Abstract
Vectors derived from murine leukemia virus (MLV) have been used in many human gene therapy clinical trials. However, insertion of the locus control regions (LCRs) derived from the β-globin gene locus or the CD2 gene into MLV vectors frequently led to vector rearrangement. Since the human immunodeficiency virus (HIV) sequence diverges significantly from the MLV sequence, we tested whether the LCR sequence is more stable in the context of an HIV vector. Clones derived from human fibrosarcoma line HT1080 cells transduced with an HIV vector containing the T-cell-specific CD2 LCR exhibit the same wide range of transgene expression as clones lacking the LCR. In contrast, Jurkat and primary T-cell clones derived from the transduction of the LCR-containing vector show, on average, a three- to fourfold increase in transgene expression relative to that of the control vector. This is consistent with previous observations that the CD2 LCR contains a T-cell-specific enhancer. In addition, the clones derived from the LCR-containing vector have a much lower clonal variation in transgene expression than those derived from the control vector. We also demonstrate that the level of transgene expression is proportional to the vector copy number. These results suggest that the human CD2 LCR sequence is compatible with HIV vector sequences and confers enhanced integration site-independent and copy number-dependent expression of the transgene. Thus, HIV vectors may represent the ideal vehicle to deliver genes controlled by various cis-acting elements such as LCRs.
Murine leukemia virus (MLV)-derived vectors have been widely used to introduce genes into mammalian cells (12, 34). Since MLV integrates randomly into the host genome, transgene expression is frequently influenced by the surrounding host chromatin (20). A large portion of MLV insertions has led to silencing or position effect variegation of gene expression either immediately after insertion or following cell expansion in culture or in vivo (11, 40, 45, 50–52). This observation poses a major obstacle for the use of retrovirus vectors in the treatment of human diseases. However, the discovery of the locus control regions (LCRs) raises the possibility that the problem associated with chromosomal position effects may be overcome: LCRs are cis-regulatory elements that confer high-level, tissue-specific expression of homologous and heterologous genes in a position-independent, copy number-dependent manner (9, 18, 19, 46). In transgenic mice studies, LCRs have been shown to both enhance transgene expression and direct integration site-independent expression in various cell types (1, 9, 10, 19, 22, 44). These observations led to the hope that incorporation of the LCR sequence may overcome the position effect of the integration sites on transgene expression from a retroviral vector.
However, insertion of the LCR from either the human CD2 (hCD2) or the β-globin gene into an MLV vector resulted in frequent rearrangement of the vector sequences and low vector titers from the producer cells. In the case of the β-globin gene, combining LCR derivatives containing only the nuclease hypersensitivity sites with the deletion of intronic segments and/or various point mutations in the gene led to increased vector titers and more stable proviral genomes (25, 29, 31, 42, 43, 48). However, gene silencing or position effect variegation continued to be observed in mice grafted with the transduced hematopoietic stem cells (HSC). These results suggest that sequences outside the nuclease hypersensitivity sites may be required for position-independent expression of the transgene. In the case of the hCD2 LCR, the vectors containing the LCR either were prone to rearrange or failed to express higher levels of the transgene compared with control vectors without the LCR (25). Thus, sequences within the LCR may be incompatible with sequences in the MLV vectors.
Human immunodeficiency virus type 1 (HIV-1)-based vectors have recently been demonstrated to efficiently deliver genes into mammalian cells. Unlike MLV vectors, HIV-1 vectors are capable of transducing nondividing cells both in culture and in vivo (5, 6, 8, 15, 24, 35, 38, 47). Compared with MLV, the HIV-1 sequence is significantly different. The observations that some of the cis-regulatory sequences inserted into HIV vectors are more stable than those in MLV vectors and that a tissue-specific promoter functions properly in the context of an HIV vector prompted us to investigate the stability and function of the hCD2 LCR in an HIV-1 vector (24, 36). Our results show that insertion of the hCD2 LCR did not generate gross rearrangement of the vector sequences, indicating that the hCD2 LCR is stable in the context of an HIV vector. The introduction of the hCD2 LCR enhanced the T-cell-specific expression of the transduced gene. Moreover, we show here for the first time that the hCD2 LCR confers position-independent and copy number-dependent expression of the transgene in human T cells. These results demonstrate the potential of using LCRs in combination with lentivirus vectors to direct stable and high-level expression of transgenes for the treatment of human diseases. Our results are consistent with a recent study by May et al. (32), demonstrating that the human β-globin LCR is stable in an HIV vector and confers increased, long-term β-globin expression in β-thalassemic mice. These results raise hope that the problem of rearrangements of the vector, as observed in MLV-based vectors, may be overcome by the use of lentivirus vectors.
MATERIAL AND METHODS
Plasmid construction.
To generate pHIV (Fig. 1A), the following fragment was cloned into a pBluescript SK(−) backbone in the unique NotI and EcoRI sites in the polylinker: a 1-kb fragment comprising the sequence from nucleotide position −53 relative to the transcription initiation site to the PstI site in pv653RSN (41), amplified by PCR using the two primers 5′-GGCGGAATTCGGAGTGGCGAGCCCTCAGATC-3′ and 5′-CATGCACTGGATGCACTCTATC-3′. The remainder of the HIV sequence in pHIV is identical to that of pv653RSN except that the U3 region of the 3′ long terminal repeat (LTR) has a 400-bp deletion that removes all the cis-regulatory elements for transcription initiation in the HIV U3 region. This deletion was generated similarly as reported by Zufferey et al. (54), leading to the production of self-inactivating lentivirus vectors. To insert the enhancer of the cytomegalovirus (CMV) immediate-early (IE) gene (2), a 590-bp fragment containing the CMV enhancer was PCR amplified using pCMV-lux (J.-K. Yee, unpublished data) as a template. The sequences of the two PCR primers used are 5′-ACATATCGATTGGCTCATGTCCAACATTACCG-3′ and 5′-GACCGAATTCCGTACACGCCTACCGCCCATT-3′.
FIG. 1.
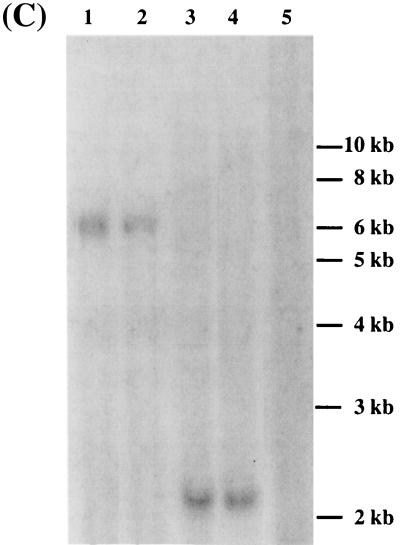
Structures of HIV vectors. (A) Structure of pHIV. Arrows represent LTRs; CMV is the enhancer of the CMV IE gene; ΔU3 symbolizes the 400-bp deletion in the 3′ LTR; ori represents the SV40 replication origin; RRE is the Rev/Rev-responsive element; BamHI is the unique cloning site in pHIV. (B) Structures of pHIV/CK-4 and pHIV/CK-3. LCR represents the 2-kb fragment carrying the hCD2 LCR; SV40 is the SV40 early promoter. The probes used for Southern blots and the expected fragment sizes are indicated. (C) Southern blot analysis of vector integrity in unselected HT1080 cells. HT1080 cells were transduced with pHIV/CK-3 at high MOI. The genomic DNA was isolated and cut with XhoI (lane 2) or SacI (lane 3). pHIV/CK-3 was digested with XhoI (lane 1) or SacI (lane 4) and used as a positive control. Genomic DNA of untransduced cells was used as a negative control (lane 5). The blot was hybridized to probe B (Fig. 1 B). The expected fragment sizes were 6.1 and 2.2 kb.
The PCR product that carried a ClaI site at the 5′ end and an EcoRI site at the 3′ end was inserted immediately upstream of the PCR-amplified 5′ LTR fragment described above in order to create a CMV-5′ LTR fusion. This fusion removed all of the HIV sequence upstream from the TATA box and replaced it with the CMV enhancer, rendering the generation of the HIV-1 vector Tat independent (26). To increase expression of the genomic RNA of the vector in 293T cells, a 200-bp BamHI/HindIII fragment containing the simian virus 40 (SV40) replication origin was isolated from pSV2Agpt (23) and inserted immediately upstream of the CMV enhancer. For the construction of pHIV/CK-3 and pHIV/CK-4, a 2-kb ClaI/SmaI fragment containing the hCD2 LCR (28), a 200-bp SmaI/HindIII fragment containing the SV40 early promoter (14), and a 3.9-kb HindIII/XbaI fragment containing the β-geo gene (13) were cloned into pBluescript SK(−) to generate pCK-3. A 6.1-kb XhoI/NotI fragment containing the hCD2 LCR and the β-geo gene under the control of the SV40 promoter was isolated from pCK-3 and inserted into the unique BamHI site in pHIV to generate pHIV/CK-3 (Fig. 1B). A 4.1-kb SalI/NotI fragment containing the β-geo gene under the control of the SV40 promoter was isolated from pCK-3 and inserted into the unique BamHI site in pHIV to generate pHIV/CK-4.
Cell culture.
HT1080 and 293T cells were maintained in high-glucose (4.5 g/liter) Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 100 mg of gentamicin/liter. Jurkat and primary human T cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum and 100 mg of gentamicin/liter. Primary human T cells were also supplemented with 25 U of recombinant human interleukin-2 (rhIL-2; Chiron, Emeryville, Calif.) per ml. Peripheral blood mononuclear cells were isolated by a Ficoll gradient and activated by OKT3 (30 ng/ml; Ortho Biotech, Raritan, N.J.) and (25 U/ml) rhIL-2 for 2 days before vector transduction.
Vector production and cell transduction.
To produce infectious vectors, 293T cells were plated at a density of 4 × 106 cells per 10-cm-diameter culture dish. The cells were cotransfected with 10 μg of pCMV-HIV-1 (16), 10 μg of pCMV-G (53), and 20 μg of pHIV/CK-3 or pHIV/CK-4 by calcium phosphate coprecipitation (17). The culture medium was replaced with fresh medium after 6 h. The supernatant was collected 16 h after the transfection and stored at −80°C. To determine the vector titers, 105 HT1080 cells were seeded in a six-well plate in the presence of 4 μg of Polybrene/ml. The cells were transduced for 5 h with various dilutions of the vector. The culture medium was replaced 48 h later with fresh medium containing G418 at a concentration of 600 μg/ml. G418-resistant colonies were counted 14 days after transduction. Jurkat and primary human T cells were similarly transduced with a multiplicity of infection (MOI) of 10. The concentrations of G418 used for selection of transduced Jurkat and primary T cells were 600 and 800 μg/ml, respectively. Primary T cells were cloned and expanded as previously described (21).
β-Gal assay.
Extracts were prepared from exponentially growing cells in six-well plates. The cells were resuspended in 100 μl of 250 mM Tris-HCl (pH 7.8) and subjected to four freeze-thaw cycles. Cell debris was removed by centrifugation, and the supernatant was used for the β-galactosidase (β-Gal) assay. To assay for β-Gal activity, 50-μl aliquots of the extracts were added to 450 μl of β-Gal buffer (0.05 M Tris-HCl) [pH 7.5], 0.1 M NaCl, 0.01 M MgCl2) containing 0.75 mg of o-nitrophenyl-β-d-galactopyranoside/ml. The samples were incubated at 37°C for 30 min, and the reaction was terminated by adding 500 μl of 1 M Na2CO3. β-Gal activity was determined by measuring the optical density at 420 nm with visible light. Units of active β-Gal were determined from a standard curve of β-Gal activity versus protein concentration, using purified β-Gal (Sigma, St. Louis, Mo.). The total protein concentration of the cell extract was determined by the Bradford method (3).
Southern blot analysis.
Genomic DNA was isolated as described previously (49). For copy number determination and integration site analysis, approximately 10 μg of the isolated DNA was digested with PstI overnight. The DNA fragments were separated on 1% agarose gels and blotted onto Nytran Supercharge membranes (Schleicher & Schuell, Dassel, Germany). The DNA probe was prepared by PCR amplification of a 740-bp fragment immediately upstream of the Rev/Rev-responsive element sequence in the vector (Fig. 1B, probe A). The sequences of the primers are 5′-ACCAGAGCTCTCTCGACGCA-3′ and 5′-CCATTCTGCAGCTTCCTCAT-3′.
The amplified fragment was labeled with [32P]dATP by using a DNA labeling kit from ICN (Costa Mesa, Calif.) and hybridized with the membranes. The membranes were washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate for 15 min at 65°C three times and subjected to autoradiography. To determine provirus integrity, the genomic DNA was digested with either XhoI or SacI overnight. The DNA was similarly separated, transferred, and hybridized to a 470-bp PCR-amplified probe containing sequences within the β-geo gene (Fig. 1, probe B). The sequences for the primers are 5′-GAATTCCGCCGATACTGACG-3′ and 5′-TTTATCAGCCGGAAAACCTA-3′
RESULTS
Generation of HIV vectors.
To test hCD2 LCR functions, we first generated a vector backbone, pHIV (Fig. 1A). This construct contained the replication origin of SV40 to enhance plasmid replication in 293T cells. For Tat-independent vector production, most of the U3 region in the 5′ LTR of pHIV was replaced with the enhancer of the CMV IE gene. In addition, the cis-regulatory sequences in the U3 region of the 3′ LTR were removed to produce self-inactivating vectors (54), which minimized the possibility of vector mobilization by wild-type HIV. A fragment containing the β-geo gene under the control of the SV40 early promoter and the hCD2 LCR was inserted into pHIV to generate pHIV/CK-3 (Fig. 1B). The presence of the β-geo gene product, a fusion between neomycin phosphotransferase and β-Gal, allows both the selection of the transduced cells in G418-containing medium and the quantification of gene expression by the β-Gal assay (13). pHIV/CK-4 lacking the hCD2 LCR was constructed to serve as a control vector. Infectious vectors were generated by transient transfection of the vector construct together with a packaging plasmid and a plasmid containing the vesicular stomatitis virus G gene into 293T cells (16). To determine titers, human fibrosarcoma HT1080 cells were transduced with the vectors and selected in G418-containing medium. Both vectors gave similar titers ranging between 3 × 105 and 5 × 105 CFU/ml, which is equivalent to approximately 3,000 transinducing units of p24/ng. These results suggest that the presence of the hCD2 LCR has very little effect on vector production.
A previous report indicated that the presence of the hCD2 LCR in MLV vectors caused frequent rearrangement of the vector genome (25). To determine whether a similar event occurred in the context of an HIV vector backbone, we transduced HT1080 cells with pHIV/CK-3 at an MOI of 10 and isolated chromosomal DNA from unselected cells. The DNA was digested with either XhoI or with SacI and subjected to Southern blot analysis using probe B derived from the β-geo gene (Fig. 1B). As shown in Fig. 1C, the probe detected a 6.1-kb fragment with XhoI digestion and a 2.2-kb fragment with SacI digestion, as predicted from the vector map (Fig. 1B). No other aberrant-size fragments were detected. At an MOI of 10, close to 100% of HT1080 cells were expected to be transduced, given that an MOI of 1 led to transduction efficiencies of greater than 95% (data not shown). Thus, we should have been able to detect any major rearrangements in the proviruses. Since the transduced HT1080 cells were not preselected for G418 resistance, we concluded that, unlike in MLV vectors, the presence of the hCD2 LCR in an HIV vector did not cause major instability of the vector genome.
Lack of hCD2 LCR functions in transduced HT1080 cells.
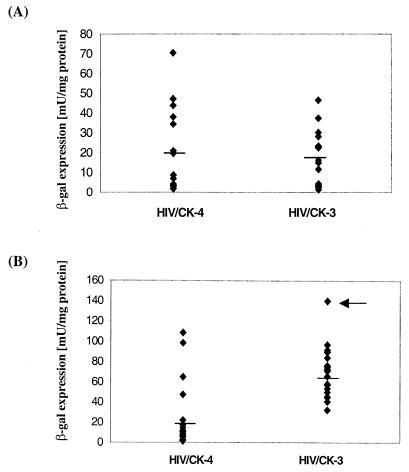
To investigate the T-cell specificity of the hCD2 LCR, HT1080 cells were transduced with either pHIV/CK-3 or pHIV/CK-4. G418-resistant cells were pooled, and the protein extracts of exponentially growing cells were prepared and assayed for β-Gal activity. As shown in Fig. 2, no significant differences in the levels of β-Gal expression were detected in either pHIV/CK-3- or pHIV/CK-4-transduced cells. To analyze clonal variation of β-Gal expression, individual G418-resistant clones were isolated from cells transduced with each vector and tested for β-Gal activity. As shown in Fig. 3A, the levels of β-Gal expression varied approximately 33-fold among 18 clones transduced with pHIV/CK-3. Clones transduced with pHIV/CK-4 showed a 39-fold variation. Thus, the hCD2 LCR confers no position-independent expression of the β-geo gene in a fibroblast cell line.
FIG. 2.
T-cell-specific stimulation of β-Gal expression mediated by the hCD2 LCR in cell pools. HT1080, Jurkat, and primary human T cells were transduced with pHIV/CK-3 or with pHIV/CK-4 at an MOI of 10. Transduced cells were selected with G418. Cell extracts were prepared by freeze-thaw cycles. β-Gal expression in the cell pools was quantified and corrected for the protein concentration of the samples. All assays were carried out at least three times with freshly prepared cell extracts of exponentially growing cells. Standard deviations are indicated.
FIG. 3.
T-cell-specific stimulation of β-Gal expression mediated by the hCD2 LCR in individual clones. HT1080 and Jurkat cells were transduced with pHIV/CK-3 or pHIV/CK-4 at an MOI of 10 (as determined in HT1080 cells). Transduced cells were selected with 600 μg of G418/ml. Individual clones were isolated after 2 weeks of selection. The expression of β-Gal in individual clones was quantified and normalized to the protein concentration of the samples. Three assays were carried out per clone with freshly prepared cell extracts of exponentially growing cells each time. Each dot represents the average β-Gal activity of three assays. The mean expression levels of all clones are indicated by horizontal lines. (A) HT1080 clones; (B) Jurkat clones. β-Gal expression varied 55-fold among clones transduced with pHIV/CK-4, while the variation in pHIV/CK-3-transduced clones was 4-fold (P < 0.001, Wilcoxon rank sum test). The arrow in panel B indicates the clone containing two vector copies, as determined by Southern blot analysis (Fig. 4A, lane 3).
hCD2 LCR confers position-independent gene expression in T cells.
To study the functions of hCD2 LCR in T cells, Jurkat cells were transduced with either of the two vectors at an MOI of 10. G418-resistant cells were pooled, and cell extracts were prepared for the β-Gal assay. As shown in Fig. 2, the level of β-Gal expression in pHIV/CK-3-transduced Jurkat cells was 3.5-fold higher than that in pHIV/CK-4-transduced cells, indicating the presence of a T-cell-specific enhancer in the hCD2 LCR. To study the effect of the LCR on position-independent gene expression, 18 individual Jurkat clones derived from the cells transduced with each vector were isolated and assayed for β-Gal activity. As shown in Fig. 3B, the average β-Gal activity of the 18 clones derived from pHIV/CK-3-transduced cells was threefold higher than that derived from pHIV/CK-4-transduced cells, consistent with the result of the pooled cells shown in Fig. 2. While the β-Gal activity among the clones derived from pHIV/CK-4-transduced cells varied 55-fold (Fig. 3B), only a 4-fold variation in β-Gal expression was observed among the clones derived from pHIV/CK-3-transduced cells (P < 0.001, Wilcoxon rank sum test). Since Jurkat cells were transduced with pHIV/CK-3 and pHIV/CK-4 at the same MOI, a difference in the proviral copy number is unlikely to account for the variation in β-Gal expression between the two groups of Jurkat clones. Thus, the presence of the hCD2 LCR in an HIV vector not only increases gene expression specifically in T cells but also reduces the influence of the host chromosomal DNA around the integration site on transgene expression.
To determine the vector copy in each clone and to confirm random vector integration, chromosomal DNA was isolated from the pHIV/CK-3-transduced clones, digested with restriction enzymes, and subjected to Southern blot analysis. PstI digested only once in the vector, and the probe used (Fig. 1B, probe A) should hybridize with DNA fragments greater than 1 kb, including the vector sequence and the flanking host chromosomal DNA. As shown in Fig. 4A, 9 out of 10 randomly picked Jurkat clones derived from the pHIV/CK-3 transduction contained one hybridized fragment, indicating the presence of one vector copy. As expected, the vector integrated randomly since the sizes of the DNA fragments varied among different clones. One clone (Fig. 4A, lane 3) clearly contained two vector copies. It is interesting that the β-Gal activity of this particular clone was twofold higher than the average activity of all other clones tested (Fig. 3B). As demonstrated above, β-Gal activity varied fourfold among the pHIV/CK-3-transduced clones. If we normalize β-Gal expression to vector copy number, this variation is reduced to only threefold.
FIG. 4.
Southern blot analysis of vector integration in individual Jurkat clones. (A) Analysis of vector integration sites and copy number. All DNA samples were digested with PstI, which cuts once in the vector sequence. The probe hybridized to the sequence upstream of the PstI site (Fig. 1B, probe A). (B) Analysis of provirus integrity. Lanes 1 to 10, individual G418-resistant clones; lane 11, untransduced Jurkat cells. All DNA samples were digested with XhoI, which generated a 6.1-kb fragment comprising the hCD2 LCR and the β-geo gene. The blot was hybridized to probe B (Fig. 1B).
To determine the integrity of the provirus, the DNA from 10 of the pHIV/CK-3-derived clones was digested with either XhoI or SacI and hybridized with probe B (Fig. 1B). XhoI digestion generates a 6.1-kb fragment containing the entire hCD2 LCR, the SV40 promoter, and the β-geo gene (Fig. 1B, probe B). As shown in Fig. 4B, all 10 clones showed the presence of the 6.1-kb fragment. As expected, SacI digestion generated a 2.2-kb fragment containing the 3′ half of the β-geo gene and most of the 3′ LTR (data not shown). These results confirm that no gross rearrangement occurred in the integrated vector structure carrying the hCD2 LCR sequence.
To determine whether hCD2 LCR can also function in primary cells, primary human T cells isolated from peripheral blood and stimulated with OKT3 and rhIL-2 were transduced with either pHIV/CK-3 or pHIV/CK-4 at an MOI of 10. G418-resistant cells were either pooled or isolated as individual clones. As shown in Fig. 2, the level of β-Gal expression in the pooled T cells transduced with pHIV/CK-3 is approximately threefold higher than that in T cells transduced with pHIV/CK-4. Thus, the T-cell-specific enhancer in hCD2 LCR activates the linked SV40 promoter to similar levels in both Jurkat cells and primary human T cells. The β-Gal activities of individual clones derived from pHIV/CK-3-transduced primary human T cells were also determined. As shown in Fig. 5, β-Gal activity varied between three- and fourfold among these clones, consistent with the results obtained from pHIV/CK-3-transduced Jurkat cells. Thus, the hCD2 LCR functions similarly in both a well-established human T-cell line and primary human T cells, conferring position-independent and T-cell-specific expression of the linked gene from a heterologous promoter.
FIG. 5.
LCR-mediated β-Gal gene expression in individual primary T-cell clones. Primary human T cells were transduced with pHIV/CK-3 at an MOI of 10. Transduced cells were selected with G418. The β-Gal activity in individual clones was quantified and corrected for the protein concentration in the samples. The average expression level is indicated by a horizontal line. Each dot represents the average β-Gal activity from three independent assays.
hCD2 LCR-mediated gene expression is copy number dependent.
To investigate the effect of multiple LCR copies on the expression level of β-Gal, one clone carrying one copy of the LCR (as confirmed by Southern blot analysis) was transduced with pHIV/CK-3 at an MOI of 20. Individual clones were isolated, and the cell extracts were assayed for β-Gal activity. As shown in Fig. 6A, a distinct pattern of β-Gal expression was revealed: the β-Gal activity of 11 clones was in the same range as that of the parental clone, the β-Gal activity of 5 clones was on average 2.5 times higher than that of the parental clone, and one clone showed β-Gal activity 3.6 times higher than that of the parental clone (Fig. 6A). The chromosomal DNA from some clones was isolated, digested with PstI, and subjected to Southern blotting. As shown in Fig. 6B, the clone showing β-Gal activity 3.6-fold higher than that of the parental clone contained three vector copies. Five clones with β-Gal activities about 2.5 times higher than the parental clone carried two copies, and three clones revealing β-Gal activities in the same range as the parental clone harbored only one vector copy. The blot also confirms that the integration of the vector occurred randomly. Since the level of β-Gal expression is proportional to the vector copy number in the clones, this result suggests that hCD2 LCR confers copy number-dependent, integration site-independent expression in the context of a lentivirus vector.
FIG. 6.
Copy number-dependent β-Gal gene expression in Jurkat cells. (A) β-Gal activities of Jurkat clones reinfected with pHIV/CK-3. A Jurkat clone carrying one copy of pHIV/CK-3 was reinfected with pHIV/CK-3 at an MOI of 20 to introduce multiple copies of pHIV/CK-3. β-Gal expression of individual clones was measured and normalized to the total protein concentration. Two assays were carried out per clone with freshly prepared cell extracts of exponentially growing cells each time. The results represent the mean β-Gal activity of two assays. The arrow indicates the β-Gal activity of the parental clone; N indicates the β-Gal activity in untransduced Jurkat cells. Clones in groups 1, 2, and 3 contain one, two, and three proviral vector copies, respectively, as determined by Southern blot analysis (B). (B) Southern blot analysis of the vector copy number in reinfected Jurkat cell clones. The genomic DNA from clones in groups 1, 2, and 3 (A) was digested with PstI, which cuts once in the vector sequence. The blot was hybridized to probe A (Fig. 1B).
DISCUSSION
For successful gene therapy approaches in the treatment of human diseases, the therapeutic gene must be delivered efficiently and expressed in a tissue-specific and constant level over time. MLV vectors have been widely used to deliver genes into mammalian cells. Since these vectors integrate randomly into host genome, the gene expression is strongly influenced by the surrounding chromatin, leading to position effect variegation and gene silencing either upon integration or following cell expansion (48). These problems were expected to be overcome with the discovery of the LCRs, given that LCRs confer tissue-specific, high-level, and integration-site independent expression of the transgene in cell lines as well as in transgenic mice (18, 28, 37). However, the introduction of the β-globin LCR or the hCD2 LCR into MLV vectors led invariably to rearrangements of the vector sequence and low vector titers (39). Combining LCR fragments with only the nuclease hypersensitivity sites or reversing the orientation of the LCR alleviated the problem of vector instabilities and increased vector titers, but transgene expression remained integration site dependent (7, 25, 29, 31, 48).
Unlike MLV vectors, HIV vectors are capable of transducing nondividing cells in culture and in vivo (4–6, 8, 15, 24, 30, 35, 38, 47). The significant divergence of the HIV sequence from the MLV sequence and the stability of some cis-regulatory elements in the context of lentivirus vectors prompted us to test the stability and function of the hCD2 LCR in an HIV vector. Southern blot analysis from pooled cells demonstrated no gross rearrangement of the proviral structure, suggesting that the hCD2 LCR is more stable in the context of an HIV vector than in an MLV vector. The hCD2 LCR did not stimulate the SV40 promoter in HT1080 cells. In Jurkat and primary human T cells, the SV40 promoter was stimulated in the presence of the LCR, demonstrating T-cell-specific enhancer activity of the hCD2 LCR in the context of a lentivirus vector. This is in direct contrast with the results reported by Kaptein et al. (25), showing no hCD2 LCR function in the context of an MLV vector. The latter study and our present study used exactly the same LCR fragment, which suggests that sequences in the MLV vector act negatively to suppress the hCD2 LCR function. This is consistent with the observation by McCune and Townes that MLV sequences down-regulated the β-globin LCR function in transgenic mice (33). Removal of the MLV enhancer and promoter from the retrovirus vector proved to be nonessential for the repression of β-globin expression in McCune and Towne's study (33). This observation rules out the possibility that promoter interference could be responsible for the repression. This suggests that the lack of an active HIV promoter in our vector did not account for the differences between our results and those of Kaptein et al. (25).
Stimulation of SV40 promoter activity by the hCD2 LCR enhancer, however, is relatively modest, ranging between three- and fourfold. This is in contrast to the study of Lake et al., demonstrating that the enhancer in hCD2 LCR stimulated a linked thymidine kinase promoter of herpes simplex virus to much higher levels in a transient transfection assay (27). The variation in promoter stimulation may be explained by the different approaches used to assay for enhancer activity, one by transient transfection and the other by stable integration. But it is also likely that the heterologous promoters used for the enhancer assay may interact with the LCR with different efficiencies. To achieve efficient and tissue-specific gene expression with the hCD2 LCR in vivo, it may be necessary to combine the authentic hCD2 promoter together with its LCR to direct gene expression in an HIV vector. In a previous study, a significant increase in gene expression levels could be obtained when the β-globin LCR was combined with its own promoter in an MLV-based vector to drive β-globin gene expression (31).
Analysis of individual Jurkat clones demonstrated that the clonal variation of β-geo gene expression among pHIV/CK-3-transduced cells is significantly lower than that among pHIV/CK-4-transduced cells. The variation in β-geo expression is most likely underestimated in our study because the Jurkat clones were preselected for G418 resistance. Only those clones containing the vector integrated into favored chromosomal sites would express sufficient amounts of the β-geo gene and survive the selection. Thus, the position effect on gene expression among pHIV/CK-4-transduced Jurkat cells may even be larger than the observed 55-fold. This variation in expression levels is consistent with results obtained for MLV vectors containing no LCR (25). On the other hand, the β-Gal expression levels in pHIV/CK-3-transduced Jurkat clones containing the LCR fell in a narrow range. The pHIV/CK-3 Jurkat clone expressing the highest β-Gal level contains two integrated HIV vectors. If the β-Gal activity of this clone is normalized to the vector copy number, the level of β-Gal expression falls within the average range of other pHIV/CK-3 transduced clones. These results strongly suggest that the hCD2 LCR confers position-independent expression of the gene inserted into an HIV vector.
Results of reintroduction of the vector into an already transduced Jurkat clone confirmed copy number-dependent gene expression conferred by the hCD2 LCR. Thus, the hCD2 LCR, when placed in the context of an HIV vector backbone, directs tissue-specific, integration site-independent and copy number-dependent expression of the gene from a heterologous promoter. Similar results were obtained with primary human T cells, suggesting that the effect of the LCR is not limited to established cell lines.
Whether the LCR function can persist upon gene delivery into HSC and subsequent differentiation of the transduced stem cells into T cells in vivo remains to be tested. But the recent observation by May et al. (32) that the silencing of gene expression occurred less frequently from lentivirus vectors than from oncoretrovirus vectors raises hope that the LCR function can indeed persist in vivo over time. The study by May et al. demonstrates that the insertion of a large fragment of the β-globin LCR into an HIV vector, followed by mouse HSC transduction, led to long-term, therapeutic levels of β-globin expression in β-thalassemic mice.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI46030.
We thank J. Sodroski for kindly providing plasmid pv653RSN and D. Kioussis for the hCD2 LCR fragment. We also thank M. Jensen for the isolation of PBMC and for helpful advice.
REFERENCES
- 1.Bonifer C, Vidal M, Grosveld F, Sippel A E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990;9:2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshart M, Weber F, Jahn G, Dorsch-Hasler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Buchschacher G L, Jr, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000;95:2499–2504. [PubMed] [Google Scholar]
- 5.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Case S S, Price M A, Jordan C T, Yu X J, Wang L, Bauer G, Haas D L, Xu D, Stripecke R, Naldini L, Kohn D B, Crooks G M. Stable transduction of quiescent CD34(+)CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang J C, Liu D, Kan Y W. A 36-base-pair core sequence of locus control region enhances retrovirally transferred human beta-globin gene expression. Proc Natl Acad Sci USA. 1992;89:3107–3110. doi: 10.1073/pnas.89.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran M A, Kaiser S M, Achacoso P L, Nolan G P. Efficient transduction of nondividing cells by optimized feline immunodeficiency virus vectors. Mol Ther. 2000;1:31–38. doi: 10.1006/mthe.1999.0007. [DOI] [PubMed] [Google Scholar]
- 9.Diaz P, Cado D, Winoto A. A locus control region in the T cell receptor alpha/delta locus. Immunity. 1994;1:207–217. doi: 10.1016/1074-7613(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 10.Festenstein R, Tolaini M, Corbella P, Mamalaki C, Parrington J, Fox M, Miliou A, Jones M, Kioussis D. Locus control region function and heterochromatin-induced position effect variegation. Science. 1996;271:1123–1125. doi: 10.1126/science.271.5252.1123. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan J R, Becker K G, Ennist D L, Gleason S L, Driggers P H, Levi B Z, Appella E, Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992;12:38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedmann T. Progress toward human gene therapy. Science. 1989;244:1275–1281. doi: 10.1126/science.2660259. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 14.Fromm M, Berg P. Transcription in vivo from SV40 early promoter deletion mutants without repression by large T antigen. J Mol Appl Genet. 1983;2:127–135. [PubMed] [Google Scholar]
- 15.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasmi M, Glynn J, Jin M J, Jolly D J, Yee J K, Chen S T. Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J Virol. 1999;73:1828–1834. doi: 10.1128/jvi.73.3.1828-1834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 18.Greaves D R, Wilson F D, Lang G, Kioussis D. Human CD2 3′-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989;56:979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- 19.Grosveld F, van Assendelft G B, Greaves D R, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoeben R C, Migchielsen A A, van der Jagt R C, van Ormondt H, van der Eb A J. Inactivation of the Moloney murine leukemia virus long terminal repeat in murine fibroblast cell lines is associated with methylation and dependent on its chromosomal position. J Virol. 1991;65:904–912. doi: 10.1128/jvi.65.2.904-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen M C, Clarke P, Tan G, Wright C, Chung-Chang W, Clark T N, Zhang F, Slovak M L, Wu A M, Forman S J, Raubitschek A. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000;1:49–55. doi: 10.1006/mthe.1999.0012. [DOI] [PubMed] [Google Scholar]
- 22.Jones B K, Monks B R, Liebhaber S A, Cooke N E. The human growth hormone gene is regulated by a multicomponent locus control region. Mol Cell Biol. 1995;15:7010–7021. doi: 10.1128/mcb.15.12.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadesch T, Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986;6:2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 25.Kaptein L C, Breuer M, Valerio D, van Beusechem V W. Expression pattern of CD2 locus control region containing retroviral vectors in hemopoietic cells in vitro and in vivo. Gene Ther. 1998;5:320–330. doi: 10.1038/sj.gt.3300583. [DOI] [PubMed] [Google Scholar]
- 26.Kim V N, Mitrophanous K, Kingsman S M, Kingsman A J. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lake R A, Wotton D, Owen M J. A 3′ transcriptional enhancer regulates tissue-specific expression of the human CD2 gene. EMBO J. 1990;9:3129–3136. doi: 10.1002/j.1460-2075.1990.tb07510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang G, Mamalaki C, Greenberg D, Yannoutsos N, Kioussis D. Deletion analysis of the human CD2 gene locus control region in transgenic mice. Nucleic Acids Res. 1991;19:5851–5856. doi: 10.1093/nar/19.21.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leboulch P, Huang G M, Humphries R K, Oh Y H, Eaves C J, Tuan D Y, London I M. Mutagenesis of retroviral vectors transducing human beta-globin gene and beta-globin locus control region derivatives results in stable transmission of an active transcriptional structure. EMBO J. 1994;13:3065–3076. doi: 10.1002/j.1460-2075.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Emery D W, Fernandez M, Han H, Stamatoyannopoulos G. Development of viral vectors for gene therapy of beta-chain hemoglobinopathies: optimization of a gamma-globin gene expression cassette. Blood. 1999;93:2208–2216. [PubMed] [Google Scholar]
- 32.May C, Rivella S, Callegari J, Heller G, Gaensler K M, Luzzatto L, Sadelain M. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- 33.McCune S L, Townes T M. Retroviral vector sequences inhibit human beta-globin gene expression in transgenic mice. Nucleic Acids Res. 1994;22:4477–4481. doi: 10.1093/nar/22.21.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller A D. Retroviral vectors. Curr Top Microbiol Immunol. 1992;158:1–24. doi: 10.1007/978-3-642-75608-5_1. [DOI] [PubMed] [Google Scholar]
- 35.Miyoshi H, Blomer U, Takahashi M, Gage F H, Verma I M. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyoshi H, Takahashi M, Gage F H, Verma I M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon A M, Ley T J. Functional properties of the beta-globin locus control region in K562 erythroleukemia cells. Blood. 1991;77:2272–2284. [PubMed] [Google Scholar]
- 38.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 39.Novak U, Harris E A, Forrester W, Groudine M, Gelinas R. High-level beta-globin expression after retroviral transfer of locus activation region-containing human beta-globin gene derivatives into murine erythroleukemia cells. Proc Natl Acad Sci USA. 1990;87:3386–3390. doi: 10.1073/pnas.87.9.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer T D, Rosman G J, Osborne W R, Miller A D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci USA. 1991;88:1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parolin C, Dorfman T, Palu G, Gottlinger H, Sodroski J. Analysis in human immunodeficiency virus type 1 vectors of cis-acting sequences that affect gene transfer into human lymphocytes. J Virol. 1994;68:3888–3895. doi: 10.1128/jvi.68.6.3888-3895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plavec I, Papayannopoulou T, Maury C, Meyer F. A human beta-globin gene fused to the human beta-globin locus control region is expressed at high levels in erythroid cells of mice engrafted with retrovirus-transduced hematopoietic stem cells. Blood. 1993;81:1384–1392. [PubMed] [Google Scholar]
- 43.Raftopoulos H, Ward M, Leboulch P, Bank A. Long-term transfer and expression of the human beta-globin gene in a mouse transplant model. Blood. 1997;90:3414–3422. [PubMed] [Google Scholar]
- 44.Reitman M, Lee E, Westphal H, Felsenfeld G. Site-independent expression of the chicken beta A-globin gene in transgenic mice. Nature. 1990;348:749–752. doi: 10.1038/348749a0. [DOI] [PubMed] [Google Scholar]
- 45.Rivella S, Sadelain M. Genetic treatment of severe hemoglobinopathies: the combat against transgene variegation and transgene silencing. Semin Hematol. 1998;35:112–125. [PubMed] [Google Scholar]
- 46.Ryan T M, Behringer R R, Martin N C, Townes T M, Palmiter R D, Brinster R L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989;3:314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- 47.Sadaie M R, Zamani M, Whang S, Sistron N, Arya S K. Towards developing HIV-2 lentivirus-based retroviral vectors for gene therapy: dual gene expression in the context of HIV-2 LTR and Tat. J Med Virol. 1998;54:118–128. doi: 10.1002/(sici)1096-9071(199802)54:2<118::aid-jmv9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Sadelain M, Wang C H, Antoniou M, Grosveld F, Mulligan R C. Generation of a high-titer retroviral vector capable of expressing high levels of the human beta-globin gene. Proc Natl Acad Sci USA. 1995;92:6728–6732. doi: 10.1073/pnas.92.15.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Speck N A, Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987;7:1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams D A, Orkin S H, Mulligan R C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci USA. 1986;83:2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu L, Yee J K, Wolff J A, Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- 53.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]