Abstract
The CC-chemokines RANTES, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β are natural ligands for the CC-chemokine receptor CCR5. MIP-1α, also known as LD78α, has an isoform, LD78β, which was identified as the product of a nonallelic gene. The two isoforms differ in only 3 amino acids. LD78β was recently reported to be a much more potent CCR5 agonist than LD78α and RANTES in inducing intracellular Ca2+ signaling and chemotaxis. CCR5 is expressed by human monocytes/macrophages (M/M) and represents an important coreceptor for macrophage-tropic, CCR5-using (R5) human immunodeficiency virus type 1 (HIV-1) strains to infect the cells. We compared the antiviral activities of LD78β and the other CC-chemokines in M/M. LD78β at 100 ng/ml almost completely blocked HIV-1 replication, while at the same concentration LD78α had only weak antiviral activity. Moreover, when HIV-1 infection in M/M was monitored by a flow cytometric analysis using p24 antigen intracellular staining, LD78β proved to be the most antivirally active of the chemokines. RANTES, once described as the most potent chemokine in inhibiting R5 HIV-1 infection, was found to be considerably less active than LD78β. LD78β strongly downregulated CCR5 expression in M/M, thereby explaining its potent antiviral activity.
Macrophage inflammatory protein 1α (MIP-1α) exists in two nonallelic isoforms, LD78α and LD78β, with high-level sequence homology. The secreted proteins differ in only 3 amino acids: the penultimate NH2-terminal residue and amino acids 39 and 47 (10, 21, 23). The biological relevance, also in terms of antiviral activity, of the NH2-terminal residues of CXC- and CC-chemokines has been convincingly demonstrated (26, 27, 29, 31–33, 42). Besides MIP-1α, the CC-chemokines RANTES and MIP-1β are natural ligands for the CC-chemokine receptor CCR5 and are inhibitors of macrophage-tropic (M-tropic) human immunodeficiency virus (HIV) strains (7).
LD78β was reported to be much more potent than LD78α and RANTES in inducing intracellular Ca2+ signaling and chemotaxis preferentially through the CC-chemokine receptor CCR5 (20, 22, 43). In these studies, the anti-HIV activity of LD78β in peripheral blood mononuclear cells (PBMCs) was investigated (20), however, its activity in human monocytes/macrophages (M/M) had not been determined.
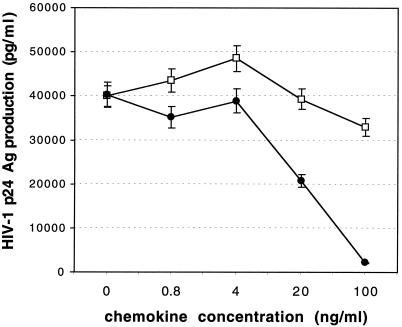
The chemokine receptor CCR5 is expressed by M/M and represents the most important coreceptor for M-tropic R5 HIV type 1 (HIV-1) strains to enter the cells (1, 13, 14, 34, 36, 39–41). Macrophages may play an important role in all phases of HIV infection. Infected macrophages are present in all body tissues of HIV patients (12, 17–19) and represent the most important cellular reservoir for the virus during antiviral therapy (4, 24, 30). In fact, M/M secreting nerve growth factor survive after HIV infection (9) and produce high and stable levels of virus for a long period of time (S. Aquaro, T. Guenci, P. Bagnarelli, M. Clementi, A, Modesti, R. Caliò, and C. F. Perno, 4th Intl. Workshop HIV, Cells of Macrophages Lineage, and Other Reservoirs, p. 29, 1999). In the central nervous system, more then 90% of the HIV-1-infected cells are M/M (8, 12, 15, 37), and CCR5 Δ32 heterozygosity prevents the development of the AIDS dementia complex (38). At the same time, the downregulation of CCR5 expression by CC-chemokines in macrophages is correlated with a reduction of virus entry and replication (11). These data demonstrate the relevance of CCR5 and thus the important role of CC-chemokines in reducing HIV entry and hence virus replication through their interaction with CCR5. Here, we have studied the antiviral efficacy of LD78β, in comparison with those of LD78α and the other CCR5-interacting CC-chemokines, RANTES and MIP-1β, in purified macrophages. To evaluate the antiviral activities of LD78α and LD78β, M/M were incubated with the chemokines for 20 min at different concentrations and then infected by the R5 HIV-1BaL strain. LD78β showed a potent dose-dependent inhibition and antiviral activity against HIV-1BaL. As shown in Fig. 1, at an LD78β concentration of 100 ng/ml, viral p24 antigen (Ag) production dropped from 40,300 pg/ml to 2,070 pg/ml (94% inhibition). In contrast, LD78α only weakly inhibited viral replication at a concentration of 100 ng/ml (roughly 20% inhibition) (Fig. 1). Thus, the antiviral activity of LD78β in M/M was far superior to that of LD78α.
FIG. 1.
Dose-dependent antiviral activity of LD78β in HIV-1-infected M/M. Macrophages were obtained from the blood of healthy HIV-seronegative donors by previously published procedures (25). Briefly, PBMCs were separated by Ficoll-Hypaque gradient centrifugation and seeded in plastic 48-well plates (Costar, Cambridge, Mass.) at a density of 1.8 × 106 cells/ml in RPMI 1640 (Gibco, Gaithersburg, Md.) supplemented with 50 U of penicillin/ml, 50 μg of streptomycin/ml, 2 mM l-glutamine, and 20% heat-inactivated, mycoplasma- and endotoxin-free fetal calf serum (HyClone, Logan, Utah) (complete medium). On the 5th day of culture, nonadherent cells were removed by repeated gentle washing with warm complete medium. Adherent cells obtained with this technique consisted of >95% differentiated M/M. After purification, M/M were cultured in a humidified chamber with 5% CO2 at 37°C in the presence of the same medium. Further details are described elsewhere (25). Macrophages were exposed to various concentrations of CC-chemokine LD78β (●) or LD78α (□) for 20 min; then they were challenged with HIV-1BaL at 300 50% cell culture infective doses per ml. After 2 h of incubation, M/M were extensively washed with warm complete medium to remove the excess virus and then cultured in the presence of chemokines under the conditions used previously. M/M were washed and fed every 5 days with fresh medium and replenished with chemokines. Supernatants were collected at day 12 after virus challenge, and virus production was determined by Ag capture assay with a commercially available p24 Ag kit (NEN Life Science Products Inc., Boston, Mass.). Human recombinant MIP-1α isoform LD78α was purchased from PeproTech Inc. (Rocky Hill, N.J.). The 7.793-kDa LD78β was synthesized by 9-fluorenylmethoxycarbonyl (fMOC) solid-phase peptide synthesis (20). Data represent the means of values from two independent experiments, each run in triplicate. Error bars show the standard deviations.
The antiviral activities of LD78α and LD78β were also evaluated by intracellular p24 Ag staining to determine the percentage of HIV-1-infected M/M. As can be seen in Fig. 2, 42% of the cells from HIV-infected M/M cultures stained positive for p24 Ag, whereas the level decreased to about 11% in the LD78β-treated cells (at 100 ng/ml). In contrast, no difference in numbers of p24 Ag-positive cells was observed between the untreated HIV-infected and LD78α-treated HIV-infected cells (Fig. 2).
FIG. 2.
Intracellular p24 Ag detection in M/M. At day 14 after infection, M/M were carefully washed with cold phosphate-buffered saline to remove excess virus and were detached by using 1 mM EDTA for 5 min followed by gentle scraping. The percentage of HIV-infected M/M was determined by intracellular staining for p24 Ag, using the fluorescein isothiocyanate (FITC)-conjugated anti-p24 mAb KC-57-FITC (Coulter, Hialeah, Fla.). Cells were analyzed by using a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). At a concentration of 100 mg of LD78β/ml, viral p24 Ag expression was strongly inhibited (D), whereas at the same concentration, LD78α showed no antiviral activity (C). (B) HIV-1BaL-infected M/M; (A) mock-infected M/M. The results of one representative experiment of three independent experiments are shown.
To compare the anti-HIV efficacy of LD78β with those of the other CCR5-binding chemokines, RANTES and MIP-1β, additional experiments were performed in M/M. The MIP-1α isoform LD78β exhibited the highest antiviral activity against HIV-1BaL. RANTES reached 92% inhibition of HIV replication at a concentration of 500 ng/ml, while LD78β suppressed HIV replication by 93% at 100 ng/ml (i.e., at a fivefold-lower concentration than RANTES); moreover, MIP-1β, considered the most specific CCR5 ligand, inhibited virus replication by about 30% at 500 ng/ml (data not shown). Therefore, as shown in Table 1, the 50% effective concentration (EC50) of LD78β against HIV-1BaL in M/M was 21 ng/ml, which is 16-fold lower than that of LD78α (EC50, 351 ng/ml). Also, a more than 10-fold difference in the EC90s of LD78α and LD78β was observed (Table 1). RANTES, with an EC50 and an EC90 of 149 and 478 ng/ml, respectively (Table 1), was six- to sevenfold less active than LD78β. With an EC50 of almost 1,000 ng/ml (Table 1), MIP-1β was found to be the least potent chemokine in inhibiting viral replication.
TABLE 1.
Antiviral activity of LD78β, LD78α, RANTES, and MIP-1β against HIV-1BaL strain and a clinical R5 HIV-1 isolate in macrophagesa
| Chemokine | Effective concn (ng/ml) against:
|
|||
|---|---|---|---|---|
| HIV-1BaL
|
HIV-1#15
|
|||
| EC50 | EC90 | EC50 | EC90 | |
| LD78β | 21 | 78 | 28 | 87 |
| LD78α | 351 | 980 | 118 | 476 |
| RANTES | 149 | 478 | 58 | 406 |
| MIP-1β | 980 | >1,000 | 480 | >1,000 |
Macrophages were isolated, infected, and treated with chemokines as described in the legend to Fig. 1. Briefly, M/M were exposed for 20 min to different concentrations of LD78α, LD78β, RANTES, or MIP-1β before infection with HIV-1BaL or HIV-1 isolate 15. After 2 h of incubation, M/M were extensively washed with warm medium to remove excess virus and then cultured in the presence of different concentrations of chemokines. Every 5 days, M/M were washed and fed with fresh medium and replenished with chemokines. Supernatants were collected on day 14 after infection, and HIV p24 Ag production was assessed. The antiviral activity was determined as the percentage of virus inhibition compared with that of untreated controls. LD78α, RANTES, and MIP-1β were obtained from Peprotech Inc. LD78β was synthesized as described elsewhere (20). Data represent the means of values from two independent experiments, each run in triplicate.
To confirm that LD78β has potent antiviral activity against M-tropic HIV strains, and not only against the cell culture-adapted virus strain HIV-1BaL, we performed additional experiments with primary R5 HIV-1 clinical isolate (HIV-1 isolate 15). This virus isolate was obtained after only one passage in PBMCs and replicated in U87.CD4.CCR5-transfected cells but not in U87.CD4.CXCR4 cells, confirming its CCR5 usage (data not shown). Here, again, the chemokine LD78β was the most active in inhibiting viral replication, with an EC50 of 28 ng/ml (Table 1). The other chemokines were somewhat more active against this clinical viral isolate than against HIV-1BaL (Table 1).
We demonstrated previously that the antiviral activity of a compound that inhibits virus entry in fresh monocytes (such as the sulfated polysaccharide dextran sulfate or the bicyclam AMD3100) is different from that in macrophages (3). Therefore, we also assessed the anti-HIV efficacy of LD78β in freshly isolated monocytes. At a concentration of 100 ng/ml, LD78β inhibited HIV replication by 85% (EC50, 35 ng/ml). In sharp contrast, at a concentration of 100 ng/ml, RANTES had no antiviral activity in fresh monocytes (data not shown). It has been previously reported that RANTES has no or only weak activity against HIV-1 in freshly isolated monocytes (28, 31). A likely explanation for this phenomenon is that only the NH2-terminally truncated form of RANTES has anti-HIV activity and that monocytes express very low, or undetectable, levels of CD26/dipeptidyl peptidase IV, which is responsible for NH2-terminal truncation of RANTES (29).
Because previous studies demonstrated that downregulation of HIV coreceptors by their natural ligands contribute to the inhibition of viral replication (2, 16), we examined the efficiency of LD78β at downregulating CCR5. As shown in Fig. 3, expression of CCR5 from the surface of monocytes is shown for LD78β, in comparison with LD78α and MIP-1β. LD78β was much more effective (after 1 h of incubation at 37°C) than LD78α or MIP-1β at downregulating CCR5; it showed a marked downregulation at 40 ng/ml, whereas for LD78α and MIP-1β a weak effect was observed only at a concentration of 200 ng/ml. This enhanced potency of LD78β in receptor binding and downregulation may explain its potent anti-HIV activity and is probably due to its greater affinity for CCR5 (20).
FIG. 3.
Downregulation of CCR5 from the surfaces of monocytes by LD78β, LD78α, and MIP-1β. PBMCs were suspended in complete medium and seeded in petri dishes at a concentration of 1.5 × 106 cells/cm2. After 2 h of incubation, all nonadherent cells were removed by gentle washing with warm medium. Adherent cells were scraped from the plates, counted, and suspended in complete medium at a concentration of 2 × 105 ml. After this purification step, more then 97% of the cells were monocytes, as determined by CD14 staining. Monocytes were incubated with LD78β (40 ng/ml) (B), LD78α (200 ng/ml) (C), or MIP-1β (200 ng/ml) (D) for 1 h at 37°C. In panel A, the cells were incubated with medium alone. Surface CCR5 was detected with CCR5 monoclonal antibody clone 2D7 (PharMingen, San Diego, Calif.) and analyzed by flow cytometry.
This study shows that the MIP-1α isoform LD78β is the most potent CC-chemokine described so far in terms of inhibiting R5 HIV-1 infection of macrophages and monocytes. The inhibitory effect of LD78β on viral replication may be ascribed to its high affinity for CCR5 and the subsequent downregulation of this coreceptor. The potent anti-HIV-1 activity of LD78β compared with that of LD78α is conferred by differences in only 3 amino acids (20), with the NH2-terminal dipeptide of LD78α seemingly important for receptor affinity (33).
Our findings that RANTES and MIP-1α/LD78α have EC50s of about 60 and 120 ng/ml, respectively, are in agreement with previously published data (35). The previously reported order for the anti-HIV activities of the CC-chemokines in M/M, RANTES > MIP-1β > MIP-1α/LD78α (5), should be changed to the following order: MIP-1α/LD78β > RANTES > MIP-1α/LD78α > MIP-1β. Further experiments are required to determine if MIP-1α/LD78α is consistently more potent than MIP-1β in inhibiting HIV-1 replication in M/M. The superior anti-HIV-1 activity of LD78β has to be interpreted in the light of the isolation from cultured T cells of MIP-1α, MIP-1β, and RANTES as suppressors of HIV-1 infection (7). Increased production of the CC-chemokines MIP-1α, MIP-1β, and RANTES in repeatedly HIV-1-exposed subjects is correlated with protection against HIV-1 infection; MIP-1α appears sooner and attains higher concentrations than MIP-1β and RANTES (6, 44). These clinical data on natural resistance to HIV-1 infection, not linked to a deletion mutation in the CCR5 gene, are in agreement with the higher antiviral potency of the LD78β isoform of MIP-1α, as previously shown in PBMCs (20, 22) and here confirmed for M/M.
Acknowledgments
We thank Sandra Claes and Erik Fonteyn for excellent technical assistance.
This work was supported by grants from the Fonds voor Wetenschappelijk Onderzoek (FWO)—Vlaanderen (Krediet no. G.0104.98) and the Geconcerteerde Onderzoeksacties (Vlaamse Gemeenschap) (Krediet no. 00/12). S.A. was supported by a grant from Istituto Superiore di Sanità, Rome, Italy.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Amara A, Le Gall S, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aquaro S, Perno C-F, Balestra E, Balzarini J, Cenci A, Francesconi M, Panti S, Serra F, Villani N, Caliò R. Inhibition of replication of HIV in primary monocyte/macrophages by different antiviral drugs and comparative efficacy in lymphocytes. J Leukoc Biol. 1997;62:54–59. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- 4.Aquaro S, Balestra E, Cenci A, Francesconi M, Caliò R, Perno C F. HIV infection in macrophage: role of long-lived cells and related therapeutical strategies. J Biol Regul Homeost Agents. 1997;11:69–73. [PubMed] [Google Scholar]
- 5.Capobianchi M R, Abbate I, Antonelli G, Turriziani O, Dolei A, Dianzani F. Inhibition of HIV type 1 BaL replication by MIP-1α, MIP-1β, and RANTES in macrophages. AIDS Res Hum Retrovir. 1998;14:233–240. doi: 10.1089/aid.1998.14.233. [DOI] [PubMed] [Google Scholar]
- 6.Casoli C, Vicenzi E, Cimarelli A, Magnani G, Ciancianaini P, Cattaneo E, Dall'Aglio P, Poli G, Bertazzoni U. HTLV-II down-regulates HIV-1 replication in IL-2-stimulated primary PBMC of coinfected individuals through expression of MIP-1α. Blood. 2000;95:2760–2769. [PubMed] [Google Scholar]
- 7.Cocchi F, De Vico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β, major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Gabuzda D H, Ho D D, de la Monte S M, Hirsch M S, Rota T R, Sobel R A. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- 9.Garaci E, Caroleo M C, Aloe L, Aquaro S, Piacentin M, Costa N, Amendola A, Micera A, Calio R, Perno C F, Levi-Montalcini R. Nerve growth factor is an autocrine factor essential for the survival of macrophages infected with HIV. Proc Natl Acad Sci USA. 1999;96:14013–14018. doi: 10.1073/pnas.96.24.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irving S G, Zipfel P F, Balke J, McBride O W, Morton C C, Burd P R, Siebenlist U, Kelly K. Two inflammatory mediator cytokine genes are closely linked and variably amplified on chromosome 17q. Nucleic Acids Res. 1990;18:3261–3270. doi: 10.1093/nar/18.11.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y, Jolly P E. Effect of β-chemokines on human immunodeficiency virus type 1 replication, binding, uncoating, and CCR5 receptor expression in human monocyte-derived macrophages. J Hum Virol. 1999;2:123–132. [PubMed] [Google Scholar]
- 12.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 13.Kutza J, Crim L, Feldman S, Hayes M P, Gruber M, Beeler J, Clouse K A. Macrophage colony-stimulating factor antagonists inhibit replication of HIV-1 in human macrophages. J Immunol. 2000;164:4955–4960. doi: 10.4049/jimmunol.164.9.4955. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Juarez J, Alali M, Dwyer D, Collman R, Cunningham A, Naif H M. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol. 1999;73:9741–9755. doi: 10.1128/jvi.73.12.9741-9755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipton S A, Gendelman H E. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 16.Mack M, Luckow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N C, Schlöndorff D, Proudfoot A E I. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattern C F, Murray K, Jensen A, Farzadegan H, Pang J, Modlin J F. Localization of human immunodeficiency virus core antigen in term human placentas. Pediatrics. 1992;89:207–209. [PubMed] [Google Scholar]
- 18.McElrath M J, Pruett J E, Cohn Z A. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci USA. 1989;86:675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meltzer M S, Nakamura M, Hansen B D, Turpin J A, Kalter D C, Gendelman H E. Macrophages as susceptible targets for HIV infection, persistent viral reservoirs in tissue, and key immunoregulatory cells that control levels of virus replication and extent of disease. AIDS Res Hum Retrovir. 1990;6:967–971. doi: 10.1089/aid.1990.6.967. [DOI] [PubMed] [Google Scholar]
- 20.Menten P, Struyf S, Schutyser E, Wuyts A, De Clercq E, Schols D, Proost P, Van Damme J. The LD78β isoform of MIP-1α is the most potent CCR5 agonist and HIV-1-inhibiting chemokine. J Clin Investig. 1999;104:R1–R5. doi: 10.1172/JCI7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakao M, Nomiyama H, Shimada K. Structures of human genes coding for cytokine LD78 and their expression. Mol Cell Biol. 1990;10:3646–3658. doi: 10.1128/mcb.10.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nibbs R J, Yang J, Landau N R, Mao J H, Graham G J. LD78β, a nonallelic variant of human MIP-1α (LD78α), has enhanced receptor interactions and potent HIV suppressive activity. J Biol Chem. 1999;274:17478–17483. doi: 10.1074/jbc.274.25.17478. [DOI] [PubMed] [Google Scholar]
- 23.Obaru K, Fukuda M, Maeda S, Shimada K. A cDNA clone used to study mRNA inducible in human tonsillar lymphocytes by a tumor promoter. J Biochem. 1986;99:885–894. doi: 10.1093/oxfordjournals.jbchem.a135549. [DOI] [PubMed] [Google Scholar]
- 24.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 25.Perno C F, Yarchoan R. Culture of HIV in monocytes and macrophages. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1993. pp. 12.4.1–12.4.11. [Google Scholar]
- 26.Proost P, De Meester I, Schols D, Struyf S, Lambeir A M, Wuyts A, Opdenakker G, De Clercq E, Scharpé S, Van Damme J. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1 infection. J Biol Chem. 1998;273:7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 27.Proost P, Struyf S, Schols D, Durinx C, Wuyts A, Lenaerts J P, De Meester I, Van Damme J. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor 1α. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 28.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 29.Schols D, Proost P, Struyf S, Wuyts A, De Meester I, Scharpé S, Van Damme J, De Clercq E. CD26-processed RANTES(3–68), but not intact RANTES, has potent anti-HIV-1 activity. Antivir Res. 1998;39:175–187. doi: 10.1016/s0166-3542(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 30.Sharkey M E, Teo I, Greenough T, Sharova N, Luzuriaga K, Sullivan J L, Bucy R P, Kostrikis L G, Haase A, Veryard C, Davaro R E, Cheeseman S H, Daly J S, Bova C, Ellison R T, Mady B, Lai K K, Moyle G, Nelson M, Gazzard B, Shaunak S, Stevenson M. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 32.Struyf S, De Meester I, Scharpé S, Lenaerts J P, Menten P, Wang J M, Proost P, Van Damme J. Natural truncation of RANTES abolishes signaling through the CC chemokine receptors CCR1 and CCR3, impairs its chemotactic potency and generates a CC chemokine inhibitor. Eur J Immunol. 1998;28:1262–1271. doi: 10.1002/(SICI)1521-4141(199804)28:04<1262::AID-IMMU1262>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 33.Struyf S, Proost P, Sozzani S, Mantovani A, Wuyts A, De Clercq E, Schols D, Van Damme J. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 34.Trkola A, Gordon C, Matthews J, Maxwell E, Ketas T, Czaplewski L, Proudfoot A E I, Moore J P. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J Virol. 1999;73:6370–6379. doi: 10.1128/jvi.73.8.6370-6379.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuttle D L, Harrison J K, Anders C, Sleasman J W, Goodenow M M. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72:4962–4969. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyor W R, Power C, Gendelman H E, Markham R B. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci USA. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rij R P, Portegies P, Hallaby T, Lange J M, Visser J, Husman A M, van't Wout A B, Schuitemaker H. Reduced prevalence of the CCR5 Δ32 heterozygous genotype in human immunodeficiency virus-infected individuals with AIDS dementia complex. J Infect Dis. 1999;180:854–857. doi: 10.1086/314940. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Roderiquez G, Oravecz T, Norcross M A. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J Virol. 1998;72:7642–7647. doi: 10.1128/jvi.72.9.7642-7647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman D, Rabin R L, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber J M, Fauci A S. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuyts A, Govaerts C, Struyf S, Lenaerts J P, Put W, Conings R, Proost P, Van Damme J. Isolation of the CXC chemokines ENA-78, GRO α and GRO γ from tumor cells and leukocytes reveals NH2-terminal heterogeneity. Functional comparison of different natural isoforms. Eur J Biochem. 1999;260:421–429. doi: 10.1046/j.1432-1327.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 43.Xin X, Shioda T, Kato A, Liu H, Sakai Y, Nagai Y. Enhanced anti-HIV-1 activity of CC-chemokine LD78β, a non-allelic variant of MIP-1α/LD78α. FEBS Lett. 1999;457:219–222. doi: 10.1016/s0014-5793(99)01035-2. [DOI] [PubMed] [Google Scholar]
- 44.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, Feldman M, O'Brien S J, Burny A, Gallo R C. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]