Abstract
BACKGROUND:
At present, 4 prescription therapies have been approved by the US Food and Drug Administration for the treatment of chronic idiopathic constipation (CIC) in adults.
OBJECTIVES:
To compare persistence with and adherence to prucalopride vs 3 other prescription medications for CIC in a US population.
METHODS:
This retrospective, observational cohort study used data from the IBM MarketScan Commercial Claims and Encounters and Medicare Supplemental Databases (January 2015-June 2020). Inclusion criteria were patients (aged ≥18 years) with at least 1 prescription fill for prucalopride, lubiprostone, linaclotide, or plecanatide on or after April 2, 2019 (commercial availability of prucalopride), and at least 1 constipation-related diagnosis code. Persistence was assessed by time to discontinuation, and adherence was assessed by the proportion of days covered (PDC) and the proportion of patients who achieved PDC of at least 80%. Adjusted hazard ratios (HRs) for discontinuation and odds ratios for adherence were calculated.
RESULTS:
A total of 14,700 patients (mean age = 48.3 years; female = 81.9%) were included (prucalopride, n = 675; lubiprostone, n = 1,591; linaclotide, n = 11,105; plecanatide, n = 1,329). After adjusting for confounding factors, the HRs for discontinuation were significantly higher for all comparator medications compared with prucalopride after 2 months (HR [95% CI]: lubiprostone, 1.70 [1.48-1.95]; linaclotide, 1.25 [1.10-1.41]; plecanatide, 1.31 [1.13-1.51], all P < 0.001). The unadjusted mean (SD) PDC was 0.53 (0.32) with prucalopride compared with 0.41 (0.31); P less than 0.001 with lubiprostone, 0.48 (0.31), P less than 0.05 with linaclotide, and 0.48 (0.29), P = 0.98 with plecanatide. The comparator medications were all associated with lower odds of achieving PDC of at least 80% relative to prucalopride (odds ratio [95% CI]: lubiprostone, 0.52 [0.40-0.69], P < 0.001; linaclotide, 0.73 [0.58-0.93], P = 0.009; plecanatide, 0.70 [0.53-0.93], P = 0.015).
CONCLUSIONS:
The findings of this study indicate that prucalopride has higher treatment persistence and adherence compared with other CIC prescription medications. This research represents the first instance of a real-world claims study showcasing such outcomes.
Plain language summary
Patients with chronic idiopathic constipation (CIC) do not always take their medication when they should. This can affect how well the medication works. This study looked at how long patients took their medication for. It also looked at how often patients took medication as their doctor had recommended. We studied 4 CIC medications and found that patients were most likely to continue taking prucalopride as recommended compared with other medications.
Implications for managed care pharmacy
Through the utilization of insurance claims, this study showed that prucalopride exhibits a higher level of persistence with and adherence to treatment compared with 3 other prescription medications for CIC in a US real-world setting. These findings may assist pharmacists, physicians, advanced practice providers, and patients in making informed treatment-related decisions, potentially leading to improved clinical outcomes and health-related quality of life, which may in turn result in more cost-effective treatment plans.
Chronic idiopathic constipation (CIC) is a common disorder of gut–brain interaction, characterized by symptoms of infrequent or difficult bowel movements and/or stool inconsistency.1,2 The prevalence of CIC is higher in women than men, increases with age, and is associated with lower socioeconomic status.1 The worldwide prevalence of functional constipation is estimated to be 11.7%.3 Given the recurrent and persistent nature of CIC, long-term therapy is often required to relieve symptoms4; management strategies for CIC include lifestyle changes and pharmacotherapy.5,6 Pharmacotherapeutic options for CIC include over-the-counter bulking agents and laxatives (osmotic or stimulant); however, when these are unsuccessful, prescription medications are recommended.5,7,8 These include prosecretory agents, such as lubiprostone, linaclotide, and plecanatide, and 5-hydroxytryptamine receptor 4 (5-HT4) agonists with prokinetic effects, such as prucalopride.5
To better understand the experiences and needs of patients with CIC, a survey was conducted between 2016 and 2017 (BURDEN-CIC) in patients with CIC and their health care professionals. Results from this survey demonstrated the consequences of CIC symptoms on patients’ health-related quality of life.9 Additionally, this study also highlighted the most substantial challenges in CIC management according to health care professionals, including insufficient response rates to currently available treatment(s), low patient adherence or compliance with treatment(s), and the scarcity of CIC-specific treatment options.9
Research suggests that persistence with and adherence to treatment are associated with better clinical outcomes for patients and consequently reduce health care costs and optimize health care resource utilization (HCRU).10 Nonadherence can result in medication waste, increased health care costs, disease progression, reduced functional abilities, decreased health-related quality of life, and increased use of medical resources.10 Understanding treatment patterns and persistence with and adherence to medications is therefore important to inform prescribing patterns and improve HCRU and clinical outcomes for patients.
Prosecretory agents for the treatment of CIC have been approved by the US Food and Drug Administration (FDA) since 2006; these agents increase chloride production within the lumen of the small intestine to encourage fluid secretion (lubiprostone, 2006; linaclotide, 2012; plecanatide, 2017).11-14 Prucalopride is a selective, high-affinity 5-HT4 receptor agonist, which increases peristalsis within the intestine.15 Prucalopride was approved by the FDA for the treatment of CIC in adults in the United States in 2018 and introduced to the US market on April 2, 2019.16,17 Although the safety and efficacy of prucalopride18 and other CIC medications19-24 have been well established in clinical studies, real-world data on the persistence and adherence to these CIC medications are limited.25,26
This study aimed to assess and compare the persistence with and adherence to prucalopride vs 3 other CIC prescription medications (lubiprostone, linaclotide, and plecanatide) in a patient population in the United States.
Methods
STUDY DESIGN
This was a retrospective, observational cohort study based on insurance claims data collected between January 1, 2015, and June 30, 2020, from the IBM MarketScan Commercial Claims and Encounters (CCAE) Database and the Medicare Supplemental (MDCR) Database. The IBM MarketScan CCAE Database comprises the combined claims for approximately 260 self-insured employers and 40 health care plans in the United States, capturing approximately 240 million covered lives. The MDCR Database captures information on the subset of Medicare beneficiaries who possess supplemental insurance paid by their employers (and their Medicare-eligible dependents); in 2010, this represented approximately 14% of the 46 million retirees with Medicare benefits in the United States.27 The following definitions are referred to throughout (Figure 1): (1) index date: the date of the first prescription fill for prucalopride, lubiprostone, linaclotide, or plecanatide on or after April 2, 2019 (date of commercial availability for prucalopride, after which all 4 prescription medications were available on the market to ensure a fair comparison); (2) index treatment: the corresponding treatment from the first prescription fill; (3) baseline period: the 6-month period before the index date; (4) follow-up period: the period from the index date to the end of continuous eligibility.
FIGURE 1.
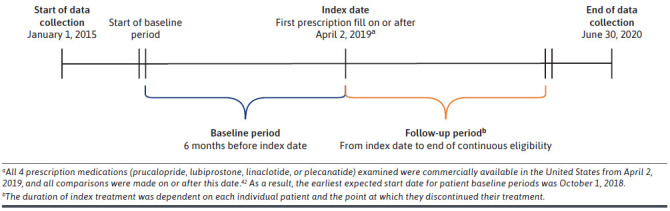
Study Design Timeline
Index date characteristics, clinical outcomes (constipation-related symptoms and complications), and HCRU outcomes were assessed during the baseline period. Treatment patterns, persistence, and adherence for prucalopride were evaluated and compared with those of lubiprostone, linaclotide, and plecanatide.
STUDY POPULATION
Patients were eligible if they were aged at least 18 years and had received at least 1 prescription fill for prucalopride or any of the 3 comparator treatments (lubiprostone, linaclotide, or plecanatide) on or after April 2, 2019 (Supplementary Table 1 (333.2KB, pdf) , available in online article). Patients were required to have had continuous health plan enrollment for at least 6 months before and at least 30 days after the index date (adherence outcomes were assessed for patients who were enrolled for at least 6 months after the index date) and at least 1 constipation-related International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis code during the baseline or follow-up period (Supplementary Table 2 (333.2KB, pdf) ). Patients were excluded if they reported at least 1 diagnosis code for irritable bowel syndrome with constipation, drug-induced constipation, opioid supply for 45 days or longer, or postoperative ileus (Supplementary Table 3 (333.2KB, pdf) ), or if they reported exposure to the index drug before treatment initiation (i.e., the index date), concurrent CIC prescription medication during the follow-up period, or not meeting continuous eligibility criteria. This study used deidentified data compliant with the Health Insurance Portability and Accountability Act, therefore formal consent was not obtained.
TREATMENT PATTERN OUTCOMES
The number of prescription fills per patient over the follow-up period was measured using the following categories: 1, 2-3, 4-5, and at least 6 prescription fills. Persistence, which is the duration of time from initiation to discontinuation of therapy,28 was assessed by raw treatment duration and time to discontinuation. Raw treatment duration was defined as the time between the patient’s first and last prescription fills for the index treatment in the follow-up period, in addition to the number of days covered by their last prescription fill. Raw treatment duration was measured continuously by the number of days and by the proportion of patients in each of the following treatment duration categories: 0-30, 31-60, 61-90, 91-180, 181-270, and at least 271 days. Time to discontinuation was defined as the time from the patient’s first prescription fill until a treatment gap of more than 90 days was reached; this gap was selected as prescriptions for CIC are often written as a 90-day supply, and this aligns with methodology used in other published studies examining persistence in chronic lower gastrointestinal conditions.6,29,30 Time to discontinuation was measured continuously by the number of days and by the proportion of patients continuing to receive index treatment at 60, 90, 180, and 365 days after the index date.
Adherence, which was the extent of treatment use and the extent to which patients followed a treatment schedule as prescribed by their health care providers,31,32 was evaluated for 6 months after the index date for patients who had continuous enrollment for at least 6 months after the index date. Adherence was measured by the proportion of days covered (PDC), defined as the total number of days of supply covered by the prescription fills during the 6 months after the index date, divided by the number of days in the 6-month period. PDC was measured continuously by the total number of days covered by the prescription supply and categorically by the proportion of patients who achieved PDC of at least 80%; this is a threshold that is generally accepted as being representative of the level of adherence that is required for an optimal treatment effect.32
STATISTICAL ANALYSES
All data analyses were conducted using R Version 3.6.3 and SAS Version 9.4 (SAS Institute, Inc.). The threshold for statistical significance was 5%.
Unadjusted Comparisons of Treatment Patterns. Patient demographics, clinical characteristics, and treatment patterns were summarized descriptively. Continuous variables evaluated in this study were treatment duration and PDC, which were described using the mean (SD) and median (range). The categorical variable evaluated in this study was PDC of at least 80%, which was described by the frequency count and percentage of patients treated with prucalopride, lubiprostone, linaclotide, or plecanatide who achieved this threshold. Time to discontinuation was assessed using a Kaplan-Meier analysis. P values from log-rank tests were provided. Pairwise statistical comparisons were conducted using the Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables to individually compare persistence and adherence outcomes in the prucalopride-treated cohort with each of the comparator medication cohorts. Pairwise comparisons were only conducted if the P value from the joint statistical comparison was significant (P < 0.05).
Adjusted Comparisons of Treatment Patterns. Multi-variable regression models were used to assess the association of treatment with prucalopride and the persistence and adherence outcomes (time to discontinuation, PDC, and PDC ≥80%) relative to the comparator medications. To allow pairwise statistical comparisons between prucalopride and each of the 3 comparator medications, the prucalopride-treated cohort was used as the reference category for each multivariable regression model. Confounding factors that were controlled for were age at index date, sex, geographical region, insurance plan type (operationalized as preferred provider organization [PPO] plan vs non-PPO plan), select comorbidities during the baseline period (baseline Charlson Comorbidity Index score ≥1, anxiety, chronic pulmonary disease, depression, diabetes, fatigue, gastroesophageal reflux disease, hyperlipidemia, hypertension, hypothyroidism, migraine, and overweight or obesity), constipation-related/gastroenterologist HCRU during the baseline period, and constipation-related treatments used during the baseline period. The type of regression model used was dependent on the outcome of interest. Time to discontinuation was modeled using multivariable Cox regression models, PDC was modeled using multivariable linear regression models, and PDC of at least 80% was modeled using multivariable logistic regression models.
Results
STUDY POPULATION
In total, 14,700 patients who had at least 1 prescription fill of prucalopride (n = 675), lubiprostone (n = 1,591), linaclotide (n = 11,105), or plecanatide (n = 1,329) were included (Supplementary Figure 1 (333.2KB, pdf) ). Of these, 8,833 patients had at least 6 months of continuous health plan enrollment and were included in the adjusted and unadjusted adherence analyses. Index and baseline demographics and clinical characteristics are shown in Table 1 (tablet strength at index date: Supplementary Table 4 (333.2KB, pdf) ). Most patients were female (81.9%) and had a mean (SD) age of 48.3 (14.9) years. Health insurance was provided by a PPO for 57.2% (8,405/14,700) of patients. Only 7.0% (1,023/14,700) of patients had been prescribed a constipation-related treatment (including lubiprostone, linaclotide, or plecanatide) before the index date. A significantly higher proportion of prucalopride-treated patients had used a prescription constipation-related treatment before the index date compared with patients who were treated with lubiprostone, linaclotide, and plecanatide at the index date (30.8% [208/675] vs 14.1% [225/1,591], 2.8% [314/11,105], and 20.8% [276/1,329], respectively, all P < 0.001). The mean [SD] length of follow-up was similar between the 4 prescription medications (linaclotide 228 [121] days, lubiprostone 246 [122] days, plecanatide 221 [117] days, and prucalopride 213 [112] days).
TABLE 1.
Patient Demographics and Clinical Characteristics Assessed at the Index Date or During the Baseline Period
| Index treatment | All patients(N = 14,700) | ||||
|---|---|---|---|---|---|
| Prucalopride(n = 675) | Lubiprostone(n = 1,591) | Linaclotide(n = 11,105) | Plecanatide(n = 1,329) | ||
| Index date characteristics | |||||
| Age, years, mean (SD) | 47.02 (14.41) | 50.89 (17.15) | 48.04 (14.74) | 47.73 (13.56) | 48.27 (14.93) |
| Sex, n (%) | |||||
| Female | 586 (86.8) | 1,276 (80.2) | 9,035 (81.4) | 1,139 (85.7) | 12,036 (81.9) |
| Male | 89 (13.2) | 315 (19.8) | 2,070 (18.6) | 190 (14.3) | 2,664 (18.1) |
| US region, n (%) | |||||
| South | 322 (47.7) | 882 (55.4) | 6,747 (60.8) | 854 (64.3) | 8,805 (59.9) |
| Northeast | 194 (28.7) | 327 (20.6) | 1,967 (17.7) | 245 (18.4) | 2,733 (18.6) |
| North central | 85 (12.6) | 275 (17.3) | 1,601 (14.4) | 149 (11.2) | 2,110 (14.4) |
| West | 72 (10.7) | 104 (6.5) | 781 (7.0) | 79 (5.9) | 1,036 (7.0) |
| Unknown | 2 (0.3) | 3 (0.2) | 9 (0.1) | 2 (0.2) | 16 (0.1) |
| Insurance plan type, n (%) | |||||
| Basic/major medical, comprehensive, or EPO | 18 (2.7) | 76 (4.8) | 441 (4.0) | 57 (4.3) | 592 (4.0) |
| HMO | 49 (7.3) | 166 (10.4) | 1,145 (10.3) | 137 (10.3) | 1,497 (10.2) |
| POS | 67 (9.9) | 94 (5.9) | 813 (7.3) | 121 (9.1) | 1,095 (7.4) |
| PPO | 401 (59.4) | 930 (58.5) | 6,348 (57.2) | 726 (54.6) | 8,405 (57.2) |
| PPO with capitation | 0 (0.0) | 31 (1.9) | 101 (0.9) | 14 (1.1) | 146 (1.0) |
| CDHP | 87 (12.9) | 160 (10.1) | 1,389 (12.5) | 155 (11.7) | 1,791 (12.2) |
| HDHP | 48 (7.1) | 111 (7.0) | 711 (6.4) | 112 (8.4) | 982 (6.7) |
| Unknown | 5 (0.7) | 23 (1.4) | 157 (1.4) | 7 (0.5) | 192 (1.3) |
| Any constipation-related treatment before the index date,a n (%) | |||||
| Yes | 208 (30.8) | 225 (14.1) | 314 (2.8) | 276 (20.8) | 1,023 (7.0) |
| Baseline characteristicsb | |||||
| CCI score, mean (SD) | 0.61 (1.16) | 0.70 (1.45) | 0.52 (1.13) | 0.51 (1.04) | 0.54 (1.16) |
| Selected comorbidities, n (%) | |||||
| Anxiety | 176 (26.1) | 344 (21.6) | 2,336 (21.0) | 289 (21.7) | 3,145 (21.4) |
| Chronic pulmonary disease | 90 (13.3) | 180 (11.3) | 1,171 (10.5) | 148 (11.1) | 1,589 (10.8) |
| Depression | 156 (23.1) | 289 (18.2) | 1,736 (15.6) | 217 (16.3) | 2,398 (16.3) |
| Diabetes | 90 (13.3) | 205 (12.9) | 1,554 (14.0) | 155 (11.7) | 2,004 (13.6) |
| Fatigue | 125 (18.5) | 245 (15.4) | 1,548 (13.9) | 221 (16.6) | 2,139 (14.6) |
| Gastroesophageal reflux disease | 217 (32.1) | 419 (26.3) | 2,519 (22.7) | 339 (25.5) | 3,494 (23.8) |
| Hyperlipidemia | 154 (22.8) | 468 (29.4) | 3,105 (28.0) | 354 (26.6) | 4,081 (27.8) |
| Hypertension | 165 (24.4) | 522 (32.8) | 3,377 (30.4) | 346 (26.0) | 4,410 (30.0) |
| Hypothyroidism | 137 (20.3) | 259 (16.3) | 1,606 (14.5) | 202 (15.2) | 2,204 (15.0) |
| Migraine | 82 (12.1) | 133 (8.4) | 909 (8.2) | 133 (10.0) | 1,257 (8.6) |
| Overweight/obesity | 83 (12.3) | 253 (15.9) | 1,691 (15.2) | 193 (14.5) | 2,220 (15.1) |
| Congestive heart failure | 16 (2.4) | 58 (3.6) | 240 (2.2) | 22 (1.7) | 336 (2.3) |
| Constipation-related HCRU,c n (%) | |||||
| Inpatient visits | 15 (2.2) | 36 (2.3) | 130 (1.2) | 12 (0.9) | 193 (1.3) |
| ED visits | 38 (5.6) | 113 (7.1) | 674 (6.1) | 67 (5.0) | 892 (6.1) |
| Outpatient visits | 531 (78.7) | 1,029 (64.7) | 6,676 (60.1) | 979 (73.7) | 9,215 (62.7) |
| Outpatient office/clinic or urgent care visits | 523 (77.5) | 1,010 (63.5) | 6,564 (59.1) | 964 (72.5) | 9,061 (61.6) |
| Outpatient hospital/surgical center visits | 20 (3.0) | 38 (2.4) | 238 (2.1) | 45 (3.4) | 341 (2.3) |
Index dates were defined as the date of the first prescription fill for prucalopride, lubiprostone, linaclotide, or plecanatide on or after April 2, 2019.
aIncludes use of lubiprostone, linaclotide, or plecanatide.
b Baseline characteristics were evaluated during the baseline period, which was defined as the 6 months before the index date.
cConstipation-related HCRU was defined as HCRU for which a diagnosis code for constipation can be found in 1 of the first 3 positions for the corresponding claim (constipation-related diagnosis codes are reported in Supplementary Table 2 (333.2KB, pdf) ).
CCI = Charlson Comorbidity Index; CDHP = consumer-directed health plan; ED = emergency department; EPO = exclusive provider organization; HCRU = health care resource utilization; HDHP = high deductible health plan; HMO = health maintenance organization; POS = point of service plan; PPO = preferred provider organization.
TREATMENT PATTERNS
Overall, 52.4% (7,710/14,700) of patients refilled their prescription of prucalopride, lubiprostone, linaclotide, or plecanatide at least once during the follow-up period (from index date to the end of continuous eligibility). The proportions of patients who refilled their prescription 2-3, 4-5, or 6 or more times were 32.4% (4,764/14,700), 11.2% (1,644/14,700), and 8.9% (1,302/14,700), respectively. The proportion of patients refilling their prescription at least once was significantly higher in those taking prucalopride compared with lubiprostone, linaclotide, and plecanatide (57.6% [389/675] vs 45.6% [726/1,591], 53.7% [5,961/11,105], and 47.7% [634/1,329], respectively; all P < 0.001).
PERSISTENCE
Unadjusted Analyses of Persistence. The mean (SD) treatment duration (days) was significantly higher in patients treated with prucalopride compared with lubiprostone (123.44 [109.42] vs 100.79 [101.46], P < 0.001), but no statistically significant differences were observed (vs prucalopride) in patients treated with linaclotide (117.18 [106.37], P = 0.300) and plecanatide (112.25 [100.26], P = 0.213). Compared with the 3 comparator medications, a higher proportion of prucalopride-treated patients had a treatment duration of 91-180 days, 181-270 days, and at least 271 days (Table 2); these findings were statistically significant.
TABLE 2.
Treatment Duration Between Prucalopride, Lubiprostone, Linaclotide, and Plecanatide During the Follow-Up Period
| Persistence of index treatment a,b | Prucalopride(n = 675) | Lubiprostone(n = 1,591) | Linaclotide(n = 11,105) | Plecanatide(n = 1,329) | All patients(N = 14,700) |
|---|---|---|---|---|---|
| Treatment duration, days | |||||
| Mean (SD) | 123.44 (109.42) | 100.79 (101.46) | 117.18 (106.37) | 112.25 (100.26) | 115.25 (105.58) |
| Median (range) | 90.0 (5.0-457.0) | 60.0 (1.0-455.0) | 89.0 (1.0-457.0) | 90.0 (10.0-457.0) | 84.0 (1.0-457.0) |
| Pairwise P valuea,c | — | <0.001 | 0.300 | 0.213 | — |
| Treatment duration category, days, n (%) | |||||
| 0-30 | 233 (34.5) | 718 (45.1) | 3,772 (34.0) | 440 (33.1) | 5,163 (35.1) |
| 31-60 | 53 (7.9) | 136 (8.5) | 963 (8.7) | 93 (7.0) | 1,245 (8.5) |
| 61-90 | 89 (13.2) | 210 (13.2) | 2,013 (18.1) | 321 (24.2) | 2,633 (17.9) |
| 91-180 | 126 (18.7) | 224 (14.1) | 1,863 (16.8) | 211 (15.9) | 2,424 (16.5) |
| 181-270 | 81 (12.0) | 154 (9.7) | 1,200 (10.8) | 126 (9.5) | 1,561 (10.6) |
| ≥271 | 93 (13.8) | 149 (9.4) | 1,294 (11.7) | 138 (10.4) | 1,674 (11.4) |
| Pairwise P valuec | — | <0.001 | <0.05 | <0.001 | — |
a Differences across treatment groups may reflect differences in the length of the follow-up period.
b Accounting for stockpiling, any days of supply that fell outside of the follow-up period were excluded.
c Pairwise statistical comparisons between prucalopride and each of the 3 comparator treatment cohorts were conducted using Wilcoxon rank-sum tests for continuous variables and chi-square tests for binary variables. Comparisons were conducted only if the P value for the joint statistical comparison was significant (P < 0.05).
An unadjusted Kaplan-Meier analysis of time to discontinuation demonstrated that a greater proportion of prucalopride-treated patients continued to receive treatment for longer compared with patients who were treated with any of these 3 comparator medications (Figure 2). Of the patients who persisted with treatment for 60 days or longer after the index date, the proportion of patients who continued to receive prucalopride was significantly higher than those treated with lubiprostone, linaclotide, and plecanatide (71.3% vs 57.2%, 68.7%, and 69.8%, respectively, P < 0.001). The proportion of prucalopride-treated patients who continued to receive index treatment after 1 year was 50.1% vs 20.8%, 31.5%, and 28.3% for lubiprostone-treated, linaclotide-treated, and plecanatide-treated patients, respectively.
FIGURE 2.
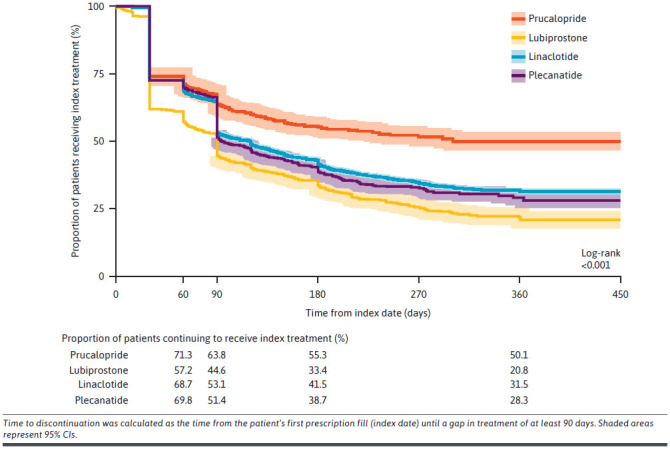
Unadjusted Kaplan-Meier Analysis Comparing Time With Index Treatment Discontinuation for Prucalopride, Lubiprostone, Linaclotide, and Plecanatide
Adjusted Analyses of Persistence. In an adjusted Cox regression model of time to discontinuation over a 12-month period after the index date, all 3 comparator medications were consistently associated with higher hazards of discontinuation relative to prucalopride; significantly higher hazards were reported from 2 months after the index date (Table 3). Over the follow-up period, the hazard ratios increased for all 3 comparator medications relative to prucalopride, more than doubling from 6 months to 1 year after the index date (hazard ratio [95% CI] at 6 months: lubiprostone, 3.02 [2.07-4.40]; linaclotide, 2.51 [1.77-3.55]; plecanatide, 2.89 [1.98-4.23]; at 1 year: lubiprostone, 7.18 [2.99-17.26]; linaclotide, 7.14 [3.17-16.07]; plecanatide, 9.52 [3.91-23.17], all, P < 0.001).
TABLE 3.
Hazard Ratios for Time to Discontinuation of Lubiprostone, Linaclotide, and Plecanatide Relative to Prucalopride During the Follow-Up Period
| Time during the follow-up period | Time to index treatment discontinuationa (N = 14,700) | ||
|---|---|---|---|
| Hazard ratiob (relative to prucalopride) | 95% CI | P | |
| At month 1 | |||
| Lubiprostone | 1.47 | 1.25-1.72 | <0.001 |
| Linaclotide | 1.05 | 0.91-1.21 | 0.517 |
| Plecanatide | 1.07 | 0.91-1.27 | 0.414 |
| At month 2 | |||
| Lubiprostone | 1.70 | 1.48-1.95 | <0.001 |
| Linaclotide | 1.25 | 1.10-1.41 | <0.001 |
| Plecanatide | 1.31 | 1.13-1.51 | <0.001 |
| At month 3 | |||
| Lubiprostone | 1.96 | 1.66-2.31 | <0.001 |
| Linaclotide | 1.49 | 1.28-1.73 | <0.001 |
| Plecanatide | 1.59 | 1.35-1.89 | <0.001 |
| At month 6 | |||
| Lubiprostone | 3.02 | 2.07-4.40 | <0.001 |
| Linaclotide | 2.51 | 1.77-3.55 | <0.001 |
| Plecanatide | 2.89 | 1.98-4.23 | <0.001 |
| At month 12 | |||
| Lubiprostone | 7.18 | 2.99-17.26 | <0.001 |
| Linaclotide | 7.14 | 3.17-16.07 | <0.001 |
| Plecanatide | 9.52 | 3.91-23.17 | <0.001 |
Statistical significance set at P < 0.05.
a Time to discontinuation was calculated as the time from the patient’s first prescription fill (index date) until a gap in treatment of at least 90 days.
b Hazard ratios were calculated using a multivariate Cox regression model, controlling for confounding factors. The model was adjusted for age at index date, sex, geographical region, insurance plan type, selected comorbidities during the baseline period, constipation-related/gastroenterologist health care resource utilization during the baseline period, and constipation-related treatments used during the baseline period. In addition, the model included interaction terms between time and each of the 3 comparator medications. A hazard ratio of greater than 1.00 indicates a higher risk of treatment discontinuation occurring in the comparator medication group compared with the prucalopride group.
ADHERENCE
Unadjusted Analyses of Adherence. Prucalopride-treated patients had a significantly higher mean (SD) PDC than lubiprostone-treated patients (0.53 [0.32] vs 0.41 [0.31], P < 0.001) and linaclotide-treated patients (0.48 [0.31], P < 0.05); however, no statistically significant difference was observed between prucalopride-treated and plecanatide-treated patients (0.48 [0.29], P = 0.098). Furthermore, of patients who had at least 6 months of follow-up, a significantly higher proportion of prucalopride-treated patients achieved a PDC of at least 80% compared with patients treated with lubiprostone (31.1% [115/370] vs 18.6% [194/1,045], P < 0.001), linaclotide (23.5% [1,564/6,658], P < 0.01), and plecanatide (23.3% [177/760], P < 0.01).
Adjusted Analyses of Adherence. In patients who had continuous health plan enrollment for at least 6 months after the index date, an adjusted linear regression of PDC showed that lubiprostone was associated with a statistically significant 10-percentage point decrease in PDC relative to prucalopride (β coefficient [95% CI]: −0.10 [−0.13 to −0.06], P < 0.001). No statistically significant differences in PDC were observed with linaclotide and plecanatide compared with prucalopride (β coefficient [95% CI]: linaclotide, −0.03 [−0.06 to 0.01], P = 0.130; plecanatide, −0.03 [−0.07 to 0.01], P = 0.134).
When adjusted for confounding factors, a logistic regression model demonstrated that relative to prucalopride, the 3 comparator medications were associated with significantly lower odds of achieving a PDC of at least 80%. Patients receiving lubiprostone had the lowest odds at 0.52 (95% CI = 0.40-0.69, P < 0.001), whereas the odds were slightly higher with linaclotide and plecanatide (linaclotide: 0.73 [95% CI = 0.58 - 0.93], P = 0.009; plecanatide: 0.70 [95% CI = 0.53-0.93], P = 0.015) (Supplementary Table 5 (333.2KB, pdf) ). A greater number of gastroenterologist visits during the baseline period was associated with significantly higher odds of achieving a PDC of at least 80% (1.09 [95% CI = 1.01 - 1.18], P = 0.025). The use of any prescription CIC medication during the baseline period was also associated with significantly higher odds of achieving a PDC of at least 80% (1.16 [95% CI = 1.02 - 1.32], P = 0.026) (Supplementary Table 5 (333.2KB, pdf) ).
Discussion
Using real-world claims data, this study demonstrated that approximately half of all patients who received any of the prescription CIC medications did not refill them. However, significantly higher proportions of patients treated with prucalopride refilled their prescription compared with those treated with lubiprostone, linaclotide, or plecanatide. Furthermore, patients with CIC treated with prucalopride were more likely to adhere to their prescription treatment compared with patients receiving the comparator prescription medications.
Our findings demonstrate relatively low persistence with treatment in this population of patients with CIC, regardless of prescription medication. This trend aligns with a previous retrospective cohort study in which discontinuation of lubiprostone and linaclotide was common among patients with CIC, particularly after 30 days of treatment.25 The results demonstrated that the proportion of patients who discontinued treatment after 90 days was generally higher for those treated with lubiprostone, linaclotide, and plecanatide (≥50%), compared with prucalopride (36%). One year after treatment initiation, half of prucalopride-treated patients continued treatment, whereas this proportion was less than one-third for the comparator medications. The adjusted analyses also showed that all 3 comparator medications were associated with a significantly higher risk of treatment discontinuation at months 2, 3, 6, and 12, compared with prucalopride.
Persistence with treatment tends to be low in many patients with chronic conditions, despite the benefits associated with long-term management of symptoms.6,33,34 A retrospective analysis of pharmacy claims data demonstrated low levels of persistence with treatment 6 months after initiation across 6 different chronic medication classes.35 The BURDEN-CIC survey demonstrated that more than half of patients receiving either lubiprostone or linaclotide were not satisfied with their current treatment.9 Early discontinuation of these 2 prescription medications for CIC has been reported in other retrospective cohort studies examining real-world patterns of pharmacotherapy, aligning with our study results.
In the BURDEN-CIC survey, reasons reported for discontinuation of CIC treatment included treatment-emergent diarrhea (53%) and lack of efficacy (36%).9 Another study by Shah and colleagues evaluated the reasons for discontinuation in patients receiving either lubiprostone or linaclotide for the treatment of irritable bowel syndrome with constipation or CIC.6 The most commonly reported reasons for early discontinuation were intolerance, (ie, side effects such as nausea, abdominal pain, and diarrhea), loss of prescription drug coverage, and insufficient efficacy.6 The current study used insurance claims data, and thus no information was available on why patients discontinued treatment. However, it is noteworthy that over time, the risk of treatment discontinuation relative to prucalopride increased for lubiprostone, linaclotide, and plecanatide. Further studies using real-world data would need to be conducted to thoroughly evaluate the reasons for discontinuation of prucalopride compared with the 3 comparator prescription medications. Additionally, studies could examine whether the trend of increasing risk of discontinuation relative to prucalopride would continue over a longer period of time.
In our study, prucalopride was also associated with increased adherence compared with the comparator medications. The unadjusted analysis demonstrated that patients treated with prucalopride had a significantly higher mean PDC compared with lubiprostone and linaclotide but not when compared with plecanatide. Additionally, a significantly higher proportion of prucalopride-treated patients achieved the adherence threshold of PDC of at least 80% compared with those treated with one of the comparator medications. The results from the adjusted PDC of at least 80% comparative analysis for the prescription CIC medications supported these findings, demonstrating that the comparator medications were associated with lower odds of achieving a PDC of at least 80%, relative to prucalopride. Our findings are important considering that the PDC of at least 80% threshold is indicative of an increased likelihood of achieving clinical benefit.32 Prucalopride has a different mechanism of action from the 3 comparator medications,11,12,16,36 providing a potential explanation for the greater adherence demonstrated by prucalopride. Additionally, as prucalopride is a highly selective 5-HT4 receptor agonist,16,17 the possible reduction in off-target side effects may have improved tolerability compared with the 3 other prescription medications.
Data reveal that poor adherence to treatment has a substantial impact on clinical outcomes.10,31 The cost associated with nonadherence is approximately US$290 billion per year in the United States (assessed in 2009).33 A study examining the costs of medication nonadherence by disease group showed that the main contributors to nonadherence-related total costs for gastrointestinal diseases were inpatient costs, outpatient costs, and pharmacy costs.37 In the present era of heightened shared decision-making between patients and their health care providers,38 discussions regarding adherence to medication are important considerations for patients and providers, given this may have an impact on discussions related to health insurance coverage and symptom management.33
A cost-effectiveness analysis in patients with a prescription for prucalopride, lubiprostone, linaclotide, or plecanatide showed that these medications decreased overall costs to patients and increased health-related quality of life compared with standard over-the-counter laxatives and typical recommendations on exercise and toileting habits.39 However, from an insurer perspective, CIC prescription medications increased costs by US$618-1,015 over a 12-week time period (2020).39 Considering the higher costs to insurers but the lower costs to patients, this exemplifies the importance of understanding adherence to treatment options, as this can help both parties make informed decisions. Future studies could evaluate how patient adherence is affected by medication coverage on health insurance and thus the cost to patients. Because of the greater adherence to prucalopride than comparator medications, as demonstrated by the findings from our study, this could potentially reduce medication waste.
The simplicity of a medication schedule, including the number of times a medication needs to be taken per day, has been shown to have an impact on adherence31 with less frequent dosing regimens associated with better compliance.40 In our study, prucalopride, linaclotide, and plecanatide are all prescribed as a once-daily dose, whereas lubiprostone is taken twice daily.11,12,16,36 The lubiprostone cohort had the lowest mean PDC out of the 4 prescription medications compared. Additionally, lubiprostone was associated with the lowest proportion of patients achieving a PDC of at least 80% compared with the other prescription medications examined. Comparisons of time to discontinuation also followed this trend. The lubiprostone cohort had the lowest proportion of patients continuing to receive their index treatment at day 60, compared with prucalopride, linaclotide, and plecanatide. The proportion of patients continuing to receive their index treatment at day 365 was lowest in those treated with lubiprostone. These findings support current literature, which suggests that the number of times a medication must be taken per day could be a barrier to medication adherence.
A strength of this study is that it is based on real-world data on the persistence with and adherence to treatment associated with 4 prescription CIC medications. This provides valuable information in addition to data collected during clinical studies, in which patients are typically more motivated to adhere to treatments, because of the attention from investigators or clinicians and the frequent data collection periods.41 Because the data analyzed in this study were collected using 2 high-quality insurance claims databases (combined claims from approximately 260 self-insured employers and 40 health plans in the United States), persistence and adherence measures are potentially more reflective of typical real-world behaviors.
LIMITATIONS
One of the limitations of this study is that because of its retrospective, observational cohort study design, it may be inherently subject to confounding variables, such as unobserved differences in health status and sociodemographic characteristics between groups. These included factors are thought to be associated with an increased risk of CIC. The IBM MarketScan CCAE Database and the MDCR Database do not contain clinical measures of disease severity. Furthermore, it is possible that this study inadvertently captured patients with constipation other than CIC, despite the exclusion criteria adopted to minimize misdiagnoses. A further limitation is that patients may not have been compliant with taking their medication, and therefore the dates and lengths of time associated with treatment initiation, persistence, and adherence are approximate. Insurance claims data were collected over a relatively short sample period of 14 months, particularly considering the length of time for which some of these medications have been approved for.11,12,16,36 This sample period also overlapped with the COVID-19 pandemic, which may have impacted the availability and access to prescription medications for CIC. Additionally, prucalopride was the most recent medication of those examined to receive FDA approval, providing a possible explanation for the high proportion of prucalopride-treated patients having previously received an alternative constipation-related treatment. A patient’s treatment sequence before the index date may have influenced medication compliance in this study; future studies could consider excluding patients who had a claim for any CIC prescription medication prior to the index date to ensure enrollment of a treatment-naive population.
Conclusions
This real-world comparative analysis of treatment patterns for CIC pharmacotherapy options demonstrates that approximately half of all patients who received a prescription medication for CIC did not refill it. Among those who refilled their medication, prucalopride was associated with improved persistence with and adherence to treatment compared with lubiprostone, linaclotide, and plecanatide. These findings have important implications for patients and prescribing health care providers because improved persistence with and adherence to treatment has the potential to improve clinical outcomes ultimately enhancing patients’ health-related quality of life. This study also provides valuable information that can help guide treatment-related decision-making for payors and health care providers. Future research is needed to determine the reasons behind the discontinuation of CIC medications and to assess how insurance plan coverage and out-of-pocket costs for patients may impact treatment adherence.
ACKNOWLEDGMENTS
The authors acknowledge Jessica Boles, PhD, and Iona MacKillop, MSc, of PharmaGenesis London (London, UK), for providing medical writing support, funded by Takeda Pharmaceuticals USA, Inc.
Funding Statement
This study was funded by Takeda Pharmaceuticals USA, Inc. Dr Cash has received consultancy and speaker fees from AbbVie, Alnylam Pharmaceuticals, Ardelyx Inc., Arena Pharmaceuticals, QOL Medical, Salix Pharmaceuticals, and Takeda Pharmaceuticals. Drs Lu and Terreri are employees of Takeda Pharmaceuticals USA, Inc., and stockholders of Takeda Pharmaceutical Company Limited. Dr Boules was a stockholder of Takeda Pharmaceutical Company Limited at the time this study was conducted.
REFERENCES
- 1.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am J Gastroenterol. 2011;106(9):1582-91. doi:10.1038/ajg.2011.164 [DOI] [PubMed] [Google Scholar]
- 2.Patel S, Doerfler B, Boutros K, Ng S, Manuel M, DeSimone E. Review of treatment options for irritable bowel syndrome with constipation and chronic idiopathic constipation. Int J Gen Med. 2021;14:1457-68. doi:10.2147/IJGM.S274568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology. 2021;160(1):99-114.e3. doi:10.1053/j.gastro.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 4.Lembo AJ, Johanson JF, Parkman HP, Rao SS, Miner PB Jr, Ueno R. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci. 2011;56(9):2639-45. doi:10.1007/s10620-011-1801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black CJ, Ford AC. Chronic idiopathic constipation in adults: Epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust. 2018;209(2):86-91. doi:10.5694/mja18.00241 [DOI] [PubMed] [Google Scholar]
- 6.Shah ED, Suresh S, Jou J, Chey WD, Stidham RW. Evaluating when and why patients discontinue chronic therapy for irritable bowel syndrome with constipation and chronic idiopathic constipation. Am J Gastroenterol. 2020;115(4):596-602. doi:10.14309/ajg.0000000000000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick D. Managing costs and care for chronic idiopathic constipation. Am J Manag Care. 2019;25(4)(suppl):S63-9. [PubMed] [Google Scholar]
- 8.Tse Y, Armstrong D, Andrews CN, et al. Treatment algorithm for chronic idiopathic constipation and constipation-predominant irritable bowel syndrome derived from a Canadian national survey and needs assessment on choices of therapeutic agents. Can J Gastroenterol Hepatol. 2017;2017:8612189. doi:10.1155/2017/8612189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris LA, Horn J, Kissous-Hunt M, Magnus L, Quigley EM. The better understanding and recognition of the disconnects, experiences, and needs of patients with chronic idiopathic constipation (BURDEN-CIC) study: Results of an online questionnaire. Adv Ther. 2017;34(12):2661-73. doi:10.1007/s12325-017-0633-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimmy B, Jose J. Patient medication adherence: Measures in daily practice. Oman Med J. 2011;26(3):155-9. doi:10.5001/omj.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amitiza (lubiprostone). Prescribing Information. Sucampo Pharma Americas, LLC; 2013. [Google Scholar]
- 12.Linzess (linaclotide). Prescribing Information. Forest Laboratories LLC; 2017. [Google Scholar]
- 13.US Food and Drug Administration. Trulance (plecanatide) approval letter. January 2017. Accessed June 17, 2024. www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/208745orig1s000ltr.pdf
- 14.Jiang C, Xu Q, Wen X, Sun H. Current developments in pharmacological therapeutics for chronic constipation. Acta Pharm Sin B. 2015;5(4):300-9. doi:10.1016/j.apsb.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanger GJ, Quigley EM. Constipation, IBs and the 5-HT4 receptor: What role for prucalopride? Clin Med Gastroenterol. 2010;3:CGast.S4136. doi:10.4137/CGast.S4136 [Google Scholar]
- 16.Motegrity (prucalopride). Prescribing Information. Shire LLC; 2018. [Google Scholar]
- 17.Takeda Pharmaceuticals Inc. Motegrity™ (prucalopride), the only serotonin-4 receptor agonist for adults with chronic idiopathic constipation (CIC), is now available in the United States. April 2, 2019. Accessed July 31, 2023. https://www.takeda.com/en-us/newsroom/news-releases/2019/motegrity-prucalopride-the-only-serotonin-4-receptor-agonist-for-adults-with-chronic-idiopathic-constipation-cic-is-now-available-in-the-united-states/
- 18.Camilleri M, Piessevaux H, Yiannakou Y, et al. Efficacy and safety of prucalopride in chronic constipation: An integrated analysis of six randomized, controlled clinical trials. Dig Dis Sci. 2016;61(8):2357-72. doi:10.1007/s10620-016-4147-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci. 2010;55(4):1090-7. doi:10.1007/s10620-009-1068-x [DOI] [PubMed] [Google Scholar]
- 20.Nee JW, Johnston JM, Shea EP, et al. Safety and tolerability of linaclotide for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation: Pooled phase 3 analysis. Expert Rev Gastroenterol Hepatol. 2019;13(4):397-406. doi:10.1080/17474124.2019.1575203 [DOI] [PubMed] [Google Scholar]
- 21.Lacy BE, Schey R, Shiff SJ, et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PLoS One. 2015;10(7):e0134349. doi:10.1371/journal.pone.0134349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365(6):527-36. doi:10.1056/NEJMoa1010863 [DOI] [PubMed] [Google Scholar]
- 23.DeMicco M, Barrow L, Hickey B, Shailubhai K, Griffin P. Randomized clinical trial: Efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol. 2017;10(11):837-51. doi:10.1177/1756283X17734697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barish CF, Griffin P. Safety and tolerability of plecanatide in patients with chronic idiopathic constipation: Long-term evidence from an open-label study. Curr Med Res Opin. 2018;34(4):751-5. doi:10.1080/03007995.2018.1430024 [DOI] [PubMed] [Google Scholar]
- 25.Nag A, Bornheimer R, Oster G. Pharmacotherapy patterns in patients with chronic idiopathic constipation beginning treatment with linaclotide or lubiprostone in the United States. Drugs Context. 2020;9:1-7. doi:10.7573/dic.2020-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner SA, Torres MR, Penna FJ, et al. Chronic functional constipation in children: Adherence and factors associated with drug treatment. J Pediatr Gastroenterol Nutr. 2014;58(5):598-602. doi:10.1097/MPG.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 27.Kulaylat AS, Schaefer EW, Messaris E, Hollenbeak CS. Truven health analytics MarketScan databases for clinical research in colon and rectal surgery. Clin Colon Rectal Surg. 2019;32(1):54-60. doi:10.1055/s-0038-1673354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: Terminology and definitions. Value Health. 2008;11(1):44-7. doi:10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 29.Jin R, Kruppert S, Scholz F, et al. Treatment persistence and switching patterns of ABP 501 in European patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2024;17:17562848231222332. doi:10.1177/17562848231222332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obando C, Ding Z, Muser E, et al. Persistence, dose titration, and health care resource utilization among Crohn’s disease patients treated with ustekinumab: A real-world analysis in the United States. Adv Ther. 2020;37(5):2127-43. doi:10.1007/s12325-020-01276-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-97. doi:10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 32.Pharmacy Quality Alliance (PQA). PQA adherence measures. April 19, 2022. Accessed February 7, 2023. https://www.pqaalliance.org/adherence-measures
- 33.New England Healthcare Institute. Thinking outside the pillbox: A system-wide approach to improving patient medication adherence for chronic disease. August 12, 2009. Accessed Accessed June 17, 2024. https://psnet.ahrq.gov/issue/thinking-outside-pillbox-system-wide-approach-improving-patient-medication-adherence-chronic
- 34.Elliott RA, Shinogle JA, Peele P, Bhosle M, Hughes DA. Understanding medication compliance and persistence from an economics perspective. Value Health. 2008;11(4):600-10. doi:10.1111/j.1524-4733.2007.00304.x [DOI] [PubMed] [Google Scholar]
- 35.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728-40. doi:10.18553/jmcp.2009.15.9.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trulance (plecanatide). Prescribing Information. Synergy Pharmaceuticals LLC; 2017. [Google Scholar]
- 37.Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open. 2018;8(1):e016982. doi:10.1136/bmjopen-2017-016982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: A model for clinical practice. J Gen Intern Med. 2012;27(10):1361-7. doi:10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah ED, Staller K, Nee J, et al. Evaluating the impact of cost on the treatment algorithm for chronic idiopathic constipation: Cost-effectiveness analysis. Am J Gastroenterol. 2021;116(10):2118-27. doi:10.14309/ajg.0000000000001403 [DOI] [PubMed] [Google Scholar]
- 40.Claxton AJ, Cramer J, Pierce C. A. systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296-310. doi:10.1016/S0149-2918(01)80109-0 [DOI] [PubMed] [Google Scholar]
- 41.van Onzenoort HA, Menger FE, Neef C, et al. Participation in a clinical trial enhances adherence and persistence to treatment: A retrospective cohort study. Hypertension. 2011;58(4):573-8. doi:10.1161/HYPERTENSIONAHA.111.171074 [DOI] [PubMed] [Google Scholar]
- 42.US Food and Drug Administrations. Motegrity (prucalopride) approval letter. December 14, 2018. In:2018. Accessed June 17, 2024. www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210166Orig1s000Approv.pdf


