Abstract
Bladder cancer is the tenth most prevalent malignancy worldwide, with a significant mortality burden. Urothelial carcinoma (UC) is the most common histological subtype, and treatment options are guided by whether the disease is muscle-invasive (MIBC) or non-muscle-invasive (NMIBC), with subsequent risk group stratification. The growing popularity of immune checkpoint inhibitors (ICIs) to treat MIBC and NMIBC as either monotherapy or combined with intravesical agents, may radically change the treatment paradigm of UC. Current treatments for NMBIC includes intravesical chemotherapy after trans-urethral resection of the bladder tumour, intravesical bacillus Calmette-Guerin (BCG) or radical cystectomy. Cisplatin-based chemotherapy is widely regarded as the first-line treatment for metastatic UC due to its beneficial response and survival rates when compared to alternative therapies. However, up to 70 % of metastatic UC patients are ineligible, and the prognosis of these patients remains poor, with a median survival of 13–16 months. For NMIBC and MIBC, ICIs provide a promising alternative for cisplatin-ineligible patients. In UC, ICIs including atezolizumab, nivolumab, avelumab, and pembrolizumab are Food and Drug Administration (FDA)-approved for monotherapy, and have demonstrated promising results, particularly in those who cannot receive cisplatin-based chemotherapy, and as a second-line treatment option for recurrent UC following platinum-based chemotherapy. It is important to consider that some patients may experience adverse events (AEs) with limited clinical benefit. Infusion-related reactions and immune-mediated AEs (imAEs) such as colitis, endocrinopathies, hepatitis, pneumonitis, interstitial lung disease, renal dysfunction, nephritis, cutaneous and neurological toxicities must be monitored for. Currently, there is no clear consensus on the role of a ‘two-year stopping rule’ in reducing the risk of imAEs, with further research on the optimal treatment duration of ICIs required. With increased ICI use, vigilance regarding their side effects is imperative. This review aims to provide an updated overview of ICI toxicities in bladder cancer, to assist clinicians in their therapeutic decision-making, with consideration of patient characteristics and the clinical context.
Keywords: Bladder Cancer, Toxicity, Immune Checkpoint Inhibitor, PD-L1, PD-1, CTLA-4
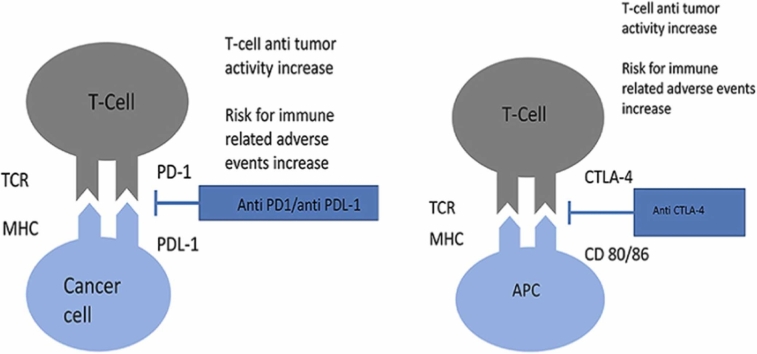
Graphical Abstract
Mechanism of action of anti CTLA-4 and anti PD-1/PD-L1 agents. Immune-mediated adverse events seem to result from an exaggerated immune response, due to the breakdown of immunological self-tolerance.
TCR: T-cell receptor, MHC: major histocompatibility complex, CD80/86: cluster of differentiation 80/87, PD1: programmed cell death protein 1, PD-L1: programmed death-ligand, CTLA-4: cytotoxic T-lymphocyte associated protein 4
Highlights
-
•
Overview of immune immune checkpoint inhibitors (ICIs) toxicities in bladder cancer.
-
•
Atezolizumab for the treatment of the bladder cancer.
-
•
Avelumab as a maintenance therapy for locally advanced, metastatic urothelial cancer.
-
•
Nivolumab as an adjuvant treatment in high risk urothelial cancer patients or after disease progression in pre-treated with platinum-based chemotherapy patients.
-
•
Pembrolizumab for second-line treatment in pre-treated patients with muscle-invasive bladder cancer.
1. Introduction
According to the Global Cancer Observatory (GLOBOCAN), bladder cancer (BC) represents the tenth most common cancer worldwide, with over 573,000 new cases and 213,000 deaths reported in 2020 [1]. Histologically, urothelial carcinoma (UC) accounts for approximately 90 % of BC cases and treatment options are guided by whether the disease is muscle-invasive (MIBC) or non-muscle-invasive bladder cancer (NMIBC), with subsequent risk group stratification [2], [3]. The majority of cases are NMIBC, in a ratio of 3:1 when compared with MIBC [3]. The European Society of Medical Oncology (ESMO), the American Urological Association (AUA) and the European Association of Urology (EAU) have produced valuable recommendations for risk-stratification criteria and initial NMIBC treatment options [3], [4]. For low-risk NMIBC, intravesical chemotherapy after trans-urethral resection of bladder tumour (TURBT) may be initiated, whilst intravesical bacillus Calmette-Guerin (BCG) or radical cystectomy is recommended for high-risk NMIBC [3], [4]. Traditionally, only intravesical chemotherapy agents have been used for adjuvant treatment following TURBT, but emerging data on the use of systemic immune checkpoint inhibitors (ICIs), either as a monotherapy or combined with intravesical agents, may radically change this treatment paradigm [4], [5]. For MIBC, multidisciplinary care is required to consider patient suitability for cisplatin-based chemotherapy and subsequent radical cystectomy with pelvic lymph node dissection (PLND) [3]. However, recent studies have also demonstrated promising results for the use of ICIs in metastatic UC (mUC) [5], [6].
Before the approval of ICIs, there were no Food and Drug Administration (FDA)-approved treatments for patients with disease progression despite platinum-based chemotherapy, which is the established first-line therapy for mUC [7]. Unfortunately, up to 70 % of patients with mUC are ineligible for this therapy, and the prognosis of these patients remains poor, with a median survival of 13–16 months [6], [7], [8], [9]. Since 2016, the FDA has approved seven different ICI drugs for various cancers: programmed cell death protein 1 (PD-1) inhibitors (nivolumab, pembrolizumab, and cemiplimab), programmed death-ligand 1 (PD-L1) inhibitors (atezolizumab, durvalumab, and avelumab), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) inhibitor (ipilimumab) [10]. For UC specifically, atezolizumab, nivolumab, avelumab, and pembrolizumab are FDA-approved [10]. ICIs bind to proteins such as PD-1, PD-L1 and CTLA-4 to overcome the tumour microenvironment inhibition of the body’s antitumoral immune response [11], (graphical abstract)]. Physiologically, PD-1 is primarily expressed on T cells, monocytes, natural killer (NK) cells, and macrophages, while PD-L1 is commonly expressed on antigen-presenting cells (APCs) and tumor cells. In tumor cells, PD-L1 expression is upregulated through crosstalk with lymphocytes in response to inflammatory signals, as well as by proliferative oncogenic signals such as the epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 3 (STAT3) [12]. This sustains innate and adaptive immune resistance in cancer [13]. Targeting PD-1/PD-L1can restore anti-tumor immune responses, mediated by CD8+ lymphocytes. Several of these drugs were approved for the high unmet need for alternative therapy among UC patients who, for various health reasons, such as pre-existing renal impairment or hearing impairment, could not receive platinum-based chemotherapy. Other approvals were for second-line therapy of recurrent UC following platinum-based chemotherapy. However, considering the aggressive nature of this malignancy, many patients who experience recurrence may be too unwell to receive immunotherapy or any other second-line therapy [14].
It is important to consider that patients may have limited clinical benefit or experience adverse events (AEs) with ICI treatment. Infusion-related reactions such as anaphylaxis and hypersensitivity may occur, as well as immune-mediated adverse events (imAEs) such as colitis, endocrinopathies, hepatitis, pneumonitis, interstitial lung disease, renal dysfunction, nephritis, cutaneous and neurological toxicities [15], [16]. Uncertainty also persists over treatment duration and the role of a ‘two-year stopping rule’ in reducing the risk of imAEs. As the popularity of ICI therapy grows, vigilance regarding their side effects is imperative. This review aims to provide an updated overview of ICI toxicities in BC, to assist clinicians in their therapeutic decision-making, with consideration of patient characteristics and the clinical context.
2. Immune checkpoint inhibitor therapy
2.1. PD-L1 checkpoint inhibitors
2.1.1. Atezolizumab
Atezolizumab was the first FDA-approved ICI for BC, based on IMvigor 210 phase II clinical trial results. Through targeting the PD-L1 protein, atezolizumab prevents PD-1 and B7–1 termination of T-cell signalling and proliferation, thereby promoting anti-tumour immunity [17]. The single arm, IMvigor 210 study treated 119 patients with one or more doses of atezolizumab, and found the objective response rate was 23 % at 20 months (95 % confidence interval (CI) 16–31) and median overall survival (OS) of 15.9 months (95 % CI 10.4 to not estimable) [17], [18]. The response was greatest amongst those whose tumour-infiltrating immune cells had the highest levels of PD-L1 protein on the surface (>5 %), as assessed with the Ventana PD-L1 (SP142) assay, which may be a valuable tool in evaluating which patients would benefit most from atezolizumab treatment [18].
IMvigor 210 reported a safety profile consistent with previous atezolizumab trials across a range of cancers and compared favourably with cytotoxic chemotherapy [19], [20], [21], [22], [23]. 119 patients between the ages 51–92 years received atezolizumab every 12 weeks until disease progression (DP), withdrawal or death. Patients treated with comparator therapy gemcitabine-carboplatin reported 21 % treatment discontinuation, with a high proportion of patients experiencing haematological toxicity (e.g. neutropenia), whereas only 9 (8 %) patients in the atezolizumab cohort discontinued treatment because of an AE, with no neutropenia reported [18]. Furthermore, no loss in median glomerular filtration rate was reported in this cohort through 27 or more treatment cycles — a finding pertinent to patients with reduced kidney function or a single kidney. Treatment-related AEs of any grade that occurred in 10 % or more patients included fatigue (36 [30 %] patients), diarrhoea (14 [12 %] patients), and pruritus (13 [11 %] patients). with one treatment-related death (sepsis) reported [18]. Immune-mediated events occurred in 14 (12 %) patients which were controlled with systemic corticosteroids [18].
The IMvigor 211 phase III clinical trial was subsequently designed to compare the OS outcomes of atezolizumab and chemotherapy among 931 patients with UC progression despite receiving at least one platinum-based chemotherapy regimen [17], [24]. Patients with transitional cell carcinoma received atezolizumab every 3 weeks for a median of 2.8 months. Median OS was 11.1 months with atezolizumab and 10.6 months with chemotherapy, resulting in a similar hazard ratio, 0.85 (95 % CI 0.73–0.99 [24]. Even for the subgroup of patients with the highest level of PD-L1 expression (expression on >5 % tumour infiltrating immune cells), median OS was not statistically significantly higher with atezolizumab (11.1 months) than with chemotherapy (10.6 months, hazard ratio 0.87; 95 % CI 0.63–1.21) [17], [24]. Furthermore, median progression-free survival (PFS) for the overall population was shorter with atezolizumab than with chemotherapy (2.1 months compared with 4.0 months), although the duration of response was longer. The trial concluded that atezolizumab was not associated with significantly longer OS than chemotherapy in patients with platinum-refractory mUC overexpressing PD-L1 [24].
Fewer patients in the atezolizumab arm of IMvigor 211 had grade 3 or 4 treatment-related AEs than in the comparator arm (20 % compared with 43 %) or discontinued treatment as a result of AEs (7 % compared with 18 %) [24]. Clinical experts explained that in their experience of using atezolizumab, it was well tolerated and reported fewer severe AEs than chemotherapy treatment. The safety profile for atezolizumab favourably compared with chemotherapy, however, there remains no available data regarding the safety of atezolizumab in paediatric patients and pregnant or breastfeeding women [17]. The National Institute for Health and Care Excellence (NICE) recommend atezolizumab for routine use in the England National Health System (NHS) for patients with previously treated locally advanced or mUC, who had undergone platinum-based chemotherapy, only if atezolizumab treatment is discontinued at two years (or earlier if there is no clinical benefit) [25].
IMvigor 130 set out to assess the OS and PFS of atezolizumab plus platinum-based chemotherapy (group A), atezolizumab monotherapy (group B), or placebo plus platinum-based chemotherapy (group C) [18], [26]. Treatment was administered in patients between the ages 61–75 years, every 3 weeks until DP or unacceptable toxicity. AEs that led to withdrawal of any agent occurred in 156 (34 %) patients in group A, 22 (6 %) patients in group B, and 132 (34 %) patients in group C [26]. Fifty (11 %) patients in group A, 21 (6 %) patients in group B, and 27 (7 %) patients in group C had AEs that led to discontinuation of atezolizumab or placebo [18], [25]. The trial supported that the addition of atezolizumab to platinum-based chemotherapy as first-line treatment prolonged PFS in patients with metastatic urothelial carcinoma [26]. The safety profile of the combination was consistent with that observed with the individual agents. Therefore, the study concluded that the use of atezolizumab plus platinum-based chemotherapy as a potential first-line treatment option for mUC would have significant benefit [26].
Patients receiving atezolizumab are at risk of developing imAEs either during therapy or after treatment discontinuation. Treatment related AEs of grade 3 or 4 occurred in 81 % of group A, 15 % of group B and 81 % of group C, with the most common being myelosuppression. ImAEs of grade 3 and 4 were treated with systemic corticosteroids in 12 % of group A, 8 % in group B and 6 % in group C. Early identification and management of imAEs are crucial in these individuals, with notable AEs highlighted in Table 1. Laboratory tests must be performed and reviewed before and during treatment with atezolizumab [26]. Moreover, close communication between the ordering physician, the pharmacist, and the nursing team about any infusion-related reactions or laboratory abnormalities related to the drug should be encouraged. Signs and symptoms of imAEs during treatment or long after discontinuation of atezolizumab should be carefully monitored, and appropriate clinical investigations must be performed to rule out other aetiologies. Detailed prescribing information for important dose management information specific to adverse reactions should be easily accessible and the relevant specialities should be promptly informed. Such a holistic approach would lead to timely recognition and management, resulting in improved outcomes.
Table 1.
AEs and laboratory tests that need to be monitored before and throughout the treatment period.
| AEs | Laboratory Tests |
|---|---|
| Exfoliative dermatitis Autoimmune colitis Endocrinopathy Immune-mediated neurological Immune-mediated cardiovascular |
Blood glucose level Renal function Liver function Thyroid function |
Abbreviations – AEs: adverse events
2.1.2. Avelumab
On June 30, 2020, the FDA approved the use of avelumab as a maintenance therapy for locally advanced, mUC that has not progressed with first-line platinum-based chemotherapy [27]. The efficacy of the drug was assessed in the JAVELIN Bladder 100 trial where 700 patients between the ages 37–90 years, were randomly assigned to receive bi-weekly Avelumab with supportive care or best supportive care (BSC) alone until DP, withdrawal or unacceptable toxicity [28]. This study concluded that the addition of avelumab to BSC significantly prolonged OS compared to BSC alone (control). OS at one year was 71.3 % (95 % CI, 66.0–76.0) in the avelumab group, compared with 58.4 % (95 % CI, 52.7–63.7) in the control; the median OS was 21.4 months (95 % CI, 18.9–26.1) and 14.3 months (95 % CI, 12.9–17.9), respectively (95 % CI, 0.56–0.86; P=0.001). Furthermore, in the PD-L1 positive population, OS was observed to be significantly longer with avelumab compared with the control, with OS at one year reported to as 79.1 % (95 % CI, 72.1–84.5) in the avelumab group, and 60.4 % (95 % CI, 52.0–67.7) with BSC (stratified hazard ratio, 0.56; 95 % CI, 0.40–0.79; repeated CI, 0.39–0.94; P<0.001). Overall, the median PFS was calculated as 3.7 months (95 % CI, 3.5–5.5) in the avelumab group and 2.0 months (95 % CI, 1.9–2.7) in the control group (stratified hazard ratio for disease progression or death, 0.62; 95 % CI, 0.52–0.75). The PD-L1-positive population observed similar findings, with a median PFS of 5.7 months (95 % CI, 3.7–7.4) in the avelumab group compared with 2.1 months (95 % CI, 1.9–3.5) in the control group (stratified hazard ratio, 0.56; 95 % CI, 0.43–0.73) [28].
In regard to the safety profile of avelumab, AEs of any grade occurred in 337 of 344 patients (98.0 %) in the avelumab group and in 268 of 345 patients (77.7 %) in the control arm, with AEs of grade 3 or higher occurring in 163 patients (47.4 %) and 87 patients (25.2 %), respectively [28]. The most common grade 3 AEs in the avelumab group includes urinary tract infection (4.4 %), anaemia (3.8 %), haematuria (1.7 %) and fatigue (1.7 %). In the avelumab arm, AEs led to treatment discontinuation in 41 patients (11.9 %). Two deaths were reported, attributed to the toxicity of the drug. One patient had sepsis after a urinary tract infection and possible catheter venous catheter infection after receiving eleven infusions of avelumab. The second patient suffered an ischaemic stroke 100 days after receiving a single dose of the trial drug and after disease progression, and AEs including limb venous thrombosis, pulmonary embolism and acute myocardial infarction [28].
In the avelumab arm, 29.4 % of patients were reported to have imAEs according to a pre-specified case definition, with 7 % experiencing a grade 3 event. No grade 4 or fatal imAEs were noted. The most common imAEs – as shown in Table 2 – included thyroid disorders, which occurred in 42 patients (12.2 %). In individuals who received avelumab, high-dose glucocorticoids (≥40 mg total daily dose of prednisone or equivalent) were administered after an imAE in 31 patients (9.0 %) [28].
Table 2.
Summary of AEs in trials assessing the safety of avelumab in patients with UC.
| AEs | Laboratory Tests |
|---|---|
| Pruritus | |
| Hypothyroidism | |
| Diarrhoea | |
| Infusion-related reaction | |
| Asthenia | |
| Fatigue | |
| Rash | Thyroid function tests |
| Chills | Full blood count |
| Nausea | Urea and electrolytes |
| Arthralgia | Amylase |
| Pyrexia | Lipase |
| Hyperthyroidism | |
| Dry skin | |
| Increased amylase | |
| Increased lipase |
Abbreviations – AEs: adverse events, UC: urothelial cancer
2.2. PD-1 checkpoint inhibitors
PD-1 inhibitors are human immunoglobulin antibodies which bind to the PD-1 receptor, blocking its interaction with its ligands: PD-L1 and PD-L2 [29]. The PD-1/PD-L1 pathway promotes immune tolerance through the inhibition of both the adaptive and innate immune system [30]. These regulatory mechanisms prevent autoimmunity by controlling immune responses within a physiological range [31]. However, negative regulation of T-cell activity due to binding of the PD-1 receptor can lead to dampening of anti-tumour immune responses [32]. Therefore, blockade of the PD-1 pathways allows tumour-reactive T-cells to generate an effective antitumour response [31], [33]. PD-1 inhibitors exert anti-tumour properties through regulating the host immune system instead of causing direct cell toxicity like chemotherapeutic agents [32], [34]. Currently, two PD-1 inhibitors have been approved by the FDA for use in urothelial cancer – nivolumab and pembrolizumab.
2.2.1. Nivolumab
Nivolumab was approved by the FDA in 2021, as an adjuvant treatment in UC patients who are at a high risk of recurrence after radical resection, or disease progression after platinum-containing chemotherapy, following the CheckMate 274 trial [35], [36]. This trial was a phase III, double-blinded, randomised control trial (RCT), designed to assess the efficacy and safety of nivolumab as an adjuvant treatment in patients with muscle-invasive urothelial carcinoma after radical surgery [37]. Seven hundred patients between the ages 30–92 years were randomly allocated receive nivolumab monotherapy (treatment arm) or the placebo arm bi-weekly for 1 year [37]. All participants were confirmed to have disease free status within 4 weeks of randomisation [38]. The two primary end-points were disease-free survival (DFS) among all patients who underwent randomisation and among those with a tumour PD-L1 expression level of 1 % or more [37].
Intention-to-treat analysis demonstrated the median DFS as 20.8 months (95 % CI, 16.5–27.6) in the nivolumab group and 10.8 months (95 % CI, 8.3–13.9) in the placebo group [37]. The percentage of disease-free patients who were alive at six-months was 74.9 % with nivolumab and 60.3 % with the placebo (hazard ratio for disease recurrence or death, 0.70; 98.22 % CI, 0.55–0.90). In those with PD-L1 expression of 1 % or more, 74.5 % were alive after six months of treatment, compared with 55.7 % in the placebo group (hazard ratio, 0.55; 98.72 % CI, 0.35–0.85; P <0.001). Additionally, patients who were free from recurrence outside the urothelial tract at six months was 77.0 % with nivolumab and 62.7 % with the placebo (hazard ratio, 0.72; 95 % CI, 0.59–0.89) [37].
AEs were evaluated according to the National Cancer Institute Common Terminology Criteria for AEs, version 4.0 (CTCAE) [39]. The most prevalent AEs of any grade in the nivolumab arm included pruritis (23.1 %), fatigue (17.4 %) and diarrhoea (16.8 %) [37]. The most common treatment-related AEs which were grade 3 or higher in the nivolumab group were elevations of serum lipase (5.1 %) and amylase (3,7 %), diarrhoea (0.9 %), colitis (0.9 %) and pneumonitis (0.9 %). Three treatment-related deaths were reported – two due to pneumonitis and one due to bowel perforation. Both patients with pneumonitis were initiated on glucocorticoid treatment at the onset of pneumonitis (3 days and 16 days after the last dose of trial therapy, respectively). The patient with bowel perforation began glucocorticoid therapy 5 days after the last dose of trial therapy [37].
No deterioration in quality of life was noted between both arms, which was assessed using the European Organisation for Research and Treatment of Cancer (EORTC) Core Quality of Life questionnaire (QLQ-C30) global health status score. Therefore, the use of nivolumab showed a significant clinical benefit compared with the placebo, both in the intention-to-treat population and in patients with a PD-L1 expression of 1 % or more. However, there was limited impact of AEs affecting patients’ health-related quality of life, which was supported by the results of the EORTC QLQ-C30 [37].
The CheckMate 032 was an open-label, phase I/II trial assessing the safety of nivolumab in patients with progression of advanced or mUC, following platinum-based chemotherapy [40]. Seventy-eight patients between the ages 31–85 years received nivolumab monotherapy every two weeks until progression or unacceptable toxicity. Patients had the option to switch to a combination therapy of nivolumab and ipilimumab (an ICI which blocks the cytotoxic lymphocyte antigen-4) following radiological disease progression outlined by investigator-assessed Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. They were then followed-up for a minimum of nine months. Interestingly enough, 21.8 % of patients experienced grade 3 or 4 treatment-related AEs, most commonly elevated lipase (5.1 %), elevated amylase (3.8 %), fatigue (2.6 %) and maculopapular rash (2.6 %). AEs of any grade which occurred in more than 10 % of patients included fatigue (36 %), pruritis (29 %), maculopapular rash (14 %), elevated lipase (11 %) and nausea (10 %). There were two treatment-related deaths due to grade 4 pneumonitis and grade 4 thrombocytopenia. Nivolumab monotherapy was generally well-tolerated in heavily pre-treated patients, suggesting a positive benefit/risk ratio [40]. However, this study had a small sample size and a short follow-up period, therefore the findings were further explored in the CheckMate 275 study [40], [41].
CheckMate 275 was a phase II trial of bi-weekly nivolumab monotherapy in 278 patients with progressive UC during or after platinum-based chemotherapy, until DP or unacceptable toxicity [41]. Nivolumab was administered after resolution of toxicities from any previous anti-cancer therapies to grade 1 or baseline. Treatment-related AEs occurred in 64 % of patients, with fatigue (17 %) reported as the most common complication of any grade, and diarrhoea (2 %) and fatigue (2 %) were the most frequent grade 3 treatment-related event. Treatment was discontinued in four patients (1 %) due to pneumonitis and in two patients due to pemphigoid. Most AEs were managed using systemic corticosteroids. There were three patient deaths related to treatment, due to pneumonitis, acute respiratory failure, and cardiovascular failure [41]. EORTC QLQ-C30 scores completed by 97 % of patients were stable throughout the trial, with improvement towards the end of week 41, suggesting a low impact of AEs on patient quality of life [41], [42]. Table 3 depicts the AEs of CheckMate 274, −032 and −275 trials.
Table 3.
Summary of AEs in trials assessing the safety of nivolumab monotherapy in patients with UC.
| Trial | Common AEs | Serious AEs |
|---|---|---|
| CheckMate 274 | Fatigue Pruritis Maculopapular rash Elevated lipase and amylase Thyroid dysfunction |
Pneumonitis Colitis Diarrhoea Elevated lipase and amylase |
| CheckMate 032 | Fatigue Pruritis Maculopapular rash Elevated lipase and amylase Nausea Thyroid dysfunction |
Pneumonitis Dyspnoea |
| CheckMate 275 | Fatigue Pruritis Diarrhoea Nausea Rash |
Pneumonitis Pemphigoid Cardiovascular failure |
Abbreviations – AEs: adverse events, UC: urothelial cancer
2.2.2. Pembrolizumab
Pembrolizumab is a PD-1 inhibitor which was approved by the FDA for second-line treatment in individuals with MIBC following the KEYNOTE-045 trial, and treatment for NMIBC with carcinoma in-situ based on data from the KEYNOTE-057 study [32], [43].
The KEYNOTE-045 was a phase III RCT, investigating pembrolizumab as second-line therapy in patients with advanced transitional UC during or following platinum-based chemotherapy [44]. Patients were randomly assigned to receive either pembrolizumab or a chemotherapy agent – paclitaxel, docetaxel or vinflunine every 3 weeks. Treatment was continued until RECIST-defined disease progression, development of unacceptable toxic effects or completion of two years of pembrolizumab therapy. The OS at 12 months in the pembrolizumab group was significantly longer at 43.9 % (95 % CI, 37.8–49.9) compared to 30.7 (95 % CI: 25.0–36.7) in the groups receiving chemotherapy, but there was no significant difference in PFS. There was, however, a significant difference in the frequency of treatment-related AEs in the pembrolizumab group compared to the chemotherapy group [44].
Overall, AEs occurred in 60.9 % of the patients who received pembrolizumab, whilst 90.2 % was reported in the chemotherapy group [44]. AEs of grade 3 or above were also less frequent, with 15.0 % in the pembrolizumab group and 49.4 % in the chemotherapy group. The most common AE associated with pembrolizumab included pruritis (19.5 %), fatigue (13.9 %), anaemia (24.7 %) and nausea (10.9 %), with the incidence of grade 3 or above AEs being no more than 5 % [44]. In the chemotherapy group, the most prevalent AEs included alopecia (37.6 %), fatigue (27.8 %) and anaemia (7.8 %), with AEs of grade 3 or above seeing more than 5 %, including: neutropenia (13.3 %) and anaemia (5.8 %). The only AEs at grade 3 in the pembrolizumab group reported pneumonitis (2.3 %), colitis (1.1 %) and nephritis (0.8 %). Moreover, four deaths were reported in the pembrolizumab group: one due to pneumonitis, one related to urinary tract obstruction, one related to cancer progression and one due to an unspecified cause. Equally, four deaths also occurred in the chemotherapy group: three related to sepsis and one unspecified cause. Thus, most AEs in the pembrolizumab group were of grade 1 or 2 severity, with overall occurrence to be considerably lower compared to the chemotherapy group, which is particularly important as patients with refractory UC are often older with complex comorbidities. The number of deaths in both groups were therefore considered to be reflective of the prognosis of the patient in the population rather than the safety profile of either treatments [44].
Accelerated FDA approval was granted for the use of pembrolizumab as first-line treatment in patients with locally advanced or mUC who were not eligible for cisplatin-containing chemotherapy, based on data from the KEYNOTE-052 study, a single-arm phase II trial [45]. Individuals who were ineligible for cisplatin-containing therapy, with confirmed unresectable transitional, mixed-transitional or non-transitional UC or mUC, received pembrolizumab every three weeks until either completion of 24 months of treatment, suffered intolerable toxicity or confirmed disease progression [46]. AEs were carefully monitored for up to 30 days after treatment completion, and for 90 days for serious AEs. Disease control was achieved in 173 patients (47 %) out of a population size of 370 treated patients [46].
Sixty-two percent of patients in the pembrolizumab arm experienced a treatment-related AE, with 16 % experiencing an AE at grade 3 or above, with the most common being fatigue (2 %), alkaline phosphatase increase (1 %), colitis (1 %) and muscle weakness (1 %) [46]. Serious AEs occurred in 10 % of patients, most commonly pyrexia (1 %), adrenal insufficiency (1 %), arthritis (1 %), diabetic ketoacidosis (1 %), and hepatitis (1 %). AEs occurred in two or more patients including hypothyroidism (6 %), hyperthyroidism (2 %), colitis (2 %), pneumonitis (2 %) and adrenal insufficiency (1 %), which resolved with high-dose corticosteroids. The most common AE which led to treatment discontinuation was colitis, and one patient died due to a treatment-related AE (myositis). Assessment of the long-term outcomes of the KEYNOTE-052 trial reported no new safety concerns, further supporting the use of pembrolizumab as first-line treatment in cisplatin-ineligible patients [47]. Further evaluation of pembrolizumab was conducted in the phase III KEYNOTE-361 trial, as both monotherapy and in combination with chemotherapy [48]. Regarding safety, the most common grade 3 or 4 AE reported in the combination therapy arm was anaemia (30 %). In the treatment arm with pembrolizumab monotherapy, the most reported AEs included diarrhoea (1 %), fatigue (1 %), and hyponatraemia (1 %) [48].
Pembrolizumab was approved by the FDA as monotherapy treatment in patients with BCG-resistant NMIBC based on the outcomes of the KEYNOTE-057 trial [49]. This single-arm phase II study treated 101 patients with at least one dose of pembrolizumab for 24 months or until DP, withdrawal or unacceptable toxicity. Patients had to have undergone complete resection by cystoscopy or TURBT within 12 weeks prior to the trial. 41 % of patients had a complete response, with no evidence of progressive disease at three months. Treatment-related AEs occurred in 67 % of patients, with the most common grade 3 or 4 AEs noted as hyponatraemia (3 %) and arthralgia (2 %). Serious treatment-related AEs occurred in 8 % of patients, including colitis, adrenal insufficiency, and hyperthyroidism, but no deaths were thought to be treatment-related. No new risks or safety concerns were highlighted in this study, when compared with the previously reported safety profile of pembrolizumab. However, imAEs reported in this study, such as hyperthyroidism, hypothyroidism and pneumonitis, are rare but life-threatening, and thus, require early recognition through imaging and blood tests is imperative for efficient management [49]. Table 4 incorporates the AEs, severe AEs, and laboratory abnormalities with pembrolizumab treatment per the FDA prescribing information [50].
Table 4.
AEs, severe AEs, and laboratory abnormalities with pembrolizumab.
| AEs | Severe AEs | Laboratory Abnormalities | |
|---|---|---|---|
| Fatigue Pruritis Nausea Diarrhoea |
Pneumonitis Colitis Hyperthyroidism Anaemia |
Haematology | Anaemia Lymphopenia |
| Biochemistry | Hyperglycaemia Hypoalbuminemia Increased ALP Increased ALT Hyponatraemia |
||
Abbreviations – AEs: adverse events, ALP: alkaline phosphatase, ALT: alanine transaminase
3. Two-year stopping rule
Research on the two-year stopping rule in the context of BC is limited and there is no clear consensus on the optimal duration of ICI therapy. In non-small cell lung cancer (NSCLC), the KEYNOTE-189 trial reported that half of patients who had completed two years of pembrolizumab experienced disease progression after stopping ICI treatment, and 32 % of patients were confirmed to have disease progression after completing 35 cycles (two years) of pembrolizumab in the KEYNOTE-010 trial [51], [52]. Another study identified that for patients who reach partial or complete response during treatment with an anti-PD-L1 ICI, discontinuation of treatment and restarting, if necessary, seems to be reasonable with preserved favourable outcomes [53]. Further research with larger, randomised data sets is required to obtain insight into optimal treatment duration of BC. This data should include the timeframe of AEs within the treatment cycle, in order to consider the duration of treatment which would minimise toxicity risk with preserved outcomes.
4. Conclusion and future perspectives
Over the past few years, tremendous developments in immunotherapy have improved outcomes for patients with BC, with continuous research successfully identifying subgroups of patients who will particularly benefit from such treatments. With any therapy, side effects and toxicities must be carefully considered to evaluate the benefits versus the risks of treatment on a case-by-case basis. Current studies have evaluated the benefit of ICIs in relation to patients with varying PD-L1 expression. An increased level of PD-L1 expression was not found to be related to improved outcomes with the use of certain ICIs such as atezolizumab and avelumab [24], [28]. The tumour microenvironment is far more complex, with UC being among the most biologically and histologically diverse cancers [54]. Therefore, further studies should consider assessing various immune markers such as CD3, CD8, CD68 and PD1, in order to improve the prognostic and predictive values of ICIs [54].
Table 5 summarises the FDA-approved ICI therapies, their indicated use and their most associated toxicities.
Table 5.
FDA-approved immune checkpoint inhibitor therapies for UC, their indications and AEs.
| Immune Checkpoint Inhibitor | Indicated Use | Associated AEs | |
|---|---|---|---|
| PD−1 inhibitor | Nivolumab | Advanced bladder cancer | Pneumonitis Colitis Diarrhoea Elevated lipase and amylase Dyspnoea Pemphigoid Cardiovascular failure |
| Pembrolizumab | Advanced bladder cancer | Pneumonitis Colitis Diarrhoea Hyperthyroidism Anaemia Pruritis Nausea Fatigue |
|
| PD-L1 inhibitor | Atezolizumab | Advanced UC | Exfoliative dermatitis Autoimmune colitis Endocrinopathy Immune-mediated neurological Immune-mediated cardiovascular |
| Avelumab | Maintenance therapy for locally advanced or metastatic disease | Pruritus Hypothyroidism Diarrhoea Infusion-related reaction Asthenia Fatigue Rash Chills Nausea Arthralgia Pyrexia Hyperthyroidism Dry skin Increased amylase Increased lipase |
|
Abbreviations – FDA: Food and Drug Administration, UC: urothelial carcinoma, AEs: adverse events, PD-1: programmed cell death protein 1, PD-L1: programmed death-ligand 1
Despite the popularity of immunotherapy, it is crucial to consider the AEs and their unsuitability for certain groups of patients. In individuals who are progression-free on ICI therapy, studies have suggested that stopping therapy at two years seems reasonable with preserved outcomes, and may reduce the incidence of imAEs, but further high-powered, long-term studies are required to obtain a clear consensus for the optimal treatment duration [51], [52], [53]. Previous trials have yet to report in which treatment cycle common or severe AEs are likely to occur. The reported timeframe of AEs would be helpful in providing guidance on the frequency of monitoring. The method of reporting AEs should also be considered. Physician led reporting can lead to an underestimation of patient symptoms compared to patient led reporting [55]. Currently, the CTCAE is a widely used standardised framework used by clinicians to assess safety in cancer clinical trials. The use of patient-reported outcomes (PRO-CTCAE) introduces patient-centred data which is complementary to exiting safety assessments [56]. This modified framework can be used in future studies for a more detailed assessment of AEs.
The outcomes of BC remain poor and alternative therapies must also be investigated. Currently, other ICIs such as camrelizumab, tremelimumab toripalimab, tislelizumab and ipilimumab are being investigated as potential therapies for BC, which may have a huge impact on patient outcomes [57], [58], [59], [60], [61].
Cadolinimab is the world’s first dual immune checkpoint bi-specific antibody, targeting both CTLA-4 and PD-1, possessing a high binding avidity [62]. Several studies have found that targeting both CTLA-4 and PD-1 significantly improved clinical outcomes compared with just targeting the PD-1 antibody alone in cancers including melanoma, colorectal and renal cell cancers [62], [63], [64], [65]. However, the use of this therapy has been limited due to its toxicity profile [66] Cadonilimab shows minimal antibody-dependent cellular cytotoxicity and cellular phagocytosis, and interleukin-6 and 8 release, features that are likely to contribute to significantly lower toxicities. Higher binding avidity of cadonilimab may also lead to better drug retention in tumours and contribute to better safety while achieving anti-tumour efficacy [62], [63]
Durvalumab was granted accelerated approval in 2017 following a phase I/II study for locally advanced or mUC, which demonstrated an objective response rate (ORR) of 17 %, with high PD-L1 expression patients demonstrating a far greater ORR (26.3 %) compared to low or no PD-L1 expression (4.1 %) [67], [68]. However, a confirmatory trial in 2020, the phase III DANUBE trial, missed its primary endpoints and durvalumab was subsequently withdrawn for its use in previously treated patients with NIMBC and MIBC due to efficacy, rather than toxicity [69]. This highlights the need for ongoing rigorous studies.
To conclude, ICI therapy has demonstrated great possibilities for the treatment of BC, improving OS and PFS in some circumstances. Testing for immunotherapy susceptibility, for example via the Ventana PD-L1 assay, may improve outcomes by providing a personalised and targeted approach to treatment. ImAEs are a leading toxicity-related concern, although toxicity rates are comparable to standard treatment with chemotherapy. Early identification of toxicity is important for prompt intervention, and consideration of treatment appropriateness for patients on a case-by-case basis, incorporating the expertise of the multi-disciplinary team. Future research on optimising patient selection for ICI therapy and their indicated use may effectively decrease this risk. Additionally, alternative ICIs should be further researched for their safety and effectiveness, in addition to alternative therapies such as oncolytic viruses and gene therapy which are currently being investigated, and may prove to be highly beneficial [70], [71].
CRediT authorship contribution statement
Avenie Mavadia: Writing – review & editing, Writing – original draft. Sunyoung Choi: Writing – review & editing, Data curation. Ayden Ismail: Writing – review & editing, Resources. Aruni Ghose: Writing – review & editing, Methodology. Joecelyn Kirani Tan: Writing – review & editing, Data curation. Vasileios Papadopoulos: Writing – review & editing, Software. Elisabet Sanchez: Writing – review & editing, Validation. Stergios Boussios: Writing – review & editing, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Data availability
Data will be made available on request.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Saginala K., Barsouk A., Aluru J.S., Rawla P., Padala S.A., Barsouk A. Epidemiology of bladder cancer. Med. Sci. (Basel). 2020;8(1):15. doi: 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powles T., Bellmunt J., Comperat E., Santis M.D., Huddart R., Loriot Y., et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33(3):244–258. doi: 10.1016/j.annonc.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Matulewicz R.S., Steinberg G.D. Non—muscle-invasive bladder cancer: overview and contemporary treatment landscape of neoadjuvant chemoablative therapies. Rev. Urol. 2020;22(2):43–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Uccello M., Adeleke S., Moschetta M., Ghose A., Boussios S. Immunotherapy for advanced urothelial carcinoma (UC): rational and current evidence. Ann. Palliat. Med. 2023;12(6):1345–1354. doi: 10.21037/apm-22-1350. [DOI] [PubMed] [Google Scholar]
- 6.Parent P., Marcq G., Adeleke S., Turpin A., Boussios S., Rassy E., et al. Predictive biomarkers for immune checkpoint inhibitor response in urothelial cancer. Ther. Adv. Med. Oncol. 2023;15 doi: 10.1177/17588359231192402. 17588359231192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Necchi A., Pond G.R., Giannatempo P., Di Lorenzo G., Eigl B.J., Locke J., et al. Cisplatin based first-line therapy for advanced urothelial carcinoma after previous perioperative cisplatin-based therapy. Clin. Genitourin. Cancer. 2015;13(2):178–184. doi: 10.1016/j.clgc.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellmunt J., von der Maase H., Mead G.M., Skoneczna I., De Santis M., Daugaard G., et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012;30(10):1107–1113. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dash A., Galsky M.D., Vickers A.J., Serio A.M., Koppie T.M., Dalbagni G., et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107(3):506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 10.Okobi T.J., Uhomoibhi T.O., Akahara D.E., Odoma V.A., Sanusi I.A., Okobi O.E., et al. Immune checkpoint inhibitors as a treatment option for bladder cancer: current evidence. Cureus. 2023;15(6)) doi: 10.7759/cureus.40031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barone B., Calogero A., Scafuri L., Ferro M., Lucarelli G., Di Zazzo E., et al. Immune checkpoint inhibitors as a neoadjuvant/adjuvant treatment of muscle-invasive bladder cancer: a systematic review. Cancers (Basel) 2022;14(10):2545. doi: 10.3390/cancers14102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eden D., Ghose A., Moschetta M., Pérez-Fidalgo J.A., Rassy E., Boussios S. Immunotherapy combined with standard therapies in head and neck squamous cell carcinoma - a meta-analysis. Anticancer. Res. 2024;44(3):861–878. doi: 10.21873/anticanres.16880. [DOI] [PubMed] [Google Scholar]
- 13.Rassy E., Boussios S., Pavlidis N. Genomic correlates of response and resistance to immune checkpoint inhibitors in carcinomas of unknown primary. Eur. J. Clin. Invest. 2021;51(9) doi: 10.1111/eci.13583. [DOI] [PubMed] [Google Scholar]
- 14.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18(1):155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boussios S., Sheriff M., Rassy E., Moschetta M., Samartzis E.P., Hallit R., et al. Immuno-oncology: a narrative review of gastrointestinal and hepatic toxicities. Ann. Transl. Med. 2021;9(5):423. doi: 10.21037/atm-20-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel Y., Babu A., Karagkouni F., Ismail A., Choi S., Boussios S. Cardiac toxicities in oncology: elucidating the dark box in the era of precision medicine. Curr. Issues Mol. Biol. 2023;45(10):8337–8358. doi: 10.3390/cimb45100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A. Aleem, H. Shah, Atezolizumab. StatPearls [Internet], Available from: 〈https://www.ncbi.nlm.nih.gov/books/NBK567758/#:~:text=Many%20immune%20and%20tumor-infiltrating%20cells%20express%20programmed%20death-ligand,cytotoxic%20mediators%20leading%20to%20inhibited%20tumor%20cell%20killing〉 [cited 2024 Apr 4].
- 18.Balar A.V., Galsky M.D., Rosenberg J.E., Powles T., Petrylak D.P., Bellmunt J., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C., et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 21.Fehrenbacher L., Spira A., Ballinger M., Kowanetz M., Vansteenkiste J., Mazieres J., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 22.McDermott D.F., Sosman J.A., Sznol M., Massard C., Gordon M.S., Hamid O., et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 2016;34(8):833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A., et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powles T., Durán I., van der Heijden M.S., Loriot Y., Vogelzang N.J. U. De Giorgi, et al., Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 25.Atezolizumab for treating locally advanced or metastatic urothelial carcinoma after platinum-containing chemotherapy [Internet], Available from: 〈https://www.nice.org.uk/guidance/ta525〉 (2018) [cited 2024 Apr 4].
- 26.Galsky M.D., Arija J.Á.A., Bamias A., Davis I.D., De Santis M., Kikuchi E., et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 27.FDA approves avelumab for urothelial carcinoma maintenance treatment [Internet], Available from: 〈https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment#:~:text=On%20June%2030%2C%202020%2C%20the,%2Dline%20platinum%2Dcontaining%20chemotherapy〉 [cited 2024 Apr 4].
- 28.Powles T., Park S.H., Voog E., Caserta C., Valderrama B.P., Gurney H., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 29.Single Technology Appraisal Nivolumab for treating resected high-risk invasive urothelial cancer [ID2694] Committee Papers [Internet], Available from: 〈https://www.nice.org.uk/guidance/ta817/evidence/committee-papers-pdf-11188108765〉 (2021) [cited 2024 Jan 29].
- 30.Han Y., Liu D., Li L. PD-1/PD-L1 pathway: current researches in cancer. Am. J. Cancer Res. 2020;10(3):727–742. [PMC free article] [PubMed] [Google Scholar]
- 31.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 32.Chiang R.S., Glover M.J., Khaki A.R., Srinivas S. Immunotherapy for urothelial carcinoma: focus on clinical utility of nivolumab. Onco. Targets Ther. 2022;15:1259–1269. doi: 10.2147/OTT.S369043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das A., Ghose A., Naicker K., Sanchez E., Chargari C., Rassy E., et al. Advances in adoptive T-cell therapy for metastatic melanoma. Curr. Res. Transl. Med. 2023;71(3) doi: 10.1016/j.retram.2023.103404. [DOI] [PubMed] [Google Scholar]
- 34.Linares C.A., Varghese A., Ghose A., Shinde S.D., Adeleke S., Sanchez E., et al. Hallmarks of the tumour microenvironment of gliomas and its interaction with emerging immunotherapy modalities. Int. J. Mol. Sci. 2023;24(17) doi: 10.3390/ijms241713215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA approves nivolumab for adjuvant treatment of urothelial carcinoma [Internet], Available from: 〈https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-adjuvant-treatment-urothelial-carcinoma〉 [cited 2024 Jan 29].
- 36.Highlights of Prescribing Information, OPVIDO (nivolumab), Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125554s097lbl.pdf〉 [cited 2024 Jan 29].
- 37.Bajorin D.F., Witjes J.A., Gschwend J.E., Schenker M., Valderrama B.P., Tomita Y., et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 2021;384(22):2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An Investigational Immuno-therapy Study of Nivolumab, Compared to Placebo, in Patients With Bladder or Upper Urinary Tract Cancer, Following Surgery to Remove the Cancer (CheckMate 274) [Internet], Available from: 〈https://classic.clinicaltrials.gov/ct2/show/study/NCT02632409〉 [cited 2024 Jan 29].
- 39.Common terminology criteria for adverse events (CTCAE) [Internet], Available from: 〈https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40〉 [cited 2024 Jan 29].
- 40.Sharma P., Callahan M.K., Bono P., Kim J., Spiliopoulou P., Calvo E., et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590–1598. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma P., Retz M., Siefker-Radtke A., Baron A., Necchi A., Bedke J., et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 42.Galsky M.D., Saci A., Szabo P.M., Han G.C., Grossfeld G., Collette S., et al. Nivolumab in patients with advanced platinum-resistant urothelial carcinoma: efficacy, safety, and biomarker analyses with extended follow-up from checkmate 275. Clin. Cancer Res. 2020;26(19):5120–5128. doi: 10.1158/1078-0432.CCR-19-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pembrolizumab (Keytruda): Advanced or Metastatic Urothelial Carcinoma [Internet], Available from: 〈https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-advanced-or-metastatic-urothelial-carcinoma〉 [cited 2024 Jan 31].
- 44.Bellmunt J., de Wit R., Vaughn D.J., Fradet Y., Lee J.L., Fong L., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzman D.L., Agrawal S., Ning Y.M., Maher V.E., Fernandes L.L., Karuri S., et al. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist. 2019;24(4):563–569. doi: 10.1634/theoncologist.2018-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balar A.V., Castellano D., O'Donnell P.H., Grivas P., Vuky J., Powles T., et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 47.Vuky J., Balar A.V., Castellano D., O'Donnell P.H., Grivas P., Bellmunt J., et al. Long-term outcomes in KEYNOTE-052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 2020;38(23):2658–2666. doi: 10.1200/JCO.19.01213. [DOI] [PubMed] [Google Scholar]
- 48.Powles T., Csőszi T., Özgüroğlu M., Matsubara N., Géczi L., Cheng S.Y., et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 49.Balar A.V., Kamat A.M., Kulkarni G.S., Uchio E.M., Boormans J.L., Roumiguié M., et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919–930. doi: 10.1016/S1470-2045(21)00147-9. [DOI] [PubMed] [Google Scholar]
- 50.Highlights of Prescribing information, KEYTRUDA (pembrolizumab) [Internet], Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s066lbl.pdf〉 [cited 2024 Jan 31].
- 51.Rodríguez-Abreu D., Powell S.F., Hochmair M.J., Gadgeel S., Esteban E., Felip E., et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 2021;32(7):881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Herbst R.S., Garon E.B., Kim D.W., Cho B.C., Perez-Gracia J.L., Han J.Y., et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J. Clin. Oncol. 2020;38(14):1580–1590. doi: 10.1200/JCO.19.02446. [DOI] [PubMed] [Google Scholar]
- 53.Sun L., Bleiberg B., Hwang W.T., Marmarelis M.E., Langer C.J., Singh A., et al. Association between duration of immunotherapy and overall survival in advanced non–small cell lung cancer. Jama Oncol. 2023;9(8):1075–1082. doi: 10.1001/jamaoncol.2023.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H., Zhang Q., Shuman L., Kaag M., Raman J.D., Merrill S., et al. Evaluation of PD-L1 and other immune markers in bladder urothelial carcinoma stratified by histologic variants and molecular subtypes. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-58351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao C., Polomano R., Bruner D.W. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs. 2013;36(6):E1–E16. doi: 10.1097/NCC.0b013e318269040f. [DOI] [PubMed] [Google Scholar]
- 56.Kluetz P.G., Chingos D.T., Basch E.M., Mitchell S.A. Patient-reported outcomes in cancer clinical trials: measuring symptomatic adverse events with the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) Am. Soc. Clin. Oncol. Educ. Book. 2016;35:67–73. doi: 10.1200/EDBK_159514. [DOI] [PubMed] [Google Scholar]
- 57.NCT04706598. Safety and Efficacy of Camrelizumab for High-risk NMIBC Failing BCG Treatment, 〈https://clinicaltrials.gov/study/NCT04706598?cond=bladder%20cancer&term=Immunotherapy&intr=Camrelizumab&rank=1〉 [cited 2024 Apr 08].
- 58.NCT02812420. Durvalumab and Tremelimumab in Treating Patients With Muscle-Invasive, High-Risk Urothelial Cancer That Cannot Be Treated With Cisplatin-Based Therapy Before Surgery, 〈https://clinicaltrials.gov/study/NCT02812420?cond=bladder%20cancer&term=Immunotherapy&intr=tremelimumab%20&rank=8〉 [cited 2024 Apr 08].
- 59.NCT04553939. Neoadjuvant Toripalimab in Combination With Gemcitabine Therapy in Cisplatin Ineligible Local Advanved Bladder Cancer, 〈https://clinicaltrials.gov/study/NCT04553939?cond=bladder%20cancer&intr=Toripalimab&rank=4〉 [cited 2024 Apr 08].
- 60.NCT04004221. Study of Tislelizumab in Participants With Locally Advanced or Metastatic Urothelial Bladder Cancer, 〈https://clinicaltrials.gov/study/NCT04004221?cond=bladder%20cancer&intr=tislelizumab%20&rank=6〉 [cited 2024 Apr 08].
- 61.NCT03520491. A Study to Test the Safety of Immunotherapy With Nivolumab Alone or With Ipilimumab Before Surgery for Bladder Cancer Patients Who Are Not Suitable for Chemotherapy. 〈https://clinicaltrials.gov/study/NCT03520491?cond=bladder%20cancer&intr=ipilimumab%20&rank=1〉 [cited 2024 Apr 08].
- 62.Pang X., Huang Z., Zhong T., Zhang P., Wang Z.M., Xia M., et al. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. Mabs. 2023;15(1) doi: 10.1080/19420862.2023.2180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morse M.A., Overman M.J., Hartman L., Khoukaz T., Brutcher E., Lenz H.J., et al. Safety of nivolumab plus low-dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist. 2019;24(11):1453–1461. doi: 10.1634/theoncologist.2019-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao X., McDermott D.F. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert. Opin. Biol. Ther. 2018;18(9):947–957. doi: 10.1080/14712598.2018.1513485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown J.E., Royle K.L., Gregory W., Ralph C., Maraveyas A., Din O., et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2023;24(3):213–227. doi: 10.1016/S1470-2045(22)00793-8. [DOI] [PubMed] [Google Scholar]
- 67.Syed Y.Y. Durvalumab: first global approval. Drugs. 2017;77(12):1369–1376. doi: 10.1007/s40265-017-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powles T., O'Donnell P.H., Massard C., Arkenau H.T., Friedlander T.W., Hoimes C.J., et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. Jama Oncol. 2017;3(9) doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powles T., van der Heijden M.S., Castellano D., Galsky M.D., Loriot Y., Petrylak D.P., et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–1588. doi: 10.1016/S1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 70.Hu H., Xia Q., Hu J., Wang S. Oncolytic viruses for the treatment of bladder cancer: advances, challenges, and prospects. J. Clin. Med. 2022;11(23) doi: 10.3390/jcm11236997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee A. Nadofaragene firadenovec: first approval. Drugs. 2023;83(4):353–357. doi: 10.1007/s40265-023-01846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.