Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide. The gut microbiome has been implicated in outcomes for HCC, and gut microbe-derived products may serve as potential non-invasive indices for early HCC detection. This study evaluated differences in plasma concentrations of gut microbiota-derived metabolites. Methods: Forty-one patients with HCC and 96 healthy controls were enrolled from surgical clinics at the Cleveland Clinic from 2016 to 2020. Gut microbiota-derived circulating metabolites detectable in plasma were compared between patients with HCC and healthy controls. Hierarchical clustering was performed for generating heatmaps based on circulating metabolite concentrations using ClustVis, with Euclidean and Ward settings and significant differences between metabolite concentrations were tested using a binary logistic regression model. Results: In patients with HCC, 25 (61%) had histologically confirmed cirrhosis. Trimethylamine (TMA)-related metabolites were found at higher concentrations in those with HCC, including choline (p < 0.001), betaine (p < 0.001), carnitine (p = 0.007), TMA (p < 0.001) and trimethylamine N-oxide (TMAO, p < 0.001). Notably, concentrations of P-cresol glucuronide (p < 0.001), indole-lactic acid (p = 0.038), 5-hydroxyindoleacetic acid (p < 0.0001) and 4-hydroxyphenyllactic acid (p < 0.001) were also increased in those with HCC compared to healthy controls. Hierarchical clustering of the metabolite panel separated patients based on the presence of HCC (p < 0.001), but was not able to distinguish between patients with HCC based on the presence of cirrhosis (p = 0.42). Conclusions: Gut microbiota-derived metabolites were differentially abundant in patients with HCC versus healthy controls. The observed perturbations of the TMAO pathway in HCC seem particularly promising as a target of future research and may have both diagnostic and therapeutic implications.
Keywords: nutrition, metabolism, microbiome, hepatocellular carcinoma, cirrhosis
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer, the third leading cause of cancer-related mortality and the second most lethal cancer per case worldwide [1,2]. Both incidence and mortality of HCC continue to rise in Western countries, in large part due to the rising burden of both non-alcoholic and alcoholic steatohepatitis in the United States and Europe [3,4]. Cirrhosis and HCC are co-morbid in over 80% of cases, and thus any patient with cirrhosis or end-stage liver disease (ESLD) is at high risk for development of HCC [5]. Therefore, any study of HCC should account for the underlying architecture of the liver parenchyma.
HCC often originates in a background of chronic cirrhosis, which is initiated by viral hepatitis, alcohol abuse or metabolic dysfunction-associated steatohepatitis (MASH) [1,2,3]. Although HCC is one of the classic examples of a viral-driven cancer, there is also strong evidence that obesity is another major risk factor for developing HCC [1,2,3]. Today, the vast majority of HCC cases are indeed driven by either hepatitis B or C (HBV or HCV) infection [1]. However, with recent availability of highly effective therapies targeting the HBV and HCV viral pathogens, it is anticipated that viral-driven HCC will decline sharply over the next decade. Despite this exciting prediction with viral-associated HCC, the incidence of obesity-driven HCC is poised to rise at an alarming rate as the obesity epidemic continues to grow in developed countries. Although obesity is driven by many genetic and environmental determinants, alterations in the gut microbiome are central to the promotion of obesity.
The interplay between obesity, HCC, ESLD/cirrhosis and the gut microbiome is a field of emerging interest [4,5,6,7,8,9,10,11,12,13,14,15,16]. The gut–liver axis has, for example, been implicated in chronic injury to hepatocytes, leading to regenerative liver injury and HCC-inducing genetic damage [6]. Further studies have correlated alterations in the gut microbiome community with progression of HCC in those with background MASH-cirrhosis [7,8]. Indeed, alterations in gut-derived microbiota have been proposed as mechanisms to diagnose HCC in high-risk patients, and as potential markers for response to immunotherapy regimens [6,9,10]. Using sequencing-based methods to profile either the gut or HCC tumor microbiome, several groups have shown clear associations between microbial diversity and HCC initiation, progression and response to therapy [4,5,6,7,8,9,10,11,12,13,14,15,16]. However, despite clear links between the microbiome and liver cancer, the gut microbe-derived factors that could serve as either diagnostic or therapeutic targets are largely unknown. Our study provides key new information, given we are not relying on sequencing-based methodologies. Instead, we quantified the level of gut microbe-derived metabolic products in the circulation of HCC patients using stable isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS).
It has long been assumed that for gut microbes to impact liver disease and HCC, bacterial cell wall products such as lipopolysaccharide (LPS) “leak” into the portal circulation to directly engage hepatic pattern-recognition receptors and drive chronic inflammation in the liver. Here, we investigate an alternative model, where gut microbial metabolism of nutrients common in high-fat diets results in the production of a wide variety of small molecule microbial metabolites that enter the systemic circulation [17,18,19]. While previous studies have demonstrated that variations in actual GI microbiota are associated with HCC, these have typically been identified either from oral or gut sequencing, and/or from multi-omics analyses of fecal/oral GI samples in patients with HCC [9,10,11,12,13,14,15,16]. Serologic analyses of metabolites known to be derived from the gut–liver axis are generally lacking in the literature. While current evidence is limited, this type of analysis may have the potential to identify potential targets for diagnosis and therapy. This study aimed to identify plasma gut microbe-associated metabolomic signatures that differ in the circulation of patients with HCC versus healthy patients, and compare patients who have HCC both with and without cirrhosis.
2. Materials and Methods
2.1. Study Population
Plasma samples from 41 patients with a diagnosis of HCC using OPTN classification were obtained from patients seen by a surgeon in the multi-disciplinary liver-tumor clinic from January 2016 to July 2020 (see Table 1). Only patients with OPTN 5 lesions and scheduled surgical resection were included in this cohort, and samples were obtained prior to surgery. This cohort was chosen to maximize our histologic understanding of the tumor, as well as to confirm or rule out any diagnosis of cirrhosis or other underlying liver disease. Patients were stratified by presence (n = 25) or absence (n = 16) of cirrhosis to obtain subgroup specific effect sizes for associations with metabolites. The etiology of cirrhosis included non-alcoholic steatohepatitis (9.8%), hepatitis B (9.8%), hepatitis C (34.1%), hemochromatosis (2.4%) and idiopathic (4,9%). Most patients were treatment naïve at the time of sample collection, though some had received loco-regional therapy as detailed below. Plasma samples from 96 healthy control patients were obtained from an institutional biorepository of patients known to have no history of malignancy, heavy alcohol use, hepatitis, cirrhosis or other known liver disease. These samples were collected at Cleveland Clinic, Cleveland, OH, USA. This study was approved by the Institutional Review Board IRB# 10-727 (CHAMPS) for healthy controls and 10-347 for HCC samples, and all patients signed informed consent prior to sample collection.
Table 1.
(a) Patient demographics for healthy control subjects. (b) Patient demographics for subjects with hepatocellular carcinoma (HCC).
| (a) | |||
| Total (n = 96) | |||
| Average Age | 50.38 | ||
| Male Sex | 44 | ||
| Race | |||
| White | 32 (72.72%) | ||
| Black | 11 (25.0%) | ||
| Asian | 1 (2.27%) | ||
| Average BMI (kg/m2) | 28.06 | ||
| Female Sex | 50 | ||
| Race | |||
| White | 45 (90.0%) | ||
| Black | 5 (10.0%) | ||
| Average BMI (kg/m2) | 27.46 | ||
| (b) | |||
| Total HCC (n = 41) | HCC + Cirrhosis (n = 25) | HCC Alone (n = 16) | |
| Median Age (IQR) | 68 (62.5–76.5) | 66 (62–72) | 73 (62.5–79.3) |
| Male Sex | 13 | 7 (28%) | 6 (37.5%) |
| Race | |||
| White | 34 (82.9%) | 19 (76%) | 15 (93.7%) |
| Black | 4 (9.8%) | 4 (16%) | - |
| Asian | 3 (7.3%) | 2 (8%) | 1 (6.3%) |
| BMI (kg/m2) | 27.3 (24–30.7) | 27.3 (24–31.3) | 27.6 (25.7–30.7) |
| Etiology of Cirrhosis | - | ||
| NASH | 4 (9.8%) | 4 (16%) | |
| Hepatitis B | 4 (9.8%) | 4 (16%) | |
| Hepatitis C | 14 (34.1%) | 14 (56%) | |
| Idiopathic | 2 (4.9%) | 2 (8%) | |
| Hemochromatosis | 1 (2.4%) | 1 (4%) | |
| Diabetes | 11 (26.8%) | 8 (32%) | 3 (18.8%) |
|
No. Lesions Median (IQR) |
1 (1–2) | 1 (1–2) | 1 (1–3) |
|
Size Largest Lesion Median cm (IQR) |
4 (2.5–6) | 3 (2–4) | 10 (4.3–14.3) |
| Treatment Pre-Sample Collection | |||
| Y90 | 3 (7.3%) | 2 (8%) | 1 (6.3%) |
| TACE | 4 (9.8%) | 2 (8%) | 2 (12.6%) |
| None | 34 (82.9%) | 21 (84%) | 13 (81.1%) |
| Grade of Differentiation | |||
| Well | 4 (9.8%) | - | 4 (25%) |
| Moderate | 34 (82.9%) | 24 (96%) | 10 (62.5%) |
| Poor | 3 (7.3%) | 1 (4%) | 2 (12.6%) |
| Vascular Invasion | 30 (73.2%) | 20 (80%) | 10 (62.5%) |
| Recurrence | 15 (36.6%) | 9 (36%) | 6 (37.5%) |
Age, race and BMI are expressed as count in percent; gut microbe-associated metabolites are altered in HCC patients compared to healthy controls. All the variables are expressed as count in percent. Age, number and size of lesions is presented as median (IQR).
2.2. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
For this study we wanted to broadly understand how gut microbe-derived metabolites originating from diverse dietary substrates are associated with HCC. Therefore, we used a stable-isotope-dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) methods for the quantitative analysis of diverse bacterially derived metabolites that originate from dietary micronutrients such as choline and carnitine, as well as aromatic amino acids including phenylalanine, tyrosine and tryptophan. A total of 20 mL of human plasma was used for extraction of metabolites using the method described by Nemet and colleagues [19]. Briefly, 80 mL of isotope labeled internal standard mix was added to 20 uL of plasma, vortexed for 1 min, followed by spin down at 20,000 g, in 4 °C for 10 min. A total of 80 mL of supernatant was transferred to a MS vial with insert and 5 mL was used for LC-MS. The plasma concentration of gut microbe-associated metabolites originating from aromatic amino acids was recently described by Nemet and colleagues [19]. Trimethylamine (TMA) related metabolites were measured as previously descibed by Wang and colleagues [20]. Detailed substrate-producte maps of the microbe and host or co-metabolites in these diverse metaorganimsal nutrient metabolism pathways under invetigation here are also available in our previous publications [19,20,21].
2.3. Statistical Analysis
All values depicted are represented as mean concentrations with standard deviations. Significant differences between metabolite concentrations were tested using a binary logistic regression model with a Bonferroni adjustment for multiple hypothesis testing [21]. For all tests, an adjusted p < 0.05 was employed as the threshold for statistical significance. Hierarchical clustering was performed on the log fold change for each metabolite between the average concentrations of each group and that of healthy controls. Heatmaps were generated using Euclidean distance and Ward’s method using ClustVis software (https://biit.cs.ut.ee/clustvis/) [22].
3. Results
Detailed healthy control and HCC patient demographics are provided in Table 1. Healthy controls have an average age of 50.38 and average BMI is 28.06 for males and 27.46 for females. Of those with HCC, 25 (61.0%) had concurrent histology-confirmed cirrhosis, while the remaining 16 (39.0%) had histologically confirmed normal hepatic parenchymal architecture. The etiology of cirrhosis was Hepatitis C Virus (HCV, n = 14, 56%), Hepatitis B Virus (HBV, n = 4, 16%), non-alcoholic steatohepatitis (NASH, n = 4, 16%), idiopathic (n = 2, 8%) and hemochromatosis (n = 1, 4%). One-quarter (n = 11) of the entire HCC cohort had a history of diabetes mellitus, all of which was type 2 diabetes. An additional 96 patients with no known liver disease or cancer of any kind were included in the study as healthy controls. Limited background information (basic demographics) on these patients is available due to regulatory design of the study but they are independent, active persons who have not been diagnosed with any systemic medical conditions, including cirrhosis, any malignancy, diabetes, obesity or other potentially confounding diagnoses. Median follow-up for the HCC cohort was 1161 days, or 3.18 years. Seven patients (17.1%) received locoregional therapy prior to sample acquisition, including trans-arterial chemoembolization (TACE, n = 4), Yttrium-90 (Y90, n = 2) or both Y90 and TACE (n = 1). The rate of recurrence for all HCC patients was 36.5% (n = 15).
3.1. Gut Microbe-Derived Metabolites Are Altered in HCC
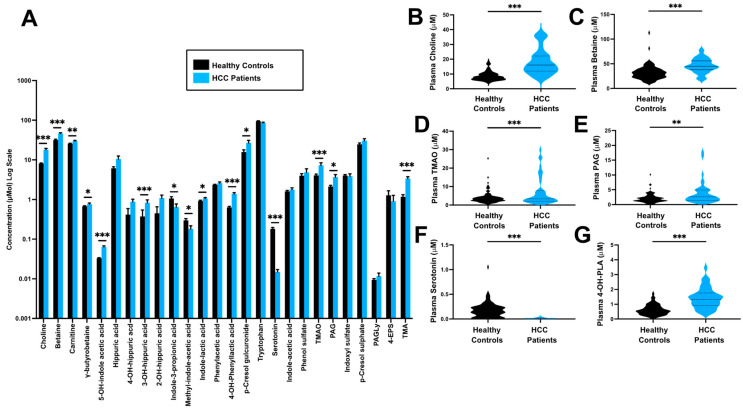
To understand whether the circulating levels of gut microbe-associated metabolites are altered in HCC, we applied a targeted stable isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) method to plasma collected either from healthy controls that had no documented history of any malignancy (n = 96) or patients with HCC (n = 41). When comparing HCC patients to healthy controls, 14/25 (56%) of the metabolites investigated were significantly different (Figure 1 and Table 2). Notably, 10/15 were increased in patients with HCC when compared to healthy controls (Adjusted, p < 0.05). Metabolites that were increased in HCC patients included choline (18.1 vs 8.5 µmol/L, p < 0.001), betaine (46.0 vs 31.7 µmol/L, p < 0.001), L-carnitine (29.5 vs 25.6 µmol/L, p = 0.007), g-butyrobetaine (0.075 vs 0.67 µmol/L, p = 0.029), 5-hydroxyindole acetic acid (0.064 vs 0.033 µmol/L, p < 0.001), methyl-indole-acetic acid (0.18 vs 0.29 µmol/L, p = 0.030), indole-lactic acid (1.06 vs 0.91 µmol/L, p = 0.038), 4-hydroxyphenyllactic acid (4-OH-PLA,1.41 vs 0.63 µmol/L, p < 0.001), P-cresol glucuronide (26.9 vs 15.8 µmol/L, p = 0.032), trimethylamine-N-oxide (TMAO, 7.11 vs 3.99 µmol/L, p < 0.001), phenylacetylglutamine (PAG, 3.66 vs 2.11 µmol/L, p = 0.002) and trimethylamine (TMA, 3.30 vs 1.17 µmol/L, p < 0.001). As shown in Figure 1B–G, several of the metabolites were elevated in HCC patients (choline, betaine, g-butyrobetaine, L-carnitine, TMA and TMAO) and these metabolites are in a common metaorganismal metabolic pathway, whereby gut microbes can initially metabolize dietary substrates such as choline, g-butyrobetaine and L-carnitine into the primary gut microbe-derived metabolite TMA. Conversely, two molecules were reduced in HCC patients compared to controls, including indole-3-propionic acid (0.63 vs 1.03 µmol/L, p = 0.038) and serotonin (0.015 vs 0.180 µmol/L, p < 0.001). The difference in metabolite abundance between HCC and healthy controls is shown in Figure 1 and Table 2.
Figure 1.
Gut microbe-derived metabolites are altered in HCC. Plasma levels of gut microbe-associated metabolites were quantified using stable isotope dilution tandem mass spectrometry (LC-MS/MS) in patients with HCC (n = 41) or healthy controls (n = 96). (A) Relative mean plasma concentrations of all metabolites measured. (B–G) Violin plots for individual metabolites that were most strikingly altered in HCC patients compared to controls. Significant differences between groups were tested using a binary logistic regression model with a Bonferroni adjustment for multiple hypothesis testing. Abbreviations: PAG, phenylacetylglutamine; PAGly phenylacetylglycine; PCS, p-cresol sulfate; TMA, trimethylamine; TMAO, trimethylamine N-oxide; 4-EPS, 4-ethylphenyl sulfate; 4-OH-PLA, 4-hydroxyphenyllactic acid. Stars in this figure indicate the level of statistical significance, * indicates a p-value less than 0.05, ** indicates a p-value less than 0.01, and ***, indicates a p-value less than 0.001.
Table 2.
Select gut microbe-derived metabolites are altered in HCC patients compared to healthy controls. Circulating concentration of gut-derived metabolites in patients with hepatocellular carcinoma split by background liver morphology. Between-group means compared using binary logistic regression model with the Tukey-b adjustment for multiple testing.
| HCC (n = 41) | Healthy Control (n = 96) | p-Value | |
|---|---|---|---|
| Choline | 18.10 (8.54) | 8.49 (2.75) | <0.001 |
| Betaine | 46.0 (14.5) | 31.70 (14.1) | <0.001 |
| Carnitine | 29.5 (9.2) | 25.60 (6.2) | 0.007 |
| g-butyrobetaine | 0.75 (0.33) | 0.67 (0.22) | 0.029 |
| 5-OH-indole acetic acid | 0.064 (0.025) | 0.033 (0.010) | <0.001 |
| Hippuric acid | 9.61 (12.2) | 6.27 (5.65) | 0.124 |
| 4-OH-hippuric acid | 0.89 (0.037) | 0.40 (1.68) | 0.106 |
| 3-OH-hippuric acid | 0.84 (0.98) | 0.36 (1.48) | <0.001 |
| 2-OH-hippuric acid | 1.09 (1.32) | 0.43 (1.91) | 0.108 |
| Indole-3-propionic acid | 0.63 (0.83) | 1.03 (1.08) | 0.028 |
| Methyl-indole-acetic acid | 0.18 (0.23) | 0.29 (0.30) | 0.030 |
| Indole-lactic acid | 1.06 (0.36) | 0.91 (0.42) | 0.038 |
| Phenylacetic acid | 2.59 (1.19) | 2.36 (0.68) | 0.105 |
| 4-OH-Phenyllactic acid | 1.41 (0.70) | 0.625 (0.36) | <0.001 |
| p-Cresol glucuronide | 26.9 (28.1) | 15.8 (20.3) | 0.032 |
| Tryptophan | 86.7 (22.3) | 94.9 (20.7) | 0.056 |
| Serotonin | 0.015 (0.014) | 0.18 (0.15) | <0.001 |
| Indole-acetic acid | 1.79 (1.22) | 1.58 (0.81) | 0.177 |
| Phenol Sulfate | 4.69 (7.09) | 3.94 (4.66) | 0.508 |
| Trimethylamine N-oxide (TMAO) | 7.11 (7.72) | 3.99 (3.26) | <0.001 |
| Phenylacetylglutamine (PAG) | 3.66 (3.73) | 2.11 (1.50) | 0.002 |
| Indoxyl sulfate | 3.80 (3.65) | 4.03 (2.20) | 0.748 |
| p-Cresol sulfate | 30.7 (23.9) | 24.70 (19.0) | 0.224 |
| Phenylacetylglycine (PAGLY) | 0.011 (0.014) | 0.0096 (0.0064) | 0.281 |
| 4-ethylphenyl sulfate (4-EPS) | 0.88 (2.33) | 1.25 (3.78) | 0.487 |
| Trimethylamine (TMA) | 3.30 (2.42) | 1.17 (1.38) | <0.001 |
3.2. Impact of Background Liver Morphology on Circulating Gut Microbe-Derived Metabolites
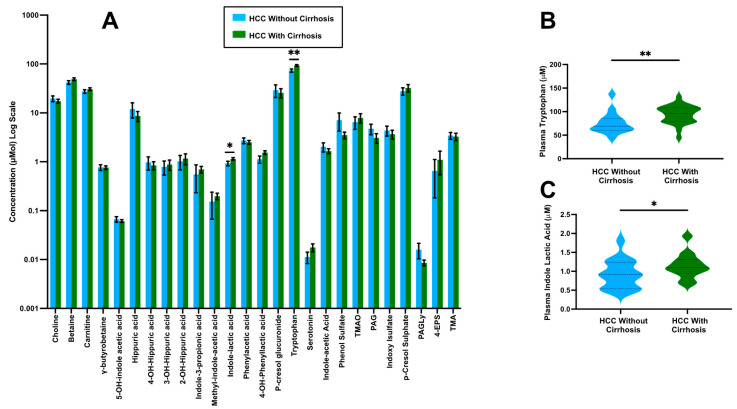
We next investigated the hypothesis that alterations in gut-derived metabolites were arising from HCC-associated changes in liver function due to background cirrhosis. Here, the concentration of gut microbe-derived metabolites was compared within the HCC cohort based upon the presence of cirrhosis versus healthy background liver (Figure 2 and Table 3). We observed significant differences in the mean concentrations of only two metabolites—tryptophan (93.9 vs. 75.5 µmol/L, p = 0.009) and indole-lactic acid (1.15 vs 0.92 µmol/L, p = 0.033), which were both found to be elevated in patients with both HCC and cirrhosis compared with patients who have HCC and normal liver parenchyma (Figure 1 and Table 3).These two metabolites play an important role in immune tolerance and response to anticancer drugs; thus, these data indicate that background cirrhosis does not have a strong impact on the circulating levels of the gut microbe-derived metabolites investigated.
Figure 2.
Gut microbe-derived metabolites in HCC patients with or without underlying cirrhosis. Plasma levels of gut microbe-associated metabolites were quantified using stable isotope dilution tandem mass spectrometry (LC-MS/MS) in patients with HCC with underlying cirrhosis (n = 25) and HCC patients without cirrhosis (n = 16). (A) Relative mean plasma concentrations of all metabolites measured. (B,C) Violin plots for individual metabolites that were significantly altered in HCC patients with or without underlying cirrhosis. Significant differences between groups were tested using a binary logistic regression model with a Bonferroni adjustment for multiple hypothesis testing. Abbreviations: PAG, phenylacetylglutamine; PAGly, phenylacetylglycine; TMAO, trimethylamine N-oxide; 4-EPS, 4-ethylphenyl sulfate. Stars in this figure indicate the level of statistical significance, * indicates a p-value less than 0.05, ** indicates a p-value less than 0.01.
Table 3.
Cirrhosis has minimal impact on circulating levels of gut microbe-associated metabolites in HCC. Circulating concentration of gut-derived metabolites in patients with hepatocellular carcinoma split by background liver morphology of HCC alone versus HCC in the background of underlying cirrhosis.
| Cirrhosis + HCC (n = 25) | HCC Alone (n = 16) | p-Value | |
|---|---|---|---|
| Choline | 17.3 (7.67) | 19.3 (9.90) | 0.321 |
| Betaine | 48.7 (14.7) | 41.9 (13.7) | 0.078 |
| Carnitine | 30.6 (9.54) | 27.6 (8.53) | 0.300 |
| g-butyrobetaine | 0.75 (0.30) | 0.75 (0.39) | 0.776 |
| 5-OH-indole acetic acid | 0.061 (0.019) | 0.067 (0.032) | 0.618 |
| Hippuric acid | 8.46 (9.70) | 11.4 (15.5) | 0.162 |
| 4-OH-hippuric acid | 0.84 (0.76) | 0.96 (1.09) | 0.744 |
| 3-OH-hippuric acid | 0.88 (1.00) | 0.74 (0.92) | 0.539 |
| 2-OH-hippuric acid | 0.866 (1.00) | 0.78 (0.94) | 0.804 |
| Indole-3-propionic acid | 0.699 (0.51) | 0.15 (0.32) | 0.645 |
| Methyl-indole-acetic acid | 0.32 (0.14) | 1.00 (1.24) | 0.620 |
| Indole-lactic acid | 1.15 (0.33) | 0.92 (0.38) | 0.033 |
| Phenylacetic acid | 2.50 (1.07) | 2.73 (1.37) | 0.546 |
| 4-OH-Phenyllactic acid | 1.54 (0.62) | 1.21 (0.79) | 0.079 |
| p-Cresol glucuronide | 28.6 (26.5) | 28.5 (31.2) | 0.507 |
| Tryptophan | 93.9 (19.4) | 75.5 (22.4) | 0.009 |
| Serotonin | 0.17 (0.16) | 0.011 (0.011) | 0.131 |
| Indole-acetic acid | 1.67 (0.90) | 2.00 (1.61) | 0.414 |
| Phenol Sulfate | 3.40 (2.61) | 6.71 (10.78) | 0.141 |
| Trimethylamine N-oxide (TMAO) | 7.7 (8.27) | 6.17 (6.93) | 0.412 |
| Phenylacetylglutamine (PAG) | 3.06 (3.31) | 4.61 (6.93) | 0.183 |
| Indoxyl sulfate | 3.56 (3.63) | 4.61 (4.26) | 0.652 |
| p-Cresol sulfate | 32.4 (27.2) | 28.1 (17.8) | 0.787 |
| Phenylacetylglycine (PAGLY) | 0.0085 (0.005) | 0.15 (0.21) | 0.136 |
| 4-ethylphenyl sulfate (4-EPS) | 0.97 (2.45) | 2.29 (6.03) | 0.372 |
| Trimethylamine (TMA) | 1.05 (2.66) | 0.611 (1.74) | 0.632 |
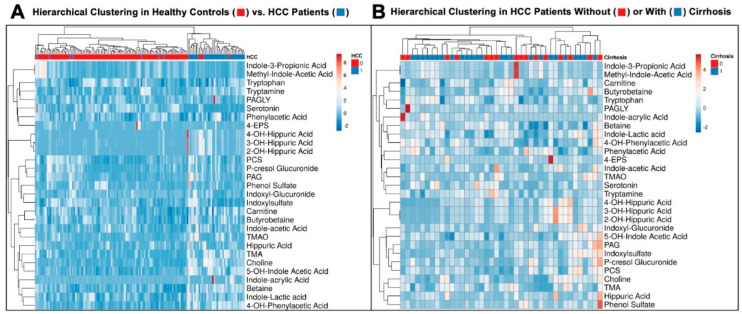
3.3. Hierarchical Clustering Clearly Distinguishes Healthy Controls from HCC Patients
Next, hierarchical clustering was performed to assess whether the overall metabolite profile was different between those with HCC and healthy controls, and a clear distinction was observed (p < 0.001—Figure 3A). An additional analysis was performed to determine whether patients with HCC and cirrhosis clustered in a manner that distinguished them from those with HCC without cirrhosis, but no significant differences were observed (Figure 3B). These results indicate that levels of the metabolites concentrations measured here were not dramatically impacted by underlying cirrhosis.
Figure 3.
Hierarchical clustering clearly distinguishes healthy controls from HCC patients. Plasma levels of gut microbe-associated metabolites were quantified using stable isotope dilution tandem mass spectrometry (LC-MS/MS) in patients with HCC and healthy controls. Shown here are Heatmaps demonstrating differential metabolite abundance. (A) Gut microbiota-derived metabolites can distinctly classify patients without HCC (healthy controls, red) from patients with HCC (blue). (B) Gut microbiota -derived metabolites are not differentially expressed in patients with HCC based on the quality of their underlying liver parenchyma.
4. Discussion
The gut–liver access is increasingly implicated in the regulation of chronic liver disease, yet its relevance to primary liver cancers is only beginning to be explored. This work demonstrates that circulating levels of several gut microbe-derived metabolites (TMA, TMAO, indole-3-propionic acid, 5-hydroxyindole acetic acid, methyl indole acetic acid, indole lactic acid, 4-hydroxyphenyllactic acid, phenylacetylglutamine) are altered in HCC patients compared to healthy controls. Although it is well known that reorganization of the gut microbiome assessed by fecal 16S rRNA or shotgun metagenomic sequencing is associated with diverse liver diseases such as MASLD, MASH, ALD and HCC [23,24,25,26,27,28,29,30,31], there is very little information on gut microbe-associated metabolomic signatures of these same liver diseases. Here we show that quantifying circulating metabolites originating from gut microbe-driven metabolism can distinguish patients with HCC from healthy controls. However, using this approach, this same set of metabolites was not able to separate patients with HCC by the presence of underlying cirrhosis. This study provides important proof of concept that gut microbe-focused metabolomic approaches may hold promise for improved detection of HCC and could provide clues into mechanistic links between metaorganismal nutrient metabolism and the development of HCC.
The liver represents the primary site of metabolism for almost all nutrients and xenobiotics we ingest. Gut microbe-derived metabolites are produced initially in the gut, absorbed into portal circulation and then delivered to the liver for downstream metabolism by the host [17,18]. As such, altered concentrations of gut microbe-derived metabolites in the peripheral circulation are a result of combinations of compounds that originate from both microbe- and host-driven metabolic processes. It is reasonable to assume that combined reorganization of the gut microbiome community coupled together with dysfunctional hepatic metabolism may together be reflected in the plasma concentrations of metabolites linked to microbial metabolism. This study represents one of the first studies to perform a targeted metabolomic investigation into metabolites that originate from the gut microbial endocrine organ [17,18]. Using this approach, we show that alterations in gut microbial metabolism of choline/L-carnitine, tyrosine, phenylalanine and tryptophan are associated with HCC. These findings warrant further investigation into whether altered gut microbial metabolism is simple associated with, or instead can be causally linked to HCC. Although there are very few examples of gut microbe-derived metabolites that have been causally linked to human disease, one powerful example can be highlighted by the metaorganismal TMAO pathway found to be altered in HCC patients here.
The TMAO pathway is initiated when nutrient precursors commonly found in high-fat foods (phosphatidylcholine, choline, L-carnitine and g-butyrobetaine) are metabolized by substrate-selective bacterial enzyme systems including cutC/D (choline-specific), yeaW/X, bbu and gbuA-E (g-butyrobetaine specific), cntA/B (L-carnitine specific) and likely others [32,33,34,35,36]. Once TMA is generated by bacteria, it can be rapidly transported via the portal vein to the liver where it can be subsequently metabolized by the host liver enzyme monooxygenases yielding TMAO [37]. The TMAO pathway has been shown to alter cholesterol and bile acid metabolism and alter cholesterol transporters in the liver and intestine that have been previously linked to liver cancer [38,39,40]. TMAO-mediated processes have also been specifically shown to regulate liver–microbiome crosstalk and thus promote or reduce liver fibrosis in MASH [41]. Most interestingly, a 2018 study by Liu et al. demonstrated a higher concentration of TMAO in patients with primary liver cancers, findings which are further supported by this work [42]. Although our studies here have focused on HCC associations, TMAO and related metabolites have also been studied in other liver disease etiologies. In agreement with our finding here, several studies have shown that plasma TMAO levels are elevated in subjects with metabolic dysfunction-associated fatty liver disease (formerly called non-alcoholic fatty liver disease) [42,43,44,45]. In contrast, we recently reported that in subjects with alcoholic hepatitis, plasma TMAO is reduced with reciprocal elevations in TMA [46]. This reciprocal alteration in TMA and TMAO in alcohol-associated hepatitis is due at least in part to reduced expression of the host hepatic enzyme flavin-containing monooxygenase 3 (FMO3) that is responsible for converting TMA to TMAO [46]. Collectively, there is strong emerging evidence that the metaorganismal TMAO pathway is altered in liver disease, but the mechanisms underlying this as well as therapeutic potential for TMAO-lowering drugs require further investigation.
Gut microbe-associated metabolites that were at higher concentrations in the HCC cohort compared to healthy controls were not differentially abundant within the HCC cohort stratified by the presence of underlying cirrhosis. Metabolites in the TMAO pathways have been linked to inflammatory or fibrotic regulation in cirrhotic pathways [36,37,38]. However, nearly identical levels of TMAO pathway metabolites were found in the cirrhotic and non-cirrhotic HCC patients here. Importantly, the metabolic signatures classified patients by presence of malignancy despite the heterogenous presence of cirrhosis in this population, which may point to a diagnostic use for this difference, especially in the high-risk cirrhotic cohort. Further, given the findings of Zhou et al. that direct TMAO infusion in the portal system can reduce liver fibrosis [41], mechanistic studies examining the impact of TMAO pathway metabolites on HCC outcomes may be of interest in ascertaining the utility of this axis moving forward.
In addition to clear alterations in the TMAO pathway, this study also found elevated levels of other interesting metabolites that originate from gut microbes in the HCC group. This includes p-cresol glucuronide, a conjugated form of p-cresol, a molecule with known uremic toxicity that results from bacterial metabolism of tyrosine [47,48]. P-cresol glucuronide also plays an anti-inflammatory role mediated by TLR4 [49], a key signaling pathway known to modulate immune response in both metabolic and alcoholic liver disease [50,51,52]. However, while p-cresol glucuronide was elevated in the HCC cohort, no differences were found within the HCC cohort by presence of cirrhosis, perhaps implicating the TLR4 pathway in the carcinogenic process. Interestingly, however, p-cresol glucuronide’s close relative, p-cresol sulfate was not elevated in HCC patients. The preferentially increased level of the glucuronidated derivative may warrant further investigation, as one might expect p-cresol sulfate to covary with this closely related pathway.
As with any study, there were limitations that warrant consideration. Most notably this study investigated a single time point of collection of the plasma. All patients had active malignancy at the time of collection, but the longitudinal impact of various treatments, including curative-intent surgical resection, cannot be ascertained. Since we did not also have samples post-resection, the concentration change after a theoretically curative surgery is likely to change, but we cannot make definitive comment. The healthy control population is known to be collected from patients without any cancer diagnosis, but additional information is not available due to nature of the samples and regulatory restrictions. Another clear limitation here is the relatively small sample size of this cohort. Validation of our findings in larger cohorts with sufficient numbers of underlying metabolic or viral associated tumors is an obvious next step. Another important limitation of this study is that the HCC patients were compared to healthy controls that did not have underlying liver disease. Follow up studies will include important liver disease controls without HCC including those with MASH, alcoholic hepatitis, as well as viral hepatitis with or without cirrhosis. Sequencing of stool was not available for these patients, which means that we could not correlate the circulating concentrations with differences in the actual bacterial colonization. However, this limitation is not particularly concerning given the fact that 16S and shotgun metagenomic sequencing very poorly predict circulating levels of gut microbe-derived metabolites [53,54]. Even with these noted limitations, we expect these results to inform downstream gut microbiome-focused metabolomic investigation in HCC and other cancers.
5. Conclusions
Gut microbiota-derived metabolites are differentially abundant in the peripheral plasma of patients with HCC compared to healthy controls, including striking changes in metabolites of the TMAO pathway. This difference was not observed between those with HCC with and without cirrhosis. Overall, these results may present an opportunity for improved diagnostic utility and could inform future interventional studies. The results from this study may also inform downstream investigation into how gut microbial metabolism can impact HCC initiation, progression and/or response to therapy. There is a growing appreciation that alterations in the gut microbiome may underlie liver disease progression towards HCC, and our study provides the first glimpse into the gut microbiota-derived metabolomic signatures of HCC. Although the current study only allows us to associate gut microbial metabolites with HCC risk, these finding could provide new clues into new therapeutic targets in HCC. For example, there are several emerging examples of small molecule inhibitors targeting gut microbial enzymes to lower the level of disease-associated metabolites [55,56]. In fact, we have recently developed mechanism-based small molecule inhibitors for the bacterial choline trimethylamine lyase cutC/D [57], and have shown that these bacterially targeted drugs can very effectively protect against common diseases associated with non-viral HCC including obesity [58], alcohol-associated [46] and metabolic dysfunction-associated fatty liver disease (MAFLD) in mice [58]. As a logical next step, it will be important to test the efficacy of these bacterially targeted TMAO-lowering drugs in preclinical animal models of HCC. Additional work is now required to determine whether the HCC-associated metabolites identified here are simply biomarkers of disease or potentially causally related.
Author Contributions
Conceptualization, O.R., J.S.Y., J.D.L., F.A. and J.M.B.; Methodology, R.B., Z.W., J.D.W., V.U., V.V., M.M., C.H., O.R., J.S.Y., J.D.L., D.M.R., S.L.H., W.H.W.T., F.A. and J.M.B.; Software, R.B., C.J.W., Z.W., V.U., V.V., M.M., C.H., D.M.R. and J.M.B.; Validation, R.B., C.J.W., Z.W., V.U., V.V., M.M. and J.M.B.; Formal analysis, R.B., C.J.W., Z.W., V.U., V.V., M.M., D.M.R. and J.M.B.; Investigation, R.B., C.J.W., Z.W., O.R., J.S.Y., J.D.L., S.L.H. and J.M.B.; Resources, R.B., C.J.W., Z.W., J.D.W., D.M.R., S.L.H., W.H.W.T. and J.M.B.; Data curation, R.B., C.J.W., Z.W., J.D.W., O.R., J.S.Y., J.D.L., D.M.R., W.H.W.T. and J.M.B.; Writing—original draft preparation, R.B., C.J.W. and J.M.B.; Writing—review and editing, R.B., J.D.W., V.U. and J.M.B.; Visualization, R.B., C.J.W. and J.M.B.; Supervision, F.A. and J.M.B.; Project administration, F.A. and J.M.B.; Funding acquisition, Z.W., S.L.H. and J.M.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The studies involving human participants were reviewed and approved by the Ethical Committee at the Cleveland Clinic IRB# 10-727 for healthy Controls and 10-347 for HCC samples.
Informed Consent Statement
Written informed consent was obtained from each subject participating in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
D.M.R. has received research support from Novo Nordisk, has an equity stake in Clarified Precision Medicine, and has intellectual property related to the clinical management of diabetes and liver cancer. Z.W. and S.L.H. report being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. S.L.H. also reports being a paid consultant for Zehna Therapeutics. S.L.H reports having received research funds from Procter & Gamble, Zehna Therapeutics and Roche Diagnostics. Z.W. and S.L.H. report being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, and Procter & Gamble, and S.L.H. from Zehna Therapeutics. All other authors declare no conflict of interest related to this work.
Funding Statement
This work was supported in part by National Institutes of Health grants 5R01 DK130227-04 (J.M.B.), RF1 NS133812 (J.M.B.), P50 AA024333 (J.M.B.), R01 HL144651 (Z.W.), and P01 HL147823 (J.M.B., Z.W., and S.L.H.), and a Cleveland Clinic Center of Excellence (O.R., J.S.Y., J.D.L., and J.M.B.). Mass spectrometry studies were performed on instrumentation housed in a facility supported in part by a Shimadzu Center of Excellence award.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marquardt J.U., Thorgeirsson S.S. SnapShot: Hepatocellular carcinoma. Cancer Cell. 2014;25:550.e1. doi: 10.1016/j.ccr.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 3.Singal A.G., Lampertico P., Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020;72:250–261. doi: 10.1016/j.jhep.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73((Suppl. 1)):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J., Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temraz S., Nassar F., Kreidieh F., Mukherji D., Shamseddine A., Nasr R. Hepatocellular carcinoma immunotherapy and the potential influence of gut microbiome. Int. J. Mol. Sci. 2021;22:7800. doi: 10.3390/ijms22157800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwabe R.F., Greten T.F. Gut microbiome in HCC—Mechanisms, diagnosis and therapy. J. Hepatol. 2020;72:230–238. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Daniel N., Genua F., Jenab M., Mayén A.L., Chrysovalantou C.A., Keski-Rankonen P., Hughes D.J. The role of the gut microbiome in the development of hepatobiliary cancers. Hepatology. 2023 doi: 10.1097/HEP.0000000000000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J., He Q., Lu F., Chen K., Ni Z., Wang H., Zhou C., Zhang Y., Chen B., Bo Z., et al. A distinct microbiota signature precedes the clinical diagnosis of hepatocellular carcinoma. Gut Microbes. 2023;15:2201159. doi: 10.1080/19490976.2023.2201159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Z., Li A., Jiang J., Zhou L., Yu Z., Lu H., Xie H., Chen X., Shao L., Zhang R., et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014–1023. doi: 10.1136/gutjnl-2017-315084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J., Yang X., Zhang B. Understanding gut dysbiosis for hepatocellular carcinoma and treatment. Trends Endocrinol. Metab. 2024;24:00163-2. doi: 10.1016/j.tem.2024.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Ha S., Wong V.W., Zhang X., Yu J. Interplay between gut microbiome, host genetics and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 2024 doi: 10.1136/gutjnl-2024-332398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elvevi A., Laffusa A., Gallo C., Invernizzi P., Massironi S. Any role for microbiota in cholangiocarcinoma? A comprehensive review. Cells. 2023;12:370. doi: 10.3390/cells12030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederer A.K., Rasel H., Kohnert E., Kreutz C., Huber R., Badr M.T., Dellweg P.K.E., Bartsch F., Lang H. Gut microbiota in diagnosis, therapy and prognosis of cholangiocarcinoma and gallbladder carcinoma—A scoping review. Microorganisms. 2023;11:2363. doi: 10.3390/microorganisms11092363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershberger C.E., Hershberger C.E., Rodarte A.I., Siddiqi S., Moro A., Acevedo-Moreno L.A., Brown J.M., Allende D.S., Aucejo F., Rotroff D.M. Salivary Metabolites are Promising Non-Invasive Biomarkers of Hepatocellular Carcinoma and Chronic Liver Disease. Liver Cancer Int. 2021;2:33–44. doi: 10.1002/lci2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acharya C., Sahingur S.E., Bajaj J.S. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017;2:e94416. doi: 10.1172/jci.insight.94416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown J.M., Hazen S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic disease. Annu. Rev. Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donia M.S., Fischbach M.A. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemet I., Li X.S., Haghikia A., Li L., Wilcox J., Romano K.A., Buffa J.A., Witkowski M., Demuth I., König M., et al. Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality. Eur. Heart J. 2023;44:3085–3096. doi: 10.1093/eurheartj/ehad333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z., Bergeron N., Levison B.S., Xi X.S., Chiu S., Jia X., Koeth R.A., Li L., Wu Y., Tang W.H.W., et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019;40:583–594. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haynes W. Bonferroni Correction. In: Dubitzky W., Wolkenhauer O., Cho K.-H., Yokota H., editors. Encyclopedia of Systems Biology. Springer; New York, NY, USA: 2013. p. 154. [Google Scholar]
- 22.Metsalu T., Vilo J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpton S.R., Schnabl B., Knight R., Loomba R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab. 2021;33:21–32. doi: 10.1016/j.cmet.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripathi A., Debelius J., Brenner D.A., Karin M., Loomba R., Schnabl B., Knight R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilg H., Adolph T.E., Dudek M., Knolle P. Non-alcoholic fatty liver disease: The interplay between metabolism, microbes and immunity. Nat. Metab. 2021;3:1596–1607. doi: 10.1038/s42255-021-00501-9. [DOI] [PubMed] [Google Scholar]
- 26.Liu T., Guo Y., Liao Y., Liu J. Mechanism-guided fine-tuned microbiome potentiates anti-tumor immunity in HCC. Front. Immunol. 2023;14:1333864. doi: 10.3389/fimmu.2023.1333864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gok Yavuz B., Datar S., Chamseddine S., Mohamed Y.I., LaPelusa M., Lee S.S., Hu Z.I., Koay E.J., Tran Cao H.S., Jalal P.K., et al. The gut microbiome as a biomarker and therapeutic target in hepatocellular carcinoma. Cancers. 2023;15:4875. doi: 10.3390/cancers15194875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranjbarian T., Schnabl B. Gut microbiome-centered therapies for alcohol-associated liver disease. Semin. Liver Dis. 2023;43:311–322. doi: 10.1055/a-2145-7331. [DOI] [PubMed] [Google Scholar]
- 29.Ladd A.D., Duarte S., Sahin I., Zarrinpar A. Mechanisms of drug resistance in HCC. Hepatology. 2023;79:926–940. doi: 10.1097/HEP.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 30.Greten T.F., Villanueva A., Korangy F., Ruf B., Yarchoan M., Ma L., Ruppin E., Wang X.W. Biomarkers for immunotherapy of hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2023;20:780–798. doi: 10.1038/s41571-023-00816-4. [DOI] [PubMed] [Google Scholar]
- 31.Muscolino P., Granata B., Omero F., De Pasquale C., Campana S., Calabrò A., D’Anna F., Drommi F., Pezzino G., Cavaliere R., et al. Potential predictive role of gut microbiota to immunotherapy in HCC patients: A brief review. Front. Oncol. 2023;13:1247614. doi: 10.3389/fonc.2023.1247614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craciun S., Marks J.A., Balskus E.P. Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem. Biol. 2014;9:1408–1413. doi: 10.1021/cb500113p. [DOI] [PubMed] [Google Scholar]
- 33.Rajakovich L.J., Fu B., Bollenbach M., Balskus E.P. Elucidation of an anaerobic pathway for metabolism of L-carnitine-derived g-butyrobetaine to trimethylamine in human gut bacteria. Proc. Natl. Acad. Sci. USA. 2021;118:e2101498118. doi: 10.1073/pnas.2101498118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koeth R.A., Levison B.S., Culley M.K., Buffa J.A., Wang Z., Gregory J.C., Org E., Wu Y., Li L., Smith J.D., et al. γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buffa J.A., Romano K.A., Copeland M.F., Cody D.B., Zhu W., Galvez R., Fu X., Ward K., Ferrell M., Dai H.J., et al. The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota L-carnitine catabolism. Nat. Microbiol. 2022;7:73–86. doi: 10.1038/s41564-021-01010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y., Jameson E., Crosatti M., Schäfer H., Rajakumar K., Bugg T.D., Chen Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. USA. 2014;111:4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossner R., Kaeberlein M., Leiser S.F. Flavin-containing monooxygenases in aging and disease: Emerging roles for ancient enzymes. J. Biol. Chem. 2017;292:11138–11146. doi: 10.1074/jbc.R117.779678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warrier M., Shih D.M., Burrows A.C., Ferguson D., Gromovsky A.D., Brown A.L., Marshall S., McDaniel A., Schugar R.C., Wang Z., et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10:326–338. doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miao J., Ling A.V., Manthena P.V., Gearing M.E., Graham M.J., Crooke R.M., Croce K.J., Esquejo R.M., Clish C.B., Morbid Obesity Study Group et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Comm. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou D., Zhang J., Xiao C., Mo C., Ding B.S. Trimethylamine-N-oxide (TMAO) mediates the crosstalk between the gut microbiota and hepatic vascular niche to alleviate liver fibrosis in nonalcoholic steatohepatitis. Front. Immunol. 2022;13:964477. doi: 10.3389/fimmu.2022.964477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z.-Y., Tan X.Y., Li Q.J., Liao G.C., Fang A.P., Zhang D.M., Chen P.Y., Wang X.Y., Luo Y., Long J.A., et al. Trimethylamine N-oxide, a gut microbiota-dependent metabolite of choline, is positively associated with the risk of primary liver cancer: A case-control study. Nutr. Metab. 2018;15:81. doi: 10.1186/s12986-018-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flores-Guerrero J.L., Post A., van Dijk P.R., Connelly M.A., Garcia E., Navis G., Bakker S.J.L., Dullaart R.P.F. Circulating trimethylamine-N-oxide is associated with all-cause mortality in subjects with nonalcoholic fatty liver disease. Liver Int. 2021;41:2371–2382. doi: 10.1111/liv.14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan X., Liu Y., Long J., Chen S., Liao G., Wu S., Li C., Wang L., Ling W., Zhu H. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2019;63:e1900257. doi: 10.1002/mnfr.201900257. [DOI] [PubMed] [Google Scholar]
- 45.Theofilis P., Vordoni A., Kalaitzidis R.G. Trimethylamine N-oxide levels in non-alcoholic fatty liver disease. Metabolites. 2022;12:1243. doi: 10.3390/metabo12121243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helsley R.N., Miyata T., Kadam A., Varadharajan V., Sangwan N., Huang E.C., Banerjee R., Brown A.L., Fung K.K., Massey W.J., et al. Gut microbial trimethylamine is elevated in alcohol-associated hepatitis and contributes to ethanol-induced liver injury in mice. eLife. 2022;11:e76554. doi: 10.7554/eLife.76554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blachier F., Andriamihaja M. Effects of the L-tyrosine-derived bacterial metabolite p-cresol on colonic and peripheral cells. Amino Acids. 2022;54:325–338. doi: 10.1007/s00726-021-03064-x. [DOI] [PubMed] [Google Scholar]
- 48.Fu H.Y., Xu J.M., Ai X., Dang F.T., Tan X., Yu H.Y., Feng J., Yang W.X., Ma H.T., Tu R.F., et al. The clostridium metabolite p-cresol sulfate relieves inflammation of primary biliary cholangitis by regulating Kupffer cells. Cells. 2022;11:3782. doi: 10.3390/cells11233782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wypych T.P., Pattaroni C., Perdijk O., Yap C., Trompette A., Anderson D., Creek D.J., Harris N.L., Marsland B.J. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat. Immunol. 2021;22:279–286. doi: 10.1038/s41590-020-00856-3. [DOI] [PubMed] [Google Scholar]
- 50.Khanmohammadi S., Kuchay M.S. Toll-like receptors and metabolic (dysfunction)-associated fatty liver disease. Pharmacol. Res. 2022;185:106507. doi: 10.1016/j.phrs.2022.106507. [DOI] [PubMed] [Google Scholar]
- 51.Roh Y.S., Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 2013;28((Suppl. 1)):38–42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Y.L., Zhu L., Tao Y., Lu W., Cheng H. Role of targeting TLR4 signaling axis in liver-related disease. Pathol. Res. Pract. 2023;244:154410. doi: 10.1016/j.prp.2023.154410. [DOI] [PubMed] [Google Scholar]
- 53.Han S., Treuren W.V., Fischer C.R., Merrill B.D., DeFelice B.C., Sanchez J.M., Higginbottom S.K., Guthrie L., Fall L.A., Dodd D., et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature. 2021;595:415–420. doi: 10.1038/s41586-021-03707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrell M., Bazeley P., Wang Z., Levison B.S., Li X.S., Jia X., Krauss R.M., Knight R., Lusis A.J., Garcia-Garcia J.C., et al. Fecal microbiome composition does not accurately predict diet-induced TMAO production in healthy adults. J. Am. Heart Assoc. 2021;10:e021934. doi: 10.1161/JAHA.121.021934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown J.M., Hazen S.L. Targeting of microbe-derived metabolites to improve human health. J. Biol. Chem. 2017;292:8560–8568. doi: 10.1074/jbc.R116.765388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo A.Y.M., Aguilar Ramos M.A., Narayan R., Richards-Corke K.C., Wang M.L., Sandoval-Espinola W.J., Balskus E.P. Targeting the human gut microbiome with small-molecule inhibitors. Nat. Rev. Chem. 2023;7:319–339. doi: 10.1038/s41570-023-00471-4. [DOI] [PubMed] [Google Scholar]
- 57.Oellgaard J., Winther S.A., Hansen T.S., Rossing P., von Scholten B.J. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017;23:3699–3712. doi: 10.2174/1381612823666170622095324. [DOI] [PubMed] [Google Scholar]
- 58.Li X., Hong J., Wang Y., Pei M., Wang K., Gong Z. Trimethylamine-N-Oxide Pathway: A Potential Target for the Treatment of MAFLD. Front. Mol. Biosci. 2021;8:733507. doi: 10.3389/fmolb.2021.733507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.