Abstract
During mammalian gonadal development, nuclear import/export of the transcription factor SOX9 is a critical step of the Sry-initiated testis-determining cascade. In this study, we identify a molecular mechanism contributing to the SOX9 nuclear translocation in NT2/D1 cells, which is mediated by the prostaglandin D2 (PGD2) signalling pathway via stimulation of its adenylcyclase-coupled DP1 receptor. We find that activation of cAMP-dependent protein kinase A (PKA) induces phosphorylation of SOX9 on its two S64 and S181 PKA sites, and its nuclear localization by enhancing SOX9 binding to the nucleocytoplasmic transport protein importin β. Moreover, in embryonic gonads, we detect a male-specific prostaglandin D synthase expression and an active PGD2 signal at the time and place of SOX9 expression. We thus propose a new step in the sex-determining cascade where PGD2 acts as an autocrine factor inducing SOX9 nuclear translocation and subsequent Sertoli cell differentiation.
Keywords: cAMP signalling, nuclear import, prostaglandin D2, Sertoli cells, SOX9
Introduction
Gonad development is a unique system in which a single rudimentary tissue can be induced to form one of two different organs, the ovary and the testis. In mammals, the choice between these two fates is initiated by the Sry gene (sex-determining region of the Y chromosome) (Sinclair et al, 1990). Expression of Sry in the undifferentiated male gonad induces a variety of morphogenic events, including cell proliferation, cell migration, Sertoli cell determination and subsequent sex cord formation (Martineau et al, 1997; Tilmann and Capel, 1999). At the molecular level, the earliest downstream effect of Sry is the upregulation of Sox9 expression in the developing gonad (Kent et al, 1996; Sekido et al, 2004), which in turn, represses Sry expression (Chaboissier et al, 2004). SOX9 is related to SRY by its high-mobility group (HMG) domain (Foster et al, 1994) and heterozygous mutations in SOX9 lead to campomelic dysplasia, a skeletal malformation syndrome associated with sex reversal in 75% of XY patients (Wagner et al, 1994). Unlike SRY which is specific to mammals, the SOX9 protein is highly conserved throughout vertebrate evolution and, as SRY, is necessary (Chaboissier et al, 2004) and sufficient to induce testis differentiation when ectopically overexpressed in female gonad (Vidal et al, 2001). Expression studies in mouse and human embryos have shown that SOX9 is present at low levels in both male and female genital ridges before SRY expression and differentiation (Morais da Silva et al, 1996). However, following SRY expression, SOX9 is upregulated in the male and turned off in the female mouse gonad. Concomitantly the SOX9 protein, which appears cytoplasmic when present in both sexes, becomes nuclear at the onset of sex determination only in pre-Sertoli cells of the male gonad, in both mouse (Morais da Silva et al, 1996) and human (de Santa Barbara et al, 2000). Recently, we showed that subcellular localization of the SOX9 protein results from a nuclear import/export equilibrium, representing a regulatory switch which prevents (in female) or triggers (in male) male-specific sexual differentiation (Gasca et al, 2002).
To date, nothing is known about the signalling pathway(s) controlling nuclear translocation of SOX9. Several signalling molecules such as platelet-derived growth factor receptor (PGDFR-α) and its ligand PDGF-A, or fibroblast growth factor (FGF9) through its receptor FGFR2, act downstream of Sry, controlling early migration and proliferation steps in the developing testis (Colvin et al, 2001; Brennan et al, 2003; Ross and Capel, 2005). In the search for genes showing dimorphic expression in the gonads, the prostaglandin D synthase (Ptgds) was identified by PCR-based cDNA substraction on 11.5 days post coïtum (dpc) embryonic gonads (Adams and McLaren, 2002) and representational difference analysis (Menke and Page, 2002) as a male-specific gene. Its expression in the mouse gonads was detected specifically in 11.5 dpc Sertoli cells and prospermatogonia throughout the testis cords (Adams and McLaren, 2002). These authors suggest that secreted prostaglandin D2 (PGD2), the product of Ptgds enzymatic activity, acts as a paracrine signal for Sertoli cell differentiation in male gonadal development. Indeed, this signalling molecule masculinized XX 11.5 dpc embryonic gonads in culture and induced anti-Müllerian hormone (AMH) expression, a Sertoli cell marker (Adams and McLaren, 2002).
The lipocalin-type Ptgds (L-Ptgds), a member of the lipocalin ligand-carrier protein gene family (Urade and Eguchi, 2002), is expressed in numerous tissues (Urade and Hayaishi, 2000), and this enzyme mediates the last regulatory step in the biosynthetic pathway of prostaglandin D2 production (Urade and Eguchi, 2002). PGD2 is synthesized in many organs and has been implicated as a signalling molecule in the mediation or the regulation of various biological processes including platelet aggregation, bronchoconstriction and allergic diseases (Matsuoka et al, 2000; Breyer et al, 2001). Secreted PGD2 interacts with two receptors, the specific membrane-bound DP receptor associated to adenylcyclase and intracellular cAMP production (or DP1) (Boie et al, 1995; Breyer and Breyer, 2001), and a novel receptor, CRTH2 (chemo attractant receptor T helper type 2 (Th2) cells) (or DP2), which is coupled to Ca2+ signalling and is expressed in Th2 cells, eosinophils and basophils in human (Hirai et al, 2001; Monneret et al, 2001) and in colorectal cancer cells (Hawcroft et al, 2004).
In the present investigation, we demonstrate that PGD2, via its DP1 receptor and the subsequent stimulation of the cAMP pathway, induces SOX9 nuclear translocation via its protein kinase A (PKA) phosphorylation in NT2/D1 cells. Expression patterns, cAMP assays and gonad culture studies suggest that PGD2 might act as an autocrine factor for SOX9 nuclear translocation, and suggest a novel role for the prostaglandin D2 signalling pathway in the regulation of SOX9 subcellular localization in the gonadal model.
Results
Stimulation of the cAMP–PKA pathway induces SOX9 nuclear translocation in NT2/D1 cells and in cultured mouse gonads
In chondrocytes, SOX9 is a target of cAMP signalling and its phosphorylation by PKA on two serines (S64 and S181) enhances its activity on the Col2a1 chondrocyte-specific enhancer (Huang et al, 2000). To further investigate the implication of this signalling pathway in the activation of SOX9 in the male gonad, we first analysed SOX9 subcellular distribution with or without cAMP stimulation in the human testicular carcinoma NT2/D1 cell line. In these Sertoli-like NT2/D1 cells, SOX9 is endogenously expressed and distributed throughout the cytoplasm and the nucleus (Figure 1A). Treatment with Br-AMP, a stable cAMP analogue, stimulated nuclear translocation of SOX9 in 60% of the cells instead of 10% in control cells. Pre-incubation with cycloheximide did not prevent translocation of SOX9 in nuclei (data not shown), indicating that pre-existing SOX9 was translocated in a protein synthesis-independent manner. To evaluate the function of this process in gonads, we then analysed the effect of Br-AMP treatment on undifferentiated mouse gonad culture. Sexual undifferentiated mesogonads from 10.5–11.5 dpc (8 to 15 tail somite (TS)) were cultured in the presence or absence of Br-AMP as described previously (Gasca et al, 2002). Female control mesogonad cultures never expressed SOX9, whereas cultures treated with Br-AMP expressed nuclear SOX9 similarly to male cultures (Figure 1B). Thus, in embryonic gonads, stimulation of the cAMP signalling pathway is able to induce male SOX9 expression pattern in a XX genetic background (i.e. without SRY).
Figure 1.
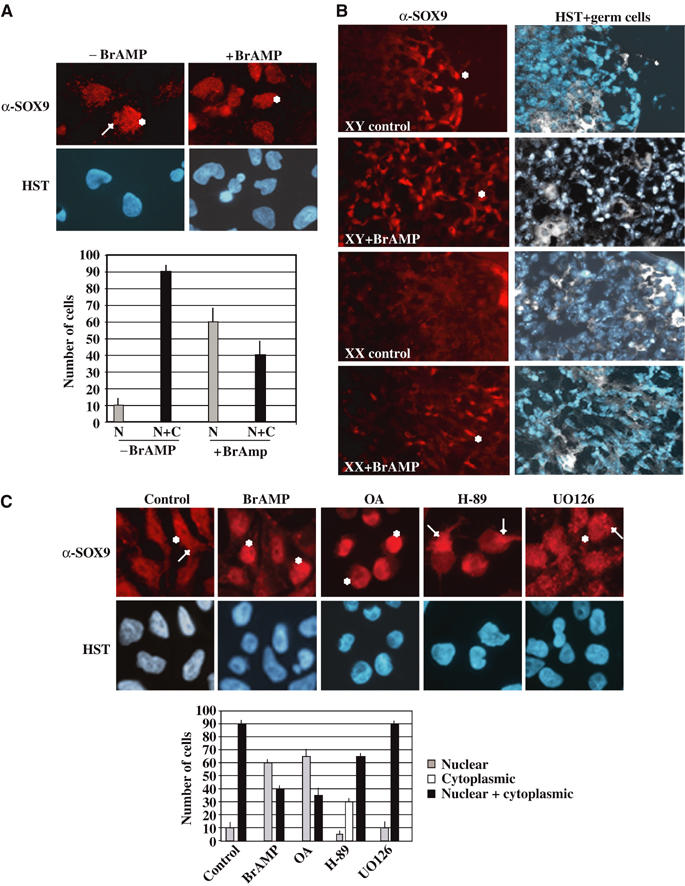
SOX9 nuclear translocation is induced by stimulation of the cAMP–PKA pathway. (A) NT2/D1 cells express SOX9 in the nuclear (*) and the cytoplasmic (→) compartments as shown by immunostaining with an anti-SOX9 antibody (α-SOX9) and DNA staining (HST) (magnification × 60). Statistical SOX9 subcellular distribution was represented on the corresponding graph: nuclear SOX9 (N, gray bars) and nuclear+cytoplasmic SOX9 (N+C, black bars). (B) cAMP pathway stimulation in cultured mouse gonads induces SOX9 nuclear localization in female gonads. Dissected pairs of mesogonads from 10.5 dpc embryos were cultured with Br-AMP for 24 h. Nuclear SOX9 (in red, *) was visualized with a specific antibody, while germ cells (in white) and nuclei (in blue) were merged on the same sections (HST+germ cells) (magnification × 40). (C) PKA activation is involved in SOX9 nuclear translocation. Following treatment of NT2/D1 cells with different drugs, protein kinase inhibitors H-89 (PKA), UO126 (MAPK), phosphatase inhibitor OA and Br-AMP, SOX9 subcellular distribution was analysed by immunostaining with an anti-SOX9 antibody (α-SOX9) and DNA staining (HST) (magnification × 60). Nuclear SOX9 is indicated by an (*) and cytoplasmic SOX9, by an (→). Statistical SOX9 subcellular distribution was represented in the corresponding graph: nuclear SOX9 (grey bars), cytoplasmic SOX9 (white bars) and nuclear+cytoplasmic SOX9 (black bars).
We next determined which kinase was involved in this cAMP-dependent SOX9 nuclear accumulation. NT2/D1 cells were treated with the specific protein kinase inhibitors, H-89 and UO126 (specific for the PKA and the MAPK pathways, respectively), or the phosphatase inhibitor, okadaic acid (OA). OA treatment mimicked Br-AMP treatment by stimulating nuclear accumulation of SOX9 in 60% of cells, whereas inhibition of the MAPK signalling pathway with the drug UO126 did not affect SOX9 subcellular localization (Figure 1C). PKA inhibitor H-89 induced complete cytoplasmic SOX9 in 30% of cells, whereas 65% of cells still showed a cytoplasmic and nuclear profile, indicating that cAMP-dependent PKA phosphorylation contributed to SOX9 nuclear localization in NT2/D1 cells.
cAMP–PKA stimulation induces SOX9 nuclear translocation by facilitating its nuclear import
There are two PKA phosphorylation sites located at the N-terminal end (Ser64) and the C-terminal end (Ser181) of the HMG box (Figure 2A) (Huang et al, 2000). We mutated these serines to alanines to generate a Flag-tagged SOX9 carrying the double S64A–S181A mutation. The effect of these mutations was verified by in vitro PKA phosphorylation of wild-type (wt) and mutant SOX9 TNT products, in the presence of γ32P-ATP, followed by anti-Flag immunoprecipitation (Figure 2B) (Huang et al, 2000). When transfected in NIH3T3 cells, the wt SOX9 protein (wt) showed both nuclear and cytoplasmic localization in more than 90% of cells (Gasca et al, 2002) (Figure 2C), whereas the mutant SOX9 protein (S64A S181A) was solely cytoplasmic in the same proportion of cells. Treatment with Br-AMP induced nuclear accumulation of the wt SOX9 protein (70% of cells), but did not modify the subcellular distribution of the mutant, indicating that phosphorylation of these sites is a mechanism implicated in the SOX9 nuclear localization process. Treatment of transfected cells with leptomycin B (LMB), which inhibits nuclear export, slightly decreased the cytoplasmic localization of the mutant SOX9 protein (70% instead of 90%), while LMB and Br-AMP co-treatment induced nuclear SOX9 expression in a few number of S64A S181A-transfected cells, confirming that a cAMP–PKA-independent mechanism is also implicated in the SOX9 nuclear import process.
Figure 2.
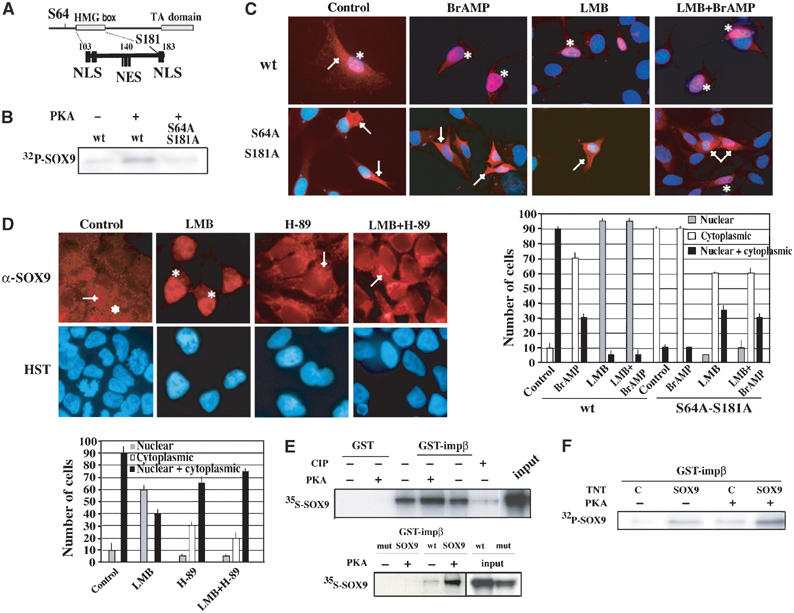
cAMP–PKA stimulation induces SOX9 nuclear translocation via facilitating its nuclear import. (A) Position and spacing of PKA phosphorylation sites (S64 and S181) in relation to the nuclear localization (NLS) and nuclear export (NES) sequences within SOX9 full sequence. (B) Phosphorylation state of wt and S64A–S181A mutant SOX9 TNT proteins was controlled by in vitro PKA labelling (+) in the presence of γ32P-ATP after α-Flag immunoprecipitation and autoradiography. (C) Subcellular distribution of wt and mutant SOX9 protein in NIH3T3 cells. Transfected cells were treated with Br-AMP (BrAMP), LMB or both (LMB+BrAMP), and SOX9 revealed by α-SOX9 immunostaining (red) was co-localized with HST staining (blue) (magnification × 60). (D) PKA phosphorylation increases SOX9 nuclear import without affecting its nuclear export. NT2/D1 cells were treated with LMB, H-89 or LMB+H-89, and SOX9 subcellular distribution was analysed by immunofluorescence with α-SOX9 antibody (red) and DNA staining (HST, blue) (magnification × 60). In both panels (C, D), nuclear SOX9 is indicated by an (*) and cytoplasmic SOX9 is indicated by an (→), and statistical SOX9 subcellular distribution is represented in the corresponding graphs. (E) Effect of SOX9 phosphorylation on its interaction with importin β. GST pulldown experiments were performed using GST alone or GST-importin β proteins and in vitro translated 35S Met-wt or S64A–S181A (mut) SOX9. Prior to the binding reaction, SOX9 protein was subjected to incubation in the presence (+) or absence (−) of PKA or calf intestine phosphatase (CIP) (panels 35S-SOX9). Input represent 50% of the SOX9 TNT proteins. (F) The phosphorylation state of SOX9 required for its interaction with importin β was confirmed in GST pulldown experiment using 32P-labelled SOX9. Cold TNT-SOX9 (SOX9) or TNT-pcDNA (C) was submitted to in vitro phosphorylation by PKA (+) in the presence of γATP prior to the binding reaction. 32P panel shows autoradiography of importin β-bound SOX9 after GST pulldown reactions.
We then tried to elucidate the molecular mechanism(s) involved in the PKA-induced SOX9 nuclear translocation. PKA can either promote SOX9 entry into the nucleus or inhibit its export. NT2/D1 cells were treated with LMB in the presence or absence of PKA inhibitor H-89. LMB treatment resulted in an accumulation of SOX9 in 60% of the nuclei, whereas H-89 induced cytoplasmic localization in 30% of cells (Figure 2D). The LMB effect was almost completely abolished when cells were co-treated with the H-89 PKA inhibitor, demonstrating that PKA activity acts upstream of LMB and is required for nuclear import. Hence, PKA phosphorylation of SOX9 appears to contribute to its nuclear import without affecting its nuclear export. The serine 181 (Ser181) residue is located next to the C-terminal NLS (Figure 2A), which mediates nuclear import via interaction with importin β (Preiss et al, 2001), a nucleocytoplasmic shuttle protein able to transport proteins from the cytoplasm to the nucleus (Moroianu, 1999). To evaluate the effect of SOX9 phosphorylation on its interaction with importin β, GST pulldown experiments were performed with purified GST-importin β fusion protein and in vitro translated wt and mutant SOX9. The interaction between GST-importin β and SOX9 was enhanced when SOX9 was subjected to in vitro PKA phosphorylation prior to the binding, but was almost completely abolished after phosphatase treatment (CIP) (Figure 2E). Moreover, the S64A–S181A mutant displayed no detectable binding to importin β. SOX9 state of phosphorylation on these both serine residues was crucial for an importin β-dependent nuclear import. The phosphorylation state of SOX9 in the importin β/SOX9 complex was confirmed by γ32P-ATP-PKA labelling of the SOX9 product before the binding reaction with GST-importin β (Figure 2F). An endogenous reticulocyte lysate phosphorylation activity on SOX9 was detectable, explaining the importin β binding to SOX9 when no exogenous PKA was added.
Prostaglandin D2 pathway induces SOX9 nuclear translocation via its PKA phosphorylation in NT2/D1 cells
The results described above prompted us to search for the signal that could regulate cAMP levels to activate SOX9 nuclear translocation. Since PGD2 mainly acts via its adenylcyclase-coupled DP1 receptor, we hypothesized that PGD2 could be the first messenger inducing cAMP production. First, we tested the ability of NT2/D1 cells to generate cAMP in response to various prostanoid stimuli. PGD2 and the DP1-specific agonist BW245C were able to induce cAMP production in NT2/D1 cells (Figure 3A). BW245C was more efficient than PGD2, as previously described in pharmacological studies (Crider et al, 1999). In contrast, the prostanoids PGI2 and PGE2 known to stimulate cAMP production through their specific receptors did not significantly stimulate cAMP production in these cells (data not shown). Stimulation by PGD2 or DP1 agonist BW245C was partially abrogated by the DP1/EP1/EP2 antagonist AH6809. These results indicate that PGD2 is able to stimulate cAMP production in NT2/D1 cells via specific activation of the DP1 receptor.
Figure 3.
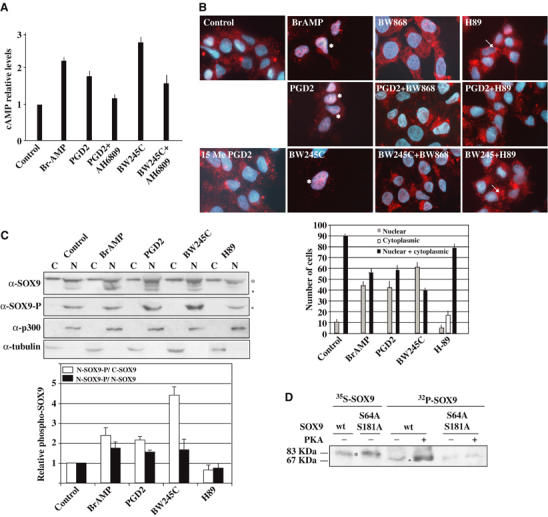
PGD2 pathway induces SOX9 nuclear translocation in NT2/D1 cells. (A) cAMP Elisa assays (R&D systems) were performed on NT2/D1 lyzates treated with Br-AMP, PGD2 and its agonist (BW245C) or antagonist (AH6809). Relative cAMP levels are expressed by nmol of produced cAMP per ng of total proteins and are normalized to the basal response of untreated cells (control). (B) SOX9 subcellular distribution in NT2/D1 cells was analysed after treatment with Br-AMP, PGD2 and its DP1-agonist (BW245C) and DP1-specific antagonist (BWA868C) or PKA inhibitor H-89. SOX9 localization was detected by α-SOX9 immunostaining (red) merged with nuclei staining (blue). Nuclear SOX9 is denoted by an (*) and cytoplasmic SOX9 by an (→) and statistical SOX9 subcellular distribution was represented in the corresponding graph. (C) SOX9 phosphorylation status was detected by Western blotting with an anti-phospho SOX9 antibody (α-SOX9-P) on sub-cellular fractions (cytoplasmic, C; nuclear, N) of treated NT2/D1 cells while α-SOX9 blot determined whole SOX9 expression. (*) and (°) indicate non-phosphorylated and phosphorylated SOX9, respectively. Relative levels of phospho-SOX9 protein induced by prostanoid treatments were quantified by the NIH Image program. Cytoplasmic or nuclear SOX9 were normalized to α-tubulin and α-p300, respectively. The ratios nuclear phospho-SOX9 (N-SOX9-P)/cytoplasmic SOX9 (C-SOX9) (white bars) and nuclear phospho-SOX9 (N-SOX9-P)/nuclear non-phospho-SOX9 (N-SOX9) (black bars) were represented on the adjacent histogram. (D) Determination of the phosphorylation state of SOX9 isoforms. Cold wt SOX9 and S64A–S181A SOX9 TNT products were subjected (+) or not (−) to in vitro PKA phosphorylation in presence of γ32P-ATP (32P panel). Migration of phosphorylated (band °) SOX9 proteins were compared to that of nonphosphorylated (band *) SOX9 proteins (35S panel).
Next, we analysed by immunofluorescence experiments whether activation of the PGD2 signalling pathway affects SOX9 subcellular distribution in NT2/D1 cells. Following Br-AMP, PGD2 or BW245C treatments, SOX9 was exclusively nuclear in 45–60% of cells compared to only 5–10% in control cells, while co-treatment with DP1-specific antagonist BWA868C or with PKA inhibitor H-89 restored the control SOX9 profile (Figure 3B). H-89 induced exclusive cytoplasmic localization of SOX9 in 20% of cells and treatment with the DP2 receptor-specific agonist 15(R)-15-methyl-PGD2 (15 Me PGD2) did not modify the SOX9 subcellular distribution. These results indicated that PGD2 induces SOX9 nuclear localization via activation of its adenylcyclase-coupled DP1 receptor. A dose-dependent response to PGD2 on SOX9 nuclear localization induction was performed (data not shown) and showed that increasing PGD2 concentrations or exposure time acted negatively on DP1 receptor activation.
We then showed that PGD2-induced SOX9 nuclear translocation occurred consecutively to SOX9 phosphorylation by the cAMP-activated PKA. SOX9 phosphorylation status in NT2/D1 subcellular fractions was analysed by Western blotting with an antiphosphorylated SOX9 (SOX9-P) antibody (Huang et al, 2000). SOX9-P was detected only in nuclear fractions (Figure 3C). As treatment with Br-AMP, prostanoid treatments (PGD2 or BW245C) increased the proportion of nuclear SOX9-P by two- to four-fold (white bars) and two-fold (black bars) compared to the cytoplasmic SOX9 and the nonphosphorylated nuclear SOX9, respectively (panel α-SOX9-P and graph). Treatment with PKA inhibitor H-89 inverted these ratios, confirming that PGD2 induced SOX9 nuclear translocation via its PKA phosphorylation. Western blotting of NT2/D1 nuclear fractions with an anti-SOX9 antibody reproducibly detected the SOX9 protein as a triplet band, indicating a covalent modification of the protein (Figure 3C). Phosphorylated SOX9 was confirmed to be the faster migrating band (band °) by incubation of SOX9-TNT products with recombinant PKA in the presence of γ32P-ATP (Figure 3D). PKA treatment of the wt SOX9 protein modified its mobility towards the faster migrating band (panel 32P-SOX9) compared to the control nonphosphorylated protein (panel 35S-SOX9, band *), thereby confirming the phosphorylation state of the faster migrating band (band °). The slight signal in the 32P-wt SOX9 protein (−PKA) was probably due to SOX9 phosphorylation by endogenous reticulocyte lysate PKA activity.
Prostaglandin D2 pathway is active in mouse embryonic gonads at the time of sexual differentiation
We surveyed the gene expression pattern of the major prostanoid receptors (DP1, DP2, EP2 and EP4) and prostaglandin synthases in the mouse gonads (Figure 4A). Expression of the receptors for PGD2 (DP1 and DP2 or CRTH2) and PGE2 (EP2 and EP4) and prostaglandin synthases (PGES and PGIS) were detected by semiquantitative RT–PCR both in male and female embryonic gonads. L-Ptgds expression was only found to be male-specific as already established (Menke and Page, 2002). Expression profile of L-Ptgds was compared to that of SOX9 by quantitative RT–PCR on RNA extracted from 10.5, 11.5 and 12.5 dpc SF-1-positive gonadic cells (Sertoli cells) (Figure 4B). These cells, purified from gonads of embryos expressing GFP under the control of SF-1 promoter (Stallings et al, 2002), prevented from contamination of mesonephric tubules cells which express SOX9 in a non-sex-specifical manner and from L-Ptgds-expressing germinal cells. L-Ptgds expression was not detected at 10.5 dpc, while SOX9 expression was low in both male and female cells. At the time of SOX9 upregulation and nuclear translocation in the 11.5 dpc male cells, a significant L-Ptgds expression was detected in the male cells, while expression in the female cells remained low, demonstrating that L-Ptgds and SOX9 are concomitantly expressed in the Sertoli cell lineage at the time when sex determination takes place. Both expressions further increased specifically in the male cell lineage (12.5 dpc). Furthermore, significant increased cAMP and PGD2 levels were measured in male 11.5 dpc gonads compared to those detected in female 11.5 dpc gonads (Figure 4C). The presence of the L-Ptgds protein in differentiating mouse gonads was further confirmed by immunostaining of 11.5 dpc (17 TS) gonad sections. The L-Ptgds protein was detected in the cytoplasm of nuclear SOX9-expressing cells, whereas no expression was detected in female gonads (Figure 4D). These results indicate that the developmental switch of SOX9 nuclear translocation, which marks the onset of male sex determination, is concomitant to L-Ptgds expression corresponding to consequent endogenous gonadal PGD2 and cAMP levels in males.
Figure 4.
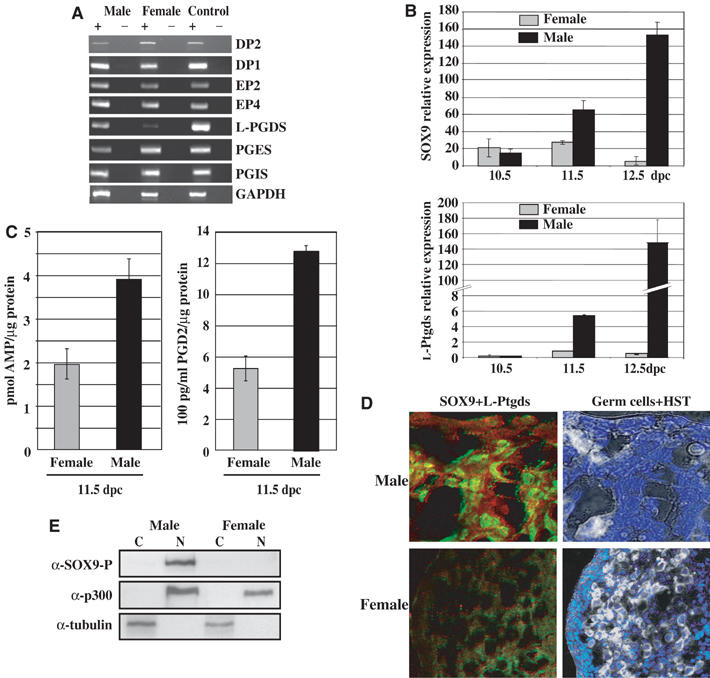
PGD2 pathway is expressed in mouse embryonic gonads at the time of sexual differentiation. (A) Prostanoid receptor and prostaglandin synthase expression was evaluated by semiquantitative RT–PCR on total RNAs isolated from 11.5 dpc embryonic gonads in the presence (+) or absence (−) of SuperScriptII and using the primers indicated in Table I. Control experiments were performed on total RNA from whole 11.5 dpc male and female mouse embryos. (B) L-Ptgds and SOX9 relative expressions were compared in SF-1-positive cells from 10.5, 11.5 and 12.5 dpc male and female gonads by quantitative RT–PCR using the primers indicated in Table I. They were measured in fluorescence units and normalized to Hprt expression, in triplicates on two independent RNA preparations. (C) Intracellular cAMP and PGD2 levels were measured in differentiating 11.5 dpc mouse gonads using the cAMP Elisa kit (R&D systems) and the Prostaglandin D2-MOX Express EIA kit (Cayman Chemical), respectively. cAMP and PGD2 levels were measured in triplicates in two experiments, relatively to protein concentration, and are represented by black bars (male) and grey bars (female). (D) L-Ptgds protein was detected in 11.5 dpc mouse male and female gonad sections by immunofluorescence with an anti-L-Ptgds antibody (1/100). Cytoplasmic L-Ptgds was detected (red) in SOX9-expressing cells (green) as visualized by confocal microscopy (magnification × 100 for male and × 40 for female). Germ cells (in white) were merged with HST DNA staining (blue). (E) Localization of phosphorylated SOX9 in subcellular fractions of 13.5 dpc male and female gonads. After fractionation, phospho-SOX9 was detected in nuclear fraction (N) using an anti-SOX9-P antibody. Western blotting with α-tubulin and α-p300 antibodies identified cytoplasmic (C) and nuclear (N) fractions, respectively.
We analysed the SOX9 phosphorylation status and its localization in vivo in gonads from 13.5 dpc male and female embryos by Western blotting. Gonads from that stage were used because they are more easily separated from adjacent mesonephros, which express SOX9 in a non-sex-dependent manner. Phosphorylated SOX9 protein was only detected in the nucleus of male gonads at this stage of sexual differentiation (Figure 4E, panel α-SOX9-P) as male-specific expression of SOX9 was largely documented (Kent et al, 1996; Morais da Silva et al, 1996).
PGD2 induces nuclear SOX9 expression in cultured female gonads
We then tested the effect of PGD2 and other agonists and antagonists of this PGD2 signalling pathway on SOX9 subcellular localization in cultured mouse gonads. The differentiation of XY mesogonads was indistinguishable in both the control and PGD2 experimental conditions since expression of nuclear SOX9 and secretion of AMH were detected in the vicinity of alkaline phosphatase-positive germ cells (Figure 5A, XY). As expected, no SOX9 expression was detected in the untreated female gonads (XX−PGD2). In contrast, PGD2-treated female mesogonads (eight out of 15) (XX+PGD2) displayed a nuclear SOX9 distribution similar to that observed in male mesogonads. Indeed, exogenous PGD2 was able to stimulate significant cAMP production in (8–15 TS) female gonads as represented on the corresponding graph (Figure 5A). Co-localization of AMH secretion in SOX9-expressing cells was confirmed by double labelling (Adams and McLaren, 2002) and confocal microscopy (Figure 5B). In parallel, PGD2 experiments performed in the presence of actinomycin D did not greatly modify the SOX9 nuclear translocation in 18-h-cultured male or female gonads (data not shown), confirming that PGD2-induced SOX9 nuclear import is independent of transcription.
Figure 5.
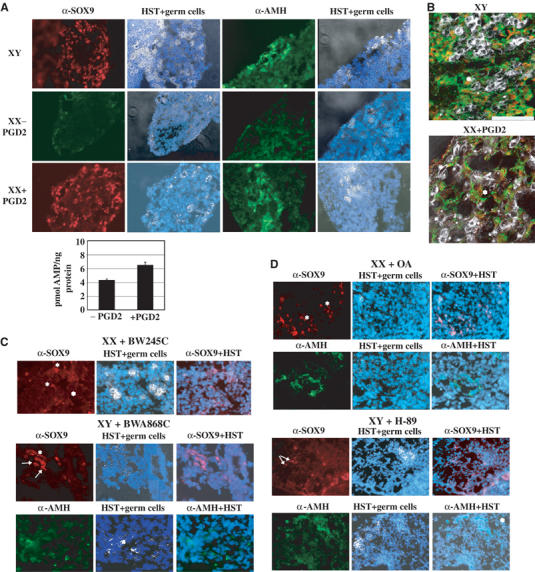
PGD2 via its DP1 receptor induces SOX9 nuclear localization and AMH expression in cultured female gonads. Dissected pairs of gonads from 8 to 15 TS (10.5–11.5 dpc) male (XY) and female (XX) embryos were cultured ex vivo (A, B) with (+PGD2) or without PGD2 (−PGD2) for 3 days, (C) with BW245C or BWA868C, (D) with OA or H-89 for 3 days. Nuclear and cytoplasmic SOX9 (red) and AMH (green) were revealed by specific antibodies and visualized by fluorescence microscopy (A, magnification × 40, C, D, magnification × 60). cAMP stimulation in PGD2-treated (8 to 15 TS) XX gonads was measured relatively to the protein concentration of the extracts and represented on the corresponding graph. (B) Expression of AMH in SOX9-positive cells was recorded on a confocal microscope after double immunostaining (Adams and McLaren, 2002) of male (XY) and treated female gonads (XX+PGD2) (scale bar=100 μm). Germ cells (white) were merged with DNA staining (HST, blue) when indicated.
DP1 agonist BW245C, but not DP2 agonist 15(R)-15-methyl PGD2 (data not shown), induced nuclear translocation of SOX9 in cultured female mesogonads (six out of 10) (Figure 5C, XX+BW245C) even though the number of SOX9-expressing cells and the SOX9 expression intensity were low, explaining probably the lack of AMH expression in these experiments (data not shown). In a reverse experiment, SOX9 nuclear distribution and AMH expression in 3 days-cultured male gonads were largely inhibited by DP1 antagonist BWA868C treatment (seven out of 8) (XY+BWA868C). Cytoplasmic SOX9 was detected in a few number of cells (XY+BWA868C, arrows). These data suggest that stimulation of PGD2 signalling pathway through activation of its DP1 receptor induces SOX9 nuclear localization in female cultured embryonic gonads. In order to verify the involvement of the cAMP–PKA pathway in this process, gonads were cultured for 3 days, in the presence of phosphatase inhibitor OA or PKA inhibitor H-89. Despite the toxicity of these drugs acting on multiple pathways, OA treatment of female mesogonads induced SOX9 nuclear expression and AMH expression in several XX cells (Figure 5D, XX+OA), whereas treatment of male mesogonads by H-89 led to cytoplasmic SOX9 and the lack of AMH expression (XY+H-89). It is noteworthy that the differentiation of the germ cell lineage was impaired in gonads cultured in the presence of the BWA868C, OA and H-89 drugs.
Discussion
In this study, we identify one of the molecular mechanisms involved in the regulation of the SOX9 nuclear translocation that acts as a molecular switch controlling the genetic cascade of testicular differentiation. The prostaglandin D synthase/PGD2 pathway, acting through the stimulation of the cAMP–PKA pathway and SOX9 phosphorylation, induces SOX9 nuclear translocation in the Sertoli-like NT2/D1 cells (Figure 6). These results demonstrate a new role for PGD2 and the DP1 receptor, which have been implicated in a variety of physiological and pathological functions (Matsuoka et al, 2000; Urade and Hayaishi, 2000). Phosphorylation modification is an usual mechanism to control import of proteins into the nucleus (Jans et al, 2000; Cyert, 2001) since it allows rapid responses to extracellular stimuli. Nuclear import of the SOX9 protein is unconventional since the nuclear transport receptor importin β directly recognizes SOX9 through the NLS at the C-terminal end of its HMG box without the need of importin α (Preiss et al, 2001). We showed that SOX9 PKA phosphorylation largely enhanced interaction between SOX9 and importin β. This modification may bring an essential negative charge to the NLS and may induce conformational changes to the HMG domain to facilitate interaction with importin β (Moroianu, 1999). The developing gonad is a unique system where dimorphic SOX9 subcellular localization has been observed. Indeed, expression studies in embryonic gonads showed that SOX9 does not undergo continual shuttling between subcellular compartments (Morais da Silva et al, 1996; de Santa Barbara et al, 2000), but the lack of SOX9 nuclear translocation induced a sex reversal phenotype in male gonads (Argentaro et al, 2003; Smith and Koopman, 2004), showing that SOX9 must be nuclear for the male sex determination process. Mechanisms that maintain SOX9 in a nuclear location after PKA phosphorylation are unknown. Interaction with nuclear proteins may retain SOX9 within the nucleus of differentiating Sertoli cells. SOX9 interacts with co-activators as CREB-binding protein CBP/p300 (Tsuda et al, 2003) and TRAP230 protein (Zhou et al, 2002), which participate in its trans-activation mechanism and may also be involved in the nuclear retention of SOX9.
Figure 6.
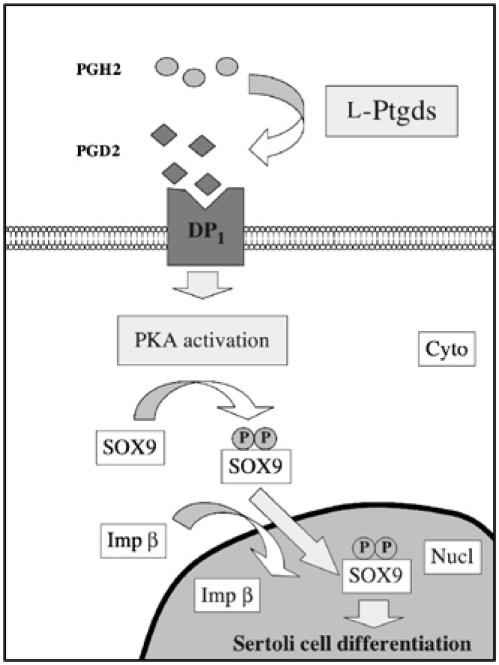
Model for the regulation of SOX9 nuclear localization through activation of PGD2/PKA pathway. Cyto and Nucl indicate cytoplasmic and nuclear compartments, respectively. P represents phosphorylated serines (Ser64 and Ser181) on SOX9 protein.
Concurrent with the increase of SOX9 expression in SF-1 positive pre-Sertoli cells between stages 10.5 and 12.5 dpc, we observed an increased L-Ptgds-specific expression in 11.5 dpc male cells that continued up to 12.5 dpc. At this time, L-Ptgds regulation may be relayed by upregulated SOX9. These results thus reveal a new piece in the mammalian sex determination puzzle: the male specific regulation of the L-Ptgds gene. At 11.5 dpc, at the time of SOX9 nuclear translocation, this L-Ptgds expression corresponds to high levels of intracellular cAMP and PGD2 in differentiating male gonad. Stimulation of XX gonads by exogenous PGD2 was able to increase this intracellular cAMP level (confirming the presence of DP1 receptor in female gonads) and induced re-localization of SOX9 and subsequent AMH expression, whereas inhibition of the PKA pathway in XY gonads reversed this nuclear localization. These data thus identified a functional PGD2/DP1/PKA signalling pathway in undifferentiated Sertoli cells and led us to hypothesize a PGD2 autocrine effect (PGD2 expressed in Sertoli cells) on SOX9 nuclear localization in these cells, at the time of sexual differentiation. Given that nuclear SOX9 is necessary for AMH gene activation (de Santa Barbara et al, 1998; Arango et al, 1999), our data bring a molecular mechanism to the previously reported AMH expression in PGD2-treated XX gonads (Adams and McLaren, 2002). This PGD2 signal may also act in a paracrine manner, possibly explaining the Sertoli differentiation of XX cells in XX–XY chimaeras mice (Palmer and Burgoyne, 1991).
Even though PGD2 might act as an autocrine signal for Sertoli cell differentiation, testis development is not disrupted in the absence of this signal, as observed in prostaglandin D synthase or DP1 receptor knocked-out mice (Eguchi et al, 1999; Matsuoka et al, 2000). The presence of multiple Ptgds enzymes and receptors with possible redundancy in relation to other prostaglandins makes it difficult to completely abolish this signal for Sertoli cell differentiation. Thus, a delay in male differentiation might occur in the absence of L-Ptgds; careful examination of gonads from these L-Ptgds or DP-null mice would be informative. As we developed, the L-Ptgds/PGD2/PKA signalling did not induce a complete phenomenon. This autocrine PGD2 induction may serve to amplify the primary induction by cell autonomous expression of Sry in somatic cells. Furthermore, a second pathway involving cAMP/PKA activation may also be stimulated during Sertoli cell differentiation. cAMP assays on embryonic gonads indicated that an L-Ptgds/PGD2-independent cAMP/PKA activation might occur in male and female gonads. On the other hand, a Ca2+-dependent SOX9 nuclear import mechanism via interaction of calmodulin with the N-terminal NLS of the HMG box has been described (Argentaro et al, 2003). This Ca2+-dependent mechanism did not involve the PGD2-Ca2+-coupled receptor CRTH2 (DP2) since treatment of NT2/D1 cells or cultured XX gonads with DP2 agonist did not modify SOX9 subcellular distribution. Both cAMP and Ca2+ mechanisms are probably required in vivo to completely induce SOX9 nuclear translocation (Argentaro et al, 2003).
As a measure of the activity of L-Ptgds, we have focused on the effects of PGD2 on SOX9 subcellular distribution. Secreted L-Ptgds during the development of the embryonic gonad possibly explains why the cAMP signal induces the arrest of mesonephric cell migration in 11.5 dpc ex vivo cultured gonads, while it did not influence Sertoli cell differentiation which still expressed SOX9 (Yao and Capel, 2002), confirming our experiments. The secondary mesonephric signal may then be a consequence of the autocrine induction of SOX9 nuclear translocation. The poor organization of nuclear SOX9 and AMH-positive XX sex reversed cells after PGD2 treatment may be a consequence of mesonephric cell migration inhibition by cAMP-coupled PGD2 signalling in the mesonephros, as a result of L-Ptgds diffusion. Together with the results of Yao and Capel (2002), our data indicate that the same cAMP pathway has antagonist effects during different stages of the same developmental process: a ‘pro-testis' effect on Sertoli cell differentiation via the subcellular control of SOX9 and an ‘anti-testis' effect via inhibition of mesonephric cell migration into the gonad.
We thus identified a novel role for the PGD2 signalling pathway as an inducer of the SOX9 nuclear translocation, a key process in the male sex determination pathway. These findings and the evolutionary conservation of prostaglandin D synthase proteins suggest that PGD2 signalling may function in sex determination in other vertebrates.
Materials and methods
Chemicals and drug treatment
All the chemicals were purchased from Sigma-Aldrich except BW245C, BWA868C and 15(R)-15-methyl-PGD2 (Cayman Chemical) and UO126 (Promega). Cells were incubated with BW245C (2 nM) for 15 min, PGD2 (500 ng/ml) for 30 min, AH6809 (2 nM) for 30 min, BWA868C (1 μM) for 15 min, 15(R)-15-methyl-PGD2 (0.3 μM) for 15 min, H-89 (10 μM) for 4 h, Br-AMP (1 mM) for 18 h, OA (10 nM) for 18 h, LMB (2.5 ng/ml) for 6 h, actinomycin D (5 μg/ml) for 3 h and cycloheximide (10 μg/ml) for 3 h prior to fixation or recovery.
Cells and organ culture
NIH3T3 and NT2/D1 cells were grown as described previously (Gasca et al, 2002). For organ culture, Swiss mouse embryos (Janvier Elevage) were collected between 10.5 and 11.5 dpc and staged according to the TS number (between 8 and 15 TS). Pairs of mesogonads were individually cultured as described previously (Gasca et al, 2002) up to 3 days, with one modification: inhere, pairs of mesogonads were longitudinally split into two halves, one gonad serving as an experimental series and the other one as a control. The culture medium supplemented or not with hormone or drugs was changed every 24 h.
Plasmids
The pcDNA-Flag tagged SOX9 wt plasmid was described previously (de Santa Barbara et al, 1998). The pcDNA-Flag-tagged SOX9 (S64A–S181A) was constructed by site-directed mutagenesis (Quickchange kit, Stratagene) and confirmed by sequencing the entire coding region of SOX9. The construct pGEX21T-importin β to produce a GST fusion protein was a gift from Dr Yoneda.
Transfection and immunofluorescence
For immunofluorescence experiments, NIH3T3 cells (40 000/well) and NT2/D1 cells (50 000/well) were cultured on glass coverslips for 18 h. NIH3T3 cells were transfected with 500 ng of pcDNA-SOX9 vectors using JetPeiII reagent (Qbiogen) and incubated for 24 h in DMEM medium in the presence or absence of drug. NT2/D1 cells were cultured for 24 h and treated as mentioned in the text. Endogenous SOX9 in NT2/D1 cells and transfected SOX9 in NIH3T3 cells were detected using an anti-SOX9 rabbit antibody (1:2000) (de Santa Barbara et al, 1998) as described previously (Gasca et al, 2002). Nuclei were labelled with 100 ng/ml Hoechst dye (HST) for 5 min and cells were examined by fluorescence microscopy. Statistical analysis of SOX9 subcellular distribution was evaluated in three fields (100 cells) of two independent experiments. The most representative SOX9 subcellular profile was shown for each experiment. Immunofluorescence studies on gonad tissue section were performed as described previously (Gasca et al, 2002). The anti-L-Ptgds goat antibody (1:100) (N20, Santa Cruz Laboratories) used as a primary antibody was incubated with a goat secondary antibody (Alexa-Ig, Molecular Probes).
In vitro protein production and GST pulldown experiments
Glutathione S-transferase (GST)-importin β fusion protein was produced and purified as described (Imamoto et al, 1995). Wt and mutated SOX9 were in vitro transcribed/translated in the presence of 35SMet in TNT-coupled reticulocyte lysate system (Promega) according to the manufacturer's instructions. GST pulldown experiments were performed in Tris-buffered saline (TBS) containing 0.2% BSA and 0.5% Triton-X100 as described previously (de Santa Barbara et al, 1998). Prior to GST pulldown binding assay, PKA phosphorylation or phosphatase treatment of TNT-SOX9 proteins was carried out using the purified recombinant catalytic subunit of PKA (Promega) or Calf Intestine Phosphatase (CIP, Biolabs) as recommended by the suppliers.
Subcellular fraction preparation and immunoblotting
Subcellular fractionation of NT2/D1 cells and gonad tissue was carried out using the Nuclear Extract Kit according to the manufacturer's instructions (Active Motif). Protein concentration was measured by Bradford reagent. To obtain a representation of SOX9 distribution, equal amounts of proteins (20 μg) from each fraction were analysed by Western blotting using anti-phospho-SOX9 (1:1000) (gift from Dr de Crombrugghe) (Huang et al, 2000) or anti-SOX9 (1:2000) (de Santa Barbara et al, 1998) rabbit antibodies and revealed with the ECL plus kit (Amersham). Blotting with the mouse monoclonal tubulin β (1:100) (gift from Dr Gauthier-Rouvière) and the rabbit p300 (1:1000) (N15, Santa Cruz Laboratories) antibodies was performed to assess the purity of cytoplasmic and nuclear fractions, respectively. The NIH programme (public domain) was used to quantify relative levels of bands obtained in blots.
Measurement of intracellular cAMP levels and endogenous L-Ptgds activity
Embryonic gonads (10.5–11.5 dpc) were collected and transferred immediately on dry ice. A 2 h PGD2-cAMP stimulation in gonads was performed in liquid DMEM medium containing 500 ng/ml PGD2 and gonads were frozen. The chromosomal sex of all embryos was determined by PCR with Sry primers (mSry forward: 5′-TGGGACTGGTGACAATTGTC and mSry reverse: 5′-GAGTACAGGTGTGCAGCTCT). NT2/D1 cells were cultured in 60-mm culture dishes for 48 h, treated as mentioned in the text and washed with phosphate-buffered saline (PBS). Intracellular cAMP level in frozen gonads (from 10 to 30 by experiment) and washed cells was measured using the cAMP Immunoassay kit, according to the manufacturer's instructions (R&D Systems). L-Ptgds activity was measured by assaying endogenous PGD2 in embryonic gonads using the Prostaglandin D2-MOX Express EIA kit (Cayman Chemical).
RNA extraction from whole gonads and semiquantitative RT–PCR
Embryonic gonads (11.5 dpc (15–21 TS)) were collected and embryos were sexed by PCR. Total RNA was extracted using RNeasy Midi kit (Qiagen) and digested from genomic DNA by treatment with DNase–RNase free (Qiagen). RT was performed using 2 μg total RNA, oligod(T) (Amersham Pharmacia) and SuperScript™ II RNase Reverse Transcriptase (Invitrogen) according to the supplied protocol. Single-strand cDNAs were subjected to 28–40 cycles of PCR amplification with Taq polymerase (Uptima, Interchim) and one specific primer set (Sigma Genosys, Table I). Amplification products were detected on a 2% agarose gel electrophoresis and ethidium bromide staining.
Table 1. Primers, hybridization temperature and expected PCR product size.
| Forward primer (5′–3′) | Reverse primer (5′–3′) | Hybridization temperature | Expected product size (bp) | |
|---|---|---|---|---|
| DP1 | CCCTGCCTTTAATTTATCGT | CTCCAGTTTCTGTAGATCAG | 49° | 205 |
| EP2 | GTGAAAGGCAAGGAGCATAT | CTGCTTATCGTGGCTGTGCT | 55° | 300 |
| EP4 | CCGCTCGTGGTGCGAGT | AAGGAGCGAGAGTGGCC | 56° | 280 |
| DP2 | GATGACATGCCAACATATCCT | CGACCCTTATCAGTTACCC | 60° | 330 |
| L-Ptgds | CAGTGCAGCCCAACTTTCAACAAG | GCAGGAAAACAATGTCCTCCTCTG | 60° | 457 |
| PGES | GCTGGTCATCAAGATGTACG | CCAGGTAGGCCACGGTGTGT | 54° | 300 |
| PGIS | TGGGCCACACAGGGGAATAT | CAAATTGTTTGATGCTGTTG | 55° | 520 |
| Gapdh | GACCACAGTCCATGCCATCACT | TCCACCACCCTGTTGCTGTAG | 55° | 450 |
| qSOX9 | GCAAGCTGGCAAAGTTGATCT | GCTGCTCAGTTCACCGATG | 65° | 106 |
| qL-Ptgds | GGGCAGCATCCACTCCGTGTC | GGGTGGCCATGCGGAAGT | 65° | 110 |
| qHprt | AGTCCCAGCGTCGTGATTAGC | CCAAATCCTCGGCATAATG | 62° | 85 |
Isolation of Sf1-eGFP-positive cells and RNA extraction
C57/B6 CBAJ female mice were bred with Sf1-BAC-eGFP transgenic male mice (Stallings et al, 2002). In all 10.5 (8ts±2ts), 11.5 (19ts±2ts), and 12.5 (30ts±3ts) dpc fluorescent (Sf1-BAC-eGFP transgene positive) urogenital ridges from individual embryos were dissected, digested with trypsin/EDTA and filtered through a 40 μm cell strainer to generate single-cell suspensions. GFP-positive cells were sorted using a FACS Vantage SE with a purity of exceeding 97%. Cells were collected in 96-well plates containing 50 μl of RNA lysis buffer from Qiagen RNeasy kit. Total RNAs were extracted using RNeasy micro kit from Qiagen according to the manufacturer's protocol. RNA integrity and quantity were assessed using RNA 6000 nanochips with an Agilent 2100 bioanalyser.
First and second RNA amplification
Approximately 50 000–100 000 GFP cells were needed to obtain the minimum 50 ng of total RNA required to reproducibly amplify total RNA using a two-amplification-steps protocol from Affymetrix. In short, 50–100 ng of RNA was converted into double-stranded cDNA using a cDNA synthesis kit (Superscript, Invitrogen) with a special oligo(dT)24 primer containing a T7 RNA promoter site added 3′ to the poly(T) tract. Following the first cRNA amplification by in vitro transcription (IVT) using the Ambion MEGAscipt T7 kit, 400 ng of cRNA was once more reverse transcribed and biotinylated cRNAs were generated from double-strand cDNAs using an IVT labelling kit from Affymetrix.
Real-time RT–PCR
Reverse transcription was performed using 1 μg of RNA from the second RNA amplification, random hexamer (Promega) and SuperScript™ II RNase Reverse Transcriptase (Invitrogen) according to supplied protocol. To quantify by real-time PCR SOX9 and L-Ptgds expression, specific primers (Table I) were designed using Oligo6 software. PCR were performed using IQ SYBR GREEN supermix on a MyIQ cycler (Biorad Laboratories). The Hprt1 housekeeping gene was systematically amplified to normalize possible variations in the yield of the RT–PCR step.
Statistical analysis
For all the quantitative analysis of immunostaining, cAMP and PGD2 assays, Western blot and real-time RT–PCR, we used the Mann–Whitney test for mean comparison experiments. Differences between groups were considered significant when the P-value was less than 0.05.
Acknowledgments
We thank N Lautredou (Centre Régional d'Imagerie Cellulaire, CRIC, Montpellier) for confocal microscopy, and Dr Helena Sim for critical reading of the manuscript. We thank Dr B de Crombrugghe (University of Texas, Houston, USA), Dr JY Picard (ENS, Montrouge) and Dr C Gauthier-Rouvière (CNRS CRBM, Montpellier) for the gifts of phospho-SOX9, AMH and tubulin β antibodies, respectively. We thank Dr Yoneda and Dr Sekimoto (Osaka University Medical School, Japan) for providing us the plasmid pGEX21T-importin β. The work was supported by the European Economic Community though the fifth framework Program, no. GLG2-CT-1999-00741. SM is a recipient of a PhD grant from the French Ministère de la Recherche et de l'Enseignement Supérieur. LT is the recipient of a PhD grant from the Ligue Départementale de l'Hérault contre le Cancer.
References
- Adams IR, McLaren A (2002) Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development 129: 1155–1164 [DOI] [PubMed] [Google Scholar]
- Arango NA, Lovell-Badge R, Behringer RR (1999) Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99: 409–419 [DOI] [PubMed] [Google Scholar]
- Argentaro A, Sim H, Kelly S, Preiss S, Clayton A, Jans DA, Harley VR (2003) A SOX9 defect of calmodulin-dependent nuclear import in campomelic dysplasia/autosomal sex reversal. J Biol Chem 278: 33839–33847 [DOI] [PubMed] [Google Scholar]
- Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M (1995) Molecular cloning and characterization of the human prostanoid DP receptor. J Biol Chem 270: 18910–18916 [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B (2003) Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 17: 800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer MD, Breyer RM (2001) G protein-coupled prostanoid receptors and the kidney. Annu Rev Physiol 63: 579–605 [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD (2001) Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690 [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, Van De Kant HJ, Wegner M, De Rooij DG, Behringer RR, Schedl A (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131: 1891–1901 [DOI] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM (2001) Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104: 875–889 [DOI] [PubMed] [Google Scholar]
- Crider JY, Griffin BW, Sharif NA (1999) Prostaglandin DP receptors positively coupled to adenylyl cyclase in embryonic bovine tracheal (EBTr) cells: pharmacological characterization using agonists and antagonists. Br J Pharmacol 127: 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS (2001) Regulation of nuclear localization during signaling. J Biol Chem 276: 20805–20808 [DOI] [PubMed] [Google Scholar]
- de Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol 18: 6653–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santa Barbara P, Moniot B, Poulat F, Berta P (2000) Expression and subcellular localization of SF-1, SOX9, WT1, and AMH proteins during early human testicular development. Dev Dyn 217: 293–298 [DOI] [PubMed] [Google Scholar]
- Eguchi N, Minami T, Shirafuji N, Kanaoka Y, Tanaka T, Nagata A, Yoshida N, Urade Y, Ito S, Hayaishi O (1999) Lack of tactile pain (allodynia) in lipocalin-type prostaglandin D synthase-deficient mice. Proc Natl Acad Sci USA 96: 726–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, David Brook J, Schafer A (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372: 525–530 [DOI] [PubMed] [Google Scholar]
- Gasca S, Canizares J, De Santa Barbara P, Mejean C, Poulat F, Berta P, Boizet-Bonhoure B (2002) A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination. Proc Natl Acad Sci USA 99: 11199–11204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawcroft G, Gardner SH, Hull MA (2004) Expression of prostaglandin D2 receptors DP1 and DP2 by human colorectal cancer cells. Cancer Lett 210: 81–84 [DOI] [PubMed] [Google Scholar]
- Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, Ichimasa M, Sugamura K, Nakamura M, Takano S, Nagata K (2001) Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med 193: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhou X, Lefebvre V, de Crombrugghe B (2000) Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol 20: 4149–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto N, Shimamoto T, Takao T, Tachibana T, Kose S, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y (1995) In vivo evidence for involvement of a 58 kDa component of nuclear pore-targeting complex in nuclear protein import. EMBO J 14: 3617–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans DA, Xiao CY, Lam MH (2000) Nuclear targeting signal recognition: a key control point in nuclear transport? BioEssays 22: 532–544 [DOI] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P (1996) A male-specific role for SOX9 in vertebrate sex determination. Development 122: 2813–2822 [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B (1997) Male-specific cell migration into the developing gonad. Curr Biol 7: 958–968 [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S (2000) Prostaglandin D2 as a mediator of allergic asthma. Science 287: 2013–2017 [DOI] [PubMed] [Google Scholar]
- Menke DB, Page DC (2002) Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Patterns 2: 359–367 [DOI] [PubMed] [Google Scholar]
- Monneret G, Gravel S, Diamond M, Rokach J, Powell WS (2001) Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood 98: 1942–1948 [DOI] [PubMed] [Google Scholar]
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R (1996) Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet 14: 62–68 [DOI] [PubMed] [Google Scholar]
- Moroianu J (1999) Nuclear import and export: transport factors, mechanisms and regulation. Crit Rev Eukaryot Gene Expr 9: 89–106 [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS (1991) In situ analysis of fetal, prepuberal and adult XX–XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development 112: 265–268 [DOI] [PubMed] [Google Scholar]
- Preiss S, Argentaro A, Clayton A, John A, Jans DA, Ogata T, Nagai T, Barroso I, Schafer AJ, Harley VR (2001) Compound effects of point mutations causing campomelic dysplasia/autosomal sex reversal upon SOX9 structure, nuclear transport, DNA binding, and transcriptional activation. J Biol Chem 276: 27864–27872 [DOI] [PubMed] [Google Scholar]
- Ross AJ, Capel B (2005) Signaling at the crossroads of gonad development. Trends Endocrinol Metab 16: 19–25 [DOI] [PubMed] [Google Scholar]
- Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R (2004) SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol 274: 271–279 [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346: 240–244 [DOI] [PubMed] [Google Scholar]
- Smith JM, Koopman PA (2004) The ins and outs of transcriptional control: nucleocytoplasmic shuttling in development and disease. Trends Genet 20: 4–8 [DOI] [PubMed] [Google Scholar]
- Stallings NR, Hanley NA, Majdic G, Zhao L, Bakke M, Parker KL (2002) Development of a transgenic green fluorescent protein lineage marker for steroidogenic factor 1. Endocr Res 28: 497–504 [DOI] [PubMed] [Google Scholar]
- Tilmann C, Capel B (1999) Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development 126: 2883–2890 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Takahashi S, Takahashi Y, Asahara H (2003) Transcriptional co-activators CREB-binding protein and p300 regulate chondrocyte-specific gene expression via association with Sox9. J Biol Chem 278: 27224–27229 [DOI] [PubMed] [Google Scholar]
- Urade Y, Eguchi N (2002) Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostagland Other Lipid Mediat 68–69: 375–382 [DOI] [PubMed] [Google Scholar]
- Urade Y, Hayaishi O (2000) Biochemical, structural, genetic, physiological, and pathophysiological features of lipocalin-type prostaglandin D synthase. Biochim Biophys Acta 1482: 259–271 [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A (2001) Sox9 induces testis development in XX transgenic mice. Nat Genet 28: 216–217 [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Yao HH, Capel B (2002) Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol 246: 356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Bonneaud N, Yuan CX, de Santa Barbara P, Boizet B, Schomber T, Scherer G, Roeder RG, Poulat F, Berta P, Tibor S (2002) SOX9 interacts with a component of the human thyroid hormone receptor-associated protein complex. Nucleic Acids Res 30: 3245–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]


