes are shown in Supplementary Figure 2.
Figure 1.
Forest plot of geriatric syndromes. *Incident delirium in the post-acute phase of COVID-19 was evaluated during the follow-up period between 1 day after the index date and the end of the 30-day follow-up. The other geriatric syndromes were evaluated during the follow-up period between 30 days after the index date and the end of the 1-year follow-up.
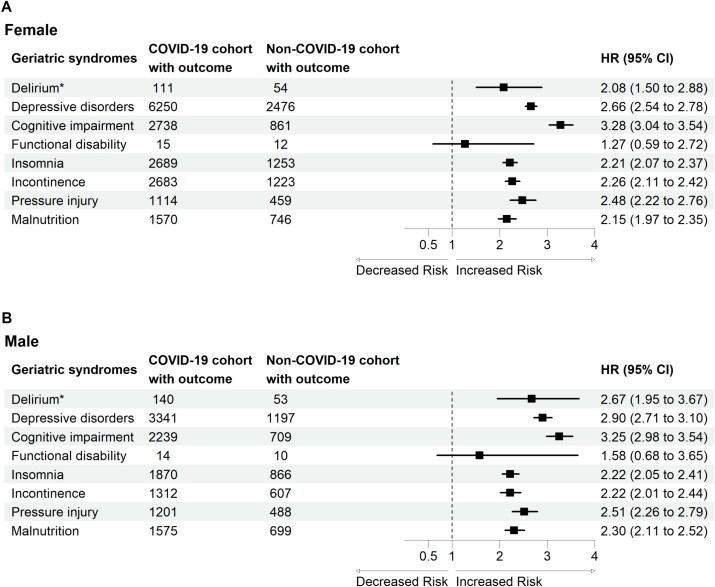
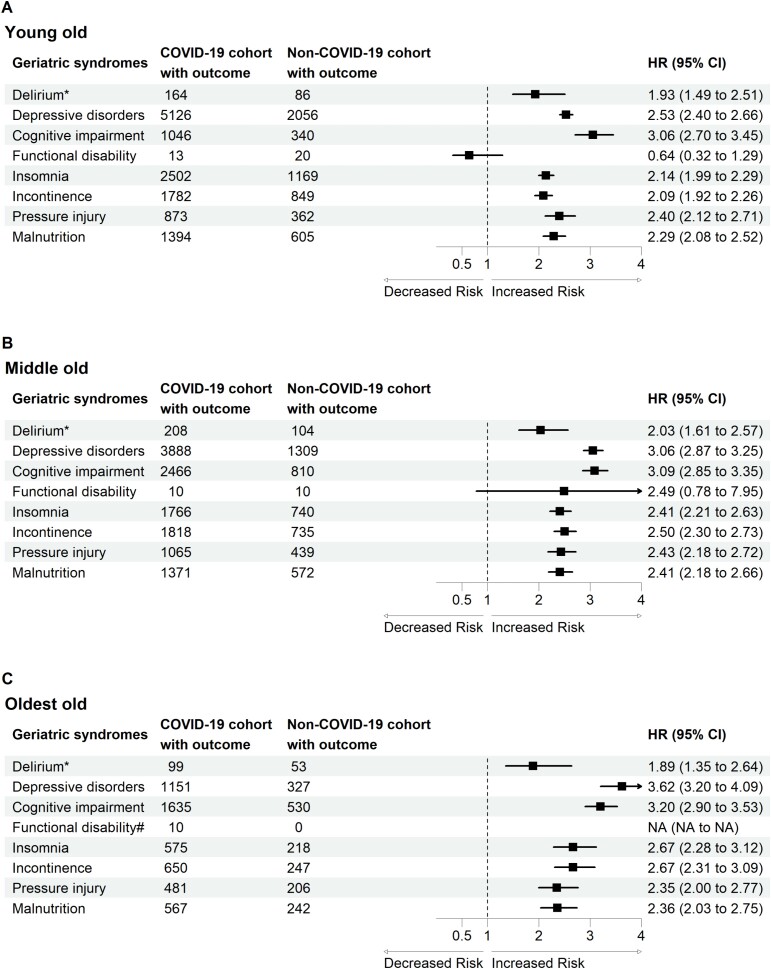
In the subgroup analysis, we first estimated the risk of developing geriatric syndromes based on sex (Figure 2) and age (Figure 3).
Figure 2.
Forest plot of geriatric syndromes by sex. *Incident delirium in the post-acute phase of COVID-19 was evaluated during the follow-up period between 1 day after the index date and the end of the 30-day follow-up. The other geriatric syndromes were evaluated during the follow-up period between 30 days after the index date and the end of the 1-year follow-up.
Figure 3.
Forest plot of geriatric syndromes by age. *Incident delirium in the post-acute phase of COVID-19 was evaluated during the follow-up period between 1 day after the index date and the end of the 30-day follow-up. The other geriatric syndromes were evaluated during the follow-up period between 30 days after the index date and the end of the 1-year follow-up.
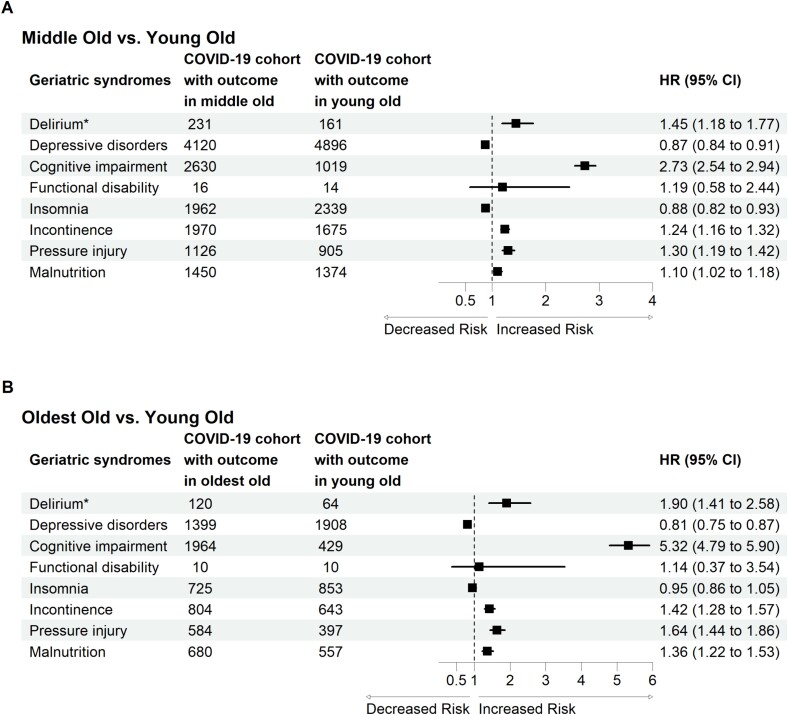
We further investigated the effect of age in the COVID-19 cohort and identified which geriatric syndromes were more likely to develop in the middle old and oldest old groups than in the young old group (Figure 4). We selected patients in the COVID-19 cohort and performed propensity score matching at a 1:1 ratio between the young old group and the middle old group and between the young old group and the oldest old group.
Figure 4.
Forest plot of the effect of age on geriatric syndrome incidence in patients with COVID-19. *Incident delirium in the post-acute phase of COVID-19 was evaluated during the follow-up period between 1 day after the index date and the end of the 30-day follow-up. The other geriatric syndromes were evaluated during the follow-up period between 30 days after the index date and the end of the 1-year follow-up.
For detailed results of subgroup analysis, please refer to the Appendix S2 (Results).
Discussion
In the present study involving 157 913 COVID-19 older adults and matched 157 913 controls, we demonstrated that older COVID-19 older adults have increased risk of developing geriatric syndromes. The risk of geriatric syndromes was significant for the different age groups (young old, middle old and oldest old) and for both sexes. The three greatest relative increases in the risk of geriatric syndromes were cognitive impairment, depressive disorder and pressure injury.
For the risk of cognitive impairment, our results are compatible with previous reports. One study showed that the risk of cognitive deficit in COVID-19 survivors was greater than that in matched controls at 6 months, with an HR of 1.36 (95% CI = 1.33–1.39) [4], and another study showed an odds ratio of 4.87 (95% CI = 3.30–7.20) for early-onset cognitive decline in COVID-19 survivors 6 months after discharge [5]. The incidence of cognitive impairment was 12.45% in the previous study [5], whereas our study yielded a figure of approximately 3%. This discrepancy can be attributed to the fact that the previous study defined cognitive impairment using a questionnaire, with a score indicating mild cognitive impairment to dementia. However, in our study, we defined cognitive impairment by ICD-10-CM of dementia, a much more rigorous definition which would result in relative low incidence. The underlying mechanisms of post-COVID-19 cognitive impairment have not been elucidated, but proposed contributors include neuroinflammation [23], hypoxia, vascular damage and latent viral reactivation [24]. To prevent and manage cognitive impairment in older adults with COVID-19, it is crucial to identify vulnerable individuals [25], and recent evidence has shown a predisposition to cognitive manifestations after COVID-19 in individuals with specific demographic/genetic risk factors, severe COVID-19, medication factors, environmental factors or comorbidities [25].
For the risk of depressive disorder, our results are consistent with those of previous studies. One study showed that the risk of anxiety/depression in COVID-19 survivors was greater than that in healthy individuals, with an HR of 1.587 (95% CI: 1.322–1.905) [26], and another study showed an HR of 1.39 (95% CI: 1.34–1.43) in COVID-19 survivors 1 month after diagnosis [17]. Although the mechanism of post-COVID-19 depressive disorder is unclear [27], there are several possibilities. The psychopathological mechanisms of post-COVID-19 depressive symptoms may be related to the inflammation triggered by the peripheral immune-inflammatory response to viral infection and to the persistent psychological burden during and after infection [27, 28]; this may also be due to social and pathological factors, including social quarantine, economic problems, stress, mitochondrial disorders, damage to the hippocampus and malnutrition [27].
For pressure injuries, the main risk factors and mechanisms associated with COVID-19 [29, 30] are wearing medical protective equipment, having a high baseline dependency level and remaining in a prone position for a long time, having a greater local pressure and friction on the skin, having a prolonged hospital stay and having a low oxygen saturation level upon arrival at the emergency department for triage. Although there are several nonmodifiable risk factors associated with the occurrence of pressure injuries in older adults with COVID-19, it is essential to pay more attention to the skin status of these high-risk groups and perform further research with consensus guidelines to reduce their occurrence.
Our results demonstrated that the differences between males and females arise from the risk of delirium and pressure injury. For delirium, our findings are compatible with one systemic review [31], which included 315 studies; male sex was identified in 15 studies as a predisposing factor, whereas female sex was identified in 4 studies. In addition, the oldest old individuals had the highest risk of developing delirium is compatible with the findings of one systemic review [31], which included 315 studies and showed that advanced age was identified as a predisposing factor in 112 of the 315 studies. Unlike other geriatric syndromes, we estimated the risk of delirium within 30 days of COVID-19 diagnosis, reflecting this important geriatric syndrome in the acute stage. Risk factors for delirium include premorbid factors, factors related to presenting illness, and post-admission factors [32]. The possible mechanisms of delirium include brain energy metabolism, inflammation, drugs, stress and neurotransmitter imbalance, neuroanatomical substrates and failure of network connectivity [32]. A multicomponent, non-pharmacological intervention to reduce modifiable risk factors for delirium, improve cognition and optimise sleep, mobility, hearing and vision in critically ill adults is suggested [32].
Since the occurrence of geriatric syndromes is associated with lower life satisfaction [14] and increased mortality in older adults [15], early detection and appropriate intervention are crucial [19, 20]. For the above goal, a multidimensional approach involving comprehensive geriatric assessment (CGA) performed by interdisciplinary hospital teams [6] in older COVID-19 survivors may provide the possibility of early detection of geriatric syndromes and the implementation of precision medicine [33]. CGA was first developed in the United Kingdom, and its concepts, indications and applications have evolved over time [34]. It is a multi-domain, multidisciplinary diagnostic and therapeutic process performed to evaluate the medical, mental, social and functional issues of older adults, therefore leading to an individualised and holistic plan for management and follow-up [35]. A CGA-based approach could strengthen the treatment goals of older adults with COVID-19 and aid in monitoring the occurrence of geriatric syndromes [33].
The rationale behind the selection of non-vaccinated individuals for the study can be attributed to several factors. Firstly, the initial cohort was predominantly non-vaccinated at the commencement of the study. Furthermore, the older adults are at greater risk of vaccine-related adverse events [36], which may result in confusion with the primary outcome in our study. Finally, it is important to consider the potential emergence of infectious diseases for which there are currently no vaccines. The management of the non-vaccinated population in the context of the post-emergence infectious diseases era is therefore a crucial issue.
Our study has several strengths to ensure that the results are sound. First, we compared the occurrence of geriatric syndromes in older adults with and without COVID-19 and performed propensity score matching to reduce confounding variables and avoid bias. Second, we carried out subgroup analysis based on age and sex. We further classified the older adults into three different age groups, namely, the young old, middle old and oldest old, to analyse the effect of age. We demonstrated the heterogeneous presentation of geriatric syndromes even though all the individuals were older than 65 years, suggesting that adults older than 65 years were not all the same. Third, to our knowledge, we are the first to report multiple geriatric syndromes following SARS-CoV-2 infections.
The current study has several limitations. First, we evaluated the occurrence of geriatric syndromes in the TriNetX of the U.S. Collaborative Network. The majority of the older adults were white and African American, and the extrapolation of our study to other races is limited. Second, we used the ICD-10-CM to define geriatric syndromes and propensity score matching to reduce bias, but underestimation of geriatric syndromes or other confounding factors could still exist because we used an electronic health database for secondary analysis. Besides, there is no ICD-10-CM of frailty to date, and we are unable to collect frailty as a geriatric syndrome from a claim-based dataset. Further studies are needed to include frailty as a geriatric syndrome. Third, the severity of COVID-19, as indicated by the use of mechanical ventilation or inotropic agents, the length of hospital stay and detailed information on the status of the older adults hospitalised with COVID-19, which may be related to the occurrence of geriatric syndromes, was not included in TriNetX. Therefore, it is possible that this information may influence our inference. Fourth, TriNetX did not include information pertaining to the specific strain of the SARS-CoV-2 virus. Fifth, TriNetX is a highly protective platform for the patients’ data. The researchers are unable to access the raw data directly, and can only analyse it by using the TriNetX platform’s built-in function. Consequently, Charlson comorbidity index and other composite comorbidity indices which require row data to estimate are not available. Finally, we conducted a study on the non-vaccinated older adults. Given the current widespread vaccination, further studies on vaccinated older adults are necessary.
Conclusions
The three greatest relative increases in the risk of geriatric syndromes in the older adults with COVID-19 were cognitive impairment, depressive disorder and pressure injury. Given the association between the occurrence of geriatric syndromes and poor outcomes, it is imperative that clinicians endeavour to prevent or minimise the development of these syndromes in the post-COVID-19 era.
Supplementary Material
Acknowledgements:
We would like to show appreciation to American Journal Experts for English editing.
Contributor Information
Chien-Chou Su, Clinical Innovation and Research Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yi-Ching Yang, Department of Geriatrics and Gerontology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; School of Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Department of Family Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yu-Huai Yu, Clinical Innovation and Research Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yu-Hsuan Tsai, Clinical Innovation and Research Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Deng-Chi Yang, Department of Geriatrics and Gerontology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; School of Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Declaration of Conflicts of Interest:
None declared.
Declaration of Sources of Funding
Funding for this study was provided by the National Cheng Kung University Hospital (intramural grant: NCKUH-V101-10). The sponsors had no involvements in the study results.
References
- 1. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021; 594: 259–64. [DOI] [PubMed] [Google Scholar]
- 2. Madjid M, Safavi-Naeini P, Solomon SDet al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020; 5: 831–40. [DOI] [PubMed] [Google Scholar]
- 3. Wang W, Wang CY, Wang SIet al. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine 2022; 53: 101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taquet M, Sillett R, Zhu Let al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1284437 patients. Lancet Psychiatry 2022; 9: 815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu YH, Chen Y, Wang QHet al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol 2022; 79: 509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aprahamian I, Cesari M. Geriatric syndromes and SARS-Cov-2: more than just being old. J Frailty Aging 2020; 9: 127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurmaev DP, Bulgakova SV, Treneva EVet al. Frailty, sarcopenia and COVID-19 in geriatric patients. Adv Gerontol 2022; 35: 726–36. [PubMed] [Google Scholar]
- 8. Tosato M, Carfì A, Martis Iet al. Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc 2021; 22: 1840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuper H, Shakespeare T. Are older people with disabilities neglected in the COVID-19 pandemic? Lancet Public Health 2021; 6: e347–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen LK. COVID-19 vaccination and frailty in older adults. Arch Gerontol Geriatr 2021; 96: 104487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dumitrascu F, Branje KE, Hladkowicz ESet al. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J Am Geriatr Soc 2021; 69: 2419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim WS, Liang CK, Assantachai Pet al. COVID-19 and older people in Asia: Asian working group for sarcopenia calls to actions. Geriatr Gerontol Int 2020; 20: 547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reuben D. Geriatric syndrome. In: Beck AC, ed. Geriatrics Review Syllabus, 2nd edition. New York: American Geriatrics Society, 1991; 117–231. [Google Scholar]
- 14. Yang DC, Lee JD, Huang CCet al. Association between multiple geriatric syndromes and life satisfaction in community-dwelling older adults: a nationwide study in Taiwan. Arch Gerontol Geriatr 2015; 60: 437–42. [DOI] [PubMed] [Google Scholar]
- 15. Huang CC, Lee JD, Yang DCet al. Associations between geriatric syndromes and mortality in community-dwelling elderly: results of a national longitudinal study in Taiwan. J Am Med Dir Assoc 2017; 18: 246–51. [DOI] [PubMed] [Google Scholar]
- 16. Huang C, Huang L, Wang Yet al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 2022; 376: e068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El-Bagalaty AE, Mohamed ME, Abdelraouf ORet al. Balance and fall risk assessment in community-dwelling older adults after recovery from COVID-19: a cross-sectional study. Sports 2023; 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morandi A, Gual N, Cesari Met al. Geriatric syndromes and functions in older adults with COVID-19 hospitalized in sub-acute care: a multicenter study. Aging Clin Exp Res 2023; 35: 221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Couture S, Lepage MA, Godard-Sebillotte Cet al. Geriatric syndromes in older adults hospitalized with COVID-19 in Montreal. Canada Can Geriatr J 2022; 25: 269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang R, Yen-Ting Chen T, Wang SIet al. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine 2023; 56: 101783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim Y, Lee E. The association between elderly people's sedentary behaviors and their health-related quality of life: focusing on comparing the young-old and the old-old. Health Qual Life Outcomes 2019; 17: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Z, Zhang Z, Zhang Zet al. Cognitive impairment after long COVID-19: current evidence and perspectives. Front Neurol 2023; 14: 1239182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Möller M, Borg K, Janson Cet al. Cognitive dysfunction in post-COVID-19 condition: mechanisms, management, and rehabilitation. J Intern Med 2023; 294: 563–81. [DOI] [PubMed] [Google Scholar]
- 25. Quan M, Wang X, Gong Met al. Post-COVID cognitive dysfunction: current status and research recommendations for high risk population. Lancet Reg Health West Pac 2023; 38: 100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu TH, Huang PY, Wu JYet al. Real-world data analysis of post-COVID-19 condition risk in older patients. Age Ageing 2023; 52: afad204. [DOI] [PubMed] [Google Scholar]
- 27. Mohammadkhanizadeh A, Nikbakht F. Investigating the potential mechanisms of depression induced-by COVID-19 infection in patients. J Clin Neurosci 2021; 91: 283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazza MG, Palladini M, Poletti Set al. Post-COVID-19 depressive symptoms: epidemiology, pathophysiology, and pharmacological treatment. CNS Drugs 2022; 36: 681–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu JN, Wu BB, Feng LPet al. COVID-19 related pressure injuries in patients and personnel: a systematic review. J Tissue Viability 2021; 30: 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sianes-Gallén M, Pujol-García AM, Rus García Met al. Pressure injuries during the SARS-CoV-2 pandemic: a retrospective, case-control study. J Tissue Viability 2021; 30: 478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ormseth CH, LaHue SC, Oldham MAet al. Predisposing and precipitating factors associated with delirium: a systematic review. JAMA Netw Open 2023; 6: e2249950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson JE, Mart MF, Cunningham Cet al. Delirium. Delirium Nat Rev Dis Primers 2020; 6: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pilotto A, Custodero C, Palmer Ket al. A multidimensional approach to older patients during COVID-19 pandemic: a position paper of the special interest group on comprehensive geriatric assessment of the European geriatric medicine society (EuGMS). Eur Geriatr Med 2023; 14: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matthews DA. Dr. Marjory Warren and the origin of British geriatrics. J Am Geriatr Soc 1984; 32: 253–8. [DOI] [PubMed] [Google Scholar]
- 35. Ellis G, Gardner M, Tsiachristas Aet al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017; 2017: Cd006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang CK, Lee WJ, Peng LNet al. COVID-19 vaccines in older adults: challenges in vaccine development and policy making. Clin Geriatr Med 2022; 38: 605–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.