Abstract
Aims
Elevated plasma branched‐chain amino acids (BCAAs) are tightly linked to incident diabetes and its complications, while lower BCAAs are associated with adverse outcomes in the elderly and heart failure (HF) patients. The interplay between body compositions and plasma BCAAs, especially under the influence of co‐morbid diabetes in HF patients, is not well understood. Here, we examined the impact of diabetes on the prognostic value of plasma BCAA and its association with body compositions in HF patients.
Methods and results
We retrospectively examined 301 HF patients (70 ± 15 years old; 59% male), among which 36% had diabetes. Blood samples for plasma BCAA measurements were collected in a fasting state after stabilization of HF and analysed using ultraperformance liquid chromatography. A dual‐energy X‐ray absorptiometry scan assessed regional body compositions, and muscle wasting was defined as appendicular skeletal muscle mass index (ASMI) < 7.00 and <5.40 kg/m2 for males and females, respectively, according to the criteria of the Asian Working Group for Sarcopenia. Although analyses of covariance revealed that plasma BCAAs were significantly higher in diabetic patients, low valine (<222.1 nmol/mL) similarly predicted adverse events defined by HF hospitalization, lethal arrhythmia, or all‐cause death in both diabetic and non‐diabetic patients independently of age, sex, and NT‐proBNP (adjusted hazard ratio [HR] 3.1, 95% confidence interval [CI] of 1.1–8.6 and adjusted HR 2.67, 95% CI 1.1–6.5, respectively; P for interaction 0.88). In multivariate linear regression analyses comprising age, sex, and regional body compositions as explanatory variables, plasma BCAAs were positively correlated with visceral adipose tissue area in non‐diabetic patients (standardized β coefficients [β] = 0.44, P < 0.001). In contrast, in diabetic patients, plasma BCAAs were correlated positively with ASMI (β = 0.49, P = 0.001) and negatively with appendicular fat mass index (AFMI; β = −0.42, P = 0.004). Co‐morbid diabetes was independently associated with muscle wasting (adjusted odds ratio 2.1, 95% CI 1.1–4.0) and significantly higher plasma 3‐methylhistidine level, a marker of myofibrillar degradation. In diabetic patients, ASMI uniquely showed a J‐shaped relationship with AFMI, and in a subgroup of HF patients with muscle wasting, diabetic patients showed 12% higher AFMI than non‐diabetic patients despite comparable ASMI reductions.
Conclusions
Despite higher plasma BCAA levels in HF patients with diabetes, the prognostic value of low valine remained consistent regardless of diabetes status. However, low BCAAs were distinctly associated with fatty muscle degeneration in the extremities in diabetic patients, suggesting the importance of targeted interventions to prevent such tissue remodelling in this population.
Keywords: Body composition, Branched‐chain amino acid, Diabetes, Heart failure, Prognosis
Introduction
Elevated plasma levels of branched‐chain amino acids (BCAAs) are highly associated with obesity, insulin resistance, and diabetes. 1 , 2 , 3 Elevation of levels of BCAAs predicts not only the development of diabetes but also future cardiovascular diseases (CVD) in general populations 4 , 5 , 6 , 7 , 8 and the development of incident heart failure (HF) in patients with diabetes. 9 On the other hand, plasma BCAA levels decrease with aging 10 and are decreased in some, but not all, HF patients. 11 , 12 Notably, recent studies have demonstrated that lower BCAA levels are associated with elevated brain natriuretic peptide (BNP) level, impaired exercise tolerance, and adverse prognosis in HF patients. 13 , 14 , 15 , 16 Using plasma amino acid profiling, we have recently shown that a lower plasma level of valine, a BCAA, independently predicts adverse events in elderly HF patients. 16 However, it remains unclear whether the prognostic value of BCAAs differs in HF patients complicated with diabetes, which is rapidly increasing worldwide, 17 because each condition could drive plasma BCAAs in opposing directions.
Changes in plasma BCAA levels are associated with body compositional changes, likely due to the significant contribution of skeletal muscle and adipose tissue to systemic BCAA metabolism. 18 In general, plasma BCAAs are positively correlated with total fat mass, visceral fat mass, and skeletal muscle mass in the general population, obese individuals, and older people. 19 However, the relative contribution of muscle and adipose tissue to plasma BCAA levels remains to be determined because the association with BCAAs was examined separately for each body composition in most previous studies. The issue would be even more complicated in diabetic patients by the coexistence of body compositional changes that affect plasma BCAA levels differently: visceral fat accumulation and predisposition of skeletal muscle loss. 20 Furthermore, there have been few studies on the relationship between BCAAs and body composition in HF patients. A deeper understanding of metabolic alterations in HF patients with diabetes could lead to novel therapeutic strategies to improve their morbidity and mortality.
In the present study, we first examined whether co‐morbid diabetes affects the prognostic value of plasma valine in HF patients. We then examined the relative contributions of skeletal muscle and fat mass to plasma BCAA levels by incorporating both indexes simultaneously in a single logistic regression model, in which analyses were conducted separately for patients with and those without diabetes. In the latter analyses, we separated fat mass into visceral and appendicular compartments because of the possibility that regional fat tissues have different effects on plasma BCAA levels.
Methods
Study subjects
This study was a single‐center, retrospective, and observational study. We enrolled 436 consecutive patients admitted to our institute for diagnosis and management of HF from 15 February 2018 to 31 July 2020 and started to follow‐up from the admission day. The inclusion criterion was HF that was diagnosed according to the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic HF. 21 Exclusion criteria were pulmonary artery hypertension, acute myocarditis, and chronic kidney disease at stage 5 that was defined as estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2, missing data for amino acid profiling and a follow‐up period of <30 days, resulting in 301 patients for final analyses as we previously described. 16 This study was conducted strictly in accordance with the principles of the Declaration of Helsinki and was approved by the Clinical Investigation Ethics Committee of Sapporo Medical University Hospital (approval number 302‐243).
Biochemical analyses, echocardiography, and body composition analyses
Data for blood tests and left ventricular ejection fraction (LVEF), measured by the modified Simpson method, were retrieved from the patients' medical records. eGFR was calculated using equations developed for Japanese subjects as follows: eGFRcre (mL/min/1.73 m2) = 194 × Scr‐1.094 × age−0.287(×0.739 if female). 22 Chronic kidney disease (CKD) was defined as eGFRcre of <60 mL/min/1.73 m2.
Whole and regional fat/lean masses of patients were analysed during hospitalization using the Horizon A DXA System (HOLOGIC, Waltham, MA, USA) as previously reported, 16 , 23 , 24 and lean mass was defined as an index of skeletal muscle mass. Appendicular skeletal muscle mass (ASM) was calculated as the sum of bone‐free lean masses in the arms and legs. Systemic fat mass (SFM) was separated into appendicular fat mass (AFM) and trunk fat mass (TFM). Visceral adipose tissue (VAT) area at the level of the L4 lumbar vertebra was also estimated, as previously reported. 25 According to previous large‐scale dual‐energy X‐ray absorptiometry (DXA) studies examining body composition reference range, 26 , 27 systemic skeletal muscle mass (SSM) index (SSMI), ASM index (ASMI), SFM index (SFMI), AFM index (AFMI), and TFM index (TFMI) were defined as SSM/height2, ASM/height2, SFM/height2, AFM/height2, and TFM/height2, respectively. Muscle wasting was defined as ASMI < 7.0 kg/m2 for males and ASMI < 5.4 kg/m2 for females according to the criteria of the Asian Working Group for Sarcopenia. 28
Measurements of plasma branched‐chain amino acids and 3‐methylhistidine concentration
Plasma concentrations of BCAAs and 3‐methylhistidine, a marker of myofibrillar degradation, were measured in a fasting state after stabilization of HF during hospitalization using ultraperformance liquid chromatography (UPLC, LSI Medience Corporation, Tokyo, Japan), and UPLC analysis was conducted using the Acquity™ UPLC system with a TUV detector and MassTrak™ AAA Solutions Kit (Waters Corporation, Milford, MA) and validated as we previously described. 16
Clinical endpoint
The clinical endpoint in this study was an adverse event that was defined as a composite of all‐cause death and unscheduled readmission due to worsening HF or lethal arrhythmia after discharge as we previously described. 16
Assessment of lipid infiltration into psoas muscle by computed tomography imaging
In a post‐hoc analysis, lipid infiltration into the psoas muscle was evaluated by single slice computed tomography (CT) imaging at L3/4 level in randomly selected 100 patients from the study subjects who underwent abdominal CT imaging within 2 months from plasma amino acid profiling. The psoas muscle area was separated into areas of lean muscle, fatty muscle, and intermuscular adipose tissue (IMAT) using CT value thresholds of 30 to 100 Hounsfield units (HU), −30 to 29 HU, and −190 to −31 HU, respectively, as previously reported. 29 Mean muscle attenuation was defined as the mean CT value of the muscle area with −30 to 100 HU. Data were analysed using SYNAPSE VINCENT (Fujifilm, Tokyo, Japan).
Statistical analysis
Data are presented as means ± standard deviation or medians (interquartile range: 25th–75th percentiles) and expressed as frequency and percentage. In comparing baseline characteristics between patients with and those without diabetes, Student's t‐test or Mann–Whitney test were used for continuous variables, and the χ 2 test was used for categorical variables. Survival curves were calculated using the Kaplan–Meier method, and the statistical significance of differences between the curves was assessed using log‐rank statistics. Multivariate Cox proportional regression analyses were used to evaluate the predictive ability of valine level adjusted for age, sex, and log NT‐proBNP, in which the interaction with the presence of diabetes was evaluated. The impact of diabetes on plasma metabolites and body compositions was assessed by analysis of covariance (ANCOVA) adjusted for potential confounders, including age, sex, New York Heart Association (NYHA) functional classification, ischaemic aetiology, hypertension, dyslipidaemia, eGFR (only in the comparison of 3‐methylhistidine), and statin use.
The associations between BCAA levels and body compositions were assessed by multivariate linear regression analyses adjusted for potential confounders including age, sex, and serum albumin. In these models, the absence of significant multicollinearity among each body composition was confirmed by variance inflation factors ranging from 1.10 to 4.38. The relative contribution of each body composition to plasma BCAA levels was assessed by calculating the standardized β coefficients. Univariate and multivariate logistic regression analyses were performed to determine factors associated with muscle wasting. The relationship between ASMI and AFMI was evaluated by linear or binomial regression analysis to obtain the highest R 2 value separately in patients with and those without diabetes. A P value < 0.05 was considered statistically significant. Statistical analyses were conducted using JMP Pro version 17.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline clinical characteristics of heart failure patients with diabetes
In our study cohort, 109 patients (36%) were diagnosed with diabetes. As shown in Table 1 , patients with diabetes were significantly older than patients without diabetes and had higher prevalences of NYHA III/IV symptoms, ischaemic aetiology, hypertension, and dyslipidaemia than patients without diabetes. Other characteristics were similar in the two groups except for higher fasting glucose and HbA1c levels in diabetic patients. Heart failure medications and device therapies were similarly used in the two groups, but statins were more frequently prescribed in patients with diabetes. Among diabetic medications, dipeptidyl peptidase 4 (DPP‐4) inhibitors were most frequently used, followed by sodium‐glucose cotransporter 2 (SGLT2) inhibitors and insulin. Because patients were enrolled in this study before the era of SGLT2 inhibitors being recommended as standard HF therapy, no patients without diabetes used SGLT2 inhibitors.
Table 1.
Baseline characteristics
| All patients | Non‐DM | DM | ||
|---|---|---|---|---|
| N = 301 | N = 192 | N = 109 | P value | |
| Age (years) | 70.2 (14.5) | 68.0 (15.3) | 74.1 (12.1) | 0.0004 |
| Male sex | 177 (59) | 110 (57) | 67 (61) | 0.4792 |
| BMI (kg/m2) | 23.2 (4.1) | 22.9 (4.0) | 23.6 (4.3) | 0.1718 |
| NYHA III or IV | 84 (28) | 46 (24) | 38 (35) | 0.0427 |
| LVEF (%) | 46.6 (16.0) | 46.2 (15.7) | 47.4 (16.5) | 0.5191 |
| LVEF <40% | 117 (39) | 75 (39) | 42 (39) | 0.9277 |
| Ischaemic aetiology | 43 (14) | 11 (6) | 32 (29) | <0.0001 |
| Co‐morbidity | ||||
| Hypertension | 185 (61) | 103 (54) | 82 (75) | 0.0002 |
| Dyslipidaemia | 163 (54) | 91 (47) | 72 (66) | 0.0018 |
| Chronic kidney disease | 174 (58) | 113 (59) | 61 (56) | 0.6255 |
| Atrial fibrillation | 114 (38) | 74 (39) | 40 (37) | 0.7512 |
| Laboratory data | ||||
| Haemoglobin (g/dL) | 12.5 (2.1) | 12.6 (2.1) | 12.4 (2.0) | 0.3750 |
| NT‐proBNP (pg/mL) | 995 (467–2,150) | 978 (477–2,176) | 996 (443–2,110) | 0.9434 |
| eGFR (mL/min/1.73 m2) | 55.5 (20.5) | 56.9 (20.0) | 53.1 (21.3) | 0.1174 |
| Albumin (g/dL) | 3.7 (0.5) | 3.7 (0.5) | 3.6 (0.5) | 0.2064 |
| Fasting glucose (mg/dL) | 96 (27) | 87 (15) | 113 (34) | <0.0001 |
| HbA1c (%) | 6.1 (0.7) | 5.8 (0.4) | 6.7 (0.8) | <0.0001 |
| Medication | ||||
| ACE‐I or ARB | 168 (56) | 101 (53) | 67 (61) | 0.1356 |
| Beta‐blocker | 199 (66) | 127 (66) | 72 (66) | 0.9872 |
| MRA | 123 (41) | 78 (41) | 45 (41) | 0.9109 |
| Loop diuretics | 165 (55) | 99 (52) | 66 (61) | 0.1321 |
| Statin | 141 (47) | 72 (38) | 69 (63) | <0.0001 |
| DPP‐4 inhibitor | 0 (0) | 46 (42) | ||
| SGLT2 inhibitor | 0 (0) | 25 (23) | ||
| Insulin | 0 (0) | 14 (13) | ||
| α‐glucosidase inhibitors | 0 (0) | 11 (10) | ||
| Metformin | 0 (0) | 7 (6) | ||
| Sulphonylureas | 0 (0) | 6 (6) | ||
| GLP‐1 receptor agonist | 0 (0) | 3 (3) | ||
| Pioglitazone | 0 (0) | 1 (1) | ||
| Glinide | 0 (0) | 1 (1) | ||
| Device | 0.9050 | |||
| Pacemaker | 24 (8) | 17 (9) | 7 (6) | |
| ICD | 41 (14) | 26 (14) | 15 (14) | |
| CRT‐P or CRT‐D | 30 (10) | 19 (10) | 11 (10) | |
Values are presented as mean (± standard deviation), median (interquartile range), or n (%) wherever appropriate. N refers to the number of patients for whom the parameter was available.
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CRT‐D, cardiac resynchronization therapy defibrillator; CRT‐P, cardiac resynchronization therapy pacemaker; DM, diabetes, DPP‐4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; SGLT2, sodium glucose cotransporter 2.
Plasma levels of BCAAs are elevated in HF patients with diabetes
In ANCOVA analyses with adjustment for potential confounders, patients with diabetes had significantly higher levels of plasma total BCAAs, valine, leucine, and isoleucine than those in patients without diabetes (Figure 1 ). Although it was reported that plasma BCAAs were increased by administration of empagliflozin in patients with type 2 diabetes (T2DM), 30 similar trends were confirmed even after excluding patients with diabetes receiving SGLT2 inhibitors (Figure S1 ).
Figure 1.
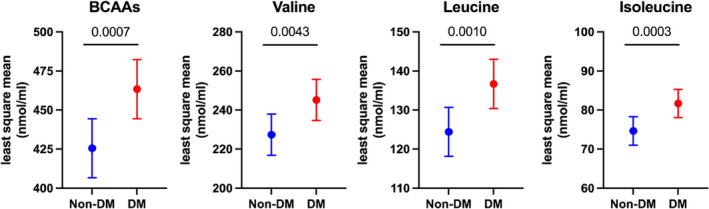
Comparison of plasma branched‐chain amino acid (BCAA) levels by analysis of covariance (ANCOVA) in patients with and those without diabetes (DM). Data are presented with the least square mean and 95% confidence interval. Age, gender, NYHA III or IV, ischaemic aetiology, hypertension, dyslipidaemia, and statin use were incorporated into the ANCOVA as potential cofounders. The P values obtained for comparisons of the groups at both ends of the line are shown.
Lower plasma valine similarly predicts adverse events in heart failure patients with and those without diabetes
During the mean follow‐up period of 379 ± 212 days, 24 (13%) of the patients without diabetes and 16 (15%) of the patients with diabetes had adverse events. When the patients were divided into two groups according to the cut‐off value of plasma valine concentration of 222.1 nmol/mL, which we derived and validated in our previous study, 16 patients with low valine had significantly lower event‐free survival rates both in patients with and those without diabetes (Figure 2 A,B ). In multivariate Cox proportional regression analysis adjusted for age, gender, and NT‐proBNP, the hazard ratios (HRs) of low plasma valine for adverse events were comparable in patients with and those without diabetes (Model 1, Figure 2 C ). The trend was consistent in another model in which NT‐proBNP was replaced by CKD [adjusted HR of 2.7, 95% confidence interval (CI) of 1.1–6.3 for non‐diabetic patients and adjusted HR of 2.5, 95% CI of 0.9–6.8 for diabetic patients, P value for interaction = 0.93]. Even after the addition of use of SGLT2 inhibitors in Model 1, there was no significant interaction between patients with and those without diabetes (P = 0.86), and lower valine was still associated with adverse events (adjusted HR: 3.3, 95% CI 1.2–9.2) in patients with diabetes. No significant interaction between patients with and those without diabetes was also consistently observed when patients receiving SLGT2 inhibitors were excluded in Model 1 (P = 0.72). Lower valine remained predictive after adjustment for age, gender, NT‐proBNP, and HbA1c in the whole study population (adjusted HR: 2.7, 95% CI 1.3–5.3), suggesting that diabetes management does not affect the prognostic value of plasma valine. These results indicate that, although diabetes is associated with elevated plasma levels of BCAAs even after HF development, it does not devalue the prognostic role of lower valine in HF patients.
Figure 2.
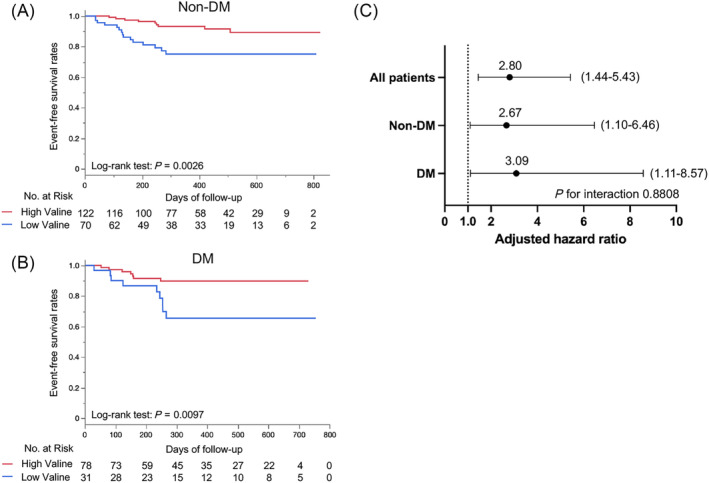
Comparison of the prognostic value of plasma valine in patients with and those without diabetes (DM). Kaplan–Meier event‐free survival curves in patients without DM (A) and those with DM (B). HF patients were divided into a high valine group (red) and a low valine group (blue) according to the cut‐off value of 222.1 nmol/mL, which we previously derived. 16 Multivariate Cox proportional regression analyses assessing the predictive value of low valine (≦222.1 nmol/mL) for adverse events with adjustment for age, gender, and log NT‐proBNP (C). Data are presented with an adjusted hazard ratio and 95% confidence interval in all patients and patients stratified by the status of DM.
Associations between plasma branched‐chain amino acids and regional body compositions are different in heart failure patients with and those without diabetes
ANCOVA analyses revealed that SSMI, ASMI, SFMI, and AFMI were comparable in patients with and those without diabetes. On the other hand, TFMI and VAT area were significantly larger in diabetic patients than in non‐diabetic patients (Figure 3 ). The least square mean of VAT area in diabetic patients (101.4 cm2, 95% CI 91.0–111.8 cm2) exceeded the cut‐off value of visceral obesity (VAT area ≥ 100 cm2) for Japanese subjects, 31 indicating that a significant proportion of HF patients with diabetes had visceral obesity.
Figure 3.
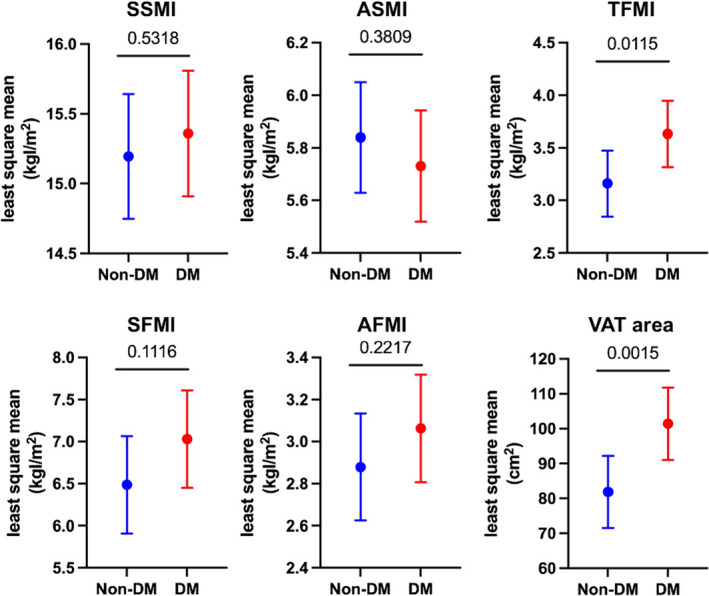
Comparison of body compositions by analysis of covariance (ANCOVA) in patients with and those without diabetes (DM). Data are presented with the least square mean and 95% confidence interval. Age, gender, NYHA III or IV, ischaemic aetiology, hypertension, dyslipidaemia, and statin use were incorporated into the ANCOVA as potential cofounders. The P values obtained for comparisons of the groups at both ends of the line are shown. AFMI, appendicular fat mass index; ASMI, appendicular skeletal muscle mass index; SFMI, systemic fat mass index; SSMI, systemic skeletal muscle mass index; TFMI, trunk fat mass index; VAT, visceral adipose tissue.
We next investigated how regional body compositions influence plasma BCAA levels in non‐diabetic and diabetic patients using multivariate logistic regression with adjustment for age and sex (Table 2 ). In non‐diabetic patients, BCAA levels were correlated positively with SFMI but not with SSMI. Conversely, in diabetic patients, BCAA levels were correlated positively with SSMI and negatively with SFMI (Model 1). Further analysis with separation of adipose tissue into AFMI and TFMI and inclusion of ASMI (Model 2) revealed that BCAA levels were correlated positively with TFMI and negatively with AFMI in non‐diabetic patients, with a more potent impact of TFMI indicated by its higher absolute β coefficients. In diabetic patients, BCAA levels showed no association with TFMI but were correlated negatively with AFMI and positively with ASMI. Similar trends persisted when TFMI was replaced with VAT area (Model 3) and after adjustment for serum albumin, a measure of nutritional status (Table S1 ). However, the associations between BCAA levels and ASMI in diabetic patients were attenuated by the adjustment for serum albumin. An adjustment for SGLT2 inhibitors, which reportedly reduce VAT in T2DM 32 and lean mass in patients with HF with reduced LVEF (HFrEF), 33 did not alter the distinct association between BCAAs and body compositions in diabetic patients (Table S2 ). An additional adjustment for HbA1c in Model 3 confirmed that diabetes management does not significantly affect the distinct association between BCAA and body compositions in diabetic patients (Table S3 ).
Table 2.
Standardized β coefficients between BCAA and body compositions assessed by DXA
| Model 1: age, sex, SSMI, SFMI | R 2 | SSMI | P value | SFMI | P value | ||||
| Non‐DM | BCAAs | 0.123 | −0.082 | 0.3941 | 0.323 | 0.0003 | |||
| Val | 0.085 | −0.102 | 0.3019 | 0.299 | 0.0011 | ||||
| Leu | 0.140 | −0.032 | 0.7359 | 0.294 | 0.0009 | ||||
| Ile | 0.104 | −0.066 | 0.7748 | 0.272 | 0.0030 | ||||
| DM | BCAAs | 0.115 | 0.463 | 0.0010 | −0.308 | 0.0095 | |||
| Val | 0.077 | 0.433 | 0.0026 | −0.307 | 0.0116 | ||||
| Leu | 0.125 | 0.434 | 0.0019 | −0.293 | 0.0131 | ||||
| Ile | 0.145 | 0.457 | 0.0010 | −0.252 | 0.0305 | ||||
| Model 2: age, sex, ASMI, AFMI, TFMI | R 2 | ASMI | P value | AFMI | P value | TFMI | P value | ||
| Non‐DM | BCAA | 0.213 | 0.076 | 0.4195 | −0.520 | 0.0001 | 0.722 | <0.0001 | |
| Val | 0.153 | 0.040 | 0.6826 | −0.464 | 0.0011 | 0.651 | <0.0001 | ||
| Leu | 0.237 | 0.121 | 0.1913 | −0.536 | <0.0001 | 0.714 | <0.0001 | ||
| Ile | 0.135 | 0.065 | 0.5093 | −0.380 | 0.0076 | 0.560 | <0.0001 | ||
| DM | BCAA | 0.126 | 0.508 | 0.0008 | −0.423 | 0.0145 | 0.188 | 0.2423 | |
| Val | 0.085 | 0.504 | 0.0011 | −0.360 | 0.0408 | 0.106 | 0.5172 | ||
| Leu | 0.149 | 0.491 | 0.0010 | −0.460 | 0.0071 | 0.232 | 0.1426 | ||
| Ile | 0.136 | 0.399 | 0.0075 | −0.398 | 0.0204 | 0.266 | 0.0962 | ||
| Model 3: age, sex, ASMI, AFMI, VAT area | R 2 | ASMI | P value | AFMI | P value | VAT area | P value | ||
| Non‐DM | BCAAs | 0.158 | 0.107 | 0.2723 | −0.236 | 0.0402 | 0.439 | <0.0001 | |
| Val | 0.110 | 0.067 | 0.4990 | −0.211 | 0.0731 | 0.401 | 0.0001 | ||
| Leu | 0.172 | 0.152 | 0.1146 | −0.234 | 0.0402 | 0.406 | <0.0001 | ||
| Ile | 0.113 | 0.088 | 0.3767 | −0.185 | 0.1155 | 0.374 | 0.0003 | ||
| DM | BCAAs | 0.139 | 0.494 | 0.0010 | −0.419 | 0.0038 | 0.225 | 0.0895 | |
| Val | 0.091 | 0.494 | 0.0014 | −0.369 | 0.0127 | 0.145 | 0.2867 | ||
| Leu | 0.162 | 0.478 | 0.0013 | −0.439 | 0.0022 | 0.254 | 0.0531 | ||
| Ile | 0.162 | 0.380 | 0.0098 | −0.392 | 0.0060 | 0.319 | 0.0155 |
AFMI, appendicular fat mass index; ASMI, appendicular skeletal muscle mass index; BCAA, branched‐chain amino acid; DM, diabetes; DXA, dual‐energy X‐ray absorptiometry; Ile, isoleucine; Leu, leucine; R 2, coefficient of determination; SFMI, systemic fat mass index; SSMI, systemic skeletal muscle mass index; TFMI, trunk fat mass index; Val, valine; VAT, visceral adipose tissue.
Appendicular skeletal muscle wasting with fat deposition is more prevalent in heart failure patients with diabetes
In the whole study population, 166 patients (55%) were diagnosed as having appendicular skeletal muscle wasting according to the criteria of the Asian Working Group for Sarcopenia. In univariate analysis, older age, diabetes, ischaemic aetiology, NYHA III or IV, higher NT‐proBNP, lower haemoglobin, lower serum albumin, and MRA use were associated with muscle wasting. In multivariate analysis, diabetes, NYHA III or IV, and lower albumin were selected as independent predictors for muscle wasting (Table 3 ). Patients with diabetes showed a significantly higher plasma 3‐methylhistidine concentration, a marker of myofibrillar degradation (Figure 4 A ). ASMI and AFMI showed a modest linear relationship in non‐diabetic patients, but a J‐shaped relationship fitted more robustly in diabetic patients (Figure 4 B ). Furthermore, in patients with muscle wasting, AFMI was significantly higher in diabetic patients than in non‐diabetic patients despite comparable ASMI levels in the two groups (Figure 4 C ). One hundred patients (33% had diabetes) were randomly selected to assess lipid infiltration into the psoas muscle using CT imaging (Figure S2 ). In non‐diabetic patients, muscle wasting reduced all areas of IMAT, fatty muscle, and lean muscle, with no change in mean muscle attenuation, suggesting that lipid content per muscle area is not altered by muscle wasting. Conversely, in diabetic patients, muscle wasting reduced only lean muscle area with lower mean muscle attenuation, suggesting that lipid content per muscle area is increased with muscle wasting. The results suggest that HF patients with diabetes are more predisposed to muscle wasting with fatty degeneration and that lower plasma levels of BCAAs in diabetic patients specifically mirror such body compositional changes.
Table 3.
Logistic regression analysis for muscle wasting
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.02 (1.00–1.04) | 0.0185 | 1.01 (0.98–1.03) | 0.5314 |
| Male | 1.14 (0.72–1.81) | 0.5744 | 1.42 (0.75–2.71) | 0.2813 |
| Diabetes mellitus | 2.30 (1.41–3.76) | 0.0009 | 2.14 (1.13–4.04) | 0.0191 |
| Smoking history | 1.02 (0.65–1.60) | 0.9422 | 0.82 (0.45–1.50) | 0.5157 |
| Ischaemic aetiology | 2.69 (1.30–5.57) | 0.0076 | 1.99 (0.79–4.99) | 0.1429 |
| NYHA III or IV | 3.93 (2.21–6.99) | <0.0001 | 3.07 (1.61–5.83) | 0.0006 |
| LVEF <40% | 1.53 (0.96–2.45) | 0.0762 | 1.47 (0.79–2.75) | 0.2225 |
| Log NT‐proBNP | 1.33 (1.11–1.60) | 0.0024 | 1.09 (0.85–1.40) | 0.4786 |
| eGFR | 0.99 (0.98–1.00) | 0.1499 | 1.01 (0.99–1.02) | 0.4972 |
| Haemoglobin | 0.89 (0.79–0.99) | 0.0346 | 0.99 (0.85–1.16) | 0.9377 |
| Albumin | 0.30 (0.18–0.52) | <0.0001 | 0.36 (0.19–0.69) | 0.0020 |
| ACE‐I or ARB | 0.82 (0.52–1.30) | 0.3944 | 0.58 (0.34–1.00) | 0.0504 |
| Beta‐blocker | 0.96 (0.59–1.55) | 0.8548 | 0.98 (0.54–1.80) | 0.9493 |
| MRA | 1.77 (1.11–2.84) | 0.0170 | 1.72 (0.97–3.04) | 0.0616 |
| SGLT2 inhibitor | 1.49 (0.64–3.49) | 0.3553 | 0.81 (0.27–2.39) | 0.7020 |
| Statin | 1.07 (0.68–1.69) | 0.7735 | 0.96 (0.55–1.69) | 0.8978 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; OR, odds ratio; SGLT2, sodium glucose cotransporter 2.
Figure 4.
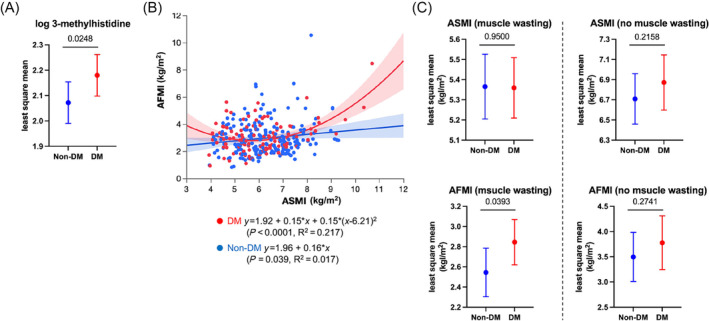
Comparison of levels of 3‐methylhistidine, a marker of myofibrillar degradation (A), correlation of ASMI with AFMI (B), and body compositions stratified by the presence of muscle wasting (C) in patients with and those without diabetes (DM). Data are presented with the least square mean (A, C), regression line or curve (B), and their 95% confidence interval. Age, gender, NYHA III or IV, ischaemic aetiology, hypertension, dyslipidaemia, and statin use were incorporated into the analysis of covariance (ANCOVA) as potential cofounders in (A) and (C). In (A), eGFR was also incorporated. The P values obtained for comparisons of the groups at both ends of the line are shown. Abbreviations are the same as those shown in the legend of Figure 3 .
Discussion
Beyond the well‐established association of elevated plasma BCAA levels with insulin resistance and diabetes, 1 , 2 , 3 , 4 , 5 , 6 diabetic patients with high plasma levels of BCAAs but with no history of CVD have been reported to be at high risk for developing myocardial infarction, stroke, coronary revascularization, and heart failure. 8 , 9 On the other hand, we found the opposite association between plasma valine level and clinical outcomes in HF patients with diabetes. Furthermore, despite significantly higher plasma BCAA levels in diabetic patients (Figure 1 ), the prognostic value of lower valine level (≦222.1 nmol/mL) was comparable to that in patients without diabetes (Figure 2 C ). Thus, the negative impact of elevated plasma levels of BCAAs in diabetes appears to be overridden once HF develops. In a secondary analysis of the ADVANCE trial in which high‐risk T2DM patients were enrolled (35% of the patients having macrovascular diseases and 5% of the patients having HF), elevated plasma levels of BCAAs had no association with macrovascular events; instead, they were associated with lower risk of all‐cause mortality. 34 Similarly, plasma metabolomics in the FIGHT trial in which the effect of liraglutide was examined in patients with advanced HFrEF, 58% of whom had T2DM, revealed that a principal component factor level comprising BCAAs correlated negatively with NT‐proBNP and positively with 6‐min walk distance regardless of co‐morbid diabetes, 14 being consistent with our findings. These results indicate that higher circulating levels of BCAAs are protective rather than detrimental in patients with advanced diabetic complications. The reversed impact of plasma BCAA levels on prognosis across the trajectory of diabetes is analogous to the ‘obesity paradox’ in HF 35 and in T2DM with established CVD, 36 suggesting that lower plasma BCAA levels might mirror adverse tissue wasting through enhanced catabolism in advanced chronic diseases.
In the present study, plasma levels of BCAAs were positively correlated with VAT area in HF patients without diabetes (Table 2 ), in line with results of previous studies conducted in community‐dwelling subjects in which significant proportions of overweight and obese subjects were included. 31 , 37 , 38 Adipose tissue is the major organ metabolizing circulating BCAAs. 39 However, obesity is associated with decreased expression of genes related to BCAA metabolism in adipose tissue (especially in VAT), 40 , 41 , 42 partly explaining the well‐established association between elevated plasma BCAA levels and obesity. In contrast, a plausible explanation for the association between BCAAs and VAT in relatively lean subjects observed in our study is that lower BCAA levels may indicate malnutrition, which is accompanied by increased lipolysis through enhanced catabolism. This hypothesis is supported by the results of a previous study showing that plasma BCAA levels positively correlate with VAT area in mostly non‐obese patients with hepatocellular carcinoma undergoing hepatectomy and that a larger VAT area is associated with a better prognosis. 43 Nevertheless, in the present study, the correlation between VAT area and BCAAs remained almost unchanged even with adjustment for serum albumin as a nutritional index (Table S1 ), suggesting that factors other than malnutrition may also be involved in the association between VAT and BCAAs.
On the other hand, in HF patients with diabetes, plasma BCAA levels showed no significant association with VAT area but were correlated positively with ASMI and negatively with AFMI. Several studies have shown correlations between BCAA and muscle mass in older adults and HF patients, 11 , 44 , 45 , 46 , 47 , 48 but the relative contribution of fat mass has not been explored. In one recent study, plasma BCAAs showed a positive correlation with both lean and fat mass index independently in older adults. 49 The discrepancy with our findings in diabetic patients might be explained by the condition termed ‘sarcopenic obesity’. Given the higher plasma BCAA levels and more prevalent visceral obesity (Figure 3 ), the effect of VAT on plasma BCAAs is possibly ‘saturated’, attenuating its impact in diabetic patients. Instead, fat deposition in the extremities, possibly through lipid infiltration into atrophic muscle, appears to become a more potent determinant of plasma BCAA levels, as discussed later. The association between ASMI and fasting plasma BCAAs in diabetic patients may stem from disturbed protein dynamics as skeletal muscle is the largest reservoir of protein (i.e., BCAAs) and has a mutual regulatory relationship with circulating BCAAs for protein synthesis. 18 The mechanistic target of rapamycin complex 1 (mTORC1), which is crucial for switching from protein breakdown to synthesis, is activated by insulin signalling and amino acids, especially leucine. Systemic insulin resistance induced by heart failure is exacerbated by co‐morbid diabetes, 50 likely intensifying the impact of latent BCAA deficiency on skeletal muscle turnover in HF patients with diabetes. This hypothesis is supported by our finding that muscle wasting was more prevalent in patients with diabetes, with higher circulating 3‐methylhistidine (Table 3 , Figure 4 A ).
Previous reports indicate that the biological effects of increased fat deposition to the extremities are context‐dependent. A study in which body composition was assessed by DXA in the general population revealed that adipose tissue expansion in the gynoid region and the extremities, especially in the legs, is metabolically protective in contrast to the detrimental effect of visceral fat accumulation. 51 In this scenario, subcutaneous adipose tissue (SAT) in the lower extremities serves as a reservoir for excess lipids, preventing ectopic fat deposition. On the other hand, lipid infiltration into skeletal muscle leads to metabolic disturbances and impaired muscle function. For example, fat infiltration into thigh skeletal muscles assessed by CT imaging has been shown to be associated with mobility limitation in the elderly, 52 insulin resistance in obese and diabetic patients, 53 and decreased peak oxygen uptake and poor prognosis in patients with HFrEF. 54 A recent large‐scale DXA study revealed that AFM increases with aging, and it has been speculated to involve this harmful fat deposition. 26 Indeed, in a small validation study with CT imaging, DXA‐estimated leg fat mass correlated more strongly with IMAT than with SAT in older men with an increased IMAT/SAT ratio. 55 Furthermore, using CT imaging, we observed a higher tendency of lipid infiltration into the psoas muscle in diabetic patients with muscle wasting (Figure S2 ). Taken together with our findings, co‐morbid diabetes in HF patients may accelerate muscle aging with fatty degeneration, with lower plasma levels of BCAAs somehow mirroring such adverse muscle remodelling.
The modest positive linear relationship between ASMI and AFMI in non‐diabetic patients may be explained by the increased catabolism of muscle and adipose tissue with HF progression. Indeed, we previously showed that a high per cent body fat was associated with a better prognosis in HF patients as an explanation for the obesity paradox, 56 and per cent body fat was strongly correlated with AFMI. In contrast, AFMI tended to increase in diabetic patients once a decline in ASMI reaches muscle wasting levels, resulting in the unique J‐shape relationship between the two parameters. This negative association between ASMI and AFMI is consistent with the findings observed in aging in community‐dwelling adults, 26 supporting the hypothesis that diabetes promotes appendicular muscle aging, as discussed above.
Our results suggest that prevention of fatty muscle degeneration is a potential therapeutic strategy in HF patients with diabetes. DXA is unsuitable for assessing body compositions in daily clinical settings. Instead, plasma BCAA levels could become simple biomarkers to screen adverse tissue remodelling. We propose that diabetic patients with low BCAA levels should be evaluated for body compositional changes quantitatively and qualitatively using imaging modalities, including DXA and CT. Identifying patients with adverse tissue remodelling, such as fatty muscle degeneration, may allow personalized cardiac rehabilitation. Since the correlation between BCAAs and ASMI in diabetic patients was partially attenuated by the adjustment for serum albumin in the present study (Table S1 ), restoring malnutrition could be one potential intervention. Resistance training has also been reported to reduce thigh intermuscular adipose tissue in older adults. 57 , 58 The hypothesis that diabetic HF patients, especially those with lower levels of BCAAs, benefit more from the combination of nutritional support and resistance training seems plausible but requires further investigations.
There are several limitations in the present study. First, selection bias might have existed because subjects were retrospectively enrolled in a single centre. Second, SGLT2 inhibitors, which reportedly affect the plasma amino acid profile and body compositions, 30 , 32 , 33 were prescribed only in diabetic patients. However, the analyses excluding patients receiving SGLT2 inhibitors or adjusting for the use of SGLT2 inhibitors did not alter the general trend of our primary results. Third, because DXA cannot separate SAT from IMAT and intramuscular fat, the compartments of fat reflected by AFMI remain to be determined. Validation studies with CT or magnetic resonance imaging in HF patients are needed. Fourth, although the logistic regression models consisting of previously reported plasma BCAA determinants (i.e., age, sex, and body compositions) were statistically valid, these variables explained <20% of the variance of plasma BCAA levels based on the R 2 values. Other factors such as protein intake and absorption, BCAA oxidation in the whole body, and gut microbiota compositions might also be involved. 18 Fifth, plasma valine had the highest prognostic value for adverse events among BCAAs in our previous study 16 but was less associated with body composition than leucine (Table 2 ). We hypothesize that plasma leucine is more reflective of skeletal muscle degeneration and dysfunction, while valine predicts broader adverse events independently of sarcopenia. Further studies are needed to elucidate whether such distinct roles exist among BCAAs. Sixth, insulin resistance could also affect aromatic amino acid metabolism. 1 , 2 The prognostic value of combined assessments of BCAAs and aromatic amino acids in HF patients has been reported, 59 , 60 , 61 though we did not explore the utility in the current study. Finally, the generalizability of our findings to other races, particularly obese Western people, remains to be examined. However, a robust correlation between BCAA dysmetabolism and insulin resistance has been well‐established in the non‐obese Asian population as well. 62
In conclusion, although co‐morbid diabetes was associated with higher plasma levels of BCAAs even in HF patients, low plasma valine level predicted adverse events similarly in patients with and those without diabetes. However, in HF patients with diabetes, low levels of BCAAs were distinctly associated with appendicular skeletal muscle wasting and fat deposition (Figure 5 ). Further studies are needed to elucidate whether such adverse tissue remodelling with plasma BCAA reductions is reversible with interventions such as comprehensive cardiac rehabilitation.
Figure 5.
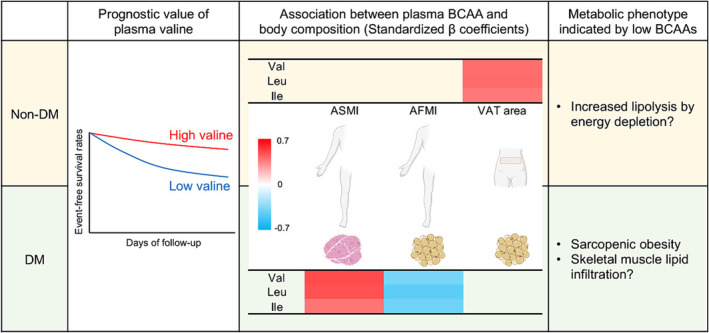
A graphical summary of the present study. Despite higher levels of plasma branched‐chain amino acids (BCAAs) in heart failure (HF) patients with diabetes (DM), low plasma valine level consistently predicts adverse events regardless of co‐morbid diabetes. However, plasma BCAAs correlate with body fat and muscle mass differently in diabetic and non‐diabetic HF patients. Low plasma BCAAs in diabetic HF patients are uniquely associated with fatty muscle degradation in the extremities. Ile, isoleucine; Leu, leucine; Val, valine. Other abbreviations are the same as those shown in the legend of Figure 3 . The figure was created partly with BioRender.com.
Funding
This study was supported by Grant 23K07481 (H.K.) from the Japan Society for the Promotion of Science, a grant from MSD Life Science Foundation, Public Interest Incorporated Foundation (H.K.).
Conflict of interest
None declared.
Supporting information
Figure S1. Comparison of plasma branched‐chain amino acid (BCAA) levels by analysis of covariance (ANCOVA) in patients with and those without diabetes (DM), excluding those receiving SGLT2 inhibitors (N = 25). Data are presented with the least square mean and 95% confidence interval. Age, gender, NYHA III or IV, ischemic aetiology, hypertension, dyslipidemia, and statin use were incorporated into the ANCOVA as potential cofounders. The p values obtained for comparisons of the groups at both ends of the line are shown.
Figure S2. Comparison of psoas muscle area stratified by the presence of diabetes (DM) in patients with and those without muscle wasting (MW) by analysis if covariance (ANCOVA). According to the computed tomography (CT) value, the psoas muscle area was separated into areas of lean muscle (30 to 100 HU), fatty muscle (−30 to 29 HU), and intermuscular adipose tissue (−190 to −31 HU). Mean muscle attenuation was defined as the mean CT value of the muscle area with −30 to 100 HU. Data are presented with the least square mean and 95% confidence interval. Age and gender were incorporated into the ANCOVA as potential cofounders. The p values obtained for comparisons of the groups at both ends of the line are shown. HU, Houndsfield units.
Table S1. Standardized β coefficients between BCAA and body compositions assessed by DXA under the adjustment for serum albumin.
Table S2. Standardized β coefficients between BCAA and body compositions assessed by DXA under the adjustment for SGLT2 inhibitors in diabetic patients.
Table S3. Standardized β coefficients between BCAA and body compositions assessed by DXA under the adjustment for HbA1c.
Kouzu, H. , Yano, T. , Katano, S. , Kawaharata, W. , Ogura, K. , Numazawa, R. , Nagaoka, R. , Ohori, K. , Nishikawa, R. , Ohwada, W. , Fujito, T. , Nagano, N. , and Furuhashi, M. (2024) Adverse plasma branched‐chain amino acid profile mirrors fatty muscle degeneration in diabetic heart failure patients. ESC Heart Failure, 11: 2941–2953. 10.1002/ehf2.14872.
References
- 1. Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811‐816. doi: 10.1056/NEJM196910092811503 [DOI] [PubMed] [Google Scholar]
- 2. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched‐chain amino acid‐related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311‐326. doi: 10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynch CJ, Adams SH. Branched‐chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014;10:723‐736. doi: 10.1038/nrendo.2014.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448‐453. doi: 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guasch‐Ferré M, Hruby A, Toledo E, Clish CB, Martínez‐González MA, Salas‐Salvadó J, et al. Metabolomics in prediabetes and diabetes: a systematic review and Meta‐analysis. Diabetes Care 2016;39:833‐846. doi: 10.2337/dc15-2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, et al. Genetic predisposition to an impaired metabolism of the branched‐chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med 2016;13:e1002179. doi: 10.1371/journal.pmed.1002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magnusson M, Lewis GD, Ericson U, Orho‐Melander M, Hedblad B, Engström G, et al. A diabetes‐predictive amino acid score and future cardiovascular disease. Eur Heart J 2013;34:1982‐1989. doi: 10.1093/eurheartj/ehs424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tobias DK, Lawler PR, Harada PH, Demler OV, Ridker PM, Manson JE, et al. Circulating branched‐chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ Genom Precis Med 2018;11:e002157. doi: 10.1161/CIRCGEN.118.002157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim L‐L, Lau ESH, Fung E, Lee H‐M, Ma RCW, Tam CHT, et al. Circulating branched‐chain amino acids and incident heart failure in type 2 diabetes: the Hong Kong diabetes register. Diabetes Metab Res Rev 2020;36:e3253. doi: 10.1002/dmrr.3253 [DOI] [PubMed] [Google Scholar]
- 10. Le Couteur DG, Ribeiro R, Senior A, Hsu B, Hirani V, Blyth FM, et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: the Concord Health and ageing in men project. J Gerontol A Biol Sci Med Sci 2020;75:1805‐1810. doi:10.1093/gerona/glz192 [DOI] [PubMed] [Google Scholar]
- 11. Tsuji S, Koyama S, Taniguchi R, Fujiwara T, Fujiwara H, Sato Y. Nutritional status of outpatients with chronic stable heart failure based on serum amino acid concentration. J Cardiol 2018;72:458‐465. doi: 10.1016/j.jjcc.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 12. Hayashi T, Yamashita T, Takahashi T, Tabata T, Watanabe H, Gotoh Y, et al. Uncovering the role of gut microbiota in amino acid metabolic disturbances in heart failure through metagenomic analysis. Front Cardiovasc Med 2021;8:789325. doi:10.3389/fcvm.2021.789325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmad T, Kelly JP, McGarrah RW, Hellkamp AS, Fiuzat M, Testani JM, et al. Prognostic implications of long‐chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol 2016;67:291‐299. doi: 10.1016/j.jacc.2015.10.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lerman JB, Giamberardino SN, Hernandez AF, Felker GM, Shah SH, McGarrah RW. Plasma metabolites associated with functional and clinical outcomes in heart failure with reduced ejection fraction with and without type 2 diabetes. Sci Rep 2022;12:9183. doi: 10.1038/s41598-022-12973-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lanfear DE, Gibbs JJ, Li J, She R, Petucci C, Culver JA, et al. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Fail 2017;5:823‐832. doi: 10.1016/j.jchf.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kouzu H, Katano S, Yano T, Ohori K, Nagaoka R, Inoue T, et al. Plasma amino acid profiling improves predictive accuracy of adverse events in patients with heart failure. ESC Heart Fail 2021;8:5045‐5056. doi: 10.1002/ehf2.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pop‐Busui R, Januzzi JL, Bruemmer D, Butalia S, Green JB, Horton WB, et al. Heart failure: an underappreciated complication of diabetes. A consensus report of the American Diabetes Association. Diabetes Care 2022;45:1670‐1690. doi: 10.2337/dci22-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol 2019;81:139‐164. doi: 10.1146/annurev-physiol-020518-114455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Couteur DG, Solon‐Biet SM, Cogger VC, Ribeiro R, de Cabo R, Raubenheimer D, et al. Branched chain amino acids, aging and age‐related health. Ageing Res Rev 2020;64:101198. doi: 10.1016/j.arr.2020.101198 [DOI] [PubMed] [Google Scholar]
- 20. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993‐1997. doi: 10.2337/dc09-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129‐2200. doi:10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 22. Ishigo T, Katano S, Yano T, Kouzu H, Ohori K, Nakata H, et al. Overestimation of glomerular filtration rate by creatinine‐based equation in heart failure patients is predicted by a novel scoring system. Geriatr Gerontol Int 2020;20:752‐758. doi: 10.1111/ggi.13959 [DOI] [PubMed] [Google Scholar]
- 23. Katano S, Yano T, Shimizu M, Ohori K, Kouzu H, Koyama M, et al. Does renin‐angiotensin system inhibition have impacts on muscle mass and bone mineral density in heart failure patients? ESC Heart Fail 2021;8:2617‐2624. doi: 10.1002/ehf2.13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katano S, Yano T, Tsukada T, Kouzu H, Honma S, Inoue T, et al. Clinical risk factors and prognostic impact of osteoporosis in patients with chronic heart failure. Circ J 2020;84:2224‐2234. doi: 10.1253/circj.CJ-20-0593 [DOI] [PubMed] [Google Scholar]
- 25. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual‐energy X‐ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity 2012;20:1109‐1114. doi: 10.1038/oby.2011.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirk B, Bani Hassan E, Brennan‐Olsen S, Vogrin S, Bird S, Zanker J, et al. Body composition reference ranges in community‐dwelling adults using dual‐energy X‐ray absorptiometry: the Australian Body Composition (ABC) Study. J Cachexia Sarcopenia Muscle 2021;12:880‐890. doi: 10.1002/jcsm.12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hinton BJ, Fan B, Ng BK, Shepherd JA. Dual energy X‐ray absorptiometry body composition reference values of limbs and trunk from NHANES 1999‐2004 with additional visualization methods. PLoS ONE 2017;12:e0174180. doi: 10.1371/journal.pone.0174180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen L‐K, Woo J, Assantachai P, Auyeung T‐W, Chou M‐Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300‐307.e2. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 29. Luetkens JA, Faron A, Geissler HL, Al‐Kassou B, Shamekhi J, Stundl A, et al. Opportunistic computed tomography imaging for the assessment of fatty muscle fraction predicts outcome in patients undergoing transcatheter aortic valve replacement. Circulation 2020;141:234‐236. doi: 10.1161/CIRCULATIONAHA.119.042927 [DOI] [PubMed] [Google Scholar]
- 30. Kappel BA, Lehrke M, Schütt K, Artati A, Adamski J, Lebherz C, et al. Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation 2017;136:969‐972. doi: 10.1161/CIRCULATIONAHA.117.029166 [DOI] [PubMed] [Google Scholar]
- 31. Yamakado M, Tanaka T, Nagao K, Ishizaka Y, Mitushima T, Tani M, et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin Obes 2012;2:29‐40. doi: 10.1111/j.1758-8111.2012.00039.x [DOI] [PubMed] [Google Scholar]
- 32. Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020‐1031. doi: 10.1210/jc.2011-2260 [DOI] [PubMed] [Google Scholar]
- 33. Jensen J, Omar M, Kistorp C, Tuxen C, Gustafsson I, Køber L, et al. Metabolic effects of empagliflozin in heart failure: a randomized, double‐blind, and placebo‐controlled trial (empire HF metabolic). Circulation 2021;143:2208‐2210. doi: 10.1161/CIRCULATIONAHA.120.053463 [DOI] [PubMed] [Google Scholar]
- 34. Welsh P, Rankin N, Li Q, Mark PB, Würtz P, Ala‐Korpela M, et al. Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: results from the ADVANCE trial. Diabetologia 2018;61:1581‐1591. doi: 10.1007/s00125-018-4619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 2013;1:93‐102. doi: 10.1016/j.jchf.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 36. Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co‐morbidity: an analysis of the PROactive study population. Int J Cardiol 2012;162:20‐26. doi: 10.1016/j.ijcard.2011.09.039 [DOI] [PubMed] [Google Scholar]
- 37. Menni C, Migaud M, Glastonbury CA, Beaumont M, Nikolaou A, Small KS, et al. Metabolomic profiling to dissect the role of visceral fat in cardiometabolic health. Obesity 2016;24:1380‐1388. doi: 10.1002/oby.21488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rietman A, Stanley TL, Clish C, Mootha V, Mensink M, Grinspoon SK, et al. Associations between plasma branched‐chain amino acids, β‐aminoisobutyric acid and body composition. J Nutr Sci 2016;5:e6. doi: 10.1017/jns.2015.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem 2010;285:11348‐11356. doi: 10.1074/jbc.M109.075184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity‐related elevations in plasma leucine are associated with alterations in enzymes involved in branched‐chain amino acid metabolism. Am J Physiol Endocrinol Metab 2007;293:E1552‐E1563. doi: 10.1152/ajpendo.00134.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, et al. Regulation of adipose branched‐chain amino acid catabolism enzyme expression and cross‐adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 2013;304:E1175‐E1187. doi: 10.1152/ajpendo.00630.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boulet MM, Chevrier G, Grenier‐Larouche T, Pelletier M, Nadeau M, Scarpa J, et al. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab 2015;309:E736‐E746. doi: 10.1152/ajpendo.00231.2015 [DOI] [PubMed] [Google Scholar]
- 43. Higashi T, Hayashi H, Kaida T, Arima K, Takeyama H, Taki K, et al. Prognostic impact of visceral fat amount and branched‐chain amino acids (BCAA) in hepatocellular carcinoma. Ann Surg Oncol 2015;22:1041‐S1047. doi: 10.1245/s10434-015-4796-5 [DOI] [PubMed] [Google Scholar]
- 44. Jourdan C, Petersen A‐K, Gieger C, Döring A, Illig T, Wang‐Sattler R, et al. Body fat free mass is associated with the serum metabolite profile in a population‐based study. PLoS ONE 2012;7:e40009. doi: 10.1371/journal.pone.0040009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lustgarten MS, Price LL, Chale A, Phillips EM, Fielding RA. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J Gerontol A Biol Sci Med Sci 2014;69:717‐724. doi: 10.1093/gerona/glt152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murphy RA, Moore SC, Playdon M, Meirelles O, Newman AB, Milijkovic I, et al. Metabolites associated with lean mass and adiposity in older black men. J Gerontol A Biol Sci Med Sci 2017;72:1352‐1359. doi: 10.1093/gerona/glw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamada M, Kimura Y, Ishiyama D, Nishio N, Tanaka T, Ohji S, et al. Plasma amino acid concentrations are associated with muscle function in older Japanese women. J Nutr Health Aging 2018;22:819‐823. doi: 10.1007/s12603-018-1014-8 [DOI] [PubMed] [Google Scholar]
- 48. Ottestad I, Ulven SM, Øyri LKL, Sandvei KS, Gjevestad GO, Bye A, et al. Reduced plasma concentration of branched‐chain amino acids in sarcopenic older subjects: a cross‐sectional study. Br J Nutr 2018;120:445‐453. doi: 10.1017/S0007114518001307 [DOI] [PubMed] [Google Scholar]
- 49. Mikkola TM, Salonen MK, Kajantie E, Kautiainen H, Eriksson JG. Associations of fat and lean body mass with circulating amino acids in older men and women. J Gerontol A Biol Sci Med Sci 2020;75:885‐891. doi:10.1093/gerona/glz126 [DOI] [PubMed] [Google Scholar]
- 50. Rame JE, Ammari H, Doc A, Pressouyre L, Rigaud H, Bochaton M, et al. Chronic heart failure: a reversible metabolic syndrome? Circulation 45:35‐42. doi: 10.1016/j.spp.2024.02.007 [DOI] [Google Scholar]
- 51. Vasan SK, Osmond C, Canoy D, Christodoulides C, Neville MJ, Di Gravio C, et al. Comparison of regional fat measurements by dual‐energy X‐ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obes (Lond) 2018;42:850‐857. doi: 10.1038/ijo.2017.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324‐333. doi: 10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 53. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885‐892. doi: 10.1093/ajcn/71.4.885 [DOI] [PubMed] [Google Scholar]
- 54. Yoshida T, Shibata A, Tanihata A, Hayashi H, Yamaguchi Y, Kitada R, et al. Thigh intramuscular fat on prognosis of patients with nonischemic cardiomyopathy. Am J Cardiol 2022;169:113‐119. doi: 10.1016/j.amjcard.2021.12.059 [DOI] [PubMed] [Google Scholar]
- 55. Scafoglieri A, Deklerck R, Tresignie J, De Mey J, Clarys JP, Bautmans I. Assessment of regional adipose tissue depots: a DXA and CT comparison in cadavers of elderly persons. Exp Gerontol 2013;48:985‐991. doi: 10.1016/j.exger.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 56. Ohori K, Yano T, Katano S, Kouzu H, Honma S, Shimomura K, et al. High percent body fat mass predicts lower risk of cardiac events in patients with heart failure: an explanation of the obesity paradox. BMC Geriatr 2021;21:16. doi:10.1186/s12877‐020‐01950‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 2010;14:362‐366. doi: 10.1007/s12603-010-0081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr 2015;101:991‐999. doi: 10.3945/ajcn.114.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Ishihara T, et al. Usefulness of the plasma branched‐chain amino acid/aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J Cardiol 2020;75:689‐696. doi: 10.1016/j.jjcc.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 60. Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Kimura Y, et al. Prognostic value of leucine/phenylalanine ratio as an amino acid profile of heart failure. Heart Vessels 2021;36:965‐977. doi: 10.1007/s00380-020-01765-z [DOI] [PubMed] [Google Scholar]
- 61. Hiraiwa H, Okumura T, Murohara T. Amino acid profiling to predict prognosis in patients with heart failure: an expert review. ESC Heart Fail 2023;10:32‐43. doi: 10.1002/ehf2.14222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tai ES, Tan MLS, Stevens RD, Low YL, Muehlbauer MJ, Goh DLM, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian‐Indian men. Diabetologia 2010;53:757‐767. doi: 10.1007/s00125-009-1637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of plasma branched‐chain amino acid (BCAA) levels by analysis of covariance (ANCOVA) in patients with and those without diabetes (DM), excluding those receiving SGLT2 inhibitors (N = 25). Data are presented with the least square mean and 95% confidence interval. Age, gender, NYHA III or IV, ischemic aetiology, hypertension, dyslipidemia, and statin use were incorporated into the ANCOVA as potential cofounders. The p values obtained for comparisons of the groups at both ends of the line are shown.
Figure S2. Comparison of psoas muscle area stratified by the presence of diabetes (DM) in patients with and those without muscle wasting (MW) by analysis if covariance (ANCOVA). According to the computed tomography (CT) value, the psoas muscle area was separated into areas of lean muscle (30 to 100 HU), fatty muscle (−30 to 29 HU), and intermuscular adipose tissue (−190 to −31 HU). Mean muscle attenuation was defined as the mean CT value of the muscle area with −30 to 100 HU. Data are presented with the least square mean and 95% confidence interval. Age and gender were incorporated into the ANCOVA as potential cofounders. The p values obtained for comparisons of the groups at both ends of the line are shown. HU, Houndsfield units.
Table S1. Standardized β coefficients between BCAA and body compositions assessed by DXA under the adjustment for serum albumin.
Table S2. Standardized β coefficients between BCAA and body compositions assessed by DXA under the adjustment for SGLT2 inhibitors in diabetic patients.
Table S3. Standardized β coefficients between BCAA and body compositions assessed by DXA under the adjustment for HbA1c.


