Abstract
The syntheses of previously unknown sulfide- and telluride-pillar[n]arenes are reported here. These macrocycles, among others, were tested as catalysts for alkylation reactions in aqueous solutions. Telluride-pillar[5]arene (P[5]-TePh) showed the best performance, emulating the behavior of the methyltransferase enzyme cofactor S-adenosyl-l-methionine. Using 1.0 mol % of P[5]-TePh, benzyl bromides reacted with NaCN/NaN3 in water, yielding organic nitriles/azides. The catalyst was recycled and efficiently reused for up to six cycles. 1H NMR experiments indicate a possible interaction between the substrate and P[5]-TePh’s cavity.
Introduction
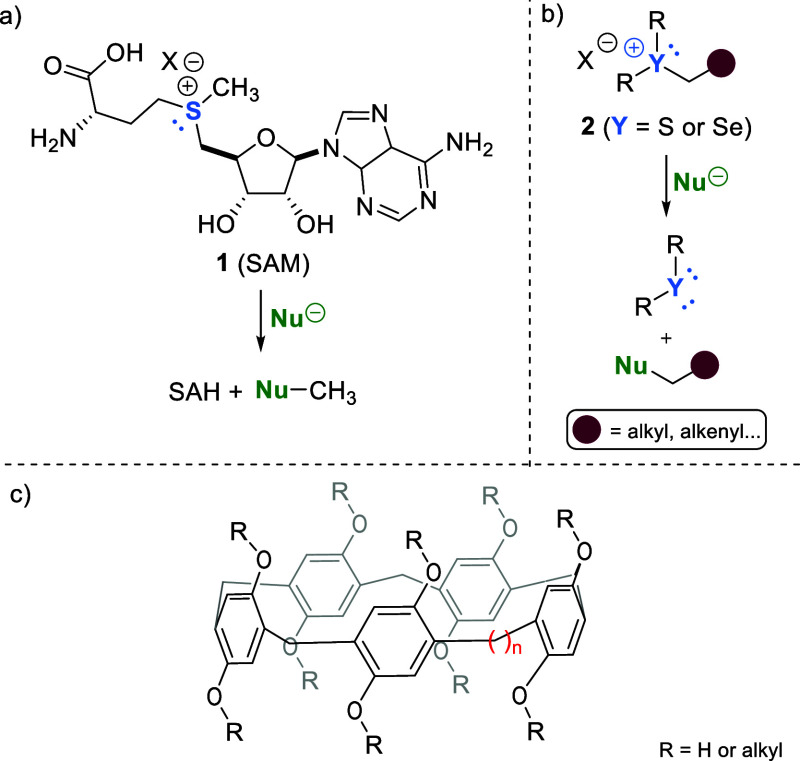
Enzymes are great catalysts with impressive efficiency, specificity, and selectivity.1,2 Methyltransferases, for instance, promote the transference of a methyl group to a variety of substrates (e.g., proteins, lipids, RNA, and DNA) in biological processes related to metabolism, biosynthesis, and detoxification of exogenous compounds.3−5 The cofactor of this enzyme is a sulfonium salt named S-adenosyl-l-methionine (SAM) 1, which is the donor of the methyl group to a given nucleophile (Figure 1a).6−9 Inspired by the transformation mediated by methyltransferases, many reports in the literature have been disclosed with a focus on the preparation and utilization of SAM and derivatives (including its selenium analog) and their application as group transfer agents in a broader sense.10−15 Structurally simpler SAM derivatives 2, most as sulfonium or selenonium salts,16−22 but rarely telluronium salts,23,24 have also been applied as alkylating agents (Figure 1b).
Figure 1.
(a) S-adenosyl-L-methionine (SAM) 1, an electrophile for methylation of nucleophiles; (b) chalcogenonium salts 2 as alkylating agents; (c) representation of a pillar[n]arene.
Synthetic macrocycles with tailored cavities can produce noncovalent bonding interactions with different substrates, resulting in the stabilization and organization of intermediates, offering a different environment for chemical reactions.25 Pillar[n]arenes have emerged as a new generation of supramolecular macrocyclic hosts in the past decade (Figure 1c).26,27 These macrocycles can form inclusion complexes with different small molecules through dipole–dipole interactions, hydrogen bonding, π–π stacking, etc.15,28−46 They have shown several promising applications for drug delivery,31−34 as nanomaterials,35−37 sensors,38−40 and as transmembrane channels.41−43 In organic synthesis, the use of pillar[n]arenes is still little explored, especially owing to their application as catalysts.44−46 Xiao and co-workers reported the synthesis of pillar[5]arenes which when combined with PdCl2(CH3CN)2, could efficiently catalyze Heck coupling reactions of styrene and aryl halides.44 In 2019, Lan and co-workers described a cross-linked porous polymeric material based on pillar[5]quinone, which was used to load Pd-catalyst and prepare a heterogeneous catalyst. The catalyst was highly efficient for Suzuki-coupling reactions and could be recycled and reused six times without any drop in reaction yield.45 Recently, a pillar[5]arene-based [2]rotaxane was designed and developed by Guo and co-workers, which, upon coordination with Pd ions, was efficiently used to catalyze Suzuki couplings.46 Although less reported, there are examples of pillararenes catalyzing reactions in aqueous environments, demonstrating their significant potential as catalysts.47,48
Accordingly, as part of our interest in developing new organochalcogen compounds with privileged molecular structures for different applications,49−53 allied to the synthesis and application of pillar[n]arenes,54−57 we report herein the synthesis of chalcogen-based pillar[5]arenes, including unknown sulfide- and telluride-based pillar[n]arenes. Additionally, we screened their catalytic activity in promoting the alkylation of nucleophiles dissolved in aqueous solutions and the possibility of recycling and reusing them.
Results and Discussion
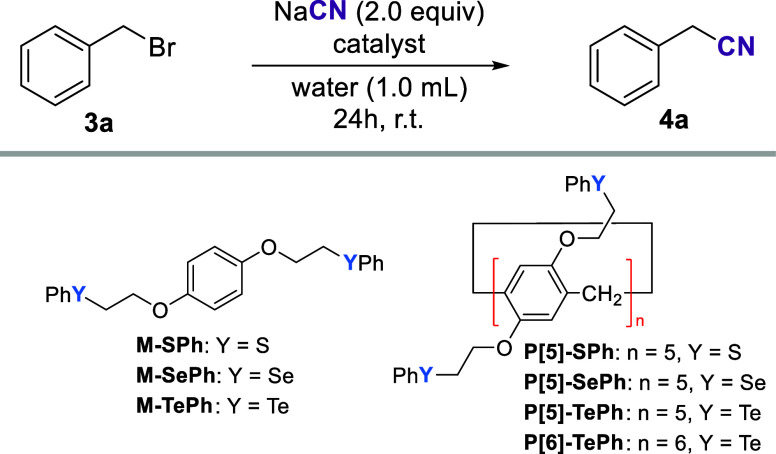
The monomeric catalysts (M-YPh, where Y = S, Se, or Te) depicted in Table 1 employed in this study were prepared from the corresponding monomeric bromides (M-Br).54 Likewise, the pillar[n]arene catalysts (P[n]-YPh, where n is 5 or 6 and Y = S, Se, or Te) were obtained from the bromide starting material. To date, only the selenium-pillar[n]arene analog is known.58 All catalysts were obtained in good yields (see the Supporting Information for the experimental details). To test their ability to promote an alkylation reaction in aqueous solution, we chose the conversion of benzyl bromide 3a to the corresponding cyanide 4a as the model experiment (Table 1).59−61 Without a catalyst, the reaction of 3a and 2 equiv of NaCN produced only 15% of product 4a after stirring the reaction at room temperature for 24 h as previously reported (entry 1).16 Addition of 5.0 mol % of monomeric catalysts accelerated product formation, especially for the tellurium analog M-TePh. In that case, 45% of benzyl nitrile 4a was obtained after 24 h (entry 4). Next, chalcogen-based pillar[n]arenes were screened. At this time, however, 1.0 mol % of the catalyst was used to keep the same amount of chalcogen in the reaction media (entries 5–10). As observed for the monomeric species, the reaction showed a clear trend between the nature of the chalcogen and the yield observed for product 4a. Telluride P[5]-TePh outperformed all other catalysts, delivering the product in near quantitative yield after 24 h of reaction (Entry 7). We performed experiments with shorter duration and observed that after 12 h the reaction was essentially done (entries 8 and 9). It is noteworthy that the contribution of the pillar[n]arene scaffold to the reaction outcome. Keeping the catalytic amount of chalcogen the same in all experiments, 4a formation was significantly increased using P[5]-TePh compared to the reaction using M-TePh (entries 4 and 7). Moreover, it was observed that the pillar[n]arene cavity size was not critical for the catalytic activity under identical reaction conditions. P[6]-TePh performed similarly to P[5]-TePh (entries 7 and 10). Therefore, we continued our study with P[5]-TePh due to its easier preparation when compared to P[6]-TePh.
Table 1. Optimization of the Reaction Conditionsa.
| entry | catalyst | mol % | yield (%)b |
|---|---|---|---|
| 1 | none | 0.0 | 15 |
| 2 | M-SPh | 5.0 | 5 |
| 3 | M-SePh | 5.0 | 29 |
| 4 | M-TePh | 5.0 | 45 |
| 5 | P[5]-SPh | 1.0 | 22 |
| 6 | P[5]-SePh | 1.0 | 55 |
| 7 | P[5]-TePh | 1.0 | 96 |
| 8c | P[5]-TePh | 1.0 | 92 |
| 9d | P[5]-TePh | 1.0 | 40 |
| 10 | P[6]-TePh | 1.0 | 85 |
Reaction conditions: benzyl bromide (0.174 mmol), catalyst (0.0–5.0 mol %), NaCN (0.348 mmol) in H2O (1.0 mL) at 25 °C for 24 h.
Isolated yield.
12 h of reaction.
6 h of reaction.
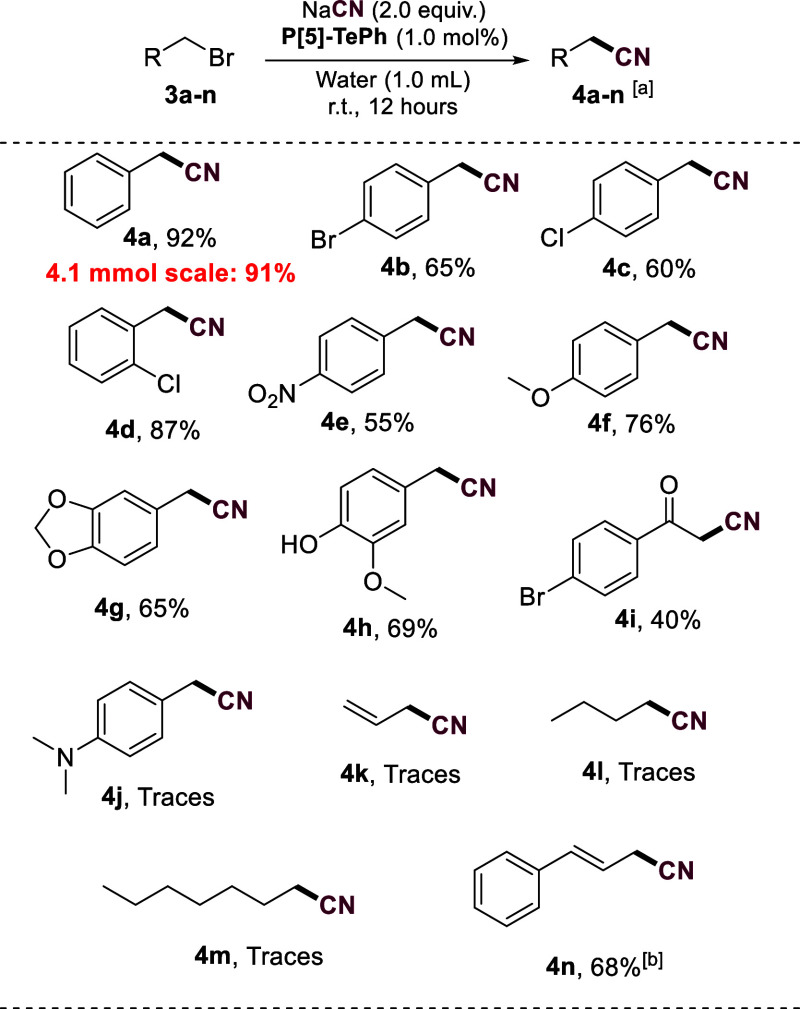
Next, we applied the best reaction conditions to convert other substrates to nitriles catalyzed by 1.0 mol % of P[5]-TePh in aqueous solution (Figure 2). First, we observed that the yield of the conversion of benzyl bromide to 3a was not affected on a larger scale. Then, aryl bromides assembled with electron-donating or electron-withdrawing groups were converted to the corresponding nitriles 4b–h in reasonable to good yields. Noteworthy, no clear correlation between the electronic properties and product yield was observed for these substrates. Product 4i obtained in 40% yield revealed that α-carbonyl bromides are feasible substrates for this transformation. Additionally, nitrile 4n was prepared in 68% yield when the same reaction conditions were applied using cinnamyl chloride as the substrate. On the contrary, products 4j-m from an electron-rich benzyl bromide, allyl bromide, or alkyl bromides could not be produced in desirable amounts. To improve the yield of product 4j, we prepared the bromide starting material and immediately used it in the reaction. However, the strong electron donation capacity of the dimethylamino substituent triggered its decomposition faster than the reaction with the nucleophile. The results collectively indicate that at the current stage, regardless of the substrate solubility in water, only activated starting materials toward displacement reactions are feasible substrates for the transformation. The reaction conditions for effective conversions of less reactive aliphatic halides or to obtain products with lower boiling points should be better designed. Finding better catalysts is crucial in this context.
Figure 2.
Substrate scope for conversion of halides to cyanides catalyzed by P[5]-TePh. aIsolated yield. bCinnamyl chloride was used as substrate.
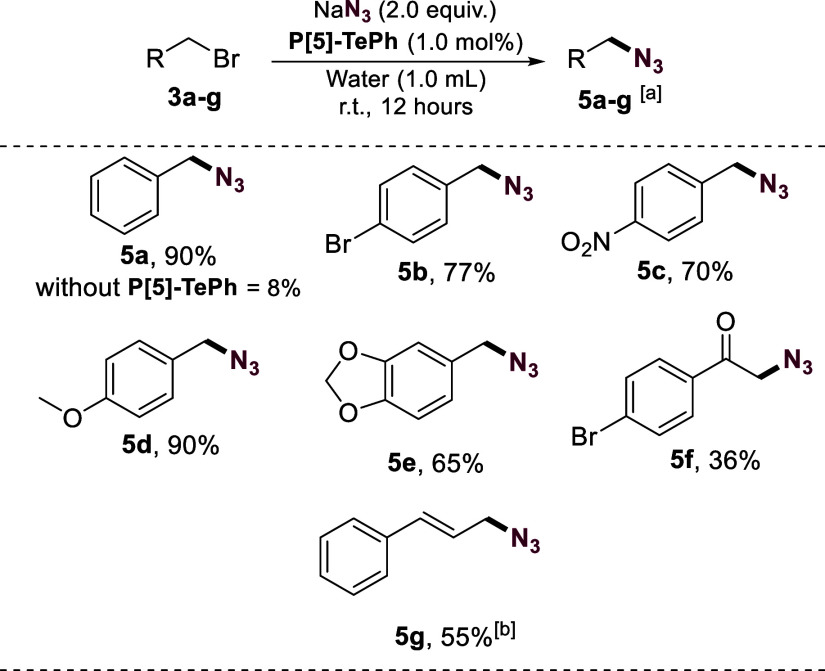
To demonstrate the effectiveness of this protocol, we studied the conversion of selected substrates to azides using NaN3 as the nucleophile (Figure 3). Gratifyingly, benzyl bromide was converted to respective azide 5a in 90% yield. Importantly, without P[5]-TePh, only 8% 5a was obtained. Other benzyl bromides containing electron-donating or electron-withdrawing groups, an α-carbonyl derivative, and cinnamyl chloride were used as substrates. Products 5b-5g were obtained in reasonable to good yields, mirroring the results obtained with cyanide as the nucleophilic species.
Figure 3.
Substrate scope for conversion of halides to azides catalyzed by P[5]-TePh. aIsolated yield. bCinnamyl chloride was used as substrate.
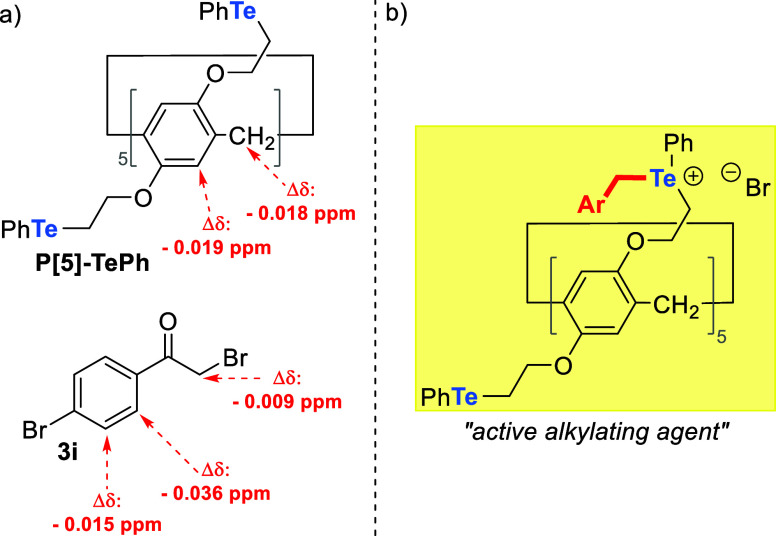
Due to their electron-rich cavities, pillar[n]arenes have excellent host–guest properties, forming stable complexes through charge transfer interactions (e.g., cation-π interactions, CH-π interactions, and π–π stacking).31−34 To further understand the interactions between substrates and P[5]-TePh in the reaction, we performed 1H NMR analysis of a mixture of P[5]-TePh and bromide 3i, chosen because it has a higher molecular weight (more experimental details and 1H NMR spectra are given in the Supporting Information). Although small, various signal shifts could be observed for catalyst and bromide 3i (Figure 4a). These results might suggest the formation of an inclusion complex between the pillar[n]arene and the substrate. In addition, since signal shifts were observed for the aromatic protons of 3i, we suggest that the aryl group of 3i could be engulfed into the P[5]-TePh cavity, while the CH2 group was not. These observations are consistent with previous reports, considering that the cavity size of the pillar[5]arenes can accommodate a benzene ring62 and lead to the formation of an efficient host–guest complex.63−69
Figure 4.
(a) Signal shifts observed by 1H NMR of a mixture P[5]-TePh and 3i in CDCl3; (b) structure of the proposed active alkylating agent.
Nevertheless, the catalytic activity of pillar[n]arene catalysts P[n]-YPh as an alkylating agent is not solely related to the size of the cavity or its host–guest interactions with the substrate. The chalcogen atom is crucial in the substrate conversion to products. Based on our previous results and reported literature,70−73 we propose that the active species is a telluronium salt, as depicted in Figure 4b.
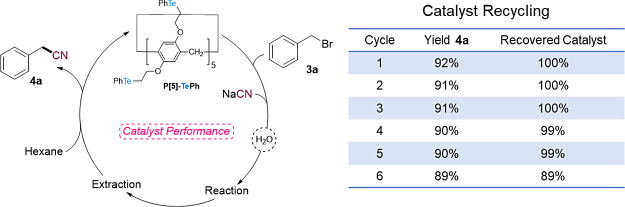
Finally, developing safer and greener organic reactions is a major goal nowadays.74,75 Accordingly, in addition to water being used as the reaction solvent, we studied the possibility of recovering and reusing catalyst P[5]-TePh. To our delight, we found that after the extraction of the reaction mixture, benzyl bromide and product 4a could be separated from P[5]-TePh by washing the crude mixture with hexanes. In this way, the catalyst could be reused for another reaction after water and NaCN addition. This process could be efficiently repeated during 5 cycles with excellent catalyst recovery and without reducing the reaction yield on 4a formation. Although the reaction yield remained constant, after the fifth cycle, a drop in the recovery of the catalyst was observed (Figure 5).
Figure 5.
Evaluation of recovery and reuse of P[5]-TePh on conversion of BnBr to 4a.
Conclusions
In conclusion, we have described, for the first time, the synthesis of sulfur- and tellurium-based pillar[n]arenes. These novel macrocycles were obtained in excellent yields via a simple nucleophilic substitution reaction from the corresponding bromide. The ability of monomeric catalysts M-YPh (5.0 mol %) and pillar[n]arene catalysts P[n]-YPh (1.0 mol %) to promote the conversion of bromides to the related nitriles or azide in an aqueous solution containing NaCN or NaN3 was investigated. It was found that the chalcogen nature and the pillar cavity size are critical for catalytic performance. 1H NMR experiments of mixtures of P[5]-TePh and a substrate indicated possible host–guest interactions in the form of an inclusion complex. Moreover, it was feasible to perform the reaction on a larger scale, and the catalyst P[5]-TePh could be recovered and reused effectively for five reaction cycles. Inspired by these results, we are pursuing further developments, including the design of new chalcogen-pillar[n]arenes and their application to a broader panel of organic transformations.
Experimental Section
General Remarks
The reactions were monitored by TLC carried out on Merck silica gel (60 F254) using UV light as a visualizing agent, an iodine chamber, and 5% vanillin in 10% H2SO4 and heat as developing agents. Baker silica gel (particle size 0.040–0.063 mm) was used for flash chromatography. Proton nuclear magnetic resonance spectra (1H NMR) were obtained on a Varian AS-400 or a Bruker Avance NEO 500 MHz employing a direct broadband probe at 500 MHz. Spectra are recorded in CDCl3 solutions. Chemical shifts are reported in parts per million, referenced to tetramethylsilane (TMS) as the internal reference. Coupling constants (J) are reported in Hertz. Abbreviations to denote the multiplicity of a particular signal are s (singlet), d (doublet), dd (doublet of doublets), ddd (doublet of doublet of doublets), q (quartet), quint (quintet), sex (sextet), t (triplet), and m (multiplet). Carbon-13 (13C{1H} NMR) nuclear magnetic resonance spectra and Carbon-13-attached proton test (13C{1H}-APT NMR) nuclear magnetic resonance spectra were obtained on a Varian AS-400 or on Bruker Avance NEO 500 MHz employing a direct broadband probe at 125 MHz. The high-resolution atmospheric pressure chemical ionization mass spectrometry (APCI-QTOF) and electrospray ionization (ESI-QTOF) mass spectrometry analyses were performed on a Bruker Daltonics micrOTOF-Q II instrument in operating positive mode. The samples were solubilized in HPLC-grade acetonitrile and injected into the source by means of a syringe pump at a flow rate of 5.0 μL min-1. The following instrument parameters were applied: capillary and cone voltages were set to +4000 and −500 V, respectively, with a desolvation temperature of 180 °C. For data acquisition, processing, and isotope simulations, Compass 1.3 for micrOTOF-Q II software (Bruker daltonics, USA) was used. Melting point (mp) values were measured in a Fisatom 430D instrument with a 0.1 °C precision. The Fourier transform infrared (FTIR) measurements were performed on a Nicolet iS50 (Thermo Fisher Scientific). UV–visible absorption spectra were obtained in the UV/visible range (from 200 to 800 nm) using a Varian Cary 50 Scan spectrophotometer and quartz cuvettes with a path length of 10 mm and 1.5 mL. UV–visible spectra were recorded using dichloromethane. Final concentrations of compounds: P[5]Br, P[5]TePh, and P[6]TePh = 1,0 μM. P[6]Br = 0,2 μM.
General Procedure for Alkylation of NaCN Catalyzed by P[5]-TePh
A 10.0 mL round-bottomed glass vial was added with the appropriate alkyl bromide 3a-n (0.174 mmol), P[5]-TePh (0.00174 mmol, 8.0 mg; 1.0 mol %), and water (1.0 mL). The resulting mixture was stirred at room temperature for 5 min. After this, NaCN (0.348 mmol, 17.1 mg) was added, and the mixture was stirred for an additional 12 h. The reactions were monitored by TLC until the total disappearance of the starting materials (the progress of the reaction could also be visually observed. See Figure S1). After that, the reaction mixture was extracted with ethyl acetate (3 × 15.0 mL). The combined organic layers were dried over Na2SO4 and concentrated under a vacuum. The residue was purified by preparative TLC using hexane/ethyl acetate (90:10) as the eluent.
Acknowledgments
This study was financed in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (VN. 310656/2021-4) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES. AYBA and EEA are grateful to Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG for the financial support of this research (grant numbers APQ-00349-22 and BPD-00482-22). VN, PCN, ICC, PC, VHMC, KV thank the Fundação Carlos Chagas Filho de Amparo à Pesquisa no Estado do Rio de Janeiro (E-26/202.911/2019, E-26/210.325/2022, and E-26/200.235/2023) and INCT- Catalysis for the financial support. The authors would like to thank the Laboratório Multiusuário de RMN da UFF – UFFLaReMN, especially to Michael Anderson P. Marques and Lívia Goneli, for the NMR analysis performed.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.4c00997.
General procedures for compounds M-SPh, M-SePh, M-TePh, P[5]-SPh, P[5]-SePh, P[5]-TePh, P[6]-TePh; general procedure for the synthesis of compounds 4a–n and 5a–g; general procedure for Gram-scale reaction; general procedure for recovery and reuse of the catalyst P[5]-TePh; experiments to detect the interactions between substrates and P[5]-TePh; and spectral data and copies of NMR spectra for all compounds (PDF)
Author Contributions
The manuscript was written with all authors’ contributions. All authors have approved the final version of the manuscript.
The Article Processing Charge for the publication of this research was funded by the Coordination for the Improvement of Higher Education Personnel - CAPES (ROR identifier: 00x0ma614).
The authors declare no competing financial interest.
Supplementary Material
References
- Hedstrom L.Enzyme Specificity and Selectivity. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd: Chichester, 2010. [Google Scholar]
- Chen K.; Arnold F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 2010, 3 (3), 203–213. 10.1038/s41929-019-0385-5. [DOI] [Google Scholar]
- Rudenko A. Y.; Mariasina S. S.; Sergiev P. V.; Polshalov V. I. Analogs of S-Adenosyl-L-Methionine in Studies of Methyltransferases. Mol. Biol. 2022, 56 (2), 229–250. 10.1134/S002689332202011X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francioso A.; Fanelli S.; d’Erme M.; Lendaro E.; Miraglia N.; Fontana M.; Carvallaro R. A.; Mosca L. Pharmacokinetic properties of a novel formulation of S-adenosyl-L-methionine phytate. Amino Acids 2021, 53 (10), 1559–1568. 10.1007/s00726-021-03076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck A.; Thompson M. L.; Wong L. S.; Micklefield J. S-Adenosyl-Methionine-Dependent Methyltransferases: Highly Versatile Enzymes in Biocatalysis, Biosynthesis and Other Biotechnological Applications. ChemBioChem. 2012, 13 (18), 2642–2655. 10.1002/cbic.201200556. [DOI] [PubMed] [Google Scholar]
- Lv Y.-B.; Chen C.; Yu Q.-M.; Lyu L.; Peng Y.-F.; Tan X.-D. Synthesis and biological evaluation of novel pentanediamide derivatives as S-adenosyl-L-homocysteine hydrolase inhibitors. Bioorg. Med. Chem. Lett. 2022, 72 (7), 128880 10.1016/j.bmcl.2022.128880. [DOI] [PubMed] [Google Scholar]
- Roje S. S-Adenosyl-L-methionine: Beyond the universal methyl group donor. Phytochemistry 2006, 67 (15), 1686–1698. 10.1016/j.phytochem.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zheng Y. G. SAM/SAH Analogs as Versatile Tools for SAM-Dependent Methyltransferases. ACS Chem. Biol. 2016, 11 (3), 583–597. 10.1021/acschembio.5b00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillson N. J.; Anderson K. E.; Reich N. O. In silico study of selective inhibition mechanism of S-adenosyl-L-methionine analogs for human DNA methyltransferase 3A. Comput. Biol. Chem. 2023, 102 (3), 107796 10.1016/j.compbiolchem.2022.107796. [DOI] [PubMed] [Google Scholar]
- Cornelissen N. V.; Michailidou F.; Muttach F.; Rau K.; Rentmeister A. Nucleoside-modified AdoMet analogues for differential methyltransferase targeting. Chem. Commun. 2020, 56 (14), 2115–2118. 10.1039/C9CC07807J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstock K.; Nilges B. S.; Ovcharenko A.; Cornelissen N. V.; Püllen N.; Lawrence-Dörner A.; Leidel S. A.; Rentmeister A. Enzymatic or In Vivo Installation of Propargyl Groups in Combination with Click Chemistry for the Enrichment and Detection of Methyltransferase Target Sites in RNA. Angew. Chem., Int. Ed. 2018, 57 (21), 6342–6346. 10.1002/anie.201800188. [DOI] [PubMed] [Google Scholar]
- Muttach F.; Rentmeister A. A Biocatalytic Cascade for Versatile One-Pot Modification of mRNA Starting from Methionine Analogues. Angew. Chem., Int. Ed. 2016, 55 (5), 1917–1920. 10.1002/anie.201507577. [DOI] [PubMed] [Google Scholar]
- Tang Q.; Grathwol C. W.; Aslan-Uzel A. S.; Wu S.; Link A.; Pavlidis I. V.; Badenhorst C. P. S.; Bornscheuer U. T. Directed Evolution of a Halide Methyltransferase Enables Biocatalytic Synthesis of Diverse SAM Analogs. Angew. Chem., Int. Ed. 2021, 60 (3), 1524–1527. 10.1002/anie.202013871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A. J.; Shepherd S. A.; Cronin V. A.; Bennett M. R.; Sung R.; Micklefield J. Engineering Orthogonal Methyltransferases to Create Alternative Bioalkylation Pathways. Angew. Chem., Int. Ed. 2020, 59 (35), 14950–14956. 10.1002/anie.202004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S.; Horne W. S.; Islam K. J. Engineering Methyllysine Writers and Readers for Allele-Specific Regulation of Protein-Protein Interactions. Am. Chem. Soc. 2019, 141 (39), 15466–15470. 10.1021/jacs.9b05725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A.; Campos P.; Alberto E. E. Synthetic application of chalcogenonium salts: beyond sulfonium. Org. Biomol. Chem. 2023, 21 (2), 223–236. 10.1039/d2ob01822e. [DOI] [PubMed] [Google Scholar]
- Martins N. S.; Ángel A. Y. B.; Anghinoni J. M.; Lenardão E. J.; Barcellos T.; Alberto E. E. From Stoichiometric Reagents to Catalytic Partners: Selenonium Salts as Alkylating Agents for Nucleophilic Displacement Reactions in Water. Adv. Synth. Catal. 2022, 364 (1), 87–93. 10.1002/adsc.202100797. [DOI] [Google Scholar]
- Taimoory S. M.; Cataldo V. A.; Schäfer A.; Trant J. F.; Guterman R. Not-So-Innocent Anions Determine the Mechanism of Cationic Alkylators. Chem.—Eur. J. 2021, 27 (10), 3440–3448. 10.1002/chem.202004208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F.; Alvarez E. M.; Frank N.; Bohdan K.; Kondratiuk M.; Torkowski L.; Engl P. S.; Barletta J.; Ritter T. Cine-Substitutions at Five-Membered Hetarenes Enabled by Sulfonium Salts. Org. Lett. 2020, 22 (14), 5671–5674. 10.1021/acs.orglett.0c02067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman R.; Miao H.; Antonietti M. J. Thioimidazolium Ionic Liquids as Tunable Alkylating Agents. Org. Chem. 2018, 83 (2), 684–689. 10.1021/acs.joc.7b02631. [DOI] [PubMed] [Google Scholar]
- Lim D.; Wen X.; Seebeck F. P. Selenoimidazolium Salts as Supramolecular Reagents for Protein Alkylation. ChemBioChem. 2020, 21, 3515–3520. 10.1002/cbic.202000557. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Helt J. P.; Autschbach J.; Detty M. R. A New Reaction for Organoselenium Compounds,: Alkyl Transfer from Diorganoselenium(IV) Dibromides to Alkenoic Acids To Give γ- and δ-Lactones. Organometallics 2009, 28 (12), 3426–3436. 10.1021/om900134p. [DOI] [Google Scholar]
- Xu C.; Lu S.; Huang X. A new synthesis of allyl ethers via allyldialkyltelluronium salts. Synth. Commun. 1993, 23 (18), 2527–2531. 10.1080/00397919308012585. [DOI] [Google Scholar]
- Xu C.; Lu S.; HUANG X. The reaction of allyldialkyltelluronium bromides with anilines. Heteroatom Chemistry 1994, 5 (1), 7–8. 10.1002/hc.520050103. [DOI] [Google Scholar]
- Wang K.; Jordan J.; Hu X.-Y.; Wang L. Supramolecular Strategies for Controlling Reactivity within Confined Nanospaces. Angew. Chem., Int. Ed. 2020, 59 (33), 13712–13721. 10.1002/anie.202000045. [DOI] [PubMed] [Google Scholar]
- Ogoshi T.; Yamagishi T.-A.; Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116 (14), 7937–8002. 10.1021/acs.chemrev.5b00765. [DOI] [PubMed] [Google Scholar]
- Ogoshi T.; Kanai S.; Fujinami S.; Yamagishi T.; Nakamoto Y. para-bridged symmetrical pillar[5]arenes:: Their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130 (15), 5022–5023. 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- Wang K.; Jordan J. A.; Velmurugan K.; Tian X.; Zuo M.; Hu X.-Y.; Wang L. Role of Functionalized Pillararene Architectures in Supramolecular Catalysis. Angew. Chem., Int. Ed. 2021, 60 (17), 9205–9214. 10.1002/anie.202010150. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Li C. Pillararene-functionalised graphene nanomaterials. RSC Adv. 2020, 10 (31), 18502. 10.1039/D0RA02964E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K.; Maeda K.; Mizuno M.; Nishina Y.; Fa S.; Ohtani S.; Ogoshi T. Room-Temperature Ring-Opening Polymerization of δ-Valerolactone and ε-Caprolactone Caused by Uptake into Porous Pillar[5]arene Crystals. Angew. Chem.Int. Ed. 2022, 61 (50), e202212874 10.1002/anie.202212874. [DOI] [PubMed] [Google Scholar]
- Zyryanov G. V.; Kopchuk D. S.; Kovalev I. S.; Santra S.; Majee A.; Ranu B. Pillararenes as Promising Carriers for Drug Delivery. Int. J. Mol. Sci. 2023, 24 (6), 5167. 10.3390/ijms24065167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Li H.; Dong J.; Chen Y.; Luan X.; Li X.; Du X. Pillararene-Based Supramolecular Vesicles for Stimuli-Responsive Drug Delivery. Chem.—Eur. J. 2022, 28 (71), e202202050 10.1002/chem.202202050. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Fan J.; Bian X.; Yao H.; Yuan X.; Han Y.; Yan C. A microenvironment sensitive pillar[5]arene-based fluorescent probe for cell imaging and drug delivery. Chin. Chem. Lett. 2022, 33 (4), 1979–1982. 10.1016/j.cclet.2021.10.040. [DOI] [Google Scholar]
- Zhou Y.; Wang Q.; Ma L.; Fan J.; Han Y.; Yan C. Complexation of pillar[5]arene-based Schiff bases with methylene blue: Formation of binary complexes with improved anticancer activity. J. Mol. Struct. 2022, 1257 (5), 132588 10.1016/j.molstruc.2022.132588. [DOI] [Google Scholar]
- Lou X.-Y.; Wang J.; Zhang G.; Yang Y.-W. Pyrene-Armed Pillararene Nanostructure-Facilitated Enhanced Fluorescence and Self-Driven Gelation. ACS Appl. Nano Mater. 2022, 5 (10), 13720–13728. 10.1021/acsanm.2c00161. [DOI] [Google Scholar]
- Zhai M. M.; Wu C.-Y.; Liu Y. A.; Hu W.-B.; Yang H.; Wen K. Hydroxy-Rich Pillar[5]arene-Based Nanoporous Aromatic Frameworks (PAFs) for Efficient CO2 Uptake under Ambient Conditions. ACS Appl. Nano Mater. 2022, 5, 14221–14226. 10.1021/acsanm.2c03045. [DOI] [Google Scholar]
- Filimonova D.; Nazarova A.; Yakimova L.; Stoikov I. Solid Lipid Nanoparticles Based on Monosubstituted Pillar[5]arenes: Chemoselective Synthesis of Macrocycles and Their Supramolecular Self-Assembly. Nanomaterials 2022, 12 (23), 4266. 10.3390/nano12234266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Zhou L.; Bei J.; Zhao Q.; Li X.; He J.; Cai Y.; Chen T.; Du Y.; Yao Y. An enhanced photo-electrochemical sensor constructed from pillar [5] arene functionalized Au NPs for ultrasensitive detection of caffeic acid. Talanta 2022, 243, 123322 10.1016/j.talanta.2022.123322. [DOI] [PubMed] [Google Scholar]
- Paudics A.; Hessz D.; Bojtár M.; Bitter I.; Horváth V.; Kállay M.; Kubinyi M. A pillararene-based indicator displacement assay for the fluorescence detection of vitamin B1. Sens. Actuators B-Chemical 2022, 369, 132364 10.1016/j.snb.2022.132364. [DOI] [Google Scholar]
- Xiao Y.; Huang C.; Li H.; Sun G.; Tu M.; Sun L.; Wang F. A novel AIEE pillar[5]arene-based conjugated oligomer as paraquat fluorescence turn-off sensor. Dyes Pigm. 2023, 210, 111027 10.1016/j.dyepig.2022.111027. [DOI] [Google Scholar]
- Zhu P.; Kong L.; Zhang Y.; Liu Q.; Liao X.; Song Y.; Yang B. Synthetic transmembrane channel molecules formed by acyclic cucurbiturils and pillararene: Tuning cation selectivity and generating membrane potential. J. Mol. Liq. 2023, 372, 121198 10.1016/j.molliq.2023.121198. [DOI] [Google Scholar]
- Xin P.; Kong H.; Sun Y.; Zhao L.; Fang H.; Zhu H.; Jiang T.; Guo J.; Zhang Q.; Dong W.; Chen C. Artificial K+ Channels Formed by Pillararene-Cyclodextrin Hybrid Molecules: Tuning Cation Selectivity and Generating Membrane Potential. Angew. Chem.Int. Ed. 2019, 58 (19), 2779–2784. 10.1002/anie.201813797. [DOI] [PubMed] [Google Scholar]
- Strilets D.; Fa S.; Hardiagon A.; Baaden M.; Ogoshi T.; Barboiu M. Biomimetic Approach for Highly Selective Artificial Water Channels Based on Tubular Pillar[5]arene Dimers. Angew. Chem., Int. Ed. 2020, 59 (51), 23213–23219. 10.1002/anie.202009219. [DOI] [PubMed] [Google Scholar]
- Xiao X.-D.; Bai Y.-L.; Liu J.-Q.; Wang J.-W. Synthesis of novel pillar[5]arene-based N-heterocyclic carbene ligands for Pd-catalysed Heck reactions. Tetrahedron Lett. 2016, 57 (30), 3385–3388. 10.1016/j.tetlet.2016.06.083. [DOI] [Google Scholar]
- Lan S.; Yang X.; Shi K.; Fan R.; Ma D. Pillarquinone-Based Porous Polymer for a Highly-Efficient Heterogeneous Organometallic Catalysis. ChemCatChem. 2019, 11 (12), 2864–2869. 10.1002/cctc.201900516. [DOI] [Google Scholar]
- Guo H.; Ye J.; Zhang Z.; Wang Y.; Yuan X.; Ou C.; Ding Y.; Yan C.; Wang J.; Yao Y. Pillar[5]arene-Based [2]Rotaxane: Synthesis, Characterization, and Application in a Coupling Reaction. Inorg. Chem. 2020, 59 (17), 11915–11919. 10.1021/acs.inorgchem.0c01752. [DOI] [PubMed] [Google Scholar]
- Ogoshi T.; Ueshima N.; Yamagishi T.-A. An Amphiphilic Pillar[5]arene as Efficient and Substrate-Selective Phase-Transfer Catalyst. Org. Lett. 2013, 15, 3742–3745. 10.1021/ol4016546. [DOI] [PubMed] [Google Scholar]
- Wanderlind E. H.; Liz D. G.; Gerola A. P.; Affeldt R. F.; Nascimento V.; Bretanha L. C.; Montecinos R.; Garcia-Rio L.; Fiedler H. D.; Nome F. Imidazole-Functionalized Pillar[5]arenes: Highly Reactive and Selective Supramolecular Artificial Enzymes. ACS Catal. 2018, 8, 3343–3347. 10.1021/acscatal.8b00901. [DOI] [Google Scholar]
- Gomes L. S.; Neto J. S. S.; Leo I.; Barbosa C. G.; Moraes C.; Freitas-Junior L. H.; Rizzuti B.; Santi C.; Nascimento V. Ecofriendly aminochalcogenation of alkenes: a green alternative to obtain compounds with potential anti-SARS-CoV-2 activity. New J. Chem. 2023, 47 (14), 6591–6601. 10.1039/D2NJ06218F. [DOI] [Google Scholar]
- Chipoline I. C.; Brasil B. F. A. B; Neto J. S. S.; Valli M.; Krogh R.; Cenci A. R.; Teixeira K. F.; Zapp E.; Brondani D.; Ferreira L. L. G.; Andricopulo A. D.; de Oliveira A. S.; Nascimento V. Synthesis and investigation of the trypanocidal potential of novel 1,2,3-triazole-selenide hybrids. Eur. J. Org. Chem. 2022, 243, 114687 10.1016/j.ejmech.2022.114687. [DOI] [PubMed] [Google Scholar]
- Portilho A. J. S.; Gomes C. B. S. M. R.; Moreira C. S.; Forezi L. S. M.; Cordeiro P.; Nascimento V.; Daniel J. P.; Vasconcellos M. C.; Moraes M. E. A.; Moreira-Nunes C. F. A.; Ferreira V. F.; Montenegro R. C.; da Rocha D. R. Synthesis, molecular docking, and biological activity of thioether derived from juglone in preclinical models of chronic myeloid leukemia. Comput. Toxicol. 2021, 20, 100197 10.1016/j.comtox.2021.100197. [DOI] [Google Scholar]
- Nascimento V.; Cordeiro P. S.; Arca M.; Marini F.; Sancineto L.; Braga A. L.; Lippolis V.; Iwaoka M.; Santi C. Fast and easy conversion of ortho amidoaryldiselenides into the corresponding ebselen-like derivatives driven by theoretical investigations. New J. Chem. 2020, 44 (22), 9444–9451. 10.1039/D0NJ01605E. [DOI] [Google Scholar]
- Ángel A. Y. B.; Campos P. R. O.; Aberto E. E. Selenonium Salt as a Catalyst for Nucleophilic Substitution Reactions in Water: Synthesis of Thiocyanites and Selenocyanates. Molecules 2023, 28 (7), 3056. 10.3390/molecules28073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A. F. M.; da Costa N. M.; Fernandes T. S.; Bessa I. A. A.; D’Amato D. L.; Senna C. A.; Lohan-Codeço M.; Nascimento V.; Palumbo A. Jr; Archanjo B. S.; Pinto L. F. R.; dos Santos T. C.; Ronconi C. M. Responsive Supramolecular Devices Assembled from Pillar[5]arene Nanogate and Mesoporous Silica for Cargo Release. ACS Appl. Nano Mater. 2022, 5 (10), 13805–13819. 10.1021/acsanm.2c01408. [DOI] [Google Scholar]
- Silveira E. V.; Wanderlind E. H.; Masson A. K.; Cordeiro P. S.; Nascimento V.; Affeldt R. F.; Micke G. Molecular recognition of methamphetamine by carboxylatopillar[5]arene: drug-dependent complexation stoichiometry and insights into medical applications. New J. Chem. 2020, 44 (7), 2701–2704. 10.1039/C9NJ06213K. [DOI] [Google Scholar]
- Silveira E. V.; Nascimento V.; Wanderlind E. H.; Affeldt R. F.; Micke G. A.; Garcia-Rio L.; Nome F. Inhibitory and Cooperative Effects Regulated by pH in Host-Guest Complexation between Cationic Pillar[5]arene and Reactive 2-Carboxyphthalanilic Acid. J. Org. Chem. 2019, 84 (15), 9684–9692. 10.1021/acs.joc.9b01377. [DOI] [PubMed] [Google Scholar]
- Santos E. C. S.; dos Santos T. C.; Fernandes T. S.; Jorge F. L.; Nascimento V.; Madriaga V. G. C.; Cordeiro P. S.; Checca N. R.; da Costa N. M.; Pinto F. R.; Ronconi C. A reversible, switchable pH-driven quaternary ammonium pillar[5]arene nanogate for mesoporous silica nanoparticles. J. Mater. Chem. B 2020, 8 (4), 703–714. 10.1039/C9TB00946A. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Jie K.; Huang F. A redox-responsive selenium-containing pillar[5]arene-based macrocyclic amphiphile: synthesis, controllable self-assembly in water, and application in controlled release. Chem. Commun. 2017, 53 (59), 8364–8367. 10.1039/C7CC04779G. [DOI] [PubMed] [Google Scholar]
- Ma X.; Liu D.; Wan X.; Zhao J. Mo(VI)-catalyzed conversion of nitriles to amides with hydrogen peroxide in ethanol. Synth. Commun. 2023, 53 (6), 503–510. 10.1080/00397911.2023.2178012. [DOI] [Google Scholar]
- Chen Y.; Yang B.; Li Q.-Y.; Lin Y.-M.; Gong L. Selectfluor®-enabled photochemical selective C(sp3)-H(sulfonyl)amidation. Chem. Commun. 2022, 59 (1), 118–121. 10.1039/D2CC05569D. [DOI] [PubMed] [Google Scholar]
- Ghadermazi M.; Molaei S. Synthesis of Sm (III) Complex Immobilized in MCM-41: A New Heterogeneous Catalyst for the Facile Synthesis of 5-substituted 1H-tetrazoles via [3 + 2] Cycloaddition of Nitriles and Sodium Azide. Inorg. Chem. 2023, 147, 110225 10.1016/j.inoche.2022.110225. [DOI] [Google Scholar]
- Ogoshi T. Synthesis of novel pillar-shaped cavitands ″Pillar[5]arenes″ and their application for supramolecular materials. J. Incl. Phenom. Macrocycl. Chem. 2012, 72 (3–4), 247–262. 10.1007/s10847-011-0027-2. [DOI] [Google Scholar]
- Ju H.; Zhu C. N.; Wang H.; Page Z. A.; Wu Z. L.; Sessler J. L.; Huang F. Paper without a Trail: Time-Dependent Encryption using Pillar[5]arene-Based Host-Guest Invisible Ink. Adv. Mater. 2022, 34 (6), 2108163 10.1002/adma.202108163. [DOI] [PubMed] [Google Scholar]
- Chen J.-F.; Yin X.; Zhang K.; Zhao Z.; Zhang S.; Zhang N.; Wang N.; Chen P. Pillar[5]arene-Based Dual Chiral Organoboranes with Allowed Host-Guest Chemistry and Circularly Polarized Luminescence. Org. Chem. 2021, 86 (18), 12654–12663. 10.1021/acs.joc.1c01175. [DOI] [PubMed] [Google Scholar]
- Hua B.; Shao L.; Zhang Z.; Liu J.; Huang F. Cooperative Silver Ion-Pair Recognition by Peralkylated Pillar[5]arenes. J. Am. Chem. Soc. 2019, 141 (38), 15008–15012. 10.1021/jacs.9b08257. [DOI] [PubMed] [Google Scholar]
- Hua B.; Zhou W.; Yang Z.; Zhang Z.; Shao L.; Zhu H.; Huang F. Supramolecular Solid-State Microlaser Constructed from Pillar[5]arene-Based Host-Guest Complex Microcrystals. J. Am. Chem. Soc. 2018, 140 (46), 15651–15654. 10.1021/jacs.8b11156. [DOI] [PubMed] [Google Scholar]
- Sun C.-L.; Peng H.-Q.; Niu L.-Y.; Chen Y.-Z.; Wu L.-Z.; Tung C.-H.; Yang Q.-Z. Artificial light-harvesting supramolecular polymeric nanoparticles formed by pillar[5]arene-based host-guest interaction. Chem. Commun. 2018, 54 (9), 1117–1120. 10.1039/C7CC09315B. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Ji X.; Xiang F.; Chi X.; Han C.; He J.; Abliz Z.; Chen W.; Huang F. A cationic water-soluble pillar[5]arene: synthesis and host-guest complexation with sodium 1-octanesulfonate. Chem. Commun. 2011, 47 (45), 12340–12342. 10.1039/c1cc15660h. [DOI] [PubMed] [Google Scholar]
- Todee B.; Sanae P.; Ruengsuk A.; Janthakit P.; Promarak V.; Tantirungrotechai J.; Sukwattanasinitt M.; Limpanuparb T.; Harding D. J.; Bunchuay T. Switchable Metal-Ion Selectivity in Sulfur-Functionalised Pillar[5]arenes and Their Host-Guest Complexes. Chem. Asi. J. 2024, 19 (1), e202300913 10.1002/asia.202300913. [DOI] [PubMed] [Google Scholar]
- Shimizu T.; Sakurai R.; Azami Y.; Hirabayashi K.; Kamigata N. Isolation and Racemization Mechanism of Optically Active Benzylmethylphenyltelluronium Salts. EuroJoc 2010, 2010 (34), 6556–6562. 10.1002/ejoc.201000893. [DOI] [Google Scholar]
- Hirabayashi K.; Nara Y.; Yamashita Y.; Kiyiota K.-I.; Kamigata N.; Shimzu T. Palladium-catalyzed Mizoroki–Heck-type reactionsof chalcogenonium trifluoromethanesulfonates. J. Sulfur Chem. 2009, 30 (3–4), 346–350. 10.1080/17415990902870927. [DOI] [Google Scholar]
- Hirabayashi K.; Nara Y.; Shimizu T.; Kamigata N. Palladium-Catalyzed Mizoroki–Heck-type Reactions Using Telluronium Salts. Chem. Lett. 2004, 33 (10), 1280–1281. 10.1246/cl.2004.1280. [DOI] [Google Scholar]
- Lenardão E. J.; Mendes S. R.; Ferreira P. C.; Perin G.; Silveira C. C.; Jacob R. G. Selenium- and tellurium-based ionic liquids and their use in the synthesis of octahydroacridines. Tetrahedron Lett. 2006, 47 (42), 7439–7442. 10.1016/j.tetlet.2006.08.049. [DOI] [Google Scholar]
- Erythropel H. C.; Zimmerman J. B.; de Winter T. M.; Petitjean L.; Melnikov F.; Lam C. H.; Lounsbury A. W.; Mellor K. E.; Janković N. Z.; Tu Q.; Pincus L. N.; Falinski M. M.; Shi W.; Coish P.; Plata D.; Anastas P. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018, 20 (9), 1929–1961. 10.1039/C8GC00482J. [DOI] [Google Scholar]
- Molnár Á.; Papp A. Catalyst recycling-A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. 10.1016/j.ccr.2017.08.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.