Abstract
Aging and age-related ailments have emerged as critical challenges and great burdens within the global contemporary society. Addressing these concerns is an imperative task, with the aims of postponing the aging process and finding effective treatments for age-related degenerative diseases. Recent investigations have highlighted the significant roles of nicotinamide adenine dinucleotide (NAD+) in the realm of anti-aging. It has been empirically evidenced that supplementation with nicotinamide mononucleotide (NMN) can elevate NAD+ levels in the body, thereby ameliorating certain age-related degenerative diseases. The principal anti-aging mechanisms of NMN essentially lie in its impact on cellular energy metabolism, inhibition of cell apoptosis, modulation of immune function, and preservation of genomic stability, which collectively contribute to the deferral of the aging process. This paper critically reviews and evaluates existing research on the anti-aging mechanisms of NMN, elucidates the inherent limitations of current research, and proposes novel avenues for anti-aging investigations.
Keywords: Nicotinamide mononucleotide (NMN), Anti-aging, Energy metabolism, Apoptosis, DNA repair
Abstract
老龄化和年龄相关疾病已成为全球当代社会面临的重大挑战和沉重负担。解决这些问题是一项势在必行的任务,其目的是延缓衰老过程,并找到与年龄有关的退行性疾病的有效治疗方法。最近的研究强调了烟酰胺腺嘌呤二核苷酸(NAD+)在抗衰老领域的重要作用。经验证明,补充烟酰胺单核苷酸(NMN)可以提高体内NAD+水平,从而改善某些与年龄相关的退行性疾病。NMN的抗衰老机制主要在于其对细胞能量代谢的影响、对细胞凋亡的抑制、对免疫功能的调节和对基因组稳定性的保持,这些共同延缓了衰老进程。本文对NMN抗衰老机制的现有研究进行了批判性的回顾和评价,阐明了当前研究的内在局限性,并提出了抗衰老研究的新途径。
Keywords: 烟酰胺单核苷酸(NMN), 抗衰老, 能量代谢, 细胞凋亡, DNA修复
1. Introduction
The escalating trend of demographic aging presents serious challenges for managing age-related degenerative diseases, with the health burden of chronic ailments and disabilities resulting from aging affecting individuals, families, and society as a whole. Within contemporary medicine, there is a pressing need to advance research to mitigate the aging process. Numerous approaches have been identified to find methods that ameliorate aging, including nutritional intervention, physical conditioning, and pharmacological therapies. Among these, nutritional intervention has garnered widespread attention, particularly through the modification of dietary structure to influence metabolism and bodily function. In recent years, nicotinamide mononucleotide (NMN) has gained prominence as a new form of nutritional intervention, with evidence of improving various aspects of aging.
Nicotinamide adenine dinucleotide (NAD+), a crucial coenzyme in redox reaction and energy metabolism, plays a pivotal role in numerous biological processes. It impacts essential cellular functions both directly and indirectly, including metabolic pathways, DNA repair, chromosomal remodeling, cell aging, and immunocyte function. These processes are critical for maintaining metabolic homeostasis and facilitating healthy aging (Covarrubias et al., 2021). Across a spectrum of organisms, NAD+ concentrations generally decline with age, closely associated with physiological deterioration and age-related pathologies (Belenky et al., 2007). Therefore, restoring NAD+ levels has become a pivotal aim to alleviate or even reverse functional decline and diseases.
However, direct supplementation faces challenges due to the limited ability of NAD+ to penetrate cellular membranes and its vulnerability to digestive enzymatic degradation. These issues result in the low bioavailability and stability of NAD+ within the body, along with potential side effects such as insomnia and fatigue (Poddar et al., 2019). As a result, researchers are actively exploring alternative methods to enhance NAD+ levels, aiming for more effective anti-aging effects. Among these alternatives, supplementation with the NAD+ precursor NMN has been considered noteworthy for impeding the aging process, as it has demonstrated the ability to alleviate and reverse age-related degenerative diseases by increasing NAD+ levels (Okabe et al., 2019).
Once inside the body, supplemented NMN undergoes a series of biochemical reactions, leading to a substantial increase in cellular NAD+ levels, NAD+/NADH ratios, and the expression of sirtuin deacetylases. This supplementation further improves mitochondrial function and alleviates cellular aging (Wang et al., 2022). However, determining the precise mechanisms of anti-aging effects, optimal dosage, and treatment safety of NMN still requires elaborate investigations. In this review, we focus on elucidating the anti-aging mechanisms of NMN and address the current research limitations, providing references and suggestions for in-depth future studies into the anti-aging effects of NMN.
2. NMN biosynthesis and depletion
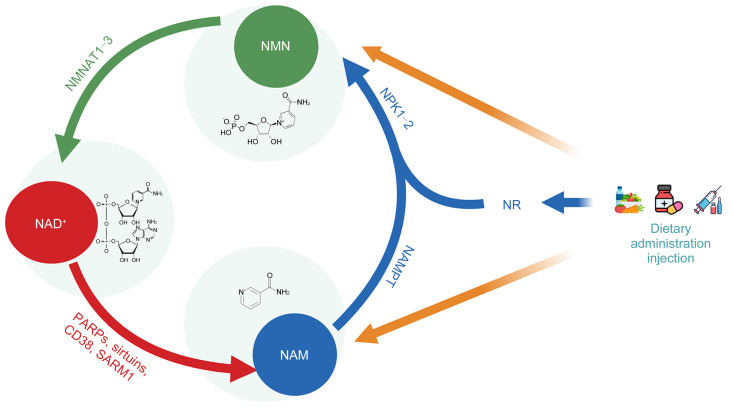
Various natural foods, such as beef, shrimp, legumes, nuts, and cereals, contain compounds related to vitamin B3, including nicotinamide (NAM), nicotinamide riboside (NR), nicotinic acid (NA), and NMN. These precursors undergo synthesis into NMN within the body through salvage pathways (Amjad et al., 2021). For example, NR is phosphorylated by nicotinamide riboside kinase (NRK) to form NMN, while NAM is catalyzed by nicotinamide phosphoribosyltransferase (NAMPT) to transform into NMN (Imai, 2011). Subsequently, NMN synthesized in the cytoplasm enters mitochondria or nuclei, where it is catalyzed by nicotinamide mononucleotide adenylyl transferases 1–3 (NMNAT1–3) to convert into NAD+ (Revollo et al., 2007; Kane and Sinclair, 2018). NAD+ can be synthesized via both a de novo pathway from tryptophan and a salvage pathway from endogenous/exogenous intermediates, with the latter being predominant in maintaining in vivo NAD+ levels.
Inside the body, NAD+-consuming enzymes, such as sirtuins, poly(adenosine diphosphate (ADP)-ribose) polymerase (PARP), and cluster of differentiation 38 (CD38), degrade NAD+ into NAM, establishing a cycle of NMN biosynthesis and degradation (Bai et al., 2011a, 2011b; Shen et al., 2021). Sirtuins are a class of NAD+-dependent deacetylases, encompassing SIRT1‒SIRT7, which play crucial roles in various metabolic pathways and cellular signaling processes. They regulate cellular functions through protein deacetylation and phosphorylation, significantly influencing the extension of lifespan and promoting the delay of age-related diseases (Zhang et al., 2020). PARP is a type of NAD+-dependent DNA repair enzyme with a DNA damage-sensing function, which is activated upon recognizing structural damage to DNA fragments and consumes NAD+ during DNA repair to convert it into NAM.
CD38 is a protein found in immune cells that consumes NAD+ and possesses intracellular signal transduction activity (Camacho-Pereira et al., 2016). It catalyzes the synthesis of NAM, ADP-ribosyl (ADPR) cyclase, and cyclic ADPR (cADPR) using NAD+ as a substrate. In turn, cADPR promotes Ca2+ release and cell apoptosis, while ADPR cyclase regulates various cellular functions through Ca2+ mobilization. These processes are closely related to immune function and inflammatory responses in the organism (Piedra-Quintero et al., 2020). The NAM resulting from NAD+ consumption subsequently re-enters the salvage pathway and thus the NAD+ synthesis cycle (Fig. 1).
Fig. 1. Biosynthesis and depletion processes of nicotinamide mononucleotide (NMN). CD38: cluster of differentiation 38; NAD+: nicotinamide adenine dinucleotide; NAM: nicotinamide; NAMPT: nicotinamide phosphoribosyltransferase; NMNAT: nicotinamide mononucleotide adenylyl transferase; NR: nicotinamide riboside; NRK: nicotinamide riboside kinase; PARP: poly(adenosine diphosphate (ADP)-ribose) polymerase; SARM1: sterile alpha and Toll/interleukin-1 receptor (TIR) motif-containing protein 1.
Recent research has unveiled that sterile alpha and Toll/interleukin-1 receptor (TIR) motif-containing protein 1 (SARM1), as the central regulatory factor associated with axonal pathological degeneration in neurodegenerative diseases, possesses NAD+ degradation activity. This is promoted via its TIR domain, whereas it is inhibited by another armadillo (ARM) domain upon binding to NAD+, an interaction that prevents axonal degeneration. However, the destruction of the NAD+-binding site ARM or the disruption of the ARM–TIR interaction results in the persistent activation of SARM1, leading to axonal degeneration. This discovery not only reveals the specific role of SARM1 in the process of NAD+ consumption but also suggests potential therapeutic targets for the treatment of neurodegenerative diseases (di Stefano et al., 2015; Jiang et al., 2020).
3. Anti-aging mechanisms of NMN
3.1. Roles of NMN in cellular energy metabolism
Comprehensive studies have revealed numerous connections between aging and energy metabolism. Endogenous NAD+ levels decline with age, impacting various physiological processes, such as energy metabolism, DNA repair, and cellular signal transduction. NMN, a precursor of NAD+, can elevate the intracellular NAD+/NADH ratio through supplementation. This enhancement of NAD+ levels leads to improved mitochondrial functionality, increased fatty acid oxidation, and heightened mitochondrial respiratory chain activity. Mitochondrial functionality here is primarily considered from the aspect of cell dependency level, encompassing mitochondrial quality control and cellular energy supply (Monzel et al., 2023). Furthermore, NAD+ modulates multiple metabolic pathways, such as those involving glucose, fatty acids, and amino acids, contributing to the maintenance of normal energy metabolism, the reduction of intracellular reactive oxygen species (ROS) levels, and the mitigation of inflammatory responses, jointly decelerating the aging process.
The incidence of metabolic disorders, such as insulin resistance and liver metabolic abnormalities related to glucose metabolism, escalates with age (Palmer and Jensen, 2022). In type 2 diabetes, an abnormal NAD+/NADH ratio is observed due to substrate over-oxidation. NAMPT, the rate-limiting enzyme in mammalian NAD+ biosynthesis, influences NAD+ biogenesis in response to a high-fat diet and aging, which is related to the pathogenesis of type 2 diabetes. NMN, a product of the NAMPT reaction and a crucial intermediary of NAD+, can ameliorate impaired glucose tolerance and hepatic insulin sensitivity by restoring the NAD+ levels reduced by diet and age. Moreover, NMN can regulate the expression of SIRT1 target transcription factors, including c-Myc, nuclear factor-κB (NF-κB), peroxisome proliferator-activated receptor γ (PPARγ), and p53, leading to the restoration of gene expression related to oxidative stress, inflammatory responses, and circadian rhythms. The beneficial effects of NMN treatment on primary hepatocytes have been further confirmed by the use of a SIRT1-specific inhibitor, indicating that SIRT1 is among the biomolecules mediating the role of NMN in improving metabolic functions. As an activator of sirtuin proteins, NMN enhances the activity of SIRT1 and other members of the sirtuin family (SIRT2–SIRT7), demonstrating significant beneficial effects. The above research findings offer novel therapeutic avenues for diabetes and elderly type 2 diabetes patients with defects in NAD+ synthesis regulated by NAMPT (Yoshino et al., 2011). Mills et al. (2016) confirmed the potential of NMN to improve insulin sensitivity and plasma lipid spectra and enhance energy metabolism while inhibiting age-related weight gain. Consistent with the observed phenotypes, NMN was found to reduce the expression of aging-related genes in key metabolic organs, such as skeletal muscle, white adipose tissue, and the liver. The administration of NMN significantly reduced age-induced changes in the expression of these genes (Mills et al., 2016).
Notably, NMN supplementation is effective not only in animal models but also in humans. A randomized controlled trial spanning ten weeks in postmenopausal women demonstrated that NMN supplementation could improve muscle insulin sensitivity, insulin signaling, and muscle remodeling in overweight or obese pre-diabetic postmenopausal women. Although NMN supplementation did not alter the concentration of NAD+ in the muscle, it increased the content of NMN metabolic products, namely, N-methyl-2-pyridone-5-carboxamide and N-methyl-4-pyridone-5-carboxamide. In this manner, NMN supplementation enhances the turnover of NAD+ in the muscle, improving muscle insulin sensitivity without raising NAD+ levels. Meanwhile, the effects on insulin sensitivity in the liver and adipose tissues were less pronounced, with NMN selectively influencing muscle insulin sensitivity. The primary mechanism of action may involve the modulation of key components of glucose uptake and muscle-remodeling signaling pathways, such as the elevation of phosphorylation levels of protein kinase B (AKT) serine-473 (Ser473) and threonine-308 (Thr308), as well as mammalian target of rapamycin (mTOR) serine-2448 (Ser2448) (Yoshino et al., 2021). In addition, NMN supplementation increased the number of myocytes in skeletal muscle and enhanced the platelet-derived growth factor (PDGF) signaling pathway. Nonetheless, further research is needed to precisely elucidate the impact of NMN on metabolic processes. The above study provides fresh insights into diabetes treatment, while more comprehensive studies and trials involving diverse populations are required to validate its efficacy and safety (Frederick et al., 2016; Fletcher et al., 2017). NMN treatment has shown extremely positive results in patients with polycystic ovary syndrome, which is characterized by similar endocrine features. The metabolic dysfunctions of these patients, such as obesity and insulin resistance, were almost entirely normalized, further validating the role of NMN in improving energy metabolism (Aflatounian et al., 2022).
Senescent cells exhibit mitochondrial dysfunction, including abnormalities in mitochondrial morphology and structure, reduced intracellular adenosine triphosphate (ATP) content, and elevated ROS levels (Fang et al., 2014, 2016). A key measure of mitochondrial activity is the number of mitochondrial DNA copies that progressively declines with age. Therefore, the regulation of mitochondrial DNA copy numbers is instrumental in mitigating mitochondrial dysfunction. Research has indicated that NMN ameliorates mitochondrial functionality by boosting nucleotide levels, as evidenced by both cellular and animal studies. NMN operates via two discrete metabolic pathways. One involves enhancing the synthesis of ubiquinone in high-energy-demand tissues such as the heart and the liver, thereby activating the de novo synthesis of pyrimidine nucleotides. The other pathway involves the stimulation of gene expression related to NMN hydrolase, like CD157, expediting the hydrolysis of NMN to ribose-5-phosphate (R5P), which enhances nucleotide synthesis. By accelerating nucleotide synthesis, mitochondrial DNA replication is promoted, leading to an increase in the number of mitochondrial DNA copies and thus fortifying mitochondrial function (Nomiyama et al., 2022).
Chronic heart failure is closely linked to mitochondrial dysfunction, primarily caused by cumulative damage from high-level reactive oxygen metabolism (Sanada et al., 2011). Despite extensive antioxidant therapies, the results have been suboptimal, necessitating novel approaches to address mitochondrial dysfunction. Studies have shown that reducing NAD+ levels in murine models hinders mitochondrial metabolism in cardiac cells, leading to heart failure in experimental animals (Walker and Tian, 2018; Zhou et al., 2020; Abdellatif et al., 2021; Tong et al., 2021; Walker et al., 2023). However, short-term supplementation with the NAD+ precursor NMN could enhance cardiac cell metabolism and improve cardiac function (Zhang et al., 2017). NMN treatment has also demonstrated similar efficacy in a mouse model of myocardial fibrosis, reducing cardiac ejection fraction and aggravating myocardial fibrosis by upregulating cardiac cell SIRT6 protein expression (Yu et al., 2020).
As women age, their oocytes develop structural and functional mitochondrial abnormalities, resulting in decreased reproductive capability. NMN supplementation could enhance the quality of aged mouse oocytes and augment ovulation by restoring NAD+ levels. Studies have revealed that NMN treatment restores ATP and SIRT1 protein levels, reduces abnormal mitochondrial distribution, and rectifies gene expression related to mitochondrial dysfunction in aged oocytes (Bertoldo et al., 2020; Huang et al., 2022; Jiang et al., 2023; Li et al., 2023; Meng et al., 2024; Singh et al., 2024). This suggests that the beneficial effects of NMN on aged oocytes are mediated through mitochondrial function (Miao et al., 2020). SIRT3, a crucial mitochondrial protein, regulates oxidative stress and mitochondrial function. Recent research has demonstrated that NMN supplementation can reverse cellular senescence and alleviate mitochondrial dysfunction in mesenchymal stem cells (MSCs) by modulating the NAD+/SIRT3 pathway (Wang et al., 2022). In addition, NMN reduces oxidative stress in aged mouse liver cells and the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream genes (Luo et al., 2022). However, in SIRT3-deficient mice, the effect of NMN was attenuated, further substantiating the pivotal role of SIRT3 in the ability of NMN to enhance mitochondrial function and regulate oxidative stress levels.
3.2. Roles of NMN in immunoregulation and cell apoptosis
As age increases, the immunoreactivity of the human body weakens, particularly regarding natural killer (NK) cells, which are essential for cancer resistance and viral infection. The NAD+ metabolic pathway plays a crucial role in the immune system, and NMN may enhance immune function and alleviate the process of immunosenescence by regulating immune cell metabolism. The immune system identifies and combats pathogens and foreign bodies invading the body, releasing inflammatory mediators to initiate an inflammatory response. However, excessive inflammatory responses can lead to tissue damage and diseases, including autoimmune and chronic inflammatory conditions. However, NMN intervention can alleviate the inflammatory response by regulating the function of immune cells.
Macrophage-mediated systemic inflammatory responses can damage various tissues and organs and are a major cause of death from sepsis and multiple organ dysfunction syndrome. Research has shown that oral or intraperitoneal injection of NMN can enhance the immunotoxicity and function of mouse NK cells (Takeda and Okumura, 2021). Inflammation leads to the accumulation of CD38 in tissues. With age, inflammation induced by senescent cells causes the accumulation of CD38+ immune cells, resulting in decreased NAD+ levels and affecting immune function. Components of the senescence-associated secretory phenotype (SASP) secreted by senescent cells may induce the accumulation of CD38+ immune cells, leading to a decline in NAD+ levels. The ectoenzyme activity of CD38 may be involved in regulating the availability of NMN to regulate cellular NAD+ levels.
During aging, the cellular NAD+ biosynthesis pathway is becoming suppressed, whereas external supplementation with NMN can enhance NAD+ levels and improve immune function (Chini et al., 2020). NMN can reprogram the phenotype of macrophages, reducing the proportion of pro-inflammatory M1 phenotype macrophage activation and enhancing the expression of anti-inflammatory M2 phenotype-specific markers (Cros et al., 2022). Consistent with a study on NMN treatment for silicosis, NMN modulates macrophage homeostasis, concurrently reducing the activation ratios of CD4+ and CD8+ T cells, thus alleviating inflammatory damage in the process of silicosis (Wang et al., 2023). Moreover, NMN can regulate stem cell differentiation and self-renewal, thereby enhancing the immune response and preventing cellular aging and tissue degeneration. Overall, NMN can regulate NAD+ levels in various immune cells, impacting their development, differentiation, and other aspects, subsequently improving the immune function.
Cell apoptosis is a critical and inevitable element of the aging process, and the anti-aging effects of NMN are associated with the regulation of cell apoptosis. Previous research has demonstrated that NMN treatment can reduce oocyte apoptosis and increase the number of oocytes, indicating its important role in regulating cell apoptosis (Miao et al., 2020). The role of NMN mainly involves enhancing PARP activity and SIRT1-mediated histone modifications to maintain chromosomal structure and stability, as PARP activity and SIRT1 expression are closely related to cell apoptosis. The regulation of cell apoptosis by NMN can also help to mitigate the toxic side effects of chemotherapy. For instance, during the treatment of late-stage hepatocellular carcinoma with the drug sorafenib, the overexpression of SIRT1 and the restoration of NAD+ levels through NMN could reduce cell apoptosis. Sorafenib induces cell apoptosis by affecting the NAD+/SIRT1/adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK) axis (Garten et al., 2019). In clinical practice, the dose-dependent toxic side effects of many anti-cancer drugs severely limit their application. To this end, the protective effect of NMN could be a potential strategy to prevent these side effects. For example, the chemotherapy drug cisplatin can induce cognitive impairment in the nervous system, while doxorubicin treatment can cause cardiac toxicity and the loss of certain physiological functions. However, combined treatment with NMN and chemotherapy drugs with toxic side effects can reduce the undesirable side effects without affecting the anti-tumor effects. The primary mechanism is that NMN inhibits the expression of genes related to cell apoptosis and DNA damage, protecting related tissues and organs from the effects of chemotherapy drugs (Yoo et al., 2021; Margier et al., 2023).
Research has also shown that NAMPT expression significantly decreases during myocardial ischemia, reperfusion, and pressure overload. Preventing the downregulation of NAMPT can reduce the death of myocardial cells in case of myocardial injury (Hsu et al., 2009). Ultraviolet (UV) radiation and metabolic toxins cause DNA damage that can block transcription and lead to cell apoptosis. For instance, high sugar-induced corneal epithelial cell damage is a key pathological process in diabetes-related eye diseases. In vitro experiments have shown that NMN treatment reduces the apoptosis of corneal epithelial cells, increases cell vitality, enhances cell migration, and restores the tight connections of cells (Pu et al., 2022). In another study, NMN could reduce the death of outer cells in the acute phase of retinal detachment and inhibit the pathological proliferation of glial cells in the late stage of retinal detachment (Chen et al., 2020). Exposure to ultraviolet B (UVB) radiation can cause corneal edema and cell apoptosis in mice; however, NMN treatment has been proven to be highly effective in preventing such damage. This beneficial effect of NMN has been verified in human corneal epithelial cells. Regarding the relevant mechanism, the activation of AKT signaling pathway and the reduction of B-cell lymphoma-2 (BCL-2)-associated X protein (BAX)/BCL-2 ratio are involved in this biological process (Zhao et al., 2020). Therefore, NMN has significant potential in the treatment of diabetes-related eye complications and other eye damage-related diseases induced by various factors. The roles of NMN in immune function and cell apoptosis are closely linked to its involvement in regulating energy metabolism and signaling pathways, while further research is necessary to explore these intricate relationships.
3.3. Roles of NMN in DNA damage repair
NAD+, as the biosynthetic product of NMN, plays a crucial role in regulating DNA repair enzymes, thereby modulating genomic stability and maintaining DNA integrity, which in turn reduces the frequency of random mutations and decelerates the aging process. Accumulated DNA damage impairs cellular functions, leading to accelerated cellular senescence and apoptosis. Members of the PARP family are essential in NAD+-mediated DNA damage repair, with PARP-3 primarily involved in repairing double-strand DNA breaks and PARP-1 and PARP-2 activated in the base excision repair triggered by single-strand DNA breaks (de Vos et al., 2012). Upon cellular DNA damage, activated PARP consumes significant amounts of NAD+ in the repair process, leading to a deficit in NAD+ supply, which negatively affects cellular metabolism and survival. Furthermore, through the accumulation of ROS, decreased NAD+ levels cause damage to mitochondrial DNA and nuclear DNA (Cohen, 2020). Several protein molecules participate in NAD+-dependent DNA repair, including those in the PARP and sirtuin families, exhibiting mutual interdependency and regulation and working in concert during the DNA repair process. When NAD+ is depleted, SIRT1 is activated, directly mediating cellular apoptosis (Fang et al., 2014).
Sirtuins play a pivotal role in regulating aging-related epigenetic modifications. They achieve gene silencing and DNA repair regulation through the deacetylation of specific lysine residues of histones, facilitating the formation of a more repressed chromatin structure related to lifespan extension (Wątroba et al., 2017). One study found that NMN supplementation bolstered DNA repair in ataxia telangiectasia neurons. Beyond a previously discovered mechanism involving NAD+-dependent SIRT1 participating in DNA repair through deacetylation and activation of relevant repair proteins in the double-strand DNA break repair process (Chalkiadaki and Guarente, 2015), it was also found that NMN supplementation can enhance neuronal DNA repair via the DNA-dependent protein kinase (DNA-PK) catalytic subunit (DNA-PKcs) regulatory pathway. Other NAD+-dependent sirtuins such as SIRT6 and SIRT7 may also contribute to DNA repair by elevating NAD+ levels (Fang et al., 2016). Moreover, increasing NAD+ levels could reduce neuronal DNA damage levels in Alzheimer’s disease mouse models, providing evidence for the protective effect of NMN supplementation on neurons (Hou et al., 2018). Miao et al. (2020) attempted to reverse aged oocyte function with NMN treatment and also found that NMN supplementation could restore the spindle/chromosome structure and kinetochore-microtubule attachment, maintaining the euploidy and maturation of aged oocytes.
UV radiation-induced DNA damage significantly elevates the risk of cellular mutations and tumor occurrences, contributing to aging and certain degenerative diseases. Recent research has highlighted the pivotal roles of NAD+ in UV radiation-induced DNA damage. Animal experiments have shown that NAM protects mice from carcinogenesis induced by UV radiation. In cell culture experiments, NAM supplementation has been found to enhance the repair capacity of primary human melanocytes for UV-induced DNA damage (Thompson et al., 2014). Furthermore, immortalized human keratinocyte (HaCaT) cells treated with NAM demonstrated an increase in both the overall nucleotide excision repair rate and the number of irradiated melanocytes undergoing DNA repair (Surjana et al., 2013). Besides, a randomized controlled trial demonstrated that oral NAM significantly reduces actinic keratosis precancerous lesions and potentially lowers the incidence rate of non-melanoma skin cancer (Surjana et al., 2012). The above studies provide valuable insights into the potential application of NMN in melanoma prevention and the exploration of melanocyte response mechanisms to DNA damage.
In summary, NAD+ plays several fundamental roles in the DNA repair process. Firstly, it acts as a critical auxiliary factor in the DNA repair pathway, interacting with various DNA repair enzymes to facilitate DNA repair. Secondly, it participates in cellular stress responses as a signaling molecule, regulating a host of gene expression and cellular metabolic processes. Thirdly, following DNA damage, cells release stress signals that modulate a range of cellular physiological processes, including DNA repair and apoptosis, with NAD+ playing a crucial regulatory role. Finally, NAD+ functions as an antioxidant, safeguarding cellular DNA from free radical damage. Therefore, NMN supplementation in vitro provides an effective approach to alleviate various forms of cellular DNA damage.
4. Conclusions and future perspectives
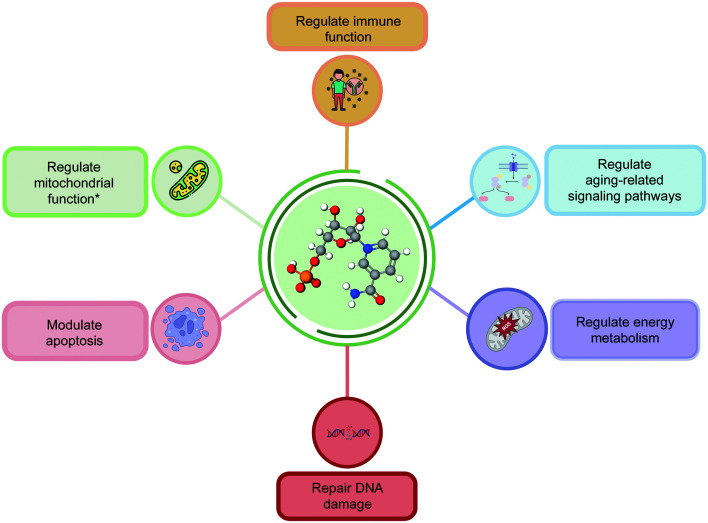
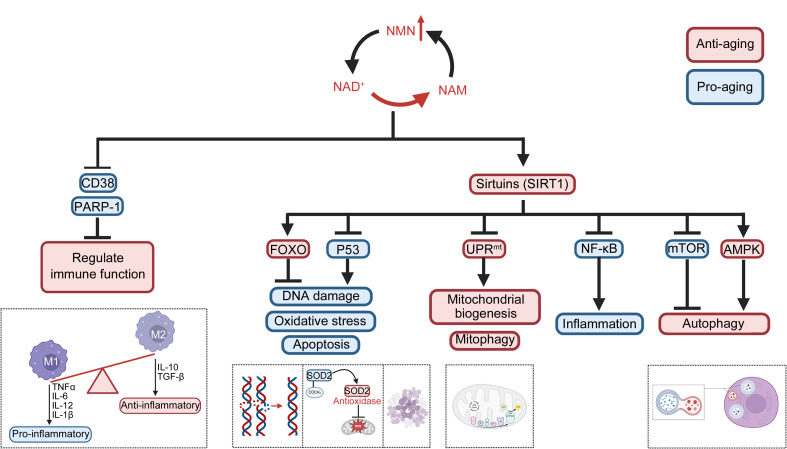
Research on the anti-aging mechanisms of NMN is continuously expanding, revealing its crucial roles in energy metabolism, mitochondrial function, immune function, cell apoptosis, and DNA repair (Fig. 2). Additionally, NMN influences certain aging-related signaling pathways, including AMPK (Zhou et al., 2021), mTOR (Zhao et al., 2023), and phosphoinositide 3-kinase (PI3K)/AKT (Verma et al., 2023), which are responsible for regulating cell metabolism, autophagy, and cell proliferation. By modulating these pathways, NMN exhibits anti-aging effects (Fig. 3). Moreover, NMN has been found to mitigate skin-aging pigmentation issues. By downregulating the cyclic adenosine monophosphate (cAMP)/Wnt signaling pathway, NMN treatment reduces melanin production in senescent melanocytes, as supported by studies using human skin models (Brito et al., 2022). In summary, the anti-aging mechanism of action of NMN is complex and involves multiple molecular pathways and biological processes. It is necessary to further explore this mechanism and evaluate clinical application prospects for treating and preventing aging-related diseases.
Fig. 2. Anti-aging mechanism of nicotinamide mononucleotide (NMN). * Mitochondrial function mainly refers to cell dependence, like the mitochondrial quality control and cell energy supply.
Fig. 3. Signal pathway of the anti-aging effect of nicotinamide mononucleotide (NMN). NMN facilitates the synthesis of nicotinamide adenine dinucleotide (NAD+), resulting in reduced levels of NAD+-consuming enzymes, such as cluster of differentiation 38 (CD38) and poly(adenosine diphosphate (ADP)-ribose) polymerase-1 (PARP-1), thereby modulating immune function and reprogramming macrophage phenotypes towards increased activation of the anti-inflammatory M2 phenotype. As the primary consumers of NAD+, the activation of sirtuins due to elevated levels of NAD+ orchestrates the regulation of critical metabolic processes, stress responses, and the biology of aging via the activation or inhibition of downstream signaling pathways, ultimately exerting an anti-aging effect. AMPK: adenosine 5'-monophosphate (AMP)-activated protein kinase; FOXO: forkhead box protein O; IL: interleukin; mTOR: mammalian target of rapamycin; NAM: nicotinamide; NF-κB: nuclear factor-κB;TGF-β: transforming growth factor-β; TNFα: tumor necrosis factor α;SOD2: superoxide dismutase 2; UPRmt: mitochondrial unfolded protein response.
Despite the numerous studies confirming the anti-aging role of NMN in recent years, existing research still has limitations. Firstly, most studies have been conducted with a small sample size and using animal models, and have not been verified in human trials. Secondly, the optimal dosage for NAD+ anti-aging has not been gauged. While NMN efficacy has been found to be dose-dependent (Mills et al., 2016), the dose of 100, 250, or 500 mg had no significant adverse effects on healthy adult males (Irie et al., 2020). Nevertheless, NMN treatment in immune cells may promote NAMPT expression, potentially exacerbating the inflammatory state in arthritis. Therefore, when using NAMPT/NAD+ signaling modulators to treat arthritis, potential adverse effects must be considered (Wang et al., 2021). To maximize the efficacy of NMN, determining its optimal dosage is crucial, as different target organs and effector molecules may require different levels and modes of action (Table 1). Further investigations into the optimal dosage and targeting mechanisms of NMN are crucial to explore its therapeutic effects more comprehensively. Moreover, studies using dual-labeled isotope NMN have revealed that orally administered NMN can be rapidly absorbed and efficiently transported into the bloodstream, converting into NAD+ in major metabolic tissues like the liver and skeletal muscle. However, this effect is transient: within 15 min of NMN supplementation, the body’s NMN and NAD+ levels quickly rise and then return to their original levels. Thus, maintaining a steady increase in systemic NMN levels and sustaining high levels for its continued functionality present a significant challenge for NMN treatment (Mills et al., 2016). Current NMN applications involve several technical challenges, such as improving the conversion rate of NMN and maximizing NAD+ levels while ensuring stability, which necessitates further investigations.
Table 1.
Effects of nicotinamide mononucleotide (NMN) in different doses and different target organs
| Administration | Duration | Subjects | Disease | Organ | Key finding | Reference |
|---|---|---|---|---|---|---|
| IP | 10 d | Mouse | High-fat diet-induced type 2 diabetes, aging-induced type 2 diabetes | Liver, WAT, SM | Enhanced insulin sensitivity | Yoshinoet al., 2011 |
| PO | 12 months | Mouse (naturally aging) | Liver, WAT, SM | Mitigated physiological decline | Mills et al., 2016 | |
| PO | 10 weeks | Human (female) | Overweight or obese postmenopausalpre-diabetes | SM, liver, AT | Increased muscle insulin sensitivity | Yoshinoet al., 2021 |
| PO | 8 weeks | Mouse | Polycystic ovary syndrome | Muscle, liver, ovaries | Normalized energy metabolism | Aflatounian et al., 2022 |
| IP | 5 d | Mouse | Cardiac-specificKruppel-like factor 4 (KLF4) deficience | Heart | Improved heart function | Zhang et al., 2017 |
| IP | 4 weeks | Mouse | Cardiac fibrosis | Heart | Improved heart function | Yu et al., 2020 |
| IP | 10 d | Mouse (maternally aging) | Ovaries | Betterfertilization | Miao et al., 2020 | |
| In vitro | 24 h | Cell (replicative aging MSCs) | MSCs | Alleviated cell aging | Wang et al., 2022 | |
| IP | 4 weeks | Mouse (naturally aging) | Liver | Reduced levels of oxidative stress | Luo et al., 2022 | |
| IP | 3 d, 4 d | Mouse | Sepsis, peritonitis | Heart, lungs | Improved survival | Cros et al., 2022 |
| PO | 7 d, 28 d | Mouse | Silicosis | Lungs | Alleviated lung damage | Wang et al., 2023 |
| In vitro | 24 h | Cell | Hepatocellular carcinoma | HCCs | Prevented cell apoptosis | Garten et al., 2019 |
| PO | 20 d (5-d injection and 5-d pause; four cycles in total) | Mouse | Chemotherapy-induced cognitive impairment | BT | Prevented cognitive dysfunctions | Yoo et al., 2021 |
| IP (acute experiment),PO (chronic experiment) | 5 d (acute experiment),60 d (chronic experiment) | Mouse | Doxo-induced cardiotoxicity and physical dysfunction | Heart, SM | Prevented damage | Margieret al., 2023 |
| In vitro | 48 h | Cell | Diabetes mellitus (DM)-related corneal epithelial dysfunction | HCECs | Increased survival | Pu et al., 2022 |
| PO | 7 d | Mouse | Retinal detachment | Eyes | Protected retinal photoreceptors | Chen et al., 2020 |
| PO | 25 d | Rat | Adjuvant-induced arthritis (AIA) | Both hind paws, spleen | Worsened severity of AIA | Wang et al., 2021 |
| In vitro | 20 d | Cell (aging melanocytes) | Melanocytes | Reduced melanin production | Brito et al., 2022 |
IP: intraperitoneal; PO: peros; In vitro: in vitro cell culture; WAT: white adipose tissue; SM: skeletal muscle; AT: adipose tissue; MSCs: mesenchymal stem cells; HCCs: hepatocellular carcinoma cells; BT: brain tissue; HCECs: human corneal epithelial cells.
To be continued
Table 1 (continued)
Author contributions
Hongjun KANG conceptualized this manuscript. Min WANG and Lianrong ZHU wrote the original draft and achieved the visualization. Min WANG, Yuan CAO, Yun LI, Lu WANG, and Yuyan LIU were all involved in the investigation. Zihui DENG was responsible for the project management. All authors contributed to the article and approved the final version.
Compliance with ethics guidelines
Min WANG, Yuan CAO, Yun LI, Lu WANG, Yuyan LIU, Zihui DENG, Lianrong ZHU, and Hongjun KANG declare that they have no conflict of interest.
This review does not contain any studies with human or animal subjects performed by any of the authors.
References
- Abdellatif M, Sedej S, Kroemer G, 2021. NAD+ metabolism in cardiac health, aging, and disease. Circulation, 144(22): 1795-1817. 10.1161/CIRCULATIONAHA.121.056589 [DOI] [PubMed] [Google Scholar]
- Aflatounian A, Paris VR, Richani D, et al. , 2022. Declining muscle NAD+ in a hyperandrogenism PCOS mouse model: possible role in metabolic dysregulation. Mol Metab, 65: 101583. 10.1016/j.molmet.2022.101583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjad S, Nisar S, Bhat AA, et al. , 2021. Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol Metab, 49: 101195. 10.1016/j.molmet.2021.101195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Canto C, Brunyánszki A, et al. , 2011a. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab, 13(4): 450-460. 10.1016/j.cmet.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, et al. , 2011b. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab, 13(4): 461-468. 10.1016/j.cmet.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C, 2007. NAD+ metabolism in health and disease. Trends Biochem Sci, 32(1): 12-19. 10.1016/j.tibs.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Bertoldo MJ, Listijono DR, Ho WHJ, et al. , 2020. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep, 30(6): 1670-1681.e7. 10.1016/j.celrep.2020.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito S, Baek JM, Cha B, et al. , 2022. Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/Wnt signaling. J Dermatol Sci, 106(3): 159-169. 10.1016/j.jdermsci.2022.05.002 [DOI] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarrago MG, Chini CCS, et al. , 2016. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab, 23(6): 1127-1139. 10.1016/j.cmet.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L, 2015. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer, 15(10): 608-624. 10.1038/nrc3985 [DOI] [PubMed] [Google Scholar]
- Chen XH, Amorim JA, Moustafa GA, et al. , 2020. Neuroprotective effects and mechanisms of action of nicotinamide mononucleotide (NMN) in a photoreceptor degenerative model of retinal detachment. Aging, 12(24): 24504-24521. 10.18632/aging.202453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini CCS, Peclat TR, Warner GM, et al. , 2020. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat Metab, 2(11): 1284-1304. 10.1038/s42255-020-00298-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, 2020. Interplay between compartmentalized NAD+ synthesis and consumption: a focus on the PARP family. Genes Dev, 34(5-6): 254-262. 10.1101/gad.335109.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias AJ, Perrone R, Grozio A, et al. , 2021. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol, 22(2): 119-141. 10.1038/s41580-020-00313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros C, Margier M, Cannelle H, et al. , 2022. Nicotinamide mononucleotide administration triggers macrophages reprogramming and alleviates inflammation during sepsis induced by experimental peritonitis. Front Mol Biosci, 9: 895028. 10.3389/fmolb.2022.895028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos M, Schreiber V, Dantzer F, 2012. The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol, 84(2): 137-146. 10.1016/j.bcp.2012.03.018 [DOI] [PubMed] [Google Scholar]
- di Stefano M, Nascimento-Ferreira I, Orsomando G, et al. , 2015. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ, 22(5): 731-742. 10.1038/cdd.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Brace LE, et al. , 2014. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reduction. Cell, 157(4): 882-896. 10.1016/j.cell.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Kassahun H, Croteau DL, et al. , 2016. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab, 24(4): 566-581. 10.1016/j.cmet.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RS, Ratajczak J, Doig CL, et al. , 2017. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol Metab, 6(8): 819-832. 10.1016/j.molmet.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DW, Loro E, Liu L, et al. , 2016. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab, 24(2): 269-282. 10.1016/j.cmet.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Grohmann T, Kluckova K, et al. , 2019. Sorafenib-induced apoptosis in hepatocellular carcinoma is reversed by SIRT1. Int J Mol Sci, 20(16): 4048. 10.3390/ijms20164048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YJ, Lautrup S, Cordonnier S, et al. , 2018. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new ad mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA, 115(8): E1876-E1885. 10.1073/pnas.1718819115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, et al. , 2009. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res, 105(5): 481-491. 10.1161/circresaha.109.203703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Zhou Y, Tang WH, et al. , 2022. Long-term treatment of Nicotinamide mononucleotide improved age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice. J Nutr Biochem, 101: 108911. 10.1016/j.jnutbio.2021.108911 [DOI] [PubMed] [Google Scholar]
- Imai SI, 2011. Dissecting systemic control of metabolism and aging in the NAD world: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett, 585(11): 1657-1662. 10.1016/j.febslet.2011.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie J, Inagaki E, Fujita M, et al. , 2020. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J, 67(2): 153-160. 10.1507/endocrj.EJ19-0313 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wang D, Zhang C, et al. , 2023. Nicotinamide mononucleotide restores oxidative stress-related apoptosis of oocyte exposed to benzyl butyl phthalate in mice. Cell Prolif, 56(8): e13419. 10.1111/cpr.13419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YF, Liu TT, Lee CH, et al. , 2020. The NAD+-mediated self-inhibition mechanism of pro-neurodegenerative SARM1. Nature, 588(7839): 658-663. 10.1038/s41586-020-2862-z [DOI] [PubMed] [Google Scholar]
- Kane AE, Sinclair DA, 2018. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res, 123(7): 868-885. 10.1161/circresaha.118.312498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Han Q, Chen Y, et al. , 2023. β-nicotinamide mononucleotide rescues the quality of aged oocyte and improves subsequent embryo development in pigs. PLoS ONE, 18(10): e0291640. 10.1371/journal.pone.0291640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CT, Ding WX, Yang CM, et al. , 2022. Nicotinamide mononucleotide administration restores redox homeostasis via the Sirt3‒Nrf2 axis and protects aged mice from oxidative stress-induced liver injury. J Proteome Res, 21(7): 1759-1770. 10.1021/acs.jproteome.2c00167 [DOI] [PubMed] [Google Scholar]
- Margier M, Kuehnemann C, Hulo N, et al. , 2023. Nicotinamide mononucleotide administration prevents doxorubicin-induced cardiotoxicity and loss in physical activity in mice. Cells, 12(1): 108. 10.3390/cells12010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Zhang YN, Du JY, et al. , 2024. Nicotinamide mononucleotide maintains cytoskeletal stability and fortifies mitochondrial function to mitigate oocyte damage induced by triocresyl phosphate. Ecotoxicol Environ Saf, 275: 116264. 10.1016/j.ecoenv.2024.116264 [DOI] [PubMed] [Google Scholar]
- Miao YL, Cui ZK, Gao Q, et al. , 2020. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep, 32(5): 107987. 10.1016/j.celrep.2020.107987 [DOI] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, et al. , 2016. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab, 24(6): 795-806. 10.1016/j.cmet.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzel AS, Enríquez JA, Picard M, 2023. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab, 5(4): 546-562. 10.1038/s42255-023-00783-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama T, Setoyama D, Yasukawa T, et al. , 2022. Mitochondria metabolomics reveals a role of β-nicotinamide mononucleotide metabolism in mitochondrial DNA replication. J Biochem, 171(3): 325-338. 10.1093/jb/mvab136 [DOI] [PubMed] [Google Scholar]
- Okabe K, Yaku K, Tobe K, et al. , 2019. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci, 26: 34. 10.1186/s12929-019-0527-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Jensen MD, 2022. Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Invest, 132(16): e158451. 10.1172/jci158451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra-Quintero ZL, Wilson Z, Nava P, et al. , 2020. CD38: an immunomodulatory molecule in inflammation and autoimmunity. Front Immunol, 11: 597959. 10.3389/fimmu.2020.597959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar SK, Sifat AE, Haque S, et al. , 2019. Nicotinamide mononucleotide: exploration of diverse therapeutic applications of a potential molecule. Biomolecules, 9: 34. 10.3390/biom9010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Q, Guo XX, Hu JJ, et al. , 2022. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed Pharmacother, 147: 112659. 10.1016/j.biopha.2022.112659 [DOI] [PubMed] [Google Scholar]
- Revollo JR, Körner A, Mills KF, et al. , 2007. Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab, 6(5): 363-375. 10.1016/j.cmet.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada S, Komuro I, Kitakaze M, 2011. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol, 301(5): H1723-H1741. 10.1152/ajpheart.00553.2011 [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhang SJ, Xue YZ, et al. , 2021. Biological synthesis of nicotinamide mononucleotide. Biotechnol Lett, 43(12): 2199-2208. 10.1007/s10529-021-03191-1 [DOI] [PubMed] [Google Scholar]
- Singh AK, Mohanty A, Kumar SL, et al. , 2024. Diminished NAD+ levels and activation of retrotransposons promote postovulatory aged oocyte (POAO) death. Cell Death Discov, 10: 104. 10.1038/s41420-024-01876-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surjana D, Halliday GM, Martin AJ, et al. , 2012. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol, 132(5): 1497-1500. 10.1038/jid.2011.459 [DOI] [PubMed] [Google Scholar]
- Surjana D, Halliday GM, Damian DL, 2013. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis, 34(5): 1144-1149. 10.1093/carcin/bgt017 [DOI] [PubMed] [Google Scholar]
- Takeda K, Okumura K, 2021. Nicotinamide mononucleotide augments the cytotoxic activity of natural killer cells in young and elderly mice. Biomed Res, 42(5): 173-179. 10.2220/biomedres.42.173 [DOI] [PubMed] [Google Scholar]
- Thompson BC, Surjana D, Halliday GM, et al. , 2014. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in primary melanocytes. Exp Dermatol, 23(7): 509-511. 10.1111/exd.12430 [DOI] [PubMed] [Google Scholar]
- Tong D, Schiattarella GG, Jiang N, et al. , 2021. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ Res, 128(11): 1629-1641. 10.1161/CIRCRESAHA.120.317046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K, Jaiswal R, Paliwal S, et al. , 2023. An insight into PI3K/Akt pathway and associated protein‒protein interactions in metabolic syndrome: a recent update. J Cell Biochem, 124(7): 923-942. 10.1002/jcb.30433 [DOI] [PubMed] [Google Scholar]
- Walker MA, Tian R, 2018. Raising NAD in heart failure: time to translate? Circulation, 137(21): 2274-2277. 10.1161/CIRCULATIONAHA.117.032626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MA, Chen H, Yadav A, et al. , 2023. Raising NAD+ level stimulates short-chain dehydrogenase/reductase proteins to alleviate heart failure independent of mitochondrial protein deacetylation. Circulation, 148(25): 2038-2057. 10.1161/CIRCULATIONAHA.123.066039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun YA, Pi CC, et al. , 2022. Nicotinamide mononucleotide supplementation improves mitochondrial dysfunction and rescues cellular senescence by NAD+/Sirt3 pathway in mesenchymal stem cells. Int J Mol Sci, 23(23): 14739. 10.3390/ijms232314739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, Zhao MY, Qian R, et al. , 2023. Nicotinamide mononucleotide ameliorates silica-induced lung injury through the Nrf2-regulated glutathione metabolism pathway in mice. Nutrients, 15(1): 143. 10.3390/nu15010143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QH, Li Y, Dou DY, et al. , 2021. Nicotinamide mononucleotide-elicited NAMPT signaling activation aggravated adjuvant-induced arthritis in rats by affecting peripheral immune cells differentiation. Int Immunopharmacol, 98: 107856. 10.1016/j.intimp.2021.107856 [DOI] [PubMed] [Google Scholar]
- Wątroba M, Dudek I, Skoda M, et al. , 2017. Sirtuins, epigenetics and longevity. Ageing Res Rev, 40: 11-19. 10.1016/j.arr.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Yoo KH, Tang JJ, Rashid MA, et al. , 2021. Nicotinamide mononucleotide prevents cisplatin-induced cognitive impairments. Cancer Res, 81(13): 3727-3737. 10.1158/0008-5472.CAN-20-3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, et al. , 2011. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab, 14(4): 528-536. 10.1016/j.cmet.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino M, Yoshino J, Kayser BD, et al. , 2021. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science, 372(6547): 1224-1229. 10.1126/science.abe9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Ma SC, Dai XC, et al. , 2020. Inhibitive effect of nicotinamide mononucleotide on angiotensin II-induced cardiac fibrosis in mice and its mechanism. Chin J Mult Organ Dis Elderly, 19(6): 452-456 (in Chinese). 10.11915/j.issn.1671-5403.2020.06.106 [DOI] [Google Scholar]
- Zhang GZ, Deng YJ, Xie QQ, et al. , 2020. Sirtuins and intervertebral disc degeneration: roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta, 508: 33-42. 10.1016/j.cca.2020.04.016 [DOI] [PubMed] [Google Scholar]
- Zhang RL, Shen YY, Zhou L, et al. , 2017. Short-term administration of nicotinamide mononucleotide preserves cardiac mitochondrial homeostasis and prevents heart failure. J Mol Cell Cardiol, 112: 64-73. 10.1016/j.yjmcc.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li WJ, Duan HY, et al. , 2020. NAD+ precursors protect corneal endothelial cells from UVB-induced apoptosis. Am J Physiol Cell Physiol, 318(4): C796-C805. 10.1152/ajpcell.00445.2019 [DOI] [PubMed] [Google Scholar]
- Zhao YZ, Li H, Guo QL, et al. , 2023. Multiple characteristic alterations and available therapeutic strategies of cellular senescence. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 24(2): 101-114. 10.1631/jzus.B2200178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wang DDH, Qiu YH, et al. , 2020. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest, 130(11): 6054-6063. 10.1172/JCI138538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XR, Du HH, Ni LY, et al. , 2021. Nicotinamide mononucleotide combined with lactobacillus fermentum TKSN041 reduces the photoaging damage in murine skin by activating AMPK signaling pathway. Front Pharmacol, 12: 643089. 10.3389/fphar.2021.643089 [DOI] [PMC free article] [PubMed] [Google Scholar]