Abstract
Objective
Selected populations of patients with chronic hepatitis B (CHB) may benefit from a combined use of pegylated interferon-alpha (pegIFN-α) and nucleos(t)ides (NUCs). The aim of our study was to assess the immunomodulatory effect of pegIFN-α on T and natural killer (NK) cell responses in NUC-suppressed patients to identify cellular and/or serological parameters to predict better T cell-restoring effect and better control of infection in response to pegIFN-α for a tailored application of IFN-α add-on.
Design
53 HBeAg-negative NUC-treated patients with CHB were randomised at a 1:1 ratio to receive pegIFN-α-2a for 48 weeks, or to continue NUC therapy and then followed up for at least 6 months maintaining NUCs. Serum hepatitis B surface antigen (HBsAg) and hepatitis B core‐related antigen (HBcrAg) levels as well as peripheral blood NK cell phenotype and function and HBV-specific T cell responses upon in vitro stimulation with overlapping HBV peptides were measured longitudinally before, during and after pegIFN-α therapy.
Results
Two cohorts of pegIFN-α treated patients were identified according to HBsAg decline greater or less than 0.5 log at week 24 post-treatment. PegIFN-α add-on did not significantly improve HBV-specific T cell responses during therapy but elicited a significant multispecific and polyfunctional T cell improvement at week 24 post-pegIFN-α treatment compared with baseline. This improvement was maximal in patients who had a higher drop in serum HBsAg levels and a lower basal HBcrAg values.
Conclusions
PegIFN-α treatment can induce greater functional T cell improvement and HBsAg decline in patients with lower baseline HBcrAg levels. Thus, HBcrAg may represent an easily and reliably applicable parameter to select patients who are more likely to achieve better response to pegIFN-α add-on to virally suppressed patients.
Keywords: Chronic HBV infection, T cell functional reconstitution, immune modulatory treatments, pegylated IFN-a, HBcrAg, HBsAg
WHAT IS ALREADY KNOWN ON THIS TOPIC
Among the current treatment options for chronic hepatitis B (CHB), pegylated interferon-alpha (pegIFN-α) remains a valuable strategy since a proportion of treated patients shows significant decline or even loss of hepatitis B surface antigen (HBsAg).
Predictive parameters are needed to tailor pegIFN-α use to the patient population who can benefit from it.
WHAT THIS STUDY ADDS
Add-on therapy of pegIFN-α to an ongoing nucleos(t)ide administration can induce better boosting of natural killer and T cell responses and better HBsAg decline in patients with lower baseline hepatitis B core‐related antigen (HBcrAg) levels.
Basal HBcrAg values may identify patients with CHB with better likelihood of response to pegIFN-α therapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The possibility to select patients who are more likely to respond better to pegIFN-α therapy may represent a significant step forward towards more efficient personalised therapies for CHB.
A validation study on a larger cohort of patients is necessary in order to confirm the clinical relevance of this parameter in the management of chronic HBV infection.
Introduction
Currently available treatments for chronic HBV infection are based on short-term interferon-alpha (IFN-α) therapy and long-term administration of nucleos(t)ide (NUC) analogues that rarely result in a functional cure.1 2 The impairment of HBV-specific T cell responses represents a substantial hurdle to the achievement of this endpoint and reconstitution of an efficient host antiviral response is believed to be a possible therapeutic approach to cure HBV infection.3 Although the impact of antigen load and duration of antigen exposure on antiviral T cell responses remain an open issue,4 5 improved T cell reactivity can be detected during long-term treatment with nucleoside analogues with better T cell restoration in patients who achieve sustained loss of serum hepatitis B surface antigen (HBsAg) and anti-HBs seroconversion.6 In addition, in an animal model of persistent HBV infection, HBsAg reduction by RNA interference significantly increased T cell response with infection control and HBsAg clearance upon subsequent heterologous therapeutic vaccination.7 Low HBsAg levels have also been reported to be significantly associated with HBsAg loss upon NUC discontinuation in patients with HBeAg-negative chronic hepatitis B (CHB).8
Among the current treatment options for CHB, pegylated IFN-α (pegIFN-α) remains a valuable strategy since it results in higher rates of HBsAg seroclearance compared with NUCs.9 10 IFN-α has been shown to have not only direct antiviral effects but also immunomodulatory properties by boosting innate immune responses.11,13 However, its ability to stimulate HBV-specific T cell responses is controversial, because the final net result of the complex interplay between the different antiviral, anti-proliferative and immune modulatory effects of IFN-α may be inhibition rather than stimulation of the antiviral T cell responses in patients with chronic HBV infection.12 14
Based on this background, we asked whether pegIFN-α given to NUC-treated patients after a prolonged period of HBV DNA suppression can stimulate a more effective HBV-specific T cell response. Identification of serological predictors of response would be extremely important to focus treatment only on the subset of patients who may be more likely to benefit from pegIFN-α treatment, thereby avoiding unpleasant side effects to patients who are unlikely to respond. Thus, our study was aimed at investigating whether parallel quantification of HBsAg, hepatitis B core‐related antigen (HBcrAg) and parameters of cellular immune response can allow selection of patients more prone to functional T cell restoration who can likely benefit more substantially from the immune-modulatory effect of pegIFN-α therapy.
Materials and methods
Patients
This phase 2, multicentre, randomised, open-label study was conducted at six Italian centres in Parma, Bologna, Pisa, Milan and Reggio Emilia. The study was performed on 53 HBeAg-negative patients with chronic hepatitis under long-term NUC treatment and with complete viral suppression for at least 2–3 years. Descriptive baseline characteristics of NUC/pegIFN-α and NUC-treated patients are shown in table 1 and online supplemental materials. Only baseline HBsAg values were statistically different between the two patient cohorts.
Table 1. Descriptive baseline characteristics of NUC/pegIFN-α-treated patients and NUC-treated patients.
| Variables | NUC/pegIFN-α-treated patients baseline* (n=29) | NUC-treated patients baseline* (n=24) | P value |
| Sex (male) | 22 (76%) | 19 (79%) | 1.000 |
| Age (years) | 57 (29–70) | 58 (43–70) | 0.368 |
| Duration of NA therapy (years) | 5 (2–9) | 5 (3–13) | 0.950 |
| NA therapy | |||
| Tenofovir | 19 (66%) | 13 (54%) | |
| Entecavir | 9 (31%) | 10 (42%) | 0.323 |
| Tenofovir and entecavir | 1 (3%) | – | |
| Lamivudine | – | 1 (4%) | |
| Baseline elastography (kPa) | 5.4 (3.3–9.2)† | 5 (3.1–9.6)‡ | 0.515 |
| Fibrosis stage | |||
| F0–F1 | 17 (77%) | 17 (89%) | |
| F1–F2 | 3 (14%) | 1 (5%) | 0.558 |
| F2–F3 | 2 (9%) | 1 (5%) | |
| HBV genotype | |||
| A | – | 2 (8%) | |
| D | 20 (69%) | 15 (63%) | 0.195 |
| n.a. | 9 (31%) | 7 (29%) | |
| AST (IU/L) | 23 (12–33) | 23 (15–74) | 0.979 |
| ALT (IU/L) | 19 (12–44) | 20 (3–39) | 0.380 |
| HBV-DNA <20 IU/mL | 29 (100%) | 24 (100%) | – |
| Serum virological markers | |||
| HBsAg (IU/mL) | 740 (105–5887) | 1375.3 (143–8721) | 0.033 |
| ≤1000 | 19 (66%) | 10 (42%) | 0.102 |
| ≤100 | – | – | |
| HBcrAg (logU/mL) | 2.5 (<2.5–5.6) | 2.5 (<2.5–4.3)† | 0.830 |
| ≥3 | 12 (41%) | 9 (41%) | 1.000 |
| <3 | 17 (59%) | 13 (59%) |
Categorical and quantitative data are expressed as n (%) and median (minimum-–maximum), respectively.
The p -values were obtained from non-parametric test comparisons between the two groups: Mann-Whitney test for continuous data, Fisher’s exact test, Chi-squareΧ2 test or Llikelihood ratio Chi-squareΧ2 test for categorical data.
Baseline refers to pegIFN-alfaα add-on.
Data available for 22 patients.ǂData available for .
Data available for 19 patients.
ALTalanine transaminaseASTaspartate transaminaseHBcrAghepatitis B core‐related antigenHBsAghepatitis B surface antigenNAnucleos(t)ide analoguen.a.not applicableNUCnucleos(t)idepegIFN-αpegylated interferon-alpha
Study design
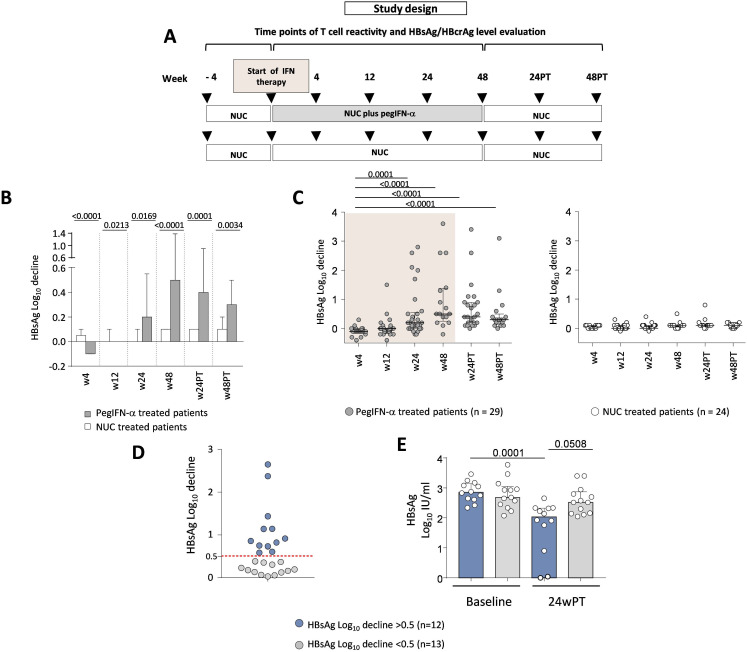
Eligible patients were randomised 1:1 in two groups: ‘add-on’ pegIFN-α for 48 weeks (180 μg/week subcutaneously; experimental arm) or continuous NUC (control arm). After 48 weeks, all patients were continued on NUC for an additional 48 weeks (total duration of the study=96 weeks). Serum HBsAg and HBcrAg levels were measured before, during and after pegIFN-α therapy (week 24 post-treatment, PT). Peripheral blood T and natural killer (NK) cell reactivity was assessed at the same time points (figure 1A and online supplemental tables S1 and S2).
Figure 1. Study design and impact of pegIFN-α therapy on serum HBsAg values. (A) Representation of the study design. (B) Mean changes in log10 IU/mL serum HBsAg from baseline through different time points in experimental and control study arms. Bars represent mean and SE values. Statistics by the Mann-Whitney test. (C) HBsAg logarithmic decline at the indicated weeks relative to baseline values in each NUC/pegIFN-α (left) and NUC-only (right) treated patient (n=29 and n=24, respectively). Statistics by the Kruskal-Wallis with Dunn’s correction test. (D) HBsAg log reductions greater (blue dots) or lower (grey dots) than 0.5 obtained comparing baseline with w24PT in each patient; each dot indicates an individual patient (n=25). (E) Median HBsAg values at baseline and w24PT in the two groups of pegIFN-α-treated patients with HBsAg decline >0.5 or <0.5 log10 IU/mL. Each dot indicates an individual patient; statistics by the Kruskal-Wallis with Dunn’s correction test. HBcrAg, hepatitis B core‐related antigen; HBsAg, hepatitis B surface antigen; IFN, interferon; log10, logarithm base 10; NUC, nucleos(t)ide; pegIFN-α, pegylated IFN-alpha; PT, post-treatment; w, week.
Serum HBV marker assessment
Serum HBcrAg levels were measured using Lumipulse G HBcrAg assay (Fujirebio Europe; lower detection and quantification limits of 2.0 and 3 logU/mL, respectively). Negative, not quantifiable HBcrAg levels below 2 logU/mL were all calculated as 2 logU/mL for statistical analysis and, in order to improve stringency, the same value was attributed also to the grey zone HBcrAg levels between 2.0 and 2.5 logU/mL.
To further maximise stringency, results were additionally assessed considering positive only HBcrAg values above 3 logU/mL and giving to all values below this threshold an estimate of 2 logU/mL for statistical analysis.
Segregation of patients with different HBsAg log decline
HBsAg logarithm (log10) decline values were calculated by comparing baseline and week 24PT in both experimental (n=29) and control (n=24) arms. For both groups, an extensive descriptive statistical analysis was conducted, including the main measures of central tendency, dispersion, shape and percentiles from 25% to 99%. We assumed that the 95th percentile, in analogy with the upper limit of the 95% CI for normal data, could be a reasonable threshold to distinguish subjects with a different log decline. In the reference population of NUC-only-treated patients, the 95th percentile in HBsAg logarithmic decline values was found to be equal to 0.48; it gives indication of the threshold above which the observed antiviral effect in the NUC/pegIFN-α combination therapy is attributable exclusively to the effect of pegIFN-α. This value was rounded up to 0.5 and assumed as the cut-off to separate patients with high versus low HBsAg logarithmic decline.
HBV-specific T cell reactivity
T cell cytokines and cytotoxic potential (CD107a) were tested by intracellular cytokine staining on short-term T cell lines (details in online supplemental materials).
Phenotypical and functional assessment of NK cells
For ex vivo NK cell phenotypical analysis, peripheral blood mononuclear cells (PBMCs) were stained with several fluorochrome-conjugated antibodies. IFN-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) cytokine production and CD107a degranulation by NK cells were measured by intracellular staining on PBMC after overnight incubation in the presence or absence of interleukin (IL)-12 and IL-18 (further details in online supplemental materials).
Statistical methods
Statistical significance between groups was assessed by the Mann-Whitney U test for non-paired samples and the Wilcoxon signed-rank test for paired data. Correlations were analysed by the Spearman’s correlation test. Multiple linear and logistic regression analyses were performed by JASP and Jamovi to assess the difference in clinical and virological baseline values in relation to immunological parameters (details in online supplemental materials).
Results
Impact of pegIFN-α therapy on HBsAg kinetics
PegIFN-α-treated patients had significant reductions in HBsAg levels starting from week 24 (weeks 24, 48, 24PT, 48PT) compared with patients in the NUC-alone group (figure 1B,C and online supplemental figure 1). One patient achieved HBsAg loss (MI-003) and a second one anti-HBs seroconversion (BOV-004) by week 24 PT. Patients in the control group did not experience significant HBsAg declines at any time point (figure 1C, right panel).
Quantification of the overall level of HBsAg logarithmic decline obtained comparing baseline with week 24PT in each patient allowed to distinguish two groups of pegIFN-α-treated patients with a log10 reduction lower or greater than 0.5, comprising 52% (13 patients) and 48% (12 patients) of the overall study population, respectively (grey and blue dots, figure 1D).
According to this segregation, baseline HBsAg values did not differ significantly between the two groups, while a statistically significant difference was evident at the follow-up time point (figure 1E).
Effect of pegIFN-α therapy on in vitro HBV-specific CD4+ and CD8+ T cell responses
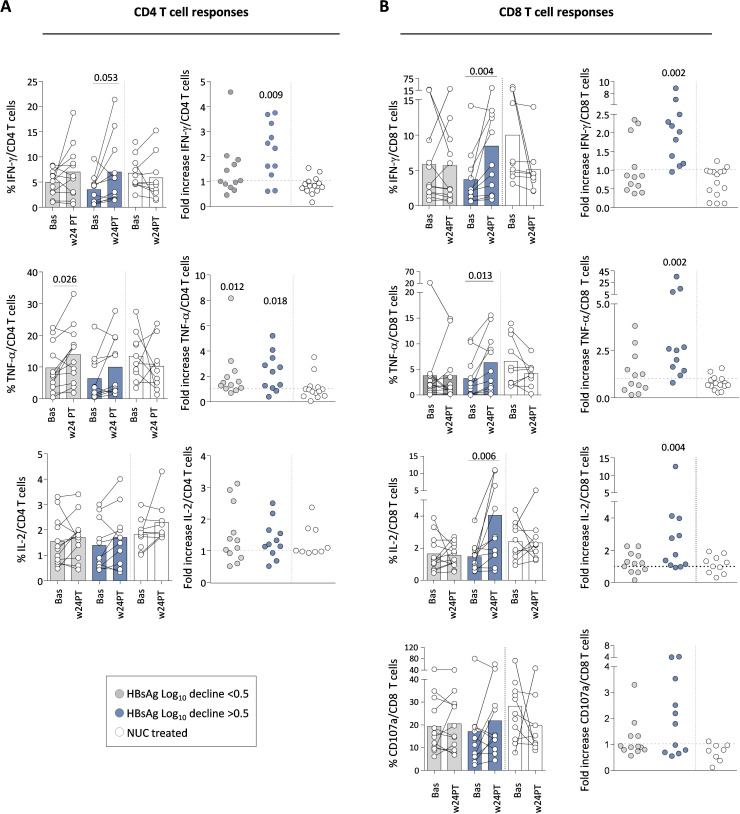
The strength and quality of CD4- and CD8-mediated HBV-specific T cell responses did not improve significantly during the 48 weeks of therapy (online supplemental figure 2). Instead, HBV-specific T cell responses at week 24 after therapy withdrawal were significantly greater than at baseline. The patient cohort with better serum HBsAg decline (>0.5 log reductions) showed maximal improvement of IFN-γ, TNF-α and IL-2 production among HBV-specific CD8 T cells and only IFN-γ, TNF-α among CD4 cells (figure 2A,B). Changes were either undetectable or much weaker among patients with a log decline <0.5 (grey bars) (figure 2A,B). Given the wide range of HBsAg decline detected among patients with fold decline values >0.5 (5 out of the 12 patients with fold decline values ranging from 1 to 3), we also investigated whether patients with an HBsAg decline >1 log exhibited a better antiviral response compared with those with a decline between 0.5 and 1 log. Higher median values of cytokine production were observed among patients with an HBsAg decline greater than 1 log, but the difference was not statistically significant (not shown). No significant correlation was observed between HBV-specific T cell responses and age which ranged from 40 to 70, as well as serological and clinical parameters (alanine transaminase, elastography and duration of NUC analogue therapy). Finally, no significant modulation of HBV-specific T cell responses was observed in the control NUC-treated group (figure 2A,B, white bars) and no changes were induced by pegIFN-α add-on on T cell responses stimulated with control peptides (cytomegalovirus, Epstein-Barr virus, influenza) (not shown).
Figure 2. Effect of pegIFN-α therapy on HBV-specific CD4 and CD8 T cell responses. Cytokine production and the ability to degranulate by CD4 and CD8 HBV-specific T cells were analysed after 10 days in vitro stimulation with HBV peptide pools in the different patient categories. (A) Percentage of IFN-γ (top), TNF-α (middle) and IL-2 cytokine production (bottom) by HBV-specific CD4 T cells at the indicated time points. Each couple of dots joined by a line indicates an individual patient, while the columns indicate mean (+SE) cytokine production at the indicated time point in each patient group. The bullet charts on the right show the ratio (fold increase) between the cytokine production detected in each patient at week 24PT relative to baseline. (B) Effect of pegIFN-α and NUC therapy on HBV-specific CD8 T cells, illustrated as in (A); in the bottom, CD107 degranulation capacity. Statistics by the Wilcoxon matched-paired test and by the Wilcoxon signed-rank test. Bas, baseline; HBsAg, hepatitis B surface antigen; IFN-γ, interferon-gamma; IL, interleukin; log10, logarithm base 10; NUC, nucleos(t)ide; pegIFN-α, pegylated IFN-alpha; PT, post-treatment; SE, standard error; TNF-α, tumour necrosis factor-alpha; w, week.
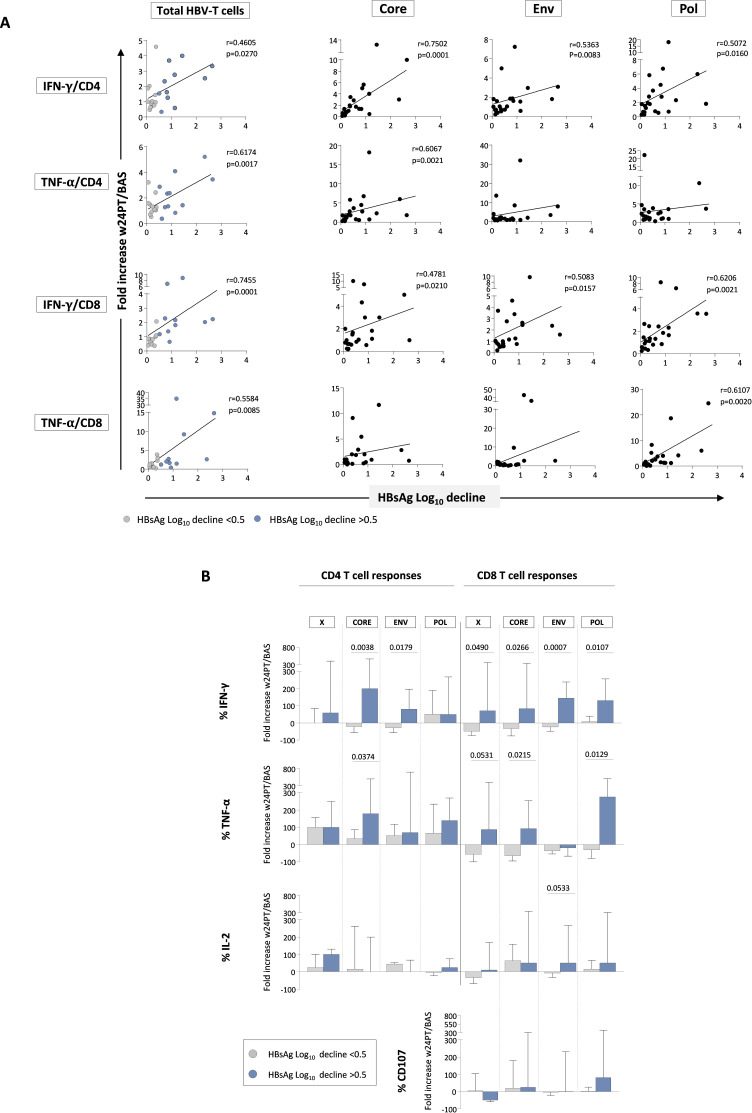
These data are further supported by the significant positive correlation between HBsAg decline and IFN-γ and TNF-α production by HBV-specific CD8+ and CD4+ T cells in the NUC/pegIFN-α-treated patients (figure 3A), but not in the NUC-treated group (not shown).
Figure 3. Improvement of HBV-specific T cell functions induced by pegIFN-α therapy is multispecific and correlates with antigen decline. (A) Correlation between HBsAg logarithmic decline (baseline vs week 24PT) and intensity of T cell responses against the whole HBV proteome (left charts) or against individual HBV antigens (HBcore, HBenv and HBpol; right charts) expressed as ratio between cytokine production by HBV-specific CD4 and CD8 T cells at w24PT and at baseline in each patient following pegIFN-α add-on. Statistics by the Spearman’s correlation test. (B) Bars represent the fold increase detected for each individual antigen at w24PT of the median cytokine production and degranulation capacity relative to baseline (patients with HBsAg log reductions <0.5 or >0.5 in grey and blue, respectively). Statistics by the Mann-Whitney U test. BAS, baseline; Env, envelope; HBsAg, hepatitis B surface antigen; IFN-γ, interferon-gamma; IL, interleukin; log10, logarithm base 10; pegIFN-α, pegylated IFN-alpha; Pol, polymerase; PT, post-treatment; TNF-α, tumour necrosis factor-alpha; w, week.
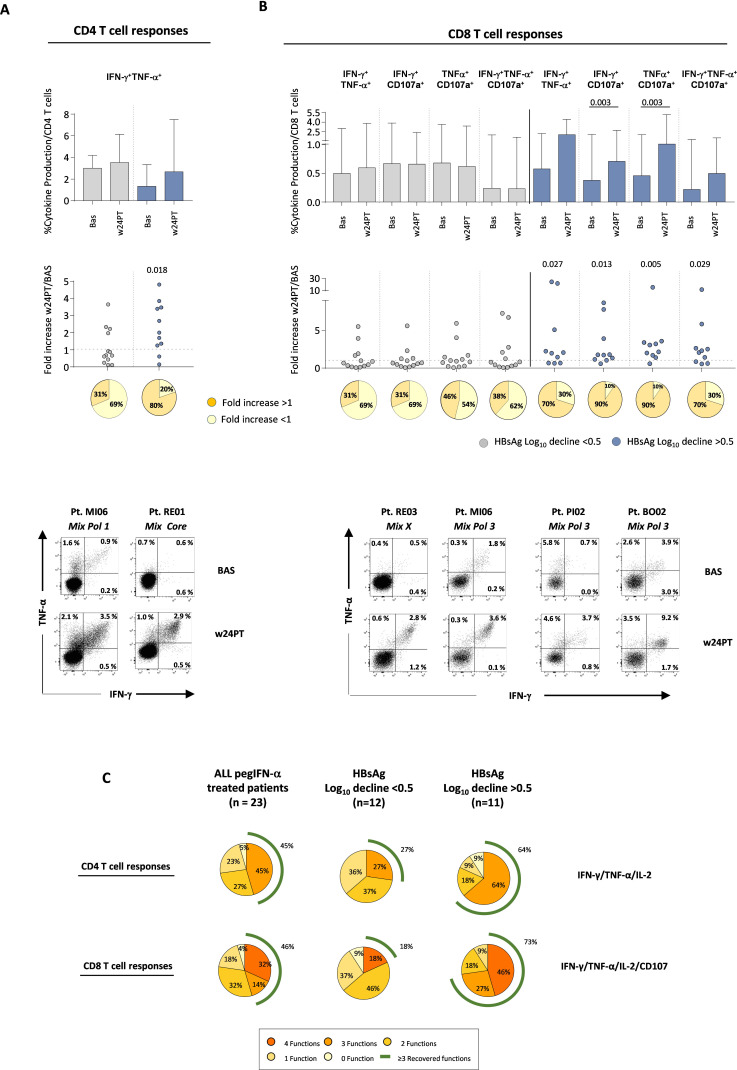
The enhancement of antiviral T cell activity was multispecific because it was sustained by CD4- and CD8-mediated T cell responses targeting all different HBV antigens (figure 3B and online supplemental figure 3). Moreover, a broader polyfunctional recovery of CD4 and CD8 T cell responses was detected in patients with a higher HBsAg decline >0.5 as indicated by frequencies of double and triple positive CD4 and CD8 T cells and by the simultaneous improvement of the different analysed functions in individual subjects (figure 4). Details on multispecificity and polyfunctionality of T cell responses are reported in the online supplemental results.
Figure 4. Polyfunctional HBV-specific T cell responses. (A) Columns show the median frequency at baseline and w24PT of HBV-specific CD4 T cells able to produce simultaneously the indicated cytokines in response to all peptide pools after in vitro stimulation (with interquartile interval) from patients receiving pegIFN-α with HBsAg log reductions lower or greater than 0.5 (grey and blue, respectively). The dotted chart shows the ratio between the cytokine production detected at w24PT relative to baseline for each patient belonging to the two different groups. Statistics by the Wilcoxon matched-paired test and by the Wilcoxon signed-rank test. Pie charts below show the percentage of patients able to improve cytokine production with a fold increase value higher than 1 (orange pies). (B) CD8 T cells able to produce simultaneously the indicated cytokines and to degranulate are illustrated as in (A). Representative dot plots of CD4 and CD8 T cell responses from treated patients are shown at the bottom of the panel. (C) Pie charts show the percentage of patients able to improve either none or multiple CD4 and CD8 HBV-specific T cell functions simultaneously. Left, middle and right pie charts refer to the whole patient population receiving pegIFN-α, and to patient subgroups with HBsAg log decline <0.5 or >0.5, respectively. BAS, baseline; HBsAg, hepatitis B surface antigen; IFN-γ, interferon-gamma; IL, interleukin; log10, logarithm base 10; pegIFN-α, pegylated IFN-alpha; PT, post-treatment; TNF-α, tumour necrosis factor-alpha; w, week.
Low baseline HBcrAg levels are associated with higher HBsAg decline values and better T cell responsiveness upon pegIFN-α therapy
In order to identify a serological predictor of better functional T cell reconstitution and virological response to pegIFN-α therapy, we analysed the behaviour of the HBcrAg in relation to HBsAg decline and T cell responses. Globally, patients under pegIFN-α treatment experienced a statistically significant HBcrAg reduction only at week 48 (p=0.015, not shown) compared with the control group, without consistent differences between the two groups with higher or lower HBsAg decline (data not shown). Remarkably, an inverse correlation was detected between HBsAg decline and baseline HBcrAg levels (p=0.001, figure 5A) that was maintained (p=0.006, r=−0.5281, not shown) also when a more stringent HBcrAg threshold of 3 instead of 2.5 logU/mL was applied for analysis. Instead, no significant association was observed between basal serum HBsAg and HBcrAg values, irrespective of the threshold stringency (2.5 or 3 logU/mL) used for calculation (figure 5B). Variable degrees of correlation, particularly in HBeAg-negative carriers, have previously been reported not only between HBsAg and HBcrAg but also between their values and those of intrahepatic viral markers, such as covalently closed circular DNA (cccDNA) levels.15,20 This may reflect the contribution of HBV integration to HBsAg production, in addition to its expression from the cccDNA template, which may account for a different sensitivity of HBsAg and HBcrAg as markers of HBV transcriptional activity.16 21
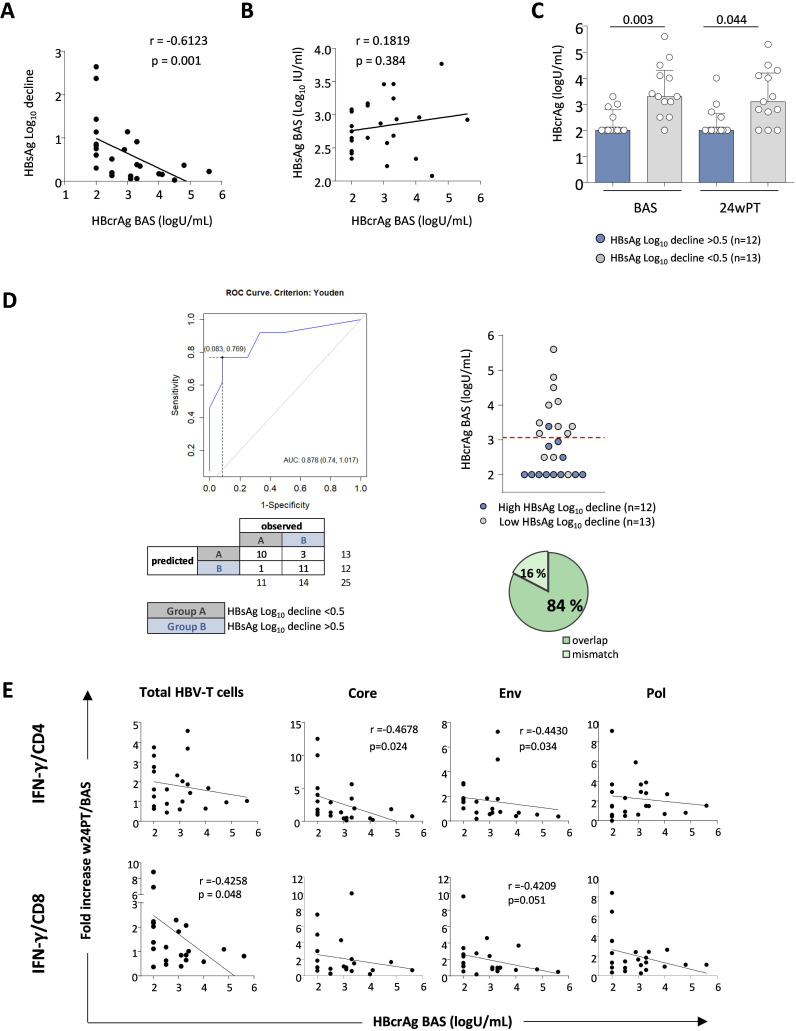
Figure 5. Predictive value of baseline HBcrAg levels on HBsAg decline and HBV-specific T cell responses. (A) Correlation between baseline HBcrAg levels and HBsAg log decline. (B) Correlation between baseline HBcrAg levels and baseline HBsAg values. (C) The columns indicate the median HBcrAg value at baseline and w24PT, while each dot shows individual patient HBcrAg values. (D) ROC analysis on segregated data derived from HBsAg log reduction values. Dotplot, confusion matrix and prediction accuracy are illustrated. (E) Correlation analyses between baseline HBcrAg values and quantity of IFN-γ-producing T cells specific for the whole HBV proteome (left charts) or specific for HBcore, HBenv and HBpol antigens (right charts) expressed as ratio between cytokine production by HBV-specific CD4 and CD8 T cells at w24PT and baseline for each patient following pegIFN-α add-on. Statistics by Spearman’s correlation test and Mann-Whitney test. As explained in the Methods section, HBcrAg values below 2.5 are indicated as 2 in all panels. AUC, area under the curve; BAS, baseline; Env, envelope; HBcrAg, hepatitis B core‐related antigen; HBsAg, hepatitis B surface antigen; IFN-γ, interferon-gamma; log10, logarithm base 10; pegIFN-α, pegylated IFN-alpha; Pol, polymerase; PT, post-treatment; ROC, receiver operating characteristic; w, week.
In addition, baseline HBcrAg levels were significantly lower in pegIFN-α-treated patients who experienced greater HBsAg decline compared with those with lower reduction (p=0.003, figure 5C, blue and grey columns, respectively), suggesting the probability of a greater HBsAg decline among patients with low HBcrAg values at baseline. In order to estimate the predictive power of baseline HBcrAg on HBsAg decline, a ROC curve analysis was performed on basal HBcrAg data segregated according to the HBsAg log reduction values. A threshold HBcrAg value of 3.05 (AUROC =0.88, p<0.001) gave the optimal discrimination between patients expressing HBsAg log decline values <0.5 or >0.5, with a classification accuracy up to 84% (21 out of 25 subjects with a concordant segregation, figure 5D). Thus, low basal HBcrAg levels represent an independent predictor of better HBsAg decline at week 24PT.
Given the significant negative correlation between basal HBcrAg levels and HBsAg reduction, which was in turn positively correlated with functional T cell improvement, we then looked for possible associations between HBV-specific T cell responses at week 24 after therapy withdrawal and baseline HBcrAg levels. An inverse correlation between IFN-γ production by total HBV-specific T cells and basal HBcrAg values was observed in NUC/pegIFN-α but not in the NUC-treated control group (not shown). In particular, core-specific and envelope (env)-specific CD4 T cell responses showed a significant inverse correlation with baseline HBcrAg values (core p=0.024; env p=0.034), whereas among CD8 T cells, a correlation was detected for the overall CD8 T cell population (p=0.048; figure 5E, left panels), with a trend very close to statistical significance for env-specific CD8 T cell responses (p=0.051, figure 5E).
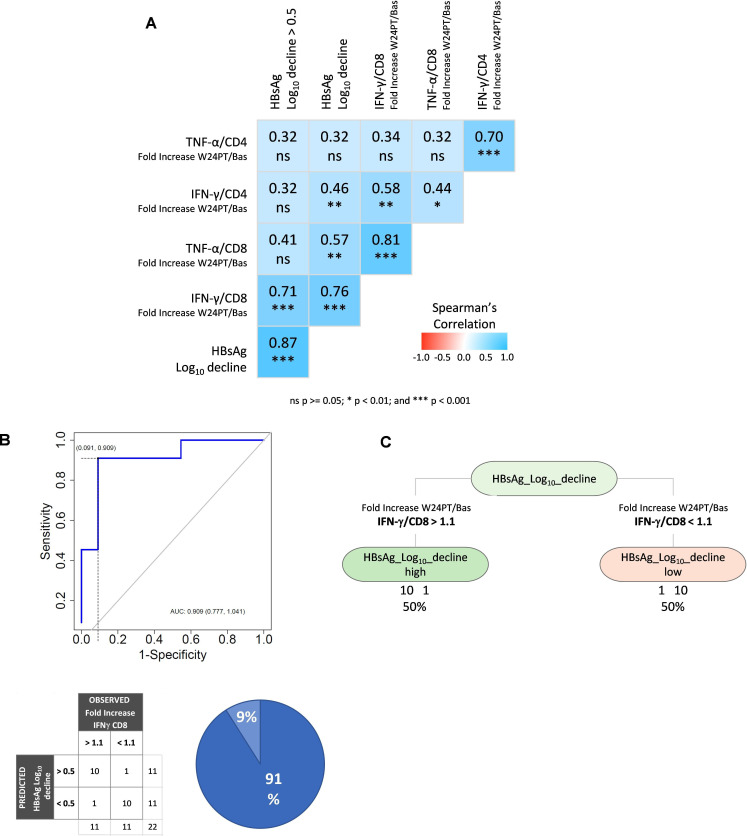
Finally, a multivariate analysis was performed between main immunological variables (TNF-α/CD4, IFN-γ/CD4, TNF-α/CD8, IFN-γ/CD8, as fold increase between week 24PT and baseline) and the HBsAg log decline in order to further confirm the relevance of our findings. IFN-γ production by HBV-specific CD8 T cells was the immunological parameter displaying the highest correlation with HBsAg decline (p<0.001) by Spearman’s correlation (figure 6A). In addition, binary logistic regression, ROC curve (figure 6B) and decision tree analyses (figure 6C) were performed on IFN-γ production by HBV-specific CD8 T cells segregated according to the HBsAg log reduction values. CD8 T cell IFN-γ was the only statistically significant variable in the logistic regression that allowed to distinguish the two cohorts of pegIFN-α-treated patients. A cut-off of 1.1 fold increase value indicating a functional T cell increase of at least 10% at week 24 post-therapy relative to baseline gave the optimal discrimination between patients expressing HBsAg log decline values greater or lower than 0.5 (AUROC=0.909; figure 6B). Notably, the optimal cut-off values obtained through ROC analysis and decision tree were found to be identical. The cut-off value of 1.1 gives a classification accuracy equal to 91%, and both positive and negative predictive values equal to 91%. These findings, of course, need to be validated on a larger cohort of patients.
Figure 6. IFN-γ production by CD8 T cells is the most relevant immune parameter associated with HBsAg decline. Multivariate analyses, including binary logistic regression and decision tree algorithms, were performed to identify the immune parameters associated with different HBsAg kinetics in pegIFN-α-treated patients. (A) Spearman’s correlation matrix of the main immunological variables (TNF-α/CD4, IFN-γ/CD4, TNF-α/CD8, IFN-γ/CD8, as fold increase between weeks 24PT and baseline) and HBsAg log decline; (B) logistic regression and ROC analysis; (C) decision tree analysis on fold increase of IFN-γ production by HBV-specific CD8 T cells segregated according to the HBsAg log reduction values. AUC, area under the curve; Bas, baseline; HBsAg, hepatitis B surface antigen; IFN-γ, interferon-gamma; log10, logarithm base 10; ns, not significant; pegIFN-α, pegylated IFN-alpha; PT, post-treatment; ROC, receiver operating characteristic; TNF-α, tumour necrosis factor-alpha; w, week.
Impact of pegIFN-α therapy on NK cell responsiveness
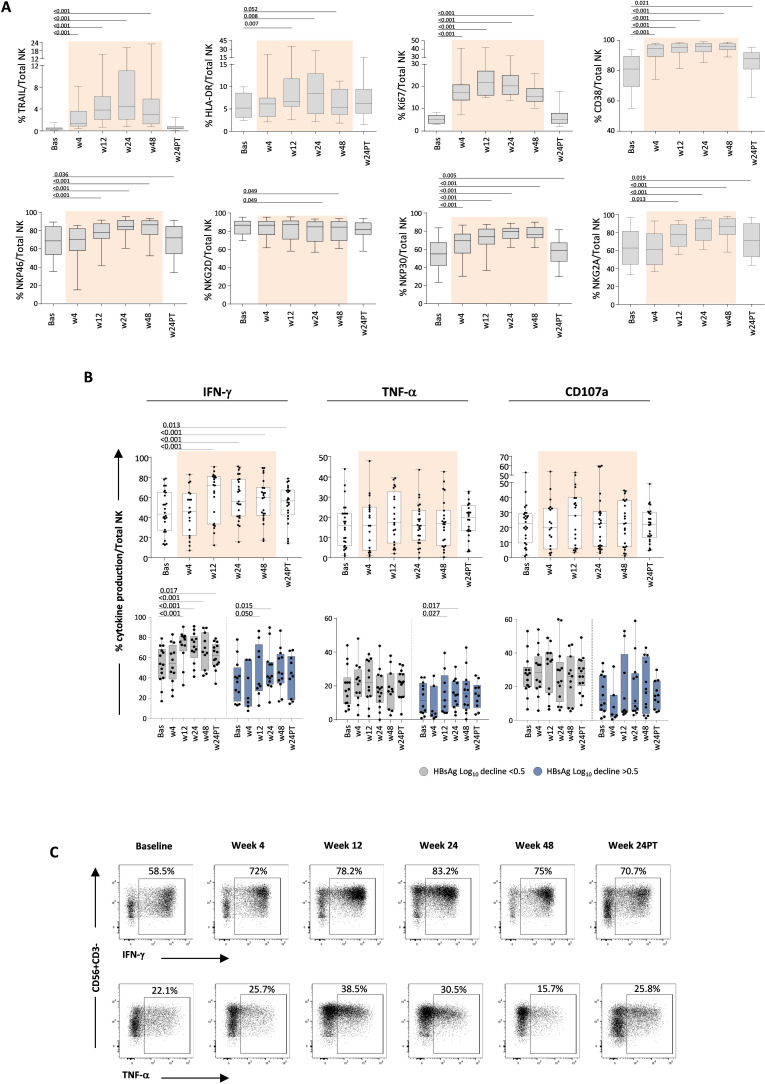
Longitudinal NK phenotypical profile analysis in pegIFN-α-treated patients highlighted a modulation of proliferation and activation markers, indicating an activating effect of treatment (figure 7A). Specifically, TRAIL, HLA-DR, Ki67 and CD38 expression on total NK cells increased progressively under IFN-α therapy. Moreover, activating receptors such as NKp46, NKG2D, NKp30 and NKG2A were significantly modulated. Levels of phenotypical changes were not correlated to different HBsAg decline levels or baseline HBcrAg values, thus confirming an overall activated phenotypical profile of the NK cell population (figure 7A). No modulation of NK cell responses was observed in the control group of patients receiving NUC alone (not shown).
Figure 7. Effect of pegIFN-α therapy on NK cell phenotype and function. (A) Expression of TRAIL, HLA-DR, Ki67, CD38, NKp46, NKG2D, NKp30 and NKG2A on NK cells before, during and after pegIFN-α treatment (n=22). Results are expressed as median percentage of each NK cell phenotypical marker on total NK cells. (B) Analysis of NK cell IFN-γ, TNF-α production and CD107a degranulation capacity before, during and after pegIFN-α treatment (n=26, top panels) in the whole patient population and according to the different patient subgroups with different HBsAg log reductions (bottom panels); each whisker plot indicates the median values of NK cell functional parameters in the indicated time points. Statistics by the Kruskal-Wallis with Dunn’s correction test. (C) Representative plots of IFN-γ and TNF-α production by NK cells from a treated patient. Bas, baseline; HBsAg, hepatitis B surface antigen; IFN-γ, interferon-gamma; log10, logarithm base 10; NK, natural killer; pegIFN-α, pegylated IFN-alpha; PT, post-treatment; TNF-α, tumour necrosis factor-alpha; w, week.
This positive phenotypical modulation was associated with an increased IFN-γ production that peaked at weeks 12–24 and was maintained until month 6 after pegIFN-α therapy suspension (figure 7B,C). Instead, TNF-α production and cytotoxic capacity (CD107a degranulation) did not show significant changes under pegIFN-α therapy. Like the phenotypical profile, functional changes were not correlated to different HBsAg decline levels or baseline HBcrAg values.
Discussion
Functional cure of CHB is achieved with current treatments in a limited proportion of patients.1 2 Thus, novel effective drugs either targeting critical steps of the HBV lifecycle, or improving innate and adaptive immune responses are needed to increase the functional cure rate.1,3 At the same time, efforts should be made to identify reliable predictors of response to available therapies in order to optimise their use in selected cohorts of patients with better chances of response.22 This may be particularly important for IFN-α therapy because a proportion of treated patients shows substantial decline or even loss of HBsAg and may thus represent the patient population that may benefit from an association of IFN-α with other drugs, perhaps through therapeutic cycles of reduced duration, to limit side effects without abrogating IFN antiviral and immune modulatory activities.9 10
Among the immunological properties of IFN-α, a positive enhancing effect on NK cell antiviral function and dendritic cell cross-priming has clearly been elucidated in chronic HBV infection,11,13 while the IFN-α boosting activity on HBV-specific T cell responses is still a matter of debate, because some studies suggest that the result of IFN-α therapy may be inhibition rather than stimulation of antiviral T cell responses.12 14 This has been speculated to represent a possible effect of the IFN-α anti-proliferative effect prevailing, at least in this specific setting, on its immune-stimulatory action.
Based on these premises, the objectives of our study were twofold: first, to assess a possible synergy between IFN-α and NUCs in enhancing HBV-specific T cell reactivity, and second, to identify cellular and/or serological predictors of response to IFN-α to focus IFN-α use only on the appropriate cohorts of potentially responsive patients.
To achieve this second goal, we analysed serum HBsAg and HBcrAg values in parallel with the evaluation of cell-mediated responses, including T cell and NK cell reactivities.
In agreement with previous studies,12 14 pegIFN-α add-on did not elicit any modulatory effects on CD4 and CD8-mediated T cell responses during treatment. These data strongly suggest that the expected immune-modulatory effect of IFN on T cell-mediated responses might be masked during therapy by other still not well-defined mechanisms, including its anti-proliferative effect, which would act in the opposite direction.
However, a significant T cell improvement was observed at week 24 post-pegIFN-α treatment compared with the baseline response, with maximal increase in patients who had a higher drop in serum HBsAg concentration and a lower basal HBcrAg values. Both CD4 and CD8 T cells were significantly modulated by IFN-α, with a slightly higher impact on CD8-mediated responses. A significant positive correlation was detected between HBsAg decline and intensity of HBV-specific CD8 and CD4 T cell responses observed in NUC/pegIFN-α-treated patients at week 24 of follow-up. Moreover, an inverse correlation was observed between basal HBcrAg—but not HBsAg—values, and IFN-γ production by HBV-specific CD4 and CD8 T cells after pegIFN-α treatment.
Importantly, among the different analysed immunological parameters, HBV-specific CD8 T cell IFN-γ production appeared to be associated with better chances of response to NUC/pegIFN-α treatment, since it turned out to be the only statistically significant variable in the logistic regression analysis that allowed to distinguish the two cohorts of pegIFN-α-treated patients previously defined according to HBsAg decline.
These results are in line with those reported in the study by Aliabadi et al5 where patients with low HBcrAg levels (<3 logIU/mL) were found to exhibit higher CD4 T cell responses specific for core and polymerase and both CD4 and CD8 T cells were more responsive to PD-L1 blockade.
Altogether, our observations suggest that low baseline HBcrAg levels are associated with greater HBsAg decline and better T cell responsiveness after pegIFN-α treatment. Of potential clinical relevance, 3.05 represents the cut-off HBcrAg value that separates patients with log HBsAg decline greater or lower than 0.5 at week 24 after therapy with 84% accuracy. Therefore, basal HBcrAg values may identify with a good level of accuracy patients with CHB with better levels of response to pegIFN-α therapy.
Importantly, pegIFN-α therapy improved T cell responses to all different HBV antigen specificities, including envelope-specific T cells, as well as their functional breadth (ie, the capacity of individual T cells to express multiple functions simultaneously), and these effects were more significantly improved among patients with higher HBsAg decline and lower baseline HBcrAg values.
This greater effect of pegIFN-α therapy on strength and quality of cell-mediated immune responses in patients with lower baseline HBcrAg levels may be relevant to explain the better effect of therapy on HBsAg elimination in this patient cohort. Indeed, HBeAg-negative patients with lower HBcrAg levels have very low or no transcriptionally active cccDNA.16 This and the evidence that in HBeAg-negative patients with CHB most of HBsAg is produced from integrated HBV DNA21 strongly suggest that the contribution of interferon mediated cccDNA silencing on HBsAg production would have been at the least marginal. Thus, the greater HBsAg decline detected among HBeAg-negative patients with lower HBcrAg at baseline is likely the result of the better intensity/quality of cell-mediated immune responses observed in these patients causing greater elimination of hepatocytes containing not only replicating but also integrated HBV DNA sequences responsible for HBsAg production.
In agreement with previous studies,12 13 23 our data showed that interferon was also able to strongly activate NK cells, enhancing their antiviral function, but no differences in NK cell phenotype and function were observed in relation to basal HBcrAg or HBsAg levels and HBsAg decline.
In summary, by an integrated analysis of virological and immunological parameters, our data reveal that add-on therapy of interferon to an ongoing NUC administration can boost both NK and T cell responses. Importantly, low basal HBcrAg values could be a reliable parameter of easy clinical applicability to select patients who may be more likely to benefit from pegIFN-α activity and with greater probability to achieve HBsAg loss or decline. A validation study on a larger cohort of patients is obviously necessary in order to confirm the clinical relevance of this parameter in the management of chronic HBV infection.
Additional studies are also needed to better explore the potential benefit of IFN-based combination therapies, including last-generation antivirals and novel immune-modulatory compounds, to personalise treatment strategies for chronic HBV infection.
supplementary material
Footnotes
Funding: This work was supported by a grant from Emilia-Romagna Region, Italy (Programma di Ricerca Regione-Università 2010–2012; PRUa1RI-2012-006), by a grant from Gilead Sciences (to CF) and by a PRIN project from the Italian Ministry of the University and Research (protocol code no. 2017MPCWPY). Pegylated IFN-α was provided free of charge by Roche.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants enrolled at distinct clinical sites and was approved by the respective competent local Ethics Committees. The Ethics Approval Number of the coordinating clinical site (Comitato Etico per Parma) is No. 3688 of January 29, 2015. Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Andrea Vecchi, Email: 2007arandy@gmail.com.
Marzia Rossi, Email: rossi.marzia@gmail.com.
Camilla Tiezzi, Email: camilla.tiezzi@unipr.it.
Paola Fisicaro, Email: pfisicaro@ao.pr.it.
Sara Doselli, Email: sara.doselli@unipr.it.
Elena Adelina Gabor, Email: elenaadelina.gabor@unipr.it.
Amalia Penna, Email: apenna@ao.pr.it.
Ilaria Montali, Email: ilaria.montali@unipr.it.
Camilla Ceccatelli Berti, Email: camilla.ceccatelliberti@unipr.it.
Valentina Reverberi, Email: valentina.reverberi@unipr.it.
Anna Montali, Email: anna.montali@unipr.it.
Simon P Fletcher, Email: simon.fletcher@gilead.com.
Elisabetta Degasperi, Email: elisabetta.degasperi@policlinico.mi.it.
Dana Sambarino, Email: dana.sambarino@policlinico.mi.it.
Diletta Laccabue, Email: diletta.laccabue@unipr.it.
Floriana Facchetti, Email: floriana.facchetti@policlinico.mi.it.
Simona Schivazappa, Email: sschivazappa@ao.pr.it.
Elisabetta Loggi, Email: bettaloggi@gmail.com.
Barbara Coco, Email: b.coco@ao-pisa.toscana.it.
Daniela Cavallone, Email: danielacavallone@hotmail.com.
Elena Rosselli Del Turco, Email: elena.rossellidelturco@aosp.bo.it.
Marco Massari, Email: marco.massari@ausl.re.it.
Giuseppe Pedrazzi, Email: giuseppe.pedrazzi@unipr.it.
Gabriele Missale, Email: gabriele.missale@unipr.it.
Gabriella Verucchi, Email: gabriella.verucchi@unibo.it.
Pietro Andreone, Email: pietro.andreone@unimore.it.
Maurizia Rossana Brunetto, Email: maurizia.brunetto@unipi.it.
Pietro Lampertico, Email: pietro.lampertico@unimi.it.
Carlo Ferrari, Email: carlo.ferrari@unipr.it.
Carolina Boni, Email: carolina.boni@unipr.it.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
References
- 1.Lim SG, Baumert TF, Boni C, et al. The scientific basis of combination therapy for chronic hepatitis B functional cure. Nat Rev Gastroenterol Hepatol. 2023;20:238–53. doi: 10.1038/s41575-022-00724-5. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Buti M, Lampertico P, et al. Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: report from the 2022 AASLD-EASL HBV-HDV treatment endpoints conference. Hepatology. 2023;78:1654–73. doi: 10.1097/HEP.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 3.Fisicaro P, Barili V, Rossi M, et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front Immunol. 2020;11:849. doi: 10.3389/fimmu.2020.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Bert N, Gill US, Hong M, et al. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection. Gastroenterology. 2020;159:652–64. doi: 10.1053/j.gastro.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Aliabadi E, Urbanek-Quaing M, Maasoumy B, et al. Impact of HBsAg and HBcrAg levels on phenotype and function of HBV-specific T cells in patients with chronic hepatitis B virus infection. Gut. 2022;71:2300–12. doi: 10.1136/gutjnl-2021-324646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boni C, Laccabue D, Lampertico P, et al. Restored function of HBV-specific T cells after long-term effective therapy with Nucleos(t)ide analogues. Gastroenterology. 2012;143:963–73. doi: 10.1053/j.gastro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Michler T, Kosinska AD, Festag J, et al. Knockdown of virus antigen expression increases therapeutic vaccine efficacy in high-titer hepatitis B virus carrier mice. Gastroenterology. 2020;158:1762–75. doi: 10.1053/j.gastro.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 8.García-López M, Lens S, Pallett LJ, et al. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol. 2021;74:1064–74. doi: 10.1016/j.jhep.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viganò M, Grossi G, Loglio A, et al. Treatment of hepatitis B: is there still a role for interferon. Liver Int. 2018;38 Suppl 1:79–83. doi: 10.1111/liv.13635. [DOI] [PubMed] [Google Scholar]
- 10.Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development. J Hepatol. 2022;76:1249–62. doi: 10.1016/j.jhep.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Isorce N, Lucifora J, Zoulim F, et al. Immune-Modulators to combat hepatitis B virus infection: from IFN-α to novel investigational Immunotherapeutic strategies. Antiviral Res. 2015;122:69–81. doi: 10.1016/j.antiviral.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Micco L, Peppa D, Loggi E, et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol. 2013;58:225–33. doi: 10.1016/j.jhep.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Bruder Costa J, Dufeu-Duchesne T, Leroy V, et al. Pegylated interferon α-2a triggers NK-cell Functionality and specific T-cell responses in patients with chronic HBV infection without HBsAg seroconversion. PLoS One. 2016;11:e0158297. doi: 10.1371/journal.pone.0158297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penna A, Laccabue D, Libri I, et al. Peginterferon-α does not improve early peripheral blood HBV-specific T-cell responses in HBeAg-negative chronic hepatitis. J Hepatol. 2012;56:1239–46. doi: 10.1016/j.jhep.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Huang D, Wu D, Wang P, et al. End-of-treatment HBcrAg and HBsAb levels identify durable functional cure after peg-IFN-based therapy in patients with CHB. J Hepatol. 2022;77:42–54. doi: 10.1016/j.jhep.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Testoni B, Lebossé F, Scholtes C, et al. Serum hepatitis B core-related antigen (HBcrAg) correlates with Covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615–25. doi: 10.1016/j.jhep.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Maasoumy B, Wiegand SB, Jaroszewicz J, et al. Hepatitis B core-related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with Genotypes A and D. Clin Microbiol Infect. 2015;21:S1198-743X(15)00298-0. doi: 10.1016/j.cmi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Chuaypen N, Posuwan N, Chittmittraprap S, et al. Predictive role of serum HBsAg and HBcrAg Kinetics in patients with HBeAg-negative chronic hepatitis B receiving pegylated interferon–based therapy. Clin Microbiol Infect. 2018;24:S1198-743X(17)30387-7. doi: 10.1016/j.cmi.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 19.van Halewijn GJ, Geurtsvankessel CH, Klaasse J, et al. Diagnostic and Analytical performance of the hepatitis B core related antigen immunoassay in hepatitis B patients. J Clin Virol. 2019;114:1–5. doi: 10.1016/j.jcv.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Brunetto MR, Carey I, Maasoumy B, et al. Incremental value of HBcrAg to classify 1582 HBeAg‐negative individuals in chronic infection without liver disease or hepatitis. Aliment Pharmacol Ther. 2021;53:733–44. doi: 10.1111/apt.16258. [DOI] [PubMed] [Google Scholar]
- 21.Wooddell CI, Yuen M-F, Chan HL-Y, et al. Rnai-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017;9:eaan0241. doi: 10.1126/scitranslmed.aan0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertoletti A. The challenges of adopting immunological biomarkers in the management of chronic HBV infection. J Hepatol. 2022;77:299–301. doi: 10.1016/j.jhep.2022.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Gill US, Peppa D, Micco L, et al. Interferon alpha induces sustained changes in NK cell responsiveness to hepatitis B viral load suppression in vivo. PLOS Pathog. 2016;12:e1005788. doi: 10.1371/journal.ppat.1005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.