Abstract
Commitment of cells to apoptosis is governed largely by the interaction between members of the Bcl-2 protein family. Its three subfamilies have distinct roles: The BH3-only proteins trigger apoptosis by binding via their BH3 domain to prosurvival relatives, while the proapoptotic Bax and Bak have an essential downstream role involving permeabilization of organellar membranes and induction of caspase activation. We have investigated the regulation of Bak and find that, in healthy cells, Bak associates with Mcl-1 and Bcl-xL but surprisingly not Bcl-2, Bcl-w, or A1. These interactions require the Bak BH3 domain, which is also necessary for Bak dimerization and killing activity. When cytotoxic signals activate BH3-only proteins that can engage both Mcl-1 and Bcl-xL (such as Noxa plus Bad), Bak is displaced and induces cell death. Accordingly, the BH3-only protein Noxa could bind to Mcl-1, displace Bak, and promote Mcl-1 degradation, but Bak-mediated cell death also required neutralization of Bcl-xL by other BH3-only proteins. The results indicate that Bak is held in check solely by Mcl-1 and Bcl-xL and induces apoptosis only if freed from both. The finding that different prosurvival proteins have selective roles has notable implications for the design of anti-cancer drugs that target the Bcl-2 family.
Keywords: Apoptosis, Bcl-2, Bcl-xL, Mcl-1, Bak, BH3-only
How the Bcl-2 family of proteins regulate programmed cell death triggered by developmental cues and in response to multiple stress signals is of intense interest (Adams 2003; Danial and Korsmeyer 2004). Whereas cell survival is promoted by Bcl-2 itself and several close relatives (Bcl-xL, Bcl-w, Mcl-1, and A1), which bear three or four conserved Bcl-2 homology (BH) regions, apoptosis is driven by two other subfamilies. The initial signal for cell death is conveyed by the diverse group of BH3-only proteins, including Bad, Bid, Bim, Puma, and Noxa, which have in common only the small BH3 interaction domain (Huang and Strasser 2000). However, Bax or Bak (multidomain proteins containing BH1-BH3) are required for commitment to cell death (Lindsten et al. 2000; Cheng et al. 2001; Wei et al. 2001; Zong et al. 2001). When activated, they can permeabilize the outer membrane of mitochondria and release proapoptogenic factors (e.g., cytochrome c) needed to activate the caspases that dismantle the cell (Adams 2003; Danial and Korsmeyer 2004; Green and Kroemer 2004).
Interactions between members of these three factions of the Bcl-2 family dictate whether a cell lives or dies. When BH3-only proteins have been activated, for example, in response to DNA damage, they can bind via their BH3 domain to a groove on their prosurvival relatives (Sattler et al. 1997). How the BH3-only and Bcl-2-like proteins control the activation of Bax and Bak, however, remains poorly understood (Adams 2003; Danial and Korsmeyer 2004). Most attention has focused on Bax. This soluble monomeric protein (Hsu et al. 1997; Wolter et al. 1997) normally has its membrane-targeting domain inserted into its groove, probably accounting for its cytosolic localization (Suzuki et al. 2000; Schinzel et al. 2004). Several unrelated peptides/proteins have been proposed to modulate Bax activity (for review, see Lucken-Ardjomande and Martinou 2005), but their physiological relevance remains to be established. Alternatively, Bax may be activated via direct engagement by certain BH3-only proteins, the best documented being the active truncated form of Bid, tBid (Wei et al. 2000; Kuwana et al. 2002; Roucou et al. 2002). As discussed elsewhere (Adams 2003), the oldest model, in which Bcl-2 directly engages Bax (Oltvai et al. 1993), has become problematic because Bcl-2 is membrane bound while Bax is cytosolic, and their interaction seems highly dependent on certain detergents used for cell lysis (Hsu and Youle 1997). Nevertheless, it is well established that the BH3 region of Bax can mediate association with Bcl-2 (Zha and Reed 1997; Wang et al. 1998) and that Bcl-2 prevents the oligomerization of Bax, even though no heterodimers can be detected (Mikhailov et al. 2001). Thus, whether the prosurvival proteins restrain Bax activation directly or indirectly remains uncertain (see Discussion).
Although Bax and Bak seem in most circumstances to be functionally equivalent (Lindsten et al. 2000; Wei et al. 2001), substantial differences in their regulation would be expected from their distinct localization in healthy cells. Unlike Bax, which is largely cytosolic, Bak resides in complexes on the outer membrane of mitochondria and on the endoplasmic reticulum of healthy cells (Wei et al. 2000; Zong et al. 2003). Nevertheless, on receipt of cytotoxic signals, both Bax and Bak change conformation, and Bax translocates to the organellar membranes, where both Bax and Bak then form homo-oligomers that can associate, leading to membrane permeabilization (Hsu et al. 1997; Wolter et al. 1997; Antonsson et al. 2001; Wei et al. 2001; Mikhailov et al. 2003).
Since Bak, unlike Bax, is normally located at its site of action, how is it kept in check to prevent inappropriate cell death? We were prompted to investigate Bak regulation by recent evidence that it can form complexes with Mcl-1 (Cuconati et al. 2003) and that Mcl-1 is degraded at an early stage of apoptosis (Cuconati et al. 2003; Nijhawan et al. 2003). Here we report evidence from binding and functional studies that Bak is subject to negative regulation specifically by Mcl-1 and Bcl-xL but not other prosurvival family members. Thus, contrary to expectation, the prototypic guardian Bcl-2 is unable to prevent Bak activation. We show that stimuli from DNA damage drive BH3-only proteins to displace Bak from Mcl-1 and Bcl-xL, allowing Bak to self-associate and trigger apoptosis. We also report that the association of Noxa with Mcl-1 can trigger Mcl-1 degradation. Our demonstration that a subset of prosurvival family members controls Bak may explain the varied phenotypes observed on disruption of the prosurvival genes (Ranger et al. 2001) and has important implications for current efforts to develop drugs that regulate apoptosis by targeting the Bcl-2 family (Cory et al. 2003).
Results
Mcl-1 degradation promotes activation of Bax and Bak
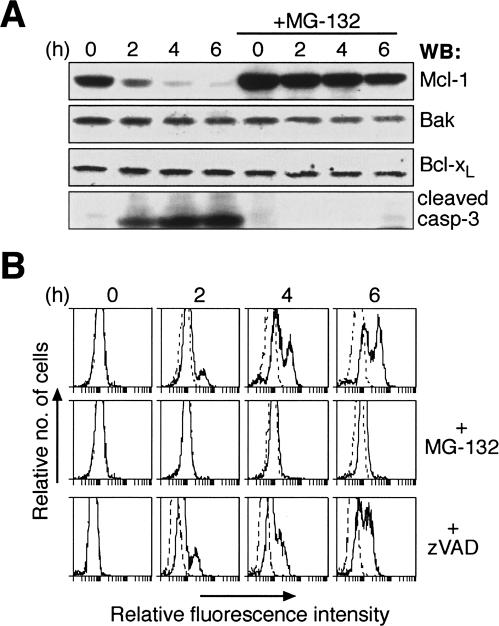
To investigate how DNA damaging agents provoke activation of Bak and Bax, we examined whether UV irradiation altered the expression of Bcl-2 family proteins in HeLa cells. Consistent with recent observations (Nijhawan et al. 2003), Mcl-1 was rapidly degraded following UV, and this was accompanied by caspase-3 processing (Fig. 1A). In contrast, the levels of Bcl-2, Bcl-xL, Bcl-w, Bax, and Bak remained unchanged (Fig. 1A; Hausmann et al. 2000; Wilson-Annan et al. 2003; data not shown). Pre-incubation of cells with the proteasome inhibitor MG-132 blocked UV-induced degradation of Mcl-1 and caspase-3 cleavage (Fig. 1A). By contrast, pretreating cells with the wide-spectrum caspase inhibitor zVAD.fmk did not impair Mcl-1 degradation (data not shown).
Figure 1.
UV irradiation promotes Mcl-1 degradation to trigger Bak activation. (A) Proteasome inhibition prevents UV-induced Mcl-1 degradation. Lysates prepared from untreated or UV-irradiated (200 J/m2) HeLa cells were resolved by SDS-PAGE and the resulting blot probed with the indicated antibodies. (Right panels) The rapid Mcl-1 degradation and caspase-3 cleavage after UV was blocked in cells pretreated with the proteasome inhibitor MG-132. (B) Abrogation of UV-induced Bak activation by proteasome inhibition. Bak activation detected by flow cytometric analysis of untreated or UV-irradiated HeLa cells stained with an antibody (Ab-1) that specifically recognizes activated Bak (Griffiths et al. 1999). Some cells were pretreated with the proteasome inhibitor MG-132 (middle) or the broad-spectrum caspase inhibitor zVAD.fmk (bottom). Controls (dotted histograms) represent cells stained with the secondary antibody alone.
As Bak and Bax levels were unaffected by UV (Fig. 1A; data not shown), we tested if their activation was some-how related to Mcl-1 degradation. Both Bak (Griffiths et al. 1999) and Bax (Hsu et al. 1997; Wolter et al. 1997; Hsu and Youle 1998) change conformation when activated by numerous stress stimuli, and these changes can be readily detected in permeabilized cells using antibodies that recognize only the activated conformers of Bak (clone Ab-1) (Griffiths et al. 1999) or Bax (clone 3) (Dewson et al. 2003). Following UV irradiation, flow cytometric analysis revealed that a population of cells harboring activated Bak appeared within 2 h and accumulated subsequently (Fig. 1B, upper panels). Strikingly, pretreatment with the proteasome but not the caspase inhibitor prevented Bak activation (Fig. 1B). Activation of Bax followed similar kinetics (Supplementary Fig. S1A), and it then translocated into the pellet fraction accompanied by cytochrome c release (Supplementary Fig. S1B). Importantly, the activation of Bax (as detected using conformation-specific antibodies), its translocation and the cytochrome c release were all prevented by proteasome inhibition (Supplementary Fig. S1). Thus, the Mcl-1 degradation triggered in HeLa cells by UV (Nijhawan et al. 2003) is closely coupled to activation of Bak and Bax.
In fibroblasts UV-induced apoptosis is mediated primarily by Bak, not Bax
As either Bax or Bak can carry out almost all cytotoxic responses (Lindsten et al. 2000; Cheng et al. 2001; Wei et al. 2001; Zong et al. 2001), we anticipated that either protein would mediate UV killing equally. Since UV irradiation of mouse embryo fibroblasts (MEFs) also resulted in Mcl-1 degradation (data not shown), we could also assess the relative roles of Bax and Bak in this response using MEFs that contained only one of these proteins.
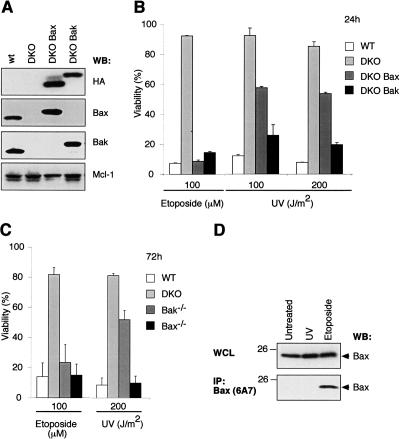
We first compared the sensitivity of immortalized wild-type MEFs with those lacking both Bax and Bak (DKO) and DKO clones engineered to express either HA-tagged Bax (DKO Bax) or Bak (DKO Bak). The levels of exogenous HA-tagged Bax and Bak in these lines were comparable, as judged by HA staining, and marginally higher than that of the endogenous proteins (Fig. 2A). As expected, loss of both Bax and Bak rendered wild-type MEFs resistant to killing induced by etoposide, while the DKO MEFs expressing either Bax or Bak regained high sensitivity (Fig. 2B). Unexpectedly however, the sensitivity of DKO MEFs to UV irradiation was restored to a much greater extent by re-expression of Bak compared with Bax (Fig. 2B), indicating that Bak has a more central role in this response.
Figure 2.
UV irradiation kills MEFs predominantly by a Bak-dependent, not Bax-dependent, mechanism. (A) Expression of Bax or Bak in MEFs. Immunoblot analysis of lysates prepared from immortalized wild-type MEFs, ones lacking Bax and Bak (DKO), or DKO subclones reconstituted with HA-tagged Bax (DKO Bax) or Bak (DKO Bak), using antibodies to HA (to specifically detect transgene expression), Bax, Bak, or Mcl-1. (B) Killing of immortalized MEFs by UV depends primarily on Bak, rather than Bax. Whereas exposure to 100 μM etoposide for 24 h caused comparable killing of Bax- or Bak-expressing MEFs (described in A), far more Bak-expressing than Bax-expressing cells died 24 h after exposure to UV (doses indicated). (C) UV-induced killing of primary MEFs is mainly mediated by Bak. Primary MEFs (derived independently from those used in A,B) were challenged with UV or etoposide. In B and C, cell viability was assessed by flow cytometric analyses after staining with propidium iodide (PI); the data represent mean ± SD from three independent experiments. (D) Unlike etoposide treatment, UV does not cause significant Bax activation in transformed MEFs. Lysates prepared from untreated Bak-/- MEFs, or 24 h after UV or etoposide treatment, were immunoblotted for total Bax (top) or for activated Bax (bottom) after immunoprecipitating with the conformation-specific antibody 6A7.
To preclude any confounding effects due to immortalization of the MEFs, we also tested freshly isolated, non-transformed MEFs. The UV-induced death of the primary fibroblasts also proceeded mainly via Bak; 3 d after a high dose of UV (200 J/m2), >90% of the fibroblasts expressing Bak alone (i.e. Bax-/- MEFs) were dead, whereas >50% of the Bax-expressing (Bak-/-) fibroblasts remained alive (Fig. 2C). Thus, whereas Bax and Bak are equally proficient in mediating apoptosis induced by etoposide, Bak plays the dominant role in UV-induced killing of MEFs. In accord with these killing assays, Bax was activated in Bak-/- fibroblasts by etoposide treatment but not by UV at this time point (Fig. 2D). Thus, in MEFs, unlike HeLa cells (Fig. 1; Supplementary Fig. S1), UV predominately activates Bak.
In healthy cells, Bak associates specifically with Mcl-1 and Bcl-xL
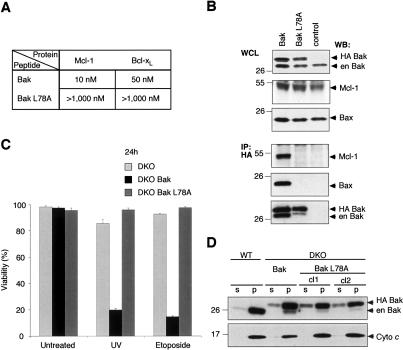
To account for the unique role of Bak in the UV-induced apoptosis of MEFs, we reasoned that Bak might be directly regulated by a restricted subset of the prosurvival Bcl-2-like proteins. If so, it seemed likely that their association would be mediated by binding of the Bak BH3 domain to the groove on the latter (Sattler et al. 1997). Therefore, we first tested, in solution competition assays using a Biacore optical biosensor, whether a (26-mer) peptide spanning the BH3 region of Bak could bind recombinant prosurvival Bcl-2-like proteins. Strikingly, the BakBH3 peptide bound tightly to Mcl-1 and Bcl-xL but only weakly to Bcl-w and not detectably to Bcl-2 (Fig. 3A).
Figure 3.
Bak is sequestered by Mcl-1 and Bcl-xL in healthy cells. (A) Tight binding of Bak BH3 to Mcl-1 and Bcl-xL. Using solution competition assays, the relative affinities (IC50 in nanomolar) of a BakBH3 peptide for prosurvival Bcl-2 proteins were determined (Chen et al. 2005). The results (plotted on an inverse log scale) are from representative experiments; the variation observed in multiple experiments was less than two-fold (using different chips or protein batches). (†) IC50 > 1000 nM. (B) Overexpressed Mcl-1 and Bcl-xL bind endogenous Bak. N-terminally Flag-tagged prosurvival proteins were overexpressed (top) in 293T cells and their capacity to bind endogenous Bak (middle) was tested by coimmunoprecipitation (bottom) using an anti-Flag affinity resin. (Control) Immunoprecipitation from untransfected cells; (en) endogenous; (★) an Mcl-1 break-down product; (★★) immunoglobulin light chain from the immunoprecipitating antibody. (C) Mcl-1, Bcl-xL, and Bak are present in the pellet fraction of healthy cells. HeLa cells, lysed in 0.025% digitonin, were fractionated into soluble (s) and pellet (p) fractions, and probed for the indicated proteins. Note that Bax, unlike Bak, is present mainly in the soluble fraction. (D) Endogenous Bcl-xL and Mcl-1 associate with endogenous Bak in healthy cells. HeLa cells (lysed in 0.025% digitonin) were fractionated into soluble (s) and pellet (p) fractions. The pellet fraction was solubilized in buffer containing Triton X-100, immunoprecipitated with anti-Mcl-1 (left), anti-Bcl-xL (right), or isotype-matched control antibodies, and examined for the presence of Bak (middle) or Bax (bottom).
Since an isolated BakBH3 peptide had high affinity for Mcl-1 and Bcl-xL, we assessed whether any of the prosurvival proteins bind full-length Bak by coimmunoprecipitation from cell lysates. Immune complexes isolated from 293T cells overexpressing comparable amounts of Mcl-1, Bcl-2, Bcl-xL, Bcl-w, or A1 were tested for associated endogenous Bak. In accord with our affinity measurements (Fig. 3A), Mcl-1 and Bcl-xL bound Bak, but no significant binding was observed between Bak and Bcl-2, Bcl-w, or A1 (Fig. 3B). Furthermore, subcellular fractionation showed that most endogenous Mcl-1 and Bcl-xL colocalized with Bak to the membrane-associated (pellet) fraction of healthy HeLa cells (Fig. 3C). In contrast to the lack of interaction between Bcl-2 and Bak (Fig. 3A,B), endogenous Bak was found in complex with endogenous Mcl-1 and Bcl-xL in the pellet fraction (Fig. 3D). As expected (Hsu et al. 1997; Wolter et al. 1997; Hsu and Youle 1998), Bax was predominantly cytosolic (Fig. 3C), and the small portion of membrane-associated Bax in healthy cells was not complexed with Mcl-1 or Bcl-xL (Fig. 3D).
The association of prosurvival proteins with Bax is promoted when nonionic detergents (e.g., Triton X-100) but not certain others (e.g., CHAPS) are used for cell lysis (Hsu and Youle 1997). No such findings have been reported for Bak, and we showed that Bak formed complexes with Mcl-1 and Bcl-xL in lysates made with Triton X-100 (Fig. 3B,D). In healthy cells, Bak has also been shown to associate with Mcl-1 in the presence of CHAPS (Cuconati et al. 2003; Leu et al. 2004), an observation we have replicated with Mcl-1 (data not shown) and Bcl-xL (Supplementary Fig. S2). Collectively, these interaction and localization studies suggest that in healthy cells Mcl-1 and Bcl-xL directly sequester Bak. In contrast, Bax was not bound by either prosurvival protein (Fig. 3D).
Bak BH3 is required for both its sequestration by Mcl-1/Bcl-xL and its dimerization and killing activity
Our binding studies (Fig. 3A) indicate that the BH3 domain of Bak mediates its association with Mcl-1 and Bcl-xL. As expected (Sattler et al. 1997), the binding of the BH3 peptide to these proteins was greatly impaired when the highly conserved leucine in the Bak BH3 (L78) was replaced by alanine (Fig. 4A). To explore whether the Bak BH3 domain is required for association of the full-length proteins, we engineered the L78A mutation into Bak. Importantly, this mutation ablated the interaction of Bak with Mcl-1 (Fig. 4B).
Figure 4.
Bak BH3 is required for interaction with Mcl-1 and Bcl-xL, and for proapoptotic function. (A) A point mutation within Bak BH3 abrogates interaction with Mcl-1 and Bcl-xL. Using solution competition assays, the relative affinities (IC50 in nanomolar) of Bak and mutant Bak L78A peptides for Mcl-1 and Bcl-xL were determined. (B) Bak L78A fails to heterodimerize with Mcl-1 or homodimerize. N-terminally HA-tagged wild-type Bak or mutant Bak L78A were transiently expressed in 293T cells (top) and tested for their ability to bind endogenous Mcl-1, Bax or Bak (bottom) by coimmunopreciptation using anti-HA affinity resin. (Control) Immunoprecipitation from untransfected cells; (en) endogenous. (C) L78A mutation inactivates Bak proapoptotic function. Viability was determined for Bax/Bak-deficient (DKO) MEFs or ones containing introduced Bak or Bak L78A, left untreated or 24 h after UV or etoposide treatment. Data represent mean ± SD from three independent experiments. (D) L78A mutant Bak, like wild-type Bak, localizes to the pellet fraction. Wild-type MEFs or Bax/Bak-deficient ones expressing wild-type Bak or mutant Bak L78A (two independent clones) were fractionated (in digitonin-containing buffer) into soluble (s) and pellet (p) fractions, and probed for Bak (top) or cytochrome c (bottom).
The BH3 region of Bak seems to be required not only for its interaction with other Bcl-2 family members but also for its proapoptotic function (Chittenden et al. 1995). Various apoptotic stimuli induce Bak to associate into homo-oligomers and to form higher-order complexes that also contain Bax (Wei et al. 2001; Mikhailov et al. 2003). Formation of these complexes is thought to be critical for the killing activity of Bak (and Bax). Interestingly, while overexpressed wild-type Bak readily associated with endogenous Bax or Bak, the Bak L78A mutant failed to do so significantly (Fig. 4B). This result suggests that the BH3 region of Bak is essential for its oligomerization (see Discussion).
In accord with the inability of Bak L78A to associate with itself or with Bax (Fig. 4B), the mutant proved to lack proapoptotic activity. Whereas wild-type Bak readily restored the sensitivity of MEFs lacking Bax and Bak (DKO MEFs) to apoptotic stimuli (Figs. 2, 4C), the L78A mutant was inert (Fig. 4C), even though it was expressed at levels comparable to the wild-type protein and was also located in the membrane-associated compartment (Fig. 4D). On the proviso that this mutation does not unexpectedly impair Bak folding, our results indicate that the BH3 domain of Bak is required not only for its sequestration by prosurvival proteins, but also for its oligomerization and hence its proapoptotic activity.
Noxa can both displace Bak from Mcl-1 and promote Mcl-1 degradation
A BakBH3 peptide binds tightly to the hydrophobic groove on Bcl-xL (Sattler et al. 1997), and the very similar hydrophobic groove demonstrated recently in Mcl-1 (Day et al. 2005) presumably is responsible for the observed Bak BH3 binding (Figs. 3A, 4A). As the BH3 regions of the BH3-only proteins also target these grooves (Liu et al. 2003), their binding to Mcl-1 may well displace Bak.
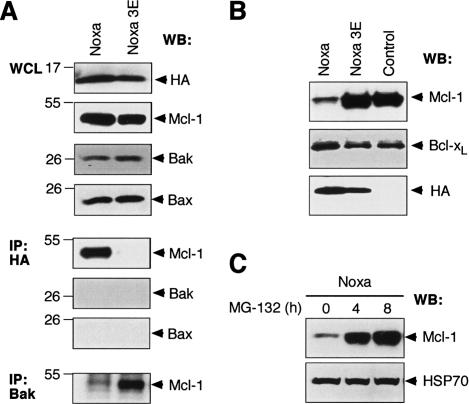
To test this model, we have focused on the BH3-only protein Noxa, because UV irradiation of MEFs leads to elevated levels of Noxa (Oda et al. 2000; Shibue et al. 2003), and we have recently shown that Noxa binds tightly to Mcl-1 but not significantly to Bcl-2, Bcl-xL, or Bcl-w (Chen et al. 2005). Hence, we explored how increased Noxa expression affected the composition of endogenous Bak immune complexes, initially in 293T cells. Consistent with the proposed model, overexpressed Noxa bound Mcl-1 tightly and displaced Bak. In contrast, the inert Noxa mutant 3E, which cannot bind Mcl-1 due to three mutations in its BH3 region (Chen et al. 2005), was unable to do so (Fig. 5A). Furthermore, a Noxa BH3 peptide can, in a dose-dependent manner, reduce binding of in vitro translated Bak to recombinant GST-Mcl-1 (Supplementary Fig. S3). Since Noxa itself does not directly bind Bak or Bax (Fig. 5A), this BH3-only protein is likely to promote Bak activation by displacing it from Mcl-1.
Figure 5.
Proapoptotic BH3-only protein Noxa displaces Bak from Mcl-1 and triggers Mcl-1 destruction. (A) Noxa displaces Bak from Mcl-1. N-terminally HA-tagged wild-type Noxa or the inert mutant Noxa 3E was transiently expressed in 293T cells, and the impact of Noxa expression on Mcl-1/Bak complex formation was assessed. (Bottom) Wild-type, but not mutant, Noxa bound Mcl-1 (fifth panel), disrupting the complex between Mcl-1 and Bak. The 293T cells were used because Mcl-1 is very stable in them. (B) Noxa triggers Mcl-1 degradation. Immunoblot of lysates prepared from Bax/Bak doubly deficient MEFs retrovirally infected with HA-tagged wild-type Noxa or mutant Noxa 3E was probed with antibodies to Mcl-1 (top), Bcl-xL (middle), or HA (bottom, to detect transgene expression). (Control) Uninfected MEFs. (D) Noxa-induced Mcl-1 degradation is proteasome dependent. A blot of lysates prepared from a Noxa-expressing fibroblast line (described in B) after treatment with the proteasome inhibitor MG-132 for different times was probed for Mcl-1 (top) and HSP70 (bottom; loading control).
Unexpectedly, enforced Noxa expression in transformed MEFs also triggered marked degradation of Mcl-1, whereas the level of Bcl-xL was unaffected (Fig. 5B). This Mcl-1 degradation, like that observed following UV treatment (Fig. 1), required proteasome activity (Fig. 5C). It also seems to require association of the proteins, because DKO MEF cells transduced with a Noxa retrovirus had lost most of their Mcl-1, whereas Mcl-1 was spared in cells infected with the nonbinding mutant Noxa 3E (Fig. 5B). Furthermore, in noxa-deficient MEFs, Mcl-1 levels were elevated and its degradation upon UV irradiation was reduced (Supplementary Fig. S4). Thus, Noxa seems to play a key role in the control of Mcl-1 turnover in healthy fibroblasts as well as during an apoptotic stimulus.
These surprising and novel findings suggest that Noxa not only displaces Bak from Mcl-1 (Fig. 5A) but also promotes Mcl-1 degradation (Fig. 5B,C), both of which can contribute to Bak activation. However, neutralization of Mcl-1 alone is not sufficient to mediate Bak activation and cell death, as neither overexpression of Noxa, which induces Mcl-1 degradation, nor its down-regulation by RNAi (Cuconati et al. 2003; Nijhawan et al. 2003) suffices to trigger apoptosis. Conversely, noxa deficiency only confers limited protection to MEFs from UV-induced apoptosis (Shibue et al. 2003), unlike the marked protection afforded by the loss of both Bax and Bak (Fig. 2). These observations suggest that Mcl-1 is unlikely to be the sole guardian of Bak, and that UV must trigger the activation of other BH3-only proteins that neutralize one or more other guardians of Bak.
Neutralization of both Mcl-1 and Bcl-xL drives efficient Bak-mediated apoptosis
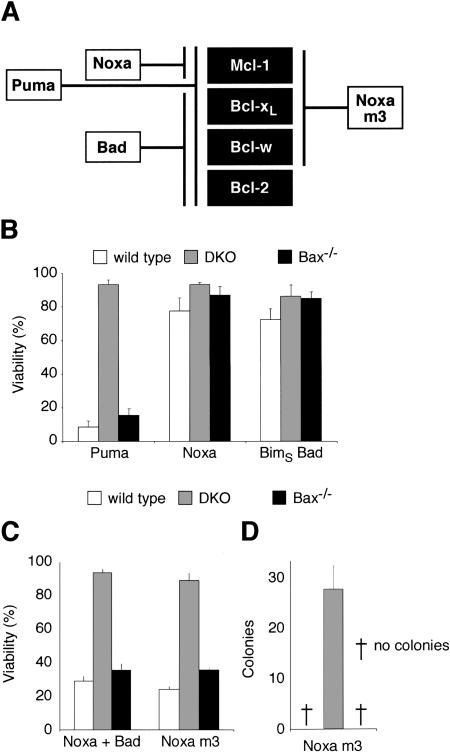
Our binding studies (Fig. 3) implicate Bcl-xL as a second prosurvival regulator of Bak, but MEFs also express Bcl-2 and Bcl-w, albeit not A1 (Supplementary Fig. S5; Chen et al. 2005). To determine which prosurvival proteins govern Bak-mediated death, we took advantage of our recent finding that different BH3-only proteins target particular subsets of the Bcl-2-like proteins (Fig. 6A). As expected, Puma, which targets all prosurvival proteins, killed Bak-expressing (Bax-/-) MEFs as effectively as wild-type cells, but Bax/Bak-deficient cells were spared (Fig. 6B). In contrast, no significant apoptosis was induced by either Noxa, which targets only Mcl-1, or by BimSBadBH3 (BimS with its BH3 replaced with that of Bad), which targets Bcl-xL, Bcl-w, and Bcl-2 (Fig. 6B), even though both of the BH3-only proteins were adequately expressed (Supplementary Fig. S6). Significantly, however, the Noxa plus Bad combination, which together neutralizes all four of these prosurvival proteins (Fig. 6A), induced potent Bak-mediated apoptosis (Fig. 6C).
Figure 6.
Neutralization of Mcl-1 and Bcl-xL triggers Bak-dependent apoptosis. (A) Selective binding profiles of Bad, Noxa, and Noxa m3, based on interaction studies (Chen et al. 2005). Puma binds all prosurvival proteins tested; Bad binds tightly to Bcl-xL, Bcl-w, and Bcl-2, whereas Noxa selectively targets Mcl-1. In addition to Mcl-1, Noxa m3 also binds Bcl-xL and Bcl-w, but its affinity for Bcl-2 is insignificant (>10,000 nM). (B) Puma, but not Noxa or BadBH3, is sufficient to induce Bak-mediated apoptosis. Wild-type MEFs, Bax and Bak doubly deficient MEFs (DKO), or MEFs lacking only Bax were infected with the indicated retroviruses. The BadBH3 was tested within an inert BimS backbone (Chen et al. 2005) to preclude any effects due to regulation of the Bad protein. Expression of each BH3-only protein was linked via an IRES to that of GFP, and the viability of GFP+ve cells was determined by PI exclusion 24 h after infection. (C) The weak killing activity of Noxa, which only targets Mcl-1, can be complemented by neutralization of Bcl-xL. The indicated MEFs were infected with retroviruses coexpressing Noxa and BimSBadBH3 (Chen et al. 2005). The combination of the BadBH3 (which neutralizes Bcl-2, Bcl-xL, and Bcl-w; see A) and Noxa gives potent Bak-dependent killing. Retroviral infection with Noxa m3 caused comparable killing of wild-type MEFs and those only expressing Bak. (A) As Noxa m3 binds Mcl-1, Bcl-xL, and Bcl-w but not Bcl-2, targeting of these prosurvival proteins suffices for Bak-mediated apoptosis, whereas neutralization of Bcl-2 is not required. (D) Bcl-2 is not required for killing by Noxa m3 in long-term colony assays. Equivalent numbers of retrovirally infected cells were plated and the number of colonies formed scored 6 d later. Data in B-D represent mean ± SD from three independent experiments.
We also tested a Noxa mutant (Noxa m3) engineered to engage Bcl-xL (and Bcl-w) in addition to Mcl-1 (Fig. 6A; Chen et al. 2005). Noxa m3 efficiently killed the fibroblasts in a Bak-dependent manner in both a short-term assay (Fig. 6C) and in a long-term assay of colony formation (Fig. 6D). Since Noxa m3 does not bind Bcl-2, we conclude that Bak can be activated and cell death induced without neutralizing Bcl-2. Moreover, both Bcl-2 and Bcl-w appear irrelevant to the direct control of Bak, because neither bound Bak (Fig. 3).
Loss of Bcl-xL, but not Bcl-2, sensitizes MEFs to Noxa killing
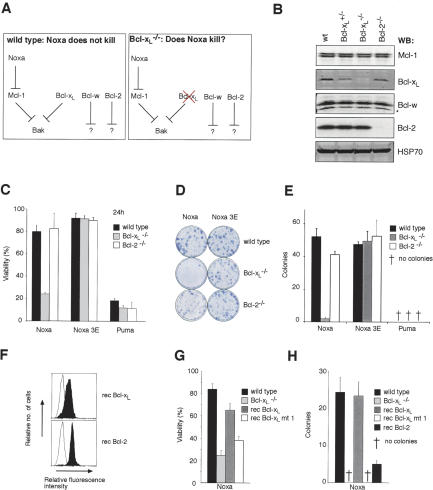
These findings led us to hypothesize that Mcl-1 and Bcl-xL are the only direct regulators of Bak. Since Noxa only antagonizes Mcl-1, the poor killing of wild-type MEFs by Noxa (Fig. 6B) would be explained if Bcl-xL acts as a second brake on Bak activation. If so, loss of Bcl-xL should sensitize MEFs to Noxa-induced killing, whereas loss of Bcl-2 should have no impact (Fig. 7A).
Figure 7.
Loss of Bcl-xL, but not Bcl-2, sensitizes MEFs to Noxa killing. (A) Hypothesis for Bak regulation. If Bak is regulated by Mcl-1 and Bcl-xL but not Bcl-2 or Bcl-w, wild-type MEFs may be resistant to Noxa killing because it only targets Mcl-1, allowing Bcl-xL to keep Bak in check. This hypothesis predicts that Noxa will kill MEFs lacking Bcl-xL but not those lacking Bcl-2. (B) Expression of Bcl-2 prosurvival proteins in MEFs. A blot of lysates prepared from wild-type, Bcl-xL+/-, Bcl-xL-/-, and Bcl-2-/- MEFs was probed with antibodies to Mcl-1, Bcl-xL, Bcl-w, Bcl-2, and HSP70 (loading control). (★) Bcl-w break-down product. (C) Noxa potently kills Bcl-xL-null MEFs. Wild-type, Bcl-xL-/-, or Bcl-2-/- MEFs were infected with the indicated retroviruses and cell viability was assessed after 24 h by flow cytometry. (D) Representative plates of colonies formed after infection with the indicated retroviruses. Noxa expression results in scant Bcl-xLdeficient colonies but does not affect Bcl-2-/- MEFs. (E) Bcl-xL-deficiency prevents the formation of Noxa-expressing colonies. Quantification of the representative data shown in D. (F) Reconstituting expression of prosurvival proteins in Bcl-xL-/- MEFs. Flow cytometric analysis for the expression of Flag-tagged Bcl-xL or Bcl-2 (filled histograms) stably expressed in Bcl-xL-/- MEFs (unfilled histogram). (G) Restoring expression of wild-type Bcl-xL, but not Bcl-xL mt 1, renders Bcl-xL-/- MEFs resistant to Noxa killing in a short-term assay. (H) Bcl-xL expression, but not overexpression of Bcl-xL mt 1 or Bcl-2, inhibits Noxa killing of Bcl-xL-/- in long-term assay of colony formation. Data in C, E, G, and H represent mean ± SD from three independent experiments.
To test this model, we compared the effect of forced Noxa expression on wild-type MEFs and MEFs lacking Bcl-xL or Bcl-2 (Fig. 7B). In accord with our hypothesis, Bcl-xL-deficient MEFs infected with a Noxa virus died rapidly (Fig. 7C) and failed to form colonies (Fig. 7D,E). Consistent with earlier findings (Fig. 5A), Noxa bound Mcl-1 but not Bax or Bak in dying Bcl-xL-/- MEFs (Supplementary Fig. S7). Importantly, Noxa failed to kill Bcl-2-deficient MEFs (Fig. 7E). When Bcl-xL was reintroduced into Bcl-xL-null cells (Fig. 7F), resistance to Noxa killing was restored (Fig. 7G) and clonogenic survival was markedly augmented (Fig. 7H). In contrast, neither a mutant of Bcl-xL that does not bind Bak (mt 1) (Cheng et al. 1996) nor Bcl-2 impacted upon survival (Fig. 7H). From these findings, we conclude that Bak can be constrained only by its direct guardians, Mcl-1 and Bcl-xL, and that Bcl-2 is irrelevant for Bak-mediated cell death (Fig. 7A).
Discussion
Although cell death depends on the multidomain proapoptotic proteins Bax and Bak (Lindsten et al. 2000; Rathmell et al. 2002), how other members of the Bcl-2 protein family control their activation has been unclear (Adams 2003; Danial and Korsmeyer 2004). Here we show that Bak is subject to a distinctive mode of regulation involving its direct sequestration by two of its prosurvival relatives. Both binding experiments (Figs. 3, 4, 5) and functional cell death assays (Figs. 6, 7) revealed that Mcl-1 and Bcl-xL keep Bak in check (Fig. 8). When cytotoxic signals activate BH3-only proteins that can engage both Mcl-1 and Bcl-xL, Bak-mediated apoptosis ensues. Accordingly, we show that inactivation of both these prosurvival proteins by the appropriate BH3-only protein, or a combination of them, is necessary and sufficient for Bak-mediated cell death. Once Bak is freed from both Mcl-1 and Bcl-xL, it can presumably oligomerize in the organellar membranes, such as the outer mitochondrial membrane, producing damage that compromises organellar function and promotes caspase activation (Fig. 8; Adams 2003; Green and Kroemer 2004).
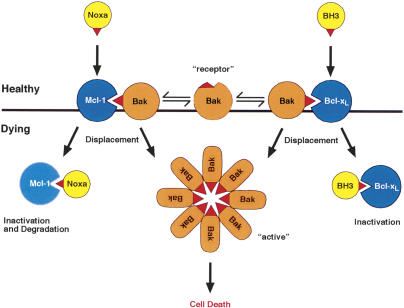
Figure 8.
Model for Bak regulation. The central proposal of the model is that both Mcl-1 and Bcl-xL, but not other prosurvival family members (e.g., Bcl-2), bind Bak in healthy cells until cytotoxic signals activate a combination of BH3-only proteins that can displace Bak (see Discussion). While Noxa can readily displace Bak from Mcl-1 and promote its degradation, another BH3-only protein that can bind Bcl-xL (BH3) is also required for Bak liberation. The Bak BH3 (red beak) is required for both Bak regulation and for formation of Bak oligomers. When freed, it might directly mediate Bak association. Alternatively, if Bak also exists (as shown) as a “receptor” conformer, dimerization of the two conformers via the exposed Bak BH3 might nucleate oligomerization (see Discussion).
Sequestration of Bak by Mcl-1 and Bcl-xL
Although Bax and Bak seem in most circumstances to be functionally equivalent (Lindsten et al. 2000), the finding that UV-induced killing of MEFs depends largely on Bak, but not Bax (Fig. 2), suggested that these two molecules must be regulated differently and that Bak might be controlled by a specific subset of its prosurvival relatives. Indeed, our binding studies, involving both in vitro studies with a BakBH3 peptide and coimmunoprecipitation of proteins from cell lysates (Figs. 3, 4), implicate direct sequestration of Bak by Mcl-1 and Bcl-xL. Notably, endogenous membrane-bound Bak was complexed with both endogenous Mcl-1 and Bcl-xL (Fig. 3D). In accord with these results, Bak has been shown to associate with Mcl-1 (Bae et al. 2000) and Bcl-xL (Farrow et al. 1995) in the yeast two-hybrid system, and in healthy mammalian cells, complexes of Bak with Mcl-1 have been detected using different antibodies and lysis conditions (Cuconati et al. 2003; Leu et al. 2004). In fact, Bak may well be the major partner of Mcl-1 in healthy cells, because in a large-scale coimmunoprecipitation experiment that used well-established methods (Verhagen et al. 2000) to isolate Mcl-1-interacting partners and identify them by mass spectroscopy, Bak was the only associated family member found (A. Verhagen, pers. commun.).
Notably, we have found no evidence that Bak associates significantly with any of the other prosurvival Bcl-2 proteins (Figs. 3, 4). That conclusion is consistent with previous reports that Bak does not bind significantly to Bcl-2 (Farrow et al. 1995; Cheng et al. 2003) or A1 (Werner et al. 2002). Thus, binding studies strongly suggest that Bak activity is regulated directly and specifically by both Mcl-1 and Bcl-xL, but not by any other Bcl-2 prosurvival family member. Pertinently, both Mcl-1 and Bcl-xL were identified biochemically as inhibitors of mitochondrial damage after DNA damage (Nijhawan et al. 2003), and we show that Bak is their critical, albeit probably not exclusive, target (see below).
The functional cell death assays with fibroblasts (Figs. 6, 7) strongly support our conclusion that Bak regulation relies on both Mcl-1 and Bcl-xL. As reported recently (Cuconati et al. 2003; Nijhawan et al. 2003), Mcl-1 degradation is often prominent early in apoptosis and essential for it to occur (Fig. 1). Mcl-1 loss, however, is clearly insufficient to trigger Bak-mediated apoptosis, because cell death does not ensue on down-regulation of Mcl-1 by either RNAi (Cuconati et al. 2003; Nijhawan et al. 2003) or overexpression of Noxa, which binds tightly to Mcl-1 and induces its degradation (Fig. 5). Strikingly, however, Bcl-xL-deficient cells were readily killed by Noxa (Fig. 7). Furthermore, a Noxa mutant (Noxa m3) engineered to bind both Mcl-1 and Bcl-xL could kill in a Bak-dependent manner (Fig. 6).
Once released from sequestration by Mcl-1 (Fig. 5) and Bcl-xL, Bak can associate with itself and with Bax, as has been reported previously (Sundararajan et al. 2001; Mikhailov et al. 2003), and induce killing, processes that depend upon its BH3 domain (Fig. 4; Chittenden et al. 1995). The binding and functional studies imply that, unlike soluble monomeric Bax, in which the BH3 domain is buried (Suzuki et al. 2000), in at least a proportion of Bak molecules the BH3 domain must protrude in healthy cells, consistent with the idea that Bak is primed to kill but prevented from doing so by Mcl-1/Bcl-xL (Fig. 8).
The basis for oligomerization of Bak (or Bax) is still unknown. However, the greatly impaired association of the Bak L78A mutant with endogenous Bak (Fig. 4B) indicates that the Bak BH3 has a critical role, and two possibilities merit consideration. One is that the exposed BH3 domains, when not bound to Mcl-1/Bcl-xL, directly associate (Fig. 8). The other is that the free BH3 promotes formation of a Bak homo-dimer by BH3-groove interaction. That is, Bak might well exist in the membrane not only as a conformer with its BH3 domain protruding (a “ligand” form) but also as one with the BH3 buried (a “receptor” form) (Fig. 8), as in the 3D structure of soluble Bax (Suzuki et al. 2000). When the ligand conformer is displaced from Mcl-1/Bcl-xL, its BH3 could bind to the groove on the receptor conformer. The Bak-Bak dimer could then nucleate oligomerization, which presumably requires interaction via another surface of Bak and/or participation of another protein.
An alternative model for Bak regulation has been proposed involving its sequestration in healthy cells by VDAC2, a voltage-dependent anion channel in the outer mitochondrial membrane (Cheng et al. 2003). Although the basis for Bak-VDAC2 association remains to be established, our results do not exclude some role for VDAC2 in Bak regulation, since Bak might be involved in more than one complex. VDAC2 might, for example, target the proposed receptor conformer of Bak. Any negative regulatory role of VDAC2 for Bak, however, presumably must be less critical than that of Mcl-1/Bcl-xL, because their combined absence provoked apoptosis (Figs. 6, 7), whereas absence of VDAC2 does not (Cheng et al. 2003). Furthermore, sequestration by VDAC2 would only account for Bak located on the outer mitochondrial membrane but not that on other membranes (Zong et al. 2003).
Does regulation of Bax share any features of Bak regulation? A model in which Bax is directly sequestered in unstressed cells by prosurvival proteins like Bcl-2 would be difficult to support, because Bax is predominantly a monomeric cytosolic protein (Hsu et al. 1997; Wolter et al. 1997). Nevertheless, the strong evidence that Bax can, under certain conditions, associate with Bcl-2 (Oltvai et al. 1993) and that its BH3 region is required for that association (Zha and Reed 1997; Wang et al. 1998) argues for some similarity with the regulation of Bak. It is tempting to speculate that, at an early stage cell stress (and perhaps even in unstressed cells), a small proportion of Bax can assume a BH3-exposed, “ligand” conformation and translocate to mitochondria. This conformer could then be engaged by certain members of the prosurvival family, until BH3-only proteins neutralize them. In this model, the control of Bax activation would be some-what akin to that proposed for Bak (Fig. 8). Although quantitative data are lacking, Bax can form heterodimers via its BH3 domain with Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1 (e.g., Wang et al. 1998). Thus most, if not all, of the prosurvival family members may well participate in Bax regulation.
Prosurvival Bcl-2 proteins are not functionally equivalent
It has generally been assumed that the mammalian prosurvival proteins are functionally equivalent and hence that the life-death decision for a given cell rests simply on the balance between their total abundance, versus the abundance of all proapoptotic family members. Our results suggest instead that only Bcl-xL and Mcl-1 control Bak, and hence that cells lacking both these guardians are fated to die (Fig. 8). Contrary to expectation, the prototypic guardian Bcl-2 is unable to prevent Bak activation (Fig. 7).
Our sequestration model for Bak helps to explain why gross developmental defects arise in mice that lack either Mcl-1 or Bcl-xL, whereas the absence of Bcl-2, Bcl-w, or A1 provokes defects in specific tissues (Ranger et al. 2001). Mice constitutively void of Mcl-1 die at the blastocyst stage (Rinkenberger et al. 2000), and we suggest that this extreme phenotype may well reflect concomitant deficiency of Bcl-xL in a critical early cell type, freeing Bak to kill cells. Similarly, the marked degeneration of lymphoid cells with mcl-1 conditionally deleted (Opferman et al. 2003) would be attributable to the minimal levels of Bcl-xL found at particular stages of B- and T-cell development (Marsden and Strasser 2003), and the decimation of myeloid progenitor and stem cells deprived of Mcl-1 (Opferman et al. 2005) can be accounted for analogously. Conversely, the massive apoptosis of erythroid cells in bcl-x-/- embryos (Motoyama et al. 1995) may well reflect absence of Mcl-1 in that cell type. We surmise that the massive cell death in all these cases reflects unleashed Bak. Nevertheless, substantial overlap in the regulation of Bak and Bax is likely. For example, Bcl-xL must also control Bax, at least in neurons, since loss of bax in this tissue ameliorates the extensive apoptosis caused by Bcl-xL deficiency (Shindler et al. 1997).
Recently, it has been suggested that Mcl-1 and Bcl-xL may function in a hierarchical manner (Nijhawan et al. 2003). Our studies are compatible with such a model, because Bak appears to bind Mcl-1 with several fold higher affinity than Bcl-xL (Fig. 3A). A backup role for Bcl-xL would be consistent with observations that, in some cells, a proportion of Bcl-xL is free (Jeong et al. 2004). How much Bak is bound to Bcl-xL in healthy cells presumably depends on the relative affinities and amounts of Mcl-1, Bcl-xL and Bak. After a death stimulus, as Mcl-1 is degraded, more Bcl-xL may bind Bak, until BH3-only proteins inactivate Bcl-xL.
BH3-only proteins can initiate apoptosis by displacing Bak from Mcl-1/Bcl-xL sequestration
As discussed elsewhere (Adams 2003), one of several plausible models for the initiation of cell death posits that, once BH3-only proteins have overwhelmed their prosurvival relatives, certain BH3-only proteins then directly bind Bax or Bak to initiate their activation (Kuwana et al. 2002; Letai et al. 2002; Roucou et al. 2002). For Bak, our results do not support that model, because Noxa, which does not bind to either Bak or Bax, in either healthy cells (Fig. 5A) or cells subjected to an apoptotic stimulus (Supplementary Fig. S7), elicits Bak-dependent apoptosis when Bcl-xL is absent (Fig. 7). Instead, all our data favor the view that Bak is controlled entirely via its negative regulation by two prosurvival family members (Fig. 8). When both Mcl-1 and Bcl-xL are inactivated by BH3-only proteins (or absent), apoptosis seems to be the default state (Figs. 6, 7). This model is somewhat akin to that favored for Caenorhabditis elegans (Horvitz 1999), where the BH3-only protein EGL-1 must bind to the sole prosurvival homolog CED-9 to initiate apoptosis and massive apoptosis ensues in the absence of CED-9. In worms, the effector molecule sequestered by CED-9, CED-4, directly controls caspase activation, whereas in mammals Bak appears to do so indirectly, via mitochondrial damage.
The BH3-only proteins therefore play the key role of determining whether Mcl-1 and Bcl-xL are available to sequester Bak (Fig. 8). We unexpectedly found that Noxa not only displaces Bak from Mcl-1 but also promotes the proteasome-dependent degradation of Mcl-1 (Fig. 5). Thus, Noxa acts to inactivate Mcl-1 by binding to it and triggering its destruction. Interestingly, UV probably also induces a Noxa-independent mode of antagonizing Mcl-1 by decreasing Mcl-1 production (Nijhawan et al. 2003), because some Mcl-1 degradation persists in Noxa-deficient MEFs (Supplementary Fig. S4). In any case, other BH3-only proteins must be activated to elicit Bak-mediated apoptosis, which requires the neutralization of both Mcl-1 and Bcl-xL (Fig. 8). Activation of distinct subsets of BH3-only proteins by UV in different cell types may explain why Bak plays the predominant role in MEFs (Fig. 2), whereas both Bax and Bak are activated in HeLa cells (Fig. 1; Supplementary Fig. S1).
Therapeutic potential of selectively activating Bak
Since BH3-only proteins are key initiators of apoptosis, there is growing interest in developing drugs that kill tumor cells by mimicking their inactivation of prosurvival targets (Cory et al. 2003). The appeal of such “BH3-mimetics” is that upstream sensors of cellular damage (e.g., p53) are often defective in tumor cells and that certain prosurvival Bcl-2 proteins, particularly Bcl-2 itself, are overexpressed in many tumors. Furthermore, their overexpression contributes to chemoresistance, a common cause of treatment failure (Kaufmann and Vaux 2003).
The studies reported here provide a new focus for attempts to develop such drugs by demonstrating that inactivation of Mcl-1 and Bcl-xL suffices for efficient Bak-mediated killing (Fig. 8). The sensitivity of multiple myeloma cells, for example, to down-regulating Mcl-1 (Zhang et al. 2002) might be due to low expression of Bcl-xL in those cells. Consequently, strategies that selectively target one or both of these two prosurvival proteins may well be particularly effective for certain types of tumors. It is noteworthy that this approach bypasses Bcl-2, because Bcl-2 has no role in regulating Bak (Figs. 6, 7). This might well prove to be a major advantage, since Bcl-2 overexpression is common in tumors. The efficient killing elicited by our engineered Noxa mutant m3 (Fig. 6) suggests that it could serve as the prototype for a strategy based upon unleashing Bak (Fig. 8).
Materials and methods
Flow cytometric analysis for Bak and Bax activation
HeLa cells were left untreated or pretreated with a proteasome inhibitor (10 μM MG-132; Calbiochem) or a wide-spectrum caspase inhibitor (100 μM zVAD.fmk; Bachem) for 1 h before UV irradiation. Following UV irradiation, cells were fixed with 1% paraformaldehyde (5 min at room temperature) and then washed with buffer supplemented with 2% fetal bovine serum. Fixed cells were then incubated with the primary antibodies: 2 μg/mL anti-Bak Ab-1 (Calbiochem) or 5 μg/mL anti-Bax clone 3 (BD) diluted in FACS buffer supplemented with 0.3% saponin for 30 min on ice. Cells were then washed, before incubation with the appropriate secondary antibody, either FITC-conjugated goat-anti-mouse IgG (10 μg/ml; SouthernBiotech) to detect Bax activation or a biotin-conjugated anti-mouse (diluted 1:200; SouthernBiotech) followed by Streptavidin-conjugated PE (diluted 1:300; Caltag) to detect Bak activation. The samples were analyzed using a FACScan (BD).
Affinity measurements and solution competition assays
Affinity measurements were performed at room temperature on a Biacore 3000 biosensor as previously described (Chen et al. 2005) using a 26-mer human (accession no. S58873) BakBH3 or mutant L78A peptide (Mimotopes):
Bak(67-92) PSSTMGQVGRQLAIIGDDINRRYDSE, where alanine replaces the highly conserved Leu 78 (underlined) in the mutant peptide L78A. All recombinant proteins used were described previously (Chen et al. 2005; Day et al. 2005).
Supplemental material
Details for expression and retroviral constructs; tissue culture; cell death induction; retroviral infections; apoptosis assays and subcellular fractionation; immunoprecipitation and immunoblotting; RNA extraction; reverse transcription and detection of A1, Bax, or Bak mRNA; and in vitro transcription/translation of Bak and interaction with Mcl-1 are provided in the Supplemental Material.
Acknowledgments
We thank H. Ierino, B. Kolevski, and L. Parma for excellent technical assistance; P. Bouillet, S. Korsmeyer, Y. Lazebnik, D. Loh, R. McCrackan, T. Lindsten, N. Motoyama, L. O'Reilly, H. Puthalakath, C. Thompson, and M. van Delft for essential reagents; A. Verhagen for informing us of her unpublished work; and C. Day, S. Cory, A. Strasser, and D. Smith for discussions. Our work is supported by the Australian NHMRC (program grant 257502), U.S. NCI (CA80188), Leukemia and Lymphoma Society (Specialized Center of Research 7015-02), Sylvia and Charles Viertel Charitable Foundation, Leukemia Foundation of Victoria, and Australian Cancer Research Foundation.
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1304105.
References
- Adams J.M. 2003. Ways of dying: Multiple pathways to apoptosis. Genes & Dev. 17: 2481-2495. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit, S., Sanchez, B., and Martinou, J.C. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276: 11615-11623. [DOI] [PubMed] [Google Scholar]
- Bae J., Leo, C.P., Hsu, S.Y., and Hsueh, A.H. 2000. Mcl-1S, a splicing variant of the antiapoptotic Bcl-2 family member Mcl-1, encodes a proapoptotic protein possessing only the BH3 domain. J. Biol. Chem. 275: 25255-25261. [DOI] [PubMed] [Google Scholar]
- Chen L., Willis, S.N., Wei, A., Smith, B.J., Fletcher, J.I., Hinds, M.G., Colman, P.M., Day, C.L., Adams, J.M., and Huang, D.C.S. 2005. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17: 393-403. [DOI] [PubMed] [Google Scholar]
- Cheng E.H.-Y., Levine, B., Boise, L.H., Thompson, C.G., and Hardwick, J.M. 1996. Bax-independent inhibition of apoptosis by Bcl-xL. Nature 379: 554-556. [DOI] [PubMed] [Google Scholar]
- Cheng E.H., Wei, M.C., Weiler, S., Flavell, T.A., Mak, T.W., Lindsten, T., and Korsmeyer, S.J. 2001. Bcl-2, Bcl-xL sequester BH3 domain-only molecules preventing Bax- and Bak-mediated mitochondrial apoptosis. Mol. Cell 8: 705-711. [DOI] [PubMed] [Google Scholar]
- Cheng E.H., Sheiko, T.V., Fisher, J.K., Craigen, W.J., and Korsmeyer, S.J. 2003. VDAC2 inhibits Bak activation and mitochondrial apoptosis. Science 301: 513-517. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Flemington, C., Houghton, A.B., Ebb, R.G., Gallo, G.J., Elangovan, B., Chinnadurai, G., and Lutz, R.J. 1995. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 14: 5589-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Huang, D.C.S., and Adams, J.M. 2003. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 22: 8590-8607. [DOI] [PubMed] [Google Scholar]
- Cuconati A., Mukherjee, C., Perez, D., and White, E. 2003. DNA damage response and Mcl-1 destruction initiate apoptosis in adenovirus-infected cells. Genes & Dev. 17: 2922-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial N.N. and Korsmeyer, S.J. 2004. Cell death: Critical control points. Cell 116: 205-219. [DOI] [PubMed] [Google Scholar]
- Day C.L., Chen, L., Richardson, S.J., Harrison, P.J., Huang, D.C.S., and Hinds, M.G. 2005. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J. Biol. Chem. 280: 4738-4744. [DOI] [PubMed] [Google Scholar]
- Dewson G., Snowden, R.T., Almond, J.B., Dyer, M.J., and Cohen, G.M. 2003. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene 22: 2643-2654. [DOI] [PubMed] [Google Scholar]
- Farrow S.N., White, J.H.M., Martinou, I., Raven, T., Pun, K.T., Grinham, C.J., Martinou, J.C., and Brown, R. 1995. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature 374: 731-733. [DOI] [PubMed] [Google Scholar]
- Green D.R. and Kroemer, G. 2004. The pathophysiology of mitochondrial cell death. Science 305: 626-629. [DOI] [PubMed] [Google Scholar]
- Griffiths G.J., Dubrez, L., Morgan, C.P., Jones, N.A., White-house, J., Corfe, B.M., Dive, C., and Hickman, J.A. 1999. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144: 903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann G., O'Reilly, L.A., van Driel, R., Beaumont, J.G., Strasser, A., Adams, J.M., and Huang, D.C.S. 2000. Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1) has a cytoplasmic localization distinct from Bcl-2 or Bcl-xL. J. Cell Biol. 149: 623-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H.R. 1999. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 59: 1701s-1706s. [PubMed] [Google Scholar]
- Hsu Y.-T. and Youle, R.T. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272: 13829-13834. [DOI] [PubMed] [Google Scholar]
- ____. 1998. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273: 10777-10783. [DOI] [PubMed] [Google Scholar]
- Hsu Y.-T., Wolter, K.G., and Youle, R.J. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-xL during apoptosis. Proc. Natl. Acad. Sci. 94: 3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.C.S. and Strasser, A. 2000. BH3-only proteins—Essential initiators of apoptotic cell death. Cell 103: 839-842. [DOI] [PubMed] [Google Scholar]
- Jeong S.Y., Gaume, B., Lee, Y.J., Hsu, Y.T., Ryu, S.W., Yoon, S.H., and Youle, R.J. 2004. Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J. 23: 2146-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S.H. and Vaux, D.L. 2003. Alterations in the apoptotic machinery and their potential role in anticancer drug resistance. Oncogene 22: 7414-7430. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Mackey, M.R., Perkins, G., Ellisman, M.H., Latterich, M., Schneiter, R., Green, D.R., and Newmeyer, D.D. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331-342. [DOI] [PubMed] [Google Scholar]
- Letai A., Bassik, M., Walensky, L., Sorcinelli, M., Weiler, S., and Korsmeyer, S. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183-192. [DOI] [PubMed] [Google Scholar]
- Leu J.I., Dumont, P., Hafey, M., Murphy, M.E., and George, D.L. 2004. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 6: 443-450. [DOI] [PubMed] [Google Scholar]
- Lindsten T., Ross, A.J., King, A., Zong, W., Rathmell, J.C., Shiels, H.A., Ulrich, E., Waymire, K.G., Mahar, P., Frauwirth, K., et al. 2000. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6: 1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Dai, S., Zhu, Y., Marrack, P., and Kappler, J.W. 2003. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity 19: 341-352. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S. and Martinou, J.C. 2005. Newcomers in the process of mitochondrial permeabilization. J. Cell. Sci. 118: 473-483. [DOI] [PubMed] [Google Scholar]
- Marsden V. and Strasser, A. 2003. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Ann. Rev. Immunol. 21: 71-105. [DOI] [PubMed] [Google Scholar]
- Mikhailov V., Mikhailova, M., Pulkrabek, D.J., Dong, Z., Venkatachalam, M.A., and Saikumar, P. 2001. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J. Biol. Chem. 276: 18361-18374. [DOI] [PubMed] [Google Scholar]
- Mikhailov V., Mikhailova, M., Degenhardt, K., Venkatachalam, M.A., White, E., and Saikumar, P. 2003. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 278: 5367-5376. [DOI] [PubMed] [Google Scholar]
- Motoyama N., Wang, F.P., Roth, K.A., Sawa, H., Nakayama, K., Nakayama, K., Negishi, I., Senju, S., Zhang, Q., Fujii, S., et al. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science 267: 1506-1510. [DOI] [PubMed] [Google Scholar]
- Nijhawan D., Fang, M., Traer, E., Zhong, Q., Gao, W., Du, F., and Wang, X. 2003. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes & Dev. 17: 1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E., Ohki, R., Murasawa, H., Nemoto, J., Shibue, T., Yamashita, T., Tokino, T., Taniguchi, T., and Tanaka, N. 2000. Noxa, a BH3-only member of the bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288: 1053-1058. [DOI] [PubMed] [Google Scholar]
- Oltvai Z.N., Milliman, C.L., and Korsmeyer, S.J. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609-619. [DOI] [PubMed] [Google Scholar]
- Opferman J.T., Letai, A., Beard, C., Sorcinelli, M.D., Ong, C.C., and Korsmeyer, S.J. 2003. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426: 671-676. [DOI] [PubMed] [Google Scholar]
- Opferman J., Iwasaki, H., Ong, C.C., Suh, H., Mizuno, S., Akashi, K., and Korsmeyer, S.J. 2005. Obligate role of anti-apoptotic Mcl-1 in the survival of hematopoietic stem cells. Science 307: 1101-1104. [DOI] [PubMed] [Google Scholar]
- Ranger A.M., Malynn, B.A., and Korsmeyer, S.J. 2001. Mouse models of cell death. Nat. Genet. 28: 113-118. [DOI] [PubMed] [Google Scholar]
- Rathmell J.C., Lindsten, T., Zong, W.X., Cinalli, R.M., and Thompson, C.B. 2002. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat. Immunol. 3: 932-939. [DOI] [PubMed] [Google Scholar]
- Rinkenberger J.L., Horning, S., Klocke, B., Roth, K., and Korsmeyer, S.J. 2000. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes & Dev. 14: 23-27. [PMC free article] [PubMed] [Google Scholar]
- Roucou X., Montessuit, S., Antonsson, B., and Martinou, J.C. 2002. Bax oligomerization in mtochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem. J. 368: 915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M., Liang, H., Nettesheim, D., Meadows, R.P., Harlan, J.E., Eberstadt, M., Yoon, H.S., Shuker, S.B., Chang, B.S., Minn, A.J., et al. 1997. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science 275: 983-986. [DOI] [PubMed] [Google Scholar]
- Schinzel A., Kaufmann, T., Schuler, M., Martinalbo, J., Grubb, D., and Borner, C. 2004. Conformational control of Bax localization and apoptotic activity by Pro168. J. Cell. Biol. 164: 1021-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T., Takeda, K., Oda, E., Tanaka, H., Murasawa, H., Takaoka, A., Morishita, Y., Akira, S., Taniguchi, T., and Tanaka, N. 2003. Integral role of Noxa in p53-mediated apoptotic response. Genes & Dev. 17: 2233-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler K.S., Latham, C.B., and Roth, K.A. 1997. Bax deficiency prevents the increased cell death of immature neurons in Bcl-x-deficient mice. J. Neurosci. 17: 3112-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan R., Cuconati, A., Nelson, D., and White, E. 2001. Tumor necrosis factor-α induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J. Biol. Chem. 276: 45120-45127. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Youle, R.J., and Tjandra, N. 2000. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell 103: 645-654. [DOI] [PubMed] [Google Scholar]
- Verhagen A.M., Ekert, P.G., Pakusch, M., Silke, J., Connolly, L.M., Reid, G.E., Moritz, R.L., Simpson, R.J., and Vaux, D.L. 2000. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing inhibitor of apoptosis (IAP) proteins. Cell 102: 43-53. [DOI] [PubMed] [Google Scholar]
- Wang K., Gross, A., Waksman, G., and Korsmeyer, S.J. 1998. Mutagenesis of the BH3 domain of Bax identifies residues critical for dimerization and killing. Mol. Cell. Biol. 18: 6083-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Lindsten, T., Mootha, V.K., Weiler, S., Gross, A., Ashiya, M., Thompson, C.B., and Korsmeyer, S.J. 2000. tBid, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & Dev. 14: 2060-2071. [PMC free article] [PubMed] [Google Scholar]
- Wei M.C., Zong, W.X., Cheng, E.H., Lindsten, T., Panoutsakopoulou, V., Ross, A.J., Roth, K.A., MacGregor, G.R., Thompson, C.B., and Korsmeyer, S.J. 2001. Proapoptotic Bax and Bak: A requisite gateway to mitochondrial dysfunction and death. Science 292: 727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A.B., de Vries, E., Tait, S.W., Bontjer, I., and Borst, J. 2002. Bcl-2 family member Bfl-1/A1 sequesters truncated Bid to inhibit is collaboration with pro-apoptotic Bak or Bax. J. Biol. Chem. 277: 22781-22788. [DOI] [PubMed] [Google Scholar]
- Wilson-Annan J., O'Reilly, L.A., Crawford, S.A., Hausmann, G., Beaumont, J.G., Parma, L.P., Chen, L., Lackmann, M., Lithgow, T., Hinds, M.G., et al. 2003. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell Biol. 162: 877-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter K.G., Hsu, Y.T., Smith, C.L., Nechushtan, A., Xi, X.G., and Youle, R.J. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139: 1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha H.B. and Reed, J.C. 1997. Heterodimerization-independent functions of cell death regulatory proteins Bax and Bcl-2 in yeast and mammalian cells. J. Biol. Chem. 272: 31482-31488. [DOI] [PubMed] [Google Scholar]
- Zhang B., Gojo, I., and Fenton, R.G. 2002. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood 99: 1885-1893. [DOI] [PubMed] [Google Scholar]
- Zong W.X., Lindsten, T., Ross, A.J., MacGregor, G.R., and Thompson, C.B. 2001. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes & Dev. 15: 1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W.X., Li, C., Hatzuvassiliou, G., Lindsten, T., Yu, Q.C., Yuan, J., and Thompson, C.B. 2003. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 162: 59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]