Abstract
Plant pathogens cause a serious menace to food production. The diseases caused by pathogens are estimated to cause a yield loss of about 14.1 %, whereas, in India, up to 26 %. Several plant pathogens like Pythium, Phytophthora, Rhizoctonia, Sclerotinia, Fusarium, and Verticillium can cause 50–75 % yield losses in cereals, cotton, and horticultural crops (fruits, vegetables, and flowers) 10–100 % in pulses, 30–60 % loses in oilseed crops and 40–50 % in plantation crops. Biochar as soil amendment is emerging as an effective environment friendly substitute for fungicides to counter plant pathogens. It has also been reported to induce resistance in plants to combat plant pathogens by activating the two important defense pathways such as salicylic acid, jasmonate/ethylene defense, and triggering the plant's antioxidant enzymatic activities. Biochar promotes soil health and consequently improves the plant health, resulting in reduced incidence of disease. This novel amendment also helps in the priming of expression of genes against foliar fungal pathogen infection. This review paper will summarize the effect of biochar incorporation in the plant disease management as well as on their growth parameters.
Keywords: Biochar, Disease, Pathogen, Management, Host plants
1. Introduction
Biochar, an organic residue formed by the pyrolysis of biomass is utilized in several fields including industrial utilization, bioremediation and as substrate to promote the soil health and plant growth. International Biochar Initiative (IBI) describes biochar as "the solid material obtained from the thermochemical conversion of biomass in an oxygen-limited environment". Biochar is a stable product and can survive in soil for thousands of years and contains ample amount of pyrogenic carbon. The word "biochar" was originated from the Greek word, “bios” (life) and "char" (production of charcoal by biomass carbonisation). Application of biochar, as an amendment in soil is gaining popularity now-a-days as its a source of carbon sequestration, and is an efficient method of utilising waste (industrial and agricultural waste). Biochar is mainly produced from agricultural wastes, animal manures, paper products and its production from agricultural waste is an eco-efficient way of utilising the waste materials (Fig. 1) [[1], [2], [3]]. Biochar incorporation in soil alters the fertility of soil by changing soil properties [4]. It enriches the soil and thus enhances plant growth leading to high crop yield. The impact of biochar on crop yield rely on a number of factors like sources of biochar, processes involved in its production, types of soil in which biochar has to be added and also the types of crop to be planted.
Fig. 1.
Sources, processes and applications of biochar.
The use of biochar dates back to an old era when pre-Columbian Amazonians used agricultural waste materials to produce biochar by smoldering (covering burning biomass with soil) in trenches or pits [[5], [6], [7]].Though their intention was not clear whether they used biochar to enhance soil fertility or productivity or for other purposes. The word terra preta de Indio was used by Europeans for biochar [8,9].The main area of interest in biochar research is its half-life period (100–1000s of years) in the soil after application, thus helping in the sequestration of carbon [6,10]. The first report of the optimistic influence of biochar in minimizing disease incidences such as wheat rust and mildew in other agricultural crops had been discussed, dated back to about 170 years ago [11] and has gained a lot of attention since last decades when researchers have studied the effects of biochar in several patho-systems worldwide [12,13].
Addition of biochar in soil not only helps in the amelioration of pollutants but the porosity of biochar also facilitates sufficient surface area for harbouring microbes such as bacteria and mycorrhizae and enhance their metabolic activities [14]. During the pyrolysis process of organic (lignocellulosic) materials, usually, complex chemical reactions take place involving depolymerization, decarboxylation, aromatization and decarboxylation of O-alkyl carbons, lignin, hemicellulose and cellulose. Besides these, several other volatile organic compounds (furan, terpenes, benzaldehyde, 2,3-butadione, phenol, acetonitrile, pyrazine) are also produced during pyrolysis, and are found to inhibit the action of Fusarium oxysporum and Rhizoctonia solani in solanaceous crops [15,16]. Hui [17] and Liu et al. [3] have found colonization of bacterial populations such as Bacillus pumilus, Streptomyces pseudovenezuelae and Pseudomonas chlororaphis, in the porous structure of animal bone biochar and they have used it to control the seedling disease of tomato caused by Pythium aphanidermatum and Fusarium oxysporum f.sp. lycopersici.
Biochar interacts with various soil microbes and helps in the plant growth by the suppression of plant pathogens. Biochar is an eco-friendly alternative for the management of plant diseases. Biochar incorporation in soil alters the nutrient availability/cycling, root exudates, and soil properties which directly or indirectly affects the plant pathogenic populations in the soil [13,18]. Also, biochar incorporation in soil induces systemic resistance, leads to the activation of active oxygen species, and stress hormone response consequently leads to the resistance of host plants against pathogens [2,19]. Alterations in microbial communities, root exudates, and induction of defense response in host plants also affect the plant parasitic nematode population in the soil. Most of the reviews highlighted the positive sides of biochar application in agri-horticultural operation such as stabilization of soil organic carbon, increased biomass and yield of plants with reduced greenhouse gas emissions. There is only a handful of literature on how biochar application can have multiple effects on the crop. It can contribute in improving, soil health, and plant health. Also, it contributes in protecting the plants from fungal, bacterial and viral diseases [7,20].
Among various cultural practices, soil amendments have been found effective in the suppression of various soil-borne diseases which are otherwise often difficult to manage [1,21].Though soil amendments have many positive effects in the majority of host-pathogen systems but due to the above-mentioned side effects and non-availability of the guidelines regarding the effect of organic amendments on soils and patho-systems, further research is required for their application in particular host-pathogen systems. Keeping this in view, biochar appears to be a recently available tool to combat various soil-borne diseases. Therefore, the main purpose of this review is to a) deal with the plant disease management aspects of biochar, prepared from different feedstocks; b) discuss most probable mechanisms involved in the management; c) highlight the direct influence of biochar in promoting the plant growth and d) elucidate the indirect effect of biochar in inducing the disease resistance in plants.
2. Biochar impacted ecosystem and microbial community dynamics
The use of biochar has been greatly emphasized now-a-days in various sectors due to the several advantages it adds to the environment as discussed elsewhere in the present review and the most significant advantage is that it can reduce the burden of waste materials on the environment. Biochar has been reported to show diverse effects on plant pathogenic microbes [22], although no exact mechanisms were found to discuss these effects [23]. The surface of the biochar has labile soil organic matter which promotes the growth of certain beneficial microbes [13,18].
Biochar alters the microbial diversity in the root zone, and promotes plant growth. Biochar based amendment in soil alters the soil properties and consequently changes the microbial dynamics of soil (Fig. 2). Ren et al. [24] and Chen et al. [25] discussed that biochar modified the fungal community and the change of biodiversity from Ascomycota and Basidiomycota divisions of fungi (which majorly includes plant pathogenic fungi) in biochar untreated soils to Zygomycota, Neocallimastigomycota and Glomeromycota (includes plant growth promoting fungi, which can suppress the disease incidence) as the composition of metabolites in soils treated with biochar were different from that with no biochar, which promoted a certain group of microbes and suppressed the other. Yan et al. [26], conducted a study for 3-years by adding biochar at the rate of 10, 20, and 40 t ha m−2 and observed that significant alteration in the fungal population Zygomycota, Ascomycota, and Basidiomycota phyla were found in majority which was about more than 90 % of the total sequences. In another study application of biochar enhanced the Ascomycota and Basidiomycota populations i.e. Aspergillus (might show antagonistic effect on plant pathogens), Conocybe, and Alternaria spp., but the abundances of Zygomycota i.e. Gibberella and Actinomucor were reduced. Bai et al. [27], conducted a study to see the effects of biochar application in soil for longer durations. They have reported that continuous applications of straw and then straw derived biochar to the field for a period of about 6 years in rice-wheat crop rotation system had resulted in the decreased fungal diversity and change in the soil aggregation capacity. Application of straw with biochar and fertilizer reduced the adverse effect of fertilizer and gave better results in the crop yield.
Fig. 2.
Biochar interaction with soil microbial communities and associated microbial responses.
Increased populations of Sphingomosnas and Pseudomonas (beneficial microbes) was seen in cotton soils which was amended with biochar, but during continuous cropping of 11 years in that soil resulted in the decreased abundances of such bacterial populations [28]. The increase of bacterial population might be due to the increase in soil pH by incorporation of biochar, as pH plays an important role in shifting microbial community in soil while a decrease with continuous cropping might be the result of reduced pH over time. Nguyen et al. [29] observed the differences in the abundances of Cyanobacteria, in freshly incorporated (1 year) and old biochar (9 year) soil. There was a significant increase in the bacterial alpha diversity, Bacillota and Pseudomonadota (non plant pathogenic and may compete with the pathogenic microbes to suppress their growth), while a decrease in the diversity was also observed in the case of Acidobacteria (plant pathogenic bacteria) which might be attributed to the changes in soil properties such as electrical conductivity, pH, extractable organic carbon and extractable nitrogen.
3. Global scenario of biochar for soil-borne and foliar plant diseases management
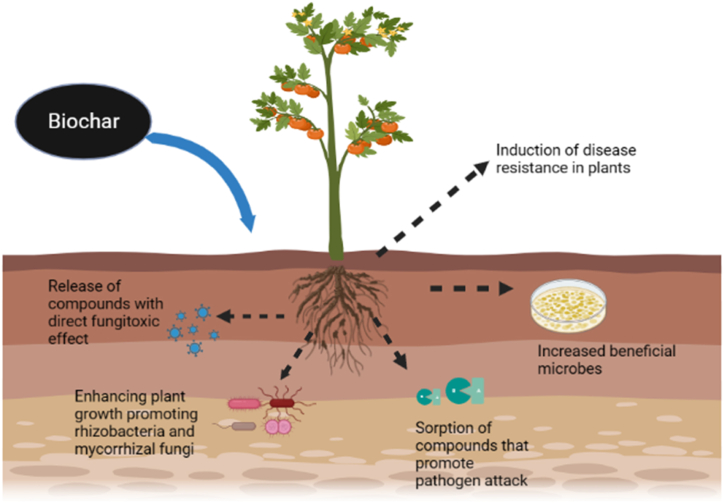
Soil borne pathogens are deadliest plant pathogens; being soil borne in nature their management becomes really difficult. They adversely influence the yield, and quality of many economically important crops around the world. The underground plant parts such as the root system (damping off, root rot, vascular wilt etc.) is severely affected, and consequently their effects can also be seen in the upper plant parts (wilting of foliage, tissue discoloration, root decay and sudden death) [30].Soil borne plant pathogens survive along with the vast variety of soil microbes which may be helpful or injurious for the plants and are highly influenced by the living and non-living factors of the soil, the kind of cultivation practices followed i.e. preparation of fields, fertilizers and manures application and irrigation [31]. Though there are several chemical management methods such as soil disinfestations [32], they have their own disadvantages such as ill effects on the beneficial microbes, increased chemical loads in the soil and high costs of application. In addition, in the case of soil application, soil being a dense medium the proper dispersion of the chemical compound is also a serious concern. Thus emphasis is now on promoting the soil health and to improve the soil properties for conquering the pathogens to minimize the losses caused by plant pathogens [7,20]. Biochar, as soil amendment, can be a potential tool to promote soil health and to suppress plant pathogens without keeping the environment at stake [33]. Stimulation of the photosynthetic activities and plant growth hormones regulating genes along with the plant growth promotion are considered as the direct and indirect effect of biochar addition to the soil which also enable quick plant responses to pathogen invasion [34,35]Addition of biochar has a direct influence on the enzymatic activity of plant pathogens such as adsorption of cell wall degrading enzymes and can suppress the disease by the soil borne plant pathogens (Table 1).
Table 1.
Biochar used for soil borne plant disease management.
| Pathogen/Disease | Host plant | Biochar feedstocks | Mechanism involved | Place of study | Reference |
|---|---|---|---|---|---|
|
Fusarium oxysporum f.spasparagi Fusarium proliferatum |
Asparagus sp | Commercial Quest biochar | Enhanced AM colonization | New York, USA | [36] |
|
Phytophthora cinnamomi P. cactorum |
Acer rubrum Quercus rubra |
Pinus taeda, P. palustris, P.echinata, P. elliotti | Induced disease resistance in plants | Washington, USA | [37] |
| Pythium ultimum |
C. annum, Ocimum basilicum |
Spruce bark | Induced host defence | Canada | [38] |
| Phytophthora infestans | Solanum tuberosum | In combination with Stremptomyces strains enriched rhizosphere | China | [39] | |
| Rhizoctonia solani |
C. sativus Phaseolus vulgaris |
Greenhouse wastes | Plant growth promotion | Israel | [40] |
| Decline disease | Bayberry | Plant biochar | Microbial diversity | China | [24] |
| Fusarium oxysporum f.sp lycopersici | L. esculatum | Wood biochar | Suppressed mycelial growth | Austria | [41] |
| Ralstonia solanacearum | Solanum tuberosum | Rice straw | Increased microbial richness | China | [35] |
| Fusarium oxysporum f. sp. radicis lycopersici | L. esculatum | Eucalyptus wood chips and greenhouse pepper plant wastes | Adsorption of cell wall degrading enzymes and toxins produced by the pathogens | Israel | [42] |
| Fusarium oxysporum f. sp. lycopersici | L. esculatum | Stems of Helianthus tuberosus | Increased beneficial microbes | China | [43] |
| P. aphanidermatum | Cucumis sativus | Eucalyptus wood chips | Increased beneficial microbes | Israel | [44] |
| Phytophthora capsici | Capsicum annum | Corn stalk | Increased abundance of potential biocontrol fungi | China | [16] |
| Stemphylium vesicarium | Allium cepa | Poultry litter biochar | – | Pakistan | [45] |
| Ralstonia solancearum | Nicotiana tabacum | Tobacco stem | Diversity of beneficial microorganisms. | China | [46] |
Globally a handful of studies have been made utilising biochar from different sources for the management of soil borne and foliar plant pathogens (Table 1, Table 2) [47,34]. Similarly, a study conducted in Israel by Jaiswal et al. [40] showed that biochar incorporation reduced the incidence of Rhizoctonia solani in cucumber by promoting plant growth. Suppression of disease incidence of wilt in tomato was observed by Murtaza et al. [48] in Austria utilising wood biochar. Zhang et al. [35] observed the reduced incidence of Ralstonia solancearum by the use of rice straw biochar in China, as it increased the beneficial microbes in soil such as Sphaerodes, Sphingobium, Sphingomonas and Caulobacter. A reduced incidence of Pythium or Phytophthora aphanidermatium in cucumber was observed in a study conducted in Israel, by increasing the population of beneficial microbes in soil [44]. In case of virus plant pathogens, a study conducted by in Italy showed a reduced incidence of Tomato spotted wilt virus by the incorporation of biochar obtained from alfalfa, through alteration of microbiome [49]. Reduced incidence of early blight of tomato was observed by the use of fruits and vegetable waste derived biochar in Pakistan by promoting plant growth through enhanced rhizobacterial activity [2]. A recent study conducted in India by showed the reduced incidence of Sclerotinia stem rot of tomato by the use of rice husk biochar [50] (Fig. 3). Both the studies concluded that biochar induced disease resistance in host plants by enhancing the production of host defense compounds, while in a study conducted against Sclerotinia sclerotiorum with rice husk biochar suppressed the carpogenic germination of sclerotia resulting in the reduction of disease incidence.
Table 2.
Biochar used for foliar plant disease management.
| Pathogen | Host plant | Biochar feedstocks | Mechanism involved | Place of study | Reference |
|---|---|---|---|---|---|
| Levilula taurica | Tomato | Citrus wood | Systemic induced resistance | Israel | [51] |
| Botrytis cinerea, Colletotrichum acutatum and Podosphaeraapahanis, | Strawberry | Wood biochar | Induced the expression of defense-related gene | Israel | [52] |
| Botrytis cinerea | L. esculatum | Green house waste | Jasmonic acid signaling | Italy | [53] |
| Tobacco mosaic virus | L. esculatum | Salix wood chips | Induce systemic resistance | Egypt | [54] |
| Tomato leaf curl virus | L. esculatum | Maize cobs | Induced plant defence | Pakistan | [55] |
| Magnaporthe oryzae | Perrenial rye grass | Rice straw biochar based Si source amendment | Increased Silicon content tissue | China | [16] |
| Tomato spotted wilt virus | L. esculatum | Alfa alfa | Altered bacterial and fungal microbiome | Italy | [56] |
| Alternaria solani | L. esculatum | Green waste biochar, and wood biochar | Enhanced rhizobacterial activity to promote plant growth | Pakistan | [2,3] |
| Gloeocercospora sorghi | Agrostis stolonifera L | Biochar compost mixture | – | US | [57] |
Fig. 3.
Picture showing stepwiseapplication of biochar in soil for plant disease management.
4. Comparison of biochar with other soil amendments in plant disease management aspects
Use of biochar in soilless substrate has been studied for several plants (Table 3). Combinations of biochar produced from different substrates with several other fertilizers, mycorrhiza, and humic acid products had also been reported in the literature [2,33]. Han et al. [8] and Jaiswal et al. [44] have evaluated the positive effects of biochar on the development of plant compared to peat media at lower concentration (30 % (v/v)) [17,58] and it was found that they were not harmful even at high dosages (>40 %) [10]. Biochar substrates with other growth media along with plant biological enhancers significantly promoted the plants growth [31,35]. Several studies which amended the biochar with plant growth promoting bacteria (PGPB), biological agents and arbuscular mycorrhizal fungi combination observed the increase in arbusular mycorrhiza and PGPB levels in soil [26,27]. Very few studies were based on the combination of biochar with biological control agents [32,59]. Also the biochar addition in the potting media may reduce the damage by the plant pathogenic nematodes [34]. One of the aspects that can be taken into consideration is that whether the applied biochar can minimize the pesticide's dosages in the nursery plantation?
Table 3.
Summary table showing synergistic and antagonistic effects of biochar on plant growth.
| Effect | Biochar (source) | Crop | Mechanism | Place of study | Reference |
|---|---|---|---|---|---|
| Synergistic | |||||
| Increased biomass and seed yield | Sawdust | Soybean | Decrease in soil pH | Pakistan | [60] |
| Improvement in growth and physiology of maize | Grapevine twigs | Maize | Enhanced beneficial microbes | Pakistan | [49] |
| Increased biomass and seed yield | Sawdust | Soybean | Decrease in soil pH | Pakistan | [61] |
| Improvement in growth and physiology of maize | Grapevine twigs | Maize | Enhanced beneficial microbes | Pakistan | [62] |
| Increased yield | Biochar | Rice | Synergistic effect with PGPR | Egypt | [63] |
| Increase in grain yield and biological yield | Cow manure | Maize | Increased Nitrogen availability | Pakistan | [64] |
| Antagonistic | |||||
| Negative yield impacts | Wheat-straw biochar | Rye grass (Lolium perenneL) | - | [65] | |
| Increased disease incidence of Rhizoctonia solani | Greenhouse waste (GHW) | Cucumber | Toxic effect | Israel | [40] |
| Reduced asparagus yield | Biochar | Asparagus | Absorbed some nutrients, such as nitrogen, | USA | [66] |
| Reduced P availability | Wheat | Suaeda salsa | Occurrence of phosphate precipitation/sorption reaction | China | [67] |
| Reduction in uptake of Ca and Mg in maize | Wheat straw and pine wood biochar | Maize | Access addition of Potassium to soil | Denmark | [68] |
4.1. Plant growth and disease response
Plant patho-systems involve soil borne and foliar pathogens [47,69]. The data to comprehend the influence of incorporation of biochar on plant diseases was summarised [24] and observed that biochar had a positive influence on 85 % of the investigations based on the minimizing disease severity. Only3% of the reported studies had showed that biochar favoured the occurrences of the plant diseases [10,20]. They had also concluded that the dosages of application of biochar were the determinative factor for enhancing or suppressing the susceptibility of the host plants against diseases (Fig. 4).
Fig. 4.
Schematic representation of the plant disease control mechanism (reduction in survival and germination of dormant structures) in biochar amended soils.
Majority of the studies had evaluated biochar at concentrations in the range of 0.5–5 % (w/v), with some of the studies had reported negative to neutral impact on plant diseases of vegetable crops when compared to control and maximum effective biochar concentrations (3–5%) [24,28,47,70]. In this case, an inverted U-shaped biochar dose curve was observed in most of the studies [1,71]. Several previous studies were conducted to test the effects of biochar in suppressing disease using a growing media showed that the disease was suppressed when biochar was used at low dosages (≤1 %) [13,24], while the use of biochar in high concentrations (3 %) was found to be ineffective in suppressing the disease [2,3]. With the further increase in biochar concentrations probably a reversed U- shaped dose response curve was obtained though the less effect was observed in the foliar disease causing pathogens [48,70] but the effect was prominent in soil-borne pathogens.
4.2. U shape effect for biochar (in soilless culture/media)
Biochar can be successfully utilized as an amendment at low concentration for a soilless media, whereas complete replacement of soil by biochar needs some thorough study as it promotes plant growth against pathogens but at high concentrations it may have some adverse effect either in the plant growth or may promote the disease incidence by pathogens (Table 4).The use of biochar as a major portion of soil media is still under question due to its mixed response. Though in plant nurseries, strict measures are taken for sanitation to reduce the disease risk from several fungal and bacterial plant pathogens such as Corynebacterium michiganesis, Xanthomonas campestris and Pythium aphanidermatum [69,72]. Arabidopsis thaliana plants when applied with high concentration (>3 %) of biochar resulted in the increased leaf area and root length, and the molecular assessment of genes showed the up-regulation of genes responsible for auxin and brassinosteroids pathways which are growth stimulators. But the down regulation of defense genes responsible for salicylic acid, jasmonic acid, and ethylene pathways, was also observed [20,39]. Depending on the substrates used for biochar preparation, it may reduce availability of nutrients to plants rendering them susceptible to disease [73]. Biochar may consist of a number of organic compounds which may have some phytotoxic effects. Some biochars showed emission of ethylene, which has a role in plant growth as well as in plant defense but it showed negative impacts at high concentrations (>20 ppm) [24,74]. Incomplete pyrolysis of biochar results in production of polycyclic aromatic hydrocarbons (PAHs), which are organic pollutants of the environment [75]. Biochar application may reduce the availability of pesticides [76,77], which would require application of higher doses of pesticides for managing pest [78]. Biochar contains volatile organic compounds (VOCs) [79] which are toxic. Some microbes produce VOCs signaling molecules [80] and can elicit plant growth by promoting rhizobacteria [81]. VOCs produced biochar may interfere with them and inhibit the fungal growth [82] and can inhibit nitrification [83].
Table 4.
Effects of biochar on plant-pathogenic fungi, arthropods, bacteria, nematodes, and oomycetes.
| Host plant | Source of biochar | Mechanism involved | Place of study | Reference | |
|---|---|---|---|---|---|
| Fungi | |||||
|
Fusarium oxysporum f.spasparagi Fusarium proliferatum |
Asparagus sp | Commercial Quest biochar | Enhanced AM colonization | USA | [42] |
| Botrytis cinerea | L. esculatum | Green house waste | Jasmonic acid signaling | Italy | [53] |
| Botrytis cinerea | L. esculatum | Green house waste | Jasmonic acid signaling | Italy | [70] |
| Fusarium oxysporum f. sp. lycopersici | L. esculatum | Eucalyptus urophylla and E. saligna | Reduced micrconidial germination | Brazil | [15] |
| Levilulataurica | L. esculatum | Citrus wood | Systemic induced resistance | Israel | [44] |
| Arthropods | |||||
| Nilaparvatalugens | Paddy | Wheat straw | Reduced egg hatching rate | China | [19] |
| Laodelphaxstriatellusas | Paddy | Corn | Decreased fecundity | China | [84] |
| Cnaphalocrocismedinalis | Paddy | Wheat straw | Mortality of larvar, reduced body weight of Mature larval, lowered consumption of rice leaves | China | [12] |
| Sitobion avenae | Wheat | Wheat, corn or rice straw | Decrease the duration of stylet penetrations | China | [9] |
| Douglas-Fir Tussock Moth | Douglas-fir | Douglas-fir | Reduced survival and weight gain | Moscow, USA | [85] |
| Bacteria | |||||
| Ralstonia solanacearum | Tomato | Peanut shell and wheat straw | Enhanced soil microbial diversity | China | [5] |
| Ralstonia solanacearum | Tomato | Pine wood biochar | Supress pathogen swarming ability | China | [86] |
| Ralstonia solancearum | Tobacco | Rice hull | Increased beneficial bacterial count | China | [9] |
| Ralstonia solancearum | Tomato | Wheat straw | Increased microbial diversity | China | [87] |
| Ralstonia solancearum | Tobacco | Tobacco stem | Diversity of beneficial microorganisms. | China | [46] |
| Nematode | |||||
| Meloidogyne graminicola | Rice | Wood biochar | Transcriptional enhancement of genes involved in the ethylene (ET) signaling pathway. | China | [88] |
| Pratylenchus penetrans | Carrot | Pinus sylvestris spelt husk biochar | Induced host defence | Germany | [34] |
| Meloidogyne incognita | Tomato | Rice husk | Reduced number of galls, egg and females | Pakistan | [89] |
| Meloidogyne incognita | Lentil | Prosopis juliflora | Reduced nematode population | India | [90] |
| Meloidogyne javanica | Tomato | Grape pomace-derived biochars | Reduced egg masses and eggs per plant | Spain | [91] |
| Oomycetes | |||||
|
Phytophthora cinnamomi P. cactorum |
Acer rubrum Quercus rubra |
Pinus taeda, P. palustris, P.echinata, P. elliotti | Induced disease resistance in plants | USA | [37] |
| Pythium ultimum |
C. annum, Ocimum Basilicum |
Spruce bark | Induced host defence | Canada | [38] |
| P.aphanidermatum | Cucumis sativus | Eucalyptus wood chips | Increased beneficial microbes | Israel | [44] |
| Phytophthora capsici | Pepper | Corn stalk | Increased abundance of potential biocontrol fungi | China | [92] |
Therefore, researchers have justified that there is no specific concentration on which the efficiency of biochar can be determined as different factors might define the effectiveness of the biochar starting from its sources to the sink (soil) [17]. For disease suppression the use of different concentrations of biochar might depend on the various mechanisms involved in that particular patho-system. Some of the probable mechanisms researchers have discussed in recent decades include the alteration of microbial communities in soil and enhanced population of beneficial microbes, supply of the nutrient contents to plants, and the hormesis effect of phytohormones [69,72,56]. The growth of most pathogens is often favoured by acidic soils (pH <6.5), but it has been reported that biochar addition enhanced soil pH which makes the nutrients unavailable to the pathogens and increases the beneficial microbial biomass to protect the plant from pathogen attacks [22,28].
4.3. Molecular assessment of biochar interaction with pathogenic fungal community
Plants have innate mechanism of defense (Induced systemic resistance), which protect plants against pathogenic fungi, bacteria, viruses, nematodes, and insect pests [70,60]. Biochar also induces resistance in host plants. Chen et al. [25] and Poveda et al. [7] described the mechanisms of inducing disease resistance in plant pathogen Botrytis cinereaa and Colletotrichum acutatum in pepper and Leveillula taurica in tomato. Increased expression of genes linked with the defense induction was observed in strawberry cultivated in potting media incorporated with biochar [20,48]. Biochar leads to the production of the phytotoxic molecules and phenols influencing the plants and also the associated microbes [49,93].Green waste biochar incorporated at concentrations of 1 % and 3 % was effective against Fusarium oxysporum f.sp. Radicis lycopersici (causal organism of crown rot in tomato), and mortality caused by disease up to 43 % and 57 %, respectively, indicating efficacy of 1 % over 3 %. Transcriptomic analysis (RNA-seq) of tomato was carried out in that study and differences were observed in the expression of genes in biochar treated plants indicating the priming effect of biochar on genes expression. Up-regulation of Jasmonic acid, lignin, flavonoid, phenylalanine and phenylpropanoid biosynthesis pathways was observed, which, were the key indicators of defense responses in plants (Fig. 5(A–D)) [19]. Rasool et al. [3] conducted study using wood and green waste biochar (at 3 % and 6 % rate of application for both) against early blight of tomato caused by Alternaria solani and observed the reduced disease incidence in the soil that was incorporated with wood biochar and 6 % green waste biochar treatment. They have observed expression of defense related genes such as Pti4 responsive to ethylene, PI2 and Tomlox D, related to jasmonic acid and PR1a, PR2 related to salicylic acid in that study. Expression of PI2, Tomlox D, PR2 and PR1a was more in the case of green waste biochar (6 %) and it was found that production of catalase, phenolics, and peroxidase reduced the infection by pathogens.Chen et al. [25] have observed that plants showed high resistance in biochar-amended soil against Fusarium oxysporum and Rhizoctonia solani due to the increased phenolic compounds and high activities of key enzymes of phenylpropanoid pathway i.e. phenyl ammonia-lyase and peroxidases, which might be associated with the enhanced lignification process.
Fig. 5.
Heat map diagram showing molecular transcripts expression of genes induced by biochar (adapted from Jaiswal et al. [19]). (A) Plant signaling pathway and JA biosynthesis induction. Significant up- and down-regulation is present in the transcripts indicated by the green and red arrows, respectively; (B) Expression heatmap of genes involved in signaling and JA biosynthesis in treatments with and without modified biochar at 72 h post-immunization. Rescaled transcripts per kilobase per million (TPM) values are represented by Z-scores; (C) Plant induction of SA biosynthesis and signaling pathway; (D) Transcript expression heatmap associated with to signaling and SA production in treatments with and without modified biochar at 72 h post-irradiation. Systemically induced signaling pathways for jasmonic acid (JA) and salicylic acid (SA) in response to the biochar and pathogen at 72 hpi. This image copyright belongs to the respective publisher and/or authors.
4.4. Effect of biochar on the survival of dormant structures of pathogens
Resting structures such as melanized mycelium, oospores, chlamydospores, and sclerotia acts as a primary source of inoculum for several plant pathogens and are responsible for the initiation of infection in the host plant. Reducing the viability and interrupting the germination of these resting structures can prevent the occurrence of disease. Soil pathogens mainly survive in the low ranges of Eh–pH [72], biochar alters the pH in the rhizosphere consequently affect in the viability of pathogens [26]. Biochar incorporation in the soil also alters the composition of root exudates of plants and thus simultaneously affects the germination of resting structures of plant pathogens. Some of the compounds which directly hamper the survival of pathogens are propylene glycol, hydroxy propionic, ethylene glycol, benzoic acid, hydroxybutyric acids, o-cresol, 2-phenoxyethanol, and quinines [47].
Jaiswal et al. [44] observed the reduced survival of Rhizoctonia solani by the use of Greenhouse pepper plant waste biochar at the rate of 3 % concentration (w/w) in pot experiments. Akhter et al. [41] observed the positive effects of the green waste biochar in controlling the wilt disease of tomato caused by the Fusarium oxysporumf. sp. lycopersici by reducing the germination of chlamydospores. Were et al. [94] used the extract of sugarcane bagasse biochar along with vermicompost to observe the suppression in the germination of sporangia and conidia of root rot pathogens. Bhatt and Sharma [50] observed suppression of carpogenic germination of sclerotia by the application of rice husk biochar under in-vitro conditions.
5. Strategy and mechanisms of plant disease control
There are varied phenomenon by which biochar protects plants against diseases such as enhancing plant growth by improving the nutrient availability, adsorption of toxic compounds produced by pathogens, alterations in the chemical composition of root exudates, inducing disease resistance [6,95] in plants. In addition, increasing the beneficial microbes count in the soil, stimulation of the specific antibacterial and antifungal compounds also contributed to the disease resistance [24,70] Several studies based on biochar against soil borne and foliar plant pathogens have demonstrated the effectiveness of biochar in minimizing disease incidence [2,3,6,8]. They have hypothesized that the suppression of the soil borne pathogens might take place through the following mechanisms such as by induction of systemic resistance in plants, Jasmonic acid signaling and by enhancing rhizobacterial activity to promote the plant growth. Fusarium is a common plant pathogen and also known to produce mycotoxin in stored plant products [25]. Elmer [66] observed that incorporation of hardwood biochar led to the increased antagonist (Pseudomonas or arbuscular mycorrhizal fungi) population against F. proliferatum and F. oxysporum f. sp. asparagi inciting crown rot and root rot of asparagus. Biochar also induced resistance against foliar plant pathogens such as Botrytis cinerea, which is a fungal plant pathogen with broad host ranges by the activation of systemic defenses [6,8]. Alterations in microbial community in rhizosphere in addition with rice husk biochar reduced F. solani and Ilyonectria destructans infection in Panax ginseng plants [96].
In case of the bacterial pathogens of plants, the majority of the studies have focused on Ralstonia solanacearum, causing bacterial wilt and is one of the serious pathogens affecting vegetable production [97]. They have used biochar from different sources thus some differences were observed in case of the disease suppression. The main underlying mechanism was increased bacterial and actinomycetes populations resulted from the improved physicochemical properties of soil and reduced root colonization and swarming motility of the pathogens [86,35]. Burned log wood biochar have been found effective against coffee nematode, Pratylenchus coffeae, by reducing its population in soil [98,99]. Incorporation of oak wood at the rate of 1.2 % (w/v) controlled the Meloidogyne graminicola, (endoparasitic root-knot nematode) in rice agrosystems, this resulted from the activation of plant defense genes by the accumulation of hydrogen peroxide (H2O2) [7,100]. Foliar accumulation of jasmonic acid by using biochar at 10 % concentration (w/w) from dolomite, deciduous trees and molasses increased rice resistance against Sojatella furcifera [101]. Likewise, biochar produced from citrus wood was found effective against Polyphagotarsonemus latus (Acari: Tarsonemidae), a pest of economic importance by inducing of systemic resistance in pepper plants [69,102]. Thus biochar from different substrates proved their efficiency against the plant pathogens and pests (Fig. 6, Table 4).
Fig. 6.
Effect of biochar application on soil, plants and microbes.
6. Future research directions and conclusions
With the negative impacts of chemical pesticides on the management of plant diseases as well as environment and emphasis on the minimizing the use of chemicals for managing plant diseases, biochar application gives a ray of hope as a sustainable disease management strategy. At the same time, the central aspect relating to the use of biochar for the disease control was addressed in a superficial manner in the past reviews (Fig. 7(a and b)). Therefore, the results from the literature reflect the need for further studies on the utilization of biochar in plant disease management with great scopes in the future.
-
a)
Biochar from different substrates may have different mechanisms and insights into these mechanisms which can be very helpful in better utilization of different substrate biochars in different pathosystems.
-
b)
Efforts should be made to comprehend the interaction of biochar with the host plants in promoting plant growth and inducing disease resistance. Very few studies are there based on molecular interactions, therefore further research needs to be carried out in terms of the activation of plant defense genes against pathogens in different host pathosystems.
-
c)
Increased populations of some specific beneficial groups of microbes and inhibition of pathogenic microbes by biochar also need our attention.
-
d)
There is a need for standardization of the doses of biochar as some of the dosages might have a negative impact on plant growth and disease suppression. Also, factors such as the types of substrate used, pyrolysis temperatures, and pH of biochar must be considered to obtain the benefits from its use. Knowledge about the compatibility of biochar with biocontrol agents and its synergistic and antagonistic effects will help us in better management of plant diseases.
Fig. 7.
a. keyword co-occurrence map showing the most frequently investigated topics using VOS viewer; b. The number of publications covering the role of biochar in the plant disease management.
Biochar utilization in plant disease management does not require much extra effort as its incorporation can be an additional cultural practice in crop production. It also provides socio-economic benefits as it can be easily produced from locally available agricultural wastes. Biochar incorporation has been found to be beneficial in minimizing the disease incidence for soil-borne and foliar plant pathogens. Studies have shown its effectiveness against nematodes [103] and insect pests using different mechanisms, indicating its further use in plant disease management. More studies need to be carried out to explore the exact mechanisms involved and dose standardization, which will help to understand the negative impacts of biochar incorporation. Very few studies have focused on the molecular mechanisms involved in the induction of disease resistance in plants by the use of biochar, thus more emphasis should be given to exploring the molecular aspect of biochar interaction with plant and soil microbes.
Consent for publication
All the authors read and agreed to publish this article.
Data availability statement
No data was used for the research described in the article.
Ethical compliance
We have meticulously followed the Guide for Authors to prepare this manuscript, ensuring compliance with the Ethics in Publishing Policy outlined in the Guide for Authors.
CRediT authorship contribution statement
Bhagyashree Bhatt: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Data curation. Satish Kumar Gupta: Writing – review & editing, Validation, Supervision, Methodology, Funding acquisition, Data curation. Santanu Mukherjee: Writing – original draft, Supervision, Funding acquisition, Data curation, Conceptualization. Ravinder Kumar: Writing – review & editing, Visualization, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are thankful to the DST-SERB (SRG grant: SRG/2021/000192) and the authorities of the Shoolini University of Biotechnology and Management Sciences, Bajhol, Solan for providing administrative and research support.
Contributor Information
Santanu Mukherjee, Email: santanu@shooliniuniversity.com, santanu_mukherjee86@yahoo.co.in.
Ravinder Kumar, Email: chauhanravinder97@gmail.com.
References
- 1.Kumar A., et al. A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar. 2022;4(1):22. [Google Scholar]
- 2.Rasool M., Akhter A., Haider M. Molecular and biochemical insight into biochar and Bacillus subtilis induced defense in tomatoes against Alternaria solani. Sci. Hortic. 2021;285 doi: 10.1016/j.scienta.2021.110203. [DOI] [Google Scholar]
- 3.Rasool M., Akhter A., Soja G. MS. Haider. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021;11(1):6092. doi: 10.1038/s41598-021-85633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awad Y.M., et al. Carbon and nitrogen mineralization and enzyme activities in soil aggregate-size classes: effects of biochar, oyster shells, and polymers. Chemosphere. 2018;198:40–48. doi: 10.1016/j.chemosphere.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z., et al. Biochar and its coupling with microbial inoculants for suppressing plant diseases: a review. Appl. Soil Ecol. 2023;190 [Google Scholar]
- 6.Poveda J., Abril-Urias P., Escobar C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020;11:992. doi: 10.3389/fmicb.2020.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poveda J., et al. The use of biochar for plant pathogen control. Phytopathology. 2021;111(9):1490–1499. doi: 10.1094/PHYTO-06-20-0248-RVW. [DOI] [PubMed] [Google Scholar]
- 8.Caseys C., et al. Quantitative interactions drive Botrytis cinerea disease outcome across the plant kingdom. bioRxiv. 2020 doi: 10.1093/g3journal/jkab175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., et al. Effects of biochar amendment to soils on stylet penetration activities by aphid Sitobion avenae and planthopper Laodelphaxstriatellus on their host plants. Pest Manag. Sci. 2020;76(1):360–365. doi: 10.1002/ps.5522. [DOI] [PubMed] [Google Scholar]
- 10.Nieto A., et al. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016;199:142–148. [Google Scholar]
- 11.Allen R.L. 1847. A Brief Compend of American Agriculture. 2nd ed. New York: C. M. Saxton. [Google Scholar]
- 12.Chen Y., et al. The effect of biochar amendment to soils on CnaphalocrocismedinalisGuenee (Lepidoptera: Pyralidae) on rice. Crop Prot. 2019;124 [Google Scholar]
- 13.Mansoor S., et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2020.129458. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann J., et al. Biochar effects on soil biota: a review. Soil Biol. Biochem. 2011;43:1812–1836. [Google Scholar]
- 15.Silva L.G., de Andrade C.A., Bettiol W. Biochar amendment increases soil microbial biomass and plant growth and suppresses Fusarium wilt in tomato. Trop Plant Pathol. 2020;45(1):73–83. [Google Scholar]
- 16.Wang M., et al. Effect of alkali-enhanced biochar on silicon uptake and suppression of gray leaf spot development in perennial ryegrass. Crop Protect. 2019;119:9–16. [Google Scholar]
- 17.Hui D. Effects of biochar application on soil properties, plant biomass production, and soil greenhouse gas emissions: a mini-review. Agric. Sci. 2021;12:213–236. [Google Scholar]
- 18.Ameloot N., et al. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013;57:401–410. [Google Scholar]
- 19.Jaiswal A.K., et al. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-70882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N., Kumar A. Plant disease management through bio-char: a review. Int JCurr Microbiol Appl Sci. 2020;11:3499–3510. [Google Scholar]
- 21.Weng Z.H., et al. Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat Clim Chan. 2017;7(5):371–376. [Google Scholar]
- 22.Palansooriya K.N., et al. Response of microbial communities to biochar-amended soils: a critical review. Biochar. 2019;1:3–22. [Google Scholar]
- 23.Gorovtsov A.V., et al. The mechanisms of biochar interactions with microorganisms in soil. EGAH. 2020;42(8):2495–2518. doi: 10.1007/s10653-019-00412-5. [DOI] [PubMed] [Google Scholar]
- 24.Ren H., et al. Improvement effect of biochar on soil microbial community structure and metabolites of decline disease bayberry. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W., et al. Biochar combined with Bacillus subtilis SL-44 as an eco-friendly strategy to improve soil fertility, reduce Fusarium wilt, and promote radish growth. Ecotoxicol. Environ. Saf. 2023;251 doi: 10.1016/j.ecoenv.2023.114509. [DOI] [PubMed] [Google Scholar]
- 26.Yan H.T., et al. Effect of biochar amendment on physicochemical properties and fungal community structures of cinnamon soil. Huan Jing Ke Xu. 2018;39(5):2412–2419. doi: 10.13227/j.hjkx.201711114. [DOI] [PubMed] [Google Scholar]
- 27.Bai N., et al. Long-term effects of straw and straw-derived biochar on soil aggregation and fungal community in a rice-wheat rotation system. PeerJ. 2019;4:6:e6171. doi: 10.7717/peerj.6171. PMID: 30631646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han G., et al. Response of soil microbial community to application of biochar in cotton soils with different continuous cropping years. Sci Rep7. 2017 doi: 10.1038/s41598-017-10427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen T.T.N., et al. The effects of short term, long term and reapplication of biochar on soil bacteria. Sci. Total Environ. 2018;636:142–151. doi: 10.1016/j.scitotenv.2018.04.278. [DOI] [PubMed] [Google Scholar]
- 30.Wang R., et al. Unraveling the characteristics of the microbial community and potential pathogens in the rhizosphere soil of Rehmanniaglutinosa with root rot disease. Appl. Soil Ecol. 2018;130:271–279. [Google Scholar]
- 31.Katan J., et al. Diseases caused by soilborne pathogens: biology, management and challenges. J. Plant Pathol. 2017;99:305–315. [Google Scholar]
- 32.Zhou X., et al. Control of Fusarium wilt of lisianthus by reassembling the microbial community in infested soilthrough reductive soil disinfestation. Microbiol. Res. 2019;220:1–11. doi: 10.1016/j.micres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Lima J.R.S., et al. Effect of biochar on physicochemical properties of a sandy soil and maize growth in a greenhouse experiment. Geoderma. 2018;319:14–23. [Google Scholar]
- 34.George C., Kohler J., Rillig M.C. Biochars reduce infection rates of the root-lesion nematode Pratylenchus penetrans and associated biomass loss in carrot. Soil Biol. Biochem. 2016;95(1):11–18. [Google Scholar]
- 35.Zhang C., et al. Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil Ecol. 2017;112:90–96. [Google Scholar]
- 36.Elmer W.H., Pignatello J.J. Effect of biochar amendments on mycorrhizal associations and fusarium crown and root rot of asparagus in replant soils. Plant Dis. 2011;95:960–966. doi: 10.1094/PDIS-10-10-0741. [DOI] [PubMed] [Google Scholar]
- 37.Zwart D.C., Kim S.H. Biochar amendment increases resistance to stem lesions caused by Phytophthora spp. in tree seedlings. Hortscience. 2012;47:1736–1740. [Google Scholar]
- 38.Gravel V., Dorais M., Menard C. Organic potted plants amended with biochar: its efect on growth and Pythium colonization. Can. J. Plant Sci. 2013;93:1217–1227. doi: 10.4141/cjps2013-315. [DOI] [Google Scholar]
- 39.Jin L., et al. Biological control of potato late blight with a combination of Streptomyces strains and biochar. Biol. Control. 2023;183 [Google Scholar]
- 40.Jaiswal A.K., et al. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 2014;69:110–118. [Google Scholar]
- 41.Akhter A., et al. Potential of Fusarium wilt-inducing chlamydospores, in vitro behaviour in root exudates and physiology of tomato in biochar and compost amended soil. Plant Soil. 2016;406:425–440. [Google Scholar]
- 42.Jaiswal A.K., et al. Immobilization and deactivation of pathogenic enzymes and toxic metabolites by biochar: a possible mechanism involved in soilborne disease suppression. Soil Biol. Biochem. 2018;121:59–66. [Google Scholar]
- 43.Jin X. Biochar amendment suppressed Fusarium wilt and altered the rhizosphere microbial composition of tomatoes. Agronomy. 2023;13(7):1811. [Google Scholar]
- 44.Jaiswal A.K., Graber E.R., Elad Y., Frenkel O. Biochar as a management tool for soilborne diseases affecting early stage nursery seedling production. Crop Prot. 2019;120:34–42. [Google Scholar]
- 45.Arif M., et al. The impact of different biochars on Stemphylium leaf blight (SLB) suppression and productivity of onion (Allium cepa L.) JKing Saud Univ. 2021;33(7) [Google Scholar]
- 46.Chengjiang Li, et al. Biochar suppresses bacterial wilt disease of flue-cured tobacco by improving soil health and functional diversity of rhizosphere microorganisms. Appl. Soil Ecol. 2022;171 [Google Scholar]
- 47.Graber E.R., et al. How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag. 2014;5(2):169–183. [Google Scholar]
- 48.Murtaza G., et al. Biochar-Soil-Plant interactions: a cross talk for sustainable agriculture under changing climate. Front. Environ. Sci. 2023;11 [Google Scholar]
- 49.Bonanomi G., et al. Decomposition and organic amendments chemistry explain contrasting effects on plant growth promotion and suppression of Rhizoctonia solani damping off. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0230925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhatt B., Sharma G. Rice husk Biochar: an effective management strategy against Sclerotinia sclerotiorum. AMA. 2023;53(7):11349–11361. [Google Scholar]
- 51.Elad Y., et al. Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology. 2010;100:913–921. doi: 10.1094/PHYTO-100-9-0913. [DOI] [PubMed] [Google Scholar]
- 52.Harel Y.M., et al. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil. 2012;357:245–257. [Google Scholar]
- 53.Mehari Z.H., et al. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil. 2015 doi: 10.1007/s11104-015-2445-1. [DOI] [Google Scholar]
- 54.Khalifa W. Biochar soil amendment induced resistance in tomato against tobacco mosaic virus. Middle East J Agric Res. 2017;6(4):1478–1489. [Google Scholar]
- 55.Zeshan M.A. Induction of resistance in tomato plants against tomato leaf curl virus by using biochar and seed priming. Pak J Phytopathol. 2018;30(1):19–25. [Google Scholar]
- 56.Bonanomi B., Ippolito F., Scala F. A“black” future for plant pathology? biochar as a new soil amendment for controlling plant diseases. J Pl Path. 2015;97(2):223–234. [Google Scholar]
- 57.Doherty J., Roberts J. Topdressing biochar compost mixtures and biological control organism applications suppress foliar pathogens in creeping bentgrass fairway turf. Plant Dis. 2023 doi: 10.1094/PDIS-07-22-1629-RE. [DOI] [PubMed] [Google Scholar]
- 58.Kolton M., et al. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. 2011;AEM77:4924–4930. doi: 10.1128/AEM.00148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukherjee S., Weihermüller L. Soil Amendments for Sustainability. CRC Press; 2018. Biochar: an anthropogenic pyrogenic amendment; pp. 145–158. [Google Scholar]
- 60.Akanmu A.O., et al. Plant disease management: leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fatima Z., et al. Synergistic effects of biochar and NPK fertilizer on soybean yield in an alkaline soil. Pedosphere. 2015;25(5):713–719. [Google Scholar]
- 62.Ahmad M.T. Synergistic effect of rhizobia and biochar on growth and physiology of maize. Agron. J. 2015;107(6):2327–2333. [Google Scholar]
- 63.Hafez E.M. Synergistic effect of biochar and plant growth promoting rhizobacteria on alleviation of water deficit in rice plants under salt-affected soil. Agronomy. 2019;9(12):847. [Google Scholar]
- 64.Zahra M.B., Aftab Z.H., Akhter A., Haider M.S. Cumulative effect of biochar and compost on nutritional profile of soil and maize productivity. J. Plant Nutr. 2021;44(11):1664–1676. [Google Scholar]
- 65.O'Toole A., Knoth de Zarruk K., Steffens M., Rasse D.P. Characterization, stability, and plant effects of kiln-produced wheat straw biochar. Plant and Environment Interaction. 2013 doi: 10.2134/jeq2012.0163. [DOI] [PubMed] [Google Scholar]
- 66.Elmer W.H. Effect of leaf mold mulch, biochar, and earthworms on mycorrhizal colonization and yield of asparagus affected by Fusarium crown and root rot. Plant Dis. 2016;100:2507–2512. doi: 10.1094/PDIS-10-15-1196-RE. [DOI] [PubMed] [Google Scholar]
- 67.Xu G., Zhang Y., Sun J., Shao H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci total environ. 2016;568:910–915. doi: 10.1016/j.scitotenv.2016.06.079. [DOI] [PubMed] [Google Scholar]
- 68.Born M.L. DS. Müller-Stöver, F. Liu. Biochar properties and soil type drive the uptake of macro- and micronutrients in maize (Zea mays L.) J. Plant Nutr. Soil Sci. 2019;182(2):149–158. [Google Scholar]
- 69.Frenkel O., et al. The effect of biochar on plant diseases: what should we learn while designing biochar substrates? J. For. Environ. 2017;25(2):105–113. [Google Scholar]
- 70.Yang Y., et al. A quantitative evaluation of the biochar's influence on plant disease suppress: a global meta-analysis. Biochar. 2022;4(1):43. [Google Scholar]
- 71.de Medeiros E.V., et al. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: a critical review. Phytoparasitica. 2021;49(4):713–726. [Google Scholar]
- 72.Frenkel O., et al. Secondary spread of Clavibactermichiganensissubsp. michiganensis in nurseries and the conditions leading to infection of tomato seedlings. Eur. J. Plant Pathol. 2016;144:569–579. [Google Scholar]
- 73.Wang Y., Zhang L., Yang H., Yan G., Xu Z., Chen C., et al. Biochar nutrient availability rather than its water holding capacity governs the growth of both C3 and C4 plants. J. Soils Sediments. 2016;16:801–810. [Google Scholar]
- 74.Ramadan M.M., Abd-Elsalam K.A. Carbon Nanomaterials for Agri-Food and Environmental Applications. 2020. Micro/Nano biochar for sustainable plant health: present status and future prospects; pp. 323–335. [Google Scholar]
- 75.Wang C., Wang Y., Herath H.M.S.K. Polycyclic aromatic hydrocarbons (PAHs) in biochar – their formation, occurrence and analysis: a review. Org. Geochem. 2017;114:1–11. [Google Scholar]
- 76.Varjani S., Kumar G., Rene E.R. Developments in biochar application for pesticide remediation: current knowledge and future research directions. J. Environ. Manag. 2019;232:505–513. doi: 10.1016/j.jenvman.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 77.Ni N., Kong D., Wu W., He J., Shan Z., Li J., et al. The role of biochar in reducing the bioavailability and migration of persistent organic pollutants in soil–plant systems: a review. Bull. Environ. Contam. Toxicol. 2020;104(2):157–165. doi: 10.1007/s00128-019-02779-8. [DOI] [PubMed] [Google Scholar]
- 78.Kookana R.S. The role of biochar in modifying the environmental fate, bioavailability, and efficacy of pesticides in soils: a review. Soil Res. 2010;48:627. [Google Scholar]
- 79.Ruzickova J., Koval S., Raclavska H., Kucbel M., Svedova B., Raclavsky K., et al. A comprehensive assessment of potential hazard caused by organic compounds in biochar for agricultural use. J. Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123644. [DOI] [PubMed] [Google Scholar]
- 80.Schöller C.E.G., Gürtler H., Pedersen R., Molin S., Wilkins K. Volatile metabolites from actinomycetes. J. Agric. Food Chem. 2002;50:2615–2621. doi: 10.1021/jf0116754. [DOI] [PubMed] [Google Scholar]
- 81.Farag M.A., Ryu C.-M., Sumner L.W., Paré P.W. GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry. 2006;67:2262–2268. doi: 10.1016/j.phytochem.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 82.Chen J., Li S., Liang C., Xu Q., Li Y., Qin H., et al. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: effect of particle size and addition rate. Sci. Total Environ. 2017;574:24–33. doi: 10.1016/j.scitotenv.2016.08.190. [DOI] [PubMed] [Google Scholar]
- 83.Bending G.D., Lincoln S.D. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biol. Biochem. 2000;32:1261–1269. [Google Scholar]
- 84.Quiang F., BaoPing L., Ling M. Effects of biochar amendment to soil on life history traits of Laodelphax striatellus (Hemiptera: Delphacidae) on rice plants. Chin. J. Rice Sci. 2018;32(2):200–206. [Google Scholar]
- 85.Marshall S.R., Cook S.P., Randall J. Impact of biochar on douglas-fir tussock moth (orgyiapseudotsugata Lepidoptera: erebidae) larvae reared on synthetic diet. Insects. 2021;12(12):1065. doi: 10.3390/insects12121065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gu Y., et al. Application of biochar reduces Ralstonia solanacearum infection via effects on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil. 2017;415:269–281. doi: 10.1007/s11104-016-3159-8. [DOI] [Google Scholar]
- 87.Ji-hui T., et al. Wheat straw biochar amendment suppresses tomato bacterial wilt caused by Ralstonia solanacearum: potential effects of rhizosphere organic acids and amino acids. J. Integr. Agric. 2021;20(9):2450–2462. [Google Scholar]
- 88.Huang W.K., et al. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Biol. 2015;15:267. doi: 10.1186/s12870-015-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arshad U., et al. Biochar application from different feedstocks enhances plant growth and resistance against Meloidogyne incognita in tomato. Int. J. Agric. Biol. 2020;24(4):961–968. [Google Scholar]
- 90.Ansari T., Asif M., Khan A., Ahmad M.T.F., Khan F., Siddiqui M.A. Effect of combined soil application of biochar and oilcakes on Meloidogyne incognita infesting lentil (Lens culinaris cv. Desi) Indian Phytopatho. 2020;l73(1):3–6. [Google Scholar]
- 91.Martínez-Gómez A., et al. Biochar from grape pomace, a waste of vitivinicultural origin, is effective for root-knot nematode control. Biochar. 2023;5:30. doi: 10.1007/s42773-023-00228-8. [DOI] [Google Scholar]
- 92.Wang G., et al. Biochar-Mediated control of Phytophthora blight of pepper is closely related to the improvement of the rhizosphere fungal community. Front. Microbiol. 2020;8(11):1427. doi: 10.3389/fmicb.2020.01427. PMID: 32733402; PMCID: PMC7360685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bonanomi G., G, et al. Mixtures of organic amendments and biochar promote beneficial soil microbiota and affect Fusarium oxysporum f. sp. lactucae, Rhizoctonia solani and Sclerotinia minor disease suppression. Plant Pathol. 2022;71(4):818–829. [Google Scholar]
- 94.Were S.A., et al. Mechanisms of biochar and vermicompost in suppression of root rot fungal disease of common bean (Phaseolus vulgaris L) Afr Biol Sci. 2021;3(2):65–86. [Google Scholar]
- 95.Tikoria R., et al. Insights into the role of biochar as potential agent in the management of disease caused by phytopathogens: a review. J. Soil Sci. Plant Nutr. 2023 doi: 10.1007/s42729-023-01489-9. [DOI] [Google Scholar]
- 96.Eo J. Effects of rice husk and rice husk biochar on root rot disease of ginseng (Panax ginseng) and on soil organisms. Biol AgricHortic. 2018;34:27–39. [Google Scholar]
- 97.Choudhary D.K., Nabi S.U., Dar M.S., Khan K.A. Ralstonia solanacearum: a wide spread and global bacterial plant wilt pathogen. Int J Pharmacogn Phytochem. 2018;7:85–90. [Google Scholar]
- 98.Vieira P., et al. Cellular and transcriptional responses of resistant and susceptible cultivars of alfalfa to the root lesion nematode. Pratylenchuspenetrans.Front Plant Sci. 2019;10:971. doi: 10.3389/fpls.2019.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muthusamy M., et al. Genome-wide identification of novel, long non-coding RNAs respon-sive to Mycosphaerellaeumusae and Pratylenchuscoffeaeinfections and their differential expression patterns in disease-resistant and sensitive banana culti-vars. Plant Biotechnol Rep. 2019;13:73–83. [Google Scholar]
- 100.Mantelin S., Bellafiore S., Kyndt T. Meloidogyne graminicola:A major threat to rice agriculture. Mol Plant Patho. 2017;l18:3–15. doi: 10.1111/mpp.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waqas M., et al. Biochar amendment changes jasmonic acid levels in two rice varieties and alters their resistance to herbivory. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Akyazi R., et al. Ecofriendly control approaches for Polyphago tarsonemuslatus(Acari:Tarsonemidae) on tea (Camellia sinensis L.) Int. J. Acarol. 2019;45:79–89. [Google Scholar]
- 103.Edussuriya R., et al. Influence of biochar on growth performances, yield of root and tuber crops and controlling plant-parasitic nematodes. Biochar. 2023;5:68. doi: 10.1007/s42773-023-00261-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.