Abstract
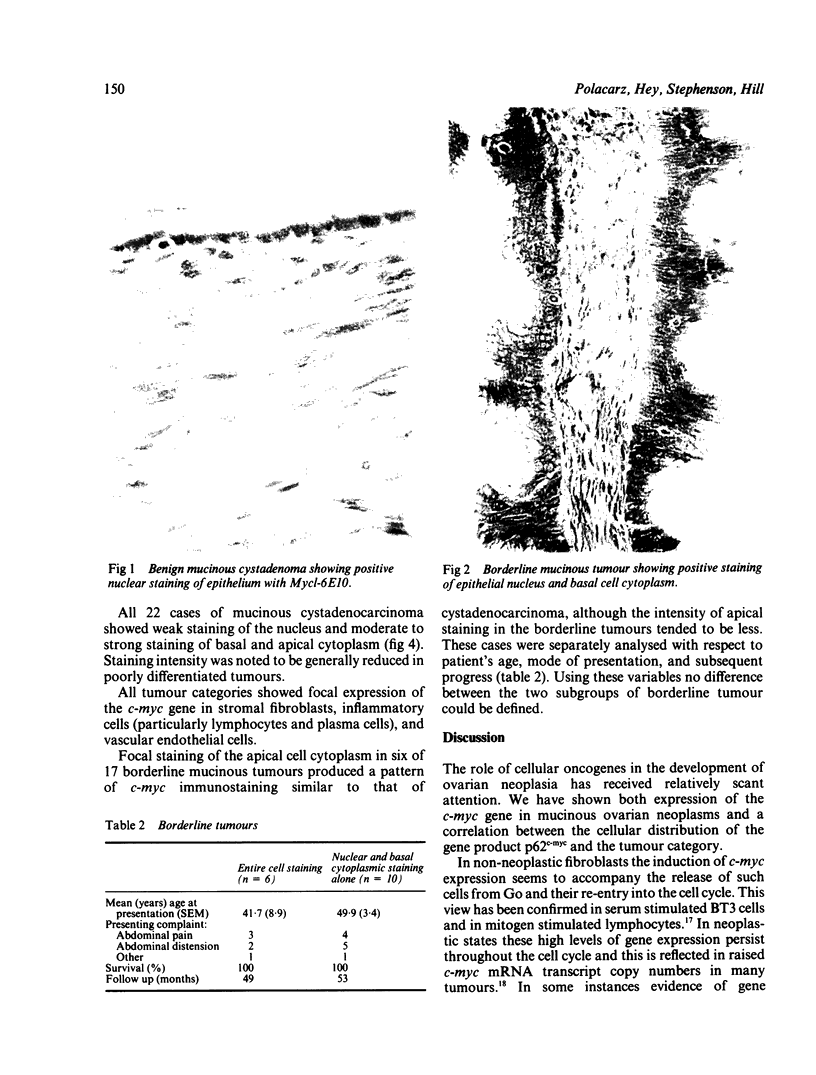
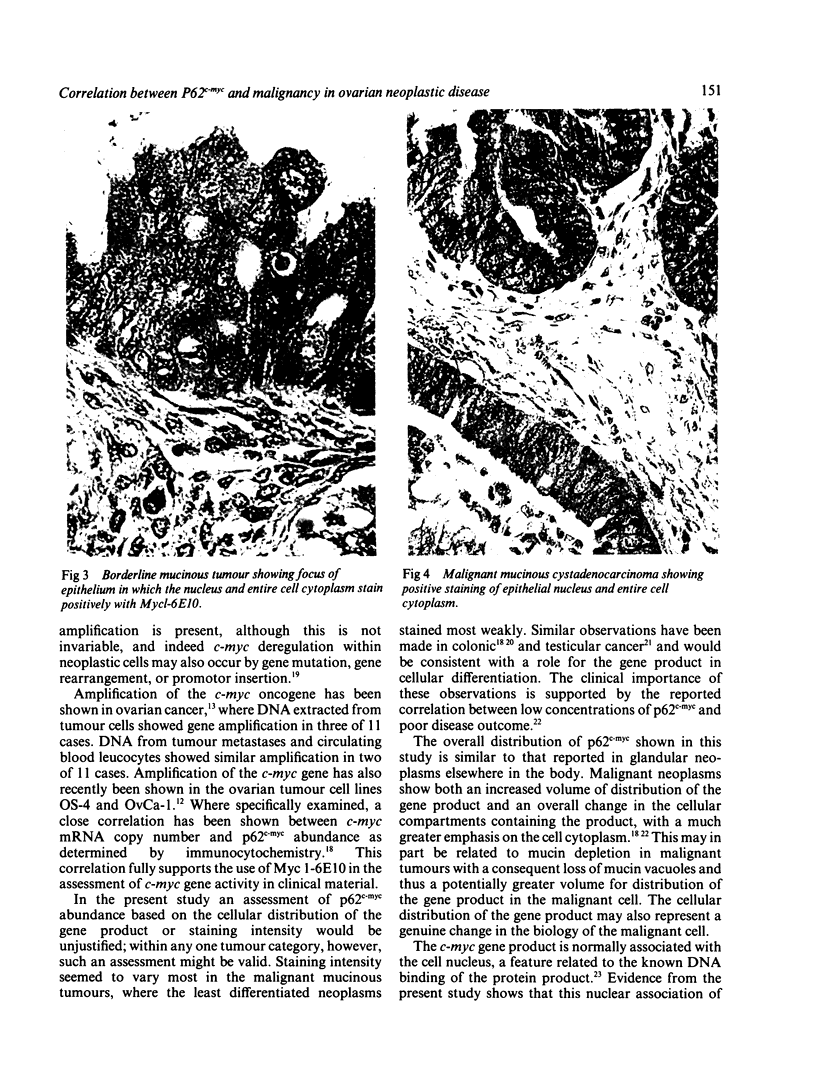
The monoclonal antibody Myc 1-6E10 was used to determine the cellular distribution of the c-myc oncogene product p62c-myc in 60 mucinous ovarian tumours. Three patterns of immunostaining were apparent: (i) nuclear staining alone; (ii) staining of the nucleus and basal cytoplasm; and (iii) staining of the entire cell. Of the 21 cases of mucinous cystadenoma, 11 showed nuclear staining alone, and a further case showed additional weak staining of the basal cytoplasm. Nuclear staining alone was not present in any of the 17 borderline mucinous tumours examined. Strong staining of the nucleus and basal cytoplasm was seen in 16 of these borderline cases, six of which also showed focal staining of the apical cytoplasm. All 22 cases of mucinous cystadenocarcinoma showed staining of the cell nucleus and entire cell cytoplasm. Focal staining of the apical cytoplasm in six of 17 borderline mucinous tumours produced a pattern of c-myc immunostaining similar to that of cystadenocarcinoma. Retrospective analysis of the clinical data showed that no significant differences between patients with borderline tumours of these two categories could be defined. Although immunostaining with Myc 1-6E10 can be used in the categorisation of mucinous ovarian tumours, it is concluded that standard histological criteria are more accurate indicators of tumour behaviour than is an assessment of c-myc expression.
Full text
PDF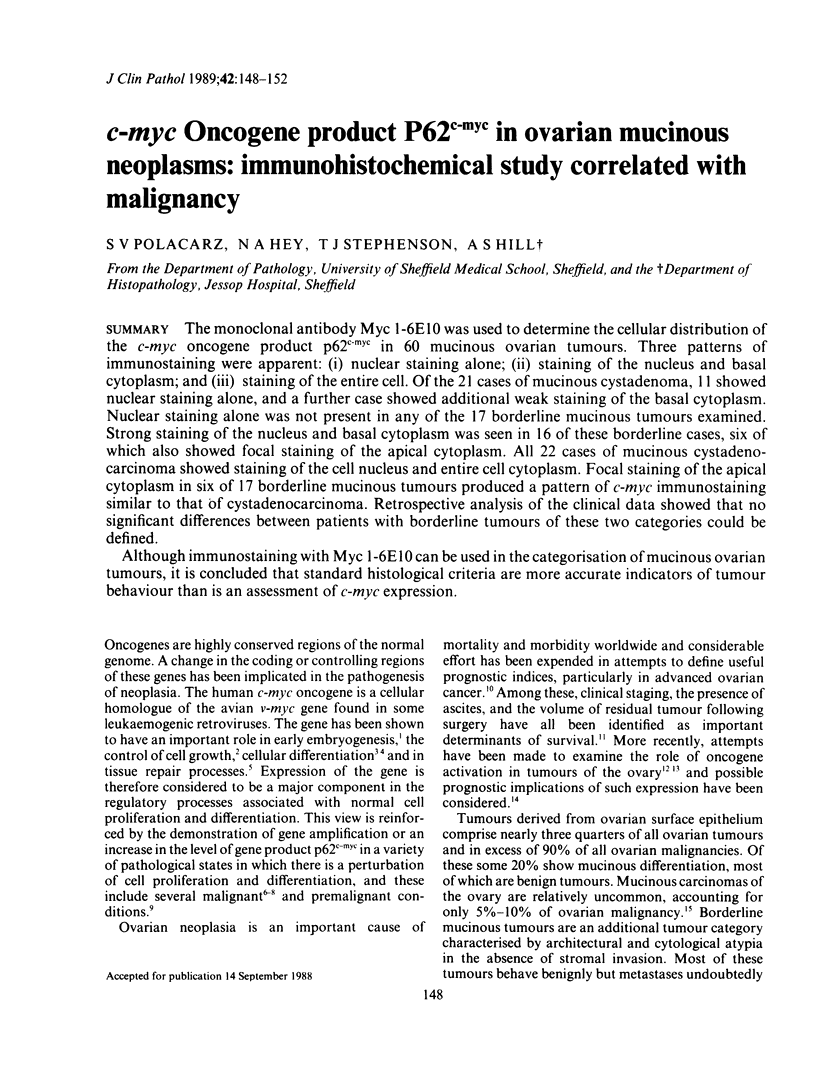


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ciclitira P. J., Macartney J. C., Evan G. Expression of c-myc in non-malignant and pre-malignant gastrointestinal disorders. J Pathol. 1987 Apr;151(4):293–296. doi: 10.1002/path.1711510409. [DOI] [PubMed] [Google Scholar]
- Ciclitira P. J., Stewart J., Evan G., Wight D. G., Sikora K. Expression of c-myc oncogene in coeliac disease. J Clin Pathol. 1987 Mar;40(3):307–311. doi: 10.1136/jcp.40.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Litwin S., Keidan R. D., Comis R. L., Astrin S. M. Noncorrelation of the expression of the c-myc oncogene in colorectal carcinoma with recurrence of disease or patient survival. Cancer Res. 1988 Mar 1;48(5):1350–1355. [PubMed] [Google Scholar]
- Evan G. I., Hancock D. C. Studies on the interaction of the human c-myc protein with cell nuclei: p62c-myc as a member of a discrete subset of nuclear proteins. Cell. 1985 Nov;43(1):253–261. doi: 10.1016/0092-8674(85)90030-3. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Hart W. R., Norris H. J. Borderline and malignant mucinous tumors of the ovary. Histologic criteria and clinical behavior. Cancer. 1973 May;31(5):1031–1045. doi: 10.1002/1097-0142(197305)31:5<1031::aid-cncr2820310501>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Koda T., Matsushima S., Sasaki A., Danjo Y., Kakinuma M. c-myc Gene amplification in primary stomach cancer. Jpn J Cancer Res. 1985 Jul;76(7):551–554. [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Neijt J. P., ten Bokkel Huinink W. W., van der Burg M. E., van Oosterom A. T., Vriesendorp R., Kooyman C. D., van Lindert A. C., Hamerlynck J. V., van Lent M., van Houwelingen J. C. Randomised trial comparing two combination chemotherapy regimens (Hexa-CAF vs CHAP-5) in advanced ovarian carcinoma. Lancet. 1984 Sep 15;2(8403):594–600. doi: 10.1016/s0140-6736(84)90594-4. [DOI] [PubMed] [Google Scholar]
- Pfeifer-Ohlsson S., Goustin A. S., Rydnert J., Wahlström T., Bjersing L., Stehelin D., Ohlsson R. Spatial and temporal pattern of cellular myc oncogene expression in developing human placenta: implications for embryonic cell proliferation. Cell. 1984 Sep;38(2):585–596. doi: 10.1016/0092-8674(84)90513-0. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Riou G., Barrois M., Lê M. G., George M., Le Doussal V., Haie C. C-myc proto-oncogene expression and prognosis in early carcinoma of the uterine cervix. Lancet. 1987 Apr 4;1(8536):761–763. doi: 10.1016/s0140-6736(87)92795-4. [DOI] [PubMed] [Google Scholar]
- Rodenburg C. J., Koelma I. A., Nap M., Fleuren G. J. Immunohistochemical detection of the ras oncogene product p21 in advanced ovarian cancer. Lack of correlation with clinical outcome. Arch Pathol Lab Med. 1988 Feb;112(2):151–154. [PubMed] [Google Scholar]
- Sikora K., Chan S., Evan G., Gabra H., Markham N., Stewart J., Watson J. c-myc oncogene expression in colorectal cancer. Cancer. 1987 Apr 1;59(7):1289–1295. doi: 10.1002/1097-0142(19870401)59:7<1289::aid-cncr2820590710>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Sikora K., Evan G., Stewart J., Watson J. V. Detection of the c-myc oncogene product in testicular cancer. Br J Cancer. 1985 Aug;52(2):171–176. doi: 10.1038/bjc.1985.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora K., Evan G., Watson J. Oncogenes and germ cell tumours. Int J Androl. 1987 Feb;10(1):57–67. doi: 10.1111/j.1365-2605.1987.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Stewart J., Evan G., Watson J., Sikora K. Detection of the c-myc oncogene product in colonic polyps and carcinomas. Br J Cancer. 1986 Jan;53(1):1–6. doi: 10.1038/bjc.1986.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V., Forgacs I. C., Wight D. G., Wilson B., Evan G. I., Watson J. V. Abnormal distribution of c-myc oncogene product in familial adenomatous polyposis. J Clin Pathol. 1987 Nov;40(11):1274–1281. doi: 10.1136/jcp.40.11.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. V., Stewart J., Evan G. I., Ritson A., Sikora K. The clinical significance of flow cytometric c-myc oncoprotein quantitation in testicular cancer. Br J Cancer. 1986 Mar;53(3):331–337. doi: 10.1038/bjc.1986.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasue H., Takeda A., Ishibashi M. Amplification of the c-myc gene and the elevation of its transcripts in human ovarian tumor lines. Cell Struct Funct. 1987 Feb;12(1):121–125. doi: 10.1247/csf.12.121. [DOI] [PubMed] [Google Scholar]