Abstract
Cytokinesis requires membrane trafficking coupled to actin remodeling and involves a number of trafficking molecules. CD2-associated protein (CD2AP) has been implicated in dynamic actin remodeling and membrane trafficking that occurs during endocytosis leading to the degradative pathway. In this study, we present several arguments for its implication in cytokinesis. First, endogenous CD2AP was found concentrated in the narrow region of the midzone microtubules during anaphase and in the midbody during late telophase. Moreover, we found that CD2AP is a membrane- and not a microtubule-associated protein. Second, the overexpression of the first two Src homology 3 domains of CD2AP, which are responsible for this localization, led to a significant increase in the rate of cell multinucleation. Third, the CD2AP small interfering RNA interfered with the cell separation, indicating that CD2AP is required for HeLa cells cytokinesis. Fourth, using the yeast two-hybrid system, we found that CD2AP interacted with anillin, a specific cleavage furrow component, and the two proteins colocalized at the midbody. Both CD2AP and anillin were found phosphorylated early in mitosis and also CD2AP phosphorylation was coupled to its delocalization from membrane to cytosol. All these observations led us to propose CD2AP as a new player in cytokinesis.
INTRODUCTION
Cytokinesis is a fundamental cellular process that leads to the separation of daughter cells after mitosis. This mechanism involves microtubules and actin remodeling that are, in part, controlled by phosphorylation, degradation, and membrane fusion events (Robinson and Spudich, 2000; Straight and Field, 2000; Glotzer, 2001). During this process, dividing cells form a plasma membrane invagination or cleavage furrow, which is created by an actomyosin-based contractile ring that assembles under the plasma membrane. At a late stage of cytokinesis a narrow cytoplasmic bridge, defined as the midbody, connects the two daughter cells. This bridge is ultimately resolved and the two cells separate, thus completing cytokinesis.
Furrow ingression is accompanied by fusion of membrane vesicles with the ingressing plasma membrane (O'Halloran, 2000; Finger and White, 2002). A number of membrane trafficking proteins were found at the division site. Septins are required for cytokinesis as well as for exocytosis in neuron (Beites et al., 1999; Field and Kellogg, 1999). Several proteins needed for internalization and endocytosis also are required for cytokinesis and/or cellularization in different species, such as syntaxins (t-SNARE), dynamin, clathrin, the clathrin adaptor AP-2, and some Rab proteins, Rab3 in sea urchin, and Rab11 and the Rab11 effector Nuf in Drosophila (O'Halloran, 2000; Finger and White, 2002; Strickland and Burgess, 2004). Furthermore, it seems that membrane fusion events are possibly linked with actin remodeling (Burgess et al., 1997; Niswonger and O'Halloran, 1997, Emoto and Umeda, 2000). Indeed, in cellularizing Drosophila embryo mutants for syntaxin 1, a protein that is essential for cellularization, the lack of membrane changes is accompanied with a complete absence of the organized actin cytoskeleton associated with the furrow canals (Burgess et al., 1997). Moreover, Dictyostelium mutants that lack clathrin fail to construct a robust contractile ring (Niswonger and O'Halloran, 1997).
CD2AP, for CD2-associated protein (Dustin et al., 1998), plays roles in endocytic degradative pathway and actin remodeling. In the immunological synapse of T-cells, its interaction with the CD2 receptor was found associated with cytoskeleton reorganization (Dustin et al., 1998). The interaction of CD2AP with actin (Lehtonen et al., 2002) and several actin binding proteins (Hutchings et al., 2003; Lynch et al., 2003) reinforced the role of CD2AP in dynamic actin remodeling process. This is also supported by its colocalization with the actin nucleation Arp2/3 complex and cortactin (Welsch et al., 2001) in actin dynamic-rich structures (leading edge of lamellipodia and comet tails). The implication of CD2AP in degradation has been shown at different steps of the endocytic pathway. The interaction of CD2AP with endophilin and potentially with AP-2 has been implicated in internalization of epidermal growth factor (EGF) receptors (Brett et al., 2002; Lynch et al., 2003). Recently, we have shown that interaction of CD2AP with Rab4 and c-Cbl was implicated in platelet-derived growth factor receptor trafficking between early and late endosomes (Cormont et al., 2003). In accordance with this observation, Lee et al. (2003) have shown that T-cells isolated from CD2AP-/- mice failed to down-regulate the T-cell receptor, although internalization was not affected (Lee et al., 2003). Finally, electron microscopic analysis of podocytes from CD2AP haploinsufficient mice revealed defects in the formation of multivesicular bodies (Kim et al., 2003). The interaction of CD2AP with CAPZ and cortactin, two actin binding proteins, has been involved in the down-regulation of the activated T-cell receptor and EGF receptor, suggesting a link between receptor endocytosis and actin polymerization (Hutchings et al., 2003; Lynch et al., 2003). Thus, similarly to cytokinesis, membrane traffic events in endocytosis seem tightly linked to actin dynamic remodeling.
Because cytokinesis requires both membrane trafficking and actin remodeling and because CD2AP has been involved in these two processes, we wondered whether CD2AP also plays a role in cytokinesis. To address this question, we looked for CD2AP distribution in dividing HeLa cells, the effect of modulating its expression, the determination of potential CD2AP-interacting molecules involved in cytokinesis and the CD2AP behavior during cell cycle.
MATERIALS AND METHODS
Antibodies
Polyclonal antibodies against CD2AP (H-290), monoclonal anti-Myc (9E10), and anti-Rho GDI (A20) were obtained from Santa Cruz Biotechnology (Santa Cruz Biotechnology, CA). Anti-CD2AP recognizes the overexpressed CD2AP but not its homologue CIN85 (Supplemental Figure 1). Polyclonal antibodies against anillin has been described previously (Oegema et al., 2000). Anti-β-tubulin-Cy3 conjugate (TUB 2.1) monoclonal antibody (mAb) was from Sigma-Aldrich (St. Louis, MO). Anti-histone mAb (clone H11-4) and anti-green fluorescent protein (GFP) antibodies were from Roche Diagnostics (Mannheim, Germany). Fluorescein isothiocyanate (FITC), Cy5, and Texas Red-conjugated as well as horseradish peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Texas Red-conjugated phalloidin was from Molecular Probes (Eugene, OR). Anti-α-tubulin mAb (clone DM1A) and anti-FLAG mAb (clone M2) were from Sigma-Aldrich (St. Louis, MO). Polyclonal anti-Polo like kinase 1 (Plk1) antibodies (06-813) were obtained from Upstate Biotechnology (Lake Placid, NY). Anti-human transferrin receptor mAb (H68,4) was obtained from Zymed Laboratories (South San Francisco, CA). Anti-hemagglutinin (HA) mAb was obtained from Covance (Berkeley, CA).
cDNA Constructs
The CD2AP and anillin full-length cDNAs (Oegema et al., 2000; Cormont et al., 2003) were subcloned into the yeast two-hybrid pLexA and pACT2 vectors (BD Biosciences Clontech, Palo Alto, CA). The cDNA sequences encoding for the N-terminally truncated forms of CD2AP and anillin were amplified by PCR and subcloned in frame with the sequence encoding the Gal4-DNA binding domain of pACT2. Various C-terminally truncated forms of CD2AP and anillin were obtained by introducing a stop codon at the desired position into pACT2-anillin or pACT2-CD2AP by using site-directed mutagenesis (QuickChange kit, Stratagene, La Jolla, CA). Full-length CD2AP cDNA as well as sequences encoding for various truncated CD2AP forms were amplified by PCR and subcloned into pEGFP-C1 (BD Biosciences Clontech, Palo Alto, CA), in frame with the sequence coding for the GFP or into pCDNA3myc. pEGFP-CD2AP-1-175 was obtained by introducing a stop codon for the amino acid at position 175 by using site-directed mutagenesis. A similar approach was used to make the GST-fusion CD2AP construct into pGEX-2T vector (Amersham Biosciences, Piscataway, NJ).
Cells transfection
HeLa cells were grown in Dulbecco's modified Eagle's medium containing glutamine and supplemented with 10% fetal bovine serum in 5% CO2 at 37°C. For cDNA transfections, HeLa cells were transiently transfected with Fu-GENE 6 (Roche Diagnostics), as indicated by the manufacturer. For time-lapse experiments, cells were transfected by electroporation. Briefly, 6–8 × 106 HeLa cells were resuspended in 250 μl of DMEM containing 10 μg of DNA and 40 mM NaCl, placed in a 0.4-cm gap cuvette and electroporated (1050 μF, 290 V) using an EasyJet electroporator system (Equibio, Ashford, United Kingdom). This transfection procedure led to 80–90% of GFP-transfected cells. For CD2AP small interfering RNA (siRNA) transfections, 40–50% confluent HeLa cells cultivated in DMEM 10% fetal calf serum were used for transfection with Oligofectamine (Invitrogen Cergy Pontoise, France), as indicated by the manufacturer. Briefly, for each 35-mm dish, 10 μl of 10 μM CD2AP siRNA (sc-29984; Santa Cruz Biotechnology) was diluted into 175 μl of Opti-MEM I (Invitrogen SARL) and incubated for 5 min. In parallel, 4 μl of Oligofectamine was diluted in 11 μl of Opti-MEM I. Diluted Oligofectamine and siRNA were mixed and incubated at room temperature for 15 min. The medium was replaced with 800 μl of medium and the siRNA/Oligofectamine mix was added onto the cells. Cells were incubated for 36 h at 37°C in a CO2 incubator before time-lapse observations.
Cell Synchronization
For synchronization experiments, HeLa cells were incubated for 16–20 h with 2 mM hydroxyurea or with 0.1 μg/ml colcemid (Invitrogen SARL) leading to a G1/S boundary arrest and prometaphase arrest, respectively (Dulic et al., 1998; Alvarez et al., 2001). Cells were then released into the cell cycle by extensive washings, first with phosphate-buffered saline (PBS), and then with complete medium. Cell cycle analysis was performed by quantifying the DNA content using propidium iodide in a flow cytometry analysis by using a FACScan apparatus (BD Biosciences, San Jose, CA), as described previously (Vindelov et al., 1983).
Time-Lapse Imaging
Cells were filmed for 20 h under constant conditions (5% CO2, 37°C) and observed by phase-contrast optics by using fully motorized Axiovert 200 microscope (Carl Zeiss, Göttingen, Germany) and a cooled, digital charge-coupled device (CCD) camera (Roper Scientific, Evry, France) by using a 20× lens. Images were recorded at 1 frame/2min and processed using MetaMorph 2.0 image analysis software (Universal Imaging, Downingtown, PA) and QuickTime pro5 software (Apple Computer, Cupertino, CA).
CD2AP Immunoprecipitation and λ Phosphatase Treatment
HeLa cells (synchronized or not in prometaphase) were harvested and lysed in 500 μl of cold 20 mM Tris-HCl, pH 7.4, lysis buffer containing 137 mM NaCl, 10 mM EDTA, 1% Triton X-100, 2 mM Na3VO4, 100 mM NaF, 10 mM Na4P2O7, and a protease inhibitor cocktail (Complete; Roche Diagnostics). The cell lysate proteins (1 mg) were incubated with 10 μg of anti-CD2AP antibody that was previously adsorbed on protein A-Sepharose beads. The immunoprecipitate was treated or not for 90 min at 30°C with 800 U of λ phosphatase (New England BioLabs, Beverly, MA) in the provided λ phosphatase buffer supplemented with 2 mM MnCl2. Immunoprecipitated proteins were resuspended in 3% SDS-containing buffer, resolved by SDS-PAGE, transferred onto polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA), and CD2AP was immunodetected using the appropriate antibody. For λ phosphatase treatment on lysate, prometaphase-synchronized cells were harvested and homogenized in the λ phosphatase buffer and incubated with λ phosphatase for 30 min at 30°C. Proteins were denatured with SDS and resolved by SDS-PAGE as described above.
HeLa Cells Fractionation Procedures
Synchronized cells were harvested and homogenized in cold sucrose containing buffer (3 mM imidazole, pH 7.4, 250 mM sucrose, Complete). Cells were broken by 20 passages through a 22-gauge needle and centrifuged for 10 min at 10,000 × g in a cooled microcentrifuge to generate a postnuclear supernatant. A high-speed supernatant and a membrane pellet were obtained after centrifugation of the postnuclear supernatant for 1 h at 100,000 × g at 4°C (Optima X100 ultracentrifuge; Beckman Coulter, Fullerton, CA). Membranes and cytosolic fractions were analyzed by immunoblotting using anti-CD2AP, anti-anillin, and anti-α-tubulin antibodies. Anti-Rho GDI and anti-transferrin receptor antibodies were used as cytosolic and membrane controls for the fractionation procedure, respectively.
Cytoskeletal fractions were obtained after extraction of cytosolic proteins as described previously (Scaife et al., 2003). Briefly, HeLa cells were incubated 2 min at 37°C in extraction buffer containing 80 mM PIPES, pH 6.7, 1 mM EGTA, 1 mM MgCl2, 10% glycerol, 0.3% Triton X-100, 5% polyethylene glycol, and Complete. Buffer containing cytosolic proteins was recovered, whereas insoluble material that remained attached on the Petri dishes was scrapped in the same volume of extraction buffer to obtain cytoskeletal proteins. Both fractions were analyzed as described above.
In vitro microtubule binding assay was performed using the microtubule binding protein spin down assay kit (Cytoskeleton, Denver, CO) following the manufacturer's instructions. Briefly, in vitro polymerized microtubules were incubated for 30 min at room temperature with 8 μg of purified MAP2, 3 μg of bovine serum albumin (BSA), or 100 μl of cytosol (200 μg of proteins) from mitotic HeLa cells. As control, the proteins were incubated in the absence of microtubules. Microtubules and soluble proteins were then separated and analyzed for the presence of MAP2, tubulin, and BSA in the pellet and soluble fraction by coloration with Coomassie Blue or for the presence of CD2AP by immunoblotting.
Fixation, Immunofluorescence, and Microscopy
For analysis of the endogenous CD2AP, HeLa cells grown on collagen (Vitrogen 100; Cohesion, Palo Alto, USA) coated cover slips were fixed with methanol for 5 min at –20°C and then washed in Tris-buffered saline (TBS) (TBS containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl). Cells were permeabilized in TBS containing 0.5% Triton X-100 for 10 min, washed with TBS 0.1% Triton X-100 before being saturated in TBS containing 0.1% Triton X-100, 2% BSA, and 0.1% sodium azide. Coverslips were then incubated for 1 h at room temperature with the primary antibodies diluted in saturation buffer i.e., 1 μg/ml anti-CD2AP antibody and 1 μg/ml anti-histone mAb. After washing, the coverslips were incubated with FITC-conjugated anti-rabbit antibodies and Texas Red-conjugated anti-mouse IgG for 45 min at room temperature. For microtubule costaining, 1 μg/ml anti-β-tubulin-Cy3 conjugate mAb was added together with the secondary antibodies. To analyze the GFP-fusion proteins, transfected HeLa cells grown on collagen-coated coverslips were fixed for 15 min in 4% paraformaldehyde in PBS and then neutralized using 10 mM NH4Cl in PBS for 15 min. Cells were subsequently washed three times with PBS, blocked, and permeabilized using PBS containing 0.1% Triton X-100 and 0.5% BSA for 15 min. The coverslips were incubated for 1 h in blocking buffer containing 1 μg/ml anti-histone antibodies and 1 μg/ml anti-anillin antibodies. After washes, they were incubated with Cy5 or Texas Red-conjugated secondary antibodies for 45 min at room temperature. For microtubules costaining, 1 μg/ml anti-β-tubulin-Cy3-conjugated mAb was incubated together with the secondary antibodies. Coverslips were finally mounted in Mowiol 4-88 (Calbiochem, Darmstadt, Germany) on glass slides. Standard cell imaging was performed on an Axiovert 200 microscope (Carl Zeiss) equipped with a Plan-Neofluar 100 × 1.3 numerical aperture oil immersion lens and a cooled digital CCD CoolSnap HQ camera (Roper Scientific) by using MetaMorph image analysis software (Universal Imaging). For colocalization analysis, cells were examined using scanning confocal fluorescence microscopy with a PL APO 63× 1.40 oil objective (TCS SP; Leica Microsystems, Deerfield, IL). The images were combined and merged using Photoshop software (Adobe Systems, Mountain View, CA).
Two-Hybrid Screening and Interaction Measurements in Yeast
The yeast two-hybrid screening was performed as described previously (Cormont et al., 2001, 2003). Briefly, the yeast reporter strain L40 was first transformed with pLexA-CD2AP by using a lithium acetate-based method and grown in synthetic medium lacking tryptophan before a second transformation with a mouse cDNA library made in pVP16 plasmid. Cells were plated on synthetic medium lacking leucine, tryptophan, and histidine. Growing colonies were tested for β-galactosidase activity. Library plasmids from positive clones were rescued into Escherichia coli HB101 cells plated on leucinefree medium and analyzed by transformation tests in L40 yeast and DNA sequencing.
For interaction measurements, cotransformed L40 yeasts were selected on medium lacking leucine and tryptophan. The induction of the reporter gene LacZ that reveals protein–protein interaction was determined on replicate filters by using 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) as substrate (Cormont et al., 2001).
In Vitro Binding Assays
Glutathione S-transferase (GST)-fused CD2AP-1-175 was expressed in E. coli and purified as described previously (Bortoluzzi et al., 1996). Ten micrograms of GST-fusion proteins was immobilized on glutathione-Sepharose beads and incubated with 45 μl of anillin (1–300) transcribed and translated in vitro in the presence of [35S]methionine by using the TnT-coupled transcription-translation kit (Promega, Madison, WI) and pcDNA3-myc-anillin (1–300) as template. After 2-h incubation at 4°C in 20 mM HEPES buffer, pH 7.8, containing 50 mM NaCl and 1 mM dithiothreitol, beads were washed twice with 20 mM HEPES buffer, pH 7.8, 50 mM NaCl, and twice with 20 mM HEPES buffer containing 250 mM NaCl. Proteins still associated with beads were eluted using 3% SDS and were then analyzed by SDS-PAGE and autoradiography.
RESULTS
Localization of CD2AP during Cell Division
To examine a potential role of CD2AP in cytokinesis, we first looked at its intracellular localization during cell division (Figure 1). HeLa cells were fixed and coimmunolabeled for the endogenous CD2AP and chromosomes (Figure 1, a–i,) or microtubules (Figure 1, j–o). During metaphase, anti-CD2AP antibody showed a diffuse labeling throughout the cell (Figure 1, a–c). During late anaphase and telophase, CD2AP was concentrated in the vicinity of the midzone microtubules (Figure 1, d–i). Moreover, CD2AP was concentrated in the middle of the midbody during late telophase (Figure 1, j–l), and numerous interphase cells contained a bright CD2AP dot that likely corresponds to the midbody remnant (Figure 1, m–o). Similarly to the endogenous protein, the full-length GFP-CD2AP protein was found localized in the vicinity of the midzone microtubules during late anaphase and concentrated in the middle of the midbody during late telophase. Likewise, numerous interphase cells were found to contain a bright GFP-CD2AP dot that may correspond to the midbody remnant (Figure 2A).
Figure 1.
Localization of CD2AP during cell division. Endogenous CD2AP localization was studied along the mitotic process in randomly growing HeLa cells. The cells were fixed with methanol, as described in Materials and Methods and incubated with anti-CD2AP and anti-histone antibodies to detect chromosomes (a–i), or anti-β-tubulin antibody coupled to Cy3 for the detection of microtubules (j–o). CD2AP labeling was revealed using anti-rabbit FITC-conjugated antibody (a, d, g, j, and m). Histone labeling was revealed using anti-mouse Texas Red-conjugated antibody (b, e, and h). The green and red images for each mitotic figure were merged using the Adobe Photoshop software (overlay c, f, i, l, and o). Bar, 10 μm.
Figure 2.
Localization of GFP-CD2AP constructs during cell division. (A) HeLa cells were transfected with the GFP-CD2AP construct, fixed with paraformaldehyde, and costained for chromosomes by using anti-histone antibodies or microtubules by using anti-β-tubulin antibody coupled to Cy3 (red). (B) HeLa cells were transfected with the GFP constructs as indicated and costained for microtubules by using anti-β-tubulin antibody coupled to Cy3 (red). Images were merged using the Adobe Photoshop software. An enlargement of the midbody region is shown (zoom/overlay). Bars, 10 μm.
The First Two Src Homology (SH)3 Domains of CD2AP Are Required for Its Localization to the Midbody
Because CD2AP is a multidomain adaptor protein, we wanted to determine which CD2AP domain is required for its midbody localization during cell division. Several CD2AP constructs were generated in fusion with GFP and transfected in HeLa cells. We first looked at the level of overexpression of the proteins, which seemed to be less than two- to threefold the level of the endogenous protein, taking into account the 50% transfection level of the cells (Supplemental Figure 2A). Furthermore, no protein fragments of lower size were generated (Supplemental Figure 2B). As shown in Figure 2B, GFP-CD2AP-(1-175), a construct corresponding to the first two SH3 domains of the protein, was concentrated in the middle of the midbody, as indicated by the colabeling with microtubules. By contrast, GFP-CD2AP-(194-639), corresponding to the converse construct, was distributed all over the cell with no particular concentration at the midbody. This experiment suggested that the first two SH3 domains of CD2AP were responsible for its targeting to the middle of the midbody.
Overexpression of CD2AP or of Its First Two SH3 Domains Affects Cytokinesis
CD2AP localization at the midbody suggested that CD2AP could play a role during cytokinesis in HeLa cells. To examine this possibility, we looked for the effects of overexpressing CD2AP or its deletion mutants on cytokinesis. HeLa cells were transfected with GFP-CD2AP, GFP-CD2AP-(1-175), GFP-CD2AP-(194-639), or GFP alone (as control). Cells were fixed 48 h posttransfection, labeled for chromosomes, and multinucleated transfected cells were counted. As shown in Figure 3, the full-length protein or the GFP-CD2AP-(1-175) overexpression led to an increase in the rate of multinucleation, compared with the control condition. This effect was modest albeit significant when the total population of the transfected cells was taken into account (Figure 3B). To substantiate this observation, we determined the fraction of multinucleated cells as a function of protein expression levels by checking the fluorescence intensity of the cells (Figure 3C). Higher number of multinucleation events was observed with higher expression levels of GFP CD2AP or 1-175 mutant proteins. By contrast, the expression of the SH3 domains of intersectin, used as a control, did not lead to increased rate of multinucleation, nor was this construct localized within the midbody (our unpublished observations). Furthermore, numerous cells in late telophase were counted upon GFP-CD2AP and GFP-CD2AP-(1-175) overexpression (Figure 3B), as determined using the typical tubulin labeling. These results further substantiated our hypothesis that CD2AP plays a role in cytokinesis, and indicated that CD2AP may be involved in the latest step, the scission. To test this proposal, cells overexpressing the CD2AP mutant 1-175 were analyzed by time-lapse videomicroscopy (Supplemental Movie 1). We observed an increased number of cytokinetic failures leading to binucleated mutant cells compared with control cells: 10% of failed divisions in the GFP-1-175–transfected cells versus 2.5% for the GFP-transfected cells were observed in a film of 20-h duration.
Figure 3.
Overexpression of CD2AP affects cytokinesis. HeLa cells were transfected to overexpress GFP proteins as indicated. Forty-eight hours posttransfection, cells were fixed and labeled for chromosomes to count the number of nuclei per cell or for microtubules to count midbodies. (A) Representative fields of cells transfected with GFP, GFP-CD2AP, and GFP-CD2AP-(1-175) costained for nuclei (in red). The arrows point out multinucleated cells. The experiment was reproduced three times, and at least 100 transfected cells were analyzed for each condition. (B) Fold increase in the number of multinucleated cells (□) or cells linked by a midbody (▪), among the transfected cells, compared with the GFP conditions expressed as 1. Results are presented as means ± SEM of three to five different experiments. (C) Rate of multinucleation as a function of GFP proteins expression level. Images of HeLa cells transfected with GFP and GFP fusion proteins were acquired using a cooled digital camera with constant exposure parameters. The level of expression of the GFP proteins was estimated by quantification of pixels in a constant square displaced from cell to cell. This allows us to classify transfected cells in three groups: a group that expresses low level of GFP proteins (<1), a group that expressed high levels of GFP proteins (>10), and an intermediary group. Cells from each group were analyzed for the presence of one or more nuclei. The figure represents the mean of two independent experiments, and results are expressed as the percentage of multinucleated cells among the transfected cells.
CD2AP Knockdown by siRNA Leads to a Late Defect in Cytokinesis
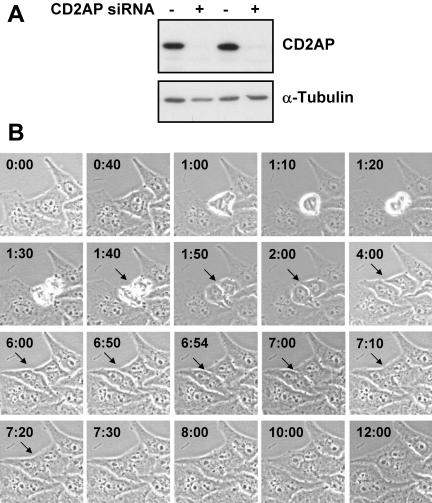
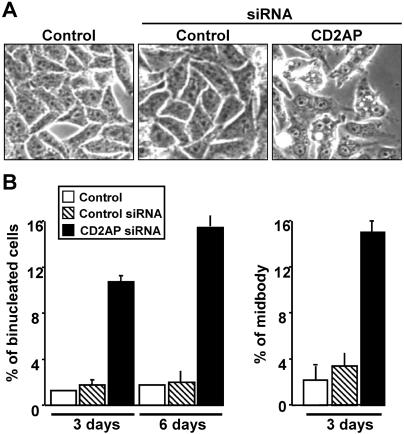
To gain insight into a role of CD2AP in cytokinesis, the expression of the endogenous protein was decreased by siRNA transfection (Figure 4A). Cells were observed 36 h (Figure 4B and Supplemental Movie 2) or 72 h (Supplemental Figure 3 and Movie 3) posttransfection by time-lapse videomicroscopy for 20 h. Although a normal division apparently occurred in a large number of cells, a careful examination showed that in some cases the two daughter cells were not completely separated. The complete process can be followed on the movies (Supplemental Movies 2 and 3), and Figure 4 (and Supplemental Figure 3) shows a selection of frames. As stated above, mitosis seemed to proceed normally (0–5 h), because at 6 h 00, the two daughter cells seemed to be totally separated. However, the images obtained at later time points clearly show that it is not the case. Indeed, a small common cytosolic region was visible at 6 h 54 and rapidly enlarged, leading to appearance of one binucleated cell at 7 h 10. This observation has been confirmed by a quantitative analysis of the siRNA-treated cells (Figure 5). A 3-d treatment of HeLa cells with the CD2AP siRNA led to a significant increase in the percentage of multinucleated cells compared with a nonrelevant siRNA treatment (10.7 ± 0.7 vs. 1.8 ± 0.5) (Figure 5, A and B) and in the percentage of cells at the midbody stage (14.4 ± 1.0 vs. 2.1 ± 0.8) (Figure 5B).
Figure 4.
CD2AP knockdown by siRNA in HeLa cells leads to a late defect in cytokinesis. HeLa cells were transfected with CD2AP siRNA by using Oligofectamine as described in Materials and Methods. (A) Expression of CD2AP was determined by Western blot in cell extracts prepared 60 h after transfection with CD2AP siRNA (+) or Oligofectamine alone (-). We observed no change in the expression of CD2AP when cells were treated with a nonrelevant siRNA, Oligofectamine alone, or remained untreated (our unpublished observation). A similar decrease in CD2AP expression has been observed 36 h after transfection (our unpublished observation). (B) Thirty-six hours after transfection, cells were submitted to time-lapse phase contrast videomicroscopy for 20 h. The figure visualized HeLa cells transfected with siRNA CD2AP at 20 different time points selected from Video 2.
Figure 5.
Quantitative analysis of the effect of CD2AP knockdown by siRNA in HeLa cells. HeLa cells were transfected every 3 d with siRNA (against CD2AP or a nonrelevant siRNA) by using Oligofectamine as described in legend to Figure 4 and in Materials and Methods. The percentage of multinucleated cells and of cells at the midbody stage was determined as described in Figure 3. (A) Representative fields of control cells (treated with Oligofectamine alone) and siRNA-treated cells in phase contrast microscopy. (B) Percentage of binucleated cells or of cells at a midbody stage 3 d (means ± SEM of 3 experiments) or 6 d (means ± the range of 2 determinations) after the siRNA treatment. Two hundred to 350 cells were analyzed in each condition of independent experiments.
Identification of Anillin as a CD2AP Interacting Protein
The structure of CD2AP as a multidomain adaptor protein led us to search for potential interacting proteins, which could participate in its function in cytokinesis. We performed a yeast two-hybrid screening by using a full-length CD2AP as bait. L40 yeast was transformed with a plasmid encoding CD2AP in fusion with the DNA binding domain of LexA, which binds to specific DNA sequences upstream of HIS3 and LacZ reporter genes. The established strain was transformed with a yeast two-hybrid mouse library fused to the VP16 transcriptional activation domain. The screening led to the isolation of three clones that strongly interacted with CD2AP, but not with lamin, and that encoded for the sequence of the first 110 amino acids of the actin binding protein anillin. Interestingly, anillin, initially identified in Drosophila melanogaster (Field and Alberts, 1995), is an evolutionary conserved protein required for cytokinesis (Field and Alberts, 1995; Oegema et al., 2000; Somma et al., 2002; Berlin et al., 2003; Tasto et al., 2003). The cDNAs of anillin and CD2AP were subsequently subcloned into the two-hybrid vectors pLexA and pAct2 to ensure that full-length proteins were able to interact with each other. Figure 6A shows that CD2AP interacted with anillin, independent of the vector used. Lamin was hereby used as a negative control. Using various constructs, we furthermore determined that the first two SH3 domains of CD2AP were crucial for interaction of CD2AP with anillin (Figure 6A).
Figure 6.
CD2AP interacts with anillin and partially colocalizes with anillin in late telophase. (A) The interacting regions of CD2AP and anillin were defined using yeast two-hybrid system. L40 reporter yeast cells were cotransformed with pLex-anillin and the indicated pAct2-CD2AP domain constructs or with pLex-CD2AP and the indicated pAct2-anillin domain constructs. Yeast cells were grown in the absence of leucine and tryptophan, and β-galactosidase activity was determined on replicate filters using X-Gal as substrate. +++, blue coloration occurred within 45 min; -, no coloration was obtained within 8 h. The expression of all constructs was verified by immunodetection (our unpublished data). (B) The first two SH3 domains of CD2AP interact with anillin 1-300 fragment in vitro. GST or GST-CD2AP-(1-175) bound to glutathione-sepharose beads were incubated with [35S]methionine-labeled in vitro-translated anillin 1-300. Bound proteins were eluted, analyzed by SDS-PAGE, and subjected to autoradiography. (C) HeLa cells expressing or not Myc-CD2AP were synchronized in late telophase, lysates were prepared, and Myc-CD2AP was immunoprecipitated using an anti-myc antibody. The presence of anillin and CD2AP was detected by immunoblotting in immunoprecipitates (IP myc). (D) GFP-CD2AP transfected cells (a, e, and i) were colabeled for anillin (b, f, and j) by using an anti-anillin antibody, followed by a Texas Red-coupled anti-rabbit antibody, and for chromosome detection with anti-histone antibody followed by a Cy5-coupled anti-mouse antibody (c, g, and k). The green, blue, and red images were merged using the Adobe Photoshop software (d, h, and l). Each picture represents the projection of eight serial confocal sections. Bars, 10 μm.
To determine which anillin domains are involved in the interaction with CD2AP, several pAct–anillin constructs were tested for their ability to bind CD2AP. We found that the anillin N-terminal fragment (1-155), which contains a proline-rich region, was required to interact with CD2AP. The PX(P/A)XXR consensus site has been recently identified as the target of SH3 domains of CD2AP or CIN85 (for c-Cbl interacting protein of 85 kDa), which is a closely related CD2AP protein (Kurakin et al., 2003). A careful examination of the anillin sequence led us to propose its 26-PTAAPR-31 sequence as a likely candidate for this interaction. Accordingly, substitution of arginine-31 to alanine (anillin-R31A) was sufficient to abolish the interaction between anillin and CD2AP in the yeast two-hybrid system (Figure 6A). Using a pull-down experiment with the GST fusion containing the first two SH3 domains of CD2AP (GST-1-175), we observed an interaction with the radiolabeled in vitro translated anillin (1-300) in accordance with the yeast two-hybrid results (Figure 6B).
We then looked whether CD2AP and anillin interaction could occur in dividing cells. We thus studied the extent of colocalization of CD2AP and anillin during mitosis. HeLa cells transfected with GFP-CD2AP cDNA were labeled for the endogenous anillin (Figure 6D, red) and chromosomes (Figure 6D, blue). During anaphase, anillin was concentrated into the contractile ring in the cell cortex (Figure 6D, b), whereas CD2AP was localized in the vicinity of the midzone (Figure 6D, a). During the late telophase, CD2AP was surrounded by an anillin ring and both proteins were colocalized at the edge of the ring (Figure 6D, e–h). This result suggested that both proteins could interact during the last step of cytokinesis. To comfort this hypothesis, we detected an interaction between the two proteins by using a coimmunoprecipitation experiment with cells synchronized in telophase (Figure 6C). After cytokinesis, the midbody anillin ring disappeared, and some anillin labeling occurred, resembling beads on a string (6D, i–l).
CD2AP and Anillin Are Phosphorylated during Mitosis
Because CD2AP seems to be implicated in cytokinesis, we investigated the expression of the protein throughout the cell cycle with respect to anillin (Figure 7A). HeLa cells were synchronized at G1/S boundary and prometaphase by using hydroxyurea and colcemid treatment, respectively, and released in the cell cycle after an extensive washout (Dulic et al., 1998; Alvarez et al., 2001). After hydroxyurea release, cells were synchronized in G1 (H0), G1/S transition (6 h of release, H6), and S/G2 transition (10h of release, H10) phases, as controlled by flow cytometry analysis (Supplemental Figure 4). They were mainly in metaphase after 1 h of colcemid release (lane C1) and in late telophase after 3–4 h (C3-C4), as confirmed by fluorescence microscopy by using an anti-β-tubulin to detect mitotic spindles and midbodies (our unpublished data). After 6 h of release (C6), cells started to return in G1 phase as controlled by flow cytometry analysis (Supplemental Figure 4). The synchronization process was validated using Plk1 expression, which markedly increased during mitosis, as reported previously (Golsteyn et al., 1994). Although the overall expression level of CD2AP did not change throughout the cycle (Figure 7A), the anillin expression was cell cycle regulated. Anillin was low in G0/G1 phase, its expression increased as cells entered the S phase, and then progressively decreased when cells reentered G1 phase (Figure 7A, lanes C6–C8). This expression profile of anillin is in agreement with the proposed model (Field and Alberts, 1995) that describes a cell cycle-dependent anillin expression and localization. Indeed, anillin expression increases with the age of the nucleus. It is very low after cell division (early G1) and increases when the cell gets closer to mitosis (S/G2).
Figure 7.
CD2AP is phosphorylated in mitosis. (A) HeLa cells were arrested using colcemid (C0-C8) or hydroxyurea (H0-H10) treatment for 16–20h, and then, released into the cell cycle with fresh drug-free medium for the indicated time. Example: H6 = 6 h after hydroxyurea release. Cells extracts (20 μg) were analyzed by western blotting with antibodies to CD2AP and anillin. Control cells were treated for one hour with colcemid. Untreated cells are randomly growing cells. Plk1 was used as a synchronization control and α-tubulin was used as a loading control. (B) HeLa cells were arrested in prometaphase with 0.1 μg/ml colcemid, washed, and incubated in drug-free medium to permit their exit from mitosis. Samples were collected up to 3 h after colcemid removal and lysed in detergent. CD2AP was immunoprecipitated and incubated in the presence or absence of λ phosphatase, as described in Materials and Methods, before immunodetection. (C) Prometaphase arrested HeLa cells were lysed and incubated with λ phosphatase as described in Materials and Methods.
Furthermore, in prometaphase (C0), CD2AP and anillin migrated as different species. These supplementary bands disappeared as cells entered telophase. The electrophoretic shift of both proteins was cell cycle dependent and not a result of microtubule depolymerization by colcemid treatment. Indeed, when cells were treated with colcemid for 1 h, a duration sufficient to observe microtubules modification, no change was observed in the mobility of either protein (Figure 7A, control lane). To determine whether the mobility shift of CD2AP and anillin was due to phosphorylation, CD2AP immunoprecipitates or lysates from mitotic cells were treated with the serine/threonine/tyrosine λ phosphatase. The mobility shift of both proteins was suppressed after λ phosphatase treatment (Figure 7, B and C) leading to the conclusion that CD2AP and anillin were phosphorylated specifically in early mitosis.
We further examined the CD2AP and anillin subcellular distribution along the cell cycle. HeLa cells were synchronized, as described above, and homogenized. Membrane fractions were separated from the cytosol, as described in experimental procedures. In untreated or S phase-synchronized cells (H0), CD2AP was mostly associated with membranes (Figure 8A, lanes H0 and untreated), whereas in prometaphase-synchronized cells (lane C0) CD2AP was enriched in the cytosol fraction. By the end of mitosis, CD2AP progressively reassociated with the membrane fraction (C1, C3, and C6). Moreover, the CD2AP present in the cytosolic fraction of mitotic cells (C0, C1, and C3) migrated with a higher apparent molecular weight, suggesting that phosphorylated form of CD2AP predominantly delocalized to the cytosol. Moreover, anillin was found mostly associated with membranes during the whole cell cycle. Rho GDI and transferrin receptor used as cytosolic and membrane controls, respectively, for the fractionation procedure did not change during the cell cycle (Figure 8A). Tubulin was found in the cytosolic fraction, a result likely due to the fact that the fractionation procedure was performed at 4°C, a temperature known to induce microtubule depolymerization.
Figure 8.
CD2AP and anillin subcellular distribution along the cell cycle. (A) Cells were synchronized at different points of the cell cycle using hydroxyurea (H0) and colcemid (C0–C6) treatment and released into cell cycle. The anillin and CD2AP subcellular distribution was monitored by immunoblotting of membrane (Mb) and cytosolic (Cyt) fractions prepared from postnuclear supernatants. Untreated cells are randomly growing cells. Rho GDI was used as a cytosolic control and transferrin receptor (TfR) as a membrane control for the fractionation procedure. Tubulin was immunodetected in parallel. (B) CD2AP did not associate with microtubules during interphase. Cells were treated or not with colcemid for 1 h, and cytosolic proteins (Sol) were separated from insoluble material (Ins) as described in Materials and Methods. Fifty micrograms of total lysates (T), soluble and insoluble proteins were immunodetected for the presence of CD2AP and tubulin. (C) Cytosolic CD2AP from mitotic synchronized HeLa cells does not associate with microtubules in vitro. In vitro polymerized microtubules (MT) were incubated with 8 μg of purified MAP2, 3 μg of BSA, or 100 μl of cytosol (200 μg) for 30 min at room temperature. As control, the proteins were incubated in the absence of microtubules (-). Microtubules and soluble proteins were then separated by centrifugation through a cushion buffer. The presence of MAP2, tubulin and BSA in the pellet (P) and soluble fractions (S) were detected by Coomassie Blue coloration, whereas the presence of CD2AP was analyzed by immunoblotting.
Because CD2AP was found enriched as the central spindle midzone, we wondered whether CD2AP could associate with microtubule. To this aim, cytosols were separated at 37°C from insoluble material as described previously (Scaife et al., 2003) (Figure 8B). CD2AP was mainly found in the insoluble fraction in control condition, whereas α-tubulin was found both in the insoluble fraction (polymerized microtubules) and in the soluble fraction (unpolymerized tubulin). When cells were treated with colcemid for 1 h (a duration sufficient to induce microtubule depolymerization), CD2AP remained in the insoluble fraction, whereas α-tubulin was shifted to the soluble fraction, suggesting that CD2AP was not bound to microtubules in interphasic cells. Last, we searched for a possible interaction of the cytosolic phosphorylated CD2AP with microtubules. To this end, a microtubule binding assay was performed using the cytosol from mitotic cells as a source of phosphorylated CD2AP (Figure 8C). MAP2, used as a control, bound to microtubules, but CD2AP did not.
DISCUSSION
In this report, we present several clues that CD2AP is implicated in cytokinesis in HeLa cells. The CD2AP localization in the middle of the midzone microtubules and the midbody, the significant increase in the rate of multinucleation in cells overexpressing CD2AP or its first two SH3 domains, the defect in late cytokinesis induced by CD2AP siRNA, the mitotic CD2AP phosphorylation, and its interaction with anillin, a specific cleavage furrow element are all in favor of this implication.
Localization of CD2AP in the Midzone Microtubules Implies a Potential Role in Cytokinesis
We found that CD2AP was recruited to the narrow region of the midzone. Only a restricted number of proteins typically required for cell division are normally found in this region. Among them are some kinesin isoforms, chromosomal passenger molecules (such as the inner centromere molecule INCENP and the telophase disk antigen TD-60), the mitotic kinases of the Plk and Aurora families, and the regulators of the Rho/Rac GTPases ECT2 and MgcRacGAP (Straight and Field, 2000; Glotzer, 2001, 2003). Unlike these molecules, we have never observed CD2AP associated either with centromere, kinetochores, or mitotic spindle in metaphase and early anaphase, indicating that CD2AP likely plays a role late in cytokinesis. The localization of CD2AP in the midzone microtubules implies that it interacts, through its two first SH3 domains, with this specialized microtubular structure. However, CD2AP did not directly bind to microtubules in vitro and was neither colocalized with microtubules in interphasic cells nor with the microtubule spindle in mitotic cells. These observations suggest that CD2AP localization in the vicinity of the midzone microtubules requires specific microtubule binding proteins. Another possibility would be that CD2AP is associated with the membrane that surrounds the central spindle. An attractive idea is that this membrane could correspond to the leading edge of the ingressing cleavage furrow, because CD2AP has been localized in the leading edge of migrating cells (Kirsch et al., 1999; Welsch et al., 2001; Lehtonen et al., 2002).
Mitotic Phosphorylation of CD2AP Implies a Potential Role in Cell Division
The mitotic phosphorylation of CD2AP supports the existence of a role of CD2AP during cell division. Mitosis requires a complex succession of events that are highly regulated by phosphorylation/dephosphorylation (Straight and Field, 2000; Glotzer, 2001, 2003). Furthermore, the mitotic kinases are essential for cytokinesis (Carmena and Earnshaw, 2003; Dai et al., 2003; Ohkura, 2003). At least two roles for mitotic CD2AP phosphorylation can be envisioned. First, it could be important for inhibition of CD2AP function in endocytosis. The phosphorylation of CD2AP is associated with its delocalization from membranes to cytosol, as described for Rab4 (Bailly et al., 1991; van der Sluijs et al., 1992). This would lead to an efficient inactivation of the trafficking pathway controlled by Rab4 and CD2AP during the first step of mitosis. Second, CD2AP phosphorylation could serve to activate a particular mitotic function of the CD2AP protein. For example, the mitotic phosphorylation of the Rho-GEF ECT2 is required for its exchange activity (Tatsumoto et al., 1999). The phosphorylation of MgcRac-GAP by Aurora B converts its RacGAP activity toward a RhoGAP activity (Minoshima et al., 2003). Rab6KIFL, the human homologue of Rabkinesin 6, plays a role in retrograde traffic along the Golgi in interphasic cells. Similarly, its phosphorylation by the kinase Plk1 during mitosis is required for the proper localization of Plk1 in the midzone (Neef et al., 2003).
CD2AP Seems to Play a Role in the Late Phase of Cytokinesis
The phenotype induced by silencing of CD2AP expression is in favor of a role for this protein in a late phase of cytokinesis, because we observed that the scission of the daughter cells was markedly hampered. This scission step successively requires the formation of a stable intercellular bridge between dividing daughter cells, the disassembly of the contractile ring, membrane remodeling events, and midbody abscission (Schweitzer and D'Souza-Schorey, 2004). Because CD2AP seems to be involved in actin and membrane trafficking in interphase cells, it is difficult to pinpoint the exact function of CD2AP in cytokinesis. CD2AP interacts with anillin in the yeast two-hybrid system, and the two proteins are in part colocalized in intact cells. Furthermore, the two proteins were shown to interact in vitro and in the cell. Anillin is a specific furrow component involved with cytokinesis, whereas other cleavage furrow components such as septins, actin, and myosin localize elsewhere and are involved in other processes (Field and Alberts, 1995; Oegema et al., 2000). The fact that PX(P/A)XXR motif required for the interaction of anillin with CD2AP is conserved between mammals, Xenopus laevis, and D. melanogaster strengthens an important role for this interaction. Anillin, like CD2AP, is phosphorylated in mitosis, and dephosphorylation of both follows a similar time course. The exact role of anillin phosphorylation and its potential implication in the binding to CD2AP are unknown. Taking into account their known molecular properties, at least three putative roles for their interaction could be proposed. First, the interaction between CD2AP and anillin could play a role in actin remodeling necessary for cytokinesis. In vitro, anillin binds and bundles filamentous actin and recruits septin proteins to actin bundles (Field and Alberts, 1995; Oegema et al., 2000; Kinoshita et al., 2002), whereas CD2AP is involved in the reorganization of actin in several cellular processes (Dustin et al., 1998; Schafer et al., 2000; Welsch et al., 2001; Lehtonen et al., 2002; Hutchings et al., 2003; Lynch et al., 2003). The interaction between CD2AP and anillin in late telophase could thus bring together all the multiprotein complexes necessary for the control of actin dynamics at the midbody. Second, this interaction could be involved in membrane trafficking events that are required for cell cleavage (O'Halloran, 2000; Finger and White, 2002). Important defects in late telophase are observed both in S2 cells lacking anillin (Somma et al., 2002) and in HeLa cells overexpressing CD2AP and CD2AP(1-175) or lacking CD2AP (this study). Anillin-deficient cells show abnormal membrane organization in the cleavage area that could be due to defective membrane traffic events of intracellular vesicles to the membrane of the cleavage furrow (Somma et al., 2002). Interestingly, syntaxin 2 and endobrevin, the two proteins involved in membrane fusion events that have been implicated in the scission (Low et al., 2003), are associated, like CD2AP, with endosomal compartments in interphasic cells (Wong et al., 1998; Quinones et al., 1999; Antonin et al., 2000). Furthermore, in budding yeast, Sla1p, the orthologue of CD2AP/CIN85, couples actin dynamics to endocytosis (Dewar et al., 2002; Warren et al., 2002), and Sla1p-deficient yeast show defect in cell division, possibly due to a defect in polarized secretion toward the site of budding (Tang et al., 2000; Li et al., 2002). The interaction between anillin and CD2AP could thus be needed for a correct coordination of vesicular membrane traffic and fusion at the level of the midbody, a process required for the scission. Third, the interaction of anillin with CD2AP could help to target anillin to a degradative pathway. Indeed, anillin expression rapidly decreased when cells went through mitosis. This is probably due to its degradation by a ubiquitin-mediated proteolysis (Straight et al., 2003). Because CD2AP as well as CIN85 are involved in protein degradation in interphasic cells, and because they bind ubiquitin ligase (Dikic, 2002), they could bring ubiquitination machinery to anillin allowing for its degradation.
No cytokinetic defect has been reported in the CD2AP knockout (KO) mice, although a phenotype has not been carefully looked at yet (Shih et al., 1999), suggesting that other(s) protein(s) could compensate for the mitotic function of CD2AP. CD2AP belongs to the same family as CIN85 (Dikic, 2002). The two proteins share similar domain organization and binding partners, although CD2AP (but not CIN85) interacts with Rab4 (unpublished data) and actin (Lehtonen et al., 2002). CIN85, which also interacted with anillin in the yeast two-hybrid system (our unpublished data), could replace CD2AP in KO mice. In accordance with this hypothesis, both CIN85 and CD2AP can compensate their roles in mediating endocytosis of receptor tyrosine kinases both in vitro and in vivo (Dikic, personal communication). Moreover mice lacking citron kinase, a protein involved in cytokinesis, can be obtained, and they display a cytokinesis defect only in some specific cells (Di Cunto et al., 2000). A careful analysis of the CD2AP KO mice would be necessary to exclude that such a defect is not occurring in some cell types.
Foot Process and Cleavage Furrow Similarity
CD2AP is a ubiquitously expressed protein, but its expression is up-regulated in specialized cells of the kidney named podocytes. Podocytes are unique cells with a complex cellular organization. They form particular actin-rich structures named foot processes that are needed for the glomerular filtration apparatus (Pavenstadt et al., 2003). CD2AP is involved in the foot process maintenance as revealed by the regression of these specialized structures in the kidney of CD2AP-/- mice (Shih et al., 1999). Interestingly, in podocyte foot processes a complete actin-based contractile apparatus is present (Asanuma and Mundel, 2003). The same protein composition (actin, myosin II, and α-actinin) is found in this podocyte apparatus and in the contractile ring apparatus of dividing cells. Moreover, CHO1/MKLP1 is required to establish the antiparallel microtubule network in podocytes necessary for process formation (Kobayashi et al., 1998) as well as to form antiparallel microtubule bundles needed in the midzone for the advancement and stable closure of the cleavage furrow (Glotzer, 2004). This suggests that the molecular mechanisms involving CD2AP in both cytokinesis and the process formation in podocytes might be, at least in part, similar.
Acknowledgments
We thank Dr. Hollenberg (Fred Hutchison Cancer Research Center, Seattle, WA) for the gift of mouse cDNA yeast two-hybrid library, Dr. K. Kirsch (Boston University, School of Medicine, Boston, MA) for the gift of human FLAG-CD2AP cDNA, Dr. S. Giordano (Institute for Cancer Research and Treatment, Turino, Italy) for the gift of CIN85 cDNA, and Dr. P. McPherson for the gift of the GFP-intersectin-SH3. We thank P. Boquet, E. Lemichez, C. Treins, J. Vukmirica, V. Kaddai, and P. Gual for stimulating discussions and critical reading of manuscript and A. Doye, T. Gonzalez, and L. Boyer for technical assistance. This work was supported by the Institut National de la Santé et de la Recherche Médicale, the University of Nice-Sophia Antipolis, The Association pour la Recherche contre le Cancer (ARC Grants 5634 and 3240), the French Association against the Myopathy (Grant 7989), the Région Provence Alpes Côte d'Azur, and the Conseil Général des Alpes Maritimes. The support of Fondation Bettencourt-Schueller is gratefully acknowledged. P. M. received fellowships successively from La Ligue contre le Cancer, The Association pour la Recherche contre le Cancer, and Fondation Bettencourt-Schueller. N. G. received support from the French Education Ministry.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-09-0773) on March 30, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Alvarez, B., Martinez, A. C., Burgering, B. M., and Carrera, A. C. (2001). Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature 413, 744-747. [DOI] [PubMed] [Google Scholar]
- Antonin, W., Holroyd, C., Tikkanen, R., Honing, S., and Jahn, R. (2000). The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol. Biol. Cell 11, 3289-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma, K., and Mundel, P. (2003). The role of podocytes in glomerular pathobiology. Clin. Exp. Nephrol. 7, 255-259. [DOI] [PubMed] [Google Scholar]
- Bailly, E., McCaffrey, M., Touchot, N., Zahraoui, A., Goud, B., and Bornens, M. (1991). Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature 350, 715-718. [DOI] [PubMed] [Google Scholar]
- Beites, C. L., Xie, H., Bowser, R., and Trimble, W. S. (1999). The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 2, 434-439. [DOI] [PubMed] [Google Scholar]
- Berlin, A., Paoletti, A., and Chang, F. (2003). Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160, 1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi, M. N., Cormont, M., Gautier, N., Van Obberghen, E., and Le Marchand-Brustel, Y. (1996). GTPase activating protein activity for Rab4 is enriched in the plasma membrane of 3T3–L1 adipocytes. Possible involvement in the regulation of Rab4 subcellular localization. Diabetologia 39, 899-906. [DOI] [PubMed] [Google Scholar]
- Brett, T. J., Traub, L. M., and Fremont, D. H. (2002). Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure 10, 797-809. [DOI] [PubMed] [Google Scholar]
- Burgess, R. W., Deitcher, D. L., and Schwarz, T. L. (1997). The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos. J. Cell Biol. 138, 861-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena, M., and Earnshaw, W. C. (2003). The cellular geography of aurora kinases. Nature Rev. Mol. Cell. Biol. 4, 842-854. [DOI] [PubMed] [Google Scholar]
- Cormont, M., Mari, M., Galmiche, A., Hofman, P., and Le Marchand-Brustel, Y. (2001). A FYVE finger-containing protein, Rabip4, is a Rab4 effector involved in early endosomal traffic. Proc. Natl. Acad. Sci. USA 98, 1637-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormont, M., Meton, I., Mari, M., Monzo, P., Keslair, F., Gaskin, C., McGraw, T. E., and Le Marchand-Brustel, Y. (2003). CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic 4, 97-112. [DOI] [PubMed] [Google Scholar]
- Dai, W., Huang, X., and Ruan, Q. (2003). Polo-like kinases in cell cycle checkpoint control. Front. Biosci. 8, d1128-d1133. [DOI] [PubMed] [Google Scholar]
- Dewar, H., Warren, D. T., Gardiner, F. C., Gourlay, C. G., Satish, N., Richardson, M. R., Andrews, P. D., and Ayscough, K. R. (2002). Novel proteins linking the actin cytoskeleton to the endocytic machinery in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 3646-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cunto, F., et al. (2000). Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 28, 115-127. [DOI] [PubMed] [Google Scholar]
- Dikic, I. (2002). CIN85/CMS family of adaptor molecules. FEBS Lett. 529, 110-115. [DOI] [PubMed] [Google Scholar]
- Dulic, V., Stein, G. H., Far, D. F., and Reed, S. I. (1998). Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol. Cell. Biol. 18, 546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin, M. L., et al. (1998). A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94, 667-677. [DOI] [PubMed] [Google Scholar]
- Emoto, K., and Umeda, M. (2000). An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J. Cell Biol. 149, 1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C. M., and Alberts, B. M. (1995). Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J. Cell Biol. 131, 165-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C. M., and Kellogg, D. (1999). Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 9, 387-394. [DOI] [PubMed] [Google Scholar]
- Finger, F. P., and White, J. G. (2002). Fusion and fission: membrane trafficking in animal cytokinesis. Cell 108, 727-730. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. (2001). Animal cell cytokinesis. Annu. Rev. Cell Dev. Biol. 17, 351-386. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. (2003). Cytokinesis: progress on all fronts. Curr. Opin. Cell Biol. 15, 684-690. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. (2004). Cleavage furrow positioning. J. Cell Biol. 164, 347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn, R. M., Schultz, S. J., Bartek, J., Ziemiecki, A., Ried, T., and Nigg, E. A. (1994). Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J. Cell Sci. 107, 1509-1517. [DOI] [PubMed] [Google Scholar]
- Hutchings, N. J., Clarkson, N., Chalkley, R., Barclay, A. N., and Brown, M. H. (2003). Linking the T cell surface protein CD2 to the actin-capping protein CAPZ via CMS and CIN85. J. Biol. Chem. 278, 22396-22403. [DOI] [PubMed] [Google Scholar]
- Kim, J. M., Wu, H., Green, G., Winkler, C. A., Kopp, J. B., Miner, J. H., Unanue, E. R., and Shaw, A. S. (2003). CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300, 1298-1300. [DOI] [PubMed] [Google Scholar]
- Kinoshita, M., Field, C. M., Coughlin, M. L., Straight, A. F., and Mitchison, T. J. (2002). Self- and actin-templated assembly of mammalian septins. Dev. Cell. 3, 791-802. [DOI] [PubMed] [Google Scholar]
- Kirsch, K. H., Georgescu, M. M., Ishimaru, S., and Hanafusa, H. (1999). CMS: an adapter molecule involved in cytoskeletal rearrangements. Proc. Natl. Acad. Sci. USA 96, 6211-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, N., Reiser, J., Kriz, W., Kuriyama, R., and Mundel, P. (1998). Nonuniform microtubular polarity established by CHO1/MKLP1 motor protein is necessary for process formation of podocytes. J. Cell Biol. 143, 1961-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakin, A. V., Wu, S., and Bredesen, D. E. (2003). Atypical recognition consensus of CIN85/SETA/Ruk SH3 domains revealed by target-assisted iterative screening. J. Biol. Chem. 278, 34102-34109. [DOI] [PubMed] [Google Scholar]
- Lee, K. H., et al. (2003). The immunological synapse balances T cell receptor signaling and degradation. Science 302, 1218-1222. [DOI] [PubMed] [Google Scholar]
- Lehtonen, S., Zhao, F., and Lehtonen, E. (2002). CD2-associated protein directly interacts with the actin cytoskeleton. Am. J. Physiol. 283, F734-F743. [DOI] [PubMed] [Google Scholar]
- Li, H., Page, N., and Bussey, H. (2002). Actin patch assembly proteins Las17p and Sla1p restrict cell wall growth to daughter cells and interact with cis-Golgi protein Kre6p. Yeast 19, 1097-1112. [DOI] [PubMed] [Google Scholar]
- Low, S. H., Li, X., Miura, M., Kudo, N., Quinones, B., and Weimbs, T. (2003). Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev. Cell. 4, 753-759. [DOI] [PubMed] [Google Scholar]
- Lynch, D. K., Winata, S. C., Lyons, R. J., Hughes, W. E., Lehrbach, G. M., Wasinger, V., Corthals, G., Cordwell, S., and Daly, R. J. (2003). A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between EGF receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 278, 21805-21813. [DOI] [PubMed] [Google Scholar]
- Minoshima, Y., et al. (2003). Phosphorylation by aurora B converts MgcRac-GAP to a RhoGAP during cytokinesis. Dev. Cell 4, 549-560. [DOI] [PubMed] [Google Scholar]
- Neef, R., Preisinger, C., Sutcliffe, J., Kopajtich, R., Nigg, E. A., Mayer, T. U., and Barr, F. A. (2003). Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswonger, M. L., and O'Halloran, T. J. (1997). A novel role for clathrin in cytokinesis. Proc. Natl. Acad. Sci. USA 94, 8575-8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema, K., Savoian, M. S., Mitchison, T. J., and Field, C. M. (2000). Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J. Cell Biol. 150, 539-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran, T. J. (2000). Membrane traffic and cytokinesis. Traffic 1, 921-926. [PubMed] [Google Scholar]
- Ohkura, H. (2003). Phosphorylation: polo kinase joins an elite club. Curr. Biol. 13, R912-R914. [DOI] [PubMed] [Google Scholar]
- Pavenstadt, H., Kriz, W., and Kretzler, M. (2003). Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253-307. [DOI] [PubMed] [Google Scholar]
- Quinones, B., Riento, K., Olkkonen, V. M., Hardy, S., and Bennett, M. K. (1999). Syntaxin 2 splice variants exhibit differential expression patterns, biochemical properties and subcellular localizations. J. Cell Sci. 112, 4291-4304. [DOI] [PubMed] [Google Scholar]
- Robinson, D. N., and Spudich, J. A. (2000). Towards a molecular understanding of cytokinesis. Trends Cell Biol. 10, 228-237. [DOI] [PubMed] [Google Scholar]
- Scaife, R. M., Job, D., and Langdon, W. Y. (2003). Rapid microtubule-dependent induction of neurite-like extensions in NIH 3T3 fibroblasts by inhibition of ROCK and Cbl. Mol. Biol. Cell 14, 4605-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D. A., D'Souza-Schorey, C., and Cooper, J. A. (2000). Actin assembly at membranes controlled by ARF6. Traffic 1, 892-903. [DOI] [PubMed] [Google Scholar]
- Schweitzer, J. K., and D'Souza-Schorey, C. (2004). Finishing the job: cytoskeletal and membrane events bring cytokinesis to an end. Exp. Cell Res. 295, 1-8. [DOI] [PubMed] [Google Scholar]
- Shih, N. Y., Li, J., Karpitskii, V., Nguyen, A., Dustin, M. L., Kanagawa, O., Miner, J. H., and Shaw, A. S. (1999). Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312-315. [DOI] [PubMed] [Google Scholar]
- Somma, M. P., Fasulo, B., Cenci, G., Cundari, E., and Gatti, M. (2002). Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Mol. Biol. Cell 13, 2448-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight, A. F., Cheung, A., Limouze, J., Chen, I., Westwood, N. J., Sellers, J. R., and Mitchison, T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743-1747. [DOI] [PubMed] [Google Scholar]
- Straight, A. F., and Field, C. M. (2000). Microtubules, membranes and cytokinesis. Curr. Biol. 10, R760-R770. [DOI] [PubMed] [Google Scholar]
- Strickland, L. I., and Burgess, D. R. (2004). Pathways for membrane trafficking during cytokinesis. Trends Cell Biol. 14, 115-118. [DOI] [PubMed] [Google Scholar]
- Tang, H. Y., Xu, J., and Cai, M. (2000). Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell. Biol. 20, 12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasto, J. J., Morrell, J. L., and Gould, K. L. (2003). An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160, 1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumoto, T., Xie, X., Blumenthal, R., Okamoto, I., and Miki, T. (1999). Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J. Cell Biol. 147, 921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs, P., Hull, M., Huber, L. A., Male, P., Goud, B., and Mellman, I. (1992). Reversible phosphorylation–dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 11, 4379-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindelov, L. L., Christensen, I. J., and Nissen, N. I. (1983). Standardization of high-resolution flow cytometric DNA analysis by the simultaneous use of chicken and trout red blood cells as internal reference standards. Cytometry 3, 328-331. [DOI] [PubMed] [Google Scholar]
- Warren, D. T., Andrews, P. D., Gourlay, C. W., and Ayscough, K. R. (2002). Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics. J. Cell Sci. 115, 1703-1715. [DOI] [PubMed] [Google Scholar]
- Welsch, T., Endlich, N., Kriz, W., and Endlich, K. (2001). CD2AP and p130Cas localize to different F-actin structures in podocytes. Am. J. Physiol. 281, F769-F777. [DOI] [PubMed] [Google Scholar]
- Wong, S. H., Zhang, T., Xu, Y., Subramaniam, V. N., Griffiths, G., and Hong, W. (1998). Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol. Biol. Cell 9, 1549-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]