Abstract
Fos protein immunodetection was used to investigate the neuronal activation elicited in some olfactory-related areas after either learning of an olfactory discrimination task or its reactivation 10 d later. Trained rats (T) progressively acquired the association between one odor of a pair and water-reward in a four-arm maze. Two groups of pseudotrained rats were used: PO rats were not water restricted and were submitted to the olfactory stimuli in the maze without any reinforcement, whereas PW rats were water-deprived and systematically received water in the maze without any odorous stimulation. When the discrimination task was well mastered, a significantly lower Fos immunoreactivity was observed in T rats compared to PW and PO rats in most of the analyzed brain areas, which could reflect the post-acquisition consolidation process. Following memory reactivation, differences in Fos immunoreactivity between trained and some pseudotrained rats were found in the anterior part of piriform cortex, CA3, and orbitofrontal cortex. We also observed that Fos labeling was significantly higher in trained rats after memory reactivation than after acquisition of the olfactory task in most of the brain areas examined. Our results support the assumption of a differential involvement of neuronal networks after either learning or reactivation of an olfactory discrimination task.
The delineation of the neuronal circuits involved in learning and memory of a defined task and in its reactivation amidst the various brain areas remains an unsettled question. For a long time, it was assumed that once consolidated, long-term memory of newly learned information was insensitive to disruption and that the neuronal network sustaining this process remained stable over time. However, several studies support the notion that memory can be considered a dynamic process with permanent reorganization related to the experiences of the animal (Przybyslawski and Sara 1997; Nader 2003; Dudai 2004). Thus, recalled memories seem to become labile again and to require subsequent reconsolidation via protein synthesis (Sara 2000; Nader 2003). In addition to the brain network sustaining initial acquisition of memories, a new network could be involved, since the events occurring during retrieval must also be memorized (Squire and Alvarez 1995; Sara 2000; Abel and Lattal 2001). A transitory interaction between hippocampal formation and neocortex is assumed to mediate the establishment of long-term cortical memory representations (Squire and Alvarez 1995), and some memories initially hippocampus-dependent become progressively insensitive to hippocampal lesion over time (Anagnostaras et al. 1999). Bontempi et al. (1999) showed a time-dependent reorganization of the neuronal circuitry underlying long-term storage of a spatial task in mice submitted to memory retrieval. In the rat, a differential involvement of various brain structures such as amygdala, lateral habenula, and neocortical areas was described after either learning or retrieval of a rapidly learned, appetitively motivated odor-discrimination task (Tronel and Sara 2002). It is notable that most of the studies of retrieval reported in the literature are based on rapidly learned tasks and especially on fear conditioning, which induces a high emotional level (for review, see Nader 2003). However, it has been emphasized that the brain areas involved in learning and reactivation could differ according to the task used (Berman and Dudai 2001). The issue of the brain networks involved in reactivation of a slowly learned task remains unresolved.
Using a slowly acquired odor-discrimination task in the rat, we previously observed that some olfactory-related brain areas exhibited a different pattern of Fos expression according to the learning stage (at the initial and intermediate learning stages or when the task was just acquired) (Roullet et al. 2005). The aim of the present study was to determine whether the same neuronal networks are involved after either learning or reactivation of such a task. The discrimination task was based on the association in a four-arm maze of one odor of a pair with water-reward, the other odor of the pair being nonrewarded but never punished (Saar et al. 1998). On average, rats needed 1 wk to master this task, although their olfactory system is a powerful model for studying memory (Slotnick 2001). Olfactory learning leads to stable memory formation that can be recalled over a long period of time (Staubli et al. 1987). In the present experiment, the neuronal networks involved after either learning or reactivation of an olfactory discrimination task were investigated using Fos immunohistochemistry. This method has been shown to be a useful tool for determining the rat brain areas involved in learning (Kaczmarek 2000; Datiche et al. 2001; Roullet et al. 2004, 2005) and memory retrieval (Tronel and Sara 2002). As a marker of brain activity and of plasticity, Fos expression allows the mapping of neuronal networks. The immediate-early gene c-fos is thought to control long-term changes in cellular functioning linked to memory processes (Herrera and Robertson 1996; Kaczmarek 2000). Here, rats were sacrificed either after learning when the olfactory discrimination task was well mastered or after a retention delay of 10 d. On the 11th day, the rats were submitted to a session in the maze (in the presence of both unconditioned and conditioned stimuli), in order to reactivate the memory of the discrimination task previously learned.
The analysis reported here focused on various central olfactory-related brain areas which might play a role in olfactory memory formation and/or retrieval. The piriform cortex (PCx), the main target of olfactory bulb projections, is assumed to be involved in learning and memory (Haberly 1985; Haberly and Bower 1989; Hasselmo 1995). In this area, long-term potentiation was described both in vitro (Jung et al. 1990) and in vivo during learning of an olfactory discrimination task (Roman et al. 1993). Changes in electrical piriform cell properties after an olfactory discrimination task (Saar et al. 1998) and in electrical responses of piriform cortex after an olfacto-mimetic learning task (Litaudon et al. 1997; Mouly and Gervais 2002) were described. Moreover, a modification in Fos expression was noted in the PCx according to the rate at which the rats learned the olfactory discrimination task (Datiche et al. 2001). Piriform outputs are relayed to the hippocampal area via the entorhinal cortex (Haberly and Price 1978). Several experiments demonstrated the role of hippocampus in olfactory learning (Hess et al. 1995a,b). Its lesion impaired olfactory discrimination learning and acquisition of an olfactory learning set in rats (Eichenbaum et al. 1989, 1996). Some hippocampal subfields seem to be involved in early memory processes, and a long-term potentiation (LTP)-like phenomenon was recorded in the dentate gyrus (DG) immediately after the first learning session of an olfactory discrimination task (Truchet et al. 2002). Moreover, by using an olfactory discrimination task, we observed that Fos expression was transiently increased in CA1 on the first day of conditioning (Roullet et al. 2005). Beside its involvement in olfactory information processing, the role of the hippocampus in consolidation and reactivation of memory has been studied extensively (Eichenbaum 2001; Debiec et al. 2002), and this area could have a time-limited role in memory storage (Bontempi et al. 1999). Lastly, a differential spatiotemporal pattern of c-fos mRNA expression was described in hippocampal subfields after either acquisition or recall of an appetitive conditioning task in mice (Bertaina and Destrade 1995).
The PCx is also interconnected to the medial and orbital frontal cortices (Datiche and Cattarelli 1996). Neocortical areas have been assumed to be crucial in long-term storage of remote memories (Squire and Alvarez 1995; Maviel et al. 2004). The orbitofrontal cortex seems critical in stimulus-reinforcer association in order to guide goal-directed behavior (Gallagher et al. 1999; Schoenbaum et al. 2003; Roullet et al. 2004). It seems to be specifically involved when an olfactory learning task is acquired by rats (Roullet et al. 2005). In monkeys, neuronal activity in the orbitofrontal cortex corresponds to the value of the expected reward (Roesch and Olson 2004). In rats, the orbitofrontal cortex neurons exhibit odor-specific firing patterns before odor stimulation when the animal arrives at the odor-associated location, and these neurons might sustain long-term memory of crossmodal associations between odors and locations (Lipton et al. 1999). The infralimbic region could be involved in the integration of rewarded cue in motivated animals performing a memory task (Pratt and Mizumori 2001). Lastly, we also analyzed the Fos expression in the lateral habenula, since this area has been shown to be involved in the retrieval of an olfactory discrimination task (Tronel and Sara 2002).
In the present experiment, the activation of these brain regions was examined when the positively reinforced and slowly acquired olfactory discrimination task was well mastered by the rats and after memory reactivation 10 d later.
Results
Behavioral data
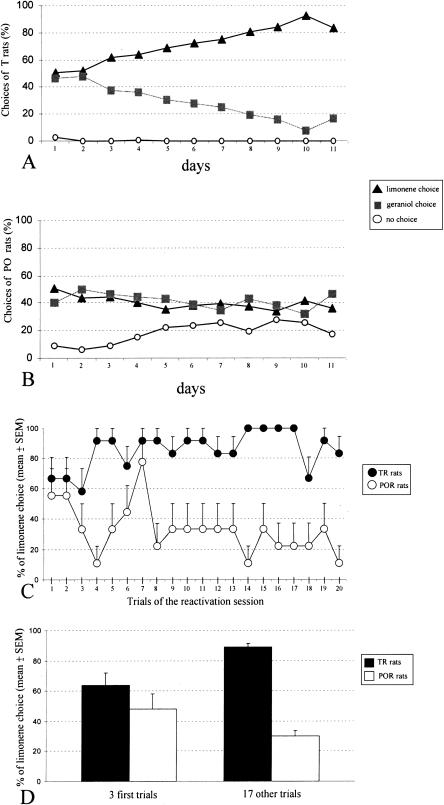
The group of rats labeled “PW” were water-deprived and systematically received water in the maze without any odorous stimulation. When placed in the maze, they could either enter in any arm to obtain water-reward or stay in the starting chamber. Since they were thirst-motivated, these rats did not often stay in the starting chamber. Trained rats (“T” group) progressively acquired the association between one odor of a pair and water-reward in the maze. “PO” rats were not water restricted and were submitted to the olfactory stimuli in the maze without any reinforcement. The T and PO rats had three possibilities on each trial in the maze: to enter the limonene-odorized arm (“limonene choice”), to enter in the geraniol-odorized arm (“geraniol choice”), or to stay in the starting chamber (“no choice”). The X2 test which took into account these three behavioral possibilities showed that the choices of the T and PO rats were different whatever the day of conditioning. Figure 1A,B illustrates the evolution of the mean number of limonene choices for T and PO rats through the daily sessions in the maze. On the first training session, the score of the T rats was 50% of “limonene choice,” and the corresponding score of the PO rats was 51%. Over the training sessions, the T rats' scores increased progressively to reach the learning criterion of 80% “limonene choice.” On average, the T rats reached the learning criterion of 80% correct choices in 8.32 (±1.99) d. One rat mastered the olfactory discrimination task in 5 d, two rats in 6 d, 7 rats in 7 d, four rats in 8 d, one rat in 9 d, two rats in 10 d, four rats in 11 d, and one rat in 12 d. In contrast, the percentage of limonene choices in the PO rats remained ∼40% over the daily sessions in the maze. As illustrated in Figure 1B, the PO rats, which were not thirst-motivated, progressively increased their time spent in the starting chamber.
Figure 1.
Behavioral data. (A) The evolution of the T rats' behavior throughout the daily sessions in the maze. On each trial, these rats could choose the geraniol-odorized arm (“geraniol choice”), the limoneneodorized arm (“limonene choice”), or to stay in the starting chamber (“no choice”) of the maze. The choices are expressed as percentages of the 20-trial daily sessions. Note that the trained rats progressively improved their performance and reached the learning criterion of 80% correct choices. (B) The evolution of PO rats' behavior throughout the daily sessions in the maze. For these rats, which were not water-deprived, the number of “no choice” responses progressively increased through the daily sessions in the maze. (C) The evolution of the percentage of “limonene choices” through the 20 trials of the reactivation session. (D) The percentage of “limonene choices” in TR and POR rats in the three first trials and the last 17 trials of the 20-trial reactivation session in the maze.
Rats in the “TR” group were conditioned to learn the olfactory discrimination task and returned to their home cages for a resting period of 10 d. “POR” rats were olfactory-stimulated without getting water, and then returned to their home cages for a 10-d resting period. After the 10-d resting period, the limonene-choice scores for the entire reactivation session of 20 trials were 85% and 33% for the TR and POR rats, respectively. The POR rats' percentage of “no choice” was 21% for the entire reactivation session.
Figure 1C illustrates the evolution of the percentage of limonene choices over the 20 trials of the reactivation session. The X2 test indicated that the scores of TR and POR rats were not significantly different on trials 1, 2, 3, 6, and 7. Of interest was the observation of the choices at the beginning of the session. Thus, the mean percentage of correct choices on the first three trials was 64% in the TR rats. After the third trial, the percentage of correct choices increased. On the 17 other trials of the session, it was 89% (Fig. 1D). Conversely, in the POR rats, the correct/limonene-choice score was 48% on the first three trials of the session and 30% on the 17 other trials (Fig. 1D).
Fos expression after learning and after reactivation
Piriform cortex
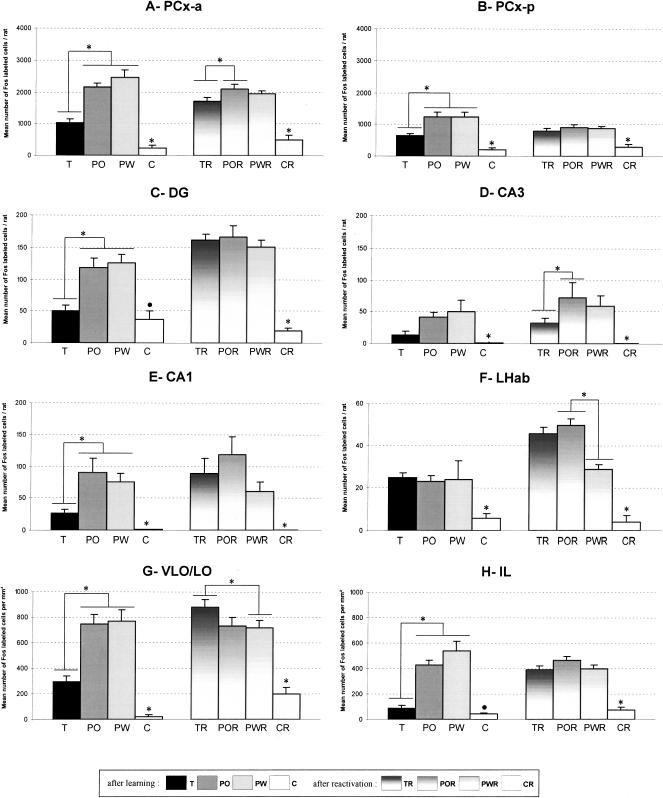
The ANOVA indicated an effect of the experimental group (F[7,53] = 24.54; P < 0.0001), an effect of the PCx subdivision (F[1,53] = 280.60; P < 0.0001), and a significant interaction (F[7,53] = 12.04; P < 0.0001). Thus, except for the home-cage control rats, all experimental groups showed a higher Fos immunoreactivity in the anterior PCx (PCx-a) compared to the posterior PCx (PCx-p) (Fig. 2A,B), after either learning or reactivation.
Figure 2.
The number of Fos-immunoreactive cells (mean ± SEM) in the brain areas analyzed in all the experimental groups after either initial conditioning in the maze or mnesic reactivation. Asterisks (*) indicate a significant difference (P < 0.05). Note that, except in LHab, Fos expression was higher in the PO and PW compared to the T rats. (A,B) Fos expression in the PCx-a and PCx-p. In all experimental groups except home-cage controls, the number of labeled cells in the PCx-a was significantly higher than in the PCx-p. In the PCx-a, the number of immunoreactive cells was significantly decreased in the TR rats compared to POR rats. (C-E) Fos immunoreactivity in the hippocampus subfields DG, CA3, and CA1, respectively. In the DG, as indicated by the circle (•), the C rats were significantly different from the PO and PW rats but not from the T rats. In the DG and CA1, no difference was found among the TR, POR, and PWR rats. In contrast, the number of immunoreactive cells in CA3 was significantly decreased in the TR rats compared to the POR rats. (F) Fos expression in the LHab. (G,H) Fos immunoreactivity in the frontal regions VLO/LO and IL, respectively. In the VLO/LO, Fos immunoreactivity was significantly increased in the TR rats compared to POR, PWR, and T rats. In the IL, as indicated by the circle (•), the C rats exhibited a significantly lower number of Fos-positive cells compared to the PO and PW rats but were not different from the T rats.
In the PCx-a, there was a significant effect of the experimental group (F[3,53] = 51.52; P < 0.0001), no significant effect of the mnesic stage, and a significant interaction (F[3,53] = 7.16; P < 0.001). Pairwise comparisons indicated that the home-cage rats (“C” group) exhibited a significantly lower Fos expression compared to the T, PO, and PW rats. After a resting period of 10 d, Fos immunoreactivity was also significantly lower in home-cage rats (“CR” group) compared to the TR, POR, and PWR rats. Fos immunoreactivity in the T rats was significantly lower compared to both the PO and PW rats (Fig. 2A). No difference was found between the PO and PW rats.
When memory reactivation occurred, pairwise comparisons showed that Fos expression in the TR group was significantly decreased compared to the POR rats (Figs. 2A,D, 3F). No significant difference was observed between the POR and PWR rats.
Figure 3.
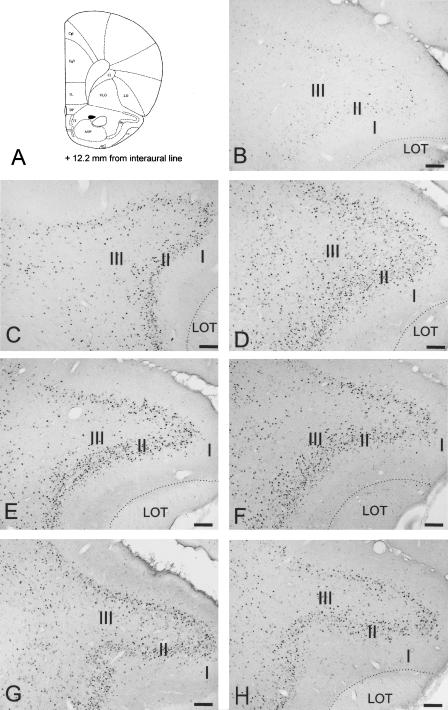
(A) Schematic drawing (Paxinos and Watson 1986) of a coronal section taken at +12.2 mm from the interaural line, illustrating the location of the PCx-a. (B-H) Photomicrographs illustrating the Fos immunoreactivity in the three layers (I, II, III) of the PCx-a in a C rat, a T rat, a TR rat, a PO rat, a POR rat, a PW rat, and a PWR rat, respectively. LOT: lateral olfactory tract. Scale bar, 100 μm.
Comparison of Fos patterns after learning and after reactivation showed a significantly higher expression in the TR and PW rats compared to the T and PWR rats, respectively (Fig. 2A, Table 1).
Table 1.
Statistical comparison of Fos expression after learning and after memory reactivation
| Brain regions
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | PCx-a | PCx-p | DG | CA3 | CA1 | LHab | VLO/LO | IL |
| T vs. TR | * | NS | * | NS | * | * | * | * |
| PO vs. POR | NS | * | * | NS | NS | * | NS | NS |
| PW vs. PWR | * | * | NS | NS | NS | NS | NS | * |
| C vs. CR | NS | NS | NS | NS | NS | NS | NS | NS |
For each brain region analyzed, the number of Fos immunoreactive cells in T, PO, PW, and C rats was compared with the Fos immunoreactivity in TR, POR, PWR, and CR rats, respectively. *: significantly different at P < 0.05; NS: no significant difference.
In the PCx-p, we observed a significant effect of the experimental group (F[3,53] = 23.70; P < 0.0001), no effect of the mnesic stage, and a significant interaction (F[3,53] = 4.40; P < 0.01). Pairwise comparisons indicated that the C and CR rats exhibited a significantly lower Fos expression compared to the T, PO, PW rats and the TR, POR, and PWR rats, respectively. In the T rats, Fos expression was significantly lower compared to both the PO and PW rats (Fig. 2B). After memory reactivation, no significant difference was noted among the TR, POR, and PWR rats. Fos immunoreactivity was significantly lower in the PO and PW rats compared to the POR and PWR rats, respectively. No difference was found between the T and TR rats (Table 1).
Hippocampus
In the DG, there was a significant effect of the experimental group (F[3,41] = 32.22; P < 0.0001), a significant effect of the mnesic stage (F[1,41] = 24.18; P < 0.0001), and a significant interaction (F[3,41] = 11.77; P < 0.0001). Pairwise comparisons indicated that the C rats had significantly less labeling than the PO and PW rats. Furthermore, Fos immunoreactivity in the T rats was significantly lower compared to both the PO and PW groups (Fig. 2C).
Pairwise comparisons indicated that the CR rats were significantly less immunoreactive than the TR, POR and PWR rats. No difference was found among the TR, PWR and POR groups.
Fos expression was significantly increased in the TR and POR rats compared to the T and PO rats, respectively (Table 1).
In the CA3 subdivision of the hippocampus, the ANOVA showed only a significant effect of the experimental group (F[3,41] = 6.65; P < 0.001). Pairwise comparisons indicated that the C and CR rats showed significantly lower Fos expression than the T, PO, and PW rats and the TR, POR, and PWR rats, respectively. Fos expression was significantly higher in the POR group compared to the TR group (Fig. 2D). No other significant difference was found between trained or pseudotrained groups.
In the CA1 subdivision, there was only a significant effect of the experimental group (F[3,41] = 6.55; P < 0.01). Pairwise comparisons indicated that the C and CR rats showed a significantly lower Fos expression than the T, PO, and PW rats and the TR, POR, and PWR rats, respectively. A significantly lower Fos immunoreactivity was further observed in the T rats compared to both the PO and PW rats (Fig. 2E). In contrast, no difference was found among the TR, PWR, and POR groups.
Comparisons of Fos patterns after learning and after reactivation showed a significantly higher immunoreactivity in TR compared to T rats (Fig. 4, Table 1).
Figure 4.
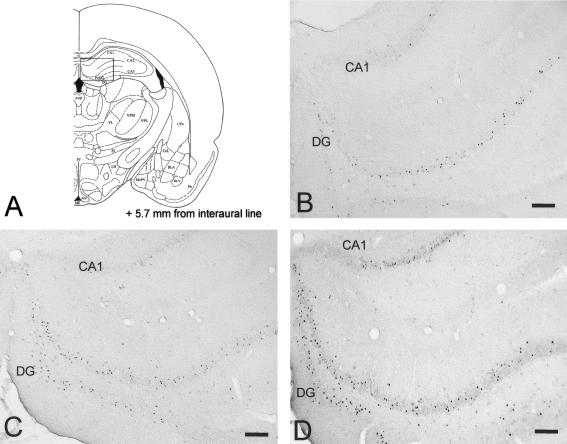
(A) Schematic drawing (Paxinos and Watson 1986) of a coronal section taken at +5.7 mm from the interaural line. The rectangle indicates the location of the hippocampal region illustrated by the photomicrographs. (B-D) Fos immunoreactivity in the hippocampus in a T rat, a TR rat, and a PO rat, respectively. Scale bar, 100 μm.
Lateral habenula
In the lateral habenula (LHab), a significant effect of the experimental group (F[3,41] = 24.91; P < 0.0001), a significant effect of the mnesic stage (F[1,41] = 22.19; P < 0.0001), and a significant interaction (F[3,41] = 6.06; P < 0.01) were found. As illustrated in the Figure 2F, both the C and CR rats exhibited significantly lower Fos expression compared to the T, PO, and PW rats and the TR, POR, and PWR rats, respectively. No difference was found among the T, PO, and PW groups. After memory reactivation, pairwise comparisons indicated only a higher Fos expression in the POR rats compared to the PWR rats.
In the lateral habenula, the number of Fos-labeled cells was significantly increased in the TR and POR rats compared to the T and PO rats, respectively (Table 1).
Ventrolateral/lateral orbitofrontal cortex
In the ventrolateral/lateral orbitofrontal cortex (VLO/LO), we observed a significant effect of the experimental group (F[3,50] = 28.07; P < 0.0001), a significant effect of the mnesic stage (F[1,50] = 12.36; P < 0.001), and a significant interaction (F[3,50] = 12.24; P < 0.0001). Pairwise comparisons indicated that the C and CR rats exhibited significantly lower Fos expression compared to the T, PO, and PW rats and the TR, POR, and PWR rats, respectively.
Pairwise comparisons further revealed that Fos immunoreactivity in T rats was significantly lower compared to both PO and PW rats (Fig. 5). No difference was found between the PO and PW rats. As illustrated in Figure 2G, Fos expression was significantly increased in the TR rats compared to the PWR rats. The number of labeled cells was also higher in the TR rats compared to the POR group, but the level of significance was not reached. No difference was found between the POR and PWR rats.
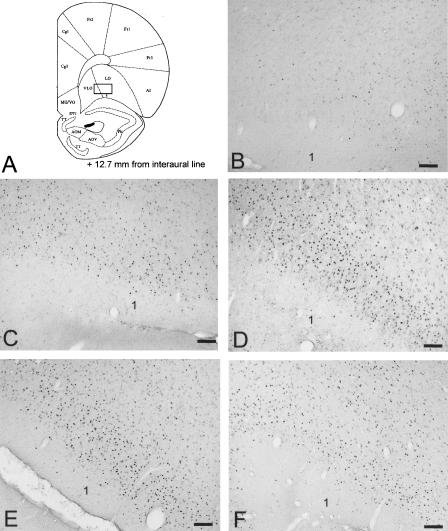
Figure 5.
(A) Schematic (Paxinos and Watson 1986) of a coronal section taken +12.7 mm from the interaural line. The rectangle illustrates the location of the sample region in which labeled cells were counted in the VLO/LO. (B-F) Photomicrographs illustrating Fos labeling in the VLO/LO in C, T, PO, TR, and PWR rats, respectively. Scale bar, 100 μm; 1, neocortical layer 1.
Comparisons of Fos patterns after learning and after reactivation showed that, in contrast to the pseudotrained rats, the immunoreactivity was significantly higher in the TR rats compared to the T rats (Table 1).
Infralimbic cortex
In the infralimbic cortex (IL), we observed a significant effect of the experimental group (F[3,48] = 50.63; P < 0.0001), a significant effect of the mnesic stage (F[1,48] = 5.22; P < 0.05), and a significant interaction (F[3,48] = 17.31; P < 0.0001).
Pairwise comparisons showed that Fos immunoreactivity was significantly lower in the C rats compared to the PO and PW rats. In the T rats, the number of Fos-labeled cells was significantly lower compared to both the PO and PW rats (Fig. 2H). Pairwise comparisons showed that Fos immunoreactivity was significantly lower in the CR rats compared to the TR, POR, and PWR rats. However, no difference was observed among the TR, POR, and PWR rats.
Pairwise comparisons further indicated that Fos expression was significantly lower in the T rats compared to the TR rats (Table 1). We also observed that Fos immunoreactivity was significantly higher in the PW rats than in the PWR rats (Fig. 2H).
Discussion
We used Fos immunocytochemistry to examine neuronal activation in some olfactory-related areas after learning of and reactivation of an olfactory discrimination task. In contrast to most of the studies carried out on memory retrieval and which relied on behavioral procedures with rapid acquisition such as fear conditioning (Nader 2003), we used a positively reinforced olfactory task that required several daily training sessions to be acquired and then well mastered by the rats.
In all the brain areas analyzed (PCx-a, PCx-p, DG, CA1, IL, VLO/LO) except in the lateral habenula and CA3, we observed that the Fos expression in T rats which mastered the olfactory discrimination task was significantly lower compared to PO and PW rats.
In some previous studies (Datiche et al. 2001; Roullet et al. 2004), we did not observe any significant changes in Fos expression between trained and pseudotrained rats. Some methodological discrepancies could explain this difference with the present findings. First, it should be noted that in Roullet et al. (2004), trained rats were sacrificed after presentation of the relevant olfactory cue in an environment different from the maze. Second, we used pseudotrained rats that were thirst-motivated and received water-reward randomly for either one or the other odor of the pair (Datiche et al. 2001; Roullet et al. 2004).
In the present paradigm, the T rats had to maintain a performance level of 80% of correct choices for 2 d to ensure that the task was well mastered. They showed a lower number of Fos-labeled cells compared to the P rats in most of the brain regions examined. It cannot be ruled out that the lower Fos expression observed in the T rats could result from a familiarization phenomenon (Montag-Sallaz and Buonviso 2002). However, since PO and PW rats were placed daily in the maze, we should also expect a decreased Fos expression in these experimental groups. Therefore, a more likely explanation can be put forward to explain the present data. The pseudotrained rats did not have to discriminate odors and did not make any decision based on detection of relevant odor cues. In these rats, Fos expression could reflect the attentional level linked to sensory stimulation, sniffing activity, and/or water-reward. Conversely, it can be assumed that since the T rats performed the task with proficiency, they should have tuned attention toward odor discrimination in order to choose the rewarded cue. In these rats, mastery of the task could result in activation of a more focused network of cells. Moreover, because the T rats, once they had reached the score of 80% correct choices, were submitted to two additional daily sessions in the maze, it can be hypothesized that the lower Fos immunoreactivity might reflect that the consolidation process either ends or is in some way terminated. Such an assumption seems in accordance with the data from a previous experiment (Roullet et al. 2005) investigating the brain Fos expression according to the learning stage. Except in the hippocampus, the Fos labeling was not significantly different between T and P rats at the initial (first day of conditioning) and at the intermediate (fourth day of conditioning) stages of learning. Moreover, when the T rats were sacrificed just after reaching the criterion of 80% correct choices, the Fos expression was not significantly different compared to P rats in most of the brain areas analyzed. However, in the VLO only, the T rats, which had just acquired the olfactory discrimination task, showed higher Fos labeling compared to the P rats (Roullet et al. 2005). Thus, it seems that the decreased Fos immunoreactivity we observed in the present study in most of the brain areas of the T rats could be linked to the two additional daily sessions in the maze they underwent after having reached the 80% correct score and to the subsequent ending of the consolidation process.
On the other hand, it is notable that the Fos expression in the T rats compared to the home-cage controls was not different in some areas, such as the DG and IL. The Fos immunoreactivity in the T rats might not reflect the plasticity underlying acquisition of the task but rather a return to a basal level of activity after learning consolidation. The present finding is in agreement with experiments showing that the repetition of an already learned task by chicks (Anokhin and Rose 1991) and a well mastered appetitive task in mice (Bertaina and Destrade 1995) induced a decrease in Fos expression.
In the present study, Fos expression elicited by memory reactivation 10 d after learning of the olfactory discrimination task was investigated. After memory reactivation, we observed significant differences between trained and some pseudotrained rats in only three of the brain areas analyzed: PCx-a, CA3, and VLO/LO. Thus, Fos immunoreactivity in PCx-a and CA3 was significantly lower in the TR rats compared to the POR rats. In contrast, Fos expression in the VLO/LO was significantly increased in the TR rats compared to the PWR rats.
Memory reactivation in the TR rats was assessed by a session that consisted of 20 reinforced trials. Retrieval seems to be more efficient when attempted in the presence of cues that were present during the acquisition (Dudai 2002). Since the T rats were sacrificed after a 20th reinforced trial session, we submitted the TR rats to a similar 20 reinforced trial sessions. Such an experimental design was used to ensure that the test session could reactivate all the neuronal networks sustaining memory of the previously learned task. In an experiment carried out on memory retrieval of a positively reinforced olfactory discrimination, Tronel and Sara (2002) did not find any marked Fos expression. Those authors assumed that it might be due to the reactivation which was not of sufficient amplitude or duration, since the rats received only a single reinforced retrieval trial. On the other hand, in a c-fos mapping study, Bertaina and Destrade (1995) assessed memory retrieval of an appetitive conditioning by submitting mice to five reinforced trials, and they observed modifications in Fos expression. In conditioning procedures based on getting a reward, it seems that the retrieval session should consist of several trials. Moreover, the reactivation trials must be reinforced in order to avoid an extinction phenomenon. This is in marked contrast with studies using negative reinforcement such as fear conditioning in which only a few trials are required for learning and retrieval is assessed by a single presentation of the sole conditioned stimulus (Nader 2003).
From a behavioral point of view, memory of the olfactory discrimination task was well retrieved by the TR rats in the present study, since they reached a mean score of 85% correct choices on the whole reactivation session of 20 trials. This is in accordance with data (Staubli et al. 1987) showing that rats can recall olfactory memories over a long period of time. Nevertheless, it should be noted that on the three first trials of the reactivation session in our study, the mean score of the TR rats was only 64%. Since we used reinforced trials, a possible relearning phenomenon cannot be ruled out. However, if the rats had forgotten which odor of the pair was associated with water-reward, it can be expected that their performance would be lower than 80% for the whole session. Therefore, in our experiment the process of relearning the odor value, if occurring, should remain minor. Nevertheless, since the present olfactory discrimination task within the maze is complex, a relearning of the task procedure by itself cannot be ruled out.
To some extent, memory reactivation might also occur in POR and PWR rats after 10 d. The POR rats became familiar with the odors and could memorize them. The PWR rats could learn the systematic presence of water-reward in the maze, and they could also memorize contextual signals. However, in contrast to the T rats, neither the PO nor the PW rats learned to discriminate between two odors of a pair in order to obtain water-reward. Even if memory reactivation could occur in all groups of rats, it remains that the nature of the information retrieved was not similar.
We also found that in most of the brain areas analyzed, except PCx-p and CA3, the Fos expression in the T rats was significantly lower compared to the TR rats. This could reflect an increased arousal level in the TR rats. After a resting period of 10 d, it can be assumed that these rats needed a high attention level to remember what was the correct odor to get the water-reward. Moreover, the reactivation session might result not only in retrieving the previously acquired memories but also in establishing new ones regarding events occurring during the session itself (Sara 2000). Such a phenomenon might also lead to increased arousal.
In both subdivisions of the PCx, we showed that Fos expression was significantly lower in the T rats compared to the PO and PW rats. It indicates a role of both the PCx-a and PCx-p in the olfactory task learning. No difference was found between the PO and PW rats in both the PCx-a and PCx-p. The PW rats showed a substantial Fos expression in the PCx, but they were not olfactory-stimulated. Such a finding is in accord with a previous study (Datiche et al. 2001) that described a noticeable Fos immunoreactivity linked to the spontaneous exploratory sniffing activity without any specific odor cue.
After memory reactivation, the TR rats showed significantly lower Fos labeling in the PCx-a compared to the POR group. In contrast, no difference in Fos expression in the PCx-p was found among the TR, POR, and PWR rats. This might indicate a differential involvement of the PCx-a and the PCx-p when an olfactory memory is reactivated. Our data support the hypothesis of a functional heterogeneity of the PCx subdivisions (Litaudon et al. 1997; Mouly and Gervais 2002). In view of its anatomic and functional features, the PCx is considered a model of associative memory (Haberly and Bower 1989). Several studies suggest its involvement in olfactory memory. Long-term potentiation has been recorded in the in vivo PCx following olfactory learning (Roman et al. 1993). Olfactory learning was also shown to elicit electrophysiological modifications such as a decreased after-hyperpolarization in PCx slices (Saar et al. 1998) and increased evoked-field potential amplitudes in the rat PCx-p (Mouly et al. 2001). From an anatomical point of view, the PCx is characterized by a dense network of intrinsic association fibers (Haberly and Price 1978). The organization of these association fibers is different in the PCx-a and the PCx-p. The association axons are caudally directed in the PCx-p, whereas long association fibers are both rostrally and caudally directed in the PCx-a (Haberly and Price 1978; Datiche et al. 2001). Such an organization could sustain recurrent activity and subsequent autoassociative processes within the PCx-a (Haberly 2001). Moreover, the PCx-a receives dense afferents from the olfactory bulb. Hasselmo (1995) hypothesized that neuromodulatory inputs might differentially influence the synapses from afferent bulbar fibers relative to the synapses of intrinsic association fibers. Thus, the relative activation of the network of intrinsic fibers versus external olfactory afferents might be involved in memory recall. It can be also underlined that the PCx-a is involved in odor detection and information transmission to other brain areas, whereas the PCx-p could be involved in odor recognition (Litaudon and Cattarelli 1996). Taken together, our study agrees with and further extends the data showing a role of the PCx in olfactory memory.
In the hippocampal DG, the number of Fos-labeled cells was not significantly different in the present study's T rats after learning compared to the home-cage controls. Moreover, no difference in Fos expression after memory reactivation was found among the TR, POR, and PWR rats. In the CA1 hippocampal subfield, Fos expression was significantly decreased in the T rats after learning compared to both the PO and PW rats. In contrast, no difference was found between the T rats and either PO or PW rats in the CA3 subfield. After memory reactivation, no difference was observed between the TR rats and either POR or PWR rats in CA1, whereas Fos immunoreactivity in CA3 was significantly decreased in the TR rats compared to the POR rats. In accordance with Hess et al. (1995a,b) who showed that CA1 and CA3 become differentially active according to behavioral circumstances, the present data support the hypothesis of a functional heterogeneity of the hippocampal subfields. The CA1 seemed involved when the task was mastered, whereas CA3 might play a role in memory reactivation. In contrast, Tronel and Sara (2002) found no significant change in Fos expression in the hippocampus after either acquisition or retrieval of a positively reinforced olfactory discrimination task. Such a discrepancy could be due to differences between the olfactory tasks (rapidly acquired vs. slowly acquired task) as well as between the retention delays (24 h versus 10 d) used in the two studies. It is notable that some authors (Kaut and Bunsey 2001) demonstrated an absence of retrograde amnesia for recently acquired olfactory discrimination after a hippocampal lesion. The role of the hippocampus in memory consolidation may depend not only on the learning task but also on the temporal aspects of mnesic processes. Thus, a differential involvement of the hippocampus might be expected after reactivation of either recent or older memories.
Our data support a role of the CA1 in learning rather than in reactivation of the olfactory task. In a previous experiment (Roullet et al. 2005) investigating brain activation with respect to learning stage, we observed higher Fos expression in the CA1 of trained rats compared to pseudotrained rats on the first day of conditioning. In contrast, when the task was just acquired, no difference between trained and pseudotrained rats was found. However, in that previous study, the rats had only to reach a criterion of 80% correct choices or to choose the water-rewarded odor in at least 80% of the last 10 trials of the conditioning session. Conversely, in the present behavioral procedure, the rats had not only to reach a score of 80% but also to maintain it for two additional days. It can be assumed that the consolidation process either ends or is in some way terminated, and this might account for the significant decrease in Fos immunoreactivity observed in the T rats.
The present data also indicate an involvement of the CA3 after reactivation. As CA3 pyramidal cells have numerous recurrent collaterals, this area is considered in some computational models as an auto-associative network for storage and retrieval of information in memory processes (Treves and Rolls 1992). Bertaina and Destrade (1995), using in situ hybridization of c-fos mRNA and an appetitive conditioning paradigm, further reported an involvement of the CA3 after memory retrieval.
In the LHab, no difference was found between trained and pseudotrained rats after either learning or retrieval in the present study. Although the LHab seems to be involved in olfaction, ingestion, and endocrine functions, its role in mnesic processes is not yet clearly defined. Vale-Martinez et al. (1997) showed no effect of an electrolytic lesion of the LHab on two-way avoidance conditioning in rats. In contrast, Tronel and Sara (2002) reported activation of the LHab after memory retrieval of a rapidly acquired olfactory task. Differences in behavioral procedures could explain why no obvious involvement of the LHab was found after reactivation in the present study. We noted only a significant increase in Fos expression in the POR rats compared to the PWR rats. This might be due to odor processing, since the LHab receives olfactory inputs (Sutherland 1982).
In the VLO/LO, a significant decrease in Fos immunoreactivity was observed in the T rats after learning compared to the PO and PW rats. After memory reactivation, Fos expression was significantly increased in the TR rats compared to the PWR rats. Fos immunoreactivity remained similar in the PO and PW rats compared to the POR and PWR rats. The POR rats did not receive any water in the maze but were submitted to both odors of the pair. In these rats, Fos labeling might reflect processing of odor cues, since the VLO/LO receives some olfactory inputs (Datiche and Cattarelli 1996). The present data did not show an involvement of the VLO/LO in mnesic reactivation of the learned odors, since the difference between the TR rats and POR rats was not significant. Nevertheless, we observed that Fos expression was significantly higher in the TR rats compared to the PWR rats. In the PWR group, Fos expression might reflect water delivery. In accord with such an assumption, the reward consumption was shown to modify VLO neuron activity in a Go/No-Go procedure based on olfactory discrimination (Schoenbaum and Eichenbaum 1995). In the TR rats studied here, Fos expression should not reflect the sole reward consumption; otherwise a similar labeling would be observed in both the TR and the PWR rats. In the TR rats, reward consumption is linked to odor discrimination, since water-reward was obtained only when the choice was correct. The Fos expression in the TR rats might reflect the increased arousal level required to reactivate the memories required to choose the rewarded cue. Thus, mnesic and reward processes linked to the odor cues seem to be intermingled in the orbitofrontal cortex. This area is reciprocally connected with the PCx-a (Datiche and Cattarelli 1996) and participates in central olfactory processing (Schoenbaum and Eichenbaum 1995). The latter authors showed that the neuronal activity was modified in the PCx-a and the orbitofrontal cortex following presentation of a relevant odor cue. It was hypothesized that these areas might cooperate to encode the significance of odor cues and subsequent recognition of relevant signals. The PCx-a might send information regarding odor identity to the VLO/LO, which in turn might provide information based on past experience in order to allow odor recognition. The connections between the VLO/LO and the PCx-a might permit the guidance of rat behavior on the basis of olfactory input detection, and the VLO/LO might play a critical role in memory representation for significant olfactory stimuli (Ramus and Eichenbaum 2000). Several lines of evidence further indicate that the prefrontal cortex could play an important role in long-term recognition memory processes. Bontempi et al. (1999) provided evidence of a time-dependent reorganization of the neuronal circuitry underlying long-term memory storage, since neocortical areas seem involved in retrieval. In agreement with previous data (Roullet et al. 2005), the present study supports a role of the VLO/LO when the rats master the olfactory discrimination task. It further underlines the complex involvement of the VLO/LO in memory reactivation, since integration of both odor significance and reward seems to be sustained by the VLO/LO.
In the IL, Fos expression in the T rats was not significantly different from that of the home-cage controls. No significant change in Fos immunoreactivity between trained and pseudotrained rats was observed after memory reactivation. Integration of rewarded cues in motivated rats during learning might be sustained in the infralimbic cortex (Pratt and Mizumori 2001). However, the present results do not support an involvement of the IL in either mastery of the olfactory discrimination task or memory reactivation.
Using a complex and progressively acquired olfactory discrimination task, the present study extends our knowledge of the neuroanatomical regions sustaining mnesic processes in rats. It is notable that the cerebral patterns of Fos expression induced by learning of the olfactory task and by memory reactivation were different. Our findings are in accord with the current hypothesis that recalled memories might not reengage neuronal circuits that were previously required for learning (Squire and Alvarez 1995).
We observed that when memory was reactivated, the pattern of activated brain areas was reduced compared with the pattern involved when the olfactory discrimination learning task is well mastered. Significantly different Fos expression was found between trained and pseudotrained rats in the CA3, PCx-a, and VLO/LO only. It can be assumed that these areas, which process olfactory inputs, might participate in a reconsolidation phenomenon after olfactory memory reactivation. However, it should be kept in mind that the present experiment cannot distinguish the effects of retrieval from those linked to the performance of the olfactory task itself. Fos mapping cannot provide the answer to such a question. On the other hand, the temporal dimension of memory retention would also be important to examine. We reactivated memory after 10 d, but it would be interesting to investigate the activation within olfactory-related brain areas after shorter and longer retention delays.
Materials and Methods
Sixty-one male SPF Wistar rats weighing 200-220 g at the beginning of the experiment were used. The experimental procedures followed the guidelines of the European Communities council directive of 24 November 1986 (86/609/EEC), and efforts were made to reduce the number of rats employed. Rats were individually housed under a 12 h/12 h inverted light:dark cycle and had food ad libitum. Rats were daily handled by the experimenter for ∼2 wks prior to training initiation.
Training apparatus
The odor training discrimination apparatus was a four-arm radial maze described by Saar et al. (1998). For each trial, the rat was placed in the starting area of the maze, and the odors (geraniol and limonene) were randomly assigned to two of the four arms. After 7 sec, the guillotine doors were lifted and the rat had to choose an arm. When the rat reached the end of the arm, the breaking of an infrared beam interrupted the trial. Olfactory training consisted of 20 trials per day. Conditioning was performed in a ventilated room, and a fan was activated between each trial.
Groups of rats
Trained rats
The trained rat group (n = 22) was water-deprived (allowed to drink for 10 min per day only). The training was olfactory conditioning: The rats had to discriminate between rewarded (limonene) and nonrewarded (geraniol) odors of the pair delivered in the maze. The discrimination task was considered as acquired when the trained rats chose the positive odor in at least 80% of the trials. In order to ascertain that the task was well mastered, the rats had to maintain such a level of performance for two additional days. Ten trained rats (T group) were sacrificed 1 h after the last session in the maze, because this delay is adequate to reach a high concentration of Fos protein (Chaudhuri et al. 2000).
In order to examine brain activation after memory reactivation, 12 trained rats (TR group) were conditioned to learn the olfactory discrimination task and then returned to their home cages for a resting period of 10 d. On the 11th day, they were submitted to a reactivation session, which consisted of 20 reinforced trials in the maze. One hour after this reactivation session, they were sacrificed for Fos immunocytochemical detection.
Pseudotrained rats
Two groups of pseudotrained rats were used for the examine Fos expression elicited by either getting water-reward (PW) or olfactory stimulation (PO). The PW rats (n = 6) were water-deprived (allowed to drink for 10 min per day only). They were daily submitted to the maze for a 20-trial session. On each trial, the PW rats had to reach the end of one or the other arm to get water, and they were systematically water-rewarded whatever the chosen arm. These rats were never stimulated by odors within the maze. The PO rats (n = 7) received water ad libitum. They were daily placed in the maze for a 20-trial session where they were stimulated by odors (geraniol and limonene). They could explore the maze freely, but never received the water reward.
For the investigation of memory reactivation, we also used PWR (n = 9) and POR (n = 7) rats that were sacrificed after a resting period of 10 d. On the 11th day, these groups were submitted to a session in the maze as previously described: The PWR rats received water systematically whatever the chosen arm, and the POR rats were olfactory-stimulated without getting water. Whatever the stage, i.e., learning or reactivation, the pseudotrained rats were always sacrificed in parallel to trained rats.
Control rats
Home-cage rats (n = 10), i.e. without any type of experimental manipulation were used. They allowed us to determine the basal level of Fos expression. The C rats (n = 3) were sacrificed in parallel to the T, PO, and PW rats, whereas the CR rats (n = 7) were sacrificed in parallel to the TR, POR, and PWR rats.
Fos immunocytochemistry
Following deep anesthesia with Equitesine (4 mL/kg i.p.), the rats were perfused transcardially with 200 mL of Ringer lactate containing 0.1% heparin followed by 500 mL of ice-cold fixative [4% paraformaldehyde, 0.1% glutaraldehyde, 0.2% picric acid in 0.1 M phosphate buffer (PB) at pH 7.4]. The brains were removed and post-fixed (2% paraformaldehyde, 0.2% picric acid in 0.1 M PB, pH 7.4) for 12 h at 4°C, then cryoprotected in a 30% sucrose PB solution for 48 h. The brains were then cut coronally (25 μm) with a cryostat, and free-floating sections were collected in 0.1 M phosphate buffer saline containing 0.3% Triton X-100 (PBST; pH 7.4) to perform Fos immunohistochemistry. Brain sections were placed in a 3% hydrogen peroxide solution for 30 min and incubated in PBST containing 3% normal goat serum for 2 h. Subsequently, sections were incubated for 2 d at 4°C with a rabbit anti-c-fos antibody (AB-5; Oncogene Science) diluted at 1:5000 in 0.1 M PBST containing 0.1% sodium azide (PBST-Az). The sections were then transferred into biotinylated anti-rabbit IgG (1: 1000 in PBST; 24 h at 4°C; Vector Laboratories). Then the sections were incubated for 1 h in avidin-biotin-horseradish peroxydase complex reagent from an ABC Elite kit (Vector Laboratories). Between all steps, sections were rinsed several times with PBST. Peroxydase activity was revealed by using 3-3′-diaminobenzidine (0.02%) and H2O2 (0.003%) in a 0.05 M Tris-HCl solution (pH 7.6). The reaction was enhanced by adding nickel ammonium sulfate (0.6%), which provides a black staining. The nickel-intensified diaminobenzidine reaction was controlled by inspecting sections under a microscope and stopped by rinses in PBST-Az. Finally, the brain sections were mounted onto gelatin-coated slides, dehydrated, and coverslipped with DePeX for light microscopy.
Fos-positive cells were identified on the basis of a black reaction product that was confined to the cell nucleus.
Data analysis
The nomenclature used in the Paxinos and Watson atlas (1986) for the rat brain was used for delimitation of cerebral regions. The PCx extends from +12.7 mm to +5.7 mm anterior to the interaural line. In each animal, we analyzed eight sections taken every 1000 μm along the whole rostrocaudal PCx extent. This paleo-cortex can be divided into PCx-a and PCx-p parts; the boundary between them is located at the level of the anterior commissure (+8.7 mm from the interaural line). The dorsal hippocampus [CA1, CA3 subfields and dentate gyrus (DG)] and lateral habenula (LHab) were analyzed on a representative section corresponding to the coronal plane located at +5.7 mm and +6.7 mm from the interaural line, respectively. We further counted Fos-labeled nuclei at the frontal cortex level in the infralimbic area (IL) on the coronal representative section located at +11.7 mm. Fos immunoreactivity was analyzed in the ventrolateral/lateral orbitofrontal area (VLO/LO) on a representative section at +12.7 mm from the interaural line (Fig. 5A). Then, Fos staining was analyzed automatically using Optilab software. All cells darker than the surrounding background were counted. Because of good delineation of the PCx, hippocampus, and LHab, labeled cells could be counted on the entire extent of these target regions within the selected coronal sections. Because we verified that the respective surface of these areas was similar in the different rats, the data are expressed as mean number of labeled cells per rat in the PCx, hippocampus, and LHab. As the borders of neocortical areas are less easily determined, labeled cells were counted in a sample region (0.66 mm2 for the VLO/LO; 1 mm2 for the IL), and the data are expressed as the number of Fos-positive cells per mm2.
Statistical analysis
Behavioral data in trained and pseudotrained rats were compared using a X2 test.
In all brain areas, a two-way analysis of variance (ANOVA) was performed. One factor was the experimental condition (T, PO, PW, or C), and the other was the mnesic stage (learning or reactivation). For PCx data, a two-way ANOVA was further used with one factor being the experimental condition (T, PO, PW, or C) and the other being the location of the immunoreactive cells in the PCx subdivisions (PCx-a and PCx-p). Post hoc pairwise group comparisons were made by an LSD (Least Significant Difference) procedure. We considered differences as significant when P < 0.05. All statistical analyses were done with the software SAS 8.2 (SAS Institute).
Acknowledgments
We thank Dominique Valentin for her helpful comments on the statistical analysis, and Edi Barkaï and his collaborators (Ben Gurion Univ., Israel) for their assistance with the maze elaboration. F.R. is a recipient of a Ministère de l'Education Nationale, de la Recherche et de la Technologie (MNERT) grant.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.89605.
References
- Abel, T. and Lattal, K.M. 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 11: 180-187. [DOI] [PubMed] [Google Scholar]
- Anagnostaras, S.G., Maren, S., and Fanselow, M.S. 1999. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J. Neurosci. 19: 1106-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin, K.V. and Rose, S.P. 1991. Learning-induced increase of immediate early-gene messenger mRNA in the chick forebrain. Eur. J. Neurosci. 3: 162-167. [DOI] [PubMed] [Google Scholar]
- Berman, D.E. and Dudai, Y. 2001. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science 291: 2417-2419. [DOI] [PubMed] [Google Scholar]
- Bertaina, V. and Destrade, C. 1995. Differential time courses of c-fos mRNA expression in hippocampal subfields following acquisition and recall testing in mice. Cog. Brain Res. 2: 269-275. [DOI] [PubMed] [Google Scholar]
- Bontempi, B., Laurent-Demir, C., Destrade, C., and Jaffard, R. 1999. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 400: 671-675. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, A., Zangenehpour, S., Rahbar-Dehgan, R., and Ye, F. 2000. Molecular maps of neural activity and quiescence. Acta Neurobiol. Exp. 60: 403-410. [DOI] [PubMed] [Google Scholar]
- Datiche, F. and Cattarelli, M. 1996. Reciprocal and topographic connections between the piriform and prefrontal cortices in the rat: A tracing study using the B subunit of the cholera toxin. Brain Res. Bull. 6: 391-398. [DOI] [PubMed] [Google Scholar]
- Datiche, F., Roullet, F., and Cattarelli, M. 2001. Expression of Fos in the piriform cortex after acquisition of olfactory learning: An immunohistochemical study in the rat. Brain Res. Bull. 55: 95-99. [DOI] [PubMed] [Google Scholar]
- Debiec, J., LeDoux, J.E., and Nader, K. 2002. Cellular and systems reconsolidation in the hippocampus. Neuron 36: 527-538. [DOI] [PubMed] [Google Scholar]
- Dudai, Y. 2002. Molecular bases of long-term memories: A question of persistence. Curr. Opin. Neurobiol. 12: 211-216. [DOI] [PubMed] [Google Scholar]
- ____. 2004. The neurobiology of consolidations, or, how stable is the engram? Ann. Rev. Psychol. 55: 51-86. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H. 2001. The long and winding road to memory consolidation. Nat. Neurosci. 4: 1057-1058. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H., Mathews, P., and Cohen, N.J. 1989. Further studies of hippocampal representation during odor discrimination learning. Behav. Neuroci. 103: 1207-1216. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H., Schoenbaum, G., Young, B., and Bunsey, M. 1996. Functional organization of the hippocampal memory system. Proc. Natl. Acad. Sci. 93: 13500-13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, M., McMahan, R.W., and Schoenbaum, G. 1999. Orbitofrontal cortex and representation of incentive value in associative learning. J. Neurosci. 19: 6610-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly, L.B. 1985. Neuronal circuitry in olfactory cortex: Anatomy and functional implications. Chem. Senses 10: 219-238. [Google Scholar]
- ____. 2001. Parallel-distributed processing in olfactory cortex: New insights from morphological and physiological analysis of neuronal circuitry. Chem. Senses 26: 551-576. [DOI] [PubMed] [Google Scholar]
- Haberly, L.B. and Bower, J.M. 1989. Olfactory cortex: Model circuit for study of associative memory? Trends Neurosci. 12: 258-264. [DOI] [PubMed] [Google Scholar]
- Haberly, L.B. and Price, J.L. 1978. Association and commissural fiber systems of the olfactory cortex of the rat. I-Systems originating in the piriform cortex and adjacent areas. J. Comp. Neurol. 178: 711-740. [DOI] [PubMed] [Google Scholar]
- Hasselmo, M.E. 1995. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav. Brain Res. 67: 1-27. [DOI] [PubMed] [Google Scholar]
- Herrera, D.G. and Robertson, H.A. 1996. Activation of c-fos in the brain. Prog. Neurobiol. 83: 83-107. [DOI] [PubMed] [Google Scholar]
- Hess, U.S., Lynch, G., and Gall, C.M. 1995a. Changes in c-fos mRNA expression in rat brain during odor discrimination learning: Differential involvement of hippocampal subfields CA1 and CA3. J. Neurosci. 15: 4786-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ____. 1995b. Regional patterns of c-fos mRNA expression in rat hippocampus following exploration of a novel environment versus performance of a well-learned discrimination. J. Neurosci. 15: 7796-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, M.W., Larson, J., and Lynch, G. 1990. Long-term potentiation of monosynaptic EPSPs in rat piriform cortex in vitro. Synapse 6: 279-283. [DOI] [PubMed] [Google Scholar]
- Kaczmarek, L. 2000. Gene expression in learning processes. Acta Neurobiol. Exp. 60: 419-424. [DOI] [PubMed] [Google Scholar]
- Kaut, K.P. and Bunsey, M.D. 2001. The effects of lesions of the rat hippocampus or rhinal cortex on olfactory and spatial memory: Retrograde and anterograde findings. Cogn. Affect. Behav. Neurosci. 1: 270-286. [DOI] [PubMed] [Google Scholar]
- Lipton, P.A., Alvarez, P., and Eichenbaum, H. 1999. Crossmodal associative memory representations in rodent orbitofrontal cortex. Neuron 22: 349-359. [DOI] [PubMed] [Google Scholar]
- Litaudon, P. and Cattarelli, M. 1996. Olfactory bulb repetitive stimulations reveal non-homogeneous distribution of the inhibitory processes in the rat piriform cortex. Eur. J. Neurosci. 8: 21-29. [DOI] [PubMed] [Google Scholar]
- Litaudon, P., Mouly, A-M., Sullivan, R., Gervais, R., and Cattarelli, M. 1997. Learning-induced changes in rat piriform cortex activity mapped using multisite recording with voltage sensitive dye. Eur. J. Neurosci. 9: 1593-1602. [DOI] [PubMed] [Google Scholar]
- Maviel, T., Durkin, T.P., Menzaghi, F., and Bontempi, B. 2004. Sites of neocortical reorganization critical for remote spatial memory. Science 305: 96-99. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz, M. and Buonviso, N. 2002. Altered odor-induced expression of c-fos and arg 3.1 immediate early genes in the olfactory system after familiarization with an odor. J. Neurobiol. 52: 61-72. [DOI] [PubMed] [Google Scholar]
- Mouly, A.M. and Gervais, R. 2002. Polysynaptic potentiation at different levels of rat olfactory pathways following learning. Learn. Mem. 9: 66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly, A.M., Fort, A., Ben-Boutayab, N., and Gervais, R. 2001. Olfactory learning induces differential long-lasting changes in rat central olfactory pathways. Neuroscience 102: 11-21. [DOI] [PubMed] [Google Scholar]
- Nader, K. 2003. Memory traces unbound. Trends Neurosci. 26: 65-72. [DOI] [PubMed] [Google Scholar]
- Paxinos, G. and Watson, C. 1986. The rat brain in stereotaxic coordinates. Academic Press, Sydney, Australia.
- Pratt, W.E. and Mizumori, J.Y. 2001. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav. Brain Res. 123: 165-183. [DOI] [PubMed] [Google Scholar]
- Przybyslawski, J. and Sara, S.J. 1997. Reconsolidation of memory after its reactivation. Behav. Brain Res. 84: 241-246. [DOI] [PubMed] [Google Scholar]
- Ramus, S.J. and Eichenbaum, H. 2000. Neural correlates of olfactory recognition memory in the rat orbitofrontal cortex. J. Neurosci. 20: 8199-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch, M.R. and Olson, C.R. 2004. Neuronal activity related to reward value and motivation in primate frontal cortex. Science 304: 307-310. [DOI] [PubMed] [Google Scholar]
- Roman, F.S., Chaillan, F.A., and Soumireu-Mourat, B. 1993. Long-term potentiation in rat piriform cortex following discrimination learning. Brain Res. 601: 265-272. [DOI] [PubMed] [Google Scholar]
- Roullet, F., Datiche, F., Liénard, F., and Cattarelli, M. 2004. Cue valence representation studied by Fos immunocytochemistry after acquisition of a discrimination learning task. Brain Res. Bull. 64: 31-38. [DOI] [PubMed] [Google Scholar]
- Roullet, F., Datiche, F., Liénard, F., and Cattarelli, M. 2005. Learning-stage dependent Fos expression in the rat brain during acquisition of an olfactory discrimination task. Behav. Brain Res. 157: 127-137. [DOI] [PubMed] [Google Scholar]
- Saar, D., Grossman, Y., and Barkai, E. 1998. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur. J. Neurosci. 10: 1518-1523. [DOI] [PubMed] [Google Scholar]
- Sara, S.J. 2000. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem. 7: 73-84. [DOI] [PubMed] [Google Scholar]
- Schoenbaum, G. and Eichenbaum, H. 1995. Information coding in the rodent prefrontal cortex. I-Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J. Neurophysiol. 74: 733-750. [DOI] [PubMed] [Google Scholar]
- Schoenbaum, G., Setlow, B., Saddoris, M.P., and Gallagher, M. 2003. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron 39: 855-867. [DOI] [PubMed] [Google Scholar]
- Slotnick, B. 2001. Animal cognition and the rat olfactory system. Trends Cogn. Sci. 5: 216-222. [DOI] [PubMed] [Google Scholar]
- Squire, L.R. and Alvarez, P. 1995. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr. Opin. Neurobiol. 5: 169-177. [DOI] [PubMed] [Google Scholar]
- Staubli, U., Schottler, F., and Nejat-Bina, D. 1987. Role of dorso-medial thalamic nucleus and piriform cortex in processing olfactory information. Behav. Brain Res. 25: 117-129. [DOI] [PubMed] [Google Scholar]
- Sutherland, R.J. 1982. The dorsal diencephalic conduction system: A review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 6: 1-13. [DOI] [PubMed] [Google Scholar]
- Treves, A. and Rolls, E.T. 1992. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2: 189-199. [DOI] [PubMed] [Google Scholar]
- Tronel, S. and Sara, S. 2002. Mapping of olfactory memory circuits: Region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learn. Mem. 9: 105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchet, B., Chaillan, F.A., Soumireu-Mourat, B., and Roman, F.S. 2002. Learning and memory of cue-reward association meaning by modifications of synaptic efficacy in dentate gyrus and piriform cortex. Hippocampus 12: 600-608. [DOI] [PubMed] [Google Scholar]
- Vale-Martinez, A., Marti-Nicolovius, M., Guillazo-Blanch, G., Coll-Andreu, M., and Morgado-Bernal, I. 1997. Effects of habenular lesions upon two-way active avoidance conditioning in rats. Neurobiol. Learn. Mem. 68: 68-74. [DOI] [PubMed] [Google Scholar]