Abstract
Objective:
To evaluate the prevalence and characteristics of concurrent bacterial sexually transmitted infections (bSTIs) among individuals with mpox.
Design:
Prospective cohort study of participants aged 18 years or older with confirmed mpox conducted in Rio de Janeiro, Brazil. This cross-sectional analysis includes only participants who underwent bSTI testing at baseline between June 2022 and January 2024.
Methods:
Participants were offered testing for chlamydia/gonorrhea (NAAT, anorectal swabs) and syphilis (active diagnosis if VDRL ≥ 1/8). Baseline prevalence of bSTIs was calculated, and participant characteristics were described based on concomitant bSTI diagnosis (yes/no). Chi-squared/Fisher's tests were used for qualitative variables, and the Wilcoxon rank-sum test for quantitative variables.
Results:
Out of 634 enrolled participants, 538 (84.9%) were tested for STIs and included in this analysis, mostly cisgender men, aged 30–39 years with postsecondary education. Overall prevalence of concomitant bSTI was 37.3%, mainly syphilis, followed by chlamydia and gonorrhea. Half of the participants were living with HIV, and one third was on HIV pre-exposure prophylaxis. Concomitant bSTI diagnosis at the time of mpox assessment was associated with being aged 30–39 years, self-identifying as cisgender men, having HIV-positive status, reporting proctitis symptoms and reporting any STI in the past 12 months.
Conclusions:
Our data reveals a notable prevalence of concomitant bSTIs among participants with confirmed mpox at a prominent infectious diseases’ referral center in Rio de Janeiro, Brazil. These findings underscore the importance of integrating mpox into the differential diagnosis of anogenital manifestations and the promotion of combination prevention strategies within sexual healthcare services.
Keywords: chlamydia, gonorrhea, mpox, sexually transmitted infections, syphilis
Introduction
Mpox remains a significant public health concern, affecting not only previous considered endemic countries in the African region, but also other countries, notably in the Americas and Europe. As of April 2024, Brazil ranked second globally with 10 967 confirmed mpox cases. Despite a decline in the number of diagnoses throughout 2023, several countries, including Brazil, Congo, Portugal, and Spain reported increases in mpox diagnoses [1]. The current situation in Congo is particularly alarming, with the country surpassing 10 000 persons with mpox in 2024, mostly affected by clade I mpox virus (MPXV). Reports indicate a new strain with transmission via sexual contact [2], a characteristic previously associated primarily with clade II outbreaks [3].
The 2022–2024 mpox multinational outbreak has highlighted the importance of including mpox in the differential diagnosis for individuals presenting with anogenital lesions. It also brought attention to a high frequency of concurrent sexually transmitted infections (STI) alongside mpox infection [4–6]. Understanding the interaction between mpox and other bacterial STI (bSTI) is crucial for guiding the implementation of combination prevention strategies and structuring integrated care delivery. In this analysis, we aim to explore the sociodemographic, clinical, and behavioral characteristics of participants with confirmed mpox assessed at a prominent Brazilian referral health service, focusing on the diagnosis of concurrent bSTI.
Methods
This was a single-center, prospective cohort study conducted at Evandro Chagas National Infectious Diseases, Oswaldo Cruz Foundation (INI/Fiocruz), the major infectious diseases referral center in Rio de Janeiro, Brazil. The study enrolled participants 18 years or older with laboratory-confirmed mpox from June 12, 2022 to April 30, 2024. We collected sociodemographic, epidemiological, behavior, clinical and laboratory data at baseline and prospectively, until the study's completion (after 28 days or lesions resolution, whichever was longer).
Procedures of INI/Fiocruz mpox cohort have been previously described [7,8]. All participants were offered anorectal swab collection for chlamydia/gonorrhea testing (Abbott RealTime CT/NG Assay or Cobas CT/NG 5800), as well as syphilis (rapid Treponema pallidum for screening, followed by VDRL/RPR titers). We performed treponemic rapid tests for syphilis screening and confirmed positive results with a Venereal Disease Research Laboratory (VDRL) test. Participants with VDRL titers equal or higher than 1 : 8, combined with a reagent rapid treponemic test, were diagnosed with active syphilis. Additionally, all participants were offered third-generation rapid HIV tests, hepatitis B surface antigen rapid test and anti-HCV rapid tests.
Our main outcome was the concomitant diagnosis of any bSTI [active syphilis, anorectal chlamydia (Ct) and/or anorectal gonorrhea (Ng)] at the baseline mpox assessment. We compared study characteristics of persons with mpox according to any concomitant bSTI diagnosis (yes/no). Qualitative variables were compared using the Chi-squared/Fisher's test, while quantitative variables were assessed using Wilcoxon rank-sum test. Results were considered statistically significant if P-value was <0.05. All analyses were performed using R-Project (4.3.2). This study was approved by the Ethics Review Board at INI-Fiocruz (CAAE #61290422.0.0000.5262). Participants provided written informed consent.
Results
From June 12, 2022 to April 30, 2024, we enrolled 634 participants with confirmed mpox in our cohort, of whom 84.9% (n = 538) were tested for any bSTI at baseline and included in this analysis. Participants tested for any bSTI more frequently exhibited clinical signs or symptoms of proctitis (26.6% vs. 16.1%, P = 0.03) and reported a higher median number of sex partners in 30 days prior to symptom onset (2 [IQR 1–4] vs. 1 [IQR 1–3], P = 0.03) compared to those not tested. There were no differences related to age, gender identity, race/skin color, educational level, HIV status or HIV pre-exposure prophylaxis (PrEP) use.
Participants diagnosed with mpox and tested for any bSTI (n = 538) were mostly aged 30–39 years (42.9%), followed by those aged 40+ years (25.5%), and 25–29 years (19.5%), with the lowest proportions among the youngest group, 18–24 years (12.1%) (Table 1). The majority of them self-identified as cisgender men (92.4%), with few participants self-identifying as cisgender women (5.0%), transgender women (2.4%) and nonbinary persons (0.2%). Self-reported race/skin color were more often Black/Pardo (60.7%), followed by White (38.5%) and Indigenous (0.8%). Participants predominantly had high educational backgrounds, with 59.5% having accessed postsecondary education, 34.1% secondary education and 6.4% only primary education. Few participants reported engaging in transactional sex (3.0%) and the median number of sex partners in 30 days before symptom onset was 2 (IQR 1–4). Among all participants, 32.7% reported a diagnosis of any bSTI in the past 12 months. Additionally, 53.2% had an HIV diagnosis, and 37.1% of those who tested negative for HIV were on PrEP. The seroprevalence of hepatitis B was 1.3%, which was lower than that of hepatitis C at 6.4%. Among the 39 participants diagnosed with hepatitis C, 48.7% (n = 19) had been previously diagnosed, and of those, 57.9% (n = 11/19) had received adequate treatment. Clinical signs and/or symptoms of proctitis were reported by 26.6% of participants.
Table 1.
Characteristics of participants with mpox according to baseline diagnosis of any concomitant bacterial sexually transmitted infection (bSTI).
| Any bSTI | ||||
| Overall N = 538 |
No n = 337 |
Yes n = 201 |
P-valuea | |
| Age (years) | ||||
| Median (IQR) | 33 (28–40) | 33 (28–40) | 33 (28–39) | 0.74 |
| 18–24 | 65/538 (12.1%) | 51/337 (15.1%) | 14/201 (6.9%) | 0.02 |
| 25–29 | 105/538 (19.5%) | 59/337 (17.5%) | 46/201 (22.9%) | |
| 30–39 | 231/538 (42.9%) | 140/337 (41.6%) | 91/201 (45.3%) | |
| ≥40 | 137/538 (25.5%) | 87/337 (25.8%) | 50/201 (24.9%) | |
| Gender identity | 0.01 | |||
| Cisgender men | 497/538 (92.4%) | 310/337 (92%) | 187/201 (93.0%) | |
| Cisgender women | 27/538 (5.0%) | 22/337 (6.5%) | 5/201 (2.5%) | |
| Transgender women | 13/538 (2.4%) | 4/337 (1.2%) | 9/201 (4.5%) | |
| Nonbinary person | 1/538 (0.2%) | 1/337 (0.3%) | 0/201 | |
| Transgender men | 0/538 | 0/337 | 0/201 | |
| Race/skin color | 0.84 | |||
| White | 190/493 (38.5%) | 121/313 (38.7%) | 69/180 (38.3%) | |
| Black/Pardo | 299/493 (60.7%) | 190/313 (60.7%) | 109/180 (60.6%) | |
| Indigenous | 4/493 (0.8%) | 2/313 (0.6%) | 2/180 (1.1%) | |
| Educational level | 0.25 | |||
| Primary | 32/499 (6.4%) | 15/315 (4.8%) | 17/184 (9.2%) | |
| Secondary | 170/499 (34.1%) | 108/315 (34.3%) | 62/184 (33.7%) | |
| Post-secondary | 297/499 (59.5%) | 192/315 (60.9%) | 105/184 (57.1%) | |
| Transactional sex | 15/506 (3.0%) | 13/318 (4.1%) | 4/188 (2.1%) | 0.24 |
| Number of sex partners | ||||
| Median (IQR) | 2 (1–4) | 2 (1–3) | 2 (1–5) | 0.11 |
| ≤1 | 174/434 (40.1%) | 114/278 (41.0%) | 60/156 (38.5%) | 0.60 |
| >1 | 260/434 (59.9%) | 164/278 (59.0%) | 96/156 (61.5%) | |
| HIV status | <0.01 | |||
| Negative | 251/536 (46.8%) | 184/336 (54.8%) | 67/200 (33.5%) | |
| Positive | 285/536 (53.2%) | 152/336 (45.2%) | 133/200 (66.5%) | |
| On PrEP (among persons with HIV negative status) | 83/224 (37.1%) | 55/184 (29.9%) | 28/67 (41.8%) | 0.07 |
| Hepatitis B seroprevalence | 7/527 (1.3%) | 3/332 (0.9%) | 4/195 (2.1%) | 0.43 |
| Hepatitis C seroprevalence | 34/532 (6.4%) | 16/334 (4.8%) | 18/198 (9.1%) | 0.05 |
| Proctitis | 142/533 (26.6%) | 74/334 (22.2%) | 68/199 (34.2%) | <0.01 |
| Reported any STI in the last 12 monthsb | 127/388 (32.7%) | 56/246 (22.8%) | 71/142 (50.0%) | <0.01 |
Wilcoxon rank sum test; Fisher's exact test; Pearson's chi-squared test.
Self-reported STI diagnosis or treatment before the onset of mpox-related symptoms.
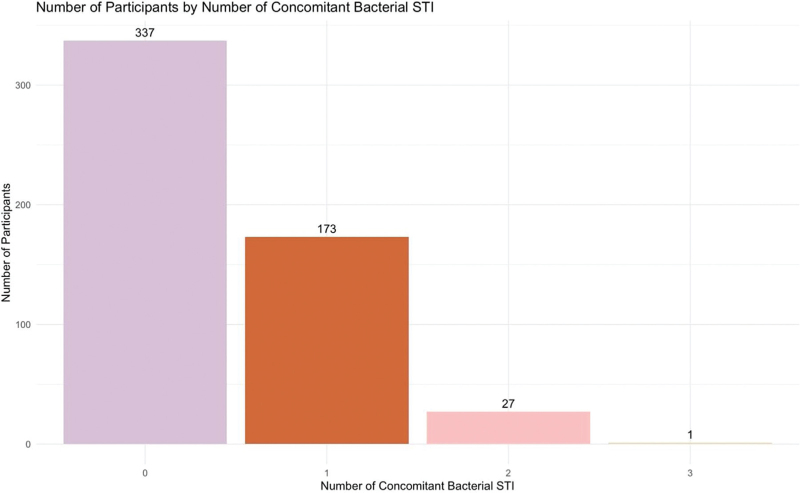
The prevalence of any concomitant bSTI at baseline mpox assessment was 37.3% (n = 201), with active syphilis being the most common (n = 128, 23.9%), followed by anorectal chlamydia (n = 51, 9.7%) and gonorrhea (n = 51, 9.7%). Twenty-seven participants were diagnosed with two or more concomitant bSTI in addition to mpox (2 STI: n = 27 [12.9%]; 3 STI: n = 1 [0.5%]) (Fig. 1).
Fig. 1.
Number of participants with confirmed mpox according to the number of concomitant bacterial sexually transmitted infections diagnosed (N = 538).
Compared to those with no bSTI at the initial mpox assessment, a higher proportion of participants with any bSTI were aged 30–39 years (45.3% vs. 41.6%, P = 0.02) or 25–29 years (22.9% vs. 17.5%, P = 0.02), while fewer were aged 18–24 years (6.9% vs. 15.1%, P = 0.02). Cisgender women were less frequently diagnosed with any bSTI (2.5% vs. 6.5%, P = 0.01), whereas there was a slightly higher number of bSTI diagnoses among transgender women (4.5% vs. 1.2%, P = 0.01). Participants living with HIV were more frequently diagnosed with any bSTI (66.5% vs. 45.2%, P < 0.01), and no differences were observed with regards to current PrEP use (41.8% vs. 29.9%, P = 0.07). Hepatitis C seroprevalence was higher among participants with bSTI (9.1% vs. 4.8%, P = 0.05). Proctitis (34.2% vs. 22.2%, P < 0.01) and a prior STI diagnosis in the last 12 months (50.0% vs. 22.8%, P < 0.01) were more common among participants diagnosed with any bSTI. No differences in educational level, race/skin color, transactional sex, number of sex partners, or hepatitis B seroprevalence were identified based on the presence of any concomitant bSTI.
Conclusions
Our study offers a deeper insight into the interaction between mpox and bSTI concomitant diagnoses among participants in a cohort from Rio de Janeiro, Brazil. Our data detail the causative pathogens, including molecular diagnoses, helping to address gaps in microbiological data on STI pathogens in a Global South country. These findings align with secondary analyses published by the Brazilian Ministry of Health, underscoring the importance of screening for other STI during initial mpox assessments [6].
However, challenges remain with accessing molecular diagnostics for STI, as testing for Ct and Ng has only recently became available through the public health system in Brazil. The high frequency of proctitis associated with concomitant bSTI diagnosis underscores the importance of empirical antimicrobial treatment covering Ct and Ng when participants report symptoms such as rectal pain, tenesmus, or anal discharge, in settings where real-time molecular testing is unavailable. Likewise, the high burden of active syphilis among our participants suggests that mpox should be considered a potential differential diagnosis in any individual presenting with genital or perianal ulcers. In this sense, mpox assessment is also an opportunity to expand access to syphilis diagnosis and treatment. These findings emphasize the necessity of a combined HIV and STI prevention approach in the context of mpox assessments.
Our findings reveal a significant prevalence of concomitant bSTI diagnosis among participants with confirmed mpox, along with frequent hepatitis C and HIV diagnoses. This highlights the impact of sexual contact on mpox transmission, and the compounded risk posed by additional STI, potentially heightening the risk of HIV acquisition. Notably, mpox appears to be concentrated among individuals aged 30–39 years, which is quite older than predominant younger age ranges of individuals with incident syphilis or HIV [9–11]. Our study primarily enrolled participants with high educational attainment, mirroring the overall profile of individuals engaged in HIV/STI prevention services in Brazil, such as PrEP [12]. However, it is crucial to emphasize the necessity of reaching out to those with lower levels of education, who may be at increased vulnerability to HIV/STI. These findings underscore the importance of understanding the complexities of sexual networks in STI transmission dynamics.
Some limitations should be acknowledged. Approximately 15% of participants affected by mpox were not included in this analysis, primarily due to organizational constraints faced in the initial months of the mpox outbreak in Brazil, affecting bSTI testing. Despite these challenges, the characteristics of the participants included in our final sample closely reflect those of our broader cohort [7]. Likewise, it is not possible to fully acknowledge temporality in terms of diagnosis of mpox and concomitant bSTI described herein. The concurrent acquisition of mpox and bSTI during the same contact, or the presence of preexisting lesions that may increase the likelihood of acquiring additional STIs, are both plausible hypotheses. Further research into these mechanisms is crucial for developing effective STI (including mpox) and HIV prevention strategies.
Delving deeper into the emergence of mpox as an STI and its complex interactions with other STI entails more than just deciphering the immunopathogenic mechanisms at play. It also necessitates examining the shared vulnerabilities within interconnected networks and their impact on STI transmission dynamics. This underscores the importance of implementing comprehensive sexual healthcare delivery strategies during mpox assessments, which can integrate individuals into a holistic prevention framework. By advocating for the integration of mpox assessment into broader sexual health initiatives, we not only broaden the scope of screening, treatment, and prevention measures for HIV and other STI but also create opportunities to engage new individuals in HIV prevention and care. This concerted effort ultimately positions these healthcare settings as pivotal hubs for enhancing surveillance and promoting comprehensive sexual healthcare.
Acknowledgements
We thank all study participants and INI-Fiocruz staff. Partial results of this manuscript were selected for poster presentation at the AIDS 2024 Conference in Munich, Germany.
B.G. was supported by the National Council of Technological and Scientific Development (CNPq; #313265/2023-2) and the Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ; #E.26/200.946/2022). T.S.T. was supported by CNPq (#402916/2021-2 and #311871/2021-6) and FAPERJ (#E-26/211.577/2021 and #E-26/201.270/2022).
Funding: National Institute of Infectious Diseases Evandro Chagas, Fiocruz, and Brazilian Ministry of Health. Funding sources had no role in study design or data analysis.
Conflicts of interest
The authors declare no conflict of interests.
References
- 1.PAHO. Monkeypox cases − region of the Americas. Available at: https://shiny.pahobra.org/monkeypox/. [Accessed 1 August 2024]. [Google Scholar]
- 2.Kibungu EM, Vakaniaki EH, Kinganda-Lusamaki E, Kalonji-Mukendi T, Pukuta E, Hoff NA, et al. Clade I-associated mpox cases associated with sexual contact, the Democratic Republic of the Congo. Emerg Infect Dis 2024; 30:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitjà O, Ogoina D, Titanji BK, Galvan C, Muyembe J-J, Marks M, et al. Monkeypox. Lancet 2022; 401:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curran KG, Eberly K, Russell OO, Snyder RE, Phillips EK, Tang EC, et al. HIV and sexually transmitted infections among persons with monkeypox — eight U.S. jurisdictions, May 17–July 22. MMWR Morb Mortal Wkly Rep 2022; 71:1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maldonado-Barrueco A, Sanz-González C, Gutiérrez-Arroyo A, Grandioso-Vas D, Roces-Álvarez P, Sendagorta-Cudos E, et al. Sexually transmitted infections and clinical features in monkeypox (mpox) patients in Madrid, Spain. Travel Med Infect Dis 2023; 52:102544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza IND, Pascom ARP, Spinelli MF, Dias GB, Barreira D, Miranda AE. Demographic and clinical characteristics of people diagnosed with active sexually transmitted infections among monkeypox cases in Brazil: the 2022 outbreak. Rev Inst Med trop S Paulo 2024; 66:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva MST, Coutinho C, Torres TS, Peixoto E, Ismério R, Lessa F, et al. Ambulatory and hospitalized patients with suspected and confirmed mpox: an observational cohort study from Brazil. Lancet Reg Health Am 2022; 17:100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva MST, Coutinho C, Torres TS, Peixoto EM, Bastos MO, Mesquita MB, et al. Mpox severity and associated hospitalizations among people with HIV and related immunosuppression in Brazil. AIDS 2024; 38:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministério da Saúde. Boletim Epidemiológico de HIV/Aids. Ministério da Saúde; 2022. [Google Scholar]
- 10.Ministério da Saúde. Boletim Epidemiológico de Sífilis. Ministério da Saúde; 2022. Available at: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2022/boletim-epidemiologico-de-sifilis-numero-especial-out-2022/view. [Accessed 1 August 2024]. [Google Scholar]
- 11.Torres TS, Teixeira SLM, Hoagland B, Konda KA, Derrico M, Moreira RI, et al. ImPrEP Seroincidence Study Group. Recent HIV infection and annualized HIV incidence rates among sexual and gender minorities in Brazil and Peru (ImPrEP seroincidence study): a cross-sectional, multicenter study. Lancet Reg Health Am 2023; 28:100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Painel PrEP. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Available at: https://www.gov.br/aids/pt-br/assuntos/prevencao-combinada/prep-profilaxia-preexposicao/painel-prep. [Accessed 1 August 2024]. [Google Scholar]