Summary
Background
Inadequate micronutrient intakes and related deficiencies are a major challenge to global public health. Analyses over the past 10 years have assessed global micronutrient deficiencies and inadequate nutrient supplies, but there have been no global estimates of inadequate micronutrient intakes. We aimed to estimate the global prevalence of inadequate micronutrient intakes for 15 essential micronutrients and to identify dietary nutrient gaps in specific demographic groups and countries.
Methods
In this modelling analysis, we adopted a novel approach to estimating micronutrient intake, which accounts for the shape of a population’s nutrient intake distribution and is based on dietary intake data from 31 countries. Using a globally harmonised set of age-specific and sex-specific nutrient requirements, we then applied these distributions to publicly available data from the Global Dietary Database on modelled median intakes of 15 micronutrients for 34 age–sex groups from 185 countries, to estimate the prevalence of inadequate nutrient intakes for 99·3% of the global population.
Findings
On the basis of estimates of nutrient intake from food (excluding fortification and supplementation), more than 5 billion people do not consume enough iodine (68% of the global population), vitamin E (67%), and calcium (66%). More than 4 billion people do not consume enough iron (65%), riboflavin (55%), folate (54%), and vitamin C (53%). Within the same country and age groups, estimated inadequate intakes were higher for women than for men for iodine, vitamin B12, iron, and selenium and higher for men than for women for magnesium, vitamin B6, zinc, vitamin C, vitamin A, thiamin, and niacin.
Interpretation
To our knowledge, this analysis provides the first global estimates of inadequate micronutrient intakes using dietary intake data, highlighting highly prevalent gaps across nutrients and variability by sex. These results can be used by public health practitioners to target populations in need of intervention.
Editorial note:
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Introduction
Micronutrient deficiencies are among the most common forms of malnutrition globally.1,2 A key pathway to micronutrient deficiencies is through inadequate intake of essential nutrients such as iron, zinc, vitamin A, iodine, and folate, among others, with deficiency in each nutrient having its own public health consequences. Iron deficiency is the most common cause of anaemia, leading to impaired cognition and adverse pregnancy outcomes.3 Vitamin A deficiency is the leading cause of preventable blindness globally, affecting mostly children and pregnant women.4 Both vitamin A and zinc have a crucial role in immunity, especially for populations facing a high burden of infectious diseases.5,6 Folate is needed early in pregnancy to reduce the risk of stillbirths and neural tube defects, and iodine is essential for pregnant and breastfeeding women because of its role in fetal and child cognitive development.7
Deficiencies in these and other micronutrients collectively contribute to a large burden of morbidity and mortality, but the scale and demographic specificities of the problem are unknown because of insufficient data.1,8 Clinical nutritional biomarkers have been used to estimate the global prevalence of micronutrient deficiencies for selected populations and micronutrients;1,4 however, substantial data gaps persist for various micronutrients, specific population groups (especially males), and many geographies. Existing data are also often outdated. The global prevalence of inadequate micronutrient supplies has been estimated using food availability data, highlighting inadequacies in food supply.9,10 Owing to scarce quantitative dietary intake data and no suitable approach with which to accurately model nutrient intake distributions, to our knowledge there have been no global estimates of inadequate micronutrient intakes. Estimates of micronutrient deficiencies, inadequate micronutrient intakes, and inadequate micronutrient supplies are all required for a comprehensive understanding of the burden of micronutrient malnutrition.
To tackle such a large-scale public health crisis, we require estimates to identify which nutrients pose the greatest risk, where, and to whom.11 Although micronutrient deficiencies are presumably widespread, data for women and children are scarce. A pooled global analysis of biomarker data found that more than one in two children younger than 5 years are deficient in either iron, zinc, or vitamin A and two in three women aged 15–49 years are deficient in either iron, zinc, or folate.1 However, we know of no global, population-wide estimates of nutrient deficiencies for a wider range of micronutrients.
The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) examines the burden of micronutrient malnutrition in 195 countries using a modelling approach that combines clinical outcomes (eg, goitre), biomarkers of micronutrient status (eg, serum retinol) and anaemia (eg, haemoglobin concentration), and inadequacy in the food supply (eg, zinc inadequacy).12 These studies include estimates of disease for only four micronutrients (iodine, iron, zinc, and vitamin A) owing to scarce data;12 however, 29 micronutrients are known to be essential.13 Although GBD models might be generated using the best available methods and data, the gaps in micronutrient status biomarkers and dietary intake data hinder the ability to comprehensively model micronutrient malnutrition. Furthermore, the GBD approach to modelling micronutrient malnutrition is not replicable because the data, methods, code, and assumed nutrient distribution shapes are not publicly available.14
Although nutritional biomarkers provide the best indication of nutritional deficiencies, these deficiencies can be caused by many factors including inadequate dietary nutrient intake, infectious diseases, or absorption issues. Therefore, the best way to identify populations at risk of diet-related malnutrition is to estimate inadequate nutrient intakes. Previous studies have estimated micronutrient adequacy of the food supply.9,10,15–17 Some of these studies have used terminology to imply that these estimates reflect nutrient intakes, including “estimated prevalence of inadequate intakes”,10 “risk of inadequate intake”,10 and “apparent consumption”.18 Such terminology could have inadvertently led to confusion that global estimates of inadequate nutrient intakes already exist. However, nutrient adequacy estimates relying on food supplies do not always fully account for household food waste, food service waste, small-scale food production, or wild harvest, and they have no information on how food is allocated across each country’s population (ie, there is no information for specific demographic groups, such as sex or age groups). Owing to these limitations, supply-based estimates are inaccurate, tending to underestimate inadequacy in high-income countries and overestimate inadequacy in many low-income and middle-income countries.19
In contrast to studies that rely primarily on food supplies, the Global Dietary Database (GDD) provides the only estimates of micronutrient intakes, using data from individual dietary intake surveys, household surveys, and national food supplies.20,21 The GDD standardises and compiles individual-level dietary datasets from 185 countries for more than 50 foods, beverages, and nutrients,21 providing the best available data to understand the amount of nutrients actually consumed by individuals rather than supplied. However, the GDD does not estimate micronutrient intake distributions or micronutrient requirements, which are needed to accurately estimate the prevalence of inadequate micronutrient intakes.
This Article provides a novel and reproducible approach to estimating the global prevalence of inadequate micronutrient intakes by accounting for the shapes of nutrient intake distributions and using globally harmonised nutrient reference values. We aimed to identify dietary nutrient gaps in specific demographic groups and countries, as well as estimate the total global burden of dietary micronutrient inadequacies for 15 essential micronutrients.
Methods
Overview
We estimated intake inadequacies for 15 micronutrients (appendix p 1) across 34 subnational age–sex groups in 185 countries. This approach required understanding nutrient intake and requirement distributions for each subnational population globally (figure 1). We developed these subnational nutrient intake distributions using estimates of distribution scale (ie, intake median) from the GDD and distribution shape (ie, intake variability) from the nutriR database (figure 1A).25 We then developed subnational nutrient requirement distributions using the harmonised average requirements defined by Allen and colleagues22 and common assumptions about variability in requirement amounts (figure 1B). We used the probability method26 to calculate intake inadequacies by comparing the derived intakes with the requirement distributions (figure 1C) and calculated the number of people with intake adequacies using subnational human population size estimates from the World Bank.24 All analyses were done using R and all data and code are available on GitHub. We used an interactive R Shiny web application to explore the results in detail.
Figure 1: Methods for estimating the prevalence of inadequate micronutrient intakes.
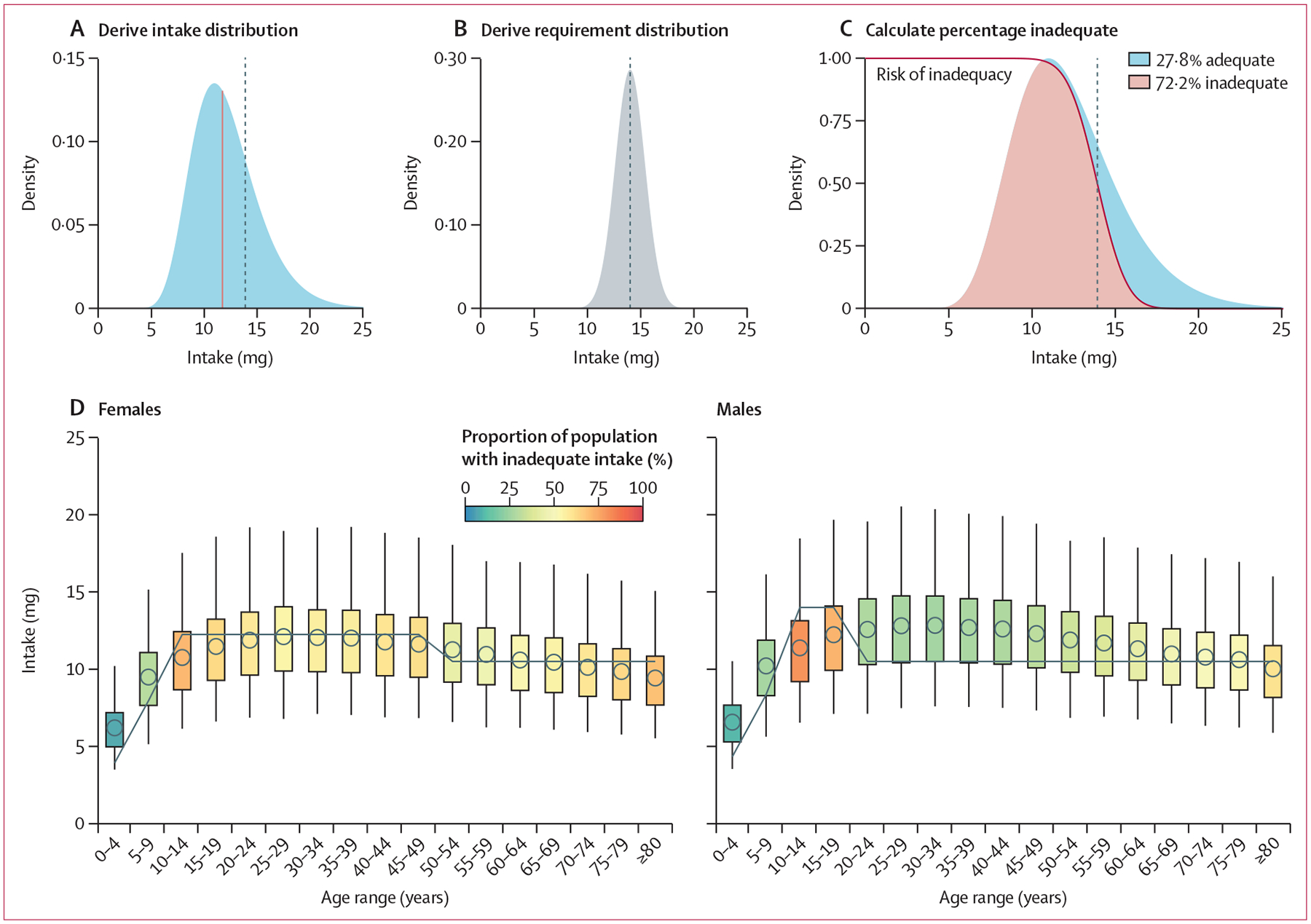
Iron intakes in Kazakhstan are used as an example. The top row illustrates the procedure for males aged 15–19 years and the bottom row illustrates the results for all age–sex groups. (A) First, we derived a skewed (gamma or log-normal) intake distribution, for which the median (blue line) of distribution was drawn from the GDD and the shape of the distribution was drawn from the nutriR database. (B) Second, we derived a normal requirement distribution, for which the mean of the distribution was drawn from the study by Allen and colleagues22 and the SD of the distribution was derived assuming a coefficient of variation of 0·25 for vitamin B12 and 0·10 for all other nutrients based on the work of Renwick and colleagues.23 (C) Finally, we derived the percentage of inadequate intake by intersecting these two distributions using the probability approach. We calculated the number of people with inadequate intakes using population estimates from the World Bank.24 In A–C, the vertical dotted line indicates the average requirement. (D) We repeated this process for every age–sex group. The points represent the median intake based on the GDD, the boxes represent the inner 50% of the intake distribution, and the whiskers represent the inner 95% of the intake distribution. The grey lines show the sex-specific and age-specific average requirements. GDD=Global Dietary Database.
Defining subnational populations
Using World Bank definitions, we estimated human population size within 34 age–sex groups (males and females in 17 age groups: 0–80 years in 5-year groups and an ≥80 years group) for the 185 evaluated countries or territories.24 We refer to these country–age–sex groups as subnational populations throughout the Article. Race and ethnicity are not reported in the World Bank population estimates or the GDD intake estimates, so results were not disaggregated by these dimensions. We used estimates for 2018, when the global population was approximately 7·57 billion people (appendix p 5), as this is the most recent year with GDD data. The 185 countries with GDD data encompass 7·52 billion people (99·3% of the global population in 2018).
Defining subnational intake medians
We developed subnational nutrient intake distributions with median intakes equivalent to the estimates provided in the GDD.20 The GDD uses datasets from household surveys and food balance sheets to estimate the median intake of 17 micronutrients from 19 food and beverage categories (appendix p 2) by subpopulation in 185 countries from 1990 to 2018 (5-year intervals from 1990 to 2015). Subpopulations are defined by 34 age–sex groups, three levels of education (ie, low, medium, and high), and two areas of residence (ie, rural and urban). We excluded two nutrients from the analysis: potassium, which does not have accepted average requirement amounts; and vitamin D, the distribution of which is highly geographically variable because the average requirement amounts can be met through sun exposure rather than dietary intake.27 This exclusion leaves 15 micronutrients (nine vitamins and six minerals) available for analysis (appendix p 1). We defined median intakes for each age–sex group using the average provided by the GDD across areas of residence and levels of education. We then averaged these intake estimates to match the 34 age–sex groups used in the Word Bank human population data (appendix p 3). Finally, to account for the supply of calcium and magnesium in drinking water, we assumed that all people consume their daily adequate intake of drinking water and that this water has an average concentration of 46 mg of calcium and 16 mg of magnesium per litre. Age-specific and sex-specific adequate intakes are from the US Institute of Medicine28 and calcium and magnesium concentrations are the average of those in global water sources from WHO.29
Defining subnational intake shapes
We defined the shape of each subnational nutrient intake distribution using estimates of subnational nutrient intake shapes provided in the nutriR database.25 Passarelli and colleagues25 assembled a database of dietary recall surveys from 31 countries and used this database to construct statistical distributions—either log-normal or gamma distributions—that describe usual intakes for 51 nutrients. The 31 countries were selected for inclusion if there was an available dataset with individual-level dietary data, calculated nutrient-level data, at least 2 days of dietary intake (for at least some participants), data based on a 24-h recall or a diet record or food diary, and a sample size of more than 200 people.25 Owing to limitations in the coverage of dietary recall surveys, distribution shapes are not available for all subnational groups, even within the 31 countries with data. We therefore matched every subnational group evaluated in this study with the shape parameters of the most similar subnational group with data. We conducted this matching with preference for shape parameters from the actual subpopulation (known), the nearest age group within the country and sex (nearest age group), the corresponding age group from the opposite sex within a country (opposite sex), and the corresponding age–sex group from the country with the most similar nutrient intakes to the country of interest (most similar country). We identified the country with the most similar nutrient intakes to the country of interest as the country with the smallest Euclidean distance in a dissimilarity matrix computed using the 2018 national nutrient intakes estimated in the GDD (appendix p 6): ie, the country with the most similar nutrient intakes in multivariate space. The extent and sources of borrowed shape information are shown in the appendix (p 7).
Defining subnational intake distributions
We specified the final usual intake distribution for each subnational group using its median value and matched shape parameters (figure 1A). The matched shape parameters describe the variability of each distribution but produce different medians to those prescribed by the GDD estimates. Therefore, we shifted the shape parameters to match the GDD median while maintaining the variability described by the matched shape parameters. For intake distributions parameterised using a log-normal distribution, we maintained the variability parameter, σ, and shifted the centrality parameter, μ. For intake distributions parameterised using a gamma distribution, we maintained the variability parameter, α, and shifted the centrality parameter, β. The shifted parameters were derived analytically for the log-normal distribution and numerically for the gamma distribution using the shift_dist() function in the nutriR package. For a conceptual illustration of these distribution shifts, see the appendix (p 8).
Estimating subnational intake inadequacy
We estimated the prevalence of intake inadequacy, also known as summary exposure value, using the probability method26 as implemented in the nutriR package. The probability method compares intake distributions against a continuous relative risk curve with a value of 1 at low intakes, 0·5 at the average intake requirement, and 0 at large intakes (figure 1C). These risk curves are defined on the basis of the cumulative normal distribution described by the average requirement and its standard deviation (figure 1B). We used the harmonised age-specific and sex-specific average requirements provided by Allen and colleagues22 as the average requirements for this analysis (appendix p 9). We assumed a coefficient of variation of 0·25 for the requirement distribution of vitamin B12 and 0·10 for the requirement distributions of all other nutrients, according to the recommendation of Renwick and colleagues.23 The coefficient of variation is used to derive the standard deviation of the requirement distribution.
We further specified country-specific average requirements for zinc and iron on the basis of dietary factors that inhibit or enhance their absorption (appendix pp 10–11). First, phytate inhibits zinc and iron absorption,30 which means that average requirements for zinc and iron increase with higher phytate intakes.22 Second, the consumption of non-dairy animal-source foods enhances iron absorption,31 which means that average requirements for iron decrease with higher intakes of such foods.22 Although calcium absorption is also affected by dietary factors such as phytate, oxalate, and dairy intakes, we were unable to account for these effects given an absence of data on global oxalate intakes—the dominant factor affecting calcium absorption.32
We derived country-specific average requirements for zinc on the basis of average country-level estimates of phytate intake from Wessels and Brown33 (appendix p 12) by linearly interpolating between the lowest average requirement and lowest phytate intake and the highest average requirement and highest phytate intake within each age–sex group (appendix p 10). We derived country-specific average requirements for iron accounting for the joint effects of phytate and non-dairy animal-source foods on iron absorption using a procedure similar to that of Beal and colleagues.10 First, we scaled the country-level phytate intakes (appendix p 12) between 0 and 1, where 0 indicates low iron absorption (high phytate intake) and 1 indicates high iron absorption (low phytate intake). Then, we scaled country-level estimates of non-dairy animal-source food intakes (ie, the sum of seafood, processed meat, unprocessed red meat, and egg intakes; unprocessed poultry meat is excluded because it is not available in the GDD; appendix pp 2, 13) from the GDD between 0 and 1, where 0 indicates low iron absorption (low non-dairy animal-source food intake) and 1 indicates high absorption (high non-dairy animal-source food intake). Next, we averaged these two indicators to create a single absorption index, in which lower values indicate lower absorption and higher values indicate higher absorption, and scaled these averages between 5% and 16% absorption, the range of real-world iron absorption values22 (appendix p 14). Finally, we derived the absorption-specific average requirements by linearly interpolating between the average requirements specified by Allen and colleagues22 (appendix p 11).
We calculated the number of people with inadequate intakes within each subnational group as the product of the number of people and the prevalence of inadequate intakes in the group.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Estimates of inadequate intake were generally high (figure 2) and were especially common for iodine (5·1 billion people; 68% of the population), vitamin E (5·0 billion people; 67% of the population), calcium (5·0 billion people; 66% of the population), and iron (4·9 billion people; 65% of the population). Niacin had the lowest estimate of inadequate intake (1·7 billion people; 22% of the population), followed by thiamin (2·2 billion people; 30% of the population) and magnesium (2·4 billion people; 31% of the population; figure 2). A few countries had estimated intake inadequacies that diverged from the general patterns. For example, in India, estimated inadequate intakes of riboflavin, folate, vitamin B6, and vitamin B12 were especially high; Madagascar and the Democratic Republic of the Congo had high inadequate niacin intakes; and Russia, Mongolia, and Kazakhstan had high inadequate selenium intakes (figure 2).
Figure 2: Estimated prevalence of intake inadequacies by country and nutrient in 2018.
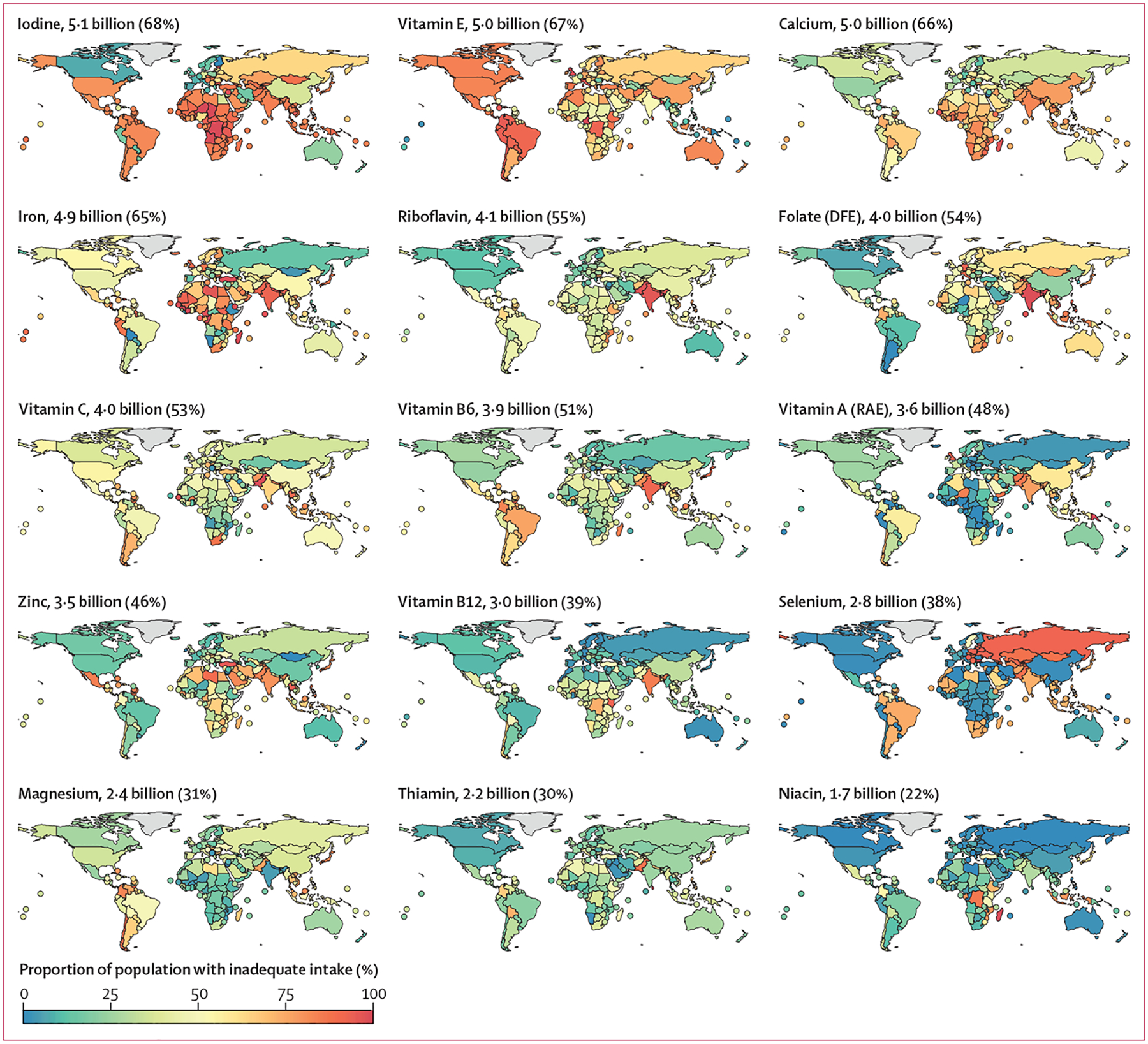
The estimated number and proportion of the global population with inadequacies is stated alongside each map. Countries with land areas of less than 25 000 km2 are shown as points to increase visibility. DFE=dietary folate equivalents. RAE=retinol activity equivalents.
Calcium intake inadequacy was highest in countries in south Asia, sub-Saharan Africa, and east Asia and the Pacific (figure 3). Intake inadequacy was high across all age–sex groups in these countries, but especially among people aged 10–30 years. Only countries in North America, Europe, and central Asia had a consistently low prevalence of inadequate calcium intake (figure 3). Low prevalence of inadequate iodine intake was observed only in Europe and Canada (figures 2, 3) and, for vitamin E, mainly in Pacific Island countries (figures 2, 3). For riboflavin and vitamin B12, high prevalences of inadequate intakes were common only in countries in south Asia and Africa (figures 2, 3).
Figure 3: Prevalence of intake inadequacies by World Bank region and nutrient in 2018.
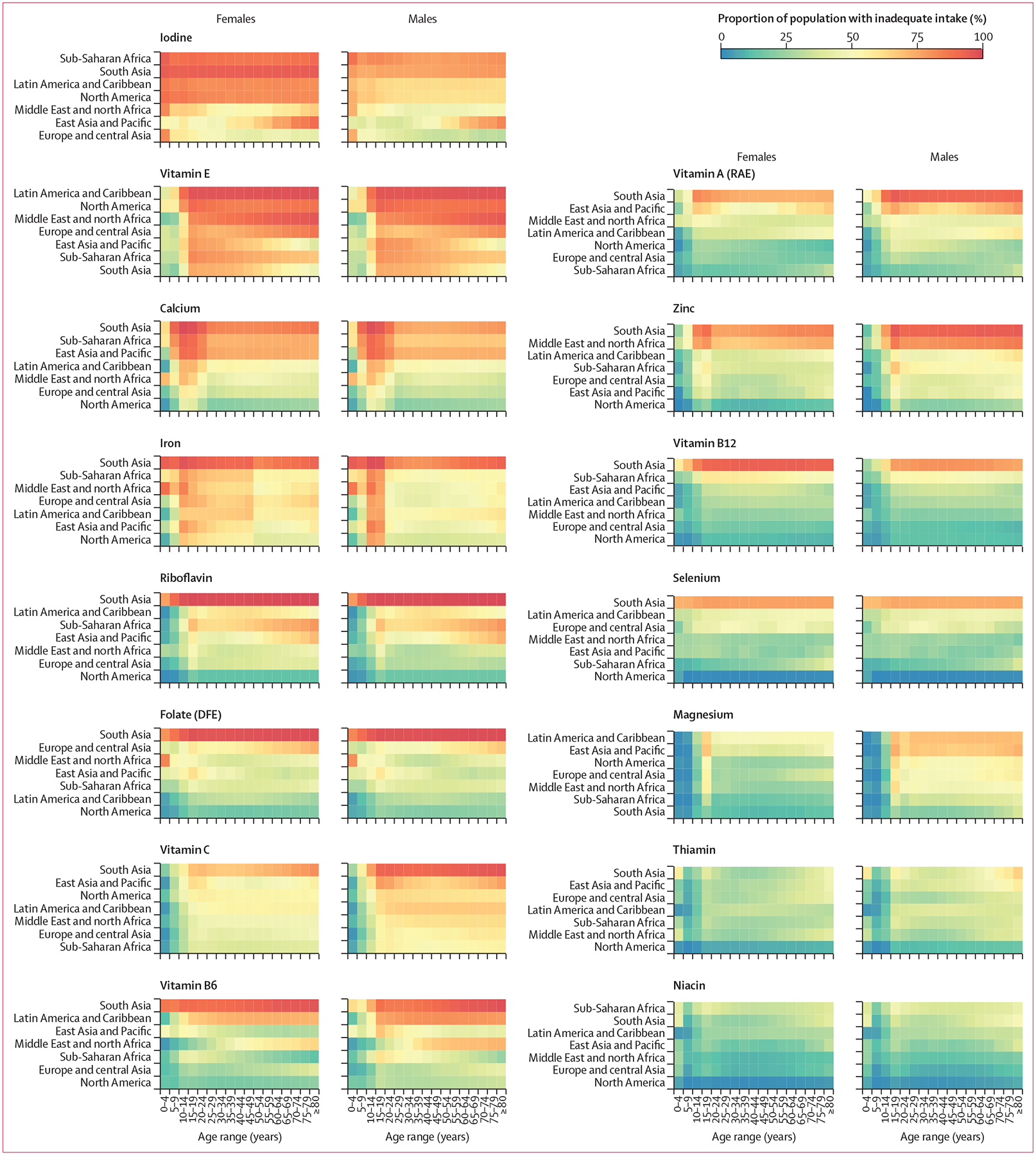
Nutrients and regions are arranged in order of decreasing prevalence of inadequate intakes. For a map of the World Bank regions, see appendix (p 15). DFE=dietary folate equivalents. RAE=retinol activity equivalents.
Globally, the prevalence of inadequate intakes was consistently higher for females than for males in the same country and age group for iodine, vitamin B12, iron, and selenium (figure 4). The prevalence was higher for females than for males in most regions for calcium, riboflavin, vitamin E, and folate. Conversely, the prevalence of inadequate intakes was consistently higher for males than for females in the same country and age group for magnesium, vitamin B6, zinc, vitamin C, vitamin A, thiamin, and niacin (figure 4).
Figure 4: Distribution of subnational differences in the prevalence of intake inadequacies between females and males by World Bank region.
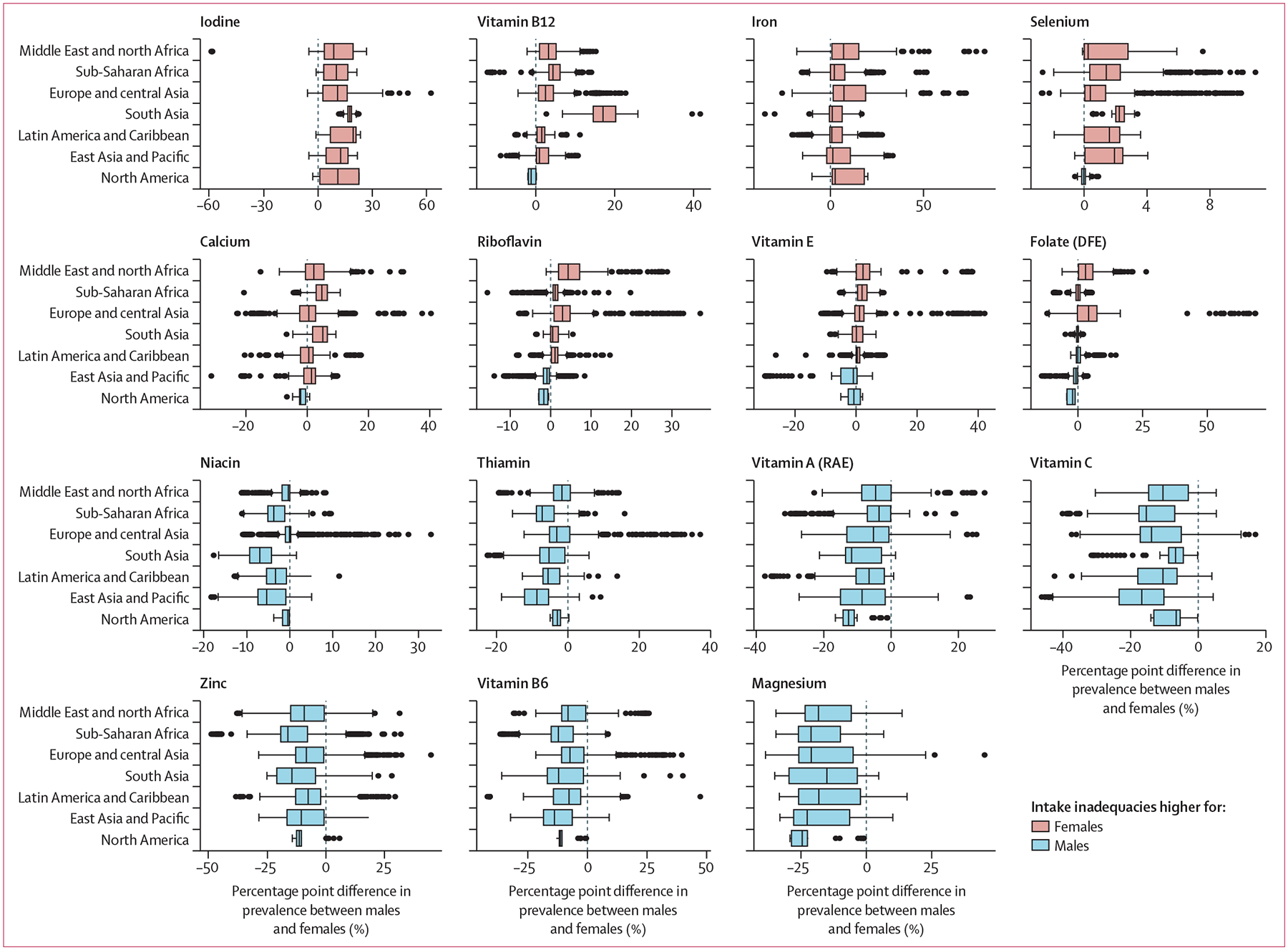
Values greater than 0 indicate a higher prevalence of intake inadequacies in females than in males in the same country and age group, whereas values less than 0 indicate a higher prevalence of intake inadequacies in males than in females in the same country and age group. In the boxplots, the solid line indicates the median, the box indicates the IQR, the whiskers indicate 1·5 times the IQR, and the points beyond the whiskers indicate outliers. For a map of the World Bank regions, see appendix (p 15). DFE=dietary folate equivalents. RAE=retinol activity equivalents.
Discussion
This analysis provides a new, replicable, and accessible method for estimating micronutrient intake inadequacy. Globally, we found that more than 5 billion people do not consume enough of each of three nutrients: iodine, vitamin E, and calcium. More than 4 billion people do not consume enough of each of another four nutrients: iron, riboflavin, folate, and vitamin C. Our analysis shows that the majority of the global population has inadequate micronutrient intake.
Globally, we found that women had a higher prevalence of inadequate intake than men for iodine, vitamin B12, iron, selenium, calcium, riboflavin, and folate. Conversely, men have higher intake inadequacies than women for magnesium, vitamin B6, zinc, vitamin C, vitamin A, thiamin, and niacin. Many of the differences observed could relate to a combination of differing dietary patterns, dietary requirements, and consumption quantities between sexes.
This Article builds on work that estimates the global prevalence of micronutrient deficiencies and inadequate nutrient supplies. Stevens and colleagues1 assessed micronutrient deficiency on the basis of biomarker data for all datasets available globally (24 nationally representative datasets) for non-pregnant women and preschool-aged (6–59 months) children, estimating that more than half of preschool-aged children and two-thirds of non-pregnant women have micronutrient deficiencies. Our estimates generally show a higher prevalence of intake inadequacy than their biomarker data. One reason for this difference might be that our estimates do not include supplements and fortified foods, so our estimates are reflective of nutrient adequacy from unfortified foods. Additionally, nutritional deficiencies (as measured by clinical biomarkers), although highly correlated with nutrient intake,34 can be strongly influenced by disease status, inflammation, the microbiome, and other contextual factors. Although many analyses have modelled inadequate nutrient supplies, ours is the first to our knowledge to estimate global inadequate intakes by applying nutrient intake distributions to estimated intake data using age-specific and sex-specific intake distributions.
Our analysis is subject to limitations—most notably, data availability (appendix p 7). There remains a scarcity of individual dietary intake data worldwide, especially nationally representative datasets and datasets with 2 or more days of intake. Although GDD coverage has increased to include more than 99% of the global population and become more precise over time, nationally representative quantitative dietary intake data within the past 10 years are scarce, which limits the ability to validate the modelled estimates across countries.35 This scarcity of data also limited the number of statistical distributions that could be estimated in the nutriR database. By basing the global intake distribution shapes on datasets from only 31 countries, some of the distribution shapes could have been incorrectly estimated, resulting in inaccurate estimates of inadequacy.
The estimates we present are of inadequate nutrient intake and do not include information on fortification or supplementation. In essence, this means that our inadequate intake estimates are likely to overestimate risk for some key nutrients (eg, iodine) in particular locations. Nonetheless, supplementation and fortification with many of these micronutrients is uncommon globally.36 Among countries with available data from the Demographic and Health Surveys programme, which operates in more than 90 low-income and middle-income countries, supplementation for selected demographic groups is somewhat common for iron, with 32% of pregnant women consuming an iron supplement for more than 90 days of their pregnancy and 14% of children aged 6–59 months consuming a supplement in the previous week. The proportion of supplementation is highest for vitamin A in children; an estimated 55% of children aged 6–59 months have received a high-dose vitamin A supplement in the previous 6 months. Data on fortification are inadequate for most nutrients except iodine; UNICEF estimates that 89% of people worldwide consume iodised salt. Therefore, iodine might be the only nutrient for which inadequate intake from food is largely overestimated.
A final limitation is that our nutrient intake estimates, with the exception of iron and zinc, do not include nutrient-to-nutrient interactions or recognition of nutrient absorption and bioavailability. Accounting for these factors would be impossible for some nutrients without knowledge of accompanying infection and inflammation status; unfortunately, nutritional science is not sufficiently advanced to accurately produce algorithms that account for these complexities.
This Article highlights the vast scale of micronutrient intake inadequacy across the world—especially for iodine, vitamin E, calcium, iron, riboflavin, and folate. Clear patterns emerged for the differing levels of estimated inadequacy for specific nutrients on the basis of sex, greater than the patterns observed across age groups within a given sex. Understanding these patterns can help us to better identify where nutritional interventions are needed, such as dietary interventions, biofortification, fortification, and supplementation. Moreover, examining which nutrient intake inadequacies are correlated with each other could help to determine which nutritional responses need to be coordinated to improve the efficiency of intervention delivery. Particular geographies warrant further investigation into the causes and severity of deficiencies before adopting fortification, supplementation, and dietary intervention policies.
This analysis represents, to our knowledge, the first estimate of inadequate micronutrient intakes globally and across diverse subpopulations, and we have made our code and underlying data publicly available so that others can use and build upon these results. We hope this analysis sheds light on crucial nutrition challenges for locations without the necessary means to collect primary data, and improves understanding of global micronutrient inadequacy so that public health interventions can more effectively address deficiencies.
Supplementary Material
Research in context.
Evidence before this study
Analyses over the past 10 years have assessed global micronutrient deficiencies and global inadequate nutrient supply, but large data gaps remain for many micronutrients and population groups. Owing to limited availability of dietary intake data and a scarcity of accurate nutrient distribution data, there have been no global estimates of inadequate micronutrient intakes.
Added value of this study
This analysis provides, to our knowledge, the first global estimates to date of inadequate global micronutrient intakes using dietary intake estimates, including for specific age and sex groups and incorporating population-specific distribution data. We estimate intake inadequacies for 15 micronutrients, adding more precision than previous estimates by specifying the shape of a population’s intake distribution. This study also uses publicly available data and provides all code to make these results accessible to researchers, practitioners, and the public.
Implications of all the available evidence
These findings show empirically that most of the global population has inadequate intake of at least one micronutrient. In combination with existing data on micronutrient deficiencies and supplies, estimates of inadequate global micronutrient intakes can help public health researchers and practitioners to identify which age and sex groups in which countries might be in greatest need of intervention for a wide range of micronutrients.
Acknowledgments
SP was supported by the National Institutes of Health Training Grant 2T32DK007703-26 in Academic Nutrition. TB was supported by contributions from the Dutch Ministry of Foreign Affairs. The open access publication fees for this Article were funded by the Swiss Agency for Development and Cooperation. The views expressed in this Article are those of the authors and do not necessarily represent the views of the US Department of State or the US Government.
Funding
The National Institutes of Health and the Dutch Ministry of Foreign Affairs.
Footnotes
Declaration of interests
We declare no competing interests.
For the Global Dietary Database see https://globaldietarydatabase.org/
For all data and code see https://github.com/cfree14/global_intake_inadequacies
For the R Shiny web application see https://emlab-ucsb.shinyapps.io/global_intake_inadequacies/
See Online for appendix
For the nutriR database see https://github.com/cfree14/nutriR
For the UNICEF estimates see https://mics.unicef.org/surveys
For data from the Demographic and Health Surveys see https://www.statcompiler.com/en/
Data sharing
All data and code are available on GitHub (https://github.com/cfree14/global_intake_inadequacies).
References
- 1.Stevens GA, Beal T, Mbuya MNN, et al. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health 2022; 10: e1590–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019; 393: 1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camaschella C Iron deficiency. Blood 2019; 133: 30–39. [DOI] [PubMed] [Google Scholar]
- 4.Stevens GA, Bennett JE, Hennocq Q, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health 2015; 3: e528–36. [DOI] [PubMed] [Google Scholar]
- 5.Imdad A, Mayo-Wilson E, Haykal MR, et al. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev 2022; 3: CD008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wessells KR, Singh GM, Brown KH. Estimating the global prevalence of inadequate zinc intake from national food balance sheets: effects of methodological assumptions. PLoS One 2012; 7: e50565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet 2021; 397: 1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown KH, Moore SE, Hess SY, et al. Increasing the availability and utilization of reliable data on population micronutrient (MN) status globally: the MN Data Generation Initiative. Am J Clin Nutr 2021; 114: 862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Dou Z, Feng S, et al. Global food nutrients analysis reveals alarming gaps and daunting challenges. Nat Food 2023; 4: 1007–17. [DOI] [PubMed] [Google Scholar]
- 10.Beal T, Massiot E, Arsenault JE, Smith MR, Hijmans RJ. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS One 2017; 12: e0175554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Development Initiatives. 2022 Global Nutrition Report: stronger commitments for greater action. 2022. https://globalnutritionreport.org/reports/2021-global-nutrition-report/ (accessed Feb 11, 2022).
- 12.Hess SY, McLain AC, Frongillo EA, et al. Challenges for estimating the global prevalence of micronutrient deficiencies and related disease burden: a case study of the Global Burden of Disease Study. Curr Dev Nutr 2021; 5: nzab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press, 2011. [PubMed] [Google Scholar]
- 14.McLain AC, Frongillo EA, Hess SY, Piwoz EG. Comparison of methods used to estimate the global burden of disease related to undernutrition and suboptimal breastfeeding. Adv Nutr 2019; 10: 380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arsenault JE, Hijmans RJ, Brown KH. Improving nutrition security through agriculture: an analytical framework based on national food balance sheets to estimate nutritional adequacy of food supplies. Food Secur 2015; 7: 693–707. [Google Scholar]
- 16.Smith MR, Micha R, Golden CD, Mozaffarian D, Myers SS. Global Expanded Nutrient Supply (GENuS) model: a new method for estimating the global dietary supply of nutrients. PLoS One 2016; 11: e0146976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lividini K, Masters WA. Tracing global flows of bioactive compounds from farm to fork in Nutrient Balance Sheets can help guide intervention towards healthier food supplies. Nat Food 2022; 3: 703–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang K, Adams KP, Ferguson EL, et al. Systematic review of metrics used to characterise dietary nutrient supply from household consumption and expenditure surveys. Public Health Nutr 2022; 25: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thar C-M, Jackson R, Swinburn B, Mhurchu CN. A review of the uses and reliability of food balance sheets in health research. Nutr Rev 2020; 78: 989–1000. [DOI] [PubMed] [Google Scholar]
- 20.Karageorgou D, Lara-Castor L, Leclercq C, et al. Harmonizing dietary datasets around the world for global diet monitoring: methods from the Global Dietary Database and the Global Individual Food Consumption Data Tool (OR06-06-19). Curr Dev Nutr 2019; 3 (suppl 1): nzz039.OR06-06-19. [Google Scholar]
- 21.Miller V, Singh GM, Onopa J, et al. Global Dietary Database 2017: data availability and gaps on 54 major foods, beverages and nutrients among 5.6 million children and adults from 1220 surveys worldwide. BMJ Glob Health 2021; 6: e003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen LH, Carriquiry AL, Murphy SP. Perspective: proposed harmonized nutrient reference values for populations. Adv Nutr 2020; 11: 469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renwick AG, Flynn A, Fletcher RJ, Müller DJG, Tuijtelaars S, Verhagen H. Risk–benefit analysis of micronutrients. Food Chem Toxicol 2004; 42: 1903–22. [DOI] [PubMed] [Google Scholar]
- 24.World Bank Group. Population, total. https://data.worldbank.org/indicator/SP.POP.TOTL(accessed May 26, 2022).
- 25.Passarelli S, Free CM, Allen LH, et al. Estimating national and subnational nutrient intake distributions of global diets. Am J Clin Nutr 2022; 116: 551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Research Council (US) Subcommittee on Criteria for Dietary Evaluation. Nutrient adequacy: assessment using food consumption surveys. Washington, DC: National Academies Press, 1986. [PubMed] [Google Scholar]
- 27.Lips P, van Schoor NM, de Jongh RT. Diet, sun, and lifestyle as determinants of vitamin D status. Ann N Y Acad Sci 2014; 1317: 92–98. [DOI] [PubMed] [Google Scholar]
- 28.Institute of Medicine. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: National Academies Press, 2005. [Google Scholar]
- 29.WHO. Calcium and magnesium in drinking-water: public health significance. Geneva: World Health Organization, 2009. [Google Scholar]
- 30.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr 2007; 137: 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurrell RF, Reddy MB, Juillerat M, Cook JD. Meat protein fractions enhance nonheme iron absorption in humans. J Nutr 2006; 136: 2808–12. [DOI] [PubMed] [Google Scholar]
- 32.Weaver CM, Wastney M, Fletcher A, Lividini K. An algorithm to assess calcium bioavailability from foods. J Nutr 2024; 154: 921–27. [DOI] [PubMed] [Google Scholar]
- 33.Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 2012; 7: e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser GE, Jaceldo-Siegl K, Henning SM, et al. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr 2016; 146: 586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beal T, Herforth A, Sundberg S, Hess SY, Neufeld LM. Differences in modelled estimates of global dietary intake. Lancet 2021; 397: 1708–09. [DOI] [PubMed] [Google Scholar]
- 36.Rohner F, Wirth JP, Zeng W, et al. Global coverage of mandatory large-scale food fortification programs: a systematic review and meta-analysis. Adv Nutr 2023; 14: 1197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code are available on GitHub (https://github.com/cfree14/global_intake_inadequacies).


