Abstract
We studied the in vivo biological properties of viruses reconstituted from the genome of murine gammaherpesvirus 68 (MHV-68) cloned as an infectious bacterial artificial chromosome (BAC). Recombinant virus RγHV68A98.01, containing BAC vector sequences, is attenuated in vivo as determined by (i) viral titers in the lungs during the acute phase of infection, (ii) the extent of splenomegaly, and (iii) the number of latently infected spleen cells reactivating virus in an ex vivo reactivation assay. Since the BAC vector sequences were flanked by loxP sites, passaging the virus in fibroblasts expressing Cre recombinase resulted in the generation of recombinant virus RγHV68A98.02, with biological properties comparable to those of wild-type MHV-68. On the basis of these data we conclude (i) that excision of BAC vector sequences from cloned MHV-68 genomes is critical for reconstitution of the wild-type phenotypic properties of this virus and (ii) that the BAC-cloned MHV-68 genome is suitable for the construction of mutants and mutant libraries whose phenotypes can be reliably assessed in vivo.
A number of herpesviruses have recently been cloned as infectious bacterial artificial chromosomes (BACs) (4, 6). This technique allows the maintenance of viral genomes by a BAC in Escherichia coli and the reconstitution of viral progeny by transfection of the BAC plasmid into eukaryotic cells. Mutagenesis of the virus genome in E. coli is possible, thereby considerably speeding up the construction of viral mutants (6). However, for in vivo pathogenesis studies of BAC-cloned viral genomes and their mutants it is necessary to preserve the wild-type phenotype of any BAC-derived virus.
We have recently cloned the genome of murine gammaherpesvirus 68 (MHV-68) as an infectious BAC and reconstituted infectious viruses (1). The BAC vector sequence was inserted at the left end of the MHV-68 genome in a manner which should not disrupt any known gene. In that study, we reported the in vitro growth properties of two recombinant BAC-derived viruses, RγHV68A98.01 (with BAC vector sequences) and RγHV68A98.02 (devoid of BAC vector sequences), which were found to be comparable to wild-type MHV-68 (1). Nonetheless, despite the fact that the presence of BAC vector sequences did not appear to affect the in vitro growth properties of recombinant viruses, it remained possible that the presence of BAC vector sequences in the BAC-derived viruses would still alter the phenotype of the reconstituted viruses in vivo.
Here, we addressed the following questions. (i) Does the presence of the BAC vector sequences in the reconstituted virus genome interfere with in vivo biological properties of MHV-68? (ii) Can infectious virus with in vivo wild-type properties be reconstituted from the BAC-cloned MHV-68? To answer these questions, we compared the in vivo biological properties of wild-type MHV-68 and two BAC-derived viruses, RγHV68A98.01 (with BAC vector sequences) and RγHV68A98.02 (devoid of BAC vector sequences) (Fig. 1).
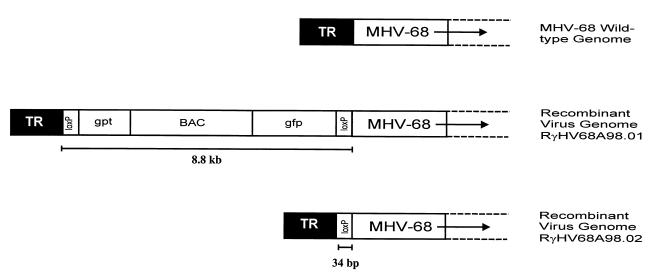
FIG. 1.
Genomic structures of wild-type MHV-68, RγHV68A98.01, and RγHV68A98.02. Top line, left end of the unique sequence of the MHV-68 wild-type genome and the adjacent terminal repeats (TR). The genome of the recombinant virus RγHV68A98.01 contains in addition to the BAC vector (BAC) the genes for the guanosine phosphoribosyltransferase (gpt) and the green fluorescent protein (gfp), flanked by loxP sites (middle line). After excision of the loxP-flanked sequences, only one loxP site is left in the genome of recombinant virus RγHV68A98.02 (bottom line).
BAC-derived virus RγHV68A98.01 (with BAC vector sequences) is attenuated in vivo.
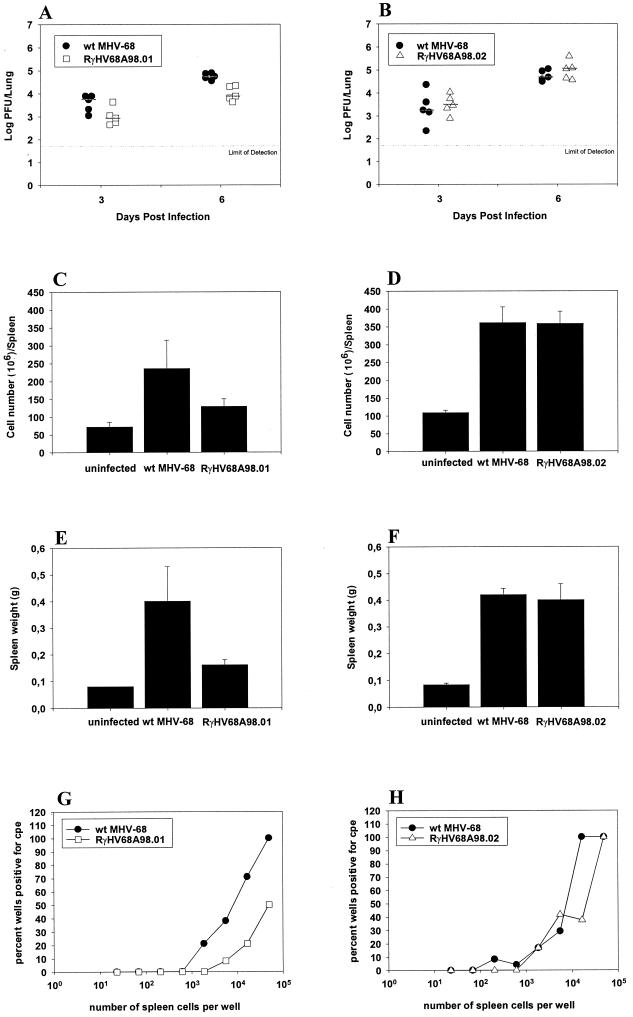
To test the in vivo properties of RγHV68A98.01, female C57BL/6 mice (8 to 10 weeks of age) (RCC Ltd., Füllinsdorf, Switzerland) were intranasally infected with 5 × 104 PFU of either wild-type MHV-68 or RγHV68A98.01 in a volume of 40 μl of phosphate-buffered saline. Lungs of mice were harvested at days 3 and 6 postinfection, and viral titers were determined from lung homogenates. Briefly, lungs were homogenized by douncing and subjected to two rounds of freezing and thawing. Virus titers were determined by plaque assay on BHK-21 cells as described previously (1). At day 3 postinfection, slightly lower titers of RγHV68A98.01 than of wild-type MHV-68 were observed, and, at day 6 postinfection, approximately 10-fold-lower titers of RγHV68A98.01 were observed (Fig. 2A). Titers of both viruses were close to or below the detection limit at day 9 postinfection, indicating that the lower titers of RγHV68A98.01 were not due to a delayed growth (data not shown). Spleens of mice were harvested at day 17 postinfection, a time point when splenomegaly occurs and latent infection has been established (13). The splenomegaly caused by MHV-68 infection was quantitated by determination of both the cell numbers per spleen and the spleen weight. As expected, mice infected with wild-type MHV-68 had pronounced splenomegaly compared to uninfected control mice (Fig. 2C and E). In contrast, splenomegaly caused by infection with RγHV68A98.01 was much less pronounced than that caused by infection with wild-type MHV-68. To determine the level of viral reactivation at day 17 postinfection, an ex vivo limiting-dilution reactivation assay was carried out (7, 19). Briefly, serial threefold dilutions of infected mouse splenocytes were plated on monolayers of 104 low-passage NIH 3T3 cells per well in 96-well tissue culture plates. Twenty-four wells were plated per dilution (starting with 5 × 104 splenocytes). NIH 3T3 cells were screened microscopically for a viral cytopathic effect for up to 3 weeks. To differentiate between latently infected cells and infectious virus in the samples, serial threefold dilutions of spleen cells were plated before or after mechanical disruption of viable cells (by two freeze-thaw cycles). No infectious virus was detected in samples of mechanically disrupted cells (data not shown). The number of spleen cells which reactivated MHV-68 was significantly lower in mice infected with RγHV68A98.01 than in mice infected with wild-type MHV-68 (Fig. 2G). Thus, BAC-derived virus RγHV68A98.01, in which the BAC vector sequences remained inserted, is attenuated in vivo in terms of viral titers in the lungs, the extent of splenomegaly, and the level of viral reactivation.
FIG. 2.
BAC-derived virus RγHV68A98.01 is attenuated in vivo, whereas BAC-derived virus RγHV68A98.02 shows in vivo properties very similar to those of wild-type (wt) MHV-68. C57BL/6 mice were intranasally infected with 5 × 104 PFU of either wild-type MHV-68, RγHV68A98.01, or RγHV68A98.02. Viral titers in the lungs were determined 3 and 6 days after infection (A and B). The extent of splenomegaly was determined by counting spleen cell numbers (C and D) and measuring the weight of the spleen 17 days after infection (E and F). The level of viral reactivation was determined by a limiting-dilution reactivation assay 17 days after infection (G and H). In panels A and B, titers of individual mice (n = 5) and median values are shown. Data shown in panels C to F are means ± standard deviations of three individual mice per group. Data in panels G and H are from pools of three mice per group. Both at day 3 and day 6 after infection, viral titers of RγHV68A98.01 are significantly lower than those of wild-type MHV-68 (P = 0.046 and 0.001, respectively; unpaired Student's t test) (A). The means of the cell numbers and the means of the spleen weights of the three groups of mice are significantly different (P = 0.0156 for cell number, and P = 0.0045 for spleen weight; one-way analysis of variance) (C and E). The number of spleen cells which reactivated MHV-68 was significantly lower in mice infected with RγHV68A98.01 than in mice infected with wild-type MHV-68 (P = 0.048; paired Student's t test) (G). cpe, cytopathic effect.
BAC-derived virus RγHV68A98.02 (devoid of BAC vector sequences) displays wild-type properties in vivo.
To test the in vivo properties of RγHV68A98.02, the set of experiments described above were performed. Wild-type MHV-68 and RγHV68A98.02 showed comparable titers in the lung at days 3 and 6 postinfection (Fig. 2B). In addition, the extent of splenomegaly caused by infection with wild-type MHV-68 was identical to that caused by infection with RγHV68A98.02 (Fig. 2D and F). Finally, the numbers of spleen cells which reactivated MHV-68 were comparable for both viruses (Fig. 2H) (P = 0.366; paired Student's t test). Thus, BAC-derived virus RγHV68A98.02 showed in vivo properties indistinguishable from those of wild-type MHV-68.
BAC-derived virus RγHV68A98.01 (with BAC vector sequences) is attenuated in SCID mice.
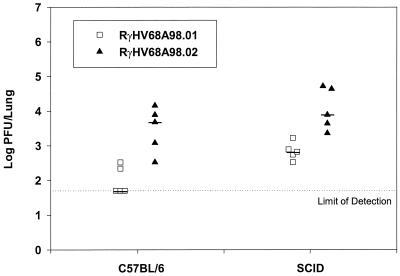
There may be several reasons why BAC-derived virus RγHV68A98.01, containing the BAC vector sequences, is attenuated in vivo. Insertion of the 8.8-kbp BAC vector sequence resulted in an overlength genome. This could affect viral replication in general. Furthermore, additional sequences included within the BAC cassette, for example, the coding sequence for green fluorescent protein (gfp), which is expressed during infection, may be recognized by the adaptive host immune response against the BAC-derived virus and thus reduce virus fitness in comparison with that of the wild-type virus. To test the latter possibility, C57BL/6 and SCID mice (female, 8 to 10 weeks of age) (Charles River Laboratories, Sulzfeld, Germany) which lack T and B cells (10) were infected intranasally with 1,000 PFU of either RγHV68A98.01 or RγHV68A98.02 in 40 μl of phosphate-buffered saline and viral titers in the lung were determined at day 6 postinfection as described above. In C57BL/6 and in SCID mice, the lung titers of RγHV68A98.01 were approximately 55-fold and 27-fold lower, respectively, than those of RγHV68A98.02 (Fig. 3). We concluded from these data that the adaptive host immune response (T and B cells) was not the major cause of the attenuated phenotype of RγHV68A98.01, but we cannot rule out a role for the innate immune response (e.g., NK cells and macrophages), which is not affected in SCID mice (10).
FIG. 3.
BAC-derived virus RγHV68A98.01 is also attenuated in SCID mice. C57BL/6 and SCID mice were intranasally infected with 1,000 PFU of RγHV68A98.01 or RγHV68A98.02. Viral titers in the lungs were determined 6 days after infection. Titers of individual mice (n = 5) and median values are shown. Both in C57BL/6 mice and in SCID mice, viral titers of RγHV68A98.01 were significantly lower than those of RγHV68A98.02 (P = 0.003 for both groups; unpaired Student's t test).
This is the first study directly comparing two BAC-derived viruses in vivo which are identical except for the presence of BAC vector sequences within the genome. Our findings are relevant for the in vivo use of BAC-derived MHV-68. First, virus with in vivo wild-type properties can be reconstituted from the BAC clone. This is important since it demonstrates that BAC-cloned MHV-68 can be used to compare wild-type MHV-68 with mutants for in vivo pathogenesis studies. For in vivo studies with BAC-derived viruses, the BAC vector sequences need to be removed. This can be achieved by propagation of BAC-derived viruses in rat fibroblasts expressing Cre recombinase (1). For testing the in vitro growth properties of the BAC-derived viruses, BHK-21 cells were used and no difference was seen (1). In vivo, lung epithelial cells, B cells, macrophages, and dendritic cells are mainly considered to harbor MHV-68 (9, 16, 17, 19, 20). Thus, slightly different growth properties of BAC-derived virus RγHV68A98.01 (with BAC vector sequences) in vitro and in vivo may reflect differences between the infected cell types. Changes created by insertion of the BAC vector sequences, for example, the oversize of the genome, apparently have a minor effect in fibroblasts but may have a more pronounced effect in cells which are infected in vivo. Viral capsid packaging limitations for oversized genomes have been reported for DNA viruses (2, 3). Additional BAC vector sequences may cause an instability of the viral genome (14) or may disrupt the expression of neighboring genes (15). However, as MHV-68 should tolerate, at least to a certain extent, the insertion of foreign sequences due to the possibility to equilibrate the number of terminal repeats of the genome, we did not expect a difference. This was also suggested by the observation that deletion of the M1 open reading frame (ORF) and the first four viral tRNA genes by insertion of a lacZ expression cassette of approximately 4 kb did not affect the ability of the mutant to establish latency and to reactivate from latency in vivo (12).
There is some evidence for position-dependent effects of sequence insertions. Recently, it has been shown that insertion of a lacZ expression cassette into the M1 ORF of MHV-68 resulted in decreased acute virus replication in the spleens of both immunocompetent and immunodeficient mice (8). As in our example, attenuation was not seen in a mutant when the same sequence was interrupted but the insertion of the lacZ expression cassette was omitted. Another MHV-68 mutant containing the lacZ expression cassette in another ORF exhibited normal virus replication in the spleen (8). Thus, the authors concluded that the attenuation observed with the M1-lacZ mutant arose from a position-dependent effect of the lacZ expression cassette (8). Whether locating the BAC vector sequences elsewhere in the genome would prevent attenuation in vivo is not known. Furthermore, the nature of such position-dependent effects is unclear to date.
Observations which emphasize the need to remove BAC vector sequences from the viral genome have been made with other BAC-cloned herpesviruses as well. First, the original BAC-derived murine cytomegalovirus (MCMV) MC96.73 was attenuated in vivo (18). In this virus, a nonessential genomic region had been deleted and replaced by BAC vector sequences to prevent the instability of the oversize genome (11). Therefore, it remained unclear whether the MCMV attenuation in vivo was due to deletion of the nonessential genomic region or to the presence of the BAC vector sequences or both. In a subsequent study, the missing MCMV sequences were reinserted and BAC vector sequences were flanked with short identical viral sequences (18). During replication in mammalian cells, homologous recombination through the viral sequence repeat resulted in the loss of the BAC vector sequences and reconstitution of the wild-type sequence and wild-type properties (18). Second, the BAC-derived pseudorabies virus (PRV) was found to be virulent and spread normally in animals but displayed a subtle growth defect in cultured cells (14). The BAC vector sequences caused an instability of the viral genome, resulting in spontaneous deletion of the BAC vector and flanking viral sequences (14). This problem was solved by a self-excising BAC plasmid containing the complete PRV genome (15). Upon delivery of the BAC-cloned PRV genome into mammalian cells, the BAC vector is removed (15). The growth properties of the resulting virus were indistinguishable from those of the wild-type virus both in vitro and in vivo (15).
Excision of the BAC vector sequences from BAC-cloned herpesvirus genomes has now been accomplished by three approaches. For MCMV, the BAC vector sequences were flanked by identical viral sequences. Complete excision of the BAC vector from the viral population required series of replication (18). For PRV, excision of the BAC vector sequences was achieved by Cre recombinase integrated into the BAC vector. Virus lacking the BAC vector sequences is isolated from transfected cells without the need of serial passage or plaque purification (15). For MHV-68, the BAC vector sequences were also flanked by loxP sites. By passaging the virus in fibroblasts expressing Cre recombinase and purification by limiting dilution using green fluorescent protein expression as a marker, recombinant viruses devoid of BAC vector sequences can be generated (1). In contrast to MCMV and PRV, where the BAC vector sequences are spontaneously excised upon delivery of the BAC-cloned genomes into mammalian cells, in the BAC-derived MHV-68 the BAC vector sequences can be removed at will. Since the sequence for gfp is included in the BAC cassette, recombinant viruses with or without gfp expression can be generated, depending on the needs of the particular experiment. Whereas in MCMV the resulting virus is completely free of bacterial sequences, in both PRV and MHV-68, a single 34-bp loxP site remains in the viral genome after excision of the BAC vector sequences (1, 15).
In conclusion, our results demonstrate the suitability of MHV-68 BAC-derived viruses for in vivo pathogenesis studies. This system will contribute to the potential of MHV-68 infection of mice as a small-animal model for gammaherpesvirus infections. Recently, we reported on a new forward-mutagenesis principle for rapid generation of virus mutant libraries from BAC-cloned herpesviruses (5). Application of this technique to MHV 68 in combination with the deletion of the BAC sequences should provide the chance for large-scale testing of random gammaherpesvirus mutants in vivo.
Acknowledgments
We thank R. Cardin for technical advice, E. T. Clambey for advice with the statistical analysis, and C. Burgmeier for excellent technical assistance. We are grateful to B. Adler for critical reading of the manuscript and to S. Efstathiou for helpful comments.
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF), Stipendienprogramm Infektionsforschung, to H.A., from the BMBF FKZ 01K1960612 to U.H.K., and from the Deutsche Forschungsgemeinschaft (DFG), SFB 455, to M.M. and U.H.K.
REFERENCES
- 1.Adler H, Messerle M, Wagner M, Koszinowski U H. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J Virol. 2000;74:6964–6974. doi: 10.1128/jvi.74.15.6964-6974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bett A J, Prevek L, Graham F L. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993;67:5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloss T A, Sugden B. Optimal lengths of DNAs encapsidated by Epstein-Barr virus. J Virol. 1994;68:8217–8222. doi: 10.1128/jvi.68.12.8217-8222.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt W J. Infectious clones of herpesviruses: a new approach for understanding viral gene function. Trends Microbiol. 2000;8:262–265. doi: 10.1016/s0966-842x(00)01747-9. [DOI] [PubMed] [Google Scholar]
- 5.Brune W, Menard C, Heesemann J, Koszinowski U H. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science. 2001;291:303–305. doi: 10.1126/science.291.5502.303. [DOI] [PubMed] [Google Scholar]
- 6.Brune W, Messerle M, Koszinowski U H. Forward with BACs—new tools for herpesvirus genomics. Trends Genet. 2000;16:254–259. doi: 10.1016/s0168-9525(00)02015-1. [DOI] [PubMed] [Google Scholar]
- 7.Christensen J P, Cardin R D, Branum K C, Doherty P C. CD4+ T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci USA. 1999;96:5135–5140. doi: 10.1073/pnas.96.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clambey E T, Virgin IV H W, Speck S H. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J Virol. 2000;74:1973–1984. doi: 10.1128/jvi.74.4.1973-1984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flano E, Husain S M, Sample J T, Woodland D L, Blackman M A. Latent murine γ-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol. 2000;165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 10.Lyon M F, Searle A G. Genetic variants and strains of the laboratory mouse. Oxford, United Kingdom: Oxford University Press; 1989. [Google Scholar]
- 11.Messerle M, Crnkovic I, Hammerschmidt W, Ziegler H, Koszinowski U H. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc Natl Acad Sci USA. 1997;94:14759–14763. doi: 10.1073/pnas.94.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simas J P, Bowden R J, Paige V, Efstathiou S. Four tRNA-like sequences and a serpin homologue encoded by murine gammaherpesvirus 68 are dispensable for lytic replication in vitro and latency in vivo. J Gen Virol. 1998;79:149–153. doi: 10.1099/0022-1317-79-1-149. [DOI] [PubMed] [Google Scholar]
- 13.Simas J P, Efstathiou S. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 1998;6:276–282. doi: 10.1016/s0966-842x(98)01306-7. [DOI] [PubMed] [Google Scholar]
- 14.Smith G A, Enquist L. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J Virol. 1999;73:6405–6414. doi: 10.1128/jvi.73.8.6405-6414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith G A, Enquist L W. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc Natl Acad Sci USA. 2000;97:4873–4878. doi: 10.1073/pnas.080502497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart J P, Usherwood E J, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunil-Chandra N P, Efstathiou S, Nash A A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 18.Wagner M, Jonjic S, Koszinowski U H, Messerle M. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J Virol. 1999;73:7056–7060. doi: 10.1128/jvi.73.8.7056-7060.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W., IV Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weck K E, Kim S S, Virgin IV H W, Speck S H. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]